Abstract
Using microarray analysis, we found that aging sarcopenia is associated with a sharp increase in the mRNA of the matricellular protein CCN1 (Cyr61/CTGF/Nov). CCN1 mRNA was upregulated 113-fold in muscle of aged vs. young rats. CCN1 protein was increased in aging muscle in both rats (2.8-fold) and mice (3.8-fold). When muscle progenitor cells (MPCs) were treated with recombinant CCN1, cell proliferation was decreased but there was no change in the myogenic marker myoD. However, the CCN1-treated MPCs did express a senescence marker (SA-βgal). Interestingly, we found CCN1 increased p53, p16Ink4A, and pRP (hypophosphorylated retinoblastoma protein) protein levels, all of which can arrest cell growth in MPCs. When MPCs were treated with aged rodent serum CCN1 mRNA increased by sevenfold and protein increased by threefold suggesting the presence of a circulating regulator. Therefore, we looked for a circulating regulator. Wnt-3a, a stimulator of CCN1 expression, was increased in serum from elderly humans (2.6-fold) and aged rodents (2.0-fold) compared with young controls. We transduced C2C12 myoblasts with wnt-3a and found that CCN1 protein was increased in a time- and dose-dependent manner. We conclude that in aging muscle, the circulating factor wnt-3a acts to increase CCN1 expression, prompting muscle senescence by activating cell arrest proteins.
Keywords: Cyr61, p50, p16ink4a, RB, wnt-3a
decreased muscle strength and power (sarcopenia) contribute to the high incidence of accidental falls and resultant injuries observed among the elderly and can compromise quality of life (17). Although aging is a normal physiologic process, identification of contributors to aging muscle loss may lead to new therapeutic strategies for the prevention and treatment of sarcopenia both in pathologic conditions and during healthy aging.
Many studies have examined aging sarcopenia. The cellular processes of sarcopenia are diverse and include the following: changes in the hormone levels (36); inflammation-linked impairment of protein synthesis and increased protein degradation (3); accumulation of reactive oxygen species (22); and mitochondrial dysfunction-impaired muscle respiration and alterations in myogenic regulatory factors that may impair the ability of aged muscle to repair damage (18). In the current study we found that myoblast senescence may affect aging sarcopenia.
Cellular senescence, the process of irreversible growth arrest, is an important mechanism for preventing the proliferation of potential cancer cells (35). It has become apparent that this process entails more than a simple cessation of cell growth. The senescence process includes cellular repair processes (14), produces chronic inflammation (3), and increases protein pathways that promote cell growth arrest. Through the above pathways, cellular senescence can impact tumor suppression (growth arrest, early life), tumor promotion (chronic inflammatory response, late life), aging tissue loss (growth arrest), and tissue repair (resolving fibrotic scar tissue). These apparently conflicting consequences suggest a complex role for senescence in aging sarcopenia.
There are a number of markers for cellular senescence. Senescent cells often express p16Ink4A, also known as multiple tumor suppressor 1, a potent inhibitor of the proliferative kinase Cdk4 and biomarker of aging (9). In some cells, p16Ink4A activates the retinoblastoma protein (RB) tumor suppressor resulting in an irreversible growth arrest that leads to senescence (30). Protein 53 (p53) is a general very potent growth inhibitor that is increased with aging and clearly critical to the process of developing senescence (28, 41). The single most accepted and widely used senescence marker is senescence-associated β-galactosidase (SA-βgal) characteristically assessed at a pH of 6.0 (11).
How cellular senescence occurs in aged muscle has not been widely studied. Recently, Jun and Lau (15) showed that the protein CCN1 (Cyr61/CTGF/Nov-1, also known as Cyr61; cysteine-rich protein 61), which is expressed at sites of wound repair, can induce fibroblast senescence. CCN1 is a member of the CCN (Cyr61/CTGF/Nov) gene family, which currently consists of six genes: CCN1, CCN2 (also named ctgf), NOV, WISP-1, WISP-2, and WISP3 (16). All members of the CCN family share a high degree of sequence homology. CCN1 is a multifunctional matricellular protein that is expressed in multiple cell types including human muscle (19). In nonmuscle cells CCN1 binds to different integrins to elicit differing responses. It promotes cell proliferation, survival, and angiogenesis by binding to integrin αvβ3 (24) and induces apoptosis and senescence by binding to integrin α6β1 and heparan sulfate proteoglycans (15, 40). However, the function of the CCN1 in muscle, and particularly in aging muscle, is largely unknown.
Several studies have demonstrated that CCN1 is a direct target gene of wnt signaling in osteoblasts (38), hepatocellular carcinoma cells (26), and human dermal papilla cells (39). Wnt proteins are secreted cysteine-rich glycosylated proteins that play a role in variety of cellular functions. They influence embryonic cell proliferation, differentiation, survival, and death (25), and they maintain embryonic stem cells in a pluripotent state (29). Wnt-3a (wingless-type MMTV integration site family member 3A), an intracellular signaling molecule found in most cell types, has multiple regulatory influences, among them, activation of the β-catenin-dependent pathway, which is implicated in numerous physiological events such as cell fate determination, apoptosis and morphogenesis (34). Two groups published studies in 2007 showing that an increase in wnt-3a activity is critical to induce aging phenomena. Brack et al. (6) showed that the enhanced wnt signaling in aged muscle and in myogenic progenitors exposed to aged serum is involved with an increase in muscle fibrosis of aging. Liu et al. (27) showed that continuous wnt-3a exposure triggered accelerated cellular senescence in a mouse model of accelerated aging.
Other studies showed that age alone does not limit the intrinsic ability of a muscle to regenerate, since extensor digitorum longus (EDL) muscles from old animals that were autografted into young rats regenerated significantly greater mass (1.8 times) and developed greater maximum contractile force (2.6 times) than EDL muscles autografted in old rats. They concluded that the poor regeneration of muscles in old animals is due to the environment for regeneration provided by the old host (7).
In this study we examine the changes of CCN1 in aging muscle and identify a potential soluble factor in the serum of aging human and rodents that contributes to CCN1-mediated effects in aging sarcopenia.
MATERIALS AND METHODS
Animals.
Fisher 344 rats were purchased from Charles River. C57BL6 mice were purchased from Jackson Laboratories. All experiments involving animals were approved by the Institutional Animal Care and Use Committee of Emory University. “Old mouse serum” (OMS) was a mixture of sera harvested from 24- to 32-mo-old mice. “Young mouse serum” (YMS) was a mixture of sera harvested from 3- to 4-mo-old mice. Hindlimb skeletal muscle was surgically removed from the living rodent under inhaled anesthesia (isoflurane), after which the animal was euthanized by cervical dislocation. Muscle was weighed and immediately freeze clamped under liquid nitrogen for later measurement of RNA and protein content or minced and processed as described below for isolation of muscle progenitor cells (MPCs).
mRNA microarray.
For the mRNA profile analysis, total RNA was extracted from the 3 young (5 mo) and 3 old (24 mo) rats muscle using TRI Reagent (Molecular Research Center; Cincinnati, OH). Following DNase treatment, the integrity of total RNA for each sample was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA); only RNA samples meeting a minimum RNA Integrity Number (RIN) value of 9 or larger (<10% degradation) were analyzed (43). Total RNA (5 μg) was analyzed on Affymetrix GeneChip Rat Genome 230 2.0, representing 30,000 transcripts and variants from over 28,000 well-substantiated rat genes. Target sample preparation, hybridization, posthybridization processing, and image scanning were performed in the Microarray Center of Emory University.
Quantitative real-time polymerase chain reaction.
Total RNA was extracted using TRI Reagent (Molecular Research Center) as described previously (42). The reverse transcription was performed with 2 μg of RNA samples using Thermoscript RT-PCR kit (Invitrogen, Carlsbad, CA). Real-time quantitative (q)PCR was performed with SYBR Green PCR Reagents (Bio-Rad, Hercules, CA) using the following cycle parameters: 94°C for 2 min and 40 cycles at 94°C for 15 s, 55°C for 30 s, and 72°C for 30 s with final extension at 72°C for 10 min. The quantification cycle (Cq) values was defined as the number of cycles required for the fluorescence signal to exceed the detection threshold. Individual mRNA expression was standardized to 18S gene, and expression was calculated as the difference between the threshold values of the two genes (2-ΔCq). Melting curve analysis was routinely performed to verify the specificity of the reaction. The following primers were used to determine mRNA expression: CCN1, forward 5′-gaa aga gac ccg gat ctg tg-3′ and reverse 5′-act gga gca tcc tgc ata ag-3′ (amplicon 133 nt); and CCN2, forward 5′-cct gtg aag ctg acc tag agt gf-3′ and reverse 5′-act ggt gca gcc aga aag-3′ (amplicon 104 nt).
MPC isolation and cell culture.
MPC were isolated from hindlimb muscles of 4-mo-old mice as described previously (45). Briefly, muscles were minced into a coarse slurry and gently agitated for 1 h at 37°C in DMEM with 25 mM HEPES (pH 7.4) plus 0.1% pronase (Calbiochem, San Diego, CA). The digest was passed through a 100-μm filter and then centrifuged through a percoll gradient (70% overlaid with 40%; Ref. 45). Cells from the gradient interface were collected and cultured on collagen-coated dishes. To get more purified MPC, new isolated MPC were preplated to uncoated dishes and incubated at 5% CO2, 37°C for 30 min; the supernatant was then transferred to a new collagen-coated dish. This procedure was also done at each initial passage. The culture medium for MPCs is Ham's F-10 Nutrient Mixture medium (Invitrogen) with 20% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Maintenance media for MPCs contained 5 ng/ml human β-fibroblast growth factor (Atlanta Biologicals, Atlanta, GA), but it was removed before the experiments. MPCs were identified using anti-myoD antibodies for positive control [Developmental Studies Hybridoma Bank (DSHB), University of Iowa] and α-smooth muscle actin (Sigma) for negative control. Cells collected were >95% MyoD positive and <5% α-smooth muscle actin positive (data not shown). C2C12 cells (ATCC, Manassas, VA), studied between passages 3 and 9, were cultured in growth medium (DMEM with 10% fetal bovine serum, 10% cow serum, 25 mM glucose, 100 u/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine). To treat cells with CCN1 (recombinant fragment, corresponding to amino acids 24/381 of human CCN1; Abcam, Cambridge, MA), 4 × 105 cells were cultured in six-well dishes with indicated medium. At 60–70% confluence, cells were treated with chemicals as indicated in individual experiments.
Cell proliferation and senescence assay.
MPCs (1 × 104/well in a 96-well microplate) were cultured in normal growth media. A cell proliferation assay kit (Millipore, Burlington, MA) was used according to the manufacturer's instructions. After 24 h, 10 μl WST-1/ECS chromogenic solution were added to each well and incubated at 37°C for an additional 4 h. The absorbance of the samples at 420 nm was determined using a TECAN microtiter plate reader with Magellan software (Morrisville, NC). Cellular senescence assay kits were purchased from Cell Biolabs (San Diego, CA) and used according to the manufacturer's instructions. This senescence kit uses acidic SA-βgal that catalyzes the hydrolysis of X-gal, producing a blue color when cells are senescent. To estimate the effect of the environment on the cell proliferation, the total cell count was determined using a hemocytometer and compared between cells exposure to YMS or OMS medium.
Cell differentiation and creatine kinase activity assay.
For measurement of MPC differentiation capacity, 4 × 105 MPCs were cultured in a Ham's F-10 medium that contains 20% FBS, 100 U/ml penicillin G, and 100 g/ml streptomycin (primary growth medium) in six-well plates that had been coated with entactin, collagen IV, and laminin (ECL matrix, Upstate Biotechnology, Temecula, CA). Differentiation was induced by switching primary growth media to the differentiation media in which FBS was replaced with either 2% house serum, 2% YMS, or 2% OMS. Cell morphology was monitored daily. The fusion index was calculated as the ratio of the number of nuclei within myotubes to the total number of nuclei in predefined fields of >450 nuclei (43). For creatine kinase activity, MPCs were lysed in Tris-MES buffer (50 mM Tris-MES and 1% Triton X-100 pH 7.7). Creatine kinase activity assay kit was purchased from Catachem (Oxford, CT) and used according to manufacturer's instructions. Enzymatic activity for creatine kinase was normalized to total protein content.
Immunoblotting, immunoprecipitation, and antibodies.
To identify protein in skeletal muscle, gastrocnemius muscles were homogenized in Gentle Lysis Buffer (10 mM Tris·HCl, 10 mM NaCl, 2 mM EDTA, 0.5% NP-40, 1% glycerol, and Na3VO4 and freshly added 0.18 μg/ml Na3VO4, 10 μg/ml PMSF, 5 μg/ml aprotinin, and 1 μg/ml leupeptin). In preparation for immunoblotting, proteins content in the soluble fraction of tissue homogenates was measured using a RC-PC protein assay kit (Bio-Rad). Equal amounts of protein were loaded on the acrylamide/bis SDS-PAGE gel. Protein was transferred to a PVDF membrane and blotted with a specific primary antibody. Alexa Fluor 680 anti-IgG (Invitrogen) was used for secondary antibody. Protein bands were scanned and quantified using the Li-cor Odyssey infrared scanning system (Li-COR Biosciences, Lincoln, NE). Primary antibodies were as follows: CCN1 (R&D System, Minneapolis, MN); CCN2, p53, and p16Ink4A (Santa Cruz Biotechnology, Santa Cruz, CA); myoD (DSHB product, Iowa, IA); RB (BD Pharmingen, San Jose, CA); and Ki-67 and GAPDH (Millipore, Burlington, MA). Wnt-3a (C64F2) rabbit monoclonal antibody (Cell Signaling, Danvers, MA) was used for immunoblotting and immunoprecipitation to identify wnt-3a levels in human and rodent serum. Serum (500 μl) was mixed with 0.5 ml of RIPA buffer and 25 μl of wnt-3a (C64F2) rabbit monoclonal antibody at 4°C with gentle mixing overnight. Protein A-Sepharose beads (25 μl) were added for additional 2 h. After being washed three times with RIPA, 50 μl of 2× Laemmli sample buffer were added and a sample was boiled for 3 min to remove the precipitated protein from the Sepharose beads and dissociate the wnt-3a from the precipitating antibody (20). Western blots, performed as previously described (20), were probed with wnt-3a antibody (1:1,000). The wnt-3a densities were normalized to the total protein concentration of each sample.
DNA concentration.
The DNA concentration was measured using a spectrofluorometer (RF-1501; Shimadzu, Columbia, MD). The standard curve was made using herring testes DNA (Sigma D6898). DNA in the soluble fraction of tissue homogenates was diluted 10 times in 3 N NaCl and then sonicated for 45 s. The reaction (500 μl sample + 1 ml 3 N NaCl + 1 ml Tris-EDTA + 1 ml of 3.5 mg/ml Hoechst dye; Sigma) was incubated in the dark for 10 min. Florescence was measured at excitation of 369 nm and emission of 450 nm.
Virus reagents.
The adenovirus Ad-wnt-3a was a gift from Dr. T. C. He (12). The adenovirus transduction unit (TU) was achieved by serial dilutions. For virus transduction, the cells were subcultured in growth medium and 5 μl of the concentrated viral preparation (109 TU) in 2-ml media were applied to each well of a six-well cluster plate. This method results in >90% of cells being successfully transduced (based on green fluorescent protein expression).
Statistical analysis.
All data are reported as the means ± the SE Comparison between groups was performed with the Kruskal-Wallis two-way ANOVA, with P < 0.05 being considered significant. For comparisons between two groups, a Student's t-test was used with P < 0.05 being considered significant. The mRNA array data were analyzed using GeneSpring GX 9.0 (Mathworks, Natick, MA) with a GC robust multiarray analysis (GC-RMA) followed by a Volcano-Benjamin analysis, which identifies the most strikingly different results.
RESULTS
Overall protein content was decreased but CCN1 was sharply increased in skeletal muscle of aged animals.
We used C57BL6 mice and Fisher 344 rats for these studies. In C57BL6 mice, we compared results from “young” (4 mo of age) to those of “old” (30 mo of age) mice. Muscle weights corrected for body weights are shown in Table 1: weights of both EDL (predominantly glycolytic, fast-twitch, white fiber) and soleus muscles (predominantly oxidative, slow-twitch, red fiber) were significantly decreased in old vs. young mice. The muscle mass index (percentage that individual muscle weight contributes to body weight) was significantly decreased in old mice vs. young mice [26.5 ± 0.1(Y) vs. 22.1 ± 0.02 (O) in soleus and 23.2 ± 0.1 (Y) vs. 20.0 ± 0.2 (O) in EDL; P < 0.05]. Protein contents (protein yield per gram muscle DNA) of both soleus and EDL muscles were also very significantly decreased in aged mice (Table 1). These results indicate that 1) protein loss is a major component of aging sarcopenia; and 2) aging induces sarcopenia in both types of muscle fibers.
Table 1.
Muscle weights and protein content from young and old mice
| 4 mo | 30 mo | P Value | |
|---|---|---|---|
| Body weight, g | 28.1 ± 1.0 | 33.5 ± 1.3 | 0.196 |
| Soleus, mg | 12.3 ± 0.4 | 10.1 ± 0.6* | 0.039 |
| EDL, mg | 11.6 ± 0.5 | 9.3 ± 0.5* | 0.037 |
| Soleus/body (×100, ratio) | 43.5 ± 0.1 | 30.1 ± 0.2* | 0.04 |
| EDL/body (×100, ratio) | 41.2 ± 0.1 | 28.0 ± 0.2* | 0.02 |
| Soleus protein content | 83.2 ± 4.6 | 75.2 ± 3.4 | 0.008 |
| EDL protein content | 89.2 ± 5.8 | 73.2 ± 6.4 | 0.007 |
Data are presented as means ± SE; n = 6/group, EDL, extensor digitorum longus.
P < 0.05 is significant (aging vs. young).
To understand how aging is associated with sarcopenia, we used microarray analyses to study differences between young and aged muscle. The mRNAs from 3 young and 3 old rats were analyzed on an Affymetrix GeneChip Rat Genome 230 microarray, encompassing 28,000 identified rat genes. We found that 1,265 genes were significantly different in young vs. aged rat mRNA populations when subjected to a parametric statistical analysis (data not shown). 36 genes were significantly different when analyzed according to the more rigorous, volcano plot-Benjamin analysis. The largest change in the 36 genes was CCN1, a matricellular protein that was increased 113-fold in aged muscles. To confirm this difference, we measured mRNAs of CCN1 using real-time qPCR and found a 16-fold increase in the CCN1 expression in muscle of aged vs. young rats (Table 2). CCN2, another member of CCN family, was also increased. There was a 4.4-fold increase by microarray analysis and a 2.1-fold increase by qPCR measurements. We replicated these measurements using mouse tissue due to a better availability of aged mouse tissue. We measured CCN1 in the muscle of C57BL6 mice from 6 young (4 mo) and 6 old (30 mo) animals and found an 18-fold increase in the CCN1 mRNA expression in the muscle of aged vs. young mice (Table 2).
Table 2.
Gene expression–verification of microarray and qPCR analysis
| Rats Microarray (n = 3) |
Rats (n = 6) | Mice (n = 6) | ||||
|---|---|---|---|---|---|---|
| Affymetix No. | Gene symbol | Protein | Microarray fold change | P value | qPCR fold change: 24 vs. 5 m (P value) | qPCR fold change: 30 vs. 4 m (P value) |
| 1368290_at | Cyr61 | CCN1 | +113.8 | <0.0001 | +16.2 (<0.001) | +18.4 (<0.001) |
| 1367631_at | Ctgf | CCN2 | +4.4 | <0.05 | +2.1 (<0.01) | +5.8 (<0.01) |
qPCR, quantitative PCR.
+Increased.
P < 0.05 is significant (aging vs. young).
CCN1 protein is increased in aged rodent muscle and inhibits muscle cell proliferation but not differentiation.
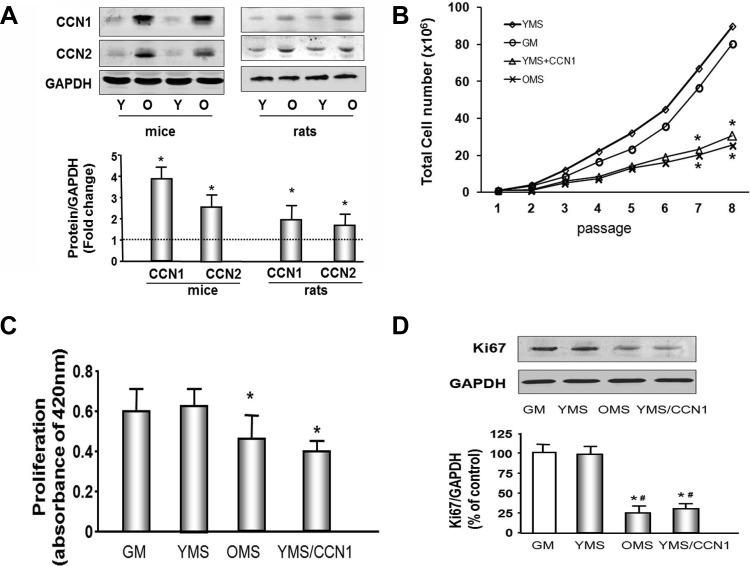
To determine if the increased mRNA levels were accompanied by an increase in the protein amount of CCN1 and CCN2 in aged muscle, we extracted protein from the hindlimb muscles of young or old rats and mice and analyzed by Western blot. The abundance of CCN1 protein was 3.8-fold increase in the muscle of aged vs. young mice and 1.9-fold increase in the muscle of aged vs. young rats (Fig. 1A). CCN2 was also increased 2.5-fold in muscle from aging mice.
Fig. 1.
CCN1 and CCN2 are increased in aging muscle and muscle progenitor cell (MPC) proliferation is decreased by CCN1 or aging mouse serum. A: representative Western blots of hindlimb muscle from old (O) and young (Y) rodents show increased CCN1 and CCN2 protein in aging muscle. Bar graph shows mean band density ± SE for CCN1 or CCN2 expressed as a fold change from levels in young rodents (set to 1-fold) after normalization to the density of GAPDH (bars: means ± SE; n = 6; *P < 0.05 vs. young). B: MPCs were cultured in Ham's F-10 medium containing 20% FBS [growth medium (GM); ○], medium with 20% young mouse serum (YMS; ◇), 20% old mouse serum (OMS; X), or YMS plus recombinant CCN1 (5 μg/ml; YMS + CCN1; △) and passaged every 3 days for 8 passages (x-axis). The cell total number was measured by hemocytometer at each passage, (n = 9; *P < 0.05 vs. GM or YMS). C: MPCs were grown in Ham's F-10 medium containing 20% FBS (GM), 20% YMS, 20% OMS, or YMS plus recombinant CCN1. The cell proliferation rate was measured using a chromogenic assay kit. (n = 9; *P < 0.05 vs. GM or YMC). D: Ki67, a proliferation marker, was assessed by Western blot in MPCs. GAPDH was determined as a loading control. Bar graph shows the mean band density of Ki67 bands expressed as a percentage of the control level (growth medium with BSA as 100%) after normalization to the density of GAPDH (bars: means ± SE; n = 6; *P < 0.05 vs. GM; #P < 0.05 vs. YMS).
To study the impact of CCN1 on muscle cell growth, MPCs were cultured in normal growth medium or growth medium in which control sera were replaced with either 20% YMS, 20% OMS, or in YMS plus 5 ug/ml CCN1 (YMS + CCN1). To maximize confidence, cell proliferation in each culture condition was determined by three separate assay methods; cell counting, chromogenic assay, and proliferation marker (Ki67) determination. First, cells were passaged and cell number counted every 3 days. Changes became statistically significant after seven passages. The myoblast number was decreased in the cells cultured with OMS and with YMS plus CCN1, when compared with either growth medium or medium with YMS. (P < 0.05; Fig. 1B). Second, cell proliferation was measured using a chromogenic assay. Muscle cell proliferation was 25% decreased in the cells cultured in medium with OMS vs. control medium (with FBS) or medium with YMS (Fig. 1C). Addition of CCN1 to the YMS also resulted in a 30% decreased proliferation vs. YMS without CCN1 treatment. Third, these results were confirmed with measurement of the proliferation marker protein Ki67 in identically cultured cells. This marker was decreased in cells cultured with OMS or with YMS plus CCN1 (Fig. 1D).
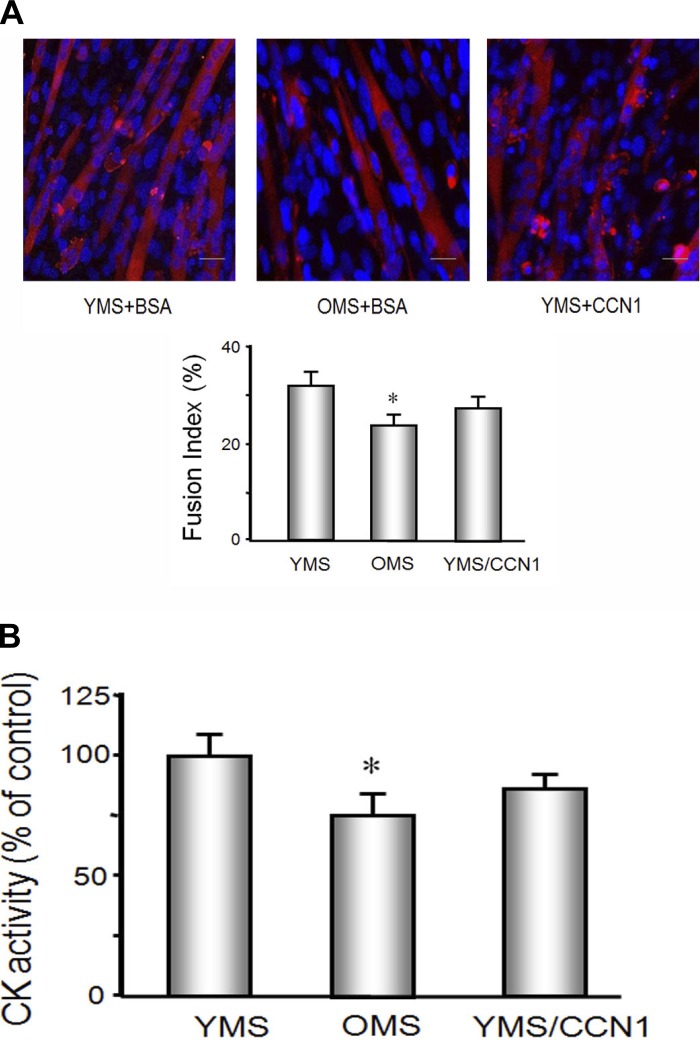
To study the effect of CCN1 and/or aged serum on muscle differentiation, MPCs were cultured in 2% OMS or 2% YMS with or without CCN1 for 5 days (Fig. 2). The responses under these serum conditions were compared with cells treated with medium containing 2% horse serum, the standard medium used to initiate differentiation in healthy myoblasts. There is no difference between the cells treated with 2% horse or 2% YMS (data not shown); both start to differentiate into myotubes on day 2 and continue to form myotubes over the 5 treatment days (Fig. 2A). The degree of myoblasts differentiation into myotubes was evaluated by calculating a fusion index on day 5. Cells that were grown in 2% OMS showed 20% less differentiation vs. cells treated with YMS. When the MPCs were cultured with YMS plus CCN1, the fusion index was no different from MPCs cultured in YMS alone suggesting that CCN1 effects proliferation but not differentiation. Creatine kinase activity, determined by colorimetric biochemical assay, was also used to identify differentiation potential. The MPCs treated with OMS had decreased creatine kinase activity compared with those cultured with YMS, either with or without CCN1 treatment (Fig. 2B).
Fig. 2.
Differentiation of MPCs was decreased by OMS but not CCN1. MPCs were seeded on entactin, collagen IV, and laminin matrix coated plates in Ham's F-10 medium contains 20% FBS. At 24 h (60–80% confluence), medium was replaced by differentiation media containing 2% YMS or 2% OMS or 2% YMS plus CCN1. A: immunohistochemistry with an antibody against eMyHC was used to assess MPCs differentiation into myotubes (red). Nuclei (blue) were stained with DAPI. The experiment was repeated an additional two times. The fusion index (bar graph) for the combined experiments was calculated from as the ratio of the number of nuclei within myotubes to the total number of nuclei in predefined fields of >450 nuclei (n = 9; *P < 0.05 vs. YMS). B: creatine kinase (CK) activity was used to identify MPCs differentiation capacity. CK activity was assessed using a chromogenic substrate and monitoring absorbance at 420 nm (materials and methods). Bars show the means ± SE (n = 9; *P < 0.05 vs. YMS).
CCN1 increases cell cycle arrest proteins and induces muscle cell senescence.
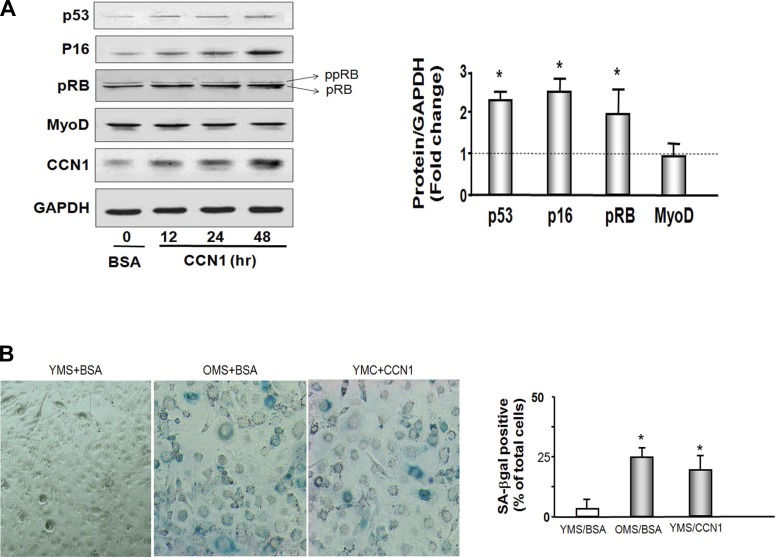
To identify the means by which CCN1 attenuates muscle cell proliferation, we treated cells with recombinant CCN1 and measured the myogenic protein markers in MPCs (Fig. 2A). There was no difference in the amount of myoD protein (myogenesis marker) in cells with or without CCN1. Interestingly, CCN1 caused changes in cell arrest proteins. The p53 tumor inhibitor protein was increased 2.1-fold after 48 h of treatment with CCN1. Another cell cycle arrest protein, p16Ink4A, was increased 2.7-fold. The RB protein is activated when hypophosphorylated. The hypophosphorylated form of RB (pRB) was 1.9-fold increased (Fig. 3A). These results indicate that CCN1 promotes muscle cell growth arrest.
Fig. 3.
CCN1 increases cell cycle arrest proteins p53, p16Ink4A, and RB and induces cellular senescence. A: p53, P16INK4A, RB, MyoD, and CCN1 were measured by Western blot in lysates from MPCs. Cells were treated with 5 μg/ml CCN1 for 12, 24, or 48 h. Cells were added 5 μg/ml BSA as a control (far left lane). RB protein was detected with 2 bands. Bottom band is the hypophosphoryated RB (pRB; MW 107 kDa), and top band is phosphorylated RB protein (ppRB; MW 112kDa). Bar graph shows the density of each 48 h protein band expressed as a fold change from control levels (control set to 1 and indicated by horizontal line in the graph). All band densities were normalized using the density of GAPDH (bars: means ± SE; n = 9; *P < 0.05 vs. BSA). B: micrographs of senescence-associated β-galactosidase (SA-βgal) staining of MPCs cultured with 20% young mouse serum with CCN1 (YMS + CCN1) or without CCN1 (YMS + BSA) or with 20% old mouse serum (OMS + BSA). Blue color shows the presence of SA-βgal indicating cellular senescence. Bar graph shows percentage of total cells that are SA-βgal positive in 5 predefined fields. Experiment was performed a total of 3 times, and each condition was determined in duplicate (bars: means ± SE; n = 6; *P < 0.05 vs. YMS + BSA).
To study the effect of CCN1 on muscle cellular senescence, MPCs were cultured in 20% OMS or 20% YMS with or without CCN1 for 10 days. Medium was change every other day with a boost of fresh CCN1. At 10 days, supplying CCN1 exogenously led to flat cells resembling fried eggs, which indicated cell senescence. Senescent cells were stained for the presence of SA-βgal and visually identified by their round structure and blue color. SA-βgal-positive cells were abundant in the cells cultured in old mouse serum (Fig. 3B). In young mouse serum SA-βgal-positive cells were almost absent, but when treated with CCN1, SA-βgal-positive cells appeared. These results indicate that CCN1 promotes muscle cellular senescence.
Serum from aging mice increased the expression of CCN1 in MPCs.
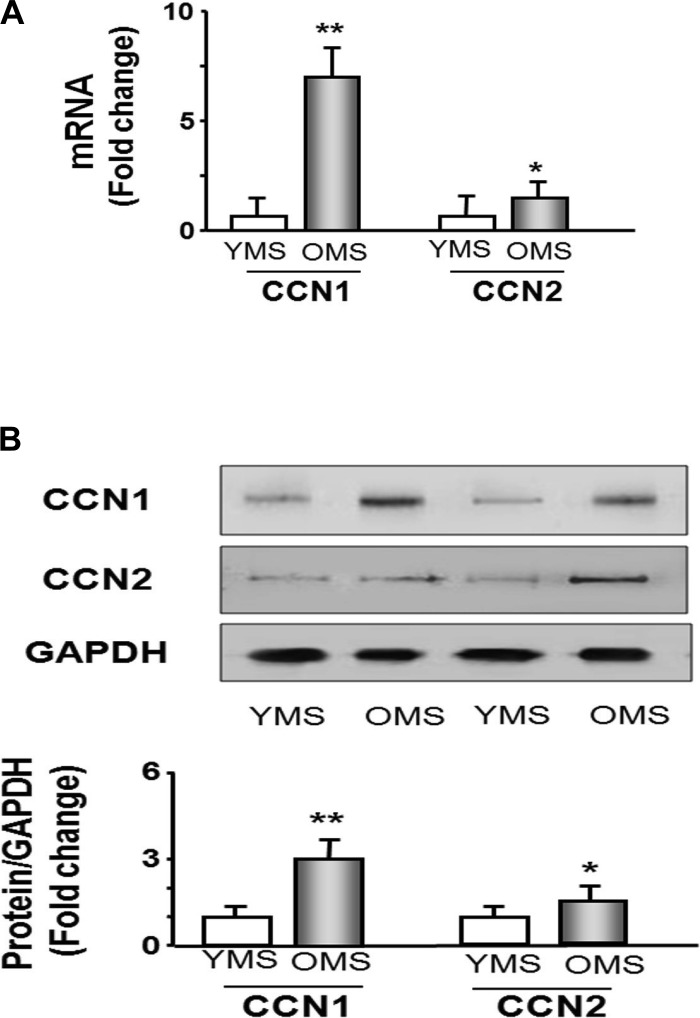
We already observed that the aging environment decreases proliferation (Fig. 1). To determine if that environment included increases in CCN1 levels, we examined CCN1 and CCN2 mRNA and protein levels in MPCs grown under the young and aging serum condition. Real-time qPCR revealed that the mRNAs of CCN1 and CCN2 were increased 7-fold and 1.8-fold, respectively, in old serum conditions compared with levels in young serum cultures (Fig. 4A). The increased mRNA was accompanied by an increase in CCN1 protein (Western blot analysis) in MPCs treated with old mice serum (3-fold increase). CCN2 protein was also increased but by a more modest 1.5-fold in cells treated with serum from old mice (Fig. 3B). These experiments indicate that aging serum stimulates increases in the levels of CCN1 in MPCs.
Fig. 4.
Extracellular environment (mimicked by old animal serum) increases CCN1 mRNA and protein in MPCs. MPCs were cultured in the medium containing 20% YMS or 20% OMS. A: RNA was isolated from each treatment of cells and used to measure CCN1 and CCN2 mRNA expression by real-time quantitative PCR; 18S was used as the control. Bar graph shows the amount of CCN1 or CCN2 mRNA expressed as a fold change from levels in cells cultured in YMS (set to 1-fold, n = 6 determinations per condition; *P < 0.05 and **P < 0.01 vs. YMS). B: protein was isolated from MPCs cultured in each serum treatment to measure CCN1 or CCN2 protein by Western blot. Bar graph shows the density of CCN1 or CCN2 protein expressed as a fold change from levels in YMS-treated cells (set to 1-fold). All bands were normalized to the density of GAPDH (bars: means ± SE; n = 6/condition; *P < 0.05 and **P < 0.001 vs. young).
Wnt-3a increases CCN1 expression in C2C12 myoblasts.
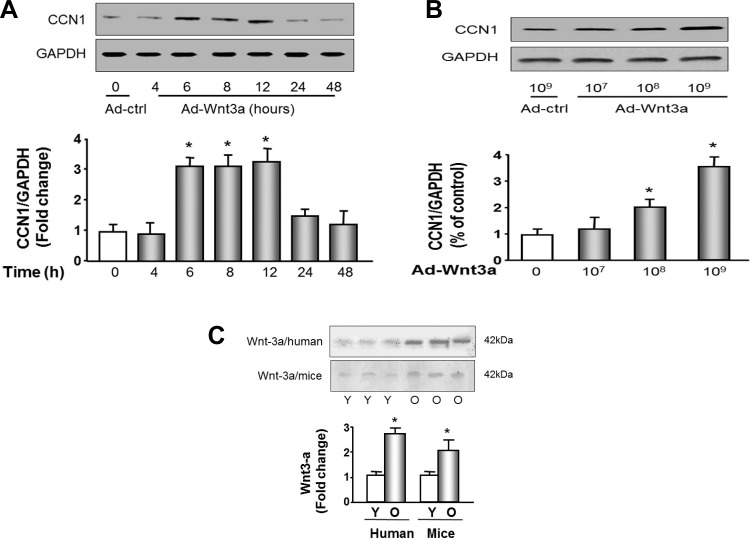
Because aging serum caused such a dramatic increase in CCN1, which in turn would cause increased muscle senescence, we looked for regulators of CCN1 abundance in the aging serum. Wnt-3a is a secreted protein that has been implicated in muscle atrophy and is increased in aging serum (6). To determine if wnt-3a was involved in muscle loss, possibly through CCN1, we used a recombinant adenovirus (Ad-wnt-3a) to mediate the overexpression of wnt-3a. C2C12 myoblasts transduced with Ad-wnt-3a adenovirus exhibited up to a threefold increase in CCN1 protein in a time (Fig. 5A)- and dose (Fig. 5B)-dependent manner. MPCs in culture showed a similar increase (data not shown). There were no significant changes in CCN2 protein abundance in response to wnt-3a in either MPCs or C2C12 cells (data not shown). To determine whether wnt-3a is a potential circulated factor in aging serum, we measured the abundance of wnt-3a in serum samples from young or aged humans and mice (Fig. 5C). Serum from human subjects was collected from 6 young (age 31.3 ± 2.4 yr old) and 6 old (age 78.9 ± 3.4 yr old) healthy males. Serum from mouse was collected from 6 young (4 mo old) and 6 old (30 mo old) male mice. Wnt-3a was increased 2.6-fold in aged human and 2-fold in aged mouse sera.
Fig. 5.
Wingless-type MMTV integration site family member 3A (wnt-3a) increases CCN1 in myoblasts; Wnt-3a is increased in old human and mouse serum. A: C2C12 myoblasts at 80% confluence were transduced with adenoviral wnt-3a (Ad-Wnt-3a; transduction unit 109) for the indicated times. Western blots were used to detect the abundance of CCN1 or GAPDH. The bar graph shows the density of CCN1 bands expressed as a fold change from 0 (with Ad-ctrl treatment, set to 1-fold) after normalization to the density of GAPDH (bars: means ± SE; n = 3; *P < 0.05 vs. Ad-ctrl treatment). B: dose response of CCN1 and CCN2 to wnt-3a. C2C12 cells at 80% confluence were transduced with recombinant adenovirus Ad-wnt-3a at the indicated titer. Western blots were used to detect the abundance of CCN1 or GAPDH. Bar graph shows the density of CCN1 bands expressed as a fold change from 0 (Ad-ctrl treatment, set to 1-fold) levels after normalization to the density of GAPDH (n = 3; *P < 0.05 vs. Ad-ctrl). C: wnt-3a protein amount was measured in young (Y) and old (O) human and mouse serum samples using immunoprecipitation followed by Western blot. Immunoprecipitated band densities were divided by the concentrations of protein in the original serum samples to correct for original protein content of the immunoprecipitation reactions. Bar graph shows the mean corrected density of wnt-3a bands expressed as a fold change from young levels (bar: means ± SE, n = 6; *P < 0.05 vs. young set to 1-fold).
DISCUSSION
Sarcopenia in skeletal muscle increases with aging and contributes to a loss of muscle integrity and functional capacity. This can cause a rapid shift from independence to a dependent life style for the aging population.
Analysis of a microarray assay on aging muscle tissue revealed that CCN1 is sharply increased in muscles of aging rats (Table 2). In further examination of CCN1 in the cultured MPCs exposed to serum from aging rodents, we demonstrated that an increase in CCN1 leads to cellular senescence (Fig. 3B). To date, little is known about the exact effect of members of the CCN family on the changes occurring in aged skeletal muscle. Bigot et al. (4) found that CCN2 was increased in senescent myoblasts isolated from human skeletal muscle. Kivela et al. (19) found that a single bout of exercise with high mechanical loading induces the expression of CCN1 in human skeletal muscle. High loading exercise frequently results in multiple small muscle injuries that then initiate muscle regeneration or repair. The increase in CCN1 is believed to induce fibroblast senescence to avoid excessive fibrosis during repair process (15). O'Connor et al. (31) showed that CCN1 increases the muscle cell migratory phenotype that is another case of wound healing type response by CCN1. Other published accounts of CCN1 in muscle cells from either young or old animals are scarce. Before this study, the ability of CCN1 to trigger cellular senescence in muscle cells was undetermined.
In nonmuscle cells, CCN1 promotes diverse and sometimes opposing cellular responses. The variety of response may be mediated through its direct binding to different trans-membrane receptors integrins (23). For example, CCN1 promotes cell survival but also triggers apoptosis and stimulates tumor growth but also suppresses tumorgenesis under different conditions. CCN1 is involved in fibroblast senescence, which restricts fibrosis in a wound healing setting (15). CCN1 induces DNA damage response pathways, cell arrest pathways, and reactive oxygen species generation pathways including the RAC1-NOX1 complex. Activation of RACI-NOX1 results in promotion of another cell arrest pathway, the p16Ink4A/pRB pathway (9). This CCN1-mediated DNA damage may contribute to the cellular senescence process, which is consistent with our results.
The morphology of senescent cells in culture is fairly distinctive displaying a large and flat morphology visually resembling a fried egg, but senescent cells in vivo lack distinctive morphological features (35). Therefore, molecular markers are necessary to identify cellular senescence in muscle tissue. The classic markers of senescence are the same proteins involved in the mechanism of cell growth arrest, such as p16Ink4A, which is considered to be the principal mediators of cellular senescence (21). Another important marker is the tumor suppressor p53, which acts to restrict proliferation in response to DNA damage by leading to the induction of cellular senescence (13). Mice with mutations that produce a constitutively active p53 display early aging-associated phenotypes (41). Mice with mutations that inactivate p53 exhibit increased cell proliferation and survival (44). The presence of the cell arrest protein markers alone suggests, but does not prove, senescence. Their presence must be coupled to evidence of reduced cellular proliferation, typically by determination of low Ki67 (proliferation marker) or bromodeoxyuridine labeling, to prove senescence. We found that the level of CCN1 rises sharply in whole muscles of aging rats and mice. This study provides three different types of evidence that CCN1 causes cellular senescence in muscle cells: 1) the senescence marker SA-βgal was significantly increased by CCN1; 2) the proliferation rate of MPCs and proliferation marker ki67 were significantly decreased by CCN1; and 3) the cell growth arrest markers p53, p16Ink4A, and RB were significantly increased by CCN1.
The increased CCN1 level measured in the whole muscle tissue preparation may not only reflect levels in the muscle cells but may also include CCN1 that is present in other cell types within the muscle such as blood, nerve, fibroblasts, or interstitial cells, including macrophages. Jun and Lau (15) found that CCN1 induces fibroblast senescence and restricts fibrosis in wound healing. Quan et al. (33) found that CCN1 contributes to skin connective tissue aging. The increased levels of CCN1 in aging that we observed would be consistent with a role for CCN1 in fibroblast senescence and connective tissue aging. However, the total levels of CCN1 measured in the muscle tissue preparation cannot be due solely from the contribution of the relatively limited amount of nonmuscle cells or tissue present in the preparation. Further study is needed to clarify the function of CCN1 in other (nonmuscle) cells within the muscle tissue.
Wnt signaling is necessary to initiate myogenesis in amniote embryos through activation of Myf5 and MyoD (10). The wnt pathway is also essential for myogenic differentiation in adult skeletal muscle. Wnt signaling appears to trigger the switch from proliferation of MPCs to differentiation of the cells (5). The timing of this switch is critical since constitutive wnt signaling during aging leads to a lack of required proliferation and results in an impaired myogenic potential. In another words, an increase in wnt signaling, therefore, impairs muscle regeneration by causing MPC differentiation before enough proliferation that can ensure sufficient cell structure to sustain functional muscle. In our study, the outcome of premature differentiation is production of senescence.
CCN1 is a direct target gene of canonical wnt signaling (26, 38, 39). Here, we report that the increased wnt-3a in the serum of aged animals could be responsible, through the stimulation of CCN1, for increased senescence in the muscles of these animals. In the muscle microarray analysis, we found that in aging muscle, wnt-3a trended toward a 2.5-fold increase (aging: 0.45 ± 0.41 vs. young: 0.18 ± 0.06; P = 0.32), but the difference did not reach statistical significance due to the large variability. We believe that wnt-3a failed to show a difference because it is mostly a circulating factor and the arrays were specifically analyzing muscle tissue components. In addition, wnt-3a is not the only factor that induces senescence; many factors that are elevated in aged serum could contribute to senescence. For example, Conboy et al. (8) found that a decline in skeletal muscle satellite cell activity due to a loss of Notch signaling results in impaired regeneration of aged muscle. TGF-β is considered to be related with senescence in primary cultured human muscle precursor cells (1). Reduced proliferation is also associated lower levels of full-length receptor for advanced glycation endproducts (RAGE; Ref. 2).
Previous studies by others found that the differentiation capacity is decreased in human aging muscle (32) and in an aging cell culture model (multiple population doublings C2C12 cells; Ref. 37). We found that differentiation potential is decreased in MPCs cultured in OMS compared with the differentiation in cells cultured in YMS. However, even though CCN1 causes a decrease in proliferation of these cells, exogenous addition of CCN1 to the YMS did not appear to change the differentiation capacity, that is, the fusion index of MPC cultured in YMS with CCN1 was not significantly different from that of MPCs in YMS alone (Fig. 2A). Similar with our finding, Bigot et al. (4) showed that senescent myoblasts, which could not reenter the cell cycle (cannot proliferate), are still able to differentiate and form multinucleated myotubes.
The data from this study show a novel pathway for the regulation of muscle mass and the development of muscle cellular senescence in aging animals. The pathway is initiated by wnt-3a, acting as a circulating factor that leads to increased CCN1 production. The elevated CCN1 contributes to myoblast senescence by upregulation of p53 and p16ink4a. Understanding how these pathways are involved in aging sarcopenia may lead to new directions in prolonging muscle health in our aging population.
GRANTS
This work was supported in part by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant 1R01-AR-060268 and National Natural Science Foundation of China (30971471 to X. H. Wang) and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-89828 (to J. D. Klein) and National Natural Science Foundation of China (31090363 to J. Du). This work was also supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R37-DK-37175 (to W. E. Mitch) from Baylor College of Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.D., F.H., J.Z., C.Z., and X.H.W. performed experiments; J.D., J.D.K., and X.H.W. analyzed data; J.D., J.D.K., and X.H.W. interpreted results of experiments; J.D., J.D.K., and X.H.W. edited and revised manuscript; J.D.K. and X.H.W. approved final version of manuscript; X.H.W. conception and design of research; X.H.W. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Yuhua Li of the Microarray Center of Emory University for providing microarray service.
REFERENCES
- 1.Alsharidah M, Lazarus NR, George TE, Agley CC, Velloso CP, Harridge SD. Primary human muscle precursor cells obtained from young and old donors produce similar proliferative, differentiation and senescent profiles in culture. Aging Cell 12: 333–344, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Beccafico S, Riuzzi F, Puglielli C, Mancinelli R, Fulle S, Sorci G, Donato R. Human muscle satellite cells show age-related differential expression of S100B protein and RAGE. Age (Dordr) 33: 523–541, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr Opin Clin Nutr Metab Care 15: 12–22, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Bigot A, Jacquemin V, Debacq-Chainiaux F, Butler-Browne GS, Toussaint O, Furling D, Mouly V. Replicative aging down-regulates the myogenic regulatory factors in human myoblasts. Biol Cell 100: 189–199, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2: 50–59, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317: 807–810, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol Cell Physiol 256: C1262–C1266, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433: 760–764, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Coppe JP, Rodier F, Patil CK, Freund A, Desprez PY, Campisi J. Tumor suppressor and aging biomarker p16(INK4a) induces cellular senescence without the associated inflammatory secretory phenotype. J Biol Chem 286: 36396–36403, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cossu G, Borello U. Wnt signaling and the activation of myogenesis in mammals. EMBO J 18: 6867–6872, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocket M, Campisi J, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92: 9363–9367, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 95: 2509–2514, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Itahana K, Dimri G, Campisi J. Regulation of cellular senescence by p53. Eur J Biochem 268: 2784–2791, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Jun JI, Lau LF. Cellular senescence controls fibrosis in wound healing. Aging (Albany, NY) 2: 627–631, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12: 676–685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov 10: 945–963, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamel HK. Sarcopenia and aging. Nutr Rev 61: 157–167, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Kim JS, Kosek DJ, Petrella JK, Cross JM, Bamman MM. Resting and load-induced levels of myogenic gene transcripts differ between older adults with demonstrable sarcopenia and young men and women. J Appl Physiol 99: 2149–2158, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kivela R, Kyrolainen H, Selanne H, Komi PV, Kainulainen H, Vihko V. A single bout of exercise with high mechanical loading induces the expression of Cyr61/CCN1 and CTGF/CCN2 in human skeletal muscle. J Appl Physiol 103: 1395–1401, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Klein JD, Blount MA, Frohlich O, Denson CE, Tan X, Sim JH, Martin CF, Sands JM. Phosphorylation of UT-A1 on serine 486 correlates with membrane accumulation and urea transport activity in both rat IMCDs and cultured cells. Am J Physiol Renal Physiol 298: F935–F940, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114: 1299–1307, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labunskyy VM, Gladyshev VN. Role of reactive oxygen species-mediated signaling in aging. Antioxid Redox Signal 19: 1362–1372, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau LF. CCN1/CYR61: the very model of a modern matricellular protein. Cell Mol Life Sci 68: 3149–3163, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leu SJ, Lam SC, Lau LF. Pro-angiogenic activities of CYR61 (CCN1) mediated through integrins alphavbeta3 and alpha6beta1 in human umbilical vein endothelial cells. J Biol Chem 277: 46248–46255, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol 21: 103–124, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li ZQ, Ding W, Sun SJ, Li J, Pan J, Zhao C, Wu WR, Si WK. Cyr61/CCN1 is regulated by Wnt/beta-catenin signaling and plays an important role in the progression of hepatocellular carcinoma. PLoS One 7: e35754, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317: 803–806, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T, Sutherland A, Thorner M, Scrable H. Modulation of mammalian life span by the short isoform of p53. Genes Dev 18: 306–319, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: aging gracefully as a protectionist? Pharmacol Ther 118: 58–81, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narita M, Nunez S, Heard E, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113: 703–716, 2003 [DOI] [PubMed] [Google Scholar]
- 31.O'Connor RS, Mills ST, Jones KA, Ho SN, Pavlath GK. A combinatorial role for NFAT5 in both myoblast migration and differentiation during skeletal muscle myogenesis. J Cell Sci 120: 149–159, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Pietrangelo T, Puglielli C, Mancinelli R, Beccafico S, Fano G, Fulle S. Molecular basis of the myogenic profile of aged human skeletal muscle satellite cells during differentiation. Exp Gerontol 44: 523–531, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Quan T, Qin Z, Robichaud P, Voorhees JJ, Fisher GJ. CCN1 contributes to skin connective tissue aging by inducing age-associated secretory phenotype in human skin dermal fibroblasts. J Cell Commun Signal 5: 201–207, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature 434: 843–850, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol 192: 547–556, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz E, Holtorf K. Hormone replacement therapy in the geriatric patient: current state of the evidence and questions for the future. Estrogen, progesterone, testosterone, and thyroid hormone augmentation in geriatric clinical practice: part 1. Clin Geriatr Med 27: 541–559, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Sharples AP, Al-Shanti N, Lewis MP, Stewart CE. Reduction of myoblast differentiation following multiple population doublings in mouse C2 C12 cells: a model to investigate ageing? J Cell Biochem 112: 3773–3785, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Si W, Kang Q, Luu HH, Park JK, Luo Q, Song WX, Jiang W, Luo X, Li X, Yin H, Montag AG, Haydon RC, He TC. CCN1/Cyr61 is regulated by the canonical Wnt signal and plays an important role in Wnt3A-induced osteoblast differentiation of mesenchymal stem cells. Mol Cell Biol 26: 2955–2964, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sung YK, Kwack MH, Kim SR, Kim MK, Kim JC. Transcriptional activation of CCN1 and CCN2, targets of canonical Wnt signal, by ascorbic acid 2-phosphate in human dermal papilla cells. J Dermatol Sci 49: 256–259, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Todorovic V, Chen CC, Hay N, Lau LF. The matrix protein CCN1 (CYR61) induces apoptosis in fibroblasts. J Cell Biol 171: 559–568, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Park SH, Thompson T, Karsenty G, Bradley A, Donehower LA. p53 mutant mice that display early ageing-associated phenotypes. Nature 415: 45–53, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 147: 4160–4168, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Wang XH, Hu Z, Klein JD, Zhang L, Fang F, Mitch WE. Decreased miR-29 suppresses myogenesis in CKD. J Am Soc Nephrol 22: 2068–2076, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445: 656–660, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L, Wang XH, Wang H, Du J, Mitch WE. Satellite cell dysfunction and impaired IGF-1 signaling cause CKD-induced muscle atrophy. J Am Soc Nephrol 21: 419–427, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]