Abstract
Winter hibernators repeatedly cycle between cold torpor and rewarming supported by nonshivering thermogenesis in brown adipose tissue (BAT). In contrast, summer animals are homeotherms, undergoing reproduction, growth, and fattening. This life history confers variability to BAT recruitment and activity. To address the components underlying prewinter enhancement and winter activation, we interrogated the BAT proteome in 13-lined ground squirrels among three summer and five winter states. We also examined mixed physiology in fall and spring individuals to test for ambient temperature and seasonal effects, as well as the timing of seasonal transitions. BAT form and function differ circannually in these animals, as evidenced by morphology and proteome dynamics. This intrinsic pattern distinguished homeothermic groups and early vs. late winter hibernators. Homeothermic variation derived from postemergence delay in growth and substrate biosynthesis. The heterothermic proteome varied less despite extreme winter physiological shifts and was optimized to exploit lipids by enhanced fatty acid binding, β-oxidation, and mitochondrial protein translocation. Surprisingly, ambient temperature did not affect the BAT proteome during transition seasons; rather, the pronounced summer-winter shift preceded environmental changes and phenotypic progression. During fall transition, differential regulation of two fatty acid binding proteins provides further evidence of recruitment and separates proteomic preparation from successful hibernation. Abundance of FABP4 correlates with torpor bout length throughout the year, clarifying its potential function in hibernation. Metabolically active BAT is a target for treating human obesity and metabolic disorders. Understanding the hibernator's extreme and seasonally distinct recruitment and activation control strategies offers untapped potential to identify novel, therapeutically relevant regulatory pathways.
Keywords: Ictidomys tridecemlineatus, axillary brown fat, aP2, 14-3-3
the annual cycle of small-bodied hibernators can be partitioned into two phases: summer homeothermy and winter heterothermy. From late spring through early fall, obligate hibernators complete reproduction, grow, and fatten to the point of becoming obese before the onset of the winter hibernation season; indeed, hibernators, including the 13-lined ground squirrels studied here rely on body lipid stores accumulated during summer to survive the winter fast (10). These energy reserves support a heterothermic period comprising repetitive torpor-arousal cycles (Fig. 1). Homeostasis is maintained throughout; therefore, execution of a torpor bout involves a dramatic change in homeostatic set point (9). The result is severe reduction of physiological processes (e.g., heart rate, respiratory rate, and body temperature) and effective quiescence in many tissues during torpor. However, activities are restored to levels at or in excess of standard euthermic rates during brief interbout arousals (43).
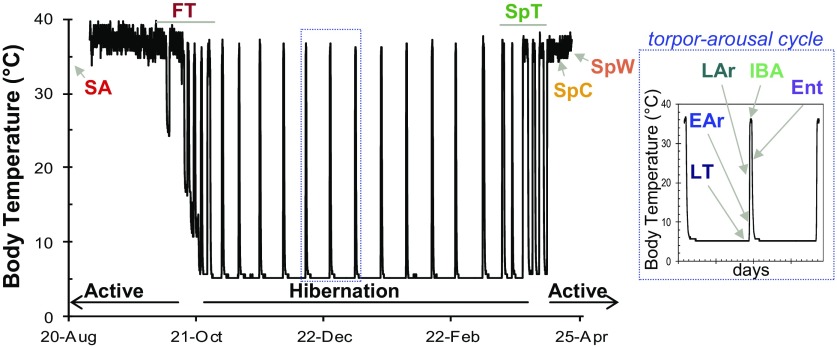
Fig. 1.
Body temperature defines sampled states. Body temperature (Tb) of a laboratory-housed 13-lined ground squirrel (Ictidomys tridecemlineatus) over ∼8 mo. The hibernation label identifies the winter heterothermic period; the annual cycle of this species is completed by a spring-fall active, homeothermic period. Sample groups for the proteomics experiment were collected from eight defined states (n = 6 animals per state) and two transition states. Summer active (SA) and spring ground squirrels represented the homeothermic portions of the annual cycle [spring-cold (SpC) and spring-warm (SpW)]. Winter states representing the heterothermic period were defined by Tb telemetry as entering torpor (Ent) at 27°C > Tb > 23°C; late torpor (LT) at 80–95% previous bout duration; early arousing (EAr) at 7°C < Tb < 12.8°C; late arousing (LAr) at 18°C < Tb < 25°C; and 3–4 h after Tb stabilized in the interbout arousal (IBA) period. Spring animals were given food and water but remained in the hibernaculum (L:D 0:24). Spring-cold (SpC) were maintained at low ambient temperature (Ta = 4°C) and were killed 11–20 days after their last recorded torpor bout. Spring-warm (SpW) were collected 7–32 days after terminal arousal (Ta = 14–18°C). Samples were also collected during the highly variable periods of fall transition (FT; n = 28) and spring transition (SpT; n = 17) transition. Opportunistic histology samples were collected from fixed tissues as represented for the proteomics experiment with the exception of SA ground squirrels. SA tissues collected for the frozen tissue bank (proteomics) were obtained Aug. 4 and 5. Fixed SA tissues for histology were obtained June 24 and 25. Sample sizes from fixed tissues were SA (n = 4), FT (n = 4), Ent (n = 1), torpid for various durations (n = 4), EAr (n = 1), LAr (n = 1), IBA (n = 3), SpT (n = 2). To supplement the fixed tissues, we cryosectioned frozen samples from an additional n = 4 SpC, SA (August) and FT proteomics animals that had not yet used torpor.
Brown adipose tissue (BAT) is the source of nonshivering thermogenesis (NST) that plays a key role in endogenous rewarming during arousals (9). BAT exploits uncoupling of oxidative phosphorylation via the specialized proton transporter, uncoupling protein 1 (UCP1) to disperse the proton-motive force generated by β-oxidation of free fatty acids. This futile catabolism produces heat as a by-product (41). BAT recruitment is generally thought to occur in response to both temperature and photoperiod (8, 25). In small-bodied obligate hibernators, however, it occurs in the fall in constant environmental conditions (9), suggesting this process is seasonally programmed in anticipation of winter need. Winter heterothermy is subsequently managed in large part by controlling the heat-producing activity of BAT to successfully execute torpor-arousal cycles. BAT activity effectively ceases during entrance into torpor to permit Tb equilibrium with the environment but can increase to defend above-freezing Tb if environmental temperature drops [reviewed by Boyer and Barnes (4)] and must increase dramatically for each arousal. BAT reactivation from cold ambient and body temperature during arousal initiates rewarming, with shivering thermogenesis contributing only after Tb reaches a given level (9).
Although not initially thought to be present in adult humans, metabolically active brown adipocytes have now been discovered (37). There is growing interest in developing therapeutic strategies based on energy expenditure by BAT for human obesity and metabolic disorders (12, 32). However, to do this, we must first determine mechanisms that promote both brown adipocyte recruitment (i.e., thermogenic capacity at maximum stimulation) and support its metabolic activation and quiescence. In light of its dynamic seasonal regulation in ground squirrels, its proliferation in advance of the hibernation season, and its critical role in endogenous rewarming from torpor, hibernator BAT provides an optimal model system in which to elucidate the molecular mechanisms underlying BAT recruitment and activity control. As an initial step, we document circannual changes in BAT morphology and histology in 13-lined ground squirrels (Ictidomys tridecemlineatus), including animals preparing to enter hibernation and those nearing spring emergence. We then merge these findings with an investigation of the annual regulation of the BAT proteome to identify seasonal differences in BAT recruitment and to deconvolute the nature and timing of the proteomic reprogramming necessary to transition between two distinct seasonal phenotypes. We identified dynamic proteins that supported the baseline physiologies of summer homeothermy vs. winter heterothermy using three time points within homeothermy, as well as five Tb-defined time points across torpor-arousal cycles. We also examined transition states (fall and spring) bordering the heterothermic period, consisting of mixed-physiology individuals to test for effects of ambient temperature, a strict segregation of summer and winter, and the timing of seasonal transitions.
METHODS
Tissue collection.
All animal care and procedures were approved by the University of Colorado School of Medicine Institutional Animal Care and Use Committee. Thirteen-lined ground squirrels were purchased from the captive breeding program at the University of Wisconsin Oshkosh and were initially housed at 18–21°C in standard rodent cages (14:10-h light-dark cycle). Ad libitum food (dry cat food and sunflower seeds) and water were provided. Summer active (SA) ground squirrels were euthanized within 2 wk of arrival at the University of Colorado. The remaining ground squirrels were surgically outfitted with an intraperitoneal Tb telemeter (MiniMitter, Respironics, Carlsbad, CA) and logger (iButton, Embedded Data Systems, Dallas, TX) to define sampling states throughout the annual cycle (see Fig. 1 for sampled states).
Animals were transferred to the hibernaculum (4°C, 0:24-h light-dark cycle) between late September and mid-October after a minimum 2-wk postsurgical recovery. Food and water were withdrawn once animals exhibited regular torpor in the hibernaculum and were returned to spring animals (SpC and SpW; Fig. 1) at least 10 days before tissue collection. We also sampled the mixed-physiology transition periods between homeothermy and heterothermy: Fall transition (FT) ground squirrels were traditionally housed or had been transferred to the hibernaculum and were in various stages of torpor or euthermy at time of tissue collection (39). Spring transition (SpT) ground squirrels were also of varied torpor status at death but remained in the hibernaculum (4°C, 0:24-h light-dark cycle) and were sampled once their torpor bouts became irregular, but prior to terminal arousal. FT and SpT groups had food and water available during these transition periods, although consumption varied among individuals. Biometrics, housing and Tb data are presented in Supplemental Table S1 for mixed-physiology FT and SpT individuals.
Animals were anesthetized with isoflurane, then exsanguinated via cardiac blood draw and perfused prior to tissue collection. For proteomics, the entire BAT axillary pads were removed after cold-saline perfusion, cleaned of connective tissue, and snap-frozen in liquid nitrogen. The entire BAT axillary depot was weighed, and samples were stored at −80°C until processing. For opportunistic histology sampling, separate animals were first perfused with 0.1 M phosphate buffer, pH 7.2, followed by 10% formalin.
Histology sample preparation and analysis.
Axillary BAT pads excised from formalin-perfused animals were stored overnight in 10% formalin (4°C) and then processed to paraffin block. Five-micrometer sections were deparaffinized and stained with hematoxylin and eosin to document general morphology. Supplemental histology samples were prepared from frozen samples collected for proteomics; pieces of axillary BAT were mounted in Tissue-Tek OCT (Sakura Finetek, Torrance, CA), at −23°C. Ten-micrometer sections were placed on glass slides, air dried, and then stained with hematoxylin. Cell densities were determined from the number of counted nuclei per unit area, using images collected from two locations within each sample.
We evaluated BAT cell proliferation by analyzing the percentage of nuclei positive for the Ki-67 antigen, again from two locations within each sample. Five-micrometer sections from formalin-perfused SpT (n = 2), SA, FT, and interbout arousal (IBA) (all n = 3) were deparaffinized, then processed for immunohistochemical detection of Ki-67. Antigens were revealed in pH 9.5 BORG solution (5 min, 125°C, 22 psi in a decloaking chamber; Biocare Medical, Concord, CA) with a 10-min ambient cool down. Immunolabeling of Ki-67 (1:500 rabbit mAb SP6, no. RM-9106-SO; Neomarkers/Pierce, Rockford, IL) was accomplished following a 32-min incubation with primary Ab at 37°C on a NexES stainer (Ventana Medical Systems, Tucson, AZ). Secondary detection employed a modification of the I-VIEW DAB detection kit (Ventana); specifically, the I-VIEW secondary antibody and enzyme were replaced with Rabbit ImmPress no. MP-7401 (Vector Laboratories, Burlingame, CA). Sections were counterstained in acidified Harris hematoxylin (1.5 min), blued in 1% ammonium hydroxide (v/v), dehydrated in graded alcohols, and cleared in xylene prior to mounting in synthetic resin.
qPCR for mitochondrial and nuclear DNA quantification.
Genomic DNA was isolated from frozen BAT samples (∼25 mg) using a ZR Genomic DNA Tissue Prep kit (no. D3050; Zymo Research, Irvine, CA), according to the manufacturer's protocol, from n = 6 of SpW and IBA, n = 5 SA, and n = 15 FT individuals. A standard curve was generated using 10-fold dilutions of plasmid clones (TopoTA cloning kit; LifeTechnologies, Carlsbad, CA) containing PCR fragments for COX1 (encoded in mitochondrial DNA) and RPPH1 (encoded in nuclear DNA). COX1 was amplified with: 5′-CTCCTCTTACTCGCTTCTTCTA and 5′-GTGAAGGGAGAAGATGGTTAG forward and reverse primers, respectively; and RRPH1 with 5′-TTAGCCGGAGCTTGGAACAGACTCA and 5′-GGGAGAGTAGTCTGAATTAGG. The quantities of COX1 and RPPH1 were calculated from their respective standard curves in the ground squirrel DNA samples using Power SYBR Green Master Mix and the absolute standard curve method (LifeTechnologies). These values were used to calculate the ratio of COX1:RPPH1, or mitochondrial to nuclear DNA.
Two-dimensional gel analysis and protein identification.
To prepare samples for 2D gel electrophoresis and proteomics, frozen samples were broken apart while submerged in liquid nitrogen; then, tissue pieces were mechanically homogenized at 4°C (Bullet Blender, Next Advance, Averill Park, NY), centrifuged to remove insoluble components, and divided into aliquots as previously described (21). Homogenate protein concentrations were determined with a BCA assay (Pierce). Following the procedures of Epperson et al. (17), homogenate samples with equal protein content were denatured and labeled with Cy3 or Cy5 fluors (GE Healthcare, Piscataway, NJ), while a pooled reference sample was labeled with Cy2. Mixed samples were run on 47 analytical 2D gels, as previously described (21), and all 141 Cy images plus images of pick gels stained with Sypro Ruby were imported into DeCyder (V7.0, GE Healthcare) for analysis. Protein spots from all Cy2 reference sample images were matched by the DeCyder software after manual landmarking (∼500 manually matched spots). Spot intensity for each Cy3 and Cy5 spot was normalized to their corresponding Cy2 reference spots.
Protein spots that differed significantly by sampled state were identified with one-way ANOVA. A Benjamini-Hochberg (3) false discovery rate correction (FDR) converted ANOVA P values to q values. Only protein spots present on ≥ 3 of 6 gels from each state were included in the ANOVA, and those spots that reached q significance were each examined and manually matched in additional gels where possible. We also examined separation among the sampled states using the machine-learning clustering tool, Random Forests (5, 31), which classifies the data based on the abundance of protein spots present in every sample. We identified the most important classifying protein spots using Random Forests variable selection, drop = 0.2; n = 100,000 iterations (15), which outputs the minimum number of variables producing the lowest out of bag clustering error among the designated groups. All analyses were performed in R v3.0.1 (42).
We attempted to identify all protein spots that were reproducible on the pick gels and were determined to be significant by ANOVA (after FDR) or Random Forests. For identification by LC-MS/MS, gel plugs (depth = 1.0 mm, diameter = 1.4 mm) were picked and digested as previously described (21). Tandem MS was performed for the four highest peaks with a 30-s dynamic exclusion. Spectral data were collected with 6300 Series TrapControl software (v6.1, Build 83, Agilent Technologies). Tryptic fragments were identified against a protein database containing predicted sequences from the published 13-lined ground squirrel genome available in Ensembl build 67 with one allowable missed residue (20,000 entries). For protein spots not initially able to be identified, we subsequently searched against a database containing the 13-lined ground squirrel proteins plus the National Center for Biotechnology Information (NCBI) mammal sequences as of April 2012 and previously published actual sequences from arctic ground squirrels, Urocitellus parryii (47) allowing two missed residues; this contained 1,071,259 entries.
We required protein identifications (IDs) to have at least two supporting peptides and a score ≥30 to be retained. After analysis of a single pick gel, protein spots resulting in a single protein ID with ≥3 supporting peptides and a score ≥30 were considered identified. The remaining protein spots were extracted from an additional 1–2 pick gels. Spectral data from multiple pick gels, as well as alignment data from multiple species (NCBI database) were merged using an in-house program, ExtracTags (v4.1). When multiple protein IDs were recovered from a single spot, the top hit was retained as the ID when it contained a ≥4-fold higher score, peptide recovery, or spectral intensity than any other hit. Single best IDs are presented in Supplemental Table S2, and the complete file output, including unresolved weak and multiple IDs, is given in Supplemental Table S3.
Additional statistical analyses.
Significant pairwise comparisons among states were determined with Tukey's post hoc tests (Supplemental Table S4). We used k-means clustering of identified protein spots to determine abundance patterns over the annual cycle. Dendrograms of k-means patterns based on Pearson correlations were generated with XCluster (19) and were plotted as a heat map with TreeView (40). Proteins within each k-means pattern were also submitted to the DAVID bioinformatics database v6.7 (28) to identify significantly enriched gene pathways. This analysis used a human background and a κ similarity of 0.85 (default settings otherwise). Functional annotation clusters were considered significant with a mean enrichment score of ≥1.3 (28). Data were also visualized with hierarchically clustered heat maps and Random Forests MDS plots (n = 50,000 trees).
Fall and spring transition ground squirrels were compared with other states using Random Forests unsupervised classification and proximity predictions. After training the algorithm with abundance data from the nontransition base states, the percent proximity to each known state was predicted for the transition animals based on the top classifying protein spots for the initial Random Forests analysis. We compared the predicted (highest proximity) state for each animal to the most appropriately analogous observed state according to Tb telemetry. We also examined the proteins comprising k-means clusters of the transition patterns in DAVID. We used ANOVA to compare the eight base sampled states with FT or SpT. Protein spots were selected for subsequent analysis if they were significant among Tukey's post hoc pairwise comparisons for the transition state and any winter state, or SA in the case of FT and SpC/W in the case of SpT (Supplemental Tables S5 and S6).
Phosphoprotein staining.
To identify posttranslational phosphorylation modifications of protein spots of interest, we examined phosphoprotein staining in BAT samples from SpC, LT, and Ent animals (n = 3 per state). One hundred-and-fifty micrograms of unlabeled protein from each sample were separated on 2D gels as outlined for the DiGE experiment, fixed overnight in 50% MeOH and 10% acetic acid, and then stained with a phosphoprotein-specific stain (ProQ Diamond; Molecular Probes, Eugene, OR) followed by a total protein stain (SYPRO Ruby), as previously described (Table 2 of Ref. 1).
Table 2.
Gene enrichment analysis of proteins in base states k-means clusters 1-5
| Annotation Term | Score | Fold | Genes |
|---|---|---|---|
| Cluster 1 | |||
| Mt membrane | 8.3 | 16 | GPD2, ACADVL, CPT2, ACSL1, SAMM50, IMMT, ETFDH, UCP1, HSPD1, HADHA, MDH2 |
| TCA cycle | 4.3 | 51 | DLST, DLD, PDHA1, IDH3A, MDH2 |
| Flavoprotein | 4.3 | 38 | GPD2, ACADVL, DLD, ETFDH, PDHA1 |
| Fatty acid metabolism | 3.9 | 16 | ACADVL, CPT2, ACSL1, ETFDH, HADHA |
| Organelle lumen | 2.7 | 3 | ACADVL, DLST, VCP, ERP29, DLD, PDHA1, HSPD1, IDH3A, HADHA, MDH2 |
| Glucose metabolism | 2.5 | 16 | GPD2, PDHA1, MDH2, ENO1 |
| Cluster 2 | |||
| Mt inner membrane | 17.1 | 22 | ACAA2, CKMT1B, OPA1, COX7A2, IMMT, PHB, STOML2, TIMM50, CRAT, UCP1, COX5A, HADHA, HADHB, ACADVL, MCCC1, HSPD1, HADH, MDH2, PC |
| Mt matrix | 10.8 | 24 | DLST, GRPEL1, ACADM, OGDH, ACADL, HADHA, IDH3A, HADHB, ACADVL, IDH3G, C1QBP, MCCC1, HSPD1, HADH, MDH2, PC |
| Fatty acid metabolism | 8.4 | 64 | ACADVL, ACAA2, ACADM, CRAT, HADH, ACADL, HADHA, HADHB |
| TCA cycle | 5.4 | 141 | DLST, IDH3G, SUCLA2, OGDH, IDH3A, MDH2 |
| Purine nucleotide binding | 3.0 | 3 | GRPEL1, ACADM, CKMT1B, OPA1, ACADL, CMPK1, ACADVL, IDH3G, MCCC1, RAB11B, HSPD1, HSPA5, SUCLA2, PC |
| Cluster 3 | |||
| Mt transit peptide | 8.2 | 30 | SDHA, UQCRC1, ACO2, PITRM1, HIBADH, IDH3A, PC, ETFA |
| Aerobic respiration | 4.8 | 155 | SDHA, UQCRC1, ACO2, IDH3A |
| Cluster 4 | |||
| Oxidative metabolism | 3.6 | 10 | GPD2, ACADVL, NDUFS8, IDH3A, MDH2, MDH1 |
| Mt transit peptide | 3.5 | 12 | GPD2, ACADVL, NDUFS8, IDH3A, PCCA, MDH2 |
| 14-3-3 | 3.5 | 476 | YWHAZ, YWHAG, YWHAB, YWHAE |
| Cytoplasmic vesicle | 3.1 | 5 | YWHAZ, YWHAB, CTSD, YWHAE, ANXA2 |
| Cluster 5 | |||
| Complex assembly | 2.7 | 6 | PPP2R1A, GSN, FGB, FKBP4, GPX3, CDA, ANXA5 |
| Mt matrix | 2.4 | 9 | DBT, HSPE1, SOD1, ACADL, ACAT1 |
| 14-3-3 | 1.8 | 486 | YWHAG, YWHAQ, YWHAE |
| Apoptosis regulation | 1.5 | 3 | PPP2R1A, HSPE1, SOD1, ANXA5, YWHAE, GSTP1 |
| Phosphoregulation | 1.4 | 4 | PRKAR2B, PPP2R1A, YWHAG, SOD1, YWHAE |
Pathways enriched in the BAT proteome (DAVID; Ref. 28) of 13-lined ground squirrels were selected from k-means clusters of the 203 identified protein spots that differed significantly among base states (Fig. 3). “Score” denotes enrichment score (significant if >1.3). “Fold” denotes pathway fold enrichment. Redundant pathways and those contained as subsets within broader pathways were removed.
Mt, mitochondria; TCA, tricarboxylic acid.
Isocitrate dehydrogenase activity assay.
The activity of NAD+-dependent isocitrate dehydrogenase (IDH3) was assessed colorimetrically. BAT tissues from SpC, SA, and IBA ground squirrels (n = 5 per state) were homogenized as described above, in Tris buffer ([final] = 50 mM, pH = 7.4) instead of potassium phosphate (1:20 wt/vol). After centrifugation, as described above, the postnuclear supernatant was collected and diluted 5× further in 100 mM Tris. The 250-μl reaction volume contained 5 mM isocitrate, 10.5 mM MnCl2, and 0.5 mM NAD+, all made up in 100 mM Tris buffer. The production of NADH at RT was followed in triplicate preparations at λ = 340 nm for 1–4 min after the reaction was initiated, using a Synergy HT microplate reader (BioTek Instruments, Winooski, VT). Reaction blanks were also conducted in triplicate from the reaction mixture minus the substrate isocitrate. The rate of production (μmol·g−1·min−1) over the linear portion of the curve was calculated by ΔA340·min−1 × 1/EC × dilution factor, where EC is the extinction coefficient of NADH at 340 nm (= 6.22). Enzyme activities, after correction for reaction blanks were normalized per microgram protein in the sample (calculated by BCA assay), as well as per gram wet weight (gww) of BAT tissue.
RESULTS
Cell size varies more among homeotherms than heterotherms.
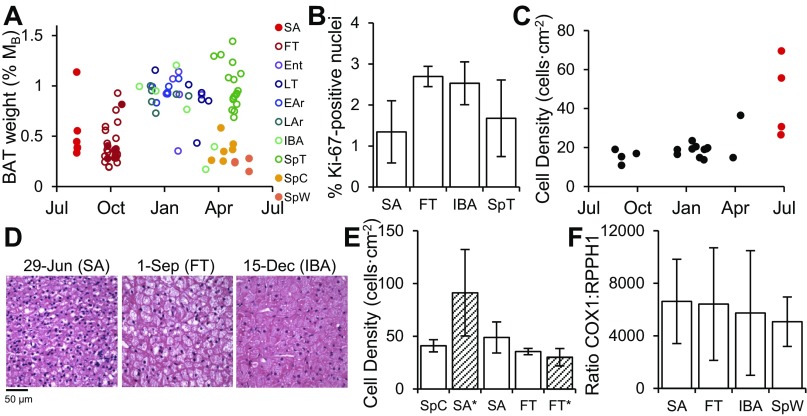
The wet weight of 13-lined ground squirrel axillary BAT was generally higher in winter compared with summer (1.8-fold greater in all heterotherms vs. all homeotherms, Fig. 2A) yet varied within seasons along an annual cycle. When normalized to individual body mass, wet weight peaked among the base states in midwinter and was lowest in spring (SpC and SpW, Fig. 2A). SpT ground squirrels, sampled within irregular pre-emergence torpor-arousal cycles had the highest mass-specific BAT weight (Fig. 2A) as a result of declining body mass prior to spring refeeding. We assessed cell proliferation in ground squirrel BAT by measuring Ki-67-positive nuclei and found a near doubling of replicating cells in FT (2.7 ± 0.3%) compared with late-June SA (1.3 ± 0.8%), which had the fewest of all samples examined in BAT (one-way ANOVA P = 0.05; Fig. 2B).
Fig. 2.
Annual cycles in 13-lined ground squirrel brown adipose morphology. A: axillary depot wet weight from tissues examined by proteomics expressed normalized to body mass (%MB); open circles represent heterotherms, while solid circles represent homeotherms. B: brown adipose tissue (BAT) cell proliferation indicated by mean ± SD. Ki-67-positive nuclei in fixed, paraffin-embedded histology samples was lowest in late-June SA ground squirrels, and peaked in FT (SA < FT Tukey P = 0.05, SA < IBA, P = 0.08; n = 3). SA, FT, and IBA squirrels were compared statistically, with n = 2 SpT animals plotted for comparison. C: cell densities, observed in paraffin-embedded, 5-μm sections of hematoxylin-and-eosin-stained BAT tissue, peaked in n = 4 summer-active (late-June) ground squirrels and were relatively stable across the remainder of opportunistically collected fixed tissues. D: representative H&E-stained paraffin sections from fixed BAT at three annual time points, 29-Jun (SA), 1-Sep (FT), and 15-Dec (IBA) illustrate high-cell density/small cell size of late-June SA ground squirrels. E: cell densities (± SD) across the homeothermic period from n = 4 SpC, SA (early August) and FT (torpor naïve) from cryosectioned samples obtained from our frozen tissue bank. Data from fixed tissues (n = 4; late-June SA and n = 3; torpor naïve FT) are represented by hatched bars and state labels with asterisk; these cell counts were normalized to the size and thickness of frozen sections for comparison. Cell densities were similar between terminally aroused SpC and early-August SA animals, which tended to be higher (i.e., have smaller cells) than FT (SA vs. FT, P = 0.06). By including the additional fixed-tissue time points, June-SA animals represent the annual minimum in cell size and indicate that SpC and SA samples used in proteomics occur in advance of and subsequent to this minimum. Cell densities for FT animals collected from both methods are included to demonstrate that the outlier June-SA densities are not based on a normalization artifact but rather reflect biological difference. F: ratio of mitochondrial to nuclear DNA content (± SD) in BAT did not vary across the annual cycle. Total DNA was isolated from frozen BAT tissue in n = 5 SA, n = 6 IBA and SpW, and n = 15 FT ground squirrels.
Cell density (nuclei/area)—and thus cell size—was relatively constant in the BAT axillary depot across the annual cycle (Fig. 2C). There were no significant differences among opportunistically sampled heterothermic states or between winter and fall or spring. The lone outliers were the four late-June ground squirrels that displayed 2.4-fold higher cell densities vs. other states on average, and thus the smallest BAT cell size (Fig. 2, C and D). We further examined this finding with tissues from our frozen bank. No additional samples were available from the June time point, so we compared three other states that span the homeothermic period: spring (SpC) along with SA animals collected in early August and fall animals, which had not yet displayed torpor (Fig. 2E). Cell densities were nearly equivalent between terminally aroused SpC ground squirrels and SA animals sampled in August. A trend toward reduced cell densities (larger cells) was apparent by FT (SA vs. FT, P = 0.06). Although histology in these two homeothermic groups appeared equivalent, when plotted alongside the data from late-June active squirrels, it was clear despite tissue processing differences that late-June SA animals represent the annual cell density maximum (cell size minimum) among all time points examined. Thus, SpC and SA sampling points for cryosections, and subsequently proteomics, occur on either side of this minimum (Fig. 2E). To analyze any annual pattern of within-cell mitochondrial proliferation in SpW, SA, FT, and IBA ground squirrels, we determined the ratio of copy number in a mitochondrial gene (COX1) vs. a nuclear gene (RPPH1) and found no significant differences among these states (Fig. 2F). Although they exhibited near-equivalent histology, we expect SpC and SA ground squirrels to exhibit distinct physiology and proteomes based on their positions in the annual cycle.
We next used a proteomics screen across homeothermic and heterothermic sampling points available in our frozen tissue bank to uncover the biochemistry underlying the observed morphology and histology cycles. Specifically, we tested whether an annual cycle is also a significant proteomic feature of 13-lined ground squirrel BAT and whether the histological differences among the homeotherms and relative lack thereof among the heterotherms are reflected by the proteome.
BAT proteome varies seasonally.
The protein complement in BAT differed significantly across the 13-lined ground squirrel's annual hibernation cycle. Of 2,982 protein spots matched in 2D gels, 323 differed among the base states, 203 of which were unequivocally identified (Supplemental Table S4) and became the object of further analyses. In partitioning those 203 differences across the states examined, the August SA animals were the most unique. They were maximally different (52%) from animals late in the process of arousing from torpor (LAr) and differed by at least 54 proteins compared with any other state; the latter, still-robust complement of pairwise differences (27%) occurred between SpW and SA. Intrawinter changes were, on the other hand, limited (0–3 significant pairwise comparisons; Table 1), demonstrating that within-season variance in the BAT proteome, consistent with changes in its histology, was higher within homeotherms than within heterotherms with SA being the farthest outlier.
Table 1.
Pairwise significant differences among the eight base hibernation states
| SA | IBA | Ent | LT | EAr | LAr | SpC | SpW | |
|---|---|---|---|---|---|---|---|---|
| SA | 95 | 98 | 70 | 88 | 105 | 61 | 54 | |
| IBA | 148 | 0 | 0 | 3 | 1 | 28 | 75 | |
| Ent | 163 | 2 | 1 | 2 | 1 | 33 | 79 | |
| LT | 111 | 0 | 2 | 1 | 3 | 5 | 25 | |
| EAr | 139 | 6 | 4 | 2 | 0 | 34 | 67 | |
| LAr | 162 | 3 | 2 | 3 | 0 | 35 | 77 | |
| SpC | 97 | 41 | 57 | 11 | 50 | 51 | 4 | |
| SpW | 88 | 105 | 123 | 37 | 102 | 110 | 4 |
Bottom left: significant comparisons for all matched spots (n = 323). Top right: significant pairwise comparisons for the identified spots (n = 203). The brown adipose tissue proteome of summer active (SA) ground squirrels is most distinct from all other groups.
IBA, interbout arousal; Ent, entering torpor; LT, late torpor; EAr, early arousing; LAr, late arousing; SpC, spring-cold; SpW, spring-warm.
Tukey highly significant difference (HSD) test was performed; P < 0.05.
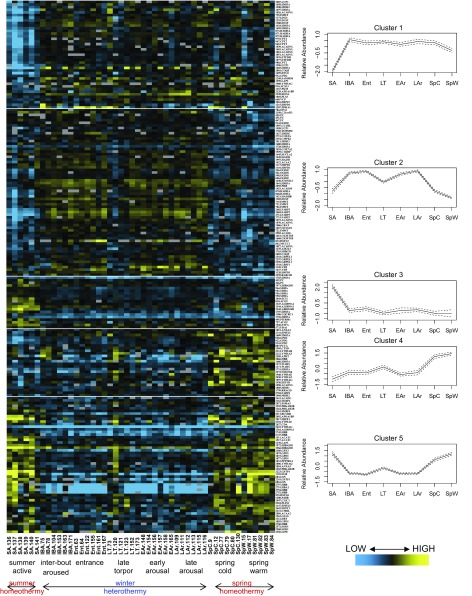
To examine global pathway shifts in the BAT proteome, we defined five k-means clusters for the 203 protein spots that differed significantly (q < 0.05) by ANOVA and were identified by LC-MS/MS. The five clusters viewed together as a heat map (Fig. 3) displayed a dominant summer-winter switch in this data set based on 57% of significant differences confined to two patterns, either increased (cluster 2) or decreased (cluster 5) during winter heterothermy. Within these two clusters, the LT ground squirrels appeared somewhat anomalous by more closely resembling the homeotherms (SA, SpC, SpW) than other winter animals (IBA, Ent, EAr, and LAr; Fig. 3). Consistent with this result, LT differed from spring and summer by the fewest significant pairwise comparisons of all winter states (Table 1). The three other patterns were SA decreased (cluster 1), SA increased (cluster 3), and both SpC and SpW increased compared with all other states (cluster 4). DAVID analysis of the proteins in each k-means cluster identified the functional components of the five patterns (Table 2). Proteins elevated throughout winter (clusters 1 and 2) represented an increased mitochondrial membrane signature, as well as pathway enrichments pertaining to energy production from lipid substrates (Table 2). The most striking feature of the spring-elevated patterns (clusters 4 and 5) was a >450-fold enrichment of 14-3-3 proteins (Table 2). Cluster 5 represents the pattern of concordant spring and summer elevation. Here, the lack of an enriched energy-production pathway that is shared in spring and summer underscores the biochemical heterogeneity of this organ across the homeothermic period.
Fig. 3.
Heat map by sampled state. Protein spots that differed significantly among hibernation states by ANOVA (q < 0.05) and could be identified by LC-MS/MS were visualized by heat map to reveal five main patterns of change. The majority of differences in Clusters 2 and 5 distinguish between the summer and winter proteome of brown adipose tissue. On the x-axis, sampled animals are grouped by state. On the y-axis, protein spots are identified (spot number.spot identity). The mean (solid line) and 95% confidence interval (dashed lines) are presented for each cluster.
Season- and state-specific biomarkers in BAT proteome.
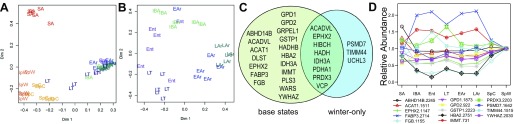
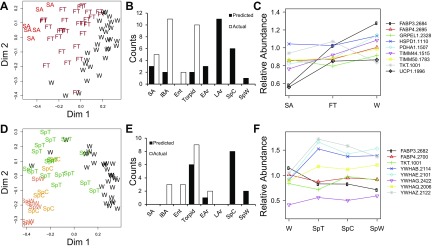
To further define critical protein differences among sampled states, we used Random Forests to determine minimum required protein spot abundance data for best group classification. This was achieved with 30 protein spots (Fig. 4A), an analysis that again separated the homeothermic states by clustering August SA ground squirrels apart. With the exception of three LT individuals that grouped closer to SpC, the winter states were largely indistinguishable (Fig. 4A). When the winter groups were considered alone, 11 protein spots provided improved, but still imperfect, group classification (Fig. 4B). By comparing these two sets of inputs (Fig. 4C), we can identify proteins unique to clustering all base-states (Fig. 4A), whose absence from winter-only clustering (Fig. 4B) suggests they contribute to seasonal, summer-vs.-winter separation, as well as distinguishing among the homeotherms. Likewise, proteins relevant only to winter clustering help us understand limited intrawinter differences.
Fig. 4.
Random Forests clustering. A: best clustering of all base states (n = 6 per state) occurred with 30 protein spots. B: better winter separation occurred by omitting spring and summer. Clustering error was lowest when only 11 protein spots were included. C: Venn diagram depicts the overlap between proteins selected to best classify all base states and those that classify winter groups only. D: abundance changes of input spots for both analyses that achieved at least a 1.3× fold change in the data set, normalized to SpW represent all five patterns from Fig. 2. If multiple isoforms for a given protein are contained within this set of inputs, only the highest fold change isoform is presented.
Only three proteins (described by gene symbolspot number) were unique to winter states clustering (Fig. 4C). TIMM441515 was highest during Ent (Fig. 4D); PSMD71642 was lowest in Ent and highest in LAr; UCHL32075 abundance peaked during LT and EAr. Considerably more protein spots were unique to the base state comparison (Fig. 4C); these classify the homeothermic states apart from winter heterothermy and illustrate again the uniqueness of the SA time point in this tissue. Not surprisingly, FABP32682/2684/2714 was winter-elevated. ABHD14B2245 and ACAT11511 were both notably highest in SA, while YWHAZ2030 elevation exemplifies the 14-3-3 signature observed in spring (Fig. 4D). GPD11873 is high in SA, and both spring states compared with winter, whereas GPD2922 abundance is lowest in SA and highest in SpC and SpW (Fig. 4D).
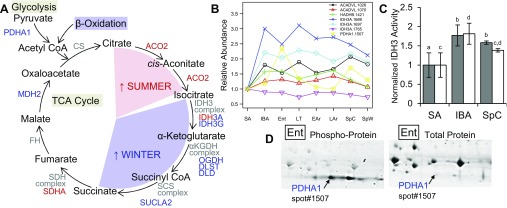
The configuration of the TCA cycle exhibits strong seasonality, as well as state-specific adjustments and the k-means patterns of Fig. 2 provide several examples of distinct regulation between SA and heterothermic winter states (Fig. 5). Summer favors citrate conversion to isocitrate, whereas winter favors the catabolism of isocitrate and succinate shuttling into the electron transport chain in addition to malate conversion to oxaloacetate (Fig. 5A). The isocitrate dehydrogenase complex straddles the proteomic boundary between summer- and winter-elevated portions of the TCA cycle, yet changes in components of this heterotetrameric enzyme are too complex to suggest a simple seasonal change in function. A single IDH3G (γ-subunit) isoform was winter-decreased (Supplemental Table S4), yet the seasonal abundance of multiple recovered IDH3A (α-subunit) isoforms varied. Nineteen IDH3A isoforms reached q significance overall (Supplemental Table S2), and three (Fig. 5B) were important for the lowest-error Random Forests clustering among the base states. An assay of IDH3 activity revealed an ∼80% increase in IBA animals compared with SA (P = 0.006 for activity normalized per microgram protein, P = 0.0008 when normalized per gram wet wt of BAT tissue; Fig. 5C). Activity did not differ between cold-exposed SpC and IBA but was significantly less in SA than in SpC (P = 0.006 per microgram protein; Fig. 5C), providing evidence in support of our proteomics data that isocitrate conversion to α-ketoglutarate is enhanced during the winter heterothermic period and remains elevated in postemergence spring time points compared with SA.
Fig. 5.
Tricarboxylic acid (TCA) cycle across the annual cycle in 13-lined ground squirrels. Several TCA cycle enzymes exhibit seasonal patterns. A: enzymes included in patterns of summer (SA) elevation (red; k-means clusters 3 and 5) or winter elevation (blue; k-means clusters 1 and 2). B: line plots of all Random Forests classifiers pertaining to substrate delivery and flux through the TCA cycle, normalized to SA. C: means ± SD activity of TCA cycle isocitrate dehydrogenase (IDH3), normalized per gram wet weight of BAT tissue (gray bars) and per microgram protein in the sample (white bars), expressed as a percentage of SA levels for n = 5 animals in each group. Letters denote significant differences by one-way ANOVA and Tukey's post hoc test within each normalization method. D: phosphoprotein staining identifies the PDHA1 isoform elevated in winter to be the phosphorylated (inhibiting) form. Two sequential staining steps of the same gel (n = 1 Ent animal) are shown to visualize PDHA1 spot 1507 in relation to total protein and phosphoproteins.
Figure 5 also documents protein changes affecting substrate delivery to the TCA cycle. The β-oxidation pathway was enriched seasonally (Table 2) and supported by an overall winter increase (vs. SA) in enzymes such as ACADVL1026/1070 and HADHB1421 (Fig. 5B). State-specific regulation of the TCA cycle also derived from fluctuating carbohydrate delivery via pyruvate dehydrogenase. PDHA11507 had the largest fold change among the 11 proteins that best separated the winter states, with a large peak in Ent (Fig. 5B). Phosphoprotein staining confirmed that this isoform was phosphorylated (Fig. 5D), a posttranslational modification that renders pyruvate dehydrogenase (E1 enzyme), and thus the pyruvate dehydrogenase complex, inactive.
The annual cycle and seasonal transitions in the BAT proteome.
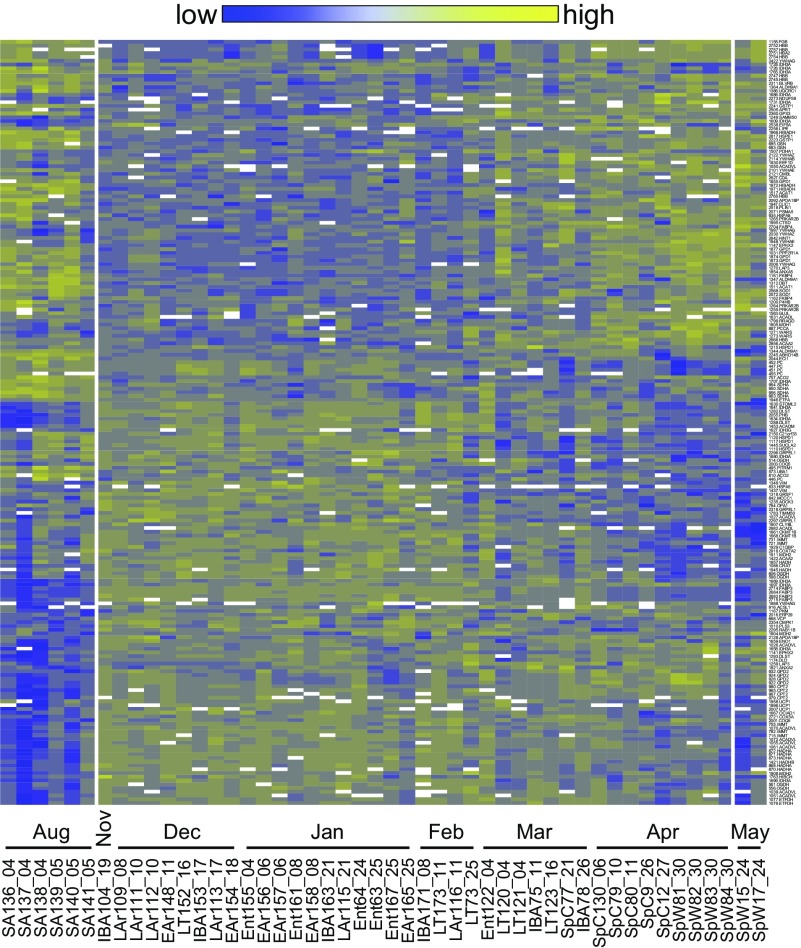
The changes in the BAT proteome across the hibernator's year are not fully explained by a seasonal switch or torpor-arousal cycles, neither of which predicts the strong separation between the Sp and SA groups or the anomalous behavior of the three LT samples. Indeed, when the individual intensities for all ANOVA q-significant, identified protein spots were ordered by sampling date rather than sampling state (Fig. 6), the 13-lined ground squirrel BAT proteome showed a striking annual cycle. The proteome remained relatively constant throughout the heterothermic period, with a late winter rapid transition toward the proteome of terminally aroused spring ground squirrels. The midpoint of this shift occurred on 04-Mar in this data set (Fig. 6). Viewing the heat map chronologically provided an explanation for the anomalous similarity of LT to spring/summer in the state-dependent heat map rather than the other winter states (Fig. 3), as well as the Random Forests clustering of three LT animals nearest to the SpC and SpW individuals (Fig. 4A); the close proximities appear to reflect the late winter collection dates of those LT samples (Fig. 6).
Fig. 6.
Chronological heat map. A hierarchically clustered heat map arranged chronologically along the x-axis displays the annual cycle of identified protein spot abundance data differing among hibernation states (y-axis, right: spot number.spot identity). Each animal sampled from a physiologically defined state (stateID_dd) is ordered chronologically (August 4–May 24). White break points in the heat map represent gaps between sampling dates longer than 1 mo.
To gain more insight into the progression between homeo- and hetherothermy, we next compared samples collected from fall and spring transition periods to the eight base sampling states. Significantly, FT and SpT are themselves not date-dependent, showing no heat map patterns when viewed in chronological sampling order (data not shown). FT was clearly intermediate to summer and winter by unsupervised classification based on the 30 protein spots from Fig. 4A that best separated all base states (Fig. 7A). The Random Forests clustering algorithm was first trained with the defined base states and then used to predict the proximity of individual transition animals to each state. Only three FT ground squirrels were most proximate to SA. Instead, FT individuals more often resembled LAr despite the complete absence of animals rewarming from torpor in the fall data set (Fig. 7B). It is also noteworthy that the state of torpor did not impose a signature on the proteome in this tissue sufficient to classify the 10 FT ground squirrels that were actually torpid at the time of tissue collection with LT (Fig. 7B).
Fig. 7.
Classification of fall and spring transition animals. Ground squirrels representing mixed physiology transition timepoints had proteomes generally intermediate to their flanking seasons. The overall proximity of transition animals (A: fall, D: spring) to flanking states (SA or SpC/SpW and winter individuals, “W”) was determined by unsupervised classification in Random Forests, using the top 30 inputs from physiologically known individuals. Counts (B: fall, E: spring) of the single most proximate state for each individual (predicted from a Random Forests training set of all base state animals) are compared with their actual physiology as measured by Tb at time of tissue collection. “Torpid” is most analogous, but not identical, to LT because, although transition animals were torpid at the time of tissue collection, Tb, Ta, and the time in torpor varied (see Fig. 1). The transition of several proteins of interest are presented by line plots (C: fall, F: spring).
We then used significant Tukey pairwise comparisons (FT vs. SA or vs. all winter states, Supplemental Table S5) to identify BAT proteins still representing the summer proteome and those that had switched to the winter phenotype. Four k-means patterns described the FT proteome. 84% of protein spots had already risen or fallen to achieve their winter abundance (2 of 4 patterns, Table 3). Fatty acid binding and catabolism were enriched pathways in these two patterns, as was the adoption of a winter configuration in the TCA cycle and electron carriers (Table 3). Uncoupling protein (UCP1, Fig. 7C) was also fall-elevated in advance of the hibernation season and reflects the general seasonal increase in mitochondrial protein content (Table 3). The remaining two clusters contained proteins that retained their high or low SA levels during FT but were too few for pathway enrichment analyses. These few proteins suggested the switch to lipid substrate preference was incomplete during FT; specifically, FABP4 remained low despite the elevation of FABP3 (both were high throughout winter) and high TKT and low phospho-PDHA1 suggested some continued SA-like reliance on carbohydrate substrates (Fig. 7C, Supplemental Table S5). FT ground squirrels had also begun proteomic adjustments toward more efficient mitochondrial protein trafficking in winter. Inner membrane translocase was winter-enhanced by more abundant motor and associated proteins (TIMM50, GREPL1, HSPD1, TIMM44). An intermediate fall condition was produced by elevation of proteins such as TIMM44, but a delayed increase in GREPL1. This fall phenotype also did not yet exhibit the winter elevation of HSPD1, which chaperones and folds newly imported mitochondrial proteins (Fig. 7C).
Table 3.
Enriched BAT proteomic pathways in k-means patterns of fall transition 13-lined ground squirrels determined from identified protein spots differing by Tukey HSD from either summer active ground squirrels or any heterothermic winter groups
| Annotation Term | Score | Fold | Genes |
|---|---|---|---|
| Fall Pathways Already in Winter Form (Decreased Abundance) | |||
| Mitochondrion | 7.1 | 6 | SDHA, PPP2R1A, UQCRC1, ACO2, GFM1, PITRM1, COQ9, SOD1, ACAT1, HIBADH, IDH3A, SOD2 |
| Aerobic respiration | 3.9 | 60 | SDHA, UQCRC1, ACO2, IDH3A |
| Complex assembly | 3.2 | 7 | PPP2R1A, GSN, FGB, FKBP4, GPX3, ANXA5, SOD2 |
| Organelle lumen | 2.5 | 3 | P4HB, CES1, ACO2, FGB, FKBP4, PITRM1, SOD1, ACAT1, IDH3A, SOD2 |
| Reactive O2 species response | 2.2 | 52 | GPX3, SOD1, SOD2 |
| Apoptosis regulation | 1.5 | 3 | PPP2R1A, SOD1, ANXA5, GSTP1, SOD2 |
| Extracellular space | 1.3 | 3 | GSN, FGB, GPX3, SOD1 |
| Fall Pathways in Already Winter Form (Increased Abundance) | |||
| Mitochondrial membrane | 11.5 | 16 | CPT2, SAMM50, IMMT, PHB, STOML2, UCP1, CRAT, OGDH, TIMM44, HADHA, HADHB, ACADVL, NDUFS8, ETFDH, MDH2 |
| Fatty acid β-oxidation | 8.0 | 121 | ACADVL, CPT2, ACADM, ETFDH, CRAT, HADHA, HADHB |
| TCA cycle | 4.1 | 39 | DLST, DLD, OGDH, IDH3A, MDH2 |
| Organelle lumen | 3.5 | 3 | DLST, ACADM, PHB, ERP29, OGDH, TIMM44, IDH3A, HADHA, HADHB, ACADVL, DLD, HSPA5, MDH2 |
| Electron carrier activity | 3.4 | 11 | ACADVL, ACADM, NDUFS8, DLD, ETFDH |
| Fatty acid binding | 1.9 | 39 | FABP3, HADHA, HADHB |
| Glucose catabolism | 1.8 | 25 | OGDH, MDH2, ENO1 |
Note: Enrichments determined by DAVID. “Score” denotes DAVID enrichment score (significance set >1.3). “Fold” denotes pathway fold enrichment. Redundant pathways and those contained as subsets within broader pathways were removed.
The BAT proteome of SpT animals shared the fall tendency to undertake a significant seasonal shift well before a phenotypic transition was complete, i.e., to look like the spring homeotherms whilst still exhibiting heterothermy. The unsupervised Random Forests classification using the top 30 protein spots in Fig. 4A placed most SpT individuals closer to spring than winter (Fig. 7D). Consistent with this, the BAT proteome from the majority of SpT animals (10 of 17) was most proximate to the terminally aroused SpC or SpW states. Although 9/17 SpT animals were actually torpid, only six of these individuals had the highest Random Forests proximity to the LT state (Fig. 7E).
From significant pairwise differences between SpT and flanking seasons (SpT vs. all winter states or vs. SpC and SpW), we determined which proteins had progressed and which lingered in their winter configurations. Four k-means patterns described the transition between winter and spring. As with the FT animals, the majority (82%) of protein spots fell into two abundance patterns that were characteristic of the subsequent phenotype, in this case terminal arousal (Table 4). Enrichment of fatty acid binding and metabolism pathways declined, while 14-3-3 proteins were already strikingly enhanced (649-fold enriched, Table 4, Fig. 7F). The final two patterns contained proteins that resembled winter rather than the terminally aroused proteome, but again, these were too few to determine significant pathway enrichments. Though limited, proteins that retained winter abundance levels though SpT are insightful for the regulation of seasonal substrate preferences and the order of substrate switches. For example, proteins involved in lipid catabolism declined from winter to spring (e.g., FABP3 and FABP4), yet SpT ground squirrels retain low TKT (Fig. 7F), suggesting carbohydrate catabolism is suppressed in the transition prior to spring emergence.
Table 4.
Enriched BAT proteomic pathways in k-means patterns of spring transition 13-lined ground squirrels determined from identified protein spots differing by Tukey HSD from either spring (SpC or SpW) ground squirrels, or any heterothermic winter groups
| Annotation Term | Score | Fold | Genes |
|---|---|---|---|
| Spring Transition Pathways Already in Terminally Aroused Form (Decreased Abundance) | |||
| Coenzyme binding | 7.1 | 26 | ACADVL, ALDH6A1, ACADM, OGDH, HADH, IDH3A, HADHA, HADHB, ETFA |
| Mitochondrial membrane | 6.3 | 12 | ACADVL, COX7A2, CKMT1B, IMMT, PHB, HSPD1, OGDH, HADH, HADHA, HADHB |
| Organelle lumen | 5.6 | 4 | GRPEL1, ACADM, PHB, OGDH, IDH3A, HADHA, HADHB, ACADVL, LONP1, C1QBP, VCP, HSPD1, HSPA5, HADH, ETFA |
| Fatty acid binding | 4.6 | 68 | ALDH6A1, FABP3, FABP4, HADHA, HADHB |
| Fatty acid metabolism | 4.3 | 55 | ACADVL, ACADM, HADH, HADHA, HADHB |
| Adenyl nucleotide binding | 3.4 | 4 | ACADVL, TRAP1, LONP1, GRPEL1, ACADM, CKMT1B, VCP, HSPA5, HSPD1, SUCLA2, ETFA |
| Endopeptidase activity regulation | 1.8 | 21 | VCP, HSPA5, HSPD1 |
| Spring Transition Pathways Already in Terminally Aroused Form (Increased Abundance) | |||
| Mitochondrion | 3.7 | 6 | GPD2, ACAA2, SHMT2, NDUFS8, ACAT1, IDH3A, PCCA |
| 14-3-3 | 3.2 | 649 | YWHAZ, YWHAB, YWHAQ, YWHAE |
| Complex assembly | 2.8 | 7 | PPP2R1A, FGB, FKBP4, NDUFS8, YWHAB, CDA |
| Phosphate metabolism regulation | 1.8 | 6 | PRKAR2B, PPP2R1A, YWHAB, FABP4, YWHAE |
| Cytoplasmic vesicle | 1.7 | 5 | YWHAZ, PRDX6, FGB, YWHAB, YWHAE |
| Apoptosis regulation | 1.5 | 4 | PPP2R1A, YWHAZ, YWHAB, YWHAE, GSTP1 |
Note: Enrichments determined by DAVID. “Score” denotes DAVID enrichment score (significance set >1.3). “Fold” denotes pathway fold enrichment. Redundant pathways and those contained as subsets within broader pathways were removed.
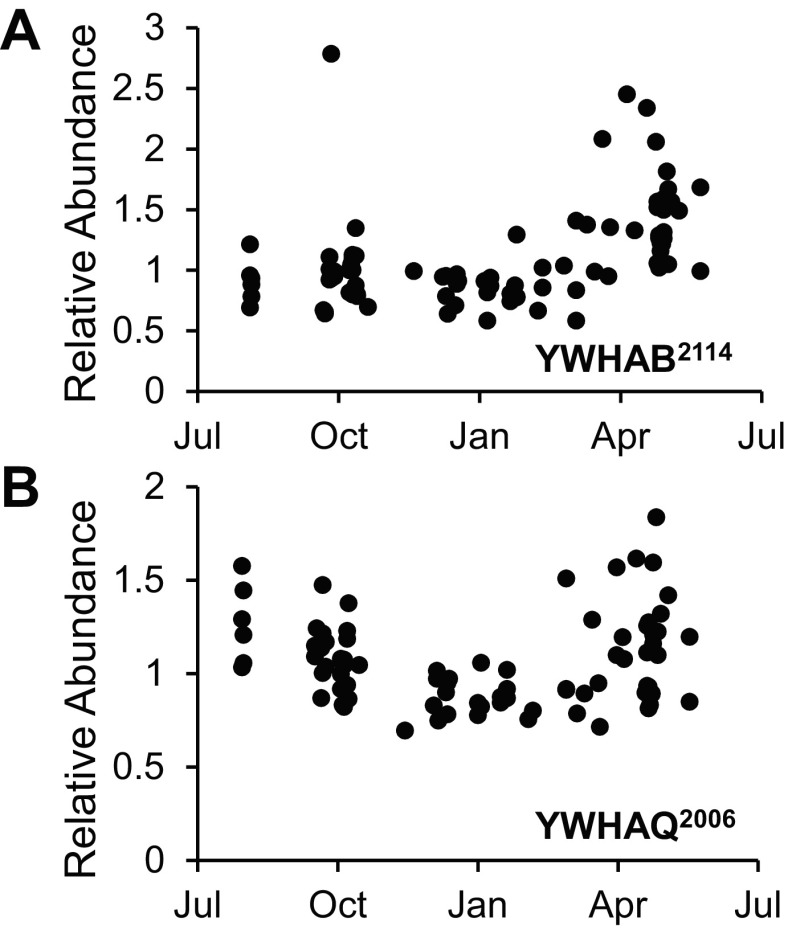
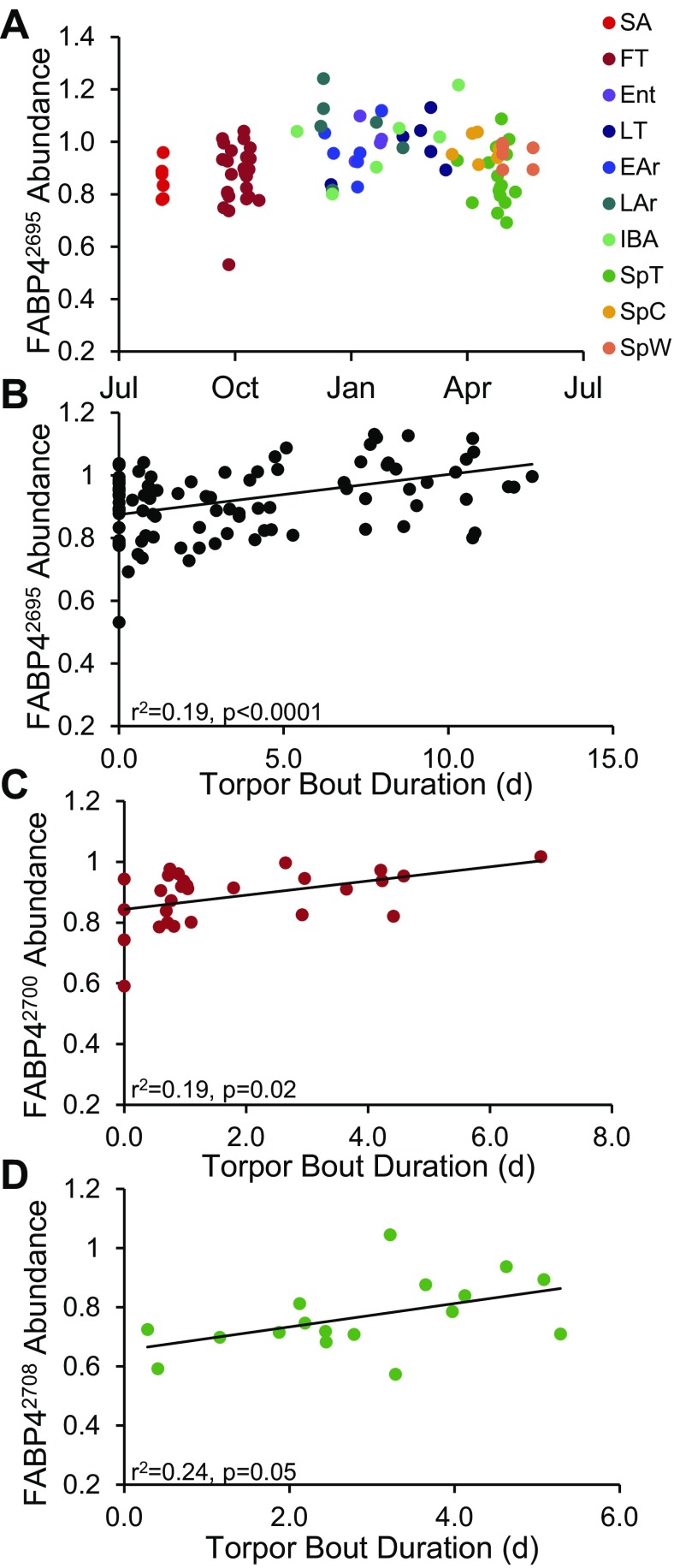
Considering the protein signatures of transition states in the context of the annual cycle exposed several potential biomarkers of hibernation form and function. Plotting the protein spots associated with the spring 14-3-3 enhancement (Fig. 8) revealed an annual cycle slightly advanced in time to that of cell density (Fig. 2C). Induction of YWHAB2114, YWHAE1948,2101, YWHAG1988,1997,2422, YWHAQ2006, and YWHAZ2030,2122 that began in SpT, persisted through the post-emergence spring states, and declined in August SA (Fig. 8; Supplemental Table S7) are proteomic examples that track the histology finding of minimum cell size in late-June ground squirrels (Fig. 2C). The transition proteomes may also provide biomarkers for the long, regular torpor-arousal cycles of winter hibernation. Many FT and all SpT ground squirrels display truncated torpor bouts compared with winter (Fig. 1, Supplemental Table S1), which can be evaluated in light of the transition proteome. Transition ground squirrels differ from winter animals in lipid handling; specifically, neither FT nor SpT had the high FABP4 (adipose-fatty acid binding protein) characteristic of winter. FABP42695,2700,2708 abundance cycles annually, with a maximum in mid–late winter (Fig. 9A). This annual peak in FABP4 abundance appears related to torpor length (Fig. 1), as abundance was positively correlated with most recent torpor bout length across the entire proteomic data set (0.16 < r2 < 0.24, P ≤ 0.0003, Fig. 9B). This linear relationship is retained for a single isoform of FABP4 when FT (FABP42700, Fig. 9C) or SpT (FABP42708, Fig. 9D) animals are considered alone.
Fig. 8.
Increased 14-3-3 protein abundance cycles annually and precedes axillary BAT atrophy. Representative plots of 14-3-3 protein abundance in all sampled animals vs. time are shown for YWHAB2114 (A) and YWHAQ2006 (B).
Fig. 9.
Fatty acid binding protein annual cycle and relationship to duration of torpor. Three isoforms of FABP4 (adipose-fatty acid binding protein2965,2700,2708) were examined by sampling date and individual animal features. A: plot of FABP42695 abundance vs. sampling date for each animal. B: plot of FABP42695 abundance vs. length of previous torpor bout for all individuals. There was a limited but significant positive linear relationship between FABP4 abundance and duration of the animal's previous torpor bout in all three isoforms (no. 2695 shown, all three: 0.16 < r2 < 0.24; P ≤ 0.0003). Duration = 0 for all homeothermic ground squirrels. A linear relationship was retained for a single FABP4 isoform in transition states considered alone (C: FT FABP42700 and D: SpT FABP42708).
DISCUSSION
Small-bodied placental hibernators undergo hypertrophy of BAT in preparation for winter and rely on tightly controlled periods of nonshivering thermogenesis during torpor-arousal cycles. This change in activity occurs in concert with an annual pattern of reproduction, fattening, and Tb regulation. Thermogenesis is a significant component of the hibernators' winter energy budget (30); therefore, energy substrates are carefully regulated during the prolonged winter fast. Here, we investigated the BAT morphology and proteome of hibernating 13-lined ground squirrels to evaluate the components and results of an annual cycle that supports specialization for fueling winter heat production despite fasting and the recalibration of fuel use during the growth and fattening phases of spring and summer homeothermy. We have identified specific proteins that underlie altered substrate reliance between summer and winter, presumably contributing to a winter-enhanced thermogenic capacity (i.e., a more recruited state), as well as those that may control the cell cycle and survival signaling in this tissue during the spring reproductive season when BAT is maximally lipid-depleted, atrophied, and cell size and proliferation are minimal (Fig. 2).
Seasonal adjustments streamline lipid catabolism in winter.
Seasonal proteomic changes separating homeotherms from heterotherms were dominant in this data set (clusters 2 and 5 in Fig. 3 and Table 2). In the face of limited intrawinter differences, this strong signal suggests that the metabolic foundations supporting the alternating periods of intense activation during winter torpor-arousal cycles are seasonally reprogrammed. Indeed, seasonal protein abundance changes are consistent with metabolic streamlining for exploiting lipid fuels. A winter switch to lipid-based catabolism is well documented in hibernators as an adaptation to most efficiently contend with the winter fast (10). In general, BAT is already specialized for lipid catabolism to support heat production (9); lipids serve both as a substrate for catabolism and modulate UCP1 activation (18). A winter proteome optimized for further lipid binding and catabolic efficiency is evidenced by elevated heart (FABP3) and adipose (FABP4) isoforms of fatty acid binding protein along with an enriched β-oxidation pathway (Table 2). Enhanced mitochondrial signatures, lipid handling, and catabolism can all be considered elements of BAT recruitment (9) and provide evidence that the thermogenic capacity of BAT is seasonally increased. The methanol/chloroform precipitation step in our protocol is necessary for 2D protein resolution; however, a limitation of this method is the exclusion of hydrophobic proteins, such as those embedded in mitochondrial and plasma membranes. Therefore, we do not expect to reveal the entirety of mitochondrial membrane dynamics across the hibernation cycle; however, we have recovered a suite of membrane-bound or associated proteins that are informative about mitochondrial dynamics.
The more recruited state of winter BAT is supported by increased components of inner mitochondrial membrane translocase (TIM23 complex). The nuclear origin of most mitochondrial proteins makes mitochondrial import critical for optimal metabolic efficiency; thus, proteins are actively translocated across both outer and inner membranes. The TIM23 complex imports nuclear-encoded proteins with a matrix-targeting signal (14). Two components of the complex, TIMM50 and GREPL1 (contained within the PAM motor complex of the translocase), are significantly winter-elevated (Table 2, Fig. 7C). One additional TIM23 component, TIMM44, trends to winter increase, with its maximum fold change occurring between Ent and SA, Supplemental Table S7). Taken together with the general enrichment of mitochondrial membrane proteins in winter-elevated k-means patterns (Table 2), our data support the notion of increased overall mitochondrial content (35) in the BAT of winter hibernators. In the absence of a seasonal increase of mitochondrial copy number (Fig. 2F), we interpret the enriched mitochondrial element observed in our data as supporting increased mitochondrial size and membrane lengthening (33). Modest but significant shifts have been documented in BAT mitochondrial size and structure in hazel dormice (Muscardinus avellanarious) between late torpor and arousal (33). Our data demonstrate within-winter stability in the soluble BAT mitochondrial proteome while distinguishing homeotherms from heterotherms. The difference between ultrastructure and proteome may be partly explained by species differences or the absence of lipophilic integral membrane components in our samples. However, the absence of torpor-arousal cycles in mitochondrial membrane-associated proteins and seasonal abundance increases of proteins with inner membrane structural function (e.g., IMMT) suggests that membrane components are seasonally programmed and remain available throughout the winter.
Carbohydrate flux into the TCA cycle is limited by pyruvate dehydrogenase. Inactive phosphorylated PDHA1 (E1 α subunit, containing the E1 active site, Fig. 5B) is specifically high in Ent, inhibiting the conversion of pyruvate to acetyl-CoA (26) and regulating substrate delivery to the TCA cycle within torpor-arousal cycles. This strategy to limit carbohydrate catabolism has been demonstrated for other tissues in this species (7, 20). TCA cycle enzymes are also seasonally adjusted in 13-lined ground squirrel BAT (Fig. 5). Enzyme abundances and isocitrate dehydrogenase activity suggest that citrate to isocitrate conversion is summer-elevated, whereas in winter, isocitrate is further processed, and malate conversion to oxaloacetate is enhanced. This winter configuration favors the shuttling of reducing equivalents derived from fatty acid hydrolysis into the electron transport chain and generally concurs with a transcriptome analysis of SA vs. winter torpid arctic ground squirrels (48). These overall SA-winter differences suggest seasonally distinct regulation of TCA cycle intermediates. It is noteworthy that the observed seasonality will produce the most metabolically meaningful differences in concentrations of isocitrate, succinate, and oxaloacetate, all able to exert allosteric or competitive effects on the activity of respiratory complex II (SDH), which is reversibly inhibited during torpor-arousal cycles (2, 6).
Within-season variance is low during heterothermy but high during homeothermy.
BAT morphology and histology from two data sets both capture an annual cycle. Although wet weight is not a clear indicator of cell proliferation in this tissue, as lipid content varies with storage and breakdown (9), we can observe general trends across the annual cycle in 13-lined ground squirrel BAT, such that the axillary depot is largest in mid-winter and is minimal after spring emergence (Fig. 2A). Concordant with morphology, histological evaluation of cell densities (a proxy of cell size) also shows a peak in early summer (Fig. 2, C and E), with cell size declining through the sampled spring states and on the increase by the August SA time point. Concomitant with these changes in BAT structure, the proteome among homeothermic states varies substantially (Table 1, Figs. 3 and 4A).
In contrast, protein abundance patterns linked to the torpor-arousal cycle, such as PDHA1, are rare in this data set, despite the dramatic changes in activity and presumably lipid mobilization that BAT undergoes in winter (34, 38). BAT thermogenesis is mediated via adrenergic signaling (9), and as our protocol excludes lipophilic proteins, it necessarily does not capture possible seasonal or intrawinter cycles in membrane receptors such as adenylyl cyclase that activate cAMP and PKA cascades in this tissue with each rewarming arousal. Only 11 proteins best separated the winter states in our analysis, still with imperfect resolution (Fig. 4B). Only three of these proteins were unique in separating these winter states without also contributing to dividing the homeotherms from the heterotherms (Fig. 4C). The limited intrawinter changes from this analysis (Table 1, Fig. 4) included two elements of protein degradation, highlighting the importance of protein turnover within torpor-arousal cycles. Mitochondrial protein import also contained a winter cycling component (Fig. 4D), with TIMM44 increasing in Ent, likely as the product of synthesis and membrane reorganization during the euthermic IBA period.
Clearly, the observed BAT proteomic variation is not fully accounted for by heterothermy vs. homeothermy or torpor vs. arousal cycle differences. When depicted chronologically, the variance sets up a striking annual cycle (Fig. 6), suggesting an underlying circannual rhythm in the BAT proteome. Three groups of homeothermic animals were studied: terminally aroused ground squirrels still in the hibernaculum at 4°C (SpC) or warming Ta (14–18°C, SpW) and early August SA ground squirrels, Ta 18–20°C. Although environmental conditions were nearly equivalent, 54 significant pairwise differences separated SA from SpW animals. This finding is strikingly different from previous proteomic investigations of the annual hibernation cycle in heart, skeletal muscle, kidney, and brain, in which postemergence spring animals strongly resemble August squirrels (20–22, 29). While the spring BAT proteome contains remnant indicators of winter energy metabolism, including elevated ACADVL, HADHA, and decreased PC compared with SA (Supplemental Tables S4 and S7), a noteworthy component of the spring to summer difference is an elevation of 14-3-3 proteins in both spring states (Supplemental Table S4 and Table S2, group 4).
14-3-3 proteins bind to and modulate hundreds of other proteins to regulate a wide range of cellular processes, which include cell signaling, cell cycle progression, intracellular trafficking, protein targeting, enzyme activity, cytoskeletal structure, transcription, and DNA replication. Their role as cofactors in regulating growth and proliferation under conditions of cell stress may be particularly significant for hibernators. Specifically, 14-3-3s facilitate signal transduction from cell nutrition and energy stress sensing by AMPK to TORC1 inhibition (reviewed by Ref. 36). We speculate that the strong 14-3-3 presence at a time point associated with refeeding following the winter fast, observed in BAT but not as clearly in other tissues (20–22, 29), affects tissue-specific regulation and inhibition of postemergence growth. An obvious functional distinction between BAT and other tissues examined by the same method is that BAT is not as critical, and, in fact, may be an energetic hindrance to successful foraging activity, reproduction, and growth in spring. Thus, BAT regrowth may be suppressed following spring emergence with the help of the increased 14-3-3 proteins, or more aggressively, marked for lipid depletion and atrophy. Later, in the homeothermic period, the 14-3-3 signal fades and SA ground squirrels recruit and expand BAT depots in an active period of differentiation and growth (Fig. 2) (9). Using Ki-67 as a proliferation marker, we observed a proliferation signal ranging from 1.3% Ki-67-positive nuclei in the late-June SA ground squirrels, reaching its peak of 2.7% in fall (Fig. 2B). When these limited time points are considered across the annual cycle, relatively high proliferation in winter IBA and a decline during SpT is consistent with our finding of a spring peak in 14-3-3 signaling. Among the limited fixed samples available for this analysis, proliferation is lowest immediately following the highest prevalence of 14-3-3s in 13-lined ground squirrel BAT.
Conspicuously absent in our data set are proteins recently shown to be important for BAT differentiation in mice (45). Although this could be because they are not sufficiently abundant or soluble to be detected by our proteomics screening method, it could also reflect a failure to capture the critical time points when differentiation and hypertrophy is initiated, or differences in the mechanisms used to recruit BAT during the hibernation cycle in ground squirrels vs. other mammals so far examined. Further studies are needed to distinguish among these possibilities. A two-state transcriptome analysis likewise failed to yield an abundance of transcripts implicated in BAT differentiation, showing winter upregulation of only two relevant mRNAs, adipophilin-Adfp and gap junction protein 1-Gja1 (48).
A further example of variance among homeotherms is differential expression between spring and summer of the two components of the glycerol phosphate shuttle. The cytosolic glycerol phosphate dehydrogenase (GPD1) is depressed in winter compared with all homeothermic states. GPD1 is important in lipid and carbohydrate metabolism, catalyzing dihydroxyacetone phosphate conversion to glycerol-3-phosphate, oxidizing NADH as a cofactor. Glycerol-3-phosphate is readily converted to glycerol, making GPD1 a key enzyme of triglyceride biosynthesis. GPD2 represents the mitochondrial component, reversing the substrate conversion while reducing FAD. The reducing equivalent FADH2 is then passed to the electron transport chain. The mitochondrial component of the shuttle is elevated only in spring, pointing to expanded reducing equivalent handling strategies employed at this time. This is likely an element of retained thermogenic capacity in spring BAT relative to SA, despite reduced fatty acid metabolism and lipid reserves. In addition to providing reducing equivalents (27), the glycerol phosphate shuttle intrinsically generates waste heat as a less-efficient ATP consumer than the malate dehydrogenase shuttle, for example, and is generally abundant in BAT (11). In summer, a change in the ratio of GPD shuttle components in favor of the cytosolic form would increase glycerol availability in the cytosol, supporting elevated FA esterification as the tissue builds in preparation for winter hibernation (27).
The annual cycle is likewise superimposed onto torpor-arousal cycles and, thus, accounts for some of the limited variability observed in protein abundance among the winter states. It also explains the seemingly anomalous heat map and Random Forests similarity between several LT individuals and the homeotherms (Fig. 3). When examined chronologically (Fig. 6), the high occurrence of late winter sampling among the LT individuals is clear. The few additional animals from other winter states sampled at late winter time points are also highlighted by this chronological heat map. The breakpoint occurs around 04-Mar, the earliest time point when we can observe irregularity in torpor-arousal cycles in our instrumented animals (Tb data from ground squirrels in this data set). Some ground squirrels are capable of sustaining torpor-arousal cycles until May; therefore, it is not clear whether this observation reflects a degradation of the winter proteome or a strategic early adoption of the spring phenotype.
The annual cycle also sets up testable questions for transition periods between summer and winter. By examining mixed-physiology fall and spring transition animals, we can ask how the BAT proteome is regulated and whether seasonal proteomic shifts precede whole-animal physiological changes or arise consequentially. The loss of a winter proteome despite continuation of apparently normal torpor-arousal cycles supports the hypothesis that BAT is fully functional at this time point, and the winter enhancements that we have documented reflect only seasonal streamlining or improved recruitment state. We observed no clear proteomic distinction between FT animals held at different ambient temperatures and only four pairwise significant differences between terminally aroused ground squirrels held under different environmental conditions (Table 1). Our inability to see the BAT proteome respond to ambient temperature in this obligate hibernator highlights the importance of an intrinsic annual cycle, rather than reliance on external environmental cues, to signal the seasonal switches. The increasing interest in translating BAT for human obesity therapies would be well served by better understanding the molecular cues that drive the intrinsic, date-dependent amplification of energy consuming ability in the tissue of this species.
BAT annual cycle defines transition periods that precede seasonal activity switches.
The majority of both fall and spring transition proteomes have progressed to that of winter hibernating or terminally aroused spring states, respectively. This tendency was more important in defining the transition state proteomes than were individual animal physiologies; we could not reliably predict the analogous physiological state from transition animal proteomes (Fig. 7, B and E).
FT animals, regardless of torpor utilization, already displayed several features of the winter proteome, including their TCA cycle configuration and enhanced fatty acid metabolism (Table 3). Ground squirrels did rely more on carbohydrate substrates in fall than they did in winter, as evidenced by high transketolase (TKT) and low phosphorylated PDHA1 levels. The retention of carbohydrate catabolic (PDHA1 supports pyruvate to acetyl-coA conversion) and anabolic capacities (TKT supports the pentose phosphate pathway) likely reflect the ongoing shift toward a fasting-tolerant winter metabolism, given that food was still available or was only recently removed.
In keeping with numerous results collected from hibernators (10), proteomic analyses of other tissues of this hibernating species show an association between winter-increased fatty acid metabolism and enhanced lipid binding and transport (21, 20, 17). BAT follows this trend in having elevated FABP3 (heart isoform) and FABP4 (adipose isoform) during hibernation; both have been previously documented in torpid hibernators at the protein and transcript level (16, 23, 24). The fall BAT had only elevated FABP3; thus, it appears incompletely converted for winter function. The heart isoform (FABP3) is necessary for BAT thermogenesis in the cold (46), while FABP4 is not (44). Significantly, FABP3 knockout mice fail to exhibit cold tolerance despite UCP1 elevation and hydrolysis of local lipid reserves (44). Moreover, an increase of FABP3 has been attributed to adaptive thermogenesis in rodents (46) and cold-tolerance in 13-lined ground squirrels specifically (24). It appears that FABP3 mediates the uptake of exogenous fatty acids delivered by the plasma from white adipose tissue hydrolysis. FABP3 knockout mice are not hampered in their ability to store and mobilize intracellular triglycerides, which falls to FABP4 (13, 44). In contrast to rats (46), abundance of both isoforms bears no direct relationship to days of cold exposure in our n = 28 mixed physiology fall ground squirrels. Thus, lipid handling provides a clear example of the transitional nature of fall, and the importance of seasonal proteomic reprogramming. Disruption of FABP4 in mice adipocytes impairs lipolysis and lipid handling, resulting in elevated intracellular fatty acid levels (13). The failure of FABP4 to achieve winter abundance during this transition period implies that BAT intracellular fatty acids are less regulated relative to winter and that hydrolysis of intracellular lipid reserves is to some degree limited until the onset of regular torpor-arousal cycles. FABP4 is also reduced in SpT vs. winter, and when considered across the annual cycle shows a correlation among three isoforms with duration of torpor (Fig. 9). Despite the highly variable nature of FT and SpT physiologies and the individual timing and patterns of transitioning between homeothermy and heterothermy (39) (Supplemental Table S1), the positive relationship between FABP4 abundance and torpor bout duration persisted in one isoform for each transitional season (Fig. 9). The r2 values for these linear relationships clearly to do not imply that fatty acid handling is the sole casual factor in maintaining torpor; however, they do suggest that isoforms of FABP4 explain a measureable portion of the variation in duration of torpor over the entire hibernation season. It is reasonable to propose a role for FABP4 in managing the prolonged torpor bouts of winter (and, by corollary, explain the irregularities of transition state cycles) through its impact on intracellular fatty acid levels. As free fatty acids directly activate UCP1 (18), FABP4 upregulation in winter may be required both to control BAT substrates and maintain UCP1 inactivation until activation signals are received.
We observed a pattern of fall increase in proteins associated with the mitochondrial membrane, including those important for protein import (TIM23 complex, Table 2). GREPL1 is a notable exception, still retaining its low summer levels, providing yet another example of the transitional nature of these mixed-physiology ground squirrels. Overall, these fall data indicate a transformation in BAT to a more active, recruited state consistent with an increased mitochondrial component, as documented previously (33, 35). Moreover, the supporting winter proteome is largely in place for the FT animals as part of an annual cycle, even for ground squirrels not yet consistently using torpor, or necessarily cold-exposed.
The proteome of SpT individuals, on the other hand, strongly resembled terminal arousal despite the animals' ongoing use of torpor. Some reversion of TCA cycle and β-oxidation enzymes to their spring/summer levels contributed to this similarity. As with terminally aroused spring ground squirrels, transition animals no longer maintaining regular torpor-arousal cycles showed strong proteomic enrichment of 14-3-3 proteins (649-fold enrichment, Table 4). The importance of regulating growth in tune with cell energy status is particularly relevant in SpT animals, as this is the annual time point at which food and water were returned to ground squirrels hibernating in the laboratory. This signal is not simply due to food availability or it would be seen in the spring proteomes of all other tissues examined (20–22, 29) and in SA. 14-3-3 appearance prior to spring emergence may reflect a suite of potentially conflicting growth and survival signals in BAT at this point in the annual cycle. Nutrient and energy status adjustments based on refeeding likely conflict with persisting cold stimulus and torpor-arousal cycles. These data show that 14-3-3 enrichment is in place well before terminal arousal and is detected at the experimental time point when regular torpor use begins to degrade and nutrient status shifts with refeeding. With foraging and reproduction being the postemergence priorities of ground squirrels (43), we do not expect energy resources to be prioritized to BAT. Enriched 14-3-3 signaling, unique to BAT, considered in context with the histology data support the hypothesis that 14-3-3 signaling is seasonally programmed to inhibit BAT protein synthesis and regrowth, or perhaps facilitate atrophy early in the homeothermic period to prioritize resources for tissues directly involved in foraging and reproduction. The contribution of 14-3-3 proteins to this process is supported by a correlation in the pattern of 14-3-3 protein abundance increases and that of lipid depletion, cell size decline, and relatively depressed cell proliferation observed between the SpT and late-June SA individuals (Fig. 2, Fig. 8). The potentially regulatory nature of the 14-3-3 signal in this process derives from the slight lag between the proteomic and histological findings.
Conclusions.
Brown adipose tissue in small hibernators such as the 13-lined ground squirrel is critical to the hibernating phenotype as it defends Tb during torpor (9) and initiates endogenous rewarming during periodic arousals. It is, therefore, likely to be seasonally optimized for heat production (i.e., highly recruited) even at cold Tb (0–4°C and below). The BAT proteome in this model hibernator reveals several pathways and cellular components that are adjusted seasonally to increase the efficiency of reducing equivalent production, including optimizing substrate use during the winter fast in favor of energy-rich lipid reserves.
The importance of annual timing, even within the physiologically explicit sampling states of winter heterothermy, for example, highlights the significance of mixed-physiology animals for unraveling the nature and timing of seasonal transitions and the annual cycle. We identified a seasonal shift that is superimposed upon a strong annual cycle in the BAT proteome—a cycle that manifests as differences among the homeothermic states and between early and late winter hibernators. In the laboratory, this cycle occurred in advance of housing and environmental changes and observed phenotypic progression. This finding indicates that hibernating ground squirrels provide a robust model system in which to define essential components for the recruitment and the control of BAT activation. These components may provide novel targets for effective treatment of human obesity.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.G.H. and S.L.M. conception and design of research; A.G.H. performed experiments; A.G.H. and S.L.M. analyzed data; A.G.H. and S.L.M. interpreted results of experiments; A.G.H. prepared figures; A.G.H. drafted manuscript; A.G.H. and S.L.M. edited and revised manuscript; A.G.H. and S.L.M. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Elaine Epperson for pioneering the sample collection and processing methods used in this experiment. We thank Julia Weis and Elaine Epperson for help with sample processing and Katherine Grabek for helpful discussion. Philip O'Neill generated the most current version of the software used to compile the protein identification and homology data (ExtracTags v4.1). We appreciate the contribution to this research made by E. Erin Smith of the University of Colorado Denver Cancer Center Research Histology Core in preparing and labeling fixed tissues for microscopy. We are also grateful to Dr. D. Orlicky for microscopy consultations and for capturing some images from the fixed tissues. 2D gel spot picking and digesting were conducted in the University of Colorado School of Medicine Proteomics Core. This work was supported by the National Institutes of Health (R21DK095180). The Institutional Proteomics Mass Spectrometry and the Tissue Biobanking & Processing Cores are supported in part by National Institutes of Health Grants to the Colorado Clinical and Translational Science Institute (UL1-RR025780) and the University of Colorado Cancer Center (P30-CA-046934).
REFERENCES
- 1.Agrawal GK, Thelen JJ. Development of a simplified, economical polyacrylamide gel staining protocol for phosphoproteins. Proteomics 5: 4684–4688, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong C, Staples JF. The role of succinate dehydrogenase and oxaloacetate in metabolic suppression during hibernation and arousal. J Comp Physiol B 180: 775–783, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57: 289–300, 1995. [Google Scholar]
- 4.Boyer BB, Barnes BM. Molecular and metabolic aspects of mammalian hibernation. Bioscience 49: 713–724, 1999. [Google Scholar]
- 5.Breiman L. Random Forests. Mach Learn 45: 5–32, 2001. [Google Scholar]
- 6.Brown JCL, Chung DJ, Cooper AN, Staples JF. Regulation of succinate-fuelled mitochondrial respiration in liver and skeletal muscle of hibernating thirteen-lined ground squirrels. J Exp Biol 216: 1736–1743, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Buck MJ, Squire TL, Andrews MT. Coordinate expression of the PDK4 gene: a means of regulating fuel selection in a hibernating mammal. Physiol Genomics 8: 5–13, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Burlington RF, Therriault DG, Hubbard RW. Lipid changes in isolated brown fat cells from hibernating and aroused thirteen-lined ground squirrels (Citellus tridecemlineatus). Comp Biochem Physiol 29: 431–437, 1969. [DOI] [PubMed] [Google Scholar]
- 9.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83: 1153–1181, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Chaffee RRJ, Pengelley ET, Allen JR, Smith RE. Biochemistry of brown fat and liver of hibernating golden-mantled ground squirrels (Citellus lateralis). Can J Physiol Pharmacol 44: 217–223, 1966. [DOI] [PubMed] [Google Scholar]
- 12.Cinti S. The role of brown adipose tissue in human obesity. Nutr Metab Cardiovasc Dis 16: 569–574, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Coe NR, Simpson MA, Bernlohr DA. Targeted disruption of the adipocyte lipid-binding protein (aP2 protein) gene impairs fat cell lipolysis and increases cellular fatty acid levels. J Lipid Res 40: 967–972, 1999. [PubMed] [Google Scholar]
- 14.Dekker PJT, Martin F, Maarse AC, Bomer U, Muller H, Guiard B, Meijer M, Rassow J, Pfanner N. The Tim core complex defines the number of mitochondrial translocation contact sites and can hold arrested preproteins in the absence of matrix Hsp70-Tim44. EMBO J 16: 5408–5419, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz-Uriarte R. varSelRF: Variable selection using random forests. R News http://CRAN.R-project.org/package=varSelRF2010.
- 16.Eddy SF, Storey KB. Up-regulation of fatty acid-binding proteins during hibernation in the little brown bat, Myotis lucifugus. Biochim Biophys Acta 1676: 63–70, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Epperson LE, Rose JC, Carey HV, Martin SL. Seasonal proteomic changes reveal molecular adaptations to preserve and replenish liver proteins during ground squirrel hibernation. Am J Physiol Regul Integr Comp Physiol 298: R329–R340, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151: 400–413, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gollub J, Sherlock G. Clustering microarray data. Methods Enzymol 411: 194–213, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Grabek KR, Karimpour-Fard A, Epperson LE, Hindle AG, Hunter LE, Martin SL. Multistate proteomics analysis reveals novel strategies used by a hibernator to precondition the heart and conserve ATP for winter heterothermy. Physiol Genomics 43: 1263–1275, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hindle AG, Karimpour-Fard A, Epperson LE, Hunter LE, Martin SL. Skeletal muscle proteomics: carbohydrate metabolism oscillates with seasonal and torpor-arousal physiology of hibernation. Am J Physiol Regul Integr Comp Physiol 301: R1440–R1452, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hindle AG, Martin SL. Cytoskeletal regulation dominates temperature-sensitive proteomic changes of hibernation in forebrain of 13-lined ground squirrels. PLos One 8: e71627, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hittel D, Storey K. Differential expression of adipose- and heart-type fatty acid binding proteins in hibernating ground squirrels. Biochim Biophys Acta 1522: 238–243, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Hittel D, Storey KB. The translation state of differentially expressed mRNAs in the hibernating 13-lined ground squirrel (Spermophilus tridecemlineatus). Arch Biochem Biophys 401: 244–254, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman RA, Hester RJ, Towns C. Effect of light and temperature on the endocrine system of the golden hamster (Mesocricetus auratus Waterhouse). Comp Biochem Physiol 15: 525–533, 1965. [DOI] [PubMed] [Google Scholar]
- 26.Holness MJ, Sugden MC. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans 31: 1143–1151, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Houšték J, Cannon B, Lindberg O. Glycerol-3-phosphate shuttle and its function in intermediary metabolism of hamster brown-adipose tissue. Eur J Biochem 54: 11–18, 1975. [DOI] [PubMed] [Google Scholar]
- 28.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protocols 4: 44–57, 2009. [DOI] [PubMed] [Google Scholar]
- 29.Jani A, Orlicky DJ, Karimpour-Fard A, Epperson LE, Russell RL, Hunter LE, Martin SL. Kidney proteome changes provide evidence for a dynamic metabolism and regional redistribution of plasma proteins during torpor-arousal cycles of hibernation. Physiol Genomics 44: 717–727, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Körtner G, Geiser F. The temporal organization of daily torpor and hibernation: circadian and carcannual rhythms. Chronobiol Int 17: 103–128, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Liaw A, Wiener M. Classification and regression by Random Forest. R News 2: 18–22, 2002. [Google Scholar]
- 32.Lidell ME, Enerback S. Brown adipose tissue-a new role in humans? Nat Rev Endocrinol 6: 319–325, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Malatesta M, Battistelli S, Rocchi MB, Zancanaro C, Fakan S, Gazzanelli G. Fine structural modifications of liver, pancreas and brown adipose tissue mitochondria from hibernating, arousing and euthermic dormice. Cell Biol Int 25: 131–138, 2001. [DOI] [PubMed] [Google Scholar]
- 34.McKee G, Andrews JF. Brown adipose tissue lipid is the main source of energy during arousal of the golden hamster (Mesocricetus auratus). Comp Biochem Physiol A Mol Integr Physiol 96: 485–488, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Milner RE, Wang LC, Trayhurn P. Brown fat thermogenesis during hibernation and arousal in Richardson's ground squirrel. Am J Physiol Regul Integr Comp Physiol 256: R42–R48, 1989. [DOI] [PubMed] [Google Scholar]
- 36.Morrison DK. The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol 19: 16–23, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nedergaard J, Bengtsson T, Cannon B. Three years with adult human brown adipose tissue. Ann NY Acad Sci 1212: E20–E36, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Nedergaard J, Cannon B. Preferential utilization of brown adipose tissue lipids during arousal from hibernation in hamsters. Am J Physiol Regul Integr Comp Physiol 247: R506–R512, 1984. [DOI] [PubMed] [Google Scholar]
- 39.Russell RL, O'Neill PH, Epperson LE, Martin SL. Extensive use of torpor in 13-lined ground squirrels in the fall prior to cold exposure. J Comp Physiol B 180: 1165–1172, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saldanha AJ. Java Treeview-extensible visualization of microarray data. Bioinformatics 20: 3246–3248, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Smith RE, Horwitz BA. Brown fat and thermogenesis. Physiol Rev 49: 330–425, 1969. [DOI] [PubMed] [Google Scholar]
- 42.R Core Development Team. R: A Language and Environment for Statistical Computing. www.R-Project.org.
- 43.van Breukelen F, Martin SL. Molecular adaptations in mammalian hibernators: unique adaptations or generalized responses? J Appl Physiol 92: 2640–2647, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Vergnes L, Chin R, Young SG, Reue K. Heart-type fatty acid-binding protein is essential for efficient brown adipose tissue fatty acid oxidation and cold tolerance. J Biol Chem 286: 380–390, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev 27: 234–250, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamashita H, Wang Z, Wang Y, Segawa M, Kusudo T, Kontani Y. Induction of fatty acid-binding protein 3 in brown adipose tissue correlates with increased demand for adaptive thermogenesis in rodents. Biochem Biophys Res Commun 377: 632–635, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Yan J, Barnes BM, Kohl F, Marr TG. Modulation of gene expression in hibernating arctic ground squirrels. Physiol Genomics 32: 170–181, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Yan J, Burman A, Nichols C, Alila L, Showe LC, Showe MK, Boyer BB, Barnes BM, Marr TG. Detection of differential gene expression in brown adipose tissue of hibernating arctic ground squirrels with mouse microarrays. Physiol Genomics 25: 346–353, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.