Abstract
Acid reflux in the esophagus can induce esophageal painful sensations such as heartburn and noncardiac chest pain. The mechanisms underlying acid-induced esophageal nociception are not clearly understood. In our previous studies, we characterized esophageal vagal nociceptive afferents and defined their responses to noxious mechanical and chemical stimulation. In the present study, we aim to determine their responses to intraluminal acid infusion. Extracellular single-unit recordings were performed in nodose ganglion neurons with intact nerve endings in the esophagus using ex vivo esophageal-vagal preparations. Action potentials evoked by esophageal intraluminal acid perfusion were compared in naive and ovalbumin (OVA)-challenged animals, followed by measurements of transepithelial electrical resistance (TEER) and the expression of tight junction proteins (zona occludens-1 and occludin). In naive guinea pigs, intraluminal infusion with either acid (pH = 2–3) or capsaicin did not evoke an action potential discharge in esophageal nodose C fibers. In OVA-sensitized animals, following esophageal mast cell activation by in vivo OVA inhalation, intraluminal acid infusion for about 20 min started to evoke action potential discharges. This effect is further confirmed by selective mast cell activation using in vitro tissue OVA challenge in esophageal-vagal preparations. OVA inhalation leads to decreased TEER and zona occludens-1 expression, suggesting an impaired esophageal epithelial barrier function after mast cell activation. These data for the first time provide direct evidence of intraluminal acid-induced activation of esophageal nociceptive C fibers and suggest that mast cell activation may make esophageal epithelium more permeable to acid, which subsequently may increase esophageal vagal nociceptive C fiber activation.
Keywords: acid, esophagus, C fiber, vagal afferent, mast cell
acid reflux in the esophagus can induce esophageal painful sensations such as heartburn and noncardiac chest pain. Proton pump inhibitor (PPI) therapy is usually effective for the symptom relief, but one-third of patients still have persistent symptoms after PPI therapy (23). A better understanding of the mechanisms of acid-evoked esophageal nociception will be helpful to develop an effective pain modulator to manage these symptoms in addition to PPIs. Currently, the underlying mechanism of acid-evoked activation of esophageal nociceptor is largely unknown (1). Recent studies have shown that several ion channels or receptors in sensory neurons are responsible for sensing acid (11). These studies provide important information on acid-evoked responses from sensory neurons, the cell bodies of sensory nerves, but the knowledge on acid-evoked responses of sensory nerve endings in peripheral tissues is still lacking.
Sensory nerves detect stimuli resulting from the physiological activity of the tissue as well as stimuli associated with impending and/or actual tissue damage (noxious stimuli). The sensory nerves capable of discriminating noxious stimuli are termed nociceptors (32). The transduction of noxious stimulation in the esophagus starts from sensory nerve terminals in the wall of the esophagus. The evoked action potential discharge is conducted through both vagal and spinal pathways into the central nervous system to generate nociception (3). Our previous studies demonstrated that esophageal vagal afferents have distinctive subtypes of nociceptive C fibers, namely nodose and jugular C fibers. They are able to discriminate noxious and innoxious esophageal distensions and respond to noxious chemical (capsaicin) stimulation (32). Whether these nociceptive C fibers are able to sense intraluminal acid has yet to be determined.
The normal esophageal epithelial barrier is usually resistant to acid exposure (6, 8). But acid combined with pepsin or bile salts can disrupt the esophageal epithelial barrier (6, 27, 28). Impaired mucosal barrier function may allow intraluminal acid to reach the sensory nerve endings underneath, activating those nociceptive afferents and inducing esophageal nociception (1). It has been reported that mast cell activation damages the intestinal epithelial barrier (18, 22, 24, 26). But the effect of mast cell activation on esophageal epithelial barrier function has not been reported. Our previous studies have established models of esophageal mast cell activation induced by either in vitro tissue antigen using ovalbumin (OVA) challenge (29) or in vivo antigen inhalation (31) in antigen-sensitized animals. Based on these present-day established models, we test the hypothesis that intraluminal acid activates esophageal vagal nodose C fibers after mast cell activation, due mainly to impaired esophageal epithelial barrier function.
METHODS
Male Hartley guinea pigs (Hilltop Laboratory Animals, Scottsdale, PA) weighing 100–300 g were used. All experiments were approved by the University of Michigan Committee on Use and Care of Animals (UCUCA, #PRO0000117).
Animal sensitization and challenge.
According to our previous studies (29, 31), guinea pigs were sensitized with three intraperitoneal injections of OVA (10 mg/kg in 0.9% saline) every 48 h. Three weeks after the last injection, guinea pigs were ready for either in vitro tissue antigen challenge or in vivo antigen inhalation. For tissue antigen challenge, the dissected esophageal-vagal preparations from OVA-sensitized animals were immersed in Krebs bicarbonate solution (KBS) containing antigen (OVA, 10 μg/ml) for 30 min to induce antigen-mediated mast cell degranulation. For antigen inhalation, OVA-sensitized animals were exposed to aerosolized antigen (0.1% OVA) in a plastic chamber for 1–10 min, depending on the development of dyspnea. The OVA was dissolved in 0.9% saline and delivered using a nebulizer driven by compressed air. The guinea pigs were closely monitored for signs of any allergic response such as gasping and increased respiratory rate. Once the guinea pigs developed such a response, they were removed and allowed to breathe ambient air. OVA inhalation was performed three times (once every 24 h) in each animal. Extracellular recordings were performed in ex vivo esophageal-vagal preparations after the last inhalation.
Extracellular single-unit recording.
In previously described esophageal-vagal preparations, extracellular single-unit recordings of action potential discharges were performed from nodose ganglia neurons with intact C fiber nerve endings in the esophagus (32). Briefly, the esophagus and trachea were dissected with intact bilateral extrinsic vagal innervations (including jugular and nodose ganglia). The tissue was pinned in a small Sylgard-lined Perspex chamber filled with KBS at 35°C, and gassed with 95% O2-5% CO2. The chamber had two compartments: the esophagus with attached trachea and the vagus were pinned in the tissue compartment, and the vagus nerves including the nodose and jugular ganglia were pinned in the recording compartment. Isobaric esophageal distension for 20 s with an intraluminal pressure of 10–60 mmHg was used to determine the distension pressure-nerve activity relationship of an esophageal afferent fiber. Extracellular single-unit recordings were performed using an aluminosilicate glass microelectrode which was placed into a nodose ganglion with an electrode holder connected directly to the headstage (A-M Systems, Everett, WA). The recorded signal was amplified (microelectrode AC amplifier 1800, A-M Systems) and filtered (low cutoff, 0.3 kHz; high cutoff, 1 kHz), and the resultant activity was displayed on an oscilloscope (TDS 340, Tektronix, Beaverton, OR) and chart recorder. The data were stored and analyzed using the software TheNerveOfIt (sampling frequency 33 kHz; PHOCIS, Baltimore, MD).
We identified esophageal vagal afferent fibers according to our previously described methods (32). Briefly, the recording electrode was micromanipulated into the nodose or jugular ganglion (left or right). A distension-sensitive unit was identified when esophageal distension (in most experiments with a rapid increase in intraluminal pressure to 10 mmHg for 5 s) evoked action potential discharge. The serosal surface of the esophagus was then searched with a punctate mechanical probe (Von Frey hair, 1 mN, filament diameter <0.5 mm) applied to the tissue. A mechanosensitive receptive field was located when the punctate stimulus evoked discharge of action potentials with waveforms identical to the action potentials evoked by distension. The receptive field was then stimulated electrically (pulse duration 1 ms, frequency 1 Hz) with a concentric electrode inserted into the esophagus with the tip positioned at the site of the mechanosensitive receptive field. The initial voltage (100 V) was gradually reduced to the lowest voltage (threshold voltage) at which each stimulation pulse was followed by a single action potential (30–90 V for most afferent nerve fibers recorded). The waveforms of the electrically evoked action potentials were identical to those induced by distension and the punctate mechanical stimulus. Conduction time was measured as the time between the stimulation pulse and the action potential (visualized by oscilloscope).
The nerve fiber was considered a C fiber if it conducted action potentials at <1 m/s. Conduction velocity was calculated by dividing the length of the approximated nerve pathway (from the recorded nodose neurons to the mechanosensitive receptive field in the esophagus) by conduction time. The peaks of action potential discharges of nodose C fibers in response to different treatments or stimuli were analyzed and compared. The compounds used in the experiment included OVA (as antigen for animal sensitization and mast cell activation), HCl (pH = 2–3, adjusted with KBS), capsaicin [transient receptor potential vanilliod-1 (TRPV1) agonist, which usually activates C fibers], ATP, prostaglandin E2 (PGE2), serotonin, histamine, and bradykinin (all from Sigma-Aldrich, MO). The compounds were diluted in KBS to final concentration on the day of use.
Histology.
Esophageal segments were fixed in 10% formaldehyde and embedded in paraffin. Sections of 5 μm were mounted on Superfrost Plus glass slides (Fisher Scientific) and stained with hematoxylin and eosin (H-E). The slides were analyzed under light microscope. The inflammation grade of the esophagus was evaluated according to a previously reported method (12), including assessments of active inflammation (neutrophil infiltration in the epithelium), the length of vascular papillae, basal-zone hyperplasia, and the number of intraepithelial eosinophils. In a separate study, esophageal segments from both naive (n = 3) and OVA-inhalation (n = 4) groups were fixed in Carnoy's solution and embedded in paraffin. Sections of 6 μm were mounted on Superfrost Plus glass slides (Fisher Scientific) and stained with toluidine blue. The slides were analyzed under a light microscope, and the numbers of mast cells in the esophageal cross-sections were counted and compared.
Transepithelial electrical resistance (TEER).
The TEER of esophageal epithelium was measured as previously described (5). Following extracellular recordings, the mucosal epithelium of the esophagus was dissected and cut into three pieces (3.5 × 3.5 mm each). Each segment was sandwiched between two Plexiglas inserts with a 3-mm-diameter central hole, introduced into Costar snapwells, and then placed in the incubator (37°C, 5% CO2) for 30 min to stabilize the pH. The TEER was measured in the micro-snapwell system by using a planar electrode (Endom SNAP electrode attached to an Evom-G WPI analyzer, World Precision Instruments, Sarasota, FL). The result was averaged from the measurements of three segments from each esophagus and expressed in ohms per square centimeter.
Western blot.
Equal amount of lysates (20 μg) freshly obtained from esophageal mucosal layers of naive (n = 4) and OVA-inhalation plus acid-infused (n = 6) animals were separated on Ready Gel 12% Tris-HCl, transferred to nitrocellulose Hybond enhanced chemiluminescence (ECL) membranes, and blotted with primary antibodies (overnight), and then secondary antibodies (1 h), followed by detection using ECL reagents (Pierce, IL). The membranes were exposed to ECL buffer for 30 s or 5 min and then high chemiluminescence film in the dark. The resulting bands were scanned and analyzed. The primary antibodies used in Western blot analysis included rabbit anti-zona occludens-1 (anti-ZO-1) antibody (1:1,000, #61–7300, Invitrogen, CA) and mouse anti-occludin antibody (1:500, #33–1500, Invitrogen, CA). The secondary antibody included goat anti-rabbit IgG-horseradish peroxidase (HRP) (1:2,000, #sc-2004, Santa Cruz, CA), and goat anti-mouse-HRP (1:4,000, #sc-2005, Santa Cruz, CA).
Data analysis.
In extracellular studies, we only analyzed the results from capsaicin-responsive nodose C fibers, which were confirmed by the end of each recording to indicate that the nerve terminals were exposed to chemical perfusion. We recorded afferent nerve activities from one nodose C fiber per animal, so the number of recorded fibers (n) equals the number of animals (N) used in the study. The chemical-evoked nerve response was quantified as peak frequency of the action potential discharge within a 5-min period, and averaged six recording periods for a total of 30 min. The peak frequency (Hz) of action potential discharges were presented as means ± SE and compared by one-way ANOVA. In the histological study, the inflammation grades from each group with different treatments were counted and compared by one-way ANOVA. In the permeability study, the TEERs were present as means ± SE and compared by one-way ANOVA. The density of each band on the Western blot was analyzed by densitometry, and normalized for quantitative comparisons by student's t-test. For all experiments, significance was defined as P < 0.05.
RESULTS
In extracellular recordings using ex vivo esophageal-vagal preparations, a total of 84 esophageal nodose C fibers from 84 animals (one from each animal) was recorded to determine their responses to intraluminal acid infusion, with or without OVA sensitization plus OVA tissue challenge in vitro or OVA inhalation in vivo. These C fibers (with conduction velocity <1 m/s) were confirmed to respond to capsaicin at the end of each recording (if intraluminal infusion did not evoke action potential discharges, capsaicin would be added through the serosal side of the esophagus to the same recorded C fiber from the esophageal-vagal preparation).
Esophageal intraluminal acid infusion activates esophageal nodose C fibers after in vivo OVA inhalation.
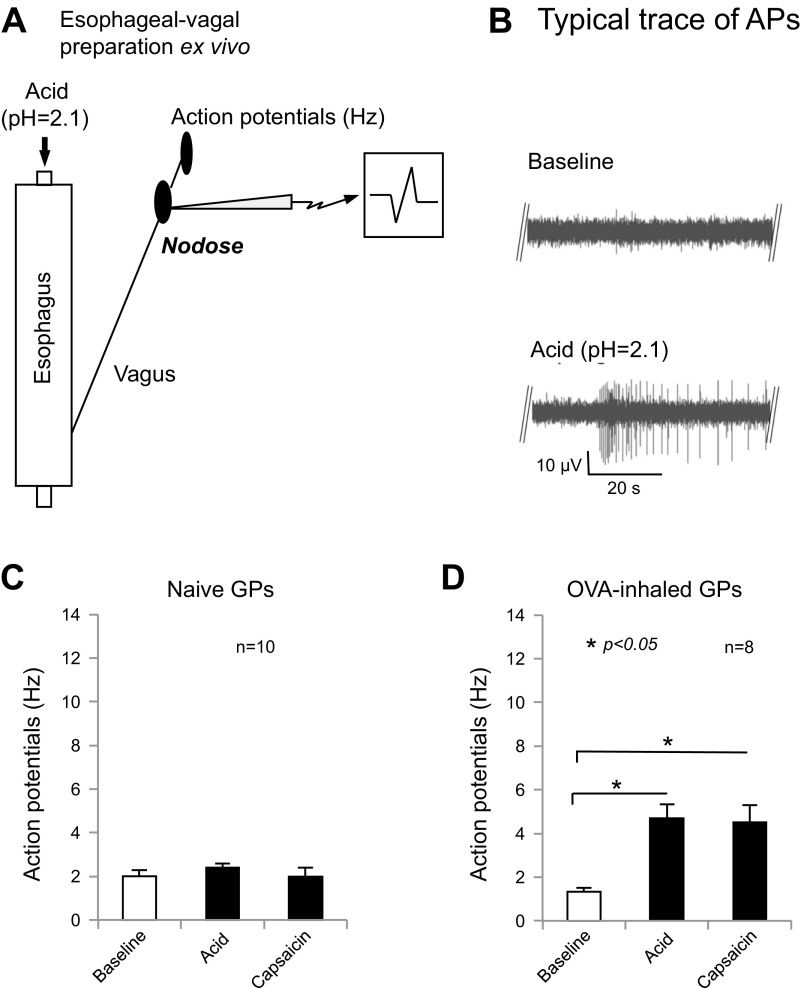
To determine the effect of acid on esophageal vagal nodose C fibers, we performed extracellular single-unit recordings from nodose ganglia neurons with intact nerve endings in the esophagus using ex vivo esophageal-vagal preparations. Esophageal distension-evoked action potentials were compared before and after intraluminal acid infusion. According to our previous studies (29, 32), we defined the response to a particular stimulus as positive when the stimulus-evoked action potential discharge had a peak frequency of at least 2–3 Hz above the baseline activity. In esophageal nodose C fibers from naive animals, acid infusion (HCl in KBS, pH = 2.1) for 30–120 min did not evoke action potential discharges (baseline vs. acid: 2 ± 0.26 vs. 2.4 ± 0.16 Hz, n = 10, P > 0.05). After washing out acid with fresh KBS (pH = 7.4) for 30 min, intraluminal infusion with capsaicin (1 μM) for 30–60 min did not evoke action potential discharges in these C fibers (baseline vs. capsaicin: 2 ± 0.26 vs. 2 ± 0.45 Hz, n = 10; Fig. 1C). In contrast, these C fibers were activated by capsaicin perfusion from the serosal side of the esophagus at the end of each recording (baseline vs. capsaicin: 2.0 ± 0.26 vs. 14.6 ± 2.7 Hz, n = 10, P < 0.05).
Fig. 1.
Intraluminal acid activates esophageal nodose C fibers after mast cell activation by in vivo ovalbumin (OVA) inhalation. A: extracellular single-unit recording from ex vivo esophageal-vagal preparation for intraluminal acid infusion. B: typical traces of action potential (AP) discharges evoked by intraluminal acid infusion in esophageal nodose C fibers before and after esophageal mast cell activation. C: in naive animals, intraluminal acid or capsaicin infusion did not evoke action potential discharge in esophageal nodose C fibers (baseline vs. acid, or vs. capsaicin = 2 ± 0.26 vs. 2.4 ± 0.16 Hz, or vs. 2 ± 0.45 Hz, both P > 0.05, n = 10). D: in OVA-inhaled animals, intraluminal acid or capsaicin infusion significantly evoked action potential discharge in esophageal nodose C fibers (baseline vs. acid, or vs. capsaicin = 1.3 ± 0.18 vs. 4.7 ± 0.63 Hz, or vs. 4.5 ± 0.8 Hz, n = 8, both *P < 0.05). GP, guinea pig.
In OVA-sensitized animals, OVA inhalation in vivo was performed three times every 24 h, then esophageal-vagal preparations were used for extracellular recording to determine intraluminal acid infusion-induced effects on esophageal nodose C fibers. Strikingly, intraluminal infusion with acid (using HCl in KBS and adjusted pH = 2.1) significantly evoked action potential discharges in esophageal nodose C fibers (baseline vs. acid: 1.3 ± 0.18 vs. 4.7 ± 0.63 Hz, n = 8, P < 0.05). These activation responses started after acid infusion for about 20 min (19.6 ± 5.4 min, n = 8). After washing out acid with fresh KBS (pH = 7.4) for 30 min, intraluminal infusion with capsaicin (1 μM) for 20–30 min also evoked action potential discharges in these C fibers (baseline vs. capsaicin: 1.3 ± 0.18 vs. 4.5 ± 0.8 Hz, n = 8, P < 0.05; Fig. 1, A, B, and D). These activation responses started after infusion with capsaicin for about 21 min (20.6 ± 4.5 min, n = 8). These data demonstrated that intraluminal acid did not activate esophageal nodose C fibers in naive animals but consistently activated nodose C fibers in the esophagus pretreated with OVA inhalations in OVA-sensitized animals. Our previous study (31) demonstrated that the mast cell is one of the most important immune cells involved in antigen inhalation-induced acute allergic reactions in the esophagus, and our present data from this model may suggest that the antigen inhalation-induced allergic response could play an important role in acid-induced activation of esophageal nodose C fibers.
Esophageal intraluminal acid infusion activates nodose C fibers after in vitro esophageal tissue challenge with OVA.
To specify the role of mast cells in acid-induced activation of esophageal nodose C fibers, we induced mast cell activation by in vitro tissue antigen challenge and determined whether mast cell activation allowed acid infusion to activate esophageal nodose C fibers.
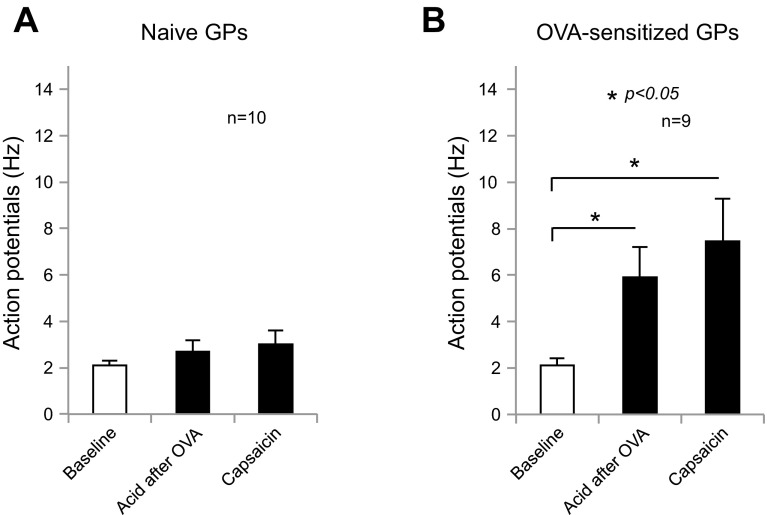
Mast cell activation by in vitro OVA tissue challenge was performed according to our previous study (30). We first determined the baseline spontaneous activities of esophageal nodose C fibers by extracellular single-unit recordings in ex vivo esophageal-vagal preparations from OVA-sensitized animals. Esophageal tissues were then perfused with OVA (10 μg/ml in KBS) for 30 min to induce mast cell degranulation. Similar to our previous study (29), mast cell degranulation itself did not evoke action potential discharges in these nodose C fibers. This was followed by intraluminal acid infusion for 30–90 min, and the responses of esophageal nodose C fibers were determined. This time, we observed that intraluminal infusion with acid (HCl in KBS with pH = 2.1) also significantly evoked action potential discharge in esophageal nodose C fibers (baseline vs. acid: 2.1 ± 0.3 vs. 5.9 ± 1.3 Hz, n = 9, P < 0.05; Fig. 2B). These activation responses started after acid infusion for about 15 min (15.4 ± 2.9 min, n = 8). After washing out acid with fresh KBS (pH = 7.4) for 30 min, intraluminal infusion with capsaicin (1 μM) for 20–30 min also evoked action potential discharges in these C fibers (baseline vs. capsaicin: 2.1 ± 0.3 vs. 7.4 ± 1.8 Hz, n = 9, P < 0.05). These activation responses started after infusion with capsaicin for about 20 min (19.9 ± 5 min, n = 7).
Fig. 2.
Intraluminal acid activates esophageal nodose C fibers after mast cell activation by in vitro tissue OVA challenge. A: intraluminal acid or capsaicin infusion did not evoke action potential discharge in esophageal nodose C fibers after esophageal tissue challenge with OVA in naive animals (baseline vs. acid, or vs. capsaicin = 2.1 ± 0.18 vs. 2.7 ± 0.45 Hz, or vs. 3 ± 0.56 Hz, both P > 0.05, n = 10). B: intraluminal acid or capsaicin infusion significantly evoked action potential discharge in esophageal nodose C fibers after esophageal tissue challenge with OVA in OVA-sensitized animals (baseline vs. acid, or vs. capsaicin = 2.1 ± 0.3 vs. 5.9 ± 1.3 Hz, or vs. 7.4 ± 1.8 Hz, both *P < 0.05, n = 9).
In naive animals, extracellular recordings from esophageal-vagal preparations were set up and esophageal tissues were perfused with OVA (10 μg/ml in KBS) for 30 min. Following this treatment, intraluminal acid infusion (HCl in KBS with pH = 2.1) for 30–90 min did not evoke action potential discharges in esophageal nodose C fibers (baseline vs. acid: 2.1 ± 0.18 vs. 2.7 ± 0.45 Hz, n = 10, P > 0.05). After washing out acid with fresh KBS (pH = 7.4) for 30 min, intraluminal infusion with capsaicin (1 μM) for 30–60 min did not evoke action potential discharges in these C fibers (baseline vs. capsaicin: 2.1 ± 0.18 vs. 3 ± 0.56 Hz, n = 10; Fig. 2A). These C fibers were activated by capsaicin perfusion from the serosal side of the esophagus at the end of each recording (baseline vs. capsaicin: 2.1 ± 0.18 vs. 12.7 ± 1.5 Hz, n = 10). These data supported a specific contribution of mast cell activation to intraluminal acid-induced activation of esophageal nodose C fibers.
Intraluminal infusion with inflammatory mediators does not activate esophageal nodose C fibers.
Previous studies reported that intraluminal acid could induce ATP release from esophageal epithelium and cause an inflammation response to produce a variety of inflammatory mediators (7, 17). It is well known that these mediators are able to activate and/or sensitize sensory afferents in the gastrointestinal tract (3, 4). To rule out that acid-induced activation of esophageal nodose C fibers is secondary for the release of these mediators, we tested the hypothesis that intraluminal infusion with these mediators themselves did not evoke action potential discharges in esophageal nodose C fibers.
We performed extracellular single-unit recordings on nodose C fibers in ex vivo esophageal-vagal preparations from naive animals. Intraluminal infusion with ATP (10–100 μM) for 30–60 min did not evoke action potential discharges in esophageal nodose C fibers (baseline vs. ATP: 1.3 ± 0.3 vs. 1.7 ± 0.3 Hz, n = 11). After ATP was washed out with fresh KBS (pH = 7.4) for 30 min, intraluminal infusion with capsaicin (1 μM) for 30–60 min did not evoke action potential discharges in these C fibers (baseline vs. capsaicin: 1.3 ± 0.3 vs. 2 ± 0.5 Hz, n = 11; Fig. 3A). In contrast, when perfused from the serosal side of the esophagus, ATP mildly increased action potential discharges of the same C fibers (baseline vs. ATP: 1.3 ± 0.3 vs. 2.9 ± 0.4 Hz, n = 11, P < 0.05). Similarly, capsaicin activated these C fibers when perfused through the serosal side (baseline vs. capsaicin: 1.3 ± 0.3 vs. 7.5 ± 1.5 Hz, n = 11, P < 0.05).
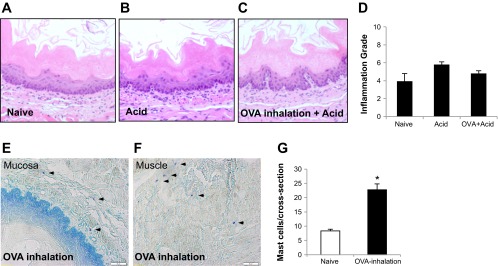
Fig. 3.
Histology of the esophagus: 1) in hematoxylin and eosin stain (A, B, C, and D), compared to control, intraluminal acid infusion in the esophagus from either naive or OVA-inhaled animals did not induce severe tissue damage (no erosion or ulceration) and only slightly increased inflammation grades (control vs. acid, or vs. OVA + acid = 3.89 ± 0.9 vs. 5.78 ± 0.3, or vs. 4.78 ± 0.3, P > 0.05, n = 4 in each group); 2) in toluidine blue stain (E, F, and G), compared with naive animals (n = 3), OVA inhalation significantly increased mast cell infiltrations (arrowheads) in the esophagus from OVA-sensitized animals (n = 4; naive vs. OVA inhalation: 8.33 ± 0.62 vs. 22.75 ± 2.07/cross-section, *P < 0.01).
Intraluminal infusion with a previously reported “inflammation soup” (IS; including bradykinin, PGE2, serotonin, and histamine, all at 10 mmol/l) (4) for 30–60 min did not evoke action potential discharges in esophageal nodose C fibers (baseline vs. IS: 2.1 ± 0.4 vs. 2.3 ± 0.3 Hz, n = 15). After IS was washed out with fresh KBS (pH = 7.4) for 30 min, intraluminal infusion with capsaicin (1 μM) for 30–60 min did not evoke action potential discharges in these C fibers (baseline vs. capsaicin: 2.1 ± 0.4 vs. 2 ± 0.4 Hz, n = 15). When perfused from the serosal side of the esophagus in 8 of these 15 C fibers, IS increased action potential discharges among those C fibers (baseline vs. IS: 1.0 ± 0.31 vs. 5.8 ± 0.8 Hz, n = 8, P < 0.05). Similarly, capsaicin activated these C fibers when perfused through the serosal side (baseline vs. capsaicin: 1.0 ± 0.31 vs. 12.8 ± 1.65 Hz, n = 8, P < 0.05).
These data indicated that intraluminal ATP and inflammatory mediators were not able to activate esophageal nodose C fibers, probably due to the resistance of the epithelial barrier, which prevented these mediators to reach the nerve terminals.
Mast cell activation induces impairment of esophageal epithelial barrier function.
Under the light microscope, we examined esophageal specimens (cross-sections with H-E staining) from animals that received treatments of KBS (pH = 7.4; Control group), ex vivo intraluminal acid (HCl in KBS, pH = 2.1) infusion (Acid group), and OVA inhalation in vivo plus ex vivo intraluminal acid infusion (OVA + Acid group). No severe injuries, including ulceration and erosion, were observed among these three groups. Based on reported methods (12), the inflammation grades were calculated from combined grades of inflammatory cells (mainly neutrophils) in the epithelial layer, basal cell hyperplasia, elongation of lamina propria papillae into the epithelium, and eosinophil infiltration. The esophageal inflammation grades mildly increased in animals treated with either acid infusion (5.78 ± 0.3) or OVA inhalation plus acid infusion (4.78 ± 0.3) compared to that from control (3.89 ± 0.9, vs. control, both P > 0.05, n = 4 in each group; Fig. 3, A-D). In contrast, increased esophageal mast cell infiltration was observed in both mucosa and muscle layers after antigen challenge in antigen-sensitized guinea pigs. Compared with those from naive animals (n = 3), OVA inhalation significantly increased mast cell numbers in the esophagus from OVA-sensitized animals (n = 4; naive vs. OVA inhalation: 8.33 ± 0.62 vs. 22.75 ± 2.07/cross-section, P < 0.01; Fig. 3, E-G).
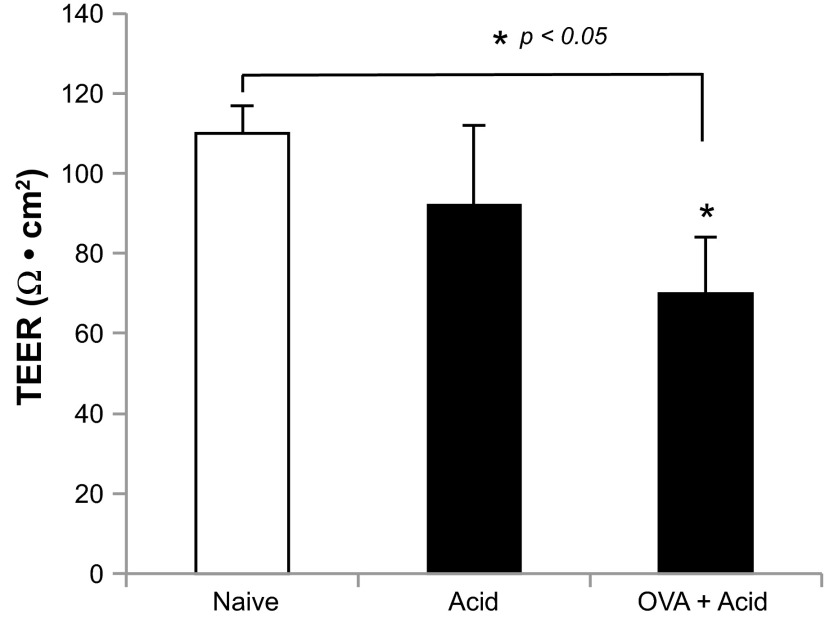
Following extracellular recordings from esophageal-vagal preparations, esophageal mucosal epithelia were carefully dissected and TEERs were measured. In naive animals, acid infusion slightly decreased TEERs of esophageal epithelia (control vs. acid: 110 ± 7 vs. 92 ± 20 Ω·cm2, n = 6–9, P > 0.05). In animals receiving OVA inhalation plus acid infusion, the TEERs of esophageal epithelia were significantly decreased (control vs. OVA + Acid: 110 ± 7 vs. 70 ± 14 Ω·cm2, n = 6–9, P < 0.05; Fig. 4).
Fig. 4.
Transepithelial electrical resistances (TEERs) of esophageal epithelium. In controls (naive animals), intraluminal acid infusion decreased TEERs of esophageal epithelium (control vs. acid: 110 ± 7 vs. 92 ± 20 Ω·cm2, P > 0.05, n = 6 in control and n = 9 in acid groups), and this decrease reached significance from animals pretreated with OVA inhalation (control vs. OVA + acid: 110 ± 7 vs. 70 ± 4 Ω·cm2, *P < 0.05, n = 6 in control and n = 6 in OVA + acid groups).
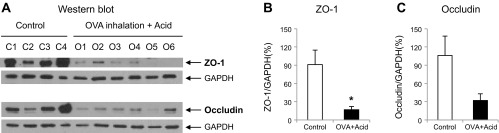
We also examined the expressions of tight junction proteins from these esophageal tissues. Compared to those from naive animals, both ZO-1 and occludin expressions were reduced in esophageal epithelia in animals receiving intraluminal acid infusion (ex vivo preparations) after in vivo OVA inhalations from OVA-sensitized animals. The ratio ZO-1/GADPH significantly reduced from 91 ± 24% (naive, n = 4) to 17 ± 5% (OVA + Acid; n = 6, P = 0.027). The ratio occludin/NADPH reduced from 106 ± 32 (naive, n = 4) to 32 ± 11% (OVA + Acid; n = 6, P = 0.052; Fig. 5, A and B).
Fig. 5.
Expressions of tight junction proteins in esophageal epithelium: A: expressions of zona occludens-1 (ZO-1) and occludin in esophageal epithelium by Western blot. B and C: compared with controls (naive animals), intraluminal acid infusion in animals pretreated with OVA inhalation reduced the expression of ZO-1 (control vs. OVA + acid: 91 ± 24 vs. 17 ± 5% of GAPDH, P = 0.027, n = 4 in control and n = 6 in OVA + acid groups) and occludin (control vs. OVA + acid: 106 ± 32 vs. 32 ± 11% of GAPDH, P = 0.052, n = 4 in control and n = 6 in OVA + acid groups) in esophageal epithelium.
These data suggested that OVA inhalation plus ex vivo intraluminal acid infusion did not induce gross esophageal epithelial damage and severe inflammation, but led to reduced expression of tight junction proteins such as ZO-1 and occludin, which might contribute to impaired epithelial barrier function.
DISCUSSION
Clinical evidence supports a strong causal relationship between acid reflux and esophageal painful sensations such as heartburn and noncardiac chest pain, which can be successfully relieved by acid inhibition therapy (15). But direct evidence of acid-induced activation on esophageal nociceptive afferent nerve terminals is still lacking. Combining our established models for extracellular single-unit recording in esophageal-vagal preparations and mast cell activation by antigen tissue challenge or antigen inhalation in vivo, the present study for the first time demonstrated that intraluminal acid infusion activates nodose C fibers when esophageal mast cell activation is induced by either antigen inhalation or antigen tissue challenge in antigen-sensitized animals. Intraluminal acid infusion in the esophagus from antigen-inhaled animals did not induce severe epithelial damage but reduced the expression of tight junction proteins and increased epithelial permeability. These changes may allow intraluminal acid to reach sensory nerve terminals underneath and evoke action potential discharges in nociceptive C fibers.
Sensory nerves detect polymodal sensory stimuli through specific groups of ion channels expressed in neuronal cell bodies and nerve terminals. This enables them to transduct different stimulations in the peripheral tissues. Recently, several groups of ion channels have been proved to be involved in sensing tissue acidity. These mainly include acid-sensing ion channels, TRPV1, ionotropic purinoceptors (P2X), several Na+, K+, and Ca2+ channels, and proton-sensing G-protein-coupled receptors (11). At present, the information on acid transduction has largely been extrapolated from the studies carried out at the level of the cell bodies of isolated neurons. These studies have provided important information on acid sensing, but have given little attention to nerve phenotype or acid delivery kinetics—two variables that are critical in acid-induced pain. In the gastrointestinal tract, sensory afferents sensing acid can be processed by different ion channels, depending on the acidity of the local tissue environment, which can be varied in different segments of the gut. But acid transduction in esophageal sensory nerve terminals is still less clear. To better understand the acid-sensing mechanism in the esophagus, we have developed an ex vivo esophageal-vagal preparation to study vagal afferent functions in normal and inflamed esophagus (29, 32). Using these approaches, in the present study we demonstrated that intraluminal infusion with either acid or capsaicin in normal esophagus does not activate esophageal nodose C fibers, and the same C fibers actually can be activated by both acid and capsaicin if they can reach the nerve terminals by delivering from the serosal side of the esophagus. This suggested that normal guinea pig esophageal epithelium might not be permeable to intraluminal acid infusion. This is in agreement with the observation from a previous study on mouse gastroesophageal vagal afferent fibers, using extracellular single fiber recordings in vitro, that application of HCl to the mucosal surface directly above the afferent receptive field did not activate either esophageal mucosal or tension receptors (20). But another study from the same group, using in vitro ferret esophageal-vagal preparations, showed that less that 20% of vagal afferents responded to only high concentrations of HCl (19). The other group using in vivo single fiber recordings on rat esophageal vagal afferents also demonstrated that about 30% vagal afferent fibers responded to intraesophageal acid infusion (21). We thought that several factors might contribute to these different observations, such as, the species of animal, the afferent subtypes, the concentration and method of acid application, and the recording systems. In the present study, we did not study acid-evoked effects in esophageal nodose Aδ fibers, which do not respond to noxious stimulation such as capsaicin (32) but might be activated by acid, as shown in airway nodose Aδ fibers (14).
Previous studies have shown that esophageal acid infusion led to tissue damage and inflammation (7, 16, 25, 28). These experiments were carried out in acute or chronic acid-infusion models either in vivo or in vitro. In the present study, intraluminal acid infusion was performed in ex vivo esophageal-vagal preparations. This minimized the effects from those mediators released in the tissue when studying the acid-sensing process in the nerve terminals. Moreover, our data from the present study show that intraluminal infusion with these mediators did not evoke action potential discharges in esophageal nodose C fibers. Thus the observed activation effect by intraluminal acid in esophageal nodose C fibers is more likely a direct response in the nerve ending induced by acid itself.
The mast cell is an important residential immune cell in the tissues. It plays an important role in initiating allergic/inflammation reactions. Mast cell activation-released mediators regulate not only sensory nerves but also epithelial barrier functions in the proximity (2). It is well known that mast cell activation increases intestinal permeability (22, 24), which may involve mast cell chymase-dependent damage on intestinal epithelial tight junction proteins (9, 10). Tight junction proteins, such as ZO-1 and occludin, have been reported to express in human esophageal epithelium (13), and may play important roles in epithelial barrier functions. Our previous study showed that esophageal mast cells contain chymase in their granulate content, which can be released after antigen-induced mast cell activation (30). Whether esophageal mast cell activation affects esophageal epithelial barrier function is still unknown. The data from the present study provided the first evidence that esophageal mast cell activation by antigen inhalation reduced the expressions of tight junction proteins and increased epithelial permeability to intraluminal acid. After in vivo antigen inhalation, intraluminal acid infusion subsequently activated esophageal nodose C fibers underneath. We further proved the specific involvement of mast cells by using a more specific tissue antigen challenge to induce esophageal mast cell activation, a well-defined model in our previous studies (29, 30), and demonstrated that intraluminal acid infusion also activated esophageal nodose C fibers afterward. The present results that acid activates esophageal nodose C fibers only after mast cell activation and that mast cell activation disrupts the esophageal epithelial barrier are novel findings. These provide us a model to further clarify acid-sensing mechanisms in esophageal vagal afferent subtypes.
We have reported that guinea pig esophagus is innervated by distinct subtypes of vagal nociceptive C fibers: the vagal jugular C fibers that are developmentally derived from the neural crest, and the vagal nodose C fibers derived from placodes (32). In the present study we only studied nodose C fiber subtypes. This is based on several considerations: first, esophageal nodose C fibers express more acid-sensitive ion channels/receptors (such as P2X) than jugular C fibers (33); second, the response of nodose C fibers to mast cell mediators in our ex vivo esophageal-vagal model is more well defined than in jugular C fibers (29); third, a previous study on acid-induced responses in guinea pig airway afferent subtypes demonstrated that citric acid application in receptive fields more frequently induced activation responses in nodose C fibers than in jugular C fibers (14). However, the manner in which neural crest vagal jugular C fibers respond to intraluminal acid infusion deserves further exploration. In the present study, intraluminal acid infusion-evoked activation of esophageal nodose C fibers only occurred after esophageal mast cell activation. One critical question may arise: whether mast cell activation-induced sensitization of esophageal nodose C fibers also contributes to acid-induced activation of these C fibers. Based on the present data, we cannot rule out this possibility. This is a very important but complicated question, because mast cell-induced sensitization of esophageal nodose C fibers may involve several ion channels or receptors downstream, and the thresholds of these ion channels or receptors to acid stimulation are different. Based on the present model and results, we will continue to elucidate the contribution of peripheral afferent sensitization to acid sensing in the esophagus in a separate study.
In summary, the present study demonstrated that intraluminal acid infusion does not activate esophageal nodose C fibers from normal esophagus, but evokes profound activation responses after mast cell activation. Esophageal mast cell activation by antigen-inhalation does not cause severe tissue damage and inflammation but increases epithelial permeability, which leads to intraluminal acid that is permeable to sensory nerve terminals underneath. These data not only provide for the first time direct evidence of acid-induced activation of nociceptive sensory nerve endings in the guinea pig esophagus but also establish a validated model to study acid-sensing processes in the esophagus. This finding may help to better understand the development of esophageal painful sensations (such as heartburn) in patients with nonerosive reflux disease, functional heartburn, or gastroesophageal reflux disease with asthma.
GRANTS
This study was supported by NIH Grant DK-087991 (to S. Yu) and DK-084039 (to C. Owyang)
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.Z., A.H., and S.Y. performed experiments; S.Z., Z.L., A.H., and S.Y. analyzed data; S.Z., Z.L., and S.Y. interpreted results of experiments; S.Z., Z.L., A.H., and S.Y. prepared figures; C.O. and S.Y. conception and design of research; S.Y. drafted manuscript; S.Y. edited and revised manuscript; S.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Undem, Myers, and Kollarik for insightful comments on the manuscript.
REFERENCES
- 1.Barlow WJ, Orlando RC. The pathogenesis of heartburn in nonerosive reflux disease: a unifying hypothesis. Gastroenterology 128: 771–778, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff SC. Physiological and pathophysiological functions of intestinal mast cells. Semin Immunopathol 31: 185–205, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil 19: 1–19, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Brumovsky PR, Feng B, Xu L, McCarthy CJ, Gebhart GF. Cystitis increases colorectal afferent sensitivity in the mouse. Am J Physiol Gastrointest Liver Physiol 297: G1250–G1258, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buzza MS, Netzel-Arnett S, Shea-Donohue T, Zhao A, Lin CY, List K, Szabo R, Fasano A, Bugge TH, Antalis TM. Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proc Natl Acad Sci USA 107: 4200–4205, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Oshima T, Tomita T, Fukui H, Watari J, Matsumoto T, Miwa H. Acidic bile salts modulate the squamous epithelial barrier function by modulating tight junction proteins. Am J Physiol Gastrointest Liver Physiol 301: G203–G209, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Cheng L, Cao W, Fiocchi C, Behar J, Biancani P, Harnett KM. HCl-induced inflammatory mediators in cat esophageal mucosa and inflammatory mediators in esophageal circular muscle in an in vitro model of esophagitis. Am J Physiol Gastrointest Liver Physiol 290: G1307–G1317, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Farré R, De Vos R, Geboes K, Verbecke K, Vanden Berghe P, Depoortere I, Blondeau K, Tack J, Sifrim D. Critical role of stress in increased oesophageal mucosa permeability and dilated intercellular spaces. Gut 56: 1191–1197, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groschwitz KR, Ahrens R, Osterfeld H, Gurish MF, Han X, Abrink M, Finkelman FD, Pejler G, Hogan SP. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc Natl Acad Sci USA 106: 22381–22386, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groschwitz KR, Wu D, Osterfeld H, Ahrens R, Hogan SP. Chymase-mediated intestinal epithelial permeability is regulated by a protease-activating receptor/matrix metalloproteinase-2-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 304: G479–G489, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holzer P. Acid sensing by visceral afferent neurones. Acta Physiol (Oxf) 201: 63–75, 2011. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology 109: 1503–1512, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Kimura Y, Shiozaki H, Hirao M, Maeno Y, Doki Y, Inoue M, Monden T, Ando-Akatsuka Y, Furuse M, Tsukita S, Monden M. Expression of occludin, tight-junction-associated protein, in human digestive tract. Am J Pathol 151: 45–54, 1997. [PMC free article] [PubMed] [Google Scholar]
- 14.Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol 543: 591–600, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacy BE, Weiser K, Chertoff J, Fass R, Pandolfino JE, Richter JE, Rothstein RI, Spangler C, Vaezi MF. The diagnosis of gastroesophageal reflux disease. Am J Med 123: 583–592, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Lanas A, Royo Y, Ortego J, Molina M, Sáinz R. Experimental esophagitis induced by acid and pepsin in rabbits mimicking human reflux esophagitis. Gastroenterology 116: 97–107, 1999. [DOI] [PubMed] [Google Scholar]
- 17.Ma J, Altomare A, Rieder F, Behar J, Biancani P, Harnett KM. ATP: a mediator for HCl-induced TRPV1 activation in esophageal mucosa. Am J Physiol Gastrointest Liver Physiol 301: G1075–G1082, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci USA 100: 7761–7766, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page AJ, Blackshaw LA. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J Physiol 512: 907–916, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol 87: 2095–2103, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Peles S, Medda BK, Zhang Z, Banerjee B, Lehmann A, Shaker R, Sengupta JN. Differential effects of transient receptor vanilloid one (TRPV1) antagonists in acid-induced excitation of esophageal vagal afferent fibers of rats. Neuroscience 161: 515–525, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scudamore CL, Thornton EM, McMillan L, Newlands GF, Miller HR. Release of the mucosal mast cell granule chymase, rat mast cell protease-II, during anaphylaxis is associated with the rapid development of paracellular permeability to macromolecules in rat jejunum. J Exp Med 182:1871–1881, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut 61: 1340–1354, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Snoek SA, Dhawan S, van Bree SH, Cailotto C, van Diest SA, Duarte JM, Stanisor OI, Hilbers FW, Nijhuis L, Koeman A, van den Wijngaard RM, Zuurbier CJ, Boeckxstaens GE, de Jonge WJ. Mast cells trigger epithelial barrier dysfunction, bacterial translocation and postoperative ileus in a mouse model. Neurogastroenterol Motil 24: 172–184, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Souza RF, Huo X, Mittal V, Schuler CM, Carmack SW, Zhang HY, Zhang X, Yu C, Hormi-Carver K, Genta RM, Spechler SJ. Gastroesophageal reflux might cause esophagitis through a cytokine-mediated mechanism rather than caustic acid injury. Gastroenterology 137: 1776–1784, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Stein J, Ries J, Barrett KE. Disruption of intestinal barrier function associated with experimental colitis: possible role of mast cells. Am J Physiol Gastrointest Liver Physiol 274: G203–G209, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Tobey NA, Hosseini SS, Argote CM, Dobrucali AM, Awayda MS, Orlando RC. Dilated intercellular spaces and shunt permeability in nonerosive acid-damaged esophageal epithelium. Am J Gastroenterol 99: 13–22, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Tobey NA, Hosseini SS, Caymaz-Bor C, Wyatt HR, Orlando GS, Orlando RC. The role of pepsin in acid injury to esophageal epithelium. Am J Gastroenterol 96: 3062–3070, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Yu S, Kollarik M, Ouyang A, Myers AC, Undem BJ. Mast cell-mediated long-lasting increases in excitability of vagal C fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 293: G850–G856, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Yu S, Li Q, Cavanaugh S, Undem BJ, Ouyang A. Characterization of mast cell subtypes, distribution, and antigen-induced activation in the guinea pig esophagus. Dis Esophagus 22: 600–605, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Yu S, Stahl E, Li Q, Ouyang A. Antigen inhalation induces mast cells and eosinophils infiltration in the guinea pig esophageal epithelium involving histamine-mediated pathway. Life Sci 82: 324–330, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Yu S, Undem BJ, Kollarik M. Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J Physiol 563: 831–842, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]