Abstract
Xenin-25 (Xen) is a neurotensin-related peptide secreted by a subset of glucose-dependent insulinotropic polypeptide (GIP)-producing enteroendocrine cells. In animals, Xen regulates gastrointestinal function and glucose homeostasis, typically by initiating neural relays. However, little is known about Xen action in humans. This study determines whether exogenously administered Xen modulates gastric emptying and/or insulin secretion rates (ISRs) following meal ingestion. Fasted subjects with normal (NGT) or impaired (IGT) glucose tolerance and Type 2 diabetes mellitus (T2DM; n = 10–14 per group) ingested a liquid mixed meal plus acetaminophen (ACM; to assess gastric emptying) at time zero. On separate occasions, a primed-constant intravenous infusion of vehicle or Xen at 4 (Lo-Xen) or 12 (Hi-Xen) pmol·kg−1·min−1 was administered from zero until 300 min. Some subjects with NGT received 30- and 90-min Hi-Xen infusions. Plasma ACM, glucose, insulin, C-peptide, glucagon, Xen, GIP, and glucagon-like peptide-1 (GLP-1) levels were measured and ISRs calculated. Areas under the curves were compared for treatment effects. Infusion with Hi-Xen, but not Lo-Xen, similarly delayed gastric emptying and reduced postprandial glucose levels in all groups. Infusions for 90 or 300 min, but not 30 min, were equally effective. Hi-Xen reduced plasma GLP-1, but not GIP, levels without altering the insulin secretory response to glucose. Intense staining for Xen receptors was detected on PGP9.5-positive nerve fibers in the longitudinal muscle of the human stomach. Thus Xen reduces gastric emptying in humans with and without T2DM, probably via a neural relay. Moreover, endogenous GLP-1 may not be a major enhancer of insulin secretion in healthy humans under physiological conditions.
Keywords: xenin, GIP, GLP-1, glucagon, incretin, gastric emptying, insulin secretion
the enteroendocrine (EE) system is composed of numerous subtypes of singly dispersed EE cells scattered throughout the gastrointestinal epithelium (6, 51). Peptides released from EE cells regulate gastrointestinal function (6, 51) and glucose homeostasis (5, 14, 33). Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are hormones that are produced predominantly by intestinal L cells in the distal intestine and K cells in the proximal small intestine, respectively. Both are released into the circulation immediately after meal ingestion in response to nutrients present in the lumen of the gut, but not to those in the blood. Both peptides amplify glucose-stimulated insulin secretion and have been coined “incretins” (5, 14, 33).
Xenin-25 (Xen) is a 25-amino acid neurotensin-related peptide produced by a subset of GIP-producing cells (4, 15). Genetic and pharmacological data indicate that effects of Xen are mediated by neurotensin receptor-1 [NTSR1 (19, 24, 35, 57)]. In animals, Xen delays gastric emptying (25), reduces food intake (2, 10, 27), increases gut motility (17), augments gall bladder contractions (23), and enhances secretion from the exocrine pancreas (16). Many of these Xen effects are known to be mediated by neurons (9, 10, 23, 25, 27). In vivo mouse studies from our (54) and another (30) laboratory demonstrated that Xen increases the effects of GIP on insulin release. We further showed that Xen does not act directly on beta cells (54). Rather, Xen initiates a cholinergic relay in the periphery without activating regions of the brain associated with afferent or efferent signaling. Thus effects of Xen on insulin release may be independent of the central nervous system. Consistent with this hypothesis, effects of Xen on gall bladder contractions are inhibited by atropine, but not vagotomy (23).
As in our mouse studies, we recently showed that administration of Xen to humans during intravenous graded glucose infusions increased the effects of exogenously administered GIP on insulin secretion rates (ISRs) in humans with normal glucose tolerance (NGT) and impaired glucose tolerance (IGT), but not in those with Type 2 diabetes mellitus [T2DM (53)]. Infusion of Xen alone had no effect in any group. With meal ingestion, GIP and other gut-derived factors are released into the circulation, suggesting that exogenously administered Xen may have different effects in conjunction with orally vs. intravenously administered nutrients. The purpose of the present study was to determine whether exogenously administered Xen modulates gastric emptying and glucose, insulin, C-peptide, glucagon, Xen, GIP, and GLP-1 levels as well as ISRs in response to meal ingestion.
MATERIALS AND METHODS
Studies in human subjects.
All protocols were approved by Washington University's Human Research Protection Office and the FDA (IND no. 103,374) and are registered with ClinicalTrials.gov (NCT00949663). Studies were performed in the Clinical Research Unit of the Institute of Clinical and Translational Sciences of Washington University after obtaining written informed consent. Male and female subjects with NGT, IGT, and mild T2DM were studied (Table 1). Glucose tolerance, inclusion/exclusion criteria, and screening protocols were as previously described (53). With respect to T2DM, subjects were required to have HbA1c ≤9%, could not be using insulin for treatment, and had no known history of symptomatic gastroparesis or peripheral neuropathy. These selection criteria were designed to exclude T2DM subjects with advanced beta cell failure.
Table 1.
Group characteristics
| NGT (n = 10) | IGT (n = 14) | T2DM (n = 12) | |
|---|---|---|---|
| 2-h Glucose, mg/dl‡ | 118 ± 11 | 162 ± 14 | 245 ± 23 |
| Fasting glucose, mg/dl‡ | 95 ± 7.2 | 97 ± 7.3 | 127 ± 20 |
| HbA1c, %† | 5.6 ± 0.3 | 5.7 ± 0.3 | 6.2 ± 0.6 |
| HbA1c, mmol/mol† | 38 ± 2.2 | 39 ± 1.9 | 44 ± 4.3 |
| Sex, men/women | 4/6 | 9/5 | 6/6 |
| Age, yr* | 40 ± 11 | 46 ± 9.1 | 51 ± 6.4 |
| BMI, kg/m2† | 29 ± 5.1 | 31 ± 5.3 | 38 ± 5.7 |
Data are group means ± SD.
P < 0.05, 0.01, and 0.0001, respectively, by 1-way ANOVA.
Study design.
Studies were performed after a 10-h overnight fast. In subjects with T2DM and taking oral diabetes medications, drugs were discontinued for 48 h before each study visit. One intravenous catheter was placed into a hand vein. This hand was kept in a thermostatically controlled box (50–55°C) for sampling arterialized venous blood (7, 31). A second intravenous line was inserted in the contralateral arm for administration of peptide. Subjects with a fasting blood sugar >120 mg/dl were given boluses of intravenous human insulin (∼0.01 U/kg) at 20-min intervals as needed to decrease the blood glucose level to 100–120 mg/dl to limit variability of initial glucose levels. Blood glucose was stable (≤120 mg/dl) for 20 min before starting the meal tolerance test.
Meal tolerance tests.
Boost Plus (Nestle Health Science, Florham Park, NJ) is a liquid mixed meal (360 calories, 14 g of fat, 45 g of carbohydrates, and 14 g of protein). From 0 to 3 min, fasted subjects ingested Boost Plus and liquid acetaminophen (ACM; 1.5 gm/15 ml; Q-PAP Infants' Drop; Qualitest Pharmaceuticals, Huntsville, AL).
Treatments.
Treatments were administered in a crossover design and involved intravenous infusions of albumin alone (Alb; i.e., no peptide) or different duration/doses of Xen. On separate visits, at least 2 wk apart, a primed constant infusion of Alb or Xen alone was administered starting at time zero and continued for 300 min. For the Lo-Xen treatments, infusion rates of 0–3, 3–7, 7–10, and 10–300 min were 10.8, 7.7, 5.6, and 4.0 pmol·kg−1·min−1. For Hi-Xen, the Xen concentration in the infusate was tripled but administered at the same relative flow rates as with Lo-Xen. Seven subjects with NGT also received Hi-Xen for 30 and 90 min followed by infusion of Alb until 300 min. Infusions were randomized and subjects were blinded to treatments.
Xenin-25.
Xen was synthesized under GMP conditions, analyzed, and prepared for infusions as previously described (53). Infusion of Lo-Xen in humans was well tolerated in our earlier study (53). Thus a threefold higher dose of Xen (Hi-Xen) was also studied during the meal tolerance tests.
Measurements.
Glucose, insulin, C-peptide, glucagon, GIP, Xen, total amylase, complete metabolic profiles, and HbA1c were determined as previously described (53). Plasma ACM was measured by the Roche Acetaminophen Assay with the Cobas c501 (Roche Diagnostics, Indianapolis, IN; lower limit of detection of 1.2 μg/ml). Plasma levels of total and active GLP-1 were measured by ELISAs (Meso Scale Discovery; Rockville, MD). The number and severity of diarrheal episodes were determined by surveys taken during the infusions and postinfusion follow-up by telephone.
Data analysis and statistics.
ISRs were derived by stochastic deconvolution of the peripheral C-peptide concentrations as previously described by using population-based estimates of C-peptide clearance kinetics (45, 46, 49). Incremental areas under the curve (iAUCs) relative to baseline were used to estimate effects and were calculated by the trapezoid method. Following meal ingestion, Xen treatment altered the temporal pattern of plasma glucose levels rather than the total amount of glucose in the blood over the 300 min of the study. Thus iAUCs for glucose, ISR, glucagon, GIP, and GLP-1 responses were calculated for the time period of 0–120 min (i.e., the crossover time point for plasma glucose levels in the NGT group). Similarly, plasma ACM levels crossed over at 240 min in all three groups. Thus ACM iAUCs were calculated for the time frame from 0–240 min.
Physiological data were analyzed by the mixed-effects models with subject as a random effect and peptide as a fixed effect by use of IBM SPSS Version 20. Within each group, comparison of 300-min infusions allowed two degrees of freedom and pairwise comparisons were limited to evaluating the effects of Hi-Xen vs. Alb and Lo-Xen vs. Alb. Within the NGT group, comparison of the 0-, 30-, 90-, and 300-min Hi-Xen infusions allowed three degrees of freedom, and pairwise comparisons were limited to evaluating the effects of 1) 300 min Hi-Xen vs. Alb, 2) 90 min Hi-Xen vs. Alb, and 3) 30 min Hi-Xen vs. Alb. Two-tailed t-tests were used for all analyses and P < 0.05 was considered significant. Differences in baseline characteristics, immunoreactive (IR)-Xen, and IR-GIP levels between groups were evaluated by one-way ANOVAs via GraphPad Prism Version 5. Contingency tables with Pearson χ2 were used to evaluate effects of treatments on occurrence of diarrhea. Fisher's exact test was used when expected cell frequencies in the contingency table were low.
Immunohistochemical studies.
Paraffin-embedded blocks of human stomach were obtained from our Department of Pathology. Sections were deparaffinized, subjected to antigen retrieval with EDTA (pH 8), blocked with CAS-Block (Invitrogen, Frederick, MD), and incubated with primary antibodies overnight at 4°C as previously described (52, 57). After washing, bound primary antibodies were detected following incubation for 45 min at room temperature with the appropriate conjugated secondary antibodies. Nuclei were counterstained with bis-benzimide. For double-label studies, single tissue sections were incubated with both primary antibodies and multiple single-color images were merged by use of Adobe Photoshop. All antibodies were diluted in Da Vinci Green Diluent (Biocare Medical, Concord, CA). The antibodies including source, catalog number, and dilution were goat anti-NTSR1 (Santa Cruz Biotechnology, Dallas, TX; no. SC-7596, 1:100), rabbit anti-PGP9.5 (Millipore; no. AB1761), and mouse anti-smooth muscle actin (Sigma Chemical; no. A5228; 1:200). Minimal cross-reacting Alexa Fluor 488- and Alexa Fluor 549-conjugated donkey anti-mouse, goat, and rabbit antibodies were obtained from Jackson ImmunoResearch (West Grove, PA) and used at a 1:500 dilution.
RESULTS
Subject characteristics.
Two-hour glucose, fasting glucose, and HbA1c levels were progressively higher in subjects with NGT vs. IGT vs. T2DM (Table 1). Body mass index was higher in subjects with T2DM. Subjects with T2DM had mild disease and no history of gastroparesis or other clinically evident neuropathies (e.g., burning or tingling in feet). Six of the 12 subjects with T2DM were treated with oral medications (three with metformin, one with metformin and glipizide, one with metformin and rosiglitazone, and one with pioglitazone). Four subjects with T2DM required insulin before all three study visits and four required insulin before only one visit. Post hoc analysis of data indicated that two subjects in the IGT group had inappropriately elevated baseline concentrations for plasma glucose, insulin, C-peptide, GIP, and GLP-1 compared with their other study visits, indicating that they had not fasted before the study visit (e.g., “fasting” GIP and GLP-1 levels were elevated ∼10-fold). Thus data for these two specific visits were excluded. For the other individuals, respective basal values for all parameters were similar for each study visit.
Xen infusion results in pharmacological levels of peptide.
Consistent with our previous results (53), IR-Xen levels were undetectable (<2 pM) in all three groups following an overnight fast (Fig. 1). After Boost Plus ingestion, IR-Xen levels remained undetectable during infusion with Alb alone. In a preliminary investigation, endogenously released IR-Xen was also undetectable in several nondiabetic subjects following ingestion of a solid meal (pizza) or oral glucose (not shown). In contrast to infusion with Alb, plasma IR-Xen levels rapidly increased to and remained at ∼200 pM and ∼600 pM during infusion of Lo-Xen and Hi-Xen, respectively, in subjects with NGT, IGT, and T2DM (Fig. 1). During infusions with Lo-Xen and Hi-Xen, the respective iAUCs for IR-Xen levels from 0 to 300 min were not different between groups (P = 0.18 for Lo-Xen and P = 0.06 for Hi-Xen), although there was a trend toward higher levels in T2DM (not shown).
Fig. 1.
Plasma xenin-25 (Xen) levels in response to Xen infusions. Immunoreactive (IR)-Xen levels were determined in plasma prepared from subjects with normal glucose tolerance (NGT; A), impaired glucose tolerance (IGT; B), and Type 2 diabetes mellitus (T2DM; C) at the indicated time after Boost Plus ingestion with a primed 300-min continuous infusion of albumin (Alb; open circles), 4 pmol·kg−1·min−1 Xen (Lo-Xen; open squares), or 12 pmol·kg−1·min−1 Xen (Hi-Xen; open triangles). Group averages ± SE are shown and n = x, y, z represents the number of Alb, Lo-Xen, and Hi-Xen infusions, respectively, included in the measurements.
Hi-Xen delays gastric emptying in humans with NGT, IGT, and T2DM.
Plasma appearance of orally administered ACM was used to indirectly measure the rate of gastric emptying (55). Compared with Alb, infusion with Hi-Xen for 300 min shifted the postprandial increase in plasma ACM levels to later times in all groups (Fig. 2, A–C). As shown in Fig. 3, A–C, the iAUCs from 0 to 240 min for ACM levels were decreased by 34% in subjects with NGT (812 ± 105 vs. 1,222 ± 105 μg·ml−1·min; P = 0.014), 26% in subjects with IGT (821 ± 90 vs. 1,106 ± 66 μg·ml−1·min; P = 0.019), and 33% in subjects with T2DM (633 ± 90 vs. 948 ± 81 μg·ml−1·min; P = 0.022) for Hi-Xen vs. Alb, respectively. Compared with Alb, infusion with Lo-Xen did not significantly affect the postprandial increase in plasma ACM levels in any group.
Fig. 2.
Infusion of Hi-Xen delays gastric emptying and reduces postprandial glucose levels in humans with NGT, IGT, and T2DM. Plasma levels of acetominophen (ACM; A–C), glucose (D–F), and C-peptide (not shown) were measured at the indicated times after Boost Plus ingestion during infusion with Alb (open blue circles), Lo-Xen (open red squares), and Hi-Xen (open green triangles). Insulin secretion rates (ISRs; G–I) were calculated by deconvolution of plasma C-peptide levels. Values represent the group means ± SE for subjects with NGT (A, D, G), IGT (B, E, H), and T2DM (C, F, I). The number of subjects (n) is shown as described in Fig. 1. Statistical analyses are shown in Fig. 3, and time frames used for calculations of incremental areas under the curve (iAUCs) are shown by the dotted lines in each panel. The lower limit of detection (LLOD) for ACM is shown in A–C. Differences in the number of subjects within each group are due to the fact that several subjects did not receive ACM with meal ingestion and some subjects did not complete all study visits.
Fig. 3.
Infusion of Hi-Xen reduces the iAUCs for gastric emptying and postprandial glucose levels in humans with NGT, IGT, and T2DM. The iAUCs and ratio of the iAUCs with infusions of Alb (open bars), Lo-Xen (hatched bars), and Hi-Xen (solid bars) from Fig. 2 were calculated for each individual for the indicated time frame following Boost Plus ingestion. Values represent group means ± SE for plasma ACM (from 0–240 min; A–C), plasma glucose (from 0–120 min; D–F), ISRs (from 0–120 min; G–I), and the ratios of the glucose and ISR iAUCs (from 0–120 min; J–L). Data for subjects with NGT (A, D, G, J), IGT (B, E, H, K), and T2DM (C, F, I, L) are shown. In K, P = 0.05 is for the 1-way ANOVA comparing the means between all 3 groups.
Hi-Xen reduces postprandial glucose levels in humans with NGT, IGT, and T2DM. Consistent with delayed gastric emptying, infusion with Hi-Xen also reduced postprandial plasma glucose levels from 0–120 min in all three groups (Fig. 2, D–F) and slightly increased plasma glucose levels after 120 min. Compared with Alb, the glucose AUC and iAUC from 0 to 300 min were unaffected by Hi-Xen infusions within each group (not shown), indicating that only the plasma glucose profile was affected by Hi-Xen. Thus, on the basis of this post hoc analysis, treatment effects for glucose and glucose-related hormones were determined for the time period of 0 to 120 min. As shown in Fig. 3, D–F, the iAUCs from 0–120 min for plasma glucose with Hi-Xen vs. Alb were decreased by 38% in subjects with NGT (1,557 ± 234 vs. 2,512 ± 234 mg·dl−1·min; P = 0.007), 26% in subjects with IGT (2,120 ± 198 vs. 2,884 ± 165 mg·dl−1·min; P = 0.001), and 27% in subjects with T2DM (3,739 ± 413 vs. 5,112 ± 362 mg·dl−1·min; P = 0.03). Compared with Alb, infusion with Lo-Xen did not significantly decrease plasma glucose levels in any group. Since Hi-Xen delayed gastric emptying in all three groups, the effects of using shorter Hi-Xen infusion times were examined only in a subset of subjects with NGT (n = 7; not shown). The 90-min infusion with Hi-Xen was equally as effective as the 300-min infusion for reducing postprandial plasma ACM and glucose levels and the respective 120-min iAUCs. In contrast, the 30-min infusion was ineffective.
Hi-Xen infusion does not affect glucose-stimulated insulin secretion.
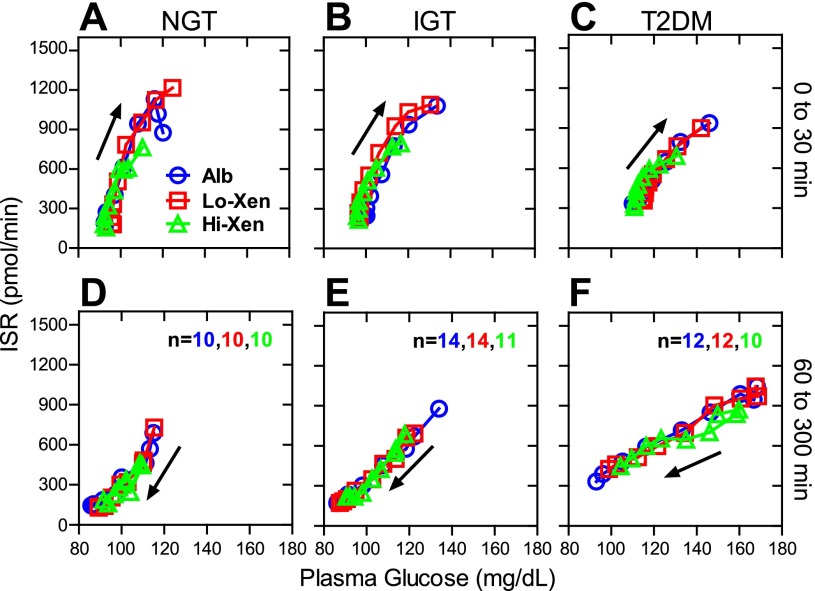
Within each group, ISRs (Fig. 2, G–I) as well as ISR iAUCs (Fig. 3, G–I) after Boost Plus ingestion tended to be lower for the first 120 min during infusion with Hi-Xen, but not Lo-Xen, compared with Alb. This difference in iAUCs was statistically significant only in the group with NGT (36,719 ± 5,094 vs. 61,640 ± 5,094 pmol·min−1·min; P = 0.003). Since Hi-Xen also reduced postprandial glucose levels (Fig. 2, D–F and 3, D–F), the iAUCs for Xen were normalized to those for plasma glucose. As shown in Fig. 3, J–L, differences in the ratios of ISR to plasma glucose iAUCs from 0–120 min were not significant within any group. To further evaluate the ability of Xen to enhance the beta cell sensitivity to glucose, ISRs were plotted as a function of plasma glucose levels (Fig. 4). Within each group, the curves from these plots were virtually identical for albumin, Lo-Xen, and Hi-Xen during the first 30 min after meal ingestion (when both glucose levels and ISRs are generally rising) and from 60–300 min (when glucose and ISRs are generally decreasing).
Fig. 4.
Infusion of Hi-Xen does not affect glucose-stimulated insulin secretion. Group average ISRs from Fig. 2, D–F, were plotted as a function of the group average plasma glucose levels from Fig. 2, G–I, at each time point. Data from 0 to 30 min (A–C) and from 60 to 300 min (D–F) are shown. X and Y error bars are the same as those in Fig. 2 but not shown. The direction of the arrow indicates that plasma glucose levels are increasing from 0 to 30 min whereas they are typically decreasing from 60 to 300 min.
Hi-Xen has little effect on GIP release.
During Alb infusion, fasting plasma IR-GIP levels were ∼10 pM, peaked at ∼82 pM 30 min after Boost Plus ingestion, and then declined similarly in all three groups (Fig. 5, A–C). In general, infusion with either dose of Xen did not alter the temporal GIP response within or between any groups. However, as noted with ISR and consistent with delayed gastric emptying, the 120-min GIP iAUC was significantly lower during infusion with Hi-Xen compared with Alb alone in the subjects with NGT (Fig. 5D; 3,459 ± 359 vs. 5,535 ± 663 pM·min; P < 0.05). There was no significant effect of Hi-Xen on the 120-min GIP iAUC in subjects with IGT and T2DM (Fig. 5, E and F).
Fig. 5.
Plasma levels of IR-glucose-dependent insulinotropic polypeptide (GIP) during Xen infusions. A–C: IR-GIP levels at the indicated times were measured in subjects with NGT, IGT, and T2DM (A–C, respectively) during infusion of Alb (open blue circles), Lo-Xen (open red squares), or Hi-Xen (open green triangles). D–F: for statistical analyses of data in A–C, IR-GIP iAUCs from 0–120 min were determined for each subject during infusion of Alb, Lo-Xen, or Hi-Xen. Group averages ± SE are shown. The number of subjects (n) is shown as described in Fig. 1. P values by 1-way ANOVA are shown in E and F.
Hi-Xen inhibits GLP-1 release.
Fasting plasma levels of intact GLP-1 were ∼0.3 pM in all three groups (Fig. 6, A–C). During Alb infusions, intact GLP-1 levels rapidly, transiently, and similarly increased following meal ingestion in all three groups. The average times (in minutes) to peak intact GLP-1 levels were 18 ± 3.0 vs. 37 ± 9.1 vs. 33 ± 6.6 in subjects with NGT vs. IGT vs. T2DM, respectively, and the 0- to 120-min iAUCs were 139 ± 21 vs. 104 ± 15 vs. 92 ± 11 pM·min, respectively (Fig. 6, D–F). However, these differences did not reach statistical significance (P = 0.16 and P = 0.14, respectively, by one-way ANOVA). Compared with Alb alone, infusion of Hi-Xen reduced the postprandial GLP-1 response [Fig. 6, D–F in subjects with NGT (24 ± 21 vs. 139 ± 21 pM·min; P < 0.001), IGT (62 ± 18 vs. 104 ± 15 pM·min; P = 0.17 by one-way ANOVA), and T2DM (37 ± 13 vs. 92 ± 11 pM·min; P < 0.01)]. Total GLP-1 levels were also measured in the 30-min time points for all infusions (not shown). As with intact GLP-1, total GLP-1 levels were also reduced by Hi-Xen infusion. Thus Hi-Xen reduces GLP-1 release rather than affecting degradation. Compared with Hi-Xen, Lo-Xen had a smaller effect on intact GLP-1 levels.
Fig. 6.
Hi-Xen reduces plasma levels of intact glucagon-like peptide-1 (GLP-1). Intact GLP-1 levels and iAUCs were determined as described in Fig. 5. P value by 1-way ANOVA is shown in E.
Hi-Xen increases the glucagon response in humans with IGT and T2DM.
Fasting plasma glucagon levels increased progressively (p = 0.04) from NGT (70 ± 5 pg/ml) to IGT (78 ± 4 pg/ml) to T2DM (90 ± 7 pg/ml). During infusions with Alb, the glucagon response (defined as the change from fasting values) rapidly increased after meal ingestion, peaked at 30 min, and then declined to subfasting levels in all three groups (Fig. 7, A–C). However, the postprandial peaks increased progressively and declined increasingly slower from NGT to IGT to T2DM. As shown in Fig. 7, D–F, iAUCs from 0–120 min were also significantly increased with Hi-Xen vs. Alb in subjects with IGT (882 ± 145 vs. 150 ± 121 pg·ml−1·min; P < 0.001) and T2DM (2,743 ± 391 vs. 1,643 ± 343 pg·ml−1·min; P = 0.05), but not in those with NGT (−32 ± 225 vs. −358 ± 225 pg·ml−1·min). When plotted vs. plasma glucose levels or ISRs, plasma glucagon levels were inappropriately high during infusions with Hi-Xen only in the subjects with IGT and T2DM (not shown). Infusion with Lo-Xen modestly, but significantly, increased the glucagon response only in subjects with IGT.
Fig. 7.
Plasma levels of glucagon during Xen infusions. Changes in plasma glucagon levels and iAUCs were determined as described in Figs. 2 and 3, respectively.
Mild diarrhea is a side effect of Xen infusions.
On the basis of symptom surveys, Xen administration for 300 min was not associated with nausea, vomiting, heart palpitations, chest pain, dizziness, shortness of breath, blurred vision, changes in salivation, sweating, or frequency of urination. Heart rate and blood pressure as well as plasma levels of potassium, bicarbonate, AST/ALT, and amylase were also unaffected (not shown). Infusion with Alb was not associated with diarrhea in any subject. In contrast, mild, self-limited diarrhea occurred in 58% (21 of 36) and 77% (24 of 31) of subjects who received Lo-Xen and Hi-Xen, respectively, for 300 min (Fig. 8A; P < 0.0001 for Lo-Xen/Hi-Xen vs. Alb). The difference between Lo-Xen and Hi-Xen was not significant (P = 0.11). The number of diarrheal episodes per affected individual was not different with Lo-Xen and Hi-Xen infusions (2.0 ± 0.33 and 1.8 ± 0.22, respectively; Fig. 8B). Hi-Xen was also infused for 30 and 90 min in a subset of subjects with NGT. There was a trend for fewer participants to experience diarrhea when the Hi-Xen was administered for shorter times (P = 0.074; Fig. 8C). Although statistically significant effects with different times or doses of Xen were not detected, we do not have sufficient power to say that differences do not exist since only seven subjects were administered 30- and 90-min infusions of Hi-Xen. With respect to the diarrhea, all episodes occurred during the study visit in 83% of the subjects in response to infusion with Hi-Xen. In the remaining subjects, diarrhea occurred within 24 h of the visit. Diarrhea was noted in all three groups. Diarrhea was nonbloody and treated with loperamide 2 mg by mouth in 7 of 31 subjects who received Hi-Xen. Infusion with Hi-Xen was terminated early in 3 of the 31 patients at their request due to diarrhea.
Fig. 8.
Xen effects on diarrhea. The percentage of subjects who experienced diarrhea in response to a particular infusion is shown (A; ****P < 0.0001). The average number of diarrhea episodes ± SE per affected subject is shown (B). Hi-Xen (Hi) infusions for shorter duration were administered only to subjects with NGT. Effects of 30-, 90-, and 300-min Hi-Xen infusions are shown (C). P value by Fisher's exact test for affect of Xen (any time or dose) vs. albumin is <0.0001. Comparison of dose and time for Xen P = 0.12 (Fisher's exact test). Lo, Lo-Xen.
Receptors for Xen are expressed on neurons in the human stomach.
As shown in Fig. 9, intense punctate staining for NTSR1 was detected within the actin-positive longitudinal muscle in the human stomach. The NTSR1 staining colocalized with a subset of PGP9.5 nerve fibers (Fig. 10). The density of NTSR1-positive puncta was greatly reduced within the circular muscle (Fig. 9) as well as in regions contacting actin-positive smooth muscle cells lining the basal membranes of glandular epithelial cells (not shown).
Fig. 9.
A high density of Xen receptors is present in the longitudinal muscle (LM) in the human stomach. A single paraffin-embedded section of human stomach was stained for smooth muscle α-actin (green) plus NTSR1 (red). Nuclei were counterstained blue. Staining was visualized by confocal microscopy with a ×40 objective. A merged image was generated in Photoshop.
Fig. 10.
Receptors for Xen are expressed on nerve fibers in the longitudinal muscle in the human stomach. A single paraffin-embedded section of human stomach was stained for PGP9.5 (red) plus NTSR1 (green). Nuclei were counterstained blue. Staining was visualized by confocal microscopy with a ×120 objective. A merged image was generated in Photoshop.
DISCUSSION
As detailed in the introduction, Xen, alone and/or in combination with GIP, has been shown to improve glucose homeostasis in animals (2, 9, 10, 25, 27, 30, 54). With the exception of a single publication from our laboratory demonstrating that Xen amplifies the effects of GIP on insulin secretion in humans without T2DM (53), nothing is known concerning the effects of Xen on glucose homeostasis in humans. Thus the present study is extremely novel. The major findings of this paper are that infusion of Hi-Xen, but not Lo-Xen, for 90 or 300 min reduced ACM appearance in the blood and thus delayed gastric emptying in humans with NGT, IGT, and T2DM. Consequently, postprandial plasma glucose levels were lower during Hi-Xen infusions. ISRs, when normalized to plasma glucose levels, were unaffected by Hi-Xen, suggesting that delayed gastric emptying alone mediates the beneficial effects of Hi-Xen on postprandial glucose levels. The GIP response, if normalized to plasma ACM levels (i.e., gastric emptying), was normal (NGT) or even elevated (IGT and T2DM) in all three groups of subjects during infusion of Hi-Xen. In stark contrast, infusion with Hi-Xen reduced postprandial levels of plasma intact GLP-1 to a much greater degree compared with ACM, suggesting that endogenously released GLP-1 may play only a minor role in regulating postprandial insulin secretion in response to ingestion of a liquid mixed meal, especially in those with NGT.
Hyperglycemia itself can delay gastric emptying (37). However, Hi-Xen was a potent antagonist of gastric emptying in all three groups even though each had varying levels of postprandial glucose. Gastric emptying in humans involves a complex interplay between the central and enteric nervous systems (20). As shown in the present study, a high density of NTSR1 was detected only on enteric neurons residing within the longitudinal muscle in the human stomach (Figs. 9 and 10). Thus the effects of Xen on gastric emptying are most likely mediated by a neural relay. Delaying gastric emptying is a therapeutic target for treating T2DM, and agonists for GLP-1 (26, 28) and amylin (40, 50) receptors work in part via this mechanism. Thus Xen alone, or in combination with other drugs that delay gastric emptying, could have therapeutic benefit for reducing postprandial glucose levels in humans with T2DM. Acute dosing of Xen, as with pramlintide (36) and GLP-1 (47), could overcome potential problems due to tachyphylaxis as noted with chronic administration of GLP-1 (32).
Hi-Xen delayed gastric emptying in all three groups but plasma levels of GIP were reduced only in the group with NGT. The reason for this differential effect on GIP levels is unknown but indicates that GIP levels do not simply reflect the rate of gastric emptying. That Hi-Xen infusion inhibited GLP-1 release is perhaps not surprising since Xen appears to act by exciting neurons, and a host of neurotransmitters and neuropeptides including ACh, bombesin/gastrin-releasing peptide, and calcitonin-related peptide are known to increase GLP-1 release (3, 11, 12, 34).
An important finding from our study is that infusion of Hi-Xen reduced plasma levels of active GLP-1 nearly sixfold without decreasing the incretin response in subjects with NGT. Several studies from other laboratories are consistent with our data since they showed that infusion of exendin-9,39 (a GLP-1 receptor antagonist) had little effect on postprandial plasma glucose, insulin, and C-peptide levels as well as static ISRs in healthy control subjects (13, 39, 44). In contrast, other studies using exendin-9,39 suggest that endogenous GLP-1 does play an important role in regulating the incretin response (38, 43, 56). However, these study protocols were significantly different from ours. For example, some were conducted by administering glucose alone, either orally or by intraduodenal infusion. However, the collective cohort as well as amounts of individual intestinal peptides that are released in response to glucose alone is not the same as that released in response to a mixed meal (6, 51). Moreover, physical ingestion of nutrients would elicit a neural response that would not be recapitulated by intraduodenal administration of the same nutrients (1). Interestingly, oral glucose elicits a greater insulin secretory response compared with an isocaloric load delivered by duodenal perfusion (42). Other studies administered a mixed meal or oral glucose in conjunction with a hyperglycemic clamp, which is quite different than conditions present when a meal is ingested in the fasted state (e.g., plasma glucagon levels would be very different). Exendin-9,39 clearly reduces the incretin response in subjects following gastric bypass surgery (22, 39, 44). However, this surgical procedure alters the anatomy of the gut and results in profoundly increased levels of postprandial plasma GLP-1 and thus does not represent normal physiology. Regardless, even when noted, exendin-9,39 typically inhibits less than 50% of the endogenous incretin response. Thus our results concerning the role of endogenous GLP-1 for the incretin response should be considered complementary rather than contradictory to these other studies but further suggest that GIP may play a more significant role in the endogenous incretin response than previously appreciated. Several alternative explanations for our results with GLP-1 include the possibilities that 1) other incretins and/or additional mechanisms (e.g., neural input) could also be important for the incretin response in humans; 2) residual GLP-1 release from L cells is still sufficient to enhance glucose stimulated insulin secretion in humans with NGT; and 3) central rather than peripheral GLP-1 production and action is important for regulating insulin release.
Glucagon secretion is dysregulated as humans progress from NGT to T2DM (21, 48). In the present study, infusion with Hi-Xen increased glucagon levels in humans with IGT and T2DM. Despite this, Hi-Xen still reduced postprandial glucose levels by a similar percentage to that noted in subjects with NGT. Although glucagon reportedly delays gastric emptying, infusion of Hi-Xen did not increase the glucagon response in humans with NGT, suggesting that increased glucagon does not account for the Xen-mediated delay in gastric emptying.
Plasma glucose, intra-islet insulin, incretins, and neural signaling regulate glucagon release. Interestingly, glucagon responses to Hi-Xen in subjects with IGT and T2DM could not be accounted for simply by changes in plasma glucose levels or ISRs. Moreover, GLP-1 levels were profoundly decreased by infusion with Hi-Xen in subjects with NGT whereas glucagon levels did not increase. Collectively, these results suggest that changes in neural input to islets may be responsible for the Xen-mediated increase in the glucagon response in humans with IGT and T2DM.
A prior study reported that postprandial Xen levels were ∼120 pM (18). However, this study measured plasma Xen by a RIA using a single antibody directed against the COOH terminus of Xen. Consistent with our previous human (53) and mouse (54) studies, fasting and/or postprandial levels of endogenous Xen were undetectable (Fig. 1, A–C). Similarly, we have not detected endogenous Xen in plasma following ingestion of a solid meal (pizza) or oral glucose. The ELISA we developed utilizes different capture and detection antibodies, requires at least 16 COOH-terminal residues of Xen, and can detect less than 2 pM peptide (54). Thus it will be important to determine the basis for the differences in endogenous Xen levels when levels are measured by different assays.
Mild diarrhea was the only side effect associated with Xen administration. Interestingly, diarrhea persisted in some subjects long after the Xen infusions were terminated despite the fact that Xen has a circulating half life of only 2.5 min in humans (53). In conscious dogs, Xen increases gall bladder contractions, which indirectly increases intestinal motility since this latter response is lost following cholecystectomy (23), which could explain the long-acting effects of Xen on diarrhea.
It is important to address several limitations to our study. First, scintigraphy is considered the gold standard for measuring gastric emptying. However, plasma appearance of orally administered ACM has been used as an indirect estimate of the rate of gastric emptying (55) since pharmacokinetic data indicate that ACM is rapidly absorbed in the duodenum, but not stomach (8). Although this assumes that gastric emptying is the rate-limiting step in ACM appearance in plasma, AUCs for plasma levels of ACM are highly correlated with the rates of gastric emptying of a liquid, but not solid, meal as measured by scintigraphy (55). However, potential errors from the ACM method can arise from large variations in ACM metabolism between individuals (41). Our results also assume that Xen infusion itself has no effect on ACM absorption or clearance. ACM clearance could also be reduced in subjects with impaired renal or liver function. Owing to selection of subjects with normal renal and liver function and the crossover design of our study, these potential artifacts are unlikely to explain how Xen reduced ACM appearance in the blood. That Hi-Xen infusion concomitantly reduced postprandial glucose levels and ACM iAUCs is also consistent with the conclusion that Hi-Xen delays gastric emptying. Secondly, it is important to note that solid, liquid, and oil phases of a meal are emptied from the stomach at different rates (29). Moreover, the specific components of each phase may also affect gastric emptying. Thus the overall effect of Hi-Xen on gastric emptying of a “normal” mixed solid-liquid meal may be different from that noted in the present study. Finally, insulin was administered to several subjects with T2DM to lower basal glucose levels before the study meal was administered, which potentially could have lowered basal glucagon levels in these subjects. However, blood glucose was stable for at least 20 min before start of the meal tolerance test. Moreover, our results suggest that neither glucagon nor insulin mediate the effects of Xen on gastric emptying, and thus insulin administration is unlikely to have affected our results.
Overall, results obtained by infusing Xen are yielding important insights concerning the differential regulation of glucose homeostasis in humans with NGT, IGT, and T2DM. Studies are currently underway to determine the role of cholinergic, noncholinergic, vagal, and nonvagal signaling for regulating Xen action in humans.
GRANTS
Portions of this research were supported by funds from the National Institute of Diabetes and Digestive and Kidney Diseases (Grant nos. 5RC1DK086163 and 1R01DK088126); the American Diabetes Association (Grants no. 1-10-CT-58 and 1-13-CE-46); the Washington University Diabetes Research and Training Center Immunoassay Core (P60 DK020579); the Washington University Nutrition Obesity Research Center Grant (P30DK056341) from the National Institute of Diabetes and Digestive and Kidney Diseases; the Washington University Digestive Disease Research Core Center (P30 DK52574-16); the Washington University Clinical and Translational Science Award (UL1 RR024992); the Biologic Therapy Core Facility of the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO (National Cancer Institute Cancer Center Support Grant P30 CA91842); the NIH National Center for Research Resources (P41 RR00954 and UL1 RR024992); and the Blum Kovler Foundation.
DISCLOSURES
Washington University is pursuing a patent related to the use of xenin-25 to treat T2DM. In the future, this could lead to personal financial benefit to B. M. Wice, K. S. Polonsky, and the University.
AUTHOR CONTRIBUTIONS
S.C., D.L.C., B.W.P., S.W., H.D.T., M.J.W., and B.M.W. analyzed data; S.C., D.N.R., D.L.C., B.W.P., D.A.R., M.J.W., and B.M.W. interpreted results of experiments; S.C., S.W., and B.M.W. prepared figures; S.C. and B.M.W. drafted manuscript; S.C., B.W.P., M.J.W., and B.M.W. edited and revised manuscript; S.C., D.N.R., D.L.C., B.W.P., E.L., S.W., H.D.T., T.A.G., D.A.R., J.D., M.J.W., J.H.L., K.S.P., and B.M.W. approved final version of manuscript; D.N.R., D.L.C., E.L., S.W., H.D.T., T.A.G., D.A.R., and J.D. performed experiments; D.L.C., B.W.P., J.H.L., K.S.P., and B.M.W. conception and design of research.
ACKNOWLEDGMENTS
The authors thank 1) the nurses of the Clinical Research Unit at Washington University for administering the screening and study protocols and 2) Dr. Elizabeth Brunt of Washington University's Department of Anatomic & Molecular Pathology for providing paraffin-embedded tissue blocks of human stomach.
REFERENCES
- 1.Ahren B, Holst JJ. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes 50: 1030–1038, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Alexiou C, Zimmermann JP, Schick RR, Schusdziarra V. Xenin—a novel suppressor of food intake in rats. Brain Res 800: 294–299, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Anini Y, Hansotia T, Brubaker PL. Muscarinic receptors control postprandial release of glucagon-like peptide-1: in vivo and in vitro studies in rats. Endocrinology 143: 2420–2426, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Anlauf M, Weihe E, Hartschuh W, Hamscher G, Feurle GE. Localization of xenin-immunoreactive cells in the duodenal mucosa of humans and various mammals. J Histochem Cytochem 48: 1617–1626, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 2131–2157, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Brand SJ, Schmidt WE. Gastrointestinal hormones. In: Textbook of Gastroenterology, edited by Yamada T. Philadelphia, PA: Lippincott, 1995, p. 25–71 [Google Scholar]
- 7.Brooks DC, Black PR, Arcangeli MA, Aoki TT, Wilmore DW. The heated dorsal hand vein: an alternative arterial sampling site. JPEN J Parenter Enteral Nutr 13: 102–105, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Clements JA, Heading RC, Nimmo WS, Prescott LF. Kinetics of acetaminophen absorption and gastric emptying in man. Clin Pharmacol Ther 24: 420–431, 1978 [DOI] [PubMed] [Google Scholar]
- 9.Cline MA, Nandar W, Rogers JO. Xenin reduces feed intake by activating the ventromedial hypothalamus and influences gastrointestinal transit rate in chicks. Behav Brain Res 179: 28–32, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Cooke JH, Patterson M, Patel SR, Smith KL, Ghatei MA, Bloom SR, Murphy KG. Peripheral and central administration of xenin and neurotensin suppress food intake in rodents. Obesity (Silver Spring) 17: 1135–1143, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Deacon CF. What do we know about the secretion and degradation of incretin hormones? Regul Pept 128: 117–124, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Dumoulin V, Dakka T, Plaisancie P, Chayvialle JA, Cuber JC. Regulation of glucagon-like peptide-1-(7-36) amide, peptide YY, and neurotensin secretion by neurotransmitters and gut hormones in the isolated vascularly perfused rat ileum. Endocrinology 136: 5182–5188, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Edwards CM, Todd JF, Mahmoudi M, Wang Z, Wang RM, Ghatei MA, Bloom SR. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9–39. Diabetes 48: 86–93, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Fehmann H, Goke R, Goke B. Cell and molecular biology of the incretin hormones glucagon-like peptide-1 and glucose-dependent insulin releasing polypeptide. Endocr Rev 16: 390–410, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Feurle GE. Xenin—a review. Peptides 19: 609–615, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Feurle GE, Hamscher G, Kusiek R, Meyer HE, Metzger JW. Identification of xenin, a xenopsin-related peptide, in the human gastric mucosa and its effect on exocrine pancreatic secretion. J Biol Chem 267: 22305–22309, 1992 [PubMed] [Google Scholar]
- 17.Feurle GE, Heger M, Niebergall-Roth E, Teyssen S, Fried M, Eberle C, Singer MV, Hamscher G. Gastroenteropancreatic effects of xenin in the dog. J Pept Res 49: 324–330, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Feurle GE, Ikonomu S, Partoulas G, Stoschus B, Hamscher G. Xenin plasma concentrations during modified sham feeding and during meals of different composition demonstrated by radioimmunoassay and chromatography. Regul Pept 111: 153–159, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Feurle GE, Metzger JW, Grudinki A, Hamscher G. Interaction of xenin with the neurotensin receptor of guinea pig enteral smooth muscles. Peptides 23: 1519–1525, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9: 286–294, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28: 84–116, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Jorgensen NB, Dirksen C, Bojsen-Moller KN, Jacobsen SH, Worm D, Hansen DL, Kristiansen VB, Naver L, Madsbad S, Holst JJ. Exaggerated glucagon-like peptide 1 response is important for improved beta-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes 62: 3044–3052, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamiyama Y, Aihara R, Nakabayashi T, Mochiki E, Asao T, Kuwano H. The peptide hormone xenin induces gallbladder contractions in conscious dogs. Neurogastroenterol Motil 19: 233–240, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Kim ER, Mizuno TM. Role of neurotensin receptor 1 in the regulation of food intake by neuromedins and neuromedin-related peptides. Neurosci Lett 468: 64–67, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Kim ER, Mizuno TM. Xenin delays gastric emptying rate and activates the brainstem in mice. Neurosci Lett 481: 59–63, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 60: 470–512, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leckstrom A, Kim ER, Wong D, Mizuno TM. Xenin, a gastrointestinal peptide, regulates feeding independent of the melanocortin signaling pathway. Diabetes 58: 87–94, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5: 262–269, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Maes BD, Hiele MI, Geypens BJ, Ghoos YF, Rutgeerts PJ. Gastric emptying of the liquid, solid and oil phase of a meal in normal volunteers and patients with Billroth II gastrojejunostomy. Eur J Clin Invest 28: 197–204, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Martin CM, Gault VA, McClean S, Flatt PR, Irwin N. Degradation, insulin secretion, glucose-lowering and GIP additive actions of a palmitate-derivatised analogue of xenin-25. Biochem Pharmacol 84: 312–319, 2012 [DOI] [PubMed] [Google Scholar]
- 31.McGuire EA, Helderman JH, Tobin JD, Andres R, Berman M. Effects of arterial vs. venous sampling on analysis of glucose kinetics in man. J Appl Physiol 41: 565–573, 1976 [DOI] [PubMed] [Google Scholar]
- 32.Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 60: 1561–1565, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nauck MA, Meier JJ. Incretins and regulation of insulin secretion. In: Pancreatic Beta Cell in Health and Disease, edited by Seino S, Bell GI. Tokyo: Springer, 2008, p. 335–378 [Google Scholar]
- 34.Persson K, Gingerich RL, Nayak S, Wada K, Wada E, Ahren B. Reduced GLP-1 and insulin responses and glucose intolerance after gastric glucose in GRP receptor-deleted mice. Am J Physiol Endocrinol Metab 279: E956–E962, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Pettibone DJ, Hess JF, Hey PJ, Jacobson MA, Leviten M, Lis EV, Mallorga PJ, Pascarella DM, Snyder MA, Williams JB, Zeng Z. The effects of deleting the mouse neurotensin receptor NTR1 on central and peripheral responses to neurotensin. J Pharmacol Exp Ther 300: 305–313, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Pullman J, Darsow T, Frias JP. Pramlintide in the management of insulin-using patients with type 2 and type 1 diabetes. Vasc Health Risk Manag 2: 203–212, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care 24: 371–381, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Salehi M, Aulinger B, Prigeon RL, D'Alessio DA. Effect of endogenous GLP-1 on insulin secretion in type 2 diabetes. Diabetes 59: 1330–1337, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salehi M, Prigeon RL, D'Alessio DA. Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 60: 2308–2314, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samsom M, Szarka LA, Camilleri M, Vella A, Zinsmeister AR, Rizza RA. Pramlintide, an amylin analog, selectively delays gastric emptying: potential role of vagal inhibition. Am J Physiol Gastrointest Liver Physiol 278: G946–G951, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Sanaka M, Kuyama Y, Yamanaka M. Guide for judicious use of the paracetamol absorption technique in a study of gastric emptying rate of liquids. J Gastroenterol 33: 785–791, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Schirra J, Katschinski M, Weidmann C, Schafer T, Wank U, Arnold R, Goke B. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest 97: 92–103, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schirra J, Nicolaus M, Roggel R, Katschinski M, Storr M, Woerle HJ, Goke B. Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut 55: 243–251, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah M, Law JH, Micheletto F, Sathananthan M, Man CD, Cobelli C, Rizza RA, Camilleri M, Zinsmeister AR, Vella A. The contribution of endogenous glucagon-like peptide-1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sparacino G, Cobelli C. A stochastic deconvolution method to reconstruct insulin secretion rate after a glucose stimulus. IEEE Trans Biomed Eng 43: 512–529, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Sparacino G, Pillonetto G, Capello M, De Nicolao G, Cobelli C. WINSTODEC: a stochastic deconvolution interactive program for physiological and pharmacokinetic systems. Comput Methods Programs Biomed 67: 67–77, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Umapathysivam MM, Lee MY, Jones KL, Annink CE, Cousins CE, Trahair LG, Rayner CK, Chapman MJ, Nauck MA, Horowitz M, Deane AM. Comparative effects of prolonged and intermittent stimulation of the glucagon-like peptide-1 receptor on gastric emptying and glycaemia. Diabetes 2013. October 2 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest 122: 4–12, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 41: 368–377, 1992 [DOI] [PubMed] [Google Scholar]
- 50.Vella A, Lee JS, Camilleri M, Szarka LA, Burton DD, Zinsmeister AR, Rizza RA, Klein PD. Effects of pramlintide, an amylin analogue, on gastric emptying in type 1 and 2 diabetes mellitus. Neurogastroenterol Motil 14: 123–131, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Walsh JH. Gastrointestinal hormones. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. New York: Raven, 1994, p. 1–128 [Google Scholar]
- 52.Wang SY, Liu J, Li L, Wice BM. Individual sub-types of enteroendocrine cells in the mouse small intestine exhibit unique patterns of inositol 1,4,5-trisphosphate receptor expression. J Histochem Cytochem 52: 53–63, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Wice BM, Reeds DR, Tran H, Crimmins DL, Patterson BW, Dunai J, Wallendorf MJ, Ladenson JH, Villareal DT, Polonsky KS. Xenin-25 amplifies GIP-mediated insulin secretion in humans with normal and impaired glucose tolerance, but not type 2 diabetes. Diabetes 61: 1793–1800, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wice BM, Wang S, Crimmins DL, Diggs-Andrews KA, Althage MC, Ford EL, Tran H, Ohlendorf M, Griest TA, Wang Q, Fisher SJ, Ladenson JH, Polonsky KS. Xenin-25 potentiates glucose-dependent insulinotropic polypeptide action via a novel cholinergic relay mechanism. J Biol Chem 285: 19842–19853, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willems M, Quartero AO, Numans ME. How useful is paracetamol absorption as a marker of gastric emptying? A systematic literature study. Dig Dis Sci 46: 2256–2262, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Woerle HJ, Carneiro L, Derani A, Goke B, Schirra J. The role of endogenous incretin secretion as amplifier of glucose-stimulated insulin secretion in healthy subjects and patients with type 2 diabetes. Diabetes 61: 2349–2358, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang S, Hyrc K, Wang S, Wice BM. Xenin-25 increases cytosolic free calcium levels and acetylcholine release from a subset of myenteric neurons. Am J Physiol Gastrointest Liver Physiol 303: G1347–G1355, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]