Abstract
Exercise-induced increase in skeletal muscle GLUT4 expression is associated with hyperacetylation of histone H3 within a 350-bp DNA region surrounding the myocyte enhancer factor 2 (MEF2) element on the Glut4 promoter and increased binding of MEF2A. Previous studies have hypothesized that the increase in MEF2A binding is a result of improved accessibility of this DNA segment. Here, we investigated the impact of fructose consumption on exercise-induced GLUT4 adaptive response and directly measured the accessibility of the above segment to nucleases. Male Wistar rats (n = 30) were fed standard chow or chow + 10% fructose or maltodextrin drinks ad libitum for 13 days. In the last 6 days five animals per group performed 3 × 17-min bouts of intermittent swimming daily and five remained untrained. Triceps muscles were harvested and used to measure 1) GLUT4, pAMPK, and HDAC5 contents by Western blot, 2) accessibility of the DNA segment from intact nuclei using nuclease accessibility assays, 3) acetylation level of histone H3 and bound MEF2A by ChIP assays, and 4) glycogen content. Swim training increased GLUT4 content by ∼66% (P < 0.05) but fructose and maltodextrin feeding suppressed the adaptation. Accessibility of the DNA region to MNase and DNase I was significantly increased by swimming (∼2.75- and 5.75-fold, respectively) but was also suppressed in trained rats that consumed fructose or maltodextrin. Histone H3 acetylation and MEF2A binding paralleled the accessibility pattern. These findings indicate that both fructose and maltodextrin modulate the GLUT4 adaptive response to exercise by mechanisms involving chromatin remodeling at the Glut4 promoter.
Keywords: fructose, GLUT4, accessibility, MEF2A binding, exercise, histone acetylation
one of the most documented effects of exercise on skeletal muscle is the adaptive increase in GLUT4, the dominant glucose transporter, during exercise (11, 38, 44). A single prolonged bout of exercise has been reported to cause up to a twofold increase in its content 18 h postexercise (42), resulting in a marked increase in glucose transport responsiveness to insulin and exercise (42, 43). As a consequence of this adaptation, muscle glycogen storage following glycogen-depleting exercise occurs more rapidly and to a greater extent in the trained than in the untrained state (12, 14, 32). However, little is known about the GLUT4 adaptive response to exercise in animals that are allowed to consume large amounts of fructose. This information is essential, because athletes and the general public are often advised to consume energy drinks that contain substantial amounts of fructose before, during, or after exercise (7, 17). A few studies have shown that nutrient availability modulates many acute and chronic adaptations to exercise by altering the activities of exercise-responsive genes (2, 13).
GLUT4 expression in response to exercise is mediated by myocyte enhancer factor 2 (MEF2)A and -D and GLUT4 enhancer factor (GEF) (4, 28), which bind to conserved DNA sequences on the Glut4 promoter to initiate transcription (28). The binding of these factors is dependent on chromatin structure in the region containing their DNA binding sequences. It is known that muscle contraction during exercise activates AMP-activated protein kinase (AMPK) (28, 33) and Ca2+/calmodulin-dependent protein kinase II (CaMKII) (16, 33–35, 46), which increase the activity of histone acetyltransferases (HATs) that acetylate histone tails within nucleosomes of the Glut4 promoter, resulting in more relaxed DNA-histone interactions (27, 29). Such remodeling of chromatin is thought to promote transcription by enhancing accessibility of binding domains to transcription factors (28). Recent evidence indicates that fructose consumption also influences the expression of some genes via histone modifications (8, 51). Whether or not fructose influences chromatin remodeling in the region surrounding the MEF2 binding domain on the Glut4 gene and affects MEF2A binding in response to exercise has not yet been studied.
Therefore, the present study was conducted to investigate the effects of ad libitum consumption of a 10% fructose solution (with caloric density comparable to that of common sugar-sweetened carbonated beverages) on the GLUT4 adaptive response to high-intensity exercise training in rat skeletal muscle. Specifically, the effects on GLUT4 expression, histone H3 acetylation, and accessibility of a 350-bp DNA segment of the Glut4 promoter containing the MEF2 binding domain, and bound Glut4 MEF2A were investigated. In the past, accessibility of the MEF2 domain on the Glut4 gene in response to exercise has been indirectly inferred from quantification of histone acetylation using chromatin immunoprecipitation (ChIP) assays (31, 47). In the present study, we describe for the first time the use of the nuclease digestion assay to directly assess accessibility of DNA segments of interest. We report that ad libitum consumption of fructose suppresses the GLUT4 adaptive response to exercise.
MATERIALS AND METHODS
Materials.
Wistar rats were purchased from the University of Cape Town Animal Unit (Cape Town, South Africa). Crystalline fructose and maltodextrin powder were purchased from Health Connection foods (Cape Town, South Africa). Pentobarbital sodium (Euthapent) was supplied by Kyron Laboratories (Johannesburg, South Africa). Micrococcal nuclease (MNase) and DNase I were purchased from New England Biolabs (Ipswich, MA). PCR primers were synthesized at the Molecular and Cellular Biology Laboratory of the University of Cape Town. Taq DNA polymerase was purchased from Solis Biodyne (Tartu, Estonia). Other reagents for PCR were from Thermo Scientific (Waltham, MA). Antibodies against GLUT4, HDAC5, AMPKα1/2, pAMPKα1/2Th172, and α-tubulin were obtained from Abcam (Cambridge, MA). Polyclonal HRP-conjugated goat anti-rabbit secondary antibody was supplied by Dako (Carpinteria, CA). Polyvinylidene difluoride (PVDF) was purchased from Amersham (Buckinghamshire, UK). Enhanced chemiluminescence (ECL) assay kit was from Thermo Scientific (Rockford, IL), and photographic film was obtained from Kodak (Rochester, NY). Complete protease inhibitors were from Roche Diagnostics (Randburg, South Africa), and all other reagents for Western blot were procured from Sigma-Aldrich (St. Louis, MO). The ChIP assay kit was purchased from Millipore (Billerica, MA). Histone H3 (Lys9/Lys14) and MEF2A antibodies for ChIP assay were obtained from Cell Signaling (Danvers, MA) and Santa Cruz Biotechnology (Dallas, TX), respectively. UN-SCAN-IT software was from Silk Scientific (Orem, UT).
Animal care.
Ninety-day-old male Wistar rats (n = 30), weighing 250–300 g, were used in the study, which was approved by the Animal Research Ethics Committee of the University of Cape Town. Animals were housed individually in cages in an environmentally controlled room (temperature: 25 ± 1°C, humidity 40–60%) with a set 12:12-h light-dark cycle. Experiments were conducted at the University's Animal Facility.
Experimental groups and dietary treatments.
On the day of arrival in the research facility, rats were assigned to one of six treatment groups (n = 5 per group) and familiarized to human handling for 4 days. All rats received standard rat chow and water ad libitum during the experiment. Ten rats were given 10% fructose, another 10 received 10% maltodextrin, and the remainder had only water. Five rats from each of these treatment groups were subjected to the swim protocol. The experimental groups were, therefore: Untrained (UT), Trained (TR), Fructose Untrained (FUT), Fructose Trained (FTR), Maltodextrin Untrained (MUT) and Maltodextrin Trained (MTR). At 8:00–9:00 AM daily, each animal was provided with 200 g of chow and 200 ml of water, and in addition some were supplied with 250 ml of 10% fructose or 10% maltodextrin solution in water. Maltodextrin, a glucose polymer, was used as a control for calories, since it is isocaloric (by wt) with fructose. The amount of water, chow, and carbohydrate (CHO) consumed per day was calculated at the same time the next day by subtracting the leftover weights from the amount initially provided. To ensure accurate chow intake calculations, all pieces of chow that were found within the bedding were collected and weighed together with the leftover chow. The decision to use 10% fructose or 10% maltodextrin (wt/vol in tap water) for these experiments was to ensure that the caloric density of these solutions was comparable to the caloric density of commercial sugar-sweetened carbonated beverages, which is ∼0.4 kcal/ml.
Exercise protocol.
After 4 days of familiarization to handling, rats in the swimming groups were introduced to swimming in a deep water bath using a protocol previously described (46). Briefly, rats performed two bouts of 17-min swims on day 5, and an additional bout was added each subsequent day until day 7. At the end of this familiarization period, these rats were able to complete four bouts of 17-min swims without any sign of distress. During the training phase (days 8–13), these animals swam 3 × 17-min bouts carrying a load equivalent to 5% of body weight. The load was securely attached to the base of the tail. Throughout the training period, rats swam in water maintained at 31 ± 1°C and were allowed 3 min of rest between exercise bouts, during which they were dried and kept warm using clean and dry towels.
Muscle harvesting and blood sampling.
On day 13, chow, fructose, and maltodextrin solutions were removed from all cages at 1800, leaving only water. At 0800–0900 the next morning, rats were weighed and anesthetized with an intraperitoneal injection of 50 mg/kg pentobarbital sodium. Triceps muscles were excised, rapidly frozen in liquid nitrogen, and stored frozen at −87°C until analyzed. Triceps muscle was used because it is involved in exercise and provides sufficient tissue for all the assays in the study. Approximately 5 ml of mixed arteriovenous blood was obtained by cardiac puncture and aliquoted into test tubes to determine free fatty acid, insulin, and glucose concentrations. Blood for assessment of insulin and nonesterified fatty acid (NEFA) concentrations was collected in tubes containing a clot activator, while blood for glucose determinations was collected in tubes containing fluoride oxalate, a glycolytic inhibitor. Immediately after blood collection, tubes were inverted a few times and kept on ice before centrifugation at 3,000 g for 15 min. Serum was collected and kept at −87°C until analysis.
Measurement of serum glucose, insulin, and free fatty acids.
Fasting serum glucose was measured using the YSI 2300 Glucose-Lactate Analyzer (YSI Ohio) according to the manufacturer's protocol. Insulin was quantified using an Advia Centaur XP Immunoassay Analyzer (Siemens, Erlangen, Germany). NEFA were quantified using the Roche free fatty acids half micro test colorimetric assay (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions.
Measurement of muscle glycogen.
Glycogen content was measured as described by Passonneau and Lauderdale (37). Briefly, ∼20 mg of frozen muscle was weighed and digested in 200 μl of 40% KOH and heated for 30 min at 95°C. Glycogen was precipitated by incubation at 4°C overnight in 800 μl of absolute ethanol. Glycogen pellets were hydrolyzed to glucose equivalents by incubation in 2 N HCl for 3 h at 95°C, and pH was neutralized with 100 μl of 0.2 M Tris·HCl (pH 7.5) and 150 μl of 2 M sodium hydroxide (NaOH). Glucose concentration was determined using a YSI 2300 glucose analyzer, and glycogen concentration was expressed as glucosyl units per kilogram.
Western blotting.
Approximately 50 mg of frozen muscle was ground to a powder in liquid nitrogen, transferred to a microtube, and mixed with 1 ml of chilled HES buffer (20 mM HEPES, 1 mM EDTA, and 250 mM sucrose containing 1× Complete protease inhibitors). Samples were homogenized on ice for 30 s using a Teflon tip dounce homogenizer attached to a drill press at slow speed. Muscle homogenates were centrifuged at 1,000 g for 10 min (4°C), and the protein concentration of the supernatants was determined using the Bradford assay (6). Twenty micrograms of total protein was used in Western blots to determine the content of GLUT4, pAMPKα1/2Th172, total AMPKα1/2, HDAC5, and α-tubulin as previously described (36). Membranes were blocked and incubated with antibodies against these proteins, as recommended by manufacturers and again incubated with the appropriate HRP-conjugated secondary antibody. Proteins were detected using enhanced chemiluminescence and exposed to X-ray film. After the film was scanned, the intensities of bands were quantified using UN-SCAN-IT gel 6.1 software. Signal intensities, representing the protein content, were normalized to α-tubulin, or AMPK for the case of pAMPK, and expressed relative to the signal from a control sample in each blot.
Isolation of nuclei.
Approximately 75 mg of triceps muscle was homogenized in 850 μl of cold homogenization buffer (10 mM Tris, 1 mM EDTA, 5 mM MgCl2 and 1.4 M sucrose) on ice using a hand-held glass tissue homogenizer with a pestle clearance of 0.7 mm for 25 turns. The resulting homogenate was centrifuged for 10 min at 600 g (4°C), and the pellet was resuspended with fresh 850 μl of cold homogenization buffer by gentle pipetting. The suspension was filtered through one layer of cheesecloth and the filtrate centrifuged again at 600 g (4°C) for 5 min. After discarding of the supernatant, the pellet was resuspended in 300 μl of cold homogenization buffer, and after centrifugation the final pellet was resuspended in 200 μl of nuclei suspension buffer (18.5% glycerol, 11.1 mM MgCl2, 14.8 mM HEPES, 0.33 mM NaCl, 75 mM KCl and 1× RCPI). Protein concentration of the nuclei suspension was first determined using the Bradford assay and the DNA concentration estimated by multiplying the protein concentration by a factor of 0.17 (9).
Nuclease digestion.
Nuclei containing ∼80 μg of DNA were diluted with either MNase digestion buffer (50 mM Tris·HCl, 5 mM CaCl2, pH 7.9) or DNase I digestion buffer (10 mM Tris·HCl, 2.5 mM MgCl2, 0.5 mM CaCl2, pH 7.6) to a final volume of 480 μl and incubated for 1 min at 37°C. An aliquot of 80 μl (nonnuclease control) was removed and added to a tube containing 8 μl of 250 mM EDTA and kept on ice. MNase or DNase I was added to the corresponding tubes to a concentration of 0.25 and 0.3 U/ml, respectively, and allowed to digest for 0, 2, 4, 6, or 10 min. At each of these time points, the tube was mixed, and 80 μl of the digest was removed and transferred to a fresh tube containing 8 μl of 250 mM EDTA and the volume brought to 200 μl with distilled water. At the end of each digestion time point, NaCl was added to each tube to a concentration of 0.4 mM and incubated on ice for 10 min to dissociate nuclear proteins. DNA was extracted using phenol-chloroform-isoamyl alcohol (25:24:1), precipitated with 70% ethanol, air-dried, suspended in 20 μl of sterile distilled water, and amplified by quantitative PCR.
ChIP assays.
ChIP assays were performed on triceps muscle for the assessment of 1) the level of acetylation of histone H3 on Lys9 and Lys14 within a 350-bp DNA containing the MEF2 binding site on the Glut4 gene promoter and 2) the binding of MEF2A to its binding site on the Glut4 gene promoter. Approximately 100 mg frozen triceps muscle was crushed to a fine power in liquid nitrogen and cross-linked using 1% formaldehyde in phosphate-buffered saline (PBS), pH 7.40, for 10 min at room temperature. Glycine was added to a concentration of 0.125 M followed by incubation with rotation for 5 min at room temperature. Samples were centrifuged at 2,000 g for 5 min, and pellets were washed twice in chilled PBS and then lysed on ice in 500 μl of SDS lysis buffer (1% SDS, 10 mM EDTA, 1× RCPI, 50 mM Tris, and pH 8.1) for 10 min on ice. Chromatin was sheared to fragments of ∼300–1,000 bp by 7 × 15-s bursts of sonication on ice at 33% of maximal power with a 1-min break between bursts. Chromatin was then separated from cell debris by centrifugation at 2,000 g for 15 min. Aliquots of the supernatant were used to 1) estimate total protein concentration using the Bradford assay and 2) isolate DNA for verifying DNA fragment size using agarose gel electrophoresis. One hundred microliters of this supernatant (∼200 μg protein) was diluted 10-fold in ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris·HCl, pH 8.1, and 167 mM NaCl) and precleared of endogenous immunoglobulins by incubating with 40 μl (∼80 μg protein) salmon sperm DNA-protein A-agarose for 1 h at 4°C. Following incubation, agarose beads were pelleted by centrifugation at 1,000 g for 1 min at 4°C. The resultant supernatant, referred to as input, was incubated with 25 μl of MEF2A antibodies or 5 μl of acetyl histone H3 (Lys9/Lys14) for 36–48 h at 4°C with gentle rotation. Antibody-protein-DNA complexes were precipitated using 60 μl of protein A-agarose (∼80 μg protein) for 1 h at 4°C. A 30-μl aliquot of the input was also saved and used as control for the ChIP assay. Reactions without antibody or with a nonspecific antibody (anti-rabbit IgG) were run in parallel to control for nonspecific binding of chromatin to the beads. Precipitated complexes were then washed in kit buffers (Millipore) and eluted in a buffer consisting of 1% SDS and 0.1 M NaHCO3 and reverse cross-linked by incubation at 65°C for 6 h after addition of 0.3 M NaCl. Proteins in the samples were digested with 0.7 U/ml proteinase K in 0.01 M EDTA and 0.08 M Tris·HCl (pH 6.5) for 1 h at 45°C. The coimmunoprecipitated DNA was extracted using a 25:24:1 phenol-chloroform-isoamyl solution, precipitated with 70% ethanol, air-dried, suspended in 20 μl of sterile distilled water, and analyzed by quantitative PCR.
Quantitative polymerase chain reaction.
DNA from endonuclease digestion experiments and from ChIP assays were amplified by quantitative PCR. A 350-bp fragment corresponding to nucleotides −284 to −634 of the rat Glut4 promoter containing the MEF2 binding site was amplified by PCR using the following primers (+ve primers): 5′-GACACGGTTCTCAGACACACG-3′ (forward) and 5′-CTGAGAGGTGGAAGAGGAGG-3′ (reverse). As a negative control for nonspecific binding of chromatin to antibodies, the following pair of primers (−ve primers) was used: 5′-GACGGACACCTTCTCTCTTAGC-3′ (forward) and 5′-CCACAGCCTAGCCACAACAC-3′ (reverse). These primers amplify a 283-bp fragment corresponding to nucleotides +4,620 to +4,903 relative to the start of transcription, which does not contain the MEF2 binding sequence. DNA from 30 μl of input sample that was not subjected to ChIP but was reverse cross-linked and purified as described above, was also amplified using the same set of primers. PCR was performed in 30-μl reactions containing 0.4 μM forward and reverse primers, 2.5 mM MgCl2, 1× reaction buffer, 0.5 U Taq DNA polymerase, and 2 mM deoxynucleotide triphosphates (dNTPs). The PCR program started with an initial denaturation step of 10 min at 94°C followed by 35 cycles, each consisting of 30 s of denaturing at 94°C, 30 s of annealing at 56°C, and 45 s of extension at 72°C. A final extension was carried on for 5 min at 72°C. Following PCR, the products were stained with SYBR Gold, resolved on a 2% agarose gel by electrophoresis, and photographed, and the densities of the bands were quantified.
Statistics.
Data are presented as means ± SD. Statistical differences between all treatments were determined using one-way ANOVA STATISTICA version 10 software (Statsoft). Significance was accepted at P < 0.05. A Fisher's least significant difference test was used for post hoc analysis when ANOVA showed a significant difference.
RESULTS
Weight gain and food consumption.
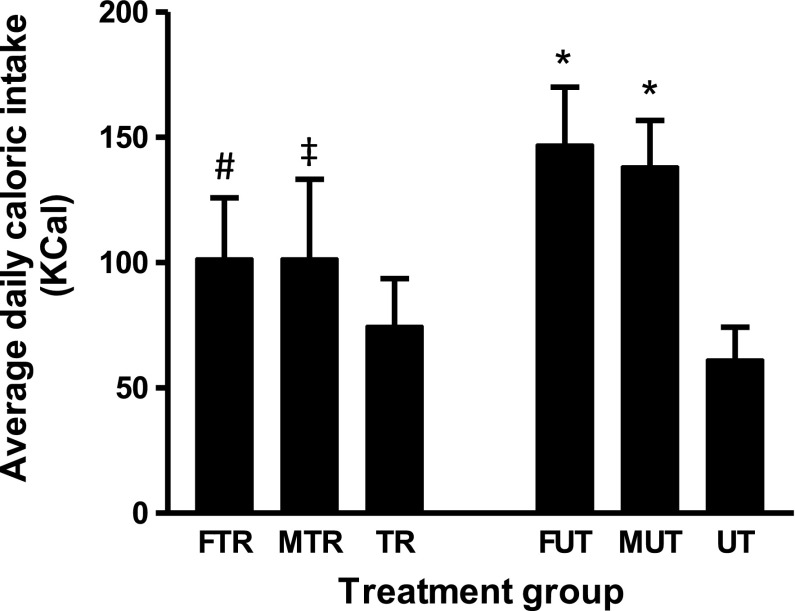
Table 1 shows the initial and final body weights of rats from all experimental groups. As expected, increases in body weight were observed in all groups over the 13 days of the experiment, but the weight gain was modulated by the different treatments. The UT group increased their body weight by 17 ± 4% over 13 days, which is consistent with normal growth of Wistar rats. The FUT group had a significantly greater percent weight gain (23 ± 2%, P < 0.05) compared with UT controls. They also consumed more calories (147 ± 22 kcal/day) than the UT controls (62 ± 8 kcal/day, P < 0.05; Fig. 1). In contrast, the weight gain of MUT was not different from UT, although they also consumed more calories, indicating that fructose, and not maltodextrin, exaggerates weight gain. Rats that were exercise trained and had access to either fructose (FTR) or maltodextrin (MTR) consumed fewer calories (Fig. 1, P < 0.05 vs. FUT; P < 0.05 vs. MUT) and experienced significantly less weight gain than their corresponding untrained counterparts (Table 1; P < 0.05 vs. FUT or P < 0.05 vs. MUT, respectively).
Table 1.
Body weight measurements
| Variables | UT | TR | FUT | FTR | MUT | MTR |
|---|---|---|---|---|---|---|
| Baseline body wt | 286 ± 7 | 258 ± 6 | 278 ± 14 | 275 ± 14 | 281 ± 6 | 280 ± 16 |
| Final body wt | 335 ± 10 | 287 ± 18 | 343 ± 19 | 320 ± 24 | 330 ± 16 | 312 ± 21 |
| Body wt gain | 48 ± 6 | 29 ± 13 | 65 ± 5* | 45 ± 11# | 48 ± 9 | 33 ± 22‡ |
| %Body wt gain | 17 ± 4 | 13 ± 1 | 23 ± 2* | 16 ± 10# | 17 ± 4 | 12 ± 8‡ |
Data are presented as means ± SD in grams; n = 5 per group. UT, untrained; TR, trained; F, fructose fed; M, maltodextrin fed. Baseline weight, final weight, and %weight gain of rats in treatment groups.
P < 0.05 vs. UT;
P < 0.05 vs. FUT;
P < 0.05 vs. MUT.
Fig. 1.
Average daily caloric intake. Data represent daily average caloric intake of rats in the various treatment groups. UT, untrained; TR, trained, F, fructose fed; M, maltodextrin fed. Data are presented as means ± SD; n = 5 per group. *P < 0.05 vs. UT; #P < 0.05 vs. FUT; ‡P < 0.05 vs. MUT.
Serum glucose, insulin, and NEFA.
The fasting levels of serum glucose, insulin, and NEFA are presented in Table 2. There were no differences in fasting glucose and insulin concentrations between the groups. Circulating NEFA concentrations were significantly lower in TR vs. UT (P < 0.05) only. Among the exercised groups, NEFA was ∼1.7 and twofold higher in FTR and MTR, respectively, compared with TR (P < 0.05).
Table 2.
Fasting Serum glucose, insulin, and NEFA
| Variables | UT | TR | FUT | FTR | MUT | MTR |
|---|---|---|---|---|---|---|
| Glucose (mmol/l) | 6.6 ± 1.4 | 6.3 ± 1.3 | 6.4 ± 0.81 | 7.00 ± 1.5 | 6.3 ± 1.5 | 4.9 ± 1.2 |
| Insulin (pmol/l) | 122 ± 24 | 156 ± 14 | 124 ± 37 | 125 ± 37 | 163 ± 28 | 127 ± 10 |
| NEFA (mmol/l) | 0.20 ± 0.04 | 0.11 ± 0.02* | 0.20 ± 0.08 | 0.18 ± 0.12# | 0.24 ± 0.03 | 0.24 ± 0.05# |
Data are expressed as mean ± SD; n = 5 per group. Samples were taken from fasted animals on the last day of the experiment.
P < 0.05 vs. UT;
P < 0.05 vs. TR.
Fructose and maltodextrin suppress the training-induced increase in GLUT4 protein content in skeletal muscle.
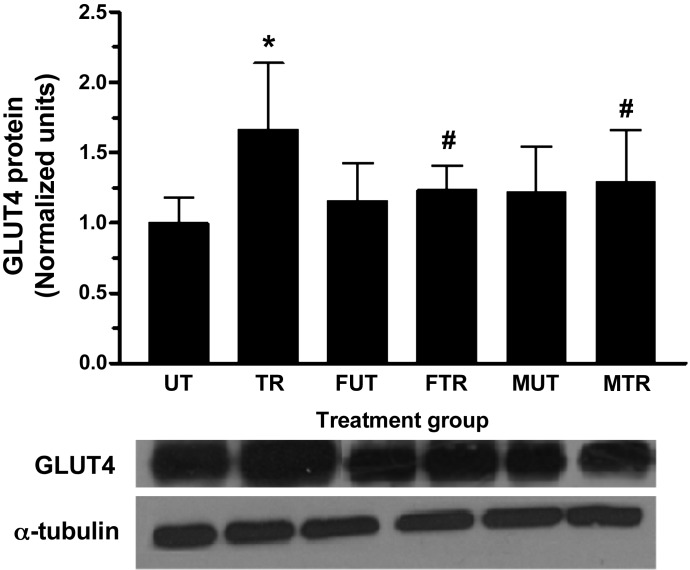
As expected, swim training significantly increased total GLUT4 content by ∼66% in triceps muscle (Fig. 2; P < 0.01 vs. UT). No change in GLUT4 content was noted as a result of fructose or maltodextrin feeding in untrained rats (P > 0.05 for FUT vs. UT and MUT vs. UT). GLUT4 content in the FTR was not significantly different from FUT, and neither was GLUT4 different between MTR and MUT. However, the contents of GLUT4 in FTR and MTR were significantly lower compared with TR (Fig. 2; P < 0.01 vs. TR). Collectively, these data indicate that both fructose and maltodextrin suppressed the exercise-induced adaptive increase in GLUT4.
Fig. 2.
Fructose and maltodextrin consumption suppress exercise-induced increase in skeletal muscle GLUT4. Total cellular GLUT4 content was determined in isolated triceps muscle from all treatment groups by Western blot. Representative GLUT4 and α-tubulin blots are shown. Data are presented as means ± SD; n = 5 per group. *P < 0.01 vs. UT; #P < 0.01 vs. TR.
Fructose attenuates exercise-induced histone H3 hyperacetylation and MEF2A binding on the Glut4 promoter.
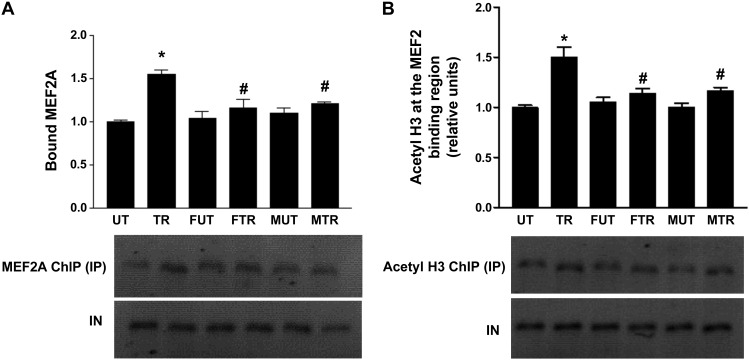
Previous studies have shown that the increase in GLUT4 expression in response to exercise training is mediated by hyperacetylation of histone H3 in the region surrounding the MEF2 binding domain on the Glut4 promoter and increased MEF2A binding (46). ChIP assays were therefore performed to assess whether fructose exerts its modulatory effects on GLUT4 expression under exercise by altering histone H3 acetylation and MEF2A binding in this region of the Glut4 gene. As expected, bound MEF2A was significantly increased (∼54%) in trained compared with untrained rats (Fig. 3A; P < 0.01 vs. UT). In contrast, the increase in MEF2A binding due to training was significantly attenuated in rats that consumed fructose or maltodextrin drinks (FTR vs. FUT and MTR vs. MUT, P > 0.05). However, MEF2A binding was significantly lower in both FRT and MRT than in TR (P < 0.05 vs. TR). Fructose or maltodextrin feeding in untrained rats did not alter MEF2A binding (FUT vs. UT and MUT vs. UT).
Fig. 3.
Fructose and maltodextrin consumption attenuate increases in exercise-induced histone acetylation and MEF2 binding at the Glut4 promoter. Chromatin immunoprecipitation (ChIP) assays were performed on triceps muscle from rats using antibodies against MEF2A (A) and acetyl histone H3 (B) as described in materials and methods. Bars represent the ratio of immunoprecipitated DNA (IP) to input DNA (IN) normalized to UT, from the various treatment groups. Gels show PCR products from chromatin using primers that amplify the MEF2 site in the Glut4 promoter (described in materials and methods). Data are presented as means ± SD; n = 5 per group. *P < 0.05 vs. UT; #P < 0.05 vs. TR.
Acetylation of histone H3 in the region containing the MEF2 binding domain within the Glut4 gene promoter followed a similar trend to that of MEF2A binding, as shown in Fig. 3B. Fructose did not alter the level of histone H3 acetylation in untrained rats (FUT vs. UT) but attenuated the ∼1.5-fold increase in histone H3 acetylation induced by exercise (P < 0.001 vs. UT) to ∼1.08-fold (FTR vs. FUT, P > 0.05). Similar to fructose, maltodextrin feeding did not change the level of histone H3 acetylation in untrained rats (MUT vs. UT) but attenuated the increase in histone H3 acetylation triggered by exercise such that acetylation in MTR was not significantly different from MUT (MRT vs. MUT, P > 0.05). Consequently, histone H3 acetylation was significantly lower in FTR and MTR than in TR (P < 0.05 vs. TR).
Nuclease accessibility of the MEF2 binding domain on the Glut4 gene.
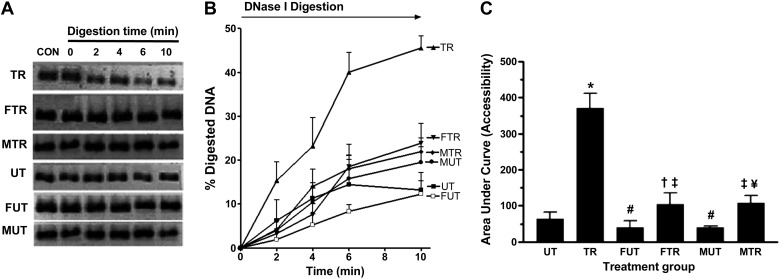
After investigating histone acetylation states and MEF2A binding, we proceeded to examine the accessibility of a 350-bp region of the Glut4 promoter containing the MEF2 binding domain. Accessibility was measured by determining the rate of digestion of the above segment by MNase and DNase I in intact nuclei that were isolated from triceps muscles from the treatment groups.
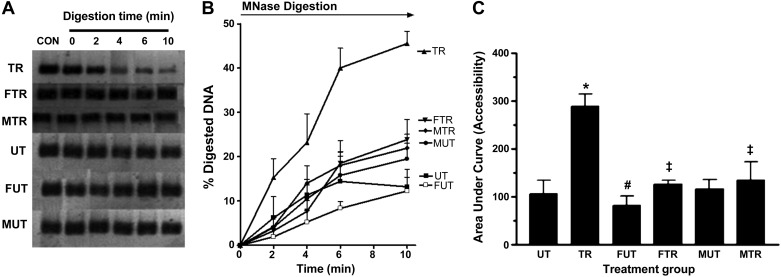
Blots in Fig. 4A were obtained by amplifying the 350-bp DNA fragment from intact nuclei after 0, 2, 4, 6, and 10 min of digestion with MNase for various treatment groups. MNase cleaves DNA preferentially in the interlinker region. The signal intensity is proportional to the amount of intact DNA template remaining at each time point. Figure 4B is a plot of the percentage of DNA that had been digested at time points 0–10 min for treatment groups. The percentage of digested DNA was calculated from the formula shown in the Fig. 4 legend. The histogram in Fig. 4C shows the area under the curve (AUC), a measure of accessibility of the interlinker segment of the 350-bp DNA after various treatments. As shown, the AUC for TR is ∼2.75-fold greater than the AUC for UT (P < 0.01), indicating that training drastically increased accessibility of the interlinker region. However, fructose suppressed the effect of training, as the increase in accessibility due to exercise is only ∼1.55-fold (FTR vs. FUT). Maltodextrin abolished the exercise effect (MTR vs. MUT).
Fig. 4.
Accessibility of interlinker DNA within the 350-bp region containing the MEF2A binding site on the Glut4 promoter. Nuclei isolated from triceps muscle were incubated with MNase for the time periods indicated. CON (Control) represents reaction without MNase. Amount of DNA present at each time point was determined by PCR using primers that amplify a 350-bp segment containing the MEF2 binding site. PCR products obtained from each time point are shown in A. Signal intensity at each time point, which corresponds to the amount of DNA present, was used to calculate %digested DNA, which was plotted against time (B). %Digested DNA at each time point was calculated as follows: %digested DNA = (I0 − In)/I0 × 100, where In = signal intensity at any time point between 0 and 10 min and I0 = signal intensity at time point 0. Area under the %digested DNA curve (AUC), a measure of accessibility, was calculated using GraphPad Prism software (v. 3.0) and plotted in C. *P < 0.01 vs. UT; #P <0.05 vs. UT; ‡P < 0.05 vs. TR; n = 5 per group.
Figure 5, A–C, shows the blots, percentage of digestion, and AUC, respectively, after digestion with DNase I for various times between 0 and 10 min. DNase I cleaves DNA preferentially in the nucleosomal region. Similarly to the interlinker region, exercise also increased the accessibility of the nucleosomal DNA within the targeted 350-bp region ∼5.75-fold (Fig. 5C, P < 0.01 vs. UT) in the absence of fructose or maltodextrin. Both carbohydrates reduced the effect of training, since the increase in accessibility is only ∼2.6-fold (P < 0.05 vs. FUT) and ∼2.70-fold (P < 0.05 vs. MUT) in rats fed fructose and maltodextrin, respectively. Fructose and maltodextrin also decreased accessibility in untrained rats (P < 0.05 vs. UT).
Fig. 5.
Accessibility of nucleosomal DNA within a 350-bp region containing the MEF2A binding site on the Glut4 promoter. Nuclei isolated from triceps muscle were incubated with DNase I for time periods indicated. CON (control) represents a reaction without added DNase I. Amount of DNA present at each time was determined by PCR using primers that amplify a 350-bp segment containing the MEF2A binding site. PCR products obtained from each time point are shown in A. Signal intensity at each time point, which corresponds to the amount of DNA present, was used to calculate %digestion, which was plotted against time (B). %Digestion at each time point was calculated as follows: %digestion = [(In − I0)/I0 × 100], where In = signal intensity at any time point between 0 and 10 min and I0 = signal intensity at time point 0. %Digestion AUC, a measure of accessibility, is shown in C, using GraphPad Prism software (v. 3.0). *P < 0.01 vs. UT; #P < 0.05 vs. UT; ‡P < 0.05 vs. TR; †P < 0.05 vs. FUT; #P < 0.05 vs. MUT.
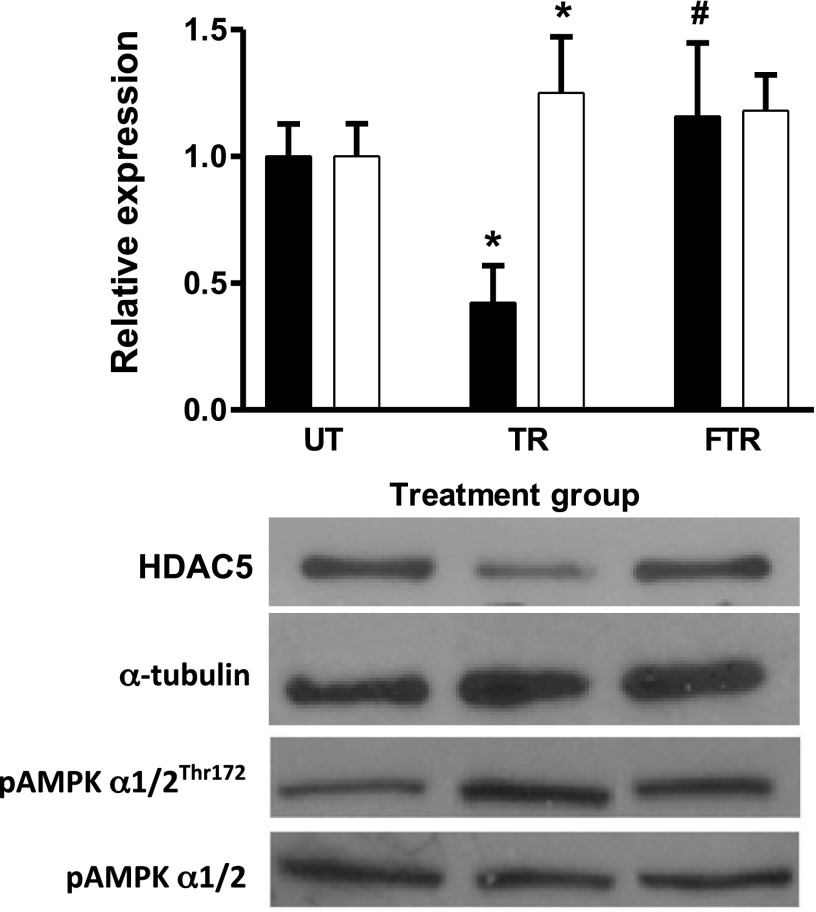
pAMPK and HDAC5.
To provide insights into the mechanisms that might be responsible for the effects of fructose and maltodextrin on the adaptive response to exercise, we measured the levels of pAMPK/AMPK and HDAC5 by Western blot. Activated AMPK (pAMPK) phosphorylates HDAC5 and causes their nuclear export and degradation, which indirectly favors increased HAT activity. Figure 6 shows that pAMPK/AMPK was significantly increased in TR but not in FTR (P < 0.05 vs. UT; open bar), indicating that fructose consumption suppressed phosphorylation of AMPK by exercise. As expected, HDAC5 was reduced by exercise training (P < 0.05 UT; solid bar), but this effect was negated by fructose consumption. These data suggest that the level of pAMPK might have played a role in the state of histone H3 acetylation and accessibility of the 350-bp GLUT4 promoter segment studied.
Fig. 6.
Fructose consumption attenuated the exercise-induced decrease in total HDAC5 content and AMPK activation. Total cellular HDAC5 and pAMPKα1/2Thr172 content were determined in isolated triceps muscle by Western blot and normalized to α-tubulin and AMPKα1/2, respectively. Representative blots are shown. Results are presented as means ± SD relative to UT; n = 5 per group. *P < 0.05 vs. UT (AMPK); #P < 0.01 vs. UT (HDAC5).
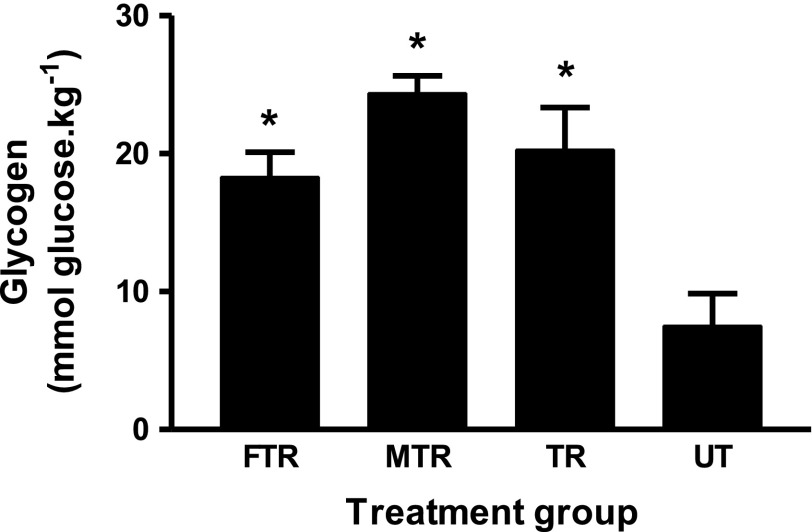
Muscle glycogen.
Previous studies (5, 48) have indicated that pAMPK content and their nuclear translocation are affected by muscle glycogen content. Therefore we also measured muscle glycogen levels in trained rats (UT, FTR, and MTR) and untrained control (UT). Figure 7 indicates that training induced more than threefold increases in glycogen levels in all groups (P < 0.01 vs. UT), and the content of glycogen in fructose-fed (FTR) and maltodextrin-fed (MTR) were not different from TR.
Fig. 7.
Muscle glycogen concentration at 24 h postexercise. Muscle glycogen content was determined as described in materials and methods; n = 5. *P < 0.01 vs. UT.
DISCUSSION
The major findings of this paper are that ad libitum consumption of a 10% fructose drink for 13 days caused significant attenuation of exercise-induced increases in GLUT4 expression and histone H3 acetylation in a 350-bp region of the Glut4 promoter containing the MEF2 binding site and MEF2A binding to the site. The study also found that exercise increased the accessibility of this region to nucleases, but fructose reduced this effect of exercise. Together, our data provide evidence that fructose might modulate the GLUT4 adaptive response to exercise by remodeling chromatin within the Glut4 promoter.
The exercise protocol used in the study caused GLUT4 protein content in triceps muscle to increase by ∼66%, consistent with previous observations (27, 29, 47). However, this adaptive response to exercise was severely attenuated in rats that consumed a 10% fructose drink ad libitum. To the best of our knowledge, this is the first report of a modulatory effect of fructose on the GLUT4 adaptive response to exercise. Since people, including athletes, are often encouraged to consume energy drinks (during and after exercise) that contain similar fructose content, our finding highlights an important starting point for further investigations of the physiological, health, and performance implications of fructose consumption.
To investigate the mechanisms that might be responsible for the modulatory effects of fructose, we examined the level of acetylation of histone H3 and MEF2A binding within a 350-bp region of the Glut4 gene in exercise-trained rats that consumed fructose or vehicle (water) ad libitum. Consistent with previous reports (27, 29, 47), exercise alone increased both histone acetylation and MEF2A binding, but consumption of fructose attenuated both effects of exercise; suggesting that the effects of fructose on exercise-induced GLUT4 expression involves remodeling of chromatin within the Glut4 promoter. We hypothesized that chromatin remodeling in this region improves accessibility of the MEF2 binding domain, which explains the increase in MEF2A binding. The HAT responsible for hyperacetylation of histones in this promoter segment has not been identified with certainty. A potential candidate that is being investigated in our laboratory is p300, the ubiquitously expressed global transcriptional coactivator that has a potent HAT domain that acetylates MEF2 transcription factors on seven lysine residues to enhance its DNA binding and transcriptional activity (1, 19, 26). In the past, accessibility of this chromatin segment has been indirectly inferred from measurements of the extent of lysine acetylation on histone proteins on nucleosomes, since acetylation of these residues neutralizes the positively charged lysine side chains and loosens their electrostatic interaction with the negatively charged DNA phosphate backbone (49) to create a more open chromatin conformation (18). In the present study, we optimized a nuclease digestion assay and used it, for the first time, to directly assess accessibility of the 350-bp region. The assay has previously been validated and used for assessment of accessibility and transcription competency of chromatin segments in plant (1, 10, 16, 17) and yeast (41) genes. Use of MNase and DNase I permitted assessment of the accessibilities of interlinker and nucleosomal DNA segments (45). We found that high-intensity intermittent exercise training (TR) increased accessibilities of both the interlinker and nucleosomal DNA regions as indicated by ∼2.75 and 5.75-fold increases [vs. untrained controls (UT)] in the rates of digestion by MNase and DNase I, respectively. Consistent with our hypothesis, fructose consumption significantly attenuated exercise-induced nuclease accessibility of this region and decreased MEF2A binding as well as GLUT4 expression. Importantly, our data show for the first time a direct correlation between the level of acetylation and accessibility of the region under investigation. The observation that MEF2A binding, histone H3 acetylation, and nuclease accessibility were increased 24 h after the last exercise session indicates that these changes are not transient responses to an exercise bout but rather long-lasting effects of training; possibly due to sustained activation of signaling molecules that regulate GLUT4 transcription. In support, Frosig et al. (10) found that a 3-wk endurance training intervention in previously untrained men increased AMPK signaling in the leg 15–55 h after the last exercise bout in contrast to an acute exercise bout in previously untrained individuals that increased AMPK activity transiently, returning to basal levels within 24 h (21, 22).
In untrained rats, accessibilities of both the nucleosomal and interlinker regions were not changed by fructose. Consistent with our hypothesis, GLUT4 protein levels, histone H3 acetylation, and MEF2A binding were also unchanged by fructose. Some earlier studies had reported that fructose consumption decreased GLUT4 expression (20, 30). We attribute that conflicting finding to the fact that those earlier studies administered a high-fructose dose for a long period of time to induce features of the metabolic syndrome (MS) in the animals. There is evidence that skeletal muscle GLUT4 expression is decreased in animals with MS (46) due to the effects of inflammatory cytokines such as TNFα and IL-6 (21, 25). In our study, the levels of fasting insulin, glucose, and free fatty acids were not elevated in all experimental groups (Table 2), suggesting the absence of MS. In light of this observation, the dramatic suppression of the GLUT4 adaptive response to exercise that we observed in this study cannot be attributed to signals arising from MS.
At this stage, the molecular mechanism responsible for the exercise-induced chromatin remodeling and modulation of GLUT4 expression by fructose are not known. However, since AMPK and CaMKII have been shown to play important roles in these events (15, 25, 28, 31, 31, 34, 47), their involvement needs to be investigated. By phosphorylating HDACs, these kinases 1) induce dissociation of MEF2A/HDAC5 complexes and promote HDAC nuclear export, 2) relieve the repression that HDACs exert on MEF2-dependent genes, and 3) promote the interaction of HATs with MEF2 factors and histones (27, 47). By the above actions, AMPK and CaMKII remodel chromatin in MEF2-dependent genes. Higaki el al. (15) and Lira et al. (24) have previously reported that activation of AMPK by exercise requires nitric oxide (NO), which is produced by the action of endothelial nitric oxide synthase (eNOS) (15) on l-arginine. Furthermore, Rahman et al. (40) showed that skeletal muscle NO is significantly reduced in rats that consumed food containing 43.6% fructose for 4 wk and that swimming exercise failed to normalize NO levels. It is therefore possible that the observed attenuation of the epigenetic modifications by exercise in fructose-fed rats might have arisen from the effects of fructose on eNOS. In support of this hypothesis, we found pAMPK in triceps muscles to be increased in the TR but not FTR group (Fig. 6); indicating that fructose suppressed exercised-induced AMPK phosphorylation. Consistent with the role of AMPK in phosphorylating HDAC5 and inducing its nuclear export and degradation, we found HDAC5 to be significantly reduced in TR but not in FTR. Potthoff et al. (39) have shown that, upon nuclear export, HDACs are degraded via a ubiquitin-mediated proteasomal pathway, and others have shown that following exercise there is a significant increase in ubiquitination of HDAC5 (27).
In this study, maltodextrin, a glucose polymer of 20–30 glucose residues that is commonly included in commercial sports drinks (23), was used as a control drink for calorie intake. It is hydrolyzed to free glucose molecules prior to being absorbed into the body (50). Figure 7, indicating daily caloric consumption by rats from TR, FTR, and MTR groups, shows that caloric consumption was more than 1.4-fold higher in the groups that were provided with carbohydrate solutions (FTR and MTR) than in the group that was not (TR). Whether or not caloric consumption per see played a role in suppressing the GLUT4 adaptive response to exercise is unclear. Steinberg et al. (48) have previously demonstrated that, during muscle contraction, AMPKα2 activity, AMPKα2 translocation to the nucleus, and GLUT4 mRNA are inversely associated with muscle glycogen content. Therefore, we measured muscle glycogen content in exercised rats to see if it could explain the suppression of the adaptive responses, but we found no such association. It is therefore unlikely that glycogen content is responsible for this modulation.
NEFA is another factor that could have mediated the observed suppression of GLUT4 adaptive response to exercise. As shown in Table 2, plasma NEFA was ∼0.7- and 2.1-fold higher in FTR and MTR, respectively, compared with TR. Armoni et al. (3) showed that patients with hyperlipidemia, including type 2 diabetics, had 30% lower GLUT4 protein levels. By using reporter studies and electromobility shift assays in H9C2 cardiomyotubes, the same authors showed that hyperlipidemia (in the form of arachidonic acid) repressed transcription of the GLUT4 promoter via binding of yet unknown nuclear proteins to the GLUT4 promoter. Importantly, these nuclear proteins bind to DNA sequences within the 350-bp region that we investigated in the current study. Suppression of exercise-induced hyperacetylation, MEF2A binding and increased accessibility that we have reported in this study would be conveniently explained if these yet unknown nuclear proteins recruit HDACs upon binding to these loci. Future studies need to investigate 1) if these nuclear proteins repress transcription by modulating histone acetylation and DNA accessibility and 2) if the above mechanism is relevant for the observed effects of fructose in skeletal muscle of exercise rats.
In summary, we have demonstrated that ad libitum consumption of 10% fructose or 10% maltodextrin drinks induces epigenetic modifications on the Glut4 promoter and suppresses the GLUT4 adaptive response to exercise. Further studies are needed to determine which signals are responsible for these effects of fructose and maltodextrin and the extent to which they affect GLUT4 transcriptional activity.
GRANTS
This research was funded by the National Research Foundation of South Africa.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
E.O.O. conception and design of research; V.G. and T.A.K. performed experiments; E.O.O., V.G., and T.A.K. analyzed data; E.O.O., V.G., and T.A.K. interpreted results of experiments; V.G. prepared figures; V.G and E.O.O. drafted the manuscript; E.O.O., V.G., and T.A.K. edited and revised manuscript; E.O.O., V.G., and T.A.K. approved the final version.
ACKNOWLEDGMENTS
We are grateful to Anita Buramu and Daniel Freitag, former members of our research group, for having helped in performing the experiments.
REFERENCES
- 1.Angelelli C, Magli A, Ferrari D, Ganassi M, Matafora V, Parise F, Razzini G, Bachi A, Ferrari S, Molinari S. Differentiation-dependent lysine 4 acetylation enhances MEF2C binding to DNA in skeletal muscle cells. Nucleic Acids Res 36: 915–928, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arkinstall MJ, Bruce CR, Clark SA, Rickards CA, Burke LM, Hawley JA. Regulation of fuel metabolism by preexercise muscle glycogen content and exercise intensity. J Appl Physiol 97: 2275–2283, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Armoni M, Harel C, Bar-Yoseph F, Milo S, Karnieli E. Free fatty acids repress the GLUT4 gene expression in cardiac muscle via novel response elements. J Biol Chem 280: 34786–34795, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Baar K, Song Z, Semenkovich CF, Jones TE, Han DH, Nolte LA, Ojuka EO, Chen M, Holloszy JO. Skeletal muscle overexpression of nuclear respiratory factor 1 increases glucose transport capacity. FASEB J 17: 1666–1673, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Barnes BR, Glund S, Long YC, Hjalm G, Andersson L, Zierath JR. 5′-AMP-activated protein kinase regulates skeletal muscle glycogen content and ergogenics. FASEB J 19: 773–779, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 7.Da Silva-Grigoletto ME, Fernandez JM, de Sa CA, Gomez-Puerto JR, Vaamonde D, Perez-Jimenez F. Fructose addition to a glucose supplement modifies perceived exertion during strength and endurance exercise. J Strength Cond Res 24: 3334–3342, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Douard V, Ferraris RP. The role of fructose transporters in diseases linked to excessive fructose intake. J Physiol 591: 401–414, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelman JC, Edelman PM, Kniggee KM, Schwartz IL. Isolation of skeletal muscle nuclei. J Cell Biol 27: 365–377, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frosig C, Jorgensen SB, Hardie DG, Richter EA, Wojtaszewski JF. 5′-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab 286: E411–E417, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Goodyear LJ, Hirshman MF, Valyou PM, Horton ES. Glucose transporter number, function, and subcellular distribution in rat skeletal muscle after exercise training. Diabetes 41: 1091–1099, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Greiwe JS, Hickner RC, Hansen PA, Racette SB, Chen MM, Holloszy JO. Effects of endurance exercise training on muscle glycogen accumulation in humans. J Appl Physiol 87: 222–226, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Hawley JA, Gibala MJ, Bermon S. Innovations in athletic preparation: role of substrate availability to modify training adaptation and performance. J Sports Sci 25, Suppl 1: S115–S124, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Hickner RC, Fisher JS, Hansen PA, Racette SB, Mier CM, Turner MJ, Holloszy JO. Muscle glycogen accumulation after endurance exercise in trained and untrained individuals. J Appl Physiol 83: 897–903, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Higaki Y, Hirshman MF, Fujii N, Goodyear LJ. Nitric oxide increases glucose uptake through a mechanism that is distinct from the insulin and contraction pathways in rat skeletal muscle. Diabetes 50: 241–247, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Hook SS, Means AR. Ca(2+)/CaM-dependent kinases: from activation to function. Annu Rev Pharmacol Toxicol 41: 471–505, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Johnson RJ, Murray R. Fructose, exercise, health. Curr Sports Med Rep 9: 253–258, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Kouzarides T. Chromatin modifications and their function. Cell 128: 693–705, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Kraniou GN, Cameron-Smith D, Hargreaves M. Effect of short-term training on GLUT-4 mRNA and protein expression in human skeletal muscle. Exp Physiol 89: 559–563, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Le KA, Faeh D, Stettler R, Debard C, Loizon E, Vidal H, Boesch C, Ravussin E, Tappy L. Effects of four-week high-fructose diet on gene expression in skeletal muscle of healthy men. Diabetes Metab 34: 82–85, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Lee-Young RS, Canny BJ, Myers DE, McConell GK. AMPK activation is fiber type specific in human skeletal muscle: effects of exercise and short-term exercise training. J Appl Physiol 107: 283–289, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Lee-Young RS, Koufogiannis G, Canny BJ, McConell GK. Acute exercise does not cause sustained elevations in AMPK signaling or expression. Med Sci Sports Exerc 40: 1490–1494, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Leese GP, Bowtell J, Mudambo S, Reynolds N, Thompson J, Srimgeour CM, Rennie MJ. Post-exercise gastric emptying of carbohydrate solutions determined using the 13C acetate breath test. Eur J Appl Physiol Occup Physiol 71: 306–310, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Lira VA, Brown DL, Lira AK, Kavazis AN, Soltow QA, Zeanah EH, Criswell DS. Nitric oxide and AMPK cooperatively regulate PGC-1 in skeletal muscle cells. J Physiol 588: 3551–3566, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lira VA, Soltow QA, Long JH, Betters JL, Sellman JE, Criswell DS. Nitric oxide increases GLUT4 expression and regulates AMPK signaling in skeletal muscle. Am J Physiol Endocrinol Metab 293: E1062–E1068, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Ma K, Chan JK, Zhu G, Wu Z. Myocyte enhancer factor 2 acetylation by p300 enhances its DNA binding activity, transcriptional activity, and myogenic differentiation. Mol Cell Biol 25: 3575–3582, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGee SL, Fairlie E, Garnham AP, Hargreaves M. Exercise-induced histone modifications in human skeletal muscle. J Physiol 587: 5951–5958, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGee SL, Hargreaves M. Exercise and skeletal muscle glucose transporter 4 expression: molecular mechanisms. Clin Exp Pharmacol Physiol 33: 395–399, 2006 [DOI] [PubMed] [Google Scholar]
- 29.McGee SL, Hargreaves M. Histone modifications and exercise adaptations. J Appl Physiol 110: 258–263, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Meeprom A, Sompong W, Suwannaphet W, Yibchok-anun S, Adisakwattana S. Grape seed extract supplementation prevents high-fructose diet-induced insulin resistance in rats by improving insulin and adiponectin signalling pathways. Br J Nutr 106: 1173–1181, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Mukwevho E, Kohn TA, Lang D, Nyatia E, Smith J, Ojuka EO. Caffeine induces hyperacetylation of histones at the MEF2 site on the Glut4 promoter and increases MEF2A binding to the site via a CaMK-dependent mechanism. Am J Physiol Endocrinol Metab 294: E582–E588, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Nakatani A, Han DH, Hansen PA, Nolte LA, Host HH, Hickner RC, Holloszy JO. Effect of endurance exercise training on muscle glycogen supercompensation in rats. J Appl Physiol 82: 711–715, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Ojuka EO. Role of calcium and AMP kinase in the regulation of mitochondrial biogenesis and GLUT4 levels in muscle. Proc Nutr Soc 63: 275–278, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Ojuka EO, Goyaram V, Smith JA. The role of CaMKII in regulating GLUT4 expression in skeletal muscle. Am J Physiol Endocrinol Metab 303: E322–E331, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Ojuka EO, Jones TE, Han DH, Chen M, Holloszy JO. Raising Ca2+ in L6 myotubes mimics effects of exercise on mitochondrial biogenesis in muscle. FASEB J 17: 675–681, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Ojuka EO, Jones TE, Nolte LA, Chen M, Wamhoff BR, Sturek M, Holloszy JO. Regulation of GLUT4 biogenesis in muscle: evidence for involvement of AMPK and Ca2+. Am J Physiol Endocrinol Metab 282: E1008–E1013, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Passonneau JV, Lowry OH. Enzymatic Analysis: A Practical Guide. Totowa, NJ: Humana, 1993 [Google Scholar]
- 38.Ploug T, Stallknecht BM, Pedersen O, Kahn BB, Ohkuwa T, Vinten J, Galbo H. Effect of endurance training on glucose transport capacity and glucose transporter expression in rat skeletal muscle. Am J Physiol Endocrinol Metab 259: E778–E786, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Potthoff MJ, Wu H, Arnold MA, Shelton JM, Backs J, McAnally J, Richardson JA, Bassel-Duby R, Olson EN. Histone deacetylase degradation and MEF2 activation promote the formation of slow-twitch myofibers. J Clin Invest 117: 2459–2467, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahman MM, Park HM, Kim SJ, Go HK, Kim GB, Hong CU, Lee YU, Kim SZ, Kim JS, Kang HS. Taurine prevents hypertension and increases exercise capacity in rats with fructose-induced hypertension. Am J Hypertens 24: 574–581, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Reese JC, Zhang H, Zhang Z. Isolation of highly purified yeast nuclei for nuclease mapping of chromatin structure. Methods Mol Biol 463: 43–53, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Ren JM, Semenkovich CF, Gulve EA, Gao J, Holloszy JO. Exercise induces rapid increases in GLUT4 expression, glucose transport capacity, and insulin-stimulated glycogen storage in muscle. J Biol Chem 269: 14396–14401, 1994 [PubMed] [Google Scholar]
- 43.Rodnick KJ, Henriksen EJ, James DE, Holloszy JO. Exercise training, glucose transporters, and glucose transport in rat skeletal muscles. Am J Physiol Cell Physiol 262: C9–C14, 1992 [DOI] [PubMed] [Google Scholar]
- 44.Rodnick KJ, Holloszy JO, Mondon CE, James DE. Effects of exercise training on insulin—regulatable glucose-transporter protein levels in rat skeletal muscle. Diabetes 39: 1425–1429, 1990 [DOI] [PubMed] [Google Scholar]
- 45.Simpson RT. Chromatin structure and analysis of mechanisms of activators and repressors. Methods 15: 283–294, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Smith JA, Collins M, Grobler LA, Magee CJ, Ojuka EO. Exercise and CaMK activation both increase the binding of MEF2A to the Glut4 promoter in skeletal muscle in vivo. Am J Physiol Endocrinol Metab 292: E413–E420, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Smith JA, Kohn TA, Chetty AK, Ojuka EO. CaMK activation during exercise is required for histone hyperacetylation and MEF2A binding at the MEF2 site on the Glut4 gene. Am J Physiol Endocrinol Metab 295: E698–E704, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Steinberg GR, Watt MJ, Mcgee SL, Chan S, Hargreaves M, Febbraio MA, Stapleton D, Kemp BE. Reduced glycogen availability is associated with increased AMPKalpha2 activity, nuclear AMPKalpha2 protein abundance, and GLUT4 mRNA expression in contracting human skeletal muscle. Appl Physiol Nutr Metab 31: 302–312, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Strahl BD, Allis CD. The language of covalent histone modifications. Nature 403: 41–45, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Wallis GA, Rowlands DS, Shaw C, Jentjens RL, Jeukendrup AE. Oxidation of combined ingestion of maltodextrins and fructose during exercise. Med Sci Sports Exerc 37: 426–432, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Yoshinaga Y, Mochizuki K, Goda T. Trimethylation of histone H3K4 is associated with the induction of fructose-inducible genes in rat jejunum. Biochem Biophys Res Commun 419: 605–611, 2012 [DOI] [PubMed] [Google Scholar]