Abstract
Intrauterine growth-restricted (IUGR) fetuses experience prolonged hypoxemia, hypoglycemia, and elevated norepinephrine (NE) concentrations, resulting in hypoinsulinemia and β-cell dysfunction. Previously, we showed that acute adrenergic blockade revealed enhanced insulin secretion responsiveness in the IUGR fetus. To determine whether chronic exposure to NE alone enhances β-cell responsiveness afterward, we continuously infused NE into fetal sheep for 7 days and, after terminating the infusion, evaluated glucose-stimulated insulin secretion (GSIS) and glucose-potentiated arginine-induced insulin secretion (GPAIS). During treatment, NE-infused fetuses had greater (P < 0.05) plasma NE concentrations and exhibited hyperglycemia (P < 0.01) and hypoinsulinemia (P < 0.01) compared with controls. GSIS during the NE infusion was also reduced (P < 0.05) compared with pretreatment values. GSIS and GPAIS were approximately fourfold greater (P < 0.01) in NE fetuses 3 h after the 7 days that NE infusion was discontinued compared with age-matched controls or pretreatment GSIS and GPAIS values of NE fetuses. In isolated pancreatic islets from NE fetuses, mRNA concentrations of adrenergic receptor isoforms (α1D, α2A, α2C, and β1), G protein subunit-αi-2, and uncoupling protein 2 were lower (P < 0.05) compared with controls, but β-cell regulatory genes were not different. Our findings indicate that chronic exposure to elevated NE persistently suppresses insulin secretion. After removal, NE fetuses demonstrated a compensatory enhancement in insulin secretion that was associated with adrenergic desensitization and greater stimulus-secretion coupling in pancreatic islets.
Keywords: adrenergic receptor, β-cell, intrauterine growth restriction, uncoupling protein 2, catecholamines
small-for-gestational age or intrauterine growth-restricted (IUGR) infants are at greater risk for developing metabolic diseases such as type 2 diabetes mellitus (29, 40, 54). Impaired insulin secretion is associated with a diabetic phenotype indicating that in utero complications can permanently compromise β-cell development and function (27, 28). A fetal sheep model with placental insufficiency-induced intruterine growth restriction shares many similarities with human IUGR fetuses, such as asymmetric growth, hypoxemia, hypoglycemia, hypoinsulinemia, and hypercatecholaminemia [epinephrine and norepinephrine (NE)] (4, 16, 18, 22, 23, 32, 41, 47). Furthermore, glucose-stimulated insulin secretion (GSIS) and β-cell mass are lower in IUGR sheep fetuses, which also replicate features in human IUGR fetuses (34–36, 41, 53). Several characteristics of the fetal IUGR environment, including hypoglycemia, hypoxemia, and hypercatecholaminemia, are proposed to cause hypoinsulinemia and pancreatic β-cell dysfunction (22).
NE inhibits insulin secretion via the α2-adrenergic receptors (ARs) in fetal sheep and other species (26, 48, 51, 56). Chronic exposure to high catecholamine concentrations often causes ARs to become desensitized by downregulation of their transcription (5, 12, 14). However, in IUGR fetal sheep islets, α2A-AR mRNA concentrations were elevated (32), and genetic variants that increase α2A-AR are associated with a higher incidence of type 2 diabetes in man (6, 17, 49). In our previous studies in IUGR sheep fetuses, we showed that elevated plasma NE concentrations chronically suppress insulin concentrations (32, 37). Strikingly, AR antagonists administered to acutely block the action of chronically elevated NE concentrations revealed an enhancement in glucose-stimulated insulin concentrations in IUGR fetuses that was equivalent to control fetuses, although β-cell mass was less in IUGR fetuses (32, 35). There was also an enhancement in fractional insulin release from isolated islets in IUGR fetuses that indicates β-cell-specific compensatory adaptations (12, 36). Therefore, chronic exposure to high NE appears to produce a β-cell compensatory response distal to the ARs and enhanced insulin stimulus-secretion coupling.
In sheep fetuses, previous experiments have demonstrated that a 7-day infusion of NE slows growth and attenuates cardiovascular and metabolite effects (3). The objective of this study was to determine whether sustained exposure to elevated NE, independent of other intrauterine growth restriction-related deficiencies, acutely enhances secretagogue-stimulated insulin secretion after the exogenous administration of NE has been terminated for ≥3 h. In addition, we associated the compensatory enhancement in insulin secretion responsiveness in NE-infused fetuses with lower expression of AR and uncoupling protein 2 (UCP2) in pancreatic islets compared with vehicle-infused controls while also demonstrating that regulatory genes for insulin secretion or β-cell function were unaffected (2, 11, 19, 42).
MATERIALS AND METHODS
Ethical approval.
Twelve Columbia-Rambouillet crossbred ewes carrying singleton fetuses were purchased from Nebeker Ranch (Lancaster, CA) and managed in compliance with the Institutional Animal Care and Use Committee at the University of Arizona, which approved our study and is accredited by the American Association for Accreditation of Laboratory Animal Care. Ewes were fed Standard-Bread Alfalfa Pellets (Sacate Pellet Mills) and provided water ad libitum.
Animal preparations.
Animals were randomly assigned to control (n = 6) or NE (n = 6) treatments. At 126 ± 1 days of gestational age (dGA), indwelling polyvinyl catheters were surgically placed in the fetus and ewe, as described previously (33). Fetal catheters for blood sampling were placed in the abdominal aorta via hindlimb pedal arteries, and infusion catheters were placed in the femoral veins via the saphenous veins. Maternal catheters were placed in the femoral artery and vein for arterial sampling and venous infusions. All catheters were tunneled subcutaneously to the ewe's flank, exteriorized through a skin incision, and kept in a plastic mesh pouch sutured to the ewe's skin. Ewes were allowed to recover for 5 days before the first GSIS and glucose-potentiated arginine-induced insulin secretion (GPAIS) studies were conducted.
Experimental design and treatment protocol.
Fetal GSIS and GPAIS studies were performed at 132 ± 1 dGA to establish pretreatment insulin secretion responsiveness. Chronic infusions of NE and vehicle (described below) were initiated following the studies (treatment day 0). On day 7, chronic infusions were terminated for ≥3 h prior to acute insulin secretion responsiveness being evaluated. GSIS studies were also completed on each NE-infused fetus on day 3 (mid-NE treatment) to evaluate the effectiveness of NE suppression during treatment.
In the chronic NE treatment group, NE bitartrate (Bedford Laboratories) diluted with 0.3% ascorbic acid and saline (0.9% NaCl) was infused continuously in the fetal vein. During the 7-day infusion period, the dose of NE concentration was started at 1 μg/min for the first 24 h, increased to 2 μg/min for days 2–4, and finally increased to 4 μg/min for days 5–7, as described previously (3). Control fetuses received an infusion of saline with 0.3% ascorbic acid. After the final GSIS study at 139 ± 1 dGA, both ewes and fetuses were euthanized with an overdose of pentobarbital sodium (86 mg/kg, Euthasol; Virbac Animal Health, Fort Worth, TX). Perirenal adipose, liver, heart, lung, and kidneys were dissected and weighed.
GSIS and GPAIS studies.
Square-wave hyperglycemic clamps were performed as described previously (21). Three baseline blood samples were collected at 5-min intervals before the hyperglycemic clamp was initiated. Following the last baseline sample, at time designated 0 min, a glucose bolus (110 mg/kg estimated fetal weight) was given to the fetuses and followed immediately by a continuous infusion of 33% dextrose solution (Abbott Laboratories, Abbott Park, IL) that was varied to maintain fetal glucose concentration at ∼2.4 mM. This is the recommended value to achieve maximum glucose-stimulated insulin concentrations in singleton sheep fetuses (21). During the hyperglycemic clamp, three fetal arterial plasma samples were collected every 5 min starting from 35 min. Following the hyperglycemic clamp, an arginine bolus (261 mg of arginine in 0.4 M sodium acetate) was administered, and plasma samples were collected at 5, 10, 20, and 30 min after the arginine bolus infusion. Maternal blood was transfused continuously into the fetus (5 ml/h; −60 min to completion) to compensate for blood collections.
Blood collection and analysis.
Fetal blood was collected in syringes lined with EDTA (Sigma-Aldrich, St. Louis, MO) and centrifuged (16,000 g) for 2 min at 4°C to separate plasma. Plasma glucose concentrations were measured immediately with an YSI model 2700 SELECT Biochemistry Analyzer (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin and NE concentrations were measured with an ovine insulin ELISA (Alpco Diagnostics, Windham, NH) and Noradrenaline ELISA (Labor Diagnostika Nord), respectively, as described previously (20). Additional fetal blood samples were collected in heparin-lined syringes (Elkins-Sinn, Cherry Hill, NJ) for blood gas and oxygen saturation measurements with an ABL 725 (Radiometer, Copenhagen, Denmark).
Pancreatic islet isolation.
Islets were isolated from the fetal pancreas after a Liberase BlendZyme III (0.175 mg/ml; Roche, Indianapolis, IN) digestion and purified with a Histopaque density gradient (Sigma-Aldrich), as described previously (36, 50). After isolation, islets were washed in Krebs-Ringer buffer containing 0.5% BSA and then cultured overnight at 37°C in 95% O2-5% CO2 in RPMI 1640 media supplemented with 2.8 mM glucose (Sigma-Aldrich), 2% fetal bovine serum, and penicillin-streptomycin-neomycin (50 U, 50 μg, and 100 μg, respectively, Sigma-Aldrich). Islets were hand-picked into a 1.5-ml tube and centrifuged at 800 g for 5 min. After removing the supernatant, the pellets were stored at −80°C until RNA was extracted.
RNA extraction.
RNA was extracted from isolated pancreatic islets with the AllPrep DNA/RNA/Protein Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Quality and concentration of the RNA were determined by measuring absorbance at 260 and 280 nm with the NanoDrop ND-1000 Spectrophotometer (NanoDrop, Wilmington, DE). RNA integrity was evaluated with an Experion Automated Electrophoresis System (Bio-Rad Laboratories, Hercules, CA). PCR amplification was performed on RNA (∼1 μg) that was reverse transcribed into cDNA with Superscript III reverse transcriptase (Invitrogen Life Technologies, Carlsbad, CA).
Primer design and cDNA cloning.
Synthetic oligonucleotide primers were designed against sequences for ovine α1(A, B, D)-ARs, α2(A, B, C)-ARs, β(1, 2, 3)-ARs (12), insulin (GenBank accession no. U00659), pancreatic duodenal homeobox 1 (PDX-1; JF728303), glucose transporter 2 (Glut2; AJ318925), Gαi-1 (JX012067), Gαi-2 (JX012068), Gαi-3 (JX012069), Gαo (JX012070), Gαq (JX012071), Gαs (forward, 5′-GAC CAG GAC CTG CTT CGC TGC-3′; reverse, 5′-AGC CTC CTG CAG ACG GTT GGT-3′), Gαz (forward, 5′-GCT GCC GGA CCA TGG GAT GTC-3′; reverse, 5′-TCT TGC CCG AGT TGC TGG TGC-3′), Gβ1 (forward, 5′-CCG GCC AGC AGA CAA CCA CAT-3′; reverse, 5′-CTG CCG GCA CAT CCC TTC TCG-3′), Gβ2 (JX012072), Gβ3 (JX012073), UCP2 (forward, 5′-TCC CCT GTC GAC GTG GTC AAG A-3′; reverse, 5′-TGC AGC TGG AGC CAA TGC TGA TCA G-3′), Kir6.2 (forward, 5′-ACA GGA CGT GTT CAC CAC GCT-3′; reverse, 5′-CGA AAA GGA GTG GAT GCT GGT AAC G-3′), and L-type voltage-dependent calcium channel α1D subunit (CACNA1D; forward, 5′-GCC GGG AAG ACC CGG AGA TAC A-3′; reverse, 5′-AGG CCG CTC AAA GTC CAA GCT G-3′). All DNA products were cloned and sequenced as described previously (12, 13).
Quantitative real-time PCR.
The relative expression levels of mRNA transcripts were determined by quantitative PCR (qPCR) using SYBR Green (Qiagen) in an iQ5 Real-Time PCR Detection System (Bio-Rad). After an initial denaturation for 15 min, all reactions went through 40 cycles at 96°C (30 s), annealing temperature (30 s), and 72°C (10 s), at which point the fluorescent measurement was collected. Optimal annealing temperatures for different primer sets were determined using a temperature gradient (54–62°C) and examined on an agarose gel for specificity. Melt-curve analysis, starting at 60°C with an increase of 0.2°C every 6 s to 96°C, was performed at the end of the amplification to confirm product homogeneity.
PCR efficiency was determined with gene-specific plasmid DNA, for which threshold cycles (CT) were linear over six orders of magnitude. Samples were run in triplicate. The results were normalized to the reference gene ribosomal protein S15, the average ΔCT was analyzed by the comparative ΔCT method (CT gene of interest − CT reference gene), and fold change was determined by the 2−ΔΔCT method (46).
Statistical analysis.
Data for body and organ weights and qPCR (ΔCT) were analyzed by one-way ANOVA, using the general linear model procedure of SAS (SAS 9.3; SAS Institute, Cary, NC). Insulin concentrations during basal and hyperglycemic steady-state periods were compared between treatments and studies. GSIS responsiveness was taken to be the difference in mean insulin concentration between the hyperglycemic and baseline states and was compared between treatments and studies. GPAIS was analyzed as the area under the curve relative to basal insulin concentrations for each sheep fetus (Prism 5.01; GraphPad Software, La Jolla, CA). Biochemical, hematological, and hormone measurements for daily, GSIS, and GPAIS studies were analyzed by ANOVA, using the MIXED procedure with fetus as a random effect (SAS 9.3). The midtreatment GSIS in NE fetuses was compared with pretreatment values with a nonparametric Kruskal-Wallis test (proc npar1way; SAS 9.3). All values are expressed as means ± SE. P values ≤0.05 were considered significant.
RESULTS
Body weights and organ weights.
Body weights were not different between control ewes (56.0 ± 4.5 kg) and ewes with NE-infused fetuses (49.8 ± 3.3 kg) or between control (3.8 ± 0.4 kg) and NE-infused fetuses (3.3 ± 0.2 kg). No treatment effects were found for fetal organ weights (Table 1).
Table 1.
Organ and tissue weights in control and NE-infused fetal sheep
| Tissue Weight, g |
||
|---|---|---|
| Control (n = 6) | NE (n = 6) | |
| Brain | 52.5 ± 1.7 | 50.9 ± 1.5 |
| Heart | 25.0 ± 2.2 | 24.4 ± 1.1 |
| Kidneys | 19.9 ± 1.5 | 18.4 ± 1.2 |
| Liver | 98.8 ± 11.6 | 88.3 ± 5.1 |
| Lungs | 123.8 ± 8.9 | 109.8 ± 5.9 |
| Perirenal adipose | 13.8 ± 1.5 | 15.2 ± 0.8 |
Values are means ± SE.
NE, norepinephrine.
Daily fetal plasma biochemical values.
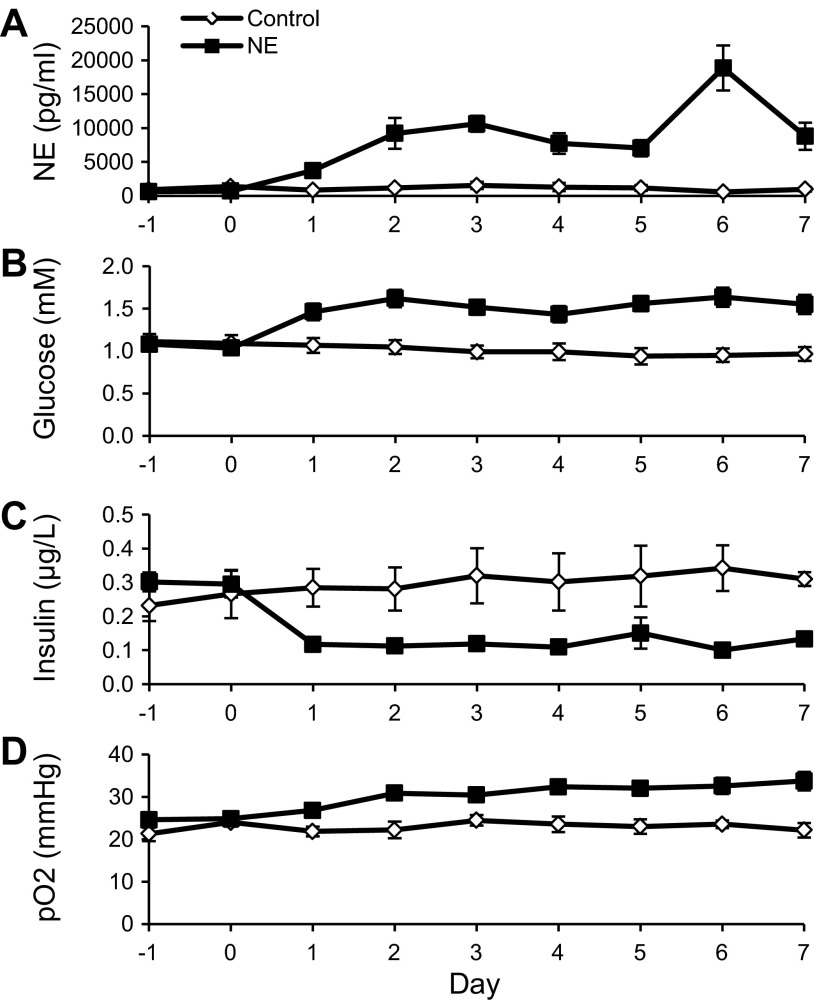
Fetal plasma glucose, insulin, and NE concentrations were not different between groups prior to treatment (Fig. 1). During the 7-day infusion, average NE concentrations were ninefold higher in NE-infused fetuses than control fetuses (9,343 ± 1,047 vs. 1,059 ± 142 pg/ml, P < 0.01; Fig. 1A). Insulin concentrations were 61% lower in NE fetuses compared with control fetuses (0.12 ± 0.01 vs. 0.31 ± 0.03 μg/l, P < 0.01; Fig. 1C). NE-infused fetuses had greater plasma glucose concentrations (1.54 ± 0.04 vs. 0.99 ± 0.03 mM, P < 0.01; Fig. 1B) and partial pressure of oxygen (Po2; 31.29 ± 0.65 vs. 22.98 ± 0.56 mmHg, P < 0.01; Fig. 1D) compared with controls. Hematocrit and blood pH were not different between treatments throughout the 7-day infusion period.
Fig. 1.
Daily biochemical values during treatment conditions. Plasma norepinephrine (NE) concentrations (A), plasma glucose concentrations (B), plasma insulin concentrations (C), and arterial blood partial pressure of oxygen (Po2; D) are presented for control (n = 6) and NE fetuses (n = 6) relative to days of treatment. The samples collected on day 0 were prior to the chronic NE or vehicle infusions being initiated. Subsequent sampling was performed during the chronic infusions initiated on day 0 following the pretreatment studies and continued through day 7. Significant differences between control and NE-infused fetuses are indicated in results.
GSIS studies.
Among the pretreatment GSIS studies, fetal plasma glucose concentrations of NE fetuses were not different from controls at baseline but were lower (P < 0.05) during the hyperglycemic clamp (Table 2). In the posttreatment GSIS studies, plasma glucose concentrations at baseline were 19% greater in NE fetuses than controls. However, posttreatment GSIS hyperglycemic glucose concentrations of NE-infused fetuses were not different from controls or from the pretreatment hyperglycemic values. During all GSIS studies, the mean glucose infusion rates needed to maintain the hyperglycemic steady state were similar between treatment groups within the study period.
Table 2.
Hematological values and glucose infusion rate during GSIS studies
| Parameter | Condition | Control | NE | P Value |
|---|---|---|---|---|
| Pre-NE infusion | ||||
| Glucose, mmol/h | Infusion rate | 10.75 ± 1.44 | 10.69 ± 1.12 | NS |
| Glucose, mM | Baseline | 1.19 ± 0.07 | 1.14 ± 0.03 | NS |
| Hyperglycemia | 2.48 ± 0.03 | 2.24 ± 0.03 | <0.05 | |
| Hematocrit, % | Baseline | 34.78 ± 0.82 | 34.00 ± 1.00 | NS |
| Hyperglycemia | 34.73 ± 0.80 | 34.22 ± 0.96 | NS | |
| Po2, mmHg | Baseline | 24.48 ± 0.96 | 24.29 ± 0.83 | NS |
| Hyperglycemia | 23.66 ± 0.99 | 23.75 ± 0.70 | NS | |
| NE, pg/ml | Baseline | 1,262.33 ± 178.76 | 588.89 ± 65.86 | NS |
| Hyperglycemia | 1,434.80 ± 98.11 | 693.23 ± 95.80 | <0.05 | |
| Post-NE infusion | ||||
| Glucose, mmol/h | Infusion rate | 14.79 ± 1.90 | 10.09 ± 1.59 | NS |
| Glucose, mM | Baseline | 0.97 ± 0.05 | 1.15 ± 0.04 | <0.01 |
| Hyperglycemia | 2.38 ± 0.03 | 2.37 ± 0.03 | NS | |
| Hematocrit, % | Baseline | 34.74 ± 0.91 | 30.53 ± 0.87 | <0.01 |
| Hyperglycemia | 35.00 ± 0.84 | 30.68 ± 0.85 | <0.01 | |
| Po2, mmHg | Baseline | 24.57 ± 0.77 | 30.52 ± 0.80 | <0.01 |
| Hyperglycemia | 24.08 ± 0.88 | 32.19 ± 1.06 | <0.01 | |
| NE, pg/ml | Baseline | 903.80 ± 140.37 | 3,189.40 ± 469.70 | <0.01 |
| Hyperglycemia | 1,329.35 ± 181.32 | 3,415.22 ± 392.04 | <0.01 |
Values are means ± SE and represent plasma glucose and NE concentrations, hematocrit, and partial pressure of blood oxygen (Po2) for treatment conditions.
GSIS, glucose-stimulated insulin secretion; NS, not significant.
Differences were determined between treatment groups for pre- and posttreatment GSIS studies.
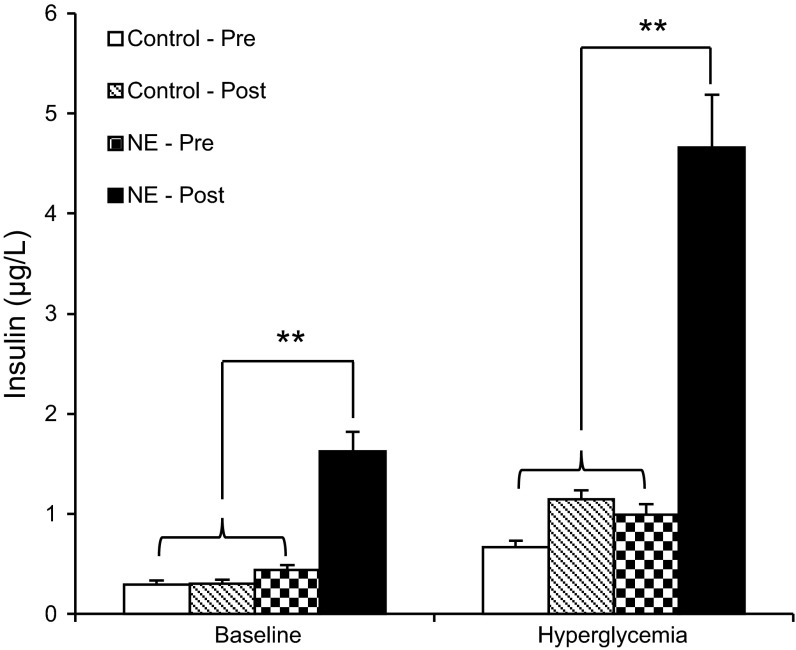
In the pretreatment GSIS studies, fetal plasma insulin concentrations at baseline and hyperglycemic steady-state periods were not different between control fetuses and NE fetuses (Fig. 2). Insulin concentrations during the NE infusion were decreased at both the baseline and hyperglycemic periods (0.09 ± 0.01 μg/l, baseline; 0.40 ± 0.16 μg/l, hyperglycemic; P < 0.01) compared with NE pretreatment values (0.44 ± 0.05 μg/l, baseline; 0.99 ± 0.10 μg/l, hyperglycemic). Three hours after the 7-day NE infusion was terminated, NE fetuses had fivefold greater (P < 0.01) insulin concentrations at baseline and fourfold greater (P < 0.01) insulin concentrations during the hyperglycemic clamp compared with their own pretreatment GSIS studies. Posttreatment baseline and hyperglycemic insulin concentrations in NE fetuses were greater than control insulin concentrations. Among controls, insulin concentrations during the GSIS studies (both at baseline and during hyperglycemic steady state) were not different between pre- and posttreatment periods (Fig. 2).
Fig. 2.
Insulin concentrations during pre- (pre) and posttreatment (post) glucose-stimulated insulin secretion (GSIS) studies. Mean plasma insulin concentrations during baseline and hyperglycemic steady-state periods for pre- and posttreatment GSIS studies are presented for control and NE-infused fetuses. **Significance, P < 0.01.
Hematocrit, Po2, and pH at baseline and hyperglycemic steady states were similar between NE and control fetuses prior to the chronic infusion (Table 2). During the posttreatment GSIS, the hematocrit was 12% lower (P < 0.01) at baseline and hyperglycemic states in NE fetuses compared with controls. Po2 was greater (P < 0.01) during the baseline and hyperglycemic clamp in NE fetuses compared with controls.
During the pretreatment GSIS studies, fetal plasma NE concentrations were similar between treatment groups at baseline, but control fetuses had higher (P < 0.05) plasma NE concentrations at hyperglycemic state compared with NE fetuses. During the posttreatment GSIS studies, plasma NE concentrations of NE fetuses were 3.5-fold greater (P < 0.01) than control fetuses at baseline and hyperglycemic steady states (Table 2).
GPAIS studies.
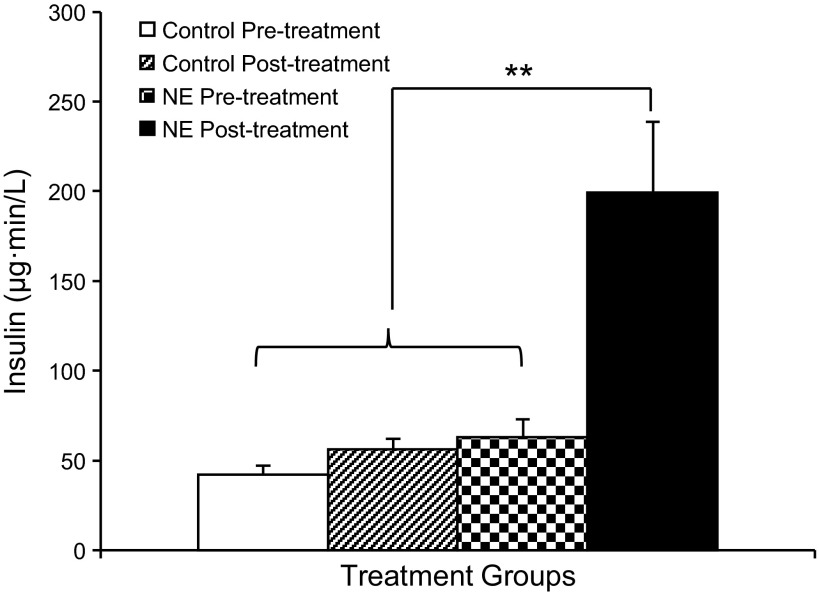
Fetal plasma insulin concentrations following the arginine bolus reached maximum concentrations after 5 min in all fetuses. The pretreatment GPAIS area under the curve measurement was not different between controls and NE fetuses. During the posttreatment GPAIS studies, insulin concentrations of NE-infused fetuses were greater (P < 0.05), and the area under the curve was 3.6-fold greater (P < 0.01) compared with NE pretreatment and control values (Fig. 3).
Fig. 3.
Insulin concentrations during pre- and posttreatment GPAIS studies. In the GPAIS studies, area under the curve calculations for plasma insulin concentration are presented for control and NE-infused fetuses during the pretreatment and posttreatment studies. **Significance, P < 0.01.
Islet gene expression.
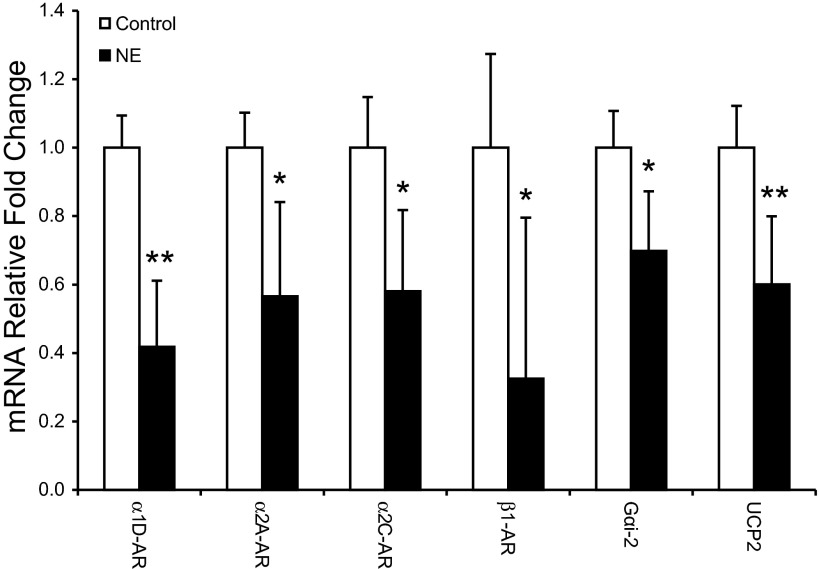
To interpret compensatory enhancement of GSIS in NE-infused fetuses, we evaluated factors regulating aspects of insulin synthesis and secretion. Pancreatic islets from NE fetuses had lower (P < 0.05) mRNA concentrations for α1D-AR, α2A-AR, α2C-AR, β1-AR, G protein subunit αi-2, and UCP2 (Fig. 4). Insulin, PDX-1, Glut2, Kir6.2, CACNA1D, other ARs, and Gαi-1, Gαi-3 Gαo, Gαq, Gαs, Gαz, and Gβ1–3 mRNA concentrations were not different in pancreatic islets (data not shown).
Fig. 4.
Expression levels in isolated pancreatic islets. The relative fold changes for adrenergic receptors (ARs), G protein subunit-αi-2, and uncoupling protein 2 (UCP2) are presented for isolated pancreatic islets from chronic NE-infused fetuses and controls. Symbols indicate significance; *P < 0.05 and **P < 0.01.
DISCUSSION
In the present study, we show that chronic infusion of NE suppressed fetal insulin concentrations throughout the 7-day treatment and was accompanied by hyperglycemia. Circulating NE concentrations resembled those observed in placental insufficiency-induced IUGR sheep fetuses, which have 69% lower insulin concentrations (36). After the NE infusion was terminated, we observed a compensatory augmentation of GSIS and GPAIS in NE fetuses, although plasma NE concentrations remained elevated compared with controls. The α2A-AR, α2C-AR, and Gαi-2 mRNA concentrations were lower in pancreatic islets from NE-infused fetuses compared with controls, indicating AR desensitization in fetal islets (45, 52). No differences were noted for measured β-cell regulatory factors; however, the reduction of UCP2 mRNA indicates improved insulin stimulus-secretion coupling in islets from NE fetuses. These findings show that chronic exposure to elevated NE induces a compensatory enhancement in β-cell insulin secretion responsiveness.
As expected, there was no difference in GSIS responsiveness between the two groups before the treatment (Fig. 2). In control fetuses, GSIS was also not different following the 7-day vehicle infusion (Fig. 2). Comparison between the two GSIS studies in control fetuses demonstrates that there is no significant developmental enhancement or maturation in β-cell function at this gestational stage (33), in contrast to that found for younger fetuses (1). During the NE treatment, insulin concentrations at basal and hyperglycemic steady states were inhibited. After removing the chronic NE infusion, basal, hyperglycemic, and GPAIS insulin concentrations were approximately fourfold higher than pretreatment measurement in NE fetuses or posttreatment measurements in control fetuses (Fig. 2). Therefore, these findings support the hypothesis that NE induces a compensatory hyperinsulin secretion state in IUGR fetuses similar to that observed after adrenergic antagonists are administered (32).
Enhanced insulin secretion responsiveness was observed despite higher than normal plasma NE concentrations 3 h after the chronic NE infusion was terminated. According to previous studies, the whole body clearance rate of NE is 178 ± 28 ml·kg−1·min−1 in near-term sheep fetuses and is not dependent on plasma catecholamine concentration (43). Since the average blood volume in fetal sheep is 120 ml/kg at 131 dGA (7), theoretically, the half-life of circulating plasma NE is <2 min. However, longer than expected clearance rates were shown in a previous long-term NE infusion study (38), indicating that chronic NE exposure decreased NE clearance, as found previously (9). In the current study, our primary objective was to look at the immediate insulin secretion responsiveness following a chronic NE infusion. Despite NE's inhibitory effect on insulin secretion, NE fetuses with relatively greater plasma NE concentrations still had enhanced insulin secretion responsiveness compared with control fetuses.
Hyperglycemia was observed throughout the NE treatment and represents a confounding factor that might also negatively influence β-cell function. The 55% increase in fetal plasma glucose concentrations is most likely due to reduced glucose oxidation in peripheral tissues of the NE fetus, which is further supported by the chronic hyperoxemia (3, 39). Previous studies in fetal sheep have found that chronic hyperglycemia for 7–10 days results in impaired β-cell function and decreased insulin secretion (10). Additionally, 2 wk of pulsatile hyperglycemia also lowers GSIS in fetal sheep and increases the rate of accumulation for reactive oxygen species in isolated pancreatic islets at 11.1 mM glucose compared with controls (20). Together, these findings indicate that fetal sheep islets exposed to hyperglycemia exhibit glucotoxicity and β-cell dysfunction (25). Interestingly, the islets from NE fetuses, also exposed to chronic hyperglycemia, were not affected in a similar fashion, as insulin secretion was enhanced after the 7 days of exposure. This shows that NE may protect islets from the ensuing hyperglycemia and subsequent glucotoxicity, possibly by inducing metabolic quiescence (30, 31).
Chronic exposure of high catecholamine concentrations has been shown to persistently reduce both mRNA and protein expression of ARs (5, 12, 14) and related G proteins (52), which is also supported in the current study (Fig. 4). NE inhibits insulin secretion principally through α2-ARs, but not α1-ARs or β-ARs (31). Thus, this lower expression of α2A-AR and α2C-AR might lead to decreased adrenergic inhibition of insulin secretion (51, 55). The decreased expression of Gαi-2 is also postulated to lower NE inhibitory effects on insulin secretion (52). Therefore, desensitization of both α2-ARs and Gαi-2 contributes to a higher insulin concentration as well as higher insulin responsiveness after the 7-day NE infusion is removed, whereas plasma NE concentrations remain greater than controls.
As the exclusive proton leak regulator in the mitochondria of the β-cell, UCP2 negatively affects β-cell responsiveness by decreasing ATP production (15, 57). Chronic hyperglycemia stimulates UCP2 expression in rat and human islet cultures (8, 44). In glucokinase knockout mice, whether incubated with or without chronic hyperglycemia, decreased rates of glucose utilization lower UCP2 expression and activity in pancreatic islets (15). Thus, expression and function of UCP2 are associated with glucose metabolism in islets, which is suppressed by α2-AR stimulation (31).
In conclusion, a 7-day infusion of NE in fetal sheep chronically suppresses plasma insulin concentrations and lowers α2-ARs and Gαi-2 mRNA in islets. After NE infusion is terminated, NE-infused fetuses have enhanced insulin secretion responsiveness to both glucose and arginine stimulation, although NE concentrations are greater than controls. These findings demonstrate that 7-day NE suppression during late gestation contributes AR desensitization and a compensatory enhancement of β-cell function. In addition, lower expression of UCP2 in pancreatic islets may facilitate enhanced insulin stimulus-secretion coupling. Therefore, these data begin to explain how endocrine factors such as catecholamines may facilitate prenatal adaptations for thriftiness in utero (24) but contribute to postnatal catchup growth, early onset obesity, and other metabolic diseases.
GRANTS
Funding for this work was from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-084842; S. W. Limesand, principal investigator). X. Chen was supported by Grant no. 20710916, Southwest University, China. D. T. Yates and A. R. Macko were supported by T32-HL-7249, and D. T. Yates was supported by a US Department of Agriculture-National Institute of Food and Agriculture Fellowship (no. 2012-67012-19855). A. S. Green was supported by F32-DK-088514. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
The authors have no conflicts of interest, financial or otherwise, to declare.
AUTHOR CONTRIBUTIONS
X.C. and S.W.L. contributed to the conception and design of the research; X.C., A.S.G., A.R.M., D.T.Y., A.C.K., and S.W.L. performed the experiments; X.C., A.S.G., A.R.M., D.T.Y., A.C.K., and S.W.L. analyzed the data; X.C., A.S.G., A.R.M., D.T.Y., A.C.K., and S.W.L. interpreted the results of the experiments; X.C. and S.W.L. prepared the figures; X.C. drafted the manuscript; X.C., A.S.G., A.R.M., D.T.Y., A.C.K., and S.W.L. edited and revised the manuscript; X.C., A.S.G., A.R.M., D.T.Y., A.C.K., and S.W.L. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Miranda J. Anderson and Mandie M. Dunham for technical assistance.
REFERENCES
- 1.Aldoretta PW, Carver TD, Hay WW., Jr. Maturation of glucose-stimulated insulin secretion in fetal sheep. Biol Neonate 73: 375–386, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annu Rev Neurosci 11: 97–118, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Bassett JM, Hanson C. Catecholamines inhibit growth in fetal sheep in the absence of hypoxemia. Am J Physiol Regul Integr Comp Physiol 274: R1536–R1545, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Bell AW, Wilkening RB, Meschia G. Some aspects of placental function in chronically heat-stressed ewes. J Dev Physiol 9: 17–29, 1987 [PubMed] [Google Scholar]
- 5.Benovic JL, Onorato JJ, Caron MG, Lefkowitz RJ. Regulation of G protein-coupled receptors by agonist-dependent phosphorylation. Soc Gen Physiol Ser 45: 87–103, 1990 [PubMed] [Google Scholar]
- 6.Boesgaard TW, Grarup N, Jørgensen T, Borch-Johnsen K; Meta-Analysis of Glucose and Insulin-Related Trait Consortium (MAGIC), Hansen T, Pedersen O. Variants at DGKB/TMEM195, ADRA2A, GLIS3 and C2CD4B loci are associated with reduced glucose-stimulated beta cell function in middle-aged Danish people. Diabetologia 53: 1647–1655, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Brace RA. Blood volume and its measurement in the chronically catheterized sheep fetus. Am J Physiol Heart Circ Physiol 244: H487–H494, 1983 [DOI] [PubMed] [Google Scholar]
- 8.Brown JE, Thomas S, Digby JE, Dunmore SJ. Glucose induces and leptin decreases expression of uncoupling protein-2 mRNA in human islets. FEBS Lett 513: 189–192, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Bzoskie L, Blount L, Kashiwai K, Humme J, Padbury JF. The contribution of transporter-dependent uptake to fetal catecholamine clearance. Biol Neonate 71: 102–110, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Carver TD, Anderson SM, Aldoretta PA, Esler AL, Hay WW., Jr Glucose suppression of insulin secretion in chronically hyperglycemic fetal sheep. Pediatr Res 38: 754–762, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Chan CB, De Leo D, Joseph JW, McQuaid TS, Ha XF, Xu F, Tsushima RG, Pennefather PS, Salapatek AM, Wheeler MB. Increased uncoupling protein-2 levels in beta-cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Diabetes 50: 1302–1310, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Fahy AL, Green AS, Anderson MJ, Rhoads RP, Limesand SW. β2-Adrenergic receptor desensitization in perirenal adipose tissue in fetuses and lambs with placental insufficiency-induced intrauterine growth restriction. J Physiol 588: 3539–3549, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Rozance PJ, Hay WW, Jr, Limesand SW. Insulin-like growth factor and fibroblast growth factor expression profiles in growth-restricted fetal sheep pancreas. Exp Biol Med (Maywood) 237: 524–529, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins S, Caron MG, Lefkowitz RJ. Regulation of adrenergic receptor responsiveness through modulation of receptor gene expression. Annu Rev Physiol 53: 497–508, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Dalgaard LT. UCP2 mRNA expression is dependent on glucose metabolism in pancreatic islets. Biochem Biophys Res Commun 417: 495–500, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Divers WA, Wilkes MM, Babaknia A, Hill LM, Quilligan EJ, Yen SS. Amniotic fluid catecholamines and metabolites in intrauterine growth retardation. Am J Obstet Gynecol 141: 608–610, 1981 [DOI] [PubMed] [Google Scholar]
- 17.Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Mägi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JR, Egan JM, Lajunen T, Grarup N, Sparsø T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proença C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O'Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Böttcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Cornelis M, Crawford G, Crisponi L, Day IN, de Geus EJ, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, Hallmans G, Hammond N, Han X, Hartikainen AL, Hassanali N, Hayward C, Heath SC, Hercberg S, Herder C, Hicks AA, Hillman DR, Hingorani AD, Hofman A, Hui J, Hung J, Isomaa B, Johnson PR, Jørgensen T, Jula A, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Lyssenko V, Mahley R, Mangino M, Manning AK, Martínez-Larrad MT, McAteer JB, McCulloch LJ, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Morken MA, Mukherjee S, Naitza S, Narisu N, Neville MJ, Oostra BA, Orrù M, Pakyz R, Palmer CN, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Perola M, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Psaty BM, Rathmann W, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Roden M, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Scott LJ, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvänen AC, Tanaka T, Thorand B, Tichet J, Tönjes A, Tuomi T, Uitterlinden AG, van Dijk KW, van Hoek M, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Walters GB, Ward KL, Watkins H, Weedon MN, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zeggini E, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC; DIAGRAM Consortium; GIANT Consortium; Global BPgen Consortium, Borecki IB, Loos RJ, Meneton P, Magnusson PK, Nathan DM, Williams GH, Hattersley AT, Silander K, Salomaa V, Smith GD, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Dedoussis GV, Serrano-Ríos M, Morris AD, Lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WH, Pankow JS, Sampson MJ, Kuusisto J, Laakso M, Hansen T, Pedersen O, Pramstaller PP, Wichmann HE, Illig T, Rudan I, Wright AF, Stumvoll M, Campbell H, Wilson JF; Anders Hamsten on behalf of Procardis Consortium; MAGIC investigators, Bergman RN, Buchanan TA, Collins FS, Mohlke KL, Tuomilehto J, Valle TT, Altshuler D, Rotter JI, Siscovick DS, Penninx BW, Boomsma DI, Deloukas P, Spector TD, Frayling TM, Ferrucci L, Kong A, Thorsteinsdottir U, Stefansson K, van Duijn CM, Aulchenko YS, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Waterworth DM, Vollenweider P, Peltonen L, Mooser V, Abecasis GR, Wareham NJ, Sladek R, Froguel P, Watanabe RM, Meigs JB, Groop L, Boehnke M, McCarthy MI, Florez JC, Barroso I. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 42: 105–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction 127: 515–526, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Gopel S, Zhang Q, Eliasson L, Ma XS, Galvanovskis J, Kanno T, Salehi A, Rorsman P. Capacitance measurements of exocytosis in mouse pancreatic alpha-, beta- and delta-cells within intact islets of Langerhans. J Physiol 556: 711–726, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green AS, Chen X, Macko AR, Anderson MJ, Kelly AC, Hart NJ, Lynch RM, Limesand SW. Chronic pulsatile hyperglycemia reduces insulin secretion and increases accumulation of reactive oxygen species in fetal sheep islets. J Endocrinol 212: 327–342, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green AS, Macko AR, Rozance PJ, Yates DT, Chen X, Hay WW, Jr, Limesand SW. Characterization of glucose-insulin responsiveness and impact of fetal number and sex difference on insulin response in the sheep fetus. Am J Physiol Endocrinol Metab 300: E817–E823, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green AS, Rozance PJ, Limesand SW. Consequences of a compromised intrauterine environment on islet function. J Endocrinol 205: 211–224, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenough A, Nicolaides KH, Lagercrantz H. Human fetal sympathoadrenal responsiveness. Early Hum Dev 23: 9–13, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303: 1019–1022, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harmon JS, Stein R, Robertson RP. Oxidative stress-mediated, post-translational loss of MafA protein as a contributing mechanism to loss of insulin gene expression in glucotoxic beta cells. J Biol Chem 280: 11107–11113, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Jackson BT, Piasecki GJ, Cohn HE, Cohen WR. Control of fetal insulin secretion. Am J Physiol Regul Integr Comp Physiol 279: R2179–R2188, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Jensen CB, Storgaard H, Dela F, Holst JJ, Madsbad S, Vaag AA. Early differential defects of insulin secretion and action in 19-year-old caucasian men who had low birth weight. Diabetes 51: 1271–1280, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE; American Diabetes Association GENNID Study Group Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes 51: 2170–2178, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Kaijser M, Bonamy AK, Akre O, Cnattingius S, Granath F, Norman M, Ekbom A. Perinatal risk factors for diabetes in later life. Diabetes 58: 523–526, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laychock SG. Alpha 2-adrenoceptor stimulation affects total glucose utilization in isolated islets of Langerhans. Mol Pharmacol 32: 241–248, 1987 [PubMed] [Google Scholar]
- 31.Laychock SG, Bilgin S. Alpha 2-adrenergic inhibition of pancreatic islet glucose utilization is mediated by an inhibitory guanine nucleotide regulatory protein. FEBS Lett 218: 7–10, 1987 [DOI] [PubMed] [Google Scholar]
- 32.Leos RA, Anderson MJ, Chen X, Pugmire J, Anderson KA, Limesand SW. Chronic exposure to elevated norepinephrine suppresses insulin secretion in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab 298: E770–E778, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Limesand SW, Hay WW., Jr Adaptation of ovine fetal pancreatic insulin secretion to chronic hypoglycaemia and euglycaemic correction. J Physiol 547: 95–105, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Limesand SW, Jensen J, Hutton JC, Hay WW., Jr Diminished β-cell replication contributes to reduced β-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 288: R1297–R1305, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Limesand SW, Rozance PJ, Macko AR, Anderson MJ, Kelly AC, Hay WW., Jr Reductions in insulin concentrations and β-cell mass precede growth restriction in sheep fetuses with placental insufficiency. Am J Physiol Endocrinol Metab 304: E516–E523, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW., Jr Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 147: 1488–1497, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Macko AR, Yates DT, Chen X, Green AS, Kelly AC, Brown LD, Limesand SW. Elevated plasma norepinephrine inhibits insulin secretion, but adrenergic blockade reveals enhanced β-cell responsiveness in an ovine model of placental insufficiency at 0.7 of gestation. J Dev Origin Health Dis 4: 402–410, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milley JR. Ovine fetal metabolism during norepinephrine infusion. Am J Physiol Endocrinol Metab 273: E336–E347, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Milley JR, Papacostas JS, Tabata BK. Effect of insulin on uptake of metabolic substrates by the sheep fetus. Am J Physiol Endocrinol Metab 251: E349–E356, 1986 [DOI] [PubMed] [Google Scholar]
- 40.Newsome CA, Shiell AW, Fall CH, Phillips DI, Shier R, Law CM. Is birth weight related to later glucose and insulin metabolism?—A systematic review. Diabet Med 20: 339–348, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Nicolini U, Hubinont C, Santolaya J, Fisk NM, Rodeck CH. Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm Metab Res 22: 426–430, 1990 [DOI] [PubMed] [Google Scholar]
- 42.Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J 12: 4251–4259, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Padbury JF, Ludlow JK, Humme JA, Agata Y. Metabolic clearance and plasma appearance rates of catecholamines in preterm and term fetal sheep. Pediatr Res 20: 992–995, 1986 [DOI] [PubMed] [Google Scholar]
- 44.Patane G, Anello M, Piro S, Vigneri R, Purrello F, Rabuazzo AM. Role of ATP production and uncoupling protein-2 in the insulin secretory defect induced by chronic exposure to high glucose or free fatty acids and effects of peroxisome proliferator-activated receptor-gamma inhibition. Diabetes 51: 2749–2756, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Peterhoff M, Sieg A, Brede M, Chao CM, Hein L, Ullrich S. Inhibition of insulin secretion via distinct signaling pathways in alpha2-adrenoceptor knockout mice. Eur J Endocrinol 149: 343–350, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regnault TR, Galan HL, Parker TA, Anthony RV. Placental development in normal and compromised pregnancies— a review. Placenta 23, Suppl A: S119–S129, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Rosengren AH, Braun M, Mahdi T, Andersson SA, Travers ME, Shigeto M, Zhang E, Almgren P, Ladenvall C, Axelsson AS, Edlund A, Pedersen MG, Jonsson A, Ramracheya R, Tang Y, Walker JN, Barrett A, Johnson PR, Lyssenko V, McCarthy MI, Groop L, Salehi A, Gloyn AL, Renstrom E, Rorsman P, Eliasson L. Reduced insulin exocytosis in human pancreatic beta-cells with gene variants linked to type 2 diabetes. Diabetes 61: 1726–1733, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosengren AH, Jokubka R, Tojjar D, Granhall C, Hansson O, Li DQ, Nagaraj V, Reinbothe TM, Tuncel J, Eliasson L, Groop L, Rorsman P, Salehi A, Lyssenko V, Luthman H, Renström E. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science 327: 217–220, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Rozance PJ, Limesand SW, Hay WW., Jr Decreased nutrient-stimulated insulin secretion in chronically hypoglycemic late-gestation fetal sheep is due to an intrinsic islet defect. Am J Physiol Endocrinol Metab 291: E404–E411, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Sharp GW. Mechanisms of inhibition of insulin release. Am J Physiol Cell Physiol 271: C1781–C1799, 1996 [DOI] [PubMed] [Google Scholar]
- 52.Urano Y, Sakurai T, Ueda H, Ogasawara J, Takei M, Izawa T. Desensitization of the inhibitory effect of norepinephrine on insulin secretion from pancreatic islets of exercise-trained rats. Metabolism 53: 1424–1432, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol 84: 751–753, 1977 [DOI] [PubMed] [Google Scholar]
- 54.Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, Barrett-Connor E, Bhargava SK, Birgisdottir BE, Carlsson S, de Rooij SR, Dyck RF, Eriksson JG, Falkner B, Fall C, Forsén T, Grill V, Gudnason V, Hulman S, Hyppönen E, Jeffreys M, Lawlor DA, Leon DA, Minami J, Mishra G, Osmond C, Power C, Rich-Edwards JW, Roseboom TJ, Sachdev HS, Syddall H, Thorsdottir I, Vanhala M, Wadsworth M, Yarbrough DE. Birth weight and risk of type 2 diabetes: a systematic review. JAMA 300: 2886–2897, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Yang J, Dolinger M, Ritaccio G, Mazurkiewicz J, Conti D, Zhu X, Huang Y. Leucine stimulates insulin secretion via down-regulation of surface expression of adrenergic alpha2A receptor through the mTOR (mammalian target of rapamycin) pathway: implication in new-onset diabetes in renal transplantation. J Biol Chem 287: 24795–24806, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yates DT, Macko AR, Chen X, Green AS, Kelly AC, Anderson MJ, Fowden AL, Limesand SW. Hypoxaemia-induced catecholamine secretion from adrenal chromaffin cells inhibits glucose-stimulated hyperinsulinaemia in fetal sheep. J Physiol 590: 5439–5447, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell 105: 745–755, 2001 [DOI] [PubMed] [Google Scholar]