Abstract
Conditions under which skeletal myoblasts are cultured in vitro are critical to growth and differentiation of these cells into mature skeletal myofibers. We examined several culture conditions that promoted human skeletal myoblast (HSkM) culture and examined the effect of microRNAs and mechanical stimulation on differentiation. Culture conditions for HSkM are different from those that enable rapid C2C12 myoblast differentiation. Culture on a growth factor-reduced Matrigel (GFR-MG)-coated surface in 2% equine serum-supplemented differentiation medium to promote HSkM differentiation under static conditions was compared with culture conditions used for C2C12 cell differentiation. Such conditions led to a >20-fold increase in myogenic miR-1, miR-133a, and miR-206 expression, a >2-fold increase in myogenic transcription factor Mef-2C expression, and an increase in sarcomeric α-actinin protein. Imposing ±10% cyclic stretch at 0.5 Hz for 1 h followed by 5 h of rest over 2 wk produced a >20% increase in miR-1, miR-133a, and miR-206 expression in 8% equine serum and a >35% decrease in 2% equine serum relative to static conditions. HSkM differentiation was accelerated in vitro by inhibition of proliferation-promoting miR-133a: immunofluorescence for sarcomeric α-actinin exhibited accelerated development of striations compared with the corresponding negative control, and Western blotting showed 30% more α-actinin at day 6 postdifferentiation. This study showed that 100 μg/ml GFR-MG coating and 2% equine serum-supplemented differentiation medium enhanced HSkM differentiation and myogenic miR expression and that addition of antisense miR-133a alone can accelerate primary human skeletal muscle differentiation in vitro.
Keywords: skeletal muscle, microRNA, myogenesis, differentiation, tissue engineering
the dynamics of primary human skeletal myoblast (HSkM) growth and differentiation into mature myofibers are critical to their use for applications in regenerative medicine, such as repair of severe muscle injuries, congenital defects, and muscular dystrophy. Most studies have focused on rodent myoblasts, and the bulk of the research has been carried out with the widely used immortalized murine C2C12 myoblast line, which is used for ease of culture, differentiation potential, and accessibility (7). Culture conditions for these cells have been optimized to ensure rapid growth and effective differentiation in vitro. These cells do not require protein-coated substrates and differentiate well in a range of equine serum-supplemented differentiation media (DM) (14, 24, 28, 29, 32). While C2C12 cells have provided invaluable information about key mechanistic steps in differentiation (31), extensive in vitro cultivation may lead to deviations from normal biological processes that are important for differentiation of myoblasts in a normal in vivo environment. Less is known about the dynamics of key differentiation factors in primary human myoblasts and how culture conditions affect the changes in these factors (3).
In vitro, rodent myoblast differentiation to myotubes is influenced by many stimuli, including growth factors, substrate composition and mechanical properties, mechanical and electrical stimulation, and addition of microRNAs (16, 23, 26, 28). Mechanical conditioning, or stretch, is an important physiological stimulus that has been applied to skeletal myofiber cultures to mimic in vivo exercise (4, 23, 26, 34). The duration and percentage of strain modulate the effects on muscle in vitro: 10% strain at 1 Hz for 1 h alternated with 23 h of relaxation effectively inhibited differentiation and promoted myoblast proliferation (18), while 10% strain at 0.5 Hz for 1 h alternated with 5 h of relaxation induced myoblast differentiation (45). The latter cyclic stretch regimen has been used to effectively accelerate the differentiation timeline for our two-dimensional C2C12 and HSkM cultures. We previously reported that this cyclic stretch regimen improved muscle differentiation and maturation compared with regimens consisting of 0.05- to 1-Hz frequency and 10–17% strain (45).
Three muscle-specific microRNAs, or miRs (miR-1, miR-133a, and miR-206), are essential to proliferation and differentiation of skeletal myoblasts (11, 22, 28, 37). MiRs are short (∼22 nt), highly conserved, noncoding RNAs (11, 28, 37) that act by negatively regulating gene expression and usually act as repressors of repressors (37). The role of miRs is essential to many important cell processes, such as proliferation, differentiation, and apoptosis (37); therefore, expression levels of these three specific miRs can be used to track development of skeletal muscle as it matures from skeletal myoblasts. MiR-1 and miR-206 promote myoblast differentiation, while miR-133a promotes proliferation (10, 11, 21, 37). Permutation of the ratios of these miRs can lead to various developmental and functional alterations, including damaged sarcomere organization and impaired contractile function of cardiac muscle in miR-1-overexpressing mice (1) and delayed formation of new neuromuscular junctions after nerve injury in miR-206-knockout mice (6, 39). Using a miR-133a-knockout model, Liu et al. (20) showed that mice developed numerous alterations: adult-onset centronuclear myopathy in fast-twitch muscle fibers, impaired mitochondrial function, and compromised fast-to-slow myofiber plasticity. Use of miR transfections to elevate myogenic miR levels has proven instructive as well. Transfections of C2C12 myoblasts with a miR-133a inhibitor led to a 30% decrease in proliferation and successfully enhanced differentiation of in vitro cultures, as measured by force production by three-dimensional engineered muscle bundles (28); transfections with miR-1 and miR-206 led to a 10-fold upregulation of miRs and accelerated differentiation, as measured by an increase in nuclear staining for MyoD (16, 21).
The goal of this study was to identify conditions for improved culture of primary human myoblasts into mature human skeletal muscle for the study of potential therapies for a number of muscular disorders, including muscular dystrophy and other genetic defects, as well as the sarcopenia of aging. Culture conditions were chosen on the basis of myoblast response to growth factor-reduced Matrigel (GFR-MG) coating, media serum level, application of cyclic stretch, and transient inhibition of miR-133a compared with widely used C2C12 cells as reference. MiR-1, miR-133a, miR-206, and transcription factor Mef-2C were measured over time to establish a timeline of myogenic gene expression and assess the extent of differentiation from myoblasts into mature muscle under static and cyclic stretch conditions. Further verification of differentiation was measured by immunofluorescence and Western blotting for sarcomeric α-actinin. This novel information will be essential to the field of human muscle tissue engineering and its potential translation into clinical studies.
METHODS AND MATERIALS
Primary HSkM isolation.
Primary HSkM were isolated from biopsies of the vastus lateralis of healthy middle-aged volunteers (male and female) under conditions of written informed consent and approval of the Duke Investigational Review Board. Biopsies were placed in growth medium (GM) for human skeletal myoblasts (hGM) consisting of low-glucose (1 g/l d-glucose) DMEM (GIBCO/Invitrogen), 8% heat-inactivated fetal bovine serum (HyClone), and SkGM SingleQuots (Lonza/Clonetics) without insulin and kept on ice until ready for isolation. The muscle biopsy (30–50 mg) was placed in Ca2+- and Mg2+-free Hanks' balanced salt solution (GIBCO/Invitrogen) and minced into fine pieces. After removal of Hanks' balanced salt solution, minced tissue was placed in warmed solution containing 0.25% trypsin, 0.1% collagenase, 0.1% BSA, and 0.05% EDTA for 30 min. Action of trypsin and collagenase was halted with addition of 10% fetal bovine serum in medium to tissue. Tissue was placed onto an uncoated T25 flask to eliminate undesired cells (i.e., fibroblasts, nerve cells, and blood cells) by adhesion to plastic. An adhesion time of 3 h was optimal for adhesion of other cell types without significant adhesion of skeletal myoblasts. After 3 h, tissue was transferred to a collagen-coated T25 flask (BD Biosciences) to allow migration of skeletal myoblasts from tissue. The hGM was changed every 5 days until the contents of the flask were 75% confluent, ∼1–2 wk. Once 75% confluent, HSkM were split into dishes for expansion. After ∼1 wk, when the cells were 85–90% confluent, they were trypsinized and plated for studies or frozen. One 30- to 50-mg biopsy yielded 15–20 × 106 cells.
Primary HSkM purity.
Primary isolated HSkM were assessed for purity by staining for desmin, a muscle-specific intermediate filament protein that has been widely used to measure primary skeletal myoblast purity (2, 3, 42). Cultures were fixed with ice-cold 100% methanol and rinsed twice with PBS. Fixed samples were blocked in 10% goat serum for 30 min at 37°C. The primary antibody against desmin (Sigma) was diluted 1:100 in 10% goat serum and incubated at 37°C for 30 min. After the samples were rinsed with PBS, they were incubated at 37°C with secondary Alexa Fluor 546 goat anti-mouse antibody (1:250 dilution; Invitrogen) for 30 min. Finally, Hoechst 33342 nuclear stain (1:1,000 dilution; Invitrogen) was added for 15 min at 37°C. Samples were thoroughly rinsed with PBS and viewed on a fluorescence microscope (Nikon Eclipse TE2000-U). ImageJ software (National Institutes of Health) was used to analyze images for percentage of desmin-positive cells per field of view.
Cell culture.
Primary HSkM cultures were expanded from 1,500 cells/cm2 in uncoated standard tissue culture plastic flasks at passages 3–6 (each passage split 1:4 in flasks) at 37°C in 5% CO2-95% air. Cells were fed hGM every other day until they were confluent. Myoblasts were plated onto six-well plastic tissue culture plates coated with 100 μg/ml GFR-MG (BD Biosciences) or left uncoated. To promote differentiation of confluent myoblasts into myotubes after 2–3 days, hGM was changed to DM consisting of high-glucose DMEM (GIBCO/Invitrogen) supplemented with 2% or 8% equine serum (HyClone) and 0.1% gentamicin (GIBCO/Invitrogen).
Immortalized murine C2C12 myoblasts (American Type Culture Collection), originally derived from C3H mouse leg muscle, were expanded and cultured on uncoated standard tissue culture plastic at passages 3–7 (each passage split 1:4) at 37°C in 5% CO2. Cells were fed murine GM (mGM) consisting of high-glucose (4.5 g/l d-glucose) DMEM, 8% calf serum (HyClone), 8% fetal bovine serum (HyClone), 0.5% chicken embryo extract (Accurate Chemicals), and 0.1% gentamicin every other day. Similar to HSkM cultures, mGM was changed to DM to promote myoblast fusion when the C2C12 cells reached confluence, in ∼2–3 days.
Myoblast size and growth rate.
At 4 h postplating, bright-field images of HSkM and C2C12 cell cultures were obtained. Areas of observation were marked on culture flasks and reexamined after 28 and 48 h. Cell growth rate was observed in the same manner as cell size; images were taken at 4, 12, 24, 36, 48, and 72 h. ImageJ software was used to assess cell size and cell number.
Immunofluorescence.
Cellular proliferation was measured with the Click-iT ethynyl-2′-deoxyuridine (EdU) imaging kit (Invitrogen) following the manufacturer's protocol. Briefly, cultures of myoblasts were incubated for 2 h at 37°C with hGM or mGM containing 1 mM EdU solution. EdU, a nucleoside analog of thymidine, is incorporated into DNA during active DNA synthesis and, therefore, preferentially stains the nucleus of proliferating cells. Detection of EdU occurs through a click reaction, i.e., a copper-catalyzed covalent reaction between an azide and an alkyne (30). The cells were fixed with ice-cold 100% methanol and rinsed with PBS. Fixed cultures were incubated for 30 min at room temperature with EdU reaction cocktail. The labeled cells were then incubated for 30 min at room temperature with Hoechst 33342. Cultures were rinsed with PBS and imaged using a fluorescence microscope (Nikon Eclipse TE2000-U). EdU-stained cells were counted with ImageJ software and quantified as a fraction of the total number of cells in the field of view. Four fields of view were imaged per well in a six-well plate.
To assess the timeline of differentiation for primary HSkM and C2C12 cells, samples were fixed every other day, starting from the day on which the medium was changed from GM to DM at 80% confluence, denoted day 0, until day 14 postdifferentiation. Cultures were fixed with 100% ice-cold methanol and stained for sarcomeric α-actinin (1:800 dilution; Sigma) and nuclei (Hoechst 33342, Invitrogen). At each time point, the proportion of nuclei contained within α-actinin-positive fibers was calculated to find the rate of fusion during myoblast differentiation.
To identify the optimal level of serum for DM, HSkM were cultured in 2% and 8% equine serum-supplemented DM and on 100 μg/ml GFR-MG-coated surfaces and uncoated tissue-treated polystyrene surfaces. Samples were fixed at day 10 postdifferentiation with 100% ice-cold methanol and stained for sarcomeric α-actinin and nuclei. Briefly, fixed samples were blocked in 10% goat serum for 30 min at 37°C. The primary antibody against sarcomeric α-actinin (Sigma) was diluted 1:800 in 10% goat serum and incubated at 37°C for 30 min. After they were rinsed with PBS, the samples were incubated at 37°C with secondary Alexa Fluor 488 goat anti-mouse antibody (1:250 dilution; Invitrogen) for 30 min. Finally, Hoechst nuclear stain was added for 15 min at 37°C. Samples were thoroughly rinsed with PBS and viewed on a fluorescence microscope (Nikon Eclipse TE2000-U). At each time, the proportion of myofibers containing striations was calculated and compared with total number of fibers per field of view to assess development of striations and muscle maturation.
Anti-miR-133a transient transfection.
Single-stranded antisense miR molecules specific for the miR-133a sequence (UUUGGUCCCCUUCAACCAGCUG, hsa-miR-133a; AM10413, Applied Biosystems) were used with siPORT NeoFX transfection agent (Ambion). Anti-miR Negative Control #1 (AM17010, Applied Biosystems), a single-stranded random miR sequence, was used as transfected control.
Briefly, siPORT NeoFX transfection agent was diluted with OptiMEM I (GIBCO) and mixed with 50 nM single-stranded anti-miR (anti-miR-133a and anti-miR negative control). The transfection mixtures were allowed to incubate in tissue culture flasks at 37°C for 10 min to allow for formation of liposomes; then HSkM cells and GM were added. Cells were transfected at 37°C in 5% CO2 for 18 h; then they were rinsed, trypsinized, and plated for studies. The day on which the culture was 80% confluent was designated day 0, when GM was exchanged for DM (effectively, time 0). Samples were fixed at days 0, 6, 10, and 14 to track the differentiation process. On the terminal day, samples were stained for sarcomeric α-actinin and with Hoechst nuclear dye and imaged on a fluorescence microscope (Nikon Eclipse TE2000-U). Resulting images were assessed with ImageJ software for extent of myoblast fusion and myofiber striation: percentage of nuclei in fibers and striated fibers per field of view were calculated, respectively.
For assessment of transfection efficiency and half-life of the anti-miR, Cy3 dye-labeled Anti-miR Negative Control #1 was used as described above. Transfected HSkM [containing the characteristic Cy3 (red) label, as viewed under fluorescence] were quantified as proportion of total cells in view.
Cyclic mechanical stretch.
Primary HSkM and C2C12 cells were cultured on flexible, Silastic membranes coated with GFR-MG until they were 80% confluent. Then the GM was replaced with DM; this time point was denoted day 0. Myoblast cultures were subjected to equibiaxial mechanical stretch via a tension system (model FX-5000T, Flexcell International, Hillsborough, NC) onto flexible Silastic membranes; unstretched Silastic membranes served as control. Previous work in our laboratory with C2C12 cells optimized parameters for stretch studies: 10% stretch at 0.5 Hz for 1 h followed by 5 h of rest for differentiation (45).
MiR/RNA isolation and quantitative real-time PCR.
Samples from static, stretched, and transfected studies were gathered at denoted days to track miR and RNA expression changes over time. The miRs were isolated using the mirVANA miR isolation kit (Applied Biosystems). Total mRNA was isolated using the Aurum total RNA mini kit (Bio-Rad).
To assess miR levels in mature human muscle tissue, fresh human muscle explants (from the vastus lateralis) were also used for miR isolation. Sterile forceps and scalpels were used to mince tissue in a glass petri dish. Isolation of miR was completed with the mirVANA miR isolation kit (Applied Biosystems).
Quantitative RT-PCR was used to assess miR and RNA expression from collected samples. Concentration and quality of miR/RNA were measured using a spectrophotometer (model ND-1000, NanoDrop, Wilmington, DE). For miR expression, cDNA was prepared using the microRNA reverse transcriptase kit (Applied Biosystems) and the MyiQ single-color real-time PCR detection system (Bio-Rad) according to the manufacturer's instructions. Hairpin primers for miRs of interest (hsa-miR-1, hsa-miR-133a, and hsa-miR-206) were purchased from Applied Biosystems. Small nucleolar RNA RNU6B (Applied Biosystems) was used as an endogenous control. All primers were tested and approved by the manufacturer for use with mouse and human cell samples. TaqMan Universal PCR Master Mix (Applied Biosystems) and hairpin primers were added to the cDNA samples according to the manufacturer's instructions and placed back into the MyiQ single-color real-time PCR detection system for PCR analysis. For RNA expression, cDNA was prepared using the iScript cDNA synthesis kit (Bio-Rad) following the manufacturer's instructions. Primers for Mef-2C and GAPDH (endogenous control) were custom-ordered from IDT for the following sequences: ACTTCCTGGAGAAGCAGAAAGGCA (forward) and AACACGTTTCCTTCTTCAGCACGC (reverse) for Mef-2C and TCAACAGCAACTCCCACTCTTCCA (forward) and ACCCTGTTGCTGTAGCCGTATTCA (reverse) for GAPDH. These primers have been previously used to measure GAPDH and Mef-2C mRNA levels (9, 43). iQ SYBR green supermix (Bio-Rad) and primers were combined with cDNA and placed into the MyiQ single-color real-time PCR detection system for PCR analysis. The 2−ΔΔCT method was used to assess the real-time mRNA and miR expression data. Total RNA from 11-day-old mouse embryo was used as the calibrator (Clontech).
PCR results were normalized to account for variability among the levels initially present in the different biological replicates. Data from all experiments, regardless of conditions, were averaged to obtain a “global average.” To identify the variability between experiments, data from each experiment were averaged. To decrease interexperiment variability while maintaining the relation between conditions within each experiment, a normalization factor was calculated by dividing the global average by the average of each experiment. New normalized values were calculated for each experiment by scaling each data point by the normalization factor of the experiment. These data were used to calculate statistical significance.
Western blotting.
Western blots were performed to determine the expression of sarcomeric α-actinin, a structural protein known to be abundant during skeletal muscle differentiation. After day 6 or 14, cells were harvested with a cell scraper and lysed using CellLytic-M (Sigma) and protease inhibitor cocktail (Sigma). Protein concentration was assessed using the bicinchoninic acid (BCA) protein assay kit (Pierce). After 10–15 μg of protein were loaded into each lane on a 7.5% Tris·HCl precast gel (Ready Gel, Bio-Rad), the proteins were separated by SDS-PAGE. Then protein was transferred onto a polyvinylidene difluoride membrane, which was blocked for 1 h in 3% BSA in Tris-buffered saline with 0.1% Tween 20 (TBST) with gentle agitation at room temperature. Primary antibodies for mouse sarcomeric α-actinin (1:2,500 dilution; clone EA-53, Sigma) and mouse β-tubulin (1:2,500 dilution; Invitrogen) were diluted in TBST solution containing 1% BSA and incubated with the membrane overnight at 4°C with gentle rotation. After the cells were rinsed to remove unbound primary antibody, the membrane was incubated with goat anti-mouse horseradish peroxidase secondary antibody (1:2,500 dilution; Invitrogen) in TBST containing 1% BSA for 1.5 h at room temperature with gentle rocking. Residual antibody was washed, and immunoreactive bands were detected by luminography using Supersignal chemiluminescence substrate (Pierce). Band density was determined by densitometry after scanning onto autoradiographic films (Kodak) and evaluated by ImageJ software. Protein expression was reported as intensity of the band of interest (sarcomeric α-actinin) normalized to the band of reference protein (β-tubulin).
Statistical analysis.
Values are means ± SE unless otherwise noted. ANOVA and Fisher's protected least significance difference post hoc tests were performed using the StatView 5.0 statistical analysis package. P < 0.05 was considered significant. HSkM isolated from separate individuals were considered distinct n values; for C2C12 cells, n represents separate cultures of the same cell line.
RESULTS
Comparison of C2C12 and HSkM growth, size, and differentiation under conditions optimal for C2C12 myofiber maturation.
The purity of subconfluent primary HSkM myoblasts was assessed at passage 3. With the percentage of desmin-positive cells used as a measure of purity, 90.4 ± 4.2% (n = 3) of subconfluent myoblasts were positive for desmin.
When grown on uncoated tissue culture plastic in 8% serum, primary HSkM were significantly larger than C2C12 cells at 8, 28, and 48 h postplating (n = 3, passage 4 all cells). Over 72 h, both cell types exhibited the characteristic exponential growth; however, the primary HSkM had a lag phase (typically 18–20 h compared with none for C2C12 cells) before reaching exponential growth. A linear fit to the logarithm of the exponential growth phase showed doubling times of 19.7 ± 0.5 h (R2 = 0.994, n = 3) and 41.4 ± 3.3 h (R2 = 0.912, n = 4) for C2C12 cells and primary HSkM, respectively.
To assess proliferation at 0 and 24 h in various culture media, an EdU cellular proliferation assay was used. The point at which the cells reached 40% confluence was designated time 0 (i.e., 0 h); a subconfluent time point was used to prevent a change in growth rate due to contact inhibition. Cell numbers were normalized to values at time 0 for individual studies to allow for comparison between studies (n = 3). ANOVA of EdU levels on the different culture days showed a significant drop in proliferation when DM was added at time 0 for 24 h compared with cultures kept in GM for 24 h (P < 0.05): C2C12 cell proliferation dropped from 16.9 ± 3.8% in mGM to 7.3 ± 0.8% in DM, and HSkM proliferation dropped from 18.8 ± 0.9% in hGM to 10.5 ± 3.3% in DM. EdU levels in HSkM were similar to those in C2C12 cells in GM and DM after 24 h.
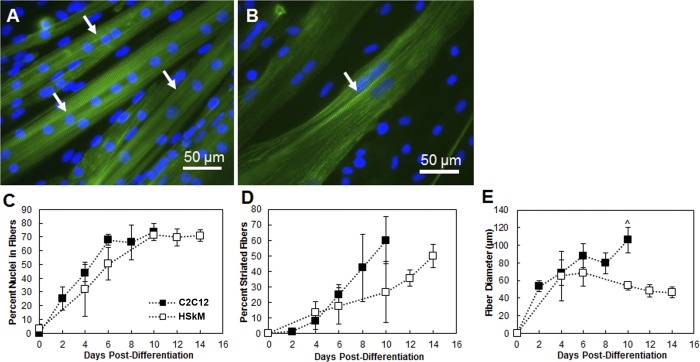
The extent of differentiation was first investigated by means of myotube formation and maturation to myofibers under conditions that were optimal for C2C12 cell differentiation (no protein coating and 8% serum in DM). Myogenesis occurs by fusion of myoblasts to myotubes and subsequent development of striations as myotubes mature to myofibers and, ultimately, striated skeletal muscle (7). The lag in development of striated HSkM relative to C2C12 cells is apparent by immunofluorescence for α-actinin, paralleling previous findings for growth rate (Fig. 1, A and B) (40). A linear fit was used to calculate the rate of fusion over the linear portion of the data shown in Fig. 1C. C2C12 cells fused at a rate of 11.13 ± 1.6% nuclei per field of view per day (R2 = 0.990) from day 0 to day 6, which was not different from the fusion rate of HSkM (6.92 ± 2.9% nuclei per field of view per day, R2 = 0.997) from day 0 to day 10. Rate of striation development, an important indicator of muscle maturity, was significantly slower in HSkM than C2C12 cells: 3.25 ± 0.78% vs. 8.65 ± 2.79% striations per field of view per day (Fig. 1D; P < 0.05 by analysis of covariance). As skeletal muscle differentiated, HSkM fiber diameter decreased, while C2C12 fiber diameter increased. At day 8 and earlier, the fiber diameters were similar. Thereafter they diverged, with diameter increasing for C2C12 cells and decreasing for HSkM; at day 10, HSkM fibers were significantly thinner (54.0 ± 4.4 μm, n = 3) than C2C12 cell fibers (105.8 ± 14.5 μm, n = 3, P < 0.05; Fig. 1E). At day 8, C2C12 cell cultures showed prominent striations, while the HSkM cultures reached a comparable maturation point at day 14 postdifferentiation. On the basis of these results, subsequent studies for HSkM were conducted to day 14 to allow for appropriate development and muscle maturation.
Fig. 1.
Myoblast fusion and striation of C2C12 cells and human skeletal muscle myoblasts (HSkM) at passage 3. A: C2C12 cell cultures stained for sarcomeric α-actinin (green) and Hoechst 33342 nuclear stain (blue) at day 8 postdifferentiation. B: HSkM cultures stained for sarcomeric α-actinin and Hoechst at day 14 postdifferentiation. Both cell types exhibit multinucleation and clear striations at the terminal differentiation day. Magnification ×40. C: fusion of myoblasts into myotubes. A linear fit was used to calculate the rate of fusion: C2C12 cells fused at a rate of 11.13 ± 1.6% nuclei per field of view per day (R2 = 0.990) from day 0 to day 6, and HSkM fused at a rate of 6.92 ± 2.9% nuclei per field of view per day (R2 = 0.997) from day 0 to day 10. D: C2C12 cells develop striations more quickly than HSkM cells. Slopes of C2C12 and HSkM regression lines are significantly different by analysis of covariance (P < 0.05). E: C2C12 myofibers are thicker than HSkM myofibers at day 10. Error bars, SE. ^P < 0.05.
Culture conditions for HSkM.
When HSkM were cultured on an uncoated tissue culture polystyrene surface for 14 days after they were shifted to 8% equine serum, immunofluorescence for sarcomeric α-actinin showed a sparse distribution of short myofibers that lacked striations (Fig. 2A). MATLAB image analysis showed an average sarcomeric α-actinin-stained area of 29.7 ± 3.5% in each field of view (4 fields of view per experiment, n = 3). Staining for von Willebrand factor showed 3.1 ± 0.8% positive staining for endothelial cells among the myoblast population. Unfused cells are a combination of unfused self-renewed satellite cells and other cell types that are not removed by the isolation process.
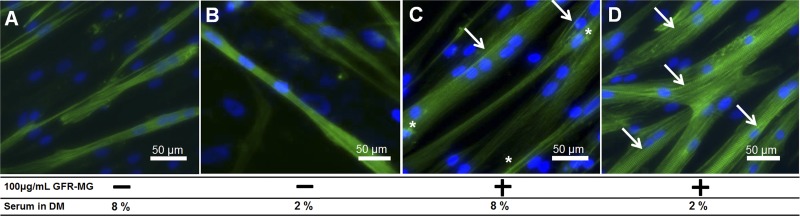
Fig. 2.
Effect of serum and growth factor-reduced Matrigel (GFR-MG) coating on formation of striated human muscle fibers 14 days after shift to differentiation medium (DM) with the indicated level of serum. Differentiation was characterized by immunofluorescence for sarcomeric α-actinin (green) and Hoechst nuclear stain (blue). A: few fibers were seen in samples cultured on an uncoated surface in 8% equine serum DM. B: few fibers were present in samples cultured on an uncoated surface in 2% equine serum; however, myofibers were longer than those cultured on an uncoated surface in 8% equine serum. C: fibers were more prevalent and robust on surfaces coated with 100 μg/ml GFR-MG in 8% equine serum DM; some cell debris was also present (*), and a few striations were present (arrows). D: robust, striated fibers were seen in HSkM attached to GFR-MG-coated surfaces and differentiated in 2% equine serum-supplemented DM (arrows). Magnification ×20.
Myofibers cultured in 2% serum on uncoated surfaces appeared longer than those cultured in 8% serum. However, myoblasts cultured under these conditions also lacked striations (Fig. 2B), and only 23.9 ± 2.2% of total stained areas were positively stained for sarcomeric α-actinin. Culture of myoblasts on a surface treated with 100 μg/ml GFR-MG, which has been shown to maintain differentiation capacity of muscle precursor cells in vitro (15), showed an increase of sarcomeric α-actinin staining on day 14 to 50.1 ± 5.2% of area. These myofibers also contained striations (Fig. 2C); however, there appeared to be scattered sarcomeric α-actinin-stained aggregates as well (Fig. 2C). HSkM grown on 100 μg/ml GFR-MG in 2% serum showed 43.4 ± 3.0% area positive for sarcomeric α-actinin with fewer stained aggregates and more striations (Fig. 2D); this result was not statistically different from 50.1 ± 5.2% for HSkM cultured on GFR-MG in 8% serum. When cultured on surfaces coated with 100 μg/ml GFR-MG in 2% serum, HSkM exhibited a greater extent of fusion and striation after 14 days (84.0 ± 2.0% of α-actinin-positive area contained striations in 2% serum vs. 13.3 ± 7.7% in 8% serum, n = 2). Therefore, 2% equine serum in DM on GFR-MG-coated surfaces appeared to facilitate HSkM differentiation relative to other tested conditions.
Effect of culture conditions on C2C12 and HSkM miR and Mef-2C expression.
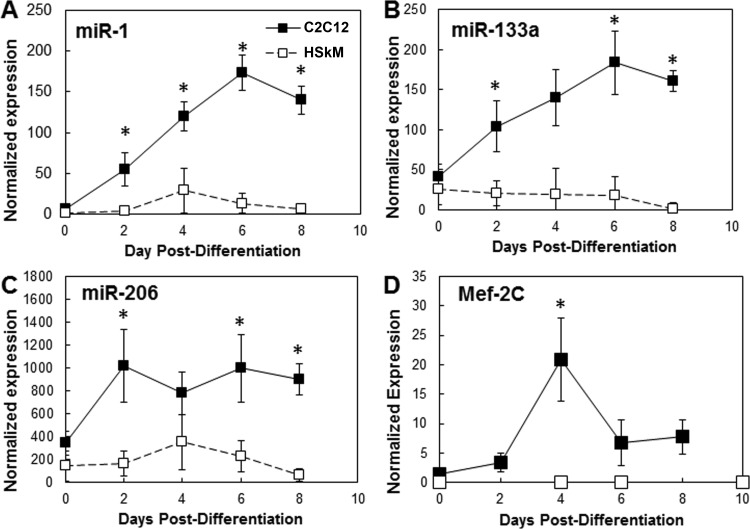
On the basis of the improved differentiation of HSkM on GFR-MG at low serum levels, we hypothesized that adding GFR-MG coating and decreasing media serum levels would significantly elevate the miR and Mef-2C gene expression of HSkM during differentiation in vitro. All miR expression was normalized to the small nucleolar RNA RNU6B as endogenous control; Mef-2C gene levels were normalized to GAPDH as endogenous control. When cultured in 8% serum during differentiation on uncoated surfaces to reproduce typical culture conditions for C2C12 cells, levels of miR-1, miR-133a, miR-206, and Mef-2C were ≥200% higher in C2C12 cells than HSkM on corresponding days (Fig. 3). C2C12 cells exhibited, on average, a 15-fold increase between miR-1 and miR-133a expression on day 6 compared with day 0 postdifferentiation for both genes. In contrast, levels of these miRs in HSkM remained stable and very low compared with those in C2C12 cells (Fig. 3, A and B). MiR-206 reached a plateau of 800–1,000 by day 2 in C2C12 cells and an average of 2.5-fold greater expression than in HSkM on day 0. However, in HSkM, miR-206 showed a maximum expression >350 on day 4 that declined to <70 on day 8 (Fig. 3C).
Fig. 3.
Comparison of microRNA (miR) and myogenic transcription factor Mef-2C mRNA levels in mouse C2C12 cells (reference) and HSkM. Under optimal conditions to promote C2C12 cell differentiation (8% serum and no Matrigel coating of plastic surface), expression of miR-1, miR-133a, and miR-206 was significantly higher in developing C2C12 myotubes. A–C: dynamic expression of miR-1, miR-133a, and miR-206 from day 0 to day 8 postdifferentiation. D: expression of Mef-2C was higher in C2C12 cell cultures than HSkM samples at all time points. Error bars, SE (n = 4). *P < 0.05 vs. HSkM.
MiR-1 represses histone deacetylase 4, which is a repressor of Mef-2C. Therefore, increases in miR-1 levels promote skeletal muscle differentiation (25). The levels of the Mef-2C gene in C2C12 cells increased 14.5-fold between day 0 and day 4 but remained consistently <1 in HSkM (Fig. 3D). MiR-1 and Mef-2C gene expression was >40-fold higher in C2C12 cells than HSkM at all times. Overall, the lower levels of key muscle miRs and Mef-2C in HSkM were consistent with reduced levels of differentiation in HSkM relative to C2C12 cells.
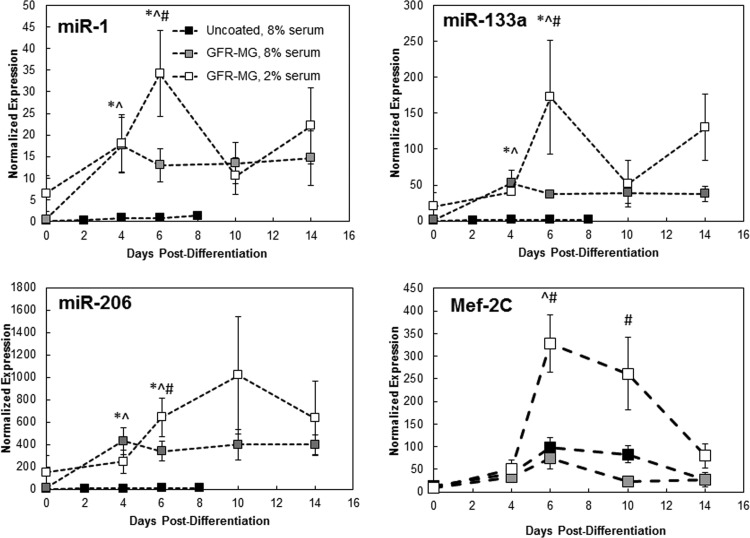
HSkM plated onto GFR-MG-coated surfaces in 8% serum exhibited ≥14-fold average increases in miR-1, miR-133a, and miR-206 levels compared with HSkM plated onto uncoated surfaces in 8% serum (Fig. 4). On day 6, miR-1 expression peaked at 26 times that on day 0 and then reached a plateau of 10–20 from day 6 to day 14; similarly, on day 4, miR-133a expression peaked at 29 times that on day 0 and then reached a plateau of ∼40 from day 6 to day 14. On day 4, miR-206 expression peaked at 28 times that on day 0 and then reached a plateau of 400 from day 6 to day 14. Mef-2C did not change much in 8% serum with the addition of GFR-MG coating: levels on day 14 with and without GFR-MG averaged 26–27. Overall, in the presence of GFR-MG, Mef-2C expression increased an average of 67% of that of uncoated HSkM in 8% equine serum-supplemented DM.
Fig. 4.
Effect of culture conditions on expression of miRs. HSkM cultures were grown on uncoated plastic or plastic incubated with 100 μg/ml GFR-MG in 2% or 8% equine serum. Levels of all miRs, were higher in cultures grown on GFR-MG in 2% equine serum than in cultures grown on uncoated plastic in 8% equine serum at terminal day (n = 3). *P < 0.05, uncoated, 8% serum vs. GFR-MG, 8% serum; ^P < 0.05, uncoated, 8% serum vs. GFR-MG, 2% serum; #P < 0.05, GFR-MG, 8% serum vs. GFR-MG, 2% serum.
While 100 μg/ml GFR-MG coating was maintained, DM was changed from 8% to 2% serum. With this change, miR levels in the HSkM cultures were compared with those in HSkM grown on a GFR-MG-coated surface in 8% serum (Fig. 4). For all times, expression increased an average of >220% between GFR-MG in 2% serum and GFR-MG in 8% serum for miR-1, miR-133a, and miR-206. Similar to the previous condition, miR expression also exhibited dynamic changes over the 2-wk period on a GFR-MG-coated surface in 2% serum. Expression of miR-1 increased fivefold between days 0 and 6 and then dropped 35% at day 14 compared with day 6. Expression of miR-133a also peaked at day 6 and then declined 24% from day 6 to day 14. Expression of miR-206 increased more than sixfold from day 0 to day 10 and then declined 38% between day 10 and day 14. Mef-2C increased 6.5-fold between day 4 and day 6 and then declined 75% from day 6 to day 14. For all times studied, Mef-2C levels were an average of fourfold higher when cultured on GFR-MG in 2% serum than when cultured on GFR-MG in 8% serum.
To assess whether the serum level also influenced the increased miR levels in HSkM cultured on a GFR-MG-coated surface, we measured miR-1, miR-133a, and miR-206 expression at day 8 postdifferentiation in HSkM cultured in 2% serum on uncoated tissue-treated polystyrene. This condition led to increases in expression of miR-1, miR-133a, and miR-206 compared with HSkM cultured on an uncoated surface in 8% serum: miR-1 increased 4.4-fold, miR-133a increased 9.0-fold, and miR-206 increased 90.6-fold. However, expression of all three miRs was markedly lower in these cells than in HSkM cultured on GFR-MG in 2% serum. Because of the elevated levels of miR-1, miR-133a, miR-206, and Mef-2C, subsequent studies with HSkM were conducted using a 100 μg/ml GFR-MG-coated substrate and 2% serum DM.
Effect of stretch on HSkM differentiation.
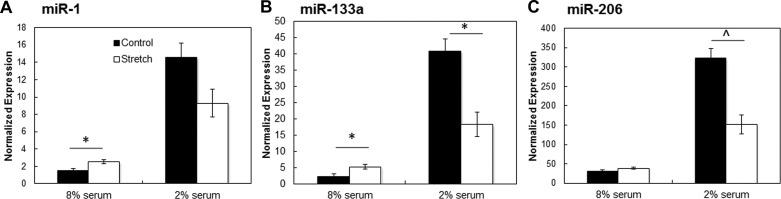
We previously found that ±10% cyclic mechanical stretch at 0.5 Hz for 1 h followed by 5 h of rest for 1 wk significantly accelerated C2C12 cell differentiation (44) and hypothesized that this mechanical stimulation regimen would also enhance HSkM differentiation when applied for 2 wk (to account for slower differentiation) to confluent monolayers. Cyclic sinusoidal stretch was applied continuously for 14 days after differentiation in 8% and 2% serum. To facilitate stretch, C2C12 cells and HSkM were cultured on softer, flexible Silastic membranes coated with 100 μg/ml GFR-MG (for both static and stretched samples). The decreased substrate rigidity compared with tissue culture plastic can account for differences in miR expression of HSkM in Fig. 5 relative to Fig. 4. After 14 days of cyclic stretch, miR-1 expression increased 40% and miR-133a expression increased 128% (P < 0.05, n = 6) in HSkM cultured in 8% serum compared with HSkM grown statically on GFR-MG-coated membranes in 8% serum. Levels of miR-206 increased 20% after stretch in 8% serum (Fig. 5) compared with static control in 8% serum. In 2% serum, all miRs showed decreased expression after 14 days of cyclic stretch compared with static controls cultured in 2% serum: miR-1 decreased 36%, miR-133a decreased 55% (P < 0.05, n = 3), and miR-206 decreased 53% (P < 0.01, n = 3).
Fig. 5.
Cyclic stretch affects miR levels. Cyclic stretch was applied to HSkM monolayers for 14 days postdifferentiation at ±10% strain and 0.5 Hz for 1 h followed by 5 h of rest. All studies were conducted on 100 μg/ml GFR-MG-coated flexible membranes. A: in 8% serum, miR-1 level increased significantly (*P < 0.05, n = 6) after 14 days of stretch; in 2% serum, miR-1 level decreased compared with static controls after 14 days of stretch. B: miR-133a increased significantly after 14 days of stretch in 8% serum (*P < 0.05, n = 6) but decreased in 2% serum (*P < 0.05, n = 3). C: after 14 days of stretch, miR-206 expression increased in HSkM in 8% serum but declined significantly in HSkM in 2% serum (^P < 0.01). Error bars, SE.
Comparison of miR levels in fresh human muscle explants and cultured HSkM.
To assess the effect of culture on HSkM miR levels, miRs were isolated directly from mature skeletal muscle explants of the vastus lateralis from adult human subjects (Table 1), and these results were compared with those obtained in vitro at day 14. Levels of miRs measured in fresh explants were 3 to >500 times greater than levels measured in HSkM cultured on an uncoated surface in 8% serum. With the addition of a GFR-MG coating prior to culture with 8% serum during differentiation, miR levels in vitro increased drastically but were still less than in explant tissue. Interestingly, miR-206 was most affected by the various culture conditions, with in vitro expression surpassing that of fresh explants by almost sixfold. In contrast, other measured miRs remained below levels in fresh explants. In HSkM cultured on GFR-MG in 2% serum, miR levels further improved; however, the increase in expression was larger with the addition of GFR-MG coating (average 14-fold increase over uncoated) than with a change in serum content (average 2.3-fold increase from 8% to 2% serum).
Table 1.
MicroRNA levels in HSkM under various conditions
| HSkM Static |
||||
|---|---|---|---|---|
| No GFR-MG |
GFR-MG |
|||
| MicroRNA | Fresh Explant | 8% serum | 8% serum | 2% serum |
| miR-1 | 590.9 ± 154.3 | 1.1 ± 0.3 | 16.1 ± 8.3 | 27.2 ± 9.4 |
| miR-133a | 708.9 ± 173.8 | 3.6 ± 1.0 | 37.3 ± 9.4 | 130.4 ± 46.7 |
| miR-206 | 64.2 ± 19.9 | 21.1 ± 6.1 | 376.6 ± 45.8 | 633.4 ± 331.9 |
HSkM, human skeletal myoblast; GFR-MG, growth factor-reduced Matrigel.
Effect of miR-133a inhibition on HSkM differentiation.
As the differentiation of HSkM appears to lag behind that of C2C12 cells, as assessed by the development of striated fibers, a miR-133a inhibitor was added to accelerate the HSkM differentiation process. On the basis of our prior studies with C2C12 cells in three-dimensional cultures (28), we hypothesized that by inhibiting the proliferation-inducing miR-133a, the extent of HSkM differentiation would be significantly accelerated.
In experiments using the Cy3-labeled negative control, the transfection efficiency of siPORT NeoFX transfection agent was 96.9 ± 1.7% after 18 h of incubation of the transfection reagent with HSkM. The percentages of miR-containing HSkM at hours 24, 48, 72, and 96 posttransfection were 93.2 ± 4.1%, 84.1 ± 5.7%, 75.0 ± 2.9%, and 52.7 ± 6.2%, respectively. This slow decay indicates a long lifetime of the miR within the cell. The data were well fit to a polynomial [%transfected = −0.0052(h)2 + 0.0801(h) + 94.156, R2 = 0.9923] from which the half-life of the miR was calculated to be 92.2 ± 1.8 h.
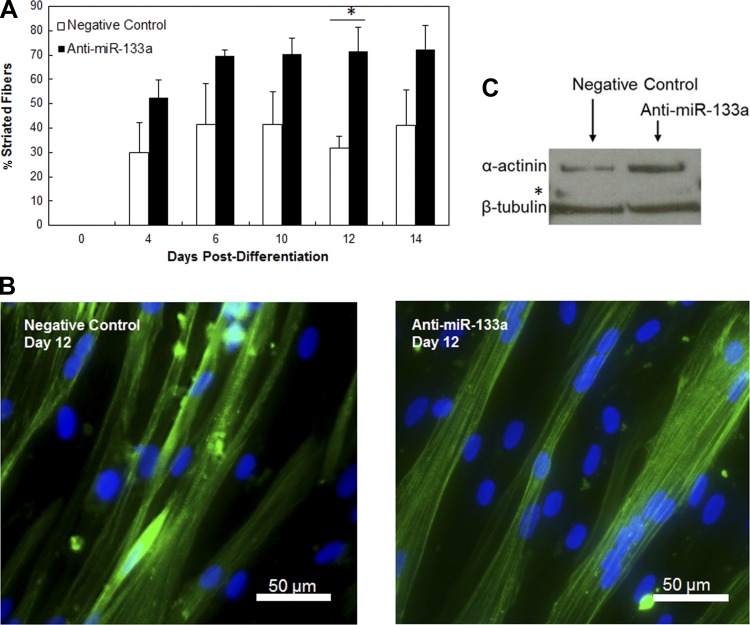
HSkM differentiation following anti-miR-133a transfection was examined after they were plated on GFR-MG and shifted to DM containing 2% equine serum for 4, 6, 10, 12, and 14 days. Immunofluorescence for sarcomeric α-actinin showed an increase in striated fibers in anti-miR-133a samples compared with the negative control miR postdifferentiation (Fig. 6, A and B; n = 3). Overall, significantly more striated fibers were observed in the anti-miR-133a-treated human cultures than the negative control (Fig. 6A; n = 3, P < 0.0001). Representative images show an increase in striated fibers in the anti-miR-133a-transfected HSkM compared with the negative control (Fig. 6B).
Fig. 6.
Inhibition of miR-133a accelerates HSkM differentiation in vitro. HSkM were transfected for 18 h with miR-133a inhibitor. At 18 h, samples were rinsed to remove transfection agent, and cells were plated in growth medium. Day on which differentiation medium was added was designated day 0. Both preparations fused at comparable rates. A: anti-miR-133a developed striated fibers more quickly postdifferentiation than negative control (*P < 0.0001, n = 3). Error bars, SE. B: anti-miR-133a cultures exhibit more robust and striated fibers by immunofluorescence for sarcomeric α-actinin (green) and 4′,6-diamidino-2-phenylindole (blue) than negative control. C: Western blot shows more sarcomeric α-actinin at day 6 than negative control. Mef-2C (*) did not exhibit significant difference in expression. β-Tubulin was used as reference.
To confirm an increase in differentiation due to addition of anti-miR-133a, protein samples at day 6 postdifferentiation were assessed for sarcomeric α-actinin protein expression by Western blotting. Samples transfected with antisense miR-133a exhibited significantly higher levels of sarcomeric α-actinin than HSkM treated with scrambled control miR. The normalized intensity of α-actinin expression was 30% higher than in the negative control samples (Fig. 6B; P < 0.05, n = 3, paired t-test).
DISCUSSION
In this study, conditions that improved differentiation of primary isolated HSkM myoblasts to form myofibers were tested. The use of 100 μg/ml GFR-MG coating and 2% equine serum-supplemented DM promoted human myoblast fusion and striated fiber formation while elevating levels of the myogenic genes miR-1, miR-133a, and miR-206 and Mef-2C in human muscle cultured in vitro. Furthermore, the addition of antisense miR-133a alone accelerated differentiation in primary HSkM, shown by a 30% increase in sarcomeric α-actinin protein expression; in previous studies, more than one miR was needed to obtain a similar outcome in primary human cells (16).
Murine myoblasts differentiate well under 8% (28), 2% (24), or 1% (7) serum and even in serum-free (14, 19) DM conditions on uncoated surfaces. The decreased serum content in DM is sufficient to trigger a halt in myoblast proliferation and a shift to myoblast fusion and myofiber formation. Few similar studies have been conducted with human myoblasts; Stern-Straeter et al. (33) reported higher rates of proliferation in primary isolated human myoblasts cultured in gelatin-coated substrates than in noncoated polystyrene and used 2% horse serum in DM. Similar to the study of Stern-Straeter et al., we showed the beneficial myogenic effect of 2% serum content and the addition of Matrigel coating to human skeletal myoblast culture and differentiation; however, the work presented here provides a more in-depth study of human myogenesis by way of miR and gene expression levels during HSkM differentiation in vitro.
Murine and human skeletal muscle cell types differ drastically in growth rate, cell size, formation of sarcomeres, and substrates required for growth (Figs. 1 and 2). This may be due, in part, to the differences between a cell line and primary isolated cells or differences in species. It has been consistently found that human cells proliferate and develop at a slower rate than murine cells in vivo and in vitro (27). The slower response of human myoblasts and myofibers may be due, in part, to the extended lifetime of humans. Humans live, on average, 30–50 times longer than mice; this translates to ∼105 more cell divisions in a human than a mouse lifetime (27). If human and murine myoblasts doubled at the same rate, mathematically, an increase in incidence of cancer and other late-onset illnesses would be expected. However, on average, 30% of rodents develop cancer at the end of their lifespan (typically 2–3 yr), and 30% of humans develop cancer at 70–80 yr of age (27). The slowed proliferation and differentiation rates of human myoblasts compared with murine myoblasts can be partially attributed to the human body's attempt to protect from rapid aging, cancer, and other illness.
Other groups measured the expression levels of miRs during a few specific stages of human muscle development: proliferation, differentiation, and maturity (16, 17); provided here is a more detailed time course of the changes in miR expression throughout the important process of muscle fusion and fiber formation, showing changes as large as 29-fold over 2 wk of myoblast differentiation. Koning et al. (17) reported significant downregulation of miR-106b, miR-25, miR-29c, and miR-320c levels between the quiescent human satellite cell stage and the fused myotube stage. Additionally, they showed miR-1 and miR-206 relative expression at general points throughout in vitro muscle growth: proliferation, confluency, differentiation (5 days after inducing differentiation), and maturity (human biopsy tissue) (16). The temporal trends of their miR-1 and miR-206 dynamic expression from confluency to differentiation are quite similar to our findings from day 0 to day 6 postdifferentiation showing an increase in miR expression. However, they concluded that transfection with miR-1 or miR-206 alone was not sufficient to trigger myotube formation (16), an important step in the process of differentiation; we show here that transfection solely with antisense miR-133a was able to achieve myotube formation.
The results presented in these studies are the first to show the effect of various culture conditions on expression of myogenic miRs during HSkM differentiation. Culture conditions clearly have a significant effect on muscle growth, as miR-1, miR-133a, miR-206, and Mef-2C levels increased drastically with the introduction of protein coating and the decrease in media serum content. Satellite cells are extremely sensitive to their surrounding environment (5); we found that the presence of GFR-MG-coated substrate improved differentiation of these cells by elevating levels of myogenic miRs and transcription factors. Lawson and Purslow (19) showed significant mouse and rat muscle cell responses to serum content in medium and substrate extracellular matrix coating. Rat L6 cells only formed myotubes in serum-containing medium and exhibited higher creatine phosphokinase activity when cultured in 2% serum medium. In C2C12 cells, however, creatine phosphokinase activity was dependent on substrate coating and media serum content (19).
We found that miR levels in cyclically stretched human myoblasts respond differently, depending on media serum content (Fig. 5). Since miRs work in concert to guide myoblast development, compensatory increases in differentiation signals may exist elsewhere, pushing the myoblast toward differentiation. Additionally, in cells with such environmental sensitivity, the response to stimuli may, indeed, be the opposite of that expected when the cells are exposed to different conditions. Carson and Booth (8) showed that α-actin gene expression in primary chicken myoblasts was dependent on serum availability and static mechanical stretch; cultures underwent 8–20% static stretch in 10% or 0.5% equine serum. They found no difference in α-actin mRNA level between stretched and static samples in 10% serum; however, the levels decreased by 26% in samples stretched in 0.5% serum (8). At that time, the effects of serum content on cyclically stretched primary human myoblasts had not been reported. We showed increased miR levels after 14 days of cyclic stretch in 8% serum, while stretch in 2% serum led to significant miR decreases.
We chose to study miRs for their potent effects on myogenesis (38), endogenous presence, and localization to the cytoplasm. The endogenous nature of miRs provides a platform whereby myogenesis can be affected without introducing viral vectors. Because of their localization to the cytoplasm, miRs allow for easier access and manipulation than nucleic acids, which reside predominantly within the cell nucleus. The miRs exert their effects through dynamic changes in expression over time compared with transcription factors, which function similar to on-off switches. Since miRs can be effectively delivered to the cellular cytoplasm via liposomes and have been shown to exert effects transiently (28), the potential of miRs to be used as therapy for individuals with muscular dystrophy or sarcopenia is large. Compared with constitutive expression, the ability to deliver gene therapy nonvirally and transiently is attractive because of lower cell cytotoxicity. However, knowledge of miR expression in normal, healthy muscle tissue is necessary to understand how best to treat disorders caused by their imbalances. Comparison of in vitro and in vivo levels of the muscle-specific miRs (Table 1) suggests that further work is needed to replicate the in vivo physiological state.
Prolonged increases or decreases in miR-133a expression have been implicated in energy deficit, muscular dystrophy, skeletal muscle hypertrophy, and heart failure (12, 13, 35, 36, 38). This study showed, however, that transient miR-133a inhibition in primary isolated HSkM positively affects the differentiation rate of HSkM in vitro, consistent with past findings using C2C12 cells (28). Transient transfection was utilized, as miRs demonstrated long retention within HSkM (half time = 92.2 h). Overexpression of miR-1 and miR-206 in murine and human cultures (16, 28) led to increased differentiation; however, it was found that both miRs must be overexpressed to trigger myotube formation in vitro (11, 41). Here, inhibition of miR-133a alone successfully triggered formation of myotubes and increased sarcomeric α-actinin staining (a measure of differentiation) by 30%.
The delivery and application of transient myogenic miRs may be a viable way to enhance the growth and differentiation of engineered skeletal muscle in vitro for basic and translational research. The research presented here shows the importance of culture conditions and the potential of miR modulation to affect human myogenesis in vitro.
GRANTS
This work was supported by National Institutes of Health Grants R21-AR-55195 and UH2-TR-000505 and the National Institutes of Health Common Fund for the Microphysiological Systems Initiative.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.S.C., W.E.K., and G.A.T. are responsible for conception and design of the research; C.S.C., Y.E.-A., K.B., and R.H.H. performed the experiments; C.S.C., Y.E.-A., and Y.-E.H. analyzed the data; C.S.C. and G.A.T. interpreted the results of the experiments; C.S.C. prepared the figures; C.S.C. drafted the manuscript; C.S.C., W.E.K., and G.A.T. edited and revised the manuscript; C.S.C., Y.E.-A., K.B., Y.-E.H., R.H.H., W.E.K., and G.A.T. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Dorothy Slentz for help in development of the primary myoblast isolation protocol.
REFERENCES
- 1.Ai J, Zhang R, Gao X, Niu HF, Wang N, Xu Y, Li Y, Ma N, Sun LH, Pan ZW, Li WM, Yang BF. Overexpression of microRNA-1 impairs cardiac contractile function by damaging sarcomere assembly. Cardiovasc Res 95: 385–393, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Baj A, Bettaccini AA, Casalone R, Sala A, Cherubino P, Toniolo AQ. Culture of skeletal myoblasts from human donors aged over 40 years: dynamics of cell growth and expression of differentiation markers. J Transl Med 3: 21, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boldrin L, Muntoni F, Morgan JE. Are human and mouse satellite cells really the same? J Histochem Cytochem 58: 941–955, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boonen KJ, Langelaan ML, Polak RB, van der Schaft DW, Baaijens FP, Post MJ. Effects of a combined mechanical stimulation protocol: value for skeletal muscle tissue engineering. J Biomech 43: 1514–1521, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Boonen KJ, Rosaria-Chak KY, Baaijens FP, van der Schaft DW, Post MJ. Essential environmental cues from the satellite cell niche: optimizing proliferation and differentiation. Am J Physiol Cell Physiol 296: C1338–C1345, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Brown RH. Medicine. A reinnervating microRNA. Science 326: 1494–1495, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Burattini S, Ferri P, Battistelli M, Curci R, Luchetti F, Falcieri E. C2C12 murine myoblasts as a model of skeletal muscle development: morpho-functional characterization. Eur J Histochem 48: 223–233, 2004 [PubMed] [Google Scholar]
- 8.Carson JA, Booth FW. Effect of serum and mechanical stretch on skeletal α-actin gene regulation in cultured primary muscle cells. Am J Physiol Cell Physiol 275: C1438–C1448, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Chang L, Noseda M, Higginson M, Ly M, Patenaude A, Fuller M, Kyle AH, Minchinton AI, Puri MC, Dumont DJ, Karsan A. Differentiation of vascular smooth muscle cells from local precursors during embryonic and adult arteriogenesis requires Notch signaling. Proc Natl Acad Sci USA 109: 6993–6998, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 38: 228–233, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JF, Tao Y, Li J, Deng Z, Yan Z, Xiao X, Wang DZ. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol 190: 867–879, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Wang K, Chen J, Guo J, Yin Y, Cai X, Guo X, Wang G, Yang R, Zhu L, Zhang Y, Wang J, Xiang Y, Weng C, Zen K, Zhang J, Zhang CY. In vitro evidence suggests that miR-133a-mediated regulation of uncoupling protein 2 (UCP2) is an indispensable step in myogenic differentiation. J Biol Chem 284: 5362–5369, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg I, Eran A, Nishino I, Moggio M, Lamperti C, Amato AA, Lidov HG, Kang PB, North KN, Mitrani-Rosenbaum S, Flanigan KM, Neely LA, Whitney D, Beggs AH, Kohane IS, Kunkel LM. Distinctive patterns of microRNA expression in primary muscular disorders. Proc Natl Acad Sci USA 104: 17016–17021, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goto S, Miyazaki K, Funabiki T, Yasumitsu H. Serum-free culture conditions for analysis of secretory proteinases during myogenic differentiation of mouse C2C12 myoblasts. Anal Biochem 272: 135–142, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Grefte S, Vullinghs S, Kuijpers-Jagtman AM, Torensma R, Von den Hoff JW. Matrigel, but not collagen I, maintains the differentiation capacity of muscle derived cells in vitro. Biomed Mater Eng 7: 055004, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Koning M, Werker PM, van der Schaft DW, Bank RA, Harmsen MC. MicroRNA-1 and microRNA-206 improve differentiation potential of human satellite cells: a novel approach for tissue engineering of skeletal muscle. Tissue Eng 18: 889–898, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Koning M, Werker PM, van Luyn MJ, Krenning G, Harmsen MC. A global downregulation of microRNAs occurs in human quiescent satellite cells during myogenesis. Differentiation 84: 314–321, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Kook SH, Son YO, Choi KC, Lee HJ, Chung WT, Hwang IH, Lee JC. Cyclic mechanical stress suppresses myogenic differentiation of adult bovine satellite cells through activation of extracellular signal-regulated kinase. Mol Cell Biochem 309: 133–141, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Lawson MA, Purslow PP. Differentiation of myoblasts in serum-free media: effects of modified media are cell line-specific. Cells Tissues Organs 167: 130–137, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Liu N, Bezprozvannaya S, Shelton JM, Frisard MI, Hulver MW, McMillan RP, Wu Y, Voelker KA, Grange RW, Richardson JA, Bassel-Duby R, Olson EN. Mice lacking microRNA 133a develop dynamin 2-dependent centronuclear myopathy. J Clin Invest 121: 3258–3268, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu N, Williams AH, Maxeiner JM, Bezprozvannaya S, Shelton JM, Richardson JA, Bassel-Duby R, Olson EN. MicroRNA-206 promotes skeletal muscle regeneration and delays progression of Duchenne muscular dystrophy in mice. J Clin Invest 122: 2054–2065, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morley JE, Perry HM, 3rd, Miller DK. Something about frailty. J Gerontol A Biol Sci Med Sci 57: M698–M704, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Nielsen S, Scheele C, Yfanti C, Akerstrom T, Nielsen AR, Pedersen BK, Laye MJ. Muscle specific microRNAs are regulated by endurance exercise in human skeletal muscle. J Physiol 588: 4029–4037, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philp A, Belew MY, Evans A, Pham D, Sivia I, Chen A, Schenk S, Baar K. The PGC-1α-related coactivator promotes mitochondrial and myogenic adaptations in C2C12 myotubes. Am J Physiol Regul Integr Comp Physiol 301: R864–R872, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potthoff MJ, Olson EN. MEF2: a central regulator of diverse developmental programs. Development 134: 4131–4140, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Powell CA, Smiley BL, Mills J, Vandenburgh HH. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am J Physiol Cell Physiol 283: C1557–C1565, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Rangarajan A, Weinberg RA. Comparative biology of mouse versus human cells: modelling human cancer in mice. Nat Rev Cancer 3: 952–959, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Rhim C, Cheng CS, Kraus WE, Truskey GA. Effect of microRNA modulation on bioartificial muscle function. Tissue Eng 16: 3589–3597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhim C, Lowell DA, Reedy MC, Slentz DH, Zhang SJ, Kraus WE, Truskey GA. Morphology and ultrastructure of differentiating three-dimensional mammalian skeletal muscle in a collagen gel. Muscle Nerve 36: 71–80, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA 105: 2415–2420, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharples AP, Al-Shanti N, Stewart CE. C2 and C2C12 murine skeletal myoblast models of atrophic and hypertrophic potential: relevance to disease and ageing? J Cell Physiol 225: 240–250, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Springer ML, Ozawa CR, Blau HM. Transient production of α-smooth muscle actin by skeletal myoblasts during differentiation in culture and following intramuscular implantation. Cell Motil Cytoskeleton 51: 177–186, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Stern-Straeter J, Bran G, Riedel F, Sauter A, Hormann K, Goessler UR. Characterization of human myoblast cultures for tissue engineering. Int J Mol Med 21: 49–56, 2008 [PubMed] [Google Scholar]
- 34.Syedain ZH, Weinberg JS, Tranquillo RT. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci USA 105: 6537–6542, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatsuguchi M, Seok HY, Callis TE, Thomson JM, Chen JF, Newman M, Rojas M, Hammond SM, Wang DZ. Expression of microRNAs is dynamically regulated during cardiomyocyte hypertrophy. J Mol Cell Cardiol 42: 1137–1141, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, Gross C, Engelhardt S, Ertl G, Bauersachs J. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation 116: 258–267, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Townley-Tilson WH, Callis TE, Wang D. MicroRNAs 1, 133, and 206: critical factors of skeletal and cardiac muscle development, function, and disease. Int J Biochem Cell Biol 42: 1252–1255, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams AH, Liu N, van Rooij E, Olson EN. MicroRNA control of muscle development and disease. Curr Opin Cell Biol 21: 461–469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams AH, Valdez G, Moresi V, Qi X, McAnally J, Elliott JL, Bassel-Duby R, Sanes JR, Olson EN. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science 326: 1549–1554, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yablonka-Reuveni Z, Seger R, Rivera AJ. Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J Histochem Cytochem 47: 23–42, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Yan D, Dong Xda E, Chen X, Wang L, Lu C, Wang J, Qu J, Tu L. MicroRNA-1/206 targets c-Met and inhibits rhabdomyosarcoma development. J Biol Chem 284: 29596–29604, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye L, Haider H, Esa WB, Law PK, Zhang W, Su L, Zhang Y, Sim EK. Nonviral vector-based gene transfection of primary human skeletal myoblasts. Exp Biol Med (Maywood) 232: 1477–1487, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Kan S, Huang B, Hao Z, Mak TW, Zhong Q. Mule determines the apoptotic response to HDAC inhibitors by targeted ubiquitination and destruction of HDAC2. Genes Dev 25: 2610–2618, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang JS, Kraus WE, Truskey GA. Stretch-induced nitric oxide modulates mechanical properties of skeletal muscle cells. Am J Physiol Cell Physiol 287: C292–C299, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Zhang SJ, Truskey GA, Kraus WE. Effect of cyclic stretch on β1D-integrin expression and activation of FAK and RhoA. Am J Physiol Cell Physiol 292: C2057–C2069, 2007 [DOI] [PubMed] [Google Scholar]