Abstract
Sjögren's syndrome (SS) is an autoimmune disorder characterized by chronic inflammation and destruction of salivary and lacrimal glands, leading to dry mouth, dry eyes, and the presence of anti-nuclear antibodies. Despite modern advances, the current therapies for SS have no permanent benefit. A potential treatment could involve the use of resolvins, which are highly potent endogenous lipid mediators that are synthesized during the resolution of inflammation to restore tissue homeostasis. Our previous studies indicate that ALX/FPR2, the receptor for RvD1, is expressed and active in the rat parotid cell line Par-C10. Specifically, activation of ALX/FPR2 with RvD1 blocked inflammatory signals caused by TNF-α and enhanced salivary epithelial integrity. The goal of this study was to investigate RvD1 receptor expression and signaling pathways in primary salivary cells. Additionally, we determined the role of the aspirin-triggered 17R analog (AT-RvD1, a more chemically stable RvD1 epimeric form) in prevention of TNF-α-mediated salivary inflammation in mouse submandibular glands (mSMG). Our results indicate that ALX/FPR2 is expressed in mSMG and is able to elicit intracellular Ca2+ responses and phosphorylation of Erk1/2, as well as Akt. Given that these signaling pathways are linked to cell survival, we investigated whether AT-RvD1 was able to prevent programmed cell death in mSMG. Specifically, we determined that AT-RvD1 prevented TNF-α-mediated caspase-3 activation. Finally, we show that ALX/FPR2 is expressed in human minor salivary glands with and without SS, indicating the potential therapeutic use of AT-RvD1 for this condition.
Keywords: resolvins, salivary glands, AT-RvD1, RvD1, Sjögren's syndrome, ALX/FPR2, GPR32
sjögren's syndrome (SS) is an autoimmune disease with a population prevalence of ∼0.5% and a female preponderance (9:1 female-to-male ratio) (12). The hallmarks of SS are xerostomia (dry mouth), keratoconjunctivitis sicca (dry eyes), and the presence of anti-nuclear antibodies (5, 11, 14). Typically, such symptoms are clinically detectable only after salivary and lacrimal glands display chronic inflammation, a point at which current therapies have no benefit (8, 12). The current treatments for dry mouth (e.g., saliva stimulants and saliva substitutes) only provide temporary relief (37). Therefore, SS greatly decreases the quality of life for patients suffering from the disease (38). The mechanisms behind the characteristic salivary gland destruction in SS patients remain unclear. However, it has been suggested that SS is characterized by an early phase in which the salivary and lacrimal tissues undergo inappropriate apoptosis (23) followed by a later phase of lymphocyte infiltration and autoimmune aggression associated with gland destruction and secretory dysfunction (23).
Apoptosis in SS may be mediated through the following mechanisms: 1) stimulation of the TNF-α receptor by TNF-α (2), 2) Fas ligand receptor interaction (29, 34), and/or 3) activation of the perforin-granzyme pathway (43). Caspase-3 plays a pivotal role in mediating programmed cell death (46). It was previously observed that increased proteolytic activity in salivary epithelial cells is mediated by caspases, supporting the concept that excessive cell death contributes to the pathogenesis of this disease (22).
Resolvins are short-lived autacoids, part of a family of bioactive lipids that are synthesized during the resolution of inflammation (39). These mediators exhibit anti-inflammatory and proresolving actions to block cell damage and enhance tissue repair after injury or infection (9, 16, 18, 26). Previously, we showed that resolvin D1 (RvD1) prevents inflammatory responses and enhances tissue integrity in the rat parotid cell line Par-C10 (30). Preincubation of cells with RvD1 abolished tight junction and cytoskeletal disruption caused by TNF-α (30). Furthermore, RvD1 enhanced lumen formation and cell polarity in Par-C10 cells cultured under two- and three-dimensional conditions through Akt signaling (30). These results indicate that RvD1 has the potential to improve salivary epithelial integrity in vitro and in vivo. Therefore, RvD1's potent anti-inflammatory actions in Par-C10 cells prompted us to investigate its impact on cell inflammation in primary cells from mouse submandibular glands (mSMG). Our results indicate that the RvD1 receptor lipoxin A4/formyl peptide receptor 2 (ALX/FPR2) is expressed in mouse and human salivary glands. We found that treatment of mSMG cells with the RvD1 epimer aspirin-triggered RvD1 (AT-RvD1) elicited intracellular Ca2+ signaling and triggered phosphorylation of Erk1/2 and Akt. Additionally, AT-RvD1 prevented TNF-α-mediated disruption of caspase-3 activation, indicating a role of this lipid mediator in cell survival. These results are significant, as they may lead to better therapeutic strategies for minimizing the autoimmune inflammation that occurs in SS.
MATERIALS AND METHODS
Experimental animals.
Adult female C57BL/6 mice at 20 wk of age were anesthetized with 80–100 mg/kg ketamine + 5 mg/kg xylazine. Mice were euthanized by abdominal exsanguination, and the mSMG was removed for preparation of dispersed cell aggregates or flash-frozen for tissue sections. All animal use, anesthesia, and surgeries were conducted under the strict guidelines and approval of The State University of New York at Buffalo Institutional Animal Care and Use Committee.
Human specimens.
Human minor salivary glands (hMSG) were obtained from the Sjörgen's International Collaborative Clinical Alliance Repository at the University of California, San Francisco. The new American College of Rheumatology criteria for SS were used for specimen classification (41). Use of the deidentified samples was approved by the Health Sciences Institutional Review Board under the Exempt Criterion 45 CFR 46.101(b) (4). Histological sections (10 μm) were prepared at the Histological Services of the Department of Pathology and Anatomical Sciences at the University at Buffalo.
Grading of hMSG histological sections was performed as described by Chisholm and Mason (4). Focus score was determined as described by Greenspan et al. (15). For our studies, a non-SS hMSG group consisting of three specimens and a SS hMSG group consisting of three specimens were used.
Preparation of dispersed cell aggregates from mSMG.
Mice were anesthetized, and freshly isolated mSMG cells were prepared as described previously (44). Glands were finely minced in dispersion medium consisting of 1:1 DMEM-Ham's F-12 medium (Hyclone, Logan, UT) and 0.2 mM CaCl2, 1% (wt/vol) BSA, 50 U/ml collagenase (Worthington Biochemical, Freehold, NJ), and 400 U/ml hyaluronidase at 37°C for 30 min with aeration (95% air-5% CO2). Cell aggregates in dispersion medium were suspended by pipetting at 20 and 30 min. The dispersed cell aggregates were washed with enzyme-free assay buffer (120 mM NaCl, 4 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 1 mM CaCl2, 10 mM glucose, and 15 mM HEPES, pH 7.4) containing 1% (wt/vol) BSA and filtered through a nylon mesh. Cells were washed again in 1:1 DMEM-Ham's F-12 medium containing 2.5% (vol/vol) fetal bovine serum (GIBCO, Gaithersburg, MD) and the following supplements: 0.1 μM retinoic acid, 80 ng/ml epidermal growth factor, 2 nM triiodothyronine, 5 mM glutamine, 0.4 μg/ml hydrocortisone, 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml sodium selenite, and freshly added 100 μg/ml Normocin (InvivoGen, San Diego, CA). Cells were centrifuged at room temperature and 1,000 rpm for 5 min, resuspended in supplemented 1:1 DMEM-Ham's F-12 medium (see above), and plated on tissue culture plates.
AT-RvD1 time course.
The RvD1 aspirin-triggered epimeric form AT-RvD1, which was previously shown to be more resistant to degradation in culture and equipotent to RvD1 at nanomolar concentrations (42), was used for these studies. mSMG cell suspensions were incubated with and without AT-RvD1 (100 ng/ml; Cayman Chemical, Ann Arbor, MI) for 0–60 min. Cells were then lysed and subjected to Western blot analyses for Erk1/2 and Akt phosphorylation.
Active caspase-3 detection.
mSMG cell suspensions were pretreated for 30 min with or without AT-RvD1 (100 ng/ml) and then incubated with or without TNF-α (100 ng/ml; Becton Dickinson Pharmingen, San Diego, CA) or staurosporine (1 μM; Sigma, St. Louis, MO) for 24 h at 37°C and subjected to an active caspase-3 ELISA (R & D Systems, Minneapolis, MN). ELISA was performed following the manufacturer's instructions. Data were acquired using an iMark microplate reader (Bio-Rad, Hercules, CA) set to 450 nm and a correction wavelength of 595 nm.
Intracellular Ca2+ measurements.
Intracellular Ca2+ release was quantified in single mSMG cells. Cells were placed on eight-well chambers mounted on German borosilicate coverglasses (no. 1, Nalge Nunc, Naperville, IL) coated with Cell-Tak (BD Biosciences, Franklin Lakes, NJ). Subsequently, cells were preloaded with fluo 4-AM (Invitrogen, Carlsbad, CA), a Ca2+-sensitive fluorescent dye, for 20 min at 37°C and washed with assay buffer [120 mM NaCl, 4 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 1 mM CaCl2, 10 mM glucose, 15 mM HEPES, pH 7.4, and 0.1% (wt/vol) BSA]. Calcium release was measured using a fluorescence microscope (model DMI6000B, Leica Microsystems, Mannheim, Germany) and stimulated with AT-RvD1 (100 ng/ml), carbachol (100 μM; Sigma), or 1× PBS (negative control) at room temperature. Fluo 4-AM fluorescence images were captured with an ORCA-R2 camera (Hamamatsu Photonics, Hamamatsu City, Japan). The graph was obtained using the averaged pixel intensity values of images taken over time.
Detection of RvD1 receptors.
hMSG or mSMG frozen sections (10 μm) were fixed in 4% paraformaldehyde for 20 min at room temperature, incubated with 0.1% Triton X-100 in PBS for 5 min, and washed with PBS. Tissues were blocked with 5% goat serum in PBS for 2 h at room temperature. The sections were incubated overnight at 4°C with rabbit anti-ALX/FPR2 antibody (Alomone Labs, Jerusalem, Israel; 1:200 dilution) or rabbit anti-G protein-coupled receptor (GPR) 32 antibody (Abcam, Cambridge, MA; 1:200 dilution). Dilutions were made with 5% goat serum in PBS. On the next day, sections were washed three times for 5 min with PBS. Sections were incubated for 1 h with Alexa Fluor 488-conjugated goat anti-rabbit (Invitrogen; 1:500 dilution in 5% goat serum) and washed three times with PBS. Tissues were stained for 30 min with an Alexa Fluor 633-conjugated phalloidin-F-actin stain (Sigma; 1:400 dilution in PBS) and then incubated for 5 min with propidium iodide (Invitrogen; 1:3,000 dilution in saline sodium citrate). Images were obtained using a confocal microscope (model 510, Zeiss, Jena, Germany). All images were analyzed using the ImageJ (National Institutes of Health, Bethesda, MD) software application.
Western blot analyses.
Cells were lysed in 120 μl of 2× Laemmli buffer [120 mM Tris·HCl, pH 6.8, 10% (vol/vol) glycerol, 2% (wt/vol) SDS, 1 mM dithiothreitol, and 0.002% (wt/vol) bromophenol blue]. Lysates were sonicated for 20 s with a sonic dismembrator (microtip, amplification 45%; model 120, Fisher Scientific, Pittsburgh, PA) and boiled for 5 min. Cell lysates were subjected to electrophoresis in 4–15% (wt/vol) Mini-Protean TGX gels (Bio-Rad) and transferred to nitrocellulose membranes. Membranes were blocked for 1 h with 3% (wt/vol) BSA in Tris-buffered saline (0.137 M NaCl and 0.025 M Tris, pH 7.4) containing 0.1% (vol/vol) Tween 20 (TBST) and immunoblotted overnight with primary antibodies at 4°C in TBST containing 3% (wt/vol) BSA. The following antibodies were utilized: rabbit phosphorylated Erk1/2, which recognizes endogenous phosphorylated Erk1/2 when phosphorylated at Thr202 and Tyr204 (Cell Signaling Technology, Danvers, MA; 1:500 dilution), and rabbit phosphorylated Akt, which recognizes endogenous phosphorylated Akt when phosphorylated at Ser473 (Cell Signaling Technology; 1:500 dilution). After incubation with the primary antibodies, membranes were washed three times for 15 min each with TBST and incubated with peroxidase-linked goat anti-rabbit IgG antibody (Cell Signaling Technology; 1:2,000 dilution) at room temperature for 1 h. The membranes were washed three times for 15 min each with TBST and treated with Clarity chemiluminescence detection reagent (Bio-Rad), and protein bands were visualized using a Chemi-Doc MP Imager (Bio-Rad). Quantification of the bands was performed using Image Lab (release 4.1, Bio-Rad). For signal normalization, membranes were treated with Restore stripping buffer (Thermo-Scientific, Waltham, MA) and reprobed with rabbit total Erk1/2 and pan-Akt (Cell Signaling Technology; 1:500 dilution), which detects endogenous levels of total analogous protein, regardless of posttranslational modifications.
Statistical analyses.
Values are means ± SE of results from three or more determinations. P < 0.05, calculated from a two-tailed t-test, represents significant differences.
RESULTS
The RvD1 receptor ALX/FPR2 is expressed in mSMG.
Our previous studies showed that the RvD1 receptor ALX/FPR2 is expressed in mSMG acinar cells and in the rat parotid gland cell line Par-C10 (30). To corroborate our previous studies and extend our observations to ductal structures, we immunostained mSMG tissue sections for ALX/FPR2 and GPR32 and used actin staining to show polar localization of the receptors. As shown in Fig. 1, ALX/FPR2 was expressed on the apical and basolateral sides of acinar and ductal cells (although the staining was stronger at the apical side of the cells), while GPR32 was absent (data not shown). A weak cytosolic staining was also observed. Phalloidin staining (F-actin) was shown throughout the entire section, but intense staining was observed at the apical actin ring, as expected.
Fig. 1.
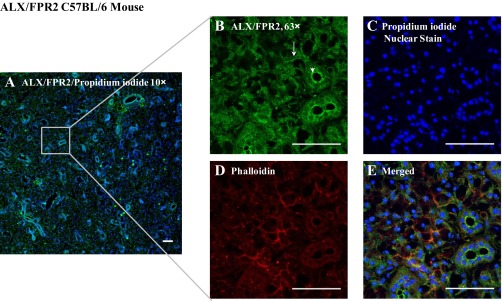
The resolvin D1 (RvD1) receptor ALX/FPR2 (lipoxin A4/formyl peptide receptor 2) is expressed in mouse submandibular glands (mSMG). Frozen sections of mSMG were fixed, and localization of ALX/FPR2 was determined using immunofluorescence microscopy: rabbit anti-ALX/FPR2 (green; A, B, and E), phalloidin (red; D and E), and propidium iodide nuclear stain (blue; A, C, and E). All images were obtained and analyzed using a confocal microscope. Arrows and arrowheads indicate acinar and ductal localization of the receptor, respectively. Scale bars, 50 μm.
ALX/FPR2 elicits intracellular Ca2+ increases in mSMG.
To determine whether ALX/FPR2 is functional in mSMG cells, we studied whether AT-RvD1 was able to induce intracellular Ca2+ release. As shown in Fig. 2A, AT-RvD1 was able to stimulate intracellular Ca2+ mobilization in mSMG cells. Additionally, progressive increases in intracellular Ca2+ mobilization were observed in mSMG cells stimulated with the muscarinic agonist carbachol (100 μM; Fig. 2B), which is known to elicit Ca2+ responses in mSMG cells (33). However, cells did not respond to the negative control buffer without agonist (Fig. 2C). These results indicate that RvD1 signaling in mSMG cells activates the classic G protein-coupled receptor second messenger system.
Fig. 2.
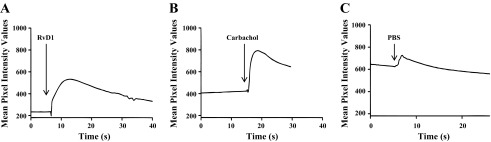
ALX/FPR2 is functional in mSMG. Cells were plated on 8-well coverglasses treated with Cell-Tak and then stimulated with aspirin-triggered RvD1 (AT-RvD1, 100 ng/ml; A), carbachol (100 μM; B), or 1× PBS (C). Changes in fluo 4-AM fluorescence intensity were recorded and analyzed using Leica Application Suite Advanced Fluorescence software. Results are representative of ≥3 experiments for all agonists.
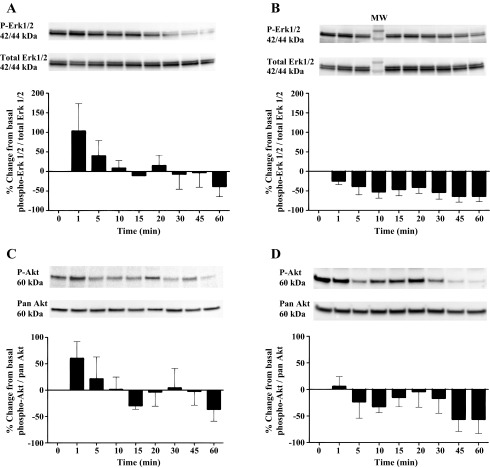
AT-RvD1 transiently phosphorylates Erk1/2 and Akt in mSMG.
To assess other RvD1-mediated intracellular signaling pathways in mSMG, we studied the activation of various protein kinases, such as Erk1/2 and Akt. Incubation with AT-RvD1 induced a transient, time-dependent increase in phosphorylation of Erk1/2 (Fig. 3A) and Akt (Fig. 3C) relative to basal levels. This phosphorylation was rapid in onset, reaching a peak at ∼1 min and subsequently dropping below basal levels at 15–60 min. In contrast to the effects of AT-RvD1 on Erk1/2 and Akt phosphorylation (Fig. 3, A and C), in cells incubated in the absence of AT-RvD1 for 0–60 min the effect on Erk1/2 and Akt phosphorylation was minimal.
Fig. 3.
Aspirin-triggered RvD1 (AT-RvD1) transiently phosphorylates Erk1/2 and Akt in mSMG. A and B: freshly isolated mSMG cells were incubated with (A) and without (B) AT-RvD1 (100 ng/ml) for 0–60 min. Cells were then lysed with Laemmli buffer, and expression of phosphorylated Erk1/2 (phospho-Erk1/2) was detected by Western blot analysis. Phosphorylated Erk1/2 data were normalized to total protein using a total Erk1/2 antibody. Values are means ± SE of results from ≥3 experiments. Results from a representative experiment are shown at top. p-Erk1/2, phosphorylated Erk1/2; MW, molecular weight. C and D: freshly isolated mSMG cells were incubated with (C) and without (D) AT-RvD1 (100 ng/ml) for 0–60 min. Cells were then lysed with Laemmli buffer, and expression of phosphorylated Akt (phospho-Akt) was detected by Western blot analysis. Phosphorylated Akt data were normalized to total protein using a pan-Akt antibody. Values are means ± SE of results from ≥3 experiments. Results from a representative experiment are shown at top. p-Akt, phosphorylated Akt.
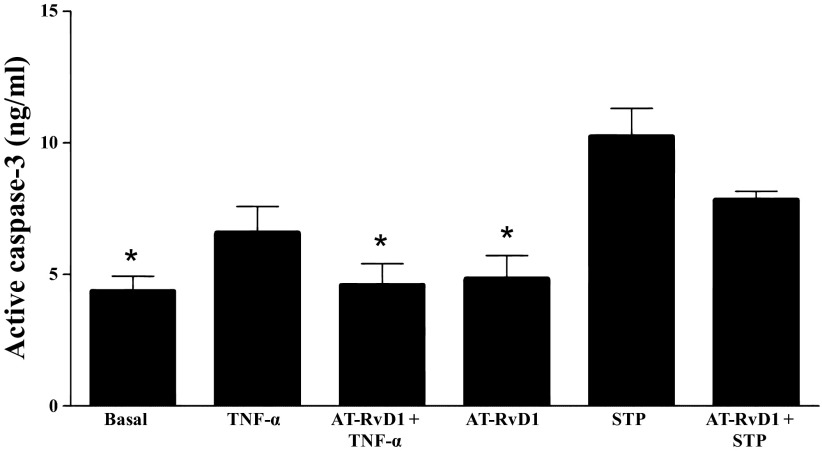
AT-RvD1 decreases active caspase-3 activity in mSMG.
Given that AT-RvD1 caused phosphorylation of survival proteins (Fig. 3, A and B), we determined whether AT-RvD1 was able to block apoptotic pathways. Particularly, we investigated whether AT-RvD1 blocked signals induced by the proapoptotic cytokine TNF-α in mSMG cells or by the protein kinase inhibitor staurosporine (used as a proapoptotic control). As shown in Fig. 4, TNF-α significantly increased caspase-3 activity compared with basal values. However, a 30-min preincubation of mSMG cells with AT-RvD1 significantly blocked the TNF-α-mediated, but not staurosporine-mediated, increases in caspase-3 activity.
Fig. 4.
AT-RvD1 decreases active caspase-3 activity in mSMG. Freshly isolated mSMG cells were incubated with and without AT-RvD1 (100 ng/ml) for 30 min and then with TNF-α or staurosporine (STP). After 24 h, cells were collected and subjected to an active caspase-3 ELISA. Values are means ± SE of results from ≥3 experiments. *P < 0.05 vs. TNF-α-treated cells.
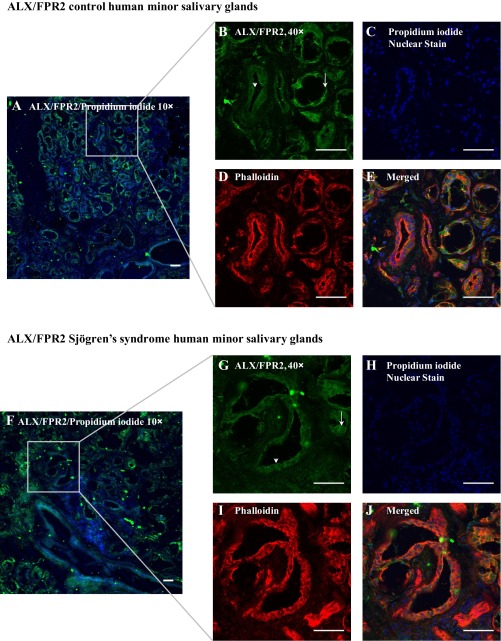
ALX/FPR2 is expressed in hMSG with and without SS.
Because RvD1 has potential therapeutic application to block inflammation associated with SS (30), we decided to test whether resolvin receptors are differentially expressed in hMSG with and without SS. As shown in Fig. 5, ALX/FPR2 was strongly localized on the apical side of acinar and ductal hMSG from SS and non-SS subjects, with a less intense basolateral and cytosolic staining. However, we observed heterogeneity of ALX/FPR2 expression in hMSG with SS. While all samples expressed ALX/FPR2, the intensity of apical expression varied (data not shown). Further analysis demonstrated that a second RvD1 receptor, GRP32, was not expressed in hMSG (data not shown).
Fig. 5.
The RvD1 receptor ALX/FPR2 is expressed in human minor salivary glands (hMSG). Frozen hMSG sections without (A–E) and with (F–J) Sjögren's syndrome (SS) were fixed, and expression of ALX/FPR2 was detected using immunofluorescence microscopy with rabbit anti-ALX/FPR2 (green; A, B, E, F, G, and J), phalloidin (red; D, E, I, and J), and propidium iodide nuclear stain (blue; A, C, E, F, H, and J). All images were obtained and analyzed using a confocal microscope. Arrows and arrowheads indicate acinar and ductal localization of ALX/FPR2, respectively. Scale bars, 50 μm.
DISCUSSION
Resolvins are highly potent, anti-inflammatory agents that control the duration and magnitude of inflammation in a variety of tissues (3, 9, 10, 19). Resolvin subtypes include the E series (RvE1 and RvE2, derived from eicosapentaenoic acid), the D series (RvD1 and RvD2, derived from docosahexanoic acid), and aspirin-triggered forms (AT-RvD1–6) (1, 21, 40). Resolvins were initially discovered as regulators of innate immune cells; for example, RvD1 and AT-RvD1 were demonstrated to be potent regulators of polymorphonuclear leukocyte (PMN) infiltration in brain, skin, and intestinal epithelium in vivo (40, 42). Further research demonstrated that resolvins also elicit their functions beyond the immune system. For instance, RvE1 triggers resolution of vascular inflammation and ADP-dependent activation in platelets (13), inhibits osteoclast growth and bone resorption in mice (20), and improves corneal staining and goblet cell density in a murine model of dry eye (7). Additionally, RvD1 and RvE1 block interstitial fibrosis in the obstructed kidney via inhibition of local fibroblast proliferation (35). More recently, we demonstrated RvD1's ability to prevent cytokine signaling and enhance tissue integrity in salivary epithelium (30). Together, these results indicate that different resolvin family members allow inflammatory resolution and restore homeostasis in a variety of tissues.
The RvD1 receptors ALX/FPR2 and GPR32 are seven-transmembrane proteins that do not seem to be linked to classic signal transduction machinery, as they do not activate Ca2+ signaling in freshly isolated human PMNs (24). Further research by our group using Par-C10 cells supports this notion (30). In the current study we were able to demonstrate that ALX/FPR2 was expressed in mSMG, with intense staining in cell membranes compared with cytosol (Fig. 1). However, AT-RvD1 was able to elicit intracellular Ca2+ responses in single mSMG cells (Fig. 2A), indicating that AT-RvD1 can activate RvD1 receptors through a classic G protein-coupled receptor signaling mechanism in these cells. We believe that this discrepancy (in terms of Ca2+ responses compared with other primary cells, i.e., PMNs) may be attributed to the fact that RvD1-mediated Ca2+ signaling varies among different cell types. From comparison of Ca2+ signaling between mSMG and Par-C10 cells, it is clear that a cell line does not completely recapitulate all the features of primary cells and that the RvD1-mediated intracellular Ca2+ response might have been lost during transformation (36).
Resolvins have been shown to activate intracellular kinases, including Erk1/2 and Akt, which regulate cell survival and integrity (25, 30, 31, 35). The results of the current study demonstrate that activation of ALX/FPR2 with AT-RvD1 also stimulates Erk1/2 and Akt phosphorylation in mSMG cells (Fig. 3, A and C) compared with cells incubated in the absence of AT-RvD1 (i.e., 0 min; Fig. 3, B and D). These results indicate that ALX/FPR2 is functional in primary salivary cells and is able to activate cell survival signals. These findings are consistent with our previous studies indicating ALX/FPR2 activity in the salivary cell line Par-C10 (30). The ability of AT-RvD1 to transiently phosphorylate Erk1/2 and Akt starting at 1 min in culture indicates that this lipid mediator initiates rapid signaling mechanisms to block stress generated by tissue culture conditions.
Several studies indicate the involvement of uncontrolled apoptosis in the pathogenesis of SS (27, 28, 45). Furthermore, enhanced apoptosis has been related to the impairment of secretory function and generation of autoantigens (17). More recently, it was shown that administration of caspase inhibitors in mouse models of SS ameliorated inflammation, indicating a critical role for caspase-mediated apoptosis (32). The decrease in TNF-α-mediated caspase-3 activation observed here suggests that AT-RvD1 blocks molecules upstream of caspase-3 (e.g., caspase-8 or TNF receptor type 1; Fig. 6) to promote cell survival in salivary cells. Interestingly, AT-RvD1 did not effectively reduce staurosporine-mediated caspase-3 activation to basal levels, suggesting a selective effect for AT-RvD1 in blocking the SS-associated proinflammatory cytokine TNF-α.
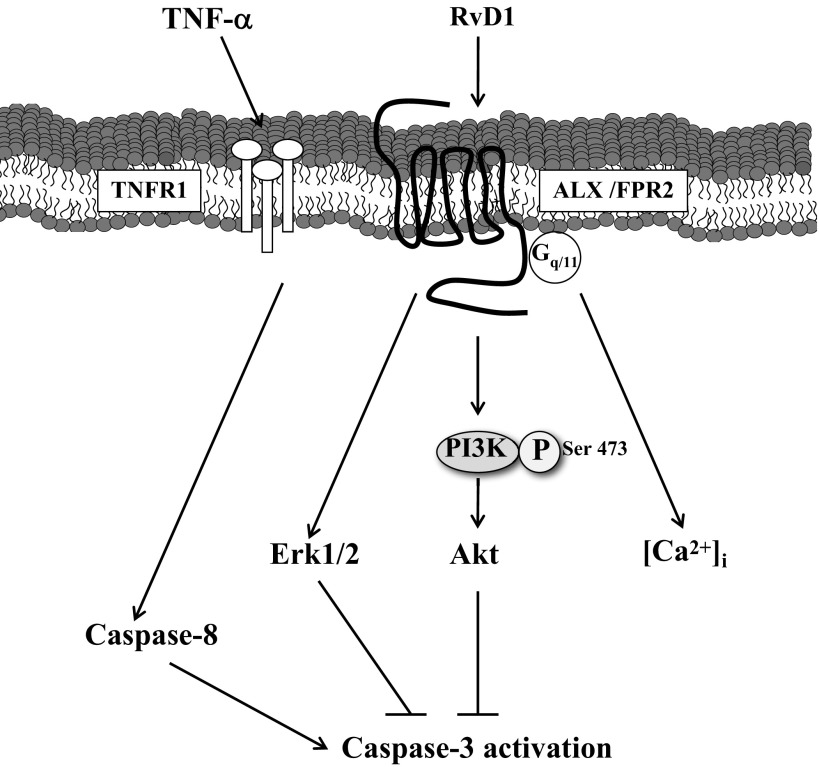
Fig. 6.
Proposed signaling mechanisms mediated by RvD1 in mSMG. RvD1 binds to ALX/FPR2 expressed on the membrane of acinar and ductal cells. RvD1 receptors signal through a classical 7-transmembrane G protein-coupled receptor, given that they activate intracellular Ca2+ ([Ca2+]i) release. After stimulation, AT-RvD1 activates Erk1/2 and Akt signaling, blocking TNF-α signaling and caspase-3 activity, leading to cell survival. TNFR1, TNF receptor type 1; PI3K, phosphatidylinositol 3-kinase.
As mentioned above, resolvins block inflammation and promote tissue repair in several in vivo and in vitro models (6, 26, 30), indicating potential therapeutic applications within the oral cavity. Our results indicate that ALX/FPR2 is expressed in human salivary glands with and without SS (Fig. 5). The fact that the receptor was highly expressed in the apical portion of minor salivary ducts suggests that resolvins are delivered to the oral cavity to promote wound healing and tissue repair through the resolution of inflammation.
In summary, the AT-RvD1 receptor is expressed in primary salivary cells and is able to increase a diverse set of intracellular signaling pathways such as Ca2+, Erk1/2, and Akt. Additionally, we were able to demonstrate that AT-RvD1 blocks TNF-α-mediated caspase-3 activation in mSMG (Fig. 6). The expression of ALX/FPR2 seems to be unaltered in hMSG with and without SS, indicating that RvD1 could be used as a therapeutic option to treat salivary gland inflammation in SS patients.
GRANTS
This work was supported by National Institute of Dental and Craniofacial Research Grants R21 DE-19721-01, 1R01 DE-022971-01, and 1R01 DE-021697-01 (to O. J. Baker). Data and specimens used in this study are from the Sjögren's International Collaborative Clinical Alliance, funded under National Institute of Dental and Craniofacial Research Contract N01 DE-32636, with funding support from the National Eye Institute and Office for Research in Women's Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.N., N.J.L., R.E.M., and A.D.M. performed the experiments; J.N. and A.A. analyzed the data; J.N., A.D.M., and O.J.B. interpreted the results of the experiments; J.N. prepared the figures; J.N. and O.J.B. drafted the manuscript; J.N., N.J.L., and O.J.B. edited and revised the manuscript; J.N., N.J.L., R.E.M., A.D.M., and O.J.B. approved the final version of the manuscript; O.J.B. is responsible for conception and design of the research.
REFERENCES
- 1.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, anti-inflammatory properties, and receptor for the ω-3 lipid mediator resolvin E1. J Exp Med 201: 713–722, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azuma M, Aota K, Tamatani T, Motegi K, Yamashita T, Harada K, Hayashi Y, Sato M. Suppression of tumor necrosis factor-α-induced matrix metalloproteinase 9 production by the introduction of a super-repressor form of inhibitor of nuclear factor κBα complementary DNA into immortalized human salivary gland acinar cells. Prevention of the destruction of the acinar structure in Sjogren's syndrome salivary glands. Arthritis Rheum 43: 1756–1767, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bento AF, Claudino RF, Dutra RC, Marcon R, Calixto JB. Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J Immunol 187: 1957–1969, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Chisholm DM, Mason DK. Labial salivary gland biopsy in Sjogren's disease. J Clin Pathol 21: 656–660, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniels TE, Fox PC. Salivary and oral components of Sjogren's syndrome. Rheum Dis Clin North Am 18: 571–589, 1992 [PubMed] [Google Scholar]
- 6.Dartt DA, Hodges RR, Li D, Shatos MA, Lashkari K, Serhan CN. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J Immunol 186: 4455–4466, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Paiva CS, Schwartz CE, Gjorstrup P, Pflugfelder SC. Resolvin E1 (RX-10001) reduces corneal epithelial barrier disruption and protects against goblet cell loss in a murine model of dry eye. Cornea 31: 1299–1303, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Delaleu N, Jonsson R, Koller MM. Sjogren's syndrome. Eur J Oral Sci 113: 101–113, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol 177: 5902–5911, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Eickmeier O, Seki H, Haworth O, Hilberath JN, Gao F, Uddin M, Croze RH, Carlo T, Pfeffer MA, Levy BD. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol 6: 256–266, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox PC, Bowman SJ, Segal B, Vivino FB, Murukutla N, Choueiri K, Ogale S, McLean L. Oral involvement in primary Sjogren syndrome. J Am Dent Assoc 139: 1592–1601, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Fox RI. Sjögren's syndrome. Lancet 366: 321–331, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Fredman G, Van Dyke TE, Serhan CN. Resolvin E1 regulates adenosine diphosphate activation of human platelets. Arterioscler Thromb Vasc Biol 30: 2005–2013, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goëb V, Salle V, Duhaut P, Jouen F, Smail A, Ducroix JP, Tron F, Le Loët X, Vittecoq O. Clinical significance of autoantibodies recognizing Sjögren's syndrome A (SSA), SSB, calpastatin and α-fodrin in primary Sjögren's syndrome. Clin Exp Immunol 148: 281–287, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenspan JS, Daniels TE, Talal N, Sylvester RA. The histopathology of Sjogren's syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol 37: 217–229, 1974 [DOI] [PubMed] [Google Scholar]
- 16.Gronert K. Lipoxins in the eye and their role in wound healing. Prostaglandins Leukot Essent Fatty Acids 73: 221–229, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Haneji N, Nakamura T, Takio K, Yanagi K, Higashiyama H, Saito I, Noji S, Sugino H, Hayashi Y. Identification of α-fodrin as a candidate autoantigen in primary Sjögren's syndrome. Science 276: 604–607, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, Petasis NA, Levy BD, Serhan CN, Van Dyke TE. RvE1 protects from local inflammation and osteoclast-mediated bone destruction in periodontitis. FASEB J 20: 401–403, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J 25: 2399–2407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrera B, Ohira T, Gao L, Omori K, Yang R, Zhu M, Muscara M, Serhan C, Van Dyke T, Gyurko R. An endogenous regulator of inflammation, resolvin E1, modulates osteoclast differentiation and bone resorption. Br J Pharmacol 155: 1214–1223, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem 278: 14677–14687, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Jimenez F, Aiba-Masago S, Al Hashimi I, Vela-Roch N, Fernandes G, Yeh CK, Talal N, Dang H. Activated caspase 3 and cleaved poly(ADP-ribose)polymerase in salivary epithelium suggest a pathogenetic mechanism for Sjogren's syndrome. Rheumatology (Oxford) 41: 338–342, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Kong L, Ogawa N, McGuff HS, Nakabayashi T, Sakata KM, Masago R, Vela-Roch N, Talal N, Dang H. Bcl-2 family expression in salivary glands from patients with primary Sjogren's syndrome: involvement of Bax in salivary gland destruction. Clin Immunol Immunopathol 88: 133–141, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci USA 107: 1660–1665, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li D, Hodges R, Jiao J, Carozza R, Shatos M, Chiang N, Serhan C, Dartt D. Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunol 6: 1119–1130, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li N, He J, Schwartz CE, Gjorstrup P, Bazan HE. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J Ocul Pharmacol Ther 26: 431–439, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manganelli P, Fietta P. Apoptosis and Sjögren syndrome. In: Seminars in Arthritis and Rheumatism. New York: Elsevier, 2003, p. 49–65 [DOI] [PubMed] [Google Scholar]
- 28.Matsumura R, Umemiya K, Kagami M, Tomioka H, Tanabe E, Sugiyama T, Sueishi M, Nakajima A, Azuma M, Okumura K. Glandular and extraglandular expression of the Fas-Fas ligand and apoptosis in patients with Sjogren's syndrome. Clin Exp Rheumatol 16: 561, 1998 [PubMed] [Google Scholar]
- 29.Nakamura H, Koji T, Tominaga M, Kawakami A, Migita K, Kawabe Y, Nakamura T, Shirabe S, Eguchi K. Apoptosis in labial salivary glands from Sjogren's syndrome (SS) patients: comparison with human T lymphotropic virus-I (HTLV-I)-seronegative and -seropositive SS patients. Clin Exp Immunol 114: 106–112, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Odusanwo O, Chinthamani S, McCall A, Duffey ME, Baker OJ. Resolvin D1 prevents TNF-α-mediated disruption of salivary epithelial formation. Am J Physiol Cell Physiol 302: C1331–C1345, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohira T, Arita M, Omori K, Recchiuti A, Van Dyke TE, Serhan CN. Resolvin E1 receptor activation signals phosphorylation and phagocytosis. J Biol Chem 285: 3451–3461, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okuma A, Hoshino K, Ohba T, Fukushi S, Aiba S, Akira S, Ono M, Kaisho T, Muta T. Enhanced apoptosis by disruption of the STAT3-IκB-ζ signaling pathway in epithelial cells induces Sjögren's syndrome-like autoimmune disease. Immunity 38: 450–460, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Pochet S, Garcia-Marcos M, Seil M, Otto A, Marino A, Dehaye JP. Contribution of two ionotropic purinergic receptors to ATP responses in submandibular gland ductal cells. Cell Signal 19: 2155–2164, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Polihronis M, Tapinos NI, Theocharis SE, Economou A, Kittas C, Moutsopoulos HM. Modes of epithelial cell death and repair in Sjogren's syndrome (SS). Clin Exp Immunol 114: 485–490, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qu X, Zhang X, Yao J, Song J, Nikolic-Paterson DJ, Li J. Resolvins E1 and D1 inhibit interstitial fibrosis in the obstructed kidney via inhibition of local fibroblast proliferation. J Pathol 228: 506–519, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Quissell D, Barzen K, Redman R, Camden J, Turner J. Development and characterization of SV40 immortalized rat parotid acinar cell lines. In Vitro Cell Dev Biol Animal 34: 58–67, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Rhodus NL. Oral pilocarpine HCl stimulates labial (minor) salivary gland flow in patients with Sjogren's syndrome. Oral Dis 3: 93–98, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Segal B, Bowman SJ, Fox PC, Vivino FB, Murukutla N, Brodscholl J, Ogale S, McLean L. Primary Sjogren's syndrome: health experiences and predictors of health quality among patients in the United States. Health Qual Life Outcomes 7: 46, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with anti-inflammatory actions generated from ω-3 fatty acids via cyclooxygenase 2-nonsteroidal anti-inflammatory drugs and transcellular processing. J Exp Med 192: 1197–1204, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of ω-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med 196: 1025–1037, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, Schiodt M, Umehara H, Vivino F, Zhao Y, Dong Y, Greenspan D, Heidenreich AM, Helin P, Kirkham B, Kitagawa K, Larkin G, Li M, Lietman T, Lindegaard J, McNamara N, Sack K, Shirlaw P, Sugai S, Vollenweider C, Whitcher J, Wu A, Zhang S, Zhang W, Greenspan J, Daniels T. American College of Rheumatology classification criteria for Sjogren's syndrome: a data-driven, expert consensus approach in the Sjogren's International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 64: 475–487, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem 282: 9323–9334, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Tapinos NI, Polihronis M, Tzioufas AG, Skopouli FN. Immunopathology of Sjogren's syndrome. Ann Med Interne 149: 17–24, 1998 [PubMed] [Google Scholar]
- 44.Turner J, Camden J. The influence of vasoactive intestinal peptide receptors in dispersed acini from rat submandibular gland on cyclic AMP production and mucin release. Arch Oral Biol 35: 103–108, 1990 [DOI] [PubMed] [Google Scholar]
- 45.Varin MM, Guerrier T, Devauchelle-Pensec V, Jamin C, Youinou P, Pers JO. In Sjögren's syndrome, B lymphocytes induce epithelial cells of salivary glands into apoptosis through protein kinase Cδ activation. Autoimmunity Rev 11: 252–258, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Walsh JG, Cullen SP, Sheridan C, Lüthi AU, Gerner C, Martin SJ. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc Natl Acad Sci USA 105: 12815–12819, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]