Abstract
Chronic heart failure (CHF) is characterized by decreased cardiac parasympathetic and increased cardiac sympathetic nerve activity. This autonomic imbalance increases the risk of arrhythmias and sudden death in patients with CHF. We hypothesized that the molecular and cellular alterations of cardiac postganglionic parasympathetic (CPP) neurons located in the intracardiac ganglia and sympathetic (CPS) neurons located in the stellate ganglia (SG) possibly link to the cardiac autonomic imbalance in CHF. Rat CHF was induced by left coronary artery ligation. Single-cell real-time PCR and immunofluorescent data showed that L (Cav1.2 and Cav1.3), P/Q (Cav2.1), N (Cav2.2), and R (Cav2.3) types of Ca2+ channels were expressed in CPP and CPS neurons, but CHF decreased the mRNA and protein expression of only the N-type Ca2+ channels in CPP neurons, and it did not affect mRNA and protein expression of all Ca2+ channel subtypes in the CPS neurons. Patch-clamp recording confirmed that CHF reduced N-type Ca2+ currents and cell excitability in the CPP neurons and enhanced N-type Ca2+ currents and cell excitability in the CPS neurons. N-type Ca2+ channel blocker (1 μM ω-conotoxin GVIA) lowered Ca2+ currents and cell excitability in the CPP and CPS neurons from sham-operated and CHF rats. These results suggest that CHF reduces the N-type Ca2+ channel currents and cell excitability in the CPP neurons and enhances the N-type Ca2+ currents and cell excitability in the CPS neurons, which may contribute to the cardiac autonomic imbalance in CHF.
Keywords: action potential, autonomic dysfunction, cardiac ganglia, ion channels
nearly 5.1 million people in the United States have chronic heart failure (CHF) (26). CHF is considered to contribute to approximately 270,000 deaths a year (26). Epidemiological studies revealed that CHF patients are susceptible to severe ventricular arrhythmias including ventricular tachycardia and ventricular fibrillation, which serve as principal underlying causes of sudden cardiac death(43). CHF is characterized by increased sympathetic tone and decreased parasympathetic nerve activity (6, 15, 23, 49, 53, 56, 61). The imbalance of sympathetic and parasympathetic nerve activity is a predictive risk factor for ventricular arrhythmia and sudden cardiac death (33) and is associated with further worsening and mortality of CHF (10, 17, 24, 29, 41). The role of sympathetic hyperactivation in CHF is highlighted by the use of pharmacological blockade of sympathetic nervous system (β-adrenergic receptor blockers) as the key approach to the current therapy of CHF (14, 22, 25, 39, 47). Direct cardiac vagal nerve stimulation has been found to suppress the ventricular tachyarrhythmia and to improve the survival rate both in animal CHF models and in patients with CHF (12, 13, 18, 19, 32, 34, 35, 40, 52, 55–57, 71). Therefore, exploring the mechanism(s) responsible for the sympathetic hyperactivity and parasympathetic hypoactivity may provide a new therapeutic strategy for improving the prognosis of CHF and reducing the mortality of CHF.
It has been shown that the afferent limb and central neural component of the autonomic nervous system contribute to the sympathetic hyperactivity and parasympathetic hypoactivity in CHF (54, 63, 66, 67, 72, 73). However, the efferent component of the autonomic nervous system might also be involved in the imbalance of sympathetic and parasympathetic nerve activity in CHF because normally, many effects of cardiac sympathetic and parasympathetic nervous systems on cardiac function are mediated via neurotransmitters released from postganglionic sympathetic and parasympathetic neurons innervating the heart (65). Although the mechanisms governing neurotransmitter release at postganglionic sympathetic and parasympathetic neuron terminals are not completely understood, Ca2+ influx through the voltage-gated Ca2+ channels (Cav) is a key trigger for the release of neurotransmitters (1, 5, 11, 74). Cell membrane depolarization is necessary for opening voltage-gated Ca2+ channels and increasing intracellular Ca2+ levels (75–77). Therefore, we investigated the alteration of voltage-gated Ca2+ channels and cell excitability in cardiac postganglionic parasympathetic (CPP) neurons of the intracardiac ganglia (ICG) and sympathetic (CPS) neurons of the stellate ganglia (SG) from sham and coronary artery ligation-induced CHF rats.1
MATERIALS AND METHODS
All experimental procedures were approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996) and the American Physiological Society's Guiding Principles for the Care and Use of Vertebrate Animals in Research and Training.
CHF animal model.
Male Sprague-Dawley rats weighing 180–200 g were assigned randomly to one of two groups: CHF (n = 49) and sham (n = 48). CHF was produced by surgical ligation of the left coronary artery, as previously described (62, 63). On the day of the terminal experiment (12–14 wk), left ventricular end-diastolic pressure was measured using a Millar pressure transducer (model SPR 524, Millar Instruments, TX). Left femoral artery was cannulated with a polyethylene-50 catheter for blood pressure (BP) and heart rate (HR) monitoring. A digital image of the left ventricle was captured using a digital camera (Canon, Japan). Infarct size was determined using a colorimetric technique coupled to a computerized planimetric analysis (Adobe Photoshop CS5). The percentage of infarct size to total left ventricle area was quantified using Adobe Photoshop CS5 (Adobe Systems).
Labeling of CPP and CPS neurons.
The axons of the SG neurons project to the heart and other target organs. Additionally, although ICG are thought to contain pure cardiac vagal neurons(9, 50, 64), it is possible that other neurons (such as interneurons and local circuit neurons) also exist in the intracardiac ganglia (2, 3, 21, 30, 51). Therefore, we used a transported florescent dye (red color DiI) to retrograde-label CPP and CPS neurons. Rats were anesthetized with 2% isoflurane and artificially ventilated. The left thoracotomy was done. Twenty to twenty-five injections (2 μl DiI for each injection) were made subepicardially into left and right atria and ventricles using a glass micropipette connected to a 1 WPI Nanoliter 2000 microinjector (48). The surgical incision was closed, and terminal experiments were performed at least 3 days after labeling surgery because we found that 3 days are needed for dye diffusion to the neurons (data not shown).
Single-cell real-time RT-PCR for calcium channel mRNA.
Single-cell real-time RT-PCR was performed as described previously (36). Briefly, SG and ICG neurons were isolated (see below) and loaded in a chamber with regular extracellular solution (in mM): 137 NaCl, 5.4 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose with pH 7.4. DiI-labeled CPP or CPS neurons were used for single-cell real-time RT-PCR. A patch-clamp pipette (1–3 MΩ resistance) was used to break the membrane of single neuronal cells. Under suction conditions, the cell and pipette's contents were expelled into a 0.2 ml PCR tube containing mRNA-preserving reagents and then mixed with RT reaction buffer. RT was performed at 42°C for 30 min, and the cDNA was then stored at −80°C. The primers (Table 1) were based on the cDNA sequences of RPL19 (housekeeping gene) and Ca2+ channel subunits (Cav1.2, Cav1.3, Cav2.1, Cav2.2, and Cav2.3). PCR reaction was performed in a 25 μl volume containing 12.5 μl iQ Syber Green Supermix (Bio-Rad), 200 nM (in the first round) or 300 nM (in the second round) of each primer. The cDNA was amplified by real-time quantitative PCR with an ABI StepOnePlus Real-Time PCR System. For quantification, Ca2+ channel genes were normalized to the expressed housekeeping gene RPL19. The data were analyzed by the 2−ΔΔCt method (38).
Table 1.
Primer sequences
| Gene Accession No. | Primer Name | Primer Sequence (5′–3′) |
|---|---|---|
| NM012517 | Cav1.2-forward | GGCAATGCGACCATCTCTACC |
| Cav1.2-reverse | CCCCTGCTTCTTGGGTTTCC | |
| Cav1.2-internal | ACTGCTGCCGCTTCCGCTGTGT | |
| NM017298 | Cav1.3-forward | TGTTCGTGGATGATGATGATGATG |
| Cav1.3-reverse | CTGGTGCCTCTTGCATAGTTTG | |
| Cav1.3-internal | CGTGGTCCTCTTGCTGCTGCCGTT | |
| NM012918 | Cav2.1-forward | GGGAATAACTTCATCAACCTGAGC |
| Cav2.1-reverse | ATCAGCAGACAGACATAAGGTAGG | |
| Cav2.1-internal | TTCTCCGCCTCTTCCGTGCTGCC | |
| NM0147141 | Cav2.2-forward | TCCTAGCCAGGTGTCCCATC |
| Cav2.2-reverse | CTTTTCCAGGGACCTCTGCTTC | |
| Cav2.2-internal | CCACCACCACCGCTGCCACCG | |
| NM019294 | Cav2.3-forward | GAGCGGAGTCTGGATGAAGG |
| Cav2.3-reverse | TCCTGAGTTCTCTCTTCTTCATGG | |
| Cav2.3-internal | TGGCTCCTTGCTCCTGTGGCTGCT | |
| NM031103 | RPL19-forward | CTGAAGGTCAAAGGGAATGTGTTC |
| RPL19-reverse | TTCGTGCTTCCTTGGTCTTAGAC | |
| RPL19-internal | TGCGAGCCTCAGCCTGGTCAGCC |
Cav, voltage-gated calcium channel.
Immunofluorescence staining for calcium channels.
Our previous study has reported that Western blot analysis could not be used as an appropriate method to measure the protein expression of the Ca2+ channel subunits in the ICG because of the limitation of the tiny ICG tissue (37). Therefore, we used immunofluorescence staining to measure protein expression of the Ca2+ channel subtypes in the ICG and SG.
Isolated SG and ICG were postfixed in 4% paraformaldehyde, followed by soaking of the SG and ICG in 30% sucrose for 12 h at 4°C for cryostat protection. The SG and ICG were cut into 10-μm-thick sections at −20°C and then mounted on precoated glass slides. The sections were incubated with 10% donkey serum for 1 h followed by incubation with rabbit antibodies against calcium channel subtypes (Cav1.2, Cav1.3, Cav2.1, Cav2.2, or Cav2.3; Alomone Labs, Jerusalem, Israel) and goat anti-choline acetyltransferase (ChAT) antibody (a cholinergic neuronal marker for ICG, Abcam, Cambridge, MA) or mouse anti-tyrosine hydroxylase (TH) antibody (an adrenergic neuronal marker for SG, Sigma-Aldrich, St. Louis, MO) overnight at 4°C. Then the sections were incubated with fluorescence-conjugated secondary antibodies (Invitrogen, Carlsbad, CA) for 60 min at room temperature. Slides were observed under a Leica fluorescent microscope with corresponding filters. Pictures were captured by a digital camera system from one sham-operated animal and one CHF animal on the same day, and fixed exposure time was used for picture capturing of each gene. No staining was seen when PBS was used instead of the primary antibody in the above procedure.
Expression of Ca2+ channel subtypes was quantified using Adobe Photoshop CS5 (Photoshop Extended). Cells were selected using a Lasso tool. After a measurement scale was set (1 pixel = 1 pixel), the integrated density (optical density, OD) of the Ca2+ channel subtype image (red color) in each ChAT- or TH-positive cell was automatically measured by clicking record measurements in the analysis menu and normalized to the background signal (black area) in each picture. The data per rat were obtained by calculating 100 cells from 10 slices.
Isolation of ICG and SG neurons.
Isolated SG and ICG were placed in ice-cold modified Tyrode's solution (in mM): 140 NaCl, 5 KCl, 10 HEPES, and 5 glucose, respectively. The SG or ICG were minced into small pieces with microscissors and incubated for 30 min at 37°C in an enzymatic modified Tyrode's solution containing 0.1% collagenase and 0.1% trypsin. The tissues were then transferred to a modified Tyrode's solution containing 0.2% collagenase and 0.5% bovine serum albumin for 30 min of incubation at 37°C. The isolated cells from SG or ICG were cultured at 37°C in a humidified atmosphere of 95% air-5% CO2 for 4 to 8 h before the patch-clamp experiments.
Whole cell recording for calcium currents and action potentials.
Cav currents and action potential were recorded only in DiI-labeled CPP or CPS neurons by the whole cell patch-clamp technique using Axopatch 200B patch-clamp amplifier (Axon Instruments) (37).
In the voltage-clamp experiment, resistance of the patch pipette was 4–6 MΩ when filled with the following solution (in mM): 120 CsCl, 1 CaCl2, 40 HEPES, 11 EGTA, 4 MgATP, 0.3 Tris-GTP, 14 creatine phosphate, and 0.1 leupeptin (pH 7.3; 305 mosM). The extracellular solution consisted of (in mM): 140 TEA-Cl, 5 BaCl2, 1 MgCl2, 10 HEPES, 0.001 TTX, 2 4-AP, and 10 glucose (pH 7.4; 310 mosM). Series resistance of 5–13 MΩ was electronically compensated 30–80%. Junction potential was calculated to be +7.9 mV using pCLAMP 10.2 software, and all values of membrane potential given throughout were corrected using this value. Current traces were sampled at 10 kHz and filtered at 5 kHz. The holding potential was −80 mV and current-voltage (I–V) relationships were elicited by 5-mV step increments to potentials between −60 and 60 mV for 500 ms. Peak currents were measured for each test potential, and current density was calculated by dividing peak current by cell membrane capacitance (32.9 ± 2.9 pF in sham and 30.6 ± 2.8 pF in CHF CPP neurons, P > 0.05; 22.7 ± 2.8 pF in sham and 23.3 ± 2.4 pF in CHF CPS neurons, P > 0.05).
In the current-clamp experiments, action potential was elicited by a ramp current injection of 0–100 pA and the current threshold-inducing action potential or threshold potential was measured at the beginning of the first action potential. Frequency of action potentials was measured in a 1-s current clamp. Input resistance was determined from the linear fit of the neuronal voltage response to hyperpolarizing current injections (20-pA step decrement from 0 to −100 pA for 1 s). The patch pipette solution was composed of (in mM) 105 K-aspartate, 20 KCl, 1 CaCl2, 5 MgATP, 10 HEPES, 10 EGTA, and 25 glucose (pH 7.2; 320 mosM). The bath solution was composed of (in mM) 140 NaCl, 5.4 KCl, 0.5 MgCl2, 2.5 CaCl2, 5.5 HEPES, 11 glucose, and 10 sucrose (pH 7.4; 330 mosM). Junction potential was calculated to be +12.3 mV and membrane potential was corrected using this value. P-clamp 10.2 program (Axon Instruments) was used for data acquisition and analysis. All experiments were done at room temperature (22–24°C).
Compounds.
ω-Conotoxin GVIA (a specific N-type Ca2+ channel blocker, Alomone Labs) was used in the electrophysiological experiments. Based on the previous study (31) and our preliminary data, the concentration of ω-conotoxin GVIA (1 μM) used in the present study is a saturating concentration for inhibiting N-type Ca2+ channels.
Data analysis.
All data are presented as means ± SE. SigmaPlot 12 was used for data analysis. Statistical significance was determined by Student's unpaired t-test for hemodynamic, expression of Ca2+ channel subtypes. A two-way ANOVA with post hoc Bonferroni test was used in the electrophysiological parameters. Statistical significance was accepted when P < 0.05.
RESULTS
Hemodynamic and morphological characteristics of sham and CHF rats.
Table 2 summarizes the hemodynamic and morphological characteristics of the sham and CHF rats. In the CHF rats, a gross examination displayed a dense scar in the anterior ventricular wall and an average myocardial infarct size over 30% of the left ventricular area. Heart weight-to-body weight ratio was significantly higher in CHF rats compared with sham rats. In addition, left ventricular end-diastolic pressure was increased in CHF rats, compared with sham rats. Heart rate was mildly increased in CHF rats (P < 0.05). These data suggest the development of CHF. However, there were no significant differences in arterial blood pressure between sham and CHF rats.
Table 2.
Hemodynamic and morphological characteristics of sham and CHF rats
| Sham | CHF | |
|---|---|---|
| n | 48 | 49 |
| Body weight, g | 403.8 ± 1.6 | 406.6 ± 2.2 |
| Mean blood pressure, mmHg | 100.5 ± 0.7 | 103.4 ± 0.8 |
| Heart rate, beats/min | 343 ± 3 | 355 ± 4* |
| Heart weight/body weight, mg/g | 3.55 ± 0.1 | 5.69 ± 0.10* |
| Infarct size, % of left ventricle | 0 | 34.9 ± 0.6* |
| LVEDP, mmHg | 1.9 ± 0.2 | 16.8 ± 0.4* |
Data are means ± SE. CHF, chronic heart failure; LVEDP, left ventricular end-diastolic pressure.
P < 0.05 vs. sham.
mRNA expression of Ca2+ channel subtypes in CPP and CPS neurons from sham and CHF rats.
CPP neurons in ICG and CPS neurons in SG were identified by DiI retrograde-labeling (see details in materials and methods). At present, five subtypes (L, P/Q, N, R, and T) of the voltage-gated Ca2+ channel α-subunits have been identified on the basis of functional properties (42). We measured the mRNA expression of L (Cav1.2 and Cav1.3), P/Q (Cav2.1), N (Cav2.2), and R (Cav2.3) subtypes of the voltage-gated Ca2+ channel α-subunits in CPP and CPS neurons from sham and CHF rats because previous studies (42, 69) have reported that the T (Cav3.1, Cav3.2, and Cav3.3) subtype of Ca2+ channels could not be detected in the rat ICG and SG.
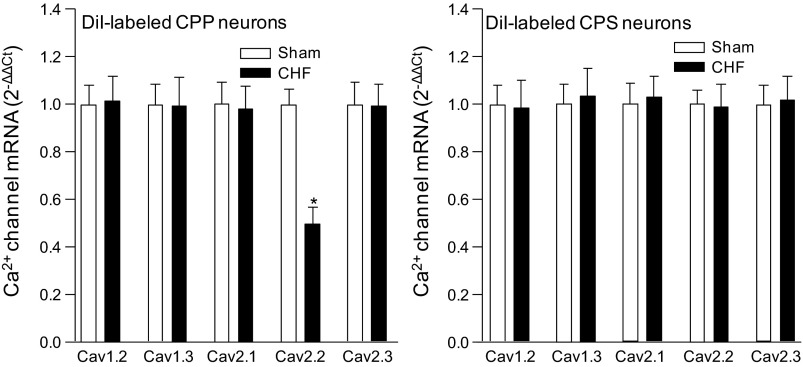
Single-cell real-time RT-PCR analysis of the Ca2+ channel α-subunits in the CPP and CPS neurons is shown in Fig. 1. The mRNA of Cav1.2, Cav1.3, Cav2.1, Cav2.2, and Cav2.3 was expressed in the CPP neurons. The mRNA expression of Cav2.2 in the CPP neurons from CHF rats was lower than that in CPP neurons from sham rats (Fig. 1). There was no significant difference in mRNA expression of the other Ca2+ channel α-subunits between sham and CHF rats.
Fig. 1.
Single-cell mRNA expression of L (Cav1.2 and Cav1.3)-, P/Q (Cav2.1)-, N (Cav2.2)-, and R (Cav2.3)-type Ca2+ channel α-subunits in the cardiac postganglionic parasympathetic (CPP) and sympathetic (CPS) neurons from sham-operated (Sham) and chronic heart failure (CHF) rats. Data are means ± SE; n = 20 neurons in each group. *P < 0.05 vs. sham rats.
In the CPS neurons, CHF did not induce the alteration in mRNA expression of the Ca2+ channel α-subunits, compared with that from sham rats (Fig. 1).
Protein expression of Ca2+ channel subtypes in ICG and SG from sham and CHF rats.
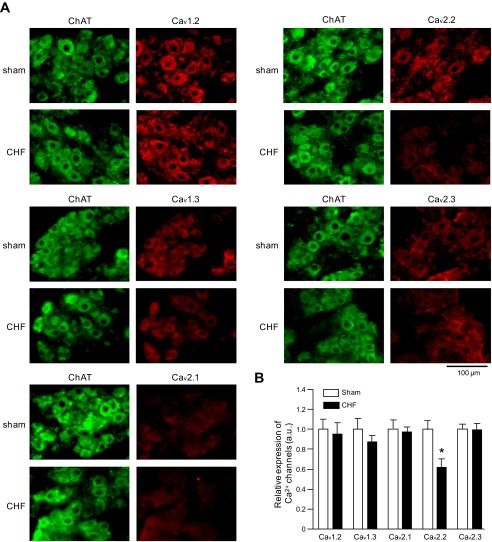
Using cholinergic neuronal marker (ChAT) and adrenergic neuronal marker (TH), we identified the postganglionic parasympathetic neurons in ICG and sympathetic neurons in SG, respectively (Figs. 2 and 3). In the postganglionic parasympathetic neurons, protein expression of voltage-gated Ca2+ channel α-subtypes (Cav1.2, Cav1.3, Cav2.1, Cav2.2, and Cav2.3) could be detected (Fig. 2). Additionally, an important finding was that CHF reduced the protein expression of N-type Ca2+ channel α-subunit (Cav2.2) but not the protein expression of other Ca2+ channel α-subtypes in the parasympathetic neurons, compared with sham parasympathetic neurons (Fig. 2).
Fig. 2.
Representative (A) and summary (B) data are shown for protein expression of the Ca2+ channel α-subunits in the intracardiac ganglia (ICG) neurons from sham and CHF rats. ChAT, choline acetyltransferase, a cholinergic neuronal marker. AU, arbitrary units. Data are means ± SE; n = 5 rats in each group (data per rat obtained by calculating 100 cells from 10 slices). *P < 0.05 vs. sham rats.
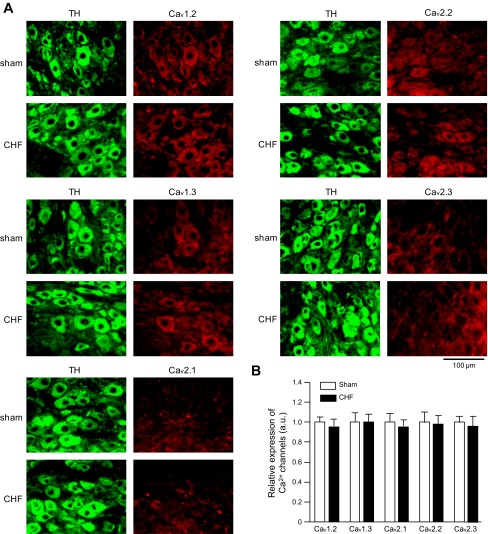
Fig. 3.
Representative (A) and summary (B) data are shown for protein expression of Ca2+ channel subtypes in the stellate ganglia neurons from sham and CHF rats. TH, tyrosine hydroxylase, an adrenergic neuronal marker. Data are means ± SE; n = 5 rats in each group (data per rat obtained by calculating 100 cells from 10 slices).
Immunofluorescence data showed that there is no significant difference in the protein expression of voltage-gated Ca2+ channel α-subtypes in sympathetic neurons between sham and CHF rats (P > 0.05, Fig. 3).
Ca2+ currents and cell excitability in CPP neurons from sham and CHF rats.
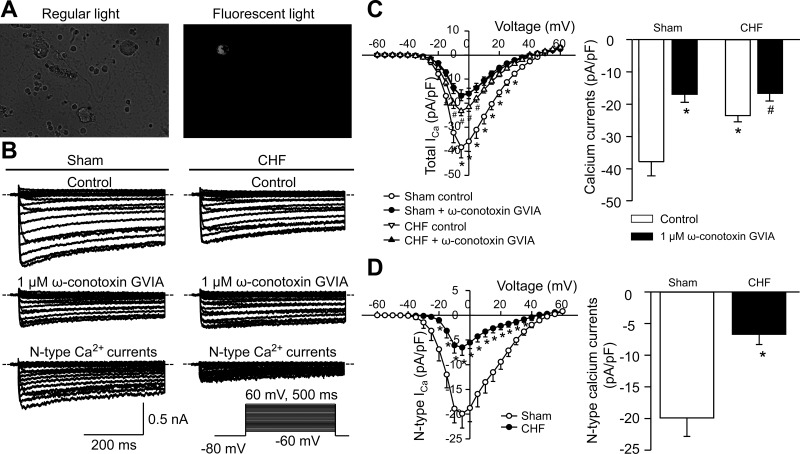
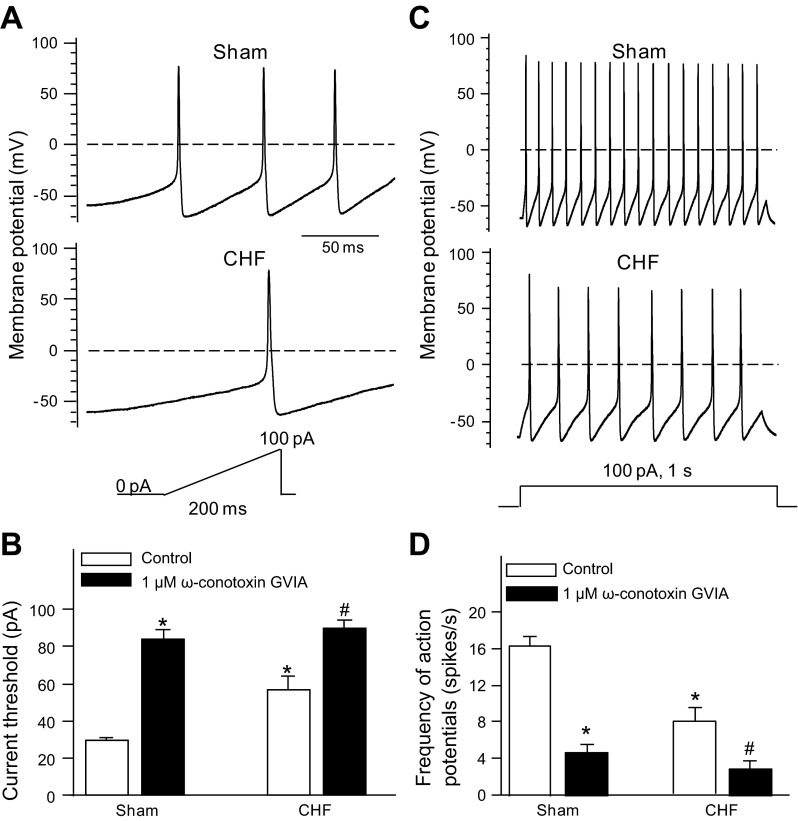
As shown in Fig. 4A, CPP neurons were labeled by DiI and identified by the microscope with fluorescent filter. Ca2+ currents (Fig. 4) and action potentials (Fig. 5) were recorded in the CPP neurons from sham and CHF rats. CHF markedly reduced total Ca2+ currents in the CPP neurons, compared with sham conditions (Fig. 4, B and C). The saturating concentration of a specific N-type Ca2+ channel blocker (1 μM ω-conotoxin GVIA) significantly decreased Ca2+ currents to the same level in the CPP neurons from sham and CHF rats, which suggests that there is no significant difference in L-, P/Q-, and R-type Ca2+ currents of the CPP neurons between sham and CHF rats (Fig. 4, B and C). The N-type Ca2+ currents were obtained by subtracting the Ca2+ currents under treatment of ω-conotoxin GVIA from total Ca2+ currents (control, Fig. 4B). N-type Ca2+ currents were lower in the CPP neurons from CHF rats (Fig. 4D). Electrophysiological data further confirmed the single-cell real-time RT-PCR and immunofluorescent results (Figs. 1 and 2) that CHF lowered the function of only the N-type Ca2+ channels in the CPP neurons.
Fig. 4.
A: DiI-labeled ICG neuron (cardiac postganglionic vagal neuron) with white color in fluorescent light. B–D: original recording (B), total Ca2+ current density (ICa; C), and N-type Ca2+ current density (D) in the cardiac postganglionic vagal neurons from sham and CHF rats. Dotted lines indicate the zero current level in B. The N-type Ca2+ current density was obtained by subtracting the Ca2+ currents under treatment of ω-conotoxin GVIA from total Ca2+ currents (control). Mean data for Ca2+ currents elicited by 500-ms test pulse at −5 mV from holding potential of −80 mV are shown in C and D. ω-Conotoxin GVIA, a specific N-type Ca2+ channel blocker. Data are means ± SE; n = 9 neurons in each group. *P < 0.05 vs. sham control; #P < 0.05 vs. CHF control.
Fig. 5.
Current threshold-inducing action potential (A and B) and frequency of action potentials (C and D) are shown before and after treatment of ω-conotoxin GVIA (1 μM) in the cardiac postganglionic vagal neurons from sham and CHF rats. Data are means ± SE, n = 9 neurons in each group. *P < 0.05 vs. sham control; #P < 0.05 vs. CHF control.
We measured current threshold-inducing action potential and frequency of action potentials to compare cell excitability of the CPP neurons between sham and CHF rats (Fig. 5). The current threshold-inducing action potential was enhanced in the CPP neurons from CHF rats (56.9 ± 6.9 pA; P < 0.05), compared with the CPP neurons from sham rats (29.3 ± 1.6 pA, Fig. 5, A and B). ω-Conotoxin GVIA (1 μM) significantly raised the current threshold-inducing action potential in the CPP neurons from sham and CHF rats (Fig. 5B). Anther parameter of cell excitability, frequency of action potentials, is lower in the CPP neurons from CHF rats (7.8 ± 1.6 spikes/s) than that in sham neurons (16.4 ± 1.0 spikes/s; P < 0.05; Fig. 5, C and D). ω-Conotoxin GVIA (1 μM) markedly decreased the frequency of action potentials in CPP neurons from sham and CHF (Fig. 5D).
Additionally, CHF also slowed down the maximum rate of depolarization of action potentials, lengthened the action potential duration at 90% repolarization, and caused the threshold potential to become less negative in the CPP neurons (Table 3). However, there was no significant difference in resting membrane potential, input resistance, overshoot of the action potential, and afterhyperpolarization amplitude in the CPP neurons between sham and CHF rats (Table 3).
Table 3.
CHF-induced electrophysiological changes on action potentials in rat cardiac postganglionic parasympathetic and sympathetic neurons
| RMP, mV | Ri, GΩ | Vmax, mV/ms | Overshoot, mV | AHP Amplitude, mV | APD90, ms | Threshold Potential, mV | |
|---|---|---|---|---|---|---|---|
| CPP neurons | |||||||
| Sham | −59.8 ± 2.0 | 0.80 ± 0.09 | 149.5 ± 6.6 | 78.3 ± 3.9 | 11.2 ± 1.0 | 3.9 ± 0.2 | −36.0 ± 1.5 |
| Sham + ω-conotoxin GVIA | −60.6 ± 2.4 | 0.77 ± 0.12 | 91.4 ± 8.9* | 75.3 ± 3.5 | 9.7 ± 1.5 | 5.1 ± 0.3* | −22.2 ± 1.6* |
| CHF | −60.4 ± 1.8 | 0.62 ± 0.11 | 111.0 ± 3.8* | 75.5 ± 2.4 | 9.7 ± 1.5 | 5.0 ± 0.5* | −25.2 ± 1.4* |
| CHF + ω-conotoxin GVIA | −61.2 ± 2.7 | 0.60 ± 0.06 | 81.9 ± 4.3# | 74.9 ± 3.7 | 8.6 ± 0.8 | 5.6 ± 0.4 | −19.8 ± 2.4# |
| CPS neurons | |||||||
| Sham | −53.5 ± 2.1 | 0.88 ± 0.10 | 124.4 ± 8.1 | 79.6 ± 2.4 | 10.4 ± 1.2 | 3.7 ± 0.1 | −26.1 ± 1.0 |
| Sham + ω-conotoxin GVIA | −53.1 ± 2.1 | 0.84 ± 0.13 | 93.6 ± 7.4* | 77.6 ± 3.3 | 9.1 ± 1.2 | 4.6 ± 0.2* | −19.7 ± 1.3* |
| CHF | −53.0 ± 2.1 | 1.05 ± 0.20 | 164.5 ± 11.2* | 81.2 ± 2.0 | 12.4 ± 2.1 | 3.1 ± 0.2* | −33.0 ± 2.0* |
| CHF + ω-conotoxin GVIA | −52.3 ± 2.0 | 1.00 ± 0.12 | 102.0 ± 11.7# | 78.5 ± 2.2 | 10.8 ± 0.9 | 4.3 ± 0.3# | −22.5 ± 1.0# |
Data are means ± SE. CPP, cardiac postganglionic parasympathetic neurons; CPS, cardiac postganglionic sympathetic neurons; RMP, resting membrane potential; Ri, input resistance; Vmax, maximum rate of depolarization of action potentials; AHP, afterhyperpolarization; APD90, action potential duration at 90% repolarization. n = 9 cells/group for CPP neurons and n = 8 cells/group for CPS neurons.
P < 0.05 vs. Sham;
P < 0.05 vs. CHF.
Ca2+ currents and cell excitability in the CPS neurons from sham and CHF rats.
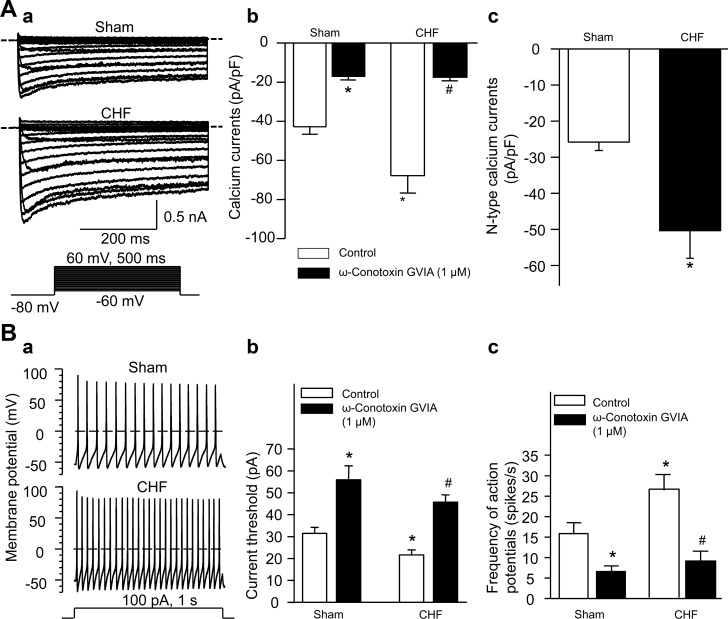
Similarly, we also measured the Ca2+ currents and cell excitability in the CPS neurons from sham and CHF rats (Fig. 6). Total Ca2+ currents were increased in the CPS neurons from CHF rats, compared with the CPS neurons from sham rats (Fig. 6, A–a and A–b). ω-Conotoxin GVIA (1 μM) inhibited the Ca2+ currents to the same level in the CPS neurons from sham and CHF rats (Fig. 6, A–b), which suggests that there is no significant difference in L-, P/Q-, and R-type Ca2+ currents of the CPS neurons between sham and CHF rats. Similarly, the N-type Ca2+ currents were obtained by subtracting the Ca2+ currents under treatment of ω-conotoxin GVIA from total Ca2+ currents (control). CHF increased the N-type Ca2+ currents in the CPS neurons, compared with sham neurons (Fig. 6, A–c).
Fig. 6.
A: original recording (a), total Ca2+ current density (b), and N-type Ca2+ current density (c) in the cardiac postganglionic sympathetic neurons (DiI-labeled stellate ganglia neurons) from sham and CHF rats. Dotted lines indicate the zero current level in A–a. The N-type Ca2+ current density was obtained by subtracting the Ca2+ currents under treatment of ω-conotoxin GVIA from total Ca2+ currents (control). Mean data for Ca2+ currents elicited by 500-ms test pulse at 0 mV from holding potential of −80 mV are shown in A–b and A–c. B: original recording of action potentials (a), current threshold-inducing action potential (b), and frequency of action potentials (c) in the cardiac postganglionic sympathetic neurons from sham and CHF rats. ω-Conotoxin GVIA, a specific N-type Ca2+ channel blocker. Data are means ± SE; n = 8 neurons in each group. *P < 0.05 vs. sham control; #P < 0.05 vs. CHF control.
CHF decreased current threshold-inducing action potential and increased frequency of action potentials in the CPS neurons (21.6 ± 2.4 pA and 26.3 ± 3.9 spikes/s), compared with the CPS neurons from sham rats (31.4 ± 2.8 pA and 15.9 ± 2.8 spikes/s; P < 0.05; Fig. 6B). ω-Conotoxin GVIA (1 μM) raised current threshold-inducing action potential and lowered frequency of action potentials in the CPS neurons from sham and CHF rats (Fig. 6, B–b and B–c).
Furthermore, CHF increased the maximum rate of depolarization of action potentials, shortened the action potential duration at 90% repolarization, and caused the threshold potential to shift to more negative in the CPS neurons (Table 3). However, CHF did not significantly alter the resting membrane potential, input resistance, overshoot of the action potential, and afterhyperpolarization amplitude in the CPS neurons (Table 3).
DISCUSSION
The findings in the present study are that 1) CHF significantly reduced mRNA, protein, and current density of the N-type Ca2+ channels and lowered the cell excitability in the CPP neurons (Figs. 1, 2, 4, and 5); and 2) although CHF did not affect mRNA and protein expression of the voltage-gated Ca2+ channels, CHF markedly increased current density of the N-type Ca2+ channels and cell excitability in the CPS neurons (Figs. 1, 3, and 6). These results suggest that CHF can induce molecular and cellular alterations of the CPP and CPS neurons. The changes of CPP and CPS neurons might contribute to the withdrawal of cardiac vagal activity and the sympathetic hyperactivity reported in the CHF state (6, 15, 23, 49, 53, 56, 61).
Parasympathetic nervous system and dysfunction of CPP neurons in CHF.
Although abundant evidence shows that overactivation of the sympathetic nervous system is associated with a worse outcome in CHF patients (46), involvement of the disordered parasympathetic function in disease progression and prognosis of CHF patients is only beginning to be recognized (44, 45).
The preganglionic parasympathetic (vagal) fibers originate within the central nervous system at the level of the brainstem (nucleus ambiguous and nucleus tractus solitaries). In the heart, the axons of these preganglionic vagal efferents extend to the ICG located in cardiac fat pads to form synapses with the CPP neurons (44, 60). In general, as a final common pathway for the vagal control of the cardiac function, the CPP neurons can generate action potentials to activate acetylcholine release (a primary neurotransmitter involved in the vagal innervation of the heart) after receiving and integrating neurohumoral inputs from cardiac preganglionic vagal efferents (2, 3, 70). Acetylcholine released from CPP neuron terminals can bind to muscarinic acetylcholine receptors to regulate cardiac function (2, 60). Our previous study has demonstrated that baroreflex sensitivity is attenuated in coronary artery ligation-induced CHF rats(63). Additionally, parasympathetic activation and its physiological role in the heart are blunted in CHF state (8, 44). The results from CHF dogs indicate that CHF-blunted vagal control of the heart is due to the abnormal peripheral efferent limb of the parasympathetic nervous system, probably a defect at the ganglion level (7). In the present study, we found that the N-type Ca2+ channel expression and activation and the cell excitability of the CPP neurons were decreased in CHF rats (Figs. 1, 2, 4, and 5). Therefore, our data further provide the evidence that the decreased CPP neuron excitability might link to the blunted vagal control of the heart in CHF state. However, we did not measure the cardiac postganglionic vagal activity in the present study because very short cardiac postganglionic vagal fiber prevents us from a direct-recording of the nerve activity. Therefore, further studies are needed to clarify the correlation between the CPP neuron dysfunction and the blunted cardiac vagal tone in CHF.
In the present study, we found that repetitive action potentials were elicited in all CPP neurons from adult rats in response to a depolarizing current injection (100 pA, 1 s; Fig. 5). However, at least two firing patterns (phasic- and tonic-firing) in rat ICG neurons have been observed by other studies (58, 68). This discrepancy could be due to one or more of the following reasons. 1) The ICG plexus is complex and contains CPP neurons, interneurons, and local circuit neurons (2, 3, 21, 30, 51). Only CPP neurons (DiI-labeled ICG neurons) were used for the present study, whereas all types of the ICG neurons were employed in previous studies (58, 68). 2) The majority of adult rat ICG neurons are tonic firing, whereas just 15% of neonatal rat ICG neurons showed the repetitive action potentials in response to sustained depolarizing currents injection (16). The CPP neurons were obtained from adults rats in the present study; however, the action potential was recorded in neonatal rat ICG neurons in Cuevas's study (68). 3) The firing rate of ICG neurons is much higher at 22°C than at 37°C (16). Our whole cell patch-clamp recording for the action potential was done at 22–24°C. 4) Animal species and experimental conditions (such as in vivo or in vitro electrophysiological recording) possibly are the influencing factors. Additionally, it has been observed that the ability of local-circuit neurons to regulate cardiodynamics was impaired in the canines with pacing-induced early-stage heart failure (4). Combining with the results in the present study, we consider that all types of the ICG neurons were impacted by CHF. Therefore, care should be taken when extrapolating the data obtained in the present study to in vivo experiments and clinical analyses.
Voltage-gated Ca2+ channels are involved in the neuronal excitability and the release of neurotransmitters including acetylcholine (1, 5, 11, 74). CHF reduced the N-type Ca2+ channel mRNA level and protein expression but did not affect other type Ca2+ channel mRNA levels and protein expressions in the CPP neurons, compared with sham rats (Figs. 1 and 2). The patch-clamp data also showed that CHF only induced a decline of the N-type Ca2+ currents (Fig. 4), which was associated with lowered cell excitability in the CPP neurons from CHF rats (Fig. 5). These data indicate that CHF modulates the transcription and/or translation of the N-type Ca2+ channels to lower the N-type Ca2+ currents. Our previous study has shown that type 2 diabetes also decreases expression and current density of the N-type Ca2+ channels in the CPP neurons (37). From these data, we consider that CHF and type 2 diabetes might share the same endogenous signaling pathway to trigger the alteration of the N-type Ca2+ channels, which should be evaluated in further study.
Sympathetic nervous system and alterations of CPS neurons in CHF.
In contrast to the parasympathetic nervous system, the sympathetic nervous system is overactivated by CHF (15, 23, 49, 53, 56, 61). Data from the direct-recording of the cardiac postganglionic sympathetic nerve activity showed that CHF significantly enhanced the cardiac sympathetic nerve activity (data not shown), which further confirms that the cardiac sympathetic hyperactivity is observed in CHF state. Much evidence demonstrates that the activity of sympathetic nerves is determined by central neurons in the medulla oblongata that receive inputs from central and peripheral sensory afferents (28, 60). However, peripheral CPS neurons cannot be ignored. These neurons also influence the cardiac sympathetic nerve activity because they represent a key relay station for the sympathetic nervous system (27). Our present results demonstrate that the N-type Ca2+ currents and cell excitability were increased in CPS neurons from CHF rats (Fig. 6). Therefore, we believe that overexcitability of the CPS neurons probably links to the cardiac sympathetic nerve hyperactivity in CHF rats.
Although CHF increased the N-type Ca2+ currents in the CPS neurons, CHF did not affect mRNA level and protein expression of the Ca2+ channel α-subtypes in these neurons (Figs. 1 and 3). The results suggest that CHF-enhanced N-type Ca2+ currents are not due to transcriptional and translational modulations of Ca2+ channel α-subtypes in the CPS neurons from CHF rats. Overactivation of the N-type Ca2+ channels is possibly induced by some posttranslational modulations, such as phosphorylation of the N-type Ca2+ channels (59) and allosteric modulation of ion channels (20). Further study is needed to clarify the mechanism(s) responsible for CHF-enhanced N-type Ca2+ currents in the CPS neurons.
Other considerations.
We cannot confirm the protein expression of Ca2+ channel subtypes in the CPP and CPS neurons due to the limitation of the method (DiI labeling was lost from cells during the immunofluorescent staining procedure). However, single-cell real-time RT-PCR and electrophysiological data were obtained in the CPP and CPS neurons (DiI-labeled neurons). Additionally, the CPP or CPS neurons are a component of the ICG or SG neurons. It is reasonable to assume that our measurement in the present study represents the protein expression of Ca2+ channel subtypes in the CPP and CPS neurons.
Although we used a transported florescent dye (red color DiI) to retrograde-label cardiac sympathetic and vagal neurons, it is possible that DiI could affect the cell excitability in ICG and SG neurons. Using isolated neurons, we checked this possibility and found that DiI did not affect the cell excitability in the ICG and SG neurons (data not shown).
We realize that voltage-gated Na+ and K+ channels also are key factors in the initiation and propagation of action potential. In the present study, CHF caused the changes in the maximum rate of depolarization of action potentials and the action potential duration at 90% repolarization in the CPP and CPS neurons (Table 3). One possibility is that CHF also induces the alterations of voltage-gated Na+ and K+ channels in the CPP and CPS neurons. Further study is needed to evaluate this.
One limitation in the present study is that we could not distinctly separate the neurons from different parts of the heart (atria and ventricles) although we retrograde-labeled the neurons projecting to the heart. It is possible that the neurons projecting to different parts of the heart are heterogeneous. This issue should be addressed in further study.
In summary, CHF decreases the N-type Ca2+ channel currents and the cell excitability in the CPP neurons, whereas CHF increases the N-type Ca2+ currents and the cell excitability in the CPS neurons. These data suggest that CHF can induce the different alterations in the different peripheral ganglionic neurons through the different signaling transduction pathways. More importantly, CHF-induced alterations in the cardiac postganglionic vagal and sympathetic neurons might be involved in the cardiac vagal hypoactivity and sympathetic hyperactivity in CHF state. The findings provide potential therapeutics such as pharmaceutical and genetic therapies targeting N-type Ca2+ channels. The potential therapeutics can reduce mortality through improving cardiac vagal and sympathetic tone and suppressing the fatal ventricular tachyarrhythmia.
GRANTS
This study was supported in part by National Heart, Lung, and Blood Institute Grant HL-098503 (to Y. L. Li).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.T., J.L., D.Z., and Y.L.L. conception and design of research; H.T., J.L., D.Z., and K.G.C. performed experiments; H.T., J.L., D.Z., H.Z., K.P.P., K.G.C., W.Z.W., R.L.M., and Y.L.L. analyzed data; H.T., J.L., D.Z., H.Z., K.P.P., K.G.C., W.Z.W., R.L.M., and Y.L.L. interpreted results of experiments; H.T., J.L., D.Z., and Y.L.L. prepared figures; H.T., J.L., D.Z., H.Z., K.P.P., K.G.C., W.Z.W., R.L.M., and Y.L.L. approved final version of manuscript; Y.L.L. drafted manuscript; Y.L.L. edited and revised manuscript.
ACKNOWLEDGMENTS
The authors thank Xuefei Liu and Kaye Talbitzer for technical assistance.
Footnotes
This article is the topic of an Editorial Focus by Javier Cuevas (16a).
REFERENCES
- 1.Akiyama T, Yamazaki T. Adrenergic inhibition of endogenous acetylcholine release on postganglionic cardiac vagal nerve terminals. Cardiovasc Res 46: 531–538, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Armour JA. Intrinsic cardiac neurons. J Cardiovasc Electrophysiol 2: 331–341, 1991 [Google Scholar]
- 3.Armour JA. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp Physiol 93: 165–176, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Arora RC, Cardinal R, Smith FM, Ardell JL, Dell'Italia LJ, Armour JA. Intrinsic cardiac nervous system in tachycardia induced heart failure. Am J Physiol Regul Integr Comp Physiol 285: R1212–R1223, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Augustine GJ. How does calcium trigger neurotransmitter release? Curr Opin Neurobiol 11: 320–326, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Benedict CR, Johnstone DE, Weiner DH, Bourassa MG, Bittner V, Kay R, Kirlin P, Greenberg B, Kohn RM, Nicklas JM. Relation of neurohumoral activation to clinical variables and degree of ventricular dysfunction: a report from the Registry of Studies of Left Ventricular Dysfunction. SOLVD Investigators. J Am Coll Cardiol 23: 1410–1420, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Bibevski S, Dunlap ME. Ganglionic mechanisms contribute to diminished vagal control in heart failure. Circulation 99: 2958–2963, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Bibevski S, Dunlap ME. Evidence for impaired vagus nerve activity in heart failure. Heart Fail Rev 16: 129–135, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Blomquist TM, Priola DV, Romero AM. Source of intrinsic innervation of canine ventricles: a functional study. Am J Physiol Heart Circ Physiol 252: H638–H644, 1987 [DOI] [PubMed] [Google Scholar]
- 10.Boogers MJ, Veltman CE, Bax JJ. Cardiac autonomic nervous system in heart failure: imaging technique and clinical implications. Curr Cardiol Rev 7: 35–42, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borst JG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature 383: 431–434, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Brack KE, Coote JH, Ng GA. Vagus nerve stimulation protects against ventricular fibrillation independent of muscarinic receptor activation. Cardiovasc Res 91: 437–446, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Brack KE, Winter J, Ng GA. Mechanisms underlying the autonomic modulation of ventricular fibrillation initiation-tentative prophylactic properties of vagus nerve stimulation on malignant arrhythmias in heart failure. Heart Fail Rev 18: 389–408, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colucci WS, Packer M, Bristow MR, Gilbert EM, Cohn JN, Fowler MB, Krueger SK, Hershberger R, Uretsky BF, Bowers JA, Sackner-Bernstein JD, Young ST, Holcslaw TL, Lukas MA. Carvedilol inhibits clinical progression in patients with mild symptoms of heart failure. US Carvedilol Heart Failure Study Group. Circulation 94: 2800–2806, 1996 [DOI] [PubMed] [Google Scholar]
- 15.Creager MA, Faxon DP, Cutler SS, Kohlmann O, Ryan TJ, Gavras H. Contribution of vasopressin to vasoconstriction in patients with congestive heart failure: comparison with the renin-angiotensin system and the sympathetic nervous system. J Am Coll Cardiol 7: 758–765, 1986 [DOI] [PubMed] [Google Scholar]
- 16.Cuevas J, Harper AA, Trequattrini C, Adams DJ. Passive and active membrane properties of isolated rat intracardiac neurons: regulation by H- and M-currents. J Neurophysiol 78: 1890–1902, 1997 [DOI] [PubMed] [Google Scholar]
- 16a.Cuevas J. Molecular mechanisms of dysautonomia during heart failure Focus on “Heart failure-induced changes of voltage-gated Ca2+ channels and cell excitability in rat cardiac postganglionic neurons”. Am J Physiol Cell Physiol (October 9, 2013). 10.1152/ajpcell.00311.2013 [DOI] [PubMed] [Google Scholar]
- 17.Cygankiewicz I, Zareba W, Vazquez R, Vallverdu M, Gonzalez-Juanatey JR, Valdes M, Almendral J, Cinca J, Caminal P, de Luna AB. Heart rate turbulence predicts all-cause mortality and sudden death in congestive heart failure patients. Heart Rhythm 5: 1095–1102, 2008 [DOI] [PubMed] [Google Scholar]
- 18.De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, Gavazzi A, Sanzo A, Dennert R, Kuschyk J, Raspopovic S, Klein H, Swedberg K, Schwartz PJ. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 32: 847–855, 2011 [DOI] [PubMed] [Google Scholar]
- 19.De Ferrari GM, Schwartz PJ. Vagus nerve stimulation: from pre-clinical to clinical application: challenges and future directions. Heart Fail Rev 16: 195–203, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Dilly S, Lamy C, Marrion NV, Liegeois JF, Seutin V. Ion-channel modulators: more diversity than previously thought. Chembiochem 12: 1808–1812, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Edwards FR, Hirst GD, Klemm MF, Steele PA. Different types of ganglion cell in the cardiac plexus of guinea-pigs. J Physiol 486: 453–471, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiuzat M, Wojdyla D, Kitzman D, Fleg J, Keteyian SJ, Kraus WE, Pina IL, Whellan D, O'Connor CM. Relationship of beta-blocker dose with outcomes in ambulatory heart failure patients with systolic dysfunction: results from the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial. J Am Coll Cardiol 60: 208–215, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol 54: 375–385, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Frenneaux MP. Autonomic changes in patients with heart failure and in post-myocardial infarction patients. Heart 90: 1248–1255, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gheorghiade M, Colucci WS, Swedberg K. Beta-blockers in chronic heart failure. Circulation 107: 1570–1575, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation 127: e6–e245, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golovac S, Mathis JM. Autonomic nerve blockade. In: Image-Guided Spine Interventions, edited by Mathis JM, Golovac S. New York: Springer, 2010 [Google Scholar]
- 28.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Hauptman PJ, Schwartz PJ, Gold MR, Borggrefe M, Van Veldhuisen DJ, Starling RC, Mann DL. Rationale and study design of the increase of vagal tone in heart failure study: INOVATE-HF. Am Heart J 163: 954–962, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Horackova M, Armour JA. Role of peripheral autonomic neurones in maintaining adequate cardiac function. Cardiovasc Res 30: 326–335, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Jeong SW, Wurster RD. Calcium channel currents in acutely dissociated intracardiac neurons from adult rats. J Neurophysiol 77: 1769–1778, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Kishi T. Heart failure as an autonomic nervous system dysfunction. J Cardiol 59: 117–122, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 59: 256–262, 1987 [DOI] [PubMed] [Google Scholar]
- 34.Klein HU, Ferrari GM. Vagus nerve stimulation: a new approach to reduce heart failure. Cardiol J 17: 638–644, 2010 [PubMed] [Google Scholar]
- 35.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 109: 120–124, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Li YL, Zheng H. Angiotensin II-NADPH oxidase-derived superoxide mediates diabetes-attenuated cell excitability of aortic baroreceptor neurons. Am J Physiol Cell Physiol 301: C1368–C1377, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Tu H, Zheng H, Zhang L, Tran TP, Muelleman RL, Li YL. Alterations of calcium channels and cell excitability in intracardiac ganglion neurons from type 2 diabetic rats. Am J Physiol Cell Physiol 302: C1119–C1127, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Nevzorov R, Porath A, Henkin Y, Kobal SL, Jotkowitz A, Novack V. Effect of beta blocker therapy on survival of patients with heart failure and preserved systolic function following hospitalization with acute decompensated heart failure. Eur J Intern Med 23: 374–378, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Ng GA, Brack KE, Patel VH, Coote JH. Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovasc Res 73: 750–760, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen M, Baig W, Flapan AD, Cowley A, Prescott RJ, Neilson JM, Fox KA. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart). Circulation 98: 1510–1516, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Nooney JM, Lambert RC, Feltz A. Identifying neuronal non-L Ca2+ channels–more than stamp collecting? Trends Pharmacol Sci 18: 363–371, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Obias-Manno D, Wijetunga M. Risk stratification and primary prevention of sudden cardiac death: sudden death prevention. AACN Clin Issues 15: 404–418, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Olshansky B, Sabbah HN, Hauptman PJ, Colucci WS. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation 118: 863–871, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Osterziel KJ, Dietz R. Improvement of vagal tone by ACE inhibition: a mechanism of cardioprotection in patients with mild-to-moderate heart failure. J Cardiovasc Pharmacol 27: S25–S30, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol 20: 248–254, 1992 [DOI] [PubMed] [Google Scholar]
- 47.Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US Carvedilol Heart Failure Study Group. N Engl J Med 334: 1349–1355, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Pardini BJ, Patel KP, Schmid PG, Lund DD. Location, distribution and projections of intracardiac ganglion cells in the rat. J Auton Nerv Syst 20: 91–101, 1987 [DOI] [PubMed] [Google Scholar]
- 49.Porter TR, Eckberg DL, Fritsch JM, Rea RF, Beightol LA, Schmedtje JF, Jr, Mohanty PK. Autonomic pathophysiology in heart failure patients Sympathetic-cholinergic interrelations. J Clin Invest 85: 1362–1371, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Priola DV. Intrinsic innervation of the canine heart. Effects on conduction in the atrium, atrioventricular node, and proximal bundle branch. Circ Res 47: 74–79, 1980 [DOI] [PubMed] [Google Scholar]
- 51.Richardson RJ, Grkovic I, Anderson CR. Immunohistochemical analysis of intracardiac ganglia of the rat heart. Cell Tissue Res 314: 337–350, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Sabbah HN, Ilsar I, Zaretsky A, Rastogi S, Wang M, Gupta RC. Vagus nerve stimulation in experimental heart failure. Heart Fail Rev 16: 171–178, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saul JP, Arai Y, Berger RD, Lilly LS, Colucci WS, Cohen RJ. Assessment of autonomic regulation in chronic congestive heart failure by heart rate spectral analysis. Am J Cardiol 61: 1292–1299, 1988 [DOI] [PubMed] [Google Scholar]
- 54.Schultz HD, Li YL, Ding Y. Arterial chemoreceptors and sympathetic nerve activity: implications for hypertension and heart failure. Hypertension 50: 6–13, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Schwartz PJ. Vagal stimulation for the treatment of heart failure: a translational success story. Heart 98: 1687–1689, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Schwartz PJ, De Ferrari GM. Sympathetic-parasympathetic interaction in health and disease: abnormalities and relevance in heart failure. Heart Fail Rev 16: 101–107, 2011 [DOI] [PubMed] [Google Scholar]
- 57.Schwartz PJ, De Ferrari GM, Sanzo A, Landolina M, Rordorf R, Raineri C, Campana C, Revera M, Ajmone-Marsan N, Tavazzi L, Odero A. Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Fail 10: 884–891, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Selyanko AA. Membrane properties and firing characteristics of rat cardiac neurones in vitro. J Auton Nerv Syst 39: 181–189, 1992 [DOI] [PubMed] [Google Scholar]
- 59.Su SC, Seo J, Pan JQ, Samuels BA, Rudenko A, Ericsson M, Neve RL, Yue DT, Tsai LH. Regulation of N-type voltage-gated calcium channels and presynaptic function by cyclin-dependent kinase 5. Neuron 75: 675–687, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas GD. Neural control of the circulation. Adv Physiol Educ 35: 28–32, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 54: 1747–1762, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Tu H, Liu J, Zhu Z, Zhang LB, Pipinos II, Li YL. Mitochondria-derived superoxide and voltage-gated sodium channels in baroreceptor neurons from chronic heart failure rats. J Neurophysiol 107: 591–602, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tu H, Zhang L, Tran TP, Muelleman RL, Li YL. Reduced expression and activation of voltage-gated sodium channels contributes to blunted baroreflex sensitivity in heart failure rats. J Neurosci Res 88: 3337–3349, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wallick DW, Martin PJ. Separate parasympathetic control of heart rate and atrioventricular conduction of dogs. Am J Physiol Heart Circ Physiol 259: H536–H542, 1990 [DOI] [PubMed] [Google Scholar]
- 65.Wallis D, Watson AH, Mo N. Cardiac neurones of autonomic ganglia. Microsc Res Tech 35: 69–79, 1996 [DOI] [PubMed] [Google Scholar]
- 66.Wang W, Chen JS, Zucker IH. Carotid sinus baroreceptor sensitivity in experimental heart failure. Circulation 81: 1959–1966, 1990 [DOI] [PubMed] [Google Scholar]
- 67.Wang W, Chen JS, Zucker IH. Carotid sinus baroreceptor reflex in dogs with experimental heart failure. Circ Res 68: 1294–1301, 1991 [DOI] [PubMed] [Google Scholar]
- 68.Xu ZJ, Adams DJ. Resting membrane potential and potassium currents in cultured parasympathetic neurones from rat intracardiac ganglia. J Physiol 456: 405–424, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu ZJ, Adams DJ. Voltage-dependent sodium and calcium currents in cultured parasympathetic neurones from rat intracardiac ganglia. J Physiol 456: 425–441, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang H, Cuevas J. Sigma receptors inhibit high-voltage-activated calcium channels in rat sympathetic and parasympathetic neurons. J Neurophysiol 87: 2867–2879, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Zheng C, Li M, Inagaki M, Kawada T, Sunagawa K, Sugimachi M. Vagal stimulation markedly suppresses arrhythmias in conscious rats with chronic heart failure after myocardial infarction. Conf Proc IEEE Eng Med Biol Soc 7: 7072–7075, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Zucker IH, Schultz HD, Patel KP, Wang W, Gao L. Regulation of central angiotensin type 1 receptors and sympathetic outflow in heart failure. Am J Physiol Heart Circ Physiol 297: H1557–H1566, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zucker IH, Wang W, Brandle M, Schultz HD, Patel KP. Neural regulation of sympathetic nerve activity in heart failure. Prog Cardiovasc Dis 37: 397–414, 1995 [DOI] [PubMed] [Google Scholar]
- 74.Zucker RS. Calcium and transmitter release. J Physiol Paris 87: 25–36, 1993 [DOI] [PubMed] [Google Scholar]
- 75.Zucker RS, Haydon PG. Membrane potential has no direct role in evoking neurotransmitter release. Nature 335: 360–362, 1988 [DOI] [PubMed] [Google Scholar]
- 76.Zucker RS, Lando L. Mechanism of transmitter release: voltage hypothesis and calcium hypothesis. Science 231: 574–579, 1986 [DOI] [PubMed] [Google Scholar]
- 77.Zucker RS, Lando L, Fogelson A. Can presynaptic depolarization release transmitter without calcium influx? J Physiol (Paris) 81: 237–245, 1986 [PubMed] [Google Scholar]