Abstract
Despite the importance of UDP-glucuronosyltransferase (UGT) 1A1*28 in irinotecan pharmacogenetics, our capability to predict drug-induced severe toxicity remains limited. We aimed at identifying novel genetic markers that would improve prediction of irinotecan toxicity and response in advanced colorectal cancer patients treated with folic acid (leucovorin), fluorouracil (5-FU), and irinotecan (camptosar)-based regimens. The relationships between UGT1A candidate markers across the gene (n = 21) and toxicity were prospectively evaluated in 167 patients. We included variants in the 3′untranscribed region (3′UTR) of the UGT1A locus, not studied in this context yet. These genetic markers were further investigated in 250 Italian FOLFIRI-treated patients. Several functional UGT1A variants, including UGT1A1*28, significantly influenced risk of severe hematologic toxicity. As previously reported in the Italian cohort, a 5-marker risk haplotype [haplotype II (HII); UGTs 1A9/1A7/1A1] was associated with severe neutropenia in our cohort [odds ratio (OR) = 2.43; P = 0.004]. The inclusion of a 3′UTR single-nucleotide polymorphism (SNP) permitted refinement of the previously defined HI, in which HIa was associated with the absence of severe neutropenia in combined cohorts (OR = 0.55; P = 0.038). Among all tested UGT1A variations and upon multivariate analyses, no UGT1A1 SNPs remained significant, whereas three SNPs located in the central region of UGT1A were linked to neutropenia grade 3–4. Haplotype analyses of these markers with the 3′UTR SNP allowed the identification of a protective HI (OR = 0.50; P = 0.048) and two risk haplotypes, HII and HIII, characterized by 2 and 3 unfavorable alleles, respectively, revealing a dosage effect (ORs of 2.15 and 5.28; P ≤ 0.030). Our results suggest that specific SNPs in UGT1A, other than UGT1A1*28, may influence irinotecan toxicity and should be considered to refine pharmacogenetic testing.
Introduction
Irinotecan (Camptosar, CPT-11), a topoisomerase I inhibitor, is a standard cytotoxic agent used for the treatment of advanced metastatic colorectal cancer. Despite its clinical efficacy, irinotecan has two major dose-limiting toxicities—myelosuppression and diarrhea—that occur with unpredictable severity (Saltz et al., 2000; Rothenberg et al., 2001). Irinotecan has a narrow therapeutic range, and adverse effects may limit the dose that can be safely administered, and subsequently compromise tumor response and clinical outcome. A greater knowledge of human genetic variations pertaining to these variable outcomes following irinotecan treatment may allow an individualized approach to therapy.
The interindividual variability of irinotecan dose/toxicity and tumor response has been attributed mainly to inherited genetic variations in the UGT1A1 gene, which encodes UDP-glucuronosyltransferase (UGT) 1A1, a key enzyme in irinotecan metabolism. The human UGT1A locus is defined by 13 first exons, which are alternatively spliced to four common exons, leading to mRNA isoforms, nine of which conduct to functionally active enzymes. Indeed, following intravenous administration, irinotecan is converted in vivo to the highly potent active metabolite 7-ethyl-10-hydroxycamptothecin (SN-38) by carboxylesterase-mediated hydrolysis (Kawato et al., 1991; Kojima et al., 1993). SN-38 is conjugated with glucuronic acid by hepatic and extrahepatic UGTs to form inactive SN-38-glucuronide. Several studies have identified specific inherited differences in irinotecan glucuronidation capacity that influence toxicity (Ando et al., 2000; Iyer et al., 2002; Innocenti et al., 2004; Marcuello et al., 2004; Mathijssen et al., 2004; Carlini et al., 2005; de Jong et al., 2006; McLeod et al., 2006; Toffoli et al., 2006). An increased number of dinucleotide repeats in the atypical TATA-box region of the UGT1A1 promoter (UGT1A1*28 allele) leads to a decreased rate of transcription initiation/expression of UGT1A1 (Beutler et al., 1998). Several studies suggest that patients homozygous for UGT1A1*28 are more likely to develop dose-dependent severe neutropenia compared with individuals with the reference genotype (*1/*1) (Iyer et al., 2002; Innocenti et al., 2004; Marcuello et al., 2004; Carlini et al., 2005; Soepenberg et al., 2005; Toffoli et al., 2006; Hoskins et al., 2007). Other genetic variations also linked to toxicity, such as the nonsynonymous coding variant G71R (UGT1A1*6 allele), are particularly prevalent in Asians (frequency of 0.13–0.25), and lead to variable enzyme activity (Jada et al., 2007). Additionally, there are few data regarding the relationship with diarrhea, the other major adverse effect (Carlini et al., 2005; Toffoli et al., 2006). Thus, the clinical value of UGT1A1 polymorphisms as predictors of irinotecan-associated toxicity has limitations, supporting the need for additional studies before implementation of individualized irinotecan dosing.
Along with UGT1A1 enzyme, several studies have revealed the importance of UGT1A9 in the hepatic conjugation of SN-38, whereas UGT1A7 is predominantly involved in its extrahepatic metabolism (Hanioka et al., 2001; Gagne et al., 2002). UGT1A6 has catalytic activity toward SN-38 in vitro (Gagne et al., 2002), but the effect on irinotecan metabolism is relatively undefined in vivo. Recent observations suggest that a combined signature of the haplotypes of UGT1A1, UGT1A6, UGT1A7, and UGT1A9 might provide more precise information about irinotecan pharmacokinetics, pharmacodynamics, and time to progression defined as the interval between the first drug [FOLFIRI; folic acid (leucovorin; Pfizer, Saint-Laurent, QC, Canada), fluorouracil (5-FU; Hospira, Montreal, QC, Canada), and irinotecan (camptosar; Pfizer)] administration and the date of first disease progression (documented by computed tomography scans of measurable lesions) or last follow-up (Cecchin et al., 2009). Therefore, clinical outcome is likely the result of complex interplay, at least in part, between key genomic variations in UGT metabolic detoxification pathways.
Here, a cohort of 167 Canadian patients treated with FOLFIRI-based regimens for metastatic colorectal cancer was prospectively studied for hematologic and gastrointestinal (GI) toxicities in relation to germline polymorphisms in the major UGT1A gene. A first series of analyses focused on specific UGT1A variants, including the UGT1A1*28, and their haplotypes that were previously associated with severe neutropenia by Cecchin et al. (2009) in an Italian cohort of 250 patients also treated with FOLFIRI. We replicated the idea that a haplotype II [HII; single-nucleotide polymorphisms (SNPs) in UGT1As 1A9/1A7/1A1) is associated with increased risk of neutropenia, as reported in the Italian cohort (Cecchin et al., 2009). We also tested the inclusion of a 3′untranscribed region (3′UTR) variant common to all UGT1As, and defined a novel haplotype associated with the absence of neutropenia (HIa) in the combined analysis of Canadian and Italian patients. In a second series of investigations, we tested a broader range of variations across the UGT1A gene (n = 21) genotyped in Canadian patients, with the aim to identify a better combination of UGT1A markers (haplotypes) associated with the presence and absence of neutropenia. We report 4-marker haplotypes (SNPs in UGTs 1A9/1A7/1A6/3′UTR) that may help to refine prediction of hematologic toxicities, and ultimately improve dosing strategies.
Materials and Methods
Study Design and Patients
This multi-institution prospective study involved patient recruitment from 2003 to 2012 at three medical centers in eastern Canada: Hotel-Dieu de Québec in Québec City, QC; Hotel-Dieu de Lévis in Lévis, QC; and The Ottawa Hospital in Ottawa, ON. The ethics committee of each participating institution approved the study protocol, and all patients signed a written informed consent before entering the study. Eligibility criteria included patients (18–90 years old) initiating their first irinotecan-based chemotherapy with a histologically confirmed metastatic colorectal cancer, a life expectancy of at least 3 months, and a good performance status (Eastern Cooperative Oncology Group ≤ 2). Table 1 summarizes patient characteristics, such as age, gender, tumor site, treatment, and toxicity. The primary objective was to assess the relationship between SNPs in candidate genes and irinotecan-induced toxicity. The second cohort is composed of 250 metastatic cases and was previously described elsewhere (Toffoli et al., 2006; Cecchin et al., 2009).
TABLE 1.
Demographic and clinical characteristics
| Characteristics | N | % |
|---|---|---|
| Total number | 167 | |
| Sex (male/female) | 110/57 | |
| Age median (years) | 61.5 | |
| Primary tumor site | ||
| Colon | 122 | 73.1 |
| Rectum | 42 | 25.1 |
| Unknown | 3 | 1.8 |
| Regimen | ||
| FOLFIRI | 167 | 41.3 |
| Cotreatment | 69 | 3.6 |
| Avastin/bevacizumab | 6 | |
| Other drugs | ||
| Toxicity | ||
| Diarrhea (grade 3-4) | 24 | 14.4 |
| Neutropenia (grade 3-4) | 28 | 16.8 |
Treatments
Patients were treated with one of the following FOLFIRI-based chemotherapies. Patients treated with the modified FOLFIRI regimen received irinotecan (180 mg/m2 i.v.) for 2 hours on day 1 plus a bolus of 5-fluorouracil (400 mg/m2) followed by continuous infusion of 5-fluorouracil (2400 mg/m2) plus leucovorin (200 mg/m2) over 46 hours. Patients received this treatment cycle every two weeks. Sixty-nine patients also received the monoclonal antibody bevacizumab (Avastin; Genentech, San Francisco, CA) in coadministration with their regimen, and 6 patients received either an experimental drug or placebo.
Efficacy Assessment.
Computed tomography scans of measurable lesions were recorded prior to irinotecan chemotherapy and every four to eight doses after the start of treatment. Objective response and duration of response were assessed by Response Evaluation Criteria in Solid Tumors. Patients were considered evaluable for response if they had at least four doses of chemotherapy.
Toxicity Assessment.
Toxicity was evaluated prospectively and according to National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 criteria. The toxicity endpoints consisted of both GI and hematologic toxicities, and were analyzed separately. For GI toxicities, all patients completed a daily report of GI toxicities during the first 14 days of each cycle to record the incidence and severity of nausea, vomiting, and diarrhea. For hematologic toxicities, laboratory parameters were collected before each cycle of chemotherapy and/or when the treatment was delayed. The most severe toxicity reported was used for data analysis. GI toxicity was evaluable for all patients except for one who died before toxicity assessment, and another who did not fill out the GI toxicity diary, while hematologic toxicity was evaluable for 166 of 167 patients. For the Italian cohort, details on eligibility, modalities of treatment, data collection, and definitions have been published previously (Toffoli et al., 2006; Cecchin et al., 2009).
Genotyping
Polymorphisms included in this study and their amplification strategies including primer sequences are described in the supplementary materials (Supplemental Tables 1 and 2). Variations linked at r2 ≥ 0.95 with another variant included or determined to be relatively rare [minor allele frequency (MAF) of <0.5%] were omitted in further analyses. At the time of patient enrollment, genomic DNA was obtained from a blood sample using a genomic DNA extraction kit (QIAamp DNA Blood Mini kit; Qiagen, Mississauga, ON, Canada). We identified polymorphisms by sequencing polymerase chain reaction products using an ABI PRISM 3730XL DNA Analyzer (Applied Biosystems, Foster City, CA). All polymerase chain reactions were carried out in a final volume of 50 µl, containing 30 ng of genomic DNA, 3 mM MgCl2, 200 nM of each deoxynucleotide triphosphate, 300 nM of each primer, 5% acetamide, and 1 U Taq polymerase. Each reaction was incubated at 94°C for 30 seconds followed by 35 cycles at 94°C for 30 seconds, 55–58°C for 30 seconds, and 72°C for 30 minutes, with a final step at 72°C for 5 minutes. All sequences were analyzed with the Staden package (Open Source Technology Group, Fairfax, VA; http://staden.sourceforge.net/) and compared with the reference sequence to assess genetic variations. Samples given an ambiguous sequencing chromatogram were systematically reamplified and resequenced. Genotyping was performed independently without knowledge of the clinical evaluations.
Data Analyses and Statistics
Deviations from the Hardy-Weinberg equilibrium of allele and genotype frequencies for the various genetic variations were assessed by Fisher's exact test. Haplotypes were inferred using the Phase version 2.1.1 program (Stephens et al., 2001; Stephens and Donnelly, 2003). Pairwise linkage disequilibrium (LD) was determined with HAPLOVIEW 3.32 (www.broad.mit.edu/mpg/haploview). Pairwise LD between polymorphisms was estimated by a log-linear model, and the extent of disequilibrium was expressed in terms of D', which is the ratio of the unstandardized coefficient to its maximal/minimal value. The possibility of genetic association was examined by testing the null hypothesis using two-tailed Fisher’s exact test, and was considered statistically significant at P ≤ 0.05 as calculated by JMP4.0.2 software (JMP Statistical Discovery, Cary, NC) or the SAS statistics package (SAS Institute, Cary, NC). When a particular variant was infrequent in the studied cohort (n ≤ 3), homozygous and heterozygous genotypes were combined for statistical analyses. Genetic variants deemed positive (P < 0.05) or with a trend (P < 0.10) by univariate analysis were included in a stepwise logistic regression analysis. No adjustments were made for multiple comparisons because of the exploratory nature of this study.
Results
Patient characteristics for the Canadian cohort are summarized in Table 1. Rates of grade 3–4 hematologic and GI toxicities prospectively evaluated were in keeping with previous reports (Schulz et al., 2009) (Supplemental Table 3). We studied 21 SNPs of the UGT1A gene genotyped in the cohort of 167 Canadian patients in relation to hematologic and GI-related toxicities. The observed allele frequency for selected SNPs was in agreement with previous analyses, and all of the SNP markers under study are in Hardy-Weinberg equilibrium, except for rs10929302 (P = 0.01) (Supplemental Table 2) (Maitland et al., 2006; Thomas et al., 2006; Menard et al., 2009). Pairwise LD analysis was performed with variations having a MAF > 0.05. As expected, the high LD observed for UGT1A variants agreed with data from a recent published analysis of a population from the same geographic region (Menard et al., 2009).
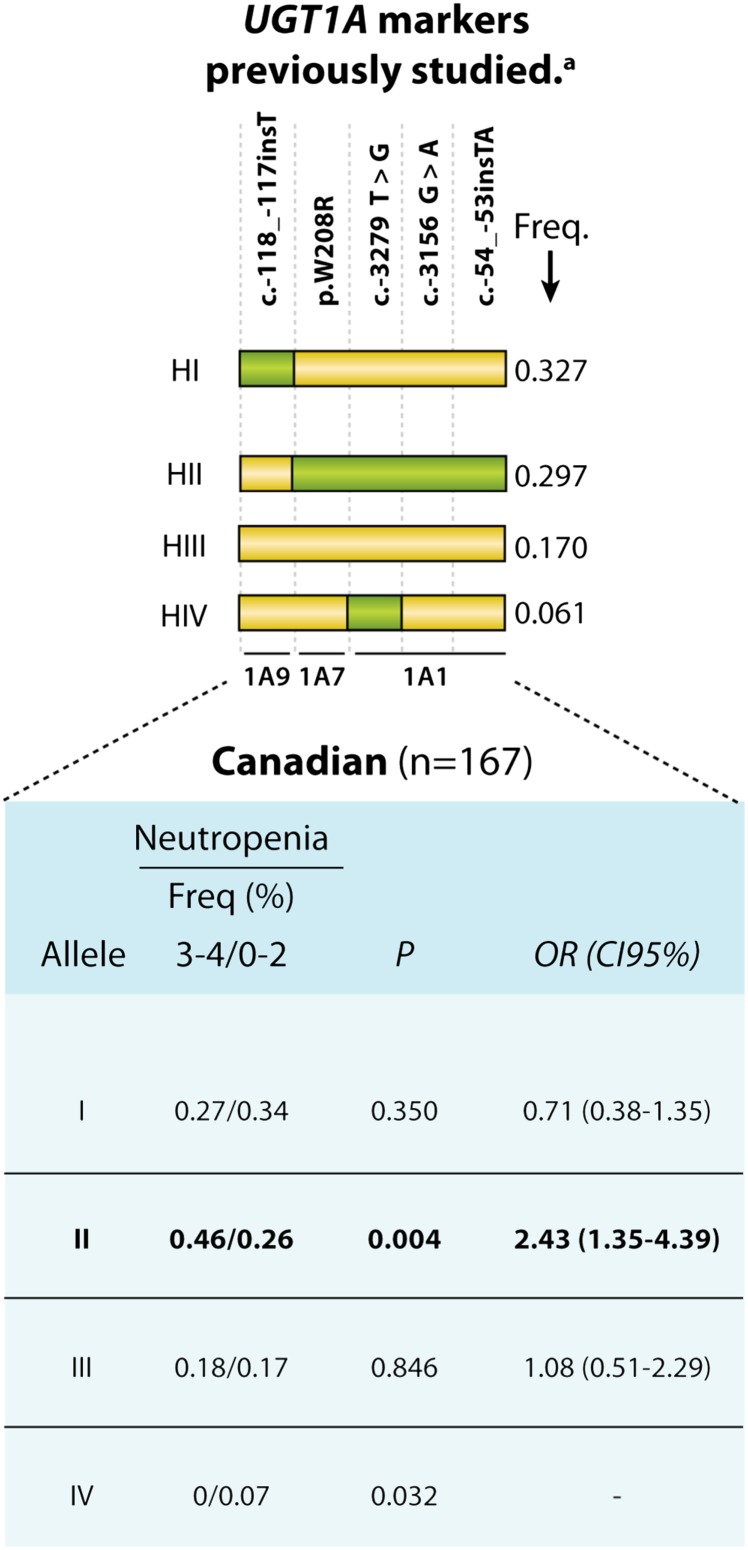
We initially tested previously reported haplotypes of the UGT1A locus named according to Cecchin et al. (2009) to thereby allow comparison between studies and avoid nomenclature confusion. Four haplotypes were inferred by 5 markers and occurred at a frequency of ≥5% in the Canadian cohort. We observed that haplotype HII, characterized by the co-occurrence of SNP susceptibility alleles including UGT1A1*28, is associated with a higher risk of severe neutropenia [odds ratio (OR) = 2.43; P = 0.004], as reported by Cecchin et al. (2009) for the Italian cohort (Fig. 1). The inclusion of an additional UGT1A-associated SNP located in the 3′UTR region resulted in two HI-related haplotype alleles, called HIa and HIb. Whereas the HIb is evenly distributed between patients who have or have not experienced neutropenia, the HIa allele is largely associated with severe neutropenia in both the Canadian and Italian cohorts (n = 417; OR = 0.55; P = 0.038).
Fig. 1.
Schematic representation of UGT1A and the haplotype analyses associated with severe neutropenia based on previously investigated markers. Variations are represented by squares: a yellow square represents a reference nucleotide, whereas a green square represents a variant (relative to the AF297093 sequence). Only haplotypes with an MAF > 5% in the present study are shown. aBased on markers studied by Cecchin et al. (2009). CI95%, 95% confidence interval; Freq., frequency.
In a second series of analyses, we included 21 variations across the UGT1A gene genotyped in the Canadian cohort. In univariate analyses of the Canadian cohort, UGT1A variants were linked to severe neutropenia but not GI toxicities (unpublished data). Severe neutropenia was associated with numerous variants with a MAF > 5% at the UGT1A locus (P < 0.05), including functional coding variants of UGT1A6 and UGT1A7; three promoter polymorphisms of UGT1A9 [c.-1212 (G/A), c.-688 (A/C), and c.-440 (C/T)], the common promoter UGT1A1*28 (c.-54_-53 TA6/7) allele; and promoter variant c.-3156 (G/A), most of which are known to impair gene expression or function (Bosma et al., 1995; Beutler et al., 1998). ORs and P values for association with hematologic toxicities are indicated in Table 2. For instance, the UGT1A1*28 allele was associated with a 1.84-fold increased risk of developing severe neutropenia (P = 0.045). Both the UGT1A6 c.181A allele (OR = 2.32; 95% confidence interval 1.03–3.30; P = 0.045) and the UGT1A7 c.208C allele (OR = 2.00 95% confidence interval 1.12–3.58; P = 0.025) were significant predictors of severe neutropenia.
TABLE 2.
Polymorphisms in the UGT1A gene positively associated with severe neutropenia under allelic or genotypic analyses
Odds ratios (OR) have been calculated under the following models as specified: Dominant (a), recessive (b).
| Gene |
Variation |
Alleles |
Neutropenia |
OR (95% CI) |
P |
Genotypes |
Neutropenia |
OR (95% CI) |
P |
Na |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–2 | 3–4 | 0–2 | 3–4 | |||||||||
| UGT1A9 | c.-1212 | G | 189 | 29 | 2.12 (1.17–3.84) | GG | 71 | 8 | 3.24b (1.32–7.98) | 0.010 | 160 | |
| A | 77 | 25 | 0.016 | GA | 47 | 13 | ||||||
| AA | 15 | 6 | ||||||||||
| c.-688 | A | 264 | 50 | 5.28 (1.28–21.81) | AA | 130 | 23 | 5.65b (1.32–24.22) | 0.028 | 161 | ||
| C | 4 | 4 | 0.030 | AC | 4 | 4 | ||||||
| CC | 0 | 0 | ||||||||||
| c.-440 | C | 182 | 29 | 1.97 (1.10–3.53) | CC | 65 | 8 | 2.36b (0.97–5.72) | 0.062 | 162 | ||
| T | 86 | 27 | 0.030 | CT | 52 | 13 | ||||||
| TT | 17 | 7 | ||||||||||
| UGT1A7 | p.W208R | T | 168 | 25 | 2.00 (1.12–3.58) | TT | 53 | 6 | 2.59c (1.03–6.51) | 0.057 | 164 | |
| C | 104 | 31 | 0.025 | TC | 62 | 13 | ||||||
| CC | 21 | 9 | ||||||||||
| UGT1A6 | p.S7A | T | 160 | 24 | 2.00 (1.12–3.57) | TT | 53 | 6 | 2.59c (1.03–6.51) | 0.057 | 164 | |
| G | 110 | 33 | 0.019 | TG | 62 | 13 | ||||||
| GG | 21 | 9 | ||||||||||
| p.T181A | A | 186 | 27 | 2.32 (1.30–4.16) | AA | 66 | 8 | 3.55c (1.38–9.18) | 0.017 | 164 | ||
| G | 86 | 29 | 0.005 | AG | 54 | 11 | ||||||
| GG | 16 | 9 | ||||||||||
| p.R184S | A | 180 | 26 | 2.26 (1.26–4.04) | AA | 62 | 8 | 3.64c (1.45–9.13) | 0.010 | 164 | ||
| C | 92 | 30 | 0.006 | AC | 56 | 10 | ||||||
| CC | 18 | 10 | ||||||||||
| UGT1A1 | c.-3156 | G | 193 | 29 | 2.28 (1.27–4.09) | GG | 73 | 9 | 3.00c (1.14–7.93) | 0.036 | 164 | |
| A | 79 | 27 | 0.007 | GA | 47 | 11 | ||||||
| AA | 16 | 8 | ||||||||||
| c.-54_-53 | 6 | 185 | 30 | 1.84 (1.03–3.30) | 66 | 66 | 9 | 2.33c (0.86–6.31) | 0.137 | 164 | ||
| 7 | 87 | 26 | 0.045 | 67 | 53 | 12 | ||||||
| 77 | 17 | 7 | ||||||||||
95% CI, 95% confidence interval.
N: number of individuals with available genotyping data and toxicity information.
Dominant model.
Recessive model.
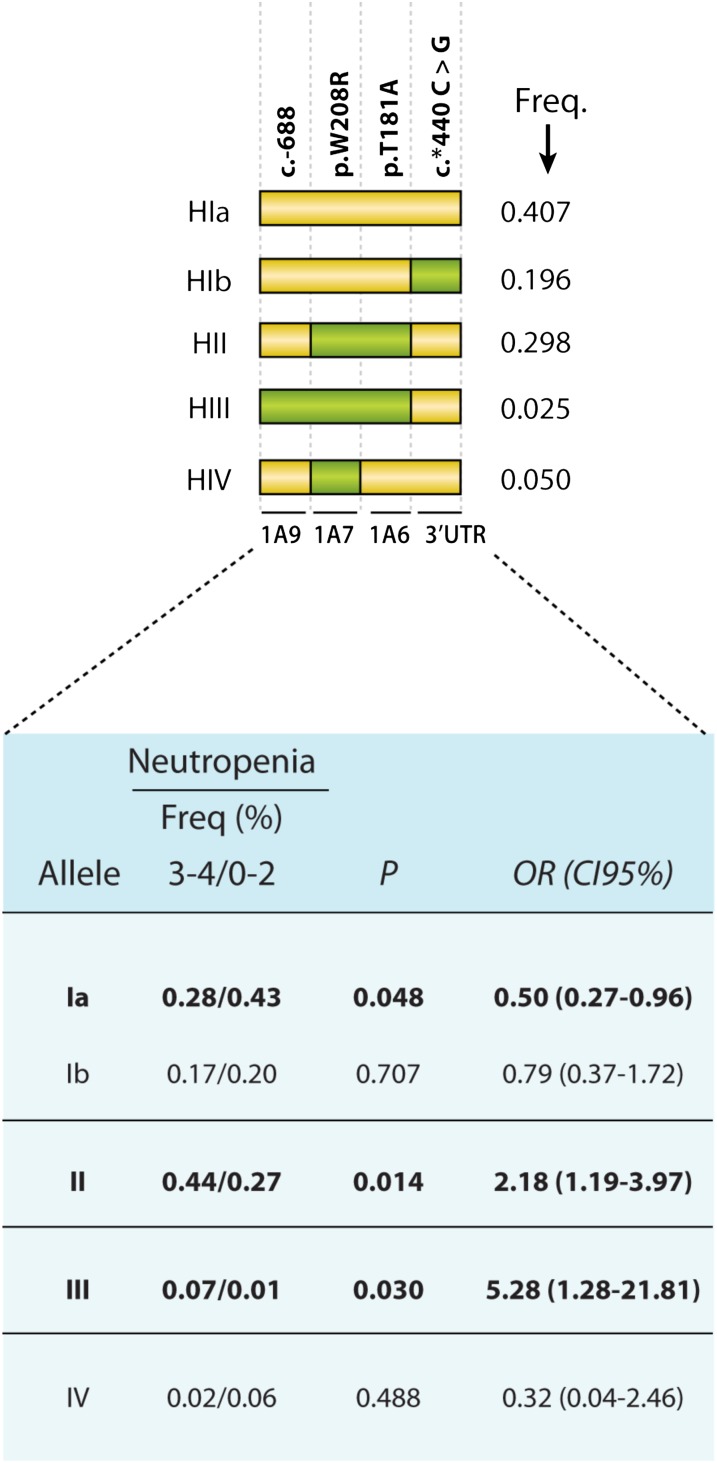
Upon multivariate analyses, no SNPs located in the UGT1A1 first exon or its promoter region, including the UGT1A1*28 (seven TA repeats) and the UGT1A1 c.-3156A alleles, remained significant in the Canadian patients. However, three markers situated in the central region of UGT1A were associated with a 2-fold increased risk of neutropenia grade 3–4 (Table 3), and are located in the UGT1A9 promoter at position −688 (MAF of 0.025), in the UGT1A7 first exon (p.W208R; MAF of 0.412), and in the UGT1A6 first exon (p.T181A; MAF of 0.352). Thus, in a second series of haplotype analysis, we tested these three SNPs with the 3′UTR SNP and revealed a protective HI (OR = 0.50; P = 0.048) and two risk haplotypes, HII and HIII, characterized by the presence of 2 (OR = 2.18; P = 0.014) and 3 (OR = 5.28; P = 0.030) unfavorable alleles, respectively, revealing a dosage effect (Fig. 2). This combination has not been tested in the Italian population due to a missing genotype for position −688 of UGT1A9.
TABLE 3.
Stepwise logistic regression model for severe neutropenia
Variables entered in the first step: UGT1A9 c.-1212, UGT1A9 c.-688, UGT1A9 c.-440, UGT1A7 p.W208R, UGT1A6 p.S7A, UGT1A6 p.T181A, UGT1A6 p.R184S, UGT1A1 c.-3156, UGT1A1 c.-54_53insTA. Age and treatment type were also included in the model.
| Severe Neutropenia | Estimate | S.E. | OR (95% CI) | P |
|---|---|---|---|---|
| UGT1A6 p.T181A | 0.741 | 0.317 | 2.10 (1.13–3.91) | 0.020 |
| UGT1A7 p.W208R | 0.701 | 0.311 | 2.00 (1.12–3.57) | 0.024 |
| UGT1A9 c.-688 | 1.509 | 0.810 | 4.52 (0.92–22.15) | 0.063 |
95% CI, 95% confidence interval; OR, odds ratio.
Fig. 2.
Schematic representation of UGT1A and the haplotype analyses associated with severe neutropenia based on markers across the UGT1A locus. Variations are represented by squares: a yellow square represents a reference nucleotide, whereas a green square represents a variant (relative to the AF297093 sequence). Only haplotypes with an MAF > 5% in the present study are shown. C, carrier; CI95%, 95% confidence interval; Freq., frequency; NC, noncarrier.
Discussion
Irinotecan combination chemotherapy causes severe and unpredictable hematologic and GI toxicities in a substantial percentage of patients (Negoro et al., 1991; Rothenberg et al., 1993, 2001; Rougier et al., 1998; Saltz et al., 2000; Vanhoefer et al., 2001; Fuchs et al., 2003). Despite several published studies on genetic markers that help predict irinotecan-associated severe neutropenia [reviewed in Hoskins et al. (2007)], much work is still required to optimize individualized treatment. Hence, better molecular markers to identify patients at risk for complications, including severe diarrhea, as well as to predict clinical response would be helpful to patients and medical oncologists. Currently, pharmacogenetic data suggest that the UGT1A1*28/*28 genotype confers the highest risk of severe neutropenia due to increased exposure to SN-38 (Ando et al., 2000; Iyer et al., 2002; Innocenti et al., 2004; Marcuello et al., 2004; Mathijssen et al., 2004; Rouits et al., 2004; de Jong et al., 2006; Massacesi et al., 2006; McLeod et al., 2006; Pillot et al., 2006; Toffoli et al., 2006; Cote et al., 2007; Hoskins et al., 2007; Kweekel et al., 2008; Ruzzo et al., 2008; Glimelius et al., 2011). Our current study confirms that this genotype is associated with an increased risk of severe neutropenia but in univariate analyses only, whereas a more comprehensive analysis of variations at the UGT1A locus suggests that other markers in the central region of the gene and in the 3′UTR region might better predict this toxicity.
As previously reported, polymorphisms at the UGT1A locus exhibit strong LD (Kohle et al., 2003; Peters et al., 2003; Menard et al., 2009). There has been sporadic conflicting information on the role of functional variants in the UGT1A1 promoter and coding regions and other UGT1A genes involved in irinotecan metabolism (Schulz et al., 2009). However, considering that UGT1A1*28 is a well-accepted predictor of severe neutropenia, and that strong LD is observed between several functional genetic variations at the UGT1A locus in diverse populations, it is thus not surprising to find an association between severe neutropenia and other common deleterious variations in UGT1A genes encoding SN-38–metabolizing enzymes (Table 2) (Iyer et al., 1998; Ciotti et al., 1999; Hanioka et al., 2001; Gagne et al., 2002).
Several UGT1A variations were individually associated with severe neutropenia, and their presence is inferred in the haplotype HII defined by a 5-marker haplotype across UGT1A first exons previously reported by Cecchin et al. (2009), and include the UGT1A1*28 allele (Fig. 2). We further described a protective UGT1A haplotype allele (HIa) defined by the reference sequence for these 5 markers, but also a variation in the 3′UTR region of the UGT1A gene common to all UGT1A-derived enzymes. Individuals with this haplotype have less chance of experiencing severe neutropenia (by 2-fold), and therefore could potentially tolerate irinotecan with less hematologic toxicity. Indeed, it has also been hypothesized that higher irinotecan doses can be safely administered to patients homozygous for the reference genotype UGT1A1*1/*1 owing to their relatively good tolerance of this drug (Schulz et al., 2009). Only the UGT1A HIa haplotype was associated with a reduced incidence of neutropenia, indicating that the simple exclusion of patients with the UGT1A1*28/*28 genotype may be insufficient to predict good tolerance to irinotecan with respect to severe neutropenia. Instead of identifying a risk haplotype and inferring that UGT1A1*28 noncarriers would be protected from severe neutropenia, the assessment of haplotype HIa seems to better identify those who have a low risk of irinotecan-induced neutropenia, presumably owing to the high glucuronidation activity of this allele. Indeed, this haplotype, as well as the haplotype HIb but with variation in the 3′UTR, contains UGT1A1*1, the reference UGT1A6/1A7, and UGT1A9, all of which are associated with high UGT expression and glucuronidation activity. These functional alleles may act synergistically to enhance SN-38 conjugation in the liver and extrahepatic tissues. It is thus tempting to speculate that genetic variations at the 3′ end affect gene expression. More studies are definitely needed to confirm these findings and elucidate the exact molecular mechanisms underlying our observation, and to assess the functionality of 3′UTR variations in UGT1A.
Additional analyses reveal that other variants in the central region of the UGT1A gene, namely, those located in UGT1A9 and in exons UGT1A7 and UGT1A6, are significant predictors of severe neutropenia with at least a 2-fold increased risk in multivariate analyses. Some of these SNPs are only partially linked to the UGT1A1*28 allele (r2 values between 0.028 and 0.82). Haplotype analyses with these markers and the 3′UTR variation, for a total of 4 markers located in UGT1A9, UGT1A7, UGT1A6, and 3′UTR, define four common haplotypes, of which one is protective and is referred to as HI = ATA (OR = 0.50) and two, HII = ACG and HIII = CCG, that are linked to a significantly higher risk of severe neutropenia. We further reveal a dosage effect with a higher risk in patients carrying 2 markers and the highest risk in those with 3 markers, also carrying the reference 3′UTR allele that does not confer protection. This set of UGT1A markers seems to improve risk prediction for severe neutropenia. Previous in vitro reports support the contribution of UGT1A9, UGT1A7, and UGT1A6 enzymes in the conjugation of SN-38 (Ciotti et al., 1999). Despite the uncertainty of the extent of the contribution of these other enzymes to SN-38 inactivation, several studies have found an association between the UGT1A7*3 allele (p.W208R) and irinotecan-induced toxicities (Ando et al., 2002; Carlini et al., 2005; Lankisch et al., 2008). UGT1A7 is one of the extrahepatic enzymes expressed mainly in the upper GI tract, whereas both UGT1A6 and UGT1A9 are expressed in the liver and other tissues (Nakamura et al., 2008). These UGT1A variations and the identified set of UGT1A markers should be carefully evaluated in future studies of irinotecan toxicity. The limitations of the study are its exploratory nature; the limited sample size, particularly for haplotype analyses; and the population studied that might be relatively genetically homogeneous. Other limitations are related to a focus on specific SNPs within the UGT1A gene, the scarcity of functional data for some of the positive markers, and a need for validation and study of additional cohorts.
In conclusion, the ultimate objective of pharmacogenetic studies is to develop tests that can be used to identify patients more likely to respond to a particular therapy and individuals who are more liable to suffer adverse reactions. In our study, we characterize UGT1A haplotypes that could potentially lead to more robust predictive tests. Additional studies that include a more comprehensive assessment of variations in UGT1A, including variations in the 3′UTR region and those across the locus, are warranted in irinotecan-containing dosage regimens, and may help clarify the role of UGT1A in the management of irinotecan toxicity and response.
Supplementary Material
Acknowledgments
The authors thank all the participants in this study as well as the research nurses from Québec and Ottawa hospitals for their contributions. The authors also thank Anne Dionne from Laval University for help in the design of the toxicity records. The authors also acknowledge the contribution of other laboratory members for work related to the support of genotyping and handling of samples.
Abbreviations
- FOLFIRI
folic acid (leucovorin), fluorouracil (5-FU), and irinotecan (camptosar) regimen
- GI
gastrointestinal
- H
haplotype
- LD
linkage disequilibrium
- MAF
minor allele frequency
- OR
odds ratio
- SN-38
7-ethyl-10-hydroxycamptothecin
- SNP
single-nucleotide polymorphism
- UGT
UDP-glucuronosyltransferase
- UTR
untranscribed region
Authorship Contributions
Participated in research design: Guillemette, Lévesque.
Conducted experiments: Harvey, Bélanger, Cecchin.
Performed data analysis: Lévesque, Harvey, Bélanger, Couture, Jonker, Innocenti, Cecchin, Toffoli, Guillemette.
Wrote or contributed to the writing of the manuscript: Lévesque, Harvey, Bélanger, Couture, Jonker, Innocenti, Cecchin, Toffoli, Guillemette.
Footnotes
This work was supported by the Canadian Institutes of Health Research [CIHR MOP-42392] (to C.G.); and Canada Research Chair Program (to C.G.). E.L. is a recipient of a CIHR clinician-scientist salary award. A.-S.B. was a recipient of a CIHR Frederick Banting and Charles Best studentship award. C.G. is the Canada Research Chair in Pharmacogenomics.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Ando M, Ando Y, Sekido Y, Ando M, Shimokata K, Hasegawa Y. (2002) Genetic polymorphisms of the UDP-glucuronosyltransferase 1A7 gene and irinotecan toxicity in Japanese cancer patients. Jpn J Cancer Res 93:591–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando Y, Saka H, Ando M, Sawa T, Muro K, Ueoka H, Yokoyama A, Saitoh S, Shimokata K, Hasegawa Y. (2000) Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res 60:6921–6926 [PubMed] [Google Scholar]
- Beutler E, Gelbart T, Demina A. (1998) Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: a balanced polymorphism for regulation of bilirubin metabolism? Proc Natl Acad Sci USA 95:8170–8174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, Lindhout D, Tytgat GN, Jansen PL, Oude Elferink RP, et al. (1995) The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med 333:1171–1175 [DOI] [PubMed] [Google Scholar]
- Carlini LE, Meropol NJ, Bever J, Andria ML, Hill T, Gold P, Rogatko A, Wang H, Blanchard RL. (2005) UGT1A7 and UGT1A9 polymorphisms predict response and toxicity in colorectal cancer patients treated with capecitabine/irinotecan. Clin Cancer Res 11:1226–1236 [PubMed] [Google Scholar]
- Cecchin E, Innocenti F, D’Andrea M, Corona G, De Mattia E, Biason P, Buonadonna A, Toffoli G. (2009) Predictive role of the UGT1A1, UGT1A7, and UGT1A9 genetic variants and their haplotypes on the outcome of metastatic colorectal cancer patients treated with fluorouracil, leucovorin, and irinotecan. J Clin Oncol 27:2457–2465 [DOI] [PubMed] [Google Scholar]
- Ciotti M, Basu N, Brangi M, Owens IS. (1999) Glucuronidation of 7-ethyl-10-hydroxycamptothecin (SN-38) by the human UDP-glucuronosyltransferases encoded at the UGT1 locus. Biochem Biophys Res Commun 260:199–202 [DOI] [PubMed] [Google Scholar]
- Côté JF, Kirzin S, Kramar A, Mosnier JF, Diebold MD, Soubeyran I, Thirouard AS, Selves J, Laurent-Puig P, Ychou M. (2007) UGT1A1 polymorphism can predict hematologic toxicity in patients treated with irinotecan. Clin Cancer Res 13:3269–3275 [DOI] [PubMed] [Google Scholar]
- de Jong FA, Kehrer DF, Mathijssen RH, Creemers GJ, de Bruijn P, van Schaik RH, Planting AS, van der Gaast A, Eskens FA, Janssen JT, et al. (2006) Prophylaxis of irinotecan-induced diarrhea with neomycin and potential role for UGT1A1*28 genotype screening: a double-blind, randomized, placebo-controlled study. Oncologist 11:944–954 [DOI] [PubMed] [Google Scholar]
- Fuchs CS, Moore MR, Harker G, Villa L, Rinaldi D, Hecht JR. (2003) Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J Clin Oncol 21:807–814 [DOI] [PubMed] [Google Scholar]
- Gagné JF, Montminy V, Belanger P, Journault K, Gaucher G, Guillemette C. (2002) Common human UGT1A polymorphisms and the altered metabolism of irinotecan active metabolite 7-ethyl-10-hydroxycamptothecin (SN-38). Mol Pharmacol 62:608–617 [DOI] [PubMed] [Google Scholar]
- Glimelius B, Garmo H, Berglund A, Fredriksson LA, Berglund M, Kohnke H, Byström P, Sørbye H, Wadelius M. (2011) Prediction of irinotecan and 5-fluorouracil toxicity and response in patients with advanced colorectal cancer. Pharmacogenomics J 11:61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanioka N, Ozawa S, Jinno H, Ando M, Saito Y, Sawada J. (2001) Human liver UDP-glucuronosyltransferase isoforms involved in the glucuronidation of 7-ethyl-10-hydroxycamptothecin. Xenobiotica 31:687–699 [DOI] [PubMed] [Google Scholar]
- Hoskins JM, Goldberg RM, Qu P, Ibrahim JG, McLeod HL. (2007) UGT1A1*28 genotype and irinotecan-induced neutropenia: dose matters. J Natl Cancer Inst 99:1290–1295 [DOI] [PubMed] [Google Scholar]
- Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, Karrison T, Janisch L, Ramírez J, Rudin CM, et al. (2004) Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 22:1382–1388 [DOI] [PubMed] [Google Scholar]
- Iyer L, Das S, Janisch L, Wen M, Ramírez J, Karrison T, Fleming GF, Vokes EE, Schilsky RL, Ratain MJ. (2002) UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenomics J 2:43–47 [DOI] [PubMed] [Google Scholar]
- Iyer L, King CD, Whitington PF, Green MD, Roy SK, Tephly TR, Coffman BL, Ratain MJ. (1998) Genetic predisposition to the metabolism of irinotecan (CPT-11). Role of uridine diphosphate glucuronosyltransferase isoform 1A1 in the glucuronidation of its active metabolite (SN-38) in human liver microsomes. J Clin Invest 101:847–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jada SR, Lim R, Wong CI, Shu X, Lee SC, Zhou Q, Goh BC, Chowbay B. (2007) Role of UGT1A1*6, UGT1A1*28 and ABCG2 c.421C>A polymorphisms in irinotecan-induced neutropenia in Asian cancer patients. Cancer Sci 98:1461–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato Y, Aonuma M, Hirota Y, Kuga H, Sato K. (1991) Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res 51:4187–4191 [PubMed] [Google Scholar]
- Köhle C, Möhrle B, Münzel PA, Schwab M, Wernet D, Badary OA, Bock KW. (2003) Frequent co-occurrence of the TATA box mutation associated with Gilbert’s syndrome (UGT1A1*28) with other polymorphisms of the UDP-glucuronosyltransferase-1 locus (UGT1A6*2 and UGT1A7*3) in Caucasians and Egyptians. Biochem Pharmacol 65:1521–1527 [DOI] [PubMed] [Google Scholar]
- Kojima A, Shinkai T, Saijo N. (1993) Cytogenetic effects of CPT-11 and its active metabolite, SN-38 on human lymphocytes. Jpn J Clin Oncol 23:116–122 [PubMed] [Google Scholar]
- Kweekel DM, Gelderblom H, Van der Straaten T, Antonini NF, Punt CJ, Guchelaar HJ, Dutch Colorectal Cancer Group study (2008) UGT1A1*28 genotype and irinotecan dosage in patients with metastatic colorectal cancer: a Dutch Colorectal Cancer Group study. Br J Cancer 99:275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankisch TO, Schulz C, Zwingers T, Erichsen TJ, Manns MP, Heinemann V, Strassburg CP. (2008) Gilbert’s Syndrome and irinotecan toxicity: combination with UDP-glucuronosyltransferase 1A7 variants increases risk. Cancer Epidemiol Biomarkers Prev 17:695–701 [DOI] [PubMed] [Google Scholar]
- Maitland ML, Grimsley C, Kuttab-Boulos H, Witonsky D, Kasza KE, Yang L, Roe BA, Di Rienzo A. (2006) Comparative genomics analysis of human sequence variation in the UGT1A gene cluster. Pharmacogenomics J 6:52–62 [DOI] [PubMed] [Google Scholar]
- Marcuello E, Altés A, Menoyo A, Del Rio E, Gómez-Pardo M, Baiget M. (2004) UGT1A1 gene variations and irinotecan treatment in patients with metastatic colorectal cancer. Br J Cancer 91:678–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massacesi C, Terrazzino S, Marcucci F, Rocchi MB, Lippe P, Bisonni R, Lombardo M, Pilone A, Mattioli R, Leon A. (2006) Uridine diphosphate glucuronosyl transferase 1A1 promoter polymorphism predicts the risk of gastrointestinal toxicity and fatigue induced by irinotecan-based chemotherapy. Cancer 106:1007–1016 [DOI] [PubMed] [Google Scholar]
- Mathijssen RH, de Jong FA, van Schaik RH, Lepper ER, Friberg LE, Rietveld T, de Bruijn P, Graveland WJ, Figg WD, Verweij J, et al. (2004) Prediction of irinotecan pharmacokinetics by use of cytochrome P450 3A4 phenotyping probes. J Natl Cancer Inst 96:1585–1592 [DOI] [PubMed] [Google Scholar]
- McLeod HL, Parodi L, Sargent DJ, Marsh S, Green E, Abreu P, Cisar LA, Goldberg RM. (2006) UGT1A1*28, toxicity and outcome in advanced colorectal cancer: Results from Trial N9741. abstract3520 J Clin Oncol 24:3520 [Google Scholar]
- Ménard V, Girard H, Harvey M, Pérusse L, Guillemette C. (2009) Analysis of inherited genetic variations at the UGT1 locus in the French-Canadian population. Hum Mutat 30:677–687 [DOI] [PubMed] [Google Scholar]
- Nakamura A, Nakajima M, Yamanaka H, Fujiwara R, Yokoi T. (2008) Expression of UGT1A and UGT2B mRNA in human normal tissues and various cell lines. Drug Metab Dispos 36:1461–1464 [DOI] [PubMed] [Google Scholar]
- Negoro S, Fukuoka M, Masuda N, Takada M, Kusunoki Y, Matsui K, Takifuji N, Kudoh S, Niitani H, Taguchi T. (1991) Phase I study of weekly intravenous infusions of CPT-11, a new derivative of camptothecin, in the treatment of advanced non-small-cell lung cancer. J Natl Cancer Inst 83:1164–1168 [DOI] [PubMed] [Google Scholar]
- Peters WH, te Morsche RH, Roelofs HM. (2003) Combined polymorphisms in UDP-glucuronosyltransferases 1A1 and 1A6: implications for patients with Gilbert’s syndrome. J Hepatol 38:3–8 [DOI] [PubMed] [Google Scholar]
- Pillot GA, Read WL, Hennenfent KL, Marsh S, Gao F, Viswanathan A, Cummings K, McLeod HL, Govindan R. (2006) A phase II study of irinotecan and carboplatin in advanced non-small cell lung cancer with pharmacogenomic analysis: final report. J Thorac Oncol 1:972–978 [PubMed] [Google Scholar]
- Rothenberg ML, Kuhn JG, Burris HA, 3rd, Nelson J, Eckardt JR, Tristan-Morales M, Hilsenbeck SG, Weiss GR, Smith LS, Rodriguez GI, et al. (1993) Phase I and pharmacokinetic trial of weekly CPT-11. J Clin Oncol 11:2194–2204 [DOI] [PubMed] [Google Scholar]
- Rothenberg ML, Kuhn JG, Schaaf LJ, Rodriguez GI, Eckhardt SG, Villalona-Calero MA, Rinaldi DA, Hammond LA, Hodges S, Sharma A, et al. (2001) Phase I dose-finding and pharmacokinetic trial of irinotecan (CPT-11) administered every two weeks. Ann Oncol 12:1631–1641 [DOI] [PubMed] [Google Scholar]
- Rougier P, Van Cutsem E, Bajetta E, Niederle N, Possinger K, Labianca R, Navarro M, Morant R, Bleiberg H, Wils J, et al. (1998) Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet 352:1407–1412 [DOI] [PubMed] [Google Scholar]
- Rouits E, Boisdron-Celle M, Dumont A, Guérin O, Morel A, Gamelin E. (2004) Relevance of different UGT1A1 polymorphisms in irinotecan-induced toxicity: a molecular and clinical study of 75 patients. Clin Cancer Res 10:5151–5159 [DOI] [PubMed] [Google Scholar]
- Ruzzo A, Graziano F, Loupakis F, Santini D, Catalano V, Bisonni R, Ficarelli R, Fontana A, Andreoni F, Falcone A, et al. (2008) Pharmacogenetic profiling in patients with advanced colorectal cancer treated with first-line FOLFIRI chemotherapy. Pharmacogenomics J 8:278–288 [DOI] [PubMed] [Google Scholar]
- Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N, et al. Irinotecan Study Group (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med 343:905–914 [DOI] [PubMed] [Google Scholar]
- Schulz C, Boeck S, Heinemann V, Stemmler HJ. (2009) UGT1A1 genotyping: a predictor of irinotecan-associated side effects and drug efficacy? Anticancer Drugs 20:867–879 [DOI] [PubMed] [Google Scholar]
- Soepenberg O, Dumez H, Verweij J, de Jong FA, de Jonge MJ, Thomas J, Eskens FA, van Schaik RH, Selleslach J, Ter Steeg J, et al. (2005) Phase I pharmacokinetic, food effect, and pharmacogenetic study of oral irinotecan given as semisolid matrix capsules in patients with solid tumors. Clin Cancer Res 11:1504–1511 [DOI] [PubMed] [Google Scholar]
- Stephens M, Donnelly P. (2003) A comparison of bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet 73:1162–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. (2001) A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68:978–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SS, Li SS, Lampe JW, Potter JD, Bigler J. (2006) Genetic variability, haplotypes, and htSNPs for exons 1 at the human UGT1A locus. Hum Mutat 27:717. [DOI] [PubMed] [Google Scholar]
- Toffoli G, Cecchin E, Corona G, Russo A, Buonadonna A, D’Andrea M, Pasetto LM, Pessa S, Errante D, De Pangher V, et al. (2006) The role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer. J Clin Oncol 24:3061–3068 [DOI] [PubMed] [Google Scholar]
- Vanhoefer U, Harstrick A, Achterrath W, Cao S, Seeber S, Rustum YM. (2001) Irinotecan in the treatment of colorectal cancer: clinical overview. J Clin Oncol 19:1501–1518 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.