Abstract
The pituitary gland has a role in puberty, reproduction, stress-adaptive responses, sodium and water balance, uterine contractions, lactation, thyroid function, growth, body composition and skin pigmentation. Ageing is marked by initially subtle erosion of physiological signalling mechanisms, resulting in lower incremental secretory-burst amplitude, more disorderly patterns of pituitary hormone release and blunted 24 h rhythmic secretion. Almost all pituitary hormones are altered by ageing in humans, often in a manner dependent upon sex, body composition, stress, comorbidity, intercurrent illness, medication use, physical frailty, caloric intake, immune status, level of exercise, and neurocognitive decline. The aim of this article is to critically discuss the mechanisms mediating clinical facets of changes in the hypothalamic–pituitary axis during ageing, and the extent to which confounding factors operate to obscure ageing effects.
Introduction
Ageing is marked by subtle incremental changes in all biological systems, including endocrine ensembles. A central regulator of endocrine axes is the hypothalamic–pituitary unit, comprising brain neurotransmitters classified as releasing and inhibitory factors that drive or restrain pituitary hormone synthesis and secretion. The mechanism by which ageing influences pituitary function is complex. Comorbidities and adaptations that accompany ageing strongly modify pituitary secretion.
In particular, the effects of age on endocrine axes depend upon hormone type, inhibitor or stimulus tested, concomitant illness, underlying stress, body composition and sex Supplementary Table online). This critical Review highlights such interactions, thus enabling clinical scientists to parse the implications of endocrine measurements in various clinical contexts in ageing individuals.
TSH axis in ageing
The TSH–thyroidal axis comprises an array of signalling centres and corresponding signals. Key components are hypothalamic TSH-releasing hormone (TRH) neurons in the paraventricular nuclei, pituitary thyrotropes (TSH-secreting cells) and thyroid hormones (T3 and T4), along with monocarboxylate T3 transporters, thyroxine binding globulin (TBG) and pre-albumin. Key mechanistic changes occur in the TSH axis as humans age (Figure 1). Whereas ageing was construed formerly as a threshold event (for example, >60 years), data in the past decade establish that ageing-related hormonal changes are continuous variables with rather distinct slopes in relation to age. Moreover, sex, exercise, fasting, concomitant illness, medications, iodine availability, brief light exposure, sleep stage, exercise training and the assay platform used to measure TSH also influence absolute TSH values. In relation to sex differences, a tendency for baseline (unstimulated) TSH concentrations and thyroid autoantibodies to rise with age is more evident in women,1–4 whereas a decrease in baseline, overnight and TRH-stimulated TSH release with age is better demonstrated in men.5–7 These collective factors contribute to the debate about absolute TSH reference ranges in the elderly. Therefore, more precise studies are needed to separate out the effects of comorbidities from those related to ageing.
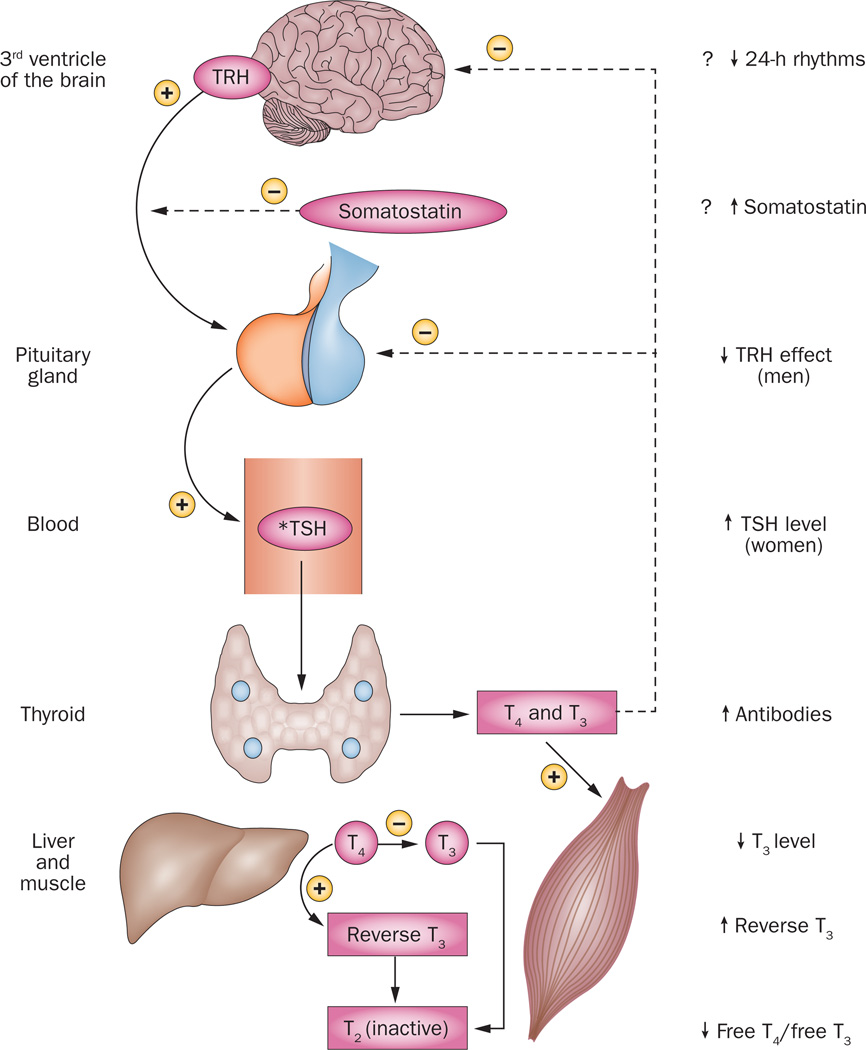
Figure 1. Human thyrotropic axis in ageing.
The thyrotropic axis exhibits multiple alterations in healthy ageing individuals, at the levels of the brain, pituitary and thyroid gland, as well as liver and muscle in which T4 is converted to T3. Key components include TRH secretion by the paraventricular nuclei surrounding the third ventricle of the brain. In turn, TRH activates pituitary TSH synthesis and secretion. By contrast, somatostatin inhibits TSH release, albeit not TSH synthesis. TSH acts upon thyroid epithelial cells to promote both synthesis and release of T3 and T4, which enter the blood and act upon peripheral tissue like bone, adipose tissue, muscle and liver. Peripheral tissues also inactivate T4 by converting it to reverse T3, and inactivate T3 by converting it to T2. Not shown is blood thyroid hormone binding globulin, which is controlled by multiple systemic factors, thus directing the local availability of free T4 and free T3. Upward arrows denote increases in the signalling factor or effector with aging, and downward arrows denote decreases observed with ageing. The asterisk denotes that the hormone value is increased by obesity, and decreased by systemic illness and undernutrition, especially in ageing individuals. The plus and minus signs denote stimulation and inhibition, respectively. The interrupted lines signify negative feedback or inhibition, and the continuous lines positive feedforward or stimulation. The hormone value is increased by obesity, and decreased by systemic illness and undernutrition, especially in ageing individuals. Abbreviation: TRH, TSH-releasing hormone.
Reduced serum T3 concentrations occur in ageing individuals of both sexes. Clinical uncertainty remains concerning the extent to which low T3 levels in ageing contribute to cognitive decline or depression.8–11 Serum T3 levels are especially in low in patients with organ failure, undernutrition, systemic inflammation or debilitating illness. Increased reverse T3 levels emerge particularly when energy intake is restricted.12 Reductions in mean and 24 h rhythmic (nycthemeral) TSH concentrations in small cohorts become prominent after the eighth decade of life.5 Typically, serum T4 levels are preserved in healthy ageing adults whilst free T3 levels fall, yielding reduced free T3:free T4 ratios. Hypothesized mechanisms mediating these changes include augmented inhibition by T3 of, or diminished hypothalamic TRH drive to, TSH output.13
A near consensus exists that, overall, TSH levels tend to rise with age.14–18 However, confounding issues in elderly adults that can inhibit TSH secretion include decreased exercise; reduced caloric intake;19 muted symptoms and signs of deficient or excessive T3:T4 production; exposure to glucocorticoids,20 iodine or L-dopa; supervening systemic illness;21 traumatic brain injury;22 inanition; and psychiatric depression. These comorbidities might mask a rise in TSH with age. Conversely, chronic fatigue syndrome, morbid obesity, type 2 diabetes mellitus, use of certain drugs and autoimmune disease2–4 might potentiate TSH secretion, thus potentially heightening an age-related populational tendency for TSH to increase.
Clinical implications for patient care include a lower threshold for evaluating the thyrotropic axis in ageing individuals than young patients. When an individual measurement is borderline, sequential TSH and free T4 measurements are helpful.21,23 However, the clinical pathological significance of minimal (<30%) laboratory deviations is quite uncertain. From a technical standpoint alone, the least significant change in TSH assays can approach 40%, and in free T4 assays, this change can approach 15% at 90% confidence intervals. In absolute terms, most thyroidologists agree that a confirmed serum TSH level of >10 mIU/l should prompt T4 replacement therapy. By contrast, therapy in patients with a TSH level >4 mIU/l but <10 mIU/l without demonstrable symptoms or signs of hypothyroidism (including high-titre thyroid antibodies and a low free T4 level) is controversial. The clinical implications of this subclinical hypothyroid state remain uncertain. The author suggests that ageing patients who have neither symptoms nor signs of thyroid disease can be re-assessed in 2–4 months.24
A common scenario in elderly patients is a normal free T4 level and low T3 level with low-normal but detectable TSH level of >0.1 mIU/l but <4 mIU/l. This laboratory triad is likely to reflect a combination of medication and illness-associated inhibition of both TSH secretion and 5’-deiodinase activity, which could normalize over 3–12 months.25 Proof that the low-normal TSH represents sick euthyroidism is retrospective, by showing normalization of TSH after recovery from acute illness, restoration of caloric intake, or reduction in cytokines, glucocorticoids, dopamine and other drugs. However, radiation-induced, traumatic and vascular hypothalamic injury, pituitary adenoma, metastatic cancer, hypophysitis, craniopharyngioma, granulomatous processes, infection, and other aetiologies of a suppressed TSH thyroidal axis must not be overlooked, requiring evaluation by an endocrinologist and relevant treatment and follow-up.
An undetectable TSH level (<0.1 mIU/l) suggests possible hyperthyroidism at any age. Further assessment includes measurements of both free T3 and free T4, as untreated hyperthyroidism, especially in elderly people, increases the risk of heart failure,26 cardiovascular disease mortality27 and bone fractures.28 Challenges to recognizing the diagnosis of hyperthyroidism in ageing individuals are that other causes of hyperthyroid-like symptoms and signs, such as fatigue, sleeplessness, proximal muscle weakness, osteopaenia, neurocognitive loss and atrial fibrillation, are more prevalent in ageing individuals even without a suppressed TSH level and elevated free T4 concentrations.8,28 Thus, close follow-up with clinical and biochemical re-assessment is essential.
What remains difficult to investigate is the exact extent to which minimal thyroid axis dysfunction contributes to the ageing phenotype and/or to comorbidity (or even mortality) in ageing individuals. Given this uncertainty, and the finite, albeit small, risk associated with treatments for thyroid disorders, consensus treatment guidelines should be helpful to practitioners.
HPA axis in ageing
The stress-responsive hypothalamic–pituitary–adrenal (HPA) axis is a vital neuroendocrine system regulating cognition, well-being, memory, behaviour, appetite, work capacity, inflammation, glucose metabolism, adipose tissue, muscle and skeletal mass, blood pressure, insulin sensitivity, immune responses, and water and electrolyte balance. The HPA axis comprises the hippocampus and frontal cortex, catecholaminergic tracts, hypothalamically released CRH (corticotropin-releasing hormone) and the antidiuretic hormone arginine vasopressin (AVP), pituitary corticotropes secreting adrenocorticotropic hormone (ACTH), and cortisol secreted from the adrenal zona fasciculata cells. No part of this dynamic array operates or functions alone. Cortisol is a potent agonist of glucocorticoid receptors and partial agonist of mineralocorticoid receptors, and the activation of these receptors mediate the tissue effects of cortisol, including negative feedback onto brain CRH and AVP neurones and onto pituitary ACTH secretion. Extensive studies reveal that the effects of age on the HPA axis are modulated by obesity and sex and the type of stress activating the system (Figure 2).
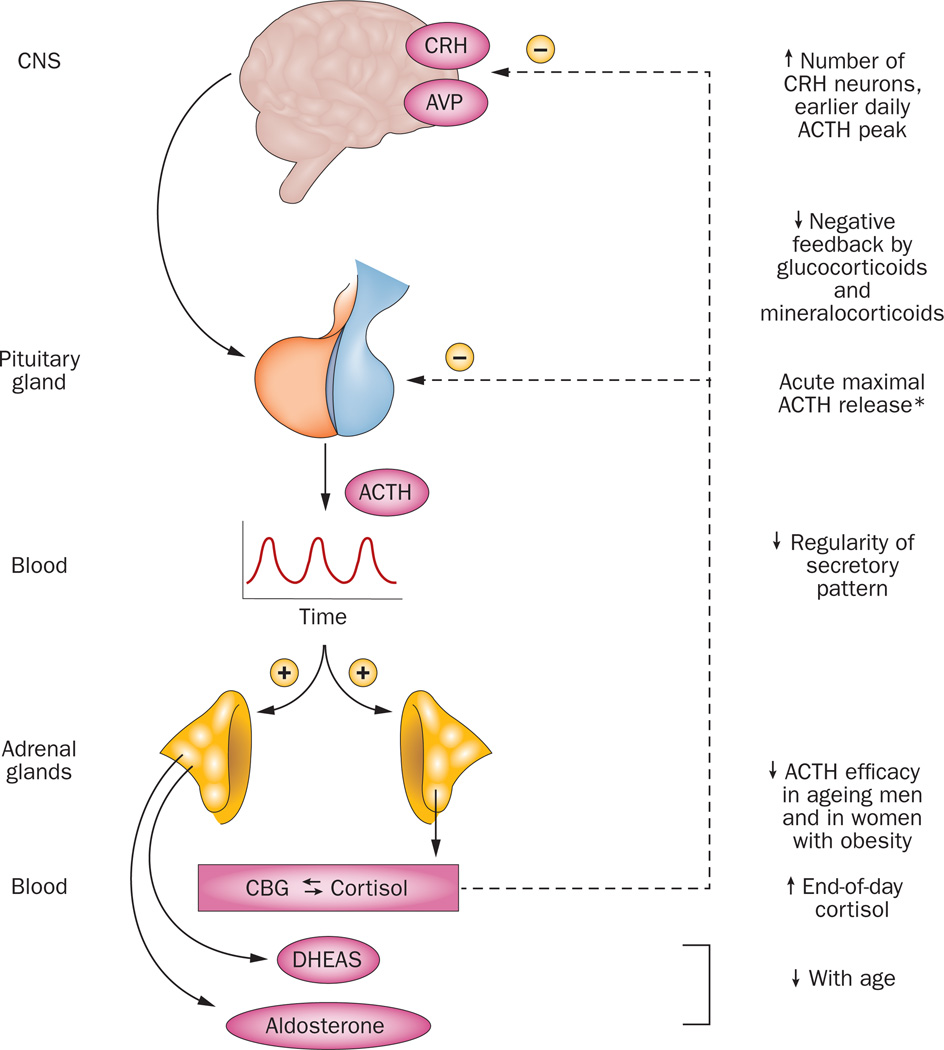
Figure 2. Adrenocorticotropic hormone secretion in ageing individuals.
The central nervous system (CNS) controls pituitary ACTH secretion into blood by synthesizing and releasing CRH and AVP. The CNS and pituitary are under negative-feedback control or inhibition by glucocorticoids and/or mineralocorticoids. However, ACTH release is decreased in aging after stimulation with CRH, a cholinergic agonist, or hypertonic saline. Conversely, ACTH release is increased in aging individuals after serotonin-receptor 1A stimulation in women and after mineralocorticoid-receptor blockade in men. Upward arrows denote feedforward drive, and downward arrows feedback inhibition. Up and down arrows indicate increases and decreases in the pathway or regulatory factor with age. The asterisk signifies that maximal ACTH release is normal after surgery, during critical illness, during hypoglycemia, and after administration of metyrapone. Abbreviations: ACTH, adrenocorticotropic hormone; AVP, arginine vasopressin; CBG, corticosteroid binding protein; CNS, central nervous system; CRH, corticotropin-releasing hormone; DHEAS, dehydroepiandrosterone sulphate.
Certain amplifiers of ACTH and/or cortisol secretion appear to be more effective in ageing than young adults, including cholinergic agonists, CRH and/or ADH injection and hypertonic saline infusion.29–31 At the mechanistic level, feedback disinhibition using a mineralocorticoid receptor antagonist also augments ACTH release more in ageing than young individuals.32 Other factors that elicit ACTH secretion are equally effective in young and ageing volunteers, namely major surgery, insulin-induced hypoglycaemia, feedback disinhibition,33–35 and opiate-receptor antagonism.33,35 Ipsapirone (a serotonin receptor agonist) stimulates more ACTH release in older women than older men,36 as do human CRH and naloxone.29 Feedback suppression via glucocorticoid receptor agonists or a mineralocorticoid receptor agonist is less effective in ageing than young volunteers.37–39 Controversy exists regarding unstressed mean ACTH concentrations, which are reportedly unchanged across the age span of 20–100 years,35,40 decreased with age or increased with age.41 Comorbidities, assay nonuniformities and sampling inconsistencies may, in part, explain the discrepant reports. Similar uncertainty applies to plasma, urinary or salivary (free) cortisol concentrations in ageing individuals.42,43 Intra-abdominal adipose tissue mass is a major confounder of ACTH and/or cortisol output with high intra-abdominal adipose tissue mass predicting increased sympathetic outflow, cortisol production and cortisol inactivation, and (in women) increased mean ACTH concentrations.42,44,45
The 24 h (circadian) rhythm of ACTH and cortisol concentrations is blunted in absolute amplitude (the algebraic difference between peak and nadir) owing to high late-day cortisol nadirs in elderly individuals.40,43 Additionally, the timing of the nycthemeral ACTH and cortisol peak and nadir is earlier in the day (by about 2 h) in ageing compared with young adults. In ageing rats, high nadirs reflect attenuation of negative feedback, arising from decreased glucocorticoid receptor and mineralocorticoid receptor expression in the brain,46 and activation of CRH and AVP neurons.47 Ageing-related changes in the HPA axis are more prominent in women than men, and in patients with Alzheimer disease or major depression.29 Diabetes mellitus, inflammation, hypertension, obstructive sleep apnoea and genetic polymorphisms can also potentiate ACTH and cortisol release.48 Confounding factors are anxiety, trauma, stress, illness, sleep loss, inanition, systemic disease, day-to-night schedule,49 and medications,50 such as benzodiazepines, antidepressants, synthetic progestins and glucocorticoids. In addition, interactions between the pituitary gland and the immune system constitute a growing focus in ageing research.51–53
Some clinical implications follow from the changes in the HPA axis that occur in the ageing population. First, valid assessment of ACTH regulation with age must include matching the patient for sex, mental health status, obesity, medication exposure, comorbidities, systemic illness and time of day. Second, higher glucocorticoid doses are not required due to ageing per se.33,54 Third, obesity often elevates urinary but not plasma free cortisol levels.42,44 Fourth, ACTH deficiency does not cause low levels of adrenal dehydroepiandrosterone (DHEA) in the ageing population.55 A broad unresolved issue is that comorbidities lead to conflicting findings in terms of the influence of ageing on the HPA axis.
Arginine vasopressin in ageing
AVP is secreted by hypothalamic neurons, and acts locally upon pituitary cells to potentiate CRH-stimulated ACTH secretion and systemically upon kidney tubules to trigger water reabsorption, thereby concentrating (increasing the osmolality of) urine. The mechanisms of AVP regulation have been extensively studied. Deficient AVP secretion or action, albeit not a cause of ACTH deficiency when CRH is present, results in diabetes insipidus with hypernatraemia and inappropriately dilute urine, unless thirst mechanisms compensate for water loss. Conversely, an excess of AVP secretion or of water intake causes (dilutional) hyponatraemia with attendant neuropsychological signs and symptoms.56–58 Disorders of sodium and water balance tend to be more frequent, less well-defined aetiologically, and are more often multifactorial and more severe in ageing individuals than in young adults.58,59 The thesis is that age reduces homeostatic adjustments to both low and high fluid or salt intake.60
In the ageing population, deficits exist in renin–aldosterone secretion,59,61 plasma volume, thirst,60 baroreceptor reflexes,62 expression of the AVP receptor and aquaporin 2,63 and hypothalamic osmoregulation (Figure 3). By contrast, exaggerated AVP (and possibly atrial natriuretic peptide) release occurs during experimentally imposed hypertonicity, water deprivation, ethanol exposure or volume contraction in humans.60,64 Thus, the risks of both low and high sodium concentrations are increased in elderly individuals, especially in relation to anaesthesia, surgery, coma, acute myocardial infarction or stroke, fever, glycosuria, diarrhoea, diuresis, emesis, burn injury, blood loss and acute tubulopathies.
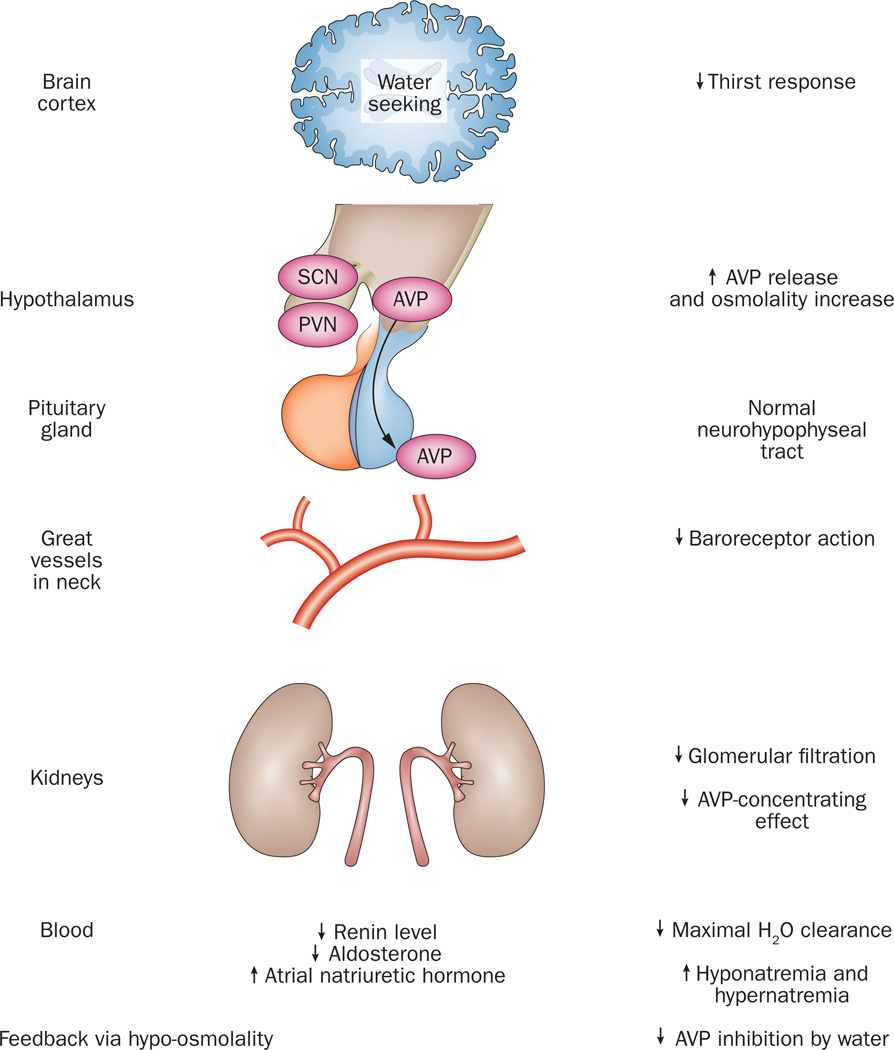
Figure 3. Effects of age on water balance.
Age influences virtually all known physiological steps in the pathway of cortical and hypothalamic regulation of thirst and water balance, including posterior pituitary secretion of AVP, baroreceptor action mediated by pressure sensors in great vessels in the neck, concentrating capacity of renal tubules, and coregulators of salt and water balance, such as renin, aldosterone, and atrial natriuretic hormone. Ageing seems to blunt water-seeking behaviour and augment brain output of AVP. Ageing is associated with a decrease in plasma volume of approximately 7%, and a decrease in plasma volume of approximately 10%. Abbreviations: AVP, arginine vasopressin; PVN, paraventricular nucleus; SCN, suprachiasmatic nucleus.
The clinical syndrome of inappropriate (autonomous) antidiuretic hormone release (SIADH), which seems more common in elderly cohorts, can cause severe dilutional hyponatraemia by impairing free water clearance.65 Causes of SIADH include medications such as cyclophosphamide and anaesthetics, neoplasms, pulmonary processes and intracranial lesions.57,66 Less severe hyponatraemia than that in SIADH occurs with elevated, but nonautonomous, AVP secretion in hypothyroidism,67 glucocorticoid deficiency, hypovolaemic and hypoperfusional states, and salt-wasting disorders.58,68 Thus, condition-specific therapy entails replacing T4 and/or cortisol (endocrine deficiency), enhancing perfusion or repleting salt (low-volume state), and restricting water intake or blocking AVP action (euvolaemic state).58,65,68 Treatment goals in elderly patients with SIADH or hyponatremia include averting and/or ameliorating signs and symptoms of hyponatraemic encephalopathy.58,68 Morbidity and mortality in hyponatraemic individuals are due to cerebral vasoconstriction, tissue hypoxia, brain oedema, and/or the underlying disease.57,66 Inasmuch as rapid reversal or overcorrection of hyponatraemia or hypernatraemia carries the risk of iatrogenic neurological injury at any age,69 repeated in-hospital assessments and attendant fluid adjustments are particularly important in frail elderly patients.69
GH–IGF-I axis in ageing
Clinical features of organic or structural growth hormone (GH) deficiency are less vivid in elderly individuals, who already often have decreased insulin-like growth factor I (IGF-I) levels, increased waist to hip ratios, a reduced quality of life, impaired glucose metabolism, unfavourable lipid profiles, and reductions in muscle and bone mass, physical endurance and fitness.70 Aged adults secrete less GH during fasting, exercise or sleep and in response to nearly all secretagogues than young individuals.71 However, in response to insulin-induced hypoglycaemia or to the triple combination of L-arginine plus GH-releasing hormone (GHRH) plus ghrelin (the naturally occurring form of GH-releasing peptides) secretion of GH in the elderly is the same as that in young individuals (Box 1).72,73 Sex, body composition and sex steroids further determine 24 h GH secretion in elderly individuals. Specifically, GH output declines more with ageing in young men than premenopausal women, and more when ageing is accompanied by abdominal visceral adiposity, hyperinsulinaemia; and oestrogen and testosterone (acting via oestrogen) deprivation.70,74–76
Box 1 Changes in human GH–IGF-I axis in ageing.
decrease in pulsatile GH secretion via decrease burst mass
decrease in IGF-I, increase IGFBP-1, increase IGFBP-3
preserved hepatic IGF-I response to exogenous GH78
decrease in GH accompanies and/or causes metabolic syndrome X
decrease in lean body mass (muscle bulk and bone mineral density)
hypopituitarism still occurs in the elderly
Abbreviations: GH, growth hormone; IGF-I, insulin-like growth factor I; IGFBP, insulin-like growth factor binding protein.
The age-related decrement in pulsatile GH secretion, and thereby IGF-I production, in ageing is due to smaller GH pulses, which reflect diminished secretory-burst mass with no change in pulse frequency.75,77 Mechanistically, GH secretory-burst mass is under the hypothalamic control of somatostatin (an inhibitory peptide), GHRH and possibly GHRP/ghrelin, which are both stimulatory, and under systemic negative feedback control by circulating GH, IGF-I and free fatty acids, and positive feedback control by gastric ghrelin.70 Thus, the reported capability of L-arginine, which is a somatostatin antagonist, combined with both GHRH and GHRP/ghrelin to normalize GH secretion acutely suggests attenuation of hypothalamic drive to pituitary somatotropes in ageing.73 However, the action of GH on the liver to generate IGF-I is preserved in ageing.78 Whether the effect of GH on the immune system, brain, bone, muscle and adipose cells change with age is unknown. This issue is an important one to resolve, as patients are concerned about both lifespan and ‘healthspan’.79
The clinical impact of age-related hyposomatotropism (decreased GH and IGF-I availability) has been explored indirectly by assessing the effects of short-term GH supplementation in ageing individuals. These investigations are deemed experimental, as relative GH deficiency in the elderly does not necessarily constitute a disease.79 Consistent effects of GH repletion in structural GH deficiency are 2–3 kg loss of total-body fat (and especially visceral fat), 1–2 kg gain of lean body mass (water, bone and muscle), and a reduction of LDL cholesterol levels and an elevation of IGF-I levels.70 In elderly GH-treated patients, balance, strength, coordination and endurance are often not improved compared with untreated, age-matched individuals.80,81 At any age, adverse effects such as oedema, heart failure, carpal tunnel syndrome, intracranial hypertension, myalgia, arthralgia or gynecomastia and glucose intolerance, are more frequent at high GH doses, prompting IGF-I-targeted GH dosing.82,83 Whether the risk of neoplasia increases with long-term supplementation with GH and IGF-I in ageing individuals, as suggested in animal models,79 is not known. Moreover, GH treatment after the eighth decade of life has not been assessed in any study. In view of these deficiencies in the field, supplementation with GH and IGF-I directly or indirectly via administration of GHRH and/or GHRP in healthy elderly adults without hypopituitarism cannot be recommended at present.84,85
Prolactin secretion in ageing
Isolated hypoprolactinaemia is rare, because prolactin deficiency usually signifies panhypopituitarism.86 By contrast, multiple factors elicit hyperprolactinaemia. Factors that stimulate prolactin secretion acutely include psychological and physical stress, breast stimulation, hypoglycaemia, certain amino acids, oestrogen, dopamine-2-receptor blockers, sexual activity, hyperthermia, TRH infusion, food intake, sleep and exercise.87–89 Pituitary stalk injury, end-stage renal disease, primary hypothyroidism, prolactinoma, pregnancy, visceral obesity and high leptin levels increase prolactin concentrations chronically.90–92
Prolactin is released approximately 50% in pulses and 50% tonically. Both modes of secretion show 24 h rhythmicity with higher output at night than during the day.93 In individuals <50 years of age, prolactin concentrations are higher in women than men.88,94 Prolactin secretion during the night-time falls by about 40% after menopause, but declines less markedly in ageing men (Figure 4).95 Results of clinical studies examining mechanisms of altered prolactin secretion in ageing indicate that TRH-stimulated prolactin secretion may decrease with age,92,96 prolactin secretion patterns become more irregular with age in men, and oestrogen and adiposity accentuate pulsatile prolactin production in ageing individuals.87,91,94 Potential mechanisms involved in changes in prolactin secretion during ageing include increased dopamine inhibition, reduced prolactin releasing-factor stimulation and/or increased adipokine inhibition of lactotropes.95
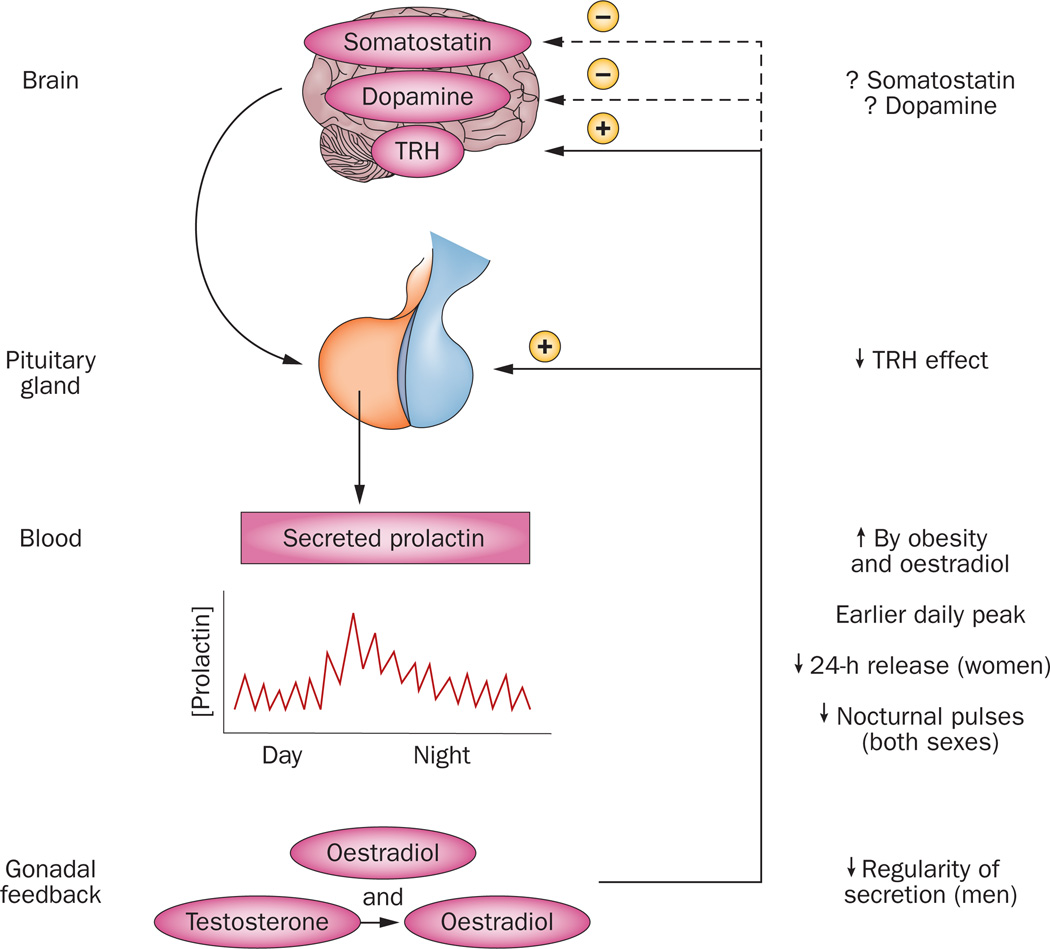
Figure 4. Age-related effects on prolactin secretion.
An ensemble comprising the brain, pituitary gland, blood, circulation, and gonadal feedback directs prolactin secretion. In general, prolactin secretion decreases with age (downward arrows), but this decrease is opposed by obesity and oestrogen (upward arrow). Precisely how somatostatin and dopamine, key negative regulators of prolactin secretion, change with age is less clear in the human, as denoted by the interrogative marks. Negative feedback is shown by the interrupted arrows and minus sign, and positive feedforward by oestrogen through the continuous arrow and positive sign. Abbreviation: TRH, TSH-releasing hormone.
A clinical implication in postmenopausal compared with premenopausal women is that generally lower prolactin concentrations should be interpreted in light of menopausal hypo-oestrogenaemia. Lower prolactin might favour longevity, given a possible increase in breast-cancer risk in ageing women in the upper versus lower quartile of prolactin concentrations,97 albeit not in frankly hyperprolactinaemic women.98 Technical caveats with regard to the findings of prolactin assays are pseudohyperprolactinaemia due to prolactin macroaggregates,99 and pseudohypoprolactinaemia caused by very high prolactin levels or heterophile antibodies.100
LH and FSH secretion in ageing
The principal known function of the gonadotropins is maintenance of gonadal steroidogenesis (oestrogen, progesterone and testosterone secretion) and gametogenesis (sperm and egg maturation). Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) rise gradually in ageing men, putatively reflecting reduced secretion of androgen and oestrogen from Leydig cells and decreased secretion of inhibin B from Sertoli cells.101 However, spermatogenesis is relatively preserved in ageing.102 FSH concentrations begin to increase in late premenopausal women owing to decreased ovarian follicle reserve, inferable by ultrasonography and falling concentrations of oestradiol, anti-Müllerian hormone and inhibin B.103 The initial elevation of FSH may precede clinical menopause by 5–10 years. After menopause, levels of LH fall by twofold-threefold and levels of FSH fall by threefold to 20-fold; these falls exceed those in men.104 A confounding factor is obesity, which increases with age and decreases LH pulse size in both sexes.105 The mechanism behind this effect in humans is not known.
The primary drive to human gonadotropes is hypothalamic neuronal secretion of gonadotropin-releasing hormone (GnRH). GnRH secretion in turn is under multifold control by sex steroids, kisspeptin, neurokinin B and dynorphin.106,107 Hypothalamic adaptations in ageing favour enhanced GnRH release.108,109 This enhanced GnRH release also occurs in gonadectomized animals, creating gonadotrope ‘castration cells’ and pituitary pericyte hyperplasia.110 The degree of menopause-associated hypergonadotropism seems to wane after age 65 years,109,111 possibly reflecting hypothalamic changes in the secretion of neurokinin B, glutamate, nitric oxide and GABA.112,113 In addition, ageing is associated with longer half-lives of more acidic LH and FSH isoforms,114 increased basal gonadotropin secretion,115,116 blunted night-time LH and FSH increments,117 frequent smaller LH pulses115,118 and less regular LH secretion patterns.119 Similar changes typify sex-steroid feedback withdrawal in young adults, which suggests that gonadoprivation is a proximate mechanism.120
Ageing of the gonadotropic axis is important clinically because sex-steroid privation adversely affects muscle and bone mass, visceral adipose tissue accumulation, insulin sensitivity, LDL metabolism, and possibly mood, libido, cognition and memory.121,122 Direct brain effects of LH and human choriogonadotropin although demonstrable in the rodent, are not inferable in women.123 Likewise, whereas high FSH levels contribute to osteopaenia in ovariectomized and aged mice,124,125 this concept does not seem to apply in women, in whom age, weight, ethnicity, inflammation and oestrogen availability are the confirmed crucial factors.126,127 Hot flushes occur in menopausal women, and their timing matches the onset of LH pulses, providing an indirect window into hypothalamic GnRH neuronal activity.128 The high FSH concentrations after menopause mean that serum FSH measurements provide suitable initial screening for suspected panhypopituitarism in older women (age >55 years), except in protracted critical illness, which suppresses gonadotropin output.129 A clinical caveat is that ageing augments secretion of free (dissociated) alpha and beta subunits in both sexes,113,130,131 necessitating age-dependent norms when assessing pituitary incidentaloma and possible gonadotropinomas in the elderly.131,132
Hypopituitarism in ageing
Hypopituitarism might be overlooked or confused with natural frailty in elderly individuals. Older patients must be treated with replacement amounts of the life-sustaining hormones, cortisol, thyroxine and AVP, when so indicated by corresponding deficiencies of ACTH, TSH and AVP. Hydrocortisone is suitable replacement for ACTH deficiency, given the absence of known extra-adrenal effects of ACTH. Low initial T4 doses (0.025 to 0.050 mg daily) with gradual increments are often preferred in older hypothyroid patients with myocardial ischemia or cardiac arrhythmias.133 The presence of congestive heart failure does not contraindicate physiological T4 replacement. However, especially in ageing patients, chronic over-replacement of thyroid hormone may be associated with atrial fibrillation, bone loss, proximal muscle weakness, tremor and glucose intolerance.134,135
Replacement of GH deficiency per se in the elderly with documented hypopituitarism is discussed elsewhere.136 Concerns in aged individuals are fluid retention, hypertension, arthralgias, glucose intolerance, which are all more common in frail elderly patients even without GH treatment. The risks and benefits of sex-steroid replacement should be discussed in clinically hypogonadal older individuals in accordance with good clinical practice.137–139
Pituitary tumours in ageing
Ageing is accompanied by an increased prevalence of pituitary tumours, including incidentaloma of the pituitary gland,132 macroadenoma, gonadotropinoma, and metastatic carcinoma. Lesions in older patients often present silently (clinically nonsecretory and without mass-lesion effects) or with hormone excess (Cushing disease, acromegaly), visual impairment, headache and/or hypopituitarism:140–142 Pituitary haemorrhage (apoplexy) heralded by acute headache and visual deficits also occurs in the elderly. Transsphenoidal endoscopic surgery remains an effective and safe treatment option in otherwise healthy older individuals,143 with similar risks of cerebrospinal-fluid leakage, meningitis, haemorrhage, tumour recurrence and hypopituitarism. Dopamine agonists may be used as primary therapy for prolactinomas, albeit cautiously because of orthostatic hypotension in the elderly. Observation alone is acceptable in some surgically high-risk patients without clinically threatening mass-lesion effects.
Limitations of pituitary studies in ageing
Target tissues of the hypothalamic–pituitary axis are regulated by combined pulsatile signalling and circadian inputs. The complex physiological dynamics can be quantified only by frequent sampling and specialized time-series analysis over 24 h.144 Such analyses have been sparse in the ageing population, especially in patients >80 years. Interpretative limitations of pituitary studies in an ageing population include prevalent comorbidities, sex-specific effects, sex hormone deficiency or replacement, disrupted sleep, altered body composition, reduced physical activity, elevated inflammatory mediators, increased medication use and methodological inconsistencies (Box 2).50 Moreover, rigorous evidence-based decisions require adequately powered, longitudinal, double-blind, randomized, placebo-controlled interventions replicated across multiple centres.
Box 2. Limitations of pituitary function ageing studies.
Technical limitations
Sex-specific effects often not examined
Few healthy individuals aged 80–100 years
Variable sex-hormone replacement regimens
Methodological inconsistencies
Comorbidities
Cardiovascular
Neural
Hepatic
Infectious
Inflammatory
Neoplastic
Sleep disturbances
Individual factors
Disability
Frailty
Reduced exercise
Poor nutrition
Medication use
Genetic factors
Body composition changes
The subtle incremental changes in hypothalamic-pituitary function with ageing are subject to confounding by technical limitations as well as comorbidities that accompany ageing. Several critical factors are listed, which must be individually and collectively controlled for to discern the underlying influence of ageing per se. In general, this stringency often required longitudinal studies, as well as precise comparisons with well-0defined control groups.
Several key unresolved queries exist in research of pituitary function changes in ageing (Box 3). Issues requiring clarification comprise the nature, degree and specificity of age-related alterations in hormone secretion, target-cell responsiveness, and feedback control. The adaptational benefits or liabilities of such alterations are also largely unknown. Incisive methods are needed to define cause-and-effect relationships between comorbidities and age-associated changes, and to elucidate the longitudinal sequence and inevitability of age-associated endocrine changes. For example, sleep-disrupting disorders alter GH, LH, testosterone, ACTH and cortisol secretion, but the exact manner and extent to which ageing and sleep apnoea interact adversely is difficult to ascertain.40,43,145 Likewise, the anorexia and hypoleptinaemia that accompany ageing could putatively affect multiple endocrine axes. Moreover, age might influence pathological responses to genetic polymorphisms in endocrine signalling. Lastly, whether seemingly minimal pituitary histologic and microcirculatory changes in ageing disrupt neuroendocrine activity is not known.146–149
Box 3. Questions related to pituitary function ageing.
Are endocrine changes that occur during ageing adaptive (for example, a decrease in IGF-I concentrations) or maladaptive (for example, stress-induced atrophy of hippocampus)?
To what degree are glandular changes that occur during ageing compensated by feedback adjustments?
What are the sequences of failure within axis components and among different axes during ageing?
What are the cellular and molecular mechanisms involved in changes in pituitary function during ageing?
How do comorbidities affect the pituitary function changes that occur during ageing?
Abbreviation: IGF-I, insulin-like growth factor I.
Conclusions
Ageing is associated with multiple subtle changes in pituitary secretion in humans. Concomitant morbidities, such as obesity, diabetes mellitus, reduced nutrition, systemic illness and medication use, alter how ageing affects individual pituitary hormones. Sex also influences the affect of ageing on pituitary function. The fundamental implications of pituitary changes with age require further investigation, as some age-related adaptation could favour longevity. To achieve maximal healthy life spans, a thorough understanding of age-related adaptation is required.
Supplementary Material
Key Points.
Growth hormone responses to most, but not all, stimuli decline markedly with ageing
Adrenocorticotropic hormone-cortisol dynamics change with age in a sex-related fashion
Water and electrolyte homeostasis is precarious in aging
TSH tends to rise with age, especially in women
Gonadotropins increase more in women during ageing than in men
Multiple factors co-determine ageing effects on pituitary hormones
Acknowledgements
The author thanks J. Smith of the Endocrine Research Unit, Mayo Clinic for support of manuscript preparation. Supported in part via AG019695, DK073148, AG029362, AG031763 and DK050456 (Metabolic Studies Core of the Minnesota Obesity Center) from the National Institutes of Health (Bethesda, MD). The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institute on ageing or the National Institutes of Health. Certain cited studies were supported by the National Center for Research Resources and by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCTS).
Biography
Johannes Veldhuis’ clinical research themes address how sex steroids and peptide-releasing factors regulate hormone secretion by the hypothalamus, pituitary gland and target tissues. His focus areas include regulatory physiology of the human pituitary-gonadal and adrenal axes, along with the somatotropic axis.
Footnotes
Competing interests
The author declares no competing interests.
Review Criteria
A PubMed search for original articles focused on the ageing pituitary that were published between 1960 and 2012 was performed. The search terms used were “pituitary” AND “hormone” AND (“age OR ageing OR old OR elderly”) AND “human”. The selection of articles reflects the authors’ opinion as to originality and importance in the context of the Review.
References
- 1.Surks MI, Sievert R. Drugs and thyroid function. N. Engl. J. Med. 1995;333:1688–1694. doi: 10.1056/NEJM199512213332507. [DOI] [PubMed] [Google Scholar]
- 2.Tietz NW, Shuey DF, Wekstein DR. Laboratory values in fit aging individuals--sexagenarians through centenarians. Clin. Chem. 1992;38:1167–1185. [PubMed] [Google Scholar]
- 3.Pinchera A, et al. Thyroid autoimmunity and ageing. Horm. Res. 1995;43:64–68. doi: 10.1159/000184239. [DOI] [PubMed] [Google Scholar]
- 4.Ligthart GJ, et al. Necessity of the assessment of health status in human immunogerontological studies: evaluation of the SENIEUR protocol. Mech. Ageing Dev. 1990;55:89–105. doi: 10.1016/0047-6374(90)90108-r. [DOI] [PubMed] [Google Scholar]
- 5.van Coevorden A, et al. Decreased basal and stimulated thyrotropin secretion in healthy elderly men. J. Clin. Endocrinol. Metab. 1989;69:177–185. doi: 10.1210/jcem-69-1-177. [DOI] [PubMed] [Google Scholar]
- 6.Erfurth EM, Norden NE, Hedner P, Nilsson A, Ek L. Normal reference interval for thyrotropin response to thyroliberin: dependence on age, sex, free thyroxin index, and basal concentrations of thyrotropin. Clin. Chem. 1984;30:196–199. [PubMed] [Google Scholar]
- 7.Roelfsema F, et al. Thyrotropin secretion profiles are not different in men and women. J. Clin. Endocrinol. Metab. 2009;94:3964–3967. doi: 10.1210/jc.2009-1155. [DOI] [PubMed] [Google Scholar]
- 8.Volpato S, et al. Serum thyroxine level and cognitive decline in euthyroid older women. Neurology. 2002;58:1055–1061. doi: 10.1212/wnl.58.7.1055. [DOI] [PubMed] [Google Scholar]
- 9.da Costa VM, Rosenthal D. Effects of aging on thyroidal function and proliferation. Curr. Aging Sci. 2008;1:101–104. doi: 10.2174/1874609810801020101. [DOI] [PubMed] [Google Scholar]
- 10.Peteranderl C, et al. Nocturnal secretion of TSH and ACTH in male patients with depression and healthy controls. J. Psychiatr. Res. 2002;36:189–196. doi: 10.1016/s0022-3956(02)00004-3. [DOI] [PubMed] [Google Scholar]
- 11.Surks MI, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–238. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 12.Burman KD, et al. Nature of suppressed TSH secretion during undernutrition: effect of fasting and refeeding on TSH responses to prolonged TRH infusions. Metabolism. 1980;29:46–52. doi: 10.1016/0026-0495(80)90097-9. [DOI] [PubMed] [Google Scholar]
- 13.Over R, Mannan S, Nsouli-Maktabi H, Burman KD, Jonklaas J. Age and the thyrotropin response to hypothyroxinemia. J. Clin. Endocrinol. Metab. 2010;95:3675–3683. doi: 10.1210/jc.2010-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waring AC, et al. Longitudinal changes in thyroid function in the oldest old and survival: the cardiovascular health study all-stars study. J. Clin. Endocrinol. Metab. 2012;97:3944–3950. doi: 10.1210/jc.2012-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gussekloo J, et al. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591–2599. doi: 10.1001/jama.292.21.2591. [DOI] [PubMed] [Google Scholar]
- 16.Atzmon G, Barzilai N, Hollowell JG, Surks MI, Gabriely I. Extreme longevity is associated with increased serum thyrotropin. J. Clin. Endocrinol. Metab. 2009;94:1251–1254. doi: 10.1210/jc.2008-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rozing MP, et al. Familial longevity is associated with decreased thyroid function. J. Clin. Endocrinol. Metab. 2010;95:4979–4984. doi: 10.1210/jc.2010-0875. [DOI] [PubMed] [Google Scholar]
- 18.Bjergved L, et al. Predictors of change in serum TSH after iodine fortification: an 11-year follow-up to the DanThyr study. J. Clin. Endocrinol. Metab. 2012;97:4022–4029. doi: 10.1210/jc.2012-2508. [DOI] [PubMed] [Google Scholar]
- 19.Costa-E-Sousa RH, Hollenberg AN. Minireview: the neural regulation of the hypothalamic pituitary thyroid axis. Endocrinol. 2012;153:4128–4135. doi: 10.1210/en.2012-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alkemade A, Unmehopa UA, Wiersinga WM, Swaab DF, Fliers E. Glucocorticoids decrease thyrotropin-releasing hormone messenger ribonucleic acid expression in the paraventricular nucleus of the human hypothalamus. J. Clin. Endocrinol. Metab. 2005;90:323–327. doi: 10.1210/jc.2004-1430. [DOI] [PubMed] [Google Scholar]
- 21.Van den Berghe G, et al. Reactivation of pituitary hormone release and metabolic improvement by infusion of growth hormone-releasing peptide and thyrotropin-releasing hormone in patients with protracted critical illness. J. Clin. Endocrinol. Metab. 1999;84:1311–1323. doi: 10.1210/jcem.84.4.5636. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman SA, Oberoi AL, Gilkison CR, Masel BE, Urban RJ. Prevalence of neuroendocrine dysfunction in patients recovering from traumatic brain injury. J. Clin. Endocrinol. Metab. 2001;86:2752–2756. doi: 10.1210/jcem.86.6.7592. [DOI] [PubMed] [Google Scholar]
- 23.Ferretti E, et al. Evaluation of the adequacy of levothyroxine replacement therapy in patients with central hypothyroidism. J. Clin. Endocrinol. Metab. 1999;84:924–929. doi: 10.1210/jcem.84.3.5553. [DOI] [PubMed] [Google Scholar]
- 24.Walsh JP, et al. Thyrotropin and thyroid antibodies as predictors of hypothyroidism: a 13-year, longitudinal study of a community-based cohort using current immunoassay techniques. J. Clin. Endocrinol. Metab. 2010;95:1095–1104. doi: 10.1210/jc.2009-1977. [DOI] [PubMed] [Google Scholar]
- 25.Parle JV, Franklyn JA, Cross KW, Jones SC, Sheppard MC. Prevalence and follow-up of abnormal thyrotrophin (TSH) concentrations in the elderly in the United Kingdom. Clin. Endocrinol. (Oxf.) 1991;34:77–83. doi: 10.1111/j.1365-2265.1991.tb01739.x. [DOI] [PubMed] [Google Scholar]
- 26.Gencer B, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126:1040–1049. doi: 10.1161/CIRCULATIONAHA.112.096024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang LB, et al. Subclinical hyperthyroidism and the risk of cardiovascular events and all-cause mortality: an updated meta-analysis of cohort studies. Eur. J. Endocrinol. 2012;167:75–84. doi: 10.1530/EJE-12-0015. [DOI] [PubMed] [Google Scholar]
- 28.Turner MR, et al. Levothyroxine dose and risk of fractures in older adults: nested case-control study. BMJ. 2011;342:d2238. doi: 10.1136/bmj.d2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heuser IJ, et al. Age-associated changes of pituitary-adrenocortical hormone regulation in humans: importance of gender. Neurobiol. Aging. 1994;15:227–231. doi: 10.1016/0197-4580(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 30.Born J, Ditschuneit I, Schreiber M, Dodt C, Fehm HL. Effects of age and gender on pituitary-adrenocortical responsiveness in humans. Eur. J. Endocrinol. 1995;132:705–711. doi: 10.1530/eje.0.1320705. [DOI] [PubMed] [Google Scholar]
- 31.Rubin RT, Rhodes ME, O'Toole S, Czambel RK. Sexual diergism of hypothalamo pituitary adrenal cortical responses to low-dose physotigmine in elderly vs. young women and men. Neuropsychopharmacology. 2002;26:672–681. doi: 10.1016/S0893-133X(01)00376-1. [DOI] [PubMed] [Google Scholar]
- 32.Giordano R, et al. Hypothalamus pituitary adrenal hyperactivity in human aging is partially refractory to stimulation by mineralocorticoid receptor blockade. J. Clin. Endocrinol. Metab. 2005;90:5656–5662. doi: 10.1210/jc.2005-0105. [DOI] [PubMed] [Google Scholar]
- 33.Blicher-Toft M, Hummer L. Immunoreactive corticotrophin reserve in old age in man during and after surgical stress. J. Geronto.l. 1976;31:539–545. doi: 10.1093/geronj/31.5.539. [DOI] [PubMed] [Google Scholar]
- 34.Otte C, et al. Mineralocorticoid receptor-mediated inhibition of the hypothalamic pituitary adrenal axis in aged humans. J. Gerontol. A Biol. Sci. Med. Sci. 2003;58:B900–B905. doi: 10.1093/gerona/58.10.b900. [DOI] [PubMed] [Google Scholar]
- 35.Blichert-Toft M, Hummer L. Serum immunoreactive corticotrophin and response to metyrapone in old age in man. Gerontology. 1977;23:236–243. doi: 10.1159/000212192. [DOI] [PubMed] [Google Scholar]
- 36.Gelfin Y, Lerer B, Lesch KP, Gorfine M, Allolio B. Complex effects of age and gender on hypothermic, adrenocorticotrophic hormone and cortisol responses to ipsapirone challenge in normal subjects. Psychopharmacology (Berl.) 1995;120:356–364. doi: 10.1007/BF02311184. [DOI] [PubMed] [Google Scholar]
- 37.Boscaro M, et al. Age-related changes in glucocorticoid fast feedback inhibition of adrenocorticotropin in man. J. Clin. Endocrinol. Metab. 1998;83:1380–1383. doi: 10.1210/jcem.83.4.4745. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson CW, et al. Human glucocorticoid feedback inhibition is reduced in older individuals: evening study. J. Clin. Endocrinol. Metab. 2001;86:545–550. doi: 10.1210/jcem.86.2.7232. [DOI] [PubMed] [Google Scholar]
- 39.Wolf OT, et al. Subjective memory complaints in aging are associated with elevated cortisol levels. Neurobiol. Aging. 2005;26:1357–1363. doi: 10.1016/j.neurobiolaging.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Sherman B, Wysham C, Pfohl B. Age-related changes in the circadian rhythm of plasma cortisol in man. J. Clin. Endocrinol. Metab. 1985;61:439–443. doi: 10.1210/jcem-61-3-439. [DOI] [PubMed] [Google Scholar]
- 41.Haus E, Nicolau G, Lakatua DJ, Sackett-Lundeen L, Petrescu E. Circadian rhythm parameters of endocrine functions in elderly subjects during the seventh to the ninth decade of life. Chronobiologia. 1989;16:331–352. [PubMed] [Google Scholar]
- 42.Purnell JQ, Brandon DD, Isabelle LM, Loriaux DL, Samuels MH. Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. J. Clin. Endocrinol. Metab. 2004;89:281–287. doi: 10.1210/jc.2003-030440. [DOI] [PubMed] [Google Scholar]
- 43.Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J. Clin. Endocrinol. Metab. 1996;81:2468–2473. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- 44.Andrew R, Phillips DI, Walker BR. Obesity and gender influence cortisol secretion and metabolism in man. J. Clin. Endocrinol. Metab. 1998;83:1806–1809. doi: 10.1210/jcem.83.5.4951. [DOI] [PubMed] [Google Scholar]
- 45.Roelfsema F, Pijl H, Keenan DM, Veldhuis JD. Diminshed adrenal sensitivity and ACTH efficacy in obese premenopausal women. Eur. J. Endocrinol. 2012;167:633–642. doi: 10.1530/EJE-12-0592. [DOI] [PubMed] [Google Scholar]
- 46.Morano MI, Vazquez DM, Akil H. The role of the hippocampal mineralocorticoid and glucocorticoid receptors in the hypothalamo pituitary adrenal axis of the aged Fisher rat. Mol. Cell Neurosci. 1994;5:400–412. doi: 10.1006/mcne.1994.1050. [DOI] [PubMed] [Google Scholar]
- 47.Swaab DF, Bao AM. (Re−)activation of neurons in aging and dementia: lessons from the hypothalamus. Exp. Gerontol. 2010;46:178–184. doi: 10.1016/j.exger.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 48.Lanfranco F, et al. Obese patients with obstructive sleep apnoea syndrome show a peculiar alteration of the corticotroph but not of the thyrotroph and lactotroph function. Clin. Endocrinol. (Oxf.) 2004;60:41–48. doi: 10.1111/j.1365-2265.2004.01938.x. [DOI] [PubMed] [Google Scholar]
- 49.Kostoglou-Athanassiou I, Treacher DF, Wheeler MJ, Forsling ML. Bright light exposure and pituitary hormone secretion. Clin. Endocrinol. (Oxf.) 1998;48:73–79. doi: 10.1046/j.1365-2265.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- 50.Naing KK, Dewar JA, Leese GP. Megestrol acetate therapy and secondary adrenal suppression. Cancer. 1999;86:1044–1049. [PubMed] [Google Scholar]
- 51.Sasayama D, et al. Modulation of cortisol responses to the DEX/CRH test by polymorphisms of the interleukin-1beta gene in healthy adults. Behav. Brain Funct. 2011;7:23. doi: 10.1186/1744-9081-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aguilera G. HPA axis responsiveness to stress: implications for healthy aging. Exp. Gerontol. 2010;46:90–95. doi: 10.1016/j.exger.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl Acad. Sci. USA. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Impallomeni M, Yeo T, Rudd A, Carr D, Aber V. Investigation of anterior pituitary function in elderly in-patients over the age of 75. Q. J. Med. 1987;63:505–515. [PubMed] [Google Scholar]
- 55.Parker L, Gral T, Perrigo V, Skowksy R. Decreased adrenal androgen sensitivity to ACTH during aging. Metabolism. 1981;30:601–604. doi: 10.1016/0026-0495(81)90139-6. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz WB, Bennett W, Curelop S, Bartler FC. A syndrome of renal sodium loss and hyponatremia probably resulting from inappropriate secretion of antidiuretic hormone. Am. J. Med. 1957;23:529–542. doi: 10.1016/0002-9343(57)90224-3. [DOI] [PubMed] [Google Scholar]
- 57.Bollanti L, Riondino G, Strollo F. Endocrine paraneoplastic syndromes with special reference to the elderly. Endocrine. 2001;14:151–157. doi: 10.1385/ENDO:14:2:151. [DOI] [PubMed] [Google Scholar]
- 58.Diringer MN, Zazulia AR. Hyponatremia in neurologic patients: consequences and approaches to treatment. Neurologist. 2006;12:117–126. doi: 10.1097/01.nrl.0000215741.01699.77. [DOI] [PubMed] [Google Scholar]
- 59.Davy KP, Seals DR. Total blood volume in healthy young and older men. J. Appl. Physiol. 1994;76:2059–2062. doi: 10.1152/jappl.1994.76.5.2059. [DOI] [PubMed] [Google Scholar]
- 60.Phillips PA, et al. Reduced thirst after water deprivation in healthy elderly men. N. Engl. J. Med. 1984;311:753–759. doi: 10.1056/NEJM198409203111202. [DOI] [PubMed] [Google Scholar]
- 61.Weidmann P, De Myttenaere-Bursztein S, Maxwell MH, de, Lima J. Effect on aging on plasma renin and aldosterone in normal man. Kidney Int. 1975;8(5):325–333. doi: 10.1038/ki.1975.120. [DOI] [PubMed] [Google Scholar]
- 62.Rowe JW, Minaker KL, Sparrow D, Robertson GL. Age-related failure of volume pressure mediated vasopressin release. J. Clin. Endocrinol. Metab. 1982;54:661–664. doi: 10.1210/jcem-54-3-661. [DOI] [PubMed] [Google Scholar]
- 63.Tian Y, Serino R, Verbalis JG. Downregulation of renal vasopressin V2 receptor and aquaporin-2 expression parallels age-associated defects in urine concentration. Am. J. Physiol. Renal Physiol. 2004;287:F797–F805. doi: 10.1152/ajprenal.00403.2003. [DOI] [PubMed] [Google Scholar]
- 64.Helderman JH, et al. The response of arginine vasopressin to intravenous ethanol and hypertonic saline in man: the impact of aging. J. Gerontol. 1978;33:39–47. doi: 10.1093/geronj/33.1.39. [DOI] [PubMed] [Google Scholar]
- 65.Sherlock M, et al. The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage. Clin. Endocrinol. (Oxf.) 2006;64:250–254. doi: 10.1111/j.1365-2265.2006.02432.x. [DOI] [PubMed] [Google Scholar]
- 66.Coupland C, et al. Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ. 2011;343:d4551. doi: 10.1136/bmj.d4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iwasaki Y, et al. Osmoregulation of plasma vasopressin in myxedema. J. Clin. Endocrinol. Metab. 1990;70:534–539. doi: 10.1210/jcem-70-2-534. [DOI] [PubMed] [Google Scholar]
- 68.Ayus JC, Arieff AI. Chronic hyponatremic encephalopathy in postmenopausal women: association of therapies with morbidity and mortality. JAMA. 1999;281:2299–2304. doi: 10.1001/jama.281.24.2299. [DOI] [PubMed] [Google Scholar]
- 69.Torres AC, Wickham EP, Biskobing DM. Tolvaptan for the management of syndrome of inappropriate antidiuretic hormone secretion: lessons learned in titration of dose. Endocr. Pract. 2011;17:e97–e100. doi: 10.4158/EP10386.CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr. Rev. 2006;27:101–140. doi: 10.1210/er.2005-0006. [DOI] [PubMed] [Google Scholar]
- 71.Broglio F, et al. The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. J. Clin. Endocrinol. Metab. 2003;88:1537–1542. doi: 10.1210/jc.2002-021504. [DOI] [PubMed] [Google Scholar]
- 72.Greenwood FC, Landon J, Stamp TC. The plasma sugar, free fatty acid, cortisol, and growth hormone response to insulin. I. In control subjects. J. Clin. Invest. 1966;45:429–436. doi: 10.1172/JCI105357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arvat E, et al. Age-related variations in the neuroendocrine control, more than impaired receptor sensitivity, cause the reduction in the GH-releasing activity of GHRP's in human aging. Pituitary. 1998;1:51–58. doi: 10.1023/a:1009970909015. [DOI] [PubMed] [Google Scholar]
- 74.Weltman A, et al. Relationship between age, percentage body, fat, fitness, and 24-hour growth hormone release in healthy young adults: effects of gender. J. Clin. Endocrinol. Metab. 1994;78:543–548. doi: 10.1210/jcem.78.3.8126124. [DOI] [PubMed] [Google Scholar]
- 75.Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr. Rev. 1998;19:717–797. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- 76.Veldhuis JD, Roelfsema F, Keenan DM, Pincus SM. Gender, age, body mass index and IGF-I individually and jointly determine distinct GH dynamics: analyses in one hundred healthy adults. J. Clin. Endocrinol. Metab. 2011;96:115–121. doi: 10.1210/jc.2010-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aimaretti G, et al. Diagnostic reliability of a single IGF-I measurement in 237 adults with total anterior hypopituitarism and severe GH deficiency. Clin. Endocrinol. (Oxf.) 2003;59:56–61. doi: 10.1046/j.1365-2265.2003.01794.x. [DOI] [PubMed] [Google Scholar]
- 78.Aimaretti G, et al. Enhancement of the peripheral sensitivity to growth hormone in adults with GH deficiency. Eur. J. Endocrinol. 2001;145:267–272. doi: 10.1530/eje.0.1450267. [DOI] [PubMed] [Google Scholar]
- 79.Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brill KT, et al. Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. J. Clin. Endocrinol. Metab. 2002;87:5649–5657. doi: 10.1210/jc.2002-020098. [DOI] [PubMed] [Google Scholar]
- 81.Blackman MR, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288:2282–2292. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- 82.Fernholm R, et al. Growth hormone replacement therapy improves body composition and increases bone metabolism in elderly patients with pituitary disease. J. Clin. Endocrinol. Metab. 2000;85:4104–4112. doi: 10.1210/jcem.85.11.6949. [DOI] [PubMed] [Google Scholar]
- 83.Hoffman AR, et al. Efficacy and tolerability of an individualized dosing regimen for adult growth hormone replacement therapy in comparison with fixed body weight-based dosing. J. Clin. Endocrinol. Metab. 2004;89:3224–3233. doi: 10.1210/jc.2003-032082. [DOI] [PubMed] [Google Scholar]
- 84.Bowers CY, et al. Sustained elevation of pulsatile growth hormone (GH) secretion and insulin-like growth factor I (IGF-I), IGF-binding protein-3 (IGFBP-3), and IGFBP-5 concentrations during 30-day continuous subcutaneous infusion of GH-releasing peptide-2 in older men and women. J. Clin. Endocrinol. Metab. 2004;89:2290–2300. doi: 10.1210/jc.2003-031799. [DOI] [PubMed] [Google Scholar]
- 85.Anawalt BD, Merriam GR. Neuroendocrine aging in men. Andropause and somatopause. Endocrinol. Metab. Clin. North Am. 2001;30:647–669. doi: 10.1016/s0889-8529(05)70206-1. [DOI] [PubMed] [Google Scholar]
- 86.Mukherjee A, Ryder WD, Jostel A, Shalet SM. Prolactin deficiency is independently associated with reduced insulin-like growth factor I status in severely growth hormone-deficient adults. J. Clin. Endocrinol. Metab. 2006;91:2520–2525. doi: 10.1210/jc.2005-2491. [DOI] [PubMed] [Google Scholar]
- 87.Veldhuis JD, Evans WS, Stumpf PG. Mechanisms subserving estradiol's induction of increased prolactin concentrations: evidence for amplitude modulation of spontaneous prolactin secretory bursts. Am. J. Obstet. Gynecol. 1989;161:1149–1158. doi: 10.1016/0002-9378(89)90654-6. [DOI] [PubMed] [Google Scholar]
- 88.Yamaji T, Shimamoto K, Ishibashi M, Kosaka K, Orimo H. Effect of age and sex on circulating and pituitary prolactin levels in human. Acta Endocrinol. (Copenh.) 1976;83:711–719. doi: 10.1530/acta.0.0830711. [DOI] [PubMed] [Google Scholar]
- 89.Kruger TH, et al. Serial neurochemical measurement of cerebrospinal fluid during the human sexual response cycle. Eur. J. Neurosci. 2006;24:3445–3452. doi: 10.1111/j.1460-9568.2006.05215.x. [DOI] [PubMed] [Google Scholar]
- 90.Quigley ME, Ropert JF, Yen SS. Acute prolactin release triggered by feeding. J. Clin. Endocrinol. Metab. 1981;52:1043–1045. doi: 10.1210/jcem-52-5-1043. [DOI] [PubMed] [Google Scholar]
- 91.Kok P, Roelfsema F, Frölich M, Meinders AE, Pijl H. Prolactin release is enhanced in proportion to excess visceral fat in obese women. J. Clin. Endocrinol. Metab. 2004;89:4445–4449. doi: 10.1210/jc.2003-032184. [DOI] [PubMed] [Google Scholar]
- 92.Arnetz BB, Lahnborg G, Eneroth P. Age-related differences in the pituitary prolactin response to thyrotropin-releasing hormone. Life Sci. 1986;39:135–139. doi: 10.1016/0024-3205(86)90447-9. [DOI] [PubMed] [Google Scholar]
- 93.Veldhuis JD, Iranmanesh A, Johnson ML, Lizarralde G. Twenty-four hour rhythms in plasma concentrations of adenohypophyseal hormones are generated by distinct amplitude and/or frequency modulation of underlying pituitary secretory bursts. J. Clin. Endocrinol. Metab. 1990;71:1616–1623. doi: 10.1210/jcem-71-6-1616. [DOI] [PubMed] [Google Scholar]
- 94.Roelfsema F, Pijl H, Keenan DM, Veldhuis JD. Prolactin secretion in healthy adults is determed by gender, age and body mass index. PLoS One. 2012;7:e31305. doi: 10.1371/journal.pone.0031305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iranmanesh A, Mulligan T, Veldhuis JD. Mechanisms subserving the physiological nocturnal relative hypoprolactinemia of healthy older men: dual decline in prolactin secretory burst mass and basal release with preservation of pulse duration, frequency, and interpulse interval. J. Clin. Endocrinol. Metab. 1999;84:1083–1090. doi: 10.1210/jcem.84.3.5514. [DOI] [PubMed] [Google Scholar]
- 96.Blackman MR, Kowatch MA, Wehmann RE, Harman SM. Basal serum prolactin levels and prolactin responses to constant infusions of thyrotropin releasing hormone in healthy aging men. J. Gerontol. 1986;41:699–705. doi: 10.1093/geronj/41.6.699. [DOI] [PubMed] [Google Scholar]
- 97.Tworoger SS, Eliassen AH, Rosner B, Sluss P, Hankinson SE. Plasma prolactin concentrations and risk of postmenopausal breast cancer. Cancer Res. 2004;64:6814–6819. doi: 10.1158/0008-5472.CAN-04-1870. [DOI] [PubMed] [Google Scholar]
- 98.Berinder K, Akre O, Granath F, Hulting AL. Cancer risk in hyperprolactinemia patients: a population-based cohort study. Eur. J. Endocrinol. 2011;165:209–215. doi: 10.1530/EJE-11-0076. [DOI] [PubMed] [Google Scholar]
- 99.McCudden CR, Sharpless JL, Grenache DG. Comparison of multiple methods for identification of hyperprolactinemia in the presence of macroprolactin. Clin. Chim. Acta. 2010;411:155–160. doi: 10.1016/j.cca.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Frieze TW, Mong DP, Koops MK. "Hook effect" in prolactinomas: case report and review of literature. Endocr. Pract. 2002;8:296–303. doi: 10.4158/EP.8.4.296. [DOI] [PubMed] [Google Scholar]
- 101.Tenover JS, et al. Decreased serum inhibin levels in normal elderly men: evidence for a decline in Sertoli cell function with aging. J. Clin. Endocrinol. Metab. 1988;67:455–459. doi: 10.1210/jcem-67-3-455. [DOI] [PubMed] [Google Scholar]
- 102.Nieschlag E, Lammers U, Freischem CW, Langer K, Wickings EJ. Reproductive functions in young fathers and grandfathers. J. Clin. Endocrinol. Metab. 1982;55:676–681. doi: 10.1210/jcem-55-4-676. [DOI] [PubMed] [Google Scholar]
- 103.Sowers MR, et al. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J. Clin. Endocrinol. Metab. 2008;93:3478–3483. doi: 10.1210/jc.2008-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tenover JS, Matsumoto AM, Plymate SR, Bremner WJ. The effects of aging in normal men on bioavailable testosterone and luteinizing hormone secretion: response to clomiphene citrate. J. Clin. Endocrinol. Metab. 1987;65:1118–1126. doi: 10.1210/jcem-65-6-1118. [DOI] [PubMed] [Google Scholar]
- 105.Veldhuis JD, Urban RJ, Lizarralde G, Johnson ML, Iranmanesh A. Attenuation of luteinizing hormone secretory burst amplitude is a proximate basis for the hypoandrogenism of healthy aging in men. J. Clin. Endocrinol. Metab. 1992;75:707–713. doi: 10.1210/jcem.75.3.1517359. [DOI] [PubMed] [Google Scholar]
- 106.Lasaga M, Debeljuk L. Tachykinins and the hypothalamo pituitary gonadal axis: An update. Peptides. 2011;32:1972–1978. doi: 10.1016/j.peptides.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 107.Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122. doi: 10.1016/j.peptides.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Downs JL, Wise PM. The role of the brain in female reproductive aging. Mol. Cell Endocrinol. 2009;299:32–38. doi: 10.1016/j.mce.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gill S, Sharpless JL, Rado K, Hall JE. Evidence that GnRH decreases with gonadal steroid feedback but increases with age in postmenopausal women. J. Clin. Endocrinol. Metab. 2002;87:2290–2296. doi: 10.1210/jcem.87.5.8508. [DOI] [PubMed] [Google Scholar]
- 110.Jindatip D, Fujiwara K, Kouki T, Yashiro T. Transmission and scanning electron microscopy study of the characteristics and morphology of pericytes and novel desmin-immunopositive perivascular cells before and after castration in rat anterior pituitary gland. Anat. Sci. Int. 2012;87:165–173. doi: 10.1007/s12565-012-0144-z. [DOI] [PubMed] [Google Scholar]
- 111.Keenan DM, Veldhuis JD. Age-dependent regression analysis of male gonadal axis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R1215–R1227. doi: 10.1152/ajpregu.90800.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bonavera JJ, Swerdloff RS, Sinha Hakim AP, Lue YH, Wang C. Aging results in attenuated gonadotropin releasing hormone-luteinizing hormone axis responsiveness to glutamate receptor agonist N methyl D-aspartate. J. Neuroendocrinol. 1998;10:93–99. doi: 10.1046/j.1365-2826.1998.00177.x. [DOI] [PubMed] [Google Scholar]
- 113.Zwart AD, Urban RJ, Odell WD, Veldhuis JD. Contrasts in the gonadotropin-releasing dose-response relationships for luteinizing hormone, follicle-stimulating hormone, and alpha-subunit release in young versus older men: appraisal with high-specificity immunoradiometric assay and deconvolution analysis. Eur. J. Endocrinol. 1996;135:399–406. doi: 10.1530/eje.0.1350399. [DOI] [PubMed] [Google Scholar]
- 114.Loreti N, et al. Carbohydrate complexity and proportion of serum FSH isoforms reflect pituitary-ovarian activity in perimenopausal women and depot medroxyprogesterone acetate users. Clin. Endocrinol. (Oxf.) 2009;71:558–565. doi: 10.1111/j.1365-2265.2009.03559.x. [DOI] [PubMed] [Google Scholar]
- 115.Keenan DM, Evans WS, Veldhuis JD. Control of LH secretory-burst frequency and interpulse-interval regularity in women. Am. J. Physiol. Endocrinol. Metab. 2003;285:E938–E948. doi: 10.1152/ajpendo.00133.2003. [DOI] [PubMed] [Google Scholar]
- 116.Keenan DM, et al. An ensemble model of the male gonadal axis: illustrative application in aging men. Endocrinol. 2006;147:2817–2828. doi: 10.1210/en.2005-1356. [DOI] [PubMed] [Google Scholar]
- 117.Mulligan T, Iranmanesh A, Kerzner R, Demers LW, Veldhuis JD. Two-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig-cell) defects in the healthy aging male gonadotropic axis. Eur. J. Endocrinol. 1999;141:257–266. doi: 10.1530/eje.0.1410257. [DOI] [PubMed] [Google Scholar]
- 118.Pincus SM, Veldhuis JD, Mulligan T, Iranmanesh A, Evans WS. Effects of age on the irregularity of LH and FSH serum concentrations in women and men. Am. J. Physiol. 1997;273:E989–E995. doi: 10.1152/ajpendo.1997.273.5.E989. [DOI] [PubMed] [Google Scholar]
- 119.Pincus SM, et al. Older males secrete luteinizing hormone and testosterone more irregularly, and jointly more asynchronously, than younger males. Proc. Natl Acad. Sci. USA. 1996;93:14100–14105. doi: 10.1073/pnas.93.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Veldhuis JD, Zwart AD, Iranmanesh A. Neuroendocrine mechanisms by which selective Leydig-cell castration unleashes increased pulsatile LH release. Am. J. Physiol. 1997;272:R464–R474. doi: 10.1152/ajpregu.1997.272.2.R464. [DOI] [PubMed] [Google Scholar]
- 121.The 2012 hormone therapy position statement of: The North American Menopause Society. Menopause. 2012;19:257–271. doi: 10.1097/gme.0b013e31824b970a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shoupe D. Individualizing hormone therapy to minimize risk: accurate assessment of risks and benefits. Womens Health (Lond. Engl.) 2011;7:475–485. doi: 10.2217/whe.11.42. [DOI] [PubMed] [Google Scholar]
- 123.Kyle CV, Griffin J, Jarrett A, Odell WD. Inability to demonstrate an ultrashort loop feedback mechanism for luteinizing hormone in humans. J. Clin Endocrinol. Metab. J. Clin Endocrinol. Metab. 1989;69:170–176. doi: 10.1210/jcem-69-1-170. [DOI] [PubMed] [Google Scholar]
- 124.Sun L, et al. FSH directly regulates bone mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 125.Iqbal J, Sun L, Kumar TR, Blair HC, Zaidi M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc. Natl Acad. Sci. USA. 2006;103:14925–14930. doi: 10.1073/pnas.0606805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gourlay ML, et al. Follicle-stimulating hormone is independently associated with lean mass but not BMD in younger postmenopausal women. Bone. 2012;50:311–316. doi: 10.1016/j.bone.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gourlay ML, Preisser JS, Hammett-Stabler CA, Renner JB, Rubin J. Follicle-stimulating hormone and bioavailable estradiol are less important than weight and race in determining bone density in younger postmenopausal women. Osteoporos Int. 2011;22:2699–2708. doi: 10.1007/s00198-010-1505-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Archer DF, et al. Menopausal hot flushes and night sweats: where are we now? Climacteric. 2011;14:515–528. doi: 10.3109/13697137.2011.608596. [DOI] [PubMed] [Google Scholar]
- 129.Van den Berghe G, et al. The combined administration of GH-releasing peptide-2 (GHRP-2), TRH and GnRH to men with prolonged critical illness evokes superior endocrine and metabolic effects compared to treatment with GHRP-2 alone. Clin. Endocrinol. (Oxf.) 2002;56:655–669. doi: 10.1046/j.1365-2265.2002.01255.x. [DOI] [PubMed] [Google Scholar]
- 130.Hall JE, Lavoie HB, Marsh EE, Martin KA. Decrease in gonadotropin-releasing hormone (GnRH) pulse frequency with aging in postmenopausal women. J. Clin. Endocrinol. Metab. 2000;85:1794–1800. doi: 10.1210/jcem.85.5.6612. [DOI] [PubMed] [Google Scholar]
- 131.Iles RK, Javid MK, Gunn LK, Chard T. Cross-reaction with luteinizing hormone beta-core is responsible for the age-dependent increase of immunoreactive beta-core fragment of human chorionic gonadotropin in women with nonmalignant conditions. Clin. Chem. 1999;45:532–538. [PubMed] [Google Scholar]
- 132.Kastelan D, Korsic M. High prevalence rate of pituitary incidentaloma: is it associated with the age-related decline of the sex hormones levels? Med. Hypotheses. 2007;69(2):307–309. doi: 10.1016/j.mehy.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 133.Khandelwal D, Tandon N. Overt and subclinical hypothyroidism: who to treat and how. Drugs. 2012;72(1):17–33. doi: 10.2165/11598070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 134.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N. Engl. J. Med. 2001;344(7):501–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 135.Murphy E, et al. Thyroid function within the upper normal range is associated with reduced bone mineral density and an increased risk of nonvertebral fractures in healthy euthyroid postmenopausal women. J. Clin. Endocrinol. Metab. 2010;95(7):3173–3181. doi: 10.1210/jc.2009-2630. [DOI] [PubMed] [Google Scholar]
- 136.Filipsson H, Johannsson G. GH replacement in adults: interactions with other pituitary hormone deficiencies and replacement therapies. Eur. J. Endocrinol. 2009;161(Suppl 1):S85–S95. doi: 10.1530/EJE-09-0319. [DOI] [PubMed] [Google Scholar]
- 137.Santen RJ, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J. Clin. Endocrinol. Metab. 2010;95(7) Suppl 1:s1–s66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wierman ME, et al. Androgen therapy in women: an Endocrine Society Clinical Practice guideline. J. Clin. Endocrinol. Metab. 2006;91(10):3697–3710. doi: 10.1210/jc.2006-1121. [DOI] [PubMed] [Google Scholar]
- 139.Bhasin S, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2010;95(6):2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 140.Turner HE, Adams CB, Wass JA. Pituitary tumours in the elderly: a 20 year experience. Eur. J. Endocrinol. 1999;140(5):383–389. doi: 10.1530/eje.0.1400383. [DOI] [PubMed] [Google Scholar]
- 141.Benbow SJ, Foy P, Jones B, Shaw D, MacFarlane IA. Pituitary tumours presenting in the elderly: management and outcome. Clin. Endocrinol. (Oxf.) 1997;46(6):657–660. doi: 10.1046/j.1365-2265.1997.1180933.x. [DOI] [PubMed] [Google Scholar]
- 142.Ferrante L, et al. Surgical treatment of pituitary tumors in the elderly: clinical outcome and long-term follow-up. J. Neurooncol. 2002;60(2):185–191. doi: 10.1023/a:1020652604014. [DOI] [PubMed] [Google Scholar]
- 143.Kurosaki M, Ludecke DK, Flitsch J, Saeger W. Surgical treatment of clinically nonsecreting pituitary adenomas in elderly patients. Neurosurgery. 2000;47(4):843–848. doi: 10.1097/00006123-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 144.Veldhuis JD, Keenan DM, Pincus SM. Motivations and methods for analyzing pulsatile hormone secretion. Endocr. Rev. 2008;29(7):823–864. doi: 10.1210/er.2008-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Latta F, et al. Sex differences in nocturnal growth hormone and prolactin secretion in healthy older adults: relationships with sleep EEG variables. Sleep. 2005;28(12):1519–1524. doi: 10.1093/sleep/28.12.1519. [DOI] [PubMed] [Google Scholar]
- 146.Hartl F, Fischer C. Morphological changes of the human adenohypophysis and their relations to age, sex and constitution. Z. Alternsforsch. 1955;8(4):301–308. [PubMed] [Google Scholar]
- 147.Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinol. 1997;138(8):3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- 148.Liu S. Peptidergic innervation in pars distalis of the human anterior pituitary. Brain Res. 2004;1008(1):61–68. doi: 10.1016/j.brainres.2003.12.055. [DOI] [PubMed] [Google Scholar]
- 149.Banks WA. Brain meets body: the blood-brain barrier as an endocrine interface. Endocrinol. 2012;153(9):4111–4119. doi: 10.1210/en.2012-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.