Abstract
Marijuana substitutes often contain blends of multiple psychoactive synthetic cannabinoids (SCBs), including the prevalent SCBs (1-pentyl-1H-indole-3-yl)-1-naphthalenyl-methanone (JWH-018) and (1-butyl-1H-indole-3-yl)-1-naphthalenyl-methanone (JWH-073). Because SCBs are frequently used in combinations, we hypothesized that coadministering multiple SCBs induces synergistic drug–drug interactions. Drug–drug interactions between JWH-018 and JWH-073 were investigated in vivo for Δ9-tetrahydrocannabinol (Δ9-THC)-like discriminative stimulus effects, analgesia, task disruption, and hypothermia. Combinations (JWH-018:JWH-073) of these drugs were administered to mice in assays of Δ9-THC discrimination, tail-immersion, and food-maintained responding, and rectal temperatures were measured. Synergism occurred in the Δ9-THC discrimination assay for two constant dose ratio combinations (1:3 and 1:1). A 1:1 and 2:3 dose ratio induced additivity and synergy, respectively, in the tail-immersion assay. Both 1:1 and 2:3 dose ratios were additive for hypothermia, whereas a 1:3 dose ratio induced subadditive suppression of food-maintained responding. In vitro drug–drug interactions were assessed using competition receptor-binding assays employing mouse brain homogenates and cannabinoid 1 receptor (CB1R)-mediated inhibition of adenylyl cyclase activity in Neuro2A wild-type cells. Interestingly, synergy occurred in the competition receptor-binding assay for two dose ratios (1:5 and 1:10), but not in the adenylyl cyclase activity assay (1:5). Altogether, these data indicate that drug–drug interactions between JWH-018 and JWH-073 are effect- and ratio-dependent and may increase the relative potency of marijuana substitutes for subjective Δ9-THC–like effects. Combinations may improve the therapeutic profile of cannabinoids, considering that analgesia but not hypothermia or task disruption was potentiated. Importantly, synergy in the competition receptor–binding assay suggests multiple CB1R-SCB binding sites.
Introduction
Marijuana substitutes that are inaccurately marketed as “all-natural herbal incense” have emerged in recent years as drugs of abuse (Seely et al., 2011). These products, often known as “K2” or “Spice” (henceforth called “K2”), are adulterated with a variable mixture of synthetic cannabinoids (SCBs) that possess cannabis-like psychoactivity. Like Δ9-tetrahydrocannabinol (Δ9-THC), the psychoactive component of marijuana, SCBs bind and activate the cannabinoid 1 receptor (CB1R) and cannabinoid 2 receptor (Aung et al., 2000). Alarmingly, the use of K2 has a seemingly high prevalence of severe adverse effects not commonly reported with marijuana, including tachycardia, hypertension, seizures, hallucinations, anxiety attacks, and psychosis (Mir et al., 2011; Schneir et al., 2011; Simmons et al., 2011; Harris and Brown, 2013).
Although SCBs were originally synthesized to study the endocannabinoid system (Huffman et al., 1994), several SCBs have become drugs of abuse, presumably because they have certain advantages over marijuana. For example, until recently, they were neither legally regulated nor detectable in standard drug urine tests (Seely et al., 2012). Since the initial 2009 report that SCBs were being abused (Auwarter et al., 2009), several groups have employed liquid or gas chromatography coupled with mass spectrometry to elucidate the composition of K2 products (Auwarter et al., 2009; Lindigkeit et al., 2009; Dresen et al., 2010; Hudson et al., 2010). These groups reported that multiple SCBs, such as (1-pentyl-1H-indole-3-yl)-1-naphthalenyl-methanone (JWH-018), (1-butyl-1H-indole-3-yl)-1-naphthalenyl-methanone (JWH-073), and CP-47,497 [2-[(1R,3S)-3-hydroxycyclohexyl]-5-(2-methyloctan-2-yl)phenol], are simultaneously present as constituents of “herbal incense.” However, such studies shed no light as to why multiple SCBs would be added to a single product. Poly-drug use was examined in a special report by the European Monitoring Centre for Drugs and Drug Addiction in 2009 (http://www.emcdda.europa.eu). Reasons for poly-drug use cited in the European Monitoring Centre for Drugs and Drug Addiction’s report include cumulative and complementary effects of multiple drugs, as well as an offsetting of adverse effects of one or more drugs. Other reasons may involve availability of drugs. For example, the inclusion of multiple SCBs in K2 may simply be due to manufacturers semi-randomly adding the most readily available SCBs in a nonspecific attempt to produce a robust cannabimimetic “high.” However, anecdotal evidence suggests that specific SCBs are purposefully combined in various proportions in an attempt to maximize desirable effects (e.g., euphoria, relaxation) while minimizing adverse effects (dysphoria, hallucinations, “bad trips”) ( http://www.drugs-forum.com). JWH-018 and JWH-073 (Fig. 1) are often co-abused in K2 products and “homemade blends” made by experienced users (http://www.drugs-forum.com), suggesting that these two specific SCBs together produce desirable effects that are highly sought by users. While use of either drug induces cannabimimetic effects, JWH-073 reportedly results in a “more rounded stoned (sic) similar to cannabis,” whereas JWH-018 is known for having higher efficacy but also more readily induces anxiety (http://www.bluelight.ru; http://www.drugs-forum.com). These differences in subjective effects may motivate users to experiment with combinations in an attempt to produce novel, desirable effects.
Fig. 1.
Structures of JWH-018 and JWH-073.
When multiple drugs with similar actions are combined, as are SCBs in K2 products, the net result produced for each effect shared by these drugs is expected to be additive. Alternatively, drug–drug interactions that result in greater-than-additive (also called “supra-additive” and “synergistic”) or less-than-additive (also called “subadditive” and “antagonistic”) pharmacologic activity can occur, significantly enhancing or reducing net effect, respectively. Potential sites of drug–drug interactions include metabolic enzymes (e.g., cytochrome P450s), excretory structures (e.g., hepatic and renal vasculature), or receptor and cellular signaling proteins.
This report is the first study to characterize the drug–drug interactions of two prevalent SCBs of abuse, JWH-018 and JWH-073, by examining the effects of different dose ratio combinations on several in vivo and in vitro endpoints. First, the subjective similarity of various constant dose ratio combinations of these SCBs to Δ9-THC was examined in mice trained to discriminate the interoceptive effects of Δ9-THC. Next, the therapeutic potential of JWH-018 and JWH-073 combinations was examined by quantifying analgesia in mice by employing a tail-immersion assay. Because hypothermia is an important, well-established physiologic measure of cannabinoid agonist activity, rectal temperature was examined for drug–drug interactions of JWH-018 and JWH-073. The effect of drug combinations on task disruption, a common adverse effect produced by cannabinoids, was also evaluated by measuring food-maintained operant responding in mice. Finally, to elucidate potential cellular mechanisms underlying these in vivo effects, we examined the drug–drug interactions of JWH-018 and JWH-073 in two in vitro assays, competition receptor binding and CB1R-mediated inhibition of adenylyl cyclase activity.
Materials and Methods
JWH-018 and JWH-073 were synthesized and validated in the laboratory of Dr. Thomas E. Prisinzano at the University of Kansas (College of Pharmacy, Lawrence, KS) as previously described (Brents et al., 2012). Δ9-THC was provided by the National Institute on Drug Abuse. For in vitro experiments, drugs were diluted in 100% ethanol to a stock concentration of 10−2 M and stored at −20°C. For in vivo experiments, drugs were diluted in a saline-based vehicle containing 7.8% Tween 80 and stored at +4°C. For all animal experiments, drugs were warmed to room temperature and injected 10 μl/g body weight i.p. Forskolin and 3-isobutyl-1-methylxanthine (IBMX) were purchased from EMD Millipore (Billerica, MA). Forskolin was diluted to a stock concentration of 10−1 M in 100% dimethylsulfoxide and stored at −20°C. Just before use in the adenylyl cyclase assay, forskolin was thawed and diluted to 10−3 M with 100% ethanol. IBMX was diluted to 25 mM in ultrapure water, with NaOH (1 M) added in a dropwise manner until all drug went into solution. [3H]Adenine (13 Ci/mmol) was purchased from ViTrax (Placentia, CA). [3H](−)-cis-3-[2-hydroxyl-4-(1,1-dimethylheptyl)phenyl]-trans-4-[3-hydroxyl-propyl] cyclohexan-1-ol (CP-55,940) (144.0 Ci/mmol) was purchased from PerkinElmer (Waltham, MA).
Animal Care and Use.
All studies were performed in accordance with the Declaration of Helsinki and with the Guide for Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. All animal protocols were approved by the Animal Care and Use Committee at the University of Arkansas for Medical Sciences.
Male NIH Swiss mice (Harlan Sprague Dawley, Inc., Indianapolis, IN) were used for all animal experiments described in the present work. Mice weighed 25 to 30 g at the start of each study and were housed 3 per Plexiglas cage (15.24 cm × 25.40 cm × 12.70 cm) in a temperature-controlled (22 ± 2°C) room with 45–50% humidity in an Association for Assessment and Accreditation of Laboratory Animal Care–accredited facility. Lights were set to a 12-hour light/dark cycle, with room lights on at 0700 and off at 1900. Animals used in operant assays were maintained at 80% their free-feeding weight, whereas animals in the temperature and analgesia experiments were free-fed. All animals consumed Laboratory Diet rodent chow (Laboratory Rodent Diet #5001; PMI Feeds, Inc., St. Louis, MO) and had ad libitum access to water.
Food-Maintained Responding.
Mice were trained or tested daily (Monday through Friday) between 1000 and 1200 hours in either two–nose-poke (for discrimination assay) or two-lever (for food-maintained responding) operant conditioning chambers (Med Associates, St. Albans, VT), which were enclosed within light- and sound-attenuating boxes. Fans mounted above the operant conditioning chambers ran during sessions and masked extraneous sounds that may have occurred during the session. Ambient light was provided by a bulb in the top center of the front panel (house light). Either two nose-poke apertures or two levers were located on the front panel of the chamber and were 12.1 cm apart, with the reinforcement aperture centered between them. A photobeam crossed the threshold of each nose-poke opening. The breaking of a photobeam in either nosepoke was registered as a response and produced an audible click. For lever chambers, downward force on the lever was required to register a response. Reinforced responses allowed mice a 5-second access period to one 0.01 ml dipper of evaporated milk (Kroger Brand, Cincinnati, OH) that was 50% diluted with water. This access period was immediately followed by a 10-second timeout in which the house light was turned off and no responses were registered. Control and data collection for training and testing sessions was accomplished with Med Associates interface and operating software.
Training sessions ended after either 60 minutes or after 60 reinforcements were delivered, whichever occurred first. Initial training sessions used a fixed ratio (FR) 1 schedule of reinforcement (FR1), meaning that a single response on either aperture/lever resulted in a milk presentation. Every 20th reinforcer earned incremented the FR by 1, and mice were thus shaped to a terminal FR10 across sessions. Testing began in the response rate assay when response rates varied no more than 20% for 3 consecutive training sessions.
Discrimination Assay.
Protocols for Δ9-THC discrimination training and testing in mice were based on previous work by McMahon et al. (2008) and Vann et al. (2009). Food-maintained responding was established as previously described (see Food-Maintained Responding), and discrimination training began after mice reliably worked at FR10 to earn all possible reinforcements in five consecutive training sessions. For discrimination training, mice were administered either vehicle or Δ9-THC (10 mg/kg i.p.) Monday through Friday between 1300 and 1500 hours and then placed in the operant conditioning chamber for a 30-minute pretreatment period before the training session began. From this point forward, another criterion for reinforcement was added: mice had to respond on the injection-appropriate aperture 10 consecutive times. The “appropriate” aperture was the left aperture when vehicle had been administered and the right aperture when the training dose of Δ9-THC had been administered. Mice were considered stably trained and ready to begin testing when, in five consecutive training sessions, ≥90% responded appropriately for the entire session and ≥75% responded appropriately for the first reinforcement of the session.
Testing sessions occurred 2 to 3 times per week, between 1400 and 1600 hours, with training sessions maintained on nontesting days. As in training sessions, test drugs were administered i.p., and mice were placed in the operant-conditioning chambers 30 minutes before the testing session began. Testing sessions were performed in extinction and lasted 2 minutes, or until 10 consecutive responses were made on one nose-poke aperture. Data from testing sessions are expressed as “percent drug-appropriate responding.”
Complete generalization of a training drug to a test drug is said to be present when (a) a mean of 80% or more of all test responses occurs on the drug-appropriate lever and (b) there is a statistically significant difference between the response distributions of the test drug and saline control sessions. An intermediate degree of generalization is defined as being present when response distributions after a test drug are less than 80% drug-appropriate but are significantly different from saline control sessions. Finally, when the response distribution after a test drug is not statistically different from that in saline control sessions, an absence of generalization of the training drug to the test drug is assumed.
Rectal Temperature and Tail Immersion Assay.
Vehicle, JWH-018, JWH-073, or combinations of the two SCBs were administered i.p. to different groups of mice using a cumulative dosing protocol of up to six injections. To reduce the number of animals used in these experiments, rectal temperature and latency to withdraw tail in the tail-immersion assay were measured in the same animals, 15 and 20 minutes, respectively, after each drug administration. Because the equieffective constant dose ratios differed for analgesia and rectal temperature, constant dose ratio combinations were selected based on the dose response curves of analgesia, which was the effect of greater interest. Temperature was registered using a Physitemp Model BAT-12 microprobe thermometer (Physitemp Instruments Inc., Clifton, NJ) that was inserted 2 cm into the rectum. In the tail-immersion assay, the body of a mouse was securely immobilized in the investigator’s hand while the tail was allowed to hang freely. The distal 5 cm of the tail was dipped into a vacuum flask containing 55°C water. The mice could remove their tails from the water at any point, and the amount of time the tails remained in the water was measured with a stopwatch. Baseline tail withdrawal latencies ranged from 2 to 4 seconds. To ensure that tail withdrawal was due specifically to the nociceptive stimulus of the 55°C water and not a learned response over the course of multiple dips, a nonnociceptive control dip of 45°C water was performed for each animal midway through the experimental session. This control resulted in the maximal latency of 15 seconds for all animals, indicating that tail withdrawal was not simply an effect of water exposure. A different control group was administered only repeated vehicle injections, and we observed that baseline values for both analgesia and temperature remained constant throughout multiple measurements in one experimental session (Supplemental Fig. 1). This experiment was performed to ensure that the effects observed in the drug groups were due to the drugs and not to such factors as stress or hyperalgesia. With the exception of Supplemental Fig. 1, tail-immersion assay data are reported as percent maximal possible effect, which is calculated as (post-treatment latency − basal latency)/(maximal possible latency − basal latency)*100, where the maximal possible latency = 15.
To reduce stress-induced effects on analgesia and temperature, mice were acclimated to the procedure 1 week before drugs were administered. The habituation procedure was performed as previously described except injections were excluded.
Membrane Preparation.
Mouse whole-brain homogenates were prepared for the competition receptor-binding assay as previously described (Brents et al., 2011). Briefly, whole brains were harvested from male and female B6SJL mice, snap frozen in liquid nitrogen, and stored at −80°C until homogenization. When needed, brains were thawed on ice, then pooled into a 40-ml Dounce homogenizer and suspended in 5 volumes of homogenization buffer containing 50 mM HEPES (pH 7.4), 3 mM MgCl2, and 1 mM EGTA. Tissues then underwent 10 strokes with the coarse grinding pestle “A,” followed by centrifugation at 40,000g for 10 minutes at 4°C. The supernatant was discarded, and the resulting pellet was resuspended in homogenization buffer and transferred to the Dounce homogenizer. The 10-stroke homogenization and centrifugation was repeated twice more. After the third centrifugation, the pellet was resuspended in buffer composed of 50 mM HEPES (pH 7.4), and homogenized using a fine grinding pestle “B.” Homogenates were aliquoted and stored at −80°C until used in the competition receptor-binding assay. Protein concentrations of the homogenates were determined using the BCA Protein Assay (Thermo Scientific, Rockford, IL).
Competition Receptor-Binding Assay.
Competition receptor binding was performed as previously described (Brents et al., 2011). Briefly, 50 μg of mouse brain homogenates, which contain abundant concentrations of CB1R and negligible cannabinoid 2 receptor (Herkenham et al., 1990; Galiegue et al., 1995), were incubated at room temperature with the following: 0.2 nM [3H]CP-55,940; 5 mM MgCl2; and either vehicle (to define total binding), 1 μM WIN-55,212-2 [(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-napthalenylmethanon] (to define nonspecific binding) or increasing concentrations of either JWH-018, JWH-073 (single competitor), or constant-ratio combinations of JWH-018:JWH-073 (1:10 and 1:5, dual competitor). Duplicate reactions were carried out in 1 ml total volume in an assay buffer containing 50 mM Tris (pH 7.4), 0.05% bovine serum albumin and 0.2% ethanol. Samples were allowed to reach equilibrium binding by incubation at room temperature for 90 minutes. Reactions were terminated by rapid vacuum filtration through Whatman GF/B glass fiber filters, followed by 5 washes of ice-cold filtration buffer [50 mM Tris (pH 7.4), 0.05% bovine serum albumin]. Filters were immediately placed in 7 ml scintillation vials to which 4 ml of ScintiVerse BD Cocktail Scintillation Fluid (Fisher Scientific, Fair Lawn, NJ) was added. Bound radioactivity was determined by liquid scintillation spectrophotometry (Tri Carb 2100 TR Liquid Scintillation Analyzer; Packard Instrument Company, Meriden, CT) after overnight incubation and shaking at room temperature. Specific binding is expressed as the amount of total binding minus nonspecific binding and is graphed for each data point as a percentage of specific binding occurring in the absence of any competitor.
Cell Culture.
After rapid thawing to room temperature from liquid nitrogen, Neuro2A wild-type (Neuro2AWT) cells, which endogenously express mouse CB1Rs, were maintained in Dulbecco’s modified Eagle’s medium (Cellgro, Manassas, VA) containing 10% FetalPlex Animal Serum Complex (Gemini Bio Products, West Sacramento, CA), and 1% penicillin/streptomycin (10,000 IU/ml pencillin, 10,000 μg/ml streptomycin; Cellgro) in a Sarstedt T175 cell culture flask. Cells were incubated at 5% CO2 in a humidified incubator at 37°C. Upon 90–100% confluency, cells were harvested with phosphate-buffered saline–EDTA, centrifuged to obtain a pellet at 1000g, and resuspended in complete media for reseeding to achieve ∼20–30% density. Adenylyl cyclase experiments were performed with cells maintained between 5 and 15 passages.
Adenylyl Cyclase Assay.
Four million Neuro2AWT cells were plated into a 24-well plate and allowed to attach overnight. At 80–90% confluency (the following morning), 0.5 ml of warm incubation media composed of Dulbecco’s modified Eagle’s medium with 0.9 g/l NaCl, 2.5 μCi/ml [3H]adenine and 0.5 mM IBMX was added to the cells. After a 4-hour incubation period at 37°C in a 5% CO2 incubator, the media was removed and the plate was briefly floated on an ice water bath while 0.5 ml of an assay mix was quickly added to the cells in triplicate. The assay mix consisted of a Krebs Ringer HEPES buffered saline solution containing 0.5 mM IBMX, 10 μM forskolin, and either vehicle (0.2% ethanol) or increasing concentrations of JWH-018, JWH-073, or the two cannabinoids in a 1:5 dose ratio of JWH-018 to JWH-073 combination. The plate was then transferred to a 37°C water bath for a 15-minute incubation, and the reaction was terminated by addition of 50 μl of 2.2 N HCl. Intracellular [3H]cAMP was separated by column chromatography employing acidic alumina. Next, 4 ml of the final eluent was added to 10 ml of ScintiVerse BD Cocktail Scintillation Fluid, and radioactivity was immediately measured employing liquid scintillation spectrophotometry (Tri Carb 2100 TR Liquid Scintillation Analyzer). Data are expressed as the percent of intracellular [3H]cAMP relative to that observed in vehicle samples.
Statistical Analyses.
Curve fitting and statistical analyses were performed using GraphPad Prism version 5.0b (GraphPad Software, Inc., San Diego, CA). Theoretical and experimental data for drug–drug combinations were compared as composite additive curves using methods previously described by Tallarida (2000) (shown in panel C of Figs. 2–7 and referred throughout the text as the “composite additive curve comparison”). In these analyses, a theoretical curve was constructed using data obtained by testing each drug separately, whereas the combination curve data were obtained by experimentally combining the two drugs. An F-ratio test was applied between the linear regression lines of the theoretical and experimental data. If either the slopes or y-intercepts of the two lines differed significantly (i.e., the data sets were better fit using two regression lines instead of one), then the combination was considered either synergistic (experimental curve shifted left of theoretical) or antagonistic (experimental curve shifted right of theoretical). Single-effect level (e.g., ED50) potency data were also graphically represented using isobolograms (panel D of each figure). These potency data were derived from the nonlinear regression of the dose-response and concentration-effect data. For these comparisons, ED50 (discrimination assays), ED10 (temperature, 10% reduction from baseline values), Ki (competition receptor binding), or IC50 (adenylyl cyclase assay) values were obtained from the respective nonlinear regression analysis of data from individual subjects. These values were then averaged together to obtain the mean and S.E.M. for each treatment group, with the exception of analgesia. For analgesia, mean and S.E.M. values were obtained from the cumulative nonlinear regression analysis of all subjects combined within each treatment group. Theoretical values listed in Tables 1–5 were derived using methods previously described by Tallarida (2000) from the individual drug data. Ki values in the competition receptor-binding assay were determined from experimental IC50 values using the Cheng-Prusoff equation (Cheng and Prusoff, 1973). Unless otherwise indicated, data are expressed as mean ± S.E.M. calculated from experiments conducted a minimum of 3 times.
Fig. 2.
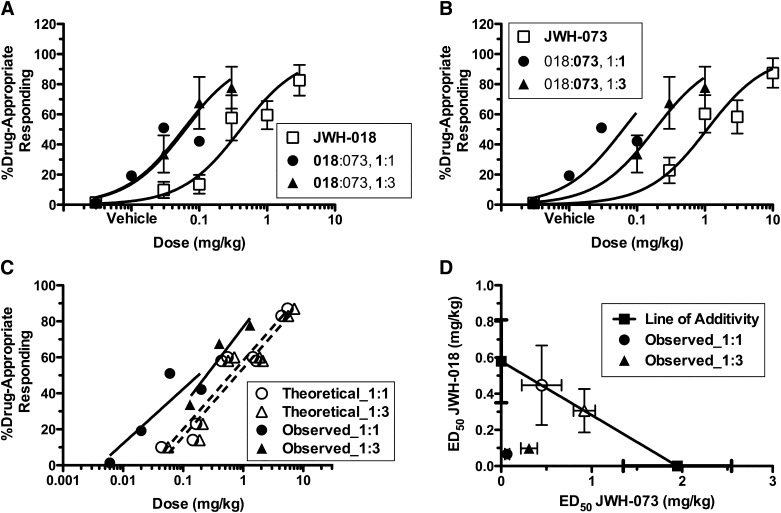
Synergistic effects of JWH-018 and JWH-073 in mice trained to discriminate 10 mg/kg Δ9-THC. Both JWH-018 (□) (A) and JWH-073 (□) (B) completely and dose-dependently substituted for Δ9-THC. Combining both synthetic cannabinoids in an ED50 equieffective constant dose ratio (1:3, JWH-018:JWH-073, ▴), as well as a 1:1 dose ratio (●), produced a leftward shift of both dose response curves, indicative of an increase in potency. Comparison of composite additive curves (C) shows a significant difference between y-intercepts of the theoretical and experimental data, indicating a synergistic drug–drug interaction. Isobolographic analysis of these data shows that the observed ED50 values fell well below the line of additivity (D), further suggesting that combining JWH-018 and JWH-073 produced synergy of substitution potency for Δ9-THC, compared with potency of substitution by each drug alone (P ≤ 0.05, n = 6–11).
Fig. 7.
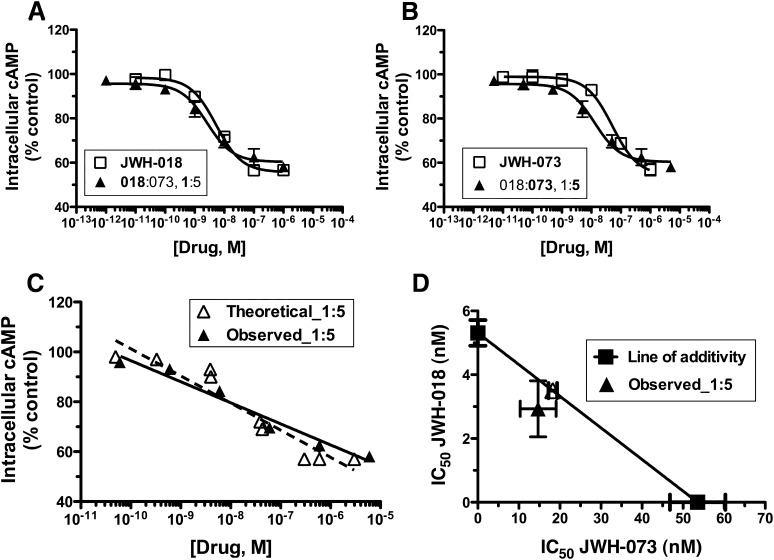
A combination of JWH-018 and JWH-073 additivly inhibits adenylyl cyclase activity by CB1Rs endogenously expressed in Neuro2AWT cells. JWH-018 (□) (A) and JWH-073 (□) (B) potently and efficaciously inhibited forskolin-stimulated intracellular cAMP accumulation in Neuro2AWT cells endogenously expressing CB1Rs. Combining JWH-018 and JWH-073 in a 1:5 constant concentration ratio (▲) produced additive inhibition of adenylyl cyclase activity, as shown in the composite additive curve comparison (C) and isobolographic representation (D). The dotted line in panel (C) emphasizes the effect level that is represented in panel (D). (F-ratio test, n = 3).
TABLE 1.
Expected and observed ED50 values of JWH-018 and JWH-073 combinations for substitution in the Δ9-THC discrimination assay
| Dose Ratio | Expected | Observed | ||
|---|---|---|---|---|
| Mean | S.E.M. | Mean | S.E.M. | |
| mg/kg | ||||
| 1:1 | 0.45:0.45 | 0.22:0.22 | 0.07:0.07 | 0.02:0.02 |
| 1:3 | 0.31:0.92 | 0.12:0.35 | 0.10:0.31 | 0.03:0.09 |
TABLE 5.
Expected and observed IC50 values of JWH-018 and JWH-073 combinations in the adenylyl cyclase activity assay in Neuro2AWT cells endogenously expressing CB1Rs
| Concentration Ratio | Expected | Observed | ||
|---|---|---|---|---|
| Mean | S.E.M. | Mean | S.E.M. | |
| nM | ||||
| JWH-018:JWH-073 | 3.50:18.3 | 0.20: 1.05 | 2.93:14.7 | 0.88:4.39 |
| 1:5 | ||||
| JWH-018:JWH-018 | 0.88:4.43 | 0.05:0.24 | 0.82:4.08 | 0.5:0.27 |
| 1:5 | ||||
Results
Combinations of JWH-018 and JWH-073 Produce a Robust Synergism for Generalization to Δ9-THC in Mice Trained to Discriminate 10 mg/kg Δ9-THC.
In mice trained to discriminate Δ9-THC (10 mg/kg i.p.), JWH-018 completely substituted for the training dose (up to 82.68% ± 10.19% drug-appropriate responding) with high potency (ED50 = 0.58 ± 0.23 mg/kg i.p., n = 6, Fig. 2A, □). JWH-073 also produced complete substitution with high potency (up to 87.41% ± 9.80% drug-appropriate responding, ED50 = 1.95 ± 0.60 mg/kg, n = 11, Fig. 2B, □). The equieffective dose ratio in the discrimination assay was thus determined to be approximately 1:3, JWH-018:JWH-073 (0.58 ÷ 1.95 = 0.30), based on the ED50 values determined separately for each drug. Doses of JWH-018:JWH-073 combinations ranging from 0.01:0.03 mg/kg to 3:10 mg/kg were administered in a 1:3 constant dose ratio combination and tested in the discrimination assay (Fig. 2, A and B, ▴). A second constant dose ratio combination (1:1) of these two SCBs was also administered (Fig. 2, A and B, ●). For each dose ratio, theoretical and experimental composite additive curves were plotted. Each experimental curve was shifted to the left of its respective theoretical curve (Fig. 2C), and the y-intercepts for the experimental versus theoretical curves differed significantly for both dose ratios (P = 0.02 and P = 0.01 for 1:3 and 1:1, respectively), indicating that JWH-018 and JWH-073 combinations produced a synergistic substitution for Δ9-THC.
As indicated by the isobolographic representation (Fig. 2D), the experimental potency values ± S.E.M. (ED50) did not overlap with the expected ED50 values ± S.E.M. for either dose ratio (Table 1), further suggesting a robust synergy of Δ9-THC substitution by these combinations.
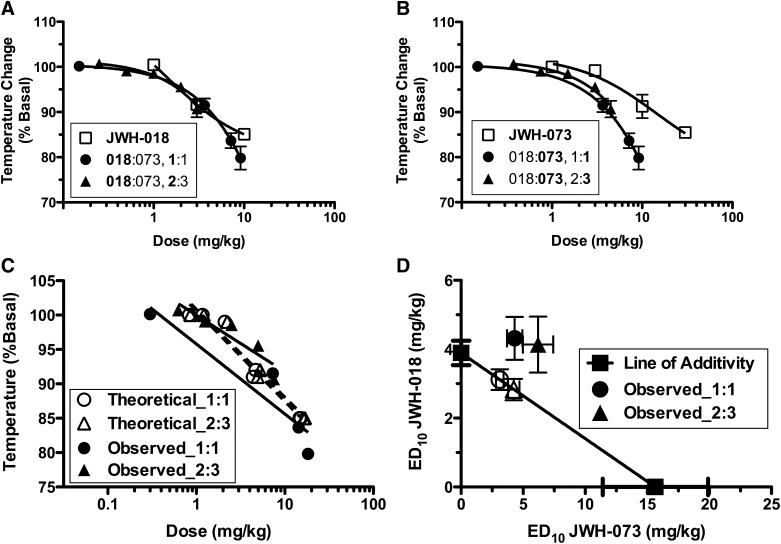
Combinations of JWH-018 and JWH-073 Are Either Synergistic or Additive for Analgesia, Depending on Constant Dose Ratio, and Additive for Hypothermia
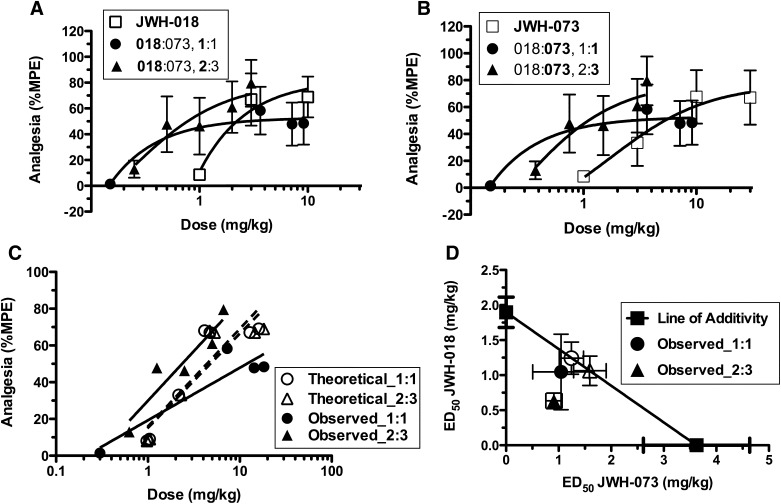
Analgesia was measured in mice as the latency of tail withdrawal from 55°C water, up to a 15-second cutoff time. Tail withdrawal latency was dose-dependently increased by JWH-018 and JWH-073, administered separately, with ED50 values of 2.52 ± 0.61 and 3.31 ± 0.76 mg/kg, respectively (n = 5, Fig. 3, A and B, □). JWH-018 and JWH-073 were combined and administered in an approximately equieffective constant dose ratio combination of 2:3 JWH-018: JWH-073, as well as a 1:1 constant dose ratio combination. Interestingly, the drug–drug interactions differed for these two dose ratios. The 1:1 constant dose ratio combination produced only an additive interaction; however, the y-intercept for the 2:3 constant dose ratio combination composite curve was significantly different (P = 0.04) and shifted leftward from the theoretical curve (Fig. 3C), indicating a synergistic effect for analgesia. As illustrated in the isobologram (Fig. 3D; Table 2), the experimental ED50 values ± S.E.M. for the 1:1, but not 2:3, constant dose ratio combination overlapped with the expected ED50 values ± S.E.M. This finding further indicated that, for this effect level, the 1:1 dose ratio was additive whereas the 2:3 dose ratio was synergistic.
Fig. 3.
Combinations of JWH-018 and JWH-073 produce either additive or synergistic analgesic effects, depending on the constant dose ratio combination employed. JWH-018 and JWH-073 dose-dependently increased the latency of mouse tail-withdrawal from 55°C water (A) and (B), indicating that both synthetic cannabinoids produce analgesia. Combining both cannabinoids in an equieffective constant dose ratio (e.g., 2:3, JWH-018:JWH-073) produced synergistic effects, as shown by comparison of the theoretical and experimental composite additive curves (C). However, combination of these drugs in a 1:1 dose ratio is only additive (C). Consistent with these observations, an isobologram of the ED50 values indicates that the observed and expected values overlap for the 1:1, but not the 2:3, dose ratio (D) (F-ratio test, P ≤ 0.05, n = 5–6). %MPE, percent maximal possible effect.
TABLE 2.
Expected and observed ED50 values of JWH-018 and JWH-073 combinations for tail withdrawal latency in the mouse tail-immersion assay
| Dose Ratio | Expected | Observed | ||
|---|---|---|---|---|
| Mean | S.E.M. | Mean | S.E.M. | |
| mg/kg | ||||
| 1:1 | 1.24:1.24 | 0.23:0.23 | 1.05:1.05 | 0.54:0.54 |
| 2:3 | 1.06:1.59 | 0.21:0.31 | 0.63:0.91 | 0.11:0.15 |
As expected of cannabinoid agonists, JWH-018 and JWH-073 administered alone dose-dependently lowered rectal temperature, with ED10 values of 3.89 ± 0.35 and 15.65 ± 4.25 mg/kg, respectively (Fig. 4, A and B, □). Administering JWH-018 with JWH-073 in 2:3 and 1:1 constant dose ratio combinations caused little or no shift in ED10 values, as shown in Table 3. The composite data comparison shows no significant difference between the theoretical and experimental curves for either dose ratio, indicating that no drug–drug interaction occurs for hypothermia (Fig. 4C). In contrast, the expected and experimental ED10 values ± S.E.M. do not overlap for either dose ratio (Fig. 4D; Table 3), and the experimental values are shifted above and rightward of the expected values. This indicates that, at the ED10 effect level, a combination of JWH-018 and JWH073 may have been antagonistic for induction of hypothermia.
Fig. 4.
A combination of JWH-018 and JWH-073 produces additive hypothermic effects. JWH-018 (A) and JWH-073 (B) robustly lowered body temperature. These two drugs combined in 1:1 and 2:3 constant dose ratio combinations were additive in producing hypothermia, as shown by composite additive curve comparison (C). An isobolograph of the ED10 effect level shows no overlap, suggesting potential antagonism at the ED10 effect level (D) (F-ratio test, n = 5–6).
TABLE 3.
Expected and observed ED10 values of JWH-018 and JWH-073 combinations for rectal temperature in mice
| Dose Ratio | Expected | Observed | ||
|---|---|---|---|---|
| Mean | S.E.M. | Mean | S.E.M. | |
| mg/kg | ||||
| 1:1 | 3.12:3.12 | 0.30:0.30 | 4.32:4.32 | 0.62:0.62 |
| 2:3 | 2.83:4.25 | 0.31:0.46 | 4.13:6.20 | 0.81:1.22 |
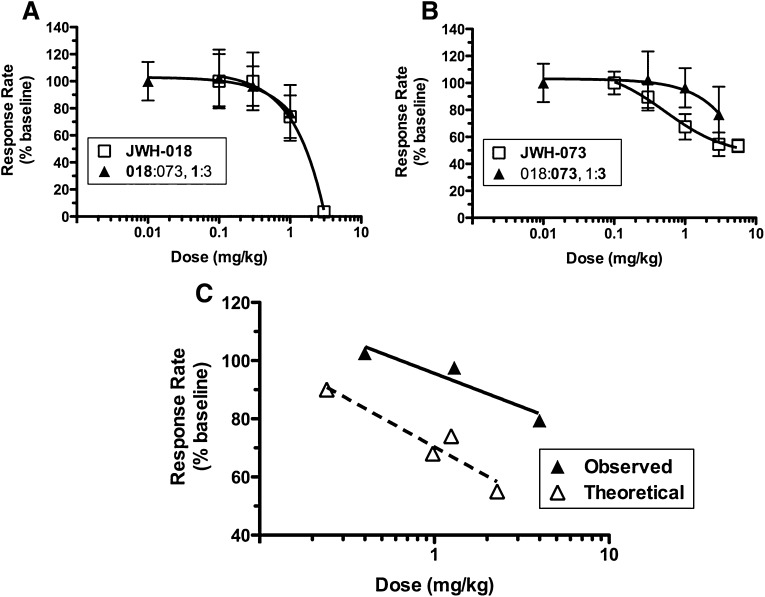
Combining JWH-018 and JWH-073 Produces a Subadditive Suppression of Food-Maintained Responding in Mice, a Model of Task Disruption.
To determine if the synergy observed with Δ9-THC discrimination and analgesia also occurs for the adverse effect of task disruption, the drug–drug interactions of JWH-018 and JWH-073 combinations were examined in an assay of food-maintained responding. As expected, JWH-018 and JWH-073 each potently suppressed response rates, with ED50 values of 1.43 ± 0.18 and 4.10 ± 0.97 mg/kg, respectively (Fig. 5, A and B, □). Interestingly, very little response rate suppression was induced by an approximately equieffective constant dose ratio of 1:3 JWH-018 and JWH-073 (1.43 ÷ 4.10 = 0.35), up to an ED25 + ED25 dosage (Fig. 5, A and B, ▴). As expected from this observation, the composite data comparison confirms sub-additivity of response rate suppression (P = 0.004; Fig. 5C). The lack of rate suppression in the combination experiments prevented the determination of new potency values; therefore, no isobologram or expected ED50 values are shown for the food-maintained response rate assay.
Fig. 5.
Coadministration of JWH-018 and JWH-073 elicits subadditive suppressive effects on food-maintained responding in mice. JWH-018 (A) and JWH-073 (B) dose-dependently suppressed food-maintained responding in mice. A 1:3 constant dose ratio combination of JWH-018 and JWH-073 produces sub-additive response rate suppression (C) (F-ratio test, P ≤ 0.05, n = 6).
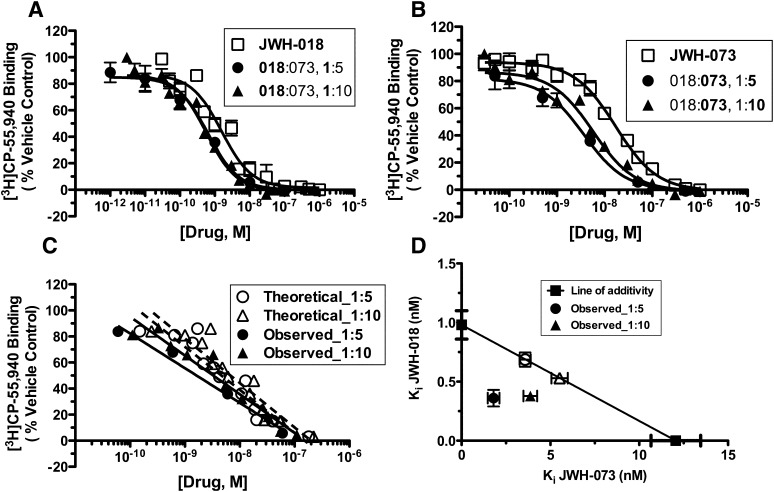
Combinations of JWH-018 and JWH-073 Produce Synergistic Displacement of the Radiolabeled Cannabinoid [3H]CP-55,940 from CB1Rs in Mouse Brain Homogenates.
We next examined the interactions of JWH-018 and JWH-073 in a competition receptor-binding assay. In agreement with previous reports (Brents et al., 2011), the radiolabeled, high-affinity CB1/CB2 cannabinoid agonist [3H]CP-55,940 [CB1R Kd = 0.38 nM, (Brents et al., 2011)] was completely displaced from CB1Rs in mouse brain homogenates by JWH-018 or JWH-073 tested separately, with each SCB exhibiting high affinity (Ki = 0.97 ± 0.12 nM and 12.06 ± 12.41 nM, respectively, n = 8–11, Fig. 6, A and B, □). The equieffective concentration ratio in this assay for these two SCBs was determined to be approximately 1:10 JWH-018:JWH-073 (0.97 ÷ 12.06 = 0.08); therefore, a 1:10 constant concentration ratio combination of JWH-018 and JWH-073 was examined in a dual-competitor competition receptor-binding assay. An additional 1:5 constant concentration ratio was also tested. Interestingly, results from these dual-competitor experiments revealed a slight leftward shift in the competition binding curve for each drug in the combination when compared with the single-drug experiments (Fig. 6, A and B, ▴ for 1:10, ● for 1:5). The composite curve comparisons indicate that this shift is statistically significant for both dose ratios (P = 0.03 for the 1:10 dose ratio and P = 0.003 for the 1:5 dose ratio; Fig. 6C). Additionally, an isobolographic representation of the Ki effect levels shows no overlap in expected and experimental values ± S.E.M., further suggesting synergistic interactions (Fig. 6D; Table 4).
Fig. 6.
Combinations of JWH-018 and JWH-073 produce synergistic competition for [3H]CP-55,940 binding to CB1Rs in mouse brain homogenates. Both JWH-018 (□) (A) and JWH-073 (□) (B) completely displaced [3H]CP-55,940 from CB1Rs with high affinity. The experimental composite curves quantifying the displacement of [3H]CP-55,940 by a 1:10 (▲) and a 1:5 (●) constant concentration ratio of JWH-018 and JWH-073 were significantly shifted left of their respective theoretical composite additive curves (C), indicating a synergistic interaction. Isobolographic representation (D) also suggests that both concentration ratios examined produced synergistic displacement of [3H]CP-55,940 binding (F-ratio test, P ≤ 0.05, n = 4–11).
TABLE 4.
Expected and observed Ki values of JWH-018 and JWH-073 combinations in the CB1R competition receptor binding assay in mouse brain homogenates
| Concentration Ratio | Expected | Observed | ||
|---|---|---|---|---|
| Mean | S.E.M. | Mean | S.E.M. | |
| nM | ||||
| JWH-018:JWH-073 | 0.69:3.58 | 0.06:0.31 | 0.36:1.80 | 0.07:0.33 |
| 1:5 | ||||
| JWH-018:JWH-073 | 0.53:5.52 | 0.04:0.46 | 0.38:3.87 | 0.03:0.39 |
| 1:10 | ||||
| JWH-018:JWH-018 | 0.09:0.89 | 0.01:0.09 | 0.12:1.19 | 0.04:0.38 |
| 1:10 | ||||
Control experiments were performed to verify that the dual-competitor competition receptor-binding assays could accurately distinguish drug–drug interactions from additive drug actions. By definition, a drug combined with itself should produce only additive effects (Wessinger and Evans, 1988); therefore, experiments combining JWH-018 with JWH-018 in a 1:10 combination were performed exactly as described for the JWH-018 plus JWH-073 combination experiments. As anticipated, composite additive curve comparison indeed demonstrated that the homogenous combination led to an additive interaction (Supplemental Fig. 2A). This finding validates the synergistic interaction observed in the competition receptor-binding assay when JWH-018 and JWH-073 are combined in 1:10 and 1:5 constant dose ratio combinations.
Combining JWH-018 and JWH-073 Produces Additive CB1R-Mediated Inhibition of Adenylyl Cyclase Activity in Neuro2AWT cells.
We next used Neuro2AWT cells to examine drug–drug interactions of JWH-018 and JWH-073 on adenylyl cyclase, a downstream effector of CB1Rs. As expected, JWH-018 and JWH-073, when examined separately, produced a potent (IC50 = 5.31 ± 0.40 nM and 53.54 ± 6.74 nM, respectively) and efficacious (43.39% ± 0.20% and 43.07% ± 2.75%, respectively) inhibition of intracellular adenylyl cyclase activity in Neuro2AWT cells (Fig. 7, A and B, □). Combining JWH-018 and JWH-073 in a 1:5 JWH-018:JWH-073 constant concentration ratio resulted in little to no shift in potency (Table 5) or efficacy (41.92% ± 1.84%) (Fig. 7, A and B, ▴). A comparison of the composite additive curves indicates no significant difference between the theoretical and experimental data (Fig. 7C), suggesting that JWH-018 and JWH-073 are additive for adenylyl cyclase inhibition. Isobolographic representation also suggests that the 1:5 constant concentration ratio combination of JWH-018 and JWH-073 is additive for inhibition of adenylyl cyclase activity, indicating that there is no interaction between the two drugs examined in this assay (Fig. 7D; Table 5). As expected, a control experiment combining JWH-018 and JWH-018 also indicated additivity (Supplemental Fig. 2B).
Discussion
The drug–drug interactions of two commonly coabused SCBs, JWH-018 and JWH-073, were examined with in vitro and in vivo assays for the first time in the present study. Because these two drugs are very similar in both chemical structure and biologic activity, their combination is expected to be additive for most or all shared effects. Instead, JWH-018 and JWH-073 can cause additive, synergistic, or antagonistic interactions, depending upon the endpoint examined and the drug dose ratio employed (Table 6).
TABLE 6.
Summary of the drug-drug interactions displayed by dose ratios of JWH-018 and JWH-073 for several cannabinoid effects, as measured using the composite additive curve comparison method (F-ratio test, P ≤ 0.05, n = 3–11)
| Assay | Drug Combos | Dose Ratio | Interaction |
|---|---|---|---|
| Δ9-THC discrimination | 018:073 | 1:1 | Synergism (P = 0.01) |
| 1:3 | Synergism (P = 0.02) | ||
| Response rate | 018:073 | 1:3 | Antagonism (P = 0.004) |
| Tail immersion | 018:073 | 1:1 | Additive |
| 2:3 | Synergism (P = 0.043) | ||
| Rectal temperature | 018:073 | 1:1 | Additive |
| 2:3 | Additive | ||
| Competition receptor binding | 018:073 | 1:5 | Synergism (P = 0.003) |
| 1:10 | Synergism (P = 0.025) | ||
| 018:018 | 1:10 | Additive | |
| Adenylyl cyclase | 018:073 | 1:5 | Additive |
| 018:018 | 1:5 | Additive |
The first significant finding of this study is the robust synergy with which combinations of JWH-018 and JWH-073 substitute for Δ9-THC in the drug discrimination assay in mice. The drug discrimination assay measures how readily test drugs substitute for a training drug in animals, which is typically an established drug of abuse. This test therefore may offer some insight into the potential abuse liability of the test drugs based on their similar subjective effects relative to the training drug. Unfortunately, because each SCB fully substituted for Δ9-THC independently, any observable synergistic efficacy in this assay was obscured by a ceiling effect. This prevented the evaluation of whether combining JWH-018 and JWH-073 increases the abuse liability of these substances by making them more Δ9-THC–like. Nonetheless, considerable synergy was observed for potency. Speculatively, users may coadminister multiple SCBs because of an apparent economic advantage over single-drug administration; that is, the total quantity of drug required for equivalent marijuana-like effects is lower when the drugs are combined. Because drug discrimination depends heavily on training drug and dose, the evaluation of possible synergistic efficacy and potential abuse liability could be accomplished in future studies by training with a JWH-018 and JWH-073 combination and testing whether each drug alone could fully substitute for the combination.
The therapeutic potential of cannabinoids would be improved if combinations of these drugs could produce a greater potency and efficacy for therapeutic effects relative to intoxicating effects. To examine this potential, the effects of various dose ratios of JWH-018 and JWH-073 on cannabinoid-mediated analgesia, as well as the off-indication effect of hypothermia, were measured. JWH-018 and JWH-073 combinations were synergistic for analgesia at a 2:3 constant dose ratio, additive for analgesia at a 1:1 constant dose ratio, and additive for hypothermia at both 1:1 and 2:3 ratios. The results indicate that by employing optimal dose ratios, greater analgesic potency can be produced without concurrently potentiating off-indication effects (e.g., hypothermia), suggesting that the therapeutic potential of cannabinoids can plausibly be enhanced. Furthermore, to model drug–drug interactions for another adverse effect of cannabinoids, we quantified the disruption of food-maintained responding in mice by JWH-018 and JWH-073 combinations. This assay is a surrogate for the variety of task-disruptive adverse effects reported by patients using cannabinoids therapeutically, including dizziness, drowsiness, and mental confusion. The assay is highly nonspecific because drug-induced disruption of food-maintained responding can be caused by a variety of factors, such as loss of appetite, stereotypies, or sedation. However, the test is extremely valuable because it mimics the cumulative net effect of multiple adverse effects that are most commonly reported by users of the clinically available CB1R agonists dronabinol (Marinol; AbbVie Inc., North Chicago, IL) and nambiximols (Sativex; GW Pharmaceuticals, Salisbury, UK): difficulty in performing simple, everyday tasks that could otherwise be performed with ease (Rog et al., 2007). Our study interestingly found that an equieffective combination of JWH-018 and JWH-073 actually antagonizes this experimental measure of task disruption produced by SCBs. These findings further support the suggestion that cannabinoid combinations may exhibit an improved therapeutic profile over mono-drug therapy.
Combinations of JWH-018 and JWH-073 synergistically displaced [3H]CP-55,940 from CB1Rs in the competition receptor-binding assay. This is intriguing because the three cannabinoids presumably share a common receptor-binding site, as evident from results of previous competition receptor-binding assays (Fig. 6, A and B) (Brents et al., 2011, 2012). As such, displacement of [3H]CP-55,940 from CB1Rs by JWH-018 and JWH-073 was anticipated to be merely additive. Instead, combining these two SCBs enhances the CB1R affinity of one or both drugs and possibly decreases the affinity of [3H]CP-55,940, resulting in the observed enhanced displacement of [3H]CP-55,940. This result is most consistent with these cannabinoids having separate binding sites on CB1Rs. These binding sites may overlap (Supplemental Fig. 3A), or may be partially or completely distinct (Supplemental Fig. 3, B and C, respectively), suggesting allosteric modulation. Determining how JWH-018 and JWH-073 interact with one another would further elucidate the receptor-level actions of these two drugs. For instance, JWH-018 displacing radiolabeled JWH-073 (or vice versa) in a competition receptor-binding assay would indicate that these two drugs share binding sites, or that there is significant overlap of the two binding sites.
We next investigated drug–drug interactions involving the downstream effector, adenylyl cyclase. Exposing Neuro2AWT cells to a 1:5 constant concentration ratio combination of JWH-018 and JWH-073 resulted in an additive inhibition of adenylyl cyclase activity by CB1Rs. Conclusions about drug combinations in the adenylyl cyclase assay should be tempered by the relatively low sample size (n = 3) and large variability from test to test. The present studies may have been underpowered to detect drug synergism. Importantly, the synergistic effects of this SCB combination may possibly occur but are mediated via CB1R-regulated intracellular effectors other than adenylyl cyclase. If so, certain SCB combinations might induce “ligand-biasing” or “agonist-directed trafficking of response” by synergistically shuttling the intracellular signal to other downstream effectors, such as the mitogen-activated protein kinase cascade (Bouaboula et al., 1995), G protein–coupled K+ channels (Baillie et al., 2013), or voltage-gated Ca2+ channels (Mackie and Hille, 1992). Examining the drug–drug interactions of JWH-018 and JWH-073 at these downstream effectors may elucidate the cellular mechanisms that underlie the in vivo synergistic effects of these two drugs observed in this study. For example, CB1R-mediated adenylyl cyclase inhibition is one mechanism contributing to analgesia (Welch et al., 1995), but spinal small conductance calcium-activated potassium channels and G protein–coupled inward rectifying potassium-2 channels also mediate cannabinoid-induced analgesia (Welch et al., 1995; Blednov et al., 2003) and thus may mediate JWH-018 and JWH-073 synergy of analgesia. Additionally, extracellular signal-regulated kinases, which are part of the mitogen-activated protein kinase cascade, are essential for CB1R-mediated reward processing (Brand et al., 2012; Guegan et al., 2013) and are involved in addiction (Valjent et al., 2000; Valjent et al., 2006). Therefore, synergy of extracellular signal-regulated kinase activation by JWH-018 and JWH-073 combinations would provide a cellular mechanism-based explanation for the motivation to co-abuse JWH-018 and JWH-073.
Coadministration of JWH-018 and JWH-073 produced differential drug–drug interactions that depend on both effect and the drug proportions in the combination (Table 6), suggesting that the mechanisms of these drug–drug interactions are complex and multileveled. The results of the present study prompt the cautious and speculative proposal of some potential hypotheses to explain the underlying causes of these differential drug–drug interactions. For instance, synergy occurred more readily and robustly for (a) effects that have high sensitivity for the drugs (i.e., effects for which the drugs are highly potent, such as drug discrimination), and (b) effects that are not strictly dependent on the drug(s) entering the central nervous system (e.g., analgesia, which has peripheral mechanisms). For example, in this study, the rank order of effect sensitivity for both JWH-018 and JWH-073 was Δ9-THC substitution >analgesia ≈ rate suppression > hypothermia. Notably, this rank order roughly follows the rank order of the drug–drug interactions observed in this study for these effects (synergy > additivity > antagonism). Because sensitivity of centrally mediated effects (i.e., Δ9-THC substitution and rate suppression) depends greatly on the entry of drugs into the brain, peripheral drug–drug interactions that reduce tissue concentrations of JWH-018 and JWH-073 (i.e., synergistic induction of metabolizing enzymes) may be of interest for future investigations to determine the mechanism of the differential drug–drug interactions observed in the present study. Specific enzymes that are involved in JWH-018 and JWH-073 metabolism include Cyp2C9 and 1A2 (Chimalakonda et al., 2012) or UGT1A1, 1A3, 1A9, 1A10, or 2B7 (Chimalakonda et al., 2011).
In conclusion, the differential drug–drug interactions exhibited by JWH-018 and JWH-073 in this study suggest that combining these two SCBs can result in unpredictable potencies for different effects; therefore, caution should be taken in coadministering JWH-018 and JWH-073, as well as other similarly structured SCBs. Furthermore, the most efficaciously analgesic doses of JWH-018 and JWH-073 were readily substituted for Δ9-THC in the discrimination assay, which suggests potential abuse liability at therapeutic doses. Despite this observation, this study demonstrates that the analgesic potential of phytocannabinoids and other low-efficacy cannabinoids, which exhibit fewer adverse effects and lower abuse potential than SCBs and commonly prescribed opioids, can likely be increased by optimizing cannabinoid combinations, possibly offering a superior alternative to current treatment strategies.
Supplementary Material
Abbreviations
- CB1R
cannabinoid 1 receptor
- CP-47,497
2-[(1R,3S)-3-hydroxycyclohexyl]-5-(2-methyloctan-2-yl)phenol
- CP-55,940
(−)-cis-3-[2-hydroxyl-4-(1,1-dimethylheptyl)phenyl]-trans-4-[3-hydroxyl-propyl] cyclohexan-1-ol
- Δ9-THC
Δ9-tetrahydrocannabinol
- FR
fixed ratio
- IBMX
3-isobutyl-1-methylxanthine
- JWH-018
(1-pentyl-1H-indole-3-yl)-1-naphthalenyl-methanone
- JWH-073
(1-butyl-1H-indole-3-yl)-1-naphthalenyl-methanone
- Neuro2AWT
Neuro2A wild-type
- SCBs
synthetic cannabinoids
- WIN-55,212-2
(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-napthalenylmethanone
Authorship Contributions
Participated in research design: Brents, Zimmerman, Prather, Fantegrossi.
Conducted experiments: Brents, Zimmerman.
Performed data analysis: Brents, Saffell, Prather, Fantegrossi.
Wrote or contributed to the writing of the manuscript: Brents, Zimmerman, Prather, Fantegrossi.
Footnotes
This work was supported by the University of Arkansas for Medical Sciences Translational Research Institute [Grant RR029884]; and the University of Arkansas for Medical Sciences Center for Translational Neuroscience [Grant RR020146].
This work was presented, in part, in L.K.B.'s doctoral dissertation defense as follows: Brents LK (2013) Active Metabolites and Drug-Drug Interactions of the Synthetic Cannabinoids JWH-018 and JWH-073 at the Cannabinoid 1 Receptor. Doctoral dissertation, University of Arkansas, Little Rock, AR.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Aung MM, Griffin G, Huffman JW, Wu M, Keel C, Yang B, Showalter VM, Abood ME, Martin BR. (2000) Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB(1) and CB(2) receptor binding. Drug Alcohol Depend 60:133–140 [DOI] [PubMed] [Google Scholar]
- Auwärter V, Dresen S, Weinmann W, Müller M, Pütz M, Ferreirós N. (2009) ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom 44:832–837 [DOI] [PubMed] [Google Scholar]
- Baillie GL, Horswill JG, Anavi-Goffer S, Reggio PH, Bolognini D, Abood ME, McAllister S, Strange PG, Stephens GJ, Pertwee RG, et al. (2013) CB(1) receptor allosteric modulators display both agonist and signaling pathway specificity. Mol Pharmacol 83:322–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Alva H, Harris RA. (2003) A pervasive mechanism for analgesia: activation of GIRK2 channels. Proc Natl Acad Sci USA 100:277–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Bourrié B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P. (1995) Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J 312:637–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand T, Spanagel R, Schneider M. (2012) Decreased reward sensitivity in rats from the Fischer344 strain compared to Wistar rats is paralleled by differences in endocannabinoid signaling. PLoS ONE 7:e31169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Gallus-Zawada A, Radominska-Pandya A, Vasiljevik T, Prisinzano TE, Fantegrossi WE, Moran JH, Prather PL. (2012) Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochem Pharmacol 83:952–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Reichard EE, Zimmerman SM, Moran JH, Fantegrossi WE, Prather PL. (2011) Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS ONE 6:e21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 [DOI] [PubMed] [Google Scholar]
- Chimalakonda KC, Bratton SM, Le VH, Yiew KH, Dineva A, Moran CL, James LP, Moran JH, Radominska-Pandya A. (2011) Conjugation of synthetic cannabinoids JWH-018 and JWH-073, metabolites by human UDP-glucuronosyltransferases. Drug Metab Dispos 39:1967–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimalakonda KC, Seely KA, Bratton SM, Brents LK, Moran CL, Endres GW, James LP, Hollenberg PF, Prather PL, Radominska-Pandya A, et al. (2012) Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/Spice: identification of novel cannabinoid receptor ligands. Drug Metab Dispos 40:2174–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresen S, Ferreirós N, Pütz M, Westphal F, Zimmermann R, Auwärter V. (2010) Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. J Mass Spectrom 45:1186–1194 [DOI] [PubMed] [Google Scholar]
- Galiègue S, Mary S, Marchand J, Dussossoy D, Carrière D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. (1995) Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem 232:54–61 [DOI] [PubMed] [Google Scholar]
- Guegan T, Cutando L, Gangarossa G, Santini E, Fisone G, Martinez A, Valjent E, Maldonado R, Martin M. (2013) Operant behavior to obtain palatable food modifies ERK activity in the brain reward circuit. Eur Neuropsychopharmacol 23:240–252 [DOI] [PubMed] [Google Scholar]
- Harris CR, Brown A. (2013) Synthetic cannabinoid intoxication: a case series and review. J Emerg Med 44:360–366 [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. (1990) Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA 87:1932–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson S, Ramsey J, King L, Timbers S, Maynard S, Dargan PI, Wood DM. (2010) Use of high-resolution accurate mass spectrometry to detect reported and previously unreported cannabinomimetics in “herbal high” products. J Anal Toxicol 34:252–260 [DOI] [PubMed] [Google Scholar]
- Huffman JW, Dai D, Martin BR, Compton DR. (1994) Design, synthesis, and pharmacology of cannabimimetic indoles. Bioorg Med Chem Lett 4:563–566 [Google Scholar]
- Lindigkeit R, Boehme A, Eiserloh I, Luebbecke M, Wiggermann M, Ernst L, Beuerle T. (2009) Spice: a never ending story? Forensic Sci Int 191:58–63 [DOI] [PubMed] [Google Scholar]
- Mackie K, Hille B. (1992) Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci USA 89:3825–3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, Ginsburg BC, Lamb RJ. (2008) Cannabinoid agonists differentially substitute for the discriminative stimulus effects of Delta(9)-tetrahydrocannabinol in C57BL/6J mice. Psychopharmacology (Berl) 198:487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir A, Obafemi A, Young A, Kane C. (2011) Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics 128:e1622–1627 [DOI] [PubMed] [Google Scholar]
- Rog DJ, Nurmikko TJ, Young CA. (2007) Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open-label, 2-year extension trial. Clin Ther 29:2068–2079 [DOI] [PubMed] [Google Scholar]
- Schneir AB, Cullen J, Ly BT. (2011) “Spice” girls: synthetic cannabinoid intoxication. J Emerg Med 40:296–299 [DOI] [PubMed] [Google Scholar]
- Seely KA, Lapoint J, Moran JH, Fattore L. (2012) Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry 39:234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely KA, Prather PL, James LP, Moran JH. (2011) Marijuana-based drugs: innovative therapeutics or designer drugs of abuse? Mol Interv 11:36–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons J, Cookman L, Kang C, Skinner C. (2011) Three cases of “spice” exposure. Clin Toxicol (Phila) 49:431–433 [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. (2000) Drug synergism and dose-effect data analysis (Stern B. ed) pp 58–87, Chapman & Hall/CRC, Boca Raton, FL [Google Scholar]
- Valjent E, Corbillé AG, Bertran-Gonzalez J, Hervé D, Girault JA. (2006) Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci USA 103:2932–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. (2000) Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci 20:8701–8709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann RE, Warner JA, Bushell K, Huffman JW, Martin BR, Wiley JL. (2009) Discriminative stimulus properties of delta9-tetrahydrocannabinol (THC) in C57Bl/6J mice. Eur J Pharmacol 615:102–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch SP, Thomas C, Patrick GS. (1995) Modulation of cannabinoid-induced antinociception after intracerebroventricular versus intrathecal administration to mice: possible mechanisms for interaction with morphine. J Pharmacol Exp Ther 272:310–321 [PubMed] [Google Scholar]
- Wessinger WD, Evans EB.(1988) Modeling Multiple Agent Interactions in Behavioral Pharmacology. J Am Coll Toxicol 7:953–962 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.