Abstract
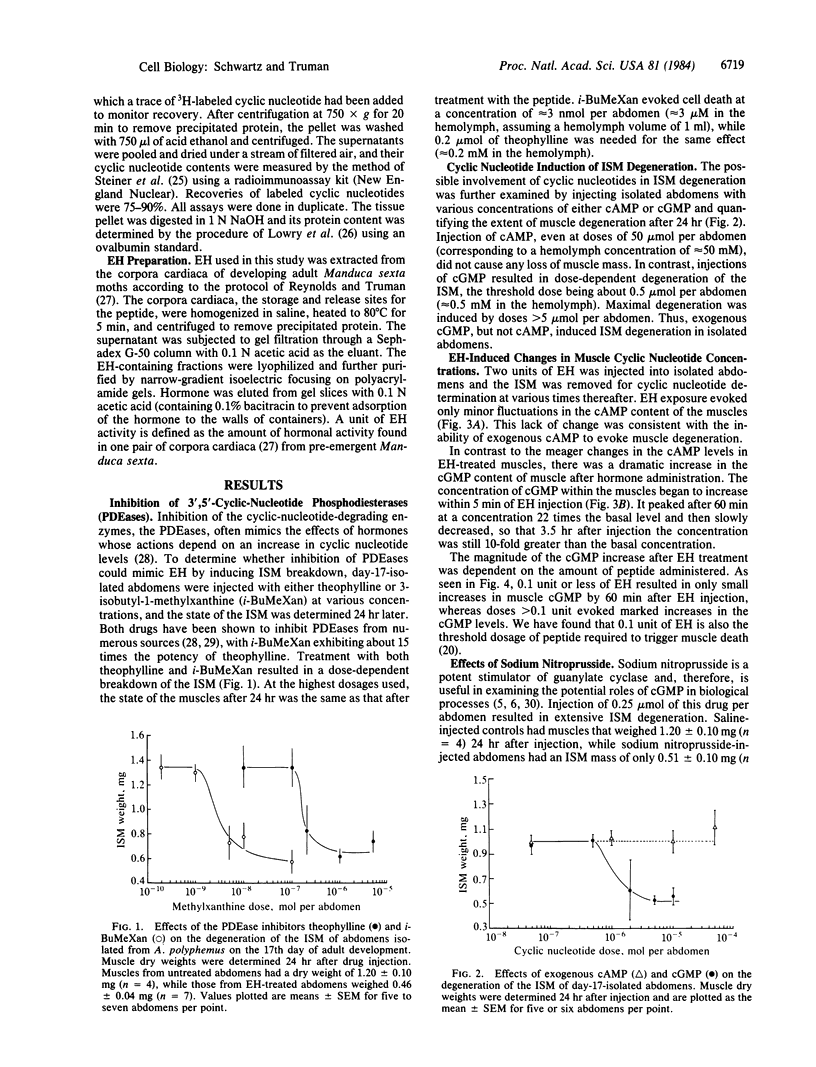
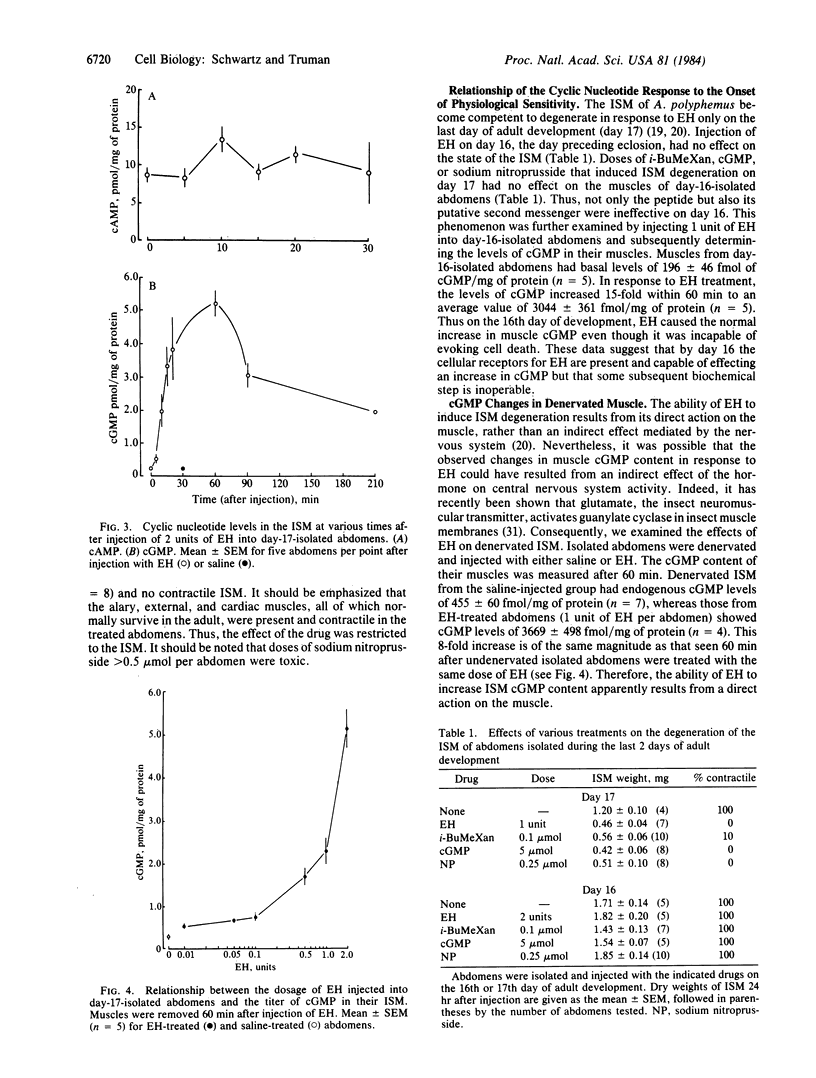
At the end of metamorphosis, the intersegmental muscles of the moth Antheraea polyphemus undergo rapid degeneration in response to the peptide eclosion hormone (EH). Muscle death was preceded by a 22-fold increase in muscle guanosine-3',5'-cyclic monophosphate (cGMP) titers, which peaked 60 min after peptide exposure; adenosine-3'5'-cyclic monophosphate (cAMP) titers remained unchanged. EH induced a dose-dependent increase in muscle cGMP content with a threshold dose similar to that needed to induce cell death. Exogenous cGMP, but not cAMP, mimicked the action of EH. Sodium nitroprusside, a potent stimulator of guanylate cyclase, and methylated xanthines, a class of 3',5'-cyclic-nucleotide phosphodiesterase inhibitors, also induced the selective death of these muscles. It is concluded that an elevation of cGMP level is involved in EH-induced muscle degeneration. The intersegmental muscles become sensitive to EH at the end of adult development in response to the declining titers of the steroid molting hormones, the ecdysteroids. At earlier times, treatment with EH, exogenous cGMP, sodium nitroprusside, or methylated xanthines was ineffective in causing cell death. Nevertheless, treatment with EH at this time resulted in a marked increase in intersegmental-muscle cGMP. Thus, the onset of physiological responsiveness to the peptide hormone presumably results from biochemical changes distal to the EH receptors and guanylate cyclase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguirre G., Acland G., Chader G. Hereditary retinal degenerations in the dog: specificity of abnormal cyclic nucleotide metabolism to diseases of arrested photoreceptor development. Birth Defects Orig Artic Ser. 1982;18(6):119–133. [PubMed] [Google Scholar]
- Albin E. E., Davison S. J., Newburgh R. W. Properties of cyclic nucleotide phosphodiesterase in the central nervous system of Manduca sexta. Biochim Biophys Acta. 1975 Feb 19;377(2):364–380. doi: 10.1016/0005-2744(75)90317-4. [DOI] [PubMed] [Google Scholar]
- Aurbach G. D. Receptor-adenylate cyclase components: abnormalities in clinical medicine. Adv Cyclic Nucleotide Res. 1980;12:1–9. [PubMed] [Google Scholar]
- Beavo J. A., Rogers N. L., Crofford O. B., Hardman J. G., Sutherland E. W., Newman E. V. Effects of xanthine derivatives on lipolysis and on adenosine 3',5'-monophosphate phosphodiesterase activity. Mol Pharmacol. 1970 Nov;6(6):597–603. [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Braughler J. M. Dissociation of increases in cyclic GMP from relaxation of arterial smooth muscle. Eur J Pharmacol. 1981 Feb 19;69(4):503–505. doi: 10.1016/0014-2999(81)90457-x. [DOI] [PubMed] [Google Scholar]
- Diamond J. Lack of correlation between cyclic GMP elevation and relaxation of nonvascular smooth muscle by nitroglycerin, nitroprusside, hydroxylamine and sodium azide. J Pharmacol Exp Ther. 1983 May;225(2):422–426. [PubMed] [Google Scholar]
- Goldberg N. D., Ames A. A., 3rd, Gander J. E., Walseth T. F. Magnitude of increase in retinal cGMP metabolic flux determined by 18O incorporation into nucleotide alpha-phosphoryls corresponds with intensity of photic stimulation. J Biol Chem. 1983 Aug 10;258(15):9213–9219. [PubMed] [Google Scholar]
- Holzmann S. Endothelium-induced relaxation by acetylcholine associated with larger rises in cyclic GMP in coronary arterial strips. J Cyclic Nucleotide Res. 1982;8(6):409–419. [PubMed] [Google Scholar]
- Janis R. A., Diamond J. Relationship between cyclic nucleotide levels and drug-induced relaxation of smooth muscle. J Pharmacol Exp Ther. 1979 Dec;211(3):480–484. [PubMed] [Google Scholar]
- Katsuki S., Arnold W. P., Murad F. Effects of sodium nitroprusside, nitroglycerin, and sodium azide on levels of cyclic nucleotides and mechanical activity of various tissues. J Cyclic Nucleotide Res. 1977 Aug;3(4):239–247. [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- LOCKSHIN R. A., WILLIAMS C. M. PROGRAMMED CELL DEATH--I. CYTOLOGY OF DEGENERATION IN THE INTERSEGMENTAL MUSCLES OF THE PERNYI SILKMOTH. J Insect Physiol. 1965 Feb;11:123–133. doi: 10.1016/0022-1910(65)90099-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lincoln T. M., Keely S. L. Effects of acetylcholine and nitroprusside on cGMP-dependent protein kinase in the perfused rat heart. J Cyclic Nucleotide Res. 1980;6(2):83–91. [PubMed] [Google Scholar]
- Lockshin R. A., Beaulaton J. Programmed cell death. electrophysiological and ultrastructural correlations in metamorphosing muscles of lepidopteran insects. Tissue Cell. 1979;11(4):803–819. doi: 10.1016/0040-8166(79)90033-8. [DOI] [PubMed] [Google Scholar]
- Lolley R. N., Rayborn M. E., Hollyfield J. G., Farber D. B. Cyclic GMP and visual cell degeneration in the inherited disorder of rd mice: a progress report. Vision Res. 1980;20(12):1157–1161. doi: 10.1016/0042-6989(80)90054-1. [DOI] [PubMed] [Google Scholar]
- Mirro M. J., Bailey J. C., Watanabe A. M. Dissociation between the electrophysiological properties and total tissue cyclic guanosine monophosphate content of guinea pig atria. Circ Res. 1979 Aug;45(2):225–233. doi: 10.1161/01.res.45.2.225. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Bitensky M. W. Light-regulated enzymes of vertebrate retinal rods. Adv Cyclic Nucleotide Res. 1979;11:265–301. [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Robinson N. L., Cox P. M., Greengard P. Glutamate regulates adenylate cyclase and guanylate cyclase activities in an isolated membrane preparation from insect muscle. Nature. 1982 Mar 25;296(5855):354–356. doi: 10.1038/296354b0. [DOI] [PubMed] [Google Scholar]
- Schultz G., Hardman J. G., Schultz K., Baird C. E., Sutherland E. W. The importance of calcium ions for the regulation of guanosine 3':5'-cyclic monophosphage levels. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3889–3893. doi: 10.1073/pnas.70.12.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz K., Schultz K., Schultz G. Sodium nitroprusside and other smooth muscle-relaxants increase cyclic GMP levels in rat ductus deferens. Nature. 1977 Feb 24;265(5596):750–751. doi: 10.1038/265750a0. [DOI] [PubMed] [Google Scholar]
- Schwartz L. M., Truman J. W. Hormonal control of rates of metamorphic development in the tobacco hornworm Manduca sexta. Dev Biol. 1983 Sep;99(1):103–114. doi: 10.1016/0012-1606(83)90257-9. [DOI] [PubMed] [Google Scholar]
- Schwartz L. M., Truman J. W. Peptide and steroid regulation of muscle degeneration in an insect. Science. 1982 Mar 12;215(4538):1420–1421. doi: 10.1126/science.6278594. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Truman J. W., Mumby S. M., Welch S. K. Involvement of cyclic GMP in the release of stereotyped behaviour patterns in moths by a peptide hormone. J Exp Biol. 1980 Feb;84:201–212. doi: 10.1242/jeb.84.1.201. [DOI] [PubMed] [Google Scholar]
- Weevers R. D. A lepidopteran saline: effects of inorganic cation concentrations on sensory, reflex and motor responses in a herbivorous insect. J Exp Biol. 1966 Feb;44(1):163–175. doi: 10.1242/jeb.44.1.163. [DOI] [PubMed] [Google Scholar]
- Williams C. M. Continuous Anesthesia for Insects. Science. 1946 Jan 11;103(2663):57–57. doi: 10.1126/science.103.2663.57. [DOI] [PubMed] [Google Scholar]
- Williams C. M. Ecdysone and ecdysone-analogues: their assay and action on diapausing pupae of the cynthia silkworm. Biol Bull. 1968 Apr;134(2):344–355. doi: 10.2307/1539610. [DOI] [PubMed] [Google Scholar]


