Abstract
As tumor cells metastasize from the primary tumor location to a distant secondary site, they encounter an array of biologically and physically heterogeneous microenvironments. While it is well established that biochemical signals guide all stages of the metastatic cascade, mounting evidence indicates that physical cues also direct tumor cell behavior, including adhesion and migration phenotypes. Physical cues acting on tumor cells in vivo include extracellular matrix mechanical properties, dimensionality, and topography, as well as interstitial flow, hydrodynamic shear stresses, and local forces due to neighboring cells. State-of-the-art technologies have recently enabled us and other researchers to engineer cell microenvironments that mimic specific physical properties of the cellular milieu. Through integration of these engineering strategies, along with physics, molecular biology, and imaging techniques, we have acquired new insights into tumor cell adhesion and migration mechanisms. In this review, we focus on the extravasation and invasion stages of the metastatic cascade. We first discuss the physical role of the endothelium during tumor cell extravasation and invasion and how contractility of endothelial and tumor cells contributes to the ability of tumor cells to exit the vasculature. Next, we examine how matrix dimensionality and stiffness coregulate tumor cell adhesion and migration beyond the vasculature. Finally, we summarize how tumor cells translate and respond to physical cues through mechanotransduction. Because of the critical role of tumor cell mechanotransduction at various stages of the metastatic cascade, targeting signaling pathways involved in tumor cell mechanosensing of physical stimuli may prove to be an effective therapeutic strategy for cancer patients.
Keywords: tumor metastasis, extravasation, three-dimensional migration, matrix stiffness, cell adhesion
cell adhesion and migration are ubiquitous events that underlie diverse physiological and pathological processes, including tissue morphogenesis, the immune response, and cancer metastasis. Recent experimental evidence has indicated that, in addition to chemical signals, physical cues from the cells' microenvironment also influence cell adhesion and motility in a range of physiological contexts. Physical cues acting on cells in vivo include extracellular matrix (ECM) mechanical properties, dimensionality, and topography, as well as hydrodynamic shear stresses and local forces due to neighboring cells. The mechanisms of cancer metastasis, in particular, have been found to depend heavily on the physical cues from the many complex microenvironments experienced by tumor cells during their metastatic journey (147) (Fig. 1). This is largely due to the process of “mechanotransduction,” where cells translate mechanical forces into cellular responses through biochemical signaling pathways. Importantly, state-of-the-art technologies have recently allowed researchers to engineer microenvironments that mimic specific physical properties of the cellular microenvironment in the context of tumor cell metastasis (Fig. 2). While no in vitro assay will ever be fully capable of exactly replicating the full in vivo situation, the technologies discussed in this review have enabled the elucidation of how cells respond to various physical cues during metastasis. Hence, our understanding of cancer metastasis is moving forward due to the integration of physics, biology, and engineering strategies.
Fig. 1.
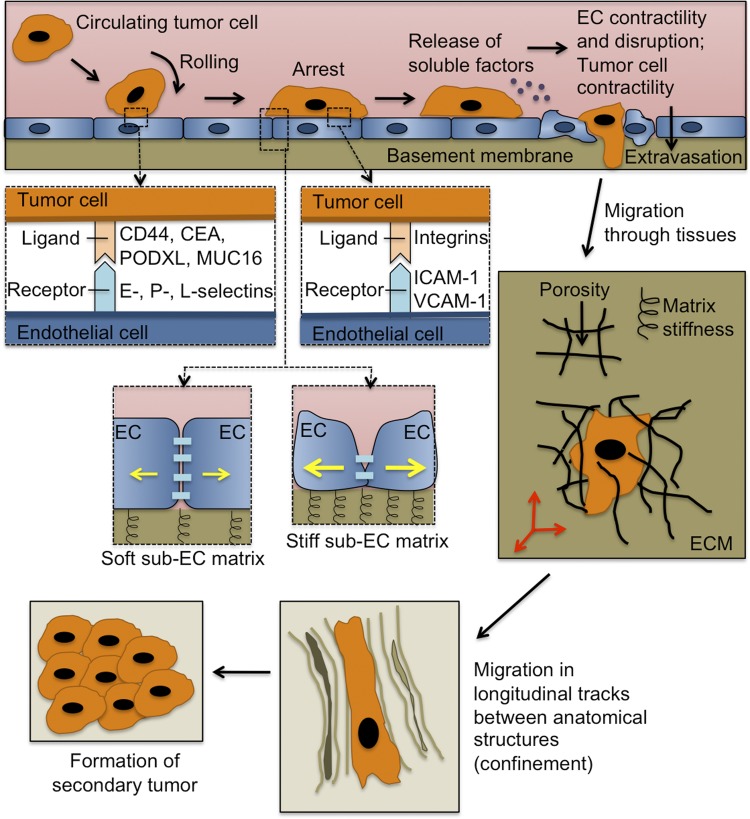
Physical cues influence the metastatic cascade. As circulating tumor cells collide with the vessel wall, transient adhesions form between various ligands on the tumor cell surface and E-, P-, and L-selectins on the vascular endothelium. The tumor cell arrests and firmly adheres to the endothelium via integrin binding with endothelial ICAM-1 and VCAM-1. Subendothelial matrix stiffness and Rho kinase/myosin light chain kinase signaling direct endothelial cell (EC) contractility (yellow arrows) and EC junction stability. The tumor cells may also release soluble factors that induce EC contractility and disruption, thus facilitating the extravasation process. Subsequently, tumor cells migrate through the extracellular matrix (ECM) of tissues and within longitudinal tracks between anatomic structures, where they are further influenced by physical cues, such as matrix stiffness and confinement. Finally, the tumor cells reach a destination where they form a secondary tumor. CEA, carcinoembryonic antigen; MUC16, mucin 16; PODXL, podocalyxin.
Fig. 2.
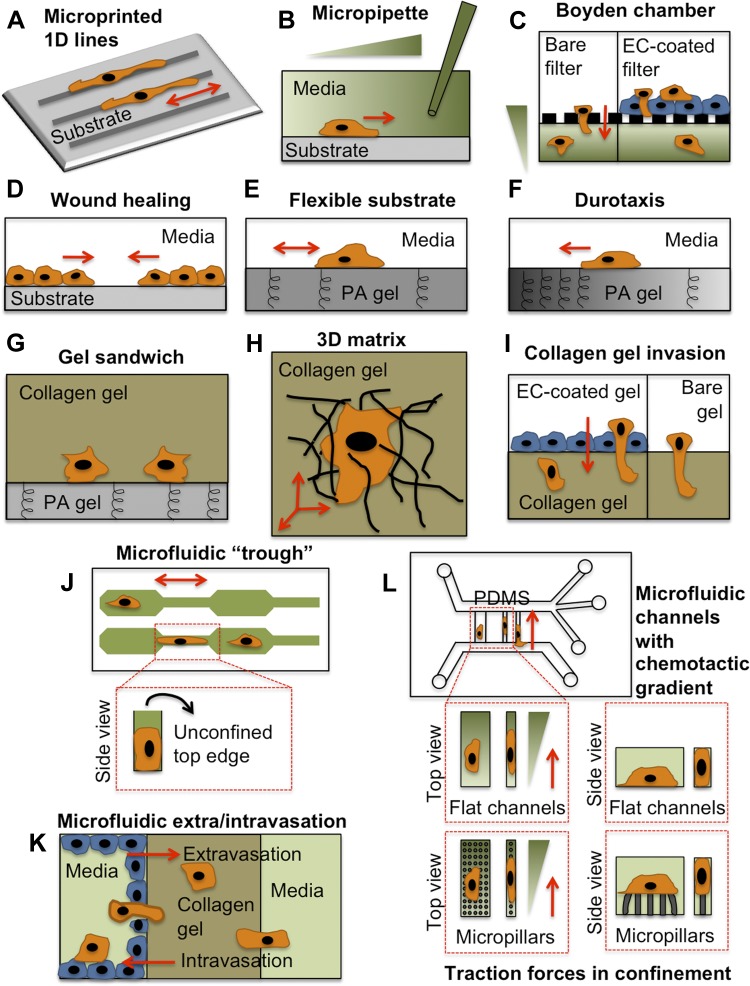
Diverse in vitro assays have been used to explore physical and biological cues affecting cell migration. Each of these assays mimics one or multiple cues presented to tumor cells (orange) and/or the endothelium (blue) during metastasis. Note that this is not an exhaustive list of migration assays. A: microphotopatterned or microcontact printed ECM protein lines (21, 33). B: micropipette chemotaxis assay (95). C: Boyden chamber chemotaxis assay, with bare filter containing nanometer- to micrometer-sized pores, or with EC-coated filter (56, 85). D: wound-healing assay. E: polyacrylamide (PA) gel flexible substrate assay (108). F: durotaxis assay using PA gel with a gradient of stiffness (87). G: collagen-PA gel sandwich assay (40). H: 3-dimensional (3D) collagen gel matrix assay (43). I: collagen gel invasion assay, with bare gel or EC-coated gel (93). J: microfluidic “trough” assay with 3 confining edges (106). K: microfluidic extra-/intravasation assay (70, 157). L: microfluidic chemotaxis assay with flat (5, 137) or micropillar-containing (114) channels. PDMS, polydimethylsiloxane. Red arrows indicate direction of cell migration.
An early step in the metastatic cascade, after metastatic cells have dissociated from the primary tumor and invaded the local tissue, is intravasation into a blood vessel. During circulation in this vascular “highway” system, tumor cells physically and biologically interact with and adhere to other cell types, including the endothelium lining blood vessels, and they also experience significant hydrodynamic shear forces due to blood flow. Subsequently, the tumor cells extravasate across the endothelium to exit the circulatory system. From there, tumor cells navigate the complex, heterogeneous ECM of the neighboring tissue before finally localizing to the site where a secondary tumor will form. This review focuses on the range of mechanical and dimensional cues experienced by tumor cells specifically during the extravasation and migration periods of the metastatic cascade (Fig. 1) and how the tumor cells translate and respond to these signals through mechanotransduction.1
Tumor Cell Extravasation
After initial interactions with the adhesion proteins on the surface of the vascular endothelium (74), tumor cells must cross the endothelial cell (EC) barrier during the next step of the metastatic cascade, i.e., extravasation. Given that the circulation is a harsh environment, subjecting tumor cells to a range of forces, including hemodynamic stress, collisions with other cell types and the vascular wall, and physical confinement in the smallest blood vessels, it is likely that extravasation from the blood vessel represents a key step during the metastatic cascade, after which tumor cells have access to ECM-rich tissues and other survival factors. However, some tumor cells may form a secondary tumor without ever leaving the vasculature, forming an “intravasculature metastasis” (1). Initially, it was believed that tumor cell extravasation could be mechanistically similar to leukocyte extravasation, which occurs during an immune response. Indeed, cancer cells have been reported to migrate through the body of ECs via the “transcellular” route (57, 73) and also between EC junctions via the “paracellular” route (145), two courses commonly used by leukocytes (2, 17, 18, 121, 130, 144, 148). During extravasation via the paracellular route, a leukocyte creates a small, localized gap in the vascular endothelial cadherin within the endothelium (121, 130). After the leukocyte has passed through the endothelium, the gap closes within several minutes, and additional leukocytes frequently pass through at the same location, where the “door has been opened” in the endothelium, so to speak. Leukocyte transendothelial migration may cause significant damage to highly contractile endothelial layers on stiffened subendothelial matrices, as would occur in atherosclerosis (128, 131). However, accumulating evidence suggests that, unlike leukocytes, which leave the endothelium mostly intact during a normal immune response, tumor cells cause significant disruption to the endothelium (10, 38, 57, 93, 159). As such, the mechanisms by which tumor cells cross the endothelium seem to be fundamentally different from immune cells, although much more research is necessary to fully understand this process.
Conventional methods for studying tumor cell extravasation have relied on 1) tail vein injection of tumor cells, followed by in vivo imaging and subsequent analysis (1, 140), 2) the Boyden assay (32, 56, 85), or 3) a three-dimensional (3D) collagen gel assay (73, 93). While each of these methods offers its own advantages, none presents an optimal combination of visualization ability and biophysical parameter control, thus limiting the degree of spatial and temporal quantitative information that can be derived from such experiments. Recently, microfluidic technologies have been utilized to address the growing need for better models of tumor cell extravasation (24, 70, 124, 141, 157, 159). In addition, optical transparency of the zebrafish has aided in real-time observation of tumor cell extravasation in vivo. Using a combination of these methods, we are beginning to understand the mechanisms by which tumor cells cross the EC barrier and the mechanical role of the endothelium during this process.
Physical role of the endothelium during tumor extravasation.
The endothelium is an important transducer of hemodynamic forces during blood vessel homeostasis and also in cardiovascular disease (29) and is far from a simple passive lining of the vasculature. Even before extravasation takes place, tumor cells physically interact with the vascular endothelium through a variety of proteins, including glycoproteins (48, 74). Similar to leukocytes, there is substantial evidence that these initial adhesive interactions are mediated by molecules expressed on the surface of tumor cells, which bind to E-, L-, or P-selectins on the endothelium (Fig. 1). We have summarized the current mechanistic knowledge of these interactions in a previous review (74). Our laboratory has reported that sialofucosylated carcinoembryonic antigen (136), podocalyxin (27, 135), and mucin 16 (22), which are expressed by numerous metastatic tumor cells, are functional E- and L-selectin ligands. Meanwhile, sialofucosylated CD44 variant isoforms represent the primary functional P-selectin ligand on the tumor cell surface (53, 101).
Not only does the endothelium facilitate initial adhesive events between the tumor cells and the vascular wall, but its mechanical state also regulates tumor cell extravasation. Endothelial cell-cell junction stability is associated with myosin light chain kinase (MLCK)- and Rho kinase-dependent EC contractility, which depends on subendothelial matrix stiffness (64, 78, 128, 131) (Fig. 1). Furthermore, the cytokine TNF-α, which is released by stromal cells during an immune response, and also oxidized LDL, which accumulates during cardiovascular disease, induce EC contractility and trigger actin polymerization and reorganization into stress fibers (12, 14, 122, 128, 131, 132). Consequently, increased EC contractility promotes leukocyte transmigration in the context of a normal immune response (128), cardiovascular disease (54, 131), and aging (64). Thus the integrity and mechanical properties of the endothelium represent key parameters in determining the efficiency of leukocyte extravasation. Mounting evidence suggests that this relationship also holds for tumor cell extravasation and that tumor cells are able to produce biological factors that optimally tune EC contractility to promote tumor cell extravasation.
One study evaluated the effectiveness of the endothelium as a vascular barrier against the invasion of 51 tumor cell lines, ranging from noninvasive to invasive, into a 3D collagen matrix (93). Only 9 tumor cell lines demonstrated decreased invasion in the presence of an EC monolayer; 17 cell lines became invasive or demonstrated significantly enhanced invasion. These results suggest that, for some tumor cells, the endothelium actually promotes invasion, rather than acting as a physical barrier and preventing tumor cell extravasation.
How then does the endothelium become hyperpermeable? Do tumor cells selectively adhere and extravasate in regions of inflamed vasculature? Alternatively, at any point in the vasculature, are tumor cells capable of producing biochemical factors that upregulate EC adhesion molecules and increase EC permeability, without a prerequisite for prior endothelium activation? While it is plausible that either scenario may occur, it is known that tumor cells secrete large amounts of VEGF (118), which increases vascular permeability, tumor cell-EC adhesion, and transendothelial migration of breast cancer cells through human brain microvascular ECs (83). Other vasoactive soluble factors secreted by metastastic tumor cells to increase vascular permeability include 12(S)-hydroxyeicosatetraenoic acid (59, 60), angiopoietin-2 (58, 62), chemokine (C-C motif) ligand 2 (150), O-glycosylated factors (100), fibrinogen (119), hepatocyte growth factor/scatter factor (90), 2,4,6,2′,6′-pentachlorobiphenyl (36), and stromal cell-derived factor-1α (82; for review see Ref. 47). Thus the tumor cells are able to change the physical state of the endothelium to promote subsequent extravasation.
In addition to soluble factors secreted by tumor cells, EC mechanical properties also change as tumor cells begin to invade the endothelium. MLCK and downstream myosin II become locally activated when MDA-MB-231 breast cancer cells begin to invade calf pulmonary artery ECs, leading to increased EC contractility, according to a 3D Förster resonance energy transfer-based study (73). Coculture of ECs with MDA-MB-231 breast cancer cells decreases EC stiffness, as measured by magnetic tweezers, and also induces EC cytoskeletal remodeling (92). These mechanical changes lead to significant endothelial damage and monolayer disruption, which likely further facilitate extravasation of tumor cells.
Tumor cell contractility in extravasation.
During transendothelial migration, neutrophils, monocytes, and T cells exert traction forces that are necessary for completion of the transmigration process (55, 130, 151). Inhibition of myosin II-, MLCK-, or RhoA-mediated contractility in these cells prevents full retraction of the leukocyte or lymphocyte cell body under the endothelium, resulting in incomplete transmigration and, thus, reduced extravasation efficiency. Meanwhile, very little is known about the role of tumor cell contractility during the extravasation step of the metastatic cascade. However, there exists some evidence that tumor cell contractility may be specifically important for extravasation. For example, inhibition of Rho-associated protein kinase (ROCK)- and MLCK-mediated traction forces in tumor cells decreases tumor cell invasion through human pulmonary microvascular ECs (92). Furthermore, tumor cell expression of Gro-β and the IL-8 receptor (CXCR2) results in increased tumor cell force generation and cytoskeletal remodeling dynamics (93), thereby physically weakening the EC barrier to promote extravasation. This process is reminiscent of how ovarian cancer spheroids produce traction forces to physically clear away the mesothelium; this process requires α5β1-integrin, talin 1, and myosin II (67). Hence, tumor cell extravasation involves contractility and retraction in the endothelium and also in the tumor cells themselves.
Migrating Beyond the Vasculature
Upon exiting the blood vessel, tumor cells must navigate a complex tissue microenvironment while seeking a hospitable location to form a secondary metastatic tumor (Fig. 1). A significant amount of our understanding of tumor cell migration (and cell migration in general) has stemmed from studies utilizing two-dimensional (2D), homogenous, stiff, ECM-coated surfaces (44, 97, 112, 117). On a 2D substrate, cell migration is driven by actin-based protrusion at the cell's leading edge, integrin-mediated adhesion to the substrate in distinct locations along the basal surface of the cell (i.e., focal adhesions), and myosin II-mediated contractile forces to promote de-adhesion at the trailing edge (81). However, the native tissue microenvironment in which tumor cells migrate in vivo is actually in 3D, or even one dimension (1D), as well as mechanically and biologically heterogeneous (44, 149). In the following sections, we review the various physical cues that direct cell migration and adhesion, with a primary focus on matrix dimensionality and mechanics. We also discuss several state-of-the-art cell migration assays used to evaluate the effects of various physical parameters within the cell's microenvironment (see Ref. 111 for a detailed review of traditional and recent developments in cell migration assays).
Microenvironment dimensional cues in tumor cell adhesion and migration.
Longitudinal tracks with bordering 2D interfaces (i.e., channels) exist between the connective tissue and the basement membrane of muscle, nerve, and epithelium (44). Furthermore, spaces between adjacent bundles of collagen fibers in fibrillar interstitial tissues also create 3D longitudinal channels (44). Similar channels may also be created by fibroblasts or leader cancer cells that degrade the matrix through proteolysis, creating a void tunnel in which follower cancer cells migrate (41, 46). The distribution of cross-sectional areas of pores/channels encountered by tumor cells in vivo is quite heterogeneous, ranging from 10 to >300 μm2 (149); thus tumor cells migrating in vivo experience varying degrees of physical confinement.
To address the fact that tumor cell migration in vivo occurs in complex environments beyond that which simple 2D assays can replicate, cell-derived and reconstituted ECM gels have been used for analysis of 3D adhesion and migration. For example, the architecture of collagen and Matrigel shares some distinct features of the native in vivo microenvironment, including the presence of pores and fibrillar structures. On 2D surfaces, cells adhere to the matrix via focal adhesions (50, 96), which promotes cell migration. However, when cells are partially embedded into a 3D matrix, focal adhesions become smaller and possess altered composition compared with those on 2D substrates (25, 26, 89). Strikingly, focal adhesions are no longer detected when cells are completely embedded into a 3D matrix (43, 45, 109), suggesting that cell adhesion to the surrounding matrix plays a reduced role during migration in 3D environments. Furthermore, in a study relating metastatic and migratory potential to adhesion strength on 2D substrates, the least adhesive (but most metastatic) cells exhibited the slowest migration speed; meanwhile, in 3D matrices, the lack of adhesion in highly metastatic cells allowed for the greatest migration speed (66). Thus, adhesion seems to affect cell migration in a dimension-sensitive manner.
In addition to altered adhesive phenotypes in 3D matrices, cells also exhibit different migration patterns as a result of matrix dimensional cues. For example, fibrosarcoma cells in 3D matrices undergo highly regular, periodic oscillatory migration patterns upon depletion of zyxin (or its binding partners α-actinin and p130Cas) (42), a protein found in focal adhesions, stress fibers, and the leading edge of many motile cells. Therefore, zyxin ensures that cells undergo random migration in 3D ECMs, although the exact mechanism is unknown. The oscillatory migration in zyxin-depleted cells is not observed on conventional 2D substrates, although it can be recapitulated on 1D micropatterned ECM stripes.
Other studies have similarly observed remarkable resemblances between migratory phenotypes in 1D and 3D, while 2D and 3D cases do not seem to be comparable. Microphotopatterning has been used to create complex ECM patterns on the surface of materials (33). This technique can be used to create 1D microphotopatterned lines that mimic the aligned fibrillar architecture in ECM matrices and that are relevant to mouse tumor (113) and in vivo metastasis models (125). In contrast to 2D, NIH 3T3 fibroblast migration along 1D lines and in 3D matrices is rapid, uniaxial, independent of ECM ligand density, and more dependent on microtubule polymerization (21, 33). Also, fibroblast adhesions along 1D lines are small, punctate, and polarized (21, 33) and are reminiscent of the diminished adhesive structures in tumor cells in 3D matrices (43) or in longitudinal channels (5). These experiments have been extended to include H-ras-transformed mouse fibroblasts (the PAP2 line, with NIH 3T3 fibroblasts as the parental cell line), which seem to be defective in dimension sensing. For example, H-ras-transformed mouse fibroblasts are more likely to exit 2D areas of matrix into 1D lines than are normal 3T3 cells; therefore, transformed cells do not accumulate on 2D regions over time, as the normal cells do (21). Thus, dimensional cues can significantly alter cell adhesion and migration phenotypes.
New microfluidic strategies to explore effects of physical confinement on tumor cells.
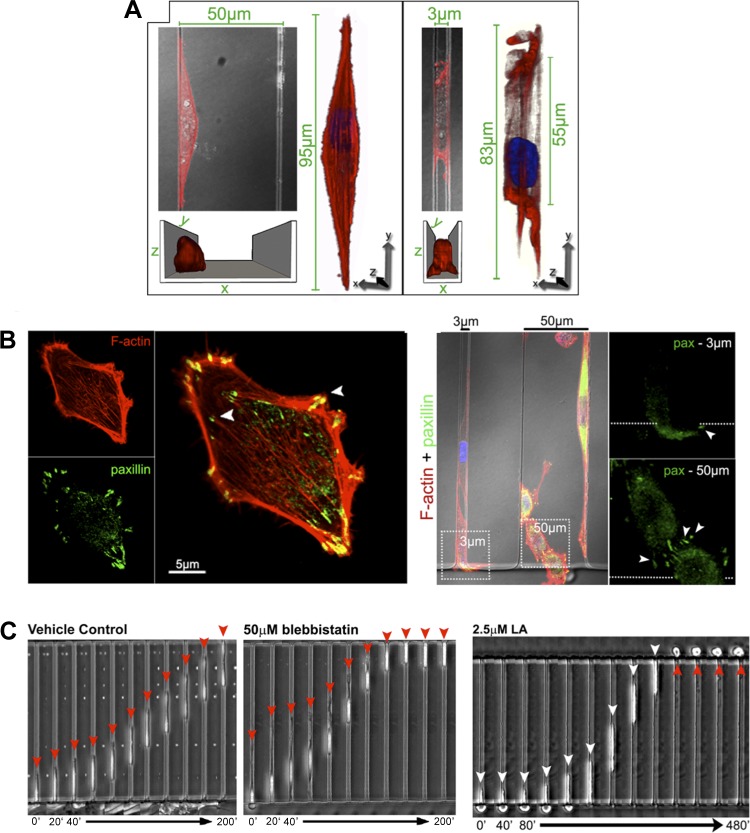
A more controlled and systematic approach to studying tumor cell migration in such complex settings is by engineering the cellular microenvironment using sophisticated microfabrication techniques, such as microfluidics or microcontact printing. Microfluidic devices are particularly useful, since they allow multiple biological and physical cues (e.g., chemotactic gradient, biological soluble factors, surface ligand density, physical confinement, topography, microenvironment stiffness, and hydrodynamic forces) to be presented and controlled simultaneously within the same experiment. A microfluidic device with tapered channels has recently been used to show that human metastatic MDA-MB-231 breast cancer cells enter physically restrictive spaces at a greater frequency than nonmetastatic cells (88). We recently developed a chemotaxis-based microfluidic device containing channels of varying width (137) and used this device to study the mechanisms of tumor cell migration in varying degrees of physical confinement (5, 23). These devices are formed from polydimethylsiloxane and coated with ECM protein (e.g., collagen or fibronectin). Tumor cell migration through wide channels (50 μm wide × 10 μm high) replicates the earmarks of typical 2D migration and depends heavily on actin polymerization, adhesion to the substrate, and contractility (5). Conversely, MDA-MB-231 metastatic breast cancer cell migration through narrow channels (3 μm wide × 10 μm high), where cells are completely confined, persists even in the absence of actin polymerization and myosin II-mediated contractility (5). When each of these processes is inhibited, cells are still capable of migrating up the chemoattractant gradient through the narrow confining channels (Fig. 3). These results align with in vivo data, where invasion of the leading fibroblasts or cancer cells requires not only proteolysis, but also Rho/ROCK activity, while migration of the following cancer cells through the precarved space is independent of Rho/ROCK activity (46). Similarly, a microfabricated collagen track assay has also demonstrated that MDA-MB-231 cell migration through void microtracks can occur independently of matrix metalloproteinase (MMP) production; these results suggest that pharmacological foci on MMPs may not inhibit all modes of tumor cell migration in vivo (77).
Fig. 3.
Physical confinement alters tumor cell adhesion and migration mechanisms. A: confocal reconstructions of the actin cytoskeleton (red; phalloidin staining) reveal significantly altered actin morphology in physically confined MDA-MB-231 metastatic breast tumor cells within narrow microchannels (3 μm wide × 10 μm high) compared with wide channels (50 μm wide × 10 μm high). B: confocal images of F-actin (red) and paxillin (green) on 2D planar surface (left) and in narrow and wide microchannels (right). Paxillin-positive punctate focal adhesions are much less pronounced in narrow than in wide channels. C: MDA-MB-231 cells migrate across narrow (3 μm wide × 10 μm high) channels, even in the absence of myosin II-mediated contractility (blebbistatin) or actin polymerization [latrunculin-A (LA)]. Time below each image series is in minutes. [From Balzer et al. (5).]
As in 3D matrices, cell migration in longitudinal channels is also less dependent on cell adhesion than is 2D migration (5, 63). As previously mentioned, tumor cells are still capable of migrating efficiently through narrow channels in the absence of ECM ligand (5, 63) or when treated with an anti-β1-integrin antibody (5). Furthermore, tumor cells completely confined in narrow channels do not form large focal adhesions at the interfaces with the four walls of the channel but, rather, form small nascent adhesions (5), reminiscent of observations in 3D matrices (43, 45, 109).
Dimensional control of cell traction forces.
Because the localization of cell traction forces usually correlates with the presence of focal adhesions, we hypothesized that tumor cell traction forces become less important during migration in confined spaces. To address this idea, we developed a novel microfluidic device that incorporates deflectable ECM-coated microposts onto the bottom surface of the four-walled (wide or narrow) channels (114); cells migrating through the channels deflect the pillars as they exert traction on the bottom wall of the channel, thus providing a way to quantitatively measure cell contractility in confined spaces. Using this device, we found that, consistent with a decreased dependence on adhesion, tumor cell migration in narrow channels leads to decreased cell traction forces. On 2D surfaces, focal adhesion formation and traction force generation are integral steps in the cell migration process (98); however, human osteosarcoma cells migrating in narrow channels exert significantly reduced traction forces compared with migration in wide, unconfining channels (114). As expected, in wide channels (50 μm wide × 4 μm high), inhibition of myosin II (via blebbistatin) diminishes cell traction forces, while inhibition of protein phosphatases (via calyculin A, which enhances myosin light chain phosphorylation) increases cell traction forces. Meanwhile, in narrow channels (10 μm wide × 4 μm high), neither blebbistatin nor calyculin A has a significant influence on cell traction forces. These results indicate that, at least for some classes of tumor cells, traction force generation plays a reduced role during migration in confined spaces in tissues. Consistent with these observations, NIH 3T3 fibroblasts also exert significantly reduced traction forces along 1D lines compared with 2D substrates, while inhibition of myosin II-dependent traction forces has a more pronounced effect on traction stress magnitude in 2D than 1D lines (21). Thus, tumor cell migration in confined spaces may occur by a divergent mechanism compared with migration on 2D surfaces, and tumor cells may be able to adapt their mode of migration in response to the physical properties of the microenvironment.
One such “plasticity” strategy could involve cross talk between Rac1 and myosin II. In normal fibroblast-like cells expressing α4β1-integrin, unconfined (2D) migration requires enhanced Rac1 activity at the leading edge of the cell, which is achieved by prevention of α4-integrin/paxillin binding (63). In contrast, in confined spaces, myosin II-mediated contractility is required and is enhanced when Rac1 activity is suppressed by α4-integrin/paxillin binding. Consistent with this cross-talk mechanism, inhibition of Rac1 reduces migration in wide channels, while inhibition of myosin II (or Rho)-dependent contractility reduces migration in narrow channels. In general, expression of α4β1-integrin promotes cell migration in unconfined and confined spaces (63). Invasive A375 melanoma cells also express α4β1-integrin and demonstrate a cross-talk mechanism similar to that of normal α4β1-integrin-expressing fibroblast-like cells. Moreover, in A375 melanoma cells in which α4β1-integrin is blocked or in normal fibroblast-like cells devoid of α4β1-integrin (e.g., NIH-3T3 and Chinese hamster ovary parental cells), unconfined migration is rescued by inhibition of myosin II contractility, which enhances Rac1 activity; meanwhile, confined migration is rescued by inhibition of Rac1, which enhances myosin II activity (63). It remains to be determined if the cross talk between Rac1 and myosin II activity in tumor cells devoid of α4β1-integrin regulates unconfined and confined migration in a similar fashion.
Matrix mechanics direct tumor cell adhesion and migration.
In addition to matrix dimensionality and microstructure, heterogeneities also exist in matrix mechanical properties in vivo (84). This is evident in the varying elastic moduli of different tissues, including breast (167 Pa) (105), brain (260–490 Pa) (94, 102), liver (640 Pa) (153), lung (5–6 kPa) (155), and skeletal muscle (100 kPa) (86). Furthermore, tissue stiffness often changes in pathological conditions. For example, while the healthy mammary gland is typically very soft, with an elastic modulus ∼167 Pa, breast tumors are much stiffer, with an elastic modulus >4,000 Pa (105). Importantly, numerous cell types, including fibroblasts (49, 108, 127), ECs (13, 15, 16, 31, 64, 80, 115, 126, 128, 131, 152, 154), epithelial cells (30, 108), cardiomyocytes (8, 34, 68), dorsal root ganglia neurons (4, 146), neutrophils (103, 129), and stems cells (35), are sensitive to matrix mechanical properties. Intriguingly, tumor cell migration and adhesion can also be driven by matrix mechanical properties on 2D substrates (75, 79, 139), which may induce metastasis (133), while some types of cancer cells may lose mechanosensitivity upon transition to a metastatic phenotype (134).
Matrix stiffness also influences migration in 3D matrices. For a given 3D Matrigel stiffness, the migration speed of DU-145 human prostate carcinoma cells is a balance between contractile and adhesive forces (156), which is similar to 2D substrates (110, 129). However, when integrins are inhibited on cells in 3D Matrigel matrices, maximal cell migration speed shifts to softer matrices (156); this is in striking contrast to current 2D models, where decreasing adhesiveness shifts maximal migration to a stiffer substrate. However, it seems that the role of matrix stiffness may also depend on the degree of transformation in the cells. MCF10A cells that co-overexpress the oncogenes ErbB2 and 14-3-3ζ (i.e., are fully transformed) are able to migrate more efficiently in stiffer 3D matrices than are partially transformed cells, which display significantly hindered migration in stiffer matrices (3).
Combinatorial effects of dimensionality and matrix stiffness.
Mounting evidence indicates that tumor cell migration is guided by dimensional (i.e., physical confinement) and mechanical (i.e., matrix stiffness) cues, which we reviewed separately in the previous sections. In particular, it seems that the role of matrix stiffness in cell migration on 2D substrates is fundamentally different from its role on 3D substrates, suggesting that the dimensionality of a cell's matrix dictates the way the cell perceives and responds to matrix stiffness. However, in 3D ECM matrices, perturbations to change matrix stiffness often concurrently change cellular confinement, making it difficult to reach firm conclusions on the combinatorial role of dimensionality and matrix stiffness in tumor cell migration. To address this issue, recent experiments have utilized a microfluidic approach to systematically and independently vary physical confinement and matrix stiffness (106, 107).
In these studies, three-walled (106) or four-walled (107) polyacrylamide-based devices were fabricated to contain channels of varying width (10, 20, or 40 μm) but with constant height (25 μm) and also varying wall stiffness (0.4, 10, or 120 kPa). For a given ECM stiffness, glioma cell migration speed increases as the cells undergo increasing physical confinement, possibly due to increased polarization of the actin cytoskeleton and traction forces (106). The ability of glioma cells to migrate through spatial restrictions has also been attributed to the volume-regulating ability of the sodium-potassium-chloride cotransporter isoform 1 (52). However, it remains to be elucidated whether traction forces generated by glioma cells are relevant in channels of even smaller cross-sectional area, since we have observed a reduced role for traction forces during osteosarcoma cell migration in channels that are 10 μm wide × 4 μm high (114). Meanwhile, in wide channels (20 μm wide × 25 μm high or 40 μm wide × 25 μm high) or on 2D substrates, glioma cell migration speed is biphasic with matrix stiffness, with optimum migration at an ECM stiffness of ∼10 kPa (106), a trend that has previously been reported for numerous cell types, including neutrophils (129), smooth muscle cells (110), and epithelial cells (30). However, in narrower channels (10 μm wide × 25 μm high), glioma cell migration speed is monotonic with matrix stiffness; that is, cells move with increasing speed as ECM stiffness increases (106). Inhibition of myosin II-mediated cell traction forces (via blebbistatin) in narrow channels abrogates this response and renders the relationship between migration speed and ECM stiffness relatively insensitive to matrix confinement. While these are interesting results, two major shortcomings are associated with this work: 1) cells are only physically confined on three sides, as the channels are actually “troughs,” rather than completely enclosed tubes; and 2) the smallest channel used in this study was 10 μm wide × 25 μm high, but future analyses should include even more physically restrictive channels (e.g., 3 μm wide × 10 μm high), as have been used in our polydimethylsiloxane-based devices (5, 63, 137), to reach a more comprehensive understanding of the connection between matrix stiffness and physical confinement. Recent improvements to this device have created a four-walled microfabricated polyacrylamide channel platform, thus enabling the discovery that MCF10A cell sensitivity to physical confinement is coregulated by transforming potential of the oncogenes ErbB2 and 14-3-3ζ and ECM stiffness (107). In other studies using 3D collagen gels vs. collagen-coated 2D substrates, cells overexpressing ErbB2 alone demonstrate the most significant decrease in cell migration speed in 3D matrices compared with 2D substrates (3). Meanwhile, cells overexpressing 14-3-3ζ alone exhibit the least sensitivity to matrix dimensionality. Thus, oncogenic lesions and ECM biophysical properties can synergistically interact to drive cell migration.
Tumor cell mechanics and metastatic potential.
On 2D substrates, human metastatic breast, prostrate, and lung cancer cells exert significantly increased traction stresses compared with their nonmetastatic counterparts, and this correlation holds for a range of ECM densities and matrix stiffnesses (76). However, we previously discussed the reduced role of traction forces during migration of some tumor cell types through physically confining ECMs (114). Therefore, it is unknown whether the correlation between traction stresses and metastatic potential in breast, prostate, and lung cancer cells also applies to migration in physically confined spaces. It is also unclear whether enhanced contractility in these metastatic cells is more important for extravasation from the blood vessel or for migration through complex ECMs within tissues. However, it is noteworthy that another study found the reverse association, such that 2D traction forces were inversely correlated with metastatic potential in a series of murine breast cancer cell lines (66). Future work that measures traction forces in a 3D setting, either in an ECM containing fluorescent marker beads or in a channel with deflectable microposts, will be necessary to further elucidate the relationship between metastatic potential and traction force generation.
Until recently, it was believed that tumor cells are necessarily more deformable than the matched normal cells, in order to facilitate squeezing through confined spaces much smaller than the size of the cell body. Indeed, many studies have found a positive correlation between tumor cell deformability and metastatic potential (28, 51, 65, 104, 116, 143). However, recently, studies on prostate cancer cells, hepatocelluar carcinoma cells, and human chondrosarcoma cells have shown an inverse, nonsignificant, or complicated correlation between mechanical compliance and metastatic potential (7, 28, 37, 158). However, it may be difficult to compare results using different experimental methods. For example, no cell-ECM adhesions exist when cell deformability is measured using optical tweezers; meanwhile, in atomic force microscopy experiments, cells are spread on and attached to a flat substrate. The presence of adhesions and altered cytoskeletal structure in this 2D setting may preclude measurement of a cell stiffness that is relevant to migratory ability. Thus, diagnostic tools that rely independently on cell compliance to predict metastatic propensity may not be the most effective; rather, diagnostic strategies based on migration ability seem to be more promising.
Tumor cell mechanotransduction.
It is clear that cells respond to various physical stimuli, including matrix dimensionality and mechanical properties, and that mechanical signaling plays a critical role in disease (69). Mechanotransduction is the process by which cells convert these extracellular physical signals into intracellular biochemical activity. Integrins are transmembrane proteins that, when activated, link the cell's ECM to the internal cytoskeleton, allowing mechanical signals from the outside to be propagated into the cell. This ECM-integrin-cytoskeleton complex acts as a molecular clutch in response to external mechanical forces (6, 20, 61). Focal adhesion kinase is recruited to the integrin complex, where it associates with other proteins, such as talin and paxillin, and is autophosphorylated at Y397 (138). Phosphorylation of focal adhesion kinase reveals a binding site for Src, leading to its activation. Indeed, Src has been implicated in cell mechanoresponses and force transmission (9, 39, 99, 123, 142), although Src's mechanotransducing ability may be dependent on its coordination with its substrate, p130Cas (120), which enhances Src activity (11). Meanwhile, tyrosine phosphorylation of p130Cas regulates actin assembly/disassembly (72), which in turn allows the cell to regulate numerous behaviors, including cell shape, motility, and stiffness. (For a complete review of the role of Src and p130Cas in cancer cell mechanotransduction, see Ref. 91.) However, with growing evidence that integrin-based cell adhesion to the ECM plays a reduced role during tumor cell migration in confined microenvironments, the exact mechanisms by which mechanotransduction occurs may need to be redefined. In addition, further work is needed to understand how tumor cells transduce other physical stimuli such as matrix dimensionality.
Summary and Future Perspectives
Cell migration in narrow microchannels, which mimics migration between anatomic structures in vivo, involves distinct mechanisms compared with unconfined migration on 2D surfaces. As the cancer biology and cell migration fields move forward, it is necessary to explore alternative mechanisms of cell migration that diverge from the classical model of 2D migration. Various aquaporins and ion channels are upregulated in cancer (19, 71). Therefore, one such mechanism could rely on the activity of ion channels and aquaporins to direct water flux through the cell membrane, thus facilitating protrusion and retraction and driving migration. An integrated experimental and mathematical framework will likely be necessary to explore this water permeation-driven mechanism of cell migration in confined spaces.
It is becoming increasingly apparent that physical cues within the cell's microenvironment directly impact tumor cell adhesion and migration during various points within the metastatic cascade. A range of physical forces act on tumor cells during circulation, extravasation through the endothelium, and migration through complex tissue architectures. Microfabrication techniques, including micropatterning and microfluidic technologies, have become valuable tools in mimicking distinct properties of the in vivo situation and have provided new perspectives on how tumor cells integrate multiple physical cues simultaneously and respond accordingly. In addition to acting as a multipurpose in vitro experimental technique for a range of applications, microfluidics may also become an important diagnostic and/or therapeutic analysis platform for metastatic cancer patients. Furthermore, because of the critical role of tumor cell mechanotransduction at various stages of the metastatic cascade, targeting signaling pathways involved in tumor cell mechanosensing of physical stimuli may prove to be an effective therapeutic strategy, although the effects on normal host cells will also need to be considered.
GRANTS
This work was supported by National Cancer Institute Grants U54 CA-143868 (to K. Konstantopoulos), T32 CA-130840 (to K. M. Stroka), and F32 CA-177756 (to K. M. Stroka), National Science Foundation Grant NSF-1159823 (to K. Konstantopoulos), and the Kleberg Foundation (to K. Konstantopoulos).
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.M.S. and K.K. conceived the idea for the review; K.M.S. designed and drafted the manuscript; K.M.S. prepared the figures; K.M.S. and K.K. edited and revised the manuscript; K.M.S. and K.K. approved the final version of the manuscript.
Footnotes
This review is part of a five-article theme series on Physical Biology in Cancer in this issue.
REFERENCES
- 1.Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med 6: 100–102, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Alcaide P, Newton G, Auerbach S, Sehrawat S, Mayadas TN, Golan DE, Yacono P, Vincent P, Kowalczyk A, Luscinskas FW. p120-Catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation. Blood 112: 2770–2779, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker EL, Srivastava J, Yu DH, Bonnecaze RT, Zaman MH. Cancer cell migration: integrated roles of matrix mechanics and transforming potential. PLos One 6: 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balgude AP, Yu X, Szymanski A, Bellamkonda RV. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials 22: 1077–1084, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Balzer EM, Tong Z, Paul CD, Hung WC, Stroka KM, Boggs AE, Martin SS, Konstantopoulos K. Physical confinement alters tumor cell adhesion and migration phenotypes. FASEB J 26: 4045–4056, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bangasser BL, Rosenfeld SS, Odde DJ. Determinants of maximal force transmission in a motor-clutch model of cell traction in a compliant microenvironment. Biophys J 105: 581–592, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastatas L, Martinez-Marin D, Matthews J, Hashem J, Lee YJ, Sennoune S, Filleur S, Martinez-Zaguilan R, Park S. AFM nano-mechanics and calcium dynamics of prostate cancer cells with distinct metastatic potential. Biochim Biophys Acta 1820: 1111–1120, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Bhana B, Iyer RK, Chen WL, Zhao RG, Sider KL, Likhitpanichkul M, Simmons CA, Radisic M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng 105: 1148–1160, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Boutahar N, Guignandon A, Vico L, Lafage-Proust MH. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in ERK activation. J Biol Chem 279: 30588–30599, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Brandt B, Heyder C, Gloria-Maercker E, Hatzmann W, Rotger A, Kemming D, Zanker KS, Entschladen F, Dittmar T. 3D-extravasation model—selection of highly motile and metastatic cancer cells. Semin Cancer Biol 15: 387–395, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Burnham MR, Bruce-Staskal PJ, Harte MT, Weidow CL, Ma A, Weed SA, Bouton AH. Regulation of c-Src activity and function by the adapter protein Cas. Mol Cell Biol 20: 5865–5878, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Byfield FJ, Aranda-Espinoza H, Romanenko VG, Rothblat GH, Levitan I. Cholesterol depletion increases membrane stiffness of aortic endothelial cells. Biophys J 87: 3336–3343, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byfield FJ, Reen RK, Shentu TP, Levitan I, Gooch KJ. Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D. J Biomech 42: 1114–1119, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byfield FJ, Tikku S, Rothblat GH, Gooch KJ, Levitan I. OxLDL increases endothelial stiffness, force generation, and network formation. J Lipid Res 47: 715–723, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Califano JP, Reinhart-King CA. A balance of substrate mechanics and matrix chemistry regulates endothelial cell network assembly. Cell Mol Bioeng 1: 122–132, 2008 [Google Scholar]
- 16.Califano JP, Reinhart-King CA. Substrate stiffness and cell area predict cellular traction stresses in single cells and cells in contact. Cell Mol Bioeng 3: 68–75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carman CV, Sage PT, Sciuto TE, de la Fuente MA, Geha RS, Ochs HD, Dvorak HF, Dvorak AM, Springer TA. Transcellular diapedesis is initiated by invasive podosomes. Immunity 26: 784–797, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol 167: 377–388, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chae YK, Woo J, Kim MJ, Kang SK, Kim MS, Lee J, Lee SK, Gong G, Kim YH, Soria JC, Jang SJ, Sidransky D, Moon C. Expression of aquaporin 5 (AQP5) promotes tumor invasion in human non small cell lung cancer. PLos One 3: 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan CE, Odde DJ. Traction dynamics of filopodia on compliant substrates. Science 322: 1687–1691, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Chang SS, Guo WH, Kim Y, Wang YL. Guidance of cell migration by substrate dimension. Biophys J 104: 313–321, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen SH, Dallas MR, Balzer EM, Konstantopoulos K. Mucin 16 is a functional selectin ligand on pancreatic cancer cells. FASEB J 26: 1349–1359, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen SH, Hung WC, Wang P, Paul C, Konstantopoulos K. Mesothelin binding to CA125/MUC16 promotes pancreatic cancer cell motility and invasion via MMP-7 activation. Sci Rep 3: 1870, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung S, Sudo R, Mack PJ, Wan CR, Vickerman V, Kamm RD. Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip 9: 269–275, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science 294: 1708–1712, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol 14: 633–639, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Dallas MR, Chen SH, Streppel MM, Sharma S, Maitra A, Konstantopoulos K. Sialofucosylated podocalyxin is a functional E- and L-selectin ligand expressed by metastatic pancreatic cancer cells. Am J Physiol Cell Physiol 303: C616–C624, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darling EM, Zauscher S, Block JA, Guilak F. A thin-layer model for viscoelastic, stress-relaxation testing of cells using atomic force microscopy: do cell properties reflect metastatic potential? Biophys J 92: 1784–1791, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev 75: 519–560, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Rooij J, Kerstens A, Danuser G, Schwartz MA, Waterman-Storer CM. Integrin-dependent actomyosin contraction regulates epithelial cell scattering. J Cell Biol 171: 153–164, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deroanne CF, Lapiere CM, Nusgens BV. In vitro tubulogenesis of endothelial cells by relaxation of the coupling extracellular matrix-cytoskeleton. Cardiovasc Res 49: 647–658, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Dimilla PA, Quinn JA, Albelda SM, Lauffenburger DA. Measurement of individual cell-migration parameters for human tissue-cells. Aiche J 38: 1092–1104, 1992 [Google Scholar]
- 33.Doyle AD, Wang FW, Matsumoto K, Yamada KM. One-dimensional topography underlies three-dimensional fibrillar cell migration. J Cell Biol 184: 481–490, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci 121: 3794–3802, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Eum SY, Lee YW, Hennig B, Toborek M. VEGF regulates PCB 104-mediated stimulation of permeability and transmigration of breast cancer cells in human microvascular endothelial cells. Exp Cell Res 296: 231–244, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Faria EC, Ma N, Gazi E, Gardner P, Brown M, Clarke NW, Snooka RD. Measurement of elastic properties of prostate cancer cells using AFM. Analyst 133: 1498–1500, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Fazakas C, Wilhelm I, Nagyoszi P, Farkas AE, Hasko J, Molnar J, Bauer H, Bauer HC, Ayaydin F, Dung NT, Siklos L, Krizbai IA. Transmigration of melanoma cells through the blood-brain barrier: role of endothelial tight junctions and melanoma-released serine proteases. PLos One 6: 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felsenfeld DP, Schwartzberg PL, Venegas A, Tse R, Sheetz MP. Selective regulation of integrin-cytoskeleton interactions by the tyrosine kinase Src. Nat Cell Biol 1: 200–206, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Fischer RS, Myers KA, Gardel ML, Waterman CM. Stiffness-controlled three-dimensional extracellular matrices for high-resolution imaging of cell behavior. Nat Protoc 7: 2056–2066, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fisher KE, Sacharidou A, Stratman AN, Mayo AM, Fisher SB, Mahan RD, Davis MJ, Davis GE. MT1-MMP- and Cdc42-dependent signaling co-regulate cell invasion and tunnel formation in 3D collagen matrices. J Cell Sci 122: 4558–4569, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fraley SI, Feng YF, Giri A, Longmore GD, Wirtz D. Dimensional and temporal controls of three-dimensional cell migration by zyxin and binding partners. Nat Commun 3: 719, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fraley SI, Feng YF, Krishnamurthy R, Kim DH, Celedon A, Longmore GD, Wirtz D. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat Cell Biol 12: 598–604, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147: 992–1009, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Friedl P, Entschladen F, Conrad C, Niggemann B, Zanker KS. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize β1-integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol 28: 2331–2343, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol 9: 1392–1400, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Roman J, Zentella-Dehesa A. Vascular permeability changes involved in tumor metastasis. Cancer Lett 335: 259–269, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Geng Y, Marshall JR, King MR. Glycomechanics of the metastatic cascade: tumor cell-endothelial cell interactions in the circulation. Ann Biomed Eng 40: 790–805, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Ghosh K, Pan Z, Guan E, Ge S, Liu Y, Nakamura T, Ren XD, Rafailovich M, Clark RA. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials 28: 671–679, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gilmore AP, Burridge K. Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4,5-bisphosphate. Nature 381: 531–535, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Guck J, Schinkinger S, Lincoln B, Wottawah F, Ebert S, Romeyke M, Lenz D, Erickson HM, Ananthakrishnan R, Mitchell D, Kas J, Ulvick S, Bilby C. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys J 88: 3689–3698, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haas BR, Sontheimer H. Inhibition of the sodium-potassium-chloride cotransporter isoform-1 reduces glioma invasion. Cancer Res 70: 5597–5606, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanley WD, Napier SL, Burdick MM, Schnaar RL, Sackstein R, Konstantopoulos K. Variant isoforms of CD44 are P- and L-selectin ligands on colon carcinoma cells. FASEB J 20: 337–339, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Hayenga HN, Aranda-Espinoza H. Stiffness increases mononuclear cell transendothelial migration. Cell Mol Bioeng 6: 253–265, 2013 [Google Scholar]
- 55.Heasman SJ, Carlin LM, Cox S, Ng T, Ridley AJ. Coordinated RhoA signaling at the leading edge and uropod is required for T cell transendothelial migration. J Cell Biol 190: 553–563, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hendrix MJ, Seftor EA, Seftor RE, Fidler IJ. A simple quantitative assay for studying the invasive potential of high and low human metastatic variants. Cancer Lett 38: 137–147, 1987 [DOI] [PubMed] [Google Scholar]
- 57.Heyder C, Gloria-Maercker E, Entschladen F, Hatzmann W, Niggemann B, Zanker KS, Dittmar T. Realtime visualization of tumor cell/endothelial cell interactions during transmigration across the endothelial barrier. J Cancer Res Clin Oncol 128: 533–538, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Holopainen T, Saharinen P, D'Amico G, Lampinen A, Eklund L, Sormunen R, Anisimov A, Zarkada G, Lohela M, Helotera H, Tammela T, Benjamin LE, Yla-Herttuala S, Leow CC, Koh GY, Alitalo K. Effects of angiopoietin-2-blocking antibody on endothelial cell-cell junctions and lung metastasis. J Natl Cancer Inst 104: 461–475, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Honn KV, Grossi IM, Diglio CA, Wojtukiewicz M, Taylor JD. Enhanced tumor-cell adhesion to the subendothelial matrix resulting from 12(S)-HETE-induced endothelial-cell retraction. FASEB J 3: 2285–2293, 1989 [DOI] [PubMed] [Google Scholar]
- 60.Honn KV, Tang DG, Grossi I, Duniec ZM, Timar J, Renaud C, Leithauser M, Blair I, Johnson CR, Diglio CA, Kimler VA, Taylor JD, Marnett LJ. Tumor cell-derived 12(S)-hydroxyeicosatetraenoic acid induces microvascular endothelial-cell retraction. Cancer Res 54: 565–574, 1994 [PubMed] [Google Scholar]
- 61.Hu K, Ji L, Applegate KT, Danuser G, Waterman-Stirer CM. Differential transmission of actin motion within focal adhesions. Science 315: 111–115, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Huang YJ, Song N, Ding YP, Yuan SP, Li XH, Cai HC, Shi HB, Luo YZ. Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Res 69: 7529–7537, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Hung WC, Chen SH, Paul CD, Stroka KM, Lo YC, Yang JT, Konstantopoulos K. Distinct signaling mechanisms regulate migration in unconfined versus confined spaces. J Cell Biol 202: 807–824, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med 3: 112ra122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Igawa S, Hayashi I, Tanaka N, Hiruma H, Majima M, Kawakami T, Hirose M, Masuda N, Kobayashi H. Nitric oxide generated by iNOS reduces deformability of Lewis lung carcinoma cells. Cancer Sci 95: 342–347, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Indra I, Undyala V, Kandow C, Thirumurthi U, Dembo M, Beningo KA. An in vitro correlation of mechanical forces and metastatic capacity. Phys Biol 8: 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iwanicki MP, Davidowitz RA, Ng MR, Besser A, Muranen T, Merritt M, Danuser G, Ince T, Brugge JS. Ovarian cancer spheroids use myosin-generated force to clear the mesothelium. Cancer Discovery 1: 144–157, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J 95: 3479–3487, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Janmey PA, Miller RT. Mechanisms of mechanical signaling in development and disease. J Cell Sci 124: 9–18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jeon JS, Zervantonakis IK, Chung S, Kamm RD, Charest JL. In vitro model of tumor cell extravasation. PLos One 8: 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jung HJ, Park JY, Jeon HS, Kwon TH. Aquaporin-5: a marker protein for proliferation and migration of human breast cancer cells. PLos One 6: 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kawauchi K, Tan WW, Araki K, Abu Bakar FB, Kim M, Fujita H, Hirata H, Sawada Y. p130Cas-dependent actin remodelling regulates myogenic differentiation. Biochem J 445: 323–332, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Khuon S, Liang L, Dettman RW, Sporn PH, Wysolmerski RB, Chew TL. Myosin light chain kinase mediates transcellular intravasation of breast cancer cells through the underlying endothelial cells: a three-dimensional FRET study. J Cell Sci 123: 431–440, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Konstantopoulos K, Thomas SN. Cancer cells in transit: the vascular interactions of tumor cells. Annu Rev Biomed Eng 11: 177–202, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Kostic A, Lynch CD, Sheetz MP. Differential matrix rigidity response in breast cancer cell lines correlates with the tissue tropism. PLos One 4: 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kraning-Rush CM, Califano JP, Reinhart-King CA. Cellular traction stresses increase with increasing metastatic potential. PLos One 7: 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kraning-Rush CM, Carey SP, Lampi MC, Reinhart-King CA. Microfabricated collagen tracks facilitate single cell metastatic invasion in 3D. Integr Biol (Camb) 5: 606–616, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krishnan R, Klumpers DD, Park CY, Rajendran K, Trepat X, van Bezu J, van Hinsbergh VW, Carman CV, Brain JD, Fredberg JJ, Butler JP, van Nieuw Amerongen GP. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. Am J Physiol Cell Physiol 300: C146–C154, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krndija D, Schmid H, Eismann JL, Lother U, Adler G, Oswald F, Seufferlein T, von Wichert G. Substrate stiffness and the receptor-type tyrosine-protein phosphatase-α regulate spreading of colon cancer cells through cytoskeletal contractility. Oncogene 29: 2724–2738, 2010 [DOI] [PubMed] [Google Scholar]
- 80.Kumar S, Maxwell IZ, Heisterkamp A, Polte TR, Lele TP, Salanga M, Mazur E, Ingber DE. Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophys J 90: 3762–3773, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell 84: 359–369, 1996 [DOI] [PubMed] [Google Scholar]
- 82.Lee BC, Lee TH, Avraham S, Avraham HK. Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1α in breast cancer cell migration through human brain microvascular endothelial cells. Mol Cancer Res 2: 327–338, 2004 [PubMed] [Google Scholar]
- 83.Lee TH, Avraham HK, Jiang SX, Avraham S. Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J Biol Chem 278: 5277–5284, 2003 [DOI] [PubMed] [Google Scholar]
- 84.Levental I, Georges PC, Janmey PA. Soft biological materials and their impact on cell function. Soft Matter 3: 299–306, 2007 [DOI] [PubMed] [Google Scholar]
- 85.Li YH, Zhu C. A modified Boyden chamber assay for tumor cell transendothelial migration in vitro. Clin Exp Metastasis 17: 423–429, 1999 [DOI] [PubMed] [Google Scholar]
- 86.Linder-Ganz E, Gefen A. Mechanical compression-induced pressure sores in rat hindlimb: muscle stiffness, histology, and computational models. J Appl Physiol 96: 2034–2049, 2004 [DOI] [PubMed] [Google Scholar]
- 87.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J 79: 144–152, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mak M, Reinhart-King CA, Erickson D. Microfabricated physical spatial gradients for investigating cell migration and invasion dynamics. PLos One 6: e20825, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mao Y, Schwarzbauer JE. Accessibility to the fibronectin synergy site in a 3D matrix regulates engagement of α5β1 versus αvβ3 integrin receptors. Cell Commun Adhes 13: 267–277, 2006 [DOI] [PubMed] [Google Scholar]
- 90.Martin TA, Mansel RE, Jiang WG. Antagonistic effect of NK4 on HGF/SF induced changes in the transendothelial resistance (TER) and paracellular permeability of human vascular endothelial cells. J Cell Physiol 192: 268–275, 2002 [DOI] [PubMed] [Google Scholar]
- 91.Matsui H, Harada I, Sawada Y. Src, p130Cas, and mechanotransduction in cancer cells. Genes Cancer 3: 394–401, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mierke CT. Cancer cells regulate biomechanical properties of human microvascular endothelial cells. J Biol Chem 286: 40025–40037, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mierke CT, Zitterbart DP, Kollmannsberger P, Raupach C, Schlotzer-Schrehardt U, Goecke TW, Behrens J, Fabry B. Breakdown of the endothelial barrier function in tumor cell transmigration. Biophys J 94: 2832–2846, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miller K, Chinzei K, Orssengo G, Bednarz P. Mechanical properties of brain tissue in-vivo: experiment and computer simulation. J Biomech 33: 1369–1376, 2000 [DOI] [PubMed] [Google Scholar]
- 95.Millius A, Weiner OD. Chemotaxis in neutrophil-like HL-60 cells. Methods Mol Biol 571: 167–177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol 131: 791–805, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mogilner A, Oster G. Cell motility driven by actin polymerization. Biophys J 71: 3030–3045, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Munevar S, Wang YL, Dembo M. Distinct roles of frontal and rear cell-substrate adhesions in fibroblast migration. Mol Biol Cell 12: 3947–3954, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci USA 105: 6626–6631, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakamori S, Okamoto H, Kusama T, Shinkai K, Mukai M, Ohigashi H, Ishikawa O, Furukawa H, Imaoka S, Akedo H. Increased endothelial cell retraction and tumor cell invasion by soluble factors derived from pancreatic cancer cells. Ann Surg Oncol 4: 361–368, 1997 [DOI] [PubMed] [Google Scholar]
- 101.Napier SL, Healy ZR, Schnaar RL, Konstantopoulos K. Selectin ligand expression regulates the initial vascular interactions of colon carcinoma cells: the roles of CD44v and alternative sialofucosylated selectin ligands. J Biol Chem 282: 3433–3441, 2007 [DOI] [PubMed] [Google Scholar]
- 102.Norman LL, Aranda-Espinoza H. Cortical neuron outgrowth is insensitive to substrate stiffness. Cell Mol Bioeng 3: 398–414, 2010 [Google Scholar]
- 103.Oakes PW, Patel DC, Morin NA, Zitterbart DP, Fabry B, Reichner JS, Tang JX. Neutrophil morphology and migration are affected by substrate elasticity. Blood 114: 1387–1395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ochalek T, Nordt FJ, Tullberg K, Burger MM. Correlation between cell deformability and metastatic potential in B16-F1 melanoma cell variants. Cancer Res 48: 5124–5128, 1988 [PubMed] [Google Scholar]
- 105.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell 8: 241–254, 2005 [DOI] [PubMed] [Google Scholar]
- 106.Pathak A, Kumar S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc Natl Acad Sci USA 109: 10334–10339, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pathak A, Kumar S. Transforming potential and matrix stiffness co-regulate confinement sensitivity of tumor cell migration. Integr Biol 5: 1067–1075, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA 94: 13661–13665, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Petroll WM, Ma L, Jester JV. Direct correlation of collagen matrix deformation with focal adhesion dynamics in living corneal fibroblasts. J Cell Sci 116: 1481–1491, 2003 [DOI] [PubMed] [Google Scholar]
- 110.Peyton SR, Putnam AJ. Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J Cell Physiol 204: 198–209, 2005 [DOI] [PubMed] [Google Scholar]
- 111.Polacheck WJ, Zervantonakis IK, Kamm RD. Tumor cell migration in complex microenvironments. Cell Mol Life Sci 70: 1335–1356, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell 112: 453–465, 2003 [DOI] [PubMed] [Google Scholar]
- 113.Provenzano PP, Inman DR, Eliceiri KW, Trier SM, Keely PJ. Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophys J 95: 5374–5384, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Raman PS, Paul CD, Stroka KM, Konstantopoulos K. Probing cell traction forces in confined microenvironments. Lab Chip 13: 4599–4607, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reinhart-King CA, Dembo M, Hammer DA. Cell-cell mechanical communication through compliant substrates. Biophys J 95: 6044–6051, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Remmerbach TW, Wottawah F, Dietrich J, Lincoln B, Wittekind C, Guck J. Oral cancer diagnosis by mechanical phenotyping. Cancer Res 69: 1728–1732, 2009 [DOI] [PubMed] [Google Scholar]
- 117.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 302: 1704–1709, 2003 [DOI] [PubMed] [Google Scholar]
- 118.Roskoski R. Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit Rev Oncol Hematol 62: 179–213, 2007 [DOI] [PubMed] [Google Scholar]
- 119.Sahni A, Arevalo MT, Sahni SK, Simpson-Haidaris PJ. The VE-cadherin binding domain of fibrinogen induces endothelial barrier permeability and enhances transendothelial migration of malignant breast epithelial cells. Int J Cancer 125: 577–584, 2009 [DOI] [PubMed] [Google Scholar]
- 120.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127: 1015–1026, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol 167: 2323–2330, 2001 [DOI] [PubMed] [Google Scholar]
- 122.Shentu TP, Singh DK, Oh MJ, Sun S, Sadaat L, Makino A, Mazzone T, Subbaiah PV, Cho M, Levitan I. The role of oxysterols in control of endothelial stiffness. J Lipid Res 53: 1348–1358, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shimizu T, Ueda J, Ho JC, Iwasaki K, Poellinger L, Harada I, Sawada Y. Dual inhibition of Src and GSK3 maintains mouse embryonic stem cells, whose differentiation is mechanically regulated by Src signaling. Stem Cells 30: 1394–1404, 2012 [DOI] [PubMed] [Google Scholar]
- 124.Shin MK, Kim SK, Jung H. Integration of intra- and extravasation in one cell-based microfluidic chip for the study of cancer metastasis. Lab Chip 11: 3880–3887, 2011 [DOI] [PubMed] [Google Scholar]
- 125.Sidani M, Wyckoff J, Xue CS, Segall JE, Condeelis J. Probing the microenvironment of mammary tumors using multiphoton microscopy. J Mammary Gland Biol Neoplasia 11: 151–163, 2006 [DOI] [PubMed] [Google Scholar]
- 126.Sieminski AL, Hebbel RP, Gooch KJ. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp Cell Res 297: 574–584, 2004 [DOI] [PubMed] [Google Scholar]
- 127.Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J 93: 4453–4461, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stroka KM, Aranda-Espinoza H. Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction. Blood 118: 1632–1640, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stroka KM, Aranda-Espinoza H. Neutrophils display biphasic relationship between migration and substrate stiffness. Cell Motil Cytoskeleton 66: 328–341, 2009 [DOI] [PubMed] [Google Scholar]
- 130.Stroka KM, Hayenga HN, Aranda-Espinoza H. Human neutrophil cytoskeletal dynamics and contractility actively contribute to trans-endothelial migration. PLos One 8: e61377, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stroka KM, Levitan I, Aranda-Espinoza H. OxLDL and substrate stiffness promote neutrophil transmigration by enhanced endothelial cell contractility and ICAM-1. J Biomech 45: 1828–1834, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stroka KM, Vaitkus JA, Aranda-Espinoza H. Endothelial cells undergo morphological, biomechanical, and dynamic changes in response to tumor necrosis factor-α. Eur Biophys J Biophys Lett 41: 939–947, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tang X, Kuhlenschmidt TB, Zhou JX, Bell P, Wang F, Kuhlenschmidt MS, Saif TA. Mechanical force affects expression of an in vitro metastasis-like phenotype in HCT-8 cells. Biophys J 99: 2460–2469, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tang X, Wen Q, Kuhlenschmidt TB, Kuhlenschmidt MS, Janmey PA, Saif TA. Attenuation of cell mechanosensitivity in colon cancer cells during in vitro metastasis. PLos One 7: 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thomas SN, Schnaar RL, Konstantopoulos K. Podocalyxin-like protein is an E-/L-selectin ligand on colon carcinoma cells: comparative biochemical properties of selectin ligands in host and tumor cells. Am J Physiol Cell Physiol 296: C505–C513, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thomas SN, Zhu F, Schnaar RL, Alves CS, Konstantopoulos K. Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E- and L-selectin in shear flow. J Biol Chem 283: 15647–15655, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tong ZQ, Balzer EM, Dallas MR, Hung WC, Stebe KJ, Konstantopoulos K. Chemotaxis of cell populations through confined spaces at single-cell resolution. PLos One 7: 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Toutant M, Costa A, Studler JM, Kadare G, Carnaud M, Girault JA. Alternative splicing controls the mechanisms of FAK autophosphorylation. Mol Cell Biol 22: 7731–7743, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ulrich TA, Pardo EM, Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res 69: 4167–4174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer 7: 659–672, 2007 [DOI] [PubMed] [Google Scholar]
- 141.Vickerman V, Blundo J, Chung S, Kamm R. Design, fabrication and implementation of a novel multi-parameter control microfluidic platform for three-dimensional cell culture and real-time imaging. Lab Chip 8: 1468–1477, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Visualizing the mechanical activation of Src. Nature 434: 1040–1045, 2005 [DOI] [PubMed] [Google Scholar]
- 143.Ward KA, Li WI, Zimmer S, Davis T. Viscoelastic properties of transformed-cells—role in tumor-cell progression and metastasis formation. Biorheology 28: 301–313, 1991 [DOI] [PubMed] [Google Scholar]
- 144.Wee H, Oh HM, Jo JH, Jun CD. ICAM-1/LFA-1 interaction contributes to the induction of endothelial cell-cell separation: implication for enhanced leukocyte diapedesis. Exp Mol Med 41: 341–348, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weis S, Cui JH, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol 167: 223–229, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Willits RK, Skornia SL. Effect of collagen gel stiffness on neurite extension. J Biomater Sci Polym Ed 15: 1521–1531, 2004 [DOI] [PubMed] [Google Scholar]
- 147.Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer 11: 512–522, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wojcikiewicz EP, Koenen RR, Fraemohs L, Minkiewicz J, Azad H, Weber C, Moy VT. LFA-1 binding destabilizes the JAM-A homophilic interaction during leukocyte transmigration. Biophys J. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, Deryugina E, Friedl P. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol 20: 931–941, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wolf MJ, Hoos A, Bauer J, Boettcher S, Knust M, Weber A, Simonavicius N, Schneider C, Lang M, Sturzl M, Croner RS, Konrad A, Manz MG, Moch H, Aguzzi A, van Loo G, Pasparakis M, Prinz M, Borsig L, Heikenwalder M. Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell 22: 91–105, 2012 [DOI] [PubMed] [Google Scholar]
- 151.Worthylake RA, Lemoine S, Watson JM, Burridge K. RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol 154: 147–160, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yamamura N, Sudo R, Ikeda M, Tanishita K. Effects of the mechanical properties of collagen gel on the in vitro formation of microvessel networks by endothelial cells. Tissue Eng 13: 1443–1453, 2007 [DOI] [PubMed] [Google Scholar]
- 153.Yeh WC, Li PC, Jeng YM, Hsu HC, Kuo PL, Li ML, Yang PM, Lee PH. Elastic modulus measurements of human liver and correlation with pathology. Ultrasound Med Biol 28: 467–474, 2002 [DOI] [PubMed] [Google Scholar]
- 154.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton 60: 24–34, 2005 [DOI] [PubMed] [Google Scholar]
- 155.Yuan HC, Kononov S, Cavalcante FS, Lutchen KR, Ingenito EP, Suki B. Effects of collagenase and elastase on the mechanical properties of lung tissue strips. J Appl Physiol 89: 3–14, 2000 [DOI] [PubMed] [Google Scholar]
- 156.Zaman MH, Trapani LM, Siemeski A, MacKellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci USA 103: 13897–13897, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci USA 109: 13515–13520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang G, Long M, Wu ZZ, Yu WQ. Mechanical properties of hepatocellular carcinoma cells. World J Gastroenterol 8: 243–246, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang Q, Liu TJ, Qin JH. A microfluidic-based device for study of transendothelial invasion of tumor aggregates in realtime. Lab Chip 12: 2837–2842, 2012 [DOI] [PubMed] [Google Scholar]