Abstract
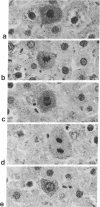
Normal rat liver cytosol was found previously to contain a 14,000-dalton polypeptide that is the principal target of the carcinogen N-2-fluorenylacetamide (2-acetyl-aminofluorene) early during hepatocarcinogenesis. By using antiserum that identifies the 14,000-dalton polypeptide in liver cytosol, an immunologically related 17,500-dalton polypeptide was shown to be present in isolated normal liver nuclei, tightly bound to chromatin. We report here that the 14,000-dalton polypeptide is associated with cell multiplication in normal adult hepatocytes. Of 150 hepatocytes in five stages of mitosis, all invariably displayed a greatly increased concentration of the 14,000-dalton polypeptide in cytoplasm, compared to hepatocytes in interphase, by immunostaining with peroxidase-antiperoxidase complex. In contrast, mitosis did not appear to affect the level of the 17,500-dalton polypeptide in nuclei. A small population of high-polyploid hepatocytes with large nuclei had elevated intensities of cytoplasmic immunostain that approached that in mitotic cells. The increased level of discernible 14,000-dalton polypeptide target of the carcinogen in cytoplasm is a marker of the rare mitotic hepatocytes in normal adult rat liver. Ingestion of the liver carcinogen N-2-fluorenylacetamide for 5 wk or an azocarcinogen, 3'-methyl-4-dimethylaminoazobenzene, for 4 wk brought about early foci of hyperplastic hepatocytes in which there was a great overload of detectable 14,000-dalton target polypeptide in their cytoplasm and a near absence of the 17,500-dalton polypeptide in most nuclei. In contrast, the cytoplasm and nuclei in surrounding morphologically nontransformed hepatocytes of livers of the carcinogen-fed rats immunostained to a much smaller degree than in normal adult hepatocytes. Removal of the azo-dye carcinogen for 4 wk resulted in the disappearance of most hyperplastic foci. A few foci did persist and had similar apparent abnormal levels of the two polypeptides. The high concentration of discernible 14,000-dalton target polypeptide in the cytoplasm of hepatocytes in the hyperplastic foci correlates with their known proliferation, whereas the low cytoplasmic level in the surrounding hepatocytes is consistent with the known inhibition of their mitosis by these carcinogens. The collected evidence appears to joint together a chemical carcinogen, a cytoplasmic principal target polypeptide, a chromatin-bound polypeptide, mitosis in normal adult hepatocytes, early and persistent hyperplastic foci caused by carcinogens, and the inhibition of mitosis by carcinogens in surrounding parenchyma in preneoplastic livers.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackburn G. R., Andrews J. P., Custer R. P., Sorof S. Early events during liver carcinogenesis involving two carcinogen:protein complexes. Cancer Res. 1981 Oct;41(10):4039–4049. [PubMed] [Google Scholar]
- Blackburn G. R., Andrews J. P., Rao K. V., Sorof S. An early event associated with liver carcinogenesis involving loss of a polypeptide that binds carcinogen. Cancer Res. 1980 Dec;40(12):4688–4693. [PubMed] [Google Scholar]
- Blackburn G. R., Schnabel S. J., Danley J. M., Hogue-Angeletti R. A., Sorof S. Principal polypeptide target of carcinogen at the beginning of liver carcinogenesis by three carcinogens. Cancer Res. 1982 Nov;42(11):4664–4672. [PubMed] [Google Scholar]
- Farber E., Cameron R. The sequential analysis of cancer development. Adv Cancer Res. 1980;31:125–226. doi: 10.1016/s0065-230x(08)60658-2. [DOI] [PubMed] [Google Scholar]
- Fausto N., Shank P. R. Oncogene expression in liver regeneration and hepatocarcinogenesis. Hepatology. 1983 Nov-Dec;3(6):1016–1023. doi: 10.1002/hep.1840030621. [DOI] [PubMed] [Google Scholar]
- Gronow M., Thackrah T. Changes in the composition of rat liver chromatin fractions during nitrosamine carcirogenesis. Eur J Cancer. 1974 Jan;10(1):21–25. doi: 10.1016/0014-2964(74)90033-4. [DOI] [PubMed] [Google Scholar]
- MacLeod M. C., Pelling J. C., Slaga T. J., Noghrei-Nikbakht P. A., Mansfield B. K., Selkirk J. K. Specificity of interaction between carcinogenic polynuclear aromatic hydrocarbons and nuclear proteins: widespread occurrence of a restricted pattern of histone binding in intact cells. Prog Nucleic Acid Res Mol Biol. 1983;29:111–115. doi: 10.1016/s0079-6603(08)60437-7. [DOI] [PubMed] [Google Scholar]
- Miller E. C., Miller J. A. Mechanisms of chemical carcinogenesis. Cancer. 1981 Mar 1;47(5 Suppl):1055–1064. doi: 10.1002/1097-0142(19810301)47:5+<1055::aid-cncr2820471302>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Miller E. C., Miller J. A. Searches for ultimate chemical carcinogens and their reactions with cellular macromolecules. Cancer. 1981 May 15;47(10):2327–2345. doi: 10.1002/1097-0142(19810515)47:10<2327::aid-cncr2820471003>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Rodman T. C., Pruslin F. H., Allfrey V. G. Protamine-DNA association in mammalian spermatozoa. Exp Cell Res. 1984 Feb;150(2):269–281. doi: 10.1016/0014-4827(84)90569-x. [DOI] [PubMed] [Google Scholar]
- Solt D. B., Medline A., Farber E. Rapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesis. Am J Pathol. 1977 Sep;88(3):595–618. [PMC free article] [PubMed] [Google Scholar]
- Teebor G. W., Becker F. F. Regression and persistence of hyperplastic hepatic nodules induced by N-2-Fluorenylacetamide and their relationship to hepatocarcinogenesis. Cancer Res. 1971 Jan;31(1):1–3. [PubMed] [Google Scholar]
- Vinores S. A., Churey J. J., Haller J. M., Schnabel S. J., Custer R. P., Sorof S. Normal liver chromatin contains a firmly bound and larger protein related to the principal cytosolic target polypeptide of a hepatic carcinogen. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2092–2096. doi: 10.1073/pnas.81.7.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]