Abstract
Large-conductance Ca2+-activated K+ channels (BK) regulate action potential (AP) properties and excitability in many central neurons. However, the properties and functional roles of BK channels in parasympathetic cardiac motoneurons (PCMNs) in the nucleus ambiguus (NA) have not yet been well characterized. In this study, the tracer X-rhodamine-5 (and 6)-isothiocyanate (XRITC) was injected into the pericardial sac to retrogradely label PCMNs in FVB mice at postnatal 7–9 days. Two days later, XRITC-labeled PCMNs in brain stem slices were identified. Using excised patch single-channel recordings, we identified voltage-gated and Ca2+-dependent BK channels in PCMNs. The majority of BK channels exhibited persistent channel opening during voltage holding. These BK channels had a conductance of 237 pS and a 50% opening probability at +27.9 mV, the channel open time constant was 3.37 ms at +20 mV, and dwell time increased exponentially as the membrane potential depolarized. At the +20-mV holding potential, the [Ca2+]50 was 15.2 μM with a P0.5 of 0.4. Occasionally, some BK channels showed a transient channel opening and fast inactivation. Using whole cell voltage clamp, we found that BK channel mediated outward currents and afterhyperpolarization currents (IAHP). Using whole cell current clamp, we found that application of BK channel blocker iberiotoxin (IBTX) increased spike half-width and suppressed fast afterhyperpolarization (fAHP) amplitude following single APs. In addition, IBTX application increased spike half-width and reduced the spike frequency-dependent AP broadening in trains and spike frequency adaption (SFA). Furthermore, BK channel blockade decreased spike frequency. Collectively, these results demonstrate that PCMNs have BK channels that significantly regulate AP repolarization, fAHP, SFA, and spike frequency. We conclude that activation of BK channels underlies one of the mechanisms for facilitation of PCMN excitability.
Keywords: nucleus ambiguus, parasympathetic cardiac motoneurons, vagal, BK channels, repolarization, afterhyperpolarization, firing frequency, adaptation
parasympathetic preganglionic cardiac motoneurons (PCMNs) in the nucleus ambiguus (NA) regulate cardiac functions (negative control of chronotropic, dromotropic, and inotropic actions) (40). Previously, we have demonstrated that lesions of the NA abolish the baroreflex control of heart rate in rats (8). Therefore, the NA plays a key role in arterial baroreflex control of heart rate. Impairment of arterial baroreflex function has been used as an indicator of potential life-threatening arrhythmia and heart failure (13, 34). Despite this, little is known concerning the cellular mechanisms responsible for the alteration of membrane properties and neuronal activity of PCMNs, which may underlie the dysfunction of arterial baroreflex in disease states. Therefore, it is very important to study the membrane properties and excitability of PCMNs in normal animals. These studies will be used as a foundation for the investigation of disease-related changes in PCMNs. In the literature, both small conductance and large conductance Ca2+-activated K+ channels (SK and BK channels, respectively) were reported to mediate the firing properties of action potentials (APs) and excitability of central neurons (14–16, 59, 67). Previously, we have characterized the functional roles of SK channels in regulating firing properties of APs and excitability of PCMNs (36). However, the membrane properties and the functional roles of BK channels of PCMNs have not yet been fully determined.
The BK channels (Maxi-K or slo channel) are both voltage gated and intracellular Ca2+ dependent (5, 33, 56). The BK channels have a single-channel conductance of >100 pS and are sensitive to the specific blockers IBTX (7, 19) or paxilline (57). When activated by membrane depolarization and Ca2+ during APs, BK channels allow the efflux of K+ out of the cell, thus repolarizing and hyperpolarizing the membrane potential. This turns off voltage-dependent Ca2+ channels and thus reduces the influx of Ca2+ into the cell. These negative feedback mechanisms allow BK channels to play key roles in regulating firing properties, such as repetitive firing of neurons (67), spike broadening during repetitive firing (59), and spike-frequency adaptation (26). There has also been evidence that BK channels can moderate neurotransmitter release and provide some protection against neuronal death (35, 51, 52, 68).
In the central nervous system, BK channels mainly contribute to AP repolarization and, to a lesser extent, to the fast (fAHP) following APs (16, 42, 48, 50, 61, 62). Activation of the BK channels was previously reported to reduce the frequency of APs and limit epileptiform bursts in rat hippocampal CA1 pyramidal neurons (3, 32). More recently, however, an opposite effect of BK channels on neuronal excitability was found in mouse dentate granule neurons that lack the β4-subunit of BK channels. Blockade of BK channels lacking the β4-subunit widened the APs and reduced the excitability (6). Granule cells from β4-knockout mice showed a gain-of-function for BK channels that sharpened APs and supported higher firing rates, which may lead to temporal lobe epilepsy. Altogether, these data indicate that activation of BK channels either exert a negative or “brake” effect on the firing rate (spike frequency) of neurons or facilitate AP firing of neurons. In an early study on PCMNs, blockade of BK channels did not show significant effects on excitability (46). In contrast, we recently found that blockade of BK channels in PCMNs slightly but significantly decreased firing rate in response to low-intensity current injection (38). As indicated by Gu et al. (26), large current injection more effectively activated BK channels, which had powerful effects in facilitating high-frequency firing and causing early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. Therefore, the physiological role of BK channels in regulating membrane properties and excitability of PCMNs appears controversial. In this study, we first identified single BK channels and assessed their voltage and Ca2+ dependence in excised patches and then examined the effects of BK channels on the membrane properties and excitability of single APs and spike trains of PCMNs in whole cell patches. In addition, we studied BK outward currents and afterhyperpolarization currents (IAHP), which may provide an underlying mechanism for AP firing properties and the control of excitability in PCMNs.
MATERIALS AND METHODS
Female (n = 12) and male FVB (or FVB/N in full name) (n = 4) mice were obtained from Jax Laboratory at age of 2–3 mo. Sixty-eight neonatal mice were used for patch-clamp recordings from brain slice preparations in experimental groups, and each group included 8–10 neurons (see results). Each neuron was only used for one test. Mice were maintained on a 12-h light/dark cycle and received food and water ad libitum. All animals were then maintained in the transgenic animal facility. When females became pregnant, they were transferred into individual cages. The procedures were approved by the University of Central Florida Animal Care and Use Committee and followed the guidelines established by National Institutes of Health.
Fluorescent Labeling of PCMNs and Brainstem Slice Preparation for Patch-Clamp Recordings
FVB neonatal mice (postnatal days 7–9) were anesthetized with 3% isoflurane (Abbott Laboratories, North Chicago, IL). When the animals were not responsive to tail and toe pinch, a right thoracotomy was performed and the fluorescent tracer X-rhodamine-5 (and 6)-isothiocyanate (XRITC, 2%, 4 μl; Molecular Probes, Eugene, OR) was injected into the pericardial sac at the base of the heart to retrogradely label cardiac motoneurons in the NA (25, 37). Previously, XRITC has been used to label NA neurons for patch-clamp study of PCMNs (25, 46, 71). After at least a 48-h recovery period, neonatal mice were deeply anesthetized with 4% isoflurane. The brainstem including PCMNs were sliced into serial sections (250 μm) using a vibrating blade microslicer (DTK-1000; Kyoto, Japan), and the brain slices were maintained in an interface chamber (36–38).
In brainstem slices, PCMNs were identified in the NA by the presence of XRITC. These slices were viewed with infrared illumination and differential interference optics (DIC; Carl Zeiss, Göttingen, Germany) under fluorescent illumination with a near-infrared sensitive, cooled charged-coupled device camera (AxioCom MRm; Carl Zeiss, Göttingen, Germany). XRITC-labeled PCMNs were identified by superimposing the fluorescent and DIC images (Fig. 1).
Fig. 1.

Identification of tracer X-rhodamine-5 (and 6)-isothiocyanate (XRITC)-labeled parasympathetic cardiac motoneurons (PCMNs) in the nucleus ambiguus (NA) in brainstem slices. A: differential interference optics imaging of PCMNs. B: fluorescent imaging of PCMNs shows XRITC-labeled PCMNs in the NA that were labeled by injection of the fluorescent tracer XRITC (2% solution, 4–8 μl; Molecular Probes) into the pericardial sac 2 days before experiment. C: merged image of A and B. Arrow indicates a recorded PCMN. Scale bar = 40 μm.
Single-channel recordings.
Single-channel currents were recorded in both inside-out and outside-out patch configurations. For inside-out patches (27), patch pipettes were made using fire-polished borosilicate glass electrodes (1.5-mm outer diameter; World Precision Instruments, Sarasota, FL) on a Flaming/Brown puller (Sutter Instruments, Novato, CA) to a final resistance of 3–4 MΩ and filled with a solution containing the following (in mM): 130 K-gluconate, 10 KCl, 1 MgCl2, 10 HEPES, and 16 dextrose, adjusted to pH 7.4 with KOH. At room temperature (20–24°C), inside-out patches were obtained by withdrawing patch pipettes from varicosities after formation of a gigaohm seal. Following excision, the cytoplasmic face was bathed in an artificial intracellular solution containing the following (in mm): 140 K-gluconate, 2 MgCl2, 15 HEPES, 1 ATPNa2, and 5 EDTA, pH adjusted to 7.3 with KOH. CaCl2 was added to give the required free Ca2+ concentration calculated using the MAXCHELATOR program (Winmaxchelator software; Dr. Chris Patton, Stanford University, Pacific Grove, CA; http://www.stanford.edu/∼cpatton/webmaxcS.htm).
In some experiments CaCl2 was added for a free Ca2+ concentration of 10 μm. To study the Ca2+ dependence of BK channels, CaCl2 was added to the artificial intracellular solution with Ca2+ concentrations adjusted from 0.5 to 100 μm. For the Ca2+-free solution, the bath solution contained no CaCl2 and was supplemented with 10 mM EGTA, giving a calculated free Ca2+ concentration of <1 nM. For outside-out patch recording, the patch was excised from the whole cell configuration at room temperature. The pipette was filled with a standard internal solution containing the following (in mM): 110 K-gluconate, 10 KCl, 5 NaCl, 2 MgCl2, 10 HEPES, 0.5 EGTA, 1 ATP, 0.2 GTP, and 0.1 leupeptin, with pH adjusted to 7.3 with KOH. The bath solution was identical to the HEPES solution for the whole cell voltage clamp recording (see below).
To identify single BK channels in inside-out or outside-out patches, the following criteria were used: 1) outward currents, 2) amplitudes >10 pA at 60 mV, 3) voltage and/or Ca2+ dependence, and/or 4) IBTX or paxilline sensitivity. In all cases of single-channel recording, the current was low-pass filtered at 1 kHz and acquired at a sampling rate of 10 kHz using a Digidata 1,300 analog-to-digital converter (Axon Instruments, Union City, CA) and the Clampex acquisition program of pCLAMP (version 9.2; Axon Instruments). Data were obtained in 1- to 3-min intervals while the patches were held at −65 mV. To determine the relationship between voltage and the channel steady-state open probability (Po), events lists were made from data files using the half-amplitude threshold criterion of the Fetchan analysis program of pCLAMP (10). The Po was analyzed and the data were fit to the Boltzmann equation Po = 1 − [1 + e(Vm−V0.5)/κ]−1, where Po is the open probability, Vm is the membrane potential, V0.5 is the half-maximal activation voltage, and κ is the slope factor.
The relationship between intracellular Ca2 concentration ([Ca2+]i) and the channel steady-state Po was fitted according to a Hill equation: Po = Pmax[Ca2+]inH/(K + [Ca2+]inH) where Po is the steady-state open probability, Pmax is the maximum open probability, nH is the slope factor, and the nth root of K gives an estimate of the midpoint of the activation curve. The slope factor provides an estimate of the number of Ca2+ ions involved in maximally activating the channel (44). Thus the required [Ca2+]i to achieve the midpoint of activation (P0.5), designated as [Ca2+]0.5, has been a widely used parameter to measure the calcium sensitivity of BK channels. To generate all-point histograms of the BK current events, single-channel current amplitudes were measured in an unbiased manner by using the Fetchan analysis program. Data were filtered to a final cut-off frequency of 500 Hz using the Fetchan digital Gaussian filter. Open times of BK channels were measured with half-amplitude threshold analysis. A single exponential function was used to fit the open time distribution curves, and the open time constant (τ) was obtained. Current dwell times were binned using a log scale bin size, plotted on linear bin amplitude coordinates, and fitted by single exponential function with a Levenberg-Marquardt curve fitting algorithm in the pCLAMP analysis suite.
Whole cell clamp recording.
For whole cell voltage-clamp recordings, electrodes were heat-polished to a final tip resistance of 2–3 MΩ and filled with the same internal solution as the outside-out patch recording pipette described above. Within the recording chamber, slices were stably held in a position using a nylon net stretched over a flattened U-shaped platinum wire and were continuously superfused at room temperature (22–25°C). To record BK currents, cells were superfused with a HEPES solution equilibrated with 95% O2-5% CO2 and containing the following (in mM): 140 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 0.0005 tetrodotoxin (TTX), 1 4-aminopyridine (4-AP), 0.005 glybenclamide, and 10 glucose, pH adjusted to 7.4 with NaOH. TTX, 4-AP, and glybenclamide were included routinely in the recording solution unless otherwise indicated. 4-AP (1 mM) was used to reduce voltage-gated K+ currents and unmask Ca2+ dependence of K+ currents. Outward currents were evoked by a series of 250-ms depolarizing steps from −70 to +40 mV in 10-mV increments. The mean current peak value of the transient outward current was plotted against membrane potential (I–V) and was fitted by the Boltzmann equation: I = Imin + (Imax − Imin)/[1 + exp − κ(V − V1/2)], where I is the current, Imax is the maximum current, Imin is the minimum current, κ is a slope factor, and V1/2 is the midpoint potential. To study IAHP, outward currents were first evoked by a 100-ms depolarizing voltage step of +10 mV from −70 mV and immediately followed by a return to −50 mV for 1.5 s. To avoid possible interference between responses, depolarizing voltage steps were delivered every 5 s. The peak amplitude of IAHP was measured following the end of the voltage step +10 mV.
With the use of whole cell current-clamp recordings, AP firing properties of PCMNs were examined. Within the recording chamber, slices were stably held in position using a nylon net stretched over a flattened U-shaped platinum wire and were continuously superfused at the room temperature (22–25°C) with artificial cerebrospinal fluid that contained the following (in mM): 126 NaCl, 2.5 KCl, 2 CaCl2, 26 NaHCO3, 1.25 NaH2PO4, 2 MgSO4, and 10 dextrose, was equilibrated with 95% O2-5% CO2, and had the pH adjusted to 7.4. Patch electrodes used for rupture patch recordings were fabricated from borosilicate glass with a Flaming/Brown horizontal puller. Electrodes were heat-polished to a final tip resistance of 3–5 MΩ and filled with intracellular solution containing the following (in mM) 120 potassium-gluconate, 20 KCl, 2 MgCl2, 10 HEPES, 0.1 EGTA, 2 ATP, and 0.25 GTP, with pH adjusted to 7.3 with KOH.
To generate a single AP, a 10-ms depolarizing current pulse of sufficient intensity (40–100 pA) from the holding potential −65 mV to trigger a single AP was applied. We used a 10-ms duration because this stimulus duration has been commonly used to evoke single APs in other central neurons (11, 17, 36). Within minutes after good recording conditions were established (i.e., resting membrane potential was less than −60 mV and AP peak amplitude was >70 mV), AP half-width was measured as the spike width at the half-maximal voltage using Clampfit 9.2. To standardize AHP recordings, neurons were depolarized to a holding potential of −65 mV by direct current (DC) application through the recording electrode. AHP was recorded after applying a 10-ms depolarizing current pulse of sufficient amplitude (40–100 pA) to generate a single AP (11). The AHP was measured by subtracting the peak amplitude of the hyperpolarizing deflection after the spike from the threshold for spike initiation. AHP amplitudes were measured at 10, 50, and 100 ms.
To investigate the AP firing frequency of PCMNs, current injection steps were applied from 100 to 340 pA in 20 pA increments. Instantaneous firing rate was measured as the inverse of interspike intervals. Input resistance was determined by Ohm's law using a linear regression within the linear range (±10 mV from the resting potential) of the voltage-current (I-V) relationship that had been established by plotting the steady-state voltage change in response to a series of depolarizing and hyperpolarizing current injections. To determine the voltage threshold of an AP, AP trains were evoked by 1.5-s, 0.2 pA/ms ramp current injections from a holding potential of −65 mV, and the threshold was determined at the membrane potential once the first AP started to fire.
Data acquisition was controlled using the ClampEx program in the pClamp 9 software package. The series resistance was in the range of 5–10 MΩ (typically 5 MΩ) and was compensated 60% on-line. Membrane potential measurements were not corrected for the liquid junction potential (∼15 mV). Leak currents were subtracted using a standard P/4 protocol. Before seals (5 GΩ) were made on cells, offset potentials were nulled. Capacitance subtraction was used in all recordings. Signals were recorded using a MultiClamp 700B patch-clamp amplifier (Axon Instruments, Foster City, CA). Responses were low-pass filtered at 3 kHz and digitized at 50 kHz with a 16-bit analog-to-digital data acquisition systems (Digidata 1322A; Axon Instruments).
Drugs and Chemicals
All channel blockers used in the present study were purchased from Sigma-Aldrich (St Louis, MO). Blockers contained in the micropipette solutions were delivered by focal ejection to the vicinity of the patches or soma (50–100 μm) from upstream of the perfusion (2 ml/min) using a pressure injector (MP8000; MDI, South Plainfield, NJ; 5 min, 5–l5 psi).
Statistics
Data were presented as means ± SE. A t-test, one-way, or two-way ANOVA with repeated measures followed by a Tukey-Kramer post hoc test was used. P < 0.05 was considered as significant.
RESULTS
Figure 1 shows that the injection of the tracer XRITC into the pericardial sac retrogradely labeled NA PCMNs. All recorded neurons were strongly labeled by XRITC (25, 36, 37).
Identification of BK Channels
To identify single BK channels and characterize their properties, BK channels were first identified and examined in outside-out (n = 10) and inside-out (n = 8) excised patches. In outside-out patches, BK channels were usually opened to a unitary current level, had a large conductance, and were mostly in the open state at +40 mV (Fig. 2A, top trace). These single-channel currents were almost completely blocked by the specific BK channel blocker paxilline (10 μM; Fig. 2A, middle trace) and these single-channel currents recovered after paxilline washout (Fig. 2A, bottom trace). In inside-out patches, we identified single BK channels as well. In most recordings, we only observed unitary current levels (Fig. 2, A and B). Occasionally, infrequent subconductance states were also observed in some traces (Fig. 2B, top trace). Outward currents were diminished in the Ca2+-free solution, but they recovered after application of an external solution containing 10 μM free Ca2+ (Fig. 2B, bottom trace).
Fig. 2.
Identification of large conductance Ca2+-activated K+ (BK) channels from PCMNs in NA. A: representative BK currents recorded from an outside-out patch held at +40 mV are seen as unitary upward current deflections. Recordings were performed in the absence (top trace), presence of 10 μM paxilline (middle trace), and paxilline washout (bottom trace). B: BK channel activity (upward current deflections) was recorded from an inside-out patch that was held at +20 mV and bathed in 10 (top trace), 0 (middle trace), and again 10 μM Ca2+ (bottom trace). In 10 μM Ca2+, unitary currents of the channel were recorded. In addition, currents in subconductance states were sometimes observed (*). Both unitary currents and subconductance states disappeared in Ca2+ free and recovered after 10 μM Ca2+.
Voltage Dependence of BK Channels and Effects of [Ca2+]i
We examined the voltage dependence of BK channels in inside-out patches. At and below −20 mV, the BK channels were mostly in a closed state and showed only brief openings (inward currents; Fig. 3A). At +20 mV, a macroscopic outward single-level current was seen and its amplitude increased with further depolarization, reversing around 0 mV as would be expected for a potassium-selective channel in symmetrical potassium ([K]i = [K]o, 140 mM; see materials and methods). As depolarized potentials increased, the current amplitude increased. The current amplitude-voltage relationship was well fitted (Fig. 3C), giving a slope conductance of 237 pS, which is consistent with the conductance value of BK channels in other neurons and smooth muscle (44). The open probability (Po) of BK channels exponentially increased as the depolarization increased (Fig. 3D). Activation was rapid, and there was no inactivation within 2–3 min of voltage steps.
Fig. 3.
Voltage dependence and Ca2+ concentration dependence of BK channel currents (unitary upward current deflections) in inside-out patches from PCMNs. A: representative currents from a single BK channel at different holding potentials in the presence of 10 μM free intracellular Ca2+ concentration ([Ca2+]i). B: representative BK channel currents recorded from an inside-out patch held at +20 mV. Cytoplasmic face was perfused with intracellular solution from 0.5 to 100 μm Ca2+. C: current vs. voltage (I-V) curve; n = 10. D: BK channel open probability (Po) was plotted as a function of the membrane potential (Vm); n = 10. E: Po vs. Ca2+ concentration curve; n = 9.
We examined Ca2+ dependence of BK channels in inside-out patches held at +20 mV in bath solutions ranging from 0.5 to 100 μM Ca2+ (Fig. 3B). At this membrane potential, Po increased as [Ca2+]i increased (Fig. 3E). Some channel openings were observed even at a cytoplasmic calcium level as low as 0.5 μM. BK channel open probability was Po = 0.199 ± 0.034 at 0.5 μM Ca2+ and Po = 0.488 ± 0.096 at 100 μM Ca2+. [Ca2+]0.5 was 15.2 ± 1.0 μM at which the estimated P0.5 was ∼0.4. Overall, BK channel activity depends both on membrane potentials and intracellular Ca2+ levels.
BK Channel Kinetics, Activation, and Inactivation
To characterize the distribution of current amplitude, opening time constant, and dwell time of BK channels, outside-out patches were used. The extracellular face was perfused with HEPES containing 2 mM Ca2+. The cytoplasmic face was exposed to an intracellular solution with 0 mM Ca2+ within the pipette. Figure 4A shows unitary BK currents recorded at holding potentials of +20 and +60 mV. All-points histograms of BK current amplitude at +20 mV (Fig. 4B) and +60 mV (Fig. 4C) had two peaks in each distribution. The amplitudes of the larger unitary current peaks were 9.45 ± 0.88 pA and 15.05 ± 0.48 pA at +20 mV and at +60 mV (n = 8), respectively. Current-voltage curves were fitted with a Boltzmann function (Fig. 4D), yielding values for half-maximal activation (V0.5 = 16.2 ± 0.7 mV) and slope factor (k = 24.8 ± 1.2). Open-time distributions were constructed from idealized data ignoring transitions of <0.5 ms duration and could be fitted well using a single exponential function as shown in Fig. 4E (+20 mV) and Fig. 4F (+60 mV). The mean time constants were not statistically different at +20 mV and +60 mV (P > 0.10, n = 15). In the 120-mM intracellular K+ condition, the dwell time of the channel opening was fitted using a single exponential function and increased exponentially as the membrane potential increased (Fig. 4G). The all-points histograms, open-time, and dwell time were obtained from those patches that contained only one BK channel based on the fact that maximum current amplitudes were unitary at high Po.
Fig. 4.
Kinetics of BK channels of PCMNs. A: representative BK channel activity recorded from an outside-out patch held at +20 and +60 mV, respectively. B and C: all-points histograms of current amplitudes at +20 and +60 mV were fitted with Gaussian function over a 2-s recording period (n = 8). Two current amplitude levels (O1, O2) are denoted to the right of their corresponding peaks. D: current-voltage curves. O2 peak current amplitude was plotted as a function of membrane potential. E and F: open-time histograms of BK channel currents at +20 and +60 mV were fitted with a single-exponential curve. τ: Open time constant. G: dwell time of BK channel currents.
In inside-out patches we observed that some BK channels displayed fast inactivation. These BK channels opened, remained open for a few hundred milliseconds, and closed (inactivated) for the remainder of the period of continuous 2- to 3-min holding membrane potentials at +20, +40, and +60 mV (Fig. 5, A1, B1, and C1). Following inactivation of such a transient opening, these channels could not be reactivated by further increasing depolarization membrane potential up to +80 mV. However, it could be reactivated by another depolarization potential (e.g., +40 mV) after the holding membrane potential completely returned to the baseline of −65 mV (not shown here). In comparison, the BK channels in the majority of patches exhibited persistent opening (i.e., high Po and a significant dwell time) over a period of 2–3 min in which holding membrane potentials were maintained (Fig. 5, A2, B2, and C2).
Fig. 5.
Inactivation of BK channels. Some BK channels showed inactivation. A1, B1, and C1: 3 representative BK channel activities recorded from inside-out patches held at +20, +40, or +60 mV in the bath solution containing 10 μM Ca2+. A2, B2, and C2: 3 representative BK channel activities from other BK channels, which did not show such inactivation in these conditions.
BK Channel-Mediated Outward Currents and Afterhyperpolarization Current (Whole Cell Patch)
To study BK channel-mediated currents, we used TTX (0.5 μM) to block Na+ and 4-AP (1 mM) to reduce voltage-dependent K+ currents but not BK currents (24). In the following experiments, TTX and 4-AP were routinely added in the bath solution. Under these conditions, outward currents and IAHP were obtained.
A family of outward currents were evoked by 250-ms voltage steps ranging from −70 to +40 mV in 10-mV increments from a holding potential of −70 mV in the control bath solution. The outward currents were composed of a rapidly inactivating transient component and a slowly inactivating persistent component (Fig. 6, A1 and B1). To dissociate the BK currents from outward currents, two specific BK channel blockers were employed. Application of 100 nM (saturation dose) of the BK channel blocker IBTX reduced outward currents (Fig. 6A2). IBTX-sensitive outward currents, including the transient and persistent components, were obtained by subtracting the suppressed currents after IBTX application from the currents evoked in the control solution (Fig. 6A3). Likewise, the paxilline-sensitive currents (Fig. 6B3) were obtained by subtracting the suppressed currents after 10 μM (saturation dose) paxilline (Fig. 6B2) from the outward currents before application (Fig. 6B1). Since the cell size of PCMNs did not vary much (conductance: 67.3 ± 0.7 pF, n = 64), we plotted I–V curve without normalizing BK current using conductance. Paxilline-sensitive outward currents were similar to IBTX-sensitive outward currents (Fig. 6C; P > 0.10, IBTX: n = 9, paxilline: n = 10).
Fig. 6.
BK channel-mediated outward currents and afterhyperpolarization currents (IAHP) in PCMNs. A1 and B1: family of outward currents were evoked by voltage steps as shown at the bottom. A2 and B2: outward currents after IBTX and paxilline. A3 and B3: IBTX-sensitive (A1 - A2) and Paxilline-sensitive (B1 - B2) currents. Insets: outward currents were irreversible after a 10 min washout for IBTX (partially) and paxilline (completely). C: IBTX and paxilline-sensitive peak currents-voltage relationships were plotted and they were not different (P > 0.10, IBTX: n = 9, paxilline: n = 10). D1 and E1: IAHP. Control: black trace; after IBTX: red traces; after paxilline: blue trace. D2 and E2: representative IBTX (red) and paxilline (blue)-sensitive IAHP by subtracting the currents in control and after IBTX or paxilline, respectively. F: average IBTX (red)- and paxilline (blue)-sensitive IAHP.
To examine the whether BK channels mediate afterhyperpolarization current (IAHP), IAHP was evoked by a 100-ms depolarizing voltage pulse from a holding potential of −70 to +10 mV followed immediately by a 1.5-s, 50-mV voltage pulse (Fig. 6, D1 and E1, black trace). Application of 100 nM IBTX (red) and 10 μM paxilline (blue) reduced the amplitude of IAHP (Fig. 6, D1 and E1). By subtracting the currents before and after IBTX or paxilline, IBTX- and paxilline-sensitive IAHP were obtained (Fig. 6, D2 and E2). The mean peak amplitude of IAHP were 40.7 ± 15.2 pA (IBTX sensitive) and 29.1 ± 13.7 pA (paxilline sensitive), respectively (Fig. 6F). They were not significantly different (P > 0.10; IBTX: n = 8, paxilline: n = 9). Thus BK channels significantly contribute to outward currents and IAHP.
BK Channels Regulated AP Repolarization and AHP in Single APs
To examine the contribution of BK channels to the AP properties, we first examined the effects of BK channel blockers on single spike repolarization. A single AP was evoked by injecting a 10-ms depolarizing current pulse from the holding potential −65 mV with an intensity sufficient to generate a single AP before (Fig. 7A, black trace) and after blockers [Fig. 7A, red (IBTX) and blue (paxilline) traces]. Spike widths were measured at the half-maximal voltage. The mean half-widths were significantly increased after IBTX and paxilline, respectively (Fig. 7B; P < 0.05, n = 9/group).
Fig. 7.
BK channels regulation of action potential (AP) repolarization and AHP in single spikes. A: single APs for control (black trace), IBTX (red trace), and paxilline (blue trace) showing the effect of blockade of BK channels on repolarization. B: average half-width of APs. C: single APs for control (black trace), IBTX (red traces) and paxilline (blue trace) showing the effect of blockade of BK channels on AHP. D and E: blockade of BK channels by IBTX (D) and paxilline (E) significantly depressed the AHP amplitude at 10 ms (*P < 0.05) but not at 50 and 100 ms. Control/IBTX: n = 9; control/paxilline: n = 8.
To investigate whether BK channel-mediated currents contribute to AHP, AP was elicited using the same protocol as above before (Fig. 7C, black trace) and after blockers [Fig. 7C, red (IBTX) and blue (paxilline) traces]. Application of IBTX reduced the amplitude of AHP by 11.3% from −20.3 ± 0.6 to −18.0 ± 0.7 mV at 10 ms relative to the beginning of AP (Fig. 7D; P < 0.05; n = 9/group). Application of paxilline reduced the amplitude by 4.8%, from −20.9 ± 0.3 to −19.9 ± 0.2 mV (Fig. 7E; P < 0.05; n = 8/group). Blockade of BK channels did not reduce the AHP at either 50 or at 100 ms (Fig. 7, D and E).
Frequency Dependence of AP Repolarization in Spike Trains
To characterize AP repolarization in spike trains, APs were evoked using current injections of different intensities. To standardize AP analysis, APs were evoked from a holding membrane potential of −65 mV and the current intensity was adjusted to trigger 4- to 20-Hz spike trains. The representative 1st APs in spike trains were shown in Fig. 8A. The half-widths of the first APs progressively increased as the firing frequency of the trains increased (P < 0.05; n = 8/group) and the first AP broadening was more pronounced at higher train frequencies (15 and 20 Hz; Fig. 8B). Within a train, the AP half-width was significantly increased as the spike order number increased (Fig. 8, C and D; P < 0.05; n = 8/group;). Compared with the same spike order number across trains, the AP half-width was broader in higher frequency spike trains (frequency-dependent broadening).
Fig. 8.
Frequency dependence of AP broadening in spike trains. A: superimposed traces of the representative 1st AP from 4-, 7-, 10-, and 15-Hz spike trains. B: half-width of 1st AP was spike-train frequency dependent. C: representative 1st, 2nd, and 5th APs for 10- and 15-Hz trains. D: AP half-width for individual spikes within the 4- to 20-Hz trains.
BK Channels Regulate AP Repolarization and Frequency-Dependent Broadening in Spike Trains
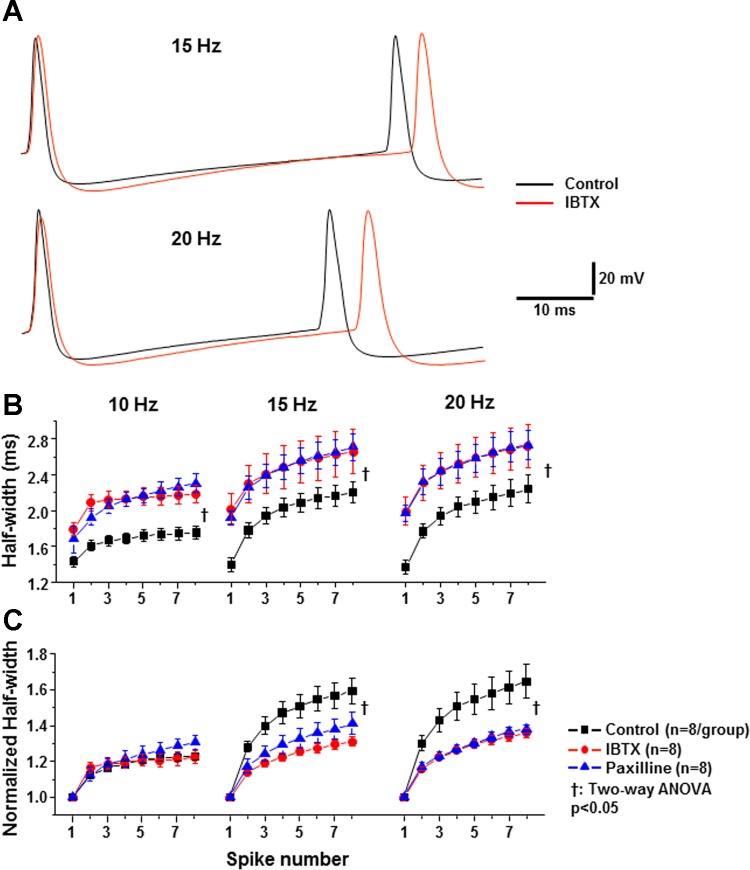
To examine whether BK channels regulate AP repolarization and the frequency-dependent broadening, 10-, 15-, and 20-Hz spike trains were generated. The first two APs in 15- and 20-Hz trains are shown in Fig. 9A. Blockade of BK channels slowed the repolarization of APs and increased the interspike interval between first and second spikes. In trains, half-width had frequency dependency. Application of IBTX (100 nM; red trace) and paxilline (10 μM, blue trace) significantly increased the half-widths of individual APs within the trains (Fig. 9B; black trace, P < 0.05; n = 8/group). This indicates that BK channels regulate the AP repolarization for each individual AP within the train. To examine whether BK channels regulate the frequency-dependent broadening, we normalized the effect of BK channel blockade on the half-width relative to the first AP half-width, i.e., the half-width of a higher order AP was divided by the half-width of the first AP (Fig. 9C). As shown in Fig. 9C, the normalized half-width-spike number curves in control and BK channels blocked groups (IBTX and Paxilline) were significantly different in 15- and 20-Hz trains (P < 0.05, n = 8/group) but only slightly different in the 10-Hz train. Thus BK channels significantly contribute to the frequency-dependent broadening in 15- and 20-Hz trains but did not to the frequency-dependent broadening in lower frequency trains.
Fig. 9.
Regulation of BK channels on repolarization and frequency dependence of APs in spike trains. A: representative 1st and 2nd spikes were evoked from a holding membrane potential of −65 mV with the current intensity adjusted to trigger generate 15- and 20-Hz spike trains in the absence (black trace) and presence IBTX (red trace). B: AP half-width significantly broadened as the spike number increased for control (black trace), IBTX (red trace), and paxillin (blue trace) in 10-, 15-, and 20-Hz spike trains (P < 0.05). Blockade of BK channels increased AP half-width for each spike within trains compared with control (†P < 0.05). C: normalized AP half-width relative to the 1st AP in the absence (black trace) and presence of IBTX (red trace) or paxilline (blue trace) in spike trains of 10, 15, and 20 Hz. IBTX and paxilline significantly reduced the frequency-dependent AP broadening in high frequency trains (†P < 0.05, n = 8/group).
Contribution of BK Channels to Spike Frequency and Spike Frequency Adaptation
To evaluate the effect of BK channels on excitability, spike trains were evoked by 1-s depolarizing currents (100–340 pA in 20-pA increments) from a holding potential of −65 mV in control and after application of IBTX. Figure 10A shows original recordings before and after IBTX. Under control conditions, spike frequency significantly increased as injected currents increased (Fig. 10C, black trace). Application of IBTX to the somatic surface significantly reduced the spike firing rate compared with control (Fig. 10C; P < 0.05; n = 8/group), indicating that activation of BK channels significantly facilitates excitability. Figure 10B shows five to seven early spikes and the effect of IBTX on the early firing of a 1-s spike train that was evoked by a depolarizing current pulse (220 pA) before (black trace) and after IBTX (red trace). Application of IBTX (100 nM) slowed the repolarization and lengthened afterhyperpolarization of APs and increased interspike intervals [Fig. 10B, red trace; spike frequency adaption (SFA)], thus reducing the instantaneous firing frequency (Fig. 10D). It should be emphasized that IBTX significantly suppressed SFA as shown in Fig. 10D. To further analyze SFA, spike instantaneous frequencies were normalized relative to the second spike instantaneous frequency. Normalized spike instantaneous frequencies significantly decreased at the third spike and remained unchanged afterwards in IBTX, while in control normalized spike instantaneous frequencies continued to decrease as the spike number increased up to the seventh spike (Fig. 10E). IBTX did not significantly change the cell input resistance nor the DC current required to hold the background potential at −65 mV (data not shown).
Fig. 10.
Contribution of BK channels to spike frequency and spike frequency adaptation (SFA). A: representative spike trains were evoked by 1-s 220 pA depolarizing current injection in the absence (black trace) and presence of IBTX (red trace). B: average firing rates in response to current injections from 100 to 340 pA were significantly reduced after application of IBTX (†P < 0.05). C: superimposed initial 5–7 representative spikes from the trains evoked by 1-s 220 pA depolarizing current injection in the absence (black trace) and presence of IBTX (red trace). Apparently, IBTX increased the interspike interval and lengthened AHP in trains. D: instantaneous frequency as the inverse of interspike intervals. IBTX significantly decreased instantaneous frequency (†P < 0.05, n = 8/group). In addition, instantaneous frequency declined in the first a few spikes as the spike number increased, indicating an early SFA in both control and IBTX groups. E: normalized instantaneous spike frequency (NISF) relative to the 2nd spike. *P < 0.01, later spikes vs. the 2nd spike in control and IBTX; #P < 0.05, IBTX vs. control; n = 8/group.
BK Channels Did Not Affect Membrane Potential Threshold and Depolarizing Input Resistance
Spike trains were elicited by a 1.5-s depolarizing ramp current from 0 to 300 pA from a holding potential of −65 mV in control and during IBTX (Fig. 11, A and B). Similar to Fig. 7, instantaneous firing rate and half-width were progressively increased during depolarizing ramp current injections in the control. IBTX significantly reduced the instantaneous firing rate, increased half-width, and suppressed the frequency dependency of spike broadening (data not shown). However, IBTX had no significant effect on the first AP threshold (Fig. 11C) and the depolarizing input resistance (Rinput; Fig. 11D; P > 0.10; n = 8/group).
Fig. 11.
Regulation of BK channels on the membrane potential threshold and depolarizing input resistance. A and B: spike trains were evoked by a 1.5-s, 0.2-pA/ms ramp current from a holding potential of −65 mV in the absence (A) and presence of IBTX (B). C: membrane potential threshold of the 1st AP was measured as denoted by an arrow which pointed to the beginning of the AP in A and B. IBTX did not change the threshold (P > 0.1; n = 8/group). D: depolarizing input resistance (Rinput). IBTX did not change the Rinput (P > 0.1; n = 8/group).
DISCUSSION
Using excised patch recordings, we first identified two types of BK channels in PCMNs. The majority of them were persistent, meaning that they did not show inactivation during the voltage holding over 2–3 min (Po was high during this period), whereas some other BK channels were transient, showing fast inactivation after a few 100 ms opening and stayed inactive during the rest of voltage holding (Po was 0 during this period). Since the majority of the BK channels that we encountered were persistent, we further tested their voltage dependency and Ca2+ dependency as well as characterized their opening and closing kinetics. BK channels of PCMNs had a conductance of 237 pS. BK channel activity exhibited a voltage and Ca2+ concentration dependence and could be reversibly blocked by specific BK blockers IBTX and paxilline. These characteristics are consistent with a variety of other cell types (see Ref. 24 for a review). Using whole cell voltage clamp, we found that there were transient and persistent components in IBTX-sensitive and paxilline-sensitive outward currents which were voltage dependent. Using whole cell current clamp, we found that blockade of BK channels markedly broadened the spike half-width in both single and train spikes, reduced the spike train frequency-dependent broadening, and decreased the amplitude of fAHP. In addition, BK channel blockers largely reduced the firing frequency and suppressed early spike-frequency adaptation. Taken together, these data indicate that BK channels in PCMNs regulate the AP properties and facilitate AP firing, possibly by regulating their repolarization, fAHP, and early spike-frequency adaptation.
Physiological Properties of BK Channels of PCMNs
BK channels are the only member of the voltage-dependent potassium channel family that is activated by both voltage and Ca2+ (44). This allows a particularly unique function of BK channels in the integration of voltage and Ca2+ signals and modulation of membrane excitability in a variety of cell types (21). In most previous studies, BK channels were shown to exhibit sustained activation (e.g., 29, 69). Likewise, Pérez et al. (49) recently found that BK channels from canine cardiac ganglia did not exhibit intrinsic inactivation. In our study, we also found fast inactivation in some BK channels in PCMNs in addition to the majority of sustained BK channels. For persistent BK channels, they had a conductance of 237 pS, which is comparable to those values for BK channels as reported in other neurons (18, 23, 49, 53, 60). In these PCMNs, BK channels were highly voltage and intracellular Ca2+ dependent, and the channel open dwell time increased exponentially as membrane potential depolarized. The relationship between BK channel activity and intracellular Ca2+ was previously studied (30, 31, 47, 66). Similar to most of these studies, we found that intracellular Ca2+ concentrations ≥0.5 μM were required to significantly induce unitary BK current and reached maximal activation at ∼50 μM.
For transient BK channels, fast inactivation of PCMNs occurred within several hundred milliseconds in response to continuous voltage holding and stayed inactivated. In previous studies (29, 43), fast inactivation of BK channels of hippocampal pyramidal neurons were observed and these channels showed fast inactivation within ∼100 ms. Using whole cell voltage-clamp recording, we found that IBTX and paxilline-sensitive currents showed both transient and persistent components, indicating that BK channels are capable of inactivation. Whether fast inactivation of transient and persistent BK currents identified in single channels contribute to the transient outward component and persistent BK channels, respectively, is an interesting issue. Alternatively, initial fast activation of all BK currents may quickly reduce Ca2+ influx, thereby reducing BK outward currents and resulting in a transient peak in outward currents.
BK Channels Contribute to AP Repolarization and fAHP
BK channels are involved in AP repolarization in other types of neurons (1, 32, 58, 63). Consistent with these reports, we showed that BK channels contribute to AP repolarization in PCMNs. After blockade of BK channels, the half-widths of individual APs in both single APs and in spike trains were increased. In addition, the broadening of each individual APs within a train was spike frequency dependent and reduced after blockade of BK channels. The contribution of BK channels to spike frequency-dependent broadening during repolarization has been found in other neurons. BK channel-mediated spike broadening in other neurons reaches a plateau after the second or third spike, possibly due to the repolarizing currents that are fully inactivated within the first few spikes (15, 22, 59). In contrast, PCMNs exhibited a progressive cumulative broadening pattern up to the 20th spike in a 20-Hz spike train. Previously, it has been shown that fast-inactivating voltage-gated K+ channel-mediated currents (A-type K+ current: IA) contribute to repolarization of APs (4, 61) and inactivation of IA eliminated frequency-dependent AP broadening in Aplysia neurons (41). Since BK outward currents showed a rapid inactivation (transient peaks), BK channels are well suited to contribute to spike frequency-dependent broadening (55).
Activation of BK channels also contributes to the fAHP in some cell types (14, 15, 54, 60). Similarly in PCMNs, blockade of BK channels reduced fAHP, but it did not affect medium (m)AHP and slow (s)AHP in single APs. This confirmed our previous observation (38). In contrast, SK channels regulate both fHAP and mAHP but not sAHP (36). Of note: blockade of BK channels with IBTX noticeably increased AHP in trains. This might contradict our finding that blockade of BK channels had reduced fAHP in single APs. Actually, blockade of BK channels with IBTX could slow repolarization and delay inactivation of Ca2+ channels. This resulted in an increase of intracellular free Ca2+ and, in turn, activation of SK channels. As we showed previously (36), activation of SK channels may increase fAHP and mAHP which may contribute to the increase of mAHP in trains after blockade of BK channels as seen in Figs. 9A and 10B.
BK Channels Facilitated Neuronal Excitability and Suppressed SFA in PCMNs
In neurons, K+ channels are commonly believed to be inhibitory, and activation of K+ channels reduces excitability (28, 63). Accordingly, the activation of BK channels was frequently reported to reduce the excitability (3, 32, 70). In the present study, however, we found that BK channel blocker IBTX inhibited the firing rate in PCMNs. This indicates that the role of activation of BK channels in PCMNs is actually to increase excitability, rather than to inhibit it. Our data are consistent with a more recent finding by Gu et al. (26) who found that BK channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. This facilitation was considered to be due to enhanced Na+ channel inactivation and the delayed rectifier K+ channel activation (26). Interestingly, paxilline blockade of BK channels lacking the β4-unit also decreased the number of APs in response to current injection in dentate gyrus neurons (6), indicating that BK channels lacking the β4-unit facilitate excitability. Furthermore, Du et al. (12) used a mutation of the α-subunit of the BK channel that enhanced BK channels, leading to increased excitability by inducing rapid repolarization of APs. More recently, Pérez et al. (49) reported that the BK current also increased excitability in isolated canine intracardiac neurons. Thus far the mechanisms for inhibitory or excitatory roles of BK channels are still not fully understood. Apparently, BK channels may have different roles in regulating AP properties and excitability in different neurons and even under different conditions.
In PCMNs, the instantaneous firing rate in the spike trains evoked by current injection was reduced dramatically during the first several initial spikes. This indicates that SFA occurred in PCMNs. Since the AP half-width increased significantly during the first several spikes, it is possible that the increased half-width may contribute to SFA. After blockade of BK channels, SFA was seen only within the first two spikes. This indicates that BK channels significantly contribute to SFA, which is consistent with Gu et al. (26) who found that BK channels cause early SFA in CA1 hippocampal pyramidal cells. Since SFA did not completely disappear after blockade, we assume that other channels may contribute to SFA as well. As reported, voltage-gated Na+ and delayed-rectifier K+ currents may contribute to SFA in other neurons (45).
BK and SK Channels: a Comparison
Another Ca2+-activated K+ channel, the SK channel, also regulates AP properties and excitability in many central neurons (2). In contrast to BK channels, SK channels are activated solely by increases in intracellular Ca2+. Previously, we have characterized the functional roles of SK channels of PCMNs (36). As a comparison, BK channels contribute to AP repolarization and fAHP, whereas SK channels contribute to AP repolarization, fAHP, and mAHP in single APs. SK channel blockade increased the spike frequency and abolished spike-frequency dependence of AHP in trains but did not alter SFA. Also, SK channel blockade increased the depolarizing resistance and decreased the AP threshold. In contrast, BK channel blockade decreased the spike frequency, frequency dependence of AP repolarization, and SFA but did not change the depolarizing resistance and AP threshold. These data suggest that BK channels and SK channels play different roles in regulating PCMN excitability: while activation of SK channels provides a negative control for PCMN excitability, possibly by mediating repolarization, fAHP, mAHP, and AP threshold, activation of BK channels facilitates PCMN excitability possibly by regulating repolarization, fAHP and SFA.
Perspectives and Significance
BK channels play a key neuroprotective role against ischemic brain damage. Liao et al. (35) showed middle cerebral artery occlusion in mice lacking BK channels (loss-of-function) produced a significantly larger infarct volume and led to more severe neurological deficits and higher postischemic mortality than control wild type mice. Previously, we have demonstrated that PCMNs in the NA play a key role in regulating cardiac functions and that chronic intermittent hypoxia (CIH; a model for sleep apnea) may reduce PCMN control over the heart rate and induce cell loss (8, 9, 72, 73, 74). However, how CIH causes impaired function and cell death in the PCMNs of the NA has not yet been elucidated. It has been reported that CIH markedly decreased open probability of BK channels in the CA1 pyramidal neurons which may account for the hippocampal injury in patients with sleep apnea (64). Further, Tjong et al. (65) demonstrated that administration of melatonin ameliorates CIH-induced deficits in the constitutive NO production and impaired BK channel activity, which may provide a potential therapeutic treatment of neuronal injury in sleep apnea patients. Since BK channels are not only voltage- and Ca2+-dependent, but are also sensitive to oxygen levels (20, 39), we speculate that CIH may impair BK channels in PCMNs, which may lead to excessive Ca2+ influx, inducing cell death in the NA (72–74). In this study, we have systematically examined the functional role of BK channels in regulating excitability and AP properties of PCMNs, which will underlie a foundation for the future investigation for disease-induced changes of PCMNs.
GRANTS
This study was supported by National Institutes of Health Grants HL-79636 and AG-21020 (to Z. J. Cheng).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.L., R.D.W., Q.-H.C., and Z.C. conception and design of research; M.L. and Z.C. performed experiments; M.L. and Z.C. analyzed data; M.L., J.T.H., Q.-H.C., and Z.C. interpreted results of experiments; M.L., J.T.H., and Z.C. prepared figures; M.L. and Z.C. drafted manuscript; M.L., J.T.H., R.D.W., Q.-H.C., and Z.C. edited and revised manuscript; M.L., J.T.H., R.D.W., Q.-H.C., and Z.C. approved final version of manuscript.
REFERENCES
- 1.Adams PR, Constanti A, Brown DA, Clark RB. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature 296: 746–749, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol 741: 245–269, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Alger BE, Williamson A. A transient calcium dependent potassium component of the epileptiform burst after-hyperpolarization in rat hippocampus. J Physiol 399: 191–205, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardoni R, Beluzzi O. Kinetic study and numerical reconstruction of A-type current in granule cells of rat cerebellar slices. J Neurophysiol 69: 2222–2231, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Barrett JN, Magleby K, Pallotta BS. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol 331: 211–230, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci 8: 1752–1759, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Candia S, Garcia ML, Latorre R. Mode of action of iberiotoxin, a potent blocker of the large conductance Ca2+-activated K+ channel. Biophys J 63: 583–590, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng Z, Zhang H, Yu Wurster RJ, Gozal D. Attenuation of baroreflex sensitivity following domoic acid lesion of the nucleus ambiguus of rats. J Appl Physiol 96: 1137–1145, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Cheng Z, Zhang H, Guo SZ, Wurster R, Gozal D. Differential control over vagal efferent postganglionic neurons in rat intrinsic cardiac ganglia by neurons in the nucleus ambiguus and the dorsal motor nucleus of the vagus: anatomical evidence. Am J Physiol Regul Integr Comp Physiol 286: R625–R633, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Colquhoun D, Sigworth FJ. Fitting and statistical analysis of single channel records. In: Single Channel Recording (2nd ed), edited by Sakmann B, Neher E. New York: Plenum, 1995, p. 483–587 [Google Scholar]
- 11.Davies PJ, Ireland DR, McLachlan EM. Sources of Ca2+ for different Ca2+-activated K+ conductances in neurones of the rat superior cervical ganglion. J Physiol 495: 353–366, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du W, Bautista JF, Yang H, Diez-Sampedro A, You SA, Wang L, Kotagal P, Lüders HO, Shi J, Cui J, Richerson GB, Wang QK. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet 37: 733–738, 2005 [DOI] [PubMed] [Google Scholar]
- 13.El-Menyar AA. Dysrhythmia and electrocardiographic changes in diabetes mellitus: pathophysiology and impact on the incidence of sudden cardiac death. J Cardiovasc Med (Hagerstown) 7: 580–855, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Faber ES, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci 22: 1618–1628, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faber ES, Sah P. Ca2+-activated K+ (BK) channel inactivation contributes to spike broadening during repetitive firing in the rat lateral amygdale. J Physiol 552: 483–497, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faber ES, Sah P. Functions of SK channels in central neurons. Clin Exp Pharmacol Physiol 34: 1077–83, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Foehring RC, Zhang XF, Lee JC, Callaway JC. Endogenous calcium buffering capacity of substantia nigral dopamine neurons. J Neurophysiol 102: 2326–2333, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franciolini F. Calcium, and voltage dependence of single Ca2+-activated K+ channels from cultured hippocampal neurons of rat. Biochim Biophys Acta 943: 419–427, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem 265: 11083–11090, 1990 [PubMed] [Google Scholar]
- 20.Gao TM, Fung ML. Decreased large conductance Ca2+-activated K+ channel activity in dissociated CA1 hippocampal neurons in rats exposed to perinatal and postnatal hypoxia. Neurosci Lett 332: 163–166, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Ghatta S, Nimmagadda D, Xu X, O'Rourke ST. Large-conductance, calcium-activated potassium channels: structural and functional implications. Pharmacol Ther 110: 103–116, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Giese KP, Storm JF, Reuter D, Fedorov NB, Shao LR, Leicher T, Pongs O, Silva AJ. Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kvbeta11-deficient mice with impaired learning. Learn Mem 5: 257–273, 1998 [PMC free article] [PubMed] [Google Scholar]
- 23.Gola M, Niel JP, Bessone R, Fayolle R. Single channel and whole-cell recordings from non-dissociated sympathetic neurons in rabbit coeliac ganglia. J Neurosci Methods 43: 13–22 1992 [DOI] [PubMed] [Google Scholar]
- 24.Goodman MB, Art JJ. Variations in the ensemble of potassium currents underlying resonance in turtle hair cells. J Physiol 497: 395–412 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorini C, Jameson HS, Mendelowitz D. Serotonergic modulation of the trigeminocardiac reflex neurotransmission to cardiac vagal neurons in the nucleus ambiguus. J Neurophysiol 102: 1443–50, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol 580: 859–882, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch Clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100, 1981 [DOI] [PubMed] [Google Scholar]
- 28.Hille B. Ion Channels of Excitable Membranes (3rd ed) Sunderland, MA: Sinauer, 2001 [Google Scholar]
- 29.Ikemoto Y, Ono K, Yoshida A, Akaike N. Delayed activation of large-conductance Ca2+-activated K channels in hippocampal neurons of the rat. Biophys J 56: 207–212, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang J, Sumners C, Posner P. Calcium-modulated inward rectification of a calcium-activated potassium channel in neurons. J Neurophysiol 72: 3023–3025, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Kang J, Huguenard JR, Prince DA. Two types of BK channels in immature rat neocortical pyramidal neurons. J Neurophysiol 76: 4194–4197, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Lancaster B, Nicoll RA. Properties of two calcium-activated hyperpolarizations in rat hippocampal neurones. J Physiol 389: 187–204, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Latorre R, Vergara C, Hidalgo C. Reconstitution in lipid bilayers of a Ca2+-dependent K+ channel from transverse tubule membranes isolated from rabbit skeletal muscle. Proc Natl Acad Sci USA 79: 805–809, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawrence IG, Weston PJ, Bennett MA, McNally PG, Burden DC, Thurston H. Is impaired baroreflex sensitivity a predictor or cause of sudden death in insulin-dependent diabetes mellitus? Diabet Med 14: 82–85, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Liao Y, Kristiansen AM, Oksvold CP, Tuvnes FA, Gu N, Rundén-Pran E, Ruth P, Sausbier M, Storm JF. Neuronal Ca2+-activated K+ channels limit brain infarction and promote survival. PLoS One 5: e15601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin M, Hatcher JT, Chen QH, Wurster RD, Cheng ZJ. Small conductance Ca2+-activated K+ channels regulate firing properties and excitability in parasympathetic cardiac motoneurons in the nucleus ambiguus. Am J Physiol Cell Physiol 299: C1285–C1298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin M, Chen QH, Wurster RD, Hatcher JT, Liu YQ, Li LH, Harden SW, Cheng ZJ. Maternal Diabetes increases small conductance Ca2+-activated K+ (SK) currents which alter action potential properties and excitability of cardiac motoneurons in the nucleus ambiguus. J Neurophysiol 104: 2125–2138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin M, Hatcher JT, Chen QH, Wurster RD, Li LH, Cheng ZJ. Maternal diabetes increases large conductance Ca2+-activated K+ outward currents that alter action potential properties but do not contribute to attenuated excitability of parasympathetic cardiac motoneurons in the nucleus ambiguus of neonatal mice. Am J Physiol Regul Integr Comp Physiol 300: R1070–R1078, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu H, Moczydlowski E, Haddad GG. O2 deprivation inhibits Ca2+-activated K+ channels via cytosolic factors in mice neocortical neurons. J Clin Invest 104: 577–588, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loewy AD, Spyer KM. Central Regulation of Autonomic Functions. New York: Oxford; 1990 [Google Scholar]
- 41.Ma M, Koester J. The role of K+ currents in frequency-dependent spike broadening in Aplysia R20 neurons: a dynamic-clamp analysis. J Neurosci 16: 4089–101, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martínez-Pinna J, Davies PJ, McLachlan EM. Diversity of channels involved in Ca2+ activation of K+ channels during the prolonged AHP in guinea-pig sympathetic neurons. J Neurophysiol 84: 346–1354, 2000 [DOI] [PubMed] [Google Scholar]
- 43.McLarnon JG. Inactivation of a high conductance calcium dependent potassium current in rat hippocampal neurons. Neurosci Lett 193: 5–8, 1995 [DOI] [PubMed] [Google Scholar]
- 44.McManus OB. Calcium-activated potassium channels: regulation by calcium. J Bioenerg Biomembr 23: 537–560, 1991 [DOI] [PubMed] [Google Scholar]
- 45.Melnick IV, Santos SF, Safronov BV. Mechanism of spike frequency adaptation in substantia gelatinosa neurones of rat. J Physiol 559: 383–395, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendelowitz D. Firing properties of identified parasympathetic cardiac neurons in the nucleus ambiguus. Am J Physiol Heart Circ Physiol 271: H2609–H2614, 1996 [DOI] [PubMed] [Google Scholar]
- 47.Pallotta BS, Hepler JR, Oglesby SA, Harden TK. A comparison of calcium-activated potassium channel currents in cell-attached and excised patches. J Gen Physiol 89: 985–997 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pedarzani P, Kulik A, Muller M, Ballanyi K, Stocker M. Molecular determinants of Ca2+-dependent K+ channel function in rat dorsal vagal neurones. J Physiol 527: 283–290, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pérez GJ, Desai M, Anderson S, Scornik FS. Large conductance calcium-activated potassium (BK) current modulates excitability in isolated canine intracardiac neurons. Am J Physiol Cell Physiol 304: C280–C286, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poolos NP, Johnston D. Calcium-activated potassium conductances contribute to action potential repolarization at the soma but not the dendrites of hippocampal CA1 pyramidal neurons. J Neurosci 19: 5205–5212, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raffaelli G, Saviane C, Mohajerani MH, Pedarzani P, Cherubini E. BK potassium channels control transmitter release at CA3-CA3 synapses in the rat hippocampus. J Physiol 557: 147–157, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robitaille R, Garcia ML, Kaczorowski GJ, Charlton MP. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron 11: 645–55, 1993 [DOI] [PubMed] [Google Scholar]
- 53.Safronov BV, Vogel W. Large conductance Ca2+-activated K+ channels in the soma of rat motoneurones. J Membr Biol 162: 9–15 1998 [DOI] [PubMed] [Google Scholar]
- 54.Sah P, McLachlan EM. Potassium currents contributing to action potential repolarization and the afterhyperpolarization in rat vagal motoneurons. J Neurophysiol 68: 1834–1841, 1992 [DOI] [PubMed] [Google Scholar]
- 55.Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol 66: 345–353, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci 7: 921–931, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Sanchez M, McManus OB. Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology 35: 963–968, 1996 [DOI] [PubMed] [Google Scholar]
- 58.Schwindt PC, Spain WJ, Foehring RC, Stafstrom CE, Chubb MC, Crill WE. Multiple potassium conductances and their functions in neurons from cat sensorimotor cortex in vitro. J Neurophysiol 59: 424–449 1988 [DOI] [PubMed] [Google Scholar]
- 59.Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol 521: 135–146, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smart TG. Single calcium-activated potassium channels recorded from cultured rat sympathetic neurones. J Physiol 389: 337–360, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol 385: 733–739, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Storm JF. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol 409: 71–190, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res 83: 161–187, 1990 [DOI] [PubMed] [Google Scholar]
- 64.Tjong YW, Li M, Hung MW, Wang K, Fung ML. Nitric oxide deficit in chronic intermittent hypoxia impairs large conductance calcium-activated potassium channel activity in rat hippocampal neurons. Free Radic Biol Med 44: 547–557, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Tjong YW, Li MF, Hung MW, Fung ML. Melatonin ameliorates hippocampal nitric oxide production and large conductance calcium-activated potassium channel activity in chronic intermittent hypoxia. J Pineal Res 44: 234–243, 2008 [DOI] [PubMed] [Google Scholar]
- 66.Tseng-Crank J, Foster CD, Krause JD, Mertz R, Godinot N, DiChiara TJ, Reinhart PH. Cloning, expression, and distribution of functionally distinct Ca2+-activated K+ channel isoforms form human brain. Neuron 13: 1315–1330, 1994 [DOI] [PubMed] [Google Scholar]
- 67.Viana F, Bayliss DA, Berger AJ. Multiple potassium conductances and their role in action potential repolarization and repetitive firing behavior of neonatal rat hypoglossal motoneurons. J Neurophysiol 69: 2150–2163, 1993 [DOI] [PubMed] [Google Scholar]
- 68.Wang ZW, Saifee O, Nonet ML, Salkoff L. SLO-1 potassium channels control quantal content of neurotransmitter release at the C. elegans neuromuscular junction. Neuron 32: 867–881, 2001 [DOI] [PubMed] [Google Scholar]
- 69.Wann KT, Richards CD. Properties of single calcium-activated potassium channels of large conductance in rat hippocampal neurones in culture. Eur J Neurosci 6: 607–617, 1994 [DOI] [PubMed] [Google Scholar]
- 70.Williamson A, Alger BE. Characterization of an early afterhyperpolarization after a brief train of action potentials in rat hippocampal neurons in vitro. J Neurophysiol 63: 72–81, 1990 [DOI] [PubMed] [Google Scholar]
- 71.Woerman AL, Mendelowitz D. Postnatal sulfur dioxide exposure reversibly alters parasympathetic regulation of heart rate. Hypertension 62: 274–80, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan B, Soukhova-O'Hare GK, Li L, Lin Y, Gozal D, Wead WB, Wurster RD, Cheng ZJ. Attenuation of heart rate control and neural degeneration in nucleus ambiguus following chronic intermittent hypoxia in young adult Fischer 344 rats. Neuroscience 153: 709–720, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Yan B, Li L, Harden SW, Gozal D, Lin Y, Wead WB, Wurster RD, Cheng ZJ. Chronic intermittent hypoxia impairs heart rate responses to AMPA and NMDA and induces loss of glutamate receptor neurons in nucleus ambiguus of F344 rats. Am J Physiol Regul Integr Comp Physiol 296: R299–R308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yan B, Li L, Harden SW, Epstein PN, Wurster RD, Cheng ZJ. Diabetes induces neural degeneration in nucleus ambiguus and attenuates heart rate control in OVE26 mice. Exp Neurol 220: 34–43, 2009 [DOI] [PubMed] [Google Scholar]