Abstract
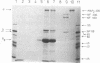
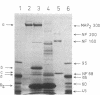
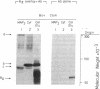
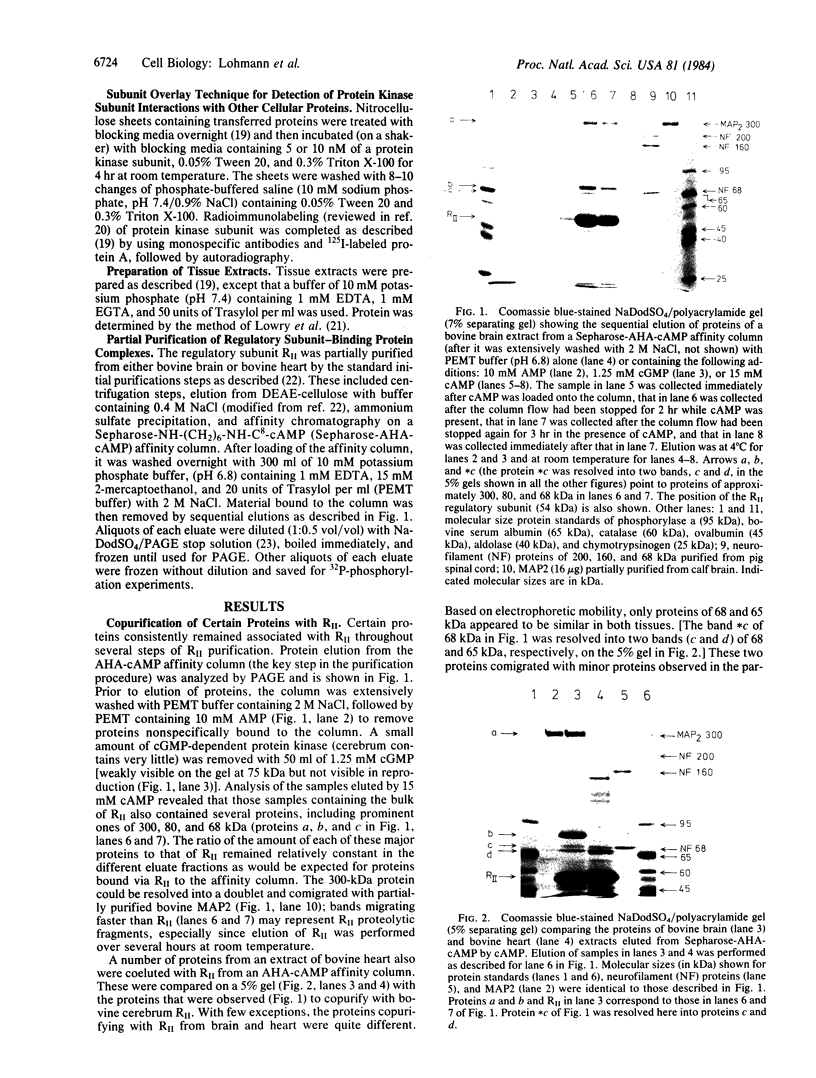
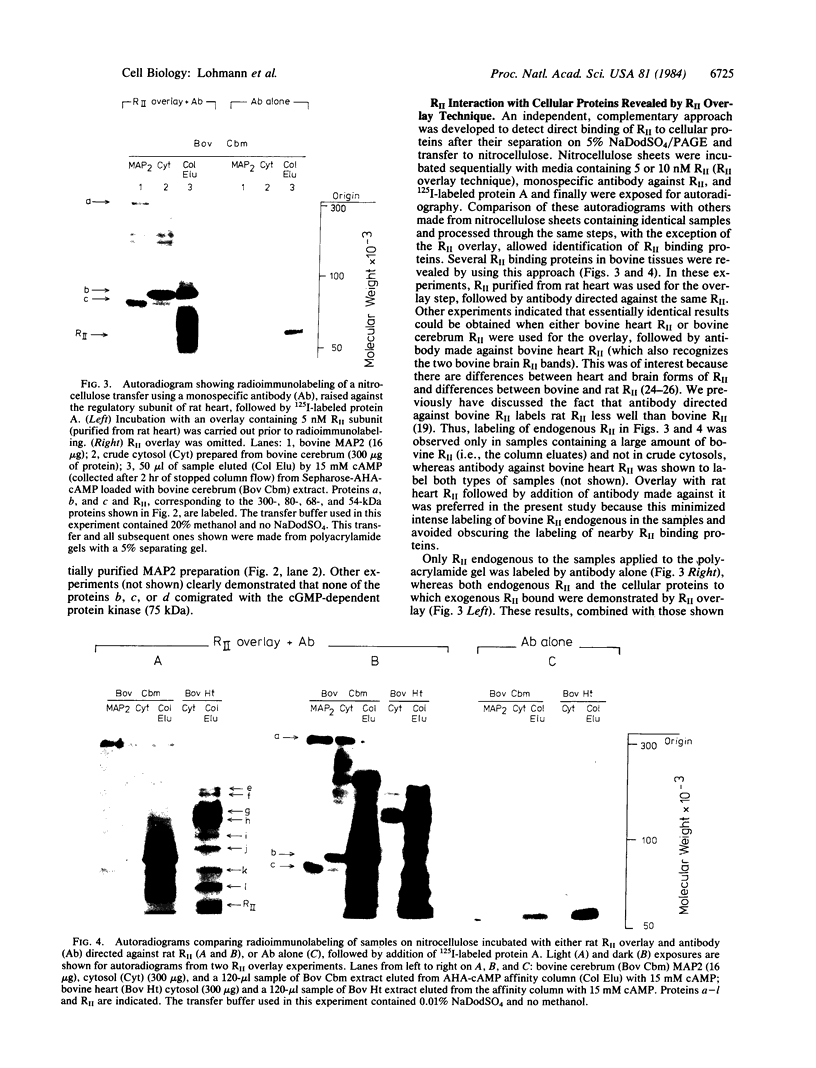
Interaction of the regulatory subunit of the type II cAMP-dependent protein kinase (RII) with tissue-specific cellular binding proteins has been demonstrated by two independent methods. Complexes of RII and its binding proteins were isolated on a cAMP analog-Sepharose affinity column, eluted from the column, and analyzed by sodium dodecyl sulfate/polyacrylamide gel electrophoresis. Alternatively, nitrocellulose blots made from polyacrylamide gels containing samples of tissue extracts or affinity column eluates were treated with sequential overlays of RII, monospecific antibody, and radioiodinated protein A. In bovine cerebrum, specific high-affinity interactions between RII and several binding proteins, including major proteins of 300, 80, and 68 kDa, were recognized by the two methods. The 300-kDa and 68-kDa proteins were identified as microtubule-associated protein 2 (300 kDa) and a protein of lower molecular weight (68 kDa) that copurifies with it. The additional major binding protein of 80 kDa requires further characterization. In addition, several binding proteins distinct from those observed in bovine cerebrum were found in bovine heart. Many of the RII binding proteins from brain and heart served to differing extents as substrates for the purified catalytic subunit of cAMP-dependent protein kinase. One hypothesis of the significance of the protein kinase regulatory subunit interaction with cellular binding proteins is that this may control the protein kinase holoenzyme localization and, thereby, define the substrate targets most accessible for phosphorylation by the activated protein kinase catalytic subunit. Alternatively, RII binding to a variety of cellular proteins might regulate their function--i.e., RII could be a regulator for multiple proteins in addition to the catalytic subunit of the cAMP-dependent protein kinase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Pato M. D., Conti M. A. The role of phosphorylation in regulating contractile proteins. Adv Cyclic Nucleotide Res. 1981;14:361–373. [PubMed] [Google Scholar]
- Cleveland D. W., Hwo S. Y., Kirschner M. W. Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol. 1977 Oct 25;116(2):227–247. doi: 10.1016/0022-2836(77)90214-5. [DOI] [PubMed] [Google Scholar]
- Cohen P., Burchell A., Foulkes J. G., Cohen P. T., Vanaman T. C., Nairn C. Identification of the Ca2+-dependent modulator protein as the fourth subunit of rabbit skeletal muscle phosphorylase kinase. FEBS Lett. 1978 Aug 15;92(2):287–293. doi: 10.1016/0014-5793(78)80772-8. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Sugden P. H., Lincoln T. M., Keely S. L. Compartmentalization of adenosine 3':5'-monophosphate and adenosine 3':5'-monophosphate-dependent protein kinase in heart tissue. J Biol Chem. 1977 Jun 10;252(11):3854–3861. [PubMed] [Google Scholar]
- Corbin J. D., Sugden P. H., West L., Flockhart D. A., Lincoln T. M., McCarthy D. Studies on the properties and mode of action of the purified regulatory subunit of bovine heart adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1978 Jun 10;253(11):3997–4003. [PubMed] [Google Scholar]
- Duerr A., Pallas D., Solomon F. Molecular analysis of cytoplasmic microtubules in situ: identification of both widespread and specific proteins. Cell. 1981 Apr;24(1):203–211. doi: 10.1016/0092-8674(81)90516-x. [DOI] [PubMed] [Google Scholar]
- Erlichman J., Sarkar D., Fleischer N., Rubin C. S. Identification of two subclasses of type II cAMP-dependent protein kinases. Neural-specific and non-neural protein kinases. J Biol Chem. 1980 Sep 10;255(17):8179–8184. [PubMed] [Google Scholar]
- Flockhart D. A., Corbin J. D. Regulatory mechanisms in the control of protein kinases. CRC Crit Rev Biochem. 1982 Feb;12(2):133–186. doi: 10.3109/10409238209108705. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. Self-assembly in Vitro of the 68,000 molecular weight component of the mammalian neurofilament triplet proteins into intermediate-sized filaments. J Mol Biol. 1981 Sep 25;151(3):565–571. doi: 10.1016/0022-2836(81)90011-5. [DOI] [PubMed] [Google Scholar]
- Gershoni J. M., Palade G. E. Protein blotting: principles and applications. Anal Biochem. 1983 May;131(1):1–15. doi: 10.1016/0003-2697(83)90128-8. [DOI] [PubMed] [Google Scholar]
- Greengard P. Intracellular signals in the brain. Harvey Lect. 1979 1980;75:277–331. [PubMed] [Google Scholar]
- Hathaway D. R., Adelstein R. S., Klee C. B. Interaction of calmodulin with myosin light chain kinase and cAMP-dependent protein kinase in bovine brain. J Biol Chem. 1981 Aug 10;256(15):8183–8189. [PubMed] [Google Scholar]
- Hofmann F., Bechtel P. J., Krebs E. G. Concentrations of cyclic AMP-dependent protein kinase subunits in various tissues. J Biol Chem. 1977 Feb 25;252(4):1441–1447. [PubMed] [Google Scholar]
- Huttner W. B., Greengard P. Multiple phosphorylation sites in protein I and their differential regulation by cyclic AMP and calcium. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5402–5406. doi: 10.1073/pnas.76.10.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebritsen T. S., Cohen P. Protein phosphatases: properties and role in cellular regulation. Science. 1983 Jul 22;221(4608):331–338. doi: 10.1126/science.6306765. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Wolff J. Calmodulin binds to both microtubule-associated protein 2 and tau proteins. J Biol Chem. 1984 Jan 25;259(2):1226–1230. [PubMed] [Google Scholar]
- Lockwood A. H. Tubulin assembly protein: immunochemical and immunofluorescent studies on its function and distribution in microtubules and cultured cells. Cell. 1978 Apr;13(4):613–627. doi: 10.1016/0092-8674(78)90212-x. [DOI] [PubMed] [Google Scholar]
- Lohmann S. M., Schwoch G., Reiser G., Port R., Walter U. Dibutyryl cAMP treatment of neuroblastoma-glioma hybrid cells results in selective increase in cAMP-receptor protein (R-I) as measured by monospecific antibodies. EMBO J. 1983;2(2):153–159. doi: 10.1002/j.1460-2075.1983.tb01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann S. M., Walter U., Greengard P. Identification of endogenous substrate proteins for cAMP-dependent protein kinase in bovine brain. J Biol Chem. 1980 Oct 25;255(20):9985–9992. [PubMed] [Google Scholar]
- Miller P., Walter U., Theurkauf W. E., Vallee R. B., De Camilli P. Frozen tissue sections as an experimental system to reveal specific binding sites for the regulatory subunit of type II cAMP-dependent protein kinase in neurons. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5562–5566. doi: 10.1073/pnas.79.18.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E. J., Greengard P. Protein phosphorylation in the brain. Nature. 1983 Oct 13;305(5935):583–588. doi: 10.1038/305583a0. [DOI] [PubMed] [Google Scholar]
- Pallas D., Solomon F. Cytoplasmic microtubule-associated proteins: phosphorylation at novel sites is correlated with their incorporation into assembled microtubules. Cell. 1982 Sep;30(2):407–414. doi: 10.1016/0092-8674(82)90238-0. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Erlichman J., Rosen O. M. Cyclic adenosine 3',5'-monophosphate-dependent protein kinase of human erythrocyte membranes. J Biol Chem. 1972 Oct 10;247(19):6135–6139. [PubMed] [Google Scholar]
- Runge M. S., Detrich H. W., 3rd, Williams R. C., Jr Identification of the major 68,000-dalton protein of microtubule preparations as a 10-nm filament protein and its effects on microtubule assembly in vitro. Biochemistry. 1979 May 1;18(9):1689–1698. doi: 10.1021/bi00576a009. [DOI] [PubMed] [Google Scholar]
- Schwechheimer K., Hofmann F. Properties of regulatory subunit of cyclic AMP-dependent protein kinase (peak I) from rabbit skeletal muscle prepared by urea treatment of the holoenzyme. J Biol Chem. 1977 Nov 10;252(21):7690–7696. [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloboda R. D., Rudolph S. A., Rosenbaum J. L., Greengard P. Cyclic AMP-dependent endogenous phosphorylation of a microtubule-associated protein. Proc Natl Acad Sci U S A. 1975 Jan;72(1):177–181. doi: 10.1073/pnas.72.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. C., Sarkar D., Rubin C. S. Tryptic peptide mapping studies on the regulatory subunits of type II protein kinases from cerebral cortex and heart. Evidence for overall structural divergence and differences in the autophosphorylation and cAMP-binding domains. J Neurochem. 1984 Feb;42(2):547–553. doi: 10.1111/j.1471-4159.1984.tb02712.x. [DOI] [PubMed] [Google Scholar]
- Vallee R. B., DiBartolomeis M. J., Theurkauf W. E. A protein kinase bound to the projection portion of MAP 2 (microtubule-associated protein 2). J Cell Biol. 1981 Sep;90(3):568–576. doi: 10.1083/jcb.90.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. Structure and phosphorylation of microtubule-associated protein 2 (MAP 2). Proc Natl Acad Sci U S A. 1980 Jun;77(6):3206–3210. doi: 10.1073/pnas.77.6.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter U., Kanof P., Schulman H., Greengard P. Adenosine 3':5'-monophosphate receptor proteins in mammalian brain. J Biol Chem. 1978 Sep 10;253(17):6275–6280. [PubMed] [Google Scholar]
- Wang C., Asai D. J., Lazarides E. The 68,000-dalton neurofilament-associated polypeptide is a component of nonneuronal cells and of skeletal myofibrils. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1541–1545. doi: 10.1073/pnas.77.3.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber I. T., Takio K., Titani K., Steitz T. A. The cAMP-binding domains of the regulatory subunit of cAMP-dependent protein kinase and the catabolite gene activator protein are homologous. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7679–7683. doi: 10.1073/pnas.79.24.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T., Fujisawa H. Phosphorylation of microtubule-associated protein 2 by calmodulin-dependent protein kinase (Kinase II) which occurs only in the brain tissues. Biochem Biophys Res Commun. 1982 Dec 15;109(3):975–981. doi: 10.1016/0006-291x(82)92035-6. [DOI] [PubMed] [Google Scholar]