Abstract
Here, we sought to compare the efficacy of combining exercise and metformin for the treatment of type 2 diabetes and nonalcoholic fatty liver disease (NAFLD) in hyperphagic, obese, type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) rats. OLETF rats (age: 20 wk, hyperglycemic and hyperinsulinemic; n = 10/group) were randomly assigned to sedentary (O-SED), SED plus metformin (O-SED + M; 300 mg·kg−1·day−1), moderate-intensity exercise training (O-EndEx; 20 m/min, 60 min/day, 5 days/wk treadmill running), or O-EndEx + M groups for 12 wk. Long-Evans Tokushima Otsuka (L-SED) rats served as nonhyperphagic controls. O-SED + M, O-EndEx, and O-EndEx + M were effective in the management of type 2 diabetes, and all three treatments lowered hepatic steatosis and serum markers of liver injury; however, O-EndEx lowered liver triglyceride content and fasting hyperglycemia more than O-SED + M. In addition, exercise elicited greater improvements compared with metformin alone on postchallenge glycemic control, liver diacylglycerol content, hepatic mitochondrial palmitate oxidation, citrate synthase, and β-HAD activities and in the attenuation of markers of hepatic fatty acid uptake and de novo fatty acid synthesis. Surprisingly, combining metformin and aerobic exercise training offered little added benefit to these outcomes, and in fact, metformin actually blunted exercise-induced increases in complete mitochondrial palmitate oxidation and β-HAD activity. In conclusion, aerobic exercise training was more effective than metformin administration in the management of type 2 diabetes and NAFLD outcomes in obese hyperphagic OLETF rats. Combining therapies offered little additional benefit beyond exercise alone, and findings suggest that metformin potentially impairs exercise-induced hepatic mitochondrial adaptations.
Keywords: exercise training, metformin, nonalcoholic fatty liver disease, hepatic mitochondria, de novo lipogenesis, Otsuka Long-Evans Tokushima Fatty rats
nonalcoholic fatty liver disease (NAFLD) is a progressive liver disease characterized by hepatic triglyceride (TG) accumulation (≥5% by weight for diagnosis) that occurs in the absence of excess alcohol consumption (>20 g/day). It encompasses a histological spectrum ranging from simple hepatic steatosis to nonalcoholic steatohepatitis, advanced fibrosis, and cirrhosis (40). NAFLD is considered the hepatic manifestation of the metabolic syndrome (11), and it affects ∼30% of the US adult population (3, 7) and ∼70% of type 2 diabetics (52). However, there are currently no clear guidelines for the treatment of NAFLD.
A component of prevention and treatment recommendations addressing NAFLD involves lifestyle modifications that alter net energy status. Indeed, both short-term (6) and longer-term caloric restriction (10, 23), as well as exercise training and increasing physical activity, have been shown to improve NAFLD in animal models (42, 46, 47) and humans (13, 18, 24, 50). Although these lifestyle interventions seem to be able to improve the disease state, many people have difficulty adhering to diet and exercise programs (16, 54). Therefore, there is a need to better understand the potential therapeutic benefits of lifestyle and/or pharmacological interventions on NAFLD.
Metformin is a drug often prescribed to individuals with type 2 diabetes; however, there is also limited evidence suggesting that it may improve NAFLD outcomes as well. Human studies have shown that continued metformin use helps sustain reductions in circulating serum alanine aminotransferase (ALT) levels in individuals at risk for developing type 2 diabetes (22) and with early liver disease (31), and these reductions may be due in part to metformin-induced reductions in body weight (22). Metformin also has been shown to improve liver TG content in some rodent models of diabetes and NAFLD (14, 25). However, it is unknown whether the efficacy of treating NAFLD in the setting of type 2 diabetes may be enhanced if metformin was taken in combination with exercise. By using an animal model of obesity, we can use more invasive measures to better understand the potential mechanisms by which metformin and/or exercise training effectively treat NAFLD.
To examine the effects of metformin and exercise on NAFLD and type 2 diabetes outcomes, we have employed the use of the Otsuka Long-Evans Tokushima Fatty (OLETF) rat, an established animal model of obesity and type 2 diabetes. These animals are selectively bred with a mutated and functionally inoperative cholecystokinin-1 receptor (34, 35), which results in hyperphagia and the progressive development of obesity, insulin resistance, type 2 diabetes, and NAFLD (43). We have demonstrated previously that these pathological metabolic events are prevented in physically active OLETF rats given daily access to voluntary running wheels (41, 42, 45, 46). However, it is unknown whether lower-volume, moderate-intensity treadmill exercise elicits similar benefits for the treatment of NAFLD and type 2 diabetes. Additionally, it is unclear whether combining metformin treatment with exercise training would confer additional benefits. Thus, we sought to determine whether 1) exercise training and metformin treatment differ in their efficacy to treat NAFLD and type 2 diabetes, 2) the combination of exercise training and metformin can further improve pathological conditions associated with NAFLD, and 3) to gain insight into the mechanisms by which NAFLD is improved by each therapy alone and in combination in the type 2 diabetic OLETF rat. Because both metformin treatment and exercise training have been shown independently to improve conditions associated with type 2 diabetes and NAFLD, it was hypothesized that the combination of the two treatments would be more effective at treating these disease states than either treatment alone.
METHODS
Animal protocol.
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri. Four-week-old OLETF male rats (Tokushima Research Institute, Otsuka Pharmaceutical, Tokushima, Japan) were housed individually in standard cages within temperature- and light-controlled animal quarters (12:12-h light-dark cycle at 21°C). The animals were given ad libitum access to standard rodent chow (Formulab 5008; Purina Mills, St. Louis, MO). All animals remained sedentary until 20 wk of age [age where sedentary OLETF rats have developed moderate NAFLD and are insulin resistant and type 2 diabetic with hyperglycemia and hyperinsulinemia (4, 43, 45)], at which time they were randomly assigned to one of the following treatment groups (n = 10/group) for 12 wk: sedentary (O-SED), SED + metformin (O-SED + M), moderate-intensity exercise training (O-EndEx), or O-EndEx + M. Nonhyperphagic control Long-Evans Tokushima Otsuka rats remained in sedentary cage conditions (L-SED). Animals in the EndEx treatments initially began treadmill running at a speed of 15 m/min on a 15% incline for 5 min/day. Duration and speed were gradually increased by 2–3 min/day and 1–2 m·min−1·wk−1 such that by week 4 the animals were running at a speed of 20 m/min on a 15% incline, 60 min/day, 5 days/wk. Metformin (Bosche Scientific) was administered in the drinking water (150 mg·kg−1·day−1 the 1st week and 300 mg·kg−1·day−1 thereafter), and the dosages were based on previous studies with chronic metformin administration (5, 32). Water intake was carefully monitored, and concentrations were adjusted accordingly to ensure that the proper dose of metformin was administered. Body mass and food intake were measured weekly throughout the study. At 32 wk of age, rats were anesthetized with pentobarbital sodium (100 mg/kg) and then exsanguinated by removal of the heart. All animals were fasted for 12 h, and water (including metformin) was removed on the morning of euthanization (∼1 h prior to euthanization). The last exercise bout for O-EndEx and O-EndEx + M animals was performed ∼18 h prior to euthanization. These conditions were chosen to better simulate conditions observed in clinical settings in which patients are typically not instructed to withdraw from physical activity or abstain from medications prior to a fasting blood draw.
Dual-energy X-ray absorptiometry.
Whole body composition was measured using a Hologic QDR-1000/w dual-energy X-ray absorptiometry machine calibrated for rats.
Serum assays.
Whole blood was collected on the day of euthanization for analysis of glycosylated hemoglobin (Hb A1c), as described previously (17). Serum glucose, insulin, TG, and nonesterified fatty acid assays were performed by Comparative Clinical Pathology Services (Columbia, MO) using commercially available assays and were completed according to the manufacturers' instructions.
Intraperitoneal glucose tolerance tests.
One week prior to euthanization, animals underwent intraperitoneal (ip) glucose tolerance tests (IPGTT) to assess glucose tolerance. Animals were fasted for 12 h prior to the test. Those animals in exercise training groups completed their last bout of exercise ∼18 h prior to the IPGTT. Whole blood samples were collected from the tail vein prior to and 15, 30, 45, 60, and 120 min following the ip glucose injection (2 g/kg), as described previously (45). The total area under the glucose curve was calculated using the trapezoidal method (51).
Tissue collection and preparation procedure.
Livers were quickly removed from anesthetized rats and either flash-frozen in liquid nitrogen, placed in 10% formalin, placed in RNAlater, or placed in ice-cold isolation buffer (100 mM KCl, 40 mM Tris·HCl, 10 mM Tris-Base, 5 mM MgCl2.6H2O, 1 mM EDTA, and 1 mM ATP, pH 7.4). Retroperitoneal, epididymal, and omental fat pads were excised from animals and weighed.
Fatty acid oxidation and measurements of mitochondrial function.
Fatty acid oxidation assays were performed in fresh hepatic tissue preparations, using radiolabeled [1-14C]palmitate (American Radiochemicals) as described previously (42). Briefly, the oxidation rate of [14C]palmitate was measured by collecting and counting the 14CO2 produced (representing complete fatty acid oxidation) and 14C-labeled acid-soluble metabolites (representing incomplete fatty acid oxidation) that were collected within a trapping device and counted with a liquid scintillation counter. Palmitate oxidation experiments were performed in the presence (100 uM) or absence of etomoxir (a specific inhibitor of mitochondrial carnitine palmitoyl-CoA transferease-1 and entry into the mitochondria) to examine the relative contribution of mitochondrial (− etomoxir) and extramitochondrial organelles (+ etomoxir) in total fatty acid oxidation, as described previously (46). Citrate synthase and β-hydroxyacyl-CoA dehydrogenase (β-HAD) activities were determined using the methods of Srere (49) and Bass et al. (1), respectively, as described previously by our group (42).
Intrahepatic lipid content and liver morphology.
To examine liver morphology, formalin-fixed, paraffin-embedded livers were sectioned and stained with haematoxylin and eosin (H & E). Biochemical intrahepatic TG content was determined as described previously (42). Hepatic diacylglycerol (DAG) content was determined after thin-layer chromatography isolation by methanolysis and measurement of fatty acid methyl esters by gas chromatography with flame ionization detection, as described previously by our group (4, 46).
Quantitative RT-PCR.
Hepatic mRNA expression was quantified using the ABI 7500 Fast Sequence Detection System and Software (Applied Biosystems, Carlsbad, CA) as described previously by our group (36). Primer sets were obtained from Sigma (St. Louis, MO) for all analyses, and analyses were conducted using Fast SYBR Green Master Mix kits (Applied Biosystems). β-Actin (forward: GCTCTCTTCCAGCCTTCCTT; reverse: CTTCTGCATCCTGTCAGCAA) was used as the housekeeping gene, and all gene expression data are represented relative to β-actin expression using the ΔΔCT method. The L-SED group was used as the referent group. Gene expression was assessed for glycerol-3-phosphate acyl transferase 1 (GPAT1; forward ATCCGCAACGCTGAAATGGAA, reverse GGCAAACATGCCCTTGTGGAC), stearoyl-CoA desaturase-1 (SCD-1; forward TGGGAAAGTGAAGCGAGCAACCG, reverse AGAGGGGCACCTTCTTCATCTTCTC), TR4 orphan nuclear receptor (forward CTCACCTCAGCGATTCAGA, reverse TCCATCTGGGCTGGTTAGGA), glucose 6-phosphatase (G6Pase; forward AGGAAGGATGGAGGAAGGAA, reverse TGGAACCAGATGGGAAAGAG), and phosphoenolpyruvate carboxykinase (PEPCK; forward: CCACAGCTGCTGCAGAACAC, reverse GAAGGGTCGCATGGCAAA).
Western blot analysis.
Western blot analyses were used to determine hepatic protein expression of markers related to mitochondrial content, fatty acid uptake, de novo lipogenesis, and TG synthesis. Peroxisome proliferator-activated receptor-γ coactivator-1α polyclonal antibody was obtained from EMD Millipore (Billerica, MA). Polyclonal antibodies for acetyl coenzyme A carboxylase (ACC), phosphorylation-specific ACC Ser79, fatty acid synthase (FAS), total AMP-activated protein kinase (AMPK)-α, phosphorylation-specific AMPK Thr172, cytochrome c, total Akt, phosphorylation-specific Akt Thr308, and phosphorylation-specific Akt Ser473 were obtained from Cell Signaling Technology (Beverly, MA). SCD-1 polyclonal antibody was from Alpha Diagnostics International (San Antonio, TX). CD36/FAT polyclonal antibody was from Santa Cruz Biotechnology (Dallas, TX). Diaclyglycerol acyltransferase (DGAT)1 and TR4 orphan nuclear receptor polyclonal antibodies were obtained from Abcam (Cambridge, MA). DGAT2 polyclonal antibody was from Novus Biologicals (Littleton, CO). Content of phosphoproteins was calculated from the density of the band of the phosphoprotein divided by the density (content) of the total protein using the appropriate antibodies (41, 42). Liver samples were homogenized using lysis buffer. Protein (20–40 μg) was loaded into a SDS-PAGE gel and probed with the primary antibody. After washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies. Protein bands were quantified using a densitometer (Bio-Rad, Hercules, CA). To control for equal protein loading and transfer, the membranes were then stained with 0.1% amido-black (Sigma), and total protein staining was quantified (42).
Statistical analysis.
Each outcome measure was examined in eight to 10 animals per group. For each outcome measure, a one-way analysis of variance was performed (SPSS/20.0; IBM, Chicago, IL), with significant interactions followed up using Fisher LSD post hoc comparisons. Values are reported as means ± SE, and statistical significance was determined as P < 0.05.
RESULTS
Metformin, exercise training, and the combination on body weight, adiposity, and food intake.
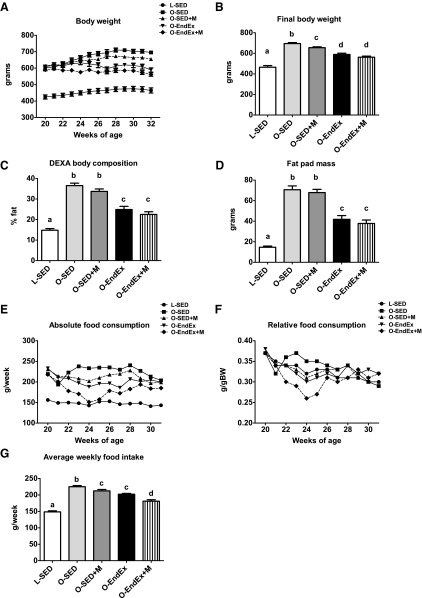
Although treatment with metformin did reduce body weight compared with O-SED, the reduction was less robust than with exercise training (5 vs. 15% reductions, respectively, P < 0.001; Fig. 1B), and metformin treatment alone had no effect on body composition or fat pad mass (Fig. 1, C and D) compared with significant reductions seen in exercising animals (Fig. 1, A and D). Only endurance exercise training elicited increases in the heart weight/body weight ratio (2.77 ± 0.08 vs. 3.25 ± 0.06 for O-SED and O-EndEx, respectively, P < 0.05). These parameters were not altered further in the combination group.
Fig. 1.
The effects of metformin, exercise training, or the combination on body weight, adiposity, and food intake. Weekly body weights (A), final body weight (B), %body fat (C), fat pad mass (omental + retroperitoneal + epididymal; D), absolute food intake (E), relative food intake (F), and average weekly food intake (G). All values are means ± SE. Values with different letters are significantly different; P < 0.05. L-SED, Long-Evans Tokushima Otsuka rats in sedentary cage conditions; O-SED, OLETF rats in sedentary cage conditions; O-SED + M, OLETF rats plus metformin; O-EndEx, OLETF rats with moderate-intensity exercise training; O-EndEx + M, OLETF rats with moderate-intensity exercise training plus metformin; DEXA, dual-energy X-ray absorptiometry.
Average weekly food consumption differed among treatment groups (P < 0.001; Fig. 1G), with O-SED consuming significantly more food than all other groups. The combination of aerobic exercise training and metformin initially reduced absolute (Fig. 1E) and relative food intake (Fig. 1F); however, this difference was normalized by the 6th wk of treatment. Both metformin treatment and EndEx reduced average weekly food intake ∼5–10% (P > 0.05, O-EndEx vs. O-Sed + M), with the combination of treatments further reducing food intake (∼10–15% lower than O-EndEx and O-Sed + M, P < 0.001).
Treatment effects on serum lipid and markers of type 2 diabetes.
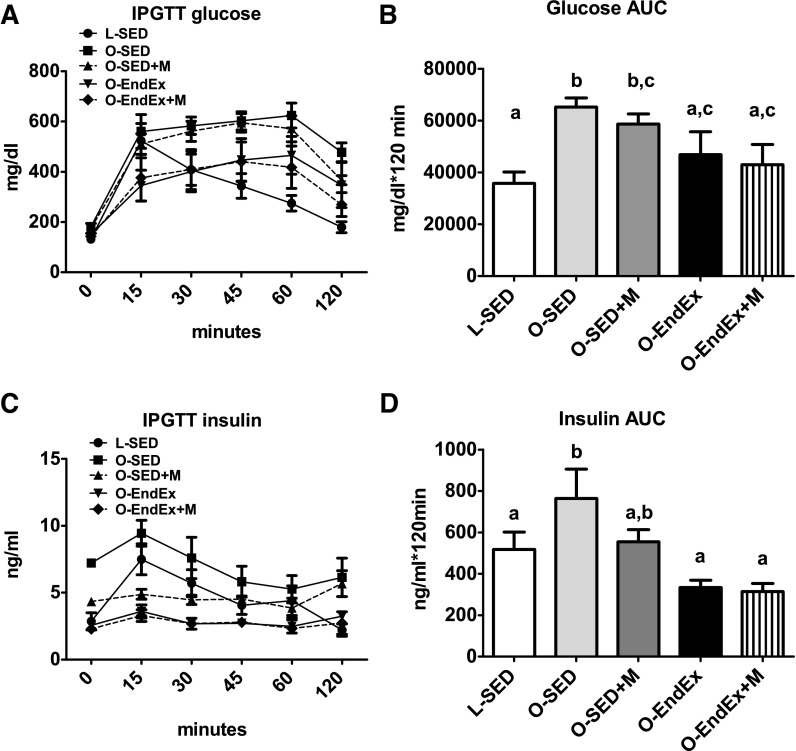
Exercise training reduced fasting serum TG and free fatty acid (FFA) concentrations compared with O-SED (P < 0.001), normalizing the former to the level witnessed in the lean LETO control animals (Table 1). Metformin treatment alone had no effect on either serum TG or FFA concentrations and, when added to EndEx training, had no additive effects. Both metformin (P < 0.001) and exercise training (P < 0.001) reduced Hb A1c levels, a marker of long-term glycemic control, compared with O-SED, with the combination of the two treatments normalizing Hb A1c to the level of the lean controls (Table 1). However, only exercise training reduced fasting glucose (P < 0.001) and insulin (P < 0.01) concentrations, and the combination of treatments provided no added benefits when compared with EndEx alone (Table 1). It should be noted that insulin values in these 32-wk-old O-SED animals reflect an ∼40% reduction compared with values at 20 wk of age (O-SED, 20 wk of insulin = 10.91 ± 1.41 ng/ml), highlighting a transition to pancreatic β-cell dysfunction and frank type 2 diabetes development not witnessed in the LETO animals or in any of the OLETF treatment groups during the 12 wk of treatment. Moreover, reductions in glucose and insulin areas under the curve after the IPGTT were seen only in the EndEx and EndEx + M groups, with no additional benefits beyond EndEx alone in the combination group (Fig. 2, B and D). These alterations were not associated with changes in basal hepatic Akt protein content or Akt Ser473 or Thr308 phosphorylation status (data not shown).
Table 1.
Fasting blood and serum parameters
| L-SED | O-SED | O-SED + M | O-EndEx | O-EndEx + M | |
|---|---|---|---|---|---|
| Triglyceride, mg/dl | 43.1 ± 3.4a | 344.0 ± 41.4b | 337.4 ± 46.1b | 121.1 ± 14.5a | 125.3 ± 25.7a |
| Free fatty acids, mmol/l | 0.28 ± 0.02a | 0.90 ± 0.07b | 0.89 ± 0.05b | 0.57 ± 0.05c | 0.53 ± 0.07c |
| Glucose, mg/dl | 152.1 ± 4.5a | 298.0 ± 13.0b | 268.9 ± 17.5b | 217.9 ± 14.4c | 189.3 ± 8.6c |
| Insulin, ng/ml | 3.14 ± 0.41a | 7.13 ± 0.83b | 7.08 ± 1.01b | 4.21 ± 0.47a | 3.70 ± 0.30a |
| Hb A1c, % glycosylated | 4.82 ± 0.11a | 6.88 ± 0.28b | 5.34 ± 0.16c | 5.40 ± 0.07c | 4.99 ± 0.08a,c |
Values are means ± SE (n = 8–10/group). L-SED, Long-Evans Tokushima Otsuka rats in sedentary cage conditions; O-SED, OLETF rats in sedentary cage conditions; O-SED + M, OLETF rats plus metformin; O-EndEx, OLETF rats with moderate-intensity exercise training; O-EndEx + M, OLETF rats with moderate-intensity exercise training plus metformin. Values with different superscripted letters are significantly different; P < 0.05.
Fig. 2.
Responses to an intraperitoneal glucose tolerance test (IPGTT) following 12 wk of treatment. Glucose curves (A), glucose area under the curve (AUC; B), insulin curves (C), and insulin AUC (D). All values are means ± SE. Values with different letters are significantly different; P < 0.05.
Treatment with exercise, metformin, or the combination on NAFLD.
Both metformin treatment and EndEx reduced hepatic lipid accumulation (representative H & E images; note that there is less lipid vacuolization in treatment groups compared with O-SED; Fig. 3A). Biochemical TG analyses revealed similar findings (Fig. 3B). O-EndEx animals did show ∼40% further reductions in hepatic TGs compared with metformin alone (P < 0.01; Fig. 3B), with no further benefit in the combination group. EndEx training also reduced hepatic DAG content ∼40% compared with the SED and SED + M groups (P < 0.01; Fig. 3C), again with no observed additional benefit when metformin was combined with EndEx. Metformin treatment tended to lower saturated DAG content (P = 0.078), whereas EndEx lowered both saturated and unsaturated DAGs to levels of the lean control animals (P < 0.01). Finally, all three treatment groups had similar effects on lowering serum ALT concentrations, a marker used to evaluate liver injury, compared with SED (Fig. 3D).
Fig. 3.
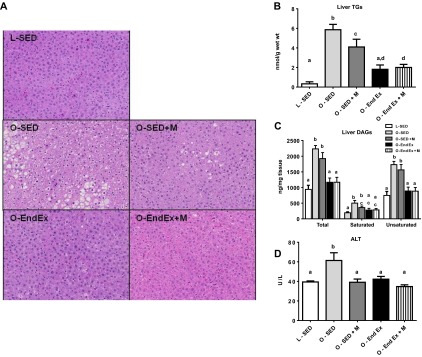
Treatment of nonalcoholic fatty liver disease with metformin, exercise training, or the combination. Intrahepatic triglyceride (TG) accumulation demonstrated by hematoxylin and eosin stain (A), biochemical analysis (B), liver diacylglycerols (DAGs; C), and serum alanine aminotransferase (ALT) concentrations (D). All values are means ± SE. Values with different letters are significantly different; P < 0.05.
TR4 and gluconeogenic gene expression.
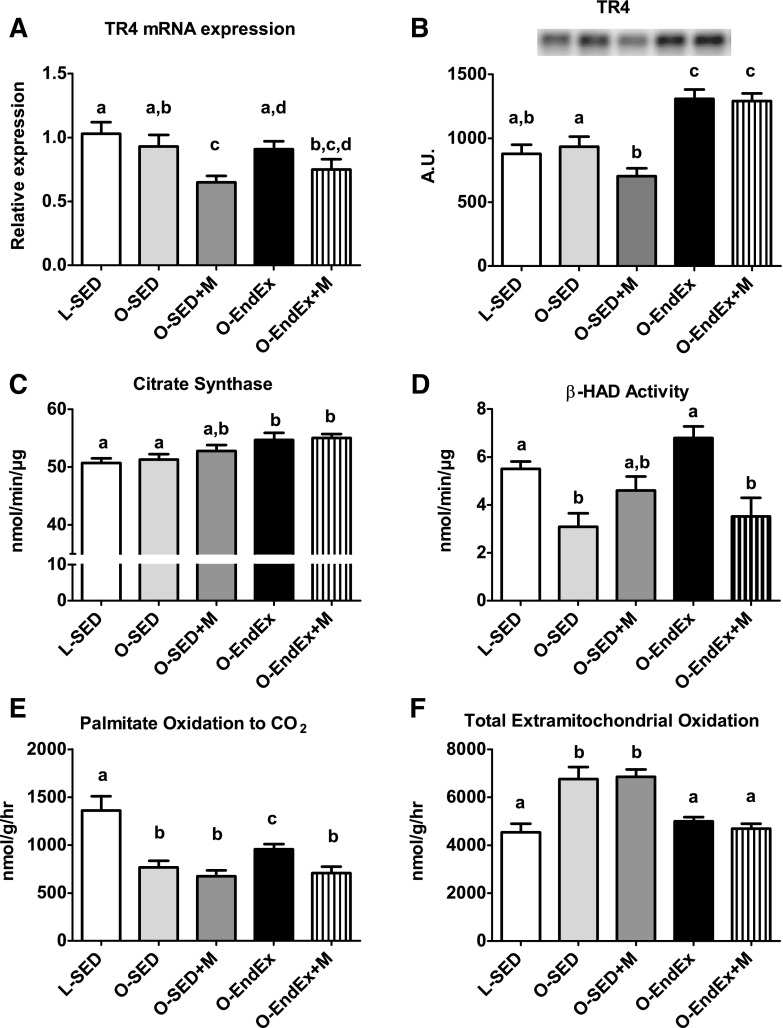
Metformin is thought to exert its antidiabetic effects by activating AMPK, subsequently inhibiting nuclear receptor TR4 and downregulating mitochondrial respiration and hepatic gluconeogenesis (20, 38). In the current study, we saw no treatment effect on AMPK activation status (AMPK Thr172 phosphorylation; data not shown). However, metformin effectively reduced TR4 mRNA expression and protein content (P < 0.05; Fig. 4, A and B). This was in contrast to the O-EndEx and O-EndEx + Met groups, where TR4 protein content was elevated compared with other groups (∼40%, P < 0.01; Fig. 4, A and B). Although we observed group differences for TR4, we found no differences between groups for gene expression of the gluconeogenic markers PEPCK or G6Pase in the fasting condition after 12 wk of treatment with treadmill exercise training, metformin, or the combination (data not shown).
Fig. 4.
TR4 and markers of hepatic mitochondrial function. Hepatic TR4 gene expression (A), TR4 protein expression (B), citrate synthase activity (C), β-hydroxyacyl-CoA dehydrogenase (β-HAD) activity (D), complete palmitate oxidation (E), and total extramitochondrial oxidation of palmitate (F). All values are means ± SE. Values with different letters are significantly different; P < 0.05.
Measurements of hepatic mitochondrial content and function.
To explore potential mechanisms by which metformin and exercise training attenuated NAFLD, we assessed several indices of hepatic mitochondrial content and function. None of the treatments provided significant benefits for the hepatic protein content of PGC-1α or cytochrome c compared with O-SED (data not shown). In addition, although metformin alone did not increase indices of hepatic mitochondrial function, exercise increased citrate synthase activity (P < 0.05), β-HAD activity (P < 0.001), and complete oxidation of palmitate to CO2 (P < 0.05). Exercise and the combination group also exhibited significant reductions in total extramitochondrial palmitate oxidation (acid-soluble metabolites + CO2, P < 0.05; Fig. 4, C–F) compared with O-SED. However, combining metformin with exercise resulted in significant impairments in exercise-induced adaptations in β-HAD activity (P < 0.001, O-EndEx vs. O-EndEx + M) and complete oxidation of palmitate (P < 0.05, O-EndEx vs. O-EndEx + M).
Treatment effects on markers of fatty acid uptake, de novo lipogenesis, and TG synthesis.
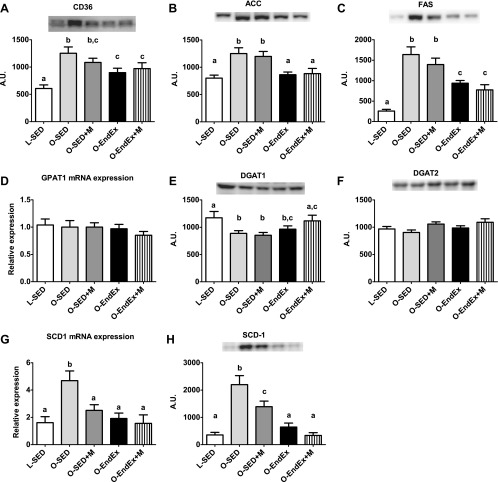
We also sought to examine other known contributing factors to NAFLD development. Exercise training was effective in lowering hepatic CD36/FAT protein content, a protein important for the cellular uptake of fatty acids (P < 0.05; Fig. 5A), as well as dramatically lowering the protein content of de novo fatty acid synthesis markers ACC and FAS (Fig. 5, B and C). These effects were not witnessed in the metformin-treated animals, and the addition of metformin to EndEx provided no further benefits compared with EndEx alone. Examination of TG synthesis markers found no differences among groups for either GPAT1 mRNA expression or DGAT2 protein content (Fig. 5, D and F). However, and somewhat surprisingly, reduced levels of DGAT1 protein content in O-SED animals compared with L-SED animals were ablated in the O-EndEx + M group (Fig. 5E), suggestive of enhanced capacity to convert hepatic DAG to TG. In addition, metformin treatment, exercise training, and the combination were effective in reducing hepatic SCD-1 mRNA expression and protein content compared with O-SED (Fig. 5, G and H), with exercise training eliciting more robust reductions (−70 vs. −40% EndEx vs. Sed + M, P < 0.05).
Fig. 5.
Regulators of lipid uptake, de novo lipogenesis, and TG synthesis. CD36 protein expression (A), acetyl-CoA carboxylase (ACC) protein expression (B), fatty acid synthase (FAS) protein expression (C), glycerol-3-phosphate acyltransferase-1 (GPAT-1) gene expression (D), diglyceride acyltransferase-1 (DGAT1) protein expression (E), DGAT2 protein expression (F), stearoyl-CoA desaturase-1 (SCD-1) gene expression (G), and SCD-1 protein expression (H). All values are means ± SE. Values with different letters are significantly different; P < 0.05. AU, arbitrary units.
DISCUSSION
This is one of the first reports to compare the effectiveness of combining two of the initial treatment strategies in the management of type 2 diabetes (metformin and exercise training) on NAFLD outcomes in an animal model of type 2 diabetes. Both metformin treatment and aerobic exercise training were effective in preventing the progression of type 2 diabetes and treating NAFLD in the OLETF rat, as indicated by improved Hb A1c and lowered hepatic lipid accumulation. However, aerobic exercise training was more effective than metformin administration in part through global improvements in both fasting and postchallenge glycemic control as well as increases in hepatic mitochondrial function and reductions in markers of hepatic fatty acid uptake and de novo fatty acid synthesis. Surprisingly, our findings highlight little evidence for the additive effects of combining metformin and aerobic exercise training on these outcomes and actually suggest potential impairments in exercise-induced hepatic mitochondrial adaptations.
It is estimated that between 70 and 80% of type 2 diabetics also have NAFLD (reviewed in Ref. 44). Current recommendations for the treatment of type 2 diabetes and NAFLD include lifestyle modifications such as exercise training and/or dietary changes. Although exercise training can help to treat NAFLD and type 2 diabetes (13, 18, 24), compliance is often difficult, and those individuals who lose weight often regain it back quickly (16, 54). Because of this trend, a better understanding of the potential use of pharmacological strategies in combination with lifestyle improvements is needed.
Metformin is one possible pharmacological candidate because limited research supports its efficacy in the treatment of NAFLD in adults at risk for type 2 diabetes (22) and with early liver disease (31). Recent clinical investigations have shown that the combination of lifestyle and metformin treatments may have greater benefits on reducing body weight (29), and recent work on rodents suggest that the combination may promote the secretion of healthy adipokines like IL-10 (17). However, the combination of these treatments may not provide additional benefits on lowering fasting insulin (29, 30), circulating TGs (30), or metabolic syndrome prevalence (30). Moreover, recent studies have demonstrated that metformin when taken in combination with lifestyle interventions blunts exercise-induced improvements in whole body insulin sensitivity (48), adipose tissue insulin sensitivity (48), and circulating FFAs (29) in humans. In addition, metformin appears to blunt exercise training adaptations in reducing serum inflammatory markers such as C-reactive protein (30). Our findings support this previous work, where we show that the combination of metformin and aerobic exercise training provided no further improvements in adiposity, blood lipids, markers of short-and long-term glycemic control, or hepatic TG or serum ALTs compared with exercise training alone. Furthermore, metformin blunted exercise-induced adaptations in hepatic mitochondrial function, as assessed by β-HAD activity and complete palmitate oxidation. These data are intriguing and warrant future investigation in both animal models and clinical trials to determine their potential health implications because they may be of great importance in the management of human NAFLD during the early stages of type 2 diabetes.
Metformin is thought to activate AMPK, inhibiting nuclear receptor TR4 transactivation and downregulating mitochondrial respiration to exert its antidiabetic effects through lower PEPCK and hepatic gluconeogenesis (20, 27, 38). Similar to previous reports, metformin reduced hepatic TR4 gene expression and protein content in the OLETF rat; however, these reductions in TR4 were not accompanied by improved glycemic control following a glucose challenge. Although metformin often improves fasting glucose, previous studies have been equivocal in metformin's ability to improve glycemic responses during glucose challenges, with metformin treatment providing no improvements in either humans (2, 19) or animal models (12, 21). This may be due in part to an inability of metformin treatment to improve insulin signaling within skeletal muscle (19) and/or reduce endogenous glucose production (2) during these trials. In the present report, no differences were observed in basal hepatic total Akt or phosphorylated Akt protein content or fasting gluconeogenic gene expression following metformin treatment, which may partially explain the lack of improvement in fasting glucose concentrations and glycemic control following a glucose challenge.
We report for the first time, to our knowledge, that aerobic exercise training increased hepatic TR4 protein content, and these increases were maintained when metformin treatment was combined with exercise training. TR4 has been shown to play a vital role in mitochondrial function (28), and although speculative, exercise-induced increases in hepatic mitochondrial function/respiration may be mediated in part through TR4-dependent mechanisms. Further investigations are needed to better understand the health implications of these exercise-induced increases in TR4.
This investigation assessed several pathways and known contributors to NAFLD, including markers of mitochondrial function, hepatic fatty acid uptake, and hepatic de novo lipogenesis (40). We have shown previously that mitochondrial dysfunction precedes hepatic steatosis development in OLETF rats under sedentary conditions (43). Observations in the current investigation support our previous work (4, 41, 42) and show that treatment of NAFLD with endurance exercise training improves hepatic mitochondrial function. However, the observed improvements in mitochondrial function were modest compared with changes obtained when we employed chronic voluntary wheel running to prevent NAFLD (41, 46). Additionally, metformin treatment alone did not elicit improvements in any of these variables of hepatic mitochondrial function, likely due to reductions in TR4 (28), and blunted exercise training adaptations when taken in combination. Together, these findings suggest that mitochondrial adaptations likely contribute to exercise-induced but not metformin-induced improvements in NAFLD. The metformin-induced reductions in β-HAD activity and TR4 likely contribute to reduced complex I activity and blunt exercise-induced improvements in mitochondrial oxidative capacity within the liver when treatments are combined. These findings are of importance given the recent focus on mitochondrial function in maintaining metabolic health (42, 43, 45).
Fatty acid uptake and de novo lipogenesis have also been linked to the development and progression of NAFLD. In fact, the fatty acid transporter CD36/FAT is upregulated in NAFLD patients (33) and in the obese OLETF rat (26), which could promote the uptake of circulating fatty acids that are associated with obesity. Additionally, recent work has indicated that >25% of hepatic TG accumulation can be accounted for by de novo lipogenesis in NAFLD patients (9). We found that only exercise training reduced CD36/FAT protein content in the hyperphagic OLETF rat. Similar to our previous findings using daily wheel running as a preventative therapy (42, 46), treatment with exercise training also suppressed the protein content of the de novo fatty acid synthesis markers FAS and ACC. These improvements were not seen in the metformin-treated animals. However, both metformin treatment and exercise training lowered SCD-1, a candidate known to contribute to the abnormal partitioning of fatty acids by increasing ACC activity and decreasing fatty acid oxidation, shunting substrates to fatty acid synthesis (8, 15). TR4 is also known to regulate SCD-1 activity (20) and induce CD36 expression (55), and metformin has been shown to reduce TR4 transactivation and prevent SCD-1 activation (20). In the present study, metformin lowered TR4 and SCD-1, possibly contributing to the observed reductions in hepatic TG accumulation in metformin-treated animals. Interestingly, exercise training increased hepatic TR4 but also lowered hepatic SCD-1, findings that suggest that metformin-induced and exercise-induced changes in SCD-1 may occur though different mechanisms. Collectively, the dramatic impact of exercise on these pathways and the modest effects seen in the metformin-treated animals are likely major contributors to differences in treatment-induced changes in hepatic TGs in these low-fat, chow-fed hyperphagic animals.
Finally, we examined the expression of several key enzymes involved in TG synthesis, because lipid intermediates such as DAGs may contribute to the metabolic dysregulation associated with NAFLD (reviewed in Ref. 37). It has been shown that DGAT1 is important for the synthesis of TGs from exogenous fatty acids, whereas DGAT2 allows for the incorporation of de novo synthesized fatty acids into TGs (39, 53). In the present report, hepatic DGAT1 content was reduced in the O-SED and O-SED + M animals and was associated with increased hepatic DAG content, whereas EndEx and the combination of EndEx + M reduced hepatic DAG content and the combination of EndEx + M normalized hepatic DGAT1 content to the lean control animals. These findings support our previous findings that hepatic DAG content is elevated in sedentary OLETF rats (4, 46) and suggest that this observed elevation in DAGs may result from a reduction in a final committed step in TG synthesis. Further investigation is needed to better understand the potential health implications of these findings in humans with NAFLD.
In summary, metformin and aerobic exercise training were effective in the management of type 2 diabetes and NAFLD progression witnessed in sedentary OLETF rats. However, aerobic exercise training was more effective than metformin treatment alone at improving fasting and postchallenge glycemic control, highlighting the importance of physical activity as a therapy in the early stages of type 2 diabetes and NAFLD. Additionally, exercise training was more effective at modulating several key pathways known to contribute to NAFLD development, including hepatic mitochondria, fatty acid uptake, and de novo fatty acid synthesis. Surprisingly, our findings highlight little evidence for additive effects of combining metformin and aerobic exercise training on these outcomes in this model of obesity, type 2 diabetes, and NAFLD and actually suggest potential impairments in exercise-induced hepatic mitochondrial adaptations.
GRANTS
This work was partially supported by National Institutes of Health Grants T32-AR-048523-07 (J. A. Fletcher), DK-088940 (J. P. Thyfault), RO1-HL-036088 (M. H. Laughlin), HL-73101-07 (J. R. Sowers), HL-107910-03 and (J. R. Sowers), Veterans Affairs-Merit System 0018 (J. R. Sowers), and VHA-CDA2 1299-01 (R. S. Rector). This work was supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.L., J.A.F., E.M.M., G.M.M., M.L.K., J.M.C., and R.S.R. performed the experiments; M.A.L. and R.S.R. analyzed the data; M.A.L., E.M.M., and R.S.R. interpreted the results of the experiments; M.A.L. and R.S.R. prepared the figures; M.A.L. and R.S.R. drafted the manuscript; M.A.L., J.A.F., E.M.M., G.M.M., M.L.K., J.M.C., M.H.L., F.W.B., J.R.S., J.A.I., J.P.T., and R.S.R. edited and revised the manuscript; M.A.L., J.A.F., E.M.M., G.M.M., M.L.K., J.M.C., M.H.L., F.W.B., J.R.S., J.A.I., J.P.T., and R.S.R. approved the final version of the manuscript; M.H.L., F.W.B., J.R.S., J.A.I., J.P.T., and R.S.R. contributed to the conception and design of the research.
ACKNOWLEDGMENTS
We gratefully acknowledge the excellent technical assistance of Pam Thorne and Kayla Kanosky. We also thank Eric Gibson, Brittany Muller, Kelcie Tacchi, Matt Brielmaier, and Nicholas Fleming for all of their hard work as animal trainers.
REFERENCES
- 1.Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem 10: 198–206, 1969 [DOI] [PubMed] [Google Scholar]
- 2.Basu R, Shah P, Basu A, Norby B, Dicke B, Chandramouli V, Cohen O, Landau BR, Rizza RA. Comparison of the effects of pioglitazone and metformin on hepatic and extra-hepatic insulin action in people with type 2 diabetes. Diabetes 57: 24–31, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med 132: 112–117, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Borengasser SJ, Rector RS, Uptergrove GM, Morris EM, Perfield JW, 2nd, Booth FW, Fritsche KL, Ibdah JA, Thyfault JP. Exercise and Omega-3 Polyunsaturated Fatty Acid Supplementation for the Treatment of Hepatic Steatosis in Hyperphagic OLETF Rats. J Nutr Metab 2012: 268680, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borst SE, Snellen HG, Lai HL. Metformin treatment enhances insulin-stimulated glucose transport in skeletal muscle of Sprague-Dawley rats. Life Sci 67: 165–174, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Browning JD, Baker JA, Rogers T, Davis J, Satapati S, Burgess SC. Short-term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr 93: 1048–1052, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 40: 1387–1395, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Dobrzyn P, Dobrzyn A, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Natl Acad Sci USA 101: 6409–6414, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115: 1343–1351, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elias MC, Parise ER, de Carvalho L, Szejnfeld D, Netto JP. Effect of 6-month nutritional intervention on non-alcoholic fatty liver disease. Nutrition 26: 1094–1099, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 43: S99–S112, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Faure P, Rossini E, Wiernsperger N, Richard MJ, Favier A, Halimi S. An insulin sensitizer improves the free radical defense system potential and insulin sensitivity in high fructose-fed rats. Diabetes 48: 353–357, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Fealy CE, Haus JM, Solomon TP, Pagadala M, Flask CA, McCullough AJ, Kirwan JP. Short-term exercise reduces markers of hepatocyte apoptosis in nonalcoholic fatty liver disease. J Appl Physiol 113: 1–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forcheron F, Abdallah P, Basset A, del Carmine P, Haffar G, Beylot M. Nonalcoholic hepatic steatosis in Zucker diabetic rats: spontaneous evolution and effects of metformin and fenofibrate. Obesity (Silver Spring) 17: 1381–1389, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab 2: 251–261, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med 168: 1550–1559; discussion 1559–1560, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkins NT, Padilla J, Arce-Esquivel AA, Bayless DS, Martin JS, Leidy HJ, Booth FW, Rector RS, Laughlin MH. Effects of endurance exercise training, metformin, and their combination on adipose tissue leptin and IL-10 secretion in OLETF rats. J Appl Physiol 113: 1873–1883, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 50: 1105–1112, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Karlsson HK, Hällsten K, Björnholm M, Tsuchida H, Chibalin AV, Virtanen KA, Heinonen OJ, Lönnqvist F, Nuutila P, Zierath JR. Effects of metformin and rosiglitazone treatment on insulin signaling and glucose uptake in patients with newly diagnosed type 2 diabetes: a randomized controlled study. Diabetes 54: 1459–1467, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Kim E, Liu NC, Yu IC, Lin HY, Lee YF, Sparks JD, Chen LM, Chang C. Metformin inhibits nuclear receptor TR4-mediated hepatic stearoyl-CoA desaturase 1 gene expression with altered insulin sensitivity. Diabetes 60: 1493–1503, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosegawa I, Katayama S, Kikuchi C, Kashiwabara H, Negishi K, Ishii J, Inukai K, Oka Y. Metformin decreases blood pressure and obesity in OLETF rats via improvement of insulin resistance. Hypertens Res 19: 37–41, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Krakoff J, Clark JM, Crandall JP, Wilson C, Molitch ME, Brancati FL, Edelstein SL, Knowler WC. Effects of metformin and weight loss on serum alanine aminotransferase activity in the diabetes prevention program. Obesity (Silver Spring) 18: 1762–1767, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larson-Meyer DE, Newcomer BR, Heilbronn LK, Volaufova J, Smith SR, Alfonso AJ, Lefevre M, Rood JC, Williamson DA, Ravussin E. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring) 16: 1355–1362, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, Arslanian S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: a randomized, controlled trial. Diabetes 61: 2787–2795, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med 6: 998–1003, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Linden MA, Meers GM, Ruebel ML, Jenkins NT, Booth FW, Laughlin MH, Ibdah JA, Thyfault JP, Rector RS. Hepatic steatosis development with four weeks of physical inactivity in previously active, hyperphagic OLETF rats. Am J Physiol Regul Integr Comp Physiol 304: R763–R771, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu NC, Lin WJ, Kim E, Collins LL, Lin HY, Yu IC, Sparks JD, Chen LM, Lee YF, Chang C. Loss of TR4 orphan nuclear receptor reduces phosphoenolpyruvate carboxykinase-mediated gluconeogenesis. Diabetes 56: 2901–2909, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Lee YF, Chou S, Uno H, Li G, Brookes P, Massett MP, Wu Q, Chen LM, Chang C. Mice lacking TR4 nuclear receptor develop mitochondrial myopathy with deficiency in complex I. Mol Endocrinol 25: 1301–1310, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malin SK, Gerber R, Chipkin SR, Braun B. Independent and combined effects of exercise training and metformin on insulin sensitivity in individuals with prediabetes. Diabetes Care 35: 131–136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malin SK, Nightingale J, Choi SE, Chipkin SR, Braun B. Metformin modifies the exercise training effects on risk factors for cardiovascular disease in impaired glucose tolerant adults. Obesity (Silver Spring) 21: 93–100, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet 358: 893–894, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto T, Noguchi E, Ishida K, Kobayashi T, Yamada N, Kamata K. Metformin normalizes endothelial function by suppressing vasoconstrictor prostanoids in mesenteric arteries from OLETF rats, a model of type 2 diabetes. Am J Physiol Heart Circ Physiol 295: H1165–H1176, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Miquilena-Colina ME, Lima-Cabello E, Sánchez-Campos S, García-Mediavilla MV, Fernández-Bermejo M, Lozano-Rodríguez T, Vargas-Castrillón J, Buqué X, Ochoa B, Aspichueta P, González-Gallego J, García-Monzón C. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut 60: 1394–1402, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Moran TH, Bi S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos Trans R Soc Lond B Biol Sci 361: 1211–1218, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moran TH, Bi S. Hyperphagia and obesity of OLETF rats lacking CCK1 receptors: developmental aspects. Dev Psychobiol 48: 360–367, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Morris EM, Meers GM, Booth FW, Fritsche KL, Hardin CD, Thyfault JP, Ibdah JA. PGC-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am J Physiol Gastrointest Liver Physiol 303: G979–G992, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 52: 774–788, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 348: 607–614, 2000 [PMC free article] [PubMed] [Google Scholar]
- 39.Qi J, Lang W, Geisler JG, Wang P, Petrounia I, Mai S, Smith C, Askari H, Struble GT, Williams R, Bhanot S, Monia BP, Bayoumy S, Grant E, Caldwell GW, Todd MJ, Liang Y, Gaul MD, Demarest KT, Connelly MA. The use of stable isotope-labeled glycerol and oleic acid to differentiate the hepatic functions of DGAT1 and -2. J Lipid Res 53: 1106–1116, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rector RS, Thyfault JP. Does physical inactivity cause nonalcoholic fatty liver disease? J Appl Physiol 111: 1828–1835, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, Chakravarthy MV, Booth FW, Ibdah JA. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol 586: 4241–4249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol 52: 727–736, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rector RS, Thyfault JP, Wei Y, Ibdah JA. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World J Gastroenterol 14: 185–192, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rector RS, Uptergrove GM, Borengasser SJ, Mikus CR, Morris EM, Naples SP, Laye MJ, Laughlin MH, Booth FW, Ibdah JA, Thyfault JP. Changes in skeletal muscle mitochondria in response to the development of type 2 diabetes or prevention by daily wheel running in hyperphagic OLETF rats. Am J Physiol Endocrinol Metab 298: E1179–E1187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rector RS, Uptergrove GM, Morris EM, Borengasser SJ, Laughlin MH, Booth FW, Thyfault JP, Ibdah JA. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol 300: G874–G883, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schultz A, Mendonca LS, Aguila MB, Mandarim-de-Lacerda CA. Swimming training beneficial effects in a mice model of nonalcoholic fatty liver disease. Exp Toxicol Pathol 64: 273–282, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Sharoff CG, Hagobian TA, Malin SK, Chipkin SR, Yu H, Hirshman MF, Goodyear LJ, Braun B. Combining short-term metformin treatment and one bout of exercise does not increase insulin action in insulin-resistant individuals. Am J Physiol Endocrinol Metab 298: E815–E823, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srere PA. Citrate synthase. Meth Enzymol 13: 3–5, 1969 [Google Scholar]
- 50.Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology 55: 1738–1745, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tai MM. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care 17: 152–154, 1994 [DOI] [PubMed] [Google Scholar]
- 52.Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 30: 1212–1218, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Villanueva CJ, Monetti M, Shih M, Zhou P, Watkins SM, Bhanot S, Farese RV., Jr Specific role for acyl CoA:Diacylglycerol acyltransferase 1 (Dgat1) in hepatic steatosis due to exogenous fatty acids. Hepatology 50: 434–442, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Lyles MF, You T, Berry MJ, Rejeski WJ, Nicklas BJ. Weight regain is related to decreases in physical activity during weight loss. Med Sci Sports Exerc 40: 1781–1788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie S, Lee YF, Kim E, Chen LM, Ni J, Fang LY, Liu S, Lin SJ, Abe J, Berk B, Ho FM, Chang C. TR4 nuclear receptor functions as a fatty acid sensor to modulate CD36 expression and foam cell formation. Proc Natl Acad Sci USA 106: 13353–13358, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]