Abstract
Background
Uganda has long been successful in controlling the HIV epidemic but there is evidence that HIV prevalence and incidence are increasing again. Data on the HIV/STI epidemic among sex workers are so far lacking from Uganda. This paper describes the baseline epidemiology of HIV/STI in a newly-established cohort of women involved in high risk sexual behaviour in Kampala, Uganda.
Methods
Women were recruited from red-light-areas in Kampala. Between April 2008-May 2009, 1027 eligible women were enrolled. Socio-demographic and behavioural information was collected; blood and genital samples were tested for HIV/STI. Risk factors for HIV-infection were examined using multivariable logistic regression.
Results
HIV seroprevalence was 37%. The prevalence of N. gonorrhoea (NG) was 13%, C. trachomatis (CT) 9%, T. vaginalis (TV) 17%, bacterial vaginosis (BV) 56% and 11% had candida infection. 80% had HSV-2 antibodies, 21% were TPHA -positive and 10% had active syphilis (RPR+TPHA+). In 3% of the genital ulcers, T. pallidum (TP) was identified, H. ducreyi (HD) in 6% and HSV-2 in 35%. Prevalent HIV was independently associated with older age, being widowed, lack of education, sex work as sole income, street based sex work, not knowing HIV-status, using alcohol and intravaginal cleansing with soap. HIV-infection was associated with NG, TV, BV, HSV-2 seropositivity and active syphilis.
Conclusions
Prevalence of HIV/STI is high among women involved in high risk sexual behaviour in Kampala. Targeted HIV prevention interventions including regular STI screening, VCT, condom promotion and counselling for reducing alcohol use are urgently needed in this population.
Keywords: HIV, STI, female sex workers, epidemiology, risk behaviour, Uganda
Introduction
Longitudinal studies from Uganda showed decreasing HIV prevalence and incidence in the general population between 1989 and 1999.1,2,3 However, there is evidence that HIV prevalence and incidence may be increasing again since 2005.4 In contrast to the well-documented HIV epidemic in the general population, there is surprisingly little data on HIV epidemiology among women involved in high-risk sexual behaviour in Uganda. Further, specific HIV prevention interventions targeting these women are scarce.
Studies from other sub-Saharan African countries have documented high rates of HIV and other STI among women involved in high-risk sexual behaviour such as female sex workers and bar workers.5,6,7,8,9,10 Several countries have responded successfully by designing HIV/STI prevention interventions for this group, including improved access for STI treatment, condom promotion, voluntary HIV testing and counseling (VCT) and risk reduction counseling. For example, HIV prevalence among attenders of a sex worker clinic in Kinshasa, DRC fell from 35% to 12% between 1988 and 2002 11, in Abidjan, Cote d’Ivoire from 80% to 32% between 1992 and 1998 12 and in Cotonou, Bénin from 53% to 41% from 1993 to 1999 13.
The first cohort of women involved in high-risk sexual behaviour in Uganda was recently established in Kampala, to better understand the dynamics of HIV/STI infection and to implement future HIV prevention intervention trials in this core group.
In this paper we report on the design of the cohort study, the baseline characteristics, the prevalence of HIV/STI and risk factors for prevalent HIV-infection at enrolment.
Methods
Setting
The project (hereafter called Good Health for Women Project, GHWP) is located in Kibuye, a densely-populated slum area in southern Kampala. A stand-alone clinic was established, offering free general and reproductive health care facilities for eligible women and their children aged under five years old. An on-site laboratory was established to perform HIV rapid tests, malaria smears and urine analysis.
Participant recruitment
The two divisions of southern Kampala (Makindye and Rubaga) were mapped for hotspots, defined as clusters of bars, night clubs, local beer breweries, eating places, lodges and guesthouses known to provide rooms for sex work, or selected street spots often frequented by sex workers in search of clients.
Collaboration was established with a local NGO, Women at Work International (WAWI), who have been offering health education and condom promotion to female sex workers in the target area since 2004. WAWI-trained peer-educators (PE) were invited to join the project and were enrolled as the first cohort participants. These PE subsequently mobilized other women involved in commercial sex or employed in surrounding entertainment facilities. The project field workers re-visited the newly mobilized women at their workplace to confirm that they belonged to the eligible study population (pre-screening) and invited them for an information meeting at the GHWP clinic. This meeting provided detailed information about the research programme, addressed questions and queries, and gave the women the opportunity to see the clinic. Women willing to join the study were scheduled for their screening visit. As the number of study participants increased, additional PE were selected among the enrolled women, based on their communication skills, commitment to the project and peer recommendation. The PE were offered a monthly allowance of 50,000 Ugandan Shillings (equivalent to £17).
Study procedures
Screening
Screening activities were conducted from March 2008 to March 2009. All women attending the clinic for screening were offered VCT, health-education counselling, free condom supplies, and free access to the general care clinic for the total duration of the project, whether or not enrolled into the cohort study. HIV-positive blood samples were sent for confirmation to the MRC clinical laboratories in Entebbe.
Women were eligible for enrolment into the cohort if aged 18 years or more, involved in commercial sex work (defined as receiving money, good or other favours in exchange for sex) or employed in entertainment facilities and living or working in Kampala. Women between 15 and 18 years old were eligible if found to be mature minors (i.e. catering for their own livelihood, being pregnant or having already children).
Eligible women were scheduled to return for the enrolment visit within one week. Women who delayed enrolment for more than one month after being screened were re-assessed for eligibility.
Cohort Enrolment
Enrolment started in April 2008. An experienced nurse-counsellor undertook the consent procedures: a comprehension check list was filled in and only after full understanding of study procedures had been established, written or thumb printed informed consent for participation in the study was obtained. Consent procedures were witnessed if participants were illiterate.
Data were collected on socio-demographic and economic status, sexual risk behaviour, alcohol and illicit drug use, intravaginal practices, reproductive health, STI symptoms, using interviewer-administered structured questionnaires. The alcohol drinking pattern was determined using CAGE indicators 16. Positive responses to at least two of the four CAGE questions are defined as indicative of problem drinking.
All consenting women were tested for HSV-2 and syphilis serology, underwent a gynaecological examination, and were examined for STI syndromes. An endocervical sample was collected to test for Neisseria gonorrhoea and Chlamydia trachomatis. A high posterior fornix swab was taken to test for Trichomonas vaginalis and a swab from the lateral vaginal walls for diagnosis of candidiasis and bacterial vaginosis. From women presenting with a genital ulcer, an additional swab was collected for identification of the etiology (Treponema pallidum, Haemophilus ducreyi, HSV-1, HSV-2 and lymphogranulorum venereum). Women diagnosed syndromically with any STI were managed following the Ugandan national guidelines and were re-assessed after the etiological diagnosis was known. Risk-reduction counselling and free condoms were provided at all visits and all participants had the opportunity to see a clinician for any health care issue.
Follow-up visits
Enrolled women were scheduled to return 3-monthly to the clinic. To ensure high retention rates, meetings were organised for all women expected for their next follow-up visit in the coming 2 weeks. The study procedures during follow-up visits were similar as for enrolment.
General and HIV care
Study participants and their children aged under five years old had free access to the general care clinic, family planning and antenatal care services and VCT throughout the study. Participants with confirmed HIV-infection had CD4-counts, complete blood, liver and renal function tests. Participants with a CD4 count <250 cells/μl were pre-counseled on ART at the GHWP clinic, and then accompanied to an HIV-care centre of their choice for initiation of antiretroviral therapy (ART). Those not eligible for ART were provided with cotrimoxazole prophylaxis and CD4-counts were repeated at subsequent follow-up visits.
Laboratory methods
A single HIV rapid test (Abbott Determine HIV-1/2) was performed at the GHWP laboratory. Negative results were given to the participant immediately. Positive samples were sent to the MRC/UVRI serology laboratory in Entebbe for confirmation using two EIA tests performed in parallel (Vironostika Uniform II plus O, Murex HIV 1.2.O). If results were discordant or equivocal, a Western Blot Test (Cambridge Calypte Western Blot) was performed to resolve the status. Syphilis serology was assessed using a quantitative rapid plasma reagin (RPR Biotec) test and the Treponema Pallidum Haemagglutination test (TPHA Biotec). Active syphilis was defined as having both RPR and TPHA tests positive, whereas a RPR titer of 1:8 or above was considered as high-titre active syphilis. HSV-2 serology was performed using a HSV type 2-IgG ELISA (Kalon Biological Ltd). N. gonorrhoea and C. trachomatis were diagnosed using the Amplicor CT/NG PCR test (Roche diagnostic Systems Inc., Branchburg, NJ).
The first vaginal swab was inoculated for culturing Trichomonas vaginalis, using InPouch (Biomed Diagnostics, San Jose, CA, USA). The second vaginal swab was used to prepare a Gram stained slide for the diagnosis of bacterial vaginosis using Nugents scoring method and to examine for candida infection.
For the identification of the etiology of GUD, single assays were run on a Roche Real Time PCR Light Cycler. HSV 1/2 was run using LightMix kit HSV-1 / 2 supplied by TIB Molbiol (TIB Molbiol Eresburgstrafse 22-23 D-12103 Berlin, Germany) while TP, LGV and HD was run using in-house assays with probes and primers also supplied by TIB Molbiol.
The inoculated Trichomonas InPouch and vaginal smears were kept at room temperature and all other specimens were stored at 4°C until they reached the MRC/UVRI laboratories in Entebbe. All samples were transported within 12 hours of collection.
Statistical methods
Sample size
A sample size of 1000 women provides good precision for the expected prevalence of all infections of interest at enrolment. It is also sufficient to estimate a 10% incidence rate of HIV after one year of follow-up with a precision of ± 3% (with 95% confidence) assuming 15% loss-to-follow-up in the same period and an initial HIV prevalence of 50%.
Analysis of baseline data
All variables were first described using summary statistics. Then factors associated with HIV prevalence were analysed using logistic regression to estimate odds ratios (OR) and 95%CI. P-values were obtained using likelihood ratio tests. A hierarchical conceptual framework was used, with variables grouped as follows: socio-demographic and economic variables, behavioural variables and other STI.17 The univariate association of HIV-infection with each socio-demographic and economic variable was assessed first. Variables associated with HIV (p<0.10) were included in a multivariable logistic regression model and were retained only if independently associated with the outcome (p<0.10) after adjusting for the other variables. This resulted in a core group of socio-demographic and economic variables independently associated with HIV. Next, the association of HIV infection with each behavioural variable, adjusted for this core group, was assessed. Behavioural variables remaining independently associated with HIV (p<0.10) after adjusting for each other and the core socio-demographic and economic variables were retained in the model. Finally, these same steps were repeated for the assessment of the association of HIV infection with STI results, after adjusting for core socio-demographic, economic and behavioural factors.
Ethical considerations
Ethical approval was obtained from the Science and Ethics Committee of the Ugandan Virus Research Institute and from the Ugandan National Committee for Science and Technology.
Results
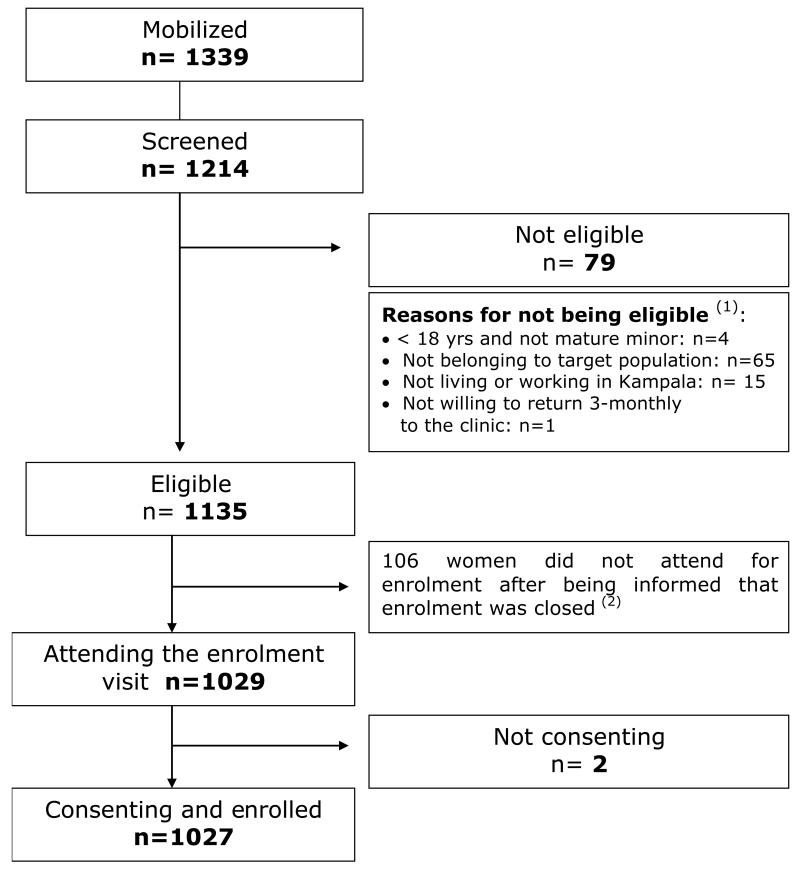
Between February 2008 and April 2009, 1339 women were mobilized, of whom 1214 (91%) attended the screening visit and 1135 (94%) of these women were eligible for cohort enrolment (Figure 1). By May 2009, 1027 women had been enrolled. The remaining 106 eligible women were not enrolled because the sample size of 1000 was met.
Figure 1. Recruitment flow.
1 Some having several reasons to be not eligible
2 It was decided to stop enrolment after the first 1027 consenting women were enrolled, as the expected sample size of the cohort was met. The 106 eligible women not invited for enrolment as well as the 2 not-consenting women got access to the free general care services offered by the GHWP.
Socio demographic and economic characteristics
The mean age of participants was 26 years (standard deviation (sd) ± 5.7 yrs), with 23 (2.2%) younger than 18 years. Only 8% of the participants were currently married or were living as married (hereafter called ‘married’) (Table 1). Most (67%) of the married women had a regular extra-spousal partner and most (71%) of the non-married women reported having a stable partner. Sex work was the sole source of income for 34% of participants, 61% had another income besides sex work and 5% were not involved in sex work (Table 1).
Table 1. Association of socio-demographic factors with HIV infection among women involved in high risk sexual behaviour in Kampala, Uganda.
| N | HIV+ (%) | OR (95%CI) | Adjusted OR5 (95%CI) |
|
|---|---|---|---|---|
| Age (y) | P<0.001 | P-trend=0.02 | ||
| <25 | 412 | 121 (29.4) | 1 | 1 |
| 25-34 | 505 | 208 (41.2) | 1.68 (1.28-2.22) | 1.35 (0.99-1.83) |
| 35+ | 110 | 53 (48.2) | 2.24 (1.45-3.44) | 1.72 (1.07-2.76) |
|
| ||||
| Religion | P=0.28 | P=0.28 | ||
| Catholic | 440 | 177 (40.2) | 1 | 1 |
| Anglican | 284 | 102 (35.9) | 0.83 (0.61-1.14) | 0.88 (0.63-1.22) |
| Muslim | 265 | 88 (33.2) | 0.74 (0.54-1.02) | 0.72 (0.51-1.00) |
| Other | 38 | 15 (39.5) | 0.97 (0.49-1.91) | 0.86 (0.41-1.82) |
|
| ||||
| Tribe 1 | P=0.17 | P=0.39 | ||
| Muganda | 608 | 213 (35.0) | 1 | 1 |
| Other Ugandan | 371 | 147 (39.6) | 1.22 (0.93-1.59) | 1.12 (0.84-1.49) |
| Non-Ugandan | 46 | 21 (45.7) | 1.56 (0.85-2.85) | 1.53 (0.79-2.94) |
|
| ||||
| Education level | P<0.001 | P-trend<0.001 | ||
| Higher than primary level | 106 | 24 (22.6) | 1 | 1 |
| Primary completed | 418 | 138 (33.0) | 1.68 (1.02-2.77) | 1.52 (0.91-2.54) |
| Primary uncompleted | 418 | 168 (40.2) | 2.30 (1.40-3.77) | 2.04 (1.22-3.41) |
| Never went to school | 85 | 52 (61.2) | 5.38 (2.87-10.11) | 4.57 (2.37-8.81) |
|
| ||||
| Current marital status | P<0.001 | P<0.001 | ||
| Married/cohabiting | 83 | 31 (37.4) | 1 | 1 |
| Widowed | 59 | 40 (67.8) | 3.53 (1.74-7.14) | 3.38 (1.61-7.10) |
| Separated/divorced | 656 | 260 (39.6) | 1.10 (0.69-1.76) | 1.03 (0.62-1.69) |
| Single | 229 | 51 (22.3) | 0.48 (0.28-0.83) | 0.58 (0.33-1.03) |
|
| ||||
| Length of stay in current location | P=0.13 | P=0.79 | ||
| ≤1 y | 264 | 94 (35.6) | 1 | 1 |
| >1 y - 3 y | 290 | 97 (33.5) | 0.91 (0.64-1.29) | 0.91 (0.63-1.32) |
| >3 y | 473 | 191 (40.4) | 1.22 (0.90-1.67) | 1.01 (0.72-1.42) |
|
| ||||
| Intending to move within the next 12 months 2 | P=0.27 | P=0.14 | ||
| No | 785 | 285 (36.3) | 1 | 1 |
| Yes | 218 | 88 (40.4) | 1.19 (0.87-1.61) | 1.28 (0.92-1.78) |
|
| ||||
| Number of people to support financially | P=0.08 | P=0.47 | ||
| Only myself | 96 | 26 (27.1) | 1 | 1 |
| ≤2 other people | 477 | 180 (37.7) | 1.63 (1.00-2.65) | 1.18 (0.69-2.00) |
| >2 other people | 454 | 176 (38.8) | 1.70 (1.05-2.78) | 0.99 (0.57-1.72) |
|
| ||||
| Reported income | P=0.009 | P=0.03 | ||
| Sex work only | 346 | 151 (43.6) | 1 | 1 |
| Sex work and other job | 632 | 213 (33.7) | 0.66 (0.50-0.86) | 0.70 (0.51-0.96) |
| Other job only | 49 | 18 (36.7) | 0.75 (0.40-1.39) | 0.81 (0.26-2.49) |
|
| ||||
| Place of recruiting male clients 3 | P=0.003 | P=0.008 | ||
| Bar, club or restaurant | 382 | 143 (37.4) | 1 | 1 |
| Street | 152 | 76 (50.0) | 1.67 (1.14-2.44) | 1.42 (0.92-2.20) |
| Home or on phone | 70 | 22 (31.4) | 0.76 (0.44-1.32) | 0.89 (0.49-1.60) |
| Several places | 374 | 123 (32.3) | 0.82 (0.61-1.10) | 0.72 (0.52-0.99) |
|
| ||||
| How often sex for money 4 | P=0.01 | P=0.01 | ||
| Daily | 482 | 184 (38.2) | 1 | 1 |
| At least once a week | 402 | 157 (39.1) | 1.04 (0.79-1.36) | 1.34 (0.98-1.82) |
| Less than once a week | 70 | 14 (20.0) | 0.40 (0.22-0.75) | 0.50 (0.26-0.99) |
|
| ||||
| Average amount of money paid per sex act 3 | P=0.05 | P=0.79 | ||
| <5000 UgSh | 231 | 99 (42.9) | 1 | 1 |
| 5000 -10 000 UgSh | 620 | 227 (36.6) | 0.77 (0.57-1.05) | 0.91 (0.65-1.27) |
| > 10 000 UgSh | 127 | 38 (29.9) | 0.57 (0.36-0.90) | 0.83 (0.50-1.39) |
Missing for 2 individuals
Missing for 24 individuals
Excludes the 49 women who did not report sex work
Excludes the 49 women who did not report sex work and another 24 women who did not answer this question
Adjusted for age, level of education, marital status, reported income, place of recruiting male clients and frequency of sex for money in past 3 months.
Risk behaviour and risk perception
The majority (85%) of participants reported more than one sexual partner in the last month and 66% of those involved in sex work reported having at least 5 paying clients in the past month (Table 2). Reported consistent condom use in the last month with paying clients was 60%, but just 6% used condoms consistently with the marital partner. The majority (78%) of participants reported using alcohol (30% on a daily basis), and 71% of users were classified as problem drinkers. Overall, 78 (8%) reported ever using marijuana and/or khat and 2 (0.2%) women ever injected heroin.
Table 2. Association of behavioural factors with HIV infection among women involved in high risk sexual behaviour in Kampala, Uganda.
| Total N |
HIV+ N (%) |
Adjusted OR3 (95%CI) |
|
|---|---|---|---|
| Sexual risk behaviour | |||
|
| |||
| Total number of lifetime partners | P=0.20 | ||
| <10 | 129 | 34 (26.4) | 1 |
| 10- 19 | 75 | 27 (36.0) | 1.38 (0.71-2.69) |
| 20- 49 | 80 | 22 (27.5) | 1.10 (0.55-2.22) |
| 50+ | 63 | 21 (33.3) | 1.14 (0.54-2.44) |
| Don’t remember | 680 | 278 (40.9) | 1.65 (0.97-2.82) |
|
| |||
| Age at first sex (y) | P=0.81 | ||
| <15 | 355 | 135 (38.0) | 1 |
| 15+ | 637 | 232 (36.4) | 1.07 (0.79-1.44) |
| Don’t remember | 35 | 15 (42.9) | 1.24 (0.60-2.59) |
|
| |||
| First sexual partner | P=0.64 | ||
| First husband/ fiancée | 257 | 112 (43.6) | 1 |
| Boyfriend | 657 | 230 (35.0) | 1.05 (0.76-1.45) |
| Casual acquaintance | 31 | 13 (41.9) | 1.01 (0.45-2.26) |
| Rapist | 82 | 27 (32.9) | 0.75 (0.43-1.30) |
|
| |||
| No. sexual partners in the last month | P=0.31 | ||
| 0-4 | 323 | 116 (35.9) | 1 |
| 5-9 | 134 | 49 (36.6) | 0.81 (0.50-1.31) |
| 10-19 | 173 | 59 (34.1) | 0.70 (0.44-1.12) |
| 20-49 | 236 | 87 (36.9) | 0.77 (0.49-1.23) |
| ≥ 50 | 87 | 33 (37.9) | 0.74 (0.40-1.37) |
| Don’t remember | 74 | 38 (51.4) | 1.29 (0.70-2.36) |
|
| |||
| No. paying clients in the last month 1 | P=0.33 | ||
| <5 | 231 | 79 (34.2) | 1 |
| 5-9 | 124 | 41 (33.1) | 0.70 (0.42-1.19) |
| 10-19 | 171 | 62 (36.3) | 0.81 (0.50-1.32) |
| 20-49 | 217 | 80 (36.9) | 0.80 (0.49-1.32) |
| ≥ 50 | 87 | 33 (37.9) | 0.76 (0.40-1.44) |
| Don’t remember | 75 | 39 (52.0) | 1.35 (0.72-2.52) |
|
| |||
| Condom use with paying clients in last month 2 | P=0.03 | ||
| Never | 43 | 16 (37.2) | 1 |
| Sometimes | 90 | 38 (42.2) | 1.09 (0.49-2.46) |
| Most of time | 230 | 97 (42.2) | 0.96 (0.45-2.03) |
| Always | 540 | 183 (33.9) | 0.64 (0.31-1.32) |
|
| |||
| HIV testing | |||
|
| |||
| Knowledge of HIV status | P<0.001 | ||
| Yes | 595 | 177 (29.8%) | 1 |
| No | 45 | 26 (57.8%) | 3.77 (1.94-7.30) |
| Never tested | 387 | 179 (46.3%) | 2.11 (1.58-2.81) |
|
| |||
| Alcohol & illicit drug use | |||
|
| |||
| Frequency of alcohol use | P=0.005 | ||
| Never | 224 | 64 (28.6) | 1 |
| Less than once a week | 71 | 36 (50.7) | 2.54 (1.42-4.55) |
| At least once a week | 489 | 193 (39.5) | 1.70 (1.18-2.46) |
| Daily | 243 | 89 (36.6) | 1.54 (1.01-2.36) |
|
| |||
| Drinking pattern following CAGE indicators | P=0.005 | ||
| Not user | 224 | 64 (28.6) | 1 |
| Not problem drinker | 231 | 93 (40.3) | 1.88 (1.23-2.85) |
| Problem drinker | 572 | 225 (39.3) | 1.67 (1.16-2.40) |
|
| |||
| Intravaginal practices | |||
|
| |||
| Cleansing inside the vagina in last 3 months | P=0.58 | ||
| No | 64 | 20 (31.3) | 1 |
| Yes | 963 | 362 (37.6) | 1.18 (0.66-2.10) |
|
| |||
| Cleansing inside vagina using soap in last 3 months | P=0.05 | ||
| No | 445 | 151 (33.9) | 1 |
| Yes | 582 | 231 (39.7) | 1.32 (1.00-1.73) |
|
| |||
| Inserting any substance inside the vagina in last 3 months | P=0.77 | ||
| No | 456 | 169 (37.1) | 1 |
| Yes | 571 | 213 (37.3) | 1.04 (0.79-1.38) |
Excluding the 49 women who were not sex workers, and 73 women with missing data for this variable
Among 903 participants with paying clients in past month
Adjusted for age, level of education, marital status, reported income, place of recruiting male clients and frequency of sex for money in past 3 months.
Vaginal cleansing, defined as cleaning inside the vagina with water or other substances, was very common (94%), mainly for daily hygiene (99%), but also to prepare for sex (58%), to clean immediately after sex (91%) or to treat vaginal irritation (58%). The insertion of substances to dry or lubricate the vagina was also common.
Only 58% of the study population knew their HIV-status when joining the project.
Prevalence of HIV and other STI
HIV prevalence was 37% (95%CI:34%-40%), increasing from 29% among those aged <25 years old to 48% among those aged ≥35 years (p<0.001) (Table 1). N. gonorrhoea prevalence was 13% (95%CI:11%-15%), C. trachomatis 9% (95%CI:7%-11%), T. vaginalis 17% (95%CI:15%-19%), bacterial vaginosis 56% (95%CI:53%-59%) and candida infection 11% (95%CI:9%-13%). Antibodies for HSV-2 were detected in 80% (95%CI:78%-82%). The prevalence of TPHA positivity was 21%, 10% were TPHA+/RPR+ and 3% had a RPR titre ≥1:8, indicating high-titre active syphilis.
T. pallidum (TP) was identified in 3% of the 62 GUD specimens, H. ducreyi (HD) in 6% and HSV2 in 35% specimens. LGV and HSV1 were not detected. In 55% of the specimens no etiology could be identified.
About 58% of the study participants reported not having symptoms of VDS. However 11% of these asymptomatic women were diagnosed with gonorrhoea, 9% with Chlamydia infection and 14% with trichomoniasis.
Factors independently associated with prevalent HIV
In the final multivariable model, HIV was strongly associated with older age (OR=1.62, 95%CI:0.99-2.42 for women aged ≥35 years vs 14-24 years, p-trend=0.02), lower levels of education (OR=4.19, 95%CI:2.12-8.28 for no education versus higher than primary level; p-trend<0.001), being widowed (OR=3.14, 95%CI:1.46-6.76 versus married women), recruiting clients on the street (OR=1.80, 95%CI:1.14-2.84), not knowing HIV status (OR=4.00, 95%CI:2.04-7.82 vs knowing HIV status), having never been tested for HIV (OR=2.06, 95%CI:.53-2.75 versus knowing HIV status); and alcohol use (OR=1.90, 95%CI:1.23-2.91 for a non-problem drinker vs non-drinker) (Table 3). HIV was less prevalent among women for whom sex work was not the sole occupation (OR=0.65, 95%CI:0.46-0.90 for those with sex work and another job vs sex work only), and those who had sex for money less than once a week (OR=0.40, 95%CI:0.20-0.81 vs those who had sex for money daily).
Table 3. Multivariable model for socio-economic and behavioural characteristics, independently associated with HIV.
| Characteristic | Adjusted OR2 |
|---|---|
| Age (y) | P-trend=0.02 |
| 14-24 | 1 |
| 25-34 | 1.41 (1.02-1.94) |
| 35+ | 1.62 (0.99-2.42) |
|
| |
| Education level | P-trend<0.001 |
| Higher than primary level | 1 |
| Primary completed | 1.42 (0.83-2.41) |
| Primary uncompleted | 1.79 (1.05-3.05) |
| Never went to school | 4.19 (2.12-8.28) |
|
| |
| Current marital status | P<0.001 |
| Married/cohabiting | 1 |
| Widowed | 3.14 (1.46-6.76) |
| Separated/divorced | 0.91 (0.54-1.54) |
| Single | 0.49 (0.27-0.88) |
|
| |
| Reported income | P=0.01 |
| Sex work only | 1 |
| Sex work and other job | 0.65 (0.46-0.90) |
| Other job only | 0.57 (0.16-2.02) |
|
| |
| Place of recruiting male clients | P=0.001 |
| Bar, club or restaurant | 1 |
| Street | 1.80 (1.14-2.84) |
| Other | 0.94 (0.50-1.76) |
| Several places | 0.76 (0.54-1.07) |
|
| |
| How often sex for money | P=0.006 |
| Daily | 1 |
| At least once a week | 1.26 (0.91-1.74) |
| Less than once a week | 0.40 (0.20-0.81) |
|
| |
| Condom use with paying clients in last month 1 | P=0.05 |
| Never | 1 |
| Sometimes | 0.99 (0.43-2.26) |
| Most of time | 0.88 (0.41-1.89) |
| Always | 0.60 (0.29-1.26) |
|
| |
| Knowledge of HIV status | P<0.001 |
| Yes | 1 |
| No | 4.00 (2.04-7.82) |
| Never tested | 2.06 (1.53-2.75) |
|
| |
| Drinking pattern: following CAGE indicators | P=0.008 |
| Not user | 1 |
| Not problem drinker | 1.90 (1.23-2.91) |
| Problem drinker | 1.64 (1.13-2.38) |
|
| |
| Cleansing inside vagina using soap in last 3 months | P=0.08 |
| No | 1 |
| Yes | 1.29 (0.97-1.70) |
Among 903 participants with paying clients in past month
Adjusted for age, level of education, marital status, main job, reported income, place of recruiting male clients, frequency of sex for money in past 3 months, condom use with paying clients in past 3 months, CAGE score, and intra-vaginal cleansing with soap.
Except for chlamydial and candida infection, all other STI were associated with HIV-infection after adjustment for socio-demographic, economic and behavioural factors (Table 4). The strongest association was with HSV-2 infection (OR=5.72, 95%CI:3.40-9.62), active syphilis (OR=1.98, 95%CI:1.25-3.13), NG (OR=2.53, 95%CI:1.67-3.82), and intermediate or positive BV (OR(int)=2.39, 95%CI:1.44-3.98; OR(pos)=2.09, 95%CI:1.53-2.87).
Table 4. Multivariable model for the association between STI and HIV, adjusted for socio-economic and behavioural characteristics.
| Total N |
HIV+ N (%) |
Adjusted OR3 (95%CI) |
|
|---|---|---|---|
| HSV-2 serology 1 | P<0.001 | ||
| Neg | 205 | 19 (9.3) | 1 |
| Pos | 821 | 362 (44.1) | 5.72 (3.40-9.62) |
|
| |||
| Syphilis 2 | P=0.005 | ||
| No infection (RPR −TPHA −) | 807 | 275 (34.1) | 1 |
| Old infection (RPR − TPHA+) | 113 | 54 (47.8) | 1.30 (0.84-2.01) |
| Active infection(RPR+TPHA+) | 103 | 53 (51.5) | 1.98 (1.25-3.13) |
|
| |||
| NG pcr 2 | P<0.001 | ||
| Neg | 889 | 304 (34.2) | 1 |
| Pos | 134 | 77 (57.5) | 2.53 (1.67-3.82) |
|
| |||
| CT pcr 1 | P=0.98 | ||
| Neg | 934 | 352 (37.7) | 1 |
| Pos | 92 | 30 (32.6) | 1.01 (0.61-1.67) |
|
| |||
| TV culture | P=0.001 | ||
| Neg | 851 | 295 (34.7) | 1 |
| Pos | 176 | 87 (49.4) | 1.83 (1.27-2.63) |
|
| |||
| BV | P<0.001 | ||
| Neg | 354 | 94 (26.6) | 1 |
| Intermediate | 100 | 43 (43.0) | 2.39 (1.44-3.98) |
| Pos | 573 | 245 (42.8) | 2.09 (1.53-2.87) |
|
| |||
| Candida | P=0.19 | ||
| Neg | 915 | 335 (36.6) | 1 |
| Pos | 112 | 47 (42.0) | 1.35 (0.86-2.11) |
excluding 1 woman with missing results
excluding 4 women with missing results
Adjusted for age, level of education, marital status, reported income, place of recruiting male clients, frequency of sex for money in past 3 months, condom use with paying clients in past 3 months, CAGE score, and intra-vaginal cleansing with soap.
Discussion
The GHWP cohort enrolled women at high risk of HIV/STI because of their likely involvement in commercial sex. We recruited either self-acknowledging sex workers or women employed in local entertainment facilities such as bars, night clubs, guesthouses and lodges. Almost all (95%) had some income from exchanging sex for money. Our study population was relatively young, with very little schooling, predominantly unmarried and living with dependent children. The prevalence of HIV is very high (37%) compared to the national prevalence (6.4%) and to the prevalence among general population women from Kampala (12%)18 but similar to prevalence in a high risk cohort in Mombasa, Kenya in 2009 (35%). 19 HIV prevalence was highest (44%) among women for whom sex work was the sole source of income but was still 34% among women reporting another income besides sex work. Among the 49 women who denied being involved in sex work, 37% were HIV positive. This would confirm that women working in entertainment facilities are equally at high risk, although it is very likely that these women may just have been reluctant to disclose their real status of income.
Other STI were also highly prevalent, suggesting inadequate STI treatment in the past, either because of poor access to existing health services and/or to inappropriate management of STI. The screening questionnaire revealed that 94% of participants had never been examined with a speculum.
HSV-2 was the main cause of GUD (35%). However some cases of TP (3%) and HD (6%) were identified, indicating that currently recommended GUD management including treatment for all three pathogens is still justified. In 55% of the GUD specimens no etiology could be identified which is in range with results from other studies 21, 22: it is likely that lesions caused by mechanical trauma or itching may have been misdiagnosed as GUD.
Among the 58% of women attending the enrolment visit without symptoms of VDS, high prevalences of curable STI were detected by the lab. Nearly half (46%) of these asymptomatic cases had confirmed VDS after gynaecological examination using speculum. Despite the known limitations of the syndromic approach especially for VDS, this target population would benefit of regular screening and treatment for STI as has already been reported before in other high risk settings.23
Alcohol use is common in this population, with over 70% of participants reporting alcohol use on a regular basis and 56% having a drinking problem as defined by the CAGE scale. Similar high prevalences of problem drinking have been reported from sex workers in Mombasa (33%) 24 and in facility workers in Moshi (35%).25 Use of alcohol, whether problem drinker or not, was independently associated with HIV–infection, as has been found in other African studies.26 Alcohol is known to reduce inhibitions and to diminish perception of risk and may therefore lead to increased unsafe sex practices. In our setting, 66% of the non drinkers consistently used condoms with their clients, compared to 61% in non problem drinkers and 57% in problem drinkers (p=0.04), although both variables are subject to misreporting. Participants expressed at several occasions their need of alcohol in order to cope with sex work. Client-centered counselling interventions have been used with some success in South-Africa to reduce alcohol intake and risky sexual behavioural patterns 27 and further such interventions are needed to assist women involved in sex work to adopt safer drinking patterns.
There was no evidence for the effect of condom use on HIV-infection. However, condom use is difficult to measure, it is subject to misreporting 28 and the reported condom use pattern may not be the same as at time of HIV-infection. Also condom use is often confounded with type and number of partners, and may be a marker for exposure to “high-risk” partners. Furthermore, nearly half of the women were not aware of their HIV-status at enrolment which may indicate low risk perception.
Intra vaginal cleansing and insertion are very common in this population. Cleansing of the vagina using soap was independently associated with HIV-infection and in range with findings from Mombasa. 29
There are several limitations of our study. Firstly, we were relying on reported behaviours. Our participants may have been reluctant to disclose information during this initial contact before rapport is established, or may not have been able to recall the precise information needed. Secondly, because of the cross-sectional design of the baseline study, we cannot analyse the temporal relationship between variables, which makes it impossible to interpret fully the associations between risk behaviour, STI and HIV infection.
Core groups with high rates of sexual partner change, such as sex workers, are thought to play an important role in the initial establishment of HIV epidemics in populations. It has been postulated that such core groups may also play an increasingly important role in later phases of HIV epidemics as prevalence declines and HIV transmission again becomes more concentrated in groups at higher risk. 30 In Uganda, with its mature HIV epidemic, this suggests that studies of the size, dynamics and determinants of the epidemic in high risk groups are very important.
The high prevalence of HIV/STI combined with the high risk behaviour among our enrolled women urges for specific HIV prevention interventions in vulnerable population groups such as sex workers. The existing general care health services may not fulfil adequately in their specific needs and accessibility may be hampered due to perceived stigma and misconceptions. The wide establishment of clinics covering and targeting high risk groups, offering regular screening and adequate treatment for STI, risk reducing counselling and providing free condoms are needed in order to reinforce the control of the HIV epidemic in Uganda.
Acknowledgments
The Medical Research Council UK and the European and Developing Countries Clinical Trials Partnership for financing the project; Women At Work International Uganda for their assistance in recruiting study participants, the GHWP study team for their dedication to the work; the study participants for their collaboration.
Sources of support:
Medical Research Council, UK
European and Developing Countries Clinical Trials Partnership
Footnotes
Dissemination meetings and dates:
18th ISSTDR, 28 June -1 July 2009, London, UK:
Prevalence of and risk factors for HIV and other STI in a cohort of women involved in high risk sexual behaviour in Kampala, Uganda. Poster presentation.
11th IUSTI World Congress, 9-12 Nov 2009, Cape Town, South Africa:
Baseline prevalence of HIV and other STI in a cohort of women involved in high risk sexual behaviour in Kampala, Uganda. Oral presentation.
References
- 1.Mulder D, Nunn A, Kamali A, et al. Decreasing HIV-1 seroprevalence in young adults in a rural Ugandan cohort. BMJ. 1995;311:833–836. doi: 10.1136/bmj.311.7009.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamali A, Carpenter LM, Whitworth JAG, et al. Seven-year trends in HIV-1 infection rates and changes in sexual behaviour among adults in rural Uganda. AIDS. 2000;14(4):427–434. doi: 10.1097/00002030-200003100-00017. [DOI] [PubMed] [Google Scholar]
- 3.Mbulaiteye SM, Mahe C, Whitworth JAG, et al. Declining HIV-1 incidence and associated prevalence over 10 years in a rural population in south-west Uganda: a cohort study. The Lancet. 2002;(360):41–46. doi: 10.1016/s0140-6736(02)09331-5. [DOI] [PubMed] [Google Scholar]
- 4.Shafer LA, Biraro S, Nakiyingi-Miiro J, et al. HIV prevalence and incidence are not longer falling in southwest Uganda: evidence from a rural population cohort 1989-2005. AIDS. 2008;22(13):1641–1649. doi: 10.1097/QAD.0b013e32830a7502. [DOI] [PubMed] [Google Scholar]
- 5.Nzila N, Laga M, Thiam MA, et al. HIV and other sexually transmitted diseases among female prostitutes in Kinshasa. AIDS. 1991 Jun;5(6):715–21. doi: 10.1097/00002030-199106000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Ghys PD, Diallo MO, Ettiègne-Traoré V, et al. Genital ulcers associated with human immunodeficiency virus-related immune suppression in female sex workers in Abidjan, Ivory Coast. J Infect Dis. 1995;172:1371–1374. doi: 10.1093/infdis/172.5.1371. [DOI] [PubMed] [Google Scholar]
- 7.Kapiga S, Sam N, Shao J, et al. HIV-1 epidemic among female bar and hotel workers in Northern Tanzania: risk factors and opportunities for prevention. JAIDS. 2002;29(4):409–417. doi: 10.1097/00126334-200204010-00013. [DOI] [PubMed] [Google Scholar]
- 8.Riedner G, Rusizoka M, Hoffmann O, et al. Baseline survey of sexually transmitted infections in a cohort of female bar workers in Mbeya region, Tanzania. Sex Transm Infect. 2003;79:382–387. doi: 10.1136/sti.79.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramjee G, Karim SS, Sturm AW. Sexually transmitted infections among sex workers in KwaZulu-Natal, South Africa. Sex Transm Dis. 1998;25(7):346–9. doi: 10.1097/00007435-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Watson-Jones D, Weiss HA, Rusizoka M, et al. Risk factors for HSV-2 and HIV among women at high risk in Northwestern Tanzania: Preparing for an HSV-2 intervention trial. J Acquir Immune Defic Syndr. 2007;46:631–642. doi: 10.1097/QAI.0b013e31815b2d9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandepitte J, Malele F, Kivuvu DM, et al. HIV and other sexually transmitted infections among female sex workers in Kinshasa, DRC in 2002. Sex Transm Dis. 2007;34(4):203–208. doi: 10.1097/01.olq.0000233743.57334.6a. [DOI] [PubMed] [Google Scholar]
- 12.Ghys PD, Diallo MO, Ettiègne-Traoré V, et al. Increase in condom use and decline in HIV and sexually transmitted infections among female sex workers in Abidjan, 1993-1999. AIDS. 2002;16:251–258. doi: 10.1097/00002030-200201250-00015. [DOI] [PubMed] [Google Scholar]
- 13.Alary M, Mukenge-Tshibaka L, Bernier F, et al. Decline in the prevalence of HIV and STD among female sex workers in Cotonou, Bénin, 1993-1999. AIDS. 2002;16:463–470. doi: 10.1097/00002030-200202150-00019. [DOI] [PubMed] [Google Scholar]
- 14.Riedner G, Hoffmann O, Rusizoka M, et al. Decline in sexually transmitted infection prevalence and HIV incidence in female bar workers attending prevention and care services in Mbeya, Tanzania. AIDS. 2006;20:609–615. doi: 10.1097/01.aids.0000210616.90954.47. [DOI] [PubMed] [Google Scholar]
- 15.Kaul R, Kimani J, Nagelkerke N, et al. Reduced HIV risk-taking and low HIV incidence after enrollment and risk-reduction counseling in a sexually transmitted disease prevention trial in Nairobi, Kenya. JAIDS. 2002;(30):69–72. doi: 10.1097/00042560-200205010-00009. [DOI] [PubMed] [Google Scholar]
- 16.Ewing JA. Detecting alcoholism: the CAGE questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- 17.Victora CG, Huttly SR, Fuchs SC, et al. The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. Int J Epid. 1997;6(1):224–227. doi: 10.1093/ije/26.1.224. [DOI] [PubMed] [Google Scholar]
- 18.Uganda AIDS Commission . A review of the trends of HIV prevalence and incidence and projections. Aug, 2006. [Google Scholar]
- 19.Luchters SM, Vanden Broeck D, Chersich MF, et al. Association of HIV infection with distribution and viral load of HPV types in Kenya: a survey with 820 female sex workers. BMC Infectious Diseases. 2010;10:18. doi: 10.1186/1471-2334-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawken MP, Melis RD, Ngombo DT, et al. Part time female sex workers in a suburban community in Kenya: a vulnerable hidden population. Sex Transm Infect. 2002 Aug;78(4):271–3. doi: 10.1136/sti.78.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeGoff J, Weiss HA, Gresenguet G, et al. Cervicovaginal HIV-1 and herpes simplex virus type 2 shedding during genital ulcer disease episodes. AIDS. 2007;21:1569–1578. doi: 10.1097/QAD.0b013e32825a69bd. [DOI] [PubMed] [Google Scholar]
- 22.Suntoke T, Hardick A, Tobian A, et al. Evaluation of multiplex real-time PCR for detection of Haemophilus ducreyi, Treponema pallidum, herpes simplex virus type 1 and 2 in the diagnosis of genital ulcer disease in the Rakai District, Uganda. Sex Transm Infect. 2009;85:97–101. doi: 10.1136/sti.2008.034207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steen R, Dallabetta G. Sexually transmitted infections control with sex workers: regular screening and presumptive treatment augment efforts to reduce risk and vulnerability. Reproductive Health Matters. 2003;11(22):74–90. doi: 10.1016/s0968-8080(03)02295-x. [DOI] [PubMed] [Google Scholar]
- 24.Chersich MF, Luchters SM, Malonza IM, et al. Heavy episodic drinking among Kenyan female sex workers is associated with unsafe sex, sexual violence and sexually transmitted infections. Int J STD AIDS. 2007 Nov;18(11):764–9. doi: 10.1258/095646207782212342. [DOI] [PubMed] [Google Scholar]
- 25.Fisher J, Cook P, Sam N, et al. Patterns of alcohol use, problem drinking, and HIV infection among high-risk African women. Sexually Transmitted Diseases. 2008 Jun;35(6):537–544. doi: 10.1097/OLQ.0b013e3181677547. [DOI] [PubMed] [Google Scholar]
- 26.Fisher JC, Bang H, Kapiga S. The Association Between HIV Infection and Alcohol Use: A Systematic Review and Meta-Analysis of African Studies. Sex Trans Dis. 2007;34(11):856–863. doi: 10.1097/OLQ.0b013e318067b4fd. [DOI] [PubMed] [Google Scholar]
- 27.Kalichman SC, Simbayi LC, Vermaak R, et al. HIV/AIDS reduction counseling for Alcohol using sexually transmitted infections clinic patients in Cape Town, South Africa. J Acquir Immune Defic Syndr. 2007;44(5):594–600. doi: 10.1097/QAI.0b013e3180415e07. [DOI] [PubMed] [Google Scholar]
- 28.Weir SS, Fox LJ, De Moya A, et al. Measuring condom use among sex workers in the Dominican Republic. Int J STD AIDS. 1998;9:223–226. doi: 10.1258/0956462981922089. [DOI] [PubMed] [Google Scholar]
- 29.McClelland RS, Lavreys L, Hassan WM, et al. Vaginal washing and increased risk of HIV-1 acquisition among African women: a 10-year prospective study. AIDS. 2006;20:269–273. doi: 10.1097/01.aids.0000196165.48518.7b. [DOI] [PubMed] [Google Scholar]
- 30.Wasserheit JN, Aral SO. The dynamic topology of sexually transmitted disease epidemics: implications for prevention strategies. Journal of Infectious Diseases. 1996;174(Suppl 2):S201–S213. doi: 10.1093/infdis/174.supplement_2.s201. [DOI] [PubMed] [Google Scholar]