Abstract
Background
The role of HSV-2 in the HIV epidemic, and the potential impact of HSV-2 suppressive therapy, have been previously explored only within the context of sub-Saharan Africa. In this analysis, modelling is used to estimate: (1) the contribution of herpes simplex virus type-2 (HSV-2) to HIV transmission from clients to female sex workers (FSWs) in a Southern Indian setting; and (2) the maximum potential impact of ‘perfect’ HSV-2 suppressive therapy on HIV incidence.
Methods
A dynamic HSV-2/HIV model was developed, parameterised and fitted to Mysore data. The model estimated the attributable fractions (AFs) of HIV infections due to HSV-2. Multivariate sensitivity analyses and regression analyses were conducted.
Results
The model suggests that 36% (95% CI: 22-62%) of FSW HIV infections were due to HSV-2, mostly through HSV-2 asymptomatic shedding. Even if HSV-2 suppressive therapy could eliminate the effect of HSV-2 on HIV infectivity among all coinfected clients, only 15% (95% CI: 3-41%) of HIV infections among FSWs would have been averted. 36% (95% CI: 18-61%) of HIV infections among HSV-2-infected FSWs could have been averted if suppressive therapy reduced their risk of HIV acquisition to that of the HSV-2-uninfected FSWs.
Conclusions
HSV-2 contributes substantially to HIV in this Southern Indian context. However, even in the best case scenario, HSV-2 suppressive therapy is unlikely to reduce HIV transmission or acquisition by more than 50% (as aimed for in recent trials), because of the limited strength of the interaction effect between HSV-2 and HIV.
Keywords: HIV, Herpes simplex virus type-2, mathematical modelling, India, female sex work
INTRODUCTION
Herpes simplex virus type-2 (HSV-2) is highly prevalent in regions with high HIV prevalence and among groups at high risk of HIV.1-2 These associations are partly due to both infections being sexually transmitted, but several lines of biological evidence support bidirectional synergistic interactions between the two viruses.3-5 In HIV-uninfected individuals, HSV-2 infection increases susceptibility to HIV through clinical and subclinical recurrences which disrupt the genital epithelium and increase the density of HIV target cells in the genital submucosa.5-7 In accord with this evidence, a systematic meta-analysis of longitudinal studies of the effect of HSV-2 on HIV acquisition showed that, after adjusting for confounding by age and at least one measure of sexual behaviour, 38-60% of new HIV infections may be attributable to prevalent HSV-2 infection in women and 8-49% in men, in the general populations reviewed.8
In HIV-infected individuals, co-infection with HSV-2 may increase HIV infectivity by upregulating the replication of HIV through different interactions, potentially resulting in increased rates of HIV transmission from these coinfected individuals.5 In fact, several cross-sectional studies have observed increased HIV genital shedding and plasma viral loads in the presence of HSV genital shedding or clinical recurrences.9-12
However, two recent randomised controlled trials evaluating the impact of HSV-2 suppressive therapy (HSVST) on HIV acquisition have shown that acyclovir 400mg twice daily had no significant effect on HIV incidence.13-14 These disappointing results may be related to the lack of effectiveness of the intervention, or the possibility that, although the treatment reduced the HSV-2 virus, it did not cause a reduction in the density of cells which enhance susceptibility to HIV in HSV-2 infected individuals.7,13-16 In contrast, results of randomised controlled trials conducted among dually infected individuals have suggested that HSV-2 enhances HIV infectivity through demonstrating that HSVST reduces HIV shedding,17-22 although perhaps not sufficiently to actually reduce HIV transmission from coinfected individuals23.
Lastly, HSV-2 may also accelerate HIV disease progression by increasing HIV plasma viral load,24 and HIV could potentially increase HSV-2 infectivity through increased genital shedding of HSV-2 among coinfected individuals.10,25 The latest trial results support this hypothesis since HSVST used by those coinfected significantly reduced HIV disease progression.26
Only four models (albeit applied to several different research questions) have incorporated both HSV-2 and HIV, and their interactions.27-34 We build on these African and US focussed analyses, and our previous work35, by using a model to explore the contribution of HSV-2 to HIV transmission for a female sex worker (FSW) population in Southern India, where the HIV epidemic is concentrated among high-risk groups. The relative importance of symptomatic versus asymptomatic HSV-2 genital shedding is explored. The implications of the findings for the maximum potential impact of HSVST are discussed.
METHODS
Dynamic deterministic models of HSV-2 and HIV were coupled to simulate the transmission of HIV and HSV-2 among FSWs and their clients. The HSV-2 model is described elsewhere,35 and includes separate compartments for initial infection (which may be symptomatic or asymptomatic), the long ‘latent’ phase with infectious asymptomatic shedding, and periodic short recurrences of infectious symptoms. The HIV model incorporates the initial short phase of high viral load, followed by the long asymptomatic phase in which viral load is low. This coupled model was programmed in C and solved numerically. Further details are supplied in the supplemental online appendix.
The FSWs were modelled as a cohort, with the HSV-2, HIV and coinfection prevalences being modelled dynamically over the duration of sex work, whereas the client prevalences were assumed to remain constant over the short timeframe of five years considered. Clients were assumed to randomly contact FSWs regardless of the duration for which each had sold/bought sex.
Enhanced transmission of HIV or HSV-2 in the presence of the other infection was modelled through the use of ‘direct cofactors’ that directly increase the probability of transmission during each (penile-vaginal) sex act. Additionally, among coinfected individuals, the symptomatic recurrence rate, proportion shedding asymptomatically, and rate of progression to AIDS were all assumed to be increased compared with those singly infected. The effect of other STIs facilitating HIV transmission was included as a constant cofactor.
Steps in analysis
Parameterisation
The parameter definitions and values used are shown in Table S1 in the supplemental online appendix, along with further details about the parameterisation process.
The model was parameterised and fitted using detailed behavioural and epidemiological data from FSWs in Mysore, Karnataka, and data on clients from Mysore when available, or elsewhere in Karnataka or other Indian states otherwise.36-37 Data came mostly from two unpublished surveys undertaken amongst FSWs (2004) and the general population (2005-6) in Mysore by the Karnataka Health Promotion Trust (KHPT), collected within the monitoring and evaluation of Avahan, the Bill and Melinda Gates Foundation funded India AIDS Initiative. The study was approved by the Ethics Review Boards of St Johns Medical College, Bangalore, India, Centre hospitalier affilié universitaire de Québec, Canada, and the University of Manitoba, Canada. The cohort model was fitted to HIV, HSV-2 and coinfection prevalences among FSWs by duration of sex work.
Uncertainty analysis and model fitting
First the model input parameter ranges were developed, distributions defined and correlations set (Table S1 in the online appendix). Latin Hypercube Sampling (LHS) was then used to randomly sample 10,000 parameter sets from these uncertainty ranges. Those simulations that were rejected in our previous analysis,35 are discarded here also, along with those predicting too low a prevalence at the start of sex work, leaving 7,536 simulations.
Any simulation that lay within the 95% confidence intervals (CIs) of the HSV-2, HIV and HSV-2/HIV coinfection prevalence data among FSWs, by duration of sex work up to five years, was accepted as a model fit. ‘Least chi-squared’ error was used to identify the best-fit. However, in addition to using prevalence data among FSWs, the mean values for the HSV-2 and HIV prevalence among the small number of men reporting visiting FSWs (42) in the unpublished general population survey from Mysore were also used to identify the best-fit.
Calculating attributable fractions
To investigate the contribution HSV-2 makes to HIV incidence, the model estimates of annual HIV incidence among FSWs after one to five years of sex work were compared with and without specific interaction cofactors included, for each model fit. These incidence values were used to calculate the attributable fraction (AF) of a specific interaction cofactor for increasing HIV transmission by using the following formula:
| (1) |
An average AF was calculated after weighting the AFs at each year of sex work by the proportion having worked in sex work for each equivalent duration. Confidence bounds were estimated by calculating the AFs for all the model fits and reporting the range spanned by 95% of the fits.
The model fits were also used to estimate the maximum potential impact that a hypothetical ‘perfect’ HSVST could have on HIV transmission in this FSW population, where ‘perfect’ refers here to completely removing the effect of HSV-2 on either HIV infectivity or HIV acquisition.
Sensitivity analysis
Multivariate sensitivity analysis using the best-fit simulation was used to identify which interaction inputs have the greatest effect on the predictions and to investigate the robustness of the model predictions to key assumptions. Each interaction model input was given the same relative uncertainty bounds (±20%), and 10,000 parameter sets were randomly sampled within these bounds using LHS. For each parameter set, the model was used to produce estimates of the percentage of incident HIV due to HSV-2. The relative importance of each non-correlated interaction parameter for the model prediction of the AFs was assessed using multilinear regression.
RESULTS
Selecting the best-fit and description of fits and correlations
Four hundred and one of the 7,536 model simulations fit within the 95% CIs of the observed HSV-2, HIV and coinfection prevalence data among FSWs. If, as in other similar analyses,27,34,38 we had only fitted to HSV-2 and HIV prevalences, 688 model fits would have been identified, leading to wider CIs on the model predictions and lower predictive power.
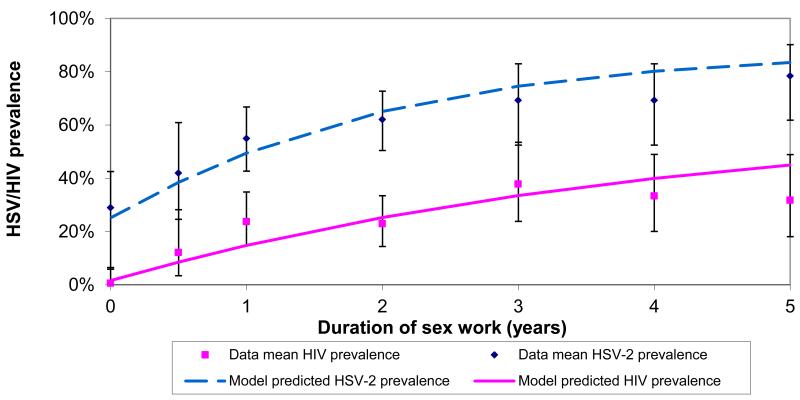
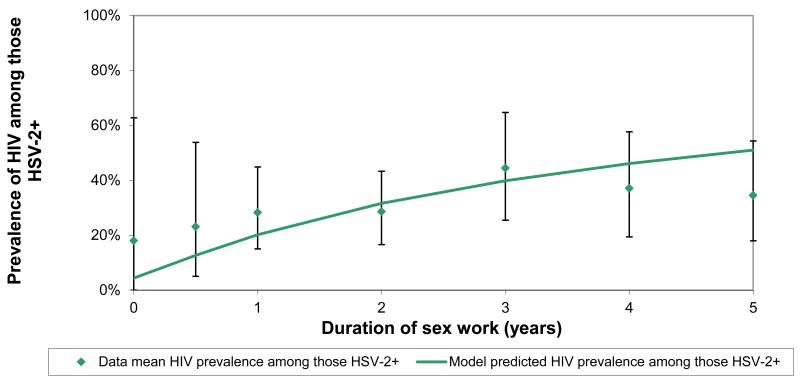
The model best-fit and the epidemiological data used to fit the model are shown in Figure 1. The input values used in the best-fit are given in Table S1 in the online appendix. The best-fit predicted an HSV-2 prevalence of 20% and HIV prevalence of 6% among clients (data estimates were 19% and 5%, respectively).
Figure 1. A comparison of the model best-fit with HSV-2 and HIV prevalence and coinfection prevalence data against the duration of sex work.
a Comparison with HSV-2 and HIV prevalence data
b Comparison with HIV/HSV-2 coinfection prevalence data
Attributable fractions
The AFs of all interaction effects of HSV-2 for HIV transmission among FSW are shown in Table 1, averaged over the first five years of sex work.
Table 1. Average attributable fractions due to specific interactions (percentage of annual HIV incidence among female sex workers (FSWs) due to interactions with HSV-2).
| Best-fit attributable fraction (95% confidence intervals from all fits) |
|
|---|---|
| Specific interaction |
% of HIV incidence among FSW due to
specific interaction with HSV-2 |
| All interactions combined | 35.5 (22.2-62.5) |
| Increased susceptibility and infectivity of HIV in the presence of asymptomatic HSV-2 shedding alone | 32.4 (20.4-59.5) |
| Increased susceptibility and infectivity of HIV in the presence of symptomatic HSV-2 recurrences alone | 3.0 (1.4-4.3) |
| Increased susceptibility to HIV among HSV-2-infected FSWs | 24.6 (11.2-46.7) |
| Increased infectivity of HIV from coinfected clients* | 10.4 (2.3-32.5) |
Includes implicitly the static effect on HIV transmission of the increased HSV-2 symptomatic recurrence rate among coinfected clients
Table 1 suggests that 35.5% (95% CI: 22.2-62.5%) of HIV infections among FSWs in Mysore are due to HSV-2, with the frequent infectious shedding of HSV-2 in the absence of symptoms contributing much more to HIV incidence than the less frequent recurrences of infectious symptoms (AF: 32.4% versus 3.0%).
If HSVST reduces the HIV infectivity of all coinfected clients to the same level as singly HIV-infected clients, then the model projects that 14.6% (95% CI: 3.3-41.2%) of HIV infections among FSWs would have been averted.
If an average cofactor is calculated that quantifies the overall effect of HSV-2 on HIV infectivity among coinfected individuals (see supplemental online appendix for details and figures), then Figure S2a suggests the impact projections are highly correlated to this average cofactor (linear regression R2=0.880). Indeed, if HSV-2 increases HIV infectivity 3-fold (up from 1.9-fold in the best-fit) then the linear regression implies that the maximum impact of ‘perfect’ HSVST for the coinfected clients would be a 34% decrease in HIV incidence in the FSWs. However, if this cofactor is between 1.25 and 1.43, coinciding with the shedding data from the trials17-20,22-23, and what their translated effect39 means for the average cofactor, then the regression suggests 7-10% of HIV infections could have been averted. This projection is in close agreement with the Partners in Prevention trial results of an 8% reduction in HIV incidence.23
If instead it is assumed that HSVST used by all FSWs singly infected with HSV-2 reduces their risk of acquiring HIV to that of the HSV-2-uninfected FSWs, the model projects that 36.0% (95% CI: 17.6-60.8%) of HIV infections among HSV-2-infected FSW could have been averted.
Again, if an average multiplicative cofactor for the effect of HSV-2 on increasing susceptibility to HIV among individuals singly-infected with HSV-2, is calculated in a similar way to that described above, then Figure S2b shows the impact projections are highly correlated to this average cofactor (linear regression R2=0.774). If HSV-2 increases a FSW’s susceptibility to HIV 5-fold (up from 3.6-fold in the best-fit) then the linear regression implies that the maximum impact of ‘perfect’ suppressive treatment for the HSV-2-infected FSWs would be a 55% decrease in HIV incidence among these FSWs. A lower average cofactor of 2, results in a lower impact estimate of 24%.
Sensitivity analysis
Table 2 shows that the AFs for HIV incidence change by at most 8% in relative terms for a 20% relative change in the interaction input parameters. The AFs are most sensitive to changes in the direct cofactors, i.e. those that increase the probability of HIV transmission per sex act in the presence of HSV-2. Other regression coefficients are very small. Table 2 presents the results when the largest cofactors from each correlated pair are entered into the multilinear regression.
Table 2. Sensitivity analysis on the effect of a 20% change in model interaction inputs on the model best-fit attributable fraction of HIV due to HSV-2 among female sex workers (FSWs).
| Model input parameters (values used for best-fit simulation in parentheses, derived from Table S1 in the supplemental online appendix) |
Relative % change in attributable fraction of HIV incidence among FSW due to HSV-2 from best-fit value of 35.54% (absolute value)** | |
|---|---|---|
| −20% relative change in parameter | +20% relative change in parameter | |
| Cofactor for increased transmission of HIV to person symptomatically shedding HSV-2 (4.5) | −8.10% (32.66%) |
+8.10% (38.42%) |
| Cofactor for increased transmission of HIV from coinfected partner symptomatically shedding HSV-2 (2.7) | −3.70% (34.22%) |
+3.70% (36.85%) |
| Cofactor for increased transmission of HSV-2 from coinfected partner with high HIV viraemia (0.76) | −1.15% (35.13%) |
+1.15% (35.95%) |
| Cofactor for increased symptomatic HSV-2 shedding rate in those with HIV (1.4) | −0.66% (35.30%) |
+0.66% (35.77%) |
| Cofactor for increased asymptomatic HSV-2 shedding in those with HIV (2.4) | −0.24% (35.45%) |
+0.24% (35.62%) |
| Cofactor for increased transmission of HSV-2 to a person with high HIV viraemia (1.7) | +0.14% (35.59%) |
−0.14% (35.49%) |
R2 value over 0.98 in the multilinear regression
DISCUSSION
The limitations of the HSV-2 model have been discussed previously,35 mainly that the model does not allow for sexual behaviour differences during symptomatic versus asymptomatic shedding.
Since the focus of this study is on the biological interactions between HIV and HSV-2, sexual behaviour among FSWs is assumed to be homogeneous (and similarly for clients), and HSV-2 is the only STI that is modelled dynamically. Non-commercial partnerships of FSWs and clients, or transmission to the general population are ignored, as is the population of men who have sex with men. Using the model as a cohort model, due to lack of data on clients, limited the use of the interaction parameters, potentially leading to underestimates in the attributable fractions.
The model suggests that approximately 36% of HIV infections among FSWs in Mysore are due to HSV-2. Similarly, another modelling analysis, by Abu-Raddad et al. (2008), predicted that 35% of HIV infections in Kisumu, Kenya, were attributable to HSV-2.27 Freeman et al. (2007), also used African data in a modelling analysis which found that the population attributable fraction of HIV due to HSV-2 increases with the maturity of the epidemic.40
Our model findings emphasise the greater contribution to the HIV epidemic of HSV-2 shedding in the absence of symptoms, than in the presence of symptoms. This is biologically plausible since, although symptomatic recurrences may be more important per shedding episode,41 asymptomatic shedding is much more frequent42.
Our analysis suggests that HSVST, used by either FSWs singly-infected with HSV-2 or clients coinfected with HIV and HSV-2, may reduce HIV incidence among FSWs, but not to any great extent (by 36% or 15%, respectively). These findings are in close agreement with White et al. (2008) for a concentrated African epidemic34 and Baggaley et al. (2009) for two generalised HIV epidemics in sub-Saharan Africa28.
For the main analysis, the values used for the cofactor effect of HSV-2 on susceptibility to HIV were calculated from reviews and pre-HSVST trial data (e.g. see references 7, 42 and 47 in the online appendix).4 These cofactor values remain valid, despite the lack of significant impact observed in the HSVST trial of HIV acquisition13-14, since the trial results do not negate the good biological plausibility and observational data that HSV-2 increases HIV susceptibility.3-6,8,43-44 The disappointing results from the HIV acquisition trials compared to the successful HIV shedding trials could be due to many reasons, including factors relating to the drug choice, dosage strategy and adherence levels13-14, different effects of the drug in coinfected individuals versus those singly infected with HSV-215-16, and/or the possible persistence of activated CD8+ T cells which enhance susceptibility to HIV in HSV-2 infected individuals but are not shut down by HSVST7.
Observing an impact of HSVST in a trial setting may also be difficult because of limited statistical power. The Partners in Prevention trial was powered to detect a 50% decrease in HIV incidence,23 yet our modelling suggests that this would have required HSV-2 to increase the per sex act HIV infectivity, among those coinfected, at least 4-fold. The HIV acquisition trials were powered at a similar level and our results suggest that an effect of HSVST would only have been observed if HSV-2 increased HIV susceptibility more than 4.5-fold. For these reasons, it is plausible that the trials observed no impact of HSVST on HIV incidence simply because the interaction between these viruses is not large enough to enable a significant impact to be observed for the statistical power set in the trials. The model strongly supports this premise for the impact of HSVST on HIV transmission (more so than acquisition), since even the upper bound on the model-predicted decrease in HIV incidence is 41% in this case, which is below the 50% that the trials were powered for. The broader scientific literature suggests that the values of these cofactors are likely to be lower than what the model suggests is required to observe a statistically significant impact in these trials (e.g. see references 7, 28, 42, 47, 58-59, 61-65 in the online appendix).45 The Partners in Prevention trial results also support these model findings since the upper bound on the impact estimate was 40%,23 implying that there may have been a small impact of acyclovir which could not achieve the statistical significance required in the trial. Any imperfections in the effect of HSVST or adherence issues in a trial would require even higher cofactors in order to observe a statistical significant result.
Given this, the modelling analyses suggest that, although HSV-2 may play an important role in both concentrated and generalised HIV epidemics, this interaction is likely too weak to warrant the promotion of HSVST specifically for HIV prevention, given the lack of effect noted in the trials. However, HSVST in an important intervention for those who are infected with HSV-2, and may offer broader benefits since it has been demonstrated in a trial to delay progression to AIDS among those coinfected.26 It also remains possible that HSVST could have a weak effect on reducing HIV acquisition or transmission by less than 50%. Larger trials may be able to observe a weak effect of HSVST on HIV incidence but the importance of conducting such a trial would need to be weighed alongside the costs and relevance of identifying a low impact product.
Conclusions
This is the first model that has been used to explore the importance of HSV-2 to a concentrated HIV epidemic in an Asian setting. The modelling suggests that HSV-2 plays a critical role in the transmission and acquisition of HIV in Mysore, and that this may be mostly due to infectious shedding of HSV-2 in the absence of symptoms.
Given the successful trial of once-daily valacyclovir to reduce the risk of transmission of genital herpes46, continuous suppressive treatment of HSV-2 could be an effective strategy among FSWs and their clients in India, for reducing the transmission of HSV-2. Over time, the HIV epidemic may be curtailed indirectly through the use of HSV-2 suppressive therapy, since a lower prevalence of HSV-2 could result in fewer HIV infections.
Increasing the potency of current HSV-2 antiviral drugs may help in further reducing HSV-2 incidence. However, the model suggests that even in the best case scenario, in which HSV-2 suppressive therapy completely removes the effect of HSV-2 on either HIV infectivity or HIV acquisition, this hypothetical ‘perfect’ therapy is unlikely to lead to a 50% reduction in HIV incidence, because of the limited strength of the interaction effect between HSV-2 and HIV.
Modelling analyses such as these should be repeated or adapted and used to explore the potential impact of other HIV/STI prevention interventions, helping to guide trial design for expected power and sample size calculations, and to retrospectively inform the interpretation of trial results.
Supplementary Material
Key Messages.
This is the first model exploring the role of HSV-2 in a concentrated HIV epidemic within an Asian setting.
The modelling suggests that HSV-2 plays an important role in the transmission and acquisition of HIV in Mysore, mostly through asymptomatic HSV-2 shedding.
Even in the best-case scenario, HSV-2 suppressive therapy is unlikely to reduce HIV transmission or acquisition by >50%, as aimed for in recent trials.
Modelling analyses can help guide trial design for expected power and sample size calculations, and retrospectively inform the interpretation of trial results.
Acknowledgements
Support for this research was provided by the Bill & Melinda Gates Foundation (BMGF) through Avahan, its India AIDS Initiative (grant no. 33978), and the Department for International Development (DFID) funded Knowledge Programme on HIV/AIDS and STI and the Research Programme Consortium for Research and Capacity Building in Sexual and Reproductive Health and HIV in Developing Countries of the LSHTM. Michel Alary is a National Researcher of the Fonds de la Recherche en Santé du Québec (grant no. 8722). The views expressed herein are those of the authors and do not necessarily reflect the official policy or position of LSHTM, the BMGF/Avahan or DFID. The Mysore data were collected as part of the monitoring and evaluation of the multisite HIV prevention intervention funded by the BMGF.
Many thanks go to the Karnataka Health Promotion Trust (KHPT) and St John’s Research Institute, Bangalore, India, and the University of Manitoba, Canada, for providing and helping interpret the Indian data. We especially thank Ashodaya Samithi, the local sex workers organisation, who agreed to the use of the data for modelling. We would also like to thank other colleagues involved in the HSV-2 therapy trials, and also Geoff Garnett at Imperial College, London, and Sevgi Aral at the US Centers for Disease Control, for their helpful input into this work.
Footnotes
Competing Interest: None declared.
REFERENCES
- 1.Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186(Suppl 1):S3–28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 2.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(Suppl 1):24A–35A. [PubMed] [Google Scholar]
- 3.Celum C, Levine R, Weaver M, et al. Genital herpes and human immunodeficiency virus: double trouble. Bull World Health Organ. 2004;82(6):447–53. [PMC free article] [PubMed] [Google Scholar]
- 4.Corey L, Wald A, Celum CL, et al. The Effects of Herpes Simplex Virus-2 on HIV-1 Acquisition and Transmission: A Review of Two Overlapping Epidemics. J Acquir Immune Defic Syndr. 2004;35(5):435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 5.Van de Perre P, Segondy M, Foulongne V, et al. Herpes simplex virus and HIV-1: deciphering viral synergy. Lancet Infectious Diseases. 2008;8:490–97. doi: 10.1016/S1473-3099(08)70181-6. [DOI] [PubMed] [Google Scholar]
- 6.Rebbapragada A, Wachihi C, Pettengell C, et al. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. AIDS. 2007;21(5):589–98. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 7.Zhu J, Koelle DM, Cao J, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204(3):595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman EE, Weiss HA, Glynn JR, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 9.Augenbraun M, Feldman J, Chirgwin K, et al. Increased Genital Shedding of Herpes Simplex Virus Type 2 in HIV-Seropositive Women. Ann Intern Med. 1995;123(11):845–847. doi: 10.7326/0003-4819-123-11-199512010-00006. [DOI] [PubMed] [Google Scholar]
- 10.Mbopi-Keou FX, Gresenguet G, Mayaud P, et al. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J Infect Dis. 2000;182(4):1090–6. doi: 10.1086/315836. [DOI] [PubMed] [Google Scholar]
- 11.McClelland RS, Wang CC, Overbaugh J, et al. Association between cervical shedding of herpes simplex virus and HIV-1. AIDS. 2002;16(18):2425–30. doi: 10.1097/00002030-200212060-00007. [DOI] [PubMed] [Google Scholar]
- 12.LeGoff J, Weiss HA, Gresenguet G, et al. Cervicovaginal HIV-1 and herpes simplex virus type 2 shedding during genital ulcer disease episodes. AIDS. 2007;21(12):1569–78. doi: 10.1097/QAD.0b013e32825a69bd. [DOI] [PubMed] [Google Scholar]
- 13.Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371(9630):2109–19. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358(15):1560–71. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lisco A, Vanpouille C, Tchesnokov EP, et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe. 2008;4(3):260–70. doi: 10.1016/j.chom.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stapleton JT, Balfour HH., Jr. Coinfection alters the playing field: herpesviruses induce acyclovir to inhibit HIV. Cell Host Microbe. 2008;4(3):194–5. doi: 10.1016/j.chom.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Baeten JM, Strick LB, Lucchetti A, et al. Herpes Simplex Virus (HSV)- Suppressive Therapy Decreases Plasma and Genital HIV-1 Levels in HSV-2/HIV-1 Coinfected Women: A Randomized, Placebo-Controlled, Cross-Over Trial. J Infect Dis. 2008;198(12):1804–8. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delany S, Mayaud P, Clayton T, et al. Impact of HSV-2 suppressive therapy on genital and plasma HIV-1 RNA in HIV-1 and HSV-2-seropositive women not taking ART: a randomized, placebo-controlled trial in Johannesburg, South Africa. AIDS. 2009;23(4):461–9. doi: 10.1097/QAD.0b013e32831db217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunne EF, Whitehead S, Sternberg M, et al. Suppressive acyclovir therapy reduces HIV cervicovaginal shedding in HIV- and HSV-2-infected women, Chiang Rai, Thailand. J Acquir Immune Defic Syndr. 2008;49(1):77–83. doi: 10.1097/QAI.0b013e3181831832. [DOI] [PubMed] [Google Scholar]
- 20.Nagot N, Ouedraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356(8):790–9. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 21.Paz-Bailey G, Sternberg M, Puren AJ, et al. Improvement in healing and reduction in HIV shedding with episodic acyclovir therapy as part of syndromic management among men: a randomized, controlled trial. J Infect Dis. 2009;200(7):1039–49. doi: 10.1086/605647. [DOI] [PubMed] [Google Scholar]
- 22.Zuckerman RA, Lucchetti A, Whittington WL, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196(10):1500–8. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 23.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362(5):427–39. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hennessey KA, Giorgi JV, Kaplan AH, et al. AIDS onset at high CD4+ cell levels is associated with high HIV load. AIDS Res Hum Retroviruses. 2000;16(2):103–7. doi: 10.1089/088922200309449. [DOI] [PubMed] [Google Scholar]
- 25.Mayaud P, Nagot N, Konate I, et al. Effect of HIV-1 and antiretroviral therapy on herpes simplex virus type 2: a prospective study in African women. Sex Transm Infect. 2008;84(5):332–7. doi: 10.1136/sti.2008.030692. [DOI] [PubMed] [Google Scholar]
- 26.Lingappa JR, Baeten JM, Wald A, et al. Daily aciclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010;375(9717):824–33. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abu-Raddad LJ, Magaret AS, Celum C, et al. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS ONE. 2008;3(5):e2230. doi: 10.1371/journal.pone.0002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baggaley RF, Griffin JT, Chapman R, et al. Estimating the public health impact of the effect of herpes simplex virus suppressive therapy on plasma HIV-1 viral load. AIDS. 2009;23(8):1005–13. doi: 10.1097/QAD.0b013e32832aadf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blower S, Ma L. Calculating the contribution of herpes simplex virus type 2 epidemics to increasing HIV incidence: treatment implications. Clin Infect Dis. 2004;39(Suppl 5):S240–7. doi: 10.1086/422361. [DOI] [PubMed] [Google Scholar]
- 30.Freeman EE, White RG, Bakker R, et al. Population-level effect of potential HSV2 prophylactic vaccines on HIV incidence in sub-Saharan Africa. Vaccine. 2009;27(6):940–6. doi: 10.1016/j.vaccine.2008.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korenromp EL, Bakker R, De Vlas SJ, et al. Can behavior change explain increases in the proportion of genital ulcers attributable to herpes in sub-Saharan Africa? A simulation modeling study. Sex Transm Dis. 2002;29(4):228–38. doi: 10.1097/00007435-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Orroth KK, White RG, Korenromp EL, et al. Empirical Observations Underestimate the Proportion of Human Immunodeficiency Virus Infections Attributable to Sexually Transmitted Diseases in the Mwanza and Rakai Sexually Transmitted Disease Treatment Trials: Simulation Results. Sex Transm Dis. 2006;33(9):536–544. doi: 10.1097/01.olq.0000204667.11192.71. [DOI] [PubMed] [Google Scholar]
- 33.White RG, Orroth KK, Korenromp EL, et al. Can population differences explain the contrasting results of the Mwanza, Rakai, and Masaka HIV/sexually transmitted disease intervention trials?: a modeling study. J Acquir Immune Defic Syndr. 2004;37(4):1500–1513. doi: 10.1097/01.qai.0000127062.94627.31. [DOI] [PubMed] [Google Scholar]
- 34.White RG, Freeman EE, Orroth KK, et al. Population-level effect of HSV-2 therapy on the incidence of HIV in sub-Saharan Africa. Sex Transm Infect. 2008;84(Suppl 2):ii12–18. doi: 10.1136/sti.2008.029918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foss AM, Vickerman PT, Chalabi Z, et al. Dynamic modelling of herpes simplex virus type 2 (HSV-2) transmission: issues in structural uncertainty. Bulletin of Mathematical Biology. 2009;71(3):720–49. doi: 10.1007/s11538-008-9379-1. [DOI] [PubMed] [Google Scholar]
- 36.Ramesh BM, Rajeswari NV, Sankangoudar S. Female commercial sex workers in Karnataka: a baseline survey, 2002. India-Canada Collaborative HIV/AIDS Project (ICHAP) and Population Research Centre; Bangalore and Dharwad: Mar, 2003. [Google Scholar]
- 37.NACO . National Baseline High Risk and Bridge Population Behavioural Surveillance Survey- Part -I (FSW and their clients) National AIDS Control Organisation (NACO); India: 2001. http://www.nacoonline.org/publication.htm [Google Scholar]
- 38.Freeman EE, Orroth KK, White RG, et al. Proportion of new HIV infections attributable to herpes simplex 2 increases over time: simulations of the changing role of sexually transmitted infections in sub-Saharan African HIV epidemics. Sex Transm Infect. 2007;83(Suppl 1):i17–24. doi: 10.1136/sti.2006.023549. [DOI] [PubMed] [Google Scholar]
- 39.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS. 2008;22(16):2179–85. doi: 10.1097/QAD.0b013e328312c756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freeman EE, Orroth K, White R, et al. The proportion of new HIV infections attributable to herpes simplex 2 increases over time: simulations of the changing role of sexually transmitted infections in sub-Saharan African HIV epidemics. Sex Transm Infect. 2007;83(Suppl 1):i17–24. doi: 10.1136/sti.2006.023549. [DOI] [PubMed] [Google Scholar]
- 41.Nagot N, Ouedraogo A, Konate I, et al. Roles of Clinical and Subclinical Reactivated Herpes Simplex Virus Type 2 Infection and Human Immunodeficiency Virus Type 1 (HIV-1)-Induced Immunosuppression on Genital and Plasma HIV-1 Levels. J Infect Dis. 2008;198(2):241–9. doi: 10.1086/589621. [DOI] [PubMed] [Google Scholar]
- 42.Mark KE, Wald A, Magaret AS, et al. 17th International Society for Sexually Transmitted Diseases Research. Seattle, Washington, USA: 2007. Rapid onset and clearance of genital HSV reactivations in immunocompetent adults: the virus is usually “on”.http://www.isstdr.org/index.php 29 July – 1 August [Abstract O-030] [Google Scholar]
- 43.Paz-Bailey G, Ramaswamy M, Hawkes SJ, et al. Genital Herpes Simplex Virus Type 2: epidemiology and management options in the developing world. Sex Transm Infect. 2007;83(1):16–22. doi: 10.1136/sti.2006.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185(1):45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 45.Mahiane SG, Legeai C, Taljaard D, et al. Transmission probabilities of HIV and herpes simplex virus type 2, effect of male circumcision and interaction: a longitudinal study in a township of South Africa. AIDS. 2009;23(3):377–383. doi: 10.1097/qad.0b013e32831c5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corey L, Wald A, Patel R, et al. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med. 2004;350(1):11–20. doi: 10.1056/NEJMoa035144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.