Abstract
The Rhesus (Rh) glycoproteins, Rh B and Rh C Glycoprotein (Rhbg and Rhcg, respectively), are ammonia-specific transporters expressed in renal distal nephron and collecting duct sites that are necessary for normal rates of ammonia excretion. The purpose of the current studies was to determine the effect of their combined deletion from the renal collecting duct (CD-Rhbg/Rhcg-KO) on basal and acidosis-stimulated acid-base homeostasis. Under basal conditions, urine pH and ammonia excretion and serum HCO3− were similar in control (C) and CD-Rhbg/Rhcg-KO mice. After acid-loading for 7 days, CD-Rhbg/Rhcg-KO mice developed significantly more severe metabolic acidosis than did C mice. Acid loading increased ammonia excretion, but ammonia excretion increased more slowly in CD-Rhbg/Rhcg-KO and it was significantly less than in C mice on days 1–5. Urine pH was significantly more acidic in CD-Rhbg/Rhcg-KO mice on days 1, 3, and 5 of acid loading. Metabolic acidosis increased phosphenolpyruvate carboxykinase (PEPCK) and Na+/H+ exchanger NHE-3 and decreased glutamine synthetase (GS) expression in both genotypes, and these changes were significantly greater in CD-Rhbg/Rhcg-KO than in C mice. We conclude that 1) Rhbg and Rhcg are critically important in the renal response to metabolic acidosis; 2) the significantly greater changes in PEPCK, NHE-3, and GS expression in acid-loaded CD-Rhbg/Rhcg-KO compared with acid-loaded C mice cause the role of Rhbg and Rhcg to be underestimated quantitatively; and 3) in mice with intact Rhbg and Rhcg expression, metabolic acidosis does not induce maximal changes in PEPCK, NHE-3, and GS expression despite the presence of persistent metabolic acidosis.
Keywords: acid-base, ammonia, collecting duct
renal ammonia1 excretion is a major mechanism through which the kidneys contribute to acid-base homeostasis and involves integrated contributions of intrarenal ammoniagenesis and renal epithelial cell ammonia transport. Our understanding of the molecular mechanisms of ammonia transport has undergone fundamental changes in the past several years. In particular, the previous paradigm that NH3 is in diffusion equilibrium in the kidney and that NH4+ is not transported, i.e., that it is “trapped,” is being replaced by one in which both NH3 and NH4+ are transported across plasma membranes by specific proteins, and that regulated expression and function of these proteins is integral to acid-base homeostasis (47, 49).
The Rhesus (Rh) glycoproteins, Rh B glycoprotein (Rhbg) and Rh C glycoprotein (Rhcg), have an important role in renal epithelial cell ammonia transport. Rhbg and Rhcg are ammonia-specific transporters (2, 31, 53) and are expressed in the distal convoluted tubule (DCT), connecting segments (CNT), initial collecting tubule, cortical collecting duct (CCD), outer medullary collecting duct (OMCD), and inner medullary collecting duct (IMCD) (11, 18, 37, 41, 46). Notably, these sites are responsible for secreting the majority of urinary ammonia (16, 38). Recent studies showed that individual deletion from the kidney of either Rhbg (4) or Rhcg (5, 27, 28) inhibited both renal ammonia excretion and the maintenance of acid-base homeostasis in response to experimental metabolic acidosis. However, because both Rhbg and Rhcg are expressed in the basolateral plasma membrane of renal epithelial cells (7, 18, 24, 40), adaptive changes in transport mediated by one of these ammonia transporter family members might compensate, at least partially, for the lack of transport by the other when only a single ammonia transporter family member is deleted.
Thus the purpose of this study was to examine the effect of genetic deletion of both Rhbg and Rhcg from the collecting duct on basal ammonia and acid-base homeostasis and on the renal response to metabolic acidosis. We generated mice with combined collecting duct-specific deletion of both Rhbg and Rhcg using Cre-loxP techniques. We then examined basal acid-base homeostasis and ammonia excretion, the response to HCl-induced metabolic acidosis, and the adaptive changes in several other proteins involved in renal ammonia metabolism. Our results show several important findings. First, Rhbg and Rhcg are critically important in the renal response to metabolic acidosis. Second, there are significantly greater changes in phosphenolpyruvate carboxykinase (PEPCK), glutamine synthetase (GS), and NHE-3 expression in acid-loaded CD-Rhbg/Rhcg-knockout (KO) compared with acid-loaded mice with intact Rhbg and Rhcg expression. This indicates the role of Rhbg and Rhcg in ammonia excretion is underestimated quantitatively, due to exaggerated changes in other proteins involved in renal ammonia metabolism. Third, in mice with intact Rhbg and Rhcg expression, acidosis-induced changes in PEPCK, GS, and NHE-3 expression are submaximal, despite the presence of persistent metabolic acidosis.
METHODS
Animals.
We generated mice with combined collecting duct-specific Rhbg and Rhcg deletion using Cre-loxP techniques. Briefly, we bred mice with floxed Rhcg alleles and expressing Cre-recombinase under control of the Ksp-cadherin promoter (Ksp-Cre) (27, 29) with mice with floxed Rhbg alleles (3, 4), and by selective breeding of offspring generated mice with floxed Rhbg and Rhcg alleles that also expressed Ksp-Cre. We genotyped all mice used in these studies using tail-clip samples, as we have described previously (3, 4, 27–29). Mice used in the current studies were offspring of mating mice with floxed Rhbg and Rhcg alleles and expressing Ksp-Cre with mice with floxed Rhbg and Rhcg alleles that did not express Ksp-Cre. Animal breeding was performed in the University of Florida College of Medicine Cancer and Genetics Transgenic Animal Core Facility by trained personnel. All animal studies were approved by the University of Florida College of Medicine and the North Florida/South Georgia Veterans Health System Institutional Animal Care and Use Committee.
Antibodies.
Affinity-purified antibodies to Rhbg and Rhcg generated in our laboratory have been characterized previously (30, 46, 48). Antibodies to PEPCK were obtained from Cayman Chemical (Ann Arbor, MI). Dr. Norman Curthoys (Colorado State University) provided antibodies to phosphate-dependent glutaminase (PDG). Antibodies to GS were obtained from Chemicon, (Temecula, CA), and antibodies to NHE-3 were obtained from StressMarq Biosciences (Victoria, BC).
Acid loading.
An acid diet was prepared as we described previously (3, 27, 29). Briefly, we added 0.4 M HCl to powdered standard rodent chow in a ratio of 1 ml/g chow. The control diet was identical, except we substituted deionized water for HCl. Adult female animals, >6 mo of age, were placed into metabolic cages (Tecniplast diuresis metabolic cage, Fisher Scientific). Animals were allowed to acclimate for 2 days while receiving the control diet and then were allocated to either the HCl diet or control diet. Daily food intake was measured. Urine was collected under mineral oil, and daily urine volume and pH were recorded. Urine samples were stored at −20°C until analyzed further.
Electrolyte measurements.
Urine ammonia was measured using a modification of a commercially available kit (A7553, Pointe Scientific, Canton, MI) as described previously (27). Urine pH was measured using a micro-pH electrode (ROSS semi-micro pH, Orion 8115BN, Thermo Scientific). Serum bicarbonate was measured as total CO2 using a commercially available kit (C750-120, Pointe Scientific) as described previously (27). Urinary titratable acid was measured using techniques described previously (27). Plasma and urine creatinine were measured using capillary electrophoresis as described previously (54), with the exception that the injection time was 10 s, the rinse used 0.1 M NaOH, LC/MS grade water, and then running buffer, with 2-min rinses between runs, and the detection wavelength was 214 nm. Plasma Na+ and K+ were measured using a flame photometer (Instrumentation Laboratory, Lexington, MA).
Tissue preparation for immunolocalization.
Mice were anesthetized with inhalant isoflurane. The kidneys were preserved by in vivo cardiac perfusion with PBS (pH 7.4) followed by periodate-lysine-2% paraformaldehyde (PLP) and then cut transversely into several 2- to 4-mm-thick slices and immersed for 48 h at 4°C in the same fixative. Kidney samples from each animal were embedded in polyester wax made using polyethylene glycol 400 distearate (Polysciences, Warrington, PA) with 10% 1-hexadecanol, and 2- or 3-μm-thick sections were cut and mounted on gelatin-coated glass slides.
Immunohistochemistry.
Immunolocalization was accomplished using immunoperoxidase procedures. The sections were dewaxed in ethanol, rehydrated, and then rinsed in PBS. Endogenous peroxidase activity was blocked by incubating the sections in 3% H2O2 in distilled water for 45 min. The sections were blocked for 15 min with Serum-Free Protein Block (DakoCytomation) and were then incubated at 4°C overnight with primary antibody. The sections were washed in PBS and incubated for 30 min with polymer-linked, peroxidase-conjugated goat anti-rabbit IgG (MACH2, Biocare Medical, Concord, CA), again washed with PBS, then exposed to diaminobenzidine (DAB) for 5 min. The sections were washed in distilled water, dehydrated with xylene, mounted, and observed by light microscopy. Comparisons of labeling were made only between sections of the same thickness from the same immunohistochemistry experiment. Sections were examined on a Nikon E600 microscope equipped with DIC optics and photographed using a DXM1200F digital camera and ACT-1 software (Nikon). Color correction was performed using Adobe Photoshop software (Adobe Systems, San Jose, CA).
Protein preparation.
Animals were anesthetized with inhalant isoflurane, and the kidneys were rinsed by in vivo cardiac perfusion with PBS (pH 7.4). The right renal vasculature was clamped, the right kidney was rapidly removed, and the cortex and outer medulla were isolated rapidly under a dissecting microscope, snap-frozen in liquid nitrogen, and then stored frozen at −70°C until used. The left kidney was perfused with PLP fixative for immunohistochemistry. Tissues were homogenized in T-PER Tissue Protein Extraction Reagent (Pierce Biotechnology, Rockford, IL) using microtube pestles (USA Scientific, Ocala, FL), and protein was extracted according to the manufacturer's recommended procedures. An aliquot was obtained for protein determination using a BCA assay, and the remainder was stored frozen at −70°C until used.
Immunoblotting procedure.
Ten micrograms of renal protein were electrophoresed on 10% PAGE ReadyGel (Bio-Rad, Hercules, CA). Gels were then transferred electrophoretically to nitrocellulose membranes, blocked with 5 g/dl nonfat dry milk in Blotto buffer (50 mM Tris, 150 mM NaCl, 5 mM Na2EDTA, and 0.05% Tween 20, pH 7.6), and incubated at 4°C overnight with primary antibody diluted in nonfat dry milk. Loading and transfer equivalence were assessed with Ponceau S staining. After washing, membranes were exposed to secondary antibody, goat anti-rabbit IgG (Millipore, Billerica, MA), conjugated to horseradish peroxidase at a dilution of 1:5,000. Sites of antibody-antigen reaction were visualized by using enhanced chemiluminescence (SuperSignal West Pico Substrate, Pierce) and a Kodak Image Station 440CF digital imaging system. Band density was quantified using Kodak 1D, version 5.0, software (Kodak Scientific Imaging, New Haven, CT). Band density was normalized such that mean density in the same region (cortex or outer medulla) in control tissues was 100.0. The absence of saturation was confirmed by examining pixel intensity distribution in all immunoblots.
Immunodot analysis.
Extracted protein was diluted with T-Per containing protease inhibitors to a final concentration of 2 μg/μl. A piece of nitrocellulose was aligned beneath a multichannel slotted gasket, and 1.0 μg of each sample was placed at regular intervals. The nitrocellulose was allowed to air dry and stained with a Ponceau S solution containing 5% concentrated acetic acid. Otherwise, protein detection was performed using similar techniques as used for the immunoblot analysis with the exception that the blocked nitrocellulose was placed in a multichannel plexiglass holder (TMR Engineering, Micanopy, FL) to analyze several antibodies at one time. In preliminary studies, we confirmed that immunodot and immunoblot analysis gave quantitatively similar results.
Statistics.
Results are presented as means ± SE. Statistical analyses were performed using Student's t-test, and P < 0.05 was taken as statistically significant; n refers to the numbers of animal studied.
RESULTS
Expression of Rhbg and Rhcg in floxed Rhbg and Rhcg, Ksp-Cre-positive mice.
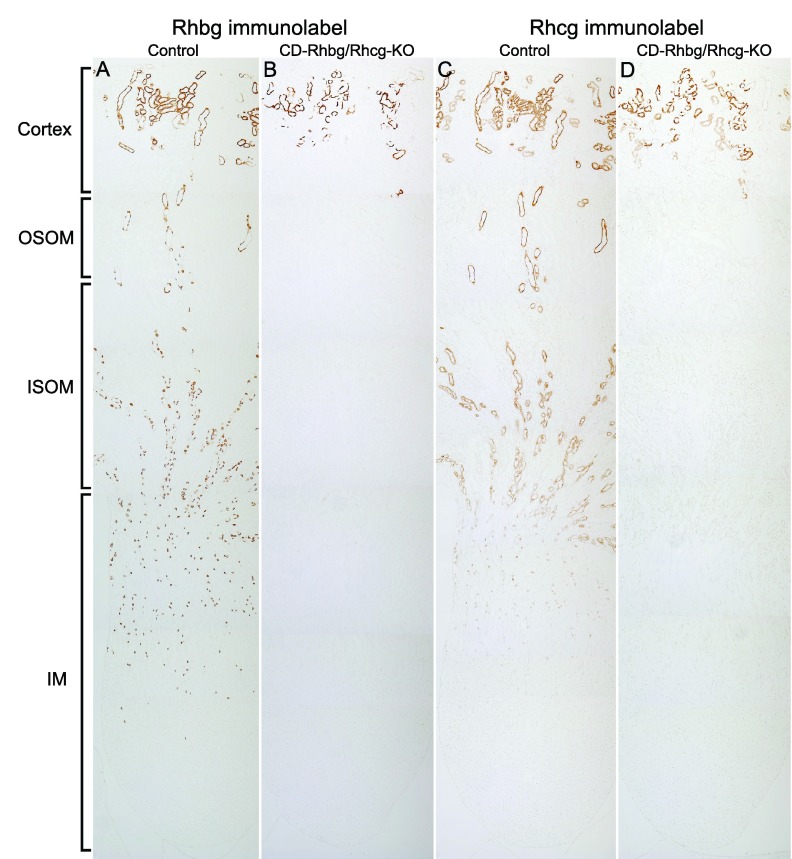
Wild-type mice express Rhbg and Rhcg in the renal distal nephron and collecting duct, from the distal convoluted tubule through the inner medullary collecting duct (11, 18, 24, 27, 37, 46). In Ksp-Cre-positive mice with floxed Rhbg and Rhcg alleles, there were dramatic changes in Rhbg and Rhcg expression. Low-power micrographs show that the number of cells expressing Rhbg and Rhcg is decreased in the cortex and that there is no detectable expression in the outer medulla and inner medulla (Fig. 1). High-power micrographs show persistent Rhbg and Rhcg immunolabel in the CNT and DCT (Fig. 2). No Rhbg and Rhcg immunolabel was evident in the CCD, outer stripe or inner stripe of the OMCD (OMCDo, OMCDi, respectively), and IMCD. Collecting duct-specific gene deletion, but not deletion in the DCT and CNT, is similar to that observed previously in single knockout studies of Rhcg (27). Thus these observations confirm collecting duct-specific deletion of Rhbg and Rhcg. In contrast, kidneys from mice with floxed Rhbg and Rhcg alleles that did not express Ksp-Cre exhibited a normal pattern of Rhbg and Rhcg immunolabel.
Fig. 1.
Low-magnification immunohistochemical localization of Rhesus (Rh) glycoproteins, Rh B and Rh C Glycoprotein (Rhbg and Rhcg, respectively), expression in control (C) and floxed Rhbg and Rhcg, Ksp-cadherin-Cre-positive (CD-Rhbg/Rhcg-KO) mice fed a normal diet. A and C: Rhbg and Rhcg immunolabel in C mice kidney. A normal distribution of Rhbg and Rhcg immunolabel in the cortex, outer stripe of outer medullary collecting duct (OSOM), inner stripe of outer medullary collecting duct (ISOM), and inner medulla (IM) is present. B and D: Rhbg and Rhcg immunolabel in CD-Rhbg/Rhcg-KO mice kidney. In CD-Rhbg/Rhcg-KO mice, Rhbg and Rhcg immunoreactivity is only present in the cortex. No Rhbg and Rhcg immunolabel appears in the OSOM, ISOM, and IM.
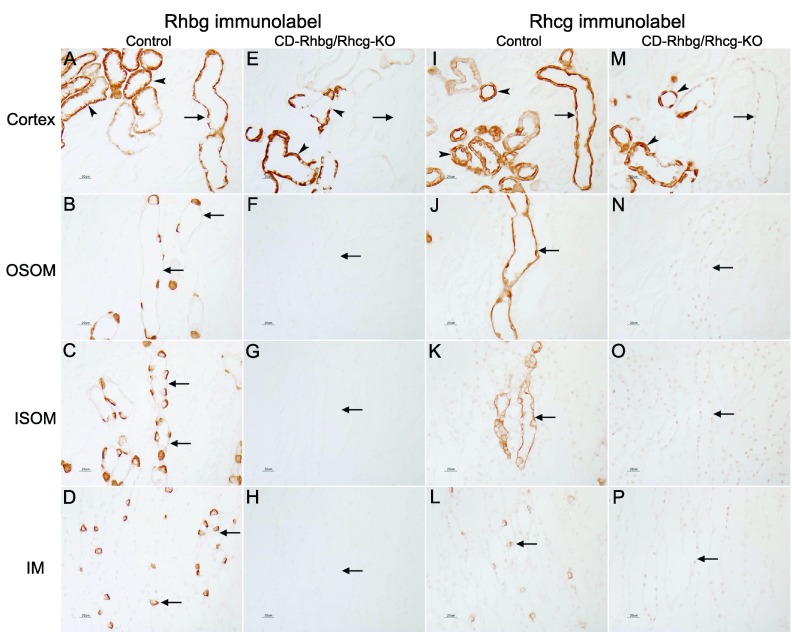
Fig. 2.
High-magnification immunohistochemical localization of Rhbg and Rhcg expression in C and CD-Rhbg/Rhcg-KO mice fed a normal diet. A and I: Rhbg and Rhcg immunolabel in C mice kidney in the cortex. A normal distribution of Rhbg and Rhcg immunolabel in the cortical collecting duct (CCD; arrows), in connecting tubule (CNT), and distal convoluted tubule (DCT; arrowheads) segments is present. E and M: Rhbg and Rhcg immunolabel in CD-Rhbg/Rhcg-KO mice kidney in the cortex. No Rhbg and Rhcg immunolabel is evident in CCD (arrows). Rhbg and Rhcg immunoreactivity is present in CNT and DCT segments (arrowheads). B–D and J–L: Rhbg and Rhcg immunolabel in OM and IM of C mice; normal Rhbg and Rhcg expression in the OSOM, ISOM, and IM (arrows). F–H and N–P: OM and IM from CD-Rhbg/Rhcg-KO mice kidney. No Rhbg or Rhcg immunolabel is detectable.
CD-Rhbg/Rhcg-KO mice on a normal diet.
To determine Rhbg and Rhcg's role in basal acid-base homeostasis, we measured serum electrolytes and urinary pH, ammonia, and titratable acid excretion in age-matched CD-Rhbg/Rhcg-KO and control mice. Table 1 summarizes these results. CD-Rhbg/Rhcg-KO did not alter urinary pH, ammonia excretion, or titratable acid excretion significantly compared with control mice. Neither serum Na+, K+, nor HCO3− differed significantly between CD-Rhbg/Rhcg-KO and control (C in figures) mice on a control diet (data not shown). We used serum creatinine and creatinine clearance to estimate glomerular filtration rate (GFR), with the understanding that endogenous creatinine clearance overestimates actual GFR due to OAT3-mediated creatinine secretion in mice (10, 44). Combined collecting duct-specific deletion of Rhbg and Rhcg did not alter creatinine clearance significantly.
Table 1.
Physiological parameters under basal conditions
| Parameter | CD-Rhbg/Rhcg-KO | C | P Value |
|---|---|---|---|
| Urine ammonia, μmol/day | 169.3 ± 15.8 (6) | 179.4 ± 39.8 (6) | NS |
| Urine pH | 6.04 ± 0.07 (6) | 6.11 ± 0.08 (6) | NS |
| Urine titratable acid, μmol/day | 94.7 ± 12.2 (6) | 84.5 ± 9.3 (6) | NS |
| Serum creatinine, mg/dl | 0.103 ± 0.010 (6) | 0.101 ± 0.004 (6) | NS |
| Creatinine clearance, μl/min | 283.3 ± 38.5 (6) | 209.3 ± 36.0 (6) | NS |
| Urine volume, ml/day | 1.86 ± 0.22 (6) | 2.05 ± 0.31 (6) | NS |
Values are means ± SE. Numbers in parentheses are numbers of animals in each group. CD-Rhbg/Rhcg-KO, collecting duct-specific Rhbg and Rhcg glycoprotein deletion; C, control; NS, not significant.
Renal response to metabolic acidosis.
We next examined the effect of acid-loading on systemic acid-base parameters. After HCl loading for 7 days, mice with collecting duct-specific Rhbg and Rhcg deletion demonstrated significantly more severe metabolic acidosis,2 assessed by serum HCO3−, than did control mice (CD-Rhbg/Rhcg-KO, 4.3 ± 1.0 vs. control, 15.9 ± 1.1 mmol/l, n = 6 in each group; P < 0.0001). Food intake, and thus the extent of acid loading, did not differ significantly between CD-Rhbg/Rhcg-KO and control mice (data not shown). Thus collecting duct Rhbg and Rhcg deletion impairs the ability to defend against metabolic acidosis from an exogenous acid load.
Urinary ammonia excretion in response to HCl-induced metabolic acidosis.
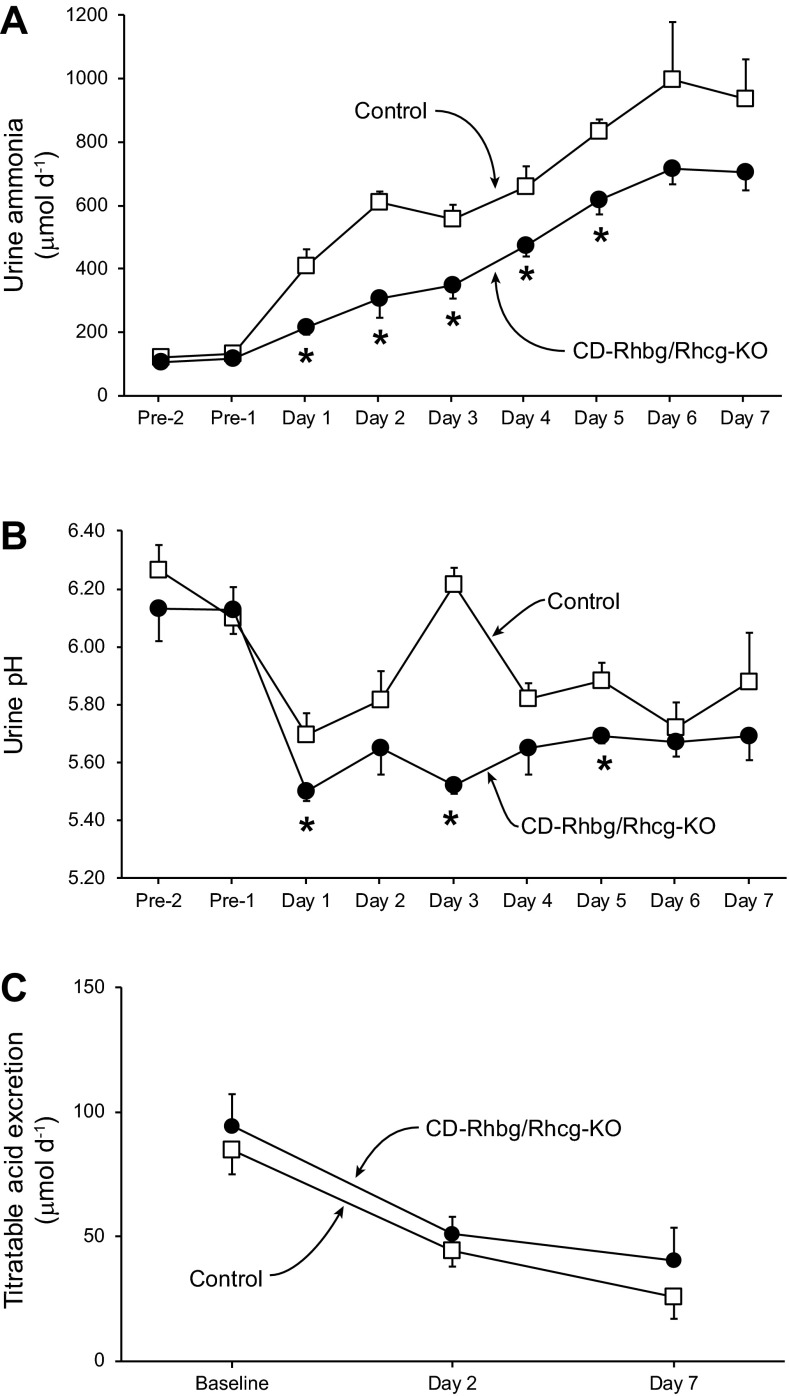
To determine whether collecting duct-specific Rhbg and Rhcg deletion in mice resulted in more severe acidosis after HCl loading due to impaired ammonia excretion, we examined urinary ammonia excretion in HCl-loaded CD-Rhbg/Rhcg-KO and control mice. Figure 3A summarizes these results. While on a normal diet, urinary ammonia excretion was not significantly different between CD-Rhbg/Rhcg-KO and control mice. After addition of HCl to their chow, urinary ammonia excretion increased significantly on the first day in control mice (Δ = +283 ± 54 μmol/day; P < 0.001, n = 6) and continued to increase to a maximum on day 6. In contrast, in CD-Rhbg/Rhcg-KO mice the increase in urinary ammonia was significantly less on the first day of acid-loading (Δ = +103 ± 27 μmol/day; P < 0.01 vs. control mice, n = 6), and urinary total ammonia remained significantly less than in control mice on days 1−5. Thus combined collecting duct-specific Rhbg and Rhcg deletion inhibited the normal changes in renal ammonia excretion in response to HCl-induced metabolic acidosis.
Fig. 3.
Urinary ammonia excretion, urine pH, and titratable acid excretion in response to HCl-induced metabolic acidosis. Mice were housed in metabolic cages, and urine was collected daily under mineral oil. A: urinary ammonia excretion. While on a normal diet, urinary ammonia excretion was not significantly different between CD-Rhbg/Rhcg-KO and C mice. After addition of HCl to their chow, urinary ammonia excretion increased significantly on the first day in C mice and continued to increase to a maximum on day 6. In CD-Rhbg/Rhcg-KO mice, urinary ammonia excretion also increased significantly on the first day of acid loading, did not peak until day 6, and remained significantly less than in C mice on days 1–5. B: urine pH. Urine pH was significantly lower in CD-Rhbg/Rhcg-KO mice than in C mice on days 1, 3, and 5 of acid loading. C: titratable acid excretion under basal conditions and in response to acid loading. There was no significant difference in titratable acid excretion between genotypes, either at baseline, or on either day 2 or 7 of acid loading. Values are means ± SE; n = 6/group. *P < 0.05 vs. C.
Urine pH in response to metabolic acidosis.
Luminal pH is an important regulator of collecting duct ammonia excretion, with increased acidification stimulating ammonia secretion. To assess whether an inability to acidify the urine contributed to the blunted urinary ammonia excretion, we measured urine pH. Figure 3B summarizes these results. Urine pH did not differ significantly before acid loading. On days 1, 3, and 5 of acid loading, CD-Rhbg/Rhcg-KO mice exhibited significantly more acidic urine than did control mice. Because acid-loaded CD-Rhbg/Rhcg-KO mice acidified the urine equally or more so than control mice, the impaired urinary ammonia excretion observed in acid-loaded CD-Rhbg/Rhcg-KO mice is not due to an inability to acidify urine. Instead, impaired secretion of the highly basic ammonia species NH3 in combined Rhbg and Rhcg deletion mice likely contributes to the development of more acidic urine. Moreover, exaggerated urine acidification, by stimulating Rhbg- and Rhcg-independent NH3 secretion, likely contributes, at least partially, to the residual increase in urinary ammonia excretion.
Titratable acid excretion in response to metabolic acidosis.
Titratable acid excretion is a second important component of renal net acid excretion. Figure 3C shows titratable acid excretion in control and KO mice under baseline conditions and in response to metabolic acidosis. Titratable acid excretion did not differ significantly between the two genotypes, either under baseline conditions or in response to metabolic acidosis. The lack of increase in titratable acid excretion with acid loading is similar to that we have observed previously (4, 27)
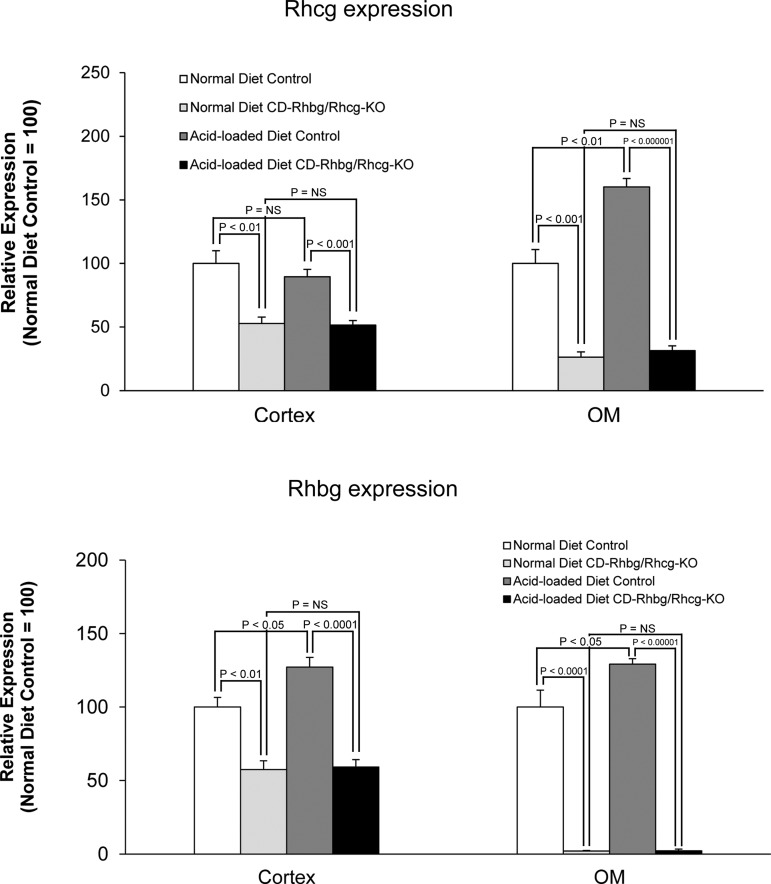
Rhcg and Rhbg expression in response to metabolic acidosis.
The blunted, but progressive, increase in urinary ammonia excretion in acid-loaded CD-Rhbg/Rhcg-KO mice suggests adaptive responses that counterbalance the lack of collecting duct Rhbg and Rhcg expression may be present. Two possible adaptive responses could be induction of Rhcg in cells that normally do not express it or increased Rhcg expression in tubules where our gene-targeting approach does not delete the Rhcg gene, i.e., DCT and CNT. To examine these possibilities, we used immunodot analysis and immunohistochemistry to determine the effect of HCl-induced metabolic acidosis on Rhcg and on Rhbg expression in CD-Rhbg/Rhcg-KO mice.
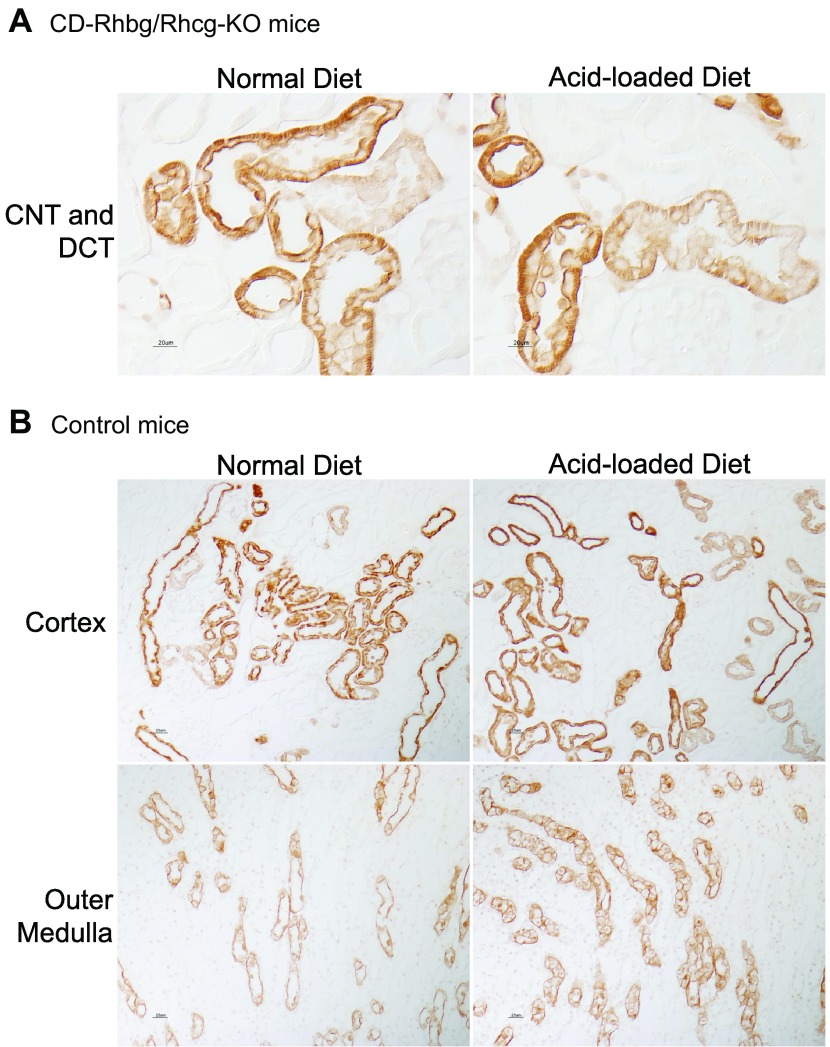
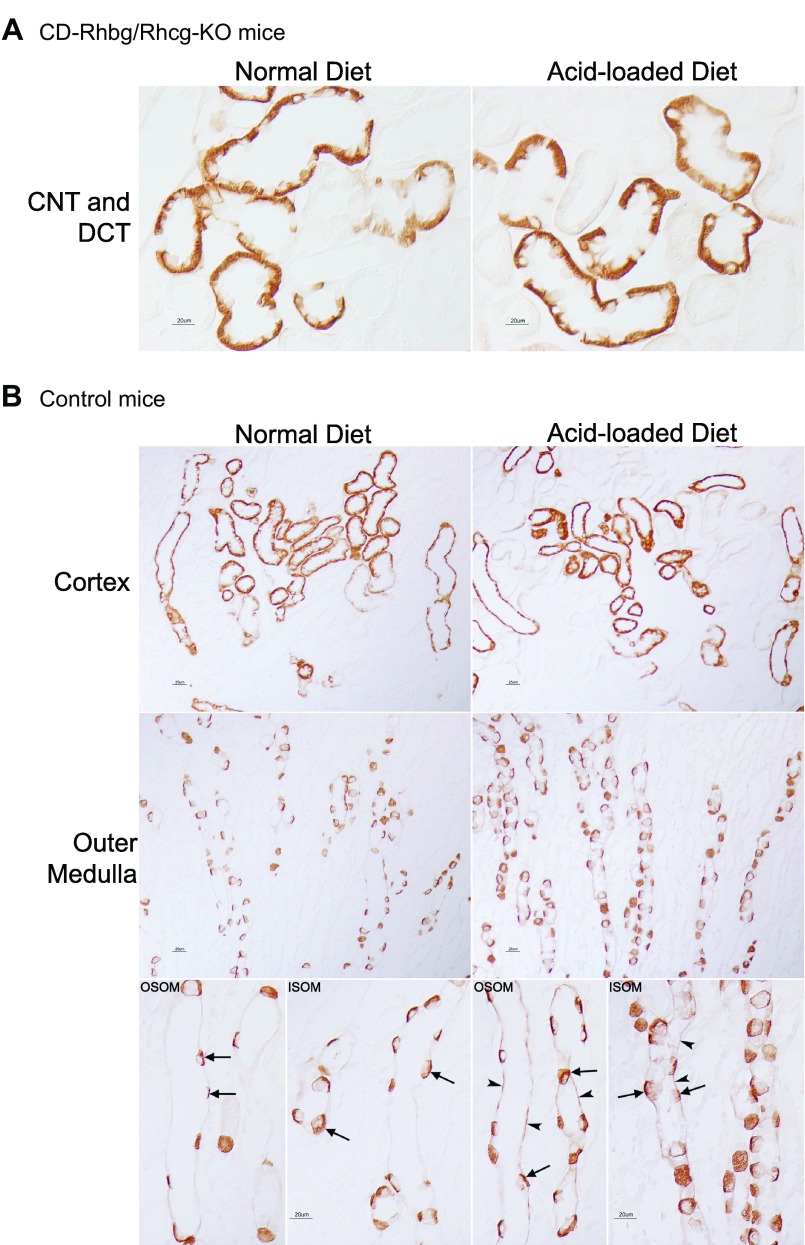
While on a normal diet, CD-Rhbg/Rhcg-KO mice express Rhcg only in the CNT and DCT. In response to metabolic acidosis, this expression pattern was unchanged, i.e., acid-loading did not induce Rhcg expression in non-collecting duct cells (Fig. 4A). In addition, metabolic acidosis did not significantly change Rhcg expression in the DCT and CNT, although there was an impression of increased basolateral Rhcg expression in the CNT. In mice with intact Rhbg and Rhcg expression, acid loading increased Rhcg expression significantly in the OMCD (Fig. 4B) but not detectably in the DCT and CNT. Figure 5 summarizes steady-state protein expression. In mice with intact Rhcg expression, metabolic acidosis increased Rhcg expression in the outer medulla but not the cortex. This is similar to what we observed previously (40). Collecting duct-specific Rhbg and Rhcg deletion resulted in significant decreases in Rhcg expression, and metabolic acidosis did not alter Rhcg expression in either the cortex or outer medulla of these mice. Thus, although Rhcg expression contributes to the increase in ammonia excretion in mice with intact Rhcg expression, changes in expression do not appear to contribute to responses in mice with collecting duct-specific Rhbg and Rhcg deletion.
Fig. 4.
Rhcg expression in normal and acid-loaded diet in C and CD-Rhbg/Rhcg-KO mice. A: Rhcg expression in CD-Rhbg/Rhcg-KO mice. After acid loading, Rhcg immunolabel in the CNT and DCT of CD-Rhbg/Rhcg-KO mice was similar to that observed in normal diet CD-Rhbg/Rhcg-KO mice. B: Rhcg expression in C mice. In the OMCD, Rhcg immunolabel intensity was significantly greater in acid-loaded than in normal diet mice.
Fig. 5.
Immunodot analysis of Rhbg and Rhcg expression. Top: summary of Rhcg protein expression in the cortex and OM. In mice with intact Rhcg expression, acid loading increases Rhcg protein expression in the OM. In mice with combined collecting duct-specific Rhbg and Rhcg deletion, Rhcg expression is greatly diminished and is not altered by acid-loading. Bottom: summary of Rhbg protein expression. In mice with intact Rhbg expression, metabolic acidosis significantly increases Rhbg expression in both the cortex and OM. In mice with collecting duct-specific Rhbg and Rhcg deletion, basal Rhbg expression is significantly decreased (residual expression reflects expression in CNT and DCT cells) and is not altered by acid loading.
The delayed increase in urinary ammonia excretion in the CD-Rhbg/Rhcg-KO mice could also result from changes in Rhbg expression. In mice with intact Rhbg and Rhcg expression, an acid-loaded diet increased Rhbg expression in the cortex and outer medulla (Fig. 6B). Immunohistochemistry demonstrated that in mice with intact Rhbg expression, acid-loading increased basolateral Rhbg immunolabel in OSOM and ISOM principal cells (Fig. 6B). In mice with CD-Rhbg/Rhcg-KO, there was no detectable change in Rhbg expression in the DCT or CNT, and no evidence of expression in the CCD or OMCD (Fig. 6A). Immunodot analysis showed increased Rhbg expression in both cortex and outer medulla in response to metabolic acidosis (Fig. 5). In contrast, in mice with collecting duct-specific Rhbg and Rhcg deletion, metabolic acidosis did not alter Rhbg expression significantly. Thus, although increased Rhbg expression contributes to the increase in ammonia excretion by the normal kidney, the delayed increase in ammonia excretion in CD-Rhbg/Rhcg-KO mice does not appear to be due to altered Rhbg expression.
Fig. 6.
Rhbg expression in normal and acid-loaded diet in C and CD-Rhbg/Rhcg-KO mice. A: Rhbg expression in CD-Rhbg/Rhcg-KO mice fed a normal and acid-loaded diet. There was no detectable change in Rhbg expression in the DCT or CNT. B: Rhbg immunolabel in C mice fed a normal and acid-loaded diet. Rhbg expression in the cortex and OM was significantly greater in acid-loaded than in normal diet mice. Basolateral Rhbg immunolabel in OMCD principal cells (arrowheads) was increased in acid-loaded C mice relative to immunolabel in normal diet C mice. Intercalated cell (arrows) basolateral Rhbg immunolabel was unchanged.
Effect of collecting duct Rhbg and Rhcg deletion on ammoniagenic enzyme expression.
An important component of the renal response to metabolic acidosis is increased ammoniagenesis. Moreover, collecting duct ammonia secretion involves both transporter-mediated ammonia movement and diffusive NH3 transport across both the apical and basolateral plasma membranes, and the rate of diffusive NH3 movement across both membranes is concentration dependent (20, 21). Thus it is possible that increased proximal tubule ammonia production could result in increased ammonia availability for diffusive collecting duct ammonia movement. To assess this possibility, we examined expression of proximal tubule ammonia-metabolizing enzymes in response to both metabolic acidosis and to collecting duct-specific Rhbg and Rhcg deletion. In particular, we determined whether exaggerated changes in expression of these proteins could develop and contribute to the increase in ammonia excretion in acid-loaded CD-Rhbg/Rhcg-KO mice.
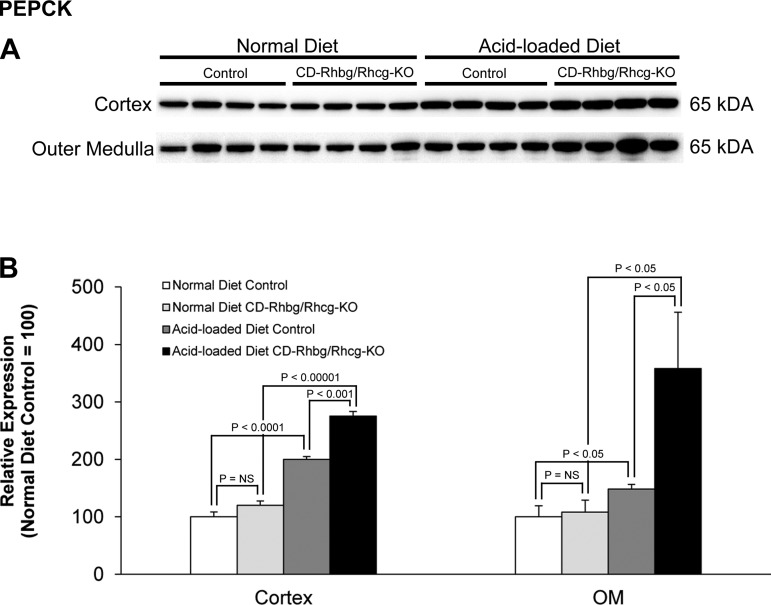
PEPCK mediates an important role in renal ammoniagenesis and gluconeogenesis (8, 9, 36, 39). With a standard diet, PEPCK expression did not differ significantly between CD-Rhbg/Rhcg-KO and control mice (Fig. 7). Acid loading increased PEPCK expression in both control and CD-Rhbg/Rhcg-KO mice, and the increase was significantly greater in CD-Rhbg/Rhcg-KO mice than in control mice in both the cortex and outer medulla. Thus increased PEPCK expression contributes to increased ammoniagenesis in response to acid loading, and exaggerated increases appear to contribute to ammonia excretion in mice with combined collecting duct-specific Rhbg and Rhcg deletion.
Fig. 7.
Phosphenolpyruvate carboxykinase (PEPCK) expression in normal and acid-loaded diet C and CD-Rhbg/Rhcg-KO kidneys. A: immunoblot analysis of PEPCK in the cortex and OM of CD-Rhbg/Rhcg-KO and C mice on a normal diet and when acid loaded. B: quantification of PEPCK expression. CD-Rhbg/Rhcg-KO did not alter PEPCK expression in either the cortex or OM in mice on a normal diet. PEPCK expression was significantly greater in acid-loaded CD-Rhbg/Rhcg-KO than in acid-loaded C mice in both the cortex and OM. Values are means ± SE; n = 4/group.
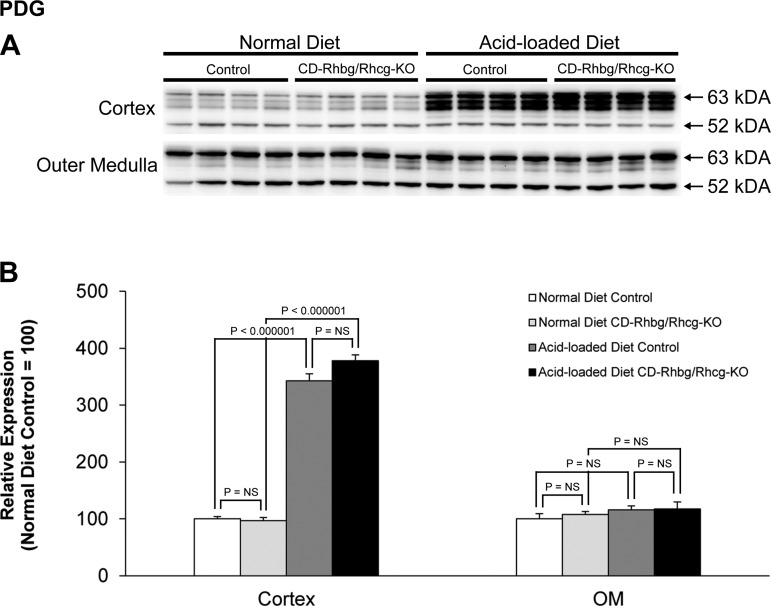
PDG is a mitochondrial protein that mediates an initial step in renal ammoniagenesis (50). With a normal diet, PDG expression did not differ significantly between control and CD-Rhbg/Rhcg-KO mice (Fig. 8). After acid loading, PDG expression increased in both control and CD-Rhbg/Rhcg-KO mice, and expression did not differ significantly between control and CD-Rhbg/Rhcg-KO mice. Thus increased PDG expression contributes to the renal response to acid loading, but exaggerated increases are not a component of the adaptive response to CD-Rhbg/Rhcg-KO.
Fig. 8.
Phosphate-dependent glutaminase (PDG) expression in normal and acid-loaded diet C and CD-Rhbg/Rhcg-KO kidneys. A: immunoblot analysis of PDG in the cortex and OM of CD-Rhbg/Rhcg-KO and C mice fed a normal diet and when acid loaded. B: quantification of PDG expression. Acid loading caused a significant increase in cortical PDG expression in both C and CD-Rhbg/Rhcg-KO mice. CD-Rhbg/Rhcg-KO did not alter PDG expression in either the cortex or OM in mice on a normal and acid-loaded diet. Values are means ± SE; n = 4/group.
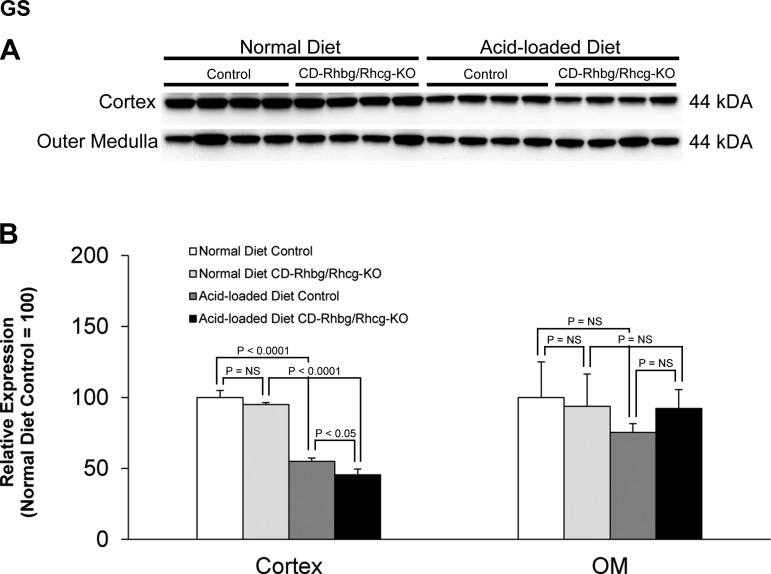
GS can contribute to the regulation of net proximal tubule ammonia production by catalyzing the reaction of NH4+ with glutamate to form glutamine, which results in decreased “net” proximal tubule ammonia production. With a normal diet, GS expression did not differ between control and CD-Rhbg/Rhcg-KO mice in either the cortex or outer medulla. Acid loading decreased GS expression significantly in the cortex in both CD-Rhbg/Rhcg-KO and control mice, and renal cortical GS expression was decreased significantly more in acid-loaded CD-Rhbg/Rhcg-KO than in acid-loaded control mice (Fig. 9). These findings indicate that adaptive decreases in GS expression can facilitate increased net ammoniagenesis in metabolic acidosis. The significantly greater changes in GS expression in acid-loaded CD-Rhbg/Rhcg-KO compared with control mice indicate that further changes in GS-mediated ammonia metabolism may contribute to renal ammonia metabolism and urinary ammonia excretion seen in the absence of collecting duct Rhbg and Rhcg expression.
Fig. 9.
Glutamine synthetase (GS) expression in normal and acid-loaded diet C and CD-Rhbg/Rhcg-KO kidneys. A: immunoblot analysis of GS in the cortex and OM of CD-Rhbg/Rhcg-KO and C mice on a normal diet and when acid loaded. B: quantification of GS expression. CD-Rhbg/Rhcg-KO did not alter GS expression in either the cortex or OM in mice on a normal diet. In the cortex, acid-loading decreased GS expression in both genotypes, and GS expression was significantly decreased in acid-loaded CD-Rhbg/Rhcg-KO compared with acid-loaded C mice. Values are means ± SE; n = 4/group.
NHE-3 expression in response to metabolic acidosis.
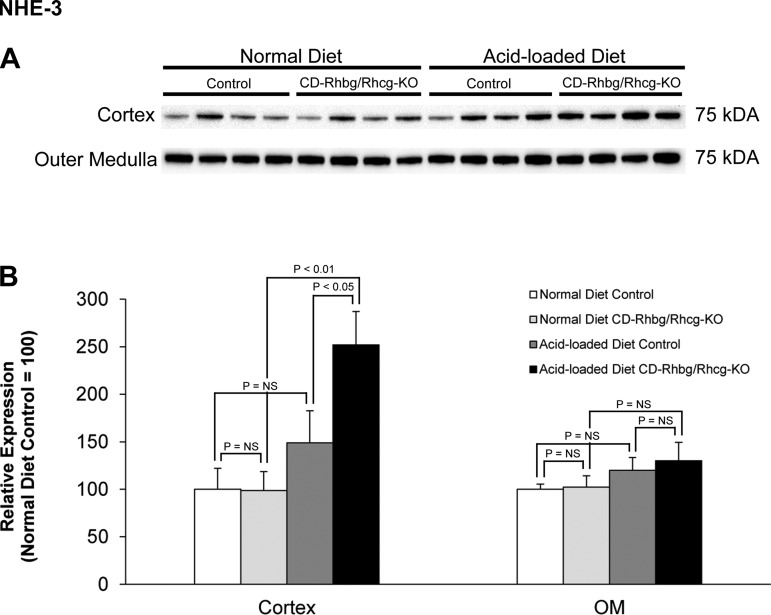
NHE-3 is a member of an extended family of Na+/H+ exchangers, is present in the apical membrane of the proximal tubule, and contributes to both luminal HCO3− reabsorption and to ammonia secretion (6, 23, 49). Previous studies have indicated that changes in NHE-3 expression and increased apical ammonia secretion are an integral response to metabolic acidosis (1, 33, 34, 52). Under basal conditions, NHE-3 expression did not differ between control and CD-Rhbg/Rhcg-KO mice (Fig. 10). Acid-loaded mice with intact Rhbg and Rhcg expression showed a trend for an increase in NHE-3 expression, which did not reach statistical significance. In contrast, in acid-loaded CD-Rhbg/Rhcg-KO mice NHE-3 expression was significantly greater than in both non-acid-loaded CD-Rhbg/Rhcg-KO mice and acid-loaded mice with intact Rhbg and Rhcg expression. Thus increased NHE-3 expression, in conjunction with increased PEPCK-mediated ammoniagenesis and decreased GS-mediated ammonia metabolism, appears to be an adaptive response in acid-loaded mice with combined collecting duct-specific Rhbg and Rhcg deletion.
Fig. 10.
Na+/H+ exchanger NHE-3 expression in normal and acid-loaded diet C and CD-Rhbg/Rhcg-KO kidneys. A: immunoblot analysis of NHE-3 in the cortex and OM of CD-Rhbg/Rhcg-KO and C mice on a normal diet and when acid loaded. B: quantification of NHE-3 expression. CD-Rhbg/Rhcg-KO did not alter NHE-3 expression in either the cortex or OM in mice on a normal diet. In the cortex, NHE-3 expression was significantly greater in acid-loaded CD-Rhbg/Rhcg-KO than in acid-loaded C mice and CD-Rhbg/Rhcg-KO fed a normal diet. Values are means ± SE; n = 4/group.
DISCUSSION
The current studies examine the molecular mechanisms of collecting duct ammonia secretion through the use of collecting duct-specific combined Rhbg and Rhcg gene deletion. Several important observations result from these studies. First, combined collecting duct Rhbg and Rhcg deletion results in substantial inhibition of urinary ammonia excretion and the development of severe metabolic acidosis in response to an oral acid load that typically is well tolerated. Second, collecting duct deletion of both Rhbg and Rhcg does not impair the ability to acidify the urine and instead results in more acidic urine in response to acid loading. Finally, acidosis-induced changes in several proteins critically important for proximal tubule ammoniagenesis and transport, including PEPCK, GS, and NHE-3, were significantly greater in collecting duct Rhbg and Rhcg deletion mice than in wild-type mice. These observations have substantial importance for our understanding of renal ammonia metabolism and of acid-base homeostasis.
The first major finding in this study is that combined collecting duct Rhbg and Rhcg deletion substantially impairs renal ammonia excretion in response to an acid-load. Compared with mice with intact Rhbg and Rhcg expression, mice with collecting duct Rhbg and Rhcg deletion had significantly less ammonia excretion. In addition, significantly more severe metabolic acidosis developed in the acid-loaded KO mice than in acid-loaded mice with intact Rhbg and Rhcg expression. Thus the decreased ability to excrete ammonia led to more severe metabolic acidosis. The results of combined Rhbg and Rhcg deletion on the renal response to metabolic acidosis are greater than observed with single deletion of either Rhbg or Rhcg. Deletion of only Rhcg from the collecting duct, in studies using an identical protocol to that used in the current study, also impaired urinary ammonia excretion, but did not result in nearly the same degree of metabolic acidosis (27). Deletion of only Rhbg impaired ammonia excretion, but did not result in greater degrees of metabolic acidosis than observed in mice with intact Rhbg expression (4). Similarly, adaptive changes in GS expression, which occurred in the current study with combined Rhbg and Rhcg deletion, did not develop in acid-loaded mice with only Rhbg or only Rhcg deletion (4, 27). Last, we demonstrate for the first time adaptive changes in NHE-3 expression in response to combined Rhbg and Rhcg deletion in acid-loaded mice. The significant inhibition of ammonia excretion and the substantial degree of metabolic acidosis observed in the current study with combined Rhbg and Rhcg deletion thus indicates the critical role of both proteins in renal ammonia excretion.
It is likely that the effect of Rhbg and Rhcg deletion on collecting duct ammonia transport was greater than the change in urinary ammonia excretion. In most species, including mice, rats, and humans, normal urine pH is relatively acidic relative to plasma, and increases in urine acidification have an important effect on collecting duct ammonia secretion (12, 17, 25, 26, 42, 43). In animals with intact Rhcg expression, we speculate that some of the stimulation is likely because luminal H+ titrates acidic residues in Rhcg's extracellular vestibule, which may alter its affinity for NH4+ (15) and thereby contribute to luminal pH-mediated regulation of collecting duct ammonia secretion. However, since extracellular acidosis inhibits Rhbg transport activity (35), the specific effect of luminal acidification on Rhcg transport of NH3 is not clear. The effects of luminal H+ concentration are also relevant to mice with collecting duct-specific Rhbg and Rhcg deletion. The apical plasma membrane of collecting duct cells exhibits passive NH3 diffusion, resulting in pH-dependent apical ammonia transport (21). Thus the greater urine acidification in the acid-loaded collecting CD-Rhbg/Rhcg KO mice, by facilitating low luminal NH3 concentration, likely contributes to collecting duct ammonia secretion and causes an underestimation of the role of Rhbg and Rhcg in acidosis-stimulated renal ammonia excretion. As proposed previously, the greater urine acidification in acid-loaded mice with combined Rhbg and Rhcg deletion than in those with intact Rhbg and Rhcg expression is likely due to impaired transport of the basic NH3 molecule (5, 27). In particular, we observed no detectable differences in H+-ATPase immunolocalization between acid-loaded mice with intact and those with collecting duct-specific Rhbg and Rhcg deletion (data not shown).
The presence of increases in ammonia secretion in response to acid-loaded in mice with collecting duct-specific deletion of both Rhcg and Rhbg suggests the presence of Rhbg-and Rhcg-independent collecting duct ammonia secretion. Previous studies show the presence of diffusive NH3 transport, in parallel with Rh glycoprotein-mediated NH3 transport, across both the apical and basolateral plasma membranes of collecting duct cells (20, 21). Studies utilizing isolated, perfused collecting duct segments from acid-loaded mice with global Rhcg gene deletion showed significant Rhcg-independent apical NH3 permeability, which may reflect diffusive NH3 movement (5). Quantitative analyses suggest that under basal conditions that as much as 70% of collecting duct NH3 transport involves diffusive, Rhcg-independent mechanisms (29). Thus it is highly likely that diffusive NH3 transport in the acid-loaded double KO mice enabled continued collecting duct ammonia excretion observed. Alternatively, it is possible that there is increased ammonia secretion in more proximal nephron segments. However, there was no detectable change in either Rhbg or Rhcg expression in either the connecting segment or DCT which could mediate changes.
Rhbg and Rhcg's role in renal ammonia metabolism was probably underestimated due to increased proximal tubule ammoniagenesis and transport. In the current study, expression of PEPCK and NHE3, key proteins involved in proximal tubule ammoniagenesis and ammonia secretion, was significantly greater, and GS expression was significantly less in acid-loaded Rhbg and Rhcg KO mice than in acid-loaded mice with intact Rhbg and Rhcg expression. Since levels of these proteins did not differ in the absence of acid loading, this indicates greater adaptation in PEPCK, NHE-3, and GS expression in response to acid loading. At least some of this greater change may result from the more severe metabolic acidosis in acid-loaded mice with collecting duct-specific Rhbg and Rhcg deletion. Importantly, greater increases in ammoniagenesis and apical NH4+ secretion resulting from these changes in PEPCK, NHE-3, and GS expression would lead to increased renal interstitial ammonia concentrations and to greater gradients for collecting duct ammonia secretion through diffusive NH3 transport. Thus the greater adaptation of PEPCK, NHE-3, and GS expression in acid-loaded Rhbg and Rhcg KO mice, and thus greater net ammoniagenesis, probably results in underestimation of the role of Rhbg and Rhcg in renal ammonia excretion.
Rhesus glycoproteins may also have roles in transport of molecules other than ammonia. Other investigators have shown that heterologous Rh glycoprotein expression in Xenopus oocytes increases CO2 transport (13, 32). However, whether Rhbg and Rhcg expression is necessary for normal CO2 transport is not clear. Many cells that transport CO2, including alveolar endothelial and epithelial cells and gastric parietal cells, do not express Rh glycoproteins (19, 22). Deletion of Rh glycoproteins from cells that do transport CO2 does not impair their function detectably. In particular, intercalated cells require CO2 transport to provide sufficient cytoplasmic CO2 for carbonic anhydrase-mediated formation of the intracellular H+ necessary for apical H+ secretion. Deletion of either Rhbg or Rhcg (4, 5, 27, 28) or both (current study) does not block the ability to acidify urine. However, it is possible that CO2 gradients in the kidney are altered by the absence of Rhbg and Rhcg, thereby enabling sufficient diffusive CO2 transport to maintain the capacity to acidify the urine. Similarly, Rhbg or Rhcg deletion may induce other, currently unidentified CO2 transport mechanisms. Thus, while the current studies do not support a role for Rhbg and Rhcg in renal CO2 transport, they also do not exclude this possibility.
Another important observation in the current study was that acidosis-induced changes in PEPCK, NHE-3, and GS expression were not maximal in acid-loaded mice with intact Rhbg and Rhcg expression despite the presence of persistent metabolic acidosis. This was demonstrated by the finding that deletion of Rhbg and Rhcg resulted in further increases in their expression. Other studies have shown that provision of exogenous glutamine, the substrate for renal ammoniagenesis, to experimental animals with metabolic acidosis further increases ammoniagenesis and urinary ammonia excretion (14, 45, 51). These findings suggest a model in which there is submaximal responsiveness to metabolic acidosis. One explanation for this observation relates to the finding that increased ammoniagenesis requires increased glutamine availability, which requires increased glutamine release from skeletal muscles, and this entails muscle catabolism. Accordingly, the submaximal response to metabolic acidosis may reflect a balance between protection against metabolic acidosis and the need to maintain skeletal muscle mass.
In summary, the current study provides important new information regarding our understanding of the molecular mechanisms of renal ammonia metabolism. During acid loading, combined deletion of Rhbg and Rhcg results in substantial inhibition of both renal ammonia excretion and maintenance of acid-base homeostasis that is greater than observed with single deletion of either Rhbg or Rhcg and thereby confirms the important role of each in renal acid-base homeostasis. Second, in acid-loaded CD-Rhbg/Rhcg-KO mice, exaggerated changes in expression of PEPCK, GS, and NHE-3 likely cause the role of Rhbg and Rhcg to be quantitatively underestimated. These findings provide important advances in our understanding of acid-base homeostasis.
GRANTS
These studies were supported by funds from the National Institutes of Health (R01-DK-045788) and the Department of Veterans Affairs (1I01BX000818-01).
AUTHOR CONTRIBUTIONS
Author contributions: I.D.W., H.W.L., and J.W.V. provided conception and design of research; H.W.L. and M.E.H. performed experiments; I.D.W., H.W.L., J.W.V., M.E.H., and K.H.H. analyzed data; I.D.W, H.W.L, J.W.V., M.E.H., and K.H.H. interpreted results of experiments; I.D.W. and H.W.L prepared figures; I.D.W. and H.W.L. drafter manuscript; I.D.W., H.W. L., J.W.V., and M.E.H. edited and revised manuscript; I.D.W., H.W.L, J.W.V., M.E.H., and K.H.H. approved final version of manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Footnotes
Ammonia exists in two molecular forms, NH3 and NH4+. When using the term “ammonia,” we are referring to both of these forms. When referring specifically to one of the molecular forms, we state specifically either “NH3” or “NH4+.”
We specifically do not assess arterial pH in these studies. Arterial pH is determined by the ratio of dissolved CO2 to HCO3−, as described by the Henderson-Hasselbach equation, pH = 6.1 + log10 ([HCO3−])/(0.03 × Pco2), where brackets indicate concentration. To obtain blood for analysis, we anesthetize and euthanize the mice, and then obtain blood by a laparotomy followed by aortic puncture. During this procedure, we believe that alterations in respiratory rate are likely to develop, and if they develop will necessarily alter Pco2. Artifactual changes in Pco2 resulting from the procedure to obtain blood will cause measured pH to incorrectly reflect pH in the absence of these procedures. Accordingly, we believe that in the absence of continuous assessment of respiratory function during the euthanasia and phlebotomy procedure that we cannot ensure that pH measurements obtained accurately reflect in vivo arterial pH. Because renal acid-base mechanisms directly regulate HCO3− concentration, and anesthesia, euthanasia, and the phlebotomy process do not affect this, we believe that direct measurement of HCO3− concentration accurately indicates the renal contribution to acid-base homeostasis.
REFERENCES
- 1.Ambuhl PM, Amemiya M, Danczkay M, Lotscher M, Kaissling B, Moe OW, Preisig PA, Alpern RJ. Chronic metabolic acidosis increases NHE3 protein abundance in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 271: F917–F925, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Bakouh N, Benjelloun F, Hulin P, Brouillard F, Edelman A, Cherif-Zahar B, Planelles G. NH3 is involved in the NH4+ transport induced by the functional expression of the human Rh C glycoprotein. J Biol Chem 279: 15975–15983, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Bishop JM, Lee HW, Handlogten ME, Han KH, Verlander JW, Weiner ID. Intercalated cell-specific Rh B Glycoprotein deletion diminishes renal ammonia excretion response to hypokalemia. Am J Physiol Renal Physiol 304: F422–F431, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop JM, Verlander JW, Lee HW, Nelson RD, Weiner AJ, Handlogten ME, Weiner ID. Role of the Rhesus glycoprotein, Rh B Glycoprotein, in renal ammonia excretion. Am J Physiol Renal Physiol 299: F1065–F1077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biver S, Belge H, Bourgeois S, Van Vooren P, Nowik M, Scohy S, Houillier P, Szpirer J, Szpirer C, Wagner CA, Devuyst O, Marini AM. A role for Rhesus factor Rhcg in renal ammonium excretion and male fertility. Nature 456: 339–343, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Bobulescu IA, Orson WM. Na+/H+ exchangers in renal regulation of acid-base balance. Semin Nephrol 26: 334–344, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourgeois S, Bounoure L, Christensen EI, Ramakrishnan SK, Houillier P, Devuyst O, Wagner CA. Haploinsufficiency of the ammonia transporter Rhcg predisposes to chronic acidosis. Rhcg is critical for apical and basolateral ammonia transport in the mouse collecting duct. J Biol Chem 288: 5518–5529, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curthoys NP, Taylor L, Hoffert JD, Knepper MA. Proteomic analysis of the adaptive response of rat renal proximal tubules to metabolic acidosis. Am J Physiol Renal Physiol 292: F140–F147, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Drewnowsk KD, Craig MR, DiGiovanni SR, McCarty JM, Moorman AF, Lamers WH, Schoolwerth AC. PEPCK mRNA localization in proximal tubule and gene regulation during metabolic acidosis. J Physiol Pharmacol 53: 3–20, 2002 [PubMed] [Google Scholar]
- 10.Eisner C, Faulhaber-Walter R, Wang Y, Leelahavanichkul A, Yuen PST, Mizel D, Star RA, Briggs JP, Levine M, Schnermann J. Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int 77: 519–526, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, Cherif-Zahar B, Cartron JP, Paillard M, Doucet A, Chambrey R. Expression of RhCG, a new putative NH3/NH4+ transporter, along the rat nephron. J Am Soc Nephrol 13: 1999–2008, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Flessner MF, Wall SM, Knepper MA. Permeabilities of rat collecting duct segments to NH3 and NH4+. Am J Physiol Renal Fluid Electrolyte Physiol 260: F264–F272, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Geyer RR, Parker MD, Burton N, Boron WF, Toye AM, Musa-Aziz R. Relative CO2/NH3 permeabilities of human RhAG, RhBG, and RhCG (Abstract). FASEB J 25: 1040.4, 2011. 21148417 [Google Scholar]
- 14.Gougoux A, Vinay P, Halperin ML. Regulation of renal ammoniagenesis in the dog with chronic metabolic acidosis: effect of a glutamine load. Am J Physiol Renal Fluid Electrolyte Physiol 249: F745–F752, 1985 [DOI] [PubMed] [Google Scholar]
- 15.Gruswitz F, Chaudhary S, Ho JD, Schlessinger A, Pezeshki B, Ho CM, Sali A, Westhoff CM, Stroud RM. Function of human Rh based on structure of RhCG at 2.1 Ä. Proc Natl Acad Sci USA 107: 9638–9643, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamm LL, Simon EE. Roles and mechanisms of urinary buffer excretion. Am J Physiol Renal Fluid Electrolyte Physiol 253: F595–F605, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Hamm LL, Trigg D, Martin D, Gillespie C, Buerkert J. Transport of ammonia in the rabbit cortical collecting tubule. J Clin Invest 75: 478–485, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han KH, Croker BP, Clapp WL, Werner D, Sahni M, Kim J, Kim HY, Handlogten ME, Weiner ID. Expression of the ammonia transporter, Rh C Glycoprotein, in normal and neoplastic human kidney. J Am Soc Nephrol 17: 2670–2679, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han KH, Mekala K, Babida V, Kim HY, Handlogten ME, Verlander JW, Weiner ID. Expression of the gas transporting proteins, Rh B Glycoprotein and Rh C Glycoprotein, in the murine lung. Am J Physiol Lung Cell Mol Physiol 297: L153–L163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Basolateral ammonium transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 287: F628–F638, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Handlogten ME, Hong SP, Westhoff CM, Weiner ID. Apical ammonia transport by the mouse inner medullary collecting duct cell (mIMCD-3). Am J Physiol Renal Physiol 289: F347–F358, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Handlogten ME, Hong SP, Zhang L, Vander AW, Steinbaum ML, Campbell-Thompson M, Weiner ID. Expression of the ammonia transporter proteins, Rh B Glycoprotein and Rh C Glycoprotein, in the intestinal tract. Am J Physiol Gastrointest Liver Physiol 288: G1036–G1047, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Karim Z, Szutkowska M, Vernimmen C, Bichara M. Renal handling of NH3/NH4+: recent concepts. Nephron Physiol 101: 77–81, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Kim HY, Verlander JW, Bishop JM, Cain BD, Han KH, Igarashi P, Lee HW, Handlogten ME, Weiner ID. Basolateral expression of the ammonia transporter family member, Rh C Glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 296: F545–F555, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knepper MA, Good DW, Burg MB. Mechanism of ammonia secretion by cortical collecting ducts of rabbits. Am J Physiol Renal Fluid Electrolyte Physiol 247: F729–F738, 1984 [DOI] [PubMed] [Google Scholar]
- 26.Knepper MA, Good DW, Burg MB. Ammonia and bicarbonate transport by rat cortical collecting ducts perfused in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 249: F870–F877, 1985 [DOI] [PubMed] [Google Scholar]
- 27.Lee HW, Verlander JW, Bishop JM, Igarashi P, Handlogten ME, Weiner ID. Collecting duct-specific Rh C Glycoprotein deletion alters basal and acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 296: F1364–F1375, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HW, Verlander JW, Bishop JM, Nelson RD, Handlogten ME, Weiner ID. Effect of intercalated cell-specific Rh C Glycoprotein deletion on basal and metabolic acidosis-stimulated renal ammonia excretion. Am J Physiol Renal Physiol 299: F369–F379, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee HW, Verlander JW, Bishop JM, Handlogten ME, Han KH, Weiner ID. Renal ammonia excretion in response to hypokalemia: effects of collecting duct-specific Rh C Glycoprotein deletion. Am J Physiol Renal Physiol 304: F410–F421, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mak DO, Dang B, Weiner ID, Foskett JK, Westhoff CM. Characterization of transport by the kidney Rh glycoproteins, RhBG and RhCG. Am J Physiol Renal Physiol 290: F297–F305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.May RC, Kelley RA, Mitch WE. Mechanisms for defects in muscle protein metabolism in rats with chronic uremia: influence of metabolic acidosis. J Clin Invest 79: 1099–1103, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musa-Aziz R, Chen LM, Pelletier MF, Boron WF. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci USA 106: 5406–5411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagami GT. Luminal secretion of ammonia in the mouse proximal tubule perfused in vitro. J Clin Invest 81: 159–164, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagami GT, Sonu CM, Kurokawa K. Ammonia production by isolated mouse proximal tubules perfused in vitro: effect of metabolic acidosis. J Clin Invest 78: 124–129, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakhoul NL, Abdulnour-Nakhoul SM, Schmidt E, Doetjes R, Rabon E, Hamm LL. pH sensitivity of ammonium transport by Rhbg. Am J Physiol Cell Physiol 299: C1386–C1397, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nowik M, Lecca MR, Velic A, Rehrauer H, Brandli AW, Wagner CA. Genome-wide gene expression profiling reveals renal genes regulated during metabolic acidosis. Physiol Genomics 32: 322–334, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Quentin F, Eladari D, Cheval L, Lopez C, Goossens D, Colin Y, Cartron JP, Paillard M, Chambrey R. RhBG and RhCG, the putative ammonia transporters, are expressed in the same cells in the distal nephron. J Am Soc Nephrol 14: 545–554, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Sajo IM, Goldstein MB, Sonnenberg H, Stinebaugh BJ, Wilson DR, Halperin ML. Sites of ammonia addition to tubular fluid in rats with chronic metabolic acidosis. Kidney Int 20: 353–358, 1982 [DOI] [PubMed] [Google Scholar]
- 39.Schoolwerth AC, deBoer PA, Moorman AF, Lamers WH. Changes in mRNAs for enzymes of glutamine metabolism in kidney and liver during ammonium chloride acidosis. Am J Physiol Renal Fluid Electrolyte Physiol 267: F400–F406, 1994 [DOI] [PubMed] [Google Scholar]
- 40.Seshadri RM, Klein JD, Kozlowski S, Sands JM, Kim YH, Handlogten ME, Verlander JW, Weiner ID. Renal expression of the ammonia transporters, Rhbg and Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F397–F408, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Seshadri RM, Klein JD, Smith T, Sands JM, Handlogten ME, Verlander JW, Weiner ID. Changes in the subcellular distribution of the ammonia transporter Rhcg, in response to chronic metabolic acidosis. Am J Physiol Renal Physiol 290: F1443–F1452, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Star RA, Burg MB, Knepper MA. Luminal disequilibrium pH and ammonia transport in outer medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 252: F1148–F1157, 1987 [DOI] [PubMed] [Google Scholar]
- 43.Star RA, Kurtz I, Mejia R, Burg MB, Knepper MA. Disequilibrium pH and ammonia transport in isolated perfused cortical collecting ducts. Am J Physiol Renal Fluid Electrolyte Physiol 253: F1232–F1242, 1987 [DOI] [PubMed] [Google Scholar]
- 44.Vallon V, Eraly SA, Rao SR, Gerasimova M, Rose M, Nagle M, Anzai N, Smith T, Sharma K, Nigam SK, Rieg T. A role for the organic anion transporter OAT3 in renal creatinine secretion in mice. Am J Physiol Renal Physiol 302: F1293–F1299, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Slyke DD, Phillips RA, Hamilton PB, Archibard RM, Futcher PH, Miller A. Glutamine as source material of urinary ammonia. J Biol Chem 150: 481–482, 1943 [Google Scholar]
- 46.Verlander JW, Miller RT, Frank AE, Royaux IE, Kim YH, Weiner ID. Localization of the ammonium transporter proteins, Rh B Glycoprotein and Rh C Glycoprotein, in the mouse kidney. Am J Physiol Renal Physiol 284: F323–F337, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Wagner C, Devuyst O, Bourgeois S, Mohebbi N. Regulated acid-base transport in the collecting duct. Pflügers Arch 458: 137–156, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Weiner ID, Miller RT, Verlander JW. Localization of the ammonium transporters, Rh B Glycoprotein and Rh C Glycoprotein in the mouse liver. Gastroenterology 124: 1432–1440, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Weiner ID, Verlander JW. Role of NH3 and NH4+ transporters in renal acid-base transport. Am J Physiol Renal Physiol 300: F11–F23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiner ID, Verlander JW. Renal acidification mechanisms. In: Brenner and Rector's The Kidney, edited by Taal M, Chertow GM, Marsden PA, Skorecki K, Yu AS, Brenner BM. Philadelphia, PA: Elsevier Saunders; 2012, p. 293–325 [Google Scholar]
- 51.Welbourne T, Weber M, Bank N. The effect of glutamine administration on urinary ammonium excretion in normal subjects and patients with renal disease. J Clin Invest 51: 1852–1860, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu MS, Biemesderfer D, Giebisch G, Aronson PS. Role of NHE3 in mediating renal brush border Na+-H+ exchange. Adaptation to metabolic acidosis. J Biol Chem 271: 32749–32752, 1996 [DOI] [PubMed] [Google Scholar]
- 53.Zidi-Yahiaoui N, Mouro-Chanteloup I, D'Ambrosio AM, Lopez C, Gane P, Kim CVAN, Cartron JP, Colin Y, Ripoche P. Human Rhesus B and Rhesus C glycoproteins: properties of facilitated ammonium transport in recombinant kidney cells. Biochem J 391: 33–40, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zinellu A, Caria MA, Tavera C, Sotgia S, Chessa R, Deiana L, Carru C. Plasma creatinine and creatine quantification by capillary electrophoresis diode array detector. Anal Biochem 342: 186–193, 2005 [DOI] [PubMed] [Google Scholar]