Abstract
The tumor microenvironment is a milieu of heterogeneous architectural features that affect tumor growth and metastatic invasion. Pore size, density, stiffness, and fiber architecture change dramatically from location to location throughout the tumor matrix. While many studies have addressed the effects of two-dimensional extracellular matrix structure and composition on cell migration, less is known about how cancer cells navigate complex, heterogeneous three-dimensional (3D) microenvironments. Mechanical structures such as actin and keratin, part of the cytoskeletal framework, and lamins, part of the nucleoskeletal framework, play a key role in migration and are altered during cancer progression. Recent evidence suggests that these changes in cytoskeletal and nucleoskeletal structures may enable cancer cells to efficiently respond to features such as pore size and stiffness to invade and migrate. Here we discuss the role of cell mechanics and the cytoskeleton in the ability of cells to navigate and respond to 3D matrix features and heterogeneities.
Keywords: extracellular matrix topography, cytoskeleton, nucleoskeleton, three-dimensional migration, actin, keratin, lamins, mechanosensing
cell migration and invasion are critical processes in tumor physiology and metastasis. Our understanding of the mechanisms underlying cell migration, primarily on two-dimensional (2D) substrates, has progressed a great deal in the last decade. It has become evident that the cytoskeleton and the mechanical characteristics defined by the cytoskeleton play a critical role in regulating cell migration (27, 75, 101, 136). Recent work has sought to expand this knowledge to three-dimensional (3D) environments and highlights the importance of the interplay between the extracellular matrix (ECM) and the cytoskeleton (39, 41, 44, 59, 137). An area that is less addressed is how cells maneuver through and interpret the inherent physical heterogeneities in the tumor microenvironment; tumor tissue contains areas of dense matrix adjacent to interstitial spaces, it has areas that are both very compliant and very stiff, and it contains fibers and pores of various sizes. To successfully metastasize, tumor cells must navigate this landscape by pushing and squeezing their way through the matrix, requiring changes in cell shape and reorganization of the cytoskeleton.
While migration is an essential step in the progression of most metastatic cancers, there are no drugs that specifically target metastasis. The lack of such drugs is likely, in part, rooted in the fact that normal cells use much of the same migration-related signaling machinery in the normal physiological process used by tumor cells during invasion and metastasis. Emerging work on scaffolding systems and proteins that regulate adhesion-mediated mechanosensing and signaling may provide new therapeutic targets of metastasis. In this review, we discuss the interplay between 3D ECM architecture within the tumor microenvironment and the cell cytoskeleton, with emphasis on mechanosensing and transformation-associated changes in signaling specific to tumor cells.1
Tumor ECM Architecture
The architecture of the ECM is multifaceted: it includes elements such as composition, density, macromolecule orientation, and extent of cross-linking (39). Together, these characteristics of the ECM define the 3D fibrous scaffold to which cells can attach (112, 147). The ECM architecture of solid tumor tissue is highly heterogeneous (45, 86, 148) in its organization and its composition, creating an intricate obstacle course for invasive cancer cells (43). For example, ECM heterogeneities are apparent in the collagen fibrous network, where both loose and dense collagen organization can exist within the same tissue (147). As part of the large-scale changes in ECM remodeling that occur during tumor progression, increased collagen bundling can be observed tangentially around the tumor, effectively forming a shell (116, 147, 148). Cancer cells reorganize the tumor ECM through cell-mediated collagen bundling, as well as deposition of new ECM components such as fibronectin (39, 43). Additionally, cells can reorganize collagen into parallel fibers radiating perpendicularly to the tumor to facilitate invasion (148). Local degradation of ECM by matrix metalloproteinase (MMP)-mediated remodeling can also occur, resulting in formation of microtracks within the collagen matrix (45). These microtracks may contribute to an increase in metastasis, as they provide a pathway for directed cell movements (78).
During tumor progression, increased deposition of matrix results in a denser, stiffer stroma (18). While collagen deposition and bundling are sufficient to increase stiffness, cross-linking reactions can further amplify changes in the mechanical properties of the ECM. Notably, increased lysyl oxidase activity in tumor cells is responsible for collagen I cross-linking, creating fibrotic regions and stiffening the tumor microenvironment (18). The rigidity of the tumor ECM can also activate contractility pathways in cancer cells that drive a tensional imbalance, which in turn mediates tumor growth and eventual cell invasion and migration (103, 112, 114). Stiffening of the tumor microenvironment may play an important role in the decision-making process that drives metastatic migration (18). As such, the relationship between ECM stiffness and cell mechanical properties appears to play an important role in tumor progression.
Key Cellular Mechanical Features That Govern Cell Migration
To support migration and maintain their structural integrity, cells need the mechanically stable, yet dynamic, scaffold provided by the cytoskeleton, which is composed of actin microfilaments, microtubules (MTs), and intermediate filaments (IFs) (Fig. 1). In addition to the cytoskeletal network, the nucleus adds an additional layer of mechanical stability, since it is significantly stiffer than the rest of the cell body (32, 57). The tensional balance provided by the cytoskeleton and the nucleus plays a central role in the cellular response to extracellular and intracellular cues. As such, the key mechanical features of each of the cytoskeletal components provide structure and function to the cell. Indeed, all these key mechanical features are interconnected to form a mechanical continuum (90). Therefore, the cell requires discrete control over the dynamics of these mechanical features to initiate and maintain cell motility.
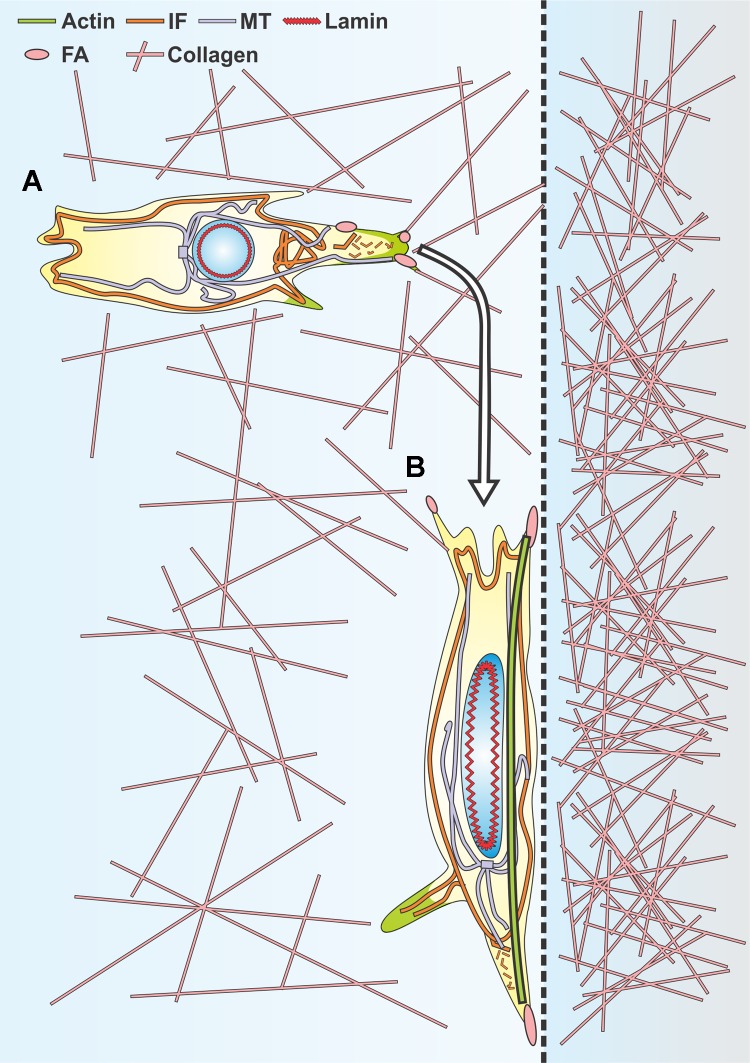
Fig. 1.
Overview of key mechanical features governing cell migration in restrictive 3-dimensional (3D) channels. A: representations of the same migrating cell inside a restrictive 3D channel showing cortical localization of actin along the wall of the channel and at both extremities of the migrating cell, intermediate filament (IF) localization at the leading edge of the migrating cell, microtubule (MT) preferential localization at the leading edge of the migrating cell, and a schematic of cytoskeletal-nucleoskeletal connections via linker of nucleoskeleton and cytoskeleton (LINC) complex coupling of lamins and actin/IFs. B: overexpression of lamin IFs stiffens the nucleus and poses a significant barrier to migration in subnuclear pore sizes, while diminished expression of lamin IFs softens the nucleus and allows for greater nuclear deformability. C: confocal image of a cell migrating inside a microfabricated 3D collagen microtrack showing actin (green) and reflectance (collagen, cyan). (See Ref. 78 for details.)
Actin stress fibers have been described as a prestressed network that supports most of the intracellular cytoskeletal tension (69). Notably, actin microfilaments provide the largest contribution to cell body stiffness when probed at adhesion sites (10, 11, 48, 67). Actin's contribution to cellular mechanical properties can largely be attributed to its inherent elastic features and the myosin-mediated contractility of actin fibers (10, 48, 69). In addition, linkage of actin to the ECM via focal adhesion (FA) (48, 66) enables actin dynamics to contribute to “fast” cell processes, including cell motility and proliferation, and long-term adaptation, as in response to ECM stress. Actin filaments are organized into different structures, including actin bundles and stress fibers. They stabilize cell architecture, including formation of lamellipodia and filopodia, which play important roles in cell motility (66, 137). Actin's rapid response to ECM stress is largely regulated through Rho GTPases (66, 67, 133). The most well-studied Rho GTPases that regulate actin dynamics are RhoA, Rac1, and Cdc42, which regulate stress fiber, lamellipodia, and filopodia formation, respectively (66). As such, these proteins are important for efficient cell migration (6, 134). Interestingly, the balance between these pathways is known to affect actin-mediated cellular mechanical properties and mechanosensing (18, 66, 134). Overall, actin participates as an internal stabilizer and a dynamic mechanical structure in cells for migration and mechanosensing.
The mechanical continuum of a cell also relies on the MT network, which provides internal structural support while contributing to the polarization and initiation of cell migration (75, 128). MTs have been described as load-bearing elements that can balance other forces in the cell (69). Notably, large-scale disruption of MTs can have dramatic mechanical consequences on cell stiffness (10). In fact, MTs can maintain their organization even under high compressive load (15). MT depolymerization and inhibition of MT dynamics effectively impair cell motility (75). Moreover, MT catastrophe occurs primarily at the rear end of the cell, while the leading edge shows a significant polarization of the MT network (75). The formation of FAs at the leading edge of migrating cells promotes repositioning of MTs toward the leading edge (74). FAs appear to be necessary for MT depolymerization, while MTs are required for FA disassembly and regulation (58, 74). These behaviors suggest that MTs may play a crucial role in cell mechanosensing of 3D ECM topography. While spatial organization of MTs influences cell migration, MTs also participate in activating signaling cascades involving Rac1, which promotes formation of lamellipodia at the leading edge and subsequent actin fiber formation for migration (142). Connections between the MT and actin network exist indirectly through the MT-stabilizing agents termed MT-associated proteins and plakin family proteins (127, 146). MT-associated proteins stabilize MTs, but some also take part in the nucleation of actin protrusions (122). As such, MTs are involved in maintaining cellular mechanical integrity and enabling mechanosensing, allowing for a cell to polarize in response to ECM cues as well as to initiate migration.
Within the mechanical continuum of the cell cytoskeleton, IFs exist as associated effectors of the cytoskeletal framework through connections with actin and MTs. IFs are the most diverse family of cytoskeletal components, comprising six types of filaments with expressions that are related to differentiation state (37). All epithelial cells express several members of the keratin IF subfamily; among those, keratin 8 (K8) constitutes a hallmark of simple epithelial tissue from which the vast majority of solid tumors originate (99, 124). The transformation process can lead to overexpression and/or loss of expression of IF proteins (73). Notably, carcinomas often begin expressing vimentin IFs, which are normally limited to mesenchymal cells (27). Carcinomas also often present variable expression of the lamin type V subfamily of IFs (19).
Cytoplasmic IFs possess unique features, including an absence of polarity, a wide subcellular dispersion between the surface membrane and the nucleus, and nonlinear viscoelastic properties. In most epithelial cells, the interconnected network formed by keratins spans the cell cortex and winds around the nucleus to provide a continuous link between ECM-cell adhesions (FA and hemidesmosome), cell-cell adhesions (desmosome), and the nucleus through the linker of nucleoskeleton and cytoskeleton (LINC) complex (33, 62). This organization is known to provide mechanical integrity to cells and tissues (31). Vimentin and keratins provide structural integrity to cells through their plectin-mediated relationship with actin filaments and other IFs (46). In functional terms, we know that IFs regulate tension within the mechanical infrastructure of a cell and participate in regulation of cellular processes such as cell migration (27). Overall, IFs constitute an important modulator of cell behavior, mainly through participation of associated proteins and their interplay with MTs, actin, and the nucleus.
Finally, the nucleus remains a central part of the cell mechanical continuum, as it exists as the largest and stiffest organelle with the ability to effect cell migration through nucleocytoskeletal connections. The nuclear membrane is a highly adaptive structure, and its underlying nucleoskeleton comprises mainly lamin IFs, a viscoelastic structure that stiffens with age (120) and softens as a result of phosphorylation during cell division (49). The morphology of the nucleus includes a dense meshwork of lamin proteins that provides the majority of the structural support to the nucleus (33), while bound heterochromatin provides a secondary mode of support (32). The nucleoskeleton exists with direct links to the cytoplasmic cytoskeleton through the LINC complex, which connects the lamin network with actin and IFs (27, 84). When cells are subjected to mechanical stress, the nucleus can sustain global deformation and changes in its subnuclear spatial organization, indicating that the nucleus is a mechanosensitive element within the cell (8, 90). While we know that the mechanosensory features of the nucleus play a role in regulating cell migration (33, 84), the exact mechanisms are unknown. Lamin IFs and the LINC complex appear to play a central role in mediating cell motility through its intimate connections to actin and IFs (33). As a mechanoresponsive and space-restricting organelle, the nucleus is a force mediator during migration through cytoskeletal connections via the LINC complex.
The mechanical properties of tumor cells are inherently different from those of healthy cells. Indeed, it has been consistently shown that adherent tumor cells are generally more compliant than normal cells while generating greater traction forces (56, 77, 103). Both of these characteristics could prove critical in understanding tumor cell migration in 3D matrices with heterogeneous topographic features. On one hand, more malleable cells might be able to squeeze through an ECM with smaller pore size. On the other hand, generating greater traction forces may facilitate cell-mediated ECM reorganization and subsequent invasion. These key mechanical features of tumor cells can be linked to alterations in cytoskeleton dynamics, organization, or interconnectivity (18, 27, 77), which in turn likely affect their mechanosensing ability and migratory behavior.
Cell Migration in 3D Confined Spaces
Given the highly heterogeneous nature of the ECM spatial organization within a solid tumor, a migrating malignant cell will encounter a variety of physical ECM structures that may facilitate or impede its movement. To overcome such migration barriers, cells can adopt different modes of migration (39, 83, 86, 104). Furthermore, simultaneous in vivo observation of tumor cell migration and ECM architecture shows that tumor cells migrate in set patterns, depending on ECM structure (29, 50, 107, 145). While understanding how cells migrate in a 3D environment represents only one aspect of the problem of metastasis, it is important to characterize the governing parameters of the ECM that support and affect migration.
Cell migration in a homogeneous 3D ECM.
Given the well-established relationship between tumor progression and local ECM density (18), efforts by our group and others have initially focused on this simple parameter. While epithelial tumor cells can freely migrate inside low-density gels, higher-density collagen can impair their migration (20, 114, 150). Similar findings hold true for other cell types, including fibroblasts and endothelial cells (93, 97). To make their way through denser ECM, evidence suggests that cells use MMPs to cleave the ECM and reorganize individual fibers (21, 148, 150), or they simply squeeze through gaps in the ECM (21, 45). In addition, increased MMP expression is frequently related to increased metastasis associated with invasion potential inside 3D Matrigel or collagen matrices (38, 40, 65, 125, 138). More specifically, MT1-MMP and MMP2 have been found in aggressive tumors, with MMP2 localized at the tumor-stroma interface (65, 116). Formation of microtracks by tumor cells and tumor-associated fibroblasts migrating inside in vitro 3D matrices has been associated with MMP activity (44, 47, 151). Interestingly, this ability of aggressive tumor cells and tumor-associated fibroblasts to use MMPs to form microtracks can enable the invasion of less aggressive cells, as evidenced by the use of cocultured spheroids embedded in collagen gels (21, 47). However, some highly aggressive tumor cells can bypass the MMP requirement and have been shown to migrate inside ECMs of different densities by adopting an MMP-independent migration mode (117, 149). Even when MMPs are inhibited, increasing the density of collagen gels effectively reduces migration of fibrosarcoma and carcinoma cells (20, 21, 150, 151).
Experimentally, there is an inherent drawback to altering hydrogel density in generating a 3D ECM: an increase in density will result in an increase in gel stiffness and a decrease in pore size (20, 97). Recent efforts to achieve independent control of these two parameters have proven invaluable in efforts to gather additional information on the regulating effects of ECM architecture on cell migration. Collagen gel pore size can be controlled using several different methods (20, 150), including varying gel thickness or using colder temperatures to limit the polymerization rate. In each case, the result remains the same: larger pore size enables faster cell migration (20, 97, 150). Alternatively, cross-linking agents, such as enzymatic glycation by ribose, can be used to increase collagen gel stiffness without affecting pore size (93). Levental and colleagues (81) showed that ErbB2-transformed MCF10A mammary cells required increased gel stiffness to invade 3D ECM. Therefore, one can assume that pore size acts as a physical barrier for migration, making it an important limiting factor, while stiffness has more of a positive modulating role.
Cell migration in heterogeneous 3D ECM.
As described above, density and cross-linking are not the only features of the ECM architecture. Macromolecular organization also plays an important role in driving cell migration. Collagen reorganization and realignment at the tumor-stroma interface have provided a strong rationale for the study of contact guidance of 3D cell migration (110). Several groups have developed methods to alter 3D ECM architecture by controlling fiber orientation within gels or by introducing matrix heterogeneities (14, 54, 96, 112, 113). Experiments with highly invasive MDA-MB-231 breast carcinoma cells have demonstrated that these cells will invade farther inside collagen gels of a highly aligned than a random-orientation fibrous network (112). Nested collagen matrices have proven a reliable method to introduce heterogeneities inside collagen gels (96, 97), where compressed collagen gels containing cells are embedded within acellular uncompressed matrices. Surprisingly, fibroblasts' ability to invade the acellular gels was found to be independent of collagen density (96). One parameter that could affect the invasion of these cells was the molecular composition of the 3D matrix: acellular collagen gels induced more extensive invasion than fibrin gels, while addition of fibronectin to either of these matrices increased cell invasion (96). Interestingly, these matrices were normalized for gel stiffness or density, suggesting that the molecular composition of the ECM is a major determinant of cell invasion. Moreover, the mode of migration (collective vs. individual migration) is determined in part by the composition of the matrix (96).
To recapitulate the spatial heterogeneities found in vivo, we pioneered a method to create a discrete 3D interface between two collagen gels without the use of gel compaction, therefore providing more control over the interface location and dimensions. Using this technique, we showed that highly invasive MDA-MB-231 cells can cross the collagen interface only toward a region of lower collagen density (14). In the reverse case, the cells migrate preferentially along the plane of the interface without crossing between the two gels. Even MCF10A cells, which are normally nonmigratory inside a 3D collagen matrix, were found to migrate along the interface. Overall, these findings underscore the importance of ECM 3D architecture in driving tumor cell migration.
Cell migration in microfabricated confined spaces.
ECM 3D architecture can create a physical barrier or provide contact guidance, depending on its structural features, raising an interesting question regarding how cells navigate the confined spaces of the stromal matrix. Several groups have adopted microfabrication techniques to create microchannel devices to mimic the spatial features within the tumor ECM, including the size of subnuclear pores or microtracks used by tumor cells (47, 151). These devices have become popular tools to analyze tumor cell behavior within the confines of rigid polydimethylsiloxane (PDMS) or silicon channels (4, 88). Through the use of tapered channels with defined physical spatial gradient junctions, we have shown that the ability of cells to permeate a spatially confined region depends on the steepness of the gradient and the cell type (89). Aggressive MDA-MB-231 cells more readily squeeze through these highly restrictive channel gradients than do noninvasive cells. Moreover, MCF10A cells lose their ability to permeate when the spatial gradient increases, while no such reduction is observed with MDA-MB-231 cells (89). It is interesting to note that the width of the channels alters cell migration in a biphasic way, with more restrictive portions of a channel resulting in significantly slower speed (70, 89, 131). For channels of similar size, tumor cells of different lineage have marked differences in cell speed (70). As the channel cross section is increased, such that its size becomes wider than the cell body, cell migration becomes more random and less persistent, which results in slower migration (4, 70).
One consistent observation in these channels is that the cytoskeleton deforms as the cell proceeds through the confined space. It is therefore not surprising that altering the stability of the actin and MT network generally impairs cell migration in most channel sizes (4, 88). Cell migration is associated with actomyosin contractility, and its inhibition prevents migration inside restrictive channels that are ≥6 μm (4). For 3-μm channels, actin contractility inhibition or actin network destabilization can prevent cells from entering the channel, but once inside, only actin disruption affects migration across the channel (4). Interestingly, recent data suggest that altering MTs alone may limit cell migration inside the 3-μm channel and greatly increase the number of times the cells change direction in the microchannel, suggesting that MTs are important for cell guidance (4). Using a similar approach, Rolli and colleagues (115) showed that sphingosylphosphorylcholine (SPC)-induced perinuclear collapse and reorganization of keratin IFs decrease the cell's ability to penetrate 7-μm channels. However, those SPC-treated cells that are able to enter the channels are less likely to stop migrating or change direction, and more cells migrate through the device (115). SPC did not affect cell speed inside the channel, whereas it increased cell speed on a 2D planar surface. In addition, cells migrating inside microchannels present an intracellular distribution of keratin IFs that appears to be predominantly facing the front of the cell. Using a micropillar-based assay to create 3D features within the cell migration path, Booth-Gauthier et al. (9) showed that lamin overexpression hinders fibroblastic cell movement through a network of PDMS pillars. In that case, migration speed and traction forces are reduced by overexpression of lamins. Although this work was not done with cancer cells, the ubiquitous nature of lamins suggests that these experiments and results are highly relevant for tumor biology.
One concern in most studies using microfabricated channels is the absolute stiffness of the devices, given that ECM stiffness is known to affect 3D cell migration and invasion (81, 93). Therefore, to better reflect the compliant nature of tissues, Pathak and Kumar (105) and Kraning-Rush et al. (78) substituted the PDMS for polyacrylamide or collagen fibrous gels. The compliance of these two materials can be limiting, since they can potentially be subject to swelling and buckling (23), which in turn may limit the smallest attainable feature size, making comparison with highly restrictive PDMS-based channels difficult. Nevertheless, feature sizes that are representative of the cellular length scale and actual reported width of microtracks can be achieved (47, 78, 151). An interesting observation reported by Pathak and Kraning-Rush and their colleagues with MCF10A and MDA-MB-231 cells, respectively, was increased migration speed and directional persistence of cells within channels compared with cell migration on 2D planar surface or inside 3D collagen gels. Microtrack-mediated cell migration inside collagen gels was independent of MMP activity (68, 78). Furthermore, it was shown that both stiffness and confinement affect actomyosin alignment in polyacrylamide channels (105). In support of those observations, inhibition of myosin activity by blebbistatin rendered cells less sensitive to confinement (105). Conversely, the effects of oncogene expression on cell sensitivity to confinement are much more pronounced on stiffer substrates (106), indicating that transforming potential and stiffness of the channels are important factors that regulate cell migration in confined spaces. Interestingly, the Rho GTPase Rac1 was found to be required for MCF10A cell motility for all stiffness and confinement spaces (106), further implicating actin dynamics in topographical feature-driven cell migration. This specific finding is particularly relevant in light of recent studies on the follow-the-leader invasion of tumor cells, where RhoA and MMP activation in leader cells is required to allow follower cell invasion along the microtracks left in the wake of leader cells (21, 47), suggesting the existence of two different migration mechanisms, depending on whether the cell is a leader or a follower. In addition, follower melanoma cells require Cdc42 activation to invade in the presence of microtracks (47), indicating that proteins from the Rho GTPase family have very distinct roles in promotion of 3D migration. Overall, these results point to an important contribution of proteins regulating actin dynamics in driving migration inside 3D confined space.
Role of Mechanosensing Machinery
Cellular mechanosensing appears to be regulated by several interlinked factors that converge on integrin-mediated FAs and the ability of cells to actively probe their surroundings. Integrin-mediated cell adhesions provide a mechanical linkage between the ECM and the cytoskeleton and a signaling platform, coupling the cell “sensing” and “responding” mechanisms (see Refs. 18, 51, and 66 for reviews). In addition, the interplay between mechanosensory proteins and cell contractility enables the cell to actively probe the ECM (51, 108). Not surprisingly, several of these mechanosensors are FA proteins and have been found to be involved in durotaxis and the contact guidance process in 2D (28, 35, 36, 48, 119, 141, 144). Remarkably, the modulating role of some of these FA proteins, such as p130Cas and zyxin, in 3D cell migration is the opposite of their known role in 2D (41). A debate has emerged concerning the formation and structure of FAs in 3D (59), but there is accumulating evidence that FA domains exist in 3D (14, 80). Increasing the density of collagen gels leads to greater activation of FA kinase (FAK) and Src in tumor cells (111). Similarly, increased stiffness induced by lysyl oxidase or ribose cross-linking of collagen promotes integrin clustering, increased FAK activation, and phosphoinositide 3-kinase signaling in vitro and in vivo (81). Moreover, FAK and zyxin downregulation has been shown to result in greater ECM remodeling (41). In addition, the 3D tumor microenvironment contains regions of low- and high-density matrices that can present 2D-like interfaces to migrating cells, which may trigger a response from FA-sensing mechanisms (Fig. 2). We recently discovered that the presence of a collagen interface stimulates cell elongation along the plane of the interface, and FAs with activated FAK can be readily identified on the interface (14). Cells migrate primarily along the interface plane (14). Work by others suggests that contact guidance causes directional migration by controlling cell polarity (109, 121). Interestingly, Raab and colleagues (113), using mesenchymal stem cells seeded on polyacrylamide gels acting as a tunable interface with a collagen overlay, where cells were more likely to migrate into the collagen gel when starting on a soft polyacrylamide gel than on a stiff gel, obtained similar findings. They also reported that directional migration is dependent on myosin contractility. Overall, these data suggest that the bidirectional relationship between the ECM, FA proteins, and cell contractility in 2D is also present in 3D.
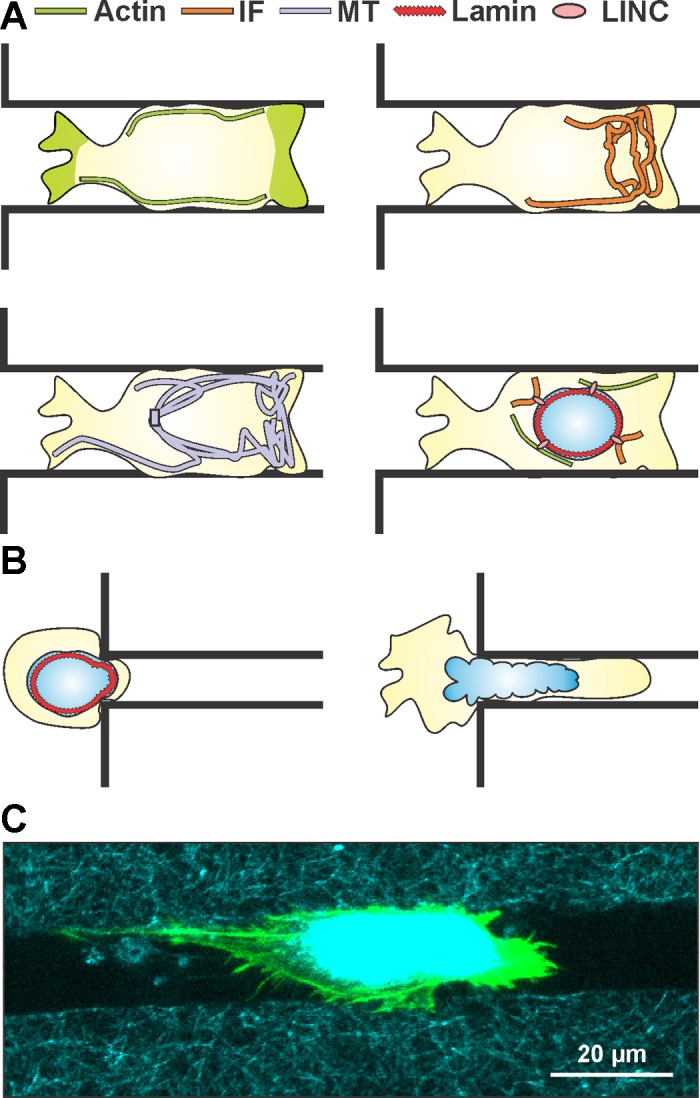
Fig. 2.
A migrating cell relies on the coordinated effort of the cytoskeleton for its mechanosensing ability in a 3D matrix. A migrating cell utilizes the polarizing features of the MT network while also relying on IF assembly and localization at the leading edge to initiate integrin binding (A). When the migrating cell reaches a stiffened interface (arrow), it has the potential to initiate localized integrin binding sites, as well as formation of stress fibers and nuclear deformation, changing the overall cell morphology to a spread shape (B).
Actin dynamics/contractility and cell adhesion are highly interdependent (66, 136), and altered actin dynamics perturb cell adhesion to the ECM and mechanosensing (13, 101–103). Changes in actin dynamics are also associated with tumorigenesis (42, 72). Several oncogenes and tumor suppressors, such as Src family kinases, phosphatase and tensin homolog, and phosphoinositide 3-kinase, are direct actin regulators (42, 66). For example, c-Src is upstream of Rho GTPases and myosin light chain kinase, which regulate actin dynamics and contractility (66). Other oncogenes can have a more indirect effect, such as Ras, which can lead to increased β1-, α5-, and α6-integrin expression (92). Indeed, expression levels of α5β1-integrins correlate with 3D migration of MDA-MB-231 cells (24, 95). However, different cell types using the same integrin dimers to bind to a given ECM substrate exhibit different cell spreading, migration, actin organization, and traction force (12, 24, 34, 36, 77, 102), raising the question: how do these differences arise if they use the same FA-sensing machinery?
Intermediate Filaments as Regulators of Tumor Cell Mechanosensing
IFs are largely understudied compared with other cytoskeletal components, in part due to their relative static nature compared with actin and MTs. As described above, IFs have been widely used as molecular markers to establish tumor cell origin and establish prognosis (73). Notably, gain in K8 expression in squamous cell carcinoma, melanoma, and some breast-derived tumors is seen as a negative prognostic, while it is seen as positive in other types of carcinomas (73, 100). Expression of vimentin in carcinomas is indicative of epithelial-to-mesenchymal transition and a highly invasive cell type (94). While IFs impart mechanical resilience to the cell (30, 91, 140), recent evidence suggests that they have several other important biological functions (for extensive review, see Refs. 22, 31, and 99). Specifically, keratin and vimentin IF expression can modulate cell signaling by acting as a scaffolding network for adaptor and signaling proteins (12, 52, 79, 85). It is widely accepted that IFs regulate different cell functions, including cell migration (1, 7, 12, 16, 143), cell surface receptor activation and trafficking (53), and actin network organization and contractility control (2, 13, 132).
Some evidence indicates that IFs are involved in mechanosensing at FAs. The K8-K18 pair is a modulator of FAK activation after integrins bind to the ECM in hepatoma cells (12). These same cells are unable to adequately sense differences in 2D substrate stiffness if they lack the K8-K18 IF pair (13). In addition, the actin stress fiber network in these K8-K18 IF-lacking cells is fragmented, and local stiffness measured at FAs is perturbed (11, 13). These observations are most likely related to altered RhoA-mediated actin dynamics and contractility in the absence of K8-K18 IFs (13). Actin network fragmentation or alteration has also been reported in other cell types following changes in K8 expression (130). Furthermore, some recent studies have revealed that K8 can interact and modulate the action of the actin-bundling proteins ezrin (52, 139) and fascin (2). Whether modulation of mechanosensing by K8 extends to other IFs remains to be seen. In terms of 3D migration, recent work by Fortier et al. (40) demonstrated that loss of K8 in carcinomas results in increased invasion through Matrigel-coated Transwell inserts, along with higher MMP expression. Finally, K19, which is the other possible partner of K8, has been reported to form a complex with cytoplasmic tissue transglutaminase (tTG) and Src (82). tTG is often overexpressed in breast cancer cells and possesses GTPase activity and acyl transferase activity (3, 25). Moreover, tTG acyl transferase activity is also known to cross-link the ECM (129). Remarkably, inhibition of tTG acyl transferase activity prevents its association with K19, while K19 silencing prevents its binding to Src (82). These findings suggest that keratin IFs may be prime candidates to modulate cell mechanosensing of 3D topography and cell migration in 3D confined spaces (Fig. 2).
Not surprisingly, vimentin expression in aggressive carcinoma is associated with a number of changes associated with epithelial-mesenchymal transition, including loss of cell-cell contact and increased migration (27, 94). Involvement of vimentin in cell migration takes several forms. Ivaska et al. (71) showed that recycling of endocytosed integrins to the plasma membrane is regulated by PKC phosphorylation of vimentin. Part of this process has been shown to be related to vimentin association with the actin cross-linking protein filamin (76). Such close association of vimentin with actin accessory proteins indicates that it may possess an actin dynamic regulatory capacity akin to keratins. Indeed, Helfand et al. (60) showed that local disruption of the vimentin network in fibroblasts induces spontaneous formation of Rac-driven lamellipodia. Furthermore, vimentin-knockout fibroblasts generate less traction and have smaller vinculin-positive FAs (5). However, it remains to be seen if these findings related to vimentin regulation of actin dynamics are also relevant to cancer cells and in 3D ECM. One recent study provides some insight into the role of vimentin in cancer cells by showing in MDA-MB-231 cells that invadopodia elongation and maturation, but not formation, require vimentin and MTs and that keratin is localized in mature invadopodia (123). Overall, given the expression patterns of IFs in carcinomas and previous reports suggesting an additive effect of vimentin-keratin coexpression on cell migration and invasion (26, 61), cooperation between both IF types could further affect mechanosensing.
Lamin IF expression is often deregulated in tumor cells, and the relation between lamins, nuclear shape, and nucleus viscoelastic properties has important consequences for tumor cell metastasis. Lamin IFs have been identified as potential malignant biomarkers in several cancers for their differential expression and localization patterns in tumor tissue specimens (19). While we lack information on the functional role of lamin expression in tumor cell migration, recent studies involving patient cells expressing the Hutchinson-Gilford progeria syndrome phenotype, a mutation of lamin A/C IFs that stiffens the nucleoskeleton, determined that a stiffened nucleus poses a significant barrier to migration in a 3D environment (9). These lamin defects are also known to affect fibroblast migration in 2D and 3D space (9, 150), suggesting that lamins play a crucial role in regulating migration. Bussolati et al. (17) showed that lamin B IF mislocalization inside the nucleus leads to nucleoskeletal invaginations that correlate with aggressive metastasis to lymph nodes and poor patient outcome. While alterations in lamin expression may contribute to nuclear deformability during migration, it has also been suggested that lamins play a mechanosensory role through their anchorage to cytoplasmic cytoskeletal components through the LINC complex (33). Other lamin-anchored nucleoproteins have also been shown to exhibit differential expression in tumor samples with connections to poor prognosis (84). While cells with lamin defects do not present apparent differences in actin organization (135), it has been shown recently that actin dynamics are directly affected by lamin expression in fibroblasts (63), suggesting a functional role for lamins. Therefore, it is likely that lamin IF expression or mutations play a significant role in regulating mechanosensing features in cancer cells, most likely through their control over nucleoskeletal dynamics, as well as their connections to the cytoplasmic cytoskeleton via the LINC complex.
Future Directions and Conclusions
An ongoing challenge is translation of these findings in vivo. Typically, tumor progression in common animal models is followed macroscopically by using bioluminescence imaging, X-ray microtomography, or magnetic resonance imaging (87, 153). These techniques do not provide information on the microenvironment architecture, nor do they have the resolution to track single metastatic cells. Recent advances in intravital imaging may provide ways to validate in vitro 3D migration data and molecular findings using current solid tumor animal models, including subcutaneous or mammary fat pad injection of tumor cells. Such imaging techniques have been successfully used to track single cells in vivo (50, 107, 145). Multiphoton imaging can provide structural information on in vivo collagen fibers through second harmonic generation and maintain the ability to track tumor cells or perform photoactivation (50, 55, 145, 152). In that context, further exploration of the role of microtracks in tumor cell dissemination in vivo is now possible. While more work needs to be done to identify relevant tumor-associated mechanosensor drug targets, these imaging advances will enable first-hand assessment of their validity in in vivo and 3D in vitro systems.
Another promising approach to studying cell behavior in 3D microenvironments relies on the use of artificial constructs of polymer hydrogels coupled to cell adhesion ECM domains. These constructs will likely offer an alternative to native ECM scaffolds to form homogeneous and heterogeneous scaffolds. Several groups have developed ways to control parameters such as stiffness, spatial organization and architecture, ECM binding sites, and MMP cleavage site availability (64, 98, 118, 126). Initial assessment of cell migration in those scaffolds looks promising, even providing information regarding the dependence of tumor cells on ligand availability in 3D (64). However, we are still a long way from recreating all the complexity of the native ECM, and because of the expertise required to create these scaffolds, their use in exploring tumor cell invasion will probably not become mainstream. Nevertheless, techniques developed to engineer these artificial scaffolds may ultimately enable better understanding and control of the physical parameters of the native ECM scaffold.
Overall, recent work has revealed that ECM topographic features in 3D space can alter cell migration. At the same time, these studies have added to our understanding of the importance of the cytoskeletal network in migration processes. More importantly, we are starting to unravel the mechanisms regulating cell migration in 3D, where FA proteins appear to again take the center stage to allow the cell to sense topographic cues provided by the ECM. Moreover, it is becoming apparent that some proteins, notably IFs, can modulate FA-mediated mechanosensing and signaling, which may affect cell tensional balance and migration. Several of the mechanisms regulating tumor-related FA-mediated mechanosensing remain to be fully tested and validated in 3D systems to correctly identify intermediary effector proteins. More in-depth exploration of that avenue may lead to identification of better prognostic markers, as well as new therapeutic targets that prevent cancer cell metastasis, by exploiting inherent cytoskeletal and scaffolding protein changes following transformation.
GRANTS
This work was supported by the Cornell Center on the Microenvironment and Metastasis through National Cancer Institute Grant U54 CA-143876 and National Science Foundation-National Institutes of Health Physical and Engineering Sciences in Oncology Grant 1233827 (C. A. Reinhart-King).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.B. and T.A.A. drafted the manuscript; F.B., T.A.A., and C.A.R.-K. edited and revised the manuscript; F.B., T.A.A., and C.A.R.-K. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Shawn P. Carey for helpful discussions and critical reading of the manuscript and Casey M. Kraning-Rush for providing the image of the cell in a microchannel.
Footnotes
This review is part of a five-article theme series on Physical Biology in Cancer in this issue.
REFERENCES
- 1.Alam H, Gangadaran P, Bhate AV, Chaukar DA, Sawant SS, Tiwari R, Bobade J, Kannan S, D'Cruz AK, Kane S, Vaidya MM. Loss of keratin 8 phosphorylation leads to increased tumor progression and correlates with clinico-pathological parameters of OSCC patients. PLos One 6: e27767, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam H, Kundu ST, Dalal SN, Vaidya MM. Loss of keratins 8 and 18 leads to alterations in α6β4-integrin-mediated signalling and decreased neoplastic progression in an oral-tumour-derived cell line. J Cell Sci 124: 2096–2106, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Antonyak MA, Li B, Regan AD, Feng Q, Dusaban SS, Cerione RA. Tissue transglutaminase is an essential participant in the epidermal growth factor-stimulated signaling pathway leading to cancer cell migration and invasion. J Biol Chem 284: 17914–17925, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balzer EM, Tong Z, Paul CD, Hung WC, Stroka KM, Boggs AE, Martin SS, Konstantopoulos K. Physical confinement alters tumor cell adhesion and migration phenotypes. FASEB J 26: 4045–4056, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharya R, Gonzalez AM, Debiase PJ, Trejo HE, Goldman RD, Flitney FW, Jones JC. Recruitment of vimentin to the cell surface by β3-integrin and plectin mediates adhesion strength. J Cell Sci 122: 1390–1400, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J 348: 241–255, 2000 [PMC free article] [PubMed] [Google Scholar]
- 7.Boczonadi V, McInroy L, Maatta A. Cytolinker cross-talk: periplakin N-terminus interacts with plectin to regulate keratin organisation and epithelial migration. Exp Cell Res 313: 3579–3591, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Booth-Gauthier EA, Alcoser TA, Yang G, Dahl KN. Force-induced changes in subnuclear movement and rheology. Biophys J 103: 2423–2431, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Booth-Gauthier EA, Du V, Ghibaudo M, Rape AD, Dahl KN, Ladoux B. Hutchinson-Gilford progeria syndrome alters nuclear shape and reduces cell motility in three dimensional model substrates. Integr Biol (Camb) 5: 569–577, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Bordeleau F, Bessard J, Marceau N, Sheng Y. Measuring integrated cellular mechanical stress response at focal adhesions by optical tweezers. J Biomed Optics 16: 095005, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Bordeleau F, Bessard J, Sheng Y, Marceau N. Keratin contribution to cellular mechanical stress response at focal adhesions as assayed by laser tweezers. Biochem Cell Biol 86: 352–359, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Bordeleau F, Galarneau L, Gilbert S, Loranger A, Marceau N. Keratin 8/18 modulation of protein kinase C-mediated integrin-dependent adhesion and migration of liver epithelial cells. Mol Biol Cell 21: 1698–1713, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bordeleau F, Myrand Lapierre ME, Sheng Y, Marceau N. Keratin 8/18 regulation of cell stiffness-extracellular matrix interplay through modulation of Rho-mediated actin cytoskeleton dynamics. PLos One 7: e38780, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bordeleau F, Tang LN, Reinhart-King CA. Topographical guidance of 3D tumor cell migration at an interface of collagen densities. Phys Biol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brangwynne CP, MacKintosh FC, Weitz DA. Force fluctuations and polymerization dynamics of intracellular microtubules. Proc Natl Acad Sci USA 104: 16128–16133, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busch T, Milena Eiseler T, Joodi G, Temme C, Jansen J, Wichert GV, Omary MB, Spatz J, Seufferlein T. Keratin 8 phosphorylation regulates keratin reorganization and migration of epithelial tumor cells. J Cell Sci 125: 2148–2159, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bussolati G, Marchio C, Gaetano L, Lupo R, Sapino A. Pleomorphism of the nuclear envelope in breast cancer: a new approach to an old problem. J Cell Mol Med 12: 209–218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer 9: 108–122, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cance WG, Chaudhary N, Worman HJ, Blobel G, Cordoncardo C. Expression of the nuclear lamins in normal and neoplastic human tissues. J Exp Clin Cancer Res 11: 233–246, 1992 [Google Scholar]
- 20.Carey SP, Kraning-Rush CM, Williams RM, Reinhart-King CA. Biophysical control of invasive tumor cell behavior by extracellular matrix microarchitecture. Biomaterials 33: 4157–4165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carey SP, Starchenko A, McGregor AL, Reinhart-King CA. Leading malignant cells initiate collective epithelial cell invasion in a three-dimensional heterotypic tumor spheroid model. Clin Exp Metastasis 30: 615–630, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang L, Goldman RD. Intermediate filaments mediate cytoskeletal crosstalk. Nat Rev Mol Cell Biol 5: 601–613, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Charest JM, Califano JP, Carey SP, Reinhart-King CA. Fabrication of substrates with defined mechanical properties and topographical features for the study of cell migration. Macromol Biosci 12: 12–20, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Chen LF, Vicente-Manzanares M, Potvin-Trottier L, Wiseman PW, Horwitz AR. The integrin-ligand interaction regulates adhesion and migration through a molecular clutch. PLos One 7: 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chhabra A, Verma A, Mehta K. Tissue transglutaminase promotes or suppresses tumors depending on cell context. Anticancer Res 29: 1909–1919, 2009 [PubMed] [Google Scholar]
- 26.Chu YW, Seftor EA, Romer LH, Hendrix MJ. Experimental coexpression of vimentin and keratin intermediate filaments in human melanoma cells augments motility. Am J Pathol 148: 63–69, 1996 [PMC free article] [PubMed] [Google Scholar]
- 27.Chung BM, Rotty JD, Coulombe PA. Networking galore: intermediate filaments and cell migration. Curr Opin Cell Biol 25: 600–612, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark K, Langeslag M, Figdor CG, van Leeuwen FN. Myosin II and mechanotransduction: a balancing act. Trends Cell Biol 17: 178–186, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer 3: 921–930, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Coulombe PA, Kerns ML, Fuchs E. Epidermolysis bullosa simplex: a paradigm for disorders of tissue fragility. J Clin Invest 119: 1784–1793, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coulombe PA, Wong P. Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat Cell Biol 6: 699–706, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Dahl KN, Engler AJ, Pajerowski JD, Discher DE. Power-law rheology of isolated nuclei with deformation mapping of nuclear substructures. Biophys J 89: 2855–2864, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahl KN, Ribeiro AJ, Lammerding J. Nuclear shape, mechanics, mechanotransduction. Circ Res 102: 1307–1318, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Rooij J, Kerstens A, Danuser G, Schwartz MA, Waterman-Storer CM. Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J Cell Biol 171: 153–164, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desai LP, White SR, Waters CM. Mechanical stretch decreases FAK phosphorylation and reduces cell migration through loss of JIP3-induced JNK phosphorylation in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 297: L520–L529, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumbauld DW, Shin H, Gallant ND, Michael KE, Radhakrishna H, Garcia AJ. Contractility modulates cell adhesion strengthening through focal adhesion kinase and assembly of vinculin-containing focal adhesions. J Cell Physiol 223: 746–756, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD. Introducing intermediate filaments: from discovery to disease. J Clin Invest 119: 1763–1771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Escaff S, Fernandez JM, Gonzalez LO, Suarez A, Gonzalez-Reyes S, Gonzalez JM, Vizoso FJ. Study of matrix metalloproteinases and their inhibitors in prostate cancer. Br J Cancer 102: 922–929, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol 17: 524–532, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Fortier AM, Asselin E, Cadrin M. Keratin 8 and 18 loss in epithelial cancer cells increases collective cell migration and cisplatin sensitivity through claudin1 up-regulation. J Biol Chem 288: 11555–11571, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fraley SI, Feng Y, Krishnamurthy R, Kim DH, Celedon A, Longmore GD, Wirtz D. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat Cell Biol 12: 598–604, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frame MC, Brunton VG. Advances in Rho-dependent actin regulation and oncogenic transformation. Curr Opin Genet Dev 12: 36–43, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol 188: 11–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedl P, Wolf K. Tube travel: the role of proteases in individual and collective cancer cell invasion. Cancer Res 68: 7247–7249, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3: 362–374, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Fuchs E, Cleveland DW. A structural scaffolding of intermediate filaments in health and disease. Science 279: 514–519, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol 9: 1392–1400, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol 159: 695–705, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerace L, Blobel G. Nuclear-envelope lamina is reversibly depolymerized during mitosis. Cell 19: 277–287, 1980 [DOI] [PubMed] [Google Scholar]
- 50.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFβ signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol 11: 1287–1296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giannone G, Sheetz MP. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol 16: 213–223, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Gilbert S, Loranger A, Lavoie JN, Marceau N. Cytoskeleton keratin regulation of FasR signaling through modulation of actin/ezrin interplay at lipid rafts in hepatocytes. Apoptosis 17: 880–894, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Gilbert S, Ruel A, Loranger A, Marceau N. Switch in Fas-activated death signaling pathway as result of keratin 8/18-intermediate filament loss. Apoptosis 13: 1479–1493, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Gillette BM, Rossen NS, Das N, Leong D, Wang M, Dugar A, Sia SK. Engineering extracellular matrix structure in 3D multiphase tissues. Biomaterials 32: 8067–8076, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gligorijevic B, Condeelis J. Stretching the timescale of intravital imaging in tumors. Cell Adh Migr 3: 313–315, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guck J, Schinkinger S, Lincoln B, Wottawah F, Ebert S, Romeyke M, Lenz D, Erickson HM, Ananthakrishnan R, Mitchell D, Kas J, Ulvick S, Bilby C. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J 88: 3689–3698, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guilak F, Tedrow JR, Burgkart R. Viscoelastic properties of the cell nucleus. Biochem Biophys Res Commun 269: 781–786, 2000 [DOI] [PubMed] [Google Scholar]
- 58.Hamadi A, Bouali M, Dontenwill M, Stoeckel H, Takeda K, Ronde P. Regulation of focal adhesion dynamics and disassembly by phosphorylation of FAK at tyrosine 397. J Cell Sci 118: 4415–4425, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Harunaga JS, Yamada KM. Cell-matrix adhesions in 3D. Matrix Biol 30: 363–368, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helfand BT, Mendez MG, Murthy SN, Shumaker DK, Grin B, Mahammad S, Aebi U, Wedig T, Wu YI, Hahn KM, Inagaki M, Herrmann H, Goldman RD. Vimentin organization modulates the formation of lamellipodia. Mol Biol Cell 22: 1274–1289, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hendrix MJ, Seftor EA, Seftor RE, Trevor KT. Experimental co-expression of vimentin and keratin intermediate filaments in human breast cancer cells results in phenotypic interconversion and increased invasive behavior. Am J Pathol 150: 483–495, 1997 [PMC free article] [PubMed] [Google Scholar]
- 62.Herrmann H, Bar H, Kreplak L, Strelkov SV, Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol 8: 562–573, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature 497: 507–511, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoffmann JC, West JL. Three-dimensional photolithographic micropatterning: a novel tool to probe the complexities of cell migration. Integr Biol (Camb) 5: 817–827, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hofmann UB, Eggert AA, Blass K, Brocker EB, Becker JC. Expression of matrix metalloproteinases in the microenvironment of spontaneous and experimental melanoma metastases reflects the requirements for tumor formation. Cancer Res 63: 8221–8225, 2003 [PubMed] [Google Scholar]
- 66.Huveneers S, Danen EH. Adhesion signaling—crosstalk between integrins, Src and Rho. J Cell Sci 122: 1059–1069, 2009 [DOI] [PubMed] [Google Scholar]
- 67.Icard-Arcizet D, Cardoso O, Richert A, Henon S. Cell stiffening in response to external stress is correlated to actin recruitment. Biophys J 94: 2906–2913, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ilina O, Bakker GJ, Vasaturo A, Hofmann RM, Friedl P. Two-photon laser-generated microtracks in 3D collagen lattices: principles of MMP-dependent and -independent collective cancer cell invasion. Phys Biol 8: 015010, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Ingber DE. Tensegrity. I. Cell structure and hierarchical systems biology. J Cell Sci 116: 1157–1173, 2003 [DOI] [PubMed] [Google Scholar]
- 70.Irimia D, Toner M. Spontaneous migration of cancer cells under conditions of mechanical confinement. Integr Biol (Camb) 1: 506–512, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ivaska J, Vuoriluoto K, Huovinen T, Izawa I, Inagaki M, Parker PJ. PKCε-mediated phosphorylation of vimentin controls integrin recycling and motility. EMBO J 24: 3834–3845, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jordan MA, Wilson L. Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr Opin Cell Biol 10: 123–130, 1998 [DOI] [PubMed] [Google Scholar]
- 73.Karantza V. Keratins in health and cancer: more than mere epithelial cell markers. Oncogene 30: 127–138, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaverina I, Rottner K, Small JV. Targeting, capture, and stabilization of microtubules at early focal adhesions. J Cell Biol 142: 181–190, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaverina I, Straube A. Regulation of cell migration by dynamic microtubules. Semin Cell Dev Biol 22: 968–974, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim H, Nakamura F, Lee W, Shifrin Y, Arora P, McCulloch CA. Filamin A is required for vimentin-mediated cell adhesion and spreading. Am J Physiol Cell Physiol 298: C221–C236, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kraning-Rush CM, Califano JP, Reinhart-King CA. Cellular traction stresses increase with increasing metastatic potential. PLos One 7: e32572, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kraning-Rush CM, Carey SP, Lampi MC, Reinhart-King CA. Microfabricated collagen tracks facilitate single cell metastatic invasion in 3D. Integr Biol (Camb) 5: 606–613, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ku NO, Michie S, Resurreccion EZ, Broome RL, Omary MB. Keratin binding to 14-3-3 proteins modulates keratin filaments and hepatocyte mitotic progression. Proc Natl Acad Sci USA 99: 4373–4378, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kubow KE, Horwitz AR. Reducing background fluorescence reveals adhesions in 3D matrices. Nat Cell Biol 13: 3–7, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139: 891–906, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li B, Antonyak MA, Druso JE, Cheng L, Nikitin AY, Cerione RA. EGF potentiated oncogenesis requires a tissue transglutaminase-dependent signaling pathway leading to Src activation. Proc Natl Acad Sci USA 107: 1408–1413, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lo AT, Mori H, Mott J, Bissell MJ. Constructing three-dimensional models to study mammary gland branching morphogenesis and functional differentiation. J Mammary Gland Biol Neoplasia 17: 103–110, 2012 [DOI] [PubMed] [Google Scholar]
- 84.Lombardi ML, Lammerding J. Keeping the LINC: the importance of nucleocytoskeletal coupling in intracellular force transmission and cellular function. Biochem Soc Trans 39: 1729–1734, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Loranger A, Gilbert S, Brouard JS, Magin TM, Marceau N. Keratin 8 modulation of desmoplakin deposition at desmosomes in hepatocytes. Exp Cell Res 312: 4108–4119, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 3: 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lyons SK. Advances in imaging mouse tumour models in vivo. J Pathol 205: 194–205, 2005 [DOI] [PubMed] [Google Scholar]
- 88.Mak M, Reinhart-King CA, Erickson D. Elucidating mechanical transition effects of invading cancer cells with a subnucleus-scaled microfluidic serial dimensional modulation device. Lab Chip 13: 340–348, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mak M, Reinhart-King CA, Erickson D. Microfabricated physical spatial gradients for investigating cell migration and invasion dynamics. PLos One 6: e20825, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA 94: 849–854, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marceau N, Loranger A, Gilbert S, Daigle N, Champetier S. Keratin-mediated resistance to stress and apoptosis in simple epithelial cells in relation to health and disease. Biochem Cell Biol 79: 543–555, 2001 [PubMed] [Google Scholar]
- 92.Maschler S, Wirl G, Spring H, Bredow DV, Sordat I, Beug H, Reichmann E. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene 24: 2032–2041, 2005 [DOI] [PubMed] [Google Scholar]
- 93.Mason BN, Starchenko A, Williams RM, Bonassar LJ, Reinhart-King CA. Tuning three-dimensional collagen matrix stiffness independently of collagen concentration modulates endothelial cell behavior. Acta Biomater 9: 4635–4644, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mendez MG, Kojima S, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial-to-mesenchymal transition. FASEB J 24: 1838–1851, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mierke CT, Frey B, Fellner M, Herrmann M, Fabry B. Integrin α5β1 facilitates cancer cell invasion through enhanced contractile forces. J Cell Sci 124: 369–383, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miron-Mendoza M, Lin X, Ma L, Ririe P, Petroll WM. Individual versus collective fibroblast spreading and migration: regulation by matrix composition in 3D culture. Exp Eye Res 99: 36–44, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miron-Mendoza M, Seemann J, Grinnell F. The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials 31: 6425–6435, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Occhetta P, Sadr N, Piraino F, Redaelli A, Moretti M, Rasponi M. Fabrication of 3D cell-laden hydrogel microstructures through photo-mold patterning. Biofabrication 5: 035002, 2013 [DOI] [PubMed] [Google Scholar]
- 99.Omary MB, Ku NO, Strnad P, Hanada S. Toward unraveling the complexity of simple epithelial keratins in human disease. J Clin Invest 119: 1794–1805, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oshima RG, Baribault H, Caulin C. Oncogenic regulation and function of keratins 8 and 18. Cancer Metastasis Rev 15: 445–471, 1996 [DOI] [PubMed] [Google Scholar]
- 101.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol 11: 633–643, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pasapera AM, Schneider IC, Rericha E, Schlaepfer DD, Waterman CM. Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol 188: 877–890, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell 8: 241–254, 2005 [DOI] [PubMed] [Google Scholar]
- 104.Pathak A, Kumar S. Biophysical regulation of tumor cell invasion: moving beyond matrix stiffness. Integr Biol (Camb) 3: 267–278, 2011 [DOI] [PubMed] [Google Scholar]
- 105.Pathak A, Kumar S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc Natl Acad Sci USA 109: 10334–10339, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pathak A, Kumar S. Transforming potential and matrix stiffness co-regulate confinement sensitivity of tumor cell migration. Integr Biol (Camb) 5: 1067–1075, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Patsialou A, Bravo-Cordero JJ, Wang Y, Entenberg D, Liu H, Clarke M, Condeelis JS. Intravital multiphoton imaging reveals multicellular streaming as a crucial component of in vivo cell migration in human breast tumors. Intravital 2: e25294, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Plotnikov SV, Pasapera AM, Sabass B, Waterman CM. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 151: 1513–1527, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poincloux R, Collin O, Lizarraga F, Romao M, Debray M, Piel M, Chavrier P. Contractility of the cell rear drives invasion of breast tumor cells in 3D Matrigel. Proc Natl Acad Sci USA 108: 1943–1948, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med 4: 38, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene 28: 4326–4343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Provenzano PP, Inman DR, Eliceiri KW, Trier SM, Keely PJ. Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophys J 95: 5374–5384, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Raab M, Swift J, Dingal PC, Shah P, Shin JW, Discher DE. Crawling from soft to stiff matrix polarizes the cytoskeleton and phosphoregulates myosin-II heavy chain. J Cell Biol 199: 669–683, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Raviraj V, Fok S, Zhao J, Chien HY, Lyons JG, Thompson EW, Soon L. Regulation of ROCK1 via Notch1 during breast cancer cell migration into dense matrices. BMC Cell Biol 13: 12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rolli CG, Seufferlein T, Kemkemer R, Spatz JP. Impact of tumor cell cytoskeleton organization on invasiveness and migration: a microchannel-based approach. PLos One 5: 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, Allen E, Chung D, Weiss SJ. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol 167: 769–781, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol 5: 711–719, 2003 [DOI] [PubMed] [Google Scholar]
- 118.Sala A, Hanseler P, Ranga A, Lutolf MP, Voros J, Ehrbar M, Weber FE. Engineering 3D cell instructive microenvironments by rational assembly of artificial extracellular matrices and cell patterning. Integr Biol (Camb) 3: 1102–1111, 2011 [DOI] [PubMed] [Google Scholar]
- 119.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127: 1015–1026, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science 312: 1059–1063, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scales TM, Jayo A, Obara B, Holt MR, Hotchin NA, Berditchevski F, Parsons M. α3β1-Integrins regulate CD151 complex assembly and membrane dynamics in carcinoma cells within 3D environments. Oncogene 32: 3965–3979, 2013 [DOI] [PubMed] [Google Scholar]
- 122.Schober JM, Kwon G, Jayne D, Cain JM. The microtubule-associated protein EB1 maintains cell polarity through activation of protein kinase C. Biochem Biophys Res Commun 417: 67–72, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol 189: 541–556, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, Maltais L, Omary MB, Parry DA, Rogers MA, Wright MW. New consensus nomenclature for mammalian keratins. J Cell Biol 174: 169–174, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Scott RW, Hooper S, Crighton D, Li A, Konig I, Munro J, Trivier E, Wickman G, Morin P, Croft DR, Dawson J, Machesky L, Anderson KI, Sahai EA, Olson MF. LIM kinases are required for invasive path generation by tumor and tumor-associated stromal cells. J Cell Biol 191: 169–185, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Soman P, Kelber JA, Lee JW, Wright TN, Vecchio KS, Klemke RL, Chen S. Cancer cell migration within 3D layer-by-layer microfabricated photocrosslinked PEG scaffolds with tunable stiffness. Biomaterials 33: 7064–7070, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sonnenberg A, Liem RK. Plakins in development and disease. Exp Cell Res 313: 2189–2203, 2007 [DOI] [PubMed] [Google Scholar]
- 128.Stamenovic D. Microtubules may harden or soften cells, depending on the extent of cell distension. J Biomech 38: 1728–1732, 2005 [DOI] [PubMed] [Google Scholar]
- 129.Stephens P, Grenard P, Aeschlimann P, Langley M, Blain E, Errington R, Kipling D, Thomas D, Aeschlimann D. Crosslinking and G-protein functions of transglutaminase 2 contribute differentially to fibroblast wound healing responses. J Cell Sci 117: 3389–3403, 2004 [DOI] [PubMed] [Google Scholar]
- 130.Toivola DM, Krishnan S, Binder HJ, Singh SK, Omary MB. Keratins modulate colonocyte electrolyte transport via protein mistargeting. J Cell Biol 164: 911–921, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tong ZQ, Balzer EM, Dallas MR, Hung WC, Stebe KJ, Konstantopoulos K. Chemotaxis of cell populations through confined spaces at single-cell resolution. PLos One 7: 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tsuruta D, Jones JC. The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. J Cell Sci 116: 4977–4984, 2003 [DOI] [PubMed] [Google Scholar]
- 133.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J 20: 4639–4647, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett 582: 2093–2101, 2008 [DOI] [PubMed] [Google Scholar]
- 135.Verstraeten VL, Ji JY, Cummings KS, Lee RT, Lammerding J. Increased mechanosensitivity and nuclear stiffness in Hutchinson-Gilford progeria cells: effects of farnesyltransferase inhibitors. Aging Cell 7: 383–393, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration—the actin connection. J Cell Sci 122: 199–206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vicente-Manzanares M, Horwitz AR. Cell migration: an overview. Methods Mol Biol 769: 1–24, 2011 [DOI] [PubMed] [Google Scholar]
- 138.Vizoso FJ, Gonzalez LO, Corte MD, Rodriguez JC, Vazquez J, Lamelas ML, Junquera S, Merino AM, Garcia-Muniz JL. Study of matrix metalloproteinases and their inhibitors in breast cancer. Br J Cancer 96: 903–911, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wald FA, Oriolo AS, Casanova ML, Salas PJ. Intermediate filaments interact with dormant ezrin in intestinal epithelial cells. Mol Biol Cell 16: 4096–4107, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang N, Stamenovic D. Contribution of intermediate filaments to cell stiffness, stiffening, and growth. Am J Physiol Cell Physiol 279: C188–C194, 2000 [DOI] [PubMed] [Google Scholar]
- 141.Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Visualizing the mechanical activation of Src. Nature 434: 1040–1045, 2005 [DOI] [PubMed] [Google Scholar]
- 142.Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Microtubule growth activates Rac1 to promote lamellipodial profusion in fibroblasts. Nat Cell Biol 1: 45–50, 1999 [DOI] [PubMed] [Google Scholar]
- 143.Weber GF, Bjerke MA, DeSimone DW. A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev Cell 22: 104–115, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wei WC, Lin HH, Shen MR, Tang MJ. Mechanosensing machinery for cells under low substratum rigidity. Am J Physiol Cell Physiol 295: C1579–C1589, 2008 [DOI] [PubMed] [Google Scholar]
- 145.Weigelin B, Bakker GJ, Friedl P. Intravital third harmonic generation microscopy of collective melanoma cell invasion: principles of interface guidance and microvesicle dynamics. Intravital 1: 32–43, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wiche G. Role of plectin in cytoskeleton organization and dynamics. J Cell Sci 111: 2477–2486, 1998 [DOI] [PubMed] [Google Scholar]
- 147.Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, Deryugina E, Friedl P. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol 20: 931–941, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wolf K, Friedl P. Mapping proteolytic cancer cell-extracellular matrix interfaces. Clin Exp Metastasis 26: 289–298, 2009 [DOI] [PubMed] [Google Scholar]
- 149.Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, Strongin AY, Brocker EB, Friedl P. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol 160: 267–277, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wolf K, Te Lindert M, Krause M, Alexander S, Te Riet J, Willis AL, Hoffman RM, Figdor CG, Weiss SJ, Friedl P. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J Cell Biol 201: 1069–1084, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, Stack MS, Friedl P. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol 9: 893–904, 2007 [DOI] [PubMed] [Google Scholar]
- 152.Wyckoff J, Gligorijevic B, Entenberg D, Segall J, Condeelis J. High-resolution multiphoton imaging of tumors in vivo. Cold Spring Harb Protoc 2011: 1167–1184, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zinn KR, Chaudhuri TR, Szafran AA, O'Quinn D, Weaver C, Dugger K, Lamar D, Kesterson RA, Wang X, Frank SJ. Noninvasive bioluminescence imaging in small animals. ILAR J 49: 103–115, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]