Abstract
Pulmonary vascular dilation and angiogenesis underlie experimental hepatopulmonary syndrome (HPS) induced by common bile duct ligation (CBDL) and may respond to receptor tyrosine kinase (RTK) inhibition. Vascular endothelial growth factor-A (VEGF-A) expression occurs in proliferating cholangiocytes and pulmonary intravascular monocytes after CBDL, the latter contributing to angiogenesis. CBDL cholangiocytes also produce endothelin-1 (ET-1), which triggers lung vascular endothelin B receptor-mediated endothelial nitric oxide synthase (eNOS) activation and pulmonary intravascular monocyte accumulation. However, whether RTK pathway activation directly regulates cholangiocyte and pulmonary microvascular alterations in experimental HPS is not defined. We assessed RTK pathway activation in cholangiocytes and lung after CBDL and the effects of the type II RTK inhibitor sorafenib in experimental HPS. Cholangiocyte VEGF-A expression and ERK activation accompanied proliferation and increased hepatic and circulating ET-1 levels after CBDL. Sorafenib decreased each of these events and led to a reduction in lung eNOS activation and intravascular monocyte accumulation. Lung monocyte VEGF-A expression and microvascular Akt and ERK activation were also found in vivo after CBDL, and VEGF-A activated Akt and ERK and angiogenesis in rat pulmonary microvascular endothelial cells in vitro. Sorafenib inhibited VEGF-A-mediated signaling and angiogenesis in vivo and in vitro and improved arterial gas exchange and intrapulmonary shunting. RTK activation in experimental HPS upregulates cholangiocyte proliferation and ET-1 production, leading to pulmonary microvascular eNOS activation, intravascular monocyte accumulation, and VEGF-A-mediated angiogenic signaling pathways. These findings identify a novel mechanism in cholangiocytes through which RTK inhibition ameliorates experimental HPS.
Keywords: common bile duct ligation, bile duct proliferation, endothelin-1, receptor tyrosine kinase, angiogenesis
the hepatopulmonary syndrome (HPS) results when pulmonary microvascular alterations cause impaired gas exchange reflected by an increased alveolar-arterial oxygen gradient (A-aPo2) in the setting of liver disease and/or portal hypertension (31, 34, 37). This syndrome develops in 5–32% of cirrhotic patients evaluated for liver transplantation and is associated with increased mortality and decreased quality of life (13, 19, 35). Currently, liver transplantation is the only available treatment option for HPS, underscoring the need to develop effective medical therapies (2–4, 13, 20).
Experimental biliary cirrhosis induced by common bile duct ligation (CBDL) in the rat reproduces the pulmonary vascular and gas exchange abnormalities of human HPS and has been extensively used as an animal model (6–8, 12). Evidence supports that pulmonary microvascular dilation and neovascularization develop in both experimental and human disease and drive the development of gas exchange abnormalities (1, 9, 11, 12, 17, 32, 33, 36, 40).
The pathogenesis of pulmonary microvascular changes in experimental HPS involves alterations in the liver that influence the lung as well as direct effects in the lung. Specifically, CBDL cholangiocytes produce endothelin-1 (ET-1), which enters the circulation and activates lung vascular endothelin B (ETB) receptors overexpressed in the setting of increased shear stress, thereby driving vascular endothelial nitric oxide synthase (eNOS)-derived NO production and vasodilation (11, 21, 25, 27, 28, 43). In addition, lung ETB receptor activation contributes to an influx of pulmonary intravascular macrophages after CBDL, and these cells produce heme oxygenase-1-derived carbon monoxide as well as vascular endothelial growth factor A (VEGF-A), which contribute to vasodilation and angiogenesis (21, 40, 41). VEGF-A is also produced in pulmonary microvascular endothelium after CBDL and signaling through its receptor tyrosine kinase (RTK) receptor (VEGFR) activates downstream angiogenic signaling pathways, including Akt and ERK, which contribute to angiogenesis (39, 41, 42).
A single report supports that RTK inhibition with sorafenib, a multispecific RTK inhibitor approved for use in hepatocellular carcinoma, improves established experimental HPS although hepatic mechanisms were not evaluated and a detailed analysis of cellular targets and RTK pathways in the pulmonary microvasculature was not included (5). Since VEGF-A is also recognized to regulate proliferation in cholangiocytes through an autocrine loop involving ERK activation (14, 15), we sought to directly evaluate RTK activation in cholangiocytes and in the pulmonary microvasculature in experimental HPS and to assess the effects of RTK inhibition with sorafenib on relevant pathways and HPS.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats (250–300 g; Charles River, Wilmington, MA) underwent sham operation (mobilization of the common bile duct without ligation) or CBDL as described (12, 21, 26). Sorafenib (Sorafenib tosylate, 5 mg/kg, gavage daily; Bayer HealthCare) was given for 2 wk beginning 2 wk after CBDL. All assessments were made at 4 wk after surgery. Eight animals were used in each group. Portal venous pressure (PVP) and spleen weight were measured. Lung, liver, and plasma were obtained for further analysis. The study was approved by the University of Texas at Houston Health Science Center Animal Welfare Committee and conformed to National Institutes of Health guidelines on the use of laboratory animals.
Arterial blood gas analysis.
Arterial blood drawn from the femoral artery as previously described (12, 21, 40) was analyzed on an ABL80 FLEX blood gas analyzer (Radiometer America, Westlake, OH). The A-aPo2 was calculated as 150 − (PaCO2/0.8) − PaO2.
Microsphere protocol.
An established technique with minor modification was used to evaluate intrapulmonary vasodilatation or shunting (12, 21, 26, 40). Cross-linked colored polystyrenedivinylbenzene microspheres (2.5 × 106, 5.0 μm, Interactive Medical Technologies) were injected into animals through a femoral vein catheter. Microspheres passing through the pulmonary microcirculation were measured in a blood sample withdrawn from a femoral arterial catheter beginning at the time of femoral vein injection. Numbers of microspheres were counted directly by use of a Nikon Eclipse E200 Binocular Microscope (Nikon). Intrapulmonary shunting was calculated as an intrapulmonary shunt fraction (%) = (total numbers of microspheres passing through the pulmonary microcirculation/total microspheres injected into the venous circulation) × 100.
Lung microvessel density quantitation.
The degree of pulmonary angiogenesis was evaluated by quantifying microvessel densities in lung sections immunostained with the endothelial marker Factor VIII as previously described (41, 42). All stained objects were counted in a blinded fashion. Vessels with thick muscular walls or larger than 100 μm in diameter were excluded. Five randomly scanned fields of three sections from each animal were investigated. The average microvessel count in each group was expressed relative to the count of sham group as fold control values.
Measurement of plasma ET-1 levels.
Plasma ET-1 levels were measured with a commercial ELISA kit (Enzo Life Sciences) according to the manufacturer's instructions.
RNA extraction and quantitative real-time RT-PCR.
Total RNA from liver was extracted with TRIzol (Invitrogen) reagent according to the manufacturer's instructions and treated with RNase-free DNase I (Invitrogen) following the manufacturer's protocol. cDNA was prepared using The High Capacity cDNA Reverse Transcription kit (Applied Biosystems). Real-time PCR analysis was performed using the StepOnePlus Real-Time PCR System and TaqMan Gene Expression Master Mix (Applied Biosystems) according to the manufacturer's recommendations. TaqMan Gene Expression Assays for rat α-smooth muscle actin, PCNA, and CK-19 were from Applied Biosystems. Expression levels were normalized to expression of 18S rRNA.
Immunofluorescence and immunohistochemistry.
Five-micrometer sections of 4% paraformaldehyde paraffin-fixed tissue were blocked and incubated with primary antibodies. For immunofluorescent staining of ED1 (a monocyte marker, AbD Serotec) and factor VIII (FVIII, Cell Marque), Texas red and fluorescein secondary antibodies (Vector Laboratories, Burlingame, CA) and mounting medium with DAPI (Vector) were used. For immunostaining of CK-19 (Santa Cruz Biotechnology), PCNA, phospho-Akt (p-Akt, Ser473), phospho-ERK (p-ERK, Thr202/Tyr204) (Cell Signaling Technology), ET-1 (Phoenix Pharmaceuticals), and VEGF-A (Abcam), biotinylated secondary antibodies were used. After incubation with streptavidin-biotin-horseradish peroxidase (VECTASTAIN Elite ABC Kit, Vector), peroxidase activity was detected with DAB Peroxidase Substrate Kit (Vector). The positive staining of p-ERK and VEGF-A was quantitated with ImageJ software provided by National Institutes of Health.
Cholangiocyte culture and treatments.
Normal rat cholangiocytes (NRCs) were maintained and subcultured in DMEM supplemented with 5% fetal bovine serum and completed growth factors at 37°C in presence of humidified 95% air and 5% CO2 as previously described (15, 28). NRCs were stimulated with recombinant rat VEGF-A (15 ng/ml, R&D Systems) for 15 h with and without pretreatment of a specific MERK/ERK inhibitor (U0126, 10 μM) for 30 min. ET-1 protein levels in culture supernate and mRNA levels in cell homogenates were measured by ELISA and quantitative real-time RT-PCR.
Endothelial cell culture and treatments.
Rat pulmonary microvascular endothelial cells (RPMVECs, Vec Technologies) were maintained and subcultured in complete media MCDB-131 (Vec Technologies) at 37°C in presence of humidified 95% air and 5% CO2. Cell passages were between 2 and 10. After incubating in EBM-2 Basal Medium (Lonza) with 0.1% fetal bovine serum for 4 h, cells were stimulated with recombinant rat VEGF-A (15 ng/ml, R&D) for 5 to 15 min with or without sorafenib (0.1 to 0.5 μM) pretreatment for 30 min. Whole cell lysates were analyzed by Western blot using Akt, p-Akt (Ser473), ERK, and p-ERK (Thr202/Tyr204) antibodies (Cell Signaling Technology).
Western blot analysis.
Equal amount of proteins from the lung or cell lysates obtained from above were fractionated on Criterion Tris·HCl Gel (4–20%) (Bio-Rad Laboratories) and transferred to PVDF membrane (Millipore). Incubation with primary antibodies against ED1, VEGF-A, Akt, p-Akt (Ser473), ERK, p-ERK (Thr202/Tyr204), and p-eNOS (Ser1177) antibodies was followed by addition of horseradish peroxidase-conjugated secondary antibodies and detection with enhanced chemiluminescence substrate Pico-West luminol reagent (Thermo Scientific Pierce). The density of autoradiographic signals was assessed with a ScanMaker i900 scanner (Microtek Lab) and quantitated with ImageJ software provided by the National Institutes of Health.
Endothelial tube formation assay.
VEGF-A stimulation of endothelial cell tubular structure formation (in vitro angiogenesis) was assessed by culturing RPMVECs on growth factor reduced Matrigel (BD Biosciences). RPMVECs (4 × 104 per well) were added to a 48-well plate coated with Matrigel and incubated (in EBM-2 Basal Medium including all growth factors + 0.1% fetal bovine serum) for 4–16 h at 37°C. VEGF-A (5 ng/ml) was administered in the presence or absence of sorafenib (2 μM). Five random fields per well were captured by use of a Nikon Eclipse E200 Binocular Microscope (Nikon). Endothelial tubular structures were skeletonized and total tube length quantified in a blinded manner and expressed as fold control values.
Statistics.
Data were analyzed with the Student's t-test or the analysis of variance with Bonferroni correction for multiple comparisons between groups. Measurements are expressed as means ± SE. Statistical significance was designated as P < 0.05.
RESULTS
Evaluation of RTK pathways in cholangiocytes and effects on ET-1 production after CBDL.
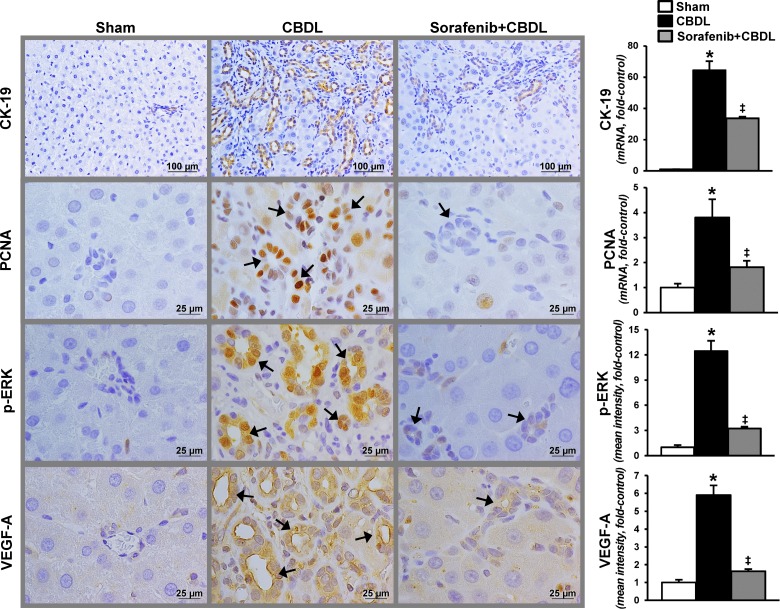
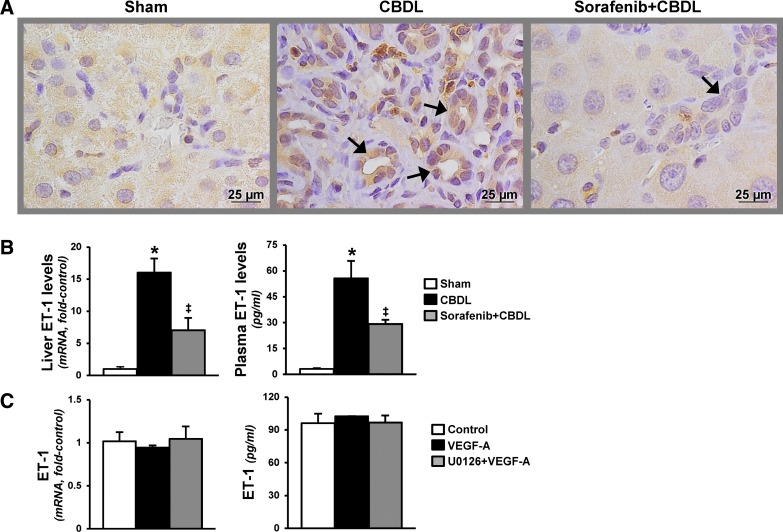
On the basis of observations that VEGF-A contributes to cholangiocyte proliferation (15), we evaluated relevant RTK signaling pathways, cholangiocyte proliferation, and ET-1 production after CBDL (Figs. 1 and 2). We found a significant increase in cholangiocyte VEGF-A levels and ERK activation by quantitative immunostaining after CBDL that was accompanied by proliferation reflected in increased CK-19 and PCNA levels and staining in cholangiocytes. These events were associated with increased cholangiocyte ET-1 production measured by hepatic and circulating levels and immunostaining. RTK inhibition with sorafenib resulted in a significant reduction in VEGF-A production and ERK activation that was accompanied by a marked decline in proliferation in cholangiocytes. In addition, cholangiocyte ET-1 staining and hepatic and circulating levels were also significantly decreased. To determine whether VEGF-A directly induces ET-1 expression in cholangiocytes, VEGF-A was administered to NRCs in the presence or absence of a MERK/ERK inhibitor (U0126). We found that cholangiocyte ET-1 protein and mRNAs levels were not influenced by the addition of VEGF-A or U0126.
Fig. 1.
Cholangiocyte proliferation, vascular endothelial growth factor-A (VEGF-A) production, and ERK activation after common bile duct ligation (CBDL) in the presence or absence of receptor tyrosine kinase (RTK) inhibition with sorafenib. Left: representative images of hepatic immunohistochemistry for CK-19 (cholangiocyte marker), PCNA (cell proliferation marker), ERK phosphorylation (p-ERK, Thr202/Tyr204), and VEGF-A production after CBDL. Arrows indicate bile ducts. Right: graphical summaries of mRNA levels for CK-19 and PCNA, and quantitation of immunohistochemical staining for p-ERK and VEGF-A. Values are expressed as means ± SE (n = 8 animals for each group). *P < 0.05 compared with sham. ‡P < 0.05 compared with CBDL.
Fig. 2.
Effects of RTK inhibition on hepatic production and circulating levels of endothelin-1 (ET-1) after CBDL. A: representative images of immunohistochemical staining for ET-1 in liver (bile ducts shown by arrows). B: liver ET-1 mRNA levels and circulating ET-1 levels. Values are expressed as means ± SE (n = 8 animals for each group). C: graphic summaries of ET-1 mRNA and supernate protein levels in VEGF-A (15 ng/ml)-treated normal rat cholangiocytes in the presence or absence of U0126 (10 μM) for 15 h. *P < 0.05 compared with sham. ‡P < 0.05 compared with CBDL.
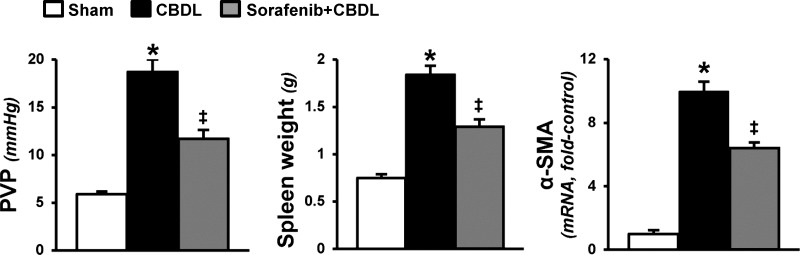
We also found that RTK inhibition improved portal hypertension (PVP and spleen weight) and hepatic fibrosis (α-smooth muscle actin levels) after CBDL, thereby confirming prior findings (29) (Fig. 3).
Fig. 3.
Effects of RTK inhibition on portal hypertension and hepatic fibrosis in experimental hepatopulmonary syndrome (HPS). Graphical summaries of portal venous pressure (PVP), spleen weight, and hepatic α-smooth muscle actin (α-SMA) mRNA levels after CBDL. Values are expressed as means ± SE (n = 8 animals for each group). *P < 0.05 compared with sham. ‡P < 0.05 compared with CBDL.
Effects of RTK inhibition on ET-1-mediated events in the pulmonary microvasculature.
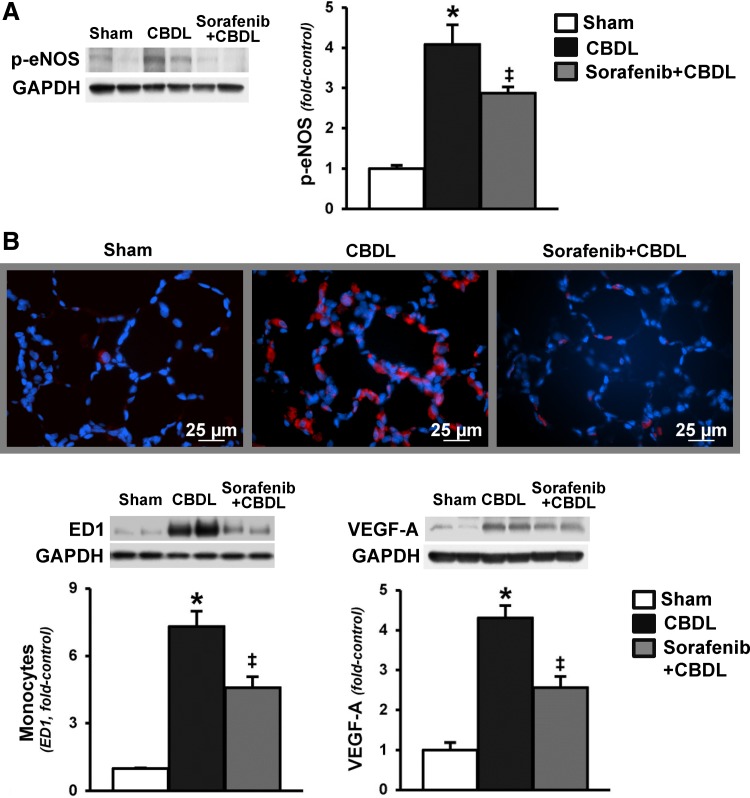
To explore whether the inhibition of bile duct proliferation and the accompanying decline in hepatic and circulating ET-1 in CBDL animals after sorafenib treatment is associated with modulation of established ET-1-driven events in the pulmonary microvasculature, we measured lung eNOS activation, vascular monocyte accumulation (ED-1 levels and immunohistochemistry), and VEGF-A levels (Fig. 4). Activation of eNOS and accumulation of monocytes in the pulmonary microvasculature were prominent after CBDL, and treatment with sorafenib resulted in significant reduction in both lung eNOS phosphorylation and monocyte accumulation. These events were also accompanied by a significant decrease in VEGF-A levels in the microvasculature.
Fig. 4.
Effects of RTK inhibition on lung endothelial nitric oxide synthase (eNOS) activation, microvascular monocyte accumulation, and VEGF levels after CBDL. A: representative immunoblots and graphical summaries of protein levels for p-eNOS (Ser1177) in lung. B: immunofluorescence staining for ED1 (monocyte marker, red) in lung, and representative immunoblots and graphical summaries of lung ED1 and VEGF-A protein levels. Values are expressed as means ± SE (n = 8 animals for each group). *P < 0.05 compared with sham. ‡P < 0.05 compared with CBDL.
Evaluation of RTK pathways in the pulmonary microvasculature and in microvascular endothelial cells and effects on angiogenesis.
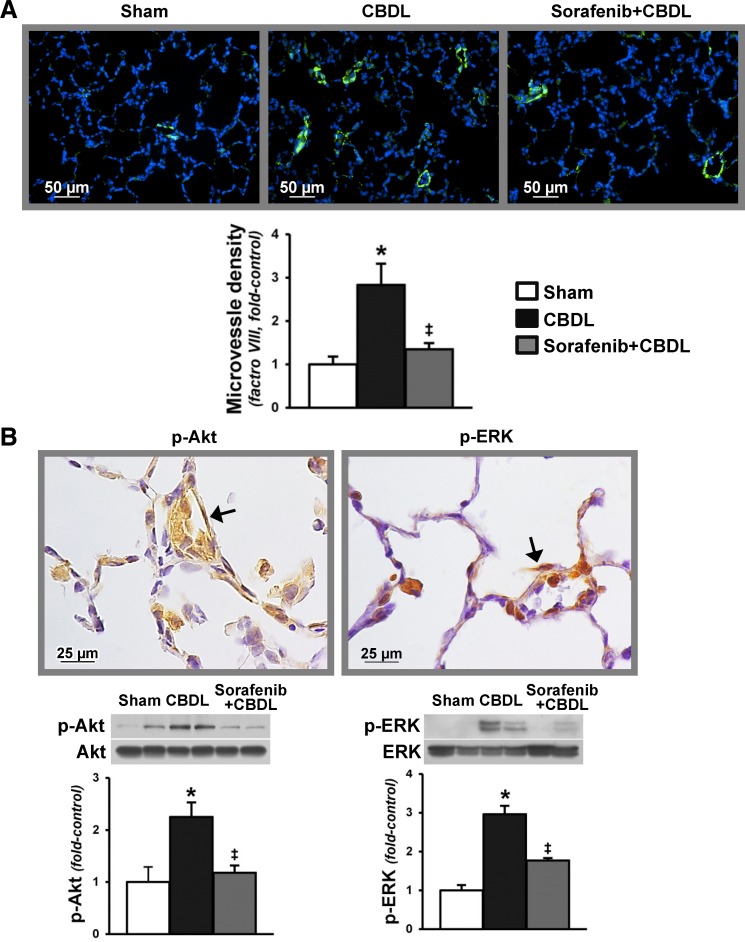
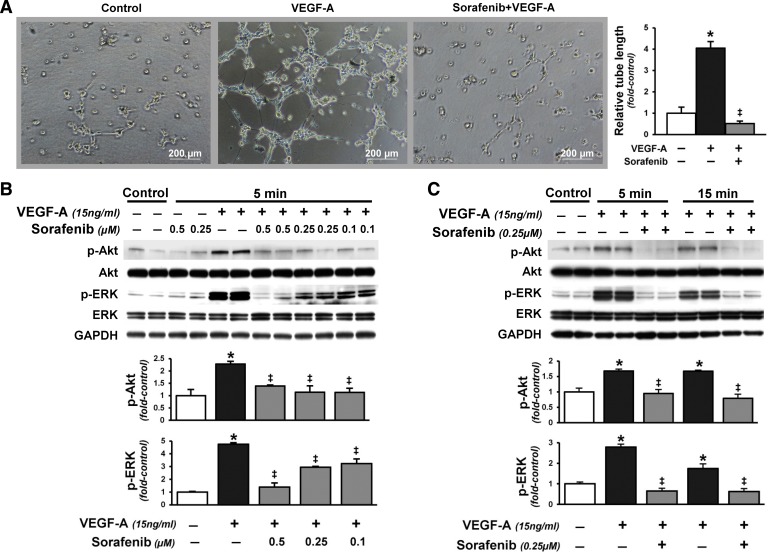
To directly assess microvascular activation of RTK signaling pathways implicated in modulation of lung angiogenesis after CBDL, we evaluated localization and activation of RTK signaling pathways (p-Akt and p-ERK) and angiogenesis in vivo (Fig. 5) and evaluated the effects of VEGF-A administration on rat pulmonary microvascular endothelial cells (RPMVECs) in vitro (Fig. 6). The development of angiogenesis in CBDL animals was accompanied by significant increases in lung Akt and ERK phosphorylation, found predominately in the microvasculature. Accordingly, VEGF-A administration to RPMVECs also increased Akt and ERK activation and was associated with increased tubular structure formation. Sorafenib administration significantly downregulated microvascular Akt and ERK activation and angiogenesis after CBDL and had similar effects on VEGF-A-mediated events in RPMVECs.
Fig. 5.
Pulmonary microvascular Akt and ERK phosphorylation after CBDL and effects of sorafenib on lung angiogenesis and RTK activation. A: representative immunofluorescence images of lung microvessel staining (factor VIII in green) and quantitation of microvessel density after CBDL. B: representative immunohistochemical staining images of p-Akt (Ser473) and p-ERK (Thr202/Tyr204) in the lung microvasculature (shown by arrows) after CBDL and representative immunoblots and graphical summaries of protein levels for p-Akt/Akt and p-ERK/ERK. Values are expressed as means ± SE (n = 8 animals for each group). *P < 0.05 compared with sham. ‡P < 0.05 compared with CBDL.
Fig. 6.
Effects of sorafenib on VEGF-A-induced in vitro tubular structure formation and Akt and ERK activation in rat pulmonary microvascular endothelial cells (RPMVECs). A: representative images of tubular structure formation in RPMVECs treated with VEGF-A (5 ng/ml) alone or in combination with sorafenib (2 μM) for 6 h and graphical summary of tubule length. Representative immunoblots and graphical summaries of p-Akt (Ser473) and p-ERK (Thr202/Tyr204) levels in VEGF-A (15 ng/ml)-treated RPMVECs in the presence or absence of sorafenib with varying doses (0.1–0.5 μM, 5 min; B) or different durations of exposure (0.25 μM, 5 or 15 min; C). Values are expressed as means ± SE (n = 3 independent experiments in duplicates). *P < 0.05 compared with control. ‡P < 0.05 compared with VEGF-A treatment.
Effects of RTK inhibition on the physiological features of established HPS.
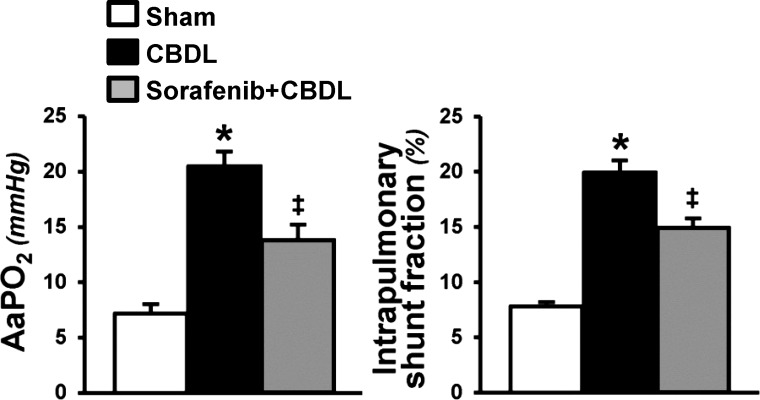
Human and experimental HPS share key diagnostic features such as arterial gas-exchange abnormalities (A-aPo2) resulting from intrapulmonary vasodilation (microsphere shunting through the lung vasculature) (41). Four-week CBDL animals developed characteristic abnormalities of HPS including intrapulmonary shunting and a significant increase in A-aPo2. RTK inhibition with sorafenib significantly improved both parameters reflected by a significant decrease in shunting and a significant improvement in A-aPo2 (Fig. 7).
Fig. 7.
Effects of sorafenib on pulmonary alterations of HPS. Graphical summaries of alveolar-arterial oxygen gradient (A-aPo2) and microsphere assessment of intrapulmonary shunting after CBDL. Values are expressed as means ± SE (n = 8 animals for each group). *P < 0.05 compared with sham. ‡P < 0.05 compared with CBDL.
DISCUSSION
In the present study, we assessed the relevance of RTK signaling pathways in cholangiocytes and in the pulmonary microvasculature in the pathogenesis of experimental HPS. We found a significant increase in VEGF-A-associated ERK activation in cholangiocytes accompanied by cell proliferation and ET-1 production and release. These events were associated with established ET-1 effects in the lung in HPS, including microvascular eNOS activation and monocyte accumulation and VEGF-A production. We also found evidence of direct activation of VEGF-A-associated signaling pathways and angiogenesis in the pulmonary microvasculature in vivo and in RPMVECs in vitro. Inhibition of RTK pathways with sorafenib administration blocked cholangiocyte ERK activation, proliferation, and ET-1 production and was associated with a decrease in eNOS activation and monocyte accumulation in the lung. Sorafenib also inhibited Akt and ERK activation and angiogenesis in the pulmonary microvasculature after CBDL and in RPMVECs and was associated with significant improvement in HPS. These findings identify a novel mechanism in cholangiocytes through which RTK inhibition ameliorates experimental HPS and establish direct RTK-mediated effects on angiogenesis in the pulmonary microvascular endothelium.
In prior work in experimental HPS, we have found that proliferating cholangiocytes produce ET-1 that is released into the circulation and drives lung microvascular eNOS activation and monocyte accumulation via ETB receptor-dependent pathways (21, 22, 25, 28). The observation here that cholangiocyte proliferation is accompanied by increased VEGF-A levels and activation of ERK and can be inhibited by sorafenib in conjunction with downregulating ERK activation is consistent with work showing that VEGF (VEGF-A and VEGF-C) and VEGF receptors are overexpressed in cholangiocytes after CBDL and upregulate proliferation through ERK signaling pathways in an autocrine fashion (14, 15). Our finding that cholangiocyte RTK inhibition also decreases hepatic and circulating ET-1 levels and blocks downstream events in the lung microvasculature reinforces the importance of cholangiocyte ET-1 production in experimental HPS. We did not find a direct effect of VEGF and ERK activation on ET-1 expression in cholangiocytes, supporting that the modulation of ET-1 production by sorafenib may occur through influencing other pathways known to drive cholangiocyte ET-1 production (28). Although other consequences of RTK inhibition with sorafenib in the liver, including effects on differing cytokines and signaling pathways and improvements in portal pressure and fibrosis, may also influence ET-1 production and the pulmonary microvasculature, our prior finding that selective ETB receptor inhibition in vivo results in similar reductions in lung eNOS activation and monocyte accumulation in experimental HPS without altering portal pressure, fibrosis, or RTK pathways supports a central role for ET-1 in experimental HPS (21).
Both intrapulmonary vasodilation and lung angiogenesis contribute to the development of abnormal oxygenation in experimental HPS and may be influenced by RTK pathways. The effect of circulating ET-1 on eNOS activation and vasodilation in experimental HPS and in RPMVECs occurs through a phospholipase-C (PLC)/inositol 1,4,5-trisphosphate (IP3)/Ca2+-dependent pathway and is phosphatidylinositol-3-kinase (PI3K)/Akt and RTK pathway independent (38). However, we have also found increased VEGF-A production in monocytes and in the pulmonary microvasculature accompanied by Akt/ERK activation in experimental HPS in the present work, and phosphorylation and activation of eNOS is also regulated by additional pathways including VEGF-A/Akt in the vasculature (10, 16, 18, 30). Therefore, eNOS activation and vasodilation may result from both RTK-independent and -dependent pathways in experimental HPS. The increase in VEGF-A production in intravascular monocytes and microvascular endothelium after CBDL with activation of microvascular Akt and ERK signaling is also associated with increased microvessel density or angiogenesis after CBDL (41, 42). We have found that RTK inhibition decreases pulmonary microvascular Akt and ERK activation and reduces microvessel density/angiogenesis. A similar effect was found on RPMVEC tubular structure formation and Akt and ERK activation in vitro. These results support that proangiogenic RTK pathways drive angiogenesis in the lung in experimental HPS. Although we have focused on VEGF-A-mediated pathways in the present study, the activation of other RTKs and their related signaling cascades may also contribute to lung vasodilation and angiogenesis in experimental HPS.
Of therapeutic and pathophysiological significance is the relative importance of RTK pathways in cholangiocytes and in the pulmonary microvasculature in contributing to experimental HPS. It is likely in established experimental HPS that initiation of RTK inhibition targets RTK pathways in both liver and lung on the basis of our present findings. However, several lines of evidence support that modulation of ET-1 production in cholangiocytes may be an initiating event in experimental HPS that triggers subsequent vasodilatory and angiogenic responses. First, ET-1 drives eNOS activation and monocyte adherence in the pulmonary microvasculature through ETB receptor activation (21, 27, 43). Monocytes, in turn, are a major source of VEGF-A production after CBDL and drive angiogenesis (41, 42). Therefore, ET-1 appears to be an upstream mediator of vascular changes. Second, direct support for the role of ET-1 in HPS comes from the observation that ETB receptor inhibition alone in established HPS ameliorates intrapulmonary vasodilation, intravascular monocyte accumulation, angiogenesis, and physiological changes (21). Third, targeted deletion of monocytes in established experimental HPS also decreases pulmonary microvascular VEGF-A production, inhibits Akt/ERK activation, blocks angiogenesis, and improves HPS (39). These observations, coupled with our finding that RTK pathways in cholangiocytes regulate proliferation and ET-1 production, support that modulation of hepatic ET-1 production is a key event in experimental HPS and a potential target for therapy.
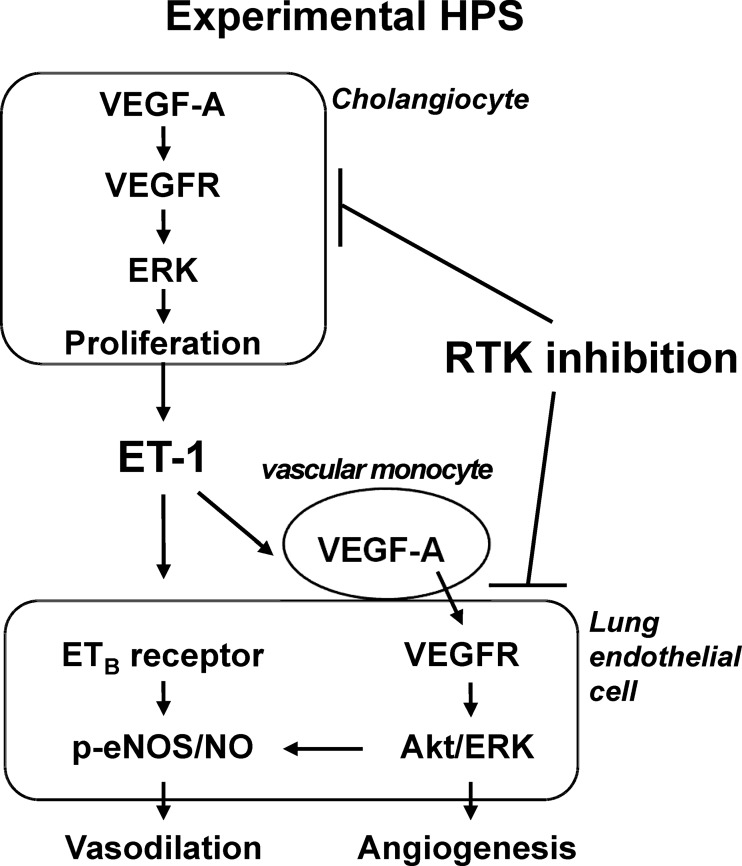
There is currently no medical therapy for HPS, a disorder that affects up to 30% of patients with cirrhosis and is associated with a doubling in mortality (2–4, 13, 20). Sorafenib targets multiple RTK pathways involved in regulating cell proliferation and angiogenesis and has been approved for the treatment of hepatocellular carcinoma (23, 24). Our observation that RTK inhibition with sorafenib improves pulmonary vascular abnormalities and oxygenation in experimental HPS through specific pathways in the liver and in the pulmonary microvasculature provides a mechanistic rationale for its use in human disease and for developing other therapeutic agents. Figure 8 summarizes our current understanding of the pathways involved in experimental HPS that are targeted by RTK inhibitors such as sorafenib.
Fig. 8.
Working model of RTK inhibition with sorafenib in experimental HPS.
GRANTS
This study was supported by 5DKR01DK056804 to M. B. Fallon. Portions of this work were supported by a VA Research Career Scientist Award and a VA Merit award to G. Alpini.
DISCLOSURES
There are no conflicts of interest for all authors and the funding source has no role in preparation of the manuscript.
AUTHOR CONTRIBUTIONS
W.Y., J.Z., G.A., and M.F. conception and design of research; W.Y., J.Z., B.H., W.W., and J.V. performed experiments; W.Y., J.Z., B.H., and M.F. analyzed data; W.Y., J.Z., B.H., and M.F. interpreted results of experiments; W.Y. and J.Z. prepared figures; W.Y. and J.Z. drafted manuscript; W.Y., J.Z., and M.F. edited and revised manuscript; W.Y., J.Z., B.H., W.W., and M.F. approved final version of manuscript.
REFERENCES
- 1.Agusti AGN, Roca J, Rodriguez-Roisin R. Mechanisms of gas exchange impairment in patients with liver cirrhosis. Clin Chest Med 17: 49–66, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Arguedas M, Abrams GA, Krowka MJ, Fallon MB. Prospective evaluation of outcomes and predictors of mortality in patients with hepatopulmonary syndrome undergoing liver transplantation. Hepatology 37: 192–197, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Arguedas M, Singh H, Faulk D, Fallon MB. Utility of pulse oximetry screening for hepatopulmonary syndrome. Clin Gastroenterol Hepatol 5: 749–754, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Battaglia SE, Pretto JJ, Irving LB, Jones RM, Angus PW. Resolution of gas exchange abnormalities and intrapulmonary shunting following liver transplantation. Hepatology 25: 1228–1232, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Chang CC, Chuang CL, Lee FY, Wang SS, Lin HC, Huang HC, Teng TH, Hsu SJ, Hsieh HG, Lee SD. Sorafenib treatment improves hepatopulmonary syndrome in rats with biliary cirrhosis. Clin Sci (Lond) 124: 457–466, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Chang SW, Ohara N. Chronic biliary obstruction induces pulmonary intravascular phagocytosis and endotoxin sensitivity in rats. J Clin Invest 94: 2009–2019, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang SW, Ohara N. Increased pulmonary vascular permeability in rats with biliary cirrhosis: role of thromboxane A2. Am J Physiol Lung Cell Mol Physiol 264: L245–L252, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Chang SW, Ohara N. Pulmonary circulatory dysfunction in rats with biliary cirrhosis. Am Rev Respir Dis 148: 798–805, 1992 [DOI] [PubMed] [Google Scholar]
- 9.Cremona G, Higenbottam T, Mayoral V, Alexander G, Demoncheaux E, Borland C, Roe P, Jones G. Elevated exhaled nitric oxide in patients with hepatopulmonary syndrome. Eur Respir J 8: 1883–1885, 1995 [DOI] [PubMed] [Google Scholar]
- 10.Desjardins F, Delisle C, Gratton JP. Modulation of the cochaperone AHA1 regulates heat-shock protein 90 and endothelial NO synthase activation by vascular endothelial growth factor. Arterioscler Thromb Vasc Biol 32: 2484–2492, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Fallon MB, Abrams GA, Luo B, Hou Z, Dai J, Ku DD. The role of endothelial nitric oxide synthase in the pathogenesis of a rat model of hepatopulmonary syndrome. Gastroenterology 113: 606–614, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Fallon MB, Abrams GA, McGrath JW, Hou Z, Luo B. Common bile duct ligation in the rat: a model of intrapulmonary vasodilatation and hepatopulmonary syndrome. Am J Physiol Gastrointest Liver Physiol 272: G779–G784, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Fallon MB, Krowka MJ, Brown RS, Trotter JF, Zacks S, Roberts KE, Shah VH, Kaplowitz N, Forman L, Wille K, Kawut SM. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology 135: 1168–1175, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Franchitto A, Onori P, Ueno Y, Marzioni M, Fava G, Venter J, Reichenbach R, Summers R, Alpini G. Administration of r-VEGF-A prevents hepatic artery ligation-induced bile duct damage in bile duct ligated rats. Am J Physiol Gastrointest Liver Physiol 291: G307–G317, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Gaudio E, Barbaro B, Alvaro D, Glaser S, Francis H, Ueno Y, Meininger CJ, Franchitto A, Onori P, Marzioni M, Taffetani S, Fava G, Stoica G, Venter J, Reichenbach R, De Morrow S, Summers R, Alpini G. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology 130: 1270–1282, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Gentile C, Muise-Helmericks RC, Drake CJ. VEGF-mediated phosphorylation of eNOS regulates angioblast and embryonic endothelial cell proliferation. Dev Biol 373: 163–175, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemprich U, Papadakos PJ, Lachmann B. Respiratory failure and hypoxemia in the cirrhotic patient including hepatopulmonary syndrome. Curr Opin Anaesthesiol 23: 133–138, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Hou HH, Hammock BD, Su KH, Morisseau C, Kou YR, Imaoka S, Oguro A, Shyue SK, Zhao JF, Lee TS. N-terminal domain of soluble epoxide hydrolase negatively regulates the VEGF-mediated activation of endothelial nitric oxide synthase. Cardiovasc Res 93: 120–129, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krowka M, Wiseman G, Burnett O, Spivey J, Therneau T, Porayko M, Wiesner R. Hepatopulmonary syndrome: a prospective study of relationships between severity of liver disease, Pao2 response to 100% oxygen, and brain uptake after 99mTc MAA lung scanning. Chest 118: 615–624, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Krowka MJ, Cortese DA. Hepatopulmonary syndrome: an evolving perspective in the era of liver transplantation. Hepatology 11: 138–141, 1990 [DOI] [PubMed] [Google Scholar]
- 21.Ling Y, Zhang J, Luo B, Song D, Liu L, Tang L, Stockard C, Grizzle W, Ku D, Fallon MB. The role of endothelin-1 and the endothelin B receptor in the pathogenesis of experimental hepatopulmonary syndrome. Hepatology 39: 1593–1602, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Zhang M, Luo B, Abrams GA, Fallon MB. Biliary cyst fluid from common bile duct ligated rats stimulates eNOS in pulmonary artery endothelial cells: a potential role in hepatopulmonary syndrome. Hepatology 33: 722–727, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 48: 1312–1327, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378–390, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Luo B, Abrams GA, Fallon MB. Endothelin-1 in the rat bile duct ligation model of hepatopulmonary syndrome: correlation with pulmonary dysfunction. J Hepatol 29: 571–578, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Luo B, Liu L, Tang L, Zhang J, Ling Y, Fallon MB. ET-1 and TNF-α in HPS: analysis in prehepatic portal hypertension and biliary and nonbiliary cirrhosis in rats. Am J Physiol Gastrointest Liver Physiol 286: G294–G303, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Luo B, Liu L, Tang L, Zhang J, Stockard C, Grizzle W, Fallon M. Increased pulmonary vascular endothelin B receptor expression and responsiveness to endothelin-1 in cirrhotic and portal hypertensive rats: a potential mechanism in experimental hepatopulmonary syndrome. J Hepatol 38: 556–563, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Luo B, Tang L, Wang Z, Zhang J, Ling Y, Feng W, Sun JZ, Stockard CR, Frost AR, Chen YF, Grizzle WE, Fallon MB. Cholangiocyte endothelin 1 and transforming growth factor beta1 production in rat experimental hepatopulmonary syndrome. Gastroenterology 129: 682–695, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mejias M, Garcia-Pras E, Tiani C, Miquel R, Bosch J, Fernandez M. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology 49: 1245–1256, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol 42: 271–279, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome — a liver-induced lung vascular disorder. N Engl J Med 358: 2378–2387, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Rolla G, Brussino L, Colagrande P, Dutto L, Polizzi S, Scappaticci E, Bergerone S, Morello M, Marzano A, Martinasso G, Salizzoni M, Bucca C. Exhaled nitric oxide and oxygenation abnormalities in hepatic cirrhosis. Hepatology 26: 842–847, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Rolla G, Bucca C, Brussino L. Methylene blue in the hepatopulmonary syndrome. N Engl J Med 331: 1098, 1994 [DOI] [PubMed] [Google Scholar]
- 34.Schenk P, Fuhrmann V, Madl C, Funk G, Lehr S, Kandel O, Muller C. Hepatopulmonary syndrome: prevalence and predictive value of various cut offs for arterial oxygenation and their clinical consequences. Gut 51: 853–859, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schenk P, Schoniger-Hekele M, Fuhrmann V, Madl C, Silberhumer G, Muller C. Prognostic significance of the hepatopulmonary syndrome in patients with cirrhosis. Gastroenterology 125: 1042–1052, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Schroeder RA, Ewing CA, Sitzmann JV, Kuo PC. Pulmonary expression of iNOS and HO-1 protein is upregulated in a rat model of prehepatic portal hypertension. Dig Dis Sci 45: 2405–2410, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Swanson K, Wiesner R, Krowka M. Natural history of hepatopulmonary syndrome: impact of liver transplantation. Hepatology 41: 1122–1129, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Tang L, Luo B, Patel RP, Ling Y, Zhang J, Fallon MB. Modulation of pulmonary endothelial endothelin B receptor expression and signaling: implications for experimental hepatopulmonary syndrome. Am J Physiol Lung Cell Mol Physiol 292: L1467–L1472, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thenappan T, Goel A, Marsboom G, Fang YH, Toth PT, Zhang HJ, Kajimoto H, Hong Z, Paul J, Wietholt C, Pogoriler J, Piao L, Rehman J, Archer SL. A central role for CD68(+) macrophages in hepatopulmonary syndrome. Reversal by macrophage depletion. Am J Respir Crit Care Med 183: 1080–1091, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Ling Y, Luo B, Tang L, Stockard C, Grizzle WE, Fallon MB. Analysis of pulmonary heme oxygenase-1 and nitric oxide synthase alterations in experimental hepatopulmonary syndrome. Gastroenterology 125: 1441–1451, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Luo B, Tang L, Wang Y, Stockard CR, Kadish I, Van Groen T, Grizzle WE, Ponnazhagan S, Fallon MB. Pulmonary angiogenesis in a rat model of hepatopulmonary syndrome. Gastroenterology 136: 1070–1080, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Yang W, Luo B, Hu B, Maheshwari A, Fallon MB. The role of CX3CL1/CX3CR1 in pulmonary angiogenesis and intravascular monocyte accumulation in rat experimental hepatopulmonary syndrome. J Hepatol 57: 752–758, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang M, Luo B, Chen SJ, Abrams GA, Fallon MB. Endothelin-1 stimulation of endothelial nitric oxide synthase in the pathogenesis of hepatopulmonary syndrome. Am J Physiol Gastrointest Liver Physiol 277: G944–G952, 1999 [DOI] [PubMed] [Google Scholar]