Abstract
Scavenger receptor class B, type I (SR-BI), the Scarb1 gene product, is a receptor associated with cholesteryl ester uptake from high-density lipoproteins (HDL), which drives cholesterol movement from peripheral tissues toward the liver for excretion, and, consequently, Scarb1 null mice are prone to atherosclerosis. Because studies have linked atherosclerosis incidence with osteoporosis, we characterized the bone metabolism in these mice. Bone morphometry was assessed through microcomputed tomography and histology. Marrow stromal cells (MSCs) were used to characterize influence of endogenous SR-BI in cell functions. Total and HDL-associated cholesterol in null mice were increased by 32–60%, correlating with its role in lipoprotein metabolism. Distal metaphyses from 2- and 4-mo-old null mice showed correspondingly 46 and 37% higher bone volume fraction associated with a higher number of trabeculae. Histomorphometric analyses in 2-mo-old null male mice revealed 1.42-fold greater osteoblast surface, 1.37-fold higher percent mineralizing surface, and 1.69-fold enhanced bone formation rate. In vitro assays for MSCs from null mice revealed 37% higher proliferation rate, 48% more alkaline phosphatase activity, 70% greater mineralization potential and a 2-fold osterix (Sp7) expression, yet a 0.5-fold decrease in caveolin-1 (Cav1) expression. Selective uptake levels of HDL-associated cholesteryl oleate and estradiol were similar between MSC from wild-type and Scarb1 null mice, suggesting that its contribution to this process is not its main role in these cells. However, Scarb1 knockout stunted the HDL-dependent regulation of Cav1 genic expression. Scarb1 null mice are not prone to osteoporosis but show higher bone mass associated with enhanced bone formation.
Keywords: osteoblasts, adrenocorticotropic hormone, high-density lipoprotein, caveolin-1
osteoporosis is of growing concern as aging of populations occurs; it is a bone disorder resulting from an imbalance in skeletal remodeling, defined by lower bone mass and higher bone fragility (53). It is considered a silent, multifactorial health issue with significant morbidity and social cost (59). Several genetic, nutritional, behavioral, and environmental parameters have been identified as risk factors, each contributing to a small fraction of total incidence (43, 52, 53). Well-documented pathophysiological cues include loss of gonadal function (e.g., menopause) (3), hyperparathyroidism (5), alterations of intestinal (34) and renal functions (19), and medical treatments such as long-term use of glucocorticoids (GC) (14). Although several of these pathologies are classically considered to be a side effect of systemic mechanisms, some direct bone alterations have been reported, such as inhibition of osteoblastogenesis, osteoblastic activity, and osteoclastic activity (17, 47). Likely most of these skeletal disorders result from the sum of systemic and bone-specific alterations.
Epidemiological studies have linked atherosclerosis incidence with the development of osteoporosis (21, 32), and a growing body of biological and genetic evidence argues for a common etiology in both conditions (7). A recurrent factor in most of these studies is high circulating cholesterol levels, and lipid-lowering agents such as statins have been shown to reduce atherosclerotic lesions and osteoporosis (45). Accordingly, most epidemiological investigations show a positive correlation between high-density lipoproteins (HDL) and bone mineral density (1). In fact, HDL are greatly responsible for the removal of cholesterol from peripheral tissues, a process termed reverse cholesterol transport (RCT) (58). The disruption of RCT in macrophages is central to the onset of atherosclerotic plaque (44). Its inhibition promotes the differentiation of macrophages to foam cells, which accumulate oxidized lipids and cause the appearance of arterial wall lesions (33, 55).
Scavenger receptor class B, type I (SR-BI), the product of the Scarb1 gene, is a glycosylated cell surface receptor highly expressed in steroidogenic organs and liver (2). This receptor binds HDL with high affinity (2). To study the role of this receptor in HDL metabolism, Rigotti et al. (49) generated Scarb1 null mice. These mice show significantly higher serum cholesterol, especially HDL-associated, and a propensity to develop atherosclerosis (29). SR-BI is considered the main physiological receptor for HDL that mediates bidirectional flux of cholesterol and other lipids between HDL and cells (41). SR-BI allows cholesterol efflux from cells and thereby contributes to the RCT from peripheral tissues toward the liver for excretion (62). Moreover, SR-BI participates in the selective uptake of cholesteryl esters (CE) from HDL in the liver and steroidogenic tissues (11, 28). In accordance, HDL-CE selective uptake is greatly reduced in Scarb1-null mice (10). Therefore, studies attribute an atheroprotective role to SR-BI by contributing to HDL-mediated RCT. Of interest, high HDL serum levels are generally correlated with lower incidence of both osteoporosis and atherosclerosis and have been proposed as a linking factor between these two conditions (1).
Scarb1 null male mice have been reported to exhibit defective GC synthesis because of lack of SR-BI-mediated cholesterol uptake in the adrenal glands; this condition was accompanied by high ACTH levels (26, 28). Because ACTH is now considered a modulator of osteoblastogenesis, Scarb1 knock out may impact bone metabolism through systemic mechanisms involving this axis (30, 61). Moreover, previous work has shown that SR-BI is expressed in osteoblast-like cell lines and primary osteoblast cultures, more specifically in caveolae-rich membrane structures (9). We have reported selective uptake of HDL-associated cholesteryl ester and estradiol by bone-forming osteoblasts (9). Although the identity of the involved receptor and the relevance of this process in osteoblasts remain to be determined, Scarb1 null osteoblasts and osteoblast precursors could also display dysfunctions altering bone growth.
Thus, the Scarb1 null mouse may prove to be a useful model in studying the role of SR-BI in bone metabolism, as well as understanding the relation between osteoporosis and atherosclerosis. The present work therefore aimed at assessing the bone phenotype in Scarb1 null mice.
MATERIALS AND METHODS
Animals.
Scarb1 null mice on a C57BL6/129 background (stock no. 003379) were purchased from Jackson Laboratories (Bar Harbor, ME) and cross-bred with wild-type (WT) C57BL/6 mice. Heterozygous (HZ) littermates (initial parental couples) were intercrossed to yield first generation (F1) WT, HZ, and null mouse pairs; because null dams show low fertility, higher reproduction rates were achieved by feeding them a 0.5% probucol diet (Research Diets, New Brunswick, NJ) 10 days before mating (40). Untreated HZ dams were mated in parallel to allow direct WT and null littermate comparison, as well as confirm that the probucol treatment did not affect the observed phenotype. Null mice of F7 to F10 generations from both HZ and null pairs were pooled (40 and 60% proportion, respectively) in age groups ranging from 2 to 4 mo, according to assay, and compared with age-matched WT groups. All individuals were kept in a 12:12-h day-night cycle with free access to food and water unless specified otherwise. All animal protocols were performed according to and were approved by the UQÀM Institutional Animal Care Committee (IACC 611). Following death, whole body, abdominal fat and epididymal fat pads were weighed. Because of significant differences in their hormonal profile and axial skeleton, some results for females are detailed elsewhere.
Serology.
Whole blood was harvested from 2- and 4-mo-old mice through cardiac punctures and collected in 3-ml heparinated tubes (68 USP; BD Bioscience, Mississauga, Ontario, Canada). Plasma was obtained by centrifugation at 2,000 g for 25 min at 4°C and stored at −80°C until analyses. Calcium, phosphate, and glucose were evaluated by QuantiChrom assays (BioAssay Systems, Hayward, CA), and alkaline phosphatase (ALP) and total/lipoprotein-associated cholesterol were quantified using EnzyChrom assays (BioAssay Systems). Briefly, plasma was incubated with p-nitrophenylphosphate for ALP activity or EnzyChrom enzyme mix (for total cholesterol) at room temperature. Very low density lipoprotein (VLDL)/low-density lipoprotein (LDL) and HDL-associated cholesterol fractions were separated by PEG-precipitation of plasma samples, and the enzyme mix was added to the pellets (for VLDL/LDL) and supernatants (for HDL). ACTH (MyBioSource, San Diego, CA), corticosterone (Enzo Life Sciences, Farmingdale, NY), and C-telopeptide of type I collagen (CTX; R&D Systems, Burlington, Ontario, Canada) were measured with ELISA kits according to the manufacturers' protocols.
Microcomputed tomography.
Following death by CO2, bones were harvested and fixed in 4% paraformaldehyde-phosphate buffered saline (PF) at 4°C for 16–20 h. Samples were scanned with a Skyscan 1172c system (Soquelec, Montreal, Quebec, Canada) at a 5-μm image pixel size, 70-kV, 100-μA, and 1,200-ms exposure. Raw images were reconstructed with the NRecon software (Skyscan, Aartselar, Belgium); trabecular and cortical volumes of interest (VOI) were defined, modelized, and analyzed with the CTAn software (Skyscan). The selected trabecular VOIs consisted of a 2-mm metaphyseal segment 0.5 mm below the growth plate reference point; the cortical VOIs were a 0.5-mm diaphyseal segment selected 2.5 mm below the growth plate reference point. The bone mineral density (BMD) was estimated using hydroxyapatite phantoms scanned under similar experimental conditions.
Static and dynamic histology.
Twenty milligrams of calcein (Sigma-Aldrich, Oakville, Ontario, Canada) per kilogram body weight were injected intraperitoneally in 2-mo-old mice on days 10 and 2 before death (day 0). PF-fixed undecalcified bones were embedded in low-temperature polymerizing polymethylmethacrylate as previously described (20), sectioned at 6 μm with a Thermofisher rotary HM 360 microtome, and used for Von Kossa (VK), ALP (Millipore, Billerica, MA), and tartrate-resistant acid phosphatase (TRAP) (K Assay; Dako, Burlington, Ontario, Canada) histochemical stainings. VK-, ALP-, or TRAP-stained bone sections were visualized with an inverted phase-contrast microscope (TE-300; Nikon, Mississauga, Ontario, Canada) and used to evaluate relative osteoblast surface and number of osteoclasts. Double calcein labeling was visualized with a Nikon FN1 Eclipse inverted fluorescence microscope and used to evaluate mineralizing surface (MS/BS), mineral apposition rate (MAR), and bone formation rate (BFR/BS). For each randomly numbered sample, three to five sections were analyzed with the ImageJ software (NIH). MS/BS was evaluated with the following formula: (dL + sL/2)/BS, were dL and sL are the double- and single-labeled bone surfaces, and BS is the whole bone surface from the analyzed area. MAR was measured as the average distance between calcein fronts in double-labeled bone surfaces divided by the time span between injections (8 days); BFR/BS was calculated by multiplying MAR and MS/BS.
Primary cultures of bone marrow stromal cells.
Long bones from 2- to 4-mo-old mice hindlimbs were harvested and sterilized in PBS containing 200 U/ml penicillin-200 μg/ml streptamycin and 1% FungiZone (all from Invitrogen, Burlington, Ontario, Canada). Epiphyses were cut off under sterile hood, and marrow was flushed out. Marrow was suspended in α-MEM culture medium supplemented with 100 U/ml penicillin-100 μg/ml streptamycin, l-glutamine, 10% fetal bovine serum (North Bio, Toronto, Ontario, Canada), and 25 μg/mL l-ascorbic acid (Sigma-Aldrich) and plated in a 100-mm culture dish; bones from one mouse were used per dish, unless otherwise specified. The cells were left to adhere for 7 days and thoroughly washed with PBS to eliminate nonadherent cells. Adherent cells were left to reach confluence before harvest and experimentation; adherent marrow stromal cell (MSC) phenotype was assessed in subsequent experiments.
MSC expansion assays.
Bone marrow was suspended in α-MEM with 10% FBS, and marrow cells were plated at either 1,000,000 or 2,000,000 cells/cm2 and left to adhere for 24 h. Adherent cells were thoroughly washed with PBS and left to grow for 10 days; the cells were then trypsinized, suspended in PBS, and counted with a hemocytometer.
Proliferation and differentiation assays.
Long bone-derived MSCs were harvested at confluence with 0.5% trypsin-0.1% EDTA (Invitrogen) and plated in 24-well plates at a density of 50,000 cells/cm2 for differentiation assays. Once confluence was reached (day 0), cells were cultured in MEM containing nonessential amino acids and 10% FBS, supplemented (osteogenic) or not (control) with 50 μg/ml ascorbic acid and 5 mM β-glycerophosphate (both from Sigma-Aldrich) three times a week for 21 days. Differentiation was evaluated on days 0 and 21 through cellular ALP activity [as described elsewhere (42)] and Alizarin Red S (Sigma-Aldrich) staining, solubilization, and spectrophotometric reading. For proliferation assays, cells were plated in 96-well plates at 20,000 cells/cm2, and viability was evaluated 24 h later (day 0) as well as on days 1, 3, 5, 7, and 10 through tetrazolium microtiter assay (Sigma-Aldrich). For experiments with ACTH, cells were cultured in medium containing 2% FBS without or with 10 nM ACTH (Tocris Bioscience, Bristol, UK). The resulting formazan crystals, indicative of cell metabolic activity, were solubilized in DMSO, and the absorbance was read at 570 nm with a microplate reader (Tecan, Männedorf, Austria). Proliferation was expressed as a ratio of metabolic activity of a given time point normalized to initial metabolic activity on day 0.
Preparation of lipoproteins.
Lipoproteins were isolated from human plasma (Bioreclamation, Hicksville, NY). Before isolation, the plasma was supplemented with 0.01% EDTA, 0.02% sodium azide, and 10 μM phenylmethylsulfonyl fluoride to prevent degradation. Human HDL3 (density 1.125–1.21 g/ml) were prepared by ultracentrifugation as described previously (8, 9). HDL3 were iodinated by a modification (36) of the iodine monochloride method of McFarlane (38) as previously reported (9). Free iodine was removed by gel filtration on Sephadex G-25, followed by dialysis in Tris-buffered saline (TBS). The specific radioactivity ranged from 98,000 to 277,750 cpm/μg protein. HDL3 were labeled with 1,2-[3H]cholesteryl oleate (CO) following established procedures (50). The labeled lipoproteins were then reisolated by ultracentrifugation. The specific activity of HDL3 labeled with CO ranged from 11,200 to 17,900 cpm/μg protein. For the labeling of HDL3 with estradiol, [2,4,6,7,16,17-3H]estradiol (E2) (87 Ci/mmol) in 0.5 M HEPES buffer (pH 7.4) was added to 1.5 mg of HDL3 diluted in 1 ml of TBS and incubated under agitation and nitrogen for 24 h. Labeled lipoproteins were reisolated by ultracentrifugation, and the specific activity ranged from 3,300 to 5,500 cpm/μg protein.
Selective uptake assays.
Cellular associations of 125I-labeled lipoprotein or [3H]CO- or [3H]E2-HDL3 (20 μg of protein/ml) were measured at 37°C for 4 h on confluent cellular monolayers in 12-well plates (Sarstedt) in α-MEM supplemented with 1% bovine serum albumin (BSA; Sigma-Aldrich) as previously described (9). Nonspecific association was assessed by the addition of 1.5 mg of protein/ml of unlabeled lipoproteins. At the end of the incubation, cells were washed two times with 1 ml PBS containing 0.2% BSA, followed by two washes with 1 ml PBS, and solubilized in 1.5 ml of 0.2 N NaOH. Radioactivity counts in the homogenates were obtained with a cobra II γ-counter (Canberra-Packard) for 125I-labeled HDL3 determination and with a β-counter (Wallack-Fisher) for measurement of [3H]CO- or [3H]E2-HDL3 content. The results are expressed in micrograms of lipoprotein protein per milligram of cellular protein. Cellular protein contents were determined by the Bradford method (7a). To compare the association of lipoproteins labeled in protein (125I) or in CO/E2 (3H), the association data of [3H]CO- or [3H]E2-HDL3 were estimated as micrograms of protein per milligram of cell protein (apparent uptake). To achieve this, the specific activity of [3H]HDL3 was expressed in counts per minute per microgram of lipoprotein protein. The specific association was calculated by subtracting the nonspecific association from the total association. Selective uptake of CO or E2 is observed when specific [3H]CO- or [3H]E2-HDL3 association minus specific 125I-HDL3 association is greater than zero.
Real-time PCR.
First-passage MSC monolayers were cultured in α-MEM containing 10% FBS to observe osteogenic marker expression at confluence, or for 24 h in MEM containing 2% lipoprotein-deficient serum with or without 150 μg of HDL3 for observation of caveolin-1 (Cav1) expression. Total RNA from MSCs was extracted using RiboZol (Amresco, Solon, OH) following the manufacturer's instructions. One microgram of RNA was reversed transcribed with AMV reverse transcriptase (Roche Diagnostics, Laval, Quebec, Canada), and the resulting cDNA was used for PCR on a MyiQ thermal cycler (Bio-Rad, Mississauga, Ontario, Canada) using SYBR Green (Bio-Rad). Primers specific for β-microglobulin (B2m) (forward: 5′-TACTCACGCCACCCACCGGAG-3′, reverse: 5′-GCTCGGCCATACTGGCATGCT-3′), collagen type 1 alpha 1 (Col1a1) (forward: 5′-ACTTCAGCTTCCTGCCTCAG-3′, reverse: 5′-GCTTCTTTTCCTTGGGGTTC-3′), Cav1 (forward: 5′-AGGTGACTGAGAAGCAAGTGTATG-3′, reverse: 5′-CAAAGTCAATCTTGACCACGTC-3′) and osterix (Sp7) (forward: 5′-TTCGCATCTGAAAGCCCACT-3′, reverse: 5′-TGCGCTGATGTTTGCTCAAG-3′) were used, and the PCR were run for 40 cycles with an annealing temperature of 58°C for 30 s. The expression of each gene was normalized to gene expression of B2m and then expressed as a null-to-WT ratio. The relative fluorescence units were analyzed with the iQ5 software (Bio-Rad).
Statistical analyses.
Statistical analyses were conducted with the Prism5 software (GraphPad, La Jolla, CA). Paired t-tests, ANOVAs, and Bonferroni post hoc tests, as indicated in the legends, were applied to determine statistical significance; a P value of 0.05 was considered as the significance threshold. All data are presented as means ± SE.
RESULTS
Cholesterol status of Scarb1 null mice.
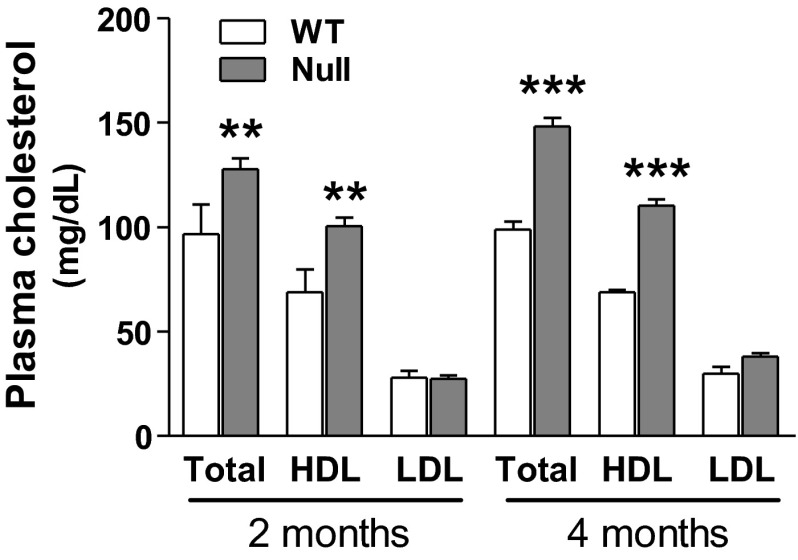
Because epidemiologic studies have linked atherosclerosis incidence with the development of osteoporosis, we took advantage of the availability of the atherogenic Scarb1 null mouse model to investigate the role of SR-BI in bone metabolism. First, we determined general morphogenic and plasmatic parameters of these null mice. Despite a trend toward an increase, no significant difference was observed in total body or abdominal fat weight between 2-mo-old WT and null male mice (Table 1), with similar results up to 12 mo of age and for female mice (data not shown). To assess whether Scarb1 knockout caused any metabolic or mineral disorder, plasmatic levels of calcium, phosphate, ALP, and glucose were measured in 2-mo-old WT and null male individuals; no significant alterations were detected under these conditions (Table 2), and similar results were obtained for female mice (data not shown). Because these null mice were reported to have a propensity to develop atherosclerosis (29, 49), we measured their cholesterol plasma profile. As expected, total plasma cholesterol and HDL-associated fractions were, respectively, increased by 32–46% and 50–60% in 2- and 4-mo-old Scarb1 null mice compared with WT mice (Fig. 1); a similar profile was observed in females (data not shown).
Table 1.
Whole body, abdominal fat, and epididymal fat pads weight in 2-mo-old male mice
| Male |
||
|---|---|---|
| WT | Null | |
| Total | 23.0 ± 1.2 | 25.0 ± 2.9 |
| Abdominal | 0.323 ± 0.129 | 0.454 ± 0.181 |
| Fat pads | 0.114 ± 0.042 | 0.077 ± 0.025 |
Values (g) are means ± SE from 3–7 mice in each group. WT, wild type.
Table 2.
Plasmatic parameters in 2-mo-old male mice
| Male |
||
|---|---|---|
| WT | Null | |
| ALP, μmol PNP · l−1 · min−1 | 556 ± 58 | 556 ± 32 |
| Ca, mg/dl | 8.6 ± 0.3 | 8.5 ± 0.5 |
| Pi, mg/dl | 10.2 ± 0.8 | 10.6 ± 0.6 |
| Ca × Pi1 | 88 ± 10 | 89 ± 10 |
| Glucose, mg/dl | 264 ± 53 | 318 ± 63 |
Values are means ± SE from 4–6 individuals/group. ALP, alkaline phosphatase; PNP, p-nitrophenyl. 1The Ca × Pi indexes were obtained by multiplying the Ca and Pi means within each group, and their relative errors were added to obtain the absolute error of the product.
Fig. 1.
Plasma cholesterol levels in 2- and 4-mo-old wild-type (WT) and Scarb1 null male mice. Total, high-density lipoprotein (HDL)-associated and low-density lipoprotein (LDL)/very low density lipoprotein (VLDL)-associated cholesterol fractions from plasma were quantified with the BioAssay Systems Enzychrom HDL, LDL/VLDL cholesterol kit. Values are means ± SE from 4–5 mice in each group. Bonferroni post hoc test: **P < 0.01 and ***P < 0.001 vs. WT of corresponding age group.
Bone architecture and histomorphometry.
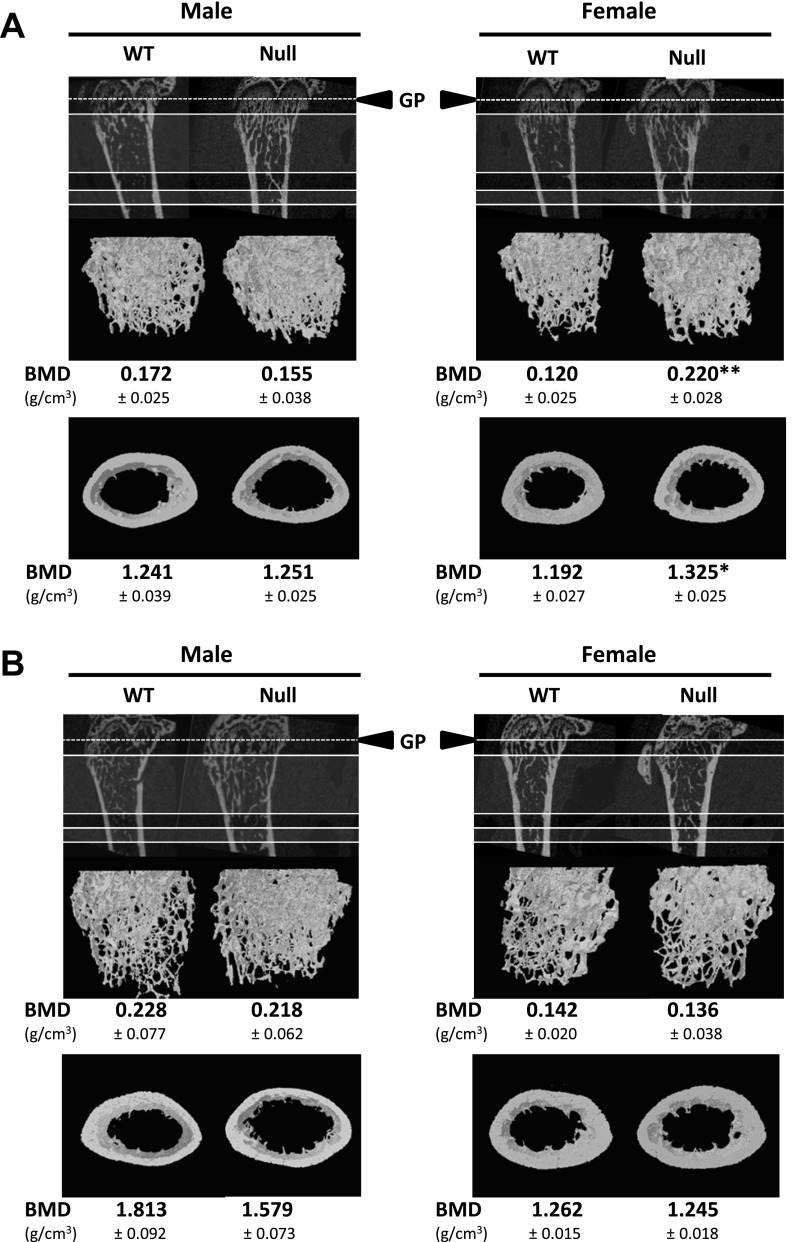
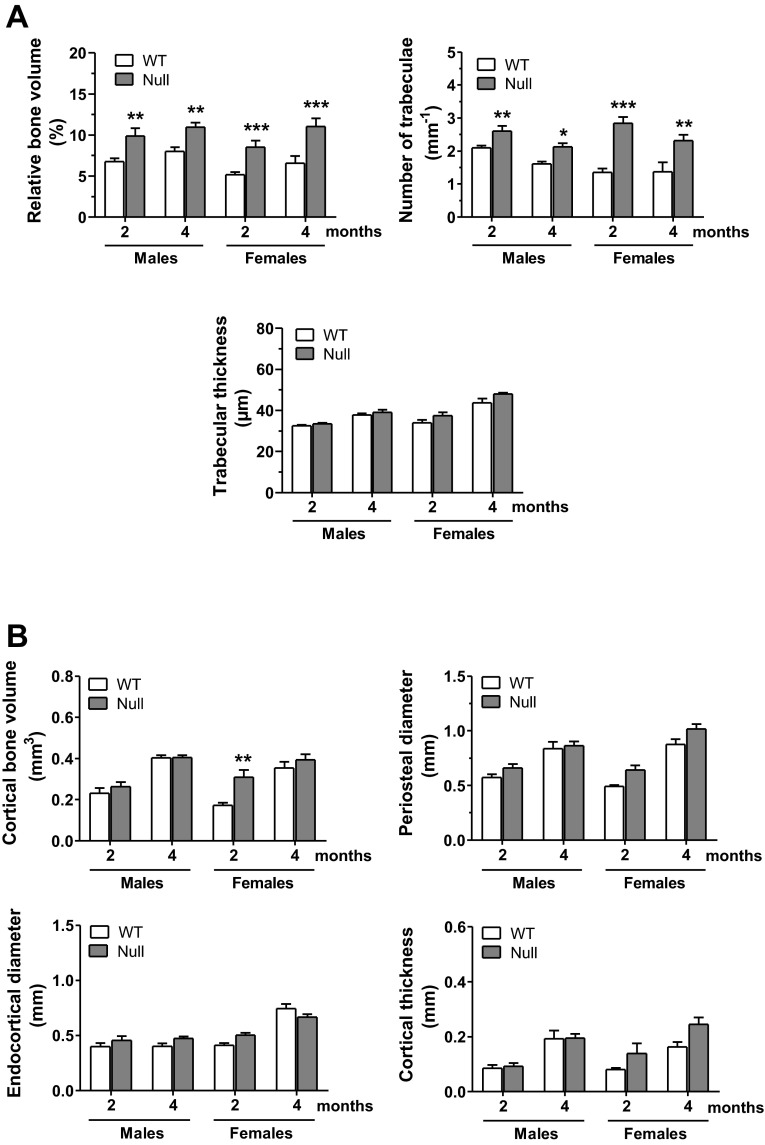
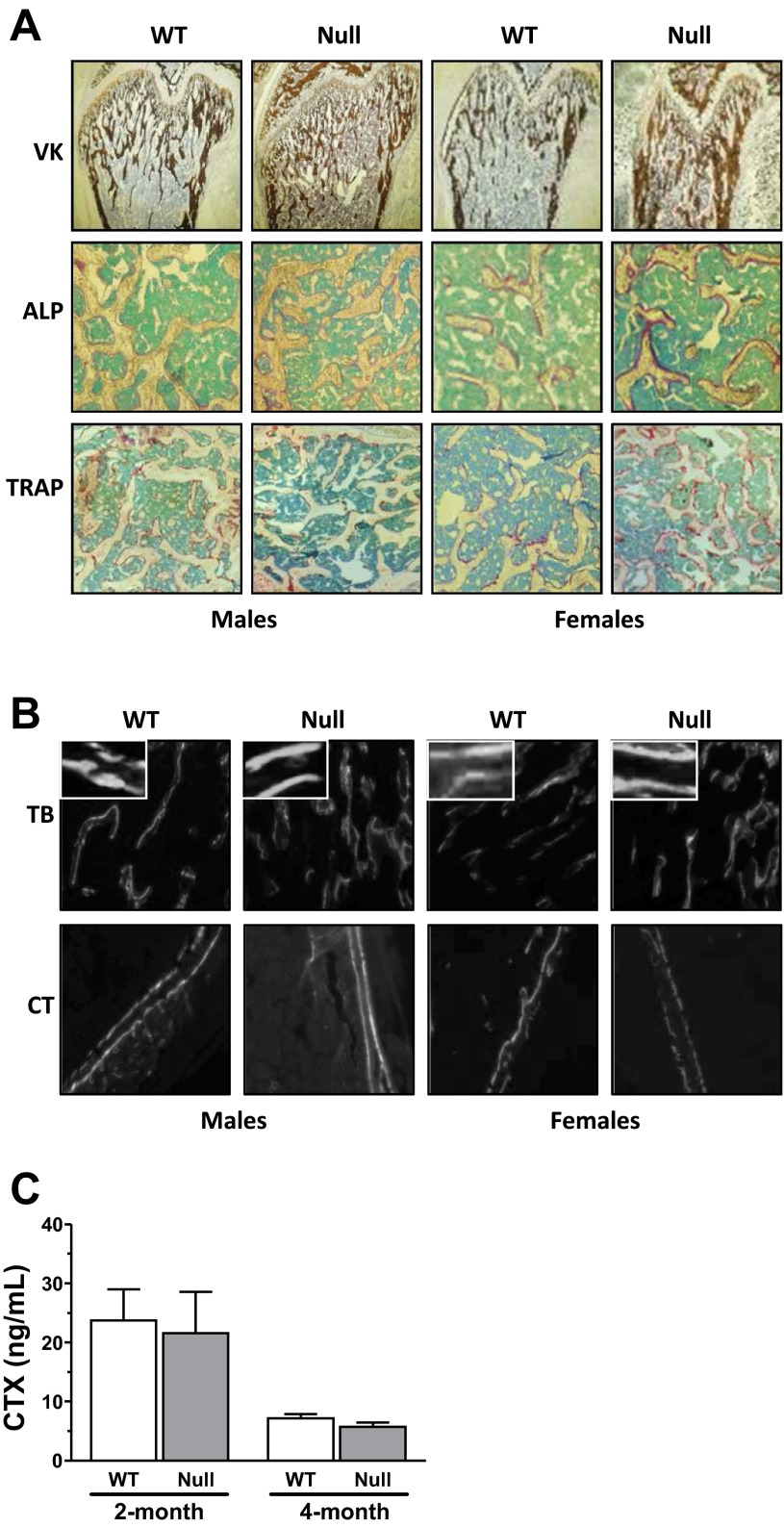
We next investigated whether Scarb1 knockout was associated with alterations of bone architecture. The visual appreciations (Fig. 2) clearly show the observed architectural alterations, particularly pronounced at a younger age [2-mo-old (Fig. 2A) and 4-mo-old (Fig. 2B) individuals depicted]; no difference was observed in BMD for Scarb1 null male mice, whereas higher BMD was measured in Scarb1 null female mice at 2-mo-old (data below Fig. 2). μCT analyses (Fig. 3) indicated that younger null male mice showed significantly higher relative trabecular bone volume, with a 46% increase in 2-mo-old mice as well as a 37% increase in 4-mo-old mice (Fig. 3A, top left). This higher bone volume was reflected by a greater number of trabeculae per millimeter (Fig. 3A, top right) and smaller distance from trabecula to trabecula (data not shown). Trabecular thickness remained unaffected under these conditions (Fig. 3A, bottom). Similar trabecular alterations were observed in Scarb1 null females (Fig. 3A). No significant alterations were observed for cortical bone in Scarb1 null males, whereas cortical volume was significantly higher in 2-mo-old females (Fig. 3B). Analysis of bone tissue sections corroborates the μCT findings (Fig. 4); VK staining of mineralized bone area was increased in femora sections from null mice compared with WT mice (Fig. 4A, top). Moreover, higher ALP-positive cell surface was observed in bone tissue of null mice (Fig. 4A, middle, and Table 3). No significant difference was noted in the number of TRAP-positive cells (Fig. 4A, bottom, and Table 3). Figure 4B shows representative interlabel distances in trabecular and cortical regions; null mice displayed accentuated trabecular mineral apposition rate (mean 1.27-fold), since a cortical mineral apposition rate only showed a slight increase (nonsignificant) in male and a significant increase (1.67-fold) in Scarb1 null female mice (Table 3). These values were accompanied in Scarb1 null male mice by greater mineralized bone surface (1.37-fold) and higher relative osteoblast surface (1.42-fold) and bone formation rate (1.69-fold) (Table 3). Dynamic histology from female femurs yielded similar results (Table 3). To assess whether osteoclastic activity was altered, plasma COOH-terminal telopeptides (CTX) were measured (Fig. 4C); no significant difference was noted.
Fig. 2.
Definition of the femoral cancellous and cortical volumes of interest (VOIs) from WT and Scarb1 null mouse bones. The growth plate (GP) reference point is represented by the dashed line; VOI top and bottom are represented by filled lines. The average bone mineral density (BMD) was determined by microCT in 2-mo-old (A) and 4-mo-old (B) individuals. Values are means ± SE from 6–10 mice in each group. *P < 0.05 and **P < 0.01.
Fig. 3.
MicroCT analyses of femoral VOIs from 2- to 4-mo-old WT and Scarb1 null mice. Morphometric parameters were evaluated in cancellous (A) and cortical (B) VOIs as defined in Fig. 2. Values are means ± SE from 6–10 mice in each group. Bonferroni post hoc test, *P < 0.05, **P < 0.01, and ***P < 0.001 vs. WT of corresponding age group.
Fig. 4.
Histological analysis of mouse femora and plasma levels of C-telopeptide of type I collagen (CTX). Polymethylmethacrylate (PMMA)-embedded bones harvested from calcein-injected mice were sectioned, deplastified, and stained for Von Kossa (VK, ×4 magnification), alkaline phosphatase (ALP, ×10 magnification), and tartrate-resistant acid phosphatase (TRAP, ×10 magnification) (A) or photographed with a fluorescence microscope to observe the calcein fronts from the trabecular (TB) and cortical (CT) areas (B). Values for dynamic histology are listed in Table 3 and are from 5–7 mice in each group. C: plasma levels of CTX in 2- and 4-mo-old WT and null mice. Values are from 3 to 6 individuals.
Table 3.
Average dynamic histomorphometric values from femora of WT and null mice
| Males |
Females |
|||
|---|---|---|---|---|
| WT | Null | WT | Null | |
| Relative OB surface, % | 20.5 ± 1.5 | 29.1 ± 3.0** | 20.5 ± 2.4 | 26.9 ± 1.4* |
| No. of Oc/mm, mm−1 | 5.2 ± 0.2 | 4.0 ± 0.3 | 5.4 ± 0.6 | 6.9 ± 0.6 |
| Trabecular mineral apposition rate, μm/day | 1.48 ± 0.04 | 1.85 ± 0.06** | 1.25 ± 0.20 | 1.61 ± 0.05* |
| Cortical mineral apposition rate, μm/day | 3.9 ± 0.5 | 5.3 ± 0.7 | 3.31 ± 0.76 | 5.50 ± 1.15* |
| Percent mineralizing surface, % | 23.3 ± 2.3 | 32.0 ± 2.9* | 22.6 ± 1.1 | 33.6 ± 0.9* |
| Bone formation rate/bone surface, μm2 · μm−1 · day−1 | 0.35 ± 0.04 | 0.59 ± 0.07*** | 0.28 ± 0.06 | 0.43 ± 0.03* |
Values are means ±SE from 5–7 mice in each group. OB, osteoblasts; Oc, osteoclasts. Bonferroni post hoc test:
P < 0.05,
P < 0.01, and
P < 0.001 vs. corresponding WT.
Functions of MSCs in null mice.
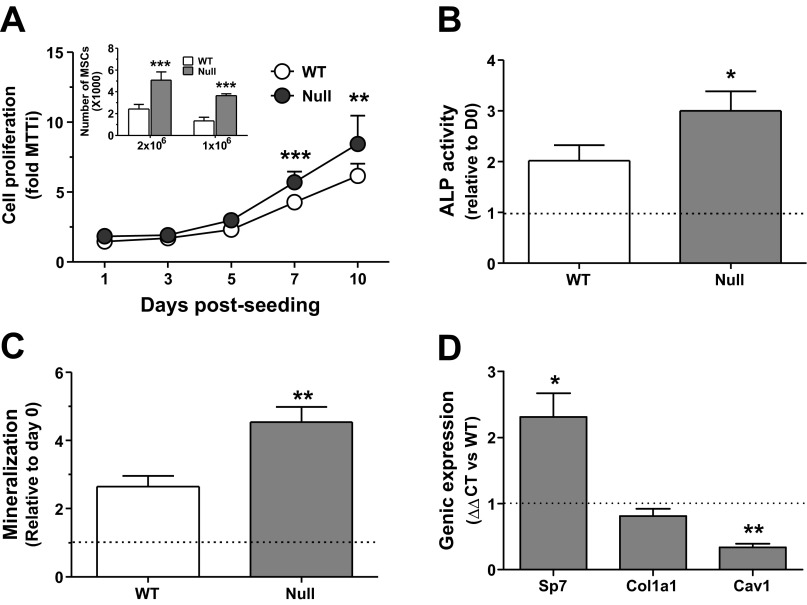
Because bone architecture and histology suggested enhanced osteoblastogenesis and/or osteoblastic cell activity, we further evaluated the expansion potential of MSCs from Scarb1 null mice. Expansion of MSCs isolated from null mice was two times that observed in WT and independent of initial plating density (Fig. 5A, inset). To discriminate whether the higher bone mass was solely linked to greater MSC number within the BM, MSCs from either genotypes were harvested at confluence, seeded at 20,000 cells/cm2, and left to proliferate in basal culture medium for 10 days. Even at similar initial plating densities, the MSCs from null mice showed an enhanced proliferation at 7 and 10 days postseeding (Fig. 5A). A greater propensity toward osteoblastic differentiation was also observed in MSCs from null mice, as determined by ALP activity (Fig. 5B) and ARS staining (Fig. 5C), as well as increased gene expression of the osteoblastic transcription factor Sp7, yet lower Cav1 expression; Col1a1 remained unaffected (Fig. 5D). MSCs extracted from long bones of null female mice behaved similarly (data not shown).
Fig. 5.
In vitro characterization of marrow-derived stromal cells (MSCs) from WT and Scarb1 null male mouse long bones. Marrow was harvested from 2-mo-old mouse femora and tibiae and characterized. A: time course of WT and null MSC proliferation; Bonferroni post hoc test, **P < 0.01 and ***P < 0.001 vs. WT. Inset, no. of adherent MSCs isolated from WT and null bone marrow; Bonferroni post hoc test, ***P < 0.001 vs. WT. B: cellular ALP activity in MSCs grown in osteogenic media for 21 days; paired Student's t-test, *P < 0.05. C: mineralization potential of MSCs grown in osteogenic media for 21 days; paired Student's t-test, **P < 0.01. D: genic expression of osterix (Sp7), collagen 1 alpha 1 (Col1a1), and caveolin-1 (Cav1) in null MSCs relative to WT MSCs; paired Student's t-tests, *P < 0.05 and **P < 0.01. All experiments are from 3–4 independent cell preparations.
Impact of high ACTH and high HDL on null MSCs.
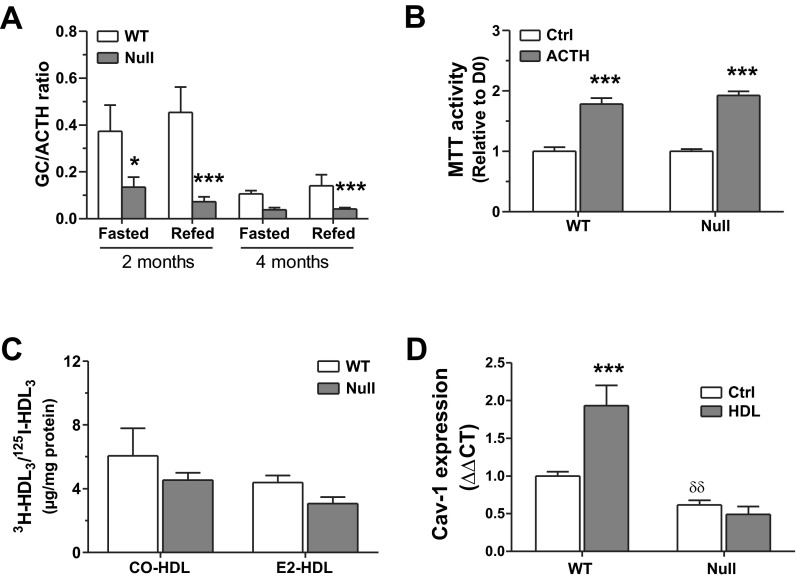
With SR-BI being associated with HDL-CE uptake by adrenal glands (28), we investigated adrenal function in null mice. Corticosterone and ACTH levels were measured in both genotypes under normal housing conditions and following a 16-h fast. The GC-to-ACTH ratio was significantly stunted in null mice, especially in 2-mo-old individuals (Fig. 6A), associated with the absence of corticosterone peak following fasting-induced stress in Scarb1 null mice and higher ACTH levels (data not shown). It was also determined how WT and null MSCs respond to in vitro exposure to ACTH; in both cases, cells proliferate more in the presence of the hormone (Fig. 6B), suggesting that SR-BI is not involved in the proliferative response of MSCs to this hormone. Similar impairment of the corticosterone/ACTH axis was observed in Scarb1 null females (data not shown).
Fig. 6.
Impact of high ACTH or high HDL3 on Scarb1 null MSCs. A: corticosterone induction index by ACTH in fasted and fed states; Bonferroni post hoc test, *P < 0.05 and ***P < 0.001 vs. WT. Plasma were harvested from 4–8 mice/group. B: proliferation response of WT and null marrow-derived MSCs grown with or without 10 nM ACTH for 10 days; Bonferroni post hoc test, ***P < 0.001. Values are from 3 independent cell preparations. C: selective cholesteryl oleate (CO) and estradiol (E2) uptake from HDL3 particles in WT and null MSCs. Data are averages ± SE from 3–6 independent cell preparations. D: gene expression of caveolin-1 (Cav1) in WT and null MSCs in control conditions (Ctrl) and following stimulation for 24 h with HDL3 (150 μg/ml). Data are averages ± SE from 4–6 independent cell preparations. ***P < 0.001, significant differences vs. Ctrl; δδP < 0.05, significant differences vs. WT; Bonferroni post hoc test.
Given that we reported cholesteryl ester and estradiol selective uptake from HDL by osteoblasts (9), we verified whether this process was impaired in Scarb1 null MSCs. Despite a decreasing trend, similar selective uptake levels for either CO or E2 were measured in MSCs from Scarb1 null and WT mice (Fig. 6C). Hence, the contribution of SR-BI to selective uptake is either not its main role in osteoblasts or is spontaneously compensated.
Because we observed that Scarb1 knockout impacts on the gene expression of Cav1 by MSC, we looked forward to the regulation of Cav1 gene expression by the HDL in Scarb1 null MSCs. Gene expression of Cav1 was increased by HDL in MSCs from WT mice (Fig. 6D), whereas HDL was without effect on its gene expression in null MSCs.
DISCUSSION
Epidemiologic studies have linked atherosclerosis incidence with the development of osteoporosis (21, 32). Because studies attribute an atheroprotective role to SR-BI by contributing to HDL-mediated RCT (11, 18, 33, 35, 57) and that Scarb1 null mice are prone to atherosclerosis, the present work therefore aimed at assessing the bone phenotype in Scarb1 null mice. In accordance with the role of SR-BI in hepatic lipoprotein metabolism, our data indicate that Scarb1 null mice show significantly higher serum cholesterol, especially HDL associated, as previously reported (41). Despite their susceptibility to atherosclerosis, our results show that Scarb1 null mice are not prone to osteoporosis but display high bone mass phenotype associated with enhanced bone formation.
Indeed bone analyses by μCT revealed that the Scarb1 null mouse model displays higher relative trabecular bone mass accounted by significantly reduced trabecular spacing (data not shown) and increased trabecular number in null mice up to 4 mo of age. Cortical bone and BMD values in both trabecular and cortical areas remained unaffected in male mice but were increased in 2-mo-old Scarb1 null female mice. Histological data corroborated the μCT analyses: there was more mineralized bone in the femora of null mice translating to higher relative mineralized bone surface. Because the high bone mass phenotype observed in Scarb1 null mice likely results from an imbalance in the cellular processes regulating bone resorption and formation, we determined the number of osteoblasts and osteoclasts in bone sections. The relative osteoblast surface was higher by 1.31- to 1.42-fold in null mice. The number of osteoclasts per millimeter was similar between WT and null mice, suggesting a prevalently osteoblast-mediated mechanism corroborated by similar plasma levels of bone resorption marker CTX in WT and null mice. Accordingly, the trabecular mineral apposition rate was significantly enhanced in these mice, suggesting greater bone formation in the absence of SR-BI. Morphometric values for cortical bone of Scarb1 null male mice were not different compared with WT mice, possibly because of higher turnover in the trabeculae relatively to the cortex (16). However, female null mice showed similar enhanced trabecular bone mass in the femora, but they differed from males in also displaying greater cortical bone volume and enhanced mineral apposition rate. This high bone mass phenotype is comparable to that observed in other mouse models deficient for key factors in cholesterol metabolism. Enhanced bone formation rate was reported in the apolipoprotein (apo) E null mouse model (54); apoE acts as a ligand in lipoprotein clearance (25), and apoE null mice develop atherosclerotic lesions (39). These indeed illustrate the significant influence of cholesterol metabolism-related genes on bone tissue.
We previously reported localization of SR-BI in caveolae of osteoblasts (9). Cav1 is a structural protein of cholesterol-rich plasma membrane microdomain termed caveolae, which are involved in intracellular cholesterol homeostasis (23) and cell signaling (46). However, SR-BI expression in nonsteroidogenic tissues such as bone (9) underscores a previously unsuspected functionality. Although associated with selective CE and estradiol uptake in human and murine osteoblasts (9), a thorough exploration of its functions in bone tissue has never been done. Given the expression of SR-BI by osteoblasts and the high bone mass phenotype of Scarb1 null mice associated with enhanced bone formation, we undertook the functional characterization of long bone-derived MSCs from Scarb1 null mice. There were two times as many MSCs in the bone marrow of null mice after 10 days of culture, which may result from both increased initial MSC number and enhanced cell proliferation. In accordance, MSCs from null mice showed enhanced proliferation compared with cells from WT mice. These findings agree with the high bone mass phenotype and enhanced bone formation in Scarb1 null mice. Moreover, enhanced proliferation of Scarb1 null cells has been also reported in other cell types such as macrophages (33) and lymphocytes (22). In contrast, Xu et al. (60) reported that genic silencing of Scarb1 resulted in lower HDL-induced proliferation of rat MSCs. In our study, the cells were grown in medium containing 10% FBS, which may account for the observed discrepancies between the two studies. Also, we observed a lower Cav1 expression in our cultures, which was not verified by Xu et al. (60). Some studies associate this protein with oncosuppressive properties because of the fact that its expression lowers the proliferation and metastatic potential of osteosarcoma cell lines (15). Moreover, neural stem cells (31) and mouse embryonic fibroblasts (48) of Cav1 null mice do display higher proliferation rates, corroborating our observations.
MSCs from null mice also displayed stronger ALP activity, and Alizarin Red S staining following osteogenic treatment, suggesting a greater differentiation potential in the absence of SR-BI. In accordance, gene expression of the osteoblast transcription factor Osx/Sp7 was increased in confluent MSCs from null mice before treatment. Therefore, the alterations in osteoblasts from null mice may significantly account for the bone phenotype observed in this study, and Scarb1 expression seemingly provides a repression mechanism to osteoblast function. Similar higher bone mass phenotype and increased bone formation have been reported in Cav1 null mice (51). Cav1 knockout has been associated with enhanced MSC osteogenic differentiation (51), which corroborates high bone mass phenotype in null mice, and it was suggested that Cav1 expression helps to maintain osteoblast progenitor cells in a less differentiated state. Accordingly, siRNA-mediated knockdown of Cav1 expression in MSCs derived from human bone marrow enhances their proliferation and osteogenic differentiation (4). Of interest, SR-BI has been reported to stabilize Cav1 expression in kidney cells (24); there may therefore be common mechanisms leading to the bone phenotype of both Cav1 and Scarb1 null models.
In addition to the cell-autonomous osteoblast alterations observed in MSC from null mice, our results indicate defective function of adrenal glands in Scarb1 null mice as illustrated by the lower GC-to-ACTH ratio. Indeed, impaired corticosterone response to fasting-induced stress associated with constitutively high levels of ACTH was observed in Scarb1 null male mice (26, 28). Impaired GC synthesis reported in Scarb1 null mice was previously associated with the lack of selective CE uptake by adrenals (28); recently, it was demonstrated that GC insufficiency can be induced in normal mice through transplantation of adrenal glands from Scarb1 null mice (27). The constitutively high ACTH levels observed in the null mice were of interest to this study; indeed, ACTH has been shown to enhance bone mass by stimulating MSC proliferation and expression of several osteogenic markers (6, 30, 61). Interestingly, MSCs isolated from either WT or null bone marrows seem to respond normally to this hormone in terms of proliferation. Therefore, high levels of ACTH associated with the adrenal gland dysfunction in the Scarb1 null mice may contribute to the observed higher bone mass phenotype. Of interest, ACTH impact in bone has been linked to vascular endothelial growth factor (VEGF) (61). On the other hand, SR-BI has been shown to be essential for HDL-induced VEGF production in ischemia-driven angiogenesis (56). The relation between ACTH, VEGF, and SR-BI seems to be quite complex; this question certainly deserves further investigation.
Because SR-BI is predominantly involved in the selective cholesterol uptake process by liver and adrenal glands (28, 62), we verified its implication in the selective uptake by MSCs. Although Scarb1 null mice show impaired selective uptake functions in adrenal glands (28) and liver (11), it does not seem to be the case in MSCs, regardless of the lipidic ligand used (e.g., CO or E2). We in fact observed a nonsignificant decreasing trend. CD36 receptor is also expressed in osteoblasts (9), and this receptor is able to selectively uptake cholesterol from HDL yet at a lower rate than SR-BI (12). Our data therefore suggest that SR-BI has a minor contribution in the selective uptake process and/or the presence of compensatory mechanisms by other receptors. Our results also indicate an upregulation of Cav1 in WT MSCs following HDL exposure; however, no modulation of Cav1 expression was observed in cells from null mice. Because SR-BI was shown to promote the stabilization of Cav1 expression in kidney cells (24), its absence may impair normal regulation of proliferation and differentiation, thus further contributing to the enhanced bone mass.
This study associates SR-BI with significant functions in bone remodeling on two levels, by modulating ACTH/GC levels through cholesterol uptake in the adrenal glands and by influencing proliferation and differentiation processes in MSCs. Although the high levels of circulating ACTH likely exert a significant influence on these mice's bone mass, one cannot overlook the effects of Scarb1 knockout in osteoblasts themselves. SR-BI seemingly slows bone formation at a cellular level, since MSCs deficient for that receptor proliferate faster and differentiate more readily toward an osteoblastic lineage, potentially through modulation of Cav1 expression. Further studies on the bone phenotype of Scarb1 null mice should focus on osteoblast-specific and adrenal cell-specific conditional deletions to discriminate between the impacts of osteoblast-mediated mechanisms vs. the effects of an altered endocrine condition on bone metabolism.
GRANTS
This work was supported by grants from the Canadian Institute of Health Research (R. Moreau). C. Martineau is a recipient of scholarships from the Natural Sciences and Engineering Research Council of Canada.
DISCLOSURES
All authors state that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Author contributions: C.M. and L.M.-F. performed experiments; C.M. and R.M. analyzed data; C.M., L.B., and R.M. interpreted results of experiments; C.M. and R.M. prepared figures; C.M., L.B., and R.M. drafted manuscript; C.M., L.M.-F., L.B., and R.M. edited and revised manuscript; C.M., L.M.-F., L.B., and R.M. approved final version of manuscript; L.B. and R.M. conception and design of research.
REFERENCES
- 1.Ackert-Bicknell CL. HDL cholesterol and bone mineral density: is there a genetic link? Bone 50: 525–533, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271: 518–520, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. Bone loss and bone size after menopause. N Engl J Med 349: 327–334, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Baker N, Zhang G, You Y, Tuan RS. Caveolin-1 regulates proliferation and osteogenic differentiation of human mesenchymal stem cells. J Cell Biochem 113: 3773–3787, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Bilezikian JP, Brandi ML, Rubin M, Silverberg SJ. Primary hyperparathyroidism: new concepts in clinical, densitometric and biochemical features. J Intern Med 257: 6–17, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Blair HC, Zaidi M, Schlesinger PH. Mechanisms balancing skeletal matrix synthesis and degradation. Biochem J 364: 329–341, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bostrom KI, Rajamannan NM, Towler DA. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ Res 109: 564–577, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein0dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 8.Brissette L, Charest MC, Falstrault L. Selective uptake of cholesteryl esters of low-density lipoproteins is mediated by the lipoprotein-binding site in HepG2 cells and is followed by the hydrolysis of cholesteryl esters. Biochem J 318: 841–847, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodeur MR, Brissette L, Falstrault L, Luangrath V, Moreau R. Scavenger receptor of class B expressed by osteoblastic cells are implicated in the uptake of cholesteryl ester and estradiol from LDL and HDL3. J Bone Miner Res 23: 326–337, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Brodeur MR, Luangrath V, Bourret G, Falstrault L, Brissette L. Physiological importance of SR-BI in the in vivo metabolism of human HDL and LDL in male and female mice. J Lipid Res 46: 687–696, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Brundert M, Ewert A, Heeren J, Behrendt B, Ramakrishnan R, Greten H, Merkel M, Rinninger F. Scavenger receptor class B type I mediates the selective uptake of high-density lipoprotein-associated cholesteryl ester by the liver in mice. Arterioscler Thromb Vasc Biol 25: 143–148, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Brundert M, Heeren J, Merkel M, Carambia A, Herkel J, Groitl P, Dobner T, Ramakrishnan R, Moore KJ, Rinninger F. Scavenger receptor CD36 mediates uptake of high density lipoproteins in mice and by cultured cells. J Lipid Res 52: 745–758, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calvo D, Gomez-Coronado D, Suarez Y, Lasuncion MA, Vega MA. Human CD36 is a high affinity receptor for the native lipoproteins HDL, LDL, and VLDL. J Lipid Res 39: 777–788, 1998 [PubMed] [Google Scholar]
- 14.Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 18: 1319–1328, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Cantiani L, Manara MC, Zucchini C, De Sanctis P, Zuntini M, Valvassori L, Serra M, Olivero M, Di Renzo MF, Colombo MP, Picci P, Scotlandi K. Caveolin-1 reduces osteosarcoma metastases by inhibiting c-Src activity and met signaling. Cancer Res 67: 7675–7685, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol 3, Suppl 3: S131–S139, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crockett JC, Rogers MJ, Coxon FP, Hocking LJ, Helfrich MH. Bone remodelling at a glance. J Cell Sci 124: 991–998, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation 113: 2548–2555, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Cunningham J, Sprague SM, Cannata-Andia J, Coco M, Cohen-Solal M, Fitzpatrick L, Goltzmann D, Lafage-Proust MH, Leonard M, Ott S, Rodriguez M, Stehman-Breen C, Stern P, Weisinger J. Osteoporosis in chronic kidney disease. Am J Kidney Dis 43: 566–571, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Erben RG. Embedding of bone samples in methylmethacrylate: an improved method suitable for bone histomorphometry, histochemistry, and immunohistochemistry. J Histochem Cytochem 45: 307–313, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Farhat GN, Cauley JA. The link between osteoporosis and cardiovascular disease. Clin Cases Miner Bone Metab 5: 19–34, 2008 [PMC free article] [PubMed] [Google Scholar]
- 22.Feng H, Guo L, Wang D, Gao H, Hou G, Zheng Z, Ai J, Foreman O, Daugherty A, Li XA. Deficiency of scavenger receptor BI leads to impaired lymphocyte homeostasis and autoimmune disorders in mice. Arterioscler Thromb Vasc Biol 31: 2543–2551, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frank PG, Cheung MW, Pavlides S, Llaverias G, Park DS, Lisanti MP. Caveolin-1 and regulation of cellular cholesterol homeostasis. Am J Physiol Heart Circ Physiol 291: H677–H686, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Frank PG, Marcel YL, Connelly MA, Lublin DM, Franklin V, Williams DL, Lisanti MP. Stabilization of caveolin-1 by cellular cholesterol and scavenger receptor class B type I. Biochemistry 41: 11931–11940, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Gonzales JC, Gordts PL, Foley EM, Esko JD. Apolipoproteins E and AV mediate lipoprotein clearance by hepatic proteoglycans. J Clin Invest 123: 2742–2751, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoekstra M, Meurs I, Koenders M, Out R, Hildebrand RB, Kruijt JK, Van Eck M, Van Berkel TJ. Absence of HDL cholesteryl ester uptake in mice via SR-BI impairs an adequate adrenal glucocorticoid-mediated stress response to fasting. J Lipid Res 49: 738–745, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Hoekstra M, van der Sluis RJ, van Eck M, Van Berkel TJ. Adrenal-specific scavenger receptor BI deficiency induces glucocorticoid insufficiency and lowers plasma very-low-density and low-density lipoprotein levels in mice. Arterioscler Thromb Vasc Biol 33: e39–e46, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Hoekstra M, Ye D, Hildebrand RB, Zhao Y, Lammers B, Stitzinger M, Kuiper J, Van Berkel TJ, Van Eck M. Scavenger receptor class B type I-mediated uptake of serum cholesterol is essential for optimal adrenal glucocorticoid production. J Lipid Res 50: 1039–1046, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huby T, Doucet C, Dachet C, Ouzilleau B, Ueda Y, Afzal V, Rubin E, Chapman MJ, Lesnik P. Knockdown expression and hepatic deficiency reveal an atheroprotective role for SR-BI in liver and peripheral tissues. J Clin Invest 116: 2767–2776, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isales CM, Zaidi M, Blair HC. ACTH is a novel regulator of bone mass. Ann NY Acad Sci 1192: 110–116, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Jasmin JF, Yang M, Iacovitti L, Lisanti MP. Genetic ablation of caveolin-1 increases neural stem cell proliferation in the subventricular zone (SVZ) of the adult mouse brain. Cell Cycle 8: 3978–3983, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Jensky NE, Hyder JA, Allison MA, Wong N, Aboyans V, Blumenthal RS, Schreiner P, Carr JJ, Wassel CL, Ix JH, Criqui MH. The association of bone density and calcified atherosclerosis is stronger in women without dyslipidemia: the multi-ethnic study of atherosclerosis. J Bone Miner Res 26: 2702–2709, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji A, Meyer JM, Cai L, Akinmusire A, de Beer MC, Webb NR, van der Westhuyzen DR. Scavenger receptor SR-BI in macrophage lipid metabolism. Atherosclerosis 217: 106–112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz S, Weinerman S. Osteoporosis and gastrointestinal disease. Gastroenterol Hepatol 6: 506–517, 2010 [PMC free article] [PubMed] [Google Scholar]
- 35.Krieger M. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J Clin Invest 108: 793–797, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langer T, Strober W, Levy RI. The metabolism of low density lipoprotein in familial type II hyperlipoproteinemia. J Clin Invest 51: 1528–1536, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McFarlane AS. Efficient trace-labelling of proteins with iodine (Abstract). Nature 182: 53, 1958 [DOI] [PubMed] [Google Scholar]
- 39.Meir KS, Leitersdorf E. Atherosclerosis in the apolipoprotein-E-deficient mouse: a decade of progress. Arterioscler Thromb Vasc Biol 24: 1006–1014, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Miettinen HE, Rayburn H, Krieger M. Abnormal lipoprotein metabolism and reversible female infertility in HDL receptor (SR-BI)-deficient mice. J Clin Invest 108: 1717–1722, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mineo C, Shaul PW. Functions of scavenger receptor class B, type I in atherosclerosis. Curr Opin Lipidol 23: 487–493, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Moreau R, Aubin R, Lapointe JY, Lajeunesse D. Pharmacological and biochemical evidence for the regulation of osteocalcin secretion by potassium channels in human osteoblast-like MG-63 cells. J Bone Miner Res 12: 1984–1992, 1997 [DOI] [PubMed] [Google Scholar]
- 43.O'Neill TW. Looking back: developments in our understanding of the occurrence, aetiology and prognosis of osteoporosis over the last 50 years. Rheumatology (Oxford) 44, Suppl 4: iv33–iv35, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Ohashi R, Mu H, Wang X, Yao Q, Chen C. Reverse cholesterol transport and cholesterol efflux in atherosclerosis. QJM 98: 845–856, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Parhami F, Garfinkel A, Demer LL. Role of lipids in osteoporosis. Arterioscler Thromb Vasc Biol 20: 2346–2348, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Quest AF, Gutierrez-Pajares JL, Torres VA. Caveolin-1: an ambiguous partner in cell signalling and cancer. J Cell Mol Med 12: 1130–1150, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raisz LG. Physiology and pathophysiology of bone remodeling. Clin Chem 45: 1353–1358, 1999 [PubMed] [Google Scholar]
- 48.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, Macaluso F, Russell RG, Li M, Pestell RG, Di Vizio D, Hou H, Jr, Kneitz B, Lagaud G, Christ GJ, Edelmann W, Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem 276: 38121–38138, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci USA 94: 12610–12615, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roberts DC, Miller NE, Price SG, Crook D, Cortese C, La Ville A, Masana L, Lewis B. An alternative procedure for incorporating radiolabelled cholesteryl ester into human plasma lipoproteins in vitro. Biochem J 226: 319–322, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubin J, Schwartz Z, Boyan BD, Fan X, Case N, Sen B, Drab M, Smith D, Aleman M, Wong KL, Yao H, Jo H, Gross TS. Caveolin-1 knockout mice have increased bone size and stiffness. J Bone Miner Res 22: 1408–1418, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Sanders KM, Nicholson GC, Ugoni AM, Seeman E, Pasco JA, Kotowicz MA. Fracture rates lower in rural than urban communities: the Geelong Osteoporosis Study. J Epidemiol Community Health 56: 466–470, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sandhu SK, Hampson G. The pathogenesis, diagnosis, investigation and management of osteoporosis. J Clin Pathol 64: 1042–1050, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Schilling AF, Schinke T, Munch C, Gebauer M, Niemeier A, Priemel M, Streichert T, Rueger JM, Amling M. Increased bone formation in mice lacking apolipoprotein E. J Bone Miner Res 20: 274–282, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Shashkin P, Dragulev B, Ley K. Macrophage differentiation to foam cells. Curr Pharm Des 11: 3061–3072, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Tan JT, Prosser HC, Vanags LZ, Monger SA, Ng MK, Bursill CA. High-density lipoproteins augment hypoxia-induced angiogenesis via regulation of post-translational modulation of hypoxia-inducible factor 1alpha. FASEB J In press [DOI] [PubMed] [Google Scholar]
- 57.Trigatti B, Rayburn H, Vinals M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Krieger M. Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc Natl Acad Sci USA 96: 9322–9327, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Velde AE, Groen AK. Shifting gears: liver SR-BI drives reverse cholesterol transport in macrophages. J Clin Invest 115: 2699–2701, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weston JM, Norris EV, Clark EM. The invisible disease: making sense of an osteoporosis diagnosis in older age. Qual Health Res 21: 1692–1704, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J, Qian J, Xie X, Lin L, Ma J, Huang Z, Fu M, Zou Y, Ge J. High density lipoprotein cholesterol promotes the proliferation of bone-derived mesenchymal stem cells via binding scavenger receptor-B type I and activation of PI3K/Akt, MAPK/ERK1/2 pathways. Mol Cell Biochem 371: 55–64, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Zaidi M, Sun L, Robinson LJ, Tourkova IL, Liu L, Wang Y, Zhu LL, Liu X, Li J, Peng Y, Yang G, Shi X, Levine A, Iqbal J, Yaroslavskiy BB, Isales C, Blair HC. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad Sci USA 107: 8782–8787, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y, Da Silva JR, Reilly M, Billheimer JT, Rothblat GH, Rader DJ. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest 115: 2870–2874, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]