Abstract
To clarify the mechanism(s) underlying intracellular Ca2+ concentration ([Ca2+]i) oscillations induced by an elevation in extracellular Ca2+ concentration ([Ca2+]e) via the extracellular Ca2+-sensing receptor (CaR), we analyzed the pattern of [Ca2+]i response in multiple (2,303) individual HEK-293 cells transfected with the human CaR. An increase in the [Ca2+]e from 1.5 to 3 mM produced oscillatory fluctuations in [Ca2+]i in 70% of the cell population. To determine the role of PKC in the generation of [Ca2+]i oscillations, cells were exposed to increasing concentrations (0.5–5 μM) of the preferential PKC inhibitor Ro-31-8220 before stimulation by extracellular Ca2+. Ro-31-8220 at 3–5 μM completely eliminated the [Ca2+]e-evoked [Ca2+]i oscillations and transformed the pattern to a peak and sustained plateau response. Treatment with other broad PKC inhibitors, including GFI or Gö6983, produced an identical response. Similarly, treatment with Ro-31-8220 or GFI eliminated [Ca2+]e-evoked [Ca2+]i oscillations in colon-derived SW-480 cells expressing the CaR. Treatment with inhibitors targeting classic PKCs, including Gö6976 and Ro-32-0432 as well as small interfering RNA-mediated knockdown of PKCα, strikingly reduced the proportion of cell displaying [Ca2+]e-evoked [Ca2+]i oscillations. Furthermore, none of the cells analyzed expressing a CaR mutant in which the major PKC phosphorylation site Thr888 was converted to alanine (CaRT888A) showed [Ca2+]i oscillations after CaR activation. Our results show that [Ca2+]i oscillations induced by activation of the CaR in response to an increase in extracellular Ca2+ or exposure to the calcimimetic R-568 result from negative feedback involving PKCα-mediated phosphorylation of the CaR at Thr888.
Keywords: G protein-coupled receptor, single cell Ca2+ imaging, PKC inhibitor Ro-31-8220, PKC downregulation, mutant CaRt888a
oscillatory changes in intracellular Ca2+ concentration ([Ca2+]i) in response to receptor stimulation are fundamental mechanisms of signaling in excitable and nonexcitable cells implicated in the regulation of a variety of targets and processes (13), including Ca2+- and calmodulin-dependent protein kinase II (45), conventional protein kinase C (PKC) isoforms (32, 37), mitochondrial function (12, 18), and nuclear transcriptional activity leading to differential gene expression (21, 22, 25, 55). Although Ca2+ oscillations have attracted intense interest for several decades, the underlying mechanisms regulating their amplitude, frequency, and duration remain incompletely understood (13). Most models proposed to explain the mechanism by which [Ca2+]i oscillations are generated in response to G protein-coupled receptor (GPCR) activation are based broadly on the periodic production of inositol 1,4,5-trisphosphate (InsP3) produced by dynamic uncoupling of Gq/phospholipase C (PLC) or on the biphasic effects of [Ca2+]i on the InsP3 receptor (15, 47). However, definitive evidence identifying the mechanism(s) involved is available in only few instances.
The extracellular Ca2+-sensing receptor (CaR), a member of the C family of GPCRs originally identified in the parathyroid gland, is expressed in many tissues and organs (4, 5), including the gastrointestinal tract (33). Accordingly, the CaR not only maintains Ca2+ homeostasis but also plays multiple diverse roles in the control of normal and abnormal cell function (16, 19, 34, 42). A number of studies of CaR activation in individual living cells have shown that [Ca2+]i oscillates upon stimulation of CaR by an elevation in extracellular Ca2+ concentration ([Ca2+]e) within a physiological range (2, 10, 35–37, 53, 54). We proposed that intracellular [Ca2+]i oscillations induced by activation of the CaR in response to an increase in [Ca2+]e result from negative feedback involving cyclic PKC-mediated phosphorylation of the CaR at the inhibitory residue Thr888 (37, 54) but the PKC isoform(s) involved was not identified. Subsequently, Ward and colleagues (10) reported that [Ca2+]i oscillations were still observed in a significant number of cells expressing the CaRT888A mutant and stimulated by a small increase in [Ca2+]e. These investigators concluded that phosphorylation of CaR at Thr888 is not the exclusive determinant of CaR-induced [Ca2+]i oscillations (10) and proposed the existence of other phosphorylation sites or mechanisms. In fact, additional phosphorylation sites for PKC (Ser895) and PKA (Ser899) have been suggested in the COOH-terminal region of the CaR (1, 46). These considerations prompted us to reexamine whether PKC-mediated phosphorylation of the CaR at Thr888 is both necessary and sufficient for generating [Ca2+]e-evoked [Ca2+]i oscillations or additional mechanisms, including protein kinases other than PKC isoforms and phosphorylation sites other than Thr888, are also involved.
To identify the mechanisms leading to [Ca2+]i oscillations induced by activation of the CaR by increases in [Ca2+]e or exposure to the calcimimetic R-568, we analyzed the pattern of [Ca2+]i response (oscillatory or nonoscillatory) evoked by submaximal concentrations of [Ca2+]e in 2,303 individual HEK-293 cells transfected with wild-type or mutant human CaR. We used multiple approaches, including PKC inhibitors with different structure and specificity, prolonged exposure to phorbol-12,13-dibutyrate (PDBu) to downregulate classic and novel PKCs, small interfering (si)RNA-mediated depletion of PKCα, and a CaR mutant in which the major PKC phosphorylation site (Thr888) was altered by substitution of alanine for threonine (CaRT888A). Our results support the notion that PKC-mediated phosphorylation of the CaR at Thr888 is necessary and sufficient for generating [Ca2+]i oscillations in response to an increase in [Ca2+]e and identify PKCα as the major PKC isoform essential in this process.
MATERIALS AND METHODS
Materials.
Fura 2-AM, DMEM, and HBSS were obtained from Invitrogen (Carlsbad, CA). Ro-31-8220, Ro-32-0432, GFI (bisindolylmaleimide I), GFV (bisindolylmaleimide V), Go6983, Go6976, and HEPES were obtained from Calbiochem (San Diego, CA). Kb NB 142–70 was obtained from Tocris (Ellsville, MO). LY317615 and AEB071 {3-(1H-indol-3-yl)-4-[2-(4-methylpiperazin-1-yl)quinazolin-4-yl]-1H-pyrrole-2,5-dione} were obtained from Selleck Chemicals (Houston, TX). H-89 and 539654 were obtained from EMD Millipore (Billerica, MA). R-568 was obtained from R&D Systems (Minneapolis, MN). HBSS was supplemented with 0.2 mM CaCl2 and 20 mM HEPES. In experiments with R-568, bovine serum albumin was added at 0.5%. The final Ca2+ concentration and pH were 1.5 mM and 7.4, respectively.
Cell culture and transfection.
HEK-293 cells were maintained in culture in DMEM supplemented with 10% FBS as described previously (30, 53). For experimentation, cells were plated onto 18-mm diameter glass coverslips inside 35-mm plastic dishes, where they could be dually transiently transfected with a plasmid encoding the human CaR or a mutant CaR receptor (CaRT888A) and as a marker a plasmid encoding the sequence for DsRed (53). A line of HEK cells stably expressing the CaR, developed in our laboratory, was also used (37). SW-480 cells, a cell line derived from human colorectal adenocarcinoma in which the expression of endogenous CaR is nondetectable by RT-PCR, were transiently cotransfected with a plasmid encoding the human CaR (pCR3.1-CaR) and a plasmid encoding a red fluorescent protein (pDsRed-Express) to facilitate the identification of the transfected cells. After 16 h, the cultures were loaded with the Ca2+ indicator as described below.
The generation of the plasmid encoding the CaRT888A mutant was previously described (54). In experiments using siRNA, pooled siRNA duplexes were purchased from Invitrogen (Carlbad, CA). PKCα siRNA pools were designed to target the mRNA of PKCα (GenBank Accession No. NM_002737.2) and consist of two different duplexes oligo1 (UUCAUCUUCACCAAAUGGAGGC) and oligo2 (GCCUCCAUUUGAAGAUGAA). With the use of the reverse transfection method, the siRNA pool obtained was mixed with Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocol and added to 35-mm dishes. Control transfections were carried out with Dharmacon (Lafayette, CO) siCONTROL nontargeting siRNA four-oligo pool (catalog no. D-001206–13). At 3 days after transfection, cells were used for experiments and subsequent Western blot analysis to verify depletion of PKCα protein.
Immunoblotting and detection of PKCα.
HEK 293 cells were lysed in 2× SDS-PAGE sample buffer (20 mM Tris·HCl, pH 6.8, 6% SDS, 2 mM EDTA, 4% 2-mercaptoethanol, and 10% glycerol) and boiled for 10 min. After SDS-PAGE, proteins were transferred to Immobilon-P membranes. The transfer was carried out at 100 V, 0.4 A at 4°C for 4 h using a Bio-Rad transfer apparatus. The transfer buffer consisted of 200 mM glycine, 25 mM Tris, 0.01% SDS, and 20% CH3OH. For detection of protein, membranes were blocked using 5% nonfat dry milk in PBS (pH 7.2) and then incubated for ≥2 h with the PKCα antibody (Cell Signaling Technologies) diluted in PBS containing 3% nonfat dry milk. Primary antibody bound to immunoreactive bands was visualized by enhanced chemiluminescence detection with horseradish peroxidase-conjugated anti-mouse, anti-rabbit antibody, and a FUJI LAS-4000 Mini Luminescent Image Analyzer.
Measurement of [Ca2+]i.
Cells on coverslips were removed from the incubator, washed twice with saline, and then incubated in saline containing 5 μM fura 2-AM for 1 h at 37°C. Coverslips were then washed with saline and mounted in an experimental chamber (volume: 0.5 ml) perfused (1 ml/min) with heated saline at 37°C. The chamber in turn was placed on the stage of an inverted microscope (Axio Observer.A1) to which was attached a digital camera (AxioCam MRm) and operated with associated software (AxioVision, all components Carl Zeiss, Thornwood, NY). Ratio images (340 nm divided by 380 nm) were obtained in ∼1 s−1, and the average ratio values from small regions (10 μm2) from each cell were stored for offline analysis. Ratio values were calibrated and converted to Ca2+concentrations by using a series of Ca2+ buffers containing fura 2 (calcium calibration kit; Molecular Probes). Data are presented as means ± SE unless otherwise stated.
Statistics.
Data are reported as percentage of cells responding in a defined category (i.e., oscillating or nonoscillating cells). Bars on graphs with percentages are 95% confidence interval for a proportion (Instat, La Jolla, CA). Bars where confidence intervals were not overlapping with control confidence intervals are marked with an asterisk.
RESULTS
[Ca2+]e-evoked [Ca2+]i oscillations: dose-response relationships.
To examine the mechanism(s) underlying [Ca2+]e-evoked [Ca2+]i oscillations via CaR, we analyzed the pattern of [Ca2+]i response in individual HEK-293 cells transfected with the human CaR, an extensively used model system in studies of CaR regulation. Cells were loaded with the fluorescent Ca2+ indicator fura 2-AM and incubated in the presence of medium containing 1.5 mM [Ca2+]e. Intracellular Ca2+ imaging revealed that most transfected cells exhibited a stable [Ca2+]i with only a small proportion of cells (3–5%) exhibiting spontaneous oscillatory activity. In line with previous studies, an increase in the [Ca2+]e from 1.5 to 3 mM produced a rapid elevation in [Ca2+]i followed by striking oscillatory fluctuations in [Ca2+]i (Fig. 1A). As shown in Fig. 1, this pattern was observed in 74% of the cell population (n = 756 cells). Most other cells (24%) displayed a rapid peak and plateau response (Fig. 1B), which in some cases was preceded or followed by [Ca2+]i spikes of diminishing amplitude (Fig. 1C). Cells were classified as nonoscillatory “plateau” if the amplitude of the secondary peaks was <10% of the amplitude of the predominant peak. The plateau response was also seen with very small increases in [Ca2+]e as shown in the lower trace of Fig. 1B. Similar results were obtained in HEK-293 cells transiently transfected with a plasmid encoding the human CaR or in cells stably expressing the CaR. In contrast, an increase in [Ca2+]e from 1.5 to 3 mM did not induce any [Ca2+]i oscillations in nontransfected HEK-293 cells or in cells transfected with vector.
Fig. 1.
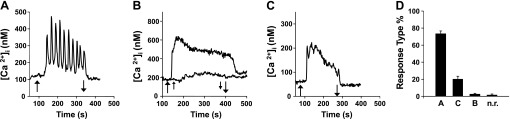
Patterns of intracellular of Ca2+ signaling in Ca2+-sensing receptor (CaR)HEK-293 cells. Starting from a resting concentration of 1.5 mM CaCl2, CaRHEK-293 cells were perfused with a solution containing 3 mM CaCl2 (upward arrow) for 200–300 s as indicated before return to perfusion containing 1.5 mM CaCl2 (downward arrow). A: typical tracing of an oscillatory response induced by increasing extracellular Ca2+ concentration ([Ca2+]e). B: peak and plateau response without intracellular Ca2+ concentration ([Ca2+]i) oscillations. Bottom trace: perfusion with 2.25 mM CaCl2. C: in some cases the pattern shown in B was preceded by [Ca2+]i spikes of diminishing amplitude. D: percentage of cells with each type of response (756 cells; n.r., not responding). In subsequent figures, the results are presented as the percentage of oscillating and nonoscillating cells in the population.
We also analyzed the pattern of [Ca2+]i response in individual HEK-293 cells transfected with the human CaR but challenged with different concentrations of extracellular Ca2+. The results are displayed in Fig. 2. An increase in the [Ca2+]e from 1.5 mM to either 2.25 mM or 3 mM produced an elevation in [Ca2+]i followed by oscillatory fluctuations in [Ca2+]i in most responding cells in the populations (Fig. 2A). In contrast, the majority of responding cells (90%) displayed a rapid peak and plateau response when the [Ca2+]e was elevated from 1.5 mM to either 5 or 10 mM (Fig. 2A). The results indicate that a small increase (0.75–1.5 mM) in [Ca2+]e elicits oscillatory fluctuations in [Ca2+]i via the CaR whereas a large increase (3.5–8.5 mM) in [Ca2+]e induces a peak and plateau response in most cells.
Fig. 2.
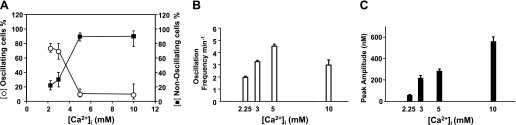
Fraction of CaRHEK-293 cells showing oscillations decreases with increasing external calcium concentration. Cells were studied after increasing the [Ca2+]e to 2.25, 3, 5, or 10 mM from the resting concentration of 1.5 mM. A: percentage of oscillating and nonoscillating cells. B: [Ca2+]i oscillation frequency as a function of extracellular Ca2+ concentration. C: amplitude of the peak [Ca2+]i in cells showing nonoscillatory pattern in response to an increase in extracellular Ca2+ concentration. Graphs reflect analysis based on 495 cells.
To further examine the pattern of [Ca2+]i response in response to increasing [Ca2+]e we also determined the average frequency of Ca2+-induced oscillations (Fig. 2B) and the amplitude of the peak in the cells that responded in a nonoscillatory manner (Fig. 2C). The average frequency of Ca2+-induced oscillations mediated by the CaR increased from 2 ± 0.1 min−1 (SE) to 3.3 ± 0.2 min−1 (SE) when the [Ca2+]e was increased from 1.5 to 2.25 and 3 mM, respectively (Fig. 2B). A further increase in [Ca2+]e to 5 or 10 mM induced a slight increase in the frequency of oscillations in the few cells that responded with this pattern. The amplitude of the [Ca2+]i peak of cells responding with a nonoscillatory pattern showed a striking dose-response relationship, as illustrated in Fig. 2C. The results displayed in Fig. 2 indicate that the pattern of [Ca2+]i response in individual HEK-293 cells transfected with the human CaR is sharply dose dependent. A small (physiological) increase in the concentration of extracellular Ca2+ elicited oscillatory [Ca2+]i fluctuations in most responding cells whereas a large increase in extracellular Ca2+, outside the range of homeostatic changes, resulted in a peak and plateau response in the vast majority of the cells. Consequently, we focused our subsequent experiments in defining the mechanism of the oscillatory response via the CaR.
Broad-spectrum PKC inhibitors eliminate [Ca2+]e-evoked [Ca2+]i oscillations.
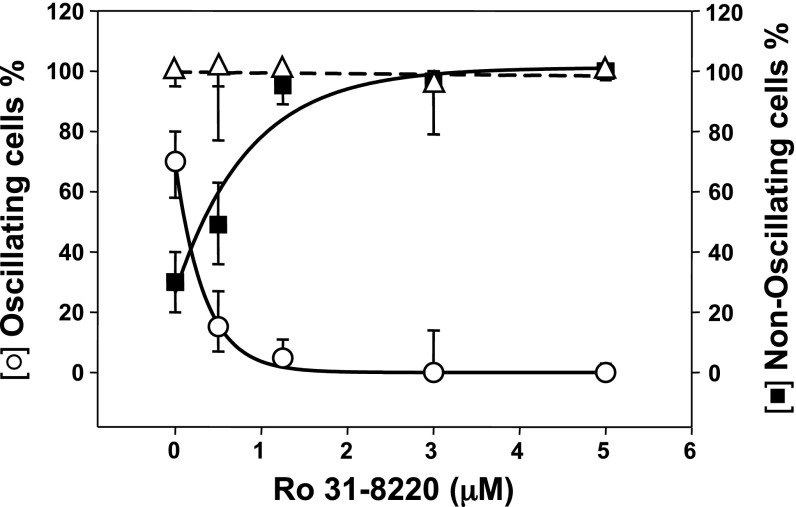
The multigene PKC family consists of classic (α, βI, βII, and γ), novel (δ, ε, η, and θ) and atypical (ζ and ι) isoforms (11). To determine the role of PKC in the generation of [Ca2+]i oscillations via the CaR, cells were exposed to increasing concentrations (0.5–5 μM) of the potent, cell-permeable PKC family inhibitor Ro-31-8220, also known as bisindolylmaleimide IX (52), for 1 h before stimulation by an increase in extracellular Ca2+. Exposure to Ro-31-8220 strikingly decreased the proportion of single responding cells displaying [Ca2+]e-evoked [Ca2+]i oscillations and produced a corresponding increase in the fraction of cells responding with a nonoscillatory pattern in a concentration-dependent manner (Fig. 3). Treatment with Ro-31-8220, at a concentration as low as 0.5 μM, reduced the proportion of cells exhibiting oscillations from 70% in the control to ∼15% in the treated cells. Cell exposure to Ro-31-8220 at higher concentrations (3–5 μM) completely eliminated the [Ca2+]e-evoked [Ca2+]i oscillations and transformed the pattern to a peak and sustained plateau response. It is noteworthy that the total number of cells responding to an elevation of [Ca2+]e was not altered by prior exposure to Ro-31-8220, at any of the concentrations tested (Fig. 3, triangles).
Fig. 3.
Exposure to the PKC inhibitor Ro-31-8220 eliminates [Ca2+]i oscillations in a dose-dependent manner. CaRHEK-293 cells were pretreated for 1 h with increasing concentrations of the PKC inhibitor Ro-31-8220. The cultures were then perfused with a solution containing 3 mM CaCl2. Results show the percentage of cells showing [Ca2+]i oscillations (○) or displaying a nonoscillatory response (■, type B + type C of Fig. 1). Also shown is the percentage of total responding cells (patterns A + B + C) in the population exposed to increasing concentrations of Ro-31-8220 (△).
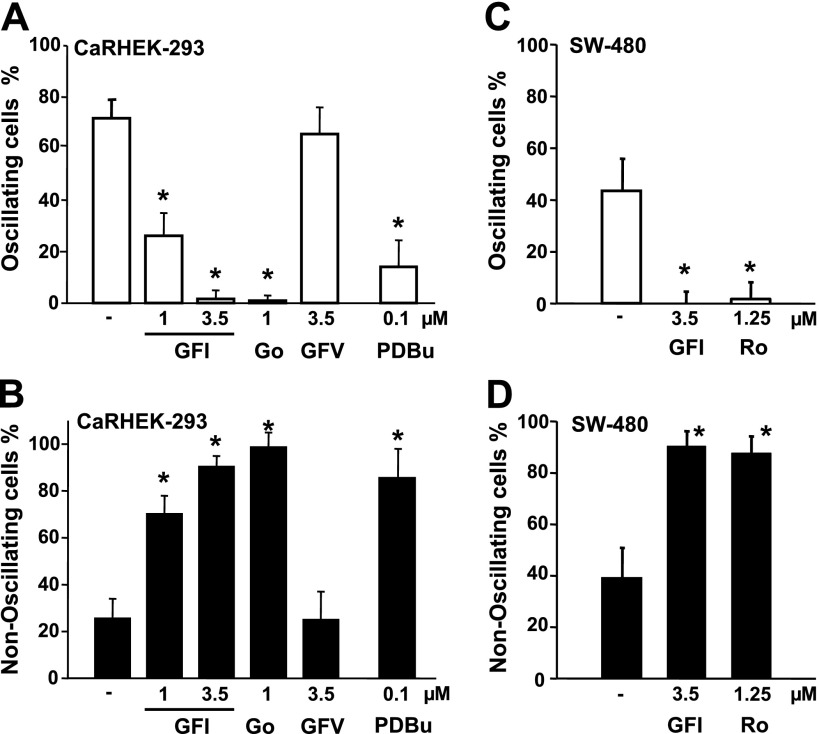
We next determined whether the conversion of the [Ca2+]i response from oscillatory to nonoscillatory elicited by an elevation of [Ca2+]e can be also induced by exposure to other inhibitors of the PKC family. Treatment with increasing concentrations of GFI (also known as GF 109203X or bisindolylmaleimide I), a different PKC inhibitor (48), produced a similar conversion of the response pattern from [Ca2+]e-evoked [Ca2+]i oscillations to peak and sustained plateau. At a concentration of 3.5 μM, treatment with GFI completely eliminated the oscillatory pattern of response in the CaRHEK-293 population (Fig. 4A). In contrast, treatment with an inactive analog, GFV (also known as bisindolylmaleimide V), did not have any discernable effect. Similar results obtained in HEK-293 cells transiently transfected with a plasmid encoding the human CaR or in cells stably expressing the CaR. To further substantiate the results obtained with Ro-31-8220 and GFI, we also determined the effect of Gö6983, another inhibitor of all isoforms of the PKC family (17). As shown in Fig. 4, A and B, exposure of CaRHEK-293 cells to Gö6983 completely eliminated the [Ca2+]e-evoked [Ca2+]i oscillations in these cells and transformed the pattern to a peak and sustained plateau response. To verify the PKC inhibitory activity of Ro-31-8220, GFI, and Gö6983 in CaRHEK-293 cells, at the concentrations used here, we determined whether exposure to these compounds prevented [Ca2+]e-evoked phosphorylation of MARCKS, a well-established substrate of PKCs (40). Treatment with these inhibitors completely suppressed [Ca2+]e-evoked MARCKS phosphorylation in CaRHEK-293 (data not shown).
Fig. 4.
Effect of PKC inhibitors GFI and Gö6983 on [Ca2+]e-evoked [Ca2+]i oscillations in CaRHEK-293 and SW-480 cells. A and B: CaRHEK-293 cells were treated with GF 109203X (GFI) at either 1 μM or 3.5 μM, GFV at 3.5 μM, and Gö6983 at 1 μM (Go) for 1 h, as indicated. Some cultures were treated with 100 nM phorbol-12,13-dibutyrate (PDBu) for 48 h. In all cases, cells were stimulated by raising [Ca2+]e to 3.0 mM. A: bars represent the percentage of cells showing [Ca2+]i oscillations. B: percentage of cells showing a nonoscillatory response. C and D: effect of PKC inhibitors GFI and Ro-31-8220 on [Ca2+]e-evoked [Ca2+]i oscillations in SW-480 cells expressing the CaR. SW-480 cells expressing the CaR were treated with the PKC inhibitors GF 109203X (GFI) at 3.5 μM or Ro-31-8220 (Ro) at 1 μM for 1 h before stimulation by raising [Ca2+]e to 3.0 mM. C: bars represent the percentage of cells showing [Ca2+]i oscillations. D: percentage of cells showing a nonoscillatory response. *Confidence intervals were not overlapping with control confidence intervals.
As pharmacological inhibitors such as Ro-31-8220, GFI, or Gö6983 may have targets other than PKC, we also decreased the protein expression of PKCs via phorbol ester-mediated downregulation. Specifically, long-term exposure of cells to phorbol esters leads to a marked and progressive reduction in the level of conventional PKCs (PKCs α, β, and γ) and novel PKCs (δ, ε, η, and θ), which require diacylglycerol (DAG) for their activation but does not affect the level of protein expression of the atypical isoforms of PKC. For this approach, CaRHEK-293 cells were treated for 48 h with 100 nM PDBu and subsequently stimulated by an elevation of [Ca2+]e. As shown in Fig. 4, A and B, PKC downregulation strikingly decreased the proportion of single cells displaying [Ca2+]e-evoked [Ca2+]i oscillations and produced a corresponding increase in the fraction of cells responding with a nonoscillatory pattern. Collectively, these results substantiated the notion that PKC activity is essential for the generation of [Ca2+]e-induced [Ca2+]i oscillations.
Broad-spectrum PKC inhibitors eliminate [Ca2+]e-evoked [Ca2+]i oscillations in SW-480 cells.
The CaR is increasingly implicated in the regulation of multiple cellular functions in the gastrointestinal tract. Using colon-derived cells expressing the CaR, including SW-480, we showed that an elevation of [Ca2+]e promoted striking intracellular Ca2+ oscillations in these cells (35). To determine the role of PKC in [Ca2+]i oscillations elicited in response to CaR stimulation in human colon-derived epithelial cells, SW-480 cells were transiently cotransfected with a plasmid encoding the human CaR (pCR3.1-CaR) and a plasmid encoding a red fluorescent protein (pDsRed-Express) to facilitate the identification of the transfected cells. In agreement with our previous results, single cell imaging of fura-2 loaded SW-480 cells expressing the CaR revealed that a rise in [Ca2+]e from 1.5 to 3.0 mM stimulated an increase in [Ca2+]i in most cells in the population (84%; n = 64 cells). Further analysis of SW-480 cells expressing the CaR indicated that a rise in [Ca2+]e induced [Ca2+]i oscillations in 44% of the population. Analysis of individual cells revealed that treatment with either Ro-31-8220 at 1.25μM (n = 57 cells) or GFI at 3.5 μM (n = 83 cells) completely eliminated the [Ca2+]e-evoked [Ca2+]i oscillations in CaR-expressing SW-480 cells and transformed the pattern to a nonoscillatory response (Fig. 4, C and D). It is noteworthy that the total number of cells responding to an elevation of [Ca2+]e was not altered by prior exposure to either Ro-31-8220 or GFI at the concentrations tested. The results presented in Figs. 3 and 4 substantiated the notion that PKC activity is essential for the generation of [Ca2+]i oscillations through the CaR in different cell types.
Preferential inhibitors of classic PKCs eliminate [Ca2+]e-evoked [Ca2+]i oscillations.
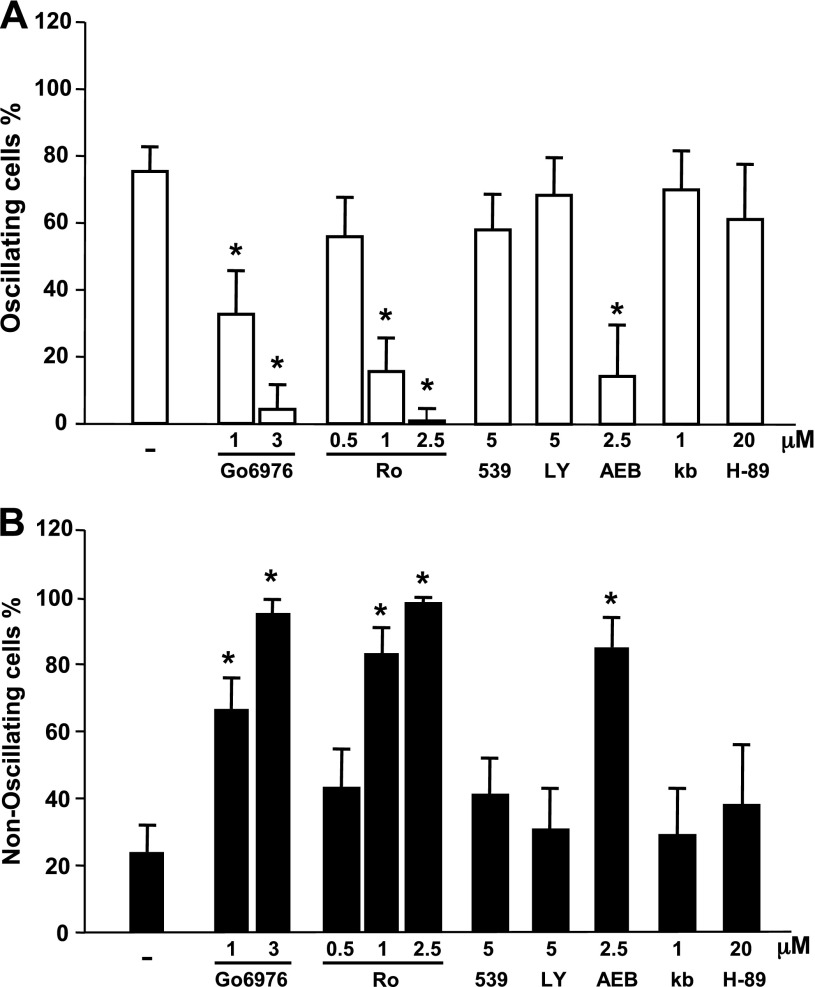
Our next objective was to identify the PKC isoform(s) involved in the negative feedback required for the generation of the [Ca2+]e-evoked [Ca2+]i oscillations. To determine whether classic PKCs mediate feedback inhibition of CaR signaling, we used Gö6976, which is a preferential inhibitor of these PKC isoforms. For example, Gö6976 potently inhibits PKCα (IC50 = 2.3 nM) but does not inhibit PKCδ, -ε, and -ζ isozymes, even at micromolar concentrations (27). Treatment of CaRHEK-293 cells with Gö6976 (1–3 μM) for 1 h eliminated the [Ca2+]e-evoked [Ca2+]i oscillations and transformed the pattern to a peak and sustained plateau response in a dose-dependent manner (Fig. 5). Similarly, cell exposure to the PKC inhibitor Ro-32-0432 (at 1.0–2.5 μM), which exhibits a 10-fold selectivity for PKCα (50), eliminated the [Ca2+]e-evoked [Ca2+]i oscillations and transformed the pattern to a peak and sustained plateau response (Fig. 5). In contrast, inhibitors preferentially targeting PKCβ (e.g., 539654 and Ly 3176155), at concentrations that were inhibitory in other systems (7, 8), had little effect on [Ca2+]e-evoked [Ca2+]i oscillations (Fig. 5.)
Fig. 5.
Inhibitors with selectivity for classic PKCs eliminated [Ca2+]e-evoked [Ca2+]i oscillations. CaRHEK-293 cells were treated for 1 h with the preferential inhibitors of classic PKCs Gö6976 (Go) and Ro-32-0432 (Ro) or inhibitors preferentially targeting PKC β, 539654 (539), Ly 3176155 (LY); an inhibitor for both classic and novel PKCs AEB071 (AEB), PKA (H-89), or PKD [kb NB 140–70 (kb)] at the indicated concentrations. Cells were then stimulated by raising [Ca2+]e to 3.0 mM. A: open bars show percentage of cells showing oscillations. B: closed bars show percentage of cells with nonoscillatory responses. *Confidence intervals were not overlapping with control confidence intervals.
AEB071 is a novel and potent inhibitor of classical and novel PKC isotypes (44). This agent has robust inhibitory activity on PKCα, PKCβ, and PKCθ and lesser inhibitory activity on PKCδ, PKCε, and PKCη. AEB071 did not inhibit ∼200 other kinases involved in cell signaling (44). As shown in Fig. 5, exposure of CaRHEK-293 cells to AEB071 at 2.5 μM, markedly reduced [Ca2+]e-evoked [Ca2+]i oscillations in these cells and transformed the pattern of [Ca2+]i signaling in these cells to a peak and sustained plateau response. We also verified that AEB071 prevented MARCKS phophorylation, a marker of PKC activity, in response to an increase in [Ca2+]e in CaRHEK-293 cells (data not shown).
The members of the PKD family are major downstream targets of PKCs in many cell types (41). Given that PKCs are upstream kinases in PKD activation and Gö6976 directly inhibits PKDs, we also examined the effect of a direct inhibitor of the PKD family on [Ca2+]e-evoked [Ca2+]i oscillations. As shown in Fig. 5, exposure to kb NB142–70 did not produce any significant change in [Ca2+]e-evoked [Ca2+]i oscillations. In addition to PKCs, it has been suggested that PKA also phosphorylates the CaR. We found that the potent PKA inhibitor H-89 at 20 μM did not produce any change in the proportion of cells displaying [Ca2+]e-evoked [Ca2+]i oscillations (Fig. 5). The results imply that neither PKD nor PKA are implicated in generating the negative feedback required for [Ca2+]i oscillatory responses induced by activation of the CaR in response to [Ca2+]e. In contrast, inhibition of classic PKC isoforms, especially PKCα, virtually eliminated [Ca2+]i oscillations in response to CaR activation in every cell in the population of CaR-transfected cells. The results suggest that PKCα activity is essential for the generation of sinusoidal [Ca2+]i oscillations via CaR activation.
Knockdown of PKCα with siRNA reduces [Ca2+]e-evoked [Ca2+]i oscillations in all cells.
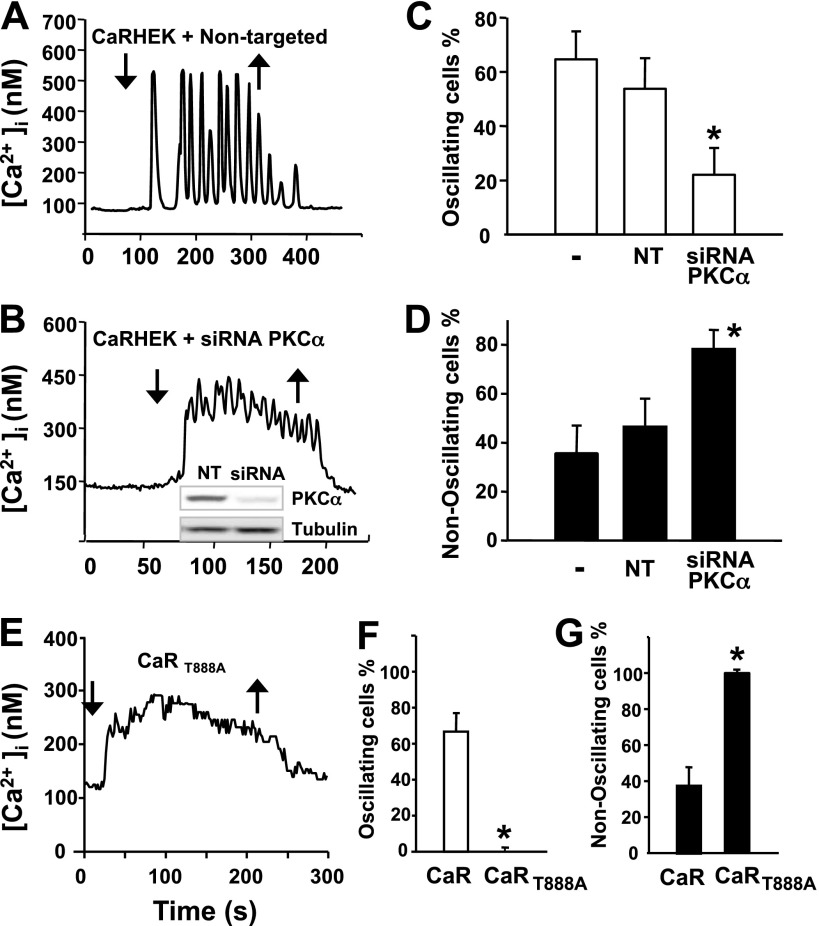
To examine whether PKCα is the predominant isoform that mediates the negative feedback required for the generation of [Ca2+]e-evoked [Ca2+]i oscillations, we depleted its expression in CaRHEK-293 via transient transfection of siRNA targeting PKCα and tested [Ca2+]e-evoked [Ca2+]i oscillations in the resulting PKCα-deficient cells. Initially, we verified that transfection of CaRHEK-293 cells with the siRNAs targeting PKCα caused a marked decrease in the level of protein expression of this PKC isoform (Fig. 6B). In contrast, transfection of the nontargeted siRNA did not produce any decrease in the level of PKCα expression in CaRHEK-293 cells (Fig. 6B). The salient feature of the results is that siRNA-mediated knockdown of PKCα in CaRHEK-293 cells strikingly reduced the proportion of cells displaying [Ca2+]e-evoked [Ca2+]i oscillations, compared with cells transfected with nontargeted siRNA (Fig. 6, B, C, and D). These results identify PKCα as the predominant isoform of the PKC family that mediates the negative feedback required for the generation of [Ca2+]e-evoked [Ca2+]i oscillations in CaRHEK-293 cells.
Fig. 6.
A–D: knockdown of PKCα reduced [Ca2+]e-evoked [Ca2+]i oscillations in CaRHEK-293 cells. From a resting [Ca2+]e of 1.5 mM, [Ca2+]e was increased to 3.0 mM (downward arrows). Upward arrows show return to 1.5 mM [Ca2+]e. A: typical individual tracing of [Ca2+]i vs. time from a CaRHEK-293 cell transfected with nontargeted oligonucleotides. B: typical [Ca2+]e-evoked [Ca2+]i response in a cell transfected with siRNA targeting PKCα. Inset: cells lysates were analyzed by SDS-PAGE and immunoblotting with a PKCα polyclonal antibody and total β-tubulin to verify equal gel loading. C and D: data summary for all 187 analyzed cells in control (-), nontargeted (NT), and siRNA PKCα (siRNA PKCα) conditions. E–G: HEK-293 cells expressing CaRT888A displayed nonoscillatory [Ca2+]i responses even in response to a small increase in [Ca2+]e. E: CaRHEK-293 cells expressing wild-type CaR and HEK-293 cells expressing CaRT888A were stimulated by raising [Ca2+]e to 2.25 mM (downward arrow). Upward arrow shows return to 1.5 mM [Ca2+]e. F and G: bars represent the data summary for 225 cells. *Confidence intervals were not overlapping with control confidence intervals.
Mutation of Thr888 of the CaR eliminates [Ca2+]e-evoked [Ca2+]i oscillations in all cells.
Motif-based profile-scanning programs recognized the sequence surrounding Thr888 (FKVAARATLRRSNVSRKRSS) as the best PKCα-putative phosphorylation site in the amino acid sequence of the CaR. Accordingly, previous results indicated that mutation of Thr888 to a nonphosphorylatable amino acid either eliminated (12) or reduced (10) the number of cells displaying [Ca2+]e-evoked [Ca2+]i oscillations. If CaR-mediated [Ca2+]i oscillations are generated by the periodic phosphorylation of Thr888 by PKCα, mutation of Thr888 to a nonphosphorylatable amino acid should abolish [Ca2+]i oscillations mediated by this receptor in all the cells in the population. To test this prediction, we extended our previous studies and analyzed multiple cells expressing a CaR in which Thr888 was mutated to alanine (T888A). As shown in Fig. 6 E, HEK-293 cells expressing CaRT888A did not show [Ca2+]i oscillations after CaR activation by increases in [Ca2+]e to 2.25 mM, while cells with the wild-type CaR continued to show oscillations. A smaller increment in [Ca2+]e (0.75 mM) was chosen to minimize the possibility that cells with CaRT888A showed only increased sensitivity to [Ca2+]e. The [Ca2+]i response consisted of a rapid rise in [Ca2+]i followed by a sustained phase of elevated [Ca2+]i. Critically, of 225 responding cells analyzed from independent preparations that responded to an increase in [Ca2+]e to 2.25 mM, all cells showed this behavior, i.e., none exhibited [Ca2+]i oscillations (Fig. 6, E-G).
[Ca2+]i oscillations in response to R-568: dependence on PKC activity.
Phenylalkylamines, including R-568, are positive allosteric modulators (calcimimetics) that activate CaR by increasing the apparent affinity of CaR for [Ca2+]e through binding to different sites from the orthosteric agonists (20, 31). A previous study showed that calcimimetics induce [Ca2+]i oscillations in the presence of a threshold concentration of extracellular Ca2+ (28), but the role of PKC in mediating the oscillatory response was not examined. Here, we determined whether R-568, like [Ca2+]e, also induces different patterns of [Ca2+]i response (i.e., oscillatory and nonoscillatory) at different concentrations. To examine this possibility, we monitored [Ca2+]i in individual CaRHEK-293 cells that were exposed to increasing concentrations of R-568 (10–500 nM). As shown in Fig. 7A, exposure of the cells to 50 nM R-568 induced striking [Ca2+]i oscillations in most responding cells in the population (∼90%). A 10-fold increase in the concentration of R-568 fold to 500 nM (Fig. 7B) did not change the pattern of the response, as most cells in that population responded with robust [Ca2+]i oscillations, characterized by an average frequency of 5.2 ± 0.2 min−1 (Fig. 7C). The effect of R-568 was mediated through the CaR since this compound did not induce any detectable change in [Ca2+]i in HEK cells that did not express the CaR.
Fig. 7.
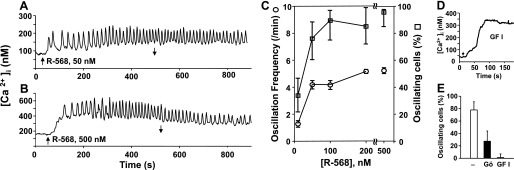
Exposure to the calcimimetic R-568 induced [Ca2+]i oscillations in CaRHEK-293 cells. A: perfusion with saline containing R-568 at 50 nM was started at the upward arrow. R-568 was removed at the time marked by a downward arrow. B: perfusion with R-568 at 500 nM. C: percentage of cells oscillating, and the oscillation frequency are plotted at R-568 concentrations of 10, 50, 100, 200, and 500 nM. Data from 145 cells. D: response to 50 nM R-568 (marked by upward arrow) after pretreatment with the PKC inhibitor GFI (1 h, 3.5 μM). E: both PKC inhibitors Gö6983 (1 h, 1 μM) and GFI inhibited the oscillatory response to 50 nM R-568 (black bars) compared with control (white bar). Data are from 101 cells.
The results demonstrating an important difference between [Ca2+]e and R-568 in their dose-dependent effects on [Ca2+]i oscillations prompted us to determine whether R-568-induced [Ca2+]i oscillations also depend critically on PKC-mediated feedback. We found, for the first time, that treatment of the CaRHEK-293 cells with broad-spectrum PKC inhibitors, including GFI and Go6983, strikingly decreased the proportion of cells displaying R-568-evoked [Ca2+]i oscillations and produced a corresponding increase in the fraction of cells responding with a nonoscillatory pattern, e.g., peak and plateau (Fig. 7, D and E). Our results imply that R-568 and small increase in the extracellular Ca2+ concentration induce [Ca2+]i oscillations via a similar mechanism involving PKC.
DISCUSSION
Multiple lines of evidence indicate that the CaR plays a critical role in maintaining Ca2+ homeostasis in the organism (5). It is increasingly recognized that the CaR also plays multiple additional roles in the control of normal and abnormal cell function (16, 19, 34, 38, 42), including pancreatic insulin secretion (43), inflammasome activation (24, 39), β-catenin signaling (34), epithelial cell proliferation (35), metastatic cancer dissemination (3), and stem cell differentiation (38). Accordingly, the mechanisms of CaR signaling are attracting intense interest in cell regulation.
Previous studies using HEK-293 and epithelial colon cells led us to propose a model to explain the mechanism by which the CaR triggers Ca2+ oscillations in response to an increase in [Ca2+]e In this model, [Ca2+]e-induced CaR activation stimulates PLCβ, which catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to produce two second messengers: InsP3 and DAG. InsP3 binds to its receptor in the endoplasmic reticulum (ER) and induces a conformational change that leads to the mobilization of Ca2+ from the ER stores whereas DAG and Ca2+ activate classic PKCs. Activated cPKCs then phosphorylate the CaR at the inhibitory Thr888 providing the negative feedback needed to cause periodic InsP3 production and sinusoidal [Ca2+]i oscillations (37, 54). However, other phosphorylation sites and/or mechanisms underlying the generation of oscillatory response have been suggested (10). Consequently, here we expanded our previous studies to determine whether PKC-mediated phosphorylation of the CaR at Thr888 is both necessary and sufficient for generating [Ca2+]e-evoked [Ca2+]i oscillations or additional mechanisms, including protein kinases other than PKC and phosphorylation sites other than Thr888, are also involved. Furthermore, we also examined the role of PKC in the generation of [Ca2+]i oscillations in response to R-568, a positive allosteric modulator of the CaR.
In the present study we continued to exploit HEK-293 cells as a model system to elucidate CaR-signaling mechanisms. We found that a small (physiological) increase in the concentration of extracellular Ca2+ (0.75–1.5 mM) elicited oscillatory [Ca2+]i fluctuations in most responding cells whereas a large increase in extracellular Ca2+, outside the range of homeostatic changes (e.g., 3.5–8.5 mM), resulted in a peak and plateau response in the vast majority of the cells. We conclude that the oscillatory pattern of response is of physiological interest, and accordingly, we extended previous studies defining the mechanism of the oscillatory response via the CaR. Based on the analysis of multiple (2,303) single cells, we conclude that [Ca2+]i oscillations induced by activation of the CaR in response to a small increase (0.75–1.5 mM) in extracellular Ca2+ result from negative feedback involving PKCα-mediated phosphorylation of the CaR at Thr888. Our evidence is as follows: 1) treatment with inhibitors that suppress the activity of all isoforms of the PKC family, including Ro-31-8220, GFI, and Gö6983, or downregulation of classic and novel PKC isoforms by prolonged treatment with PDBu completely eliminated [Ca2+]e-evoked [Ca2+]i oscillations and transformed the pattern to a peak and sustained plateau response; 2) similar results were obtained with human colon-derived SW-480 epithelial cells, transfected with a plasmid encoding the human CaR; 3) treatment of HEK 293 cells expressing CaR with either Gö6976, a potent inhibitor of classic PKCs (27), or Ro-32-0432, which exhibits a 10-fold selectivity for PKCα (50), also eliminated the [Ca2+]e-evoked [Ca2+]i oscillations and transformed the pattern to a peak and sustained plateau response; 4) crucially, siRNA-mediated knockdown of PKCα in CaRHEK-293 cells strikingly reduced the proportion of cell displaying [Ca2+]e-evoked [Ca2+]i oscillations, compared with cells transfected with nontargeted siRNA; these results identified, for the first time, PKCα as the PKC isoform necessary for the generation of [Ca2+]e-evoked [Ca2+]i oscillations; and 5) analysis of cells expressing a CaR mutant in which Thr888 was converted to alanine (CaRT888A) demonstrated that all cells responded displaying a peak and sustained plateau response rather than [Ca2+]i oscillations, even when in this case the cells were challenged with a small increase in [Ca2+]e to compensate for an increase in agonist sensitivity of this mutant. Collectively, our results support a model that envisages [Ca2+]i oscillations induced by activation of the CaR in response to a physiological increase in [Ca2+]e as a consequence of negative feedback mediated by PKCα-mediated phosphorylation of the CaR at Thr888.
In accord with this model, the structure of PKCα is ideally suited to mediate periodic phosphorylation of the CaR at Thr888 in response to repetitive spikes of [Ca2+]i. It is well established that the regulatory region of PKCα contains a C2 domain that binds Ca2+ and mediates PKCα membrane translocation (9). Indeed, Newton and colleagues (49) demonstrated that oscillations in [Ca2+]i induce oscillations in PKCα activity, emphasizing the role of Ca2+ binding to the C2 domain in the control of the activity and membrane localization of PKCα in the cell. Our own previous results demonstrated that [Ca2+]e-evoked [Ca2+]i oscillations in HEK-293 cells expressing CaR are associated with periodic InsP3 production and oscillatory translocations of PKCα to the plasma membrane (37). All these findings support a critical role of PKCα in mediating [Ca2+]i oscillations via its C2 domain. CaR also generates [Ca2+]i oscillations in response to aromatic amino acids in the presence of a threshold [Ca2+]e that are characterized by lower frequency Ca2+ spikes that return to the baseline levels (53). Amino acid-induced [Ca2+]i oscillations via the CaR are thought to be generated by periodic changes in TRPC1 activity (36) and thus differ from [Ca2+]e-evoked [Ca2+]i oscillations mediated by PKCα.
There is considerable pharmacological interest in the development of allosteric modulators of CaR function. Although these agents also elicit [Ca2+]i oscillations, the mechanism(s) involved has not been elucidated. Here, we show that the positive allosteric modulator R-568 induced [Ca2+]i oscillations even at high concentrations. Our results demonstrate that [Ca2+]i oscillations induced by R-568 were dependent on PKC activity. Thus we propose that allosteric modulators by increasing the affinity of the CaR for [Ca2+]e induce [Ca2+]i oscillations via periodic feedback inhibition by PKC.
Interestingly, a mutation in Thr888 has been recently discovered in human patients with autosomal dominant hypocalcemia (23), demonstrating the functional importance of PKC-mediated phosphorylation of this site and the generation of [Ca2+]i oscillations in vivo. Members of the PKC family have been the subject of intense interest as potential drug targets in a variety of diseases (14, 26, 51). For example, AEB071 is being tested as a therapeutic agent in a variety of clinical settings, including autoimmune diseases (14) and ocular melanoma (51). However, the results of clinical trials using PKC inhibitors have not been successful, largely owing to inadequate therapeutic effects and/or unanticipated adverse reactions (29). In view of the results presented here, it is important to consider that administration of inhibitors of PKCα in vivo will cause an increased responsiveness of the CaR to [Ca2+]e potentially leading to alterations in Ca2+ homeostasis.
GRANTS
This work was supported by the Department of Veterans Affair Grant 1I01BX001473, Veterans Affairs Greater Los Angeles Healthcare System (to E. Rozengurt) and in part supported by National Institutes of Health Grants P30-DK-41301 and P01-CA-163200. O. Rey is the recipient of a PICT 2012–0875, FONCyT-MINCyT, Argentina.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.H.Y. and O.R. performed experiments; S.H.Y., O.R., and J.S.-S. analyzed data; S.H.Y., O.R., J.S.-S., and E.R. interpreted results of experiments; S.H.Y. and J.S.-S. prepared figures; S.H.Y. and E.R. drafted manuscript; S.H.Y., O.R., J.S.-S., and E.R. edited and revised manuscript; E.R. conception and design of research; E.R. approved final version of manuscript.
ACKNOWLEDGMENTS
E. Rozengurt holds the Ronald S. Hirshberg Chair in Pancreatic Cancer Research.
REFERENCES
- 1.Bai M, Trivedi S, Lane CR, Yang Y, Quinn SJ, Brown EM. Protein kinase C phosphorylation of threonine at position 888 in Ca2+o-sensing receptor (CaR) inhibits coupling to Ca2+ store release. J Biol Chem 273: 21267–21275, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Breitwieser GE, Gama L. Calcium-sensing receptor activation induces intracellular calcium oscillations. Am J Physiol Cell Physiol 280: C1412–C1421, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Brennan SC, Thiem U, Roth S, Aggarwal A, Fetahu I, Tennakoon S, Gomes AR, Brandi ML, Bruggeman F, Mentaverri R, Riccardi D, Kallay E. Calcium sensing receptor signaling in physiology and cancer. Biochim Biophys Acta 1833: 1732–1744, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 366: 575–580, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81: 239–297, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Buitrago L, Bhavanasi D, Dangelmaier C, Manne BK, Badolia R, Borgognone A, Tsygankov AY, McKenzie SE, Kunapuli SP. Tyrosine phosphorylation on spleen tyrosine kinase (syk) is differentially regulated in human and murine platelets by protein kinase C isoforms. J Biol Chem 288: 29160–29169, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Zhang X, Jia C, Xu J, Gao H, Zhang G, Du X, Zhang H. Membrane depolarization increases membrane PtdIns(4,5)P2 levels through mechanisms involving PKC betaII and PI4 kinase. J Biol Chem 286: 39760–39767, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho W, Stahelin RV. Membrane binding and subcellular targeting of C2 domains. Biochim Biophys Acta 1761: 838–849, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Davies SL, Ozawa A, McCormick WD, Dvorak MM, Ward DT. Protein kinase C-mediated phosphorylation of the calcium-sensing receptor is stimulated by receptor activation and attenuated by calyculin-sensitive phosphatase activity. J Biol Chem 282: 1504815056, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol 279: L429–L438, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Drago I, Pizzo P, Pozzan T. After half a century mitochondrial calcium in- and efflux machineries reveal themselves. EMBO J 30: 4119–4125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dupont G, Combettes L, Bird GS, Putney JW. Calcium oscillations. Cold Spring Harb Perspect Biol 3: a004226, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evenou JP, Wagner J, Zenke G, Brinkmann V, Wagner K, Kovarik J, Welzenbach KA, Weitz-Schmidt G, Guntermann C, Towbin H, Cottens S, Kaminski S, Letschka T, Lutz-Nicoladoni C, Gruber T, Hermann-Kleiter N, Thuille N, Baier G. The potent protein kinase C-selective inhibitor AEB071 (sotrastaurin) represents a new class of immunosuppressive agents affecting early T-cell activation. J Pharmacol Exp Ther 330: 792–801, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87: 593–658, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geibel JP, Hebert SC. The functions and roles of the extracellular Ca2+-sensing receptor along the gastrointestinal tract. Annu Rev Physiol 71: 205–217, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Gschwendt M, Dieterich S, Rennecke J, Kittstein W, Mueller HJ, Johannes FJ. Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett 392: 77–80, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82: 415–424, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol 4: 530–538, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Hu J, Reyes-Cruz G, Chen W, Jacobson KA, Spiegel AM. Identification of acidic residues in the extracellular loops of the seven-transmembrane domain of the human Ca2+ receptor critical for response to Ca2+ and a positive allosteric modulator. J Biol Chem 277: 46622–46631, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Q, Deshpande S, Irani K, Ziegelstein RC. [Ca2+]i oscillation frequency regulates agonist-stimulated NF-kappaB transcriptional activity. J Biol Chem 274: 33995–33998, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Kar P, Nelson C, Parekh AB. CRAC channels drive digital activation and provide analog control and synergy to Ca2+-dependent gene regulation. Curr Biol 22: 242–247, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Lazarus S, Pretorius CJ, Khafagi F, Campion KL, Brennan SC, Conigrave AD, Brown EM, Ward DT. A novel mutation of the primary protein kinase C phosphorylation site in the calcium-sensing receptor causes autosomal dominant hypocalcemia. Eur J Endocrinol 164: 429–435, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492: 123–127, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature 392: 936–941, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Liu Q, Chen X, Macdonnell SM, Kranias EG, Lorenz JN, Leitges M, Houser SR, Molkentin JD. Protein kinase Cα, but not PKCβ or PKCγ, regulates contractility and heart failure susceptibility: implications for ruboxistaurin as a novel therapeutic approach. Circ Res 105: 194–200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem 268: 9194–9197, 1993 [PubMed] [Google Scholar]
- 28.Miedlich S, Gama L, Breitwieser GE. Calcium sensing receptor activation by a calcimimetic suggests a link between cooperativity and intracellular calcium oscillations. J Biol Chem 277: 49691–49699, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Mochly-Rosen D, Das K, Grimes KV. Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov 11: 937–957, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Needham LK, Rozengurt E. Gα12 and Gα13 stimulate Rho-dependent tyrosine phosphorylation of focal adhesion kinase, paxillin and p130 Crk-associated substrate. J Biol Chem 273: 14626–14632, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Nemeth EF, Steffey ME, Hammerland LG, Hung BC, Van Wagenen BC, DelMar EG, Balandrin MF. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc Natl Acad Sci USA 95: 4040–4045, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oancea E, Meyer T. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell 95: 307–318, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Quarles LD. Extracellular calcium-sensing receptors in the parathyroid gland, kidney, and other tissues. Curr Opin Nephrol Hypertens 12: 349–355, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Rey O, Chang W, Bikle D, Rozengurt N, Young SH, Rozengurt E. Negative cross-talk between calcium-sensing receptor and beta-catenin signaling systems in colonic epithelium. J Biol Chem 287: 1158–1167, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rey O, Young SH, Jacamo R, Moyer MP, Rozengurt E. Extracellular calcium sensing receptor stimulation in human colonic epithelial cells induces intracellular calcium oscillations and proliferation inhibition. J Cell Physiol 225: 73–83, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rey O, Young SH, Papazyan R, Shapiro MS, Rozengurt E. Requirement of the TRPC1 cation channel in the generation of transient Ca2+ oscillations by the calcium-sensing receptor. J Biol Chem 281: 38730–38737, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Rey O, Young SH, Yuan J, Slice L, Rozengurt E. Amino acid-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor are mediated by a phospholipase C/inositol 1,4,5-trisphosphate-independent pathway that requires G12, Rho, filamin-A, and the actin cytoskeleton. J Biol Chem 280: 22875–22882, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Riccardi D, Kemp PJ. The calcium-sensing receptor beyond extracellular calcium homeostasis: conception, development, adult physiology, and disease. Annu Rev Physiol 74: 271–297, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Rossol M, Pierer M, Raulien N, Quandt D, Meusch U, Rothe K, Schubert K, Schoneberg T, Schaefer M, Krugel U, Smajilovic S, Brauner-Osborne H, Baerwald C, Wagner U. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nat Commun 3: 1329, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rozengurt E. Signal transduction pathways in the mitogenic response to G protein-coupled neuropeptide receptor agonists. J Cell Physiol 177: 507–517, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem 280: 13205–13208, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Saidak Z, Mentaverri R, Brown EM. The role of the calcium-sensing receptor in the development and progression of cancer. Endocr Rev 30: 178–195, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Sassmann A, Gier B, Grone HJ, Drews G, Offermanns S, Wettschureck N. The Gq/G11-mediated signaling pathway is critical for autocrine potentiation of insulin secretion in mice. J Clin Invest 120: 2184–2193, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skvara H, Dawid M, Kleyn E, Wolff B, Meingassner JG, Knight H, Dumortier T, Kopp T, Fallahi N, Stary G, Burkhart C, Grenet O, Wagner J, Hijazi Y, Morris RE, McGeown C, Rordorf C, Griffiths CE, Stingl G, Jung T. The PKC inhibitor AEB071 may be a therapeutic option for psoriasis. J Clin Invest 118: 3151–3159, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soderling TR, Chang B, Brickey D. Cellular signaling through multifunctional Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 276: 3719–3722, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Stepanchick A, McKenna J, McGovern O, Huang Y, Breitwieser GE. Calcium sensing receptor mutations implicated in pancreatitis and idiopathic epilepsy syndrome disrupt an arginine-rich retention motif. Cell Physiol Biochem 26: 363–374, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor CW, Tovey SC. IP(3) receptors: toward understanding their activation. Cold Spring Harb Perspect Biol 2: a004010, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toullec D, Pianetti P, Coste H, Bellevergue P, Grandperret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. The bisindolylmaleimide Gf-109203x is a potent and selective inhibitor of protein kinase-C. J Biol Chem 266: 15771–15781, 1991 [PubMed] [Google Scholar]
- 49.Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol 161: 899–909, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J 294: 335–337, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu X, Li J, Zhu M, Fletcher JA, Hodi FS. Protein kinase C inhibitor AEB071 targets ocular melanoma harboring GNAQ mutations via effects on the PKC/Erk1/2 and PKC/NF-kappaB pathways. Mol Cancer Ther 11: 1905–1914, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeo EJ, Exton JH. Stimulation of phospholipase D by epidermal growth factor requires protein kinase C activation in Swiss 3T3 cells. J Biol Chem 270: 3980–3988, 1995 [DOI] [PubMed] [Google Scholar]
- 53.Young SH, Rozengurt E. Amino acids and Ca2+ stimulate different patterns of Ca2+ oscillations through the Ca2+-sensing receptor. Am J Physiol Cell Physiol 282: C1414–C1422, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Young SH, Wu SV, Rozengurt E. Ca2+-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor require negative feedback by protein kinase C. J Biol Chem 277: 46871–46876, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Zhu L, Song S, Pi Y, Yu Y, She W, Ye H, Su Y, Hu Q. Cumulated Ca2(+) spike duration underlies Ca2(+) oscillation frequency-regulated NFkappaB transcriptional activity. J Cell Sci 124: 2591–2601, 2011 [DOI] [PubMed] [Google Scholar]