Abstract
For decades, the Brattleboro rat has been a useful model in kidney physiology. These animals manifest central diabetes insipidus (lack of circulating vasopressin) due to a mutation in the vasopressin-neurophysin gene. V2 receptor-mediated vasopressin actions in the kidney can be assessed in these animals by infusing the V2-selective vasopressin analog 1-desamino-8-d-arginine vasopressin (dDAVP). However, the major commercial supplier in the United States has ceased production, creating the need for another reliable experimental model of V2 receptor-mediated vasopressin action in rodents. We designed an in vivo protocol to investigate vasopressin responses in the rat kidney using osmotic minipumps loaded with tolvaptan, a nonpeptide competitive inhibitor of the vasopressin V2 receptor. Tolvaptan-infused rats had a mean urinary osmolality of <300 vs. >2,000 mosmol/kgH2O in vehicle-infused rats. The tolvaptan infusion produced large decreases in the renal abundance of aquaporin-2 (AQP2), aquaporin-3 (AQP3), the β-subunit of the epithelial sodium channel (β-ENaC), and γ-ENaC that were comparable to the differences seen in vehicle-infused vs. vasopressin-infused Brattleboro rats. Thus we conclude that tolvaptan infusion in rats provides an additional model (besides dDAVP-infusion in the Brattleboro rat) for the assessment of V2 receptor-mediated vasopressin actions in the kidney. We also provide ancillary in vitro data in rat inner-medullary-collecting-duct suspensions showing that tolvaptan can block vasopressin's effects on phosphorylation of the water channel AQP2 in vitro. Specifically, tolvaptan almost completely inhibited the ability of vasopressin to increase AQP2 phosphorylation at Ser256, Ser264, and Ser269, while strongly inhibiting a vasopressin-induced decrease in AQP2 phosphorylation at Ser261.
Keywords: aquaporin-2, Brattleboro, osmotic minipump, urea channel, vasopressin
brattleboro rats have been a standard rodent model for the study of renal physiology for decades since their discovery by Valtin and colleagues (36). These animals manifest central diabetes insipidus (lack of circulating vasopressin) due to a mutation in the vasopressin-neurophysin gene (31). Over 1,100 publications have utilized the Brattleboro rat to investigate various aspects of vasopressin action (PubMed terms “Brattleboro vasopressin”). Among these publications are many that identify V2 receptor-mediated vasopressin actions in the kidney by comparing untreated Brattleboro rats with Brattleboro rats that are infused with vasopressin or the V2-receptor-selective vasopressin analog 1-desamino-8-d-arginine vasopressin (dDAVP).
The major commercial supplier of the Brattleboro rat in the United States has ceased production, although these animals can still be obtained from the Rat Resource and Research Center (Columbia, MO; http://www.rrrc.us/Strain/?x=445). This facility, which is supported by a National Institutes of Health (NIH) contract, is designed primarily to maintain rat strains for breeding at other locations, and its ability to supply Brattleboro rats of a specific size and gender at a given time is uncertain. Furthermore, obtaining Brattleboro rats from this facility is administratively complex and in our experience requires several months' lead time. Therefore, there is the need for another reliable experimental model, besides the Brattleboro rat, to investigate V2 receptor-mediated vasopressin actions in the kidney.
Here, we introduce a rat model based on the use of the nonpeptide vasopressin V2-receptor antagonist tolvaptan. This is a member of a relatively new class of drugs (the vaptans) that are in clinical use for treatment of hyponatremic syndromes, such as is seen in congestive heart failure, cirrhosis, and syndrome of inappropriate antidiuretic hormone secretion (SIADH) (12, 30). Also, recent investigations have provided promising evidence for the efficacy of tolvaptan in autosomal dominant polycystic kidney disease, slowing kidney growth and functional decline (35). The goal of the present study is to establish a “subtractive” model in which V2 receptor-mediated vasopressin actions in the kidney can be assessed by comparing vehicle-treated rats (high V2 receptor activation) with tolvaptan-treated rats (low V2 receptor activation). Comparison between these two states can be viewed as analogous to a comparison between dDAVP-treated Brattleboro rats (high V2 receptor activation) vs. vehicle-infused Brattleboro rats (low V2 receptor activation). Here, we show that continuous subcutaneous infusion of tolvaptan using osmotic minipumps produces large decreases in the renal abundance of aquaporin-2 (AQP2), aquaporin-3, β-subunit of the epithelial sodium channel (ENaC), and γ-ENaC that are comparable to the differences between vehicle- and AVP-infused Brattleboro rats. We also provide ancillary data on the action of tolvaptan to block vasopressin's effects on phosphorylation of the water channel aquaporin-2 in vitro. The evidence presented shows that the major molecular targets of vasopressin in the kidney revealed in Brattleboro rats are also modified in a consistent manner by tolvaptan.
METHODS
Rats.
Animal procedures were approved by the National Heart, Lung, and Blood Institute Animal Care and Use Committee (protocol H-0110R2). Pathogen-free male Sprague-Dawley rats (Taconic Farms, Germantown, NY), weighing 190–215 g, were used for these studies. A few studies, where indicated, employed Brattleboro rats obtained from the Rat Resource and Research Center. Rats were euthanized by decapitation without anesthesia. Kidneys were rapidly removed and chilled on ice-cold normal saline before processing for either immunoblotting of kidney homogenates or preparation of inner medullary collecting duct (IMCD) suspensions.
Osmotic minipump infusions.
Rats were anesthetized with isoflurane and implanted subcutaneously with osmotic minipumps (nominal delivery rate 10 μl/h, capacity 2 ml; model 2ML1, Alzet, Palo Alto, CA) with different amounts of tolvaptan (Selleckchem, Houston, TX) dissolved in 50% DMSO and 50% glycerol. After preliminary studies testing different concentrations of tolvaptan, a dose of 2.5 mg·200 g body wt−1·day−1 was chosen for all subsequent studies (tolvaptan concentration, 10.4 mg/ml). Minipumps were loaded the morning of the experiment and placed in the animal without extensive priming in saline to avoid precipitation at the minipump outlet (see appendix 1 for full protocol).
Similar procedures were followed for minipump infusion of AVP in Brattleboro rats. Brattleboro rats were anesthetized with isoflurane and implanted subcutaneously with osmotic minipumps (nominal delivery rate 1 μl/h; model 2001, Alzet) loaded with vehicle or arginine vasopressin (product no. H1780, Bachem) dissolved in saline. The concentration of AVP was set so the pumps delivered AVP at a rate of 105 ng/h. Minipumps were loaded the morning of the experiment and placed in the animal without extensive priming in saline.
Animal diets.
Rats were fed with normal autoclaved rat chow unless specifically noted. In some experiments, animals were given a gelled-agar diet to control water intake (8). The gel food contained 20 ml water·200 g body wt−1·day−1 and 15 g powdered food·200 g body wt−1·day−1. This diet allows the animals to maintain their body fluid balance when they were not given free access to a water bottle.
Urine collection and analysis.
Urine was collected every morning by holding the animal over a piece of parafilm. Urine osmolality was measured by a vapor pressure osmometer (Vapro model 5520; Wescor, Logan, UT).
Immunoblotting of kidney regions.
Kidneys were dissected using a razor blade to obtain the cortex, outer medulla, and inner medulla as previously described (34). Tissue was homogenized by a Polytron Homogenizer (Omni 1000 fitted with a micro-sawtooth generator) in ice-cold isolation solution (pH 7.6) containing 250 mM sucrose, 10 mM triethanolamine, and 1× Halt protease and phosphatase inhibitor cocktail (Thermo Scientific, Rockford, IL). The protein concentration was determined by the BCA method (Thermo Scientific) and adjusted to 2 μg/μl. The samples were diluted in 5× Laemmli sample buffer (7.5% SDS, 30% glycerol, 50 mM Tris, 1:4 ratio) followed by heating to 60°C for 15 min. Then, an SDS-PAGE gel was stained with Coomassie blue to check for equal loading before immunoblotting (20). This “total-protein-profile” approach has been the standard for equal-loading controls for vasopressin studies in our laboratory, because of studies showing that vasopressin alters the abundances of single proteins frequently used as loading controls, viz. GAPDH (29), β-actin (29), and γ-actin (37).
For immunoblotting, proteins were resolved by SDS-PAGE on 4–20% Tris·HCl polyacrylamide gels (Criterion, Bio-Rad) and transferred electrophoretically onto nitrocellulose membranes. Membranes were blocked for 1 h with Odyssey blocking buffer (Li-Cor, Lincoln, NE), rinsed, and probed overnight at 4°C with the respective affinity-purified antibodies at proper dilution (in Odyssey blocking buffer containing 0.1% Tween 20). After a 1-h incubation with secondary antibody (Alexa Fluor 680-nm goat anti-rabbit immunoglobulin G; Invitrogen, Carlsbad, CA) at 1:5,000 dilution, sites of antibody-antigen reaction were detected and quantified using an Odyssey infrared imager (Li-Cor). Band densities were normalized and analyzed statistically as described in the figure legends.
Antibodies.
All primary antibodies used in this study are previously described affinity-purified rabbit polyclonal antibodies. The following holoprotein-directed antibodies were used: AQP2 (14), AQP3 (5), AQP4 (33), urea channel UT-A1 (27), β-ENaC (25), γ-ENaC (25), Na-K-2Cl cotransporter (NKCC2) (26), Na/H exchanger NHE3 (19), and NKCC1 (a generous gift from Dr. J. Turner, NIH) were used. The following phospho-specific antibodies were used: phospho-S256-AQP2 (16), phospho-S261-AQP2 (15), phospho-S264-AQP2 (14) and phospho-S269-AQP2 (14), phospho-S84-UT-A1 (18), phospho-S486-UT-A1 (18), and phospho-T203/phospho-T208-NKCC1 (9). Phospho-antibodies phospho-T202/phospho-Y204-ERK1/2, phospho-T180/phospho-Y204-p38, and phospho-T183/phospho-Y185-JNK were purchased (Cell Signaling Technology, Danvers, MA).
Preparation of IMCD suspensions.
IMCD suspensions were prepared as previously described with slight modification (2). Kidney inner medullas were dissected, minced, and digested by incubation at 37°C for 70–90 min in digestion solution (250 mM sucrose, 10 mM triethanolamine, pH 7.6) containing collagenase B (3 mg/ml; Roche, Indianapolis, IN) and hyaluronidase (1,400 U/ml; Worthington, Lakewood, NJ). The suspensions were centrifuged (70 g, 30 s), and the supernatant was discarded. The pellet was resuspended with IMCD solution that had been preequilibrated with 95% air- 5% CO2 (repeated twice). The composition of the IMCD solution was (in mM) 118 NaCl, 25 NaHCO3, 5 KCl, 4 Na2HPO4, 2 CaCl2, 1.2 MgSO4, 5.5 glucose, and 5 Na acetate (290 mosmol/kgH2O). Samples were incubated with arginine vasopressin (product no. H1780, Bachem) and/or tolvaptan (product no. S2593. Selleckchem) as described in the figure legends.
Upon completion of the incubation, the suspensions were pelleted by centrifugation at 14,000 rpm for 1 min to harvest the IMCD segments. Pellets were resuspended in 1× Laemmli buffer (1.5% SDS, 10 mM Tris, pH 6.8) containing protease and phosphatase inhibitors (see above) and passed through a DNA shredder (Qiagen, Valencia, CA). After protein concentration determination by the BCA method (Thermo Scientific), samples were used for immunoblotting as described above.
Statistical comparisons.
Statistical comparisons were done with either analysis of variance or simple t-tests as indicated in the figure legends.
RESULTS
Optimization of tolvaptan administration to rats.
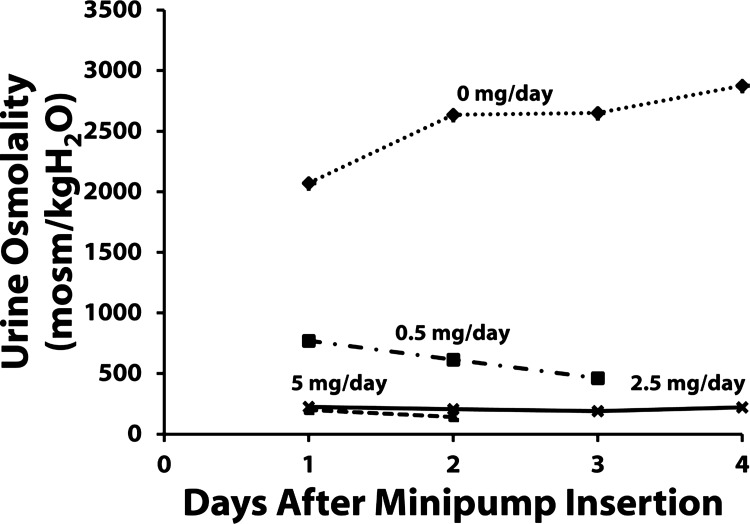
We considered three ways of administering tolvaptan to rats: 1) via drinking water, 2) by adding it to the food, and 3) via osmotic minipumps. Adding tolvaptan to the drinking water proved impractical because of the cost of the drug and the difficulty in maintaining a constant tolvaptan intake in the midst of changing water intake. Adding tolvaptan to the food proved impractical because the animals ate sporadically, and consequently we found highly variable urine osmolalities at different times of day. The third alternative, osmotic minipumps, proved the most practical approach. Initially, we tested different doses of tolvaptan (Fig. 1). A maximal decrease in urine osmolality was achieved with 2.5 mg/day, and we used this dose in further studies.
Fig. 1.
Preliminary experiments to determine effective tolvaptan minipump loading. Daily urine osmolality measurements were obtained during the 4 days after subcutaneous minipump insertion. Minipumps contained concentrations of tolvaptan to give infusion rates of 0, 0.5, 2.5, and 5.0 mg/day.
Effects of tolvaptan infusion on renal abundances of channels and transporters.
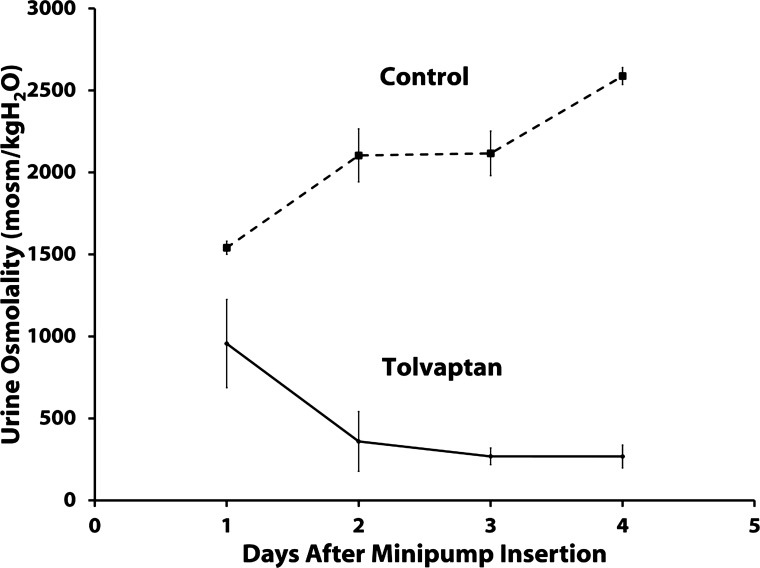
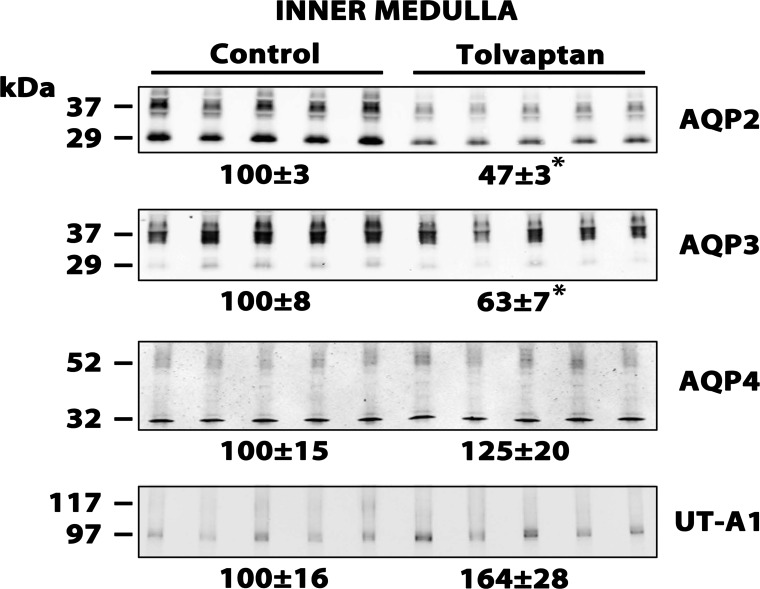
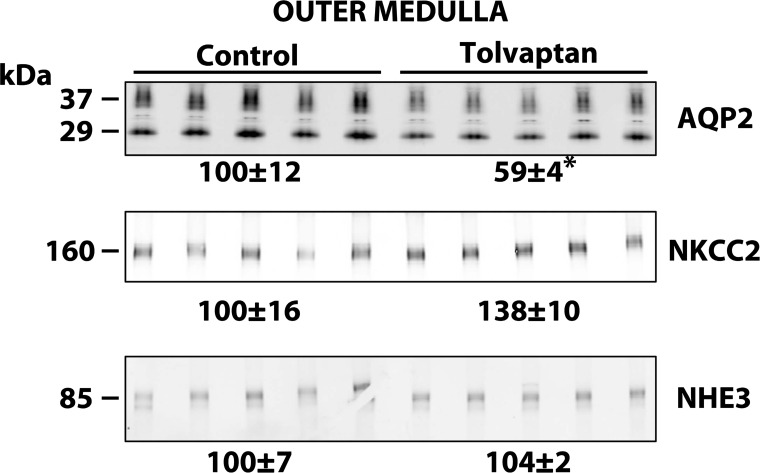
Figure 2 shows the urine osmolalities obtained in the rats we used for immunoblotting. Again, tolvaptan decreased urine osmolality to below 300 mosmol/kgH2O. Figure 3 shows inner medullary immunoblots for the same animals. As may be predicted from prior studies with Brattleboro rats (34), the expression of both AQP2 and AQP3 was reduced by tolvaptan infusion, whereas AQP4 and UT-A1 were not reduced and indeed showed a tendency to increase. As shown in Fig. 4, AQP2 was similarly decreased by tolvaptan in the outer medulla. NHE3 abundance did not change in response to tolvaptan. Interestingly, there was also no significant change in the abundance of NKCC2 in response to tolvaptan. Previous studies in Brattleboro rats were at odds with regard to NKCC2 expression. In one study, dDAVP infusion in Brattleboro rats increased NKCC2 abundance in the outer medulla (20), whereas in another study infusion of a different agent, AVP, did not increase NKCC2 abundance (6).
Fig. 2.
Urine osmolality measurements in the in vivo tolvaptan model. Shown is urine osmolality (means ± SE) for tolvaptan (2.5 mg/day infusion rate) and control groups after minipump insertion, for 4 days; n = 5 for both control and tolvaptan groups.
Fig. 3.
Immunoblots of inner medullas from control and tolvaptan-treated rats. Each lane was loaded with a sample from a different rat. Tolvaptan (2.5 mg/day) or vehicle was infused for 4 days. Loading: aquaporin-2 (AQP2), 15 μg; AQP3, 20 μg; AQP4, 25 μg; and urea transporter UT-A1, 25 μg. Values are normalized band densities covering all bands (means ± SE). *Statistical significance (P < 0.05, t-test).
Fig. 4.
Immunoblots of outer medullas from control and tolvaptan-treated rats. Each lane represents a sample from a different rat. Tolvaptan (2.5 mg/day) or vehicle was infused for 4 days. Loading: AQP2, 15 μg; Na-K-2Cl cotransporter (NKCC2), 20 μg; and Na/H exchanger (HNHE3), 20 μg. Values are normalized band densities covering all bands (means ± SE). *Statistical significance (P < 0.05, t-test).
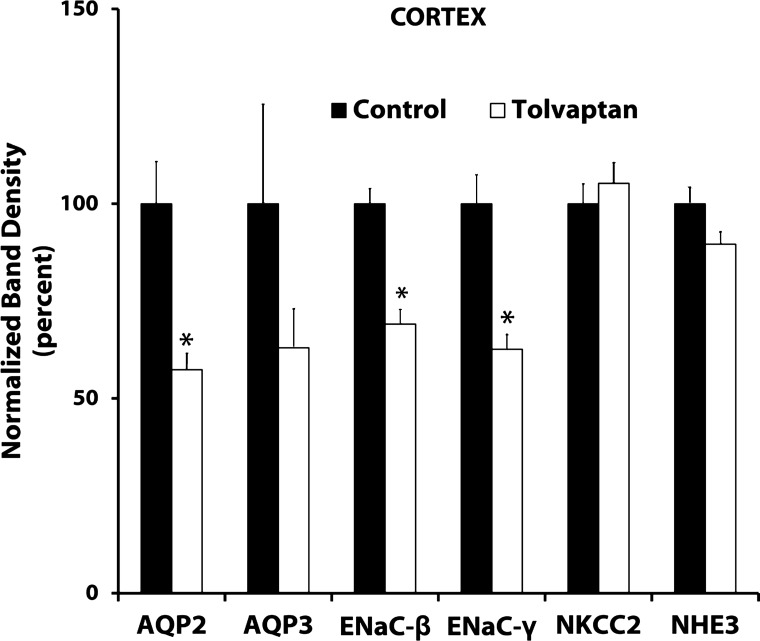
Figure 5 shows a summary of immunoblotting results for the rat renal cortex. As in the other two regions, AQP2 expression was decreased by nearly 50% in response to tolvaptan. AQP3 expression appeared to fall as well, although statistical significance was not attained. Previous studies have shown that abundances of the β- and γ-subunits of ENaC increase in response to dDAVP in rats (4). Consistent with this, Fig. 5 shows that tolvaptan reduced β- and γ-ENaC abundance. Neither NKCC2 nor NHE3 abundance was altered by tolvaptan administration.
Fig. 5.
Summary of immunoblots from cortex of control and tolvaptan-treated rats. Values are normalized band densities (means ± SE). Tolvaptan (2.5 mg/day) or vehicle was infused for 4 days; n = 5 for both control and tolvaptan groups. *Statistical significance (P < 0.05, t-test).
Brattleboro rats.
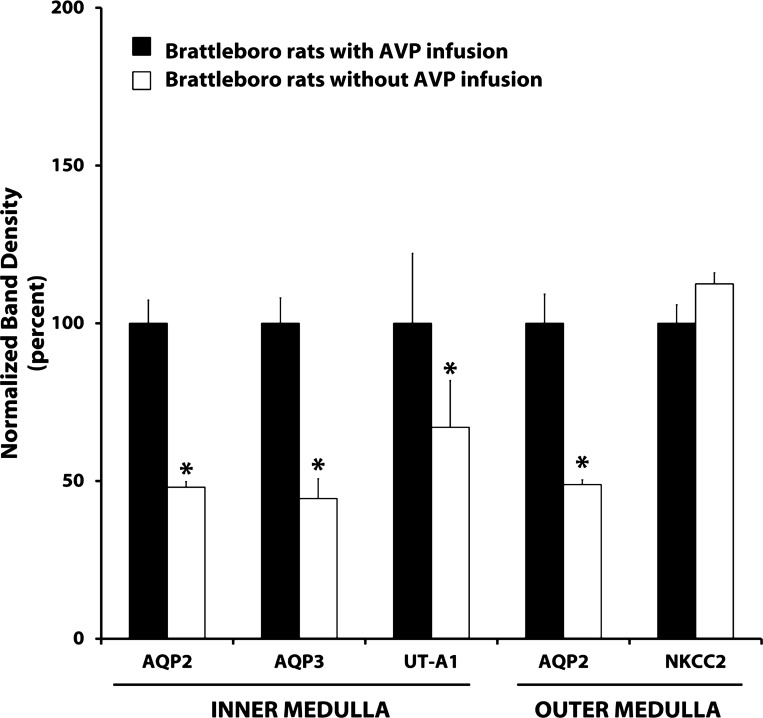
To provide data in the Brattleboro rat obtained with identical immunoblotting techniques, we carried out the additional studies shown in Fig. 6. AVP was infused into Brattleboro rats in a manner similar to that of Kishore et al. (21), delivering 105 ng/h of AVP. Both AQP2 and AQP3 in the inner medulla and AQP2 in the outer medulla showed an ∼50% lower expression level in the vehicle-infused Brattleboro rats vs. the AVP-infused rats. Interestingly, there was a small but significant decrease in urea channel UT-A1 expression in vehicle- vs. AVP-infused rats, contrasting with the findings with tolvaptan infusion. As seen previously (6), NKCC2 expression was unchanged by AVP infusion.
Fig. 6.
Summary of immunoblots of inner and outer medullas from Brattleboro rats. Values are normalized band densities (means ± SE). AVP (105 ng/h) or vehicle was infused for 5 days; n = 4 for all groups. *Statistical significance (P < 0.05, t-test).
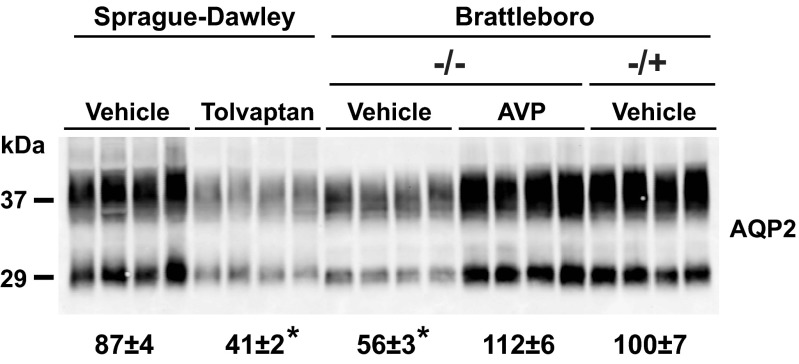
Figure 7 shows a comparison of AQP2 abundances in inner medullas of Sprague-Dawley rats, homozygous Brattleboro rats (−/−), and heterozygous Brattleboro rats (+/−). The heterozygous rats have one intact AVP-neurophysin allele, allowing normal or near normal levels of circulating AVP. As indicated by the densitometry values at the bottom, AQP2 abundances were similar in vehicle-treated Sprague-Dawley rats, AVP-treated −/− Brattleboro rats, and vehicle-treated −/+ Brattleboro rats. Consistent with the data reported above, the AQP2 abundance was markedly suppressed in vehicle-treated −/− Brattleboro rats and tolvaptan-treated Sprague-Dawley rats.
Fig. 7.
Comparison of AQP2 abundances in inner medullas of Sprague-Dawley rats, homozygous Brattleboro rats (−/−) and heterozygous Brattleboro rats (+/−). Here, −/− means that both AVP-neurophysin gene alleles are inactivated, producing a lack of circulating AVP, and −/+ means that 1 AVP-neurophysin allele is intact, allowing normal or near normal levels of circulating AVP. As can be seen, AQP2 abundances are similar in vehicle-treated Sprague-Dawley, AVP-treated −/− Brattleboro, and vehicle-treated −/+ Brattleboro rats. In contrast, the AQP2 inner medullary abundance is markedly suppressed in vehicle-treated −/− Brattleboro rats and tolvaptan-treated Sprague-Dawley rats. Densitometry values (bottom) are normalized to the vehicle-treated −/+ Brattleboro rats.
Effect of vasopressin dose on AQP2 phosphorylation in vitro.
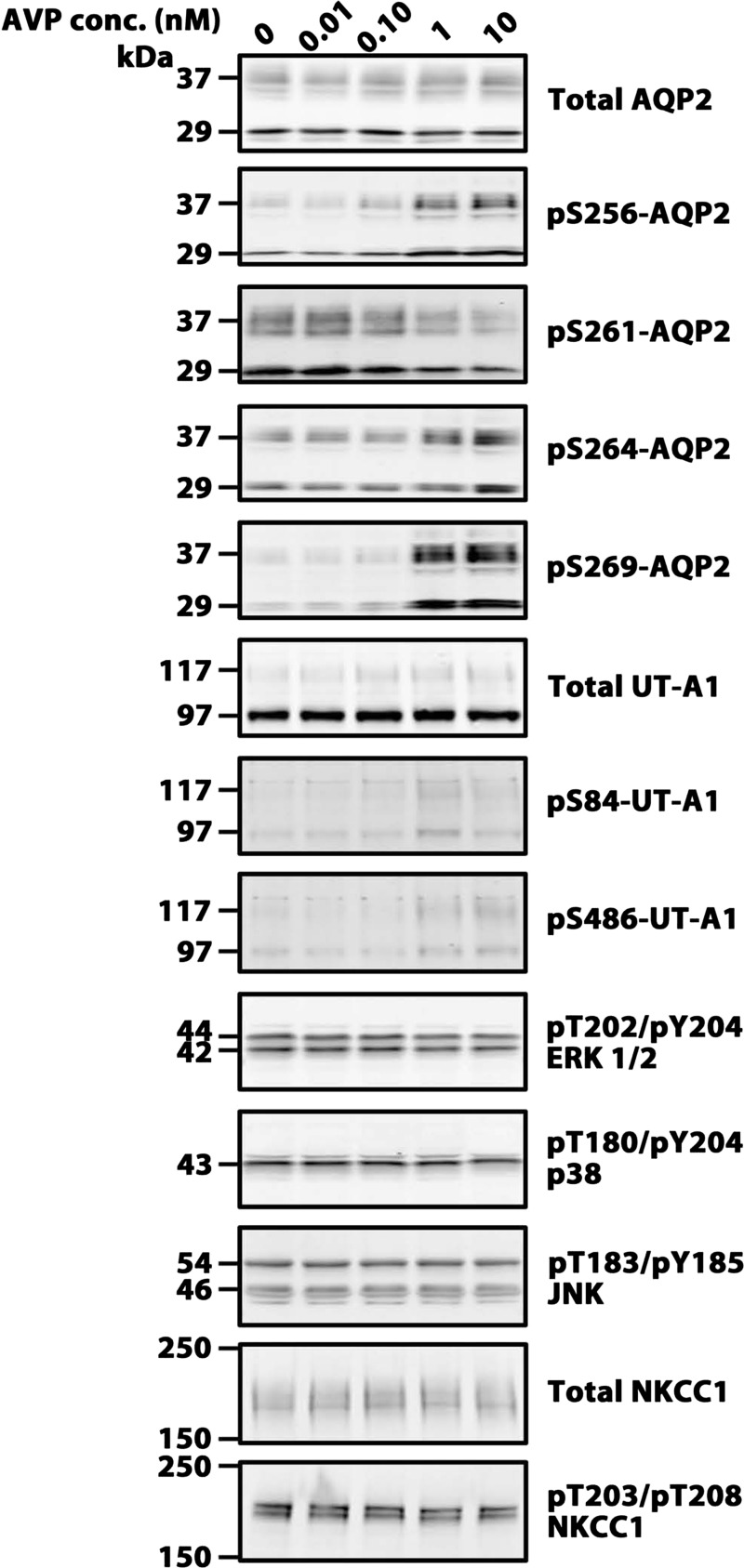
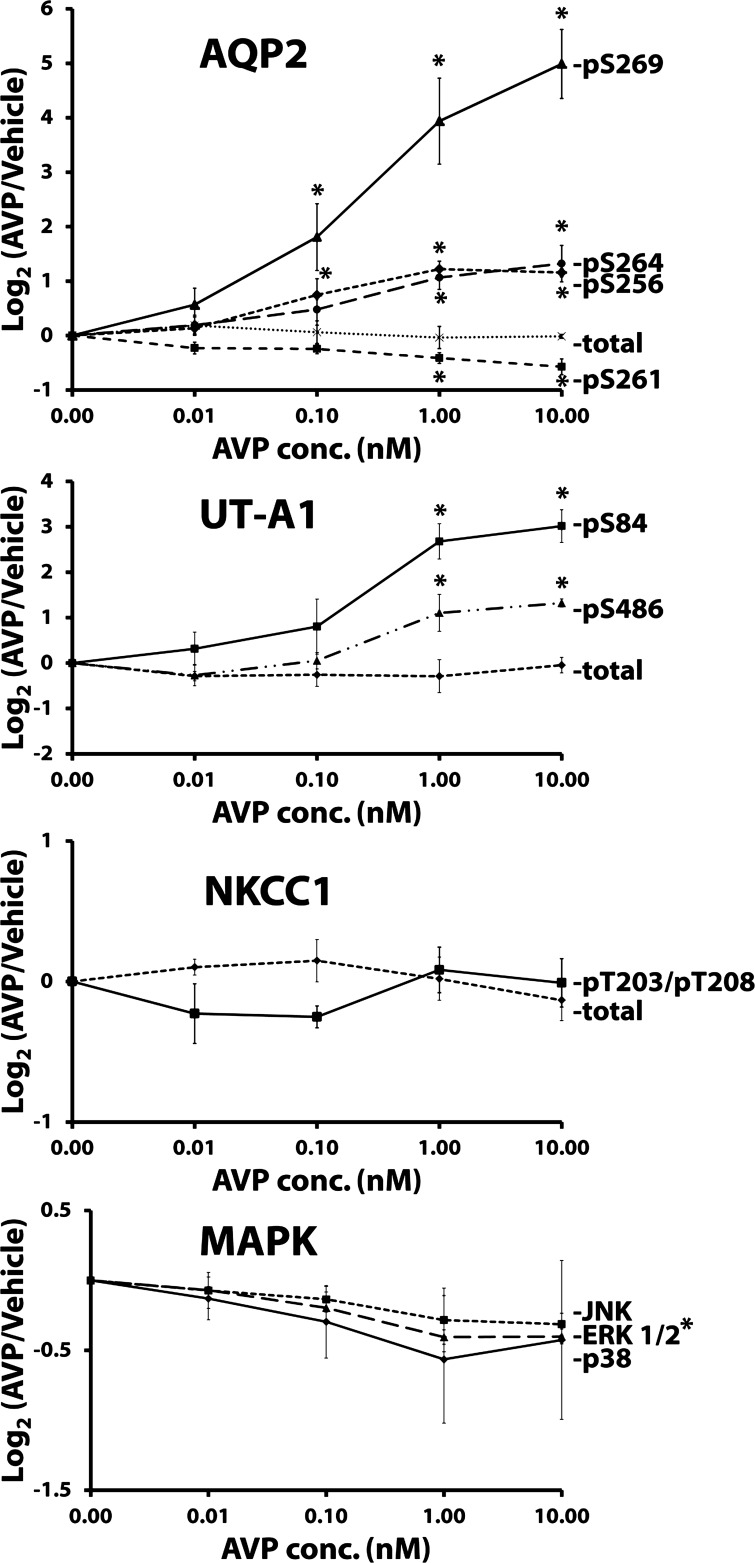
To provide additional data on tolvaptan effects in the collecting duct, we carried out studies in IMCD suspensions in which both AVP and tolvaptan were varied. The readout was phosphorylation levels of AQP2, UT-A1, NKCC1, and MAPK family members. Figure 8 shows immunoblots with dose-response data for AVP in one set of experiments, and Fig. 9 shows a summary of all the data. For AQP2, all four of the known phosphorylation sites were assessed: three of the sites (Ser256, Ser 264, Ser269) showed increases in phosphorylation as seen previously (14). However, the magnitude of the response continues to increase above 0.1 nM AVP, which is roughly the circulating level of AVP seen in high-AVP states (32). In addition, we confirmed the decrease in Ser261 phosphorylation seen previously (15, 17), and again the response increased beyond the physiological concentration of AVP. UT-A1 phosphorylation at Ser84 and Ser486 also showed increases in response to AVP as seen previously (18). In contrast, AVP did not significantly affect NKCC1 phosphorylation. There was a tendency toward a fall in phosphorylation of JNK, ERK1/2, and p38 in response to AVP. However, statistical significance was only seen in ERK1/2.
Fig. 8.
Immunoblots of vasopressin dose-response experiments in isolated inner medullary collecting duct (IMCD) suspensions. Each lane represents a different vasopressin concentration (30-min incubation). Loading: AQP2, 5 μg; pS256, 20 μg; pS261, 20 μg; pS264, 15 μg; pS269, 20 μg; UT-A1, 30 μg; pS84, 30 μg; pS486, 30 μg; ERK 1/2, 20 μg; p38, 20 μg; JNK, 20 μg; NKCC1, 20 μg; and pNKCC1, 25 μg. Summary of all data is shown in Fig. 9.
Fig. 9.
Vasopressin dose-response in isolated IMCD suspensions. Vasopressin incubation period was 30 min in all cases. Values are normalized band densities covering all bands (means ± SE); n = 3 for all groups. *Statistical significance (P < 0.05, ANOVA with Bonferroni contrast). ERK 1/2 is statistically significant at AVP concentrations of 1.00 and 10.00 nM.
Effect of tolvaptan dose on AQP2 phosphorylation in vitro.
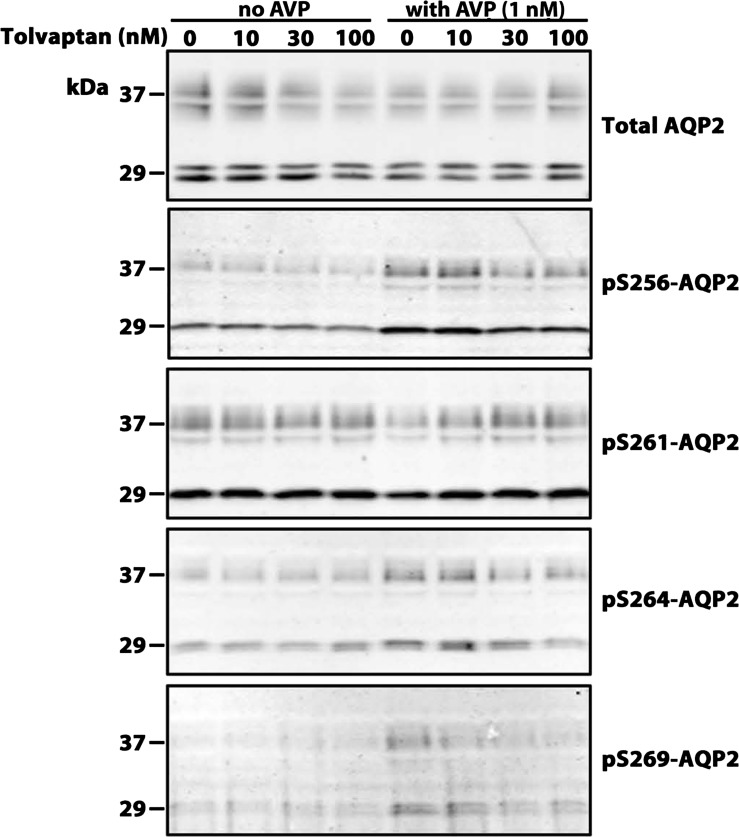
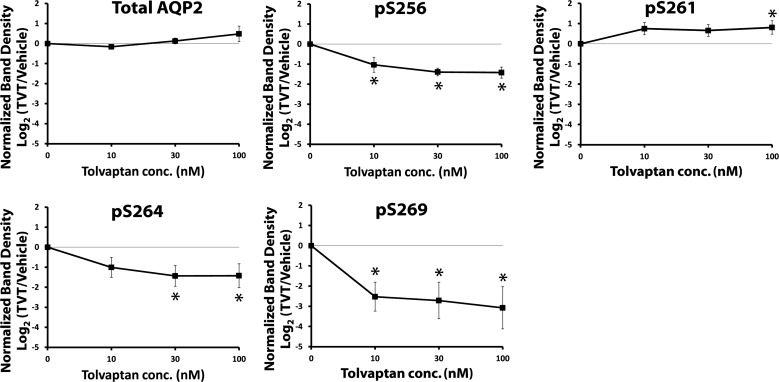
Figure 10 shows immunoblots with dose-response data for tolvaptan in the presence and absence of AVP in one set of experiments, and Fig. 11 shows a summary of all the data. In the presence of AVP, tolvaptan showed a dose-dependent decrease in AQP2 phosphorylation at Ser256, Ser264, and Ser269. It showed a dose-dependent increase in AQP2 phosphorylation at Ser261. There was no effect of tolvaptan in the absence of AVP. Thus the action of tolvaptan in isolated IMCD suspensions was consistent with expectations based on simple inhibition of the V2 receptor.
Fig. 10.
Immunoblots of tolvaptan dose-response experiments in isolated IMCD suspensions. Each lane represents a different tolvaptan concentration in the presence and absence of vasopressin. Tolvaptan/vehicle preincubation period was 30 min followed by addition of AVP at the indicated concentration or vehicle. Loading: AQP2, 5 μg; pS256, 20 μg; pS261, 20 μg; pS264, 15 μg; and pS269, 20 μg. Summary of all data is given in Fig. 11.
Fig. 11.
AQP2 phosphorylation in isolated IMCD suspensions in the presence of vasopressin, tolvaptan dose-response. Tolvaptan/vehicle preincubation period was 30 min followed by addition of AVP at the indicated concentration or vehicle. Values are normalized band densities covering all bands (means ± SE); n = 4. *Statistical significance (P < 0.05, ANOVA with Bonferroni contrast).
DISCUSSION
The conclusions that we can draw from this study are the following. 1) The tolvaptan-infusion model described in this paper is an effective means of assessing V2 receptor actions in the kidney, providing an alternative to dDAVP infusion in Brattleboro rats. 2) In an in vitro setting, tolvaptan nearly completely inhibits the action of vasopressin to alter phosphorylation of collecting duct proteins AQP2, UT-A1, and ERK1/2. In the remainder of this discussion, we analyze the evidence for these conclusions.
The evidence for the first conclusion came from immunoblotting studies of kidney tissue from tolvaptan- and vehicle-infused Sprague-Dawley rats showing marked decreases in the expression levels of AQP2, AQP3, β-ENaC, γ-ENaC, all of which are expressed in the collecting duct system. The decrease in renal AQP2 abundance in the tolvaptan model was ∼50% of the control. We wanted to know how this compares with the difference between vehicle-infused vs. vasopressin-infused Brattleboro rats (Fig. 6). Indeed, the difference in AQP2 expression in vasopressin-deficient vs. vasopressin-replete Brattleboro rats was of a similar magnitude to that seen with the tolvaptan model. Thus we expect the tolvaptan infusion model introduced here to provide a sensitive means of detecting other vasopressin-regulated proteins in the kidney. To aid investigators in setting up this model, we have provided the protocol for preparation of the osmotic minipumps for tolvaptan infusion as an appendix to this paper.
Of course, as long as Brattleboro rats continue to be available through noncommercial sources, the Brattleboro rat model will most likely continue to be a valuable tool in renal physiology. The tolvaptan infusion model provides an additional animal model that can be added to the armamentarium of tools for the study of the physiological effects of V2 receptor occupation. Instead of an “additive” model, as is dDAVP-infusion in Brattleboro rats, the tolvaptan-infusion model can be viewed as a “subtractive” model in which V2 receptor effects are deduced from a comparison of vehicle-infused rats (high V2 receptor occupancy) and tolvaptan-infused rats (low V2 receptor occupancy). The success of this model is dependent on the propensity of normal laboratory rats to spontaneously maintain a state of antidiuresis. Indeed, the vehicle-infused control rats in these studies had urinary osmolalities that were generally >2,000 mosmol/kgH2O, that is, around seven times more concentrated than blood plasma. In reducing the urinary osmolality from ∼2,100 to ∼300 mosmol/kgH2O, we estimate that the tolvaptan infusion reduced total collecting duct water reabsorption by roughly 50% (appendix 2), which is similar to the percent decrease in AQP2 abundance found.
Although the tolvaptan model described here is useful as a subtractive model of V2 receptor-mediated vasopressin effects, the tolvaptan-infused rat cannot be considered a “replacement” (per se) for the Brattleboro rat in all regards. In particular, unlike vasopressin infusions in Brattleboro rats, vasopressin infusions into tolvaptan-treated rats are unlikely to create measurable responses except when responses are mediated by receptors other than V2, e.g., the oxytocin receptor or the vasopressin V1a receptor.
An important factor in an investigator's decision to use the tolvaptan model in studies of V2 receptor action in the kidney is cost. The current cost of tolvaptan is quite high ($940 for enough to infuse 1 set of 5 rats for 4–8 days). The cost is pegged to the cost of the drug when used clinically as a therapeutic agent (currently ∼$250/day in humans in the United States), which is presumably set at a high level to recoup the costs of drug development and testing. Although forecasts can be dangerous, we predict that the cost of tolvaptan will fall with time, depending on future regulatory decisions, competition, and ultimately patent expiration. However, the model should be attractive to renal physiologists even at the current cost because of the simplicity of setting up the experiments vs. the current complexity involved in obtaining Brattleboro rats. Alternatively, considerable cost savings could be attained through use of mice rather than rats, since it is likely that much less vasopressin would be required to achieve an equal lowering of urinary osmolality.
Of course, the effect of tolvaptan to decrease AQP2 expression in the kidney cannot be considered a surprise as extensive studies with a closely related V2 receptor antagonist called OPC-31260 in rats showed a marked decrease in AQP2 mRNA (10, 24, 39) and AQP2 protein (24, 39) in kidney tissue. More recently, a study in rats with congestive heart failure showed that long-term oral administration of tolvaptan markedly reduced the high renal AQP2 levels associated with elevated circulating vasopressin levels (38). To our knowledge, the effects of tolvaptan to decrease the renal abundances of AQP3, β-ENaC, and γ-ENaC have not previously been reported, although they are not surprising because of prior results with dDAVP infusion in Brattleboro rats (4, 34).
One result that was surprising was the lack of response of NKCC2, a thick ascending limb Na-K-Cl cotransporter, to tolvaptan since we have previously reported that dDAVP infusion in Brattleboro rats increases NKCC2 expression (20). However, another paper did not show a significant change in NKCC2 expression in response to a different agent, AVP (6). Since dDAVP is V2 selective, we would have expected a significant decrease in NKCC2 expression with tolvaptan. We have no explanation for this finding from the current data. Prior studies (3, 7) indicate that the V2 receptor in the thick ascending limb undergoes marked desensitization, not seen in the collecting duct, when animals are treated for several days with dDAVP. Subsequent studies have implicated β-arrestin in G protein-coupled receptor internalization (28), raising the possibility that the β-arrestin branch of V2 receptor signaling may differ between the thick ascending limb and collecting duct. Alternatively, it seems possible that tolvaptan has effects in the thick ascending limb other than blockade of the V2 receptor, which could nullify its V2 effect. An additional possibility is that changes in local osmolality brought about by tolvaptan in the outer medulla affects NKCC2 expression. Vasopressin inhibition by water loading has been recognized to decrease medullary osmolality in rats (1). Therefore, we would expect tolvaptan to dissipate outer medullary osmolality. The work of Hao et al. (13) in cultured thick ascending limb cells showed that lowering the osmolality decreases NKCC2 abundance rather than increasing it, contrary to the above hypothesis.
The second conclusion was that in an in vitro setting (isolated rat inner medullary collecting duct suspensions), tolvaptan nearly completely inhibits the action of vasopressin to alter phosphorylation of collecting duct proteins AQP2, UT-A1, and ERK1/2. We did in vitro studies in IMCD suspensions primarily to look for vasopressin-independent effects of tolvaptan. In fact, for all of the phosphorylation sites examined in these studies, tolvaptan had no demonstrable effects in the absence of vasopressin. In the presence of AVP, tolvaptan inhibited changes in phosphorylation at phosphorylation sites previously demonstrated to be vasopressin regulated in AQP2, UT-A1, and ERK1/2. In contrast, there were no effects of tolvaptan on NKCC1 phosphorylation, which is not recognized to be sensitive to vasopressin.
We did preliminary studies in IMCD suspensions to assess phosphorylation changes in response to variations in AVP concentration. An interesting result from this set of experiments was that phosphorylation changes in AQP2 continued to increase at increasing AVP concentrations >0.1 nM, the concentration that gives maximal water permeability responses in isolated, perfused IMCD (32). Previous studies have also shown that cAMP levels continue to rise as AVP is increased above 0.1 nM (32). These results suggest that AQP2 phosphorylation is not the sole determinant of collecting duct water permeability, and other factors may limit the number of AQP2 water channels in the apical plasma membrane.
GRANTS
The study was carried out in the intramural program of the National Heart, Lung, and Blood Institute (Project ZO1-HL001285, M. A. Knepper). C. A. Miranda was a member of the National Institutes of Health (NIH) Medical Research Scholars Program, a public-private partnership supported jointly by the NIH, and generous contributions to the Foundation for the NIH from Pfizer, Inc., The Leona M. and Harry B. Helmsley Charitable Trust, and the Howard Hughes Medical Institute, as well as other private donors.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.A.M., J.W.L., C.-L.C., and M.A.K. provided conception and design of research; C.A.M. and C.-L.C. performed experiments; C.A.M., C.-L.C., and M.A.K. analyzed data; C.A.M., J.W.L., C.-L.C., and M.A.K. interpreted results of experiments; C.A.M. and C.-L.C. prepared figures; C.A.M., C.-L.C., and M.A.K. drafted manuscript; C.A.M., J.W.L., C.-L.C., and M.A.K. edited and revised manuscript; C.A.M., J.W.L., C.-L.C., and M.A.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Animal Surgery and Resources Core at the National Heart, Lung, and Blood Institute (Dr. J. Hawkins, Director) for providing resources needed for osmotic minipump implantation.
APPENDIX 1
The following is the protocol used for preparation and insertion of tolvaptan-containing osmotic minipumps into Sprague-Dawley rats, which can be modified for use in other studies.
Animals
1. Obtain 10 male Sprague-Dawley rats weighing ∼200 g.
2. Randomly divide the two groups equally: control and tolvaptan.
Preparation of Tolvaptan and Minipump
1. Weigh out 104 mg of tolvaptan, enough for the 5 rats in the tolvaptan group, and dissolve in 10 ml of 50% DMSO and 50% glycerol to make a final concentration of 10.4 mg/ml.
2. Load 10 osmotic minipumps (Alzet model 2ML1, capacity 2 ml) as per instructions from the manufacturer. Load five minipumps with vehicle only and five minipumps with tolvaptan.
3. Place the minipumps in separate, prelabeled 50-ml conical-shaped tubes containing normal saline at 37°C. Use within 1 h to avoid occlusion due to precipitation at outlet.
Subcutaneous Minipump Insertion
1. Anesthetize rats with vaporized isoflurane and shave dorsal neck from ears to shoulders.
2. Properly secure rat to surgical table and sterilize shaved skin with isopropyl alcohol and betadine, alternating three times each.
3. Using sterile surgical instruments, make a 2-cm incision and create a small subcutaneous pouch for implantation of the minipump.
4. Place minipump with vehicle or tolvaptan in the pouch and cap outlet facing caudally. Note: check the minipump outlet for precipitate and remove with sterile probe.
5. Close incision with surgical staples.
APPENDIX 2
Here, we provide a simple estimate of the amount of water absorption in the collecting duct system (connecting tubule, cortical collecting duct, outer medullary collecting duct, inner medullary collecting duct) in the vehicle-treated vs. tolvaptan-treated Sprague-Dawley rats in this paper. The calculation assumes that the glomerular filtration rate (GFR), tubule fluid flow out of the distal convoluted tubule, and osmolality in the distal convoluted tubule are not altered by the tolvaptan infusion. We assume the following values from the literature for rats (normalized to 200-g body wt): GFR, 1,000 μl/min (22); tubule-fluid-to-plasma inulin ratio in early distal tubule (TF/Pinulin), 6 (23); and osmolality (mosmol/kgH2O) in early distal tubule (ODCT), 150 (11). If we make the simplifying assumption that increases in osmolality between the early distal tubule and the final urine are due predominantly to water reabsorption along the collecting ducts, we can estimate the net collecting duct water reabsorption rate (Rwater) for both cases using the equation
where Ourine is the final urinary osmolality. The values for Ourine were: 2,100 mosmol/kgH2O for vehicle-infused rats and 300 mosmol/kgH2O for tolvaptan-infused rats (Fig. 2, day 2). Evaluating the equation for the vehicle-infused rats (Ourine = 2,100 mosmol/kgH2O), Rwater is 155 μl/min. Evaluating the equation for the tolvaptan-infused rats (Ourine = 300 mosmol/kgH2O), Rwater is 83 μl/min, or slightly more than half of the vehicle control. Thus we estimate from this simple calculation that a long-term tolvaptan infusion reduced water reabsorption in the collecting duct system by roughly 46%.
REFERENCES
- 1.Atherton JC, Hai MA, Thomas S. The time course of changes in renal tissue composition during water diuresis in the rat. J Physiol (Lond) 197: 429–443, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chou CL, Rapko SI, Knepper MA. Phosphoinositide signaling in rat inner medullary collecting duct. Am J Physiol Renal Physiol 274: F564–F572, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Dublineau I, Elalouf JM, Pradelles P, DeRouffignac C. Independent desensitization of rat renal thick ascending limbs and collecting ducts to ADH. Am J Physiol Renal Fluid Electrolyte Physiol 256: F656–F663, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA. Vasopressin-mediated regulation of ENaC abundance in rat kidney. Am J Physiol Renal Physiol 279: F46–F53, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Ecelbarger CA, Terris J, Frindt G, Echevarria M, Marples D, Nielsen S, Knepper MA. Aquaporin-3 water channel localization and regulation in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 269: F663–F672, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Ecelbarger CA, Terris J, Hoyer JR, Nielsen S, Wade JB, Knepper MA. Localization and regulation of the rat renal Na+-K+-2Cl− cotransporter, BSC-1. Am J Physiol Renal Fluid Electrolyte Physiol 271: F619–F628, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Elalouf JM, DiStefano A, DeRouffignac C. Sensitivities of rat kidney thick ascending limbs and collecting ducts to vasopressin in vivo. Proc Natl Acad Sci USA 83: 2276–2280, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenton RA, Chou CL, Stewart GS, Smith CP, Knepper MA. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci USA 101: 7469–7474, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flemmer AW, Gimenez I, Dowd BF, Darman RB, Forbush B. Activation of the Na-K-Cl cotransporter NKCC1 detected with a phospho-specific antibody. J Biol Chem 277: 37551–37558, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Fujita N, Ishikawa SE, Sasaki S, Fujisawa G, Fushimi K, Marumo F, Saito T. Role of water channel AQP-CD in water retention in SIADH and cirrhotic rats. Am J Physiol Renal Fluid Electrolyte Physiol 269: F926–F931, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Gottschalk CW, Mylle M. Micropuncture study of the mammalian urinary concentrating mechanism: evidence for the countercurrent hypothesis. Am J Physiol 196: 927–936, 1959 [DOI] [PubMed] [Google Scholar]
- 12.Greenberg A, Verbalis JG. Vasopressin receptor antagonists. Kidney Int 69: 2124–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Hao S, Zhao H, Darzynkiewicz Z, Battula S, Ferreri NR. Differential regulation of NFAT5 by NKCC2 isoforms in medullary thick ascending limb (mTAL) cells. Am J Physiol Renal Physiol 300: F966–F975, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, Yu MJ, Pisitkun T, Chen F, Knepper MA. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem 283: 24617–24627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffert JD, Nielsen J, Yu MJ, Pisitkun T, Schleicher SM, Nielsen S, Knepper MA. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol 292: F691–F700, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Hoffert JD, Pisitkun T, Saeed F, Song JH, Chou CL, Knepper MA. Dynamics of the G protein-coupled vasopressin V2 receptor signaling network revealed by quantitative phosphoproteomics. Mol Cell Proteomics 11: M111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103: 7159–7164, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang S, Gunaratne R, Rinschen MM, Yu MJ, Pisitkun T, Hoffert JD, Fenton RA, Knepper MA, Chou CL. Vasopressin increases phosphorylation of Ser84 and Ser486 in Slc14a2 collecting duct urea transporters. Am J Physiol Renal Physiol 299: F559–F567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim GH, Ecelbarger C, Knepper MA, Packer RK. Regulation of thick ascending limb ion transporter abundance in response to altered acid/base intake. J Am Soc Nephrol 10: 935–942, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Kim GH, Ecelbarger CA, Mitchell C, Packer RK, Wade JB, Knepper MA. Vasopressin increases Na-K-2Cl cotransporter expression in thick ascending limb of Henle's loop. Am J Physiol Renal Physiol 276: F96–F103, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Kishore BK, Terris JM, Knepper MA. Quantitation of aquaporin-2 abundance in microdissected collecting ducts: axial distribution and control by AVP. Am J Physiol Renal Fluid Electrolyte Physiol 271: F62–F70, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Knepper MA, Danielson RA, Saidel GM, Johnston KH. Effects of dietary protein restriction and glucocorticoid administration on urea excretion in rats. Kidney Int 8: 303–315, 1975 [DOI] [PubMed] [Google Scholar]
- 23.Lassiter WE, Gottschalk CW, Mylle M. Micropuncture study of net transtubular movement of water and urea in nondiuretic mammalian kidney. Am J Physiol 200: 1139–1146, 1961 [DOI] [PubMed] [Google Scholar]
- 24.Marples D, Christensen BM, Frokiaer J, Knepper MA, Nielsen S. Dehydration reverses vasopressin antagonist-induced diuresis and aquaporin-2 downregulation in rats. Am J Physiol Renal Physiol 275: F400–F409, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC α, β, and γ subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen S, Maunsbach AB, Ecelbarger CA, Knepper MA. Ultrastructural localizatioon of Na-K-2Cl cotransporter in thick ascending limb and macula densa of rat kidney. Am J Physiol Renal Physiol 275: F885–F893, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Nielsen S, Terris J, Smith CP, Hediger MA, Ecelbarger CA, Knepper MA. Cellular and subcellular localization of the vasopressin-regulated urea transporter in rat kidney. Proc Natl Acad Sci USA 93: 5495–5500, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierce KL, Lefkowitz RJ. Classical and new roles of beta-arrestins in the regulation of G-protein-coupled receptors. Nat Rev Neurosci 2: 727–733, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Pisitkun T, Bieniek J, Tchapyjnikov D, Wang G, Wu WW, Shen RF, Knepper MA. High-throughput identification of IMCD proteins using LC-MS/MS. Physiol Genomics 25: 263–276, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson GL. Vaptans for the treatment of hyponatremia. Nat Rev Endocrinol 7: 151–161, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Schmale H, Richter D. Single base deletion in the vasopressin gene is the cause of diabetes insipidus in Brattleboro rats. Nature 308: 705–709, 1984 [DOI] [PubMed] [Google Scholar]
- 32.Star RA, Nonoguchi H, Balaban R, Knepper MA. Calcium and cyclic adenosine monophosphate as second messengers for vasopressin in the rat inner medullary collecting duct. J Clin Invest 81: 1879–1888, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terris J, Ecelbarger CA, Marples D, Knepper MA, Nielsen S. Distribution of aquaporin-4 water channel expression within rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 269: F775–F785, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Terris J, Ecelbarger CA, Nielsen S, Knepper MA. Long-term regulation of four renal aquaporins in rat. Am J Physiol Renal Fluid Electrolyte Physiol 271: F414–F422, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valtin H, Schroeder HA, Benirschke K, Sokol HW. Familial hypothalamic diabetes insipidus in rats. Nature 196: 1109–1110, 1962 [DOI] [PubMed] [Google Scholar]
- 37.van Balkom BW, Hoffert JD, Chou CL, Knepper MA. Proteomic analysis of long-term vasopressin action in the inner medullary collecting duct of the Brattleboro rat. Am J Physiol Renal Physiol 286: F216–F224, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Veeraveedu PT, Watanabe K, Ma M, Palaniyandi SS, Yamaguchi K, Suzuki K, Kodama M, Aizawa Y. Effects of nonpeptide vasopressin V2 antagonist tolvaptan in rats with heart failure. Biochem Pharmacol 74: 1466–1475, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Xu DL, Martin PY, Ohara M, St JJ, Pattison T, Meng X, Morris K, Kim JK, Schrier RW. Upregulation of aquaporin-2 water channel expression in chronic heart failure rat. J Clin Invest 99: 1500–1505, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]