Abstract
Chronic ethanol consumption increases sensitivity of the mitochondrial permeability transition (MPT) pore induction in liver. Ca2+ promotes MPT pore opening, and genetic ablation of cyclophilin D (CypD) increases the Ca2+ threshold for the MPT. We used wild-type (WT) and CypD-null (CypD−/−) mice fed a control or an ethanol-containing diet to investigate the role of the MPT in ethanol-mediated liver injury. Ca2+-mediated induction of the MPT and mitochondrial respiration were measured in isolated liver mitochondria. Steatosis was present in WT and CypD−/− mice fed ethanol and accompanied by increased terminal deoxynucleotidyl transferase dUTP-mediated nick-end label-positive nuclei. Autophagy was increased in ethanol-fed WT mice compared with ethanol-fed CypD−/− mice, as reflected by an increase in the ratio of microtubule protein 1 light chain 3B II to microtubule protein 1 light chain 3B I. Higher levels of p62 were measured in CypD−/− than WT mice. Ethanol decreased mitochondrial respiratory control ratios and select complex activities in WT and CypD−/− mice. Ethanol also increased CypD protein in liver of WT mice. Mitochondria from control- and ethanol-fed WT mice were more sensitive to Ca2+-mediated MPT pore induction than mitochondria from their CypD−/− counterparts. Mitochondria from ethanol-fed CypD−/− mice were also more sensitive to Ca2+-induced swelling than mitochondria from control-fed CypD−/− mice but were less sensitive than mitochondria from ethanol-fed WT mice. In summary, CypD deficiency was associated with impaired autophagy and did not prevent ethanol-mediated steatosis. Furthermore, increased MPT sensitivity was observed in mitochondria from ethanol-fed WT and CypD−/− mice. We conclude that chronic ethanol consumption likely lowers the threshold for CypD-regulated and -independent characteristics of the ethanol-mediated MPT pore in liver mitochondria.
Keywords: liver, mitochondria, alcohol, cyclophilin D, permeability transition pore
chronic ethanol consumption causes liver injury, with some of the earliest pathological changes observed in the mitochondrion (31, 59). These ethanol-mediated alterations include changes in mitochondrial morphology (e.g., enlarged, misshapen mitochondria with fewer cristae) (26) and increased production of reactive oxygen species (ROS) from the organelle (6). Increased mitochondrial ROS production is recognized as a critical component in the cellular stress response induced by ethanol (18, 60). Chronic ethanol consumption also damages mitochondrial DNA and ribosomes, inhibits mitochondrial protein synthesis, decreases oxidative phosphorylation, and depresses ATP synthesis (59). Studies by Pastorino and colleagues (66, 67) show that chronic ethanol consumption stimulates formation of the mitochondrial permeability transition (MPT) pore. However, the mechanisms responsible for this effect remain poorly defined.
MPT pore induction is characterized as increased permeability of the inner mitochondrial membrane to water and solutes (39, 41). This causes depolarization of the inner mitochondrial membrane, swelling of mitochondria, and subsequent rupture of the outer membrane, which indirectly leads to release of mitochondrial proteins (e.g., cytochrome c) that participate in cell death programs (12, 50). Although the specific protein components of the MPT pore remain elusive (8), cyclophilin D (CypD) is thought to be a key regulator of MPT pore function. Studies in mice lacking CypD [peptidylprolyl cis-trans isomerase f (ppif)] have revealed an important role for CypD in induction of the MPT pore (9, 64). Baines et al. (9) reported that CypD-null (CypD−/−) mice were protected from ischemia-reperfusion-induced cell death in vivo. In addition, mitochondria isolated from liver, heart, and brain of CypD−/− mice were resistant to mitochondrial swelling and MPT in vitro (9). These findings suggest that CypD-dependent regulation of the MPT pore may play a crucial role in cellular responses to stresses relevant to human disease, especially diseases induced by Ca2+ overload and oxidative stress.
Previously, we reported that chronic ethanol consumption increased CypD at gene and protein levels in liver, whereas other proposed MPT pore components, including the adenine nucleotide translocator (ANT), remained unchanged (47). We also observed that chronic ethanol consumption enhanced sensitivity to Ca2+-dependent opening of the MPT pore in isolated rat liver mitochondria (47). These data suggest that increased CypD levels may predispose liver mitochondria to undergo MPT pore formation and opening in response to chronic ethanol consumption. On the basis of studies showing ameliorated phenotypes and/or delayed symptoms of disease and toxicity in CypD−/− mice, we hypothesized that chronic ethanol-mediated mitochondrial dysfunction and liver injury would be attenuated in mice lacking CypD. To this end, we assessed liver injury, markers of autophagy, mitochondrial bioenergetics, and Ca2+-mediated induction of the MPT pore in liver mitochondria from wild-type (WT) and CypD−/− mice fed an ethanol-containing diet. Interestingly, ethanol-mediated steatosis and mitochondrial alterations were largely unaffected by genetic depletion of CypD. However, autophagy responses appeared to be dampened in CypD−/− compared with WT mice. Moreover, results support the concept that CypD-dependent and -independent mechanisms are responsible for the enhanced sensitivity of mitochondria to MPT induction in ethanol-fed mice.
MATERIALS AND METHODS
Mice and chronic ethanol-feeding protocol.
Eight-week-old male C57BL/6J [wild-type (WT)] and CypD−/− (10) mice were fed Lieber-DeCarli control and ethanol-containing liquid diets (55) formulated by Bio-Serv (Frenchtown, NJ). WT mice were purchased from Jackson Laboratory (Bar Harbor, MA), and CypD−/− mice were obtained from a colony maintained by Dr. M. Lesort at the University of Alabama at Birmingham. CypD−/− mice were backcrossed onto the C57BL/6J genetic background, with isogenic heterozygotes intercrossed to generate congenic homozygous CypD−/− littermates (10). The ethanol diet contained 28.8% of total daily calories as ethanol, 35% as fat, 18.2% as carbohydrate, and 18% as protein; the control diet was an identical formulation, with ethanol calories substituted by carbohydrate (maltose-dextrin) calories. Control animals were pair-fed to their ethanol counterparts, so that each pair of control- and ethanol-fed WT and CypD−/− mice was isocaloric. Animals were maintained on the feeding protocols for ≥31 days before experiments. Animal protocols were approved by the Institutional Animal Care and Use Committee, and the experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th ed., National Academy of Sciences, 2011).
Liver histology and terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling.
Liver sections were fixed in 10% formalin, sectioned, and stained with hematoxylin-eosin for assessment of fatty liver. Steatosis (percentage of hepatocytes containing lipid droplets) was scored by a pathologist blinded to the experimental design. Terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) was performed in liver sections to determine whether cell death was increased by chronic ethanol consumption (47). Briefly, deparaffinized and rehydrated liver sections were subjected to antigen retrieval by incubation in 0.1 M citrate buffer (pH 6.0) followed by blocking for 1 h at room temperature with 0.1 M Tris·HCl (pH 7.5) containing 5% (wt/vol) BSA. Fifty microliters of the TUNEL reaction reagent (Roche, Indianapolis, IN) were added, and sections were incubated in a humidified chamber in darkness for 60 min at 37°C. After the sections were washed in PBS, alkaline phosphatase was added, and the sections were incubated for 30 min at 37°C. The sections were washed and then incubated with nitro blue tetrazolium chloride-5-bromo-4-chloro-3′-indolyphosphate p-toluidine salt solution (100 μl) for 10 min at 25°C in darkness. Sections were washed with PBS and mounted in PBS-glycerol and visualized under light microscopy. For analysis, the number of TUNEL-positive cells was counted per liver sample from 20 random high-power fields at ×400 magnification.
Biochemical measurements on serum and liver.
Serum samples were assayed for alanine aminotransferase (ALT) activity and alcohol content using appropriate reagent sets from Pointe Scientific (Canton, MI). Triglyceride levels were measured in serum and cytosolic liver extracts using an enzymatic-coupled assay that measures glycerol released from triglyceride (Pointe Scientific).
Western blots.
Immunoblotting was performed by loading equal amounts of protein onto 10%, 12%, or 4–20% SDS-polyacrylamide gels, with separated proteins transferred to nitrocellulose membranes (73). Purified mitochondrial fractions were used to detect CypD and aldehyde dehydrogenase 2 (ALDH2), and whole liver homogenate was used to detect cytochrome P-450 2E1 (CYP2E1), microtubule protein 1 light chain 3B I (LC3B-I) and LC3B-II, and sequestosome 1 p62 (p62). CypD was detected using a 1:5,000 dilution of antibody (Calbiochem, Gibbstown, NJ), ALDH2 was detected using a 1:500 dilution of antibody (laboratory of Dr. Henry Weiner, Purdue University, West Lafayette, IN), CYP2E1 was detected using a 1:5,000 dilution of antibody (Millipore, Billerica, MA), LC3B-I and LC3B-II were detected using a 1:1,000 dilution of antibody (Abcam, Cambridge, MA), and p62 was detected using a 1:2,000 dilution of antibody (Abnova, Taipei City, Taiwan). Nitrocellulose membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (Sigma, St. Louis, MO), and proteins were visualized using enhanced chemiluminescence. Membranes were then stripped and incubated with a loading control antibody. Levels of pyruvate dehydrogenase (PDH; MitoSciences, Eugene, OR) were detected using a 1:5,000 dilution and used as a loading control for CypD and ALDH2. Levels of β-actin (Sigma) were detected using a 1:5,000 dilution and used as a loading control for CYP2E1 and p62. Levels of GAPDH (Cell Signaling Technology, Beverly, MA) were detected using a 1:3,000 dilution and used as a control to show equal protein loading across gel lanes for LC3B. Detection methods and intensity of immunoreactive protein bands were quantified using Quantity One software (Bio-Rad Laboratories, Hercules, CA) and expressed as a ratio to appropriate loading controls.
Mitochondria isolation, respiration, and complex activity measurements.
Liver mitochondria were isolated by differential centrifugation techniques (71). Briefly, liver was homogenized in a buffer containing 250 mM sucrose, 5 mM HEPES, 3 mM MgCl2, and 1 mM EGTA (pH 7.4). After each centrifugation step, the lipid layer at the top of the supernatant fraction (postnuclear, postmitochondrial, and 3 subsequent wash steps) was removed to prevent contamination of the mitochondrial fraction with cytosolic lipid. Extensive and careful washing of mitochondria is important, because fatty acids promote MPT pore activation (68). Pearson's correlation test showed no significant relationship between the onset of mitochondrial swelling and the degree of hepatic steatosis in vivo (r = −0.28, P > 0.05). This suggests little, to no, impact on MPT swelling of cytosolic lipid contamination, if present. Oxygen consumption (i.e., respiration) of isolated liver mitochondria was monitored using a Clark-type oxygen electrode (Hansatech Instruments, Amesbury, MA), as described elsewhere (46). The buffer used for these respiration measurements contained 130 mM KCl, 2 mM KH2PO4, 3 mM HEPES, 2 mM MgCl2, and 1 mM EGTA (pH 7.2). Respiratory capacity was assessed by measuring state 3 (ADP-dependent) and state 4 (ADP-independent) respiration with the complex I-linked substrate glutamate-malate or the complex II-linked substrate succinate (in the presence of 1 μM rotenone). The respiratory control ratio (RCR) was calculated by determining the ratio of state 3 to state 4 respiration rate. Mitochondrial complex activities were determined by spectrophotometric methods, as previously described (25). Complex (I, II-III, IV, and V) activities were normalized to citrate synthase activity.
Mitochondrial swelling assay.
Mitochondria (prepared as described in Mitochondria isolation, respiration, and complex activity measurements) were resuspended in an EGTA-free buffer containing 250 mM sucrose, 5 mM HEPES, and 3 mM MgCl2 (pH 7.4) and centrifuged for 7 min at 10,000 g at 4°C. After three repeated washes in this HEPES-based buffer, the final mitochondrial protein pellet was resuspended in a KCl-based buffer [150 mM KCl, 25 mM NaHCO3, 1 mM MgCl2, 3 mM KH2PO4, and 20 mM HEPES (pH 7.4)]. Protein concentration was determined by the Bradford protein assay, with albumin used as the standard (15). Isolated mitochondria (1.0 mg/ml) were incubated in the KCl-based buffer and energized with the oxidizable substrate glutamate-malate (1 mM). Ca2+ (8 or 40 nmol) was added, and swelling was monitored by continuous measurement of changes in optical density at 540 nm in a 96-well plate reader (Synergy HT, Bio-Tek Instruments). Cyclosporin A (CsA, 1 μM) was added to select samples before the addition of Ca2+, and swelling was monitored as described elsewhere (20). CsA was used to assess involvement of the MPT pore, because CsA competes with Ca2+ for CypD binding and, thus, delays MPT pore activation (37). Inclusion of CsA allows for increased Ca2+ accumulation before induction of the MPT pore (16, 22).
Statistical analysis.
Values are means ± SE for three to eight pairs of mice per group. The level of statistical significance was set at P ≤ 0.05. Statistical differences were determined using t-tests or two-factor ANOVA with SigmaStat software (Systat, Chicago, IL). The Holm-Sidak method for pair-wise comparisons was used to identify the groups that were statistically different within the two-factor ANOVA.
RESULTS
Effect of ethanol and genotype on various serum, metabolic, and liver parameters.
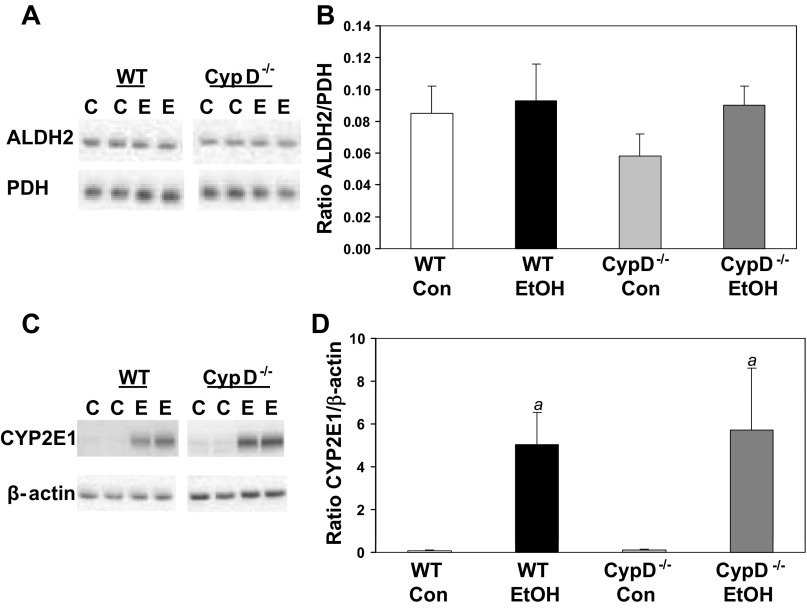
Mice were pair-fed control or ethanol-containing diets for 5 wk to induce steatosis and mitochondrial dysfunction (5). There was no significant difference in ethanol consumption between WT and CypD−/− mice (P = 0.46). Blood alcohol levels were measured in samples taken at the time of tissue collection (∼10 AM) and ranged from undetectable to 150 mg/dl, with no differences between ethanol-fed WT and ethanol-fed CypD−/− mice (P = 0.44). These results are similar to those collected in previous studies from our laboratory (76). Diet and genotype had no effect on protein levels of ALDH2 in liver (Fig. 1, A and B). Moreover, chronic ethanol consumption led to an equivalent induction of CYP2E1 protein in WT and CypD−/− mice (Fig. 1, C and D). Together, these results show no differences in ethanol consumption or metabolism between WT and CypD−/− mice.
Fig. 1.
Effect of chronic ethanol consumption and genotype on aldehyde dehydrogenase 2 (ALDH2) and cytochrome P-450 2E1 (CYP2E1) protein. Liver ALDH2 and CYP2E1 protein were measured by Western blot technique and expressed as ratio to pyruvate dehydrogenase (PDH) or β-actin, respectively. A and C: representative Western blots for wild-type (WT) and cyclophilin D (CypD)-null (CypD−/−) mice fed control (C) or ethanol (E)-containing diets. Samples from WT and CypD−/− mice were run on separate gels; a white space is visible between gels. B and D: protein levels expressed as ratio of ALDH2 to PDH and ratio of CYP2E1 to β-actin. Con, control; EtOH, ethanol. Diet and genotype had no effect on total PDH and β-actin levels (data not shown). Values are means ± SE; n = 5–6 pairs of mice per treatment group. Results from 2-factor ANOVA: ALDH2/PDH-diet, P = 0.26; genotype, P = 0.41; diet × genotype, P = 0.49. CYP2E1/β-actin-diet, P = 0.004; genotype, P = 0.82; diet × genotype, P = 0.84. aP < 0.05 compared with corresponding control diet groups.
At the beginning of the study, body weights of all mice were similar: 28.2 ± 0.5, 28.4 ± 0.4, 27.6 ± 0.9, and 28.4 ± 0.7 g for control-fed WT, ethanol-fed WT, control-fed CypD−/−, and ethanol-fed CypD−/− mice, respectively. However, at the end of the feeding period, there was a significant effect of diet on body weight: ethanol-fed WT mice weighed significantly more than control-fed WT mice (Table 1). There was a significant genotype effect on the liver weight-to-body weight ratio (Table 1). Specifically, the liver weight-to-body weight ratio was significantly higher in ethanol-fed CypD−/− than ethanol-fed WT and control-fed CypD−/− mice (Table 1). Ethanol feeding had a significant effect on serum and liver triglyceride levels (Table 1). Serum triglyceride levels were significantly higher in ethanol-fed WT than control-fed WT mice (Table 1). Furthermore, serum triglyceride levels were significantly higher in ethanol-fed CypD−/− mice than control-fed CypD−/− and ethanol-fed WT mice (Table 1). Liver triglyceride levels were significantly higher in both genotypes of ethanol-fed mice than in their control counterparts (Table 1). In addition, liver triglyceride levels were significantly higher in ethanol-fed CypD−/− than ethanol-fed WT mice (Table 1). Serum ALT levels were also elevated in both genotypes of mice fed ethanol compared with their control-fed counterparts; however, this increase was only statistically significant in ethanol-fed WT mice (Table 1).
Table 1.
Effect of chronic ethanol consumption and genotype on body, liver, and serum measurements
| Group | Body Wt, g | Liver Wt, g | Liver Wt/Body Wt, % | Serum ALT, IU/l | Serum TGs, mg/dl | Liver TGs, mg/mg protein |
|---|---|---|---|---|---|---|
| WT Con | 29.3 ± 1.0 | 1.30 ± 0.08 | 4.5 ± 0.20 | 34 ± 2.5 | 86 ± 6.4 | 1.5 ± 0.38 |
| WT EtOH | 32.2 ± 0.8a | 1.49 ± 0.06 | 4.6 ± 0.14 | 50 ± 6.8a | 142 ± 15a | 3.5 ± 0.40a |
| CypD−/− Con | 29.0 ± 2.0 | 1.45 ± 0.10 | 5.0 ± 0.13 | 40 ± 6.0 | 78 ± 16 | 1.7 ± 0.20 |
| CypD−/− EtOH | 31.5 ± 1.2 | 1.80 ± 0.13b,c | 5.7 ± 0.30b,c | 65 ± 10 | 187 ± 26b,c | 5.3 ± 0.70b,c |
| 2-Factor ANOVA (P values) | ||||||
| Diet | 0.028 | 0.028 | 0.103 | 0.007 | <0.001 | <0.001 |
| Genotype | 0.383 | 0.054 | 0.001 | 0.850 | 0.168 | 0.109 |
| Interaction (diet × genotype) | 0.506 | 0.369 | 0.101 | 0.672 | 0.088 | 0.113 |
Values are means ± SE; n = 6–8 mice per group.
WT, wild-type; CypD−/−, cyclophilin D-null; Con, control diet; EtOH, ethanol-containing diet; ALT, alanine aminotransferase; TGs, triglycerides.
Results from post hoc statistical analyses P < 0.05 vs. WT Con;
P < 0.05 vs. CypD−/− Con;
P < 0.05 vs. WT EtOH.
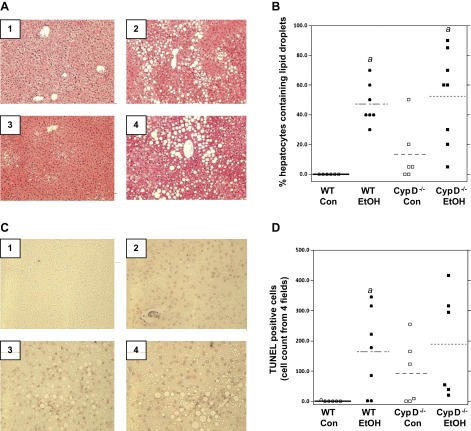
Histological examination of livers (hematoxylin-eosin-stained liver sections) indicated no steatosis in control-fed WT mice (Fig. 2, A1 and B), whereas livers from ethanol-fed WT mice showed accumulation of macro- and microvesicular fat (Fig. 2, Fig. 2A2 and Fig. 2B). Steatosis was observed in some livers of control-fed CypD−/− mice (Fig. 2, Fig. 2A3 and Fig. 2B) and was further increased by ethanol feeding (Fig. 2, Fig. 2A4 and Fig. 2B). Similar results were observed for TUNEL staining, a marker of DNA fragmentation (33) associated with late apoptotic and/or necrotic cell death (36, 42). While there was a wide range in these measurements, chronic ethanol feeding increased the number of TUNEL-positive cells in both genotypes of mice compared with corresponding controls (Fig. 2C), with a statistically significant increase in ethanol-fed WT compared with control-fed WT mice (Fig. 2D). Interestingly, large numbers of TUNEL-positive cells were observed in three of the six control-fed CypD−/− mice, and a similar trend was observed in ethanol-fed CypD−/− mice (Fig. 2, Fig. 2C3 and Fig. 2C4). However, these trends of increased TUNEL staining in the CypD−/− mice did not reach statistical significance (Fig. 2D).
Fig. 2.
Chronic ethanol consumption causes steatosis and cell death. Liver sections were fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin-eosin for steatosis (A) and with terminal deoxynucleotidyl transferase dUTP-mediated nick-end label (TUNEL) for cell death (C). A: representative liver histology for control-fed WT (A1), ethanol-fed WT (A2), control-fed CypD−/− (A3), and ethanol-fed CypD−/− (A4) mice. Magnification ×200. B: steatosis scoring. C: representative liver TUNEL staining for control-fed WT (C1), ethanol-fed WT (C2), control-fed CypD−/− (C3), and ethanol-fed CypD−/− (C4) mice. Magnification ×400. D: TUNEL-positive cells. Values are means ± SE; n = 6–8 mice for group. Results for 2-factor ANOVA: steatosis-diet, P < 0.001; genotype, P = 0.25; diet × genotype, P = 0.62. TUNEL-diet, P = 0.016; genotype, P = 0.25; diet × genotype, P = 0.52. aP < 0.05 vs. corresponding control diet group.
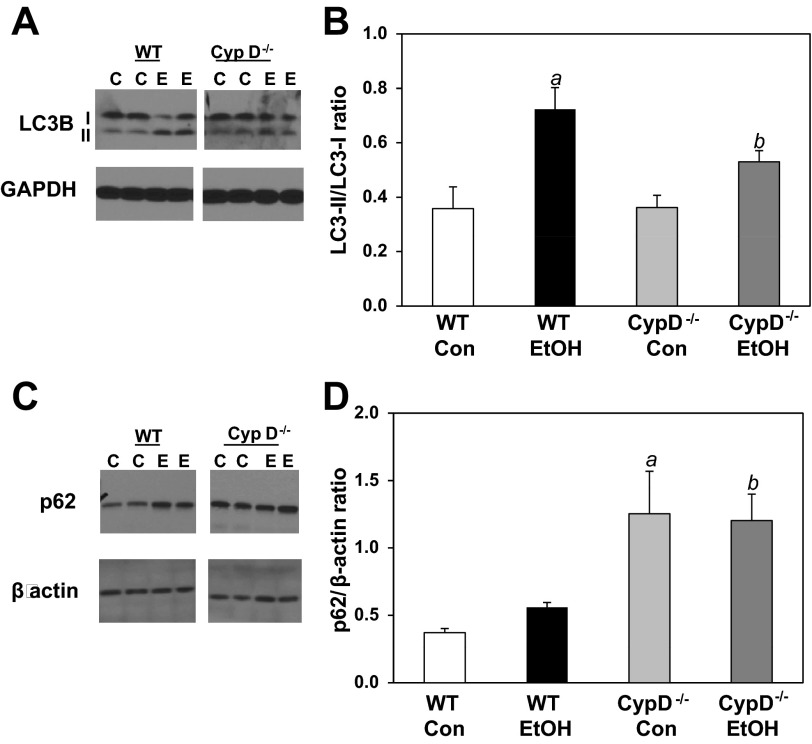
Effect of ethanol and genotype on autophagy parameters.
To determine whether chronic ethanol or the absence of CypD−/−, or both, had an impact on autophagy, we measured levels of autophagy marker proteins by Western blotting. We measured LC3B-I and LC3B-II, which can serve as markers of autophagic flux and/or autophagosome numbers (43, 44). Chronic ethanol consumption in WT mice significantly increased the ratio of LC3B-II to LC3B-I, suggesting an increase in autophagy in response to ethanol-mediated stress (Fig. 3, A and B). In contrast, the ratio of LC3B-II to LC3B-I was unchanged by ethanol in CypD−/− mice (Fig. 3, A and B). Furthermore, there was no difference in the ratio of LC3B-II to LC3B-I between control-fed WT and control-fed CypD−/− mice; however, the ratio was increased in ethanol-fed WT compared with ethanol-fed CypD−/− mice (Fig. 3B). To complement these measurements, we assessed levels of p62, a protein that accumulates in tissues when autophagy is inhibited or when there are defects in autophagic degradation (13). Levels of p62 were higher in the CypD−/− than WT mice, with no impact of chronic ethanol consumption in either genotype (Fig. 3, C and D).
Fig. 3.
Effect of chronic ethanol consumption and genotype on markers of autophagy. A and C: representative Western blots for WT and CypD−/− mice fed control or ethanol-containing diet. WT and CypD−/− samples were run on different regions of a large format gel. A white space is used to note that the WT and CypD−/− samples shown here were not run in a contiguous fashion. A and B: liver light chain 3B I and II (LC3B-I and LC3B-II) measured by Western blot technique and expressed as ratio of LC3B-II to LC3B-I. C and D: liver p62 measured by Western blot technique and expressed as ratio to β-actin. Values are means ± SE; n = 3 pairs of mice per treatment group. Results from 2-factor ANOVA: LC3B-II/LC3B-I ratio-diet, P < 0.001; genotype, P = 0.163; diet × genotype, P = 0.146. p62-diet, P = 0.134; genotype, P < 0.001; diet × genotype, P = 0.919. aP < 0.05 vs. WT Con; bP < 0.05 vs. WT EtOH.
Effect of ethanol and genotype on Ca2+-induced mitochondrial swelling.
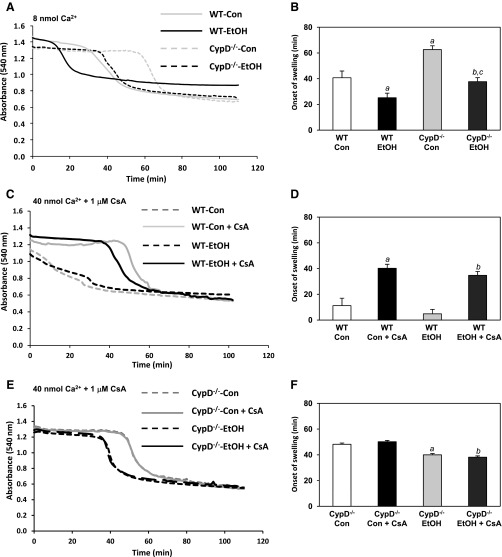
To determine whether chronic ethanol consumption changes the sensitivity of MPT induction, we measured mitochondrial swelling in response to Ca2+, a well-established inducer of the MPT pore. Shown in Fig. 4A is a representative trace of freshly isolated liver mitochondria from control-fed WT, ethanol-fed WT, control-fed CypD−/−, and ethanol-fed CypD−/− mice incubated with 8 nmol of Ca2+ to induce the MPT pore. Swelling of mitochondria (detected as a decrease in absorbance at 540 nm) is indicative of MPT pore induction (41). Mitochondria from ethanol-fed WT mice were more sensitive to Ca2+-mediated swelling than those from control-fed WT mice (Fig. 4B). These data support previous findings from our laboratory using a rat model of ethanol feeding (47). In comparison, mitochondria from control- and ethanol-fed CypD−/− mice were more resistant to Ca2+-mediated swelling than mitochondria from control- and ethanol-fed WT mice, respectively (Fig. 4B). However, onset of swelling was earlier in mitochondria from ethanol-fed CypD−/− than control-fed CypD−/− mice (Fig. 4B). This result suggests a CypD-independent component mediating the Ca2+-induced increase in swelling sensitivity in mitochondria from ethanol-fed mice. To test whether swelling onset was CypD-dependent, higher concentrations of Ca2+ (40 nmol) and CsA, a CypD inhibitor that delays onset of the MPT (37), were used. Mitochondria from control- and ethanol-fed WT mice were much more sensitive to swelling than mitochondria from CypD−/− mice when higher concentrations of Ca2+ were used (Fig. 4, D and F). For example, mitochondria from ethanol-fed WT mice had a much earlier onset of swelling at 4.8 ± 3.5 min (Fig. 4D) than mitochondria from ethanol-fed CypD−/− mice, which had an onset of swelling at 40 ± 1.3 min (Fig. 4F; P < 0.001). Pretreatment with 1 μM CsA significantly delayed Ca2+-induced swelling in mitochondria isolated from control- and ethanol-fed WT mice (Fig. 4, C and D). Importantly, pretreatment with CsA in mitochondria from CypD−/− mice did not delay onset of Ca2+-induced swelling (Fig. 4, E and F), which is consistent with previous results (10). Results from two-factor ANOVA are provided in Table 2.
Fig. 4.
Chronic ethanol consumption increases sensitivity to Ca2+-mediated mitochondrial swelling. Isolated mitochondria were incubated in a KCl-based buffer and energized with oxidizable substrate. A: representative results of swelling in mitochondria incubated in 8 nmol Ca2+ and monitored at 540-nm absorbance. C: representative results of swelling in mitochondria from WT mice incubated in 40 nmol Ca2+ with and without 1 μM cyclosporin A (CsA) and monitored at 540-nm absorbance. E: representative results of swelling in mitochondria from CypD−/− mice incubated in 40 nmol Ca2+ with and without 1 μM CsA and monitored at 540-nm absorbance. Decrease in absorbance was followed for 100–110 min. B, D, and F: summary statistics of onset (i.e., start time) of mitochondrial swelling for data in A, C, and E, respectively. Values are means ± SE; n = 4–6 mice for group (B, D, and F). Results for 2-factor ANOVA are presented in Table 2. B: aP < 0.05 vs. WT Con; bP < 0.05 vs. CypD−/− Con; cP < 0.05 vs. WT EtOH. D: aP < 0.05 vs. WT Con; bP < 0.05 vs. WT EtOH. F: aP < 0.05 vs. CypD−/− Con; bP < 0.05 vs. CypD−/− Con + CsA.
Table 2.
P values from two-factor ANOVA on mitochondrial swelling following chronic ethanol consumption
| 40 nmol Ca2+ ± CsA |
|||
|---|---|---|---|
| 8 nmol Ca2+ | WT | CypD−/− | |
| Diet | <0.001 | 0.184 | <0.001 |
| Genotype | <0.001 | ||
| Interaction | <0.001 | 0.675 | |
| Diet × genotype | 0.224 | ||
| Diet × drug | 0.920 | 0.531 | |
Results are from 2-factor ANOVA of data in Fig. 4. CsA, cyclosporin A. Results for 8 nmol Ca2+ pertain to data in Fig. 4B. Two-factor ANOVA tested for diet (control vs. ethanol), genotype (WT vs. CypD−/−), and interaction (diet × genotype). Results for 40 nmol Ca2+ pertain to data in Fig. 4, Fig. 4D and Fig. 4F. Two-factor ANOVA tested for diet (control vs. ethanol), drug (untreated vs. CsA-treated), and interaction (diet × drug).
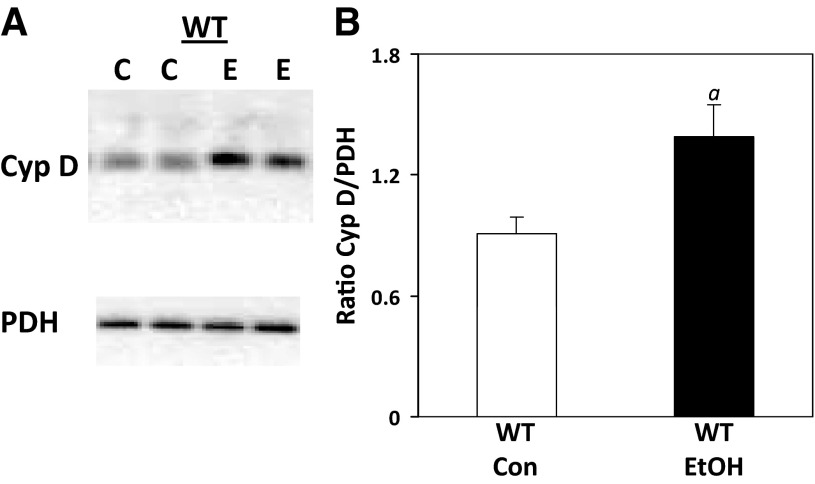
Chronic ethanol consumption increases CypD protein.
Levels of CypD protein were measured to determine whether alterations could contribute to an ethanol-dependent increase in pore induction. As shown in Fig. 5, A and B, ethanol consumption caused a significant increase in CypD protein compared with levels detected in mitochondria from control-fed mice. CypD was undetectable in liver from CypD−/− mice (data not shown).
Fig. 5.
Effect of chronic ethanol consumption on CypD protein in liver. Liver CypD was measured by Western blot technique and normalized to PDH. A: representative Western blots for control- and ethanol-fed WT mice. B: protein levels expressed as ratio of CypD to PDH. Diet had no effect on PDH (data not shown). Values are means ± SE; n = 5 pairs of mice per treatment group. aP = 0.027 vs. WT Con.
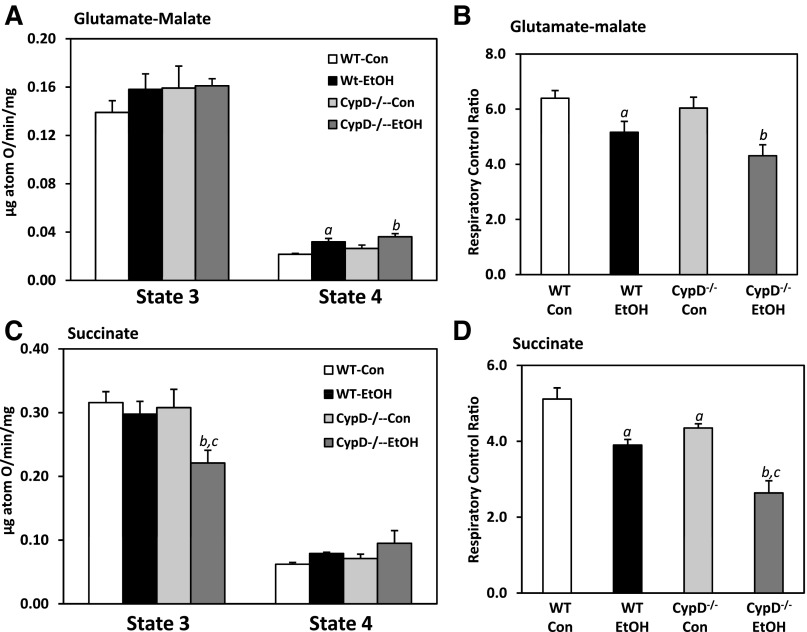
Chronic ethanol consumption alters mitochondrial respiratory function.
Mitochondria from WT and CypD−/− mice fed control or ethanol-containing diets were incubated in the presence of different oxidizable substrates and ADP for assessment of mitochondrial respiratory function following chronic ethanol feeding. Data from these measurements are shown in Fig. 6, with two-factor ANOVA results provided in Table 3. As shown in Fig. 6A, state 3 (ADP-dependent) respiration was unaffected by diet and genotype when glutamate-malate (complex I-linked substrates) was used as an oxidizable substrate for mitochondria. In contrast, ethanol feeding and the lack of CypD significantly increased state 4 (ADP-independent) respiration compared with corresponding controls (Fig. 6A, Table 3). Ethanol also significantly decreased the RCR compared with that measured in control-fed mice (Fig. 6B). Use of the complex II-linked substrate succinate resulted in some differences in respiratory function with regard to diet and genotype effects. Importantly, there were diet and genotype effects on state 3 respiration (Table 3), with a significant decrease in state 3 respiration in ethanol-fed CypD−/− mice compared with all other groups (Fig. 6C). While state 4 respiration was increased by ethanol feeding in WT and CypD−/− mice (Fig. 6C), these increases were not statistically different from those in control-fed WT and CypD−/− mice (Table 3). Diet and genotype effects on the RCR were also observed when succinate was used to energize mitochondria (Table 3). Specifically, the RCR was significantly decreased in mitochondria isolated from ethanol-fed WT compared with control-fed WT mice (Fig. 6D). Ethanol feeding had a similar effect on the RCR in mitochondria from CypD−/− mice. Interestingly, the RCR was also affected by genotype, with the RCR being significantly lower in the CypD−/− groups than in their corresponding WT counterparts (Fig. 6D, Table 3).
Fig. 6.
Effect of chronic ethanol consumption and genotype on mitochondrial bioenergetic function. State 3 and 4 respiration rates were measured using glutamate-malate (A) and succinate (C) as oxidizable substrates for WT and CypD−/− mice fed control and ethanol-containing diets. Respiratory control ratio (state 3/state 4) was calculated for all groups using both substrates [glutamate-malate (B) and succinate (D)]. Values are means ± SE; n = 6 pairs of mice per group. Results for 2-factor ANOVA are provided in Table 3. aP < 0.05 vs. WT Con; bP < 0.05 vs. CypD−/− Con; cP < 0.05 vs. WT EtOH.
Table 3.
P values from two-factor ANOVA on mitochondrial respiration following chronic ethanol consumption
| State 3 | State 4 | RCR | |
|---|---|---|---|
| Glutamate-malate | |||
| Diet | 0.410 | <0.001 | <0.001 |
| Genotype | 0.370 | 0.040 | 0.130 |
| Interaction (diet × genotype) | 0.500 | 0.700 | 0.520 |
| Succinate | |||
| Diet | 0.050 | 0.070 | <0.001 |
| Genotype | 0.025 | 0.330 | <0.001 |
| Interaction (diet × genotype) | 0.054 | 0.840 | 0.340 |
Mitochondrial respiration (state 3 and state 4) was measured using the complex I-linked substrate glutamate-malate or the complex II-linked substrate succinate. RCR, respiratory control ratio (ratio of state 3 to state 4 respiration). Results are from 2-factor ANOVA of data in Fig. 6B.
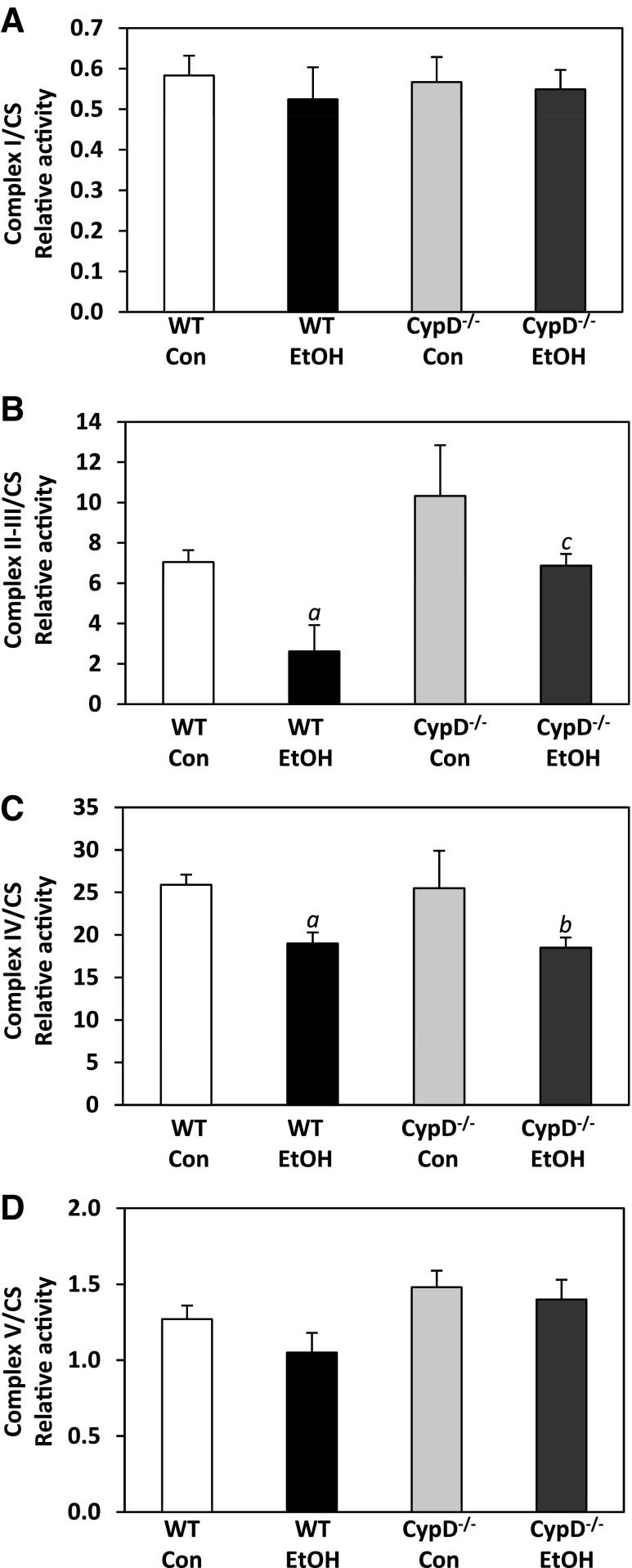
Chronic ethanol- and genotype-dependent alterations in respiratory complex activities were observed in isolated liver mitochondria. Complex activities were normalized to activity of citrate synthase, a mitochondrial matrix enzyme. Chronic ethanol feeding and genotype had no effect on citrate synthase activity (data not shown; statistics in Table 4). Diet and genotype effects on complex II-III activities were observed (Fig. 7B), whereas an effect of diet on complex IV activity was observed (Fig. 7C). Results for two-factor ANOVA are given in Table 4. Complex II-III and complex IV activities were significantly decreased in ethanol-fed WT compared with control-fed WT mice (Fig. 7, B and C). Chronic ethanol consumption also significantly decreased complex IV activity in the CypD−/− group (Fig. 7C). While ethanol consumption decreased complex II-III activity in CypD−/− mice (ethanol- compared with control-fed), this decrease was not statistically significant (Fig. 7B). Complex II-III activities were higher in control- and ethanol-fed CypD−/− mice than in their WT counterparts (Fig. 7B, Table 4). A similar effect was observed with complex V activity (Fig. 7D); however, the increase in complex V activity in CypD−/− compared with WT groups just missed statistical significance (Table 4). Complex I activity was unaffected by diet and genotype (Fig. 7A, Table 4).
Table 4.
P values from two-factor ANOVA on mitochondrial complex activities following chronic ethanol consumption
| Complex I | Complex II-III | Complex IV | Complex V | CS | |
|---|---|---|---|---|---|
| Diet | 0.569 | 0.012 | 0.004 | 0.276 | 0.416 |
| Genotype | 0.951 | 0.016 | 0.822 | 0.051 | 0.269 |
| Interaction (diet × genotype) | 0.758 | 0.728 | 0.993 | 0.600 | 0.444 |
Mitochondrial respiratory complex activities (I, II-III, IV, and V) and citrate synthase (CS) activity were measured in mitochondria isolated from liver of WT and CypD−/− mice fed control and ethanol-containing diets. Results are from 2-factor ANOVA of data in Fig. 7.
Fig. 7.
Effect of chronic ethanol consumption and genotype on respiratory complex activities. Activities for complex I (A), complex II-III (B), complex IV (C), and complex V (D) were measured in mitochondria isolated from WT and CypD−/− mice fed control and ethanol-containing diets. Complex activities (nmol·s−1·mg protein−1) were normalized to citrate synthase (CS) activity (nmol·s−1·mg protein−1) and expressed as a ratio. Diet and genotype had no effect on CS activity (data not shown). Values are means ± SE; n = 3–7 pairs of mice. Results for 2-factor ANOVA are provided in Table 4. aP < 0.05 vs. WT Con; bP < 0.05 vs. CypD−/− Con; cP < 0.05 vs. WT EtOH.
DISCUSSION
It is well documented that chronic ethanol consumption causes structural and functional alterations in liver mitochondria (2, 32, 62). Such ethanol-dependent changes include enlarged mitochondria, impaired β-oxidation, increased ROS production, decreased respiratory complex proteins, and diminished ATP production (59). These alterations in mitochondrial function and others have been implicated in the pathogenesis of alcoholic steatohepatitis and fibrosis (69). Specifically, it has been proposed that the inability to provide and maintain sufficient ATP needed for metabolism, detoxification, and repair following chronic ethanol exposure renders hepatocytes vulnerable to secondary stressors, leading to hepatocyte cell death and, ultimately, liver disease (23, 72).
Induction of the MPT pore is believed to play an integral role in mediating cell death from multiple pathological insults. The MPT pore is a multiprotein complex embedded in the mitochondrial inner membrane that allows for passage of ≤1.5-kDa solutes into and out of the mitochondrion (39, 41). While the precise components of the pore remain elusive, a growing consensus supports a new model in which CypD remains an established member of the MPT pore, with ANT serving in a regulatory role and the voltage-dependent anion channel thought to be dispensable (8). Opening of the MPT pore by various triggers (e.g., reactive species and Ca2+ overload) results in loss of membrane potential, decreased oxidative phosphorylation and ATP production, mitochondrial matrix swelling, rupture of the outer membrane, and release of pro-cell death proteins. As CypD remains one of the only firm MPT pore components, it continues to serve as a focal point for understanding the role of the MPT pore in disease processes (30, 77).
CypD, a peptidylprolyl cis-trans isomerase, functions as a chaperone for proteins imported into the mitochondrial matrix (1). In the context of the MPT pore, CypD is believed to interact with the ANT, which promotes opening of the MPT pore and solute passage (34). Ca2+ promotes binding of CypD to the ANT and may be responsible for induction of the MPT pore (61); however, recent studies implicate interactions among CypD, ANT, and the inorganic phosphate carrier in pore formation (53), as well as the ATP synthase (14, 35). CypD has also been suggested to serve as a redox sensor and to initiate and enhance pore opening by ROS (57, 65). Higher CypD levels have been shown to sensitize mitochondria to undergo the MPT (63). In light of our previous studies showing higher CypD levels following chronic ethanol consumption (47), we chose to further investigate the role of CypD in alcoholic liver injury by using mice lacking CypD.
On the basis of a large body of literature showing disease prevention or attenuation in CypD−/− mice, we hypothesized that genetic deficiency of CypD would prevent or lessen ethanol-dependent mitochondrial dysfunction and tissue injury. However, despite increased resistance to undergo MPT (Fig. 4), CypD−/− mice were not protected against alcoholic steatosis and cell death (Fig. 2). As expected, mitochondria isolated from CypD−/− mice were more resistant to Ca2+-induced swelling than were mitochondria isolated from WT mice (Fig. 4). Mitochondria from ethanol-fed WT mice also were the most sensitive to Ca2+-induced swelling, thus supporting previous findings (47, 67). While increased sensitivity may be linked, in part, to higher levels of CypD in mitochondria of ethanol-fed WT mice, Shulga and Pastorino (70) report that chronic ethanol-dependent acetylation of CypD increases its interaction with the ANT and enhances pore opening. This increased sensitivity results from an ethanol-mediated inhibition of the mitochondrial NAD+-dependent deacetylase sirtuin 3 (70). Interestingly, even in the absence of CypD, mitochondria isolated from ethanol-fed CypD−/− mice were more sensitive to Ca2+-induced swelling than mitochondria isolated from their control-fed counterparts, suggesting a CypD-independent mechanism for increased pore sensitivity. While the mechanisms responsible for this sensitivity are not known, it is possible that sites downstream of CypD might be responsible for this effect. For example, experiments with bonkrekic acid or decylubiquinone may reveal effects of ethanol on the MPT downstream of the CypD regulatory site (11, 66, 78, 80). Recent work by Li et al. (54) showed that rotenone also can be an effective inhibitor of the MPT in tissues with low levels of CypD, suggesting a novel role for complex I in MPT pore regulation. Furthermore, the data presented in the current study also support the concept proposed by He and Lemasters (40) that two mechanisms are involved in MPT pore opening: one stimulated by Ca2+ and blocked by CsA and the other “unregulated.” In the unregulated model, aggregations of misfolded mitochondrial membrane proteins damaged by reactive oxygen, nitrogen, and/or lipid species contribute to the MPT. These oxidatively damaged proteins form protein cluster pores within membranes that are initially plugged by chaperone-like proteins, thereby blocking passage of water and other solutes to prevent swelling. However, when the amount of damaged protein clusters exceeds protective chaperones, these unregulated pores “open” and swelling occurs. Therefore, it is possible that increased sensitivity of MPT pore induction in mitochondria from ethanol-fed mice (independent of genotype) may comprise two components: 1) the classic CsA-sensitive MPT pore and 2) the unregulated pore that forms in the absence of CypD binding and is associated with ethanol-mediated oxidative damage to mitochondrial membranes and proteins (4, 7). Similar results were reported in a model of high-dose acetaminophen hepatotoxicity, in which genetic depletion of CypD did not protect from reactive nitrogen species-mediated toxicity (58).
In addition to assessing effects on pore opening, we also examined ethanol-dependent effects on mitochondrial respiration and respiratory complex activities. It is important to point out that previous studies show similar rates of basal, ADP, and uncoupler-stimulated respiration in mitochondria from WT and CypD−/− mice (10), suggesting that CypD itself does not affect energy metabolism under normal conditions. Additionally, complex IV activities and ATP levels are similar in CypD−/− and WT mice fed normal chow diets (28). Similarly, we saw little difference in respiration rates between control-fed WT and CypD−/− mice (Fig. 6).
Previously, we showed that chronic ethanol feeding decreases mitochondrial state 3 (ADP-dependent) respiration and results in little change in state 4 (ADP-independent) respiration (2, 47, 75). These studies indicate that the primary ethanol-mediated detrimental effect on mitochondria is at the level of the respiratory complexes adversely affecting electron transport and is not due to proton leak and uncoupling (24). However, these previous results were obtained using a rat model of chronic ethanol consumption; few studies have been done using mouse models (38, 76). In contrast to experiments using rats, we show here that state 3 respiration measured using complex I-linked substrates is largely unaffected by ethanol, whereas state 4 respiration is higher in ethanol-fed WT and CypD−/− mice, indicating an uncoupling effect of chronic ethanol consumption.
Studies by Han et al. (38) also show different responses in mitochondrial respiration between rat and mouse models of chronic ethanol consumption. For example, chronic ethanol feeding decreased state 3 respiration in rats (38), which is in agreement with previous work (7, 47, 75). However, Han et al. showed increased state 3 respiration in two models of murine ethanol exposure (oral feeding and intragastic infusion), with state 4 respiration significantly increased only in mice intragastrically infused with ethanol. While the reasons for the differences between the findings of Han et al. and the work presented here (Fig. 6) are not known, they may be due to the difference in total ethanol dose. We used a lower dose of ethanol (28.8% of total daily calories) for 4 wk, whereas Han et al. used a higher dose (39% of total daily calories) for 5 wk. It is possible that the higher dose of ethanol used by Han et al. triggered adaptation to the metabolic and/or toxic stress of ethanol, which stimulated mitochondrial biogenesis, resulting in increased mitochondrial function (i.e., increased state 3 respiration).
Similarly, the biological mechanisms responsible for how alcohol differentially affects mitochondrial function in rats and mice are also unclear but likely due to differences in physiology, metabolism, and adaptive mechanisms to toxicant stress. The ethanol-dependent decrease in the RCR in mice in the current study is largely the same as that observed in mitochondria isolated from ethanol-fed rats; therefore, the overall impact of chronic ethanol feeding on mitochondrial bioenergetics may be similar. We did, however, observe a significant decrease in complex II-linked state 3 respiration in ethanol-fed CypD−/− compared with control-fed CypD−/− and ethanol-fed WT mice. It is not known why a defect in state 3 respiration was revealed when a complex II-linked substrate (succinate) vs. a complex I-linked substrate (glutamate-malate) was used. However, complex II-III and complex IV respiratory activities were significantly lower in mitochondria isolated from ethanol- than control-fed mice, with the lowest level of complex IV activity observed in ethanol-fed CypD−/− mice (Fig. 7, Table 4). Importantly, significant diet and genotype effects were observed for succinate-mediated state 3 respiration, with the interaction just missing statistical significance (Table 3). In contrast, complex I activities were unaffected by ethanol and genotype. It is likely that an ethanol-mediated depression of a respiratory component downstream of complex II is limiting succinate-driven state 3 respiration but may retain sufficient functionality to facilitate electron flow from NADH-linked substrates. Also, whereas glutamate-malate-driven respiration is limited by flow of electrons through complex I, succinate-driven respiration is likely limited by a downstream lesion in the respiratory chain. Investigations of the oxidative phosphorylation proteome may reveal a molecular basis for the ethanol- and genotype-mediated differences in respiration in the different genotypes and response to chronic ethanol.
One consequence of MPT pore induction is initiation of cell death programs (48, 50, 52). Formation of the MPT pore causes mitochondrial depolarization and increases ROS production, which can trigger autophagy in cells (45, 51). Autophagy is recognized as a major intracellular pathway responsible for degradation and removal of long-lived cytoplasmic proteins, lipid droplets, and damaged organelles (74). Indeed, mitochondria can be degraded by the autophagosomal-lysosomal pathway (i.e., mitophagy), but the basis for targeting individual mitochondria for autophagic removal remains unclear (3). Autophagy is necessary to control the quality of proteins and organelles to maintain cellular function; thus autophagy can be viewed as a protective mechanism limiting toxicity. Indeed, several groups have shown that inhibition of autophagy by pharmacological agents increased ethanol-induced hepatocyte cell death (27, 56, 79), whereas stimulation of autophagy reduced alcoholic steatosis and toxicity (27, 56).
We observed that chronic ethanol consumption increased the ratio of LC3B-II to LC3B-I, suggesting an adapative increase in autophagy to remove cellular components damaged by ethanol-mediated toxicity. Interestingly, this autophagic response appears to be blunted in CypD−/− mice, as shown by increased levels of p62 in control- and ethanol-fed CypD−/− mice and no increase in the ratio of LC3B-II to LC3B-I as a result of chronic ethanol consumption. Important to the current study is work by Carreira et al. (17) showing no increase in autophagy in response to starvation in cardiomyocytes isolated from CypD−/− mice. Furthermore, cardiomyocytes isolated from mice overexpressing CypD exhibited enhanced levels of autophagy (17). This may help explain why CypD deficiency did not prevent chronic ethanol hepatotoxicity, as the protective process of autophagy was less functional in CypD−/− mice. Therefore, damaged hepatocytes, cellular proteins, and organelles would not be cleared, and liver injury might ensue and be sustained. This concept is supported by observations of increased steatosis and TUNEL-positive cells in control- and ethanol-fed CypD−/− mice (Fig. 2). Together, these data suggest a new physiological role of CypD in autophagy.
Along these same lines, Eliseev et al. (29) reported that a CypD-Bcl-2 interaction is important for limiting cytochrome c release from mitochondria and inhibiting cytochrome c-dependent apoptosis (29). Furthermore, Baines et al. (9) showed that Bcl-2 family member-induced cytotoxicity does not depend on CypD, as CypD−/− fibroblasts are susceptible to staurosporine- and TNF-α-induced cell death. This finding could be important for the current study, because TNF-α is critical for alcoholic liver disease (21). Previously, we found increased Bcl-2 protein in mitochondria from ethanol-treated animals compared with controls and were unable to detect cytochrome c in cytosol from ethanol-treated and control groups (47). Thus CypD may play a role as a survival molecule, possibly acting on targets other than the MPT pore. Recognition of these newly proposed functions for CypD (30) is important, as they help explain unpredictable responses to established pharmacological treatments.
Conclusion.
On the basis of numerous studies showing a protective role of CypD deficiency in other disease models and our previous work showing increased MPT pore opening in response to chronic ethanol consumption (47), we proposed that CypD deficiency would prevent and/or attenuate ethanol hepatotoxicity. However, CypD deficiency did not prevent alcoholic steatosis and cell death. Furthermore, these findings suggest that a secondary mechanism, potentially independent of CypD, contributes to ethanol-mediated sensitivity to undergo MPT (40). This is supported by observations that MPT sensitivity was enhanced in mitochondria from WT and CypD−/− mice chronically fed ethanol. It is likely that chronic ethanol-mediated enhancement of the MPT contributes to the progression of pathology by sensitizing the alcoholic fatty liver to other secondary oxidant, metabolic, viral, and environmental stressors. Mitochondria unable to maintain sufficient energy likely drop below a threshold of normal mitochondrial health into a pathological range and are unable to support cellular repair mechanisms, leading to cell death. Indeed, the combination of chronic ethanol and cigarette smoke or hepatitis C causes severe mitochondrial dysfunction and metabolic stress, exacerbating steatosis and initiating fibrogenesis (5, 19, 49). Clearly, gaps remain in our understanding of the role of CypD and the MPT pore in normal liver physiology and pathophysiology. More studies are needed to improve understanding of the importance of mitochondrial dysfunction in alcoholic liver disease and its interaction with other key cellular processes, such as autophagy. It is critical that we continue our investigations into the importance of mitochondrial dysfunction, with the goal to provide new treatments for alcoholic liver disease.
GRANTS
This work was supported in part by National Institutes of Health Grants AA-015172 and AA-018841 (to S. M. Bailey) and NS-071168 to (M. Lesort). A. L. King was supported by a Research Supplement to Promote Diversity in Health-Related Research linked to the parent grant (AA-015172).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.L.K., C.E.G., M.J.L., and S.M.B. are responsible for conception and design of the research; A.L.K., T.M.S., Z.M., U.S.U., C.R.O., and A.M.B. performed the experiments; A.L.K., T.M.S., U.S.U., C.R.O., A.M.B., D.R.C., and S.M.B. analyzed the data; A.L.K., C.E.G., M.J.L., and S.M.B. interpreted the results of the experiments; A.L.K. and S.M.B. prepared the figures; A.L.K. and S.M.B. drafted the manuscript; A.L.K., M.J.L., and S.M.B. edited and revised the manuscript; A.L.K., M.J.L., and S.M.B. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. M. A. Forte for providing the CypD−/− mice through a materials transfer agreement with the Oregon Health and Science University.
Current addresses: A. L. King, Department of Biology, Southern Polytechnic State University, Marietta, GA; A. M. Betancourt, Division of Animal Feeds, US Food and Drug Administration, Rockville, MD.
REFERENCES
- 1.Andreeva L, Heads R, Green CJ. Cyclophilins and their possible role in the stress response. Int J Exp Pathol 80: 305–315, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andringa KK, King AL, Eccleston HB, Mantena SK, Landar A, Jhala NC, Dickinson DA, Squadrito GL, Bailey SM. Analysis of the liver mitochondrial proteome in response to ethanol and S-adenosylmethionine treatments: novel molecular targets of disease and hepatoprotection. Am J Physiol Gastrointest Liver Physiol 298: G732–G745, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20: 31–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey SM, Cunningham CC. Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology 28: 1318–1326, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Bailey SM, Mantena SK, Millender-Swain T, Cakir Y, Jhala NC, Chhieng D, Pinkerton KE, Ballinger SW. Ethanol and tobacco smoke increase hepatic steatosis and hypoxia in the hypercholesterolemic apoE−/− mouse: implications for a “multihit” hypothesis of fatty liver disease. Free Radic Biol Med 46: 928–938, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey SM, Pietsch EC, Cunningham CC. Ethanol stimulates the production of reactive oxygen species at mitochondrial complexes I and III. Free Radic Biol Med 27: 891–900, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Bailey SM, Robinson G, Pinner A, Chamlee L, Ulasova E, Pompilius M, Page GP, Chhieng D, Jhala N, Landar A, Kharbanda KK, Ballinger S, Darley-Usmar V. S-adenosylmethionine prevents chronic alcohol-induced mitochondrial dysfunction in the rat liver. Am J Physiol Gastrointest Liver Physiol 291: G857–G867, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Baines CP. The molecular composition of the mitochondrial permeability transition pore. J Mol Cell Cardiol 46: 850–857, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem 280: 18558–18561, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Belliere J, Devun F, Cottet-Rousselle C, Batandier C, Leverve X, Fontaine E. Prerequisites for ubiquinone analogs to prevent mitochondrial permeability transition-induced cell death. J Bioenerg Biomembr 44: 207–212, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, Forte MA. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J 273: 2077–2099, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Bjorkoy G, Lamark T, Pankiv S, Overvatn A, Brech A, Johansen T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol 452: 181–197, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Bonora M, Bononi A, De Marchi E, Giorgi C, Lebiedzinska M, Marchi S, Patergnani S, Rimessi A, Suski JM, Wojtala A, Wieckowski MR, Kroemer G, Galluzzi L, Pinton P. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle 12: 674–683, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 16.Broekemeier KM, Dempsey ME, Pfeiffer DR. Cyclosporin A is a potent inhibitor of the inner membrane permeability transition in liver mitochondria. J Biol Chem 264: 7826–7830, 1989 [PubMed] [Google Scholar]
- 17.Carreira RS, Lee Y, Ghochani M, Gustafsson AB, Gottlieb RA. Cyclophilin D is required for mitochondrial removal by autophagy in cardiac cells. Autophagy 6: 462–472, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol 83: 519–548, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Choi J. Oxidative stress, endogenous antioxidants, alcohol, and hepatitis C: pathogenic interactions and therapeutic considerations. Free Radic Biol Med 52: 1135–1150, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Choo YS, Johnson GV, MacDonald M, Detloff PJ, Lesort M. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum Mol Genet 13: 1407–1420, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Cohen JI, Chen X, Nagy LE. Redox signaling and the innate immune system in alcoholic liver disease. Antioxid Redox Signal 15: 523–534, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J 255: 357–360, 1988 [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham CC, Bailey SM. Ethanol consumption and liver mitochondria function. Biol Signals Recept 10: 271–282, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Cunningham CC, Coleman WB, Spach PI. The effects of chronic ethanol consumption on hepatic mitochondrial energy metabolism. Alcohol Alcohol 25: 127–136, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Darley-Usmar VM, Capaldi RA, Takamiya S, Millet F, Wilson MT, Malatesta F, Sarti P. In: Mitochondria: A Practical Approach, edited by Darley-Usmar VM, Rickwood D, Wilson MT. Oxford, UK: IRL, 1987, p. 113–152 [Google Scholar]
- 26.Das S, Hajnoczky N, Antony AN, Csordas G, Gaspers LD, Clemens DL, Hoek JB, Hajnoczky G. Mitochondrial morphology and dynamics in hepatocytes from normal and ethanol-fed rats. Pflügers Arch 464: 101–109, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W, Lu B, Stolz DB, Clemens DL, Yin XM. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology 139: 1740–1752, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med 14: 1097–1105, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eliseev RA, Malecki J, Lester T, Zhang Y, Humphrey J, Gunter TE. Cyclophilin D interacts with Bcl2 and exerts an anti-apoptotic effect. J Biol Chem 284: 9692–9699, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elrod JW, Molkentin JD. Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore. Circ J 77: 1111–1122, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione: hepatocellular survival-death switch. J Gastroenterol Hepatol 21 Suppl 3: S3–S6, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Ruiz I, Rodriguez-Juan C, Diaz-Sanjuan T, del Hoyo P, Colina F, Munoz-Yague T, Solis-Herruzo JA. Uric acid and anti-TNF antibody improve mitochondrial dysfunction in ob/ob mice. Hepatology 44: 581–591, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119: 493–501, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giorgio V, Soriano ME, Basso E, Bisetto E, Lippe G, Forte MA, Bernardi P. Cyclophilin D in mitochondrial pathophysiology. Biochim Biophys Acta 1797: 1113–1118, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci USA 110: 5887–5892, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology 21: 1465–1468, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Halestrap AP, Davidson AM. Inhibition of Ca2+-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J 268: 153–160, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han D, Ybanez MD, Johnson HS, McDonald JN, Mesropyan L, Sancheti H, Martin G, Martin A, Lim AM, Dara L, Cadenas E, Tsukamoto H, Kaplowitz N. Dynamic adaptation of liver mitochondria to chronic alcohol feeding in mice: biogenesis, remodeling, and functional alterations. J Biol Chem 287: 42165–42179, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys 195: 460–467, 1979 [DOI] [PubMed] [Google Scholar]
- 40.He L, Lemasters JJ. Regulated and unregulated mitochondrial permeability transition pores: a new paradigm of pore structure and function? FEBS Lett 512: 1–7, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem 251: 5069–5077, 1976 [PubMed] [Google Scholar]
- 42.Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 125: 1246–1257, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19: 5720–5728, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karim MR, Kanazawa T, Daigaku Y, Fujimura S, Miotto G, Kadowaki M. Cytosolic LC3 ratio as a sensitive index of macroautophagy in isolated rat hepatocytes and H4-II-E cells. Autophagy 3: 553–560, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462: 245–253, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King AL, Bailey SM. Assessment of mitochondrial dysfunction arising from treatment with hepatotoxicants. Curr Protoc Toxicol 44: 14.18.11–14.18.29, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King AL, Swain TM, Dickinson DA, Lesort MJ, Bailey SM. Chronic ethanol consumption enhances sensitivity to Ca2+-mediated opening of the mitochondrial permeability transition pore and increases cyclophilin D in liver. Am J Physiol Gastrointest Liver Physiol 299: G954–G966, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kinnally KW, Peixoto PM, Ryu SY, Dejean LM. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim Biophys Acta 1813: 616–622, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korenaga M, Wang T, Li Y, Showalter LA, Chan T, Sun J, Weinman SA. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol Chem 280: 37481–37488, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev 87: 99–163, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 441: 523–540, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim Biophys Acta 1787: 1395–1401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leung AW, Varanyuwatana P, Halestrap AP. The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition. J Biol Chem 283: 26312–26323, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li B, Chauvin C, De Paulis D, De Oliveira F, Gharib A, Vial G, Lablanche S, Leverve X, Bernardi P, Ovize M, Fontaine E. Inhibition of complex I regulates the mitochondrial permeability transition through a phosphate-sensitive inhibitory site masked by cyclophilin D. Biochim Biophys Acta 1817: 1628–1634, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Lieber CS, DeCarli LM. The feeding of alcohol in liquid diets: two decades of applications and 1982 update. Alcohol Clin Exp Res 6: 523–531, 1982 [DOI] [PubMed] [Google Scholar]
- 56.Lin CW, Zhang H, Li M, Xiong X, Chen X, Chen X, Dong XC, Yin XM. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J Hepatol 58: 993–999, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linard D, Kandlbinder A, Degand H, Morsomme P, Dietz KJ, Knoops B. Redox characterization of human cyclophilin D: identification of a new mammalian mitochondrial redox sensor? Arch Biochem Biophys 491: 39–45, 2009 [DOI] [PubMed] [Google Scholar]
- 58.LoGuidice A, Boelsterli UA. Acetaminophen overdose-induced liver injury in mice is mediated by peroxynitrite independently of the cyclophilin D-regulated permeability transition. Hepatology 54: 969–978, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Mantena SK, King AL, Andringa KK, Eccleston HB, Bailey SM. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic Biol Med 44: 1259–1272, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mantena SK, King AL, Andringa KK, Landar A, Darley-Usmar V, Bailey SM. Novel interactions of mitochondria and reactive oxygen/nitrogen species in alcohol mediated liver diseases. World J Gastroenterol 13: 4967–4973, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McStay GP, Clarke SJ, Halestrap AP. Role of critical thiol groups on the matrix surface of the adenine nucleotide translocase in the mechanism of the mitochondrial permeability transition pore. Biochem J 367: 541–548, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitchell T, Chacko B, Ballinger SW, Bailey SM, Zhang J, Darley-Usmar V. Convergent mechanisms for dysregulation of mitochondrial quality control in metabolic disease: implications for mitochondrial therapeutics. Biochem Soc Trans 41: 127–133, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naga KK, Sullivan PG, Geddes JW. High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition. J Neurosci 27: 7469–7475, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434: 652–658, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Nguyen TT, Stevens MV, Kohr M, Steenbergen C, Sack MN, Murphy E. Cysteine 203 of cyclophilin D is critical for cyclophilin D activation of the mitochondrial permeability transition pore. J Biol Chem 286: 40184–40192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pastorino JG, Hoek JB. Ethanol potentiates tumor necrosis factor-α cytotoxicity in hepatoma cells and primary rat hepatocytes by promoting induction of the mitochondrial permeability transition. Hepatology 31: 1141–1152, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Pastorino JG, Marcineviciute A, Cahill A, Hoek JB. Potentiation by chronic ethanol treatment of the mitochondrial permeability transition. Biochem Biophys Res Commun 265: 405–409, 1999 [DOI] [PubMed] [Google Scholar]
- 68.Petronilli V, Cola C, Massari S, Colonna R, Bernardi P. Physiological effectors modify voltage sensing by the cyclosporin A-sensitive permeability transition pore of mitochondria. J Biol Chem 268: 21939–21945, 1993 [PubMed] [Google Scholar]
- 69.Sastre J, Serviddio G, Pereda J, Minana JB, Arduini A, Vendemiale G, Poli G, Pallardo FV, Vina J. Mitochondrial function in liver disease. Front Biosci 12: 1200–1209, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Shulga N, Pastorino JG. Ethanol sensitizes mitochondria to the permeability transition by inhibiting deacetylation of cyclophilin-D mediated by sirtuin-3. J Cell Sci 123: 4117–4127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Spach PI, Parce JW, Cunningham CC. Effect of chronic ethanol administration on energy metabolism and phospholipase A2 activity in rat liver. Biochem J 178: 23–33, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Syn WK, Teaberry V, Choi SS, Diehl AM. Similarities and differences in the pathogenesis of alcoholic and nonalcoholic steatohepatitis. Semin Liver Dis 29: 200–210, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Twig G, Shirihai OS. The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal 14: 1939–1951, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Venkatraman A, Landar A, Davis AJ, Chamlee L, Sanderson T, Kim H, Page G, Pompilius M, Ballinger S, Darley-Usmar V, Bailey SM. Modification of the mitochondrial proteome in response to the stress of ethanol-dependent hepatotoxicity. J Biol Chem 279: 22092–22101, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Venkatraman A, Shiva S, Wigley A, Ulasova E, Chhieng D, Bailey SM, Darley-Usmar VM. The role of iNOS in alcohol-dependent hepatotoxicity and mitochondrial dysfunction in mice. Hepatology 40: 565–573, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Waldmeier PC, Zimmermann K, Qian T, Tintelnot-Blomley M, Lemasters JJ. Cyclophilin D as a drug target. Curr Med Chem 10: 1485–1506, 2003 [DOI] [PubMed] [Google Scholar]
- 78.Walter L, Nogueira V, Leverve X, Heitz MP, Bernardi P, Fontaine E. Three classes of ubiquinone analogs regulate the mitochondrial permeability transition pore through a common site. J Biol Chem 275: 29521–29527, 2000 [DOI] [PubMed] [Google Scholar]
- 79.Wu D, Wang X, Zhou R, Yang L, Cederbaum AI. Alcohol steatosis and cytotoxicity: the role of cytochrome P4502E1 and autophagy. Free Radic Biol Med 53: 1346–1370, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zamzami N, Marchetti P, Castedo M, Hirsch T, Susin SA, Masse B, Kroemer G. Inhibitors of permeability transition interfere with the disruption of the mitochondrial transmembrane potential during apoptosis. FEBS Lett 384: 53–57, 1996 [DOI] [PubMed] [Google Scholar]