Abstract
Efficient repair of epithelial tissue, which is frequently exposed to insults, is necessary to maintain its functional integrity. It is therefore necessary to better understand the biological and molecular determinants of tissue regeneration and to develop new strategies to promote epithelial repair. Interestingly, a growing body of evidence indicates that many members of the large and widely expressed family of K+ channels are involved in regulation of cell migration and proliferation, key processes of epithelial repair. First, we briefly summarize the complex mechanisms, including cell migration, proliferation, and differentiation, engaged after epithelial injury. We then present evidence implicating K+ channels in the regulation of these key repair processes. We also describe the mechanisms whereby K+ channels may control epithelial repair processes. In particular, changes in membrane potential, K+ concentration, cell volume, intracellular Ca2+, and signaling pathways following modulation of K+ channel activity, as well as physical interaction of K+ channels with the cytoskeleton or integrins are presented. Finally, we discuss the challenges to efficient, specific, and safe targeting of K+ channels for therapeutic applications to improve epithelial repair in vivo.
Keywords: epithelia, injury and repair, K+ channels, cell migration, cell proliferation
epithelial tissue acts as a protective barrier and plays a major role in ion and liquid homeostasis. This tissue is exposed to frequent aggressions, which may cause acute or chronic injuries and also trigger tissue remodeling. Epithelial regeneration, necessary to restore epithelial integrity and function, depends on several processes that are engaged sequentially after injury, including cell migration, proliferation, and differentiation, to restore a polarized and functional epithelium (Fig. 1) (16, 32, 61). These complex processes integrate many proteins and mechanisms, which are regulated by various components, including growth factors and downstream signaling effectors (32, 61). Another class of proteins, i.e., K+ channels, that exert their main recognized function in epithelia as the control of membrane potential and the maintenance of driving force for transepithelial ion/liquid transport (11, 14, 53) seems also to regulate cell migration and proliferation processes of various cell types, including epithelial cells.
Fig. 1.
Epithelial injury and repair processes. Among the causes of epithelial injury are infections, inflammation, toxic compounds, and trauma. Several mechanisms are then sequentially engaged in epithelial repair: spreading, dedifferentiation, and migration of healthy epithelial cells, followed by proliferation and redifferentiation of progenitor cells. Inset: α- and β-integrin heterodimers link actin filaments to the extracellular matrix (ECM), favoring protrusion of the front edge of migrating cells, while proteinases [matrix metalloproteinase (MMP) and a disintegrin and metalloprotease domain (ADAM)] degrade the ECM, decreasing cell adhesion and promoting retraction of the rear edge of migrating cells. MMPs are also involved in release of growth factors, such as EGF. Moreover, integrin and EGF receptor (EGFR) trigger intracellular signaling, which is crucial for cell migration and proliferation.
The purpose of our review is, first, to briefly summarize the major mechanisms and proteins responsible for epithelial repair. We then report evidence demonstrating that K+ channels are involved in the regulation of repair processes, especially cell migration and proliferation. We also present an overview of the mechanisms whereby K+ channels may control these processes. Finally, we report some data indicating a protective role of K+ channels after injury in vivo and discuss the challenges to efficient, specific, and safe targeting of K+ channels for therapeutic applications to improve epithelial regeneration.
Mechanisms of Epithelial Injury and Repair
Covering epithelial tissue, lining the outside surface of the body (skin and cornea) and the lumen of internal organs (gastrointestinal, urinary, and respiratory tracts), is frequently exposed to insults from external (e.g., pathogens, noxious chemicals, mechanical stress, and trauma) and internal (e.g., sepsis, ischemia-reperfusion, and exacerbated/chronic immune and inflammatory responses) sources. These aggressions trigger cascades of cellular events, culminating in epithelial damage and/or remodeling. The nature and severity of morphological/histological alterations may vary, depending on the source, duration (acute vs. chronic), and intensity of the injury and epithelium localization/type. However, despite this diversity, epithelial tissue will usually respond to damage by engaging multiple cellular events, including cell adhesion, migration, proliferation, and differentiation processes, in an attempt to restore its structural and functional integrity (Fig. 1).
Cell migration is one of the first mechanisms of epithelial repair. This complex phenomenon integrates well-established processes, including cytoskeleton reorganization, membrane protrusion formation, and focal adhesion to the extracellular matrix (ECM) at the front edge and release of adhesion sites at the rear edge of migrating cells (Fig. 1) (74). Cell movement on provisional ECM and on denuded basement membranes depends on a fine equilibrium via concerted actions of adhesion and de-adhesion proteins (e.g., cadherin, integrins, and proteases) (4, 52, 56, 71, 75, 139). Indeed, numerous matrix metalloproteinases (MMPs) and a disintegrin and metalloprotease domain (ADAM) are involved in ECM remodeling, as well as in release of growth factors, regulating repair processes (46, 75, 81, 110, 168). Integrins not only create links between the ECM and the cytoskeleton, but they also interact with multiple proteins [e.g., EGF receptor (EGFR), focal adhesion kinase (FAK), Src, and MEK/ERK] to trigger signaling pathways regulating repair mechanisms (4, 22, 52, 71, 139).
Subsequent phases that promote healing comprise cell proliferation and differentiation. The nature and turnover of cells, regarded as progenitors for natural renewal or regeneration after damage, are varied, depending on the epithelial tissue studied. Epithelial tissue of the skin, cornea, and intestine is characterized by continuous renewal through migration and proliferation of progenitor cells. In contrast, the rate of alveolar type (AT) II cell turnover in alveoli is low, and most ATII cells are quiescent in healthy adult lungs (148). However, these cells have the potential to dedifferentiate, proliferate (1, 24, 89, 136), and then redifferentiate into ATI or ATII cells (148) for alveolar restoration after injury. In fact, ongoing research is gradually identifying new stem cell niches participating in the regeneration of epithelial tissues.
Cell migration, proliferation, and differentiation processes depend on many cellular events (Ca2+ signaling, changes in cell volume/shape, and membrane potential), proteins (e.g., integrins and cyclins), and signaling pathways (22, 44, 73, 100, 106, 127, 135, 165). Growth factors [e.g., EGF, transforming growth factor-α, and hepatocyte growth factor (HGF)] play a prominent role by acting via paracrine and/or autocrine pathways and downstream signaling cascades, inducing mitogenic, as well as motogenic and morphogenic, cellular responses (48, 90, 144, 145, 151, 165).
Thus a large number of studies have contributed to knowledge of the mechanisms responsible for epithelial repair and have identified major proteins, factors, and signaling pathways regulating them. However, in addition to these well-defined components, other regulators of epithelial repair have emerged during the last two decades. Among them, ion channels and transporters have been shown to modulate the migration and proliferation of many cells. In the following sections, we present proof of the role of K+ channels in the control of repair processes, focusing particularly on epithelial cells.
Control of Repair Processes by K+ Channels
Molecular diversity, regulatory mechanisms, and most recognized functions of epithelial K+ channels.
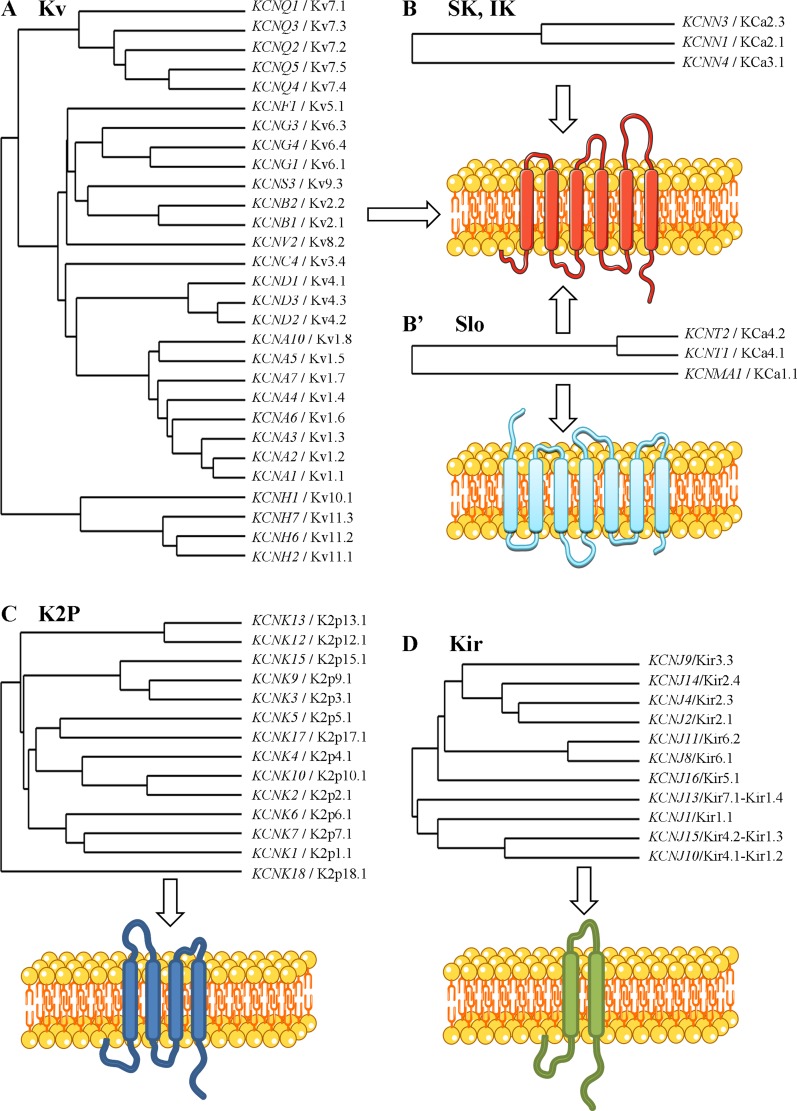
K+ channels, coded by ∼90 different genes, belong to the largest ion channel family and are widely expressed in all organs. An impressive number (∼60) of different K+ channel subtypes have been detected in epithelial cells (Fig. 2). Only pore-forming α-subunits are represented on phylogenic trees in Fig. 2; however, numerous auxiliary β-subunits are involved in the formation of K+ channels, further increasing their molecular diversity. These channels are classified into different subfamilies on the basis of their structural and functional properties (Fig. 2). Their molecular identity, regulatory mechanisms, and major functions, particularly in the control of membrane potential and maintenance of driving force for transepithelial ion/liquid transport, have been reported in several reviews (11, 14, 53, 55, 93, 96, 157) and are briefly summarized below.
Fig. 2.
Molecular diversity of epithelial K+ channels. Phylogenic trees have been generated (see http://www.phylogeny.fr, “treeviewers,” Drawtree) after alignment (with ClustalW software) of amino acid sequences of pore-forming α-subunits of K+ channels reported in epithelial cells. Different classes of K+ channels are distinguished as voltage-dependent (Kv; A) and Ca2+-activated [small-conductance (SK), intermediate-conductance (IK), and Slo; B and B′] 6-transmembrane domain (TMD) channels, 2-pore 4-TMD (K2P) channels (C), and inwardly rectified, 2-TMD [inwardly rectified (Kir)] channels (D). The exception, KCNMA1 [large-conductance Ca2+-activated K+ (BKCa), or Slo1], is formed from 7 TMDs. Although KCNT1 and KCNT2 are classified in the Slo Ca2+-activated K+ channel subfamily, these channels are atypical because of their activation by Na+, instead of Ca2+. K+ channel members are identified by their HUGO Gene Nomenclature Committee designations and International Union of Pharmacology names.
Voltage-dependent K+ channels.
A large number of voltage-dependent (Kv) channels, each formed from four subunits of six transmembrane domains (TMDs), have been detected in epithelial cells (Fig. 2A). However, the functional role of most of these channels in epithelial tissue remains undefined. KvLQT1 (Kv7.1, KCNQ1) channels have probably been the most characterized in epithelial cells, and their functions have been well established. These channels have been associated with control of Cl− secretion in airway, as well as colon and intestinal, epithelial cells (31, 39, 50, 72, 83, 149), while they have been shown to participate in Na+ and fluid absorption through alveolar epithelial cells (10, 77). Moreover, pharmacological KvLQT1 channel inhibition in vivo or KCNQ1 gene knockout (KO) in mice has been shown to impair gastric acid secretion (49, 102, 149).
The electrophysiological properties of the KvLQT1 channel are determined by the regulatory subunits (KCNE members) involved in its formation. In epithelial tissues, several lines of evidence indicate that the KCNE3 subunit could be associated with KCNQ1, leading to constitutively activated KvLQT1 channels (17, 50, 125). This channel is inhibited by chromanol 293B and clofilium and is activated by cAMP. Moreover, it has been shown in epithelial cells that the KvLQT1 channel is downregulated by AMP-activated protein kinase (3) or estrogens (97) and upregulated through the EGF-EGFR pathway (144, 145). Growth factors also regulate other Kv channels, for example, in the corneal epithelium, where the 4-aminopyridine (4-AP)-sensitive Kv channel has been shown to be stimulated by EGF and FBS (120).
Ca2+-activated K+ channels.
Ca2+-activated K+ (KCa) channels, formed from six TMDs (small- and intermediate-conductance channels; Fig. 2B) or seven TMDs (large-conductance Slo1 channels; Fig. 2B′), are expressed in epithelial cells. Many studies have focused on intermediate-conductance KCa3.1 (KCNN4) channels, which are involved in transepithelial ion transport. Indeed, KCa3.1 channel inhibition has been shown to impair Ca2+-activated Cl− secretion through airway epithelia (13, 31, 82). Conversely, KCa3.1 channel activation has been found to stimulate CFTR- and Ca2+-activated Cl− channel-mediated Cl− transport in airway and jejunum epithelial cells (39, 54, 82, 138). Large-conductance KCa (also called maxi-KCa, BKCa, Slo1, and KCa1.1) channels, identified in epithelial cells, play a role in K+ secretion in colonic and airway epithelia (84, 123, 172), where they have been demonstrated to secondarily favor Cl− secretion and maintain adequate airway surface liquid volume (84).
As indicated by their name, members of the KCa channel family are activated by Ca2+, except for the atypical KCa4.1 and KCa4.2 (or Slo2.1, Slo2.2) channels, which are activated by Na+ and Cl− (107). Changes in cell volume and secondary Ca2+ influx, as well as modifications of pH, regulate BKCa channels (41, 160). Moreover, KCa3.1 and BKCa channels are regulated by many growth factors [e.g., HGF/scatter factor (SF), fibroblast growth factor 2 (FGF-2), EGF, and platelet-derived growth factor], as well as signaling proteins (MEK and Ras), also identified as mitogenic and motogenic regulators (51, 59, 64, 68, 80, 116).
Two-pore domain K+ channels.
Another class of K+ channels, the two-pore domain K+ (K2P) channel, is characterized by a two-dimensional structure with four TMDs and two pores. Although several members of this subfamily are represented in epithelia (Fig. 2C), their function has been studied less extensively. For example, it has been reported that these channels modulate Cl− secretion and Na+ absorption in airway epithelial cells (174). Furthermore, metabolic acidosis due to deficient renal bicarbonate reabsorption has been observed in TASK-2 (KCNK5) KO mice (158), while impaired regulation of phosphate and water transport in the kidneys has been reported in TWIK-1 (KCNK1) KO mice (91).
K2P channel function is regulated by a wide range of parameters, including intra- and extracellular pH, membrane stretch, temperature, oxygen, and signaling pathways (42, 92, 134).
Inwardly rectified K+ channels.
Finally, several epithelial K+ channels belong to the inwardly rectified, two-TMD K+ (Kir) channel subfamily (Fig. 2D). Although different K+ channels are expressed in the kidneys, renal outer medullary K+ (ROMK, Kir1.1) channels are probably the best characterized renal K+ channels, and their role in NaCl absorption has been well established (156). ATP-sensitive K+ (KATP) channels, encoded by the KCNJ8 gene, are also important in epithelial function. In alveolar cells, these channels control Na+ and fluid absorption in vitro (10, 76, 77), and KATP channel activation has been shown to favor lung liquid clearance in an ex vivo model (121).
Epithelial KATP channels are sensitive not only to intracellular ATP, but also to pH (12, 60, 85, 146), as well as intracellular taurine, involved in volume regulatory decrease (19, 94). Conversely, we have reported stimulation of the alveolar and bronchial epithelial KATP channel through an autocrine EGF-EGFR loop (144, 145). Activities of members of the Kir channel subfamily are also controlled via phosphorylation by different protein kinases (i.e., PKA, PKC, and tyrosine kinase) (85, 166, 170).
In summary, epithelial K+ channels are controlled by a wide range of parameters, which are, in large part, also involved in the regulation of repair processes. We have described some examples of major epithelial K+ channel functions, especially in transepithelial ion and fluid transport. In subsequent sections, we review the main evidence implicating K+ channels in epithelial repair processes, especially cell migration and proliferation.
Regulation of cell migration by K+ channels.
Although the function of K+ channels has been less studied in nontumoral cell migration than in cancer cells, several reports (see below) indicate that different types of K+ channels, including Kv, KCa, and Kir subfamilies, could control epithelial cell motility.
In intestinal epithelial (IEC-6) cells, derived from rat intestinal crypts, Kv current inhibition with 4-AP or decreased Kv1.1 expression after polyamine depletion reduced cell migration after wounding (154). Wang et al. (154) also demonstrated that a highly differentiated phenotype in stably Cdx2-transfected IEC-6 cells (IEC-Cdx2L1) was associated with heightened Kv (Kv1.1, Kv1.5) channel expression and enhanced cell migration (117). Furthermore, polyamine depletion was followed by decreased K+ channel expression and reduced IEC-Cdx2L1 cell migration (117).
It appears that growth factor-induced cell migration could be controlled by K+ channels. Indeed, in an epithelial tubular cell line (HK2 cells), application of 4-AP or dendrotoxin prevented HGF-induced cell migration (115). Similarly, an effect of 4-AP on basal and EGF-stimulated cell migration was observed in gastric epithelial RGM-1 cells during wound healing (163). In the same cell model, nicotine exposure downregulated Kv1.1 expression and slowed cell migration (137). Our group reported previously that another Kv channel, KvLQT1 (Kv7.1, KCNQ1), regulates the migration of alveolar and bronchial epithelial cells under control and EGF-stimulated conditions (144, 145). Interestingly, upregulation of K+ channels, especially Kv2.1, has been found to promote bone marrow stem cell migration (58), which enhances therapeutic interest in targeting K+ channels, considering the importance of stem cells in epithelial repair.
A role of KCa3.1 (SK4, intermediate-conductance Ca2+-activated K+, and KCNN4) channels in Madin-Darby canine kidney (MDCK) cells has been well established (64, 68, 128, 132, 133). It was first demonstrated that Ba2+, tetraethylammonium, and charybdotoxin (a KCa channel inhibitor) inhibited MDCK-F cell migration (133). A relationship between Ca2+ oscillation and KCa3.1 channel function was then reported in migrating MDCK-F cells (128). Time-lapse videomicroscopy experiments also highlighted a link between FGF-2 signaling and KCa3.1 channel function for the stimulation of MDCK-F cell migration (68). Moreover, Jin et al. (64) determined that HGF-stimulated migration of transformed MDCK-II cells was inhibited by charybdotoxin, clotrimazole, and iberiotoxin, indicating the involvement of KCa3.1 and BKCa channels. Furthermore, we observed reduced EGF-stimulated migration of normal and cystic fibrosis bronchial cells after KCa3.1 channel inhibition (145). Other smaller-conductance Ca2+-activated KCa2.3 (or SK3, KCNN3) channels seem to participate in normal colon cell migration after wounding (112).
Few studies have examined the function of Kir channels in epithelial cell migration. In alveolar epithelial cells, we noted that KATP channel activation with pinacidil significantly enhanced cell migration, while glibenclamide reduced it (144). We then confirmed the role of these channels in the migration of normal and cystic fibrosis bronchial cells (145). The regulation of cell migration by Kir channels has also been investigated in expression systems. For example, Kir4.2 silencing [with short hairpin RNA (shRNA)] decreased mouse embryonic fibroblast cell migration induced by α9β1-integrin expression; this inhibitory effect was reversed after wild-type Kir4.2 rescue (37).
To the best of our knowledge, no evidence of K2P channel involvement in epithelial cell migration has been reported.
Regulation of cell proliferation and differentiation by K+ channels.
One of the first indicators of a mitogenic role of K+ channels probably came from studies on T lymphocytes by DeCoursey and Chandy and their colleagues (26, 36). Subsequently, involvement of K+ in the proliferation of tumoral and nontumoral cells has been extensively documented. We will focus on examples from studies conducted on epithelial cells. Roderick et al. (120) demonstrated that Kv current inhibition by 4-AP reduces rabbit corneal epithelial cell proliferation. 4-AP also prevented HGF-induced growth of HK2 kidney cells (115). In gastric epithelial cells, it has been shown that nicotine exposure is associated with decreased Kv1.1 expression and cell proliferation; both effects are reversed by spermidine (137). In alveolar and bronchial cells, we have shown the role of another Kv channel, KvLQT1, as well as a KATP channel, in the control of cell proliferation (144, 145). Conversely, Schwingshackl et al. (134) reported that Trek-1 silencing with shRNA in MLE-12 mouse alveolar cells resulted in enhanced cell growth.
The impact of K+ channel activation on epithelial cell proliferation has been assessed as well. Interestingly, pinacidil treatment to activate KATP channels significantly stimulated alveolar cell proliferation (144). Similarly, Braun et al. (18) demonstrated that KATP channel activation favors the growth of ureteric bud/nephron culture in vitro.
Of interest for tissue regeneration, K+ channels seem likewise to regulate stem cell growth, as indicated by data showing that human ether-à-go-go (hEAG) and BKCa channel inhibition or silencing significantly decreased the proliferation rates of mesenchymal stem cells (171, 173).
Cell proliferation and differentiation are interrelated, and the balance between these two processes is finely regulated. Accordingly, it would be interesting to determine if there is a relationship between K+ channel activity/expression and epithelial cell differentiation/maturation. Kv channel inhibition with 4-AP and dendrotoxin prevented the morphogenic response to HGF (differentiation and formation of tubular structures) in kidney epithelial cells (115). In addition, modification of KATP and ROMK channel expression profiles has been observed during kidney development. More precisely, Kir6.1 (KATP) mRNA levels are downregulated through the embryonic to postnatal periods, whereas Kir1.1b (ROMK2) expression is markedly stimulated during cortical collecting duct maturation (18). Similarly, Nüsing et al. (95) described heightened ROMK protein expression during kidney maturation. These two studies thus indicated differentiation-dependent ROMK expression. Furthermore, it has been shown that highly differentiated intestinal epithelial cells (stably Cdx2-transfected IEC-6 cells) express higher basal levels of Kv1.1 and Kv1.5 mRNA and protein than parental IEC-6 cells (117). However, it is not always clear if modifications of K+ channel expression/function occur subsequent to changes in cell phenotype or, conversely, if K+ channel modulation could participate in cell differentiation and tissue development processes. In fact, the relationship between K+ channels and differentiation states has also been studied in nonepithelial cells. Köhler et al. (70) showed that a proliferating phenotype in vascular smooth muscle cells is accompanied by a switch of KCa channel expression profiles from BKCa to KCa3.1. Similarly, an upregulation of Kir6.2 expression has been detected upon osteogenic differentiation of mesenchymal stem cells (40). Hofmann et al. (57) reported data indicating that activation of hEAG-related (hERG) K+ currents (Kv11.1, KCNH2) is a determinant signal for osteoclastic differentiation of leukemic FLG 29.1 cells after adhesion on fibronectin (FN) and integrin upregulation. Similarly, hyperpolarization of human cardiomyocyte progenitor cells after low K+ exposure or coculture with Kir2.1-overexpressing cells permitted cardiogenic differentiation (150). This accumulation of evidence indicates a link between K+ channel function and cell differentiation; however, further studies are necessary to clearly define the role of these channels in epithelial cell differentiation and maturation.
Mechanisms responsible for control of cell migration/proliferation by K+ channels.
Blockade of K+ channels by pharmacological inhibitors and/or molecular silencing has demonstrated the crucial function of these channels in epithelial repair processes. Because of the many different K+ channels detected in the same cell type, it may have been expected that inhibition of one particular subtype of K+ channel should be compensated by another. However, this paradox may be explained by the specific electrophysiological and functional characteristics of each type of K+ channel, in particular, its spatial, temporal, and intrinsic regulatory mechanisms (see below). In addition, an impairment of cell migration/proliferation after K+ channel inhibition/silencing implies that these channels are already active and/or that they can be recruited/activated following early mitogenic or motogenic signals induced after injury.
In an intact, polarized epithelium, membrane K+ channels are localized on the apical or basolateral side, where they elicit distinct functions. After injury, a change in cell polarity in healthy migrating and proliferating cells is accompanied by relocalization of many membrane proteins at the front and rear edges of the cell, thus creating functionally distinct cellular domains. Kv1.4 channels stably expressed in migrating MDCK-F cells are preferentially distributed at the leading edge (119). On the other hand, local applications of charybdotoxin allowed Schwab and colleagues (129, 131) to demonstrate that KCa channels localized at the rear edge of MDCK-F cells are involved in their movement.
In addition to such spatial distribution, K+ channel expression/function is also temporally regulated, including during the cell cycle. For example, the electrophysiological properties of EAG channels in heterologous expression systems (Xenopus oocytes and Chinese hamster ovary cells) are modulated during the cell cycle (25, 105). Similarly, changes in EAG and KCa3.1 currents have been observed among cell cycle phases of tumoral epithelial breast cancer cells, and their inhibition interfered with cell cycle progression and proliferation (101). There is proof that K+ channel inhibition impaired epithelial cell proliferation (115, 120, 144, 145), but, to the best of our knowledge, extended evidence of K+ channel modulation during the epithelial cell cycle is still lacking.
There are many reasons to believe that K+ channels are modulated by factors, also described as mitogenic, motogenic, and morphogenic signals, regulating epithelial repair. Indeed, cell migration, proliferation, and differentiation processes are under the control of many proteins, including receptor protein tyrosine kinases (such as growth factor receptors, e.g., EGFR) and nonreceptor kinases (e.g., FAK, Src, and MEK/ERK) (4, 90, 100, 106, 139, 144, 145, 147, 165). An autocrine EGF-EGFR loop, subsequently triggering downstream signaling pathways, has been identified during epithelial wound repair (144, 145, 147, 165). We have shown that EGF stimulation was able to upregulate KvLQT1 and KATP channels in alveolar and bronchial cells (144, 145). It has also been reported that EGF and FGF-2 stimulate 4-AP-sensitive Kv currents in rabbit corneal epithelial cells (120) and KCa3.1 channels in MDCK cells (68), respectively. In addition to these signaling pathways, each K+ channel subfamily is also controlled by specific signals, such as Ca2+ or pH (see Molecular diversity, regulatory mechanisms, and most recognized functions of epithelial K+ channels), which are also involved in the regulation of cell migration and proliferation. Regardless of the signals stimulating K+ channels during healing, one could question how their function could then trigger epithelial repair processes.
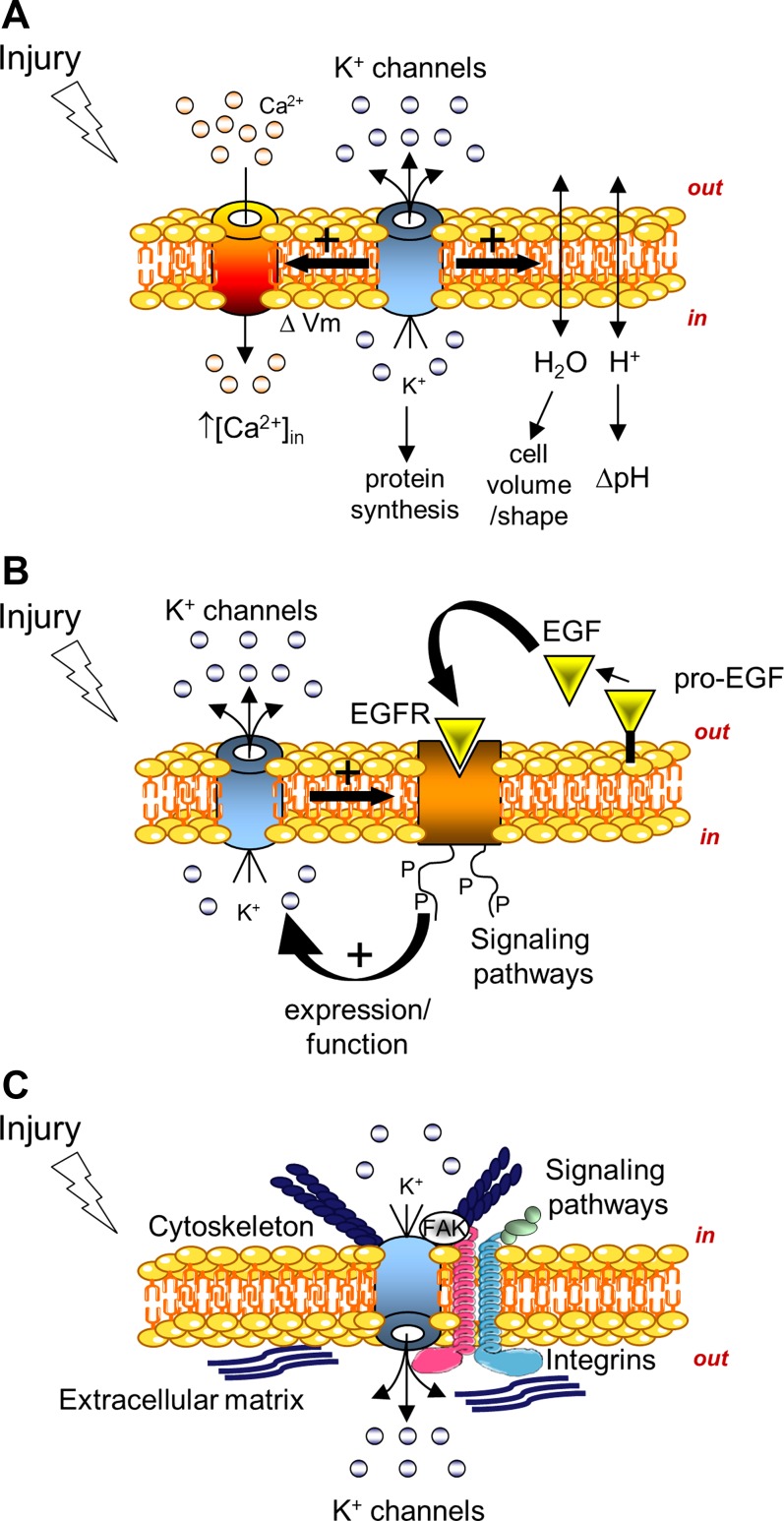
Multiple mechanisms could participate in the regulation of cell migration and proliferation processes by K+ channels. One of the first consequences of K+ channel modulation is a change in membrane potential (Fig. 3A). It has been proposed that transient hyperpolarization after K+ channel activation could be essential for G1 phase progression (162). Thus, cell cycle control by K+ channels could be one of the mechanisms by which they are involved in cell growth regulation. As reviewed in detail by Felipe et al. (43), K+ channel activity is not strictly related to G1 phase progression, since some members may be implicated in the subsequent S and G2 phases. The coupling between K+ channel function and proteins regulating the cell cycle has been further evaluated in mesenchymal stem cells. More precisely, it has been observed that BKCa and EAG channel downregulation affected cell proliferation and cycle progression, probably through inhibition of cyclin D1, cyclin E, ERK, and Akt signaling (173). Cellular assays where membrane potential or K+ gradient has been modified (by application of a K+ ionophore or by decreasing/increasing external K+) have demonstrated a link between K+ and the proliferation/migration of different cells, including epithelial cells (27, 30, 79, 111, 152, 155). It has been proposed that K+ could modulate renal epithelial cell proliferation in another way, i.e., through growth factor release, thus engaging an autocrine stimulation loop (141). In addition, it has been proposed that K+, per se, could be considered a second messenger (99), involved in, for example, protein synthesis (23). Changes in extracellular K+ concentration, membrane potential, and K+ channel activity will also induce pH variations (8, 62), which could, for example, affect integrin function (103). Another consequence of K+ flux modulation could be Ca2+ fluctuations, another key event for cell migration, proliferation, and differentiation (Fig. 3A). Drawing from their data on intestinal epithelial cells, Wang et al. (154) proposed a model of Kv channel upregulation by polyamines, inducing membrane hyperpolarization, which increases the driving force for Ca2+ influx and, consequently, enhances cell migration capacity after wounding. A relationship between cell migration dynamics, Ca2+ signaling, and KCa3.1 channel activity has also been established (for review see Refs. 126 and 127). However, experiments on human embryonic kidney (HEK-293) cells expressing a nonconducting KCa3.1 mutant have indicated that KCa3.1 protein, per se, may also directly regulate cell proliferation through ERK1/2 and JNK signaling (88).
Fig. 3.
Schematic models of possible mechanisms by which K+ channels could control cell migration and proliferation processes. A: changes in membrane potential (ΔVm), cell volume/shape, pH (ΔpH), Ca2+ signal {intracellular Ca2+ concentration ([Ca2+]in)}, or other signaling pathways could trigger the effect of K+ channel modulation on cell migration/proliferation. B: bidirectional autocrine loop linking K+ channels to growth factor receptors, such EGFR, may be another control mechanism of epithelial repair by K+ channels. C: direct interaction between K+ channels and proteins of the migratory machinery such as integrins and cytoskeleton may also occur during wound repair.
Cell shape and volume changes induced, at least in part, by K+ channels are crucial for cell movement and division as well (104, 126). It has been shown that KCa3.1 channels are implicated in cell volume regulation and cytoskeleton rearrangement (132, 153). In migrating cells, K+ efflux through KCa3.1 channel activation has been proposed to cause shrinkage at the pole of migrating cells, with reorganization of the actin cytoskeleton, promoting their movement (126). Coordinated action of different ion/water channels and transporters is necessary for integrated cell volume control at the front and rear edges of migrating cells (for review see Refs. 127 and 130). The relationship between K+ channels and cytoskeleton components seems to be physical and functional. Tian et al. (140) demonstrated interactions between the COOH-terminal pore-forming α-subunit of BKCa channels and cortactin, which created bridges with the cortical actin cytoskeleton. Moreover, the actin-cortactin-BKCa channel complex was shown to regulate BKCa channel activity (140). Similarly, cortactin was found to interact with Kv1.2 channels and to modulate their function and trafficking in HEK-293 cells (161). EAG K+ (Kv10.1, KCNH1) channels established relationships with cytoskeleton elements during the cell cycle. More precisely, microtubule disruption was demonstrated to modify channel rectification during the G2/M phase, while actin cytoskeleton disturbance was accompanied by heightened current density in expression systems (25). In corneal epithelial cells, FN coating enhanced cell adhesion/migration and favored focal adhesion formation at the leading edge. In parallel, FN coating was associated with increased Kv2.1 channels at the leading edge, compared with BSA coating (69).
Activation of growth factor receptors and downstream signaling pathways may be involved in the control of repair processes by K+ channels (Fig. 3B). A relationship between K+ channels and growth factor signaling has been seen in many cell types. It has been reported that EGF potentiates acetylcholine-induced K+ currents in mucous cells from freshly isolated porcine tracheal glands (63). In our laboratory, we have shown that EGF upregulates KATP and KvLQT1 function in alveolar and bronchial cells (144, 145). Moreover, EGF/EGFR autocrine stimulation of cell migration, proliferation, and wound healing depends on KATP and KvLQT1 channel activity (144, 145). Similarly, EGF-stimulated wound healing of rat gastric epithelial cell monolayers is reduced after Kv channel blockade with 4-AP (163). Proliferation of rabbit corneal epithelial cells is regulated by 4-AP-sensitive Kv channels, stimulated by FBS or EGF (120). Similarly, a relationship between HGF/SF and K+ channels has been described. Indeed, HGF/SF has been found to kindle Ca2+-activated K+ currents, necessary for HGF/SF-stimulated MDCK-F cell migration (64). The signaling cascade between Kv channels and IGF-I has been studied in HEK-293 cells. Phosphatidylinositol 3-kinase appears to be involved in Kv channel activity and upregulation of mRNA expression, a prerequisite for stimulation of cell proliferation (45). Other kinases, such as ERK and JNK, were also reported to participate in coupling between K+ channels and mitogenic responses (88).
Several hypotheses have been proposed to explain how K+ channels regulate cell migration and proliferation, for example, through modulation of membrane potential, cell volume, intracellular Ca2+, and signaling pathways (Fig. 3, A and B). However, because these mechanisms are interlinked, it is difficult to isolate the direct consequence of K+ channel modulation on a particular mechanism regulating epithelial repair processes. Further increasing this complexity, it appears that K+ channels may also control cell motility by coupling with migratory machinery proteins, such as integrins and associated signaling molecules (Fig. 3C). Indeed, different types of K+ channels, for example, BKCa, Kv1.3, hERG, GIRK, and Kir4.2, interact with integrins (28, 29, 37, 57, 67, 78, 86, 164, 167). Several studies, especially with expression systems, indicate that coupling between K+ channels and integrins is functional and bidirectional (for review see Refs. 5, 20, 35, and 109). First, K+ channel-integrin complex assembly seems to be modulated by their respective activities. Indeed, it has been shown that interaction between the Kv1.3 channel and β1-integrin, estimated by resonance energy transfer, is promoted by cell adhesion on FN and inhibited by Kv channel blockers (6). In addition, the association between hERG and β1-integrin is strengthened after β1-integrin activation (28). Furthermore, β1- and/or β3-integrin activation has been found to potentiate, for example, hERG and BKCa currents (28, 57, 67, 164, 167). Integrin might regulate K+ channels through Ca2+ signaling, tyrosine (FAK, Src, EGFR, protein tyrosine kinase-2, and JAK2) or serine/threonine (PKC) phosphorylation, phosphatidylinositol 3-kinase, or G proteins (5, 34, 35, 57, 67, 167). On the other hand, K+ channels have been discerned to modulate integrin expression/activation, and it has been postulated that channel opening could be directly transmitted to integrin via conformational coupling or through kinases (FAK and Rac), forming macromolecular complexes with integrin and K+ channels (5, 28, 159). Cherubini et al. (28) reported that hERG inhibition prevented FAK phosphorylation and Rac1 activation after β1-integrin-mediated adhesion on FN. Kv2.1-FAK complex formation, promoted by FN/integrin, has been identified (159). Interestingly, Kv2.1 channel silencing (or mutations in the NH2-terminal domain interacting with FAK) reduces FAK phosphorylation, cell migration, and wound closure in vitro, as well as in a mouse model of corneal repair (159). Such functional coupling between K+ channels and migration machinery proteins has not been explored extensively in epithelial cells.
K+ channels as a therapeutic target in epithelial repair?
As described above, data from the literature clearly indicate that K+ channels are regulators of migration/proliferation of epithelial, as well as progenitor, cells in vitro. These channels could act directly via physical interaction, for example, with integrins, or indirectly through cascades of cellular events, including changes in membrane potential, cell shape/volume, intracellular Ca2+, and other signaling pathways. On the basis of this evidence, it may be postulated that K+ channels play a pivotal role in epithelial regeneration for normal renewal, as well as repair after injury. K+ channels may thus be identified as potential therapeutic targets, with K+ channel activators as promoters of epithelial regeneration. To confirm this hypothesis, good cell and animal models of epithelial repair are necessary. The wound-healing assay, with mechanical or chemical injury, is the most frequently used in vitro model (7, 15, 143, 147, 169). This approach has confirmed that K+ channel activities regulate the wound-healing rates of different epithelial cell types (21, 112, 117, 144, 145, 163). The disadvantage of the assay, performed on epithelial cell monolayers grown on plastic supports, is the absence of cell polarity and differentiation. An alternative procedure consists of epithelial cells cultured on permeant filters for better differentiation and follow-up of epithelial regeneration over a longer time period (15). To the best of our knowledge, direct evaluation of the role of K+ channels in healing of differentiated epithelial cultures has not been reported. Nevertheless, a recent study by Buchanan et al. (21) showed that lipoxin A4 increases the wound-healing rate, as well as KATP currents, in polarized bronchial cells cultured at the air-liquid interface. Further evaluation in nonpolarized cultures on plastic supports indicated involvement of KATP channels in the lipoxin A4-induced stimulation of wound healing and cell migration and proliferation (21).
Thus, direct or indirect in vitro evidence has provided a proof-of-concept that K+ channels could be part of a complex network of regulatory factors influencing wound repair mechanisms. What about their actual involvement in vivo? Several animal models have been developed to study epithelial regeneration after injury, but, to the best of our knowledge, there are no data in the literature proving the role of K+ channels in epithelial tissue regeneration using K+ channel KO mice. Moreover, regarding the huge diversity of K+ channels expressed in epithelia, it is conceivable that alternative K+ channels could compensate for the extinction of one K+ channel type in vivo. However, Wei et al. (159) showed that treatment of mice with Kv2.1 shRNA markedly reduces healing of cornea epithelium after damage. There are also some reports that pharmacological modulation of one class of K+ channels has an impact on epithelial injury/repair parameters in vivo. In a model of murine skin injury, it has been shown that openers of KATP and KCa channels or exposure to the K+ ionophore valinomycin increased the barrier recovery rate, whereas K+ channel blockers slowed it (38). The impact of KATP channel modulation could be mediated, at least in part, through tight junction regulation. Indeed, analysis of the subcellular distribution of KATP channel subunits revealed a colocalization with tight junction proteins in gastric, intestinal, and kidney epithelial tissues (65). The same study also showed that the paracellular intestinal permeability of the intestinal epithelial barrier was altered after KATP channel inhibition, whereas KATP channel activation improved it. In fact, KATP channel function has been quite extensively studied in several gastrointestinal models. The KATP channel opener diazoxide has been shown to attenuate the damage and/or accelerate the restitution of indomethacin- and ethanol-induced intestinal and gastric injury in rats and mice (2, 87, 98, 113, 114, 122, 142), whereas KATP channel inhibition worsened damage parameters. Moreover, the gastroprotective effect of different compounds, such as the steroid saponin hecogenin (122), the antidepressant drug citalopram (124), or prostaglandins (108), seems dependent on KATP channel function. Even if a protective role of the KATP channel has been well established, it is difficult to clearly establish which mechanisms are involved in the phenomenon. Because of the wide distribution of KATP channels, in an animal model, it is indeed complex to clearly define if this beneficial effect is due to the function of KATP channels located at the plasma membrane or in mitochondria, as well as the relative contribution of the KATP channel, for example, on epithelial tissue, the cardiovascular system, or immune cell function. Moreover, this ubiquitous expression of some members of the K+ channel family could further increase the possibility of side effects. Thus the development of new approaches for the tissue-specific administration of K+ modulators would represent significant progress, with a therapeutic strategy targeting K+ channels. Because of the diversity of K+ channels in epithelial cells, it will also be important to define the precise role and relative contribution of each K+ channel to target the best candidates in each tissue. Finally, before K+ channel activators can be administered therapeutically to improve epithelial repair, much work is needed to bypass the lack of specificity of some of these drugs. Indeed, further studies of K+ channel three-dimensional structure will allow a better understanding of their binding sites and development of drugs that will be more efficient and specific to each K+ channel subtype. Because of the large number of publications and patents involving the use of K+ channels as targets in several different diseases (9, 33, 47, 66, 118), there is no doubt that improved tools targeting K+ channels will progressively be developed. In parallel, knowledge of K+ channel function in epithelial repair and tissue regeneration will probably increase exponentially within the next decade.
GRANTS
This work was supported by Canadian Institutes of Health Research Grant MOP-111054, Cystic Fibrosis Canada, the Natural Sciences and Engineering Research Council of Canada, a scholarship from the Centre de Recherche du Centre Hospitalier de l'Université de Montréal (CRCHUM) and Université de Montréal (E. Brochiero), and fellowships from the Canadian Institutes of Health Research training program of the Respiratory Health Network and Fonds de la Recherche du Québec en Santé (A. Girault).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.G. and E.B. prepared the figures; A.G. and E.B. drafted the manuscript; A.G. and E.B. edited and revised the manuscript; A.G. and E.B. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors acknowledge logistical assistance from the Research Support Office at the Centre de Recherche du Centre Hospitalier de l'Université de Montréal (CRCHUM).
REFERENCES
- 1.Adamson IY, Bowden DH. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest 30: 35–42, 1974 [PubMed] [Google Scholar]
- 2.Akar F, Uydes-Dogan BS, Buharalioglu CK, Abban G, Heinemann A, Holzer P, Van de Voorde J. Protective effect of cromakalim and diazoxide and proulcerogenic effect of glibenclamide on indomethacin-induced gastric injury. Eur J Pharmacol 374: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Alzamora R, Gong F, Rondanino C, Lee JK, Smolak C, Pastor-Soler NM, Hallows KR. AMP-activated protein kinase inhibits KCNQ1 channels through regulation of the ubiquitin ligase Nedd4-2 in renal epithelial cells. Am J Physiol Renal Physiol 299: F1308–F1319, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andre F, Rigot V, Thimonier J, Montixi C, Parat F, Pommier G, Marvaldi J, Luis J. Integrins and E-cadherin cooperate with IGF-I to induce migration of epithelial colonic cells. Int J Cancer 83: 497–505, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Arcangeli A, Becchetti A. Complex functional interaction between integrin receptors and ion channels. Trends Cell Biol 16: 631–639, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Artym VV, Petty HR. Molecular proximity of Kv1.3 voltage-gated potassium channels and β1-integrins on the plasma membrane of melanoma cells: effects of cell adherence and channel blockers. J Gen Physiol 120: 29–37, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atabai K, Ishigaki M, Geiser T, Ueki I, Matthay MA, Ware LB. Keratinocyte growth factor can enhance alveolar epithelial repair by nonmitogenic mechanisms. Am J Physiol Lung Cell Mol Physiol 283: L163–L169, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Austin C, Wray S. Changes of intracellular pH in rat mesenteric vascular smooth muscle with high-K+ depolarization. J Physiol 469: 1–10, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balut CM, Hamilton KL, Devor DC. Trafficking of intermediate (KCa3.1) and small (KCa2x) conductance, Ca2+-activated K+ channels: a novel target for medicinal chemistry efforts? Chem Med Chem 7: 1741–1755, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bardou O, Prive A, Migneault F, Roy-Camille K, Dagenais A, Berthiaume Y, Brochiero E. K+ channels regulate ENaC expression via changes in promoter activity and control fluid clearance in alveolar epithelial cells. Biochim Biophys Acta 1818: 1682–1690, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Bardou O, Trinh NT, Brochiero E. Molecular diversity and function of K+ channels in airway and alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 296: L145–L155, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Beck JS, Hurst AM, Lapointe JY, Laprade R. Regulation of basolateral K channels in proximal tubule studied during continuous microperfusion. Am J Physiol Renal Fluid Electrolyte Physiol 264: F496–F501, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Bernard K, Bogliolo S, Soriani O, Ehrenfeld J. Modulation of calcium-dependent chloride secretion by basolateral SK4-like channels in a human bronchial cell line. J Membr Biol 196: 15–31, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Bleich M, Shan QX. Epithelial K+ channels: driving force generation and K+ recycling for epithelial transport with physiological and clinical implications. Sheng Li Xue Bao 59: 443–453, 2007 [PubMed] [Google Scholar]
- 15.Bonnomet A, Terryn C, Cutrona J, Jonquet A, Birembaut P, Zahm JM. Analysis of cell dispersion and migration by video-microscopy. Methods Enzymol 505: 233–254, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest 121: 4210–4221, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boucherot A, Schreiber R, Kunzelmann K. Regulation and properties of KCNQ1 (KVLQT1) and impact of the cystic fibrosis transmembrane conductance regulator. J Membr Biol 182: 39–47, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Braun GS, Veh RW, Segerer S, Horster MF, Huber SM. Developmental expression and functional significance of Kir channel subunits in ureteric bud and nephron epithelia. Pflügers Arch 445: 321–330, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Brochiero E, Wallendorf B, Gagnon D, Laprade R, Lapointe JY. Cloning of rabbit Kir6.1, SUR2A, and SUR2B: possible candidates for a renal KATP channel. Am J Physiol Renal Physiol 282: F289–F300, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Brown SB, Dransfield I. Electric fields and inflammation: may the force be with you. Sci World J 8: 1280–1294, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchanan PJ, McNally P, Harvey BJ, Urbach V. Lipoxin A4-mediated KATP potassium channel activation results in cystic fibrosis airway epithelial repair. Am J Physiol Lung Cell Mol Physiol 305: L193–L201, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Cabodi S, Di Stefano P, Leal MP, Tinnirello A, Bisaro B, Morello V, Damiano L, Aramu S, Repetto D, Tornillo G, Defilippi P. Integrins and signal transduction. Adv Exp Med Biol 674: 43–54, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Cahn F, Lubin M. Inhibition of elongation steps of protein synthesis at reduced potassium concentrations in reticulocytes and reticulocyte lysate. J Biol Chem 253: 7798–7803, 1978 [PubMed] [Google Scholar]
- 24.Cakarova L, Marsh LM, Wilhelm J, Mayer K, Grimminger F, Seeger W, Lohmeyer J, Herold S. Macrophage tumor necrosis factor-α induces epithelial expression of granulocyte-macrophage colony-stimulating factor: impact on alveolar epithelial repair. Am J Respir Crit Care Med 180: 521–532, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Camacho J, Sanchez A, Stuhmer W, Pardo LA. Cytoskeletal interactions determine the electrophysiological properties of human EAG potassium channels. Pflügers Arch 441: 167–174, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Chandy KG, DeCoursey TE, Cahalan MD, McLaughlin C, Gupta S. Voltage-gated potassium channels are required for human T lymphocyte activation. J Exp Med 160: 369–385, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chantome A, Girault A, Potier M, Collin C, Vaudin P, Pages JC, Vandier C, Joulin V. KCa2.3 channel-dependent hyperpolarization increases melanoma cell motility. Exp Cell Res 315: 3620–3630, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Cherubini A, Hofmann G, Pillozzi S, Guasti L, Crociani O, Cilia E, Di Stefano P, Degani S, Balzi M, Olivotto M, Wanke E, Becchetti A, Defilippi P, Wymore R, Arcangeli A. Human ether-á-go-go-related gene 1 channels are physically linked to β1-integrins and modulate adhesion-dependent signaling. Mol Biol Cell 16: 2972–2983, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cherubini A, Pillozzi S, Hofmann G, Crociani O, Guasti L, Lastraioli E, Polvani S, Masi A, Becchetti A, Wanke E, Olivotto M, Arcangeli A. HERG K+ channels and β1-integrins interact through the assembly of a macromolecular complex. Ann NY Acad Sci 973: 559–561, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Chilton L, Ohya S, Freed D, George E, Drobic V, Shibukawa Y, MacCannell KA, Imaizumi Y, Clark RB, Dixon IM, Giles WR. K+ currents regulate the resting membrane potential, proliferation, and contractile responses in ventricular fibroblasts and myofibroblasts. Am J Physiol Heart Circ Physiol 288: H2931–H2939, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Cowley EA, Linsdell P. Characterization of basolateral K+ channels underlying anion secretion in the human airway cell line Calu-3. J Physiol 538: 747–757, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 298: L715–L731, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Amico M, Gasparoli L, Arcangeli A. Potassium channels: novel emerging biomarkers and targets for therapy in cancer. Recent Pat Anticancer Drug Discov 8: 53–65, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Gui P, Hill MA, Wilson E. Regulation of ion channels by protein tyrosine phosphorylation. Am J Physiol Heart Circ Physiol 281: H1835–H1862, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Gui P, Hill MA, Wilson E. Regulation of ion channels by integrins. Cell Biochem Biophys 36: 41–66, 2002 [DOI] [PubMed] [Google Scholar]
- 36.DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature 307: 465–468, 1984 [DOI] [PubMed] [Google Scholar]
- 37.deHart GW, Jin T, McCloskey DE, Pegg AE, Sheppard D. The α9β1-integrin enhances cell migration by polyamine-mediated modulation of an inward-rectifier potassium channel. Proc Natl Acad Sci USA 105: 7188–7193, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denda M, Tsutsumi M, Inoue K, Crumrine D, Feingold KR, Elias PM. Potassium channel openers accelerate epidermal barrier recovery. Br J Dermatol 157: 888–893, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Devor DC, Bridges RJ, Pilewski JM. Pharmacological modulation of ion transport across wild-type and ΔF508 CFTR-expressing human bronchial epithelia. Am J Physiol Cell Physiol 279: C461–C479, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Diehlmann A, Bork S, Saffrich R, Veh RW, Wagner W, Derst C. KATP channels in mesenchymal stromal stem cells: strong up-regulation of Kir6.2 subunits upon osteogenic differentiation. Tissue Cell 43: 331–336, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Dube L, Parent L, Sauve R. Hypotonic shock activates a maxi K+ channel in primary cultured proximal tubule cells. Am J Physiol Renal Fluid Electrolyte Physiol 259: F348–F356, 1990 [DOI] [PubMed] [Google Scholar]
- 42.Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J 16: 5464–5471, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Felipe A, Vicente R, Villalonga N, Roura-Ferrer M, Martinez-Marmol R, Sole L, Ferreres JC, Condom E. Potassium channels: new targets in cancer therapy. Cancer Detect Prev 30: 375–385, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Finigan JH, Downey GP, Kern JA. HER receptor signaling in acute lung injury. Am J Respir Cell Mol Biol 47: 395–404, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gamper N, Fillon S, Huber SM, Feng Y, Kobayashi T, Cohen P, Lang F. IGF-1 up-regulates K+ channels via PI3-kinase, PDK1 and SGK1. Pflügers Arch 443: 625–634, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Gilles C, Polette M, Coraux C, Tournier JM, Meneguzzi G, Munaut C, Volders L, Rousselle P, Birembaut P, Foidart JM. Contribution of MT1-MMP and of human laminin-5 γ2-chain degradation to mammary epithelial cell migration. J Cell Sci 114: 2967–2976, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Girault A, Haelters JP, Potier-Cartereau M, Chantome A, Jaffres PA, Bougnoux P, Joulin V, Vandier C. Targeting SKCa channels in cancer: potential new therapeutic approaches. Curr Med Chem 19: 697–713, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Gomperts BN, Belperio JA, Fishbein MC, Keane MP, Burdick MD, Strieter RM. Keratinocyte growth factor improves repair in the injured tracheal epithelium. Am J Respir Cell Mol Biol 37: 48–56, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grahammer F, Herling AW, Lang HJ, Schmitt-Graff A, Wittekindt OH, Nitschke R, Bleich M, Barhanin J, Warth R. The cardiac K+ channel KCNQ1 is essential for gastric acid secretion. Gastroenterology 120: 1363–1371, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Grahammer F, Warth R, Barhanin J, Bleich M, Hug MJ. The small conductance K+ channel, KCNQ1: expression, function, and subunit composition in murine trachea. J Biol Chem 276: 42268–42275, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Grgic I, Kiss E, Kaistha BP, Busch C, Kloss M, Sautter J, Muller A, Kaistha A, Schmidt C, Raman G, Wulff H, Strutz F, Grone HJ, Kohler R, Hoyer J. Renal fibrosis is attenuated by targeted disruption of KCa3.1 potassium channels. Proc Natl Acad Sci USA 106: 14518–14523, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grose R, Hutter C, Bloch W, Thorey I, Watt FM, Fassler R, Brakebusch C, Werner S. A crucial role of β1-integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 129: 2303–2315, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Hamilton KL, Devor DC. Basolateral membrane K+ channels in renal epithelial cells. Am J Physiol Renal Physiol 302: F1069–F1081, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamilton KL, Meads L, Butt AG. 1-EBIO stimulates Cl− secretion by activating a basolateral K+ channel in the mouse jejunum. Pflügers Arch 439: 158–166, 1999 [DOI] [PubMed] [Google Scholar]
- 55.Heitzmann D, Warth R. Physiology and pathophysiology of potassium channels in gastrointestinal epithelia. Physiol Rev 88: 1119–1182, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Herard AL, Pierrot D, Hinnrasky J, Kaplan H, Sheppard D, Puchelle E, Zahm JM. Fibronectin and its α5β1-integrin receptor are involved in the wound-repair process of airway epithelium. Am J Physiol Lung Cell Mol Physiol 271: L726–L733, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Hofmann G, Bernabei PA, Crociani O, Cherubini A, Guasti L, Pillozzi S, Lastraioli E, Polvani S, Bartolozzi B, Solazzo V, Gragnani L, Defilippi P, Rosati B, Wanke E, Olivotto M, Arcangeli A. HERG K+ channels activation during β1-integrin-mediated adhesion to fibronectin induces an up-regulation of αvβ3-integrin in the preosteoclastic leukemia cell line FLG 29.1. J Biol Chem 276: 4923–4931, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Hu X, Wei L, Taylor TM, Wei J, Zhou X, Wang JA, Yu SP. Hypoxic preconditioning enhances bone marrow mesenchymal stem cell migration via Kv2.1 channel and FAK activation. Am J Physiol Cell Physiol 301: C362–C372, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang Y, Rane SG. Potassium channel induction by the Ras/Raf signal transduction cascade. J Biol Chem 269: 31183–31189, 1994 [PubMed] [Google Scholar]
- 60.Hurst AM, Beck JS, Laprade R, Lapointe JY. Na+ pump inhibition downregulates an ATP-sensitive K+ channel in rabbit proximal convoluted tubule. Am J Physiol Renal Fluid Electrolyte Physiol 264: F760–F764, 1993 [DOI] [PubMed] [Google Scholar]
- 61.Iizuka M, Konno S. Wound healing of intestinal epithelial cells. World J Gastroenterol 17: 2161–2171, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ikuma M, Binder HJ, Geibel J. Role of apical H-K exchange and basolateral K channel in the regulation of intracellular pH in rat distal colon crypt cells. J Membr Biol 166: 205–212, 1998 [DOI] [PubMed] [Google Scholar]
- 63.Iwase N, Sasaki T, Oshiro T, Tamada T, Nara M, Sasamori K, Hattori T, Shirato K, Maruyama Y. Differential effect of epidermal growth factor on serous and mucous cells in porcine airway submucosal gland. Respir Physiol Neurobiol 132: 307–319, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Jin M, Defoe DM, Wondergem R. Hepatocyte growth factor/scatter factor stimulates Ca2+-activated membrane K+ current and migration of MDCK II cells. J Membr Biol 191: 77–86, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Jons T, Wittschieber D, Beyer A, Meier C, Brune A, Thomzig A, Ahnert-Hilger G, Veh RW. K+-ATP-channel-related protein complexes: potential transducers in the regulation of epithelial tight junction permeability. J Cell Sci 119: 3087–3097, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Judge SI, Smith PJ. Patents related to therapeutic activation of KATP and K2P potassium channels for neuroprotection: ischemic/hypoxic/anoxic injury and general anesthetics. Expert Opin Ther Pat 19: 433–460, 2009 [DOI] [PubMed] [Google Scholar]
- 67.Kawasaki J, Davis GE, Davis MJ. Regulation of Ca2+-dependent K+ current by αvβ3-integrin engagement in vascular endothelium. J Biol Chem 279: 12959–12966, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Kessler W, Budde T, Gekle M, Fabian A, Schwab A. Activation of cell migration with fibroblast growth factor-2 requires calcium-sensitive potassium channels. Pflügers Arch 456: 813–823, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Kimura K, Kawano S, Mori T, Inoue J, Hadachi H, Saito T, Nishida T. Quantitative analysis of the effects of extracellular matrix proteins on membrane dynamics associated with corneal epithelial cell motility. Invest Ophthalmol Vis Sci 51: 4492–4499, 2010 [DOI] [PubMed] [Google Scholar]
- 70.Köhler R, Wulff H, Eichler I, Kneifel M, Neumann D, Knorr A, Grgic I, Kampfe D, Si H, Wibawa J, Real R, Borner K, Brakemeier S, Orzechowski HD, Reusch HP, Paul M, Chandy KG, Hoyer J. Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation 108: 1119–1125, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Koivisto L, Larjava K, Hakkinen L, Uitto VJ, Heino J, Larjava H. Different integrins mediate cell spreading, haptotaxis and lateral migration of HaCaT keratinocytes on fibronectin. Cell Adhes Commun 7: 245–257, 1999 [DOI] [PubMed] [Google Scholar]
- 72.Kunzelmann K, Hubner M, Schreiber R, Levy-Holzman R, Garty H, Bleich M, Warth R, Slavik M, von Hahn T, Greger R. Cloning and function of the rat colonic epithelial K+ channel KVLQT1. J Membr Biol 179: 155–164, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Lang F, Foller M, Lang K, Lang P, Ritter M, Vereninov A, Szabo I, Huber SM, Gulbins E. Cell volume regulatory ion channels in cell proliferation and cell death. Methods Enzymol 428: 209–225, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell 84: 359–369, 1996 [DOI] [PubMed] [Google Scholar]
- 75.Legrand C, Gilles C, Zahm JM, Polette M, Buisson AC, Kaplan H, Birembaut P, Tournier JM. Airway epithelial cell migration dynamics. MMP-9 role in cell-extracellular matrix remodeling. J Cell Biol 146: 517–529, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leroy C, Dagenais A, Berthiaume Y, Brochiero E. Molecular identity and function in transepithelial transport of KATP channels in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 286: L1027–L1037, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Leroy C, Prive A, Bourret JC, Berthiaume Y, Ferraro P, Brochiero E. Regulation of ENaC and CFTR expression with K+ channel modulators and effect on fluid absorption across alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 291: L1207–L1219, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Levite M, Cahalon L, Peretz A, Hershkoviz R, Sobko A, Ariel A, Desai R, Attali B, Lider O. Extracellular K+ and opening of voltage-gated potassium channels activate T cell integrin function: physical and functional association between Kv13 channels and β1-integrins. J Exp Med 191: 1167–1176, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.MacFarlane SN, Sontheimer H. Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia 30: 39–48, 2000 [DOI] [PubMed] [Google Scholar]
- 80.Magni M, Meldolesi J, Pandiella A. Ionic events induced by epidermal growth factor. Evidence that hyperpolarization and stimulated cation influx play a role in the stimulation of cell growth. J Biol Chem 266: 6329–6335, 1991 [PubMed] [Google Scholar]
- 81.Maille E, Trinh NT, Prive A, Bilodeau C, Bissonnette E, Grandvaux N, Brochiero E. Regulation of normal and cystic fibrosis airway epithelial repair processes by TNF-α after injury. Am J Physiol Lung Cell Mol Physiol 301: L945–L955, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Mall M, Gonska T, Thomas J, Schreiber R, Seydewitz HH, Kuehr J, Brandis M, Kunzelmann K. Modulation of Ca2+-activated Cl− secretion by basolateral K+ channels in human normal and cystic fibrosis airway epithelia. Pediatr Res 53: 608–618, 2003 [DOI] [PubMed] [Google Scholar]
- 83.Mall M, Wissner A, Schreiber R, Kuehr J, Seydewitz HH, Brandis M, Greger R, Kunzelmann K. Role of KVLQT1 in cyclic adenosine monophosphate-mediated Cl− secretion in human airway epithelia. Am J Respir Cell Mol Biol 23: 283–289, 2000 [DOI] [PubMed] [Google Scholar]
- 84.Manzanares D, Gonzalez C, Ivonnet P, Chen RS, Valencia-Gattas M, Conner GE, Larsson HP, Salathe M. Functional apical large conductance, Ca2+-activated, and voltage-dependent K+ channels are required for maintenance of airway surface liquid volume. J Biol Chem 286: 19830–19839, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mauerer UR, Boulpaep EL, Segal AS. Regulation of an inwardly rectifying ATP-sensitive K+ channel in the basolateral membrane of renal proximal tubule. J Gen Physiol 111: 161–180, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McPhee JC, Dang YL, Davidson N, Lester HA. Evidence for a functional interaction between integrins and G protein-activated inward rectifier K+ channels. J Biol Chem 273: 34696–34702, 1998 [DOI] [PubMed] [Google Scholar]
- 87.Menozzi A, Pozzoli C, Poli E, Passeri B, Gianelli P, Bertini S. Diazoxide attenuates indomethacin-induced small intestinal damage in the rat. Eur J Pharmacol 650: 378–383, 2011 [DOI] [PubMed] [Google Scholar]
- 88.Millership JE, Devor DC, Hamilton KL, Balut CM, Bruce JI, Fearon IM. Calcium-activated K+ channels increase cell proliferation independent of K+ conductance. Am J Physiol Cell Physiol 300: C792–C802, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294: L152–L160, 2008 [DOI] [PubMed] [Google Scholar]
- 90.Myhre GM, Toruner M, Abraham S, Egan LJ. Metalloprotease disintegrin-mediated ectodomain shedding of EGFR ligands promotes intestinal epithelial restitution. Am J Physiol Gastrointest Liver Physiol 287: G1213–G1219, 2004 [DOI] [PubMed] [Google Scholar]
- 91.Nie X, Arrighi I, Kaissling B, Pfaff I, Mann J, Barhanin J, Vallon V. Expression and insights on function of potassium channel TWIK-1 in mouse kidney. Pflügers Arch 451: 479–488, 2005 [DOI] [PubMed] [Google Scholar]
- 92.Noel J, Sandoz G, Lesage F. Molecular regulations governing TREK and TRAAK channel functions. Channels (Austin) 5: 402–409, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Noulin JF, Brochiero E, Coady MJ, Laprade R, Lapointe JY. Molecular identity and regulation of renal potassium channels. Jpn J Physiol 51: 631–647, 2001 [DOI] [PubMed] [Google Scholar]
- 94.Noulin JF, Brochiero E, Lapointe JY, Laprade R. Two types of K+ channels at the basolateral membrane of proximal tubule: inhibitory effect of taurine. Am J Physiol Renal Physiol 277: F290–F297, 1999 [DOI] [PubMed] [Google Scholar]
- 95.Nüsing RM, Pantalone F, Grone HJ, Seyberth HW, Wegmann M. Expression of the potassium channel ROMK in adult and fetal human kidney. Histochem Cell Biol 123: 553–559, 2005 [DOI] [PubMed] [Google Scholar]
- 96.O'Grady SM, Lee SY. Molecular diversity and function of voltage-gated (Kv) potassium channels in epithelial cells. Int J Biochem Cell Biol 37: 1578–1594, 2005 [DOI] [PubMed] [Google Scholar]
- 97.O'Mahony F, Alzamora R, Betts V, LaPaix F, Carter D, Irnaten M, Harvey BJ. Female gender-specific inhibition of KCNQ1 channels and chloride secretion by 17β-estradiol in rat distal colonic crypts. J Biol Chem 282: 24563–24573, 2007 [DOI] [PubMed] [Google Scholar]
- 98.Oliveira IS, da Silva FV, Viana AF, dos Santos MR, Quintans-Junior LJ, Martins MC, Nunes PH, Oliveira FA, Oliveira RC. Gastroprotective activity of carvacrol on experimentally induced gastric lesions in rodents. Naunyn Schmiedebergs Arch Pharmacol 385: 899–908, 2012 [DOI] [PubMed] [Google Scholar]
- 99.Orlov SN, Hamet P. Intracellular monovalent ions as second messengers. J Membr Biol 210: 161–172, 2006 [DOI] [PubMed] [Google Scholar]
- 100.Osaki LH, Gama P. MAPKs and signal transduction in the control of gastrointestinal epithelial cell proliferation and differentiation. Int J Mol Sci 14: 10143–10161, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ouadid-Ahidouch H, Roudbaraki M, Delcourt P, Ahidouch A, Joury N, Prevarskaya N. Functional and molecular identification of intermediate-conductance Ca2+-activated K+ channels in breast cancer cells: association with cell cycle progression. Am J Physiol Cell Physiol 287: C125–C134, 2004 [DOI] [PubMed] [Google Scholar]
- 102.Pan Q, Ma J, Zhou Q, Li J, Tang Y, Liu Y, Yang Y, Xiao J, Peng L, Li P, Liang D, Zhang H, Chen YH. KCNQ1 loss-of-function mutation impairs gastric acid secretion in mice. Mol Biol Rep 37: 1329–1333, 2010 [DOI] [PubMed] [Google Scholar]
- 103.Paradise RK, Lauffenburger DA, Van Vliet KJ. Acidic extracellular pH promotes activation of integrin-αvβ3. PLoS One 6: e15746, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pardo LA. Voltage-gated potassium channels in cell proliferation. Physiology (Bethesda) 19: 285–292, 2004 [DOI] [PubMed] [Google Scholar]
- 105.Pardo LA, Bruggemann A, Camacho J, Stuhmer W. Cell cycle-related changes in the conducting properties of r-eag K+ channels. J Cell Biol 143: 767–775, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parrales A, Lopez E, Lee-Rivera I, Lopez-Colome AM. ERK1/2-dependent activation of mTOR/mTORC1/p70S6K regulates thrombin-induced RPE cell proliferation. Cell Signal 25: 829–838, 2013 [DOI] [PubMed] [Google Scholar]
- 107.Paulais M, Lachheb S, Teulon J. A Na+- and Cl−-activated K+ channel in the thick ascending limb of mouse kidney. J Gen Physiol 127: 205–215, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peskar BM, Ehrlich K, Peskar BA. Role of ATP-sensitive potassium channels in prostaglandin-mediated gastroprotection in the rat. J Pharmacol Exp Ther 301: 969–974, 2002 [DOI] [PubMed] [Google Scholar]
- 109.Pillozzi S, Arcangeli A. Physical and functional interaction between integrins and hERG1 channels in cancer cells. Adv Exp Med Biol 674: 55–67, 2010 [DOI] [PubMed] [Google Scholar]
- 110.Planus E, Galiacy S, Matthay M, Laurent V, Gavrilovic J, Murphy G, Clerici C, Isabey D, Lafuma C, d'Ortho MP. Role of collagenase in mediating in vitro alveolar epithelial wound repair. J Cell Sci 112: 243–252, 1999 [DOI] [PubMed] [Google Scholar]
- 111.Potier M, Joulin V, Roger S, Besson P, Jourdan ML, Leguennec JY, Bougnoux P, Vandier C. Identification of SK3 channel as a new mediator of breast cancer cell migration. Mol Cancer Ther 5: 2946–2953, 2006 [DOI] [PubMed] [Google Scholar]
- 112.Potier M, Tran TA, Chantome A, Girault A, Joulin V, Bougnoux P, Vandier C, Pierre F. Altered SK3/KCa2.3-mediated migration in adenomatous polyposis coli (Apc) mutated mouse colon epithelial cells. Biochem Biophys Res Commun 397: 42–47, 2010 [DOI] [PubMed] [Google Scholar]
- 113.Rahgozar M, Pazokitoroudi H, Bakhtiarian A, Djahanguiri B. Diazoxide, a KATP opener, accelerates restitution of ethanol or indomethacin-induced gastric ulceration in rats independent of polyamines. J Gastroenterol Hepatol 16: 290–296, 2001 [DOI] [PubMed] [Google Scholar]
- 114.Rahgozar M, Toroudi HR, Bakhtiarian A, Djahanguiri B. Diazoxide, a KATP channel opener, prevented ethanol-induced gastric ulceration in rats. Iranian J Pharmacol Ther 1: 2002 [Google Scholar]
- 115.Rampino T, Gregorini M, Guidetti C, Broggini M, Marchini S, Bonomi R, Maggio M, Roscini E, Soccio G, Tiboldo R, Dal CA. KCNA1 and TRPC6 ion channels and NHE1 exchanger operate the biological outcome of HGF/scatter factor in renal tubular cells. Growth Factors 25: 382–391, 2007 [DOI] [PubMed] [Google Scholar]
- 116.Rane SG. A Ca2+-activated K+ current in ras-transformed fibroblasts is absent from nontransformed cells. Am J Physiol Cell Physiol 260: C104–C112, 1991 [DOI] [PubMed] [Google Scholar]
- 117.Rao JN, Platoshyn O, Li L, Guo X, Golovina VA, Yuan JX, Wang JY. Activation of K+ channels and increased migration of differentiated intestinal epithelial cells after wounding. Am J Physiol Cell Physiol 282: C885–C898, 2002 [DOI] [PubMed] [Google Scholar]
- 118.Rapposelli S. Novel adenosine 5′-triphosphate-sensitive potassium channel ligands: a patent overview (2005–2010). Expert Opin Ther Pat 21: 355–379, 2011 [DOI] [PubMed] [Google Scholar]
- 119.Reinhardt J, Golenhofen N, Pongs O, Oberleithner H, Schwab A. Migrating transformed MDCK cells are able to structurally polarize a voltage-activated K+ channel. Proc Natl Acad Sci USA 95: 5378–5382, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roderick C, Reinach PS, Wang L, Lu L. Modulation of rabbit corneal epithelial cell proliferation by growth factor-regulated K+ channel activity. J Membr Biol 196: 41–50, 2003 [DOI] [PubMed] [Google Scholar]
- 121.Sakuma T, Takahashi K, Ohya N, Nakada T, Matthay MA. Effects of ATP-sensitive potassium channel opener on potassium transport and alveolar fluid clearance in the resected human lung. Pharmacol Toxicol 83: 16–22, 1998 [DOI] [PubMed] [Google Scholar]
- 122.Santos CG, dos Santos e Silva, Rios VE, Fragoso de Freitas AP, Arcanjo MB, Silveira MD, Lopes SA, Barbosa Filho JM, de Almeida Leal LK, de Castro Brito GA, Souccar C, de Barros Viana GS. Effects of hecogenin and its possible mechanism of action on experimental models of gastric ulcer in mice. Eur J Pharmacol 683: 260–269, 2012 [DOI] [PubMed] [Google Scholar]
- 123.Sausbier M, Matos JE, Sausbier U, Beranek G, Arntz C, Neuhuber W, Ruth P, Leipziger J. Distal colonic K+ secretion occurs via BK channels. J Am Soc Nephrol 17: 1275–1282, 2006 [DOI] [PubMed] [Google Scholar]
- 124.Saxena B, Singh S. Investigations on gastroprotective effect of citalopram, an antidepressant drug, against stress and pyloric ligation induced ulcers. Pharmacol Rep 63: 1413–1426, 2011 [DOI] [PubMed] [Google Scholar]
- 125.Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch TJ. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature 403: 196–199, 2000 [DOI] [PubMed] [Google Scholar]
- 126.Schwab A. Ion channels and transporters on the move. News Physiol Sci 16: 29–33, 2001 [DOI] [PubMed] [Google Scholar]
- 127.Schwab A, Fabian A, Hanley PJ, Stock C. Role of ion channels and transporters in cell migration. Physiol Rev 92: 1865–1913, 2012 [DOI] [PubMed] [Google Scholar]
- 128.Schwab A, Finsterwalder F, Kersting U, Danker T, Oberleithner H. Intracellular Ca2+ distribution in migrating transformed epithelial cells. Pflügers Arch 434: 70–76, 1997 [DOI] [PubMed] [Google Scholar]
- 129.Schwab A, Gabriel K, Finsterwalder F, Folprecht G, Greger R, Kramer A, Oberleithner H. Polarized ion transport during migration of transformed Madin-Darby canine kidney cells. Pflügers Arch 430: 802–807, 1995 [DOI] [PubMed] [Google Scholar]
- 130.Schwab A, Nechyporuk-Zloy V, Fabian A, Stock C. Cells move when ions and water flow. Pflügers Arch 453: 421–432, 2007 [DOI] [PubMed] [Google Scholar]
- 131.Schwab A, Oberleithner H. Plasticity of renal epithelial cells: the way a potassium channel supports migration. Pflügers Arch 432: R87–R93, 1996 [PubMed] [Google Scholar]
- 132.Schwab A, Schuricht B, Seeger P, Reinhardt J, Dartsch PC. Migration of transformed renal epithelial cells is regulated by K+ channel modulation of actin cytoskeleton and cell volume. Pflügers Arch 438: 330–337, 1999 [DOI] [PubMed] [Google Scholar]
- 133.Schwab A, Wojnowski L, Gabriel K, Oberleithner H. Oscillating activity of a Ca2+-sensitive K+ channel. A prerequisite for migration of transformed Madin-Darby canine kidney focus cells. J Clin Invest 93: 1631–1636, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schwingshackl A, Teng B, Ghosh M, West AN, Makena P, Gorantla V, Sinclair SE, Waters CM. Regulation and function of the two-pore-domain (K2P) potassium channel Trek-1 in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 302: L93–L102, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sheppard D. Epithelial integrins. Bioessays 18: 655–660, 1996 [DOI] [PubMed] [Google Scholar]
- 136.Shimabukuro DW, Sawa T, Gropper MA. Injury and repair in lung and airways. Crit Care Med 31: S524–S531, 2003 [DOI] [PubMed] [Google Scholar]
- 137.Shin VY, Liu ES, Koo MW, Luo JC, So WH, Cho CH. Nicotine suppresses gastric wound repair via the inhibition of polyamine and K+ channel expression. Eur J Pharmacol 444: 115–121, 2002 [DOI] [PubMed] [Google Scholar]
- 138.Singh S, Syme CA, Singh AK, Devor DC, Bridges RJ. Benzimidazolone activators of chloride secretion: potential therapeutics for cystic fibrosis and chronic obstructive pulmonary disease. J Pharmacol Exp Ther 296: 600–611, 2001 [PubMed] [Google Scholar]
- 139.Su Y, Yang J, Besner GE. HB-EGF promotes intestinal restitution by affecting integrin-extracellular matrix interactions and intercellular adhesions. Growth Factors 31: 39–55, 2013 [DOI] [PubMed] [Google Scholar]
- 140.Tian L, Chen L, McClafferty H, Sailer CA, Ruth P, Knaus HG, Shipston MJ. A noncanonical SH3 domain binding motif links BK channels to the actin cytoskeleton via the SH3 adapter cortactin. FASEB J 20: 2588–2590, 2006 [DOI] [PubMed] [Google Scholar]
- 141.Toback FG, Walsh-Reitz MM, Kartha S. Signals that release growth factors from renal epithelial cells. Am J Kidney Dis 17: 622–626, 1991 [DOI] [PubMed] [Google Scholar]
- 142.Toroudi HP, Rahgozar M, Bakhtiarian A, Djahanguiri B. Potassium channel modulators and indomethacin-induced gastric ulceration in rats. Scand J Gastroenterol 34: 962–966, 1999 [DOI] [PubMed] [Google Scholar]
- 143.Trinh NT, Bardou O, Prive A, Maille E, Adam D, Lingée S, Ferraro P, Desrosiers M, Coraux C, Brochiero E. Improvement of defective cystic fibrosis airway epithelial wound repair after CFTR rescue. Eur Respir J 40: 1390–1400, 2012 [DOI] [PubMed] [Google Scholar]
- 144.Trinh NT, Prive A, Kheir L, Bourret JC, Hijazi T, Amraei MG, Noel J, Brochiero E. Involvement of KATP and KvLQT1 K+ channels in EGF-stimulated alveolar epithelial cell repair processes. Am J Physiol Lung Cell Mol Physiol 293: L870–L882, 2007 [DOI] [PubMed] [Google Scholar]
- 145.Trinh NT, Prive A, Maille E, Noel J, Brochiero E. EGF and K+ channel activity control normal and cystic fibrosis bronchial epithelia repair. Am J Physiol Lung Cell Mol Physiol 295: L866–L880, 2008 [DOI] [PubMed] [Google Scholar]
- 146.Tsuchiya K, Wang W, Giebisch G, Welling PA. ATP is a coupling modulator of parallel Na,K-ATPase-K-channel activity in the renal proximal tubule. Proc Natl Acad Sci USA 89: 6418–6422, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Turchi L, Chassot AA, Rezzonico R, Yeow K, Loubat A, Ferrua B, Lenegrate G, Ortonne JP, Ponzio G. Dynamic characterization of the molecular events during in vitro epidermal wound healing. J Invest Dermatol 119: 56–63, 2002 [DOI] [PubMed] [Google Scholar]
- 148.Uhal BD. Cell cycle kinetics in the alveolar epithelium. Am J Physiol Lung Cell Mol Physiol 272: L1031–L1045, 1997 [DOI] [PubMed] [Google Scholar]
- 149.Vallon V, Grahammer F, Volkl H, Sandu CD, Richter K, Rexhepaj R, Gerlach U, Rong Q, Pfeifer K, Lang F. KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc Natl Acad Sci USA 102: 17864–17869, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.van Vliet P, de Boer TP, van der Heyden MA, El Tamer MK, Sluijter JP, Doevendans PA, Goumans MJ. Hyperpolarization induces differentiation in human cardiomyocyte progenitor cells. Stem Cell Rev 6: 178–185, 2010 [DOI] [PubMed] [Google Scholar]
- 151.Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, Welsh MJ. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 422: 322–326, 2003 [DOI] [PubMed] [Google Scholar]
- 152.Walsh-Reitz MM, Toback FG. Kidney epithelial cell growth is stimulated by lowering extracellular potassium concentration. Am J Physiol Cell Physiol 244: C429–C432, 1983 [DOI] [PubMed] [Google Scholar]
- 153.Wang J, Morishima S, Okada Y. IK channels are involved in the regulatory volume decrease in human epithelial cells. Am J Physiol Cell Physiol 284: C77–C84, 2003 [DOI] [PubMed] [Google Scholar]
- 154.Wang JY, Wang J, Golovina VA, Li L, Platoshyn O, Yuan JX. Role of K+ channel expression in polyamine-dependent intestinal epithelial cell migration. Am J Physiol Cell Physiol 278: C303–C314, 2000 [DOI] [PubMed] [Google Scholar]
- 155.Wang SP, Wang JA, Luo RH, Cui WY, Wang H. Potassium channel currents in rat mesenchymal stem cells and their possible roles in cell proliferation. Clin Exp Pharmacol Physiol 35: 1077–1084, 2008 [DOI] [PubMed] [Google Scholar]
- 156.Wang T. Renal outer medullary potassium channel knockout models reveal thick ascending limb function and dysfunction. Clin Exp Nephrol 16: 49–54, 2012 [DOI] [PubMed] [Google Scholar]
- 157.Warth R. Potassium channels in epithelial transport. Pflügers Arch 446: 505–513, 2003 [DOI] [PubMed] [Google Scholar]
- 158.Warth R, Barriere H, Meneton P, Bloch M, Thomas J, Tauc M, Heitzmann D, Romeo E, Verrey F, Mengual R, Guy N, Bendahhou S, Lesage F, Poujeol P, Barhanin J. Proximal renal tubular acidosis in TASK2 K+ channel-deficient mice reveals a mechanism for stabilizing bicarbonate transport. Proc Natl Acad Sci USA 101: 8215–8220, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wei JF, Wei L, Zhou X, Lu ZY, Francis K, Hu XY, Liu Y, Xiong WC, Zhang X, Banik NL, Zheng SS, Yu SP. Formation of Kv-FAK complex as a mechanism of FAK activation, cell polarization and enhanced motility. J Cell Physiol 217: 544–557, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wen D, Cornelius RJ, Yuan Y, Sansom SC. Regulation of BK-α expression in the distal nephron by aldosterone and urine pH. Am J Physiol Renal Physiol 305: F463–F476, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Williams MR, Markey JC, Doczi MA, Morielli AD. An essential role for cortactin in the modulation of the potassium channel Kv1.2. Proc Natl Acad Sci USA 104: 17412–17417, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wonderlin WF, Strobl JS. Potassium channels, proliferation and G1 progression. J Membr Biol 154: 91–107, 1996 [DOI] [PubMed] [Google Scholar]
- 163.Wu WK, Li GR, Wong TM, Wang JY, Yu L, Cho CH. Involvement of voltage-gated K+ and Na+ channels in gastric epithelial cell migration. Mol Cell Biochem 308: 219–226, 2008 [DOI] [PubMed] [Google Scholar]
- 164.Wu X, Yang Y, Gui P, Sohma Y, Meininger GA, Davis GE, Braun AP, Davis MJ. Potentiation of large conductance, Ca2+-activated K+ (BK) channels by α5β1-integrin activation in arteriolar smooth muscle. J Physiol 586: 1699–1713, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Xu KP, Ding Y, Ling J, Dong Z, Yu FS. Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells. Invest Ophthalmol Vis Sci 45: 813–820, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xu ZC, Yang Y, Hebert SC. Phosphorylation of the ATP-sensitive, inwardly rectifying K+ channel, ROMK, by cyclic AMP-dependent protein kinase. J Biol Chem 271: 9313–9319, 1996 [DOI] [PubMed] [Google Scholar]
- 167.Yang Y, Wu X, Gui P, Wu J, Sheng JZ, Ling S, Braun AP, Davis GE, Davis MJ. α5β1-Integrin engagement increases large conductance, Ca2+-activated K+ channel current and Ca2+ sensitivity through c-src-mediated channel phosphorylation. J Biol Chem 285: 131–141, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yin J, Yu FS. ERK1/2 mediate wounding- and G-protein-coupled receptor ligands-induced EGFR activation via regulating ADAM17 and HB-EGF shedding. Invest Ophthalmol Vis Sci 50: 132–139, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zahm JM, Kaplan H, Herard AL, Doriot F, Pierrot D, Somelette P, Puchelle E. Cell migration and proliferation during the in vitro wound repair of the respiratory epithelium. Cell Motil Cytoskeleton 37: 33–43, 1997 [DOI] [PubMed] [Google Scholar]
- 170.Zhang C, Wang L, Thomas S, Wang K, Lin DH, Rinehart J, Wang WH. Src-family protein tyrosine kinase regulates the basolateral K channel in the distal convoluted tubule (DCT) by phosphorylation of KCNJ10. J Biol Chem 288: 26135–26146, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang J, Chan YC, Ho JC, Siu CW, Lian Q, Tse HF. Regulation of cell proliferation of human induced pluripotent stem cell-derived mesenchymal stem cells via ether-á-go-go 1 (hEAG1) potassium channel. Am J Physiol Cell Physiol 303: C115–C125, 2012 [DOI] [PubMed] [Google Scholar]
- 172.Zhang J, Halm ST, Halm DR. Role of the BK channel (KCa1.1) during activation of electrogenic K+ secretion in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 303: G1322–G1334, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang YY, Yue J, Che H, Sun HY, Tse HF, Li GR. BKCa and hEag1 channels regulate cell proliferation and differentiation in human bone marrow-derived mesenchymal stem cells. J Cell Physiol 229: 202–212, 2014 [DOI] [PubMed] [Google Scholar]
- 174.Zhao KQ, Xiong G, Wilber M, Cohen NA, Kreindler JL. A role for two-pore K+ channels in modulating Na+ absorption and Cl− secretion in normal human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 302: L4–L12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]