Abstract
Brain-derived neurotrophic factor (BDNF) belongs to the neurotrophin family of proteins best known for its role in neuronal survival, differentiation, migration, and synaptic plasticity in central and peripheral neurons. BDNF is also widely expressed in nonneuronal tissues including the gastrointestinal tract. The role of BDNF in intestinal smooth muscle contractility is not well defined. The aim of this study was to identify the role of BDNF in carbachol (CCh)- and substance P (SP)-induced contraction of intestinal longitudinal smooth muscle. BDNF, selective tropomyosin-related kinase B (TrkB) receptor agonists, and pharmacological inhibitors of signaling pathways were examined for their effects on contraction of rabbit intestinal longitudinal muscle strips induced by CCh and SP. BDNF activation of intracellular signaling pathways was examined by Western blot in homogenates of muscle strips and isolated muscle cells. One-hour preincubation with BDNF enhanced intestinal muscle contraction induced by CCh but not by SP. The selective synthetic TrkB agonists LM 22A4 and 7,8-dihydroxyflavone produced similar effects to BDNF. The Trk antagonist K-252a, a TrkB antibody but not p75NTR antibody, blocked the effect of BDNF. The enhancement of CCh-induced contraction by BDNF was blocked by the phospholipase C (PLC) antagonist U73122, but not by ERK1/2 or Akt antagonists. Direct measurement in muscle strips and isolated muscle cells showed that BDNF caused phosphorylation of TrkB receptors and PLC-γ, but not ERK1/2 or Akt. We conclude that exogenous BDNF augments the CCh-induced contraction of longitudinal muscle from rabbit intestine by activating TrkB receptors and subsequent PLC activation.
Keywords: neurotrophins, smooth muscle contraction, Trk receptors
brain derived-neurotrophic factor (BDNF) belongs to the neurotrophin family of peptides that includes nerve growth factor, neurotrophin-3, and neurotrophin-4/5. Neurotrophins are best known for their prolonged effects and role in neuronal survival, development, differentiation, migration, and synaptic plasticity (47, 56). BDNF mediates its biological functions by activating two distinct cell membrane receptors: p75NTR, a low-affinity receptor to which all neurotrophins bind, and tropomyosin-related kinase B (TrkB), a high-affinity receptor that preferentially binds BDNF (46). BDNF binding to TrkB induces autophosphorylation of the receptor intracellular tyrosine kinase domain that leads to activation of one or more of three canonical and independent intracellular signaling pathways: the Ras/extracellular signal-regulated kinase (ERK) pathway, the phosphatidylinositol-3-OH kinase (PI3K)/Akt pathway, and the phospholipase C-γ1 (PLC-γ1) pathway (52).
There have been relatively few studies of the effects of BDNF on gastrointestinal motility even though several sources of BDNF are present in the gut including gastrointestinal smooth muscle (4, 16–18, 24, 35), vascular smooth muscle in the gut wall (17), mucosal cells including some enteroendocrine cells (7, 16, 29, 34, 35, 38), and neuronal and/or glial components of the myenteric and submucosal plexus (7, 24–26, 29, 34, 35). Where studied, however, BDNF has been shown to enhance excitation. In rat, exogenous BDNF enhances the sensory limb of the colonic peristaltic reflex by augmenting the release of serotonin and calcitonin gene-related peptide and to augment the frequency, amplitude, and duration of colonic spike bursts (9, 22, 23). In mice, partial depletion of BDNF (BDNF+/−) results in decreased velocity of fecal pellet propulsion (22). In humans, BDNF enhanced gut motility, accelerated colonic transit, and increased stool frequency without changing stool consistency, suggesting a role in motility rather than secretion (8, 12, 59). The mechanisms underlying the excitatory effect of BDNF on gut motility have not been fully characterized.
BDNF also has a potential role in the gut pathophysiology because expression of BDNF is upregulated in inflammation (13, 29–31, 54, 60, 61). In a similar manner, BDNF is upregulated in airway smooth muscle during airway inflammation and causes a hypercontractility of airway smooth muscle (1, 40, 48, 49, 57). This raises the possibility that the increased BDNF expression in gut smooth muscle may account in part for the hypercontractility of the gut muscularis during inflammation.
In the present study, we have tested the hypothesis that BDNF causes an increased contractile response in intestinal smooth muscle analogous to its effects in airway smooth muscle. The effects of BDNF on carbachol (CCh)- and substance P (SP)-induced contractions were studied in longitudinal muscle-myenteric plexus (LM-MP) strips isolated from rabbit jejunum in the presence and absence of selective antagonists of signaling pathways. In addition, we measured the phosphorylation of intracellular signaling molecules in rabbit longitudinal muscle strips and isolated muscle cells in response to BDNF. The results demonstrate that BDNF enhances CCh- but not SP-induced contraction of longitudinal smooth muscle by activation of PLC.
MATERIALS AND METHODS
Preparation of muscle strips.
New Zealand White rabbits (weight: 4–5 lbs) were purchased from RSI Biotechnology, Clemmons, NC and housed in the animal facility administered by the Division of Animal Resources, Virginia Commonwealth University. All procedures were approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University.
Rabbits were killed by injection of Euthasol (100 mg/kg), and intestinal muscle strips were prepared from the jejunum. The jejunum was removed, flushed with Krebs buffer, and placed in warmed (37°C) Krebs buffer of the following composition (in mM): 118 NaCl, 4.75 KCl, 1.19 KH2PO4, 1.2 MgSO4, 2.54 CaCl2, 25 NaHCO3, 11 mM glucose (pH 7.4) and bubbled with 95% O2-5% CO2. Two- to 3-cm-long segments were freed of fat and mesenteric attachments. The longitudinal layer was peeled off carefully with wet Kim wipes to form a LM-MP preparation and held in oxygenated Krebs buffer until use. The same strip preparation was used for strips designated for tension recording, measurement of signaling molecules, or generation of smooth muscle cultures; separate strips were used for each experiment and procedure.
Muscle strips were tied in the orientation of the longitudinal muscle layer at both ends with surgical silk. One end was tied to a glass hook and the strip was placed in a vertical orientation in a 2- or 5-ml organ bath (Radnoti, Monrovia, CA) containing oxygenated and warmed Krebs buffer. The other end of the muscle strip was then attached to a model FT03C force-displacement transducer (Grass Technologies, Quincy, MA). The bath fluid was changed at 15-min intervals during equilibration and after each test agent. Force recordings were amplified by a 15A12 model amplifier (contained within a model 15LT amplifier system) and relayed to a PVA-16 Polyview adaptor (Grass Instruments, Quincy, MA).
Experimental design and data analysis for tension experiments.
The strips were allowed to equilibrate at 1 g tension for at least 1 h before any experiment. Exposure to test agents, inhibitors, CCh, and SP was done while strips were suspended in the organ bath. In each case, control responses to CCh and SP were first established before subsequent addition of test agonists. Each strip served as control for its own treatment.
Experiments were conducted in the following manner. Two strips were assigned for each experimental condition and examined in parallel in separate organ baths after standard responses were obtained. Control strips were first tested with a defined concentration of either CCh or SP for 3 min, then washed a total of three times with 15-min intervals. A second dose of CCh or SP was applied after that and a second cycle of three washings was applied. The strips were maintained in Krebs for 1 h and the response to CCh or SP was repeated. For BDNF-treated strips, the control response to CCh or SP was obtained in the absence of BDNF as in control strips, then BDNF was added to the bath for a 1-h equilibration period, after which the strips were exposed to CCh or SP for 3 min. From preliminary studies presented under results in which strips were preincubated for varying lengths of time (15 min to 24 h), it was determined that 1 h preincubation was optimal and all subsequent studies were done at 1 h preincubation. For strips that were assigned for antagonist treatment, the antagonist was added to the bath 15 min before BDNF. These included antagonists of neurotransmission (tetrodotoxin), the TrkB receptor (K252a), Erk 1/2 (PD98059), PI3K/Akt (LY294002), and PLC (U73122). For strips assigned to treatment with antibodies to either TrkB or p75NTR, the antibody was added at the same time as BDNF and remained in the organ bath for the 1 h of incubation. Control strips were incubated with the antagonists or antibodies in the same manner in the absence of BDNF. Strips were randomly assigned to the experimental groups.
Results were analyzed by using the Polyview software program, where basal tone was measured as the mean tension during a 3-min period following at least 30 min of equilibration (control conditions) or 15 min of inhibitor incubation prior to addition of CCh or SP. The basal recording was obtained during the interval just preceding CCh or BDNF administration. Peak contraction was measured as amplitude (peak to peak) of contraction above basal (in grams) during the 2-min period following administration of CCh or SP. The contraction amplitudes were compared between treatment conditions and controls; thus each strip served as its own control. Statistical analysis was done on the data as recorded in grams force using ANOVA and a post hoc Tukey's test for multiple comparisons with GraphPad (PRISM/GraphPad Software, La Jolla, CA). A paired Student's t-test was used when control and experimental data were obtained from the same strip and a single comparison was made. A probability of P < 0.05 was considered significant. Values are reported as grams or as data normalized to percentage of control CCh-induced contraction in the same strip and are means ± SE. As separate strips were used from separate animals for each experiment, n values represent the number of experiments, strips, and animals.
Preparation and culture of isolated smooth muscle cells.
LM-MP strips were prepared from jejunum as described above for tension measurements, and smooth muscle cells were isolated and grown in culture as described previously (45, 58). Briefly, strips were incubated for 30 min in buffer at 31°C containing 0.1% collagenase (300 U/ml) and 0.01% soybean trypsin inhibitor (wt/vol). The partly digested tissues were washed twice with 50-ml of collagenase-free buffer and the muscle cells were allowed to disperse spontaneously for 30 min in collagenase-free medium. Cells were harvested by filtration through 500 μm Nitex and centrifuged twice at 350 g for 10 min to eliminate broken cells and organelles. The muscle cells were resuspended in Dulbecco's modified Eagle's medium (DMEM) containing penicillin (200 U/ml), streptomycin (200 μg/ml), gentamycin (100 μg/ml), amphotericin B (2.5 μg/ml), and 10% fetal bovine serum (DMEM-10), plated at a concentration of 5 × 105 cells/ml, and incubated at 37°C in a CO2 incubator. DMEM-10 medium was replaced every 3 days for 2–3 wk until confluence was attained. The muscle cells in confluent primary cultures were trypsinized (0.5 mg trypsin/ml), replated at a concentration of 2.5 × 105 cells/ml, and cultured under the same conditions. All experiments were done on cells in the first passage. Previous studies determined the purity of cultured muscle cells with smooth muscle-specific γ-actin and absence of non-smooth muscle cell types (58). Cultured muscle cells were starved in serum-free medium for 24 h before use to measure the effect of BDNF on expression of signaling molecules as described under Western immunoblotting below.
Protein extraction.
To determine the cellular signaling pathways mediating the response to BDNF, LM-MP and cultured smooth muscle cells from the longitudinal muscle layer were incubated in the presence of 10 nM BDNF to match the tension experiments and then the protein was extracted. In this manner the signaling pathways data and the contractile data for augmentation by BDNF were comparable.
LM-MP strips were homogenized with solubilization buffer of the following composition: 50 mM Tris·HCl, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 100 mM NaF. Cultures of muscle cells were solubilized in lysis buffer containing 20 mM Tris·HCl, 1 mM dithiothreitol, 100 mM NaCl, and 0.5% sodium dodecyl sulfate. Both solubilizing solutions contained a protease inhibitor cocktail and phosphatase inhibitor cocktail (100 μg/ml PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 30 mM sodium fluoride, and 3 mM sodium vanadate). After sonication for 15 s and centrifugation at 2,000 g for 10 min at 4°C, the protein concentrations in the supernatant was determined by use of a protein assay kit (Bio-Rad, Hercules, CA).
Western immunoblotting.
Equal amounts of proteins were separated by SDS-PAGE electrophoresis using 10% and 15% (wt/vol) acrylamide resolving gel and electrophoretically transferred onto a nitrocellulose membrane. The membrane was blocked by incubation with 5% (wt/vol) nonfat dried milk-TBS-T (Tris-buffered saline, pH 7.6 plus 0.1% Tween-20) for 1 h at room temperature with gentle shaking. Then the membrane was incubated for 25 h at 4°C with different primary antibodies diluted in TBS-T 1% (wt/vol) nonfat dried milk. After a washing with TBS-T (3×5 min), the membrane was incubated with horseradish peroxidase-conjugated matching secondary antibody (1: 2,000) in TBS-T or IRDye secondary antibody with gentle shaking at room temperature for 1 h. After washing the membrane with TBS-T (3×5 min), the immunoreactive blot was visualized by SuperSignal Femto maximum sensitivity substrate kit or with the ODYSSEY infrared imaging system (LI-COR Biosciences, Lincoln, NE). This approach was used with all homogenates from LM-MP for all phosphorylated signaling proteins and for measurement of p-Akt and p-ERK1/2 from isolated muscle cells.
For measurement of p-PLCγ and p-TrkB in cultures of isolated muscle cells, homogenates were first immunoprecipitated with one antibody and then immunoblotted with the other antibody following treatment of the cells with 10 nM BDNF or 1 μM LM22A4. Cultured cells were homogenized in lysis buffer and centrifuged at 15,000 g for 30 min. The supernatant was precleared by incubation with 5 μl of PLC-γ antibody or 5 μl of TrkB antibody for 2 h at room temperature followed by incubation overnight with 40 μl of protein-A/G agarose. The immunoprecipitates were separated by SDS-PAGE and transferred electrophoretically to PVDF membranes. The membranes were incubated overnight at 4°C with phospho-PLC-γ (1:1,000) in 5% wt/vol nonfat dry milk, 1× TBS, or phospho-TrkB (1:500) in 5% wt/vol nonfat dry milk, 1× TBS, 0.1% Tween 20. The membranes were then incubated at room temperature for 2 h in secondary antibodies conjugated to IRDye 800CW or 680 (LI-COR) diluted to 1:15,000 in PBS containing 0.1% Tween 20 and 0.01% SDS.
To ensure equal amount of protein loading, membranes were stripped and reblotted against the housekeeping protein GAPDH or antibody to the nonphosphorylated form of the kinase examined. Densitometric quantification of protein bands was done by using the software FluoroChem 8800 or with the ODYSSEY infrared imaging system (LI-COR Biosciences). The average band intensity was normalized to that of the control of the same lane. Statistical difference between the basal value and values after BDNF treatment was determined by paired Student's t-tests.
Materials.
U73122 (PLC inhibitor), PD98059 (ERK1/2 inhibitor), and LY294002 (PI3K/Akt inhibitor) were obtained from Enzo Life Sciences, Farmingdale, NY; SP was from Bachem, Torrance, CA; K252a (TrkB inhibitor) was obtained from Calbiochem, Cambridge, MA; LM22A4 and 7,8-dihydroxyflavone were obtained from Tocris Bioscience (Minneapolis, MN); BDNF was from Promega-Fisher, Madison, WI; and all other chemicals were from Sigma-Aldrich, St. Louis, MO.
Antibodies.
p75NTR (AB#1554) was from Millipore, Billerica, MA; TrkB (SC-8316), PLC-γ1 (SC-81), p-PLC-γ1(pY783.27) (SC-136186), and GAPDH (SC-25778) were from Santa Cruz Biotechnology, Santa Cruz, CA; and p-TrkB(Y516)/TrkA(Y490) (AB#4619), p-Akt (S473) (AB#4051), Akt (AB#9272), and p-ERK1/2 (Thr202/Y204) (AB#9106) were from Cell Signaling (Boston, MA).
RESULTS
CCh induced concentration-dependent contraction.
In rabbit intestinal LM-MP strips, CCh caused a concentration-dependent contraction in the range of concentrations tested (10 nM–100 μM) (data not shown) consistent with the well-known effects of cholinergic agonists on gut smooth muscle. The maximal response was obtained at 100 μM CCh (1.5 ± 0.08 g; n = 8). The effect of BDNF and all other agonists and inhibitors on the contractile responses was investigated by using a submaximal concentration of 10 μM CCh to induce muscarinic contraction. Contraction after test agent administration was compared with 10 μM CCh peak contraction during the control period and values were expressed as percentage of the control contraction induced by CCh with each strip serving as its own control.
Effect of BDNF on basal tone.
Exogenous BDNF did not significantly change basal tone of LM-MP strips on acute addition to the organ bath, and basal tone of the strips did not change during the course of the experiment regardless of the presence or absence of BDNF alone. The basal tension before addition of 10 nM BDNF was 0.82 ± 0.03 g, and following 1 h incubation in the presence of 10 nM BDNF the basal tone was 0.92 ± 0.04 g (P > 0.05, n = 18) and was 0.88 ± 0.05 g (P > 0.05, n = 15) following 1-h incubation in the absence of BDNF.
Effect of BDNF on peak contraction induced by CCh.
Incubation of rabbit LM-MP strips with 10 nM BDNF for 1-h significantly enhanced the contraction induced by 10 μM CCh from 1.45 ± 0.20 g above basal during the control period in the absence of BDNF to 2.75 ± 0.31 g above basal following pretreatment with BDNF (Fig. 1). This represents an augmentation of CCh-induced contraction of LM-MP by 88.0 ± 5.9% after pretreatment with 10 nM BDNF (n = 7, P < 0.05) (Fig. 1). In preliminary studies, preincubation of LM-MP with BDNF for shorter time periods of 15 and 30 min did not significantly augment CCh-induced contractions (9 ± 6 and 12 ± 7% above control contractions, respectively), and incubation for a longer period of 24 h caused a lesser but significant increase in CCh-induced contraction (67 ± 11% above control contraction; n = 4, P < 0.05). All subsequent studies were therefore done at 1 h preincubation with BDNF. LM-MP contraction induced by 10 μM CCh did not change after 1-h incubation with Krebs buffer alone (Fig. 1; initial 1.51 ± 0.16 vs. 1.56 ± 0.15 g after 1 h in Krebs buffer alone, n = 15, P > 0.05). In control studies, the effect of BDNF on CCh-induced contractions was determined to be due to an action at the level of the smooth muscle since the neural blocker, tetrodotoxin (1 μM), had no effect on the augmentation induced by BDNF (79.1 ± 6.3% augmentation in the absence of TTX vs. 85.9 ± 8.7% augmentation in the presence of TTX; P > 0.05; n = 4; data not shown).
Fig. 1.
Effect of brain-derived neurotrophic factor (BDNF) on carbachol (CCh)-induced contraction. A, top: strip 1 is a representative tracing illustrating that 10 μM CCh-induced contraction was unchanged by incubation in Krebs buffer alone. The strip was treated with 10 μM CCh (left), washed 3 times, and incubated for 1 h in Krebs buffer alone, after which 10 μM CCh addition was repeated (right). A, bottom: strip 2 is a representative trace illustrating 10 μM CCh-induced contraction before and after incubation with 10 nM BDNF for 1 h. The strip was treated with 10 μM CCh (left), washed 3 times, and incubated for 1 h in 10 nM BDNF, after which 10 μM CCh addition was repeated (right). B: summary graph shows that BDNF pretreatment significantly increased the contraction induced by 10 μM CCh. Left: data expression in grams of force. Right: data normalized to preincubation CCh contraction. Values are mean ± SE percent of CCh-induced contraction, *P < 0.05, n = 7.
Effects of BDNF on contraction of LM-MP strips induced by SP.
SP caused contraction of rabbit LM-MP in dose-dependent manner (10 nM to 1 μM) (data not shown). Contraction of LM-MP induced by 1 μM SP after 10 nM BDNF pretreatment for 1 h was compared with that induced by 1 μM SP before incubation. In contrast to the effect of BDNF incubation on CCh-induced contraction, 10 nM BDNF incubation for 1 h did not alter the contraction induced by 1 μM SP. The maximal contraction induced by SP in the absence of BDNF was 1.00 ± 0.07 g, and following 1-h incubation with 10 nM BDNF the contraction-induced by 1 μM SP was 1.1 ± 0.1 g (P > 0.05; n = 7).
Effect of TrkB inhibition on BDNF enhancement of CCh-induced contraction.
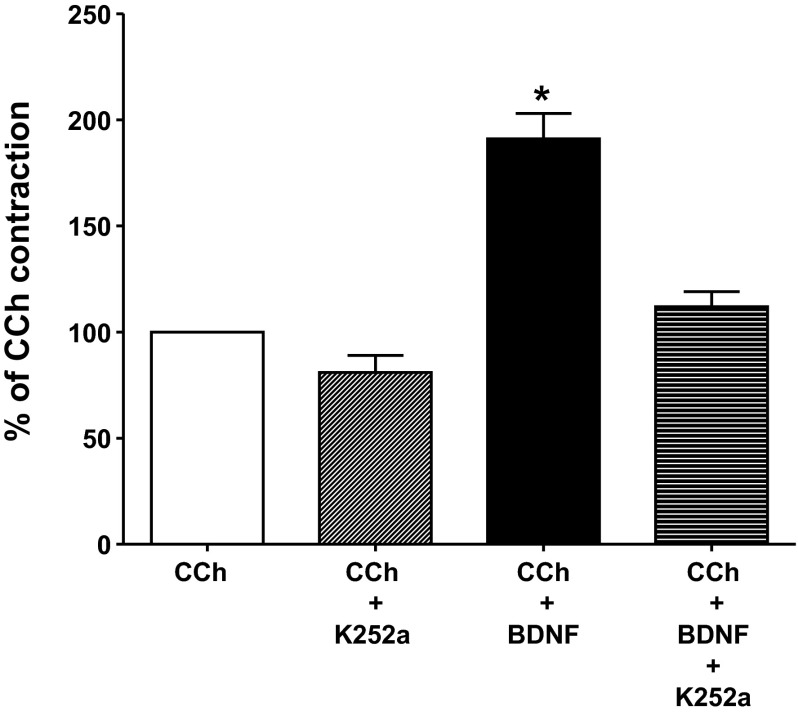
The physiological effect of BDNF is mediated by two cell surface receptors: the BDNF-preferring TrkB receptor and the nonselective p75NTR. Therefore, the effect of BDNF on cholinergic-induced contraction could be attributed to either or both receptors. To test the role of TrkB, we used K252a, an agent that selectively inhibits Trk kinase activity (7, 11, 40, 46, 49, 57, 62). K252a (1 μM) was added 15 min before addition of BDNF and was present during the 1-h incubation period with BDNF. K252a abolished the enhancement of CCh-induced contraction due to BDNF pretreatment, returning CCh-induced contraction to pre-BDNF incubation levels (Fig. 2). In contrast, incubation for 1 h in the presence of K252a alone at the same concentration (1 μM) had no significant effect on baseline tension or on peak contraction to 10 μM CCh. Peak contraction following K252a incubation alone for 1 h was 81 ± 8% of control peak contraction obtained before the incubation period (P > 0.05) (Fig. 2).
Fig. 2.
Effect of tropomyosin-related kinase B (TrkB) antagonist K252a on BDNF-mediated augmentation of intestinal longitudinal muscle-myenteric plexus (LM-MP) contraction induced by CCh. The Trk receptor inhibitor K252a (1 μM) did not significantly affect the contraction induced by 10 μM CCh. Incubation of LM-MP strips with 10 nM of BDNF resulted in a significant 88% augmentation of contraction induced by 10 μM CCh. K252a incubation for 15 min before and during BDNF incubation resulted in an inhibition of the augmentation of CCh-induced contraction by BDNF. The contraction in the presence of BDNF plus K252a was not significantly different from that elicited by 10 μM CCh alone. Data represent the percent of contraction relative to CCh. Values are means ± SE. *P < 0.05, n = 7.
The differential role of the TrkB and p75NTR was additionally tested with selective antibodies to each receptor. In separate strips, a selective antibody to TrkB (SC8316, 1:100) or p75NTR (AB1444, 1:100) was added at the same time as BDNF and was present during the 1-h incubation period with BDNF. Neither antibody had any effect on baseline tone of the muscle strips The TrkB antibody abolished the augmentation of CCh-induced contraction by BDNF (73 ± 10% augmentation in the absence of BDNF antibody vs. 10 ± 12% inhibition in the presence of TrkB antibody) but had no effect on the CCh-induced contraction in the absence of BDNF (Fig. 3). In contrast, the p75NTR antibody had no significant effect on the augmentation of CCh-induced contraction by BDNF (73 ± 10% augmentation in the absence of p75NTR antibody vs. 58 ± 11% augmentation in the presence of p75NTR antibody) or on the CCh-induced contraction in the absence of BDNF (Fig. 3). This is consistent with the effects of K252a, suggesting that the effect of BDNF was mediated by TrkB receptor activation rather than p75NTR activation.
Fig. 3.
Effect of TrkB and p75NTR antibodies on BDNF-mediated augmentation of intestinal LM-MP contraction induced by CCh. A selective antibody to TrkB (SC8316, 1:100) but not to p75NTR (AB1444, 1:100) receptor inhibited the augmentation of CCh-induced contraction by 10 nM BDNF. The antibodies were added at the same time as BDNF and were present during the 1-h incubation period with BDNF. Data represent the percent of contraction relative to CCh. Values are means ± SE. *P < 0.05, n = 4–5.
Effect of selective TrkB agonists on CCh-induced contraction.
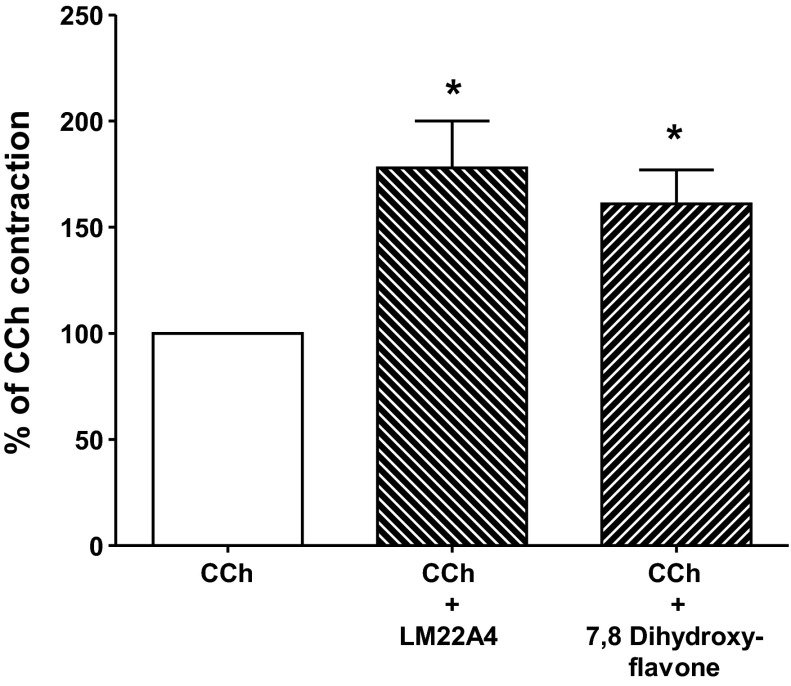
The participation of TrkB was further tested with two selective TrkB agonists LM 22A4 and 7,8-dihydroxyflavone, which do not interact with other Trk receptors and have been shown to be effective TrkB agonists in other systems (1, 5, 28, 37). The same approach was used with these agonists as described for BDNF. Incubation of LM-MP with LM22A4 (1 μM) or 7,8-dihydroxyflavone (1 μM) 60 min before challenging with CCh enhanced the contraction by 78 ± 22 and 61 ± 16%, respectively, above peak CCh-induced contraction obtained before incubation, P < 0.05 (Fig. 4). LM22A4 and 7,8-dihydroxyflavone alone had no contractile or relaxant effect on basal tone (data not shown).
Fig. 4.
TrkB activation by selective agonists enhances the contraction induced by CCh. Activation of TrkB with selective synthetic agonists led to significant increase in contraction induced by 10 μM CCh similar to that elicited by BDNF (cf. Figs. 2 and 3). Application of 1 μM of either LM22A4 or 7,8-dihydroxyflavone for 60 min augmented CCh-induced contraction. Data represent percentage of control, preincubation CCh-induced contraction. Values are means ± SE. *P < 0.05, n = 6.
Phosphorylation of TrkB by BDNF and a TrkB-selective ligand in isolated smooth muscle cells.
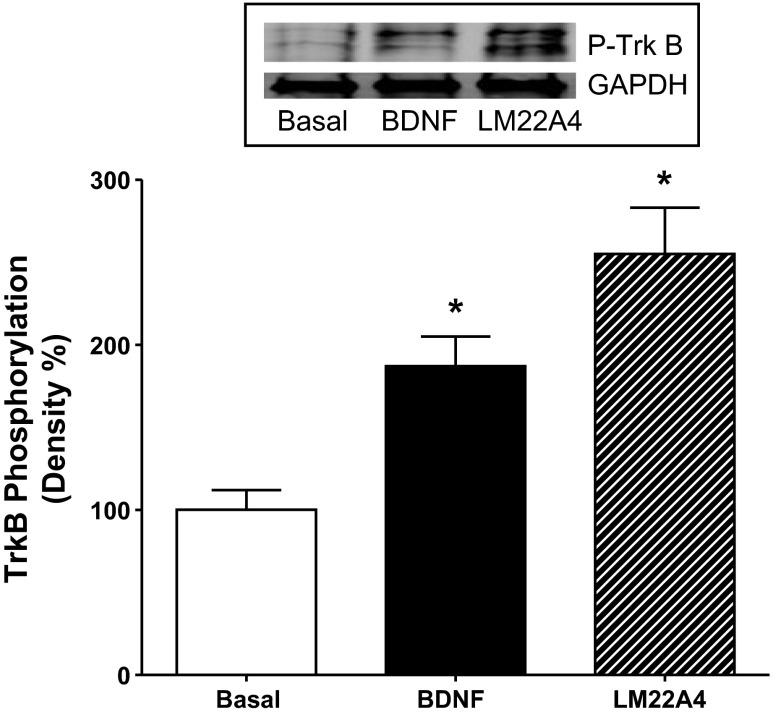
The data with the Trk antagonist K252a (Fig. 2), the TrkB antibody (Fig. 3), and the selective TrkB agonists (Fig. 4) in LM-MP strips strongly support the hypothesis that the BDNF-induced augmentation of contractions in response to CCh is mediated by activation of TrkB receptors on smooth muscle. This notion was directly tested in smooth muscle cells isolated from the rabbit intestinal longitudinal muscle layer. Addition of 10 nM BDNF to cultures of isolated smooth muscle cells caused an 87 ± 21% increase (P < 0.05) in TrkB phosphorylation and addition of the selective TrkB agonist LM22A4 (1 μM) caused a 155 ± 30% increase in TrkB phosphorylation (Fig. 5).
Fig. 5.
Effect of BDNF and TrkB selective agonist on TrkB phosphorylation in cultured muscle cells. Application of 10 nM BDNF or 1 μM LM22A4, a selective TrkB agonist, resulted in phosphorylation of TrkB in smooth muscle cells isolated from the longitudinal muscle layer of rabbit intestine. Smooth muscle cells were grown in culture and used in first passage. Following homogenization, TrkB protein was immunoprecipitated and then immunoblotted with phospho-TrkB antibody as described in materials and methods. Graph denotes integrated intensity of scanned blots and inset illustrates representative immunoblot. Values are means ± SE. *P < 0.05, n = 4.
The signaling pathways involved in the effect of BDNF on CCh-induced contraction.
In general, three signaling pathways (ERK1/2, PLC-γ, and PI3K/Akt) are responsible for BDNF-mediated signal transduction in a variety of tissues (reviewed in Refs. 41, 47, 52). To deduce the signaling pathways involved in the effect of BDNF on CCh-induced contraction in rabbit LM-MP, we used pharmacological inhibitors to block the key enzymes in these pathways. Furthermore, we measured the level of phosphorylation (i.e., activation) of kinases in response to BDNF in muscle strips and cultured smooth muscle cells by Western blot utilizing specific antibodies to these kinases.
Role of ERK1/2 in mediating BDNF-induced enhancement of CCh-induced contraction.
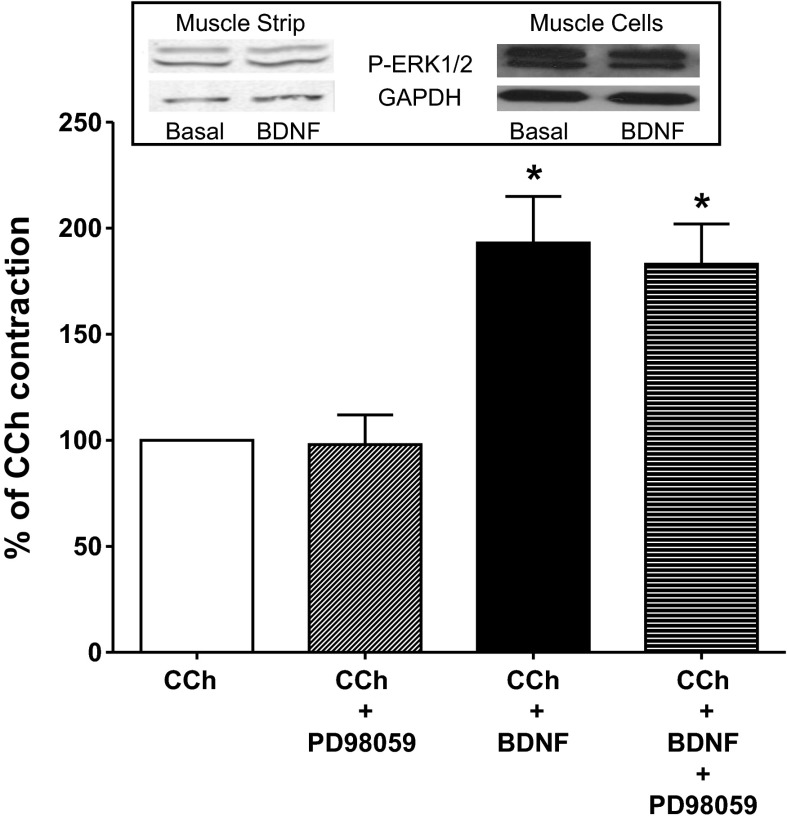
The role of ERK1/2 was assessed with the ERK1/2 specific inhibitor, PD98059. These studies were conducted as described for determination of the effect of BDNF on CCh-induced contractions except that 10 μM PD98059 was added 15 min before BDNF and remained in the organ bath during the 1-h incubation with BDNF. Parallel control LM-MP were incubated with 10 μM PD98059 alone. PD98059 itself had no effect on basal tone of the LM-MP (basal tone: 0.81 ± 0.03 g in the absence and 0.78 ± 0.06 g in the presence of PD 98059, P > 0.05, n = 8). Incubation for 1 h in the presence of 10 μM PD98059 had no effect on the subsequent response to 10 μM CCh. Peak contraction following ERK1/2 inhibition with PD 98059 was 97.6 ± 13.6% of the preincubation control peak contraction (P > 0.05, n = 6) (Fig. 6).
Fig. 6.
Effect of ERK1/2 inhibition on the augmentation of CCh-induced contraction by BDNF. Administration of 10 μM PD98059 did not significantly inhibit contraction induced by 10 μM CCh. PD98059 introduced 15 min before and during BDNF incubation did not significantly reduce the augmentation of CCh-induced by BDNF. Bar graph indicates CCh-induced contraction of muscle strips as a percentage of the control CCh-induced contraction obtained before treatment of muscle strips. Inset indicates representative immunoblot of phospho-ERK1/2 and GAPDH from muscle strip and from cultured muscle cells. Values are means ± SE. *P < 0.05, n = 6–9.
PD98059 also had no effect on the augmentation of CCh-induced contraction induced by 1-h incubation with BDNF. CCh-induced contraction following 1-h incubation with BDNF alone was 193 ± 22% of the CCh-induced contraction obtained prior to incubation with BDNF (P < 0.05; n = 9) (Fig. 6). In parallel LM-MP strips, 15-min pretreatment with 10 μM PD98059 before BDNF incubation and during the 1-h incubation with BDNF did not affect the augmentation by BDNF. CCh-induced contraction following incubation in BDNF plus PD98059 was 183 ± 19% of the contraction obtained prior to incubation with BDNF (P < 0.05; n = 8) (Fig. 6). The augmentations following incubation with BDNF alone and following BDNF plus PD 98059 were not significantly different and indicated that the effect of BDNF on CCh-induced contraction does not proceed through ERK1/2 activation.
To directly test the effect of BDNF on ERK1/2 activation in intestinal smooth muscle, we measured the level of p-ERK1/2 in rabbit LM-MP and cultured smooth muscle cells by Western blot analysis with antibody specific to p-ERK1/2. GAPDH was used to normalize for protein loading of each lane in the gel. BDNF treatment of rabbit intestinal smooth muscle for 1 h had no significant effect on the level of ERK1/2 phosphorylation (3 ± 5% decrease from basal in cultured muscle cells and 7 ± 8% increase in muscle strips) (Fig. 6), consistent with lack of effect of the ERK1/2 antagonist on BDNF-induced augmentation of CCh-induced contraction.
Role of PI3K/Akt in mediating BDNF-induced enhancement of CCh-induced contraction.
The role of PI3K/Akt was assessed with the PI3K specific inhibitor, LY294002. These studies were conducted as described for determination of the effect of BDNF on CCh-induced contractions, except that 10 μM LY294002 was added 15 min before BDNF and remained in the organ bath during the 1-h incubation with BDNF. Parallel control LM-MP strips were incubated with 10 μM LY294002 alone. LY294002 itself had no effect on basal tone of the LM-MP (basal tone: 0.6 ± 0.1 g in the absence and 0.7 ± 0.1 g in the presence of LY294002, P > 0.05, n = 5). Incubation for 1 h in the presence of 10 μM LY294002 had no effect on the subsequent response to 10 μM CCh. Peak contraction following PI3K inhibition with LY294002 was 102 ± 10% of the preincubation control peak contraction (P > 0.05, n = 5) (Fig. 7).
Fig. 7.
Effect of phosphatidylinositol-3-OH kinase (PI3K)/Akt inhibition on the augmentation of CCh-induced contraction by BDNF. Administration of 10 μM LY294002 did not significantly inhibit contraction induced by 10 μM CCh. LY294002 introduced 15 min before and during BDNF incubation did not significantly reduce the augmentation of CCh-induced contraction by BDNF. Bar graph indicates CCh-induced contraction of muscle strips as a percentage of the control CCh-induced contraction obtained before treatment of muscle strips. Inset indicates representative immunoblot of phospho-Akt and GAPDH from muscle strip and from cultured muscle cells. Values are means ± SE. *P < 0.05, n = 5.
LY294002 also had no effect on the augmentation of CCh-induced contraction induced by 1-h incubation with BDNF. CCh-induced contraction following 1-h incubation with BDNF alone was 167 ± 8% of the CCh-induced contraction obtained prior to incubation with BDNF (P < 0.05; n = 5) (Fig. 7). In parallel LM-MP strips, 15-min pretreatment with 10 μM LY294002 before BDNF incubation and during the 1-h incubation with BDNF did not affect the augmentation by BDNF. CCh-induced contraction following incubation in BDNF plus LY294002 was 158 ± 9% of the contraction obtained prior to incubation with BDNF (P < 0.05; n = 5) (Fig. 7). The augmentations following incubation with BDNF alone and following BDNF plus LY294002 were not significantly different and indicated that the effect of BDNF on CCh-induced contraction does not proceed through PI3K/Akt activation.
To directly test the effect of BDNF on Akt activation in intestinal smooth muscle, we measured the level of p-Akt in rabbit LM-MP strips and cultured smooth muscle cells by Western blot analysis with antibody specific to p-Akt. GAPDH was used to normalize for protein loading of each lane in the gel. BDNF treatment of rabbit intestinal smooth muscle cells for 1 h had no significant effect on the level of Akt phosphorylation (12 ± 6% decrease from basal in cultured muscle cells and 9 ± 13% decrease in muscle strips) (Fig. 7), consistent with lack of effect of the PI3K antagonist on BDNF-induced augmentation of CCh-induced contraction.
Role of PLC in mediating BDNF-induced enhancement of CCh-induced contraction.
The role of PLC was assessed with the PLC specific inhibitor, U73122. These studies were conducted as described for determination of the effect of BDNF on CCh-induced contractions except that 10 μM U73122 was added 15 min before BDNF and remained in the organ bath during the 1-h incubation with BDNF. Parallel control LM-MP strips were incubated with 10 μM U73122 alone. U73122 itself had no effect on basal tone of the LM-MP (basal tone: 0.83 ± 0.03 g in the absence and 0.81 ± 0.08 g in the presence of U73122, P > 0.05, n = 12). Incubation for 1 h in the presence of 10 μM U73122 had no significant effect on the subsequent response to 10 μM CCh. Peak contraction following PLC inhibition with U73122 was 117 ± 14% of the preincubation control peak contraction (P > 0.05, n = 6) (Fig. 8).
Fig. 8.
Effect of PLC inhibition on the augmentation of CCh-induced contraction by BDNF. Incubation of longitudinal muscle strips with U73122 alone had no significant effect on contraction induced by 10 μM CCh. U73122 15 min before and during BDNF incubation significantly reduced the effect of BDNF on CCh-induced contraction. CCh-induced contraction in the presence of U73122 plus BDNF was not significantly different from CCh-induced contraction before BDNF treatment. Bar graph indicates CCh-induced contraction of muscle strips as a percentage of the control CCh-induced contraction obtained before treatment of muscle strips. Inset indicates representative immunoblot of phospho-PLC-γ and either GAPDH for cultured muscle cells or total PLC-γ for muscle strip. Values are means ± SE. *P < 0.05, n = 7–12.
In contrast to the ERK1/2 antagonist PD98059 and the PI3K antagonist LY294002, the PLC antagonist U73122 significantly inhibited the augmentation of CCh-induced contraction induced by 1-h incubation with BDNF. CCh-induced contraction following 1-h incubation with BDNF alone was 198% ± 27% of the CCh-induced contraction obtained prior to incubation with BDNF (P < 0.05; n = 7) (Fig. 8). In parallel LM-MP strips, 15-min pretreatment with 10 μM U73122 before BDNF incubation and during the 1-h incubation with BDNF inhibited the augmentation by BDNF. CCh-induced contraction following incubation in BDNF plus U73122 was significantly reduced to 111 ± 18% of the contraction obtained prior to incubation with BDNF (P > 0.05; n = 7) (Fig. 8). The inhibition of the augmentatory effect of BDNF by U73122 indicated that the effect of BDNF on CCh-induced contraction proceeds through PLC, likely PLC-γ, the PLC isoform coupled to neurotrophin signaling including TrkB.
To directly test the effect of BDNF on PLC-γ activation in intestinal smooth muscle, we measured the level of phosphorylated PLC-γ in rabbit LM-MP strips and cultured smooth muscle cells by Western blot analysis with antibody specific to p-PLC-γ. Immunoblots were normalized to total PLC-γ in muscle strips and GAPDH in cultured muscle cells. BDNF treatment of rabbit LM-MP for 1 h significantly increased (P < 0.05) the amount of p-PLC-γ (62 ± 11% increase above basal in cultured muscle cells and 68 ± 9% above basal in muscle strips) (Fig. 8), consistent with a primary role of PLC-γ in mediating the BDNF-induced augmentation of CCh-induced contraction.
DISCUSSION
In this study, we provide evidence demonstrating a functional role of BDNF in gut contractility. BDNF enhanced smooth muscle contraction induced by muscarinic but not tachykinin receptor stimulation. The effect of BDNF is mediated through activation of TrkB receptors and the subsequent phosphorylation of PLC, most likely PLC-γ.
As outlined in the introduction, BDNF is present in the gastrointestinal tract and has been shown to enhance motility, transit, and stool output in both animal and human studies (8, 12, 22, 59). Additionally, mice deficient in BDNF (BDNF+/−) have reduced peristalsis and slower velocity of propulsion of fecal pellets (22). Despite this, little is known of the exact mechanism(s) by which BDNF acts. Several potential sites of action of BDNF in enhancing motility have been suggested. Grider et al. (22, 23) demonstrated that BDNF is released from mucosal enteroendocrine cells and from intrinsic primary afferent neurons innervating the mucosa of rat and mouse colon in response to mucosal mechanical stimulation. In this context BDNF acts to augment the peristaltic reflex leading to enhanced propulsive motility. In guinea pig ileum, Boesmans et al. (7) showed that BDNF enhanced the increase in Ca2+ in myenteric neurons elicited by other excitatory transmitters, thus facilitating synaptic transmission within the enteric nervous system and promoting motility. Chai et al. (9) demonstrated that in the rat colon BDNF increased the frequency, amplitude, and duration of spike bursts recorded from the muscle layer, suggesting the possibility of a site of action on smooth muscle or interstitial cell of Cajal.
The present study in rabbit longitudinal muscle strips and cells, and a study by Chen et al. (10) in mouse longitudinal muscle suggest a smooth muscle site of action, which would also explain the enhanced propulsion by BDNF. In both studies, BDNF augmented smooth muscle contraction elicited by other excitatory neurotransmitters without initiating contraction or altering baseline tone on its own. This is consistent with studies by Grider et al. (22, 23) and by Boesmans (7) in gut, and by Goggi (21) in rat brain in which BDNF enhanced neural activity (i.e., peristalsis, neurotransmitter release, synaptic activity, and elevation of intracellular Ca2+) without having a direct effect on its own. These findings suggest that BDNF should be viewed as a true modulatory agent.
Although both our study and that of Chen et al. (10) support the notion that BDNF can enhance motility by a direct effect on smooth muscle, they differ dramatically in some aspects. In our study, the augmentation by BDNF was limited to the muscarinic agonist since a 60-min preincubation with 10 nM BDNF had no effect on contraction elicited by SP. This is in contrast to the study of Chen et al. in longitudinal strips from mouse intestine and colon in which response to SP (and CGRP) but not acetylcholine was significantly enhanced. The reason for these differences is unclear; however, the studies differ in several ways. We used LM-MP strips devoid of circular muscle from rabbit intestine whereas Chen et al. used full-thickness muscularis strips from mouse intestine and colon containing both circular and longitudinal muscle layers but hung in the longitudinal orientation. Both studies used 10 nM BDNF, but we used a 60-min preincubation whereas Chen et al. used a 10-min incubation period. Finally, we measured the peak tonic contraction in response to CCh and SP, whereas in the mouse intestine and colon the contractile agonists elicited an increase in ongoing phasic contractions. It is noteworthy that similar to our findings in gut smooth muscle, preexposure of rat and human airway smooth muscle to BDNF causes an augmentation of muscarinic contractions (1, 40, 48, 49, 62).
We next identified the mechanism by which BDNF augmented muscarinic contractions. In contrast to seven-transmembrane, G protein-coupled receptors such as those that mediate the response to muscarinic agonists and SP, neurotrophins including BDNF act via two receptors: a specific, high-affinity Trk receptor TrkB in the case of BDNF, and a nonspecific, low-affinity receptor, p75NTR. The canonical intracellular signaling pathways activated by these receptors are the ERK1/2 pathway, the PI3K/Akt pathway, and the PLC-γ pathway. In the present study, we examined the participation of the TrkB and p75NTR receptor and each of these neurotrophin signaling pathways in mediating the BDNF-induced augmentation of muscarinic contractions.
TrkB and p75NTR receptors have been identified in enteric neurons and glia of guinea pig ileum (7), human intestine (14, 24, 25, 26, 29), mouse stomach and intestine (35), and frog and fish intestine (38, 50), although the proportions vary with the species. Additionally, these receptors have been identified in epithelial cells, often enteroendocrine cells, of the mucosa of guinea pig ileum (7), human intestine (14, 15, 26, 29, 55), and frog and fish intestine (38, 50). Most studies have used immunohistochemical approaches and have failed to identify TrkB or p75NTR receptors in gut muscle with the exception of the presence of TrkB receptors in upper esophagus of the mouse (35) and in smooth muscle of blood vessels in the intestinal muscularis (15).
In the present study, we demonstrated by several mechanisms that the ability of BDNF to augment muscarinic contraction is mediated through TrkB receptors. First we used the Trk-specific inhibitor K252a to block TrkB receptors. Although K252a is a general Trk inhibitor, it has been used in a variety of studies with BDNF to differentiate TrkB-mediated responses from p75NTR-mediated responses (7, 11, 40, 42, 49, 57, 62), K252a blocked the augmentatory effect of BDNF on CCh peak contraction in LM-MP strips (Fig. 2). Secondly, immunoneutralization of TrkB with TrkB antibody abolished the effect of BDNF whereas a p75NTR antibody had no effect on the ability of BDNF to augment the CCh-induced contraction (Fig. 3). This suggests that the effects of BDNF were mediated largely, if not solely, by TrkB receptors. Chen et al. (10) also found that a TrkB antibody blocked the effect of BDNF in mouse intestine. Thirdly, we used two TrkB-selective agonists, LM22A4 and 7,8-dihydroxyflavone (1, 5, 28), and showed that these resulted in augmentation of peak CCh contraction similar to that seen with BDNF (Fig. 4). These results support the conclusion that BDNF augments CCh-induced contraction by activating TrkB receptors. In line with this finding, several reports indicate that BDNF mediates its biological effect in muscle through TrkB receptor activation. For example, BDNF and 7,8-dihydroxyflavone enhanced cholinergic neurotransmission via activation of TrkB rather than the p75NTR receptor in mice diaphragm muscle and rat and human airway smooth muscle (1, 37, 40) and the augmentation of cholinergic contraction by BDNF in airway smooth muscle is strongly inhibited by K252a and a TrkB antibody (49, 57). Finally, we directly measured the ability of BDNF to activate (i.e., phosphorylate) TrkB receptors in cultured rabbit jejunal smooth muscle cells devoid of other cell types. Both BDNF and the selective TrkB agonist LM22A4 increased phospho-TrkB levels (Fig. 5), supporting the data derived from the use of the antagonists and antibodies that the action of BDNF in rabbit intestinal smooth muscle is mediated through activation of TrkB receptors.
Since TrkB receptors have been demonstrated immunohistochemically on enteric neurons and mucosal enteroendocrine cells, and because we (22, 23) and others (7) have shown augmentation of motility via these mechanisms, it is possible that the effects described in the present study could be mediated via neuronal or paracrine actions. This is unlikely since in control studies tetrodotoxin had no effect on the augmentation of CCh-induced contraction by BDNF and because the LM-MP are devoid of mucosa. Additionally, if there were a direct effect of BDNF to release an excitatory neurotransmitter in these strips, BDNF would have had an effect by itself and it would have augmented the contraction induced by SP. Finally, our studies in cultured smooth muscle cells demonstrate a direct activation (i.e., phosphorylation) of the TrkB receptor in isolated cultured smooth muscle cells devoid of neurons, glia, and endothelial cells.
The present study demonstrates that BDNF acts to augment CCh peak contraction in rabbit intestine by activating PLC. This notion is supported by the finding that a specific PLC inhibitor, U73122 (1–10 μM), abolished the augmentation by BDNF of CCh-induced contraction (Fig. 8). In contrast neither the ERK1/2 inhibitor PD98059 (Fig. 6) nor the PI3K/Akt inhibitor LY294002 (Fig. 7) had any effect on the augmentation of CCh-induced contraction by BDNF. This is in contrast to human airway smooth muscle, where PD98059 partially inhibited the augmentatory effects of BDNF (1). This difference may be explained by the fact that the effects of PD98059 in airway smooth muscle were the result of a reduction in Ca2+ release from internal stores rather than a decrease in Ca2+ influx mechanisms whereas the main source of the increase in Ca2+ in response to muscarinic agonists in intestinal longitudinal muscle is influx rather than release from internal stores (44, 45). Similar to the present study, inhibition of the Akt pathway was less effective in reducing the augmentatory effect of BDNF in human airway smooth muscle. Consistent with the lack of effect of inhibitors of ERK1/2 and PI3K/Akt in blunting the augmentation of CCh-induced contraction by BDNF, direct measurement of phosphorylated ERK1/2 and Akt in LM-MP and in cultured smooth muscle cells demonstrated that BDNF does not activate these signaling pathways in jejunal smooth muscle.
The PLC inhibitor used in this study, U73122, is selective for PLC but does not distinguish isoforms. Although there is at present no selective PLC-γ antagonist to definitively test the role of this isoform, it is most likely the PLC-γ isoform that mediates the augmentation of CCh-induced contraction by BDNF. This notion is suggested by several points. First, in the present study, BDNF addition to LM-MP and to cultured smooth muscle cells caused a robust PLC-γ phosphorylation indicative of activation (Fig. 8). Secondly, PLC-γ is one of the three canonical signaling pathways coupled to TrkB in a variety of tissues and the TrkB-PLC-γ signaling pathway has been shown to be coupled to increase in Ca2+ and augmentation of responses in mouse Purkinje cells, rat visual cortex neurons, and rat DRG neurons (11, 19, 42). Finally, PLC inhibition with U73122 had no effect on CCh-induced contraction in the absence of BDNF (Fig. 8). This is consistent with the lack of PLC-β activation in intestinal longitudinal muscle cells (reviewed in Ref. 44). Thus the augmentatory effect of BDNF on CCh-induced contraction in the present study most likely reflects the activation of PLC-γ, a mechanism that is usually absent in intestinal longitudinal muscle. This may also explain the lack of an acute effect of BDNF in longitudinal smooth muscle and the requirement for preincubation for an hour before its augmentatory effects are evident.
The augmentation of muscarinic contraction of intestinal longitudinal muscle by BDNF may explain, in part, the increased contractility of smooth muscle associated with inflammation of the gut (reviewed in Refs. 32, 36, 39, 53). Inflammation is associated with structural and functional changes in the contractile apparatus of gut that leads to altered motor function (6, 43). Hypercontractility, especially of longitudinal smooth muscle, has been reported in IBD patients, especially Crohn's patients, and is a characteristic of a variety of models of gut inflammation (2, 3, 20, 27, 33, 43, 51). Similarly, hypercontractility of airway smooth muscle is also a hallmark of lung inflammation and BDNF upregulation during airway inflammation has been shown to mediate this hypercontractility (1, 28, 40, 49, 57). In the same manner, the increase in BDNF expression induced by cytokines during gut inflammation (30, 60) may, in part, explain the hypercontractility and altered motor function associated with gut inflammation.
In conclusion, BDNF preincubation augments the CCh-induced contraction of smooth muscle of the longitudinal muscle layer of rabbit intestine. The effect of BDNF is the result of TrkB activation and subsequent phosphorylation of PLC. These results provide new insight into the mechanisms of neurotrophin (BDNF) modulation of gut function, which may lead to new therapeutic avenues for treatment of gastrointestinal disorders, and explain some of the pathological motility changes associated with inflammation.
GRANTS
This work was supported by Grant DK34153 from the National Institutes of Diabetes and Digestive and Kidney Diseases. J. R. Grider was supported by DK34153, K. S. Murthy was supported by DK15564, and H. I. Akbarali was supported by DK46367. M. Al-Qudah was supported by a grant from Jordan University of Science and Technology, Irbid, Jordan.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.A.-Q., H.I.A., K.S.M., and J.R.G. conception and design of research; M.A.-Q., C.D.A., S.M., and Z.L.B. performed experiments; M.A.-Q. and J.R.G. analyzed data; M.A.-Q. and J.R.G. interpreted results of experiments; M.A.-Q. prepared figures; M.A.-Q. drafted manuscript; M.A.-Q., S.M., H.I.A., K.S.M., and J.R.G. edited and revised manuscript; M.A.-Q., C.D.A., S.M., Z.L.B., H.I.A., K.S.M., and J.R.G. approved final version of manuscript.
REFERENCES
- 1.Abcejo AJ, Sathish V, Smelter DF, Aravamudan B, Tompson MA, Hartman WR, Pabelick CM, Prakash YS. Brain-derived neurotrophic factor enhances calcium regulatory mechanisms in human airway smooth muscle. PLoS One 7: 44343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiho H, Khan WI, Al-Kaabi A, Blennerhassett P, Deng Y, Collins SM. Cytokine modulation of muscarinic receptors in the murine intestine. Am J Physiol Gastrointest Liver Physiol 293: G250–G255, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Akiho H, Lovato P, Deng Y, Ceponis PJM, Blennerhassett P, Collins SM. Interleukin-4- and -13-induced hypercontractility of human intestinal muscle cells—implication for motility changes in Crohn's disease. Am J Physiol Gastrointest Liver Physiol 288: G609–G615, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Al-Qudah M, Mahavadi S, Bradley ZL, Kay JC, Murthy KS, Grider JR. Pituitary adenylate cyclase-activating peptide (PACAP) and substance P (SP) induce the release of brain-derived neurotropic factor (BDNF) from the longitudinal muscle layer of the intestine (Abstract). Exp Biol Proc: D699, 367, 2011 [Google Scholar]
- 5.Andero R, Heldt SA, Ye K, Liu X, Armario A, Ressler KJ. Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. Am J Psychiatry 168: 163–172, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bercik P, De Giorgio R, Blennerhassett P, Verdu EF, Barbara G, Collins SM. Immune-mediated neural dysfunction in a murine model of chronic Helicobacter pylori infection. Gastroenterology 123: 1205–1215, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Boesmans W, Gomes P, Janssens J, Tack J, Vanden Berght P. Brain-derived neurotrophic factor amplifies neurotransmitter responses and promotes synaptic communication in the enteric nervous system. Gut 57: 314–322, 2008 [DOI] [PubMed] [Google Scholar]
- 8.BDNF Study Group (Phase III) A controlled trial of recombinant methionyl human BDNF in ALS. Neurology 52: 1427–1433, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Chai NL, Dong L, Li ZF, Du KX, Wang JH, Yan LK, Dong XL. Effects of neurotrophins on gastrointestinal myoelectric activities of rats. World J Gastroenterol 9: 1874–1877, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen FX, Yu YB, Yuan XM, Zou AL, Li YQ. Brain-derived neurotrophic factor enhances the contraction of intestinal muscle strips induced by SP and CGRP in mice. Regul Pept 178: 86–94, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Cheng Q, Yeh HH. PLCγ signaling underlies BDNF potentiation of Purkinje cell responses to GABA. J Neurosci Res 79: 616–627, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Coulie B, Szarka LA, Camilleri M, Burton DD, McKinzie S, Stambler N, Cedarbaum JM. Recombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humans. Gastroenterology 119: 41–50, 2000 [DOI] [PubMed] [Google Scholar]
- 13.de Araujo-Martins L, de Oliveira RM, dos Santos GV, dos Santos RC, dos Santos AA, de Araujo E. Treatment in vitro of retinal cells with IL-4 increases the survival of retinal ganglion cells: the involvement of BDNF. Neurochem Res 38: 162–173, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Esteban I, Levanti B, Garcia-Suarez O, Germana G, Ciriaco E, Naves JF, Vega JA. A neuronal subpopulation in the mammalian enteric nervous system expresses TrkA and TrkC neurotrophin receptor-like proteins. Anat Rec 251: 360–370, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Esteban I, Hannestad J, Levanti B, Del Valle ME, Naves JF, Vega JA. Neurotrophin receptor proteins immunoreactivity in human gastrointestinal endocrine cells. Brain Res Bull 38: 539–543, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Fox EA. A genetic approach for investigating vagal sensory roles in regulation of gastrointestinal function and food intake. Auton Neurosci 126: 9–29, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Fox EA, Murphy MC. Factors regulating vagal sensory development: potential role in obesities of developmental origin. Physiol Behav 94: 90–104, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox EA, Biddinger JE. Early postnatal over nutrition: potential roles of gastrointestinal vagal afferents and brain-derived neurotrophic factor. Physiol Behav 106: 400–412, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garraway SM, Petruska JC, Mendell LM. BDNF sensitizes the response of lamina II neurons to high threshold primary afferent inputs. Eur J Neurosci 18: 2467–2476, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Ghoshal UC, Ranjan P. Post-infectious irritable bowel syndrome: the past, the present and the future. J Gastroenterol Hepatol 26: 94–101, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Goggi J, Pullar IA, Carney SL, Bradford HF. Modulation of neurotransmitter release induced by brain-derived neurotrophic factor in rat brain striatal slices in vitro. Brain Res 941: 34–42, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Grider JR, Piland BE, Gulick MA, Qiao LY. Brain-derived neurotrophic factor augments peristalsis by augmenting 5-HT and calcitonin gene-related peptide release. Gastroenterology 130: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Grider JR, Piland BE. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am J Physiol Gastrointest Liver Physiol 292: G429–G437, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Guarino N, Yoneda A, Shima H, Puir P. Selective neurotrophin deficiency in infantile hypertrophic pyloric stenosis. J Pediatr Surg 36: 1280–1284, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Hoehner JC, Wester T, Pahlman S, Olsen L. Localization of neurotrophin and their high-affinity receptors during human enteric nervous system development. Gastroenterology 110: 756–567, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Hoehner JC, Wester T, Pahlman S, Olsen L. Alterations in neurotrophin and neurotrophin-receptor localization in Hirschsprung's disease. J Pediatr Surg 31: 1542–1529, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Ihara E, Chappellaz M, Turner SR, MacDonald JA. The contribution of protein kinase C and CPI-17 signaling pathways to hypercontractility in murine experimental colitis. Neurogastroenterol Motil 24: e15–e26, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci USA 107: 2687–2692, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson M, Norrgård O, Forsgren S. Study of expression patterns and levels of neurotrophins and neurotrophin receptors in ulcerative colitis. Inflamm Bowel Dis 13: 398–409, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Johansson M, Jonnsson M, Norrgård O, Forsgren S. New aspects concerning ulcerative colitis and colonic carcinoma: analysis of levels of neuropeptides, neurotrophins, and TNFalpha/TNF receptor in plasma and mucosa in parallel with histological evaluation of the intestine. Inflamm Bowel Dis 14: 1331–1340, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Joo YE. Increased expression of brain-derived neurotrophic factor in irritable bowel syndrome and its correlation with abdominal pain. Neurogastroenterol Motil 19: 109–111, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan WI, Collins SM. Gut motor function: immunological control in enteric infection and inflammation. Clin Exp Immunol 143: 389–397, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinoshita K, Hori M, Fujisawa M, Sato K, Ohama T, Momotani E, Ozaki H. Role of TNF-α in muscularis inflammation and motility disorder in a TNBS-induced colitis model: clues from TNF-α-deficient mice. Neurogastroenterol Motil 18: 578–588, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Lucini C, Maruccio L, de Girolamo P, Vega JA, Castaldo L. Localisation of neurotrophin-containing cells in higher vertebrate intestine. Anat Embryol (Berl) 205: 135–140, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Lommatzsch M, Braun A, Mannsfeldt A, Botchkarev VA, Botchkareva NV, Paus R, Fischer A, Lewin GR, Renz H. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. Am J Pathol 155: 1183–1193, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malykhina AP, Akbarali HI. Inflammation-induced “channelopathies” in the gastrointestinal smooth muscle. Cell Biochem Biophys 41: 319–330, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Mantilla CB, Ermilov LG. The novel TrkB receptor agonist 7,8-dihydroxyflavone enhances neuromuscular transmission. Muscle Nerve 45: 274–276, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maruccio L, Castaldo L, de Girolamo P, Lucini C. Neurotrophin and trk receptor-like immunoreactivity in the frog gastrointestinal tract. Histol Histopathol 19: 349–356, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Mawe GM, Collins SM, Shea-Donohue T. Changes in enteric neural circuitry and smooth muscle in the inflamed and infected gut. Neurogastroenterol Motil 16, Suppl 1: 133–136, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Meuchel LW, Stewart A, Smelter DF, Abcejo AJ, Thompson MA, Zaidi SI, Martin RJ, Prakash YS. Neurokinin-neurotrophin interactions in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 301: L91–L98, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci 10: 850–860, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Mizoguchi Y, Nabekura J. Sustained intracellular Ca2+ elevation induced by a brief BDNF application in rat visual cortex neurons. NeuroReport 14: 1481–1483, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Mizutani T, Akiho H, Khan WI, Murao H, Ogino H, Kanayama K, Nakamura K, Takanagi R. Persistent gut motor dysfunction in a murine model of T-cell-induced enteropathy. Neurogastroenterol Motil 22: 196–203, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Murthy KS, Zhou H, Grider JR, Brautigan DL, Eto M, Makhlouf GM. Differential signalling by muscarinic receptors in smooth muscle: m2-mediated inactivation of myosin light chain kinase via Gi3, Cdc42/Rac1 and p21-activated kinase 1 pathway, and m3-mediated MLC20 (20 kDa regulatory light chain of myosin II) phosphorylation via Rho-associated kinase/myosin phosphatase targeting subunit 1 and protein kinase C/CPI-17 pathway. Biochem J 374: 145–155, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol 11: 272–280, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Poo M. Neurotrophins as synaptic modulators. Nat Rev Neurosci 2: 24–32, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Prakash YS, Tompson MA, Meuchei L, Pabelick CM, Mantilla CB, Zaidi SIA, Martin RJ. Neurotrophins in lung health and disease. Expert Rev Respir Med 4: 395–411, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prakash YS, Iyanoye A, Ay B, Mantilla CB, Pabelick CM. Neurotrophin effect on intracellular Ca2+ and force in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 291: L447–L456, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Radaelli G, Domeneghini C, Arrighi S, Castaldo L, Lucini C, Mascarello F. Neurotransmitters, neuromodulators, and neurotrophin receptors in the gut of pantex, a hybrid sparid fish (Pagrus major x Dentex dentex). Localization in the enteric nervous and endocrine systems. Histol Histopathol 16: 845–853, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Radojevic N, McKay DM, Merger M, Vallance BA, Collins SM, Croitoru K. Characterization of enteric functional changes evoked by in vivo anti-CD3 T cell activation. Am J Physiol Regul Integr Comp Physiol 276: R715–R723, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc B Biol Sci 361: 1545–1564, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarna SK. Lessons learnt from post-infections IBS. Front Physiol 2: 49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schulte-Herbrüggen O, Nassenstein C, Lommatzsch M, Quarcoo D, Renx H, Braun A. Tumor necrosis factor-α and interleukin-6 regulate secretion of brain-derived neurotrophic factor in human monocytes. J Neuroimmunol 160: 204–209, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Shibayama E, Koizumi H. Cellular localization of the Trk neurotrophin receptor family in human non-neuronal tissues. Am J Pathol 148: 1807–1818, 1996 [PMC free article] [PubMed] [Google Scholar]
- 56.Skaper SD. The neurotrophin family of neurotrophic factors: an overview. Methods Mol Biol 846: 1–12, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Sopi RB, Martin RJ, Haxhiu MA, Dreshaj Yao Q, Jafri A, Zaidi SIA. Role of brain-derived neurotrophic factor in hyperoxia-induced enhancement of contractility and impairment of relaxation in lung parenchyma. Am J Physiol Lung Cell Mol Physiol 295: L348–L355, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teng BQ, Murthy KS, Kuemmerle JF, Grider JR, Sase K, Michel T, Makhlouf GM. Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 275: G342–G351, 1998 [DOI] [PubMed] [Google Scholar]
- 59.Wellmer A, Misra VP, Sharief MK, Kopelman PG, Anand P. A double-blind placebo-controlled clinical trial of recombinant human brain-derived neurotrophic factor (rhBDNF) in diabetic polyneuropathy. J Peripher Nerv Syst 6: 204–210, 2001 [DOI] [PubMed] [Google Scholar]
- 60.Yu SJ, Grider JR, Gulick MA, Xia CM, Shen S, Qiao LY. Up-regulation of brain-derived neurotrophic factor is regulated by extracellular signal-regulated protein kinase 5 and by nerve growth factor retrograde signaling in colonic afferent neurons in colitis. Exp Neurol 238: 209–217, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu YB, Zuo XL, Zhao QJ, Chen FX, Yang J, Dong YY, Wang P, Li YO. Brain-derived neurotrophic factor contributes to abdominal pain in irritable bowel syndrome. Gut 61: 685–694, 2012 [DOI] [PubMed] [Google Scholar]
- 62.Zhou P, Porcionatto M, Pilapil M, Chen Y, Choi Y, Tolias KF, Bikoff JB, Hong EJ, Greenberg ME, Segal RA. Polarized signaling endosomes coordinate BDNF-induced chemotaxis of cerebellar precursors. Neuron 55: 53–68, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]