Abstract
Low-grade systemic inflammation is a common manifestation of hypertension; however, the exact mechanisms that initiate this pathophysiological response, thereby contributing to further increases in blood pressure, are not well understood. Aberrant vascular inflammation and reactivity via activation of the innate immune system may be the first step in the pathogenesis of hypertension. One of the functions of the innate immune system is to recognize and respond to danger. Danger signals can arise from not only pathogenic stimuli but also endogenous molecules released following cell injury and/or death [damage-associated molecular patterns (DAMPs)]. In the short-term, activation of the innate immune system is beneficial in the vasculature by providing cytoprotective mechanisms and facilitating tissue repair following injury or infection. However, sustained or excessive immune system activation, such as in autoimmune diseases, may be deleterious and can lead to maladaptive, irreversible changes to vascular structure and function. An initial source of DAMPs that enter the circulation to activate the innate immune system could arise from modest elevations in peripheral vascular resistance. These stimuli could subsequently lead to ischemic- or pressure-induced events aggravating further cell injury and/or death, providing more DAMPs for innate immune system activation. This review will address and critically evaluate the current literature on the role of the innate immune system in hypertension pathogenesis. The role of Toll-like receptor activation on somatic cells of the vasculature in response to the release of DAMPs and the consequences of this activation on inflammation, vasoreactivity, and vascular remodeling will be specifically discussed.
Keywords: innate immunity, vascular dysfunction, vascular remodeling
this article is part of a collection on Pathophysiology of Hypertension. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Introduction
Hypertension is a chronic condition characterized by elevated systemic blood pressure and is the most common and important risk factor preceding the development of other cardiovascular diseases (102, 121). Normal systemic blood pressure is defined as systolic blood pressure <120 mmHg and diastolic blood pressure <80 mmHg. Prehypertension and hypertension are systemic pressures equal to and above these values (25). Hypertension can be classified as either primary/essential, in which the disease is idiopathic, or secondary, in which the cause of the disease is known or “secondary” to another identifiable cause (e.g., renal disease), respectively. Despite the elusive nature of essential hypertension, it is known that its etiology is heterogeneous and multifactorial.
Although there is substantial debate regarding the relative contribution of kidneys, autonomic nervous system, and vasculature to the development and maintenance of hypertension, it has been established that the immune system and its aberrant activation also play a role. Low-grade systemic inflammation in these organs leads to the commencement or acceleration of pathological processes and worsening of cardiovascular risks (1, 46, 136). However, the initiation of this inflammatory response, thereby contributing to further increases in blood pressure, has not been elucidated (34).
Inflammation is one of the first responses of the immune system to danger. Clinically, inflammation can be described as dolor (pain), calor (heat), rubor (redness), tumor (swelling), or function laesa (loss of function) (122). More specifically, inflammation is the increased production of pro-inflammatory cytokines whose expression pattern guides the adaptive immune response (e.g., IL-6, TNF-α, and IFN-γ). Additionally, inflammation can be characterized as increased chemokines (chemotactic cytokines) that guide the migration of immune cells to target tissues [e.g., monocyte chemoattractant protein-1 (MCP-1)] and cell adhesion molecules that promote the binding, rolling, and infiltration of immune cells into the vascular wall and translocation to end organs (e.g., ICAM-1 and VCAM-1) (86).
The danger that stimulates inflammation can occur from not only pathogens [pathogen-associated molecular patterns (PAMPs)] but also host-derived endogenous molecules that arise due to cell death and/or injury [damage-associated molecule patterns (DAMPs)] (95, 96). This initial inflammatory response by the innate immune system subsequently signals for the adaptive immune system to elicit a more robust defense. The participation of the adaptive immune system and different lymphocyte populations in the development of hypertension has been supported for various animal models of hypertension, such as spontaneously hypertensive rats (SHRs), DOCA-salt induced hypertension, and ANG II-induced hypertension (8, 47, 64, 89, 93). However, the role of the innate immune response to DAMPs, and its subsequent effects on the activation of the adaptive immune system in the context of the pathogenesis of hypertension, is currently unknown.
Many DAMPs are present in hypertension due to chronic cell injury and death, contributing to persistent inflammation (16). Recognition of these host-derived molecules by innate immune receptors such as Toll-like receptors (TLRs), and subsequent TLR activation, may be an important link between hypertension and activation of the adaptive immune system. Accordingly, the focus of the following review will be on the emerging role of TLRs in the etiology of vascular dysfunction, vascular remodeling, and hypertension. The current literature on TLRs in cardiovascular disease will be summarized and gaps in our current knowledge regarding the potential role of TLRs in hypertension will be identified.
Vascular Dysfunction and Vascular Remodeling: Physiological Adaptations With Pathophysiological Consequences
Vascular remodeling of both conduit and resistance vessels is a hallmark of hypertension that generally precedes disease development and exacerbates the disease phenotype (37, 109, 129). Although initial remodeling of the vascular wall occurs as an adaptation to normalize exacerbated shear stress, chronic shear stress results in maladaptive changes in vessel structure (43). Pressure-dependent remodeling of large artery composition is characterized by vessel stiffening (decreased compliance and elasticity) and outward hypertrophic remodeling (105, 125, 126), whereas small artery pressure-dependent remodeling is defined as either inward eutrophic remodeling or inward hypertrophic remodeling, depending on whether the media cross-sectional area is enlarged (51, 110, 130).
Compliant conduit vessels, such as the aorta, provide important buffering actions for each ventricular contraction that ameliorates pulse pressure. Augmented pulsatile hemodynamics that occur with stiffening contribute to increased cardiac afterload, decreased diastolic blood flow to tissues, and further detrimental vascular remodeling of resistance vessels and organ damage (20, 104). Remodeling of resistance vessels can lead to decreased tissue perfusion and organ damage (129).
Vascular dysfunction is another deleterious characteristic of hypertension and prehypertension (31, 136). Vascular dysfunction has multiple characteristics including increased contractility and/or decreased relaxation (via modified production and responsiveness to vasoconstrictors and vasodilators), increased expression of pro-inflammatory and/or decreased anti-inflammatory cytokines, cell death (apoptosis, necrosis, aberrant autophagy), adhesion of immune cells to the endothelium (and subsequent infiltration into the vascular wall), increased production of reactive oxygen species (ROS), and vascular smooth muscle cell (VSMC) hypertrophy, proliferation, and migration (136). Many of these manifestations of vascular dysfunction occur in parallel, and elucidation of the initial insult on the vasculature is usually difficult and multifactorial in nature.
Vascular remodeling is the summation of dysfunctional vasomotor events that ultimately reorganize the structural composition of the vessel. In other words, vascular remodeling is thought to be initiated in vessels with chronic dysfunction, either as exacerbated vasoconstriction and/or attenuated vasorelaxation (75). There is evidence that such abnormal vasoreactivity works in conjunction with renal dysfunction and sympathetic overactivity to drive the vascular remodeling process (31). A role for augmented vasoconstrictors and/or attenuated vasodilators in the genesis and maintenance of vascular remodeling is supported by the fact that many of these vasoactive agents [e.g., ANG II, endothelin-1 (ET-1), vasopressin, prostaglandins, and nitric oxide (NO)] are pleiotropic in their effects. As a result, these vasoactive factors can also contribute to vascular remodeling through other mechanisms including VSMC proliferation, inflammation, apoptosis, and fibrosis (55).
Vascular remodeling and the progression of hypertension subsequently becomes the result of a positive feedback loop of augmented shear stress-induced changes via exacerbated vasoconstriction and attenuated vasorelaxation. Under these conditions, the maintenance of tissue perfusion through chronically constricted vessels results in increased force to the vessel wall. This force subsequently leads to remodeling of the vessels as a mechanism of normalizing this high shear stress. Eventually, a new steady state of tissue blood flow is sustained at elevated pressures by structural remodeling, rather than vasoreactivity mechanisms. Temporally, altered vascular structure in response to vascular dysfunction has been shown to occur rapidly in resistance arteries. However, these hemodynamic changes only reach pathophysiological significance ∼2 to 3 days after initiation of increased pressure, and need at least a week to be fully completed (87, 92).
A novel and potentially significant focus of research recently has been the observation that TLRs are able to modulate vascular function (15, 19, 82), and genomic analysis has revealed that different vascular beds exhibit distinctive TLR profiles (120). Specifically, TLR2 and TLR4 were ubiquitously present within the vasculature, whereas TLR7 and TLR9 were sparse, and TLR1, TLR3, TLR5, TLR6, and TLR8 were expressed in selective patterns. However, to the best of our knowledge, there are only two investigations illustrating a role of TLRs mediating vascular dysfunction and low-grade inflammation, subsequently contributing to hypertension (15, 82), as well as one investigation on a potential involvement of TLRs (via MyD88 adaptor protein) in vascular remodeling (142). Therefore, TLRs could be significant contributors to the etiology of vascular dysfunction, and consequently, vascular remodeling and hypertension.
The Innate Immune System: The First Line of Defense Against Danger
The innate immune system is the early warning system of the body that rapidly detects danger and damage. This consequently allows time for the adaptive immune system to mount an antigen-specific response. The pattern of inflammatory cytokine response after activation of the innate immune receptors diverts the adaptive immune system toward either the cell-mediated T helper 1 (TH1) response or the humoral/antibody T helper 2 (TH2) response.
Components of the innate immune system include antimicrobial chemicals on epithelial surfaces (skin and mucosa), phagocytes (macrophages and neutrophils), natural killer cells, and polymorphonuclear leukocytes, which include neutrophils, eosinophils, basophils, and mast cells. Additionally, the complement system is known to be an essential component of the innate immune system for the destruction of pathogens and clearing of cellular debris. Finally, TLRs and NOD-like receptors (nucleotide-binding oligomerization domain receptors) are sentinel pattern recognition receptors of the innate immune system that help initiate the inflammatory response. Although short-term inflammation is necessary for tissue defense, chronic and/or excessive activation of the innate immune system results in deleterious maladaptations (e.g., autoimmune diseases), and the salutary effects of this evolutionarily conserved system are negated (Fig. 1).
Fig. 1.
Paradoxical effects of Toll-like receptor (TLR) activation on vascular function. Short-term perturbations in the vasculature from either damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs) activate TLRs present on innate immune system cells such as phagocytes, polymorphonuclear leukocytes, and natural killer cells, as well as somatic cells of the vasculature [vascular smooth muscle cells (VSMCs) and endothelial cells (ECs)]. This short-term interaction initiates an inflammatory response to restore homeostasis. However, chronic and/or excess activation of these receptors due to the continuous presence of DAMPs from cell death and remodeling negates the beneficial effects of these receptors and contributes to a pro-inflammatory state and blood pressure elevation.
Currently, there is limited knowledge of the role of the innate immune system in the pathogenesis of hypertension and how the inflammation associated with this condition is initiated. As a result, various components of the innate immune system, including TLRs, are becoming a significant research focus in the field of hypertension (15, 49).
The Adaptive Immune System In Hypertension
Upon initiation of the innate immune response by pattern recognition receptors, antigen-presenting cells migrate to lymph nodes and present antigenic molecules to cells of the adaptive immune system. The adaptive immune response is then initiated by the recognition of antigens by T and B lymphocytes. Proliferation and differentiation of naive lymphocytes into effector cells is necessary for elimination of the antigen and production of memory cells, which protect the host upon subsequent encounters with the antigen (3).
T and B lymphocytes are the two major cell types of the adaptive immune system. The participation of T lymphocytes in the pathogenesis of experimental hypertension was demonstrated by Guzik et al. (47). This investigation found that mice lacking T lymphocytes are resistant to the development of both ANG II and DOCA-salt-induced hypertension. Adoptive transfer of T lymphocytes, but not B lymphocytes, restored hypertension in these animals (47). In corroboration of these findings, mice with severe combined immunodeficiency and lacking both T and B lymphocytes have a blunted blood pressure response and reduced sodium retention during ANG II-induced hypertension (29). However, it has also been demonstrated that T lymphocyte activation occurs due to a blood pressure elevation, since anti-hypertensive drug hydralazine was able to prevent activation of T lymphocytes in an ANG II model of hypertension (93). Therefore, if aberrant inflammation is involved in the initiation of hypertension, in contrast with its progression, elucidation of this mechanism has not been demonstrated.
Given that T lymphocytes play a critical role in hypertension, the subset of T lymphocytes that contributes to this disease has been a growing area of investigation. T-helper cells have been cited as a possibility since these cells differentiate into distinct subsets generally classified as either the cell-mediated “TH1” response or the humoral/antibody “TH2” response. These T-helper subsets perform different functions and elicit unique patterns of cytokine secretion (107). As such, imbalances between these subsets are implicated in a variety of autoimmune (74) and cardiovascular pathologies (98, 132). T-helper 17 cells are a subset of T-helper cells that produce the pro-inflammatory cytokine IL-17. The profound effects of IL-17 on the etiology of hypertension were demonstrated by Madhur et al. (89). It was revealed that ANG II increased IL-17 production from T lymphocytes and that ANG II-induced elevations in blood pressure were not sustained in IL-17−/− mice. Moreover, IL-17−/− mice showed improved vascular reactivity, decreased superoxide anion production, and less T-lymphocyte infiltration into the vascular wall (89).
Another type of T lymphocyte revealed to play a role in hypertension is T-regulatory cells (Treg). Tregs suppress innate and adaptive immune responses, as opposed to exacerbating them. In fact, adoptive transfer of Tregs prevented ANG II-induced blood pressure elevation, vascular stiffness, vascular inflammation and oxidative stress, endothelial dysfunction, and immune cell infiltration (11). The vasculoprotective effects of Tregs have also been observed in aldosterone-induced vascular dysfunction and hypertension (64).
Although the importance of the adaptive immune system in hypertension is not in question, how exactly the adaptive immune response is first activated is not well understood. TLRs are known to be expressed on immune cells such as T and B lymphocytes (54, 62) and antigen-presenting cells. Furthermore, TLRs are expressed by somatic cells of the vasculature [e.g., VSMCs and endothelial cells (ECs)]. Therefore, TLRs may be molecular links between DAMPs, chronic vascular inflammation, and hypertension (Fig. 2).
Fig. 2.
The collective contribution of the innate and adaptive immune systems to vascular dysfunction, vascular remodeling, and hypertension. After an initial reflexive spike due to the presence of DAMPs in the circulation (e.g., those that may arise due to prehypertensive stimuli such as ANG II, high salt, or chronic stress), the innate immune system resets to new level of homeostasis due to the ever increasing levels of DAMPs in the circulation from continued pressure- and ischemic-induced cell death and remodeling. The adaptive immune system responds accordingly, reacting to the inflammatory stimuli initiated by the innate immune system. Because TLRs and other innate and adaptive immune system effectors remain in a state of sustained activation henceforth, chronic inflammation ensues, and blood pressure rises in concert with other dysregulated systems (e.g., increased sympathetic drive and decreased renal function).
TLRs and DAMPs: A New Source of Danger
Researchers studying the development of the fruit fly Drosophila melanogaster first discovered the Toll receptor when they found that a mutation in the Toll gene resulted in abnormal development (6). The mutated flies were termed Toll (German for “wow”) after embryos carrying the mutation were remarkably dissimilar to wild-type flies. A more closely related human homologue to Drosophila Toll was subsequently identified (101), and the human Toll was then renamed TLR4 because it was “Toll-like.”
At least 13 TLRs have been reported in mammals (1–10 in humans and 11–13 in mice) (91). TLRs that primarily recognize bacterial and fungal components are localized on the cell surface (TLR1, TLR2, TLR4, TLR5, and TLR6), whereas TLRs that primarily recognize viral or microbial nucleic acids are localized to intracellular membranes such as endosomes or phagosomes (TLR3, TLR7, TLR8, and TLR9) (52). Recently, human TLR10 has been discovered (26); however, its function and specific ligand have yet to be determined. Moreover, the TLR11 gene is known to be encoded in humans; however, it contains at least one stop codon, and the protein is not expressed (156).
TLRs are responsible for recognizing and initiating an inflammatory response to dangerous molecules (95, 96). As such, potential PAMPs and DAMPs are varied and numerous and can include pathogen-derived cell wall components (e.g., LPS), DNA, and metabolic byproducts. Endogenous (host-derived) molecules that arise from injured and dying cells and activate TLRs include extracellular matrix components (e.g., hyaluronan), plasma membrane, nuclear, and cytosolic proteins (e.g., high-mobility group box protein 1), and elements of damaged/fragmented organelles [e.g., mitochondrial DNA (mtDNA)]. Table 1 provides several examples of DAMPs specific to hypertension and their corresponding TLRs [updated from reference (16)]. This list is by no means exhaustive, since numerous other yet unknown molecules may fulfill our inclusion criteria of being TLR ligands that are elevated in hypertension.
Table 1.
Damage associated molecular patterns that are able to activate toll-like receptors on cell types pertinent to vascular function, potentially contributing to hypertension [updated from Ref. 16]
| Damage Associated Molecular Patterns | Toll-like Receptors | Cell Type (Reference) |
|---|---|---|
| Asymmetric dimethylarginine | 4 | Adipocytes (154) |
| ANG II | 4 | VSMCs (58, 59) |
| Biglycan | 2 | Aortic valve interstitial cells (134) |
| CpG DNA/mitochondrial DNA | 9 | Plasma (149), VSMCs (44) |
| C-reactive protein | 4 | VSMCs (83, 84) |
| Fibrinogen | 4 | Cardiomyocytes (81), monocytes (73) |
| HDL (modified) | 2 | ECs (135) |
| High mobility group box-1 | 2, 4 | ECs (70), macrophages (117) |
| Heat shock protein 60 | 2, 4 | Cardiomyocytes (69), VSMCs (33) |
| Heat shock protein 70 | 2, 4 | Cardiomyocytes (94), monocytes (7) |
| Hyaluronan | 2, 4 | ECs (143), macrophages (128), (61) |
| IL-1α | 4 | VSMCs (131) |
| Oxidized LDL | 4 | ECs (138), macrophages (103) |
| Uric acid | 2, 4 | Macrophages (85) |
EC, endothelial cells; VSMC, vascular smooth muscle cells.
Another possibility for aberrant TLR activation, inflammation, and the development of cardiovascular pathologies (5) could be genetic abnormalities of innate immune system components (39, 112) and endogenous molecules (thus converting them to DAMPs). These anomalies may include an irregular expression of various TLR, polymorphisms and mutations, the aberrant gene expression of various cytokines, and distribution of immune cell populations. Also, TLR-induced epigenetic and chromatin modifications may be important (38). As such, these genetic components of TLRs and DAMPs may also contribute to the pro-inflammatory state seen in hypertension.
TLR Signaling: Interactions With Known Vascular Signaling
In addition to being expressed in immune cells, TLRs are expressed in other tissues, such as those of the cardiovascular system (40, 91). Expression of TLRs in the cardiovascular system, as well as immune cells, is consistent with the theory that tissues tailor their own immune responses (97). TLRs are type I transmembrane proteins that possess an amino-terminal leucine-rich repeat domain for ligand binding, a single transmembrane domain, and a carboxyl-terminal intracellular signaling domain that is similar to IL-1. This common intracellular signaling motif is termed the “Toll-IL-1 receptor” (TIR) homology domain.
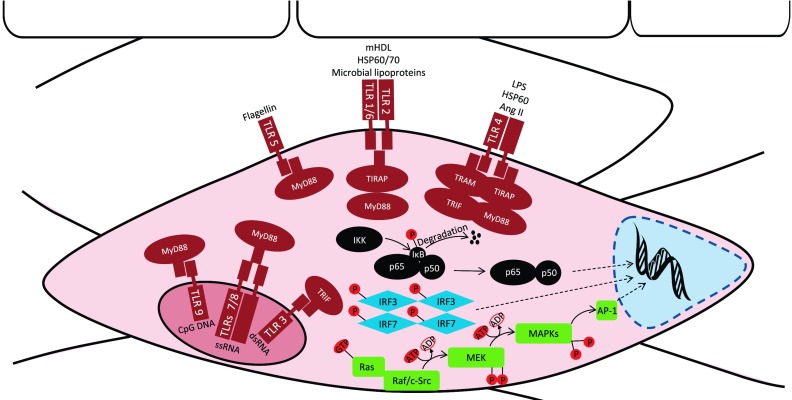
A number of different signaling pathways are activated by TLRs, and some of these are unique to particular TLRs. This differential signaling is dependent on which adaptor molecules are present to associate with the respective TLR. These adaptors, all of which contain TIR domains, include myeloid differentiation primary response protein (MyD88), TIR domain-containing adaptor protein (TIRAP), TIR-domain-containing adaptor inducing interferon-β (TRIF), and TRIF-related adaptor molecule (TRAM) (Fig. 3). TLR signaling has been extensively reviewed previously [(40) and (91)] and will not be extensively discussed here. These signaling events result in the upregulation of pro-inflammatory mediators (cytokines, chemokines, and adhesion molecules) either through a MyD88-dependent pathway (TLR1, TLR2, TLR4, TLR5, TLR6, TLR7, TLR8, and TLR9) or a MyD88-independent(/TRIF-dependent) pathway (TLR3 and TLR4). The MyD88-dependent pathway involves induction of the NF-κB gene, stimulation of the transcription factor activator protein 1 (AP-1) by MAPKs, or activation of interferon regulatory factor (IRF)7. The MyD88-independent pathway involves IRF3 activation, as well as NF-κB signaling (4, 65). The unique signaling cascades for each TLR allow for the induction of specific responses. This specificity may be attributed to the type of cell where the TLR is expressed (32) and/or the defense needed for that particular tissue (97). The ability of TLRs to discriminate particular stimuli expanded the defensive repertoire of the innate immune system.
Fig. 3.
TLR ligands/DAMPs, cellular location, and signaling in the vasculature. Evolutionarily conserved similarities between TLRs on immune cells have been extended to somatic cells of the vasculature (i.e., VSMCs and ECs). TLR1, TLR2, TLR4, TLR5, and TLR6 are expressed on the plasma membrane, and TLR3, TLR7, TLR8, and TLR9 are expressed on endosomal vacuoles. Activation of these receptors by DAMPs and PAMPs leads to complex cellular signaling cascades mediated by myeloid differentiation primary response protein (MyD88), Toll-IL-1 receptor (TIR)-domain-containing adaptor inducing interferon β (TRIF), TIR domain-containing adaptor protein (TIRAP), and TRIF-related adaptor molecule (TRAM). These adaptor molecules signal via MyD88-dependent or MyD88-independent pathways that result in the upregulation of pro-inflammatory mediators (cytokines, chemokines, and adhesion molecules). The MyD88-dependent pathway includes NF-κB translocation to the nucleus to regulate inflammatory gene expression. TLR signaling activates the endogenous NF-κB inhibitor IKK complex, which phosphorylates IκB and leads to its ubiquitylation and degradation by the proteasome. IκB degradation relieves the inhibitory influence on NF-κB, and NF-κB is then able to translocate from the cytoplasm into the nucleus. MAPK regulation of pro-inflammatory mediators is also MyD88 dependent. The MAPK module contains at least 3 protein kinases in series that culminate in the activation of a multifunctional MAPK (ERK1/2, JNK/SAPK, and p38). These MAPKs subsequently result in the activation of the transcription factor activator protein (AP-1), which then translocates to the nucleus. Interferon regulatory factor (IRF)7 is also MyD88 dependent, but is only found downstream of TLR9. Phosphorylation and dimerization of IRF7 activate its translocation to the nucleus. The MyD88-independent(/TRIF-dependent) pathway downstream of TLR3 and TLR4 involves IRF3, as well as NF-κB activation. Like IRF7, IRF3 undergoes phosphorylation and dimerization for activation and translocation to the nucleus. dsRNA, double-stranded RNA; HSP, heat shock protein; mHDL, (pathophysiologically) modified HDL; ssRNA, single stranded RNA.
As stated above, NF-κB activation is downstream of MyD88-dependent signaling. The NF-κB family is composed of homo- and heterodimers of Rel proteins [NF-κB1 (p50), NF-κB2 (p52), RelA (p65), RelB, and c-Rel (Rel)]. The NF-κB (p50/p65) transcription factor is a ubiquitous, constitutive, and inducible heterodimer with domains for dimerization and DNA binding, a nuclear translocation signal, and a binding site for the inhibitor of κB (IκB) (17, 23). Along with regulating pro-inflammatory cytokines, NF-κB activation mediates the expression of inducible NO synthase (iNOS), cyclooxygenase 2, growth factors, inhibitors of apoptosis, and effector enzymes in response to ligation of TLRs and other receptors involved in immunity, including T lymphocyte receptors and B lymphocyte receptors (17).
Synthesis of NF-κB is not de novo; therefore, its transcriptional activity is silenced by interactions with inhibitory IκB proteins present in the cytoplasm. Downstream of specific TLR signaling, the inhibitor IKK complex phosphorylates IκB, which leads to its ubiquitylation and subsequent degradation by the proteasome. As a result, the inhibitory influence on NF-κB is alleviated and NF-κB is then able to translocate from the cytoplasm into the nucleus to regulate gene expression (17, 23) (Fig. 3).
In VSMCs and ECs, ANG II activates NF-κB (99, 140, 148), and sustained inhibition of NF-κB with pyrrolidine dithiocarbamate prevents inflammation and hypertension (108, 123). Because NF-κB has been established in the development of inflammation and hypertension, investigation of the pro-inflammatory process upstream of NF-κB activation is warranted; thus TLRs represent an ideal candidate.
Another mechanism of MyD88-dependent signaling is MAPK activation of AP-1 transcription factor. Mitogen-activated protein kinases are serine/threonine-specific protein kinases involved in directing cellular responses to a diverse array of stimuli. Mitogen-activated protein kinase expression and signaling is initiated through a MAPK module, which functions to prolong transient cell signals into sustained ones that can be relayed downstream to the nucleus to alter the pattern of gene expression. This module contains at least three protein kinases in series that culminate in the activation of a multifunctional MAPK (Fig. 3). Once activated, the MAPK relays the signal downstream by phosphorylating various proteins within the cell, including gene regulatory proteins and other protein kinases. Mitogen-activated protein kinases are major components of pathways controlling embryogenesis, cell differentiation, cell proliferation, and cell death (119). After TLR activation, MAPK signaling leads to translocation of AP-1 transcription factor to the nucleus and upregulation of the expression of pro-inflammatory mediators (66) (Fig. 3).
Prominent MAPKs include ERK1 and ERK2, JNK/SAPK, and p38 MAPK pathways. The ERK1/2 phosphorylation cascade involves MEK1/2 (MAP/ERK kinase), whereas the signaling processes leading to JNK/SAPK and p38 MAPK activation involve MEK4/7 and MEK3/6, respectively (119). Further upstream signaling of MAPKs, within the MAPK module, is recognized to be primarily dependent on the nonreceptor tyrosine kinase c-Src (56, 88, 147). However, c-Src-independent regulators of MEK, such as the Ras-Raf pathway, have also been reported (72, 155).
In VSMCs and ECs, MAPKs are involved in a multitude of physiological and pathophysiological actions (e.g., contraction, migration, adhesion, collagen deposition, growth, differentiation, and survival) (68, 119, 136). Inhibition of ERK1/2 abolishes sustained contraction and normalizes the ANG II effects on mesenteric VSMCs from SHRs (145), and rapid tyrosine phosphorylation of ERKs occurs in human VSMCs treated with ANG II (146). The hypertension-associated effects of ERK are not limited to ANG II-dependent hypertension. Basal aortic tone of DOCA-salt hypertensive rats was significantly attenuated by ERK inhibition (67), and DOCA treatment resulted in augmented aortic contractile responses mediated by increased phosphorylation of ERK1/2 and decreased expression of mitogen-activated protein kinase phosphatase 1 (42).
In addition to ERK, JNK/SAPK and p38 MAPKs also contribute to vascular dysfunction and the hypertensive phenotype. The role of p38 is unequivocal, with its inhibition improving indexes of inflammation, oxidative stress, endothelial function, vascular reactivity, cardiac hypertrophy, renal function, and blood pressure, as well as morbidity/mortality (10, 13, 80, 111, 115). In addition, inhibition of JNK/SAPK signaling attenuates contractile responses in isolated aortic segments (79, 113, 158), suggesting a role of JNK/SAPK in vasoconstriction mechanisms. These findings collectively indicate a role of MAPKs in the etiology of vascular dysfunction and hypertension. Therefore, novel upstream mediators of MAPK signaling, such as TLRs, should be investigated.
The final MyD88-dependent signaling transcription factor is IRF7. IRF7 activation is specific to TLR9 (65), and it requires phosphorylation and dimerization to trigger its translocation to the nucleus and induce type I IFNs and IFN-inducible genes (Fig. 3). Currently, knowledge of the contribution of IRF7 on vascular function and hypertension is not known. However, in one investigation that used human ECs infected with bacteria Chlamydophila pneumonia, IRF7-dependent signaling was activated, as well as IRF3-dependent and mitochondrial antiviral signaling. This signaling induced type I IFN expression that successfully inhibited bacterial growth. We subsequently hypothesized that type I IFNs, produced via IRF signaling, may contribute to the control of infectious-vascular lesions in atherosclerosis (18).
MyD88-independent signaling is associated only with TLR3 and TLR4 and results in the induction of type I IFNs and IFN-inducible genes (4, 65). Because this signaling involves the recruitment of the adaptor proteins TRIF and TRAM instead of MyD88, it is also known as TRIF-dependent signaling. MyD88-independent signaling results in the phosphorylation and dimerization of IRF3, as well as activation of NF-κB signaling (Fig. 3).
Research into the contribution of MyD88-independent signaling to vascular dysfunction and hypertension is limited. However, it has been demonstrated in VSMCs that C-reactive protein stimulates IL-6 production and inhibits peroxisome proliferator-activated receptor-γ expression via TLR4-MyD88-independent signaling (84). Additionally, using an in vivo model of hindlimb ischemia-reperfusion, TLR4 on myocytes mediated pro-inflammatory responses through MyD88, whereas regenerative processes occurred through TLR4 and TRIF (124). Although, these investigations observed a roll of TLR-MyD88-independent signaling in vascular dysfunction, more are still required to establish its roll in hypertension pathogenesis.
TLRs in Cardiovascular Disease Etiology
Unlike other TLRs, which are functionally active as homomers, TLR2 has developed the unique ability to signal via heterodimers with TLR1 or TLR6. In fact, signaling does not appear to occur downstream of TLR2 homodimers (116). TLR2 is required for functional recognition of gram-positive, gram-negative, fungi, viral, and mycoplasma lipoproteins, and peptidoglycan, and heterodimerization of TLR2 with TLR1 and TLR6 allows it to attain specificity for a diverse range of ligands (141). In addition to microbial factors, TLR2 can bind pathophysiologically modified HDL (135), as well as endogenous heat shock proteins (e.g., heat shock proteins 60 and 70) (7, 33).
Illustrating its potential for cardiovascular disease pathogenesis, TLR2 activation induces a dedifferentiated migratory and proliferative phenotype in VSMCs (33, 78). In ECs, “abnormal” HDL from patients with chronic kidney disease acted upon TLR2 (via a TLR1- or TLR6-coreceptor-independent pathway) and reduced NO bioavailability, leading to impaired endothelial repair and enhancing endothelial pro-inflammatory activation (135). These data in cells provide evidence as to why TLR2 activation has been associated with the development of atherosclerosis (30). Nonetheless, the contribution of TLR2 to vascular dysfunction in hypertension remains to be clarified.
TLR3 recognizes double-stranded RNA, and TLR7 and TLR8 recognize single-stranded RNA released from viruses (or retroviruses) or necrotic cells. TLR3 has been shown to have a protective effect on the vascular wall after mechanical and hypercholesterolemia-induced arterial injury (27). This protective effect may stem from the ability of TLR3 to induce the expression of cytoprotective and anti-inflammatory glycoprotein clusterin/apolipoprotein J to counteract the progression of atherosclerosis (9). On the other hand, maternal hypertension (from here on referred to as preeclampsia) was developed in rodents treated with agonists of TLR3, -7, and -7/8 (combined) (21, 22, 144). In pulmonary artery VSMCs, TLR3 induction was found to release IL-8, IFN-γ-induced protein 10 (also known as CXCL10), and ET-1 (41). Yang et al. (153) identified that TLR3 activation induced the expression of chemokine MCP-1 and pro-inflammatory cytokine IL-6 in human coronary VSMCs. Finally, activation of TLR7/8 with its agonist (Clo97) exacerbated the pro-inflammatory effects exerted by ANG II and nicotine in SHR splenocytes (49). These effects were replicated in vivo and may have important implications for ANG II and nicotinic modulation of the autonomic nervous system and sympathetic drive in hypertension (1, 93). Accordingly, these data illustrate that TLR3, -7, and -8, and their endogenous ligand (RNA), could contribute to the development of hypertension and other hypertensive disorders (e.g., preeclampsia).
TLR4 has been well characterized in various cardiovascular diseases (40), and it has been observed to induce a pro-inflammatory and proliferative phenotype to endogenous molecules in VSMCs (33, 131, 152). Endogenous agonists of TLR4 include vasoactive molecules heat shock protein 60 and ANG II (36).
TLR4 was the first TLR to be implicated in the etiology of vascular dysfunction and hypertension (15). Specifically, Bomfim et al. (15) showed that TLR4 is elevated in SHRs and that treatment with anti-TLR4 antibody attenuated aortic contractility, serum IL-6 levels, and mean arterial pressure. This ability of TLR4 to mediate heightened aortic contractility in SHRs opposes the findings that TLR4 activation with gram-negative (Escherichia coli) bacteria causes vascular dysfunction via attenuation of murine aortic contractility through the induction of iNOS (19). However, it should be noted that the goal of Cartwright et al. (19) was to emulate septic shock. More recently, it was reported that TLR4 mutation protected obese, type II diabetic mice against endothelial dysfunction in conduit and resistance vessels, hyperglycemia, and hypertension via downregulation of NAD(P)H oxidase isoforms 1 and 4 and cyclooxygenase 1 activity (82).
In support of these vascular-specific data, impaired functioning of other cell types that play a role in the pathogenesis of hypertension has been attributed to TLR 4. For example, TLR4 expression was elevated in cardiomyocytes of SHRs compared with Wistar-Kyoto (WKY) controls, NOS inhibition upregulated the expression of TLR4, and high-doses of angiotensin-converting enzyme inhibitor (ACEi) ameliorated the expression of TLR4 (35). In human aortic VSMCs, induction of TLR4 expression by LPS increased iNOS expression and NO production. Furthermore, LPS upregulated the expression of IL-8, vascular endothelial growth factor (VEGF), ICAM-1, and VCAM-1 (53).
Despite the fact that TLR4 polymorphisms have not been shown to be a significant predictor of coronary heart disease in healthy men (106), the abovementioned investigations illustrate a significant contribution of TLR4 to the development of cardiovascular pathologies, including hypertension. As a result, the contribution of other TLRs in hypertension and cardiovascular disease development should be investigated, as well as progressing TLR4-targeted therapy.
TLR5 functions similarly to TLR2 in that it recognizes flagella (2, 133). Specifically, TLR5 recognizes flagellin, a principal component of flagella from both gram-positive and gram-negative bacteria (50). Although an endogenous molecule or DAMP specific for TLR5 has not been reported, TLR5 deficiency leads to altered gut microbiota and metabolic syndrome (150), which can encompass hypertension.
Unmethylated CpG dinucleotides activate TLR9. Although these unmethylated CpG motifs are most common of microbial DNA, unmethylated CpG motifs can also be found in mtDNA, which can be released during cell injury and/or death. Circulating mtDNA released due to trauma is then able to act on TLR9 and induce an inflammatogenic response (157). This ability occurs due to the evolutionarily conserved similarities between mitochondria and saprophytic bacteria, which at one point during evolution entered the eukaryotic cell and became an intracellular organelle (127). It has been demonstrated that pressure-overload released mtDNA that escapes autophagic degradation, leads to TLR9-mediated inflammatory responses in cardiomyocytes, inducing myocarditis and dilated cardiomyopathy (114). Additionally, activation of TLR9 with its synthetic agonist (ODN 2395) exacerbated the pro-inflammatory effects exerted by ANG II and nicotine in SHR splenocytes (49). Nonetheless, the role of TLR9 on vascular function, vascular remodeling, and hypertension remains to be elucidated, and this hypothesis will be proposed later in this review.
Interestingly, it has been shown the TLR adapter molecule MyD88 is necessary for vascular remodeling of carotid arteries. Specifically, inward remodeling was associated with MyD88-dependent and superoxide anion initiated cytokine and chemokine generation, as well as macrophage infiltration and activation in the vascular wall. (142). However, an association between MyD88 and a specific TLR was not deduced, since MyD88 is downstream of various TLRs (Fig. 3).
An endogenous peptide that is dysregulated during the development and establishment of hypertension is ANG II. Along with its profound effects on vasoconstriction, aldosterone and antidiuretic hormone (ADH) secretion, sympathetic activation, and fluid and ion reabsorption, ANG II is also able to influence various TLRs and their signaling. For example, ANG II upregulated TLR4 protein and mRNA expression, as well as TLR4-induced myeloperoxidase secretion in murine macrophage (RAW264.7) cells (60). Activation of TLRs7/8 and TLR9 exacerbated the IL-6 secretion to ANG II in SHR splenocytes, but not WKY, and this pro-inflammatory cytokine production in SHRs following ANG II and TLR7/8 agonist administration was further replicated in vivo (49). Inhibition of ANG II by antagonism of the ANG II type 1 receptor with olmesartan was able to reduce the TLR2/TLR4-mediated inflammatory action in ApoE−/− mice, subsequently improving indexes of vascular disease (e.g., inhibition of intimal neovascularization and matrix metalloproteinase activation), decreasing atherosclerotic plaque growth, and increasing plaque instability (24). Finally, given that TLRs are expressed by T lymphocytes (62) and T lymphocytes mediate ANG II-induced hypertension (47), it is conceivable that ANG II alters TLR expression on T lymphocytes and contributes to the aberrant inflammation seen in hypertension. Overall, these findings illustrate a novel effect of ANG II on various TLRs. Therefore, further exploration of the effects of ANG II on TLRs is justified, as well as investigation into the TLR interactions with other DAMPs that are upregulated due to the pathogenesis of hypertension.
TLR9 and Mitochondrial DNA: Novel Contributors to Hypertension?
As mentioned earlier, TLR9 has affinity for unmethylated CpG dinucleotides common of bacteria and viruses but not for methylated CpG dinucleotides common of vertebrate DNA. This ability of the immune system to discriminate the methylation pattern of DNA is important for preventing TLR9-dependent autoimmunity (12, 137). However, unmethylated CpG dinucleotides are also found in mtDNA, as mitochondria evolved from saprophytic bacteria to become intracellular organelles (127). Recently, the inflammatogenic properties of mtDNA, as a result of TLR9 activation, have been demonstrated (114, 157). Although the existence of TLR-independent pathways activated by nucleic acids have been previously described (151), emerging data suggest that activation of TLR9 by mtDNA could be a novel mechanism of vascular dysfunction, vascular remodeling, and hypertension. In support of this hypothesis, previous investigations have shown that cell-free CpG DNA is increased in patients with essential hypertension (149) and preeclampsia (28).
TLR9 is expressed in various immune cells such as B lymphocytes, monocytes, macrophages, and plasmacytoid dendritic cells (52), as well as vascular tissues (44). In immune cells, immature TLR9 is localized to the endoplasmic reticulum, and upon CpG internalization via class III phosphatidylinositol 3-kinase-dependent mechanism (71), translocates to endosomal vacuoles (76). Activation of TLR9 by CpG motifs involves an intracytoplasmic signaling cascade that proceeds through MyD88, IL-1-receptor-activated kinase (IRAK), and TNF receptor -associated factor 6 (TRAF6) cascade. This signaling leads to the activation of both MAPKs and IKK complexes and culminates in the upregulation of pro-inflammatory transcription factors, including NF-κB, AP-1 via MAPKs, and IRF7, stimulating the production of a TH1 response (71). Given that NF-κB and MAPKs are also involved in the pathogenesis of vascular dysfunction and hypertension, it is conceivable that TLR9 may play a role in augmented expression of pro-inflammatory cytokines, chemokines, and adhesion molecules seen in association with vascular remodeling. We have recently found that TLR9 is expressed in both conduit (aortic) and resistance (mesenteric) VSMCs, and activation of TLR9 with its synthetic ligand, ODN 2395, results in augmented contractile responses in isolated conduit (100) and resistance (45) arteries.
TLR-mediated generation of pro-inflammatory cytokines, chemokines, and adhesion molecules have been extensively investigated in immune cells (157), and recently our laboratory (15, 44), and others (19, 82), have extended some of these findings into the vasculature. Plausibly, vascular remodeling and hypertension may be promoted via mtDNA activation of TLR9 in VSMCs, ECs, and immune cells (e.g., monocytes and macrophages) in concert. For example, TLR9 activation and the presence of mitochondrial DAMPs increase endothelial permeability (57, 139). We propose it would only take a minor insult to cause cellular injury and initiate the deleterious cascade of mtDNA release and TLR9 activation (e.g., prehypertensive stimuli such as ANG II, high salt, or chronic stress). This release of mtDNA and other DAMPs could lead to further pressure- and ischemia-induced cell injury and death as vascular remodeling ensues and blood pressure rises.
In summary, although TLR9 promotor polymorphisms have not been shown to play a role in atherogenesis (48), the inflammatogenic properties of circulating mtDNA could be the missing link between TLR9 activation, vascular dysfunction, vascular remodeling, hypertension, and further cardiovascular pathologies (Fig. 4).
Fig. 4.
Schematic demonstrating the novel hypothesis that circulating mitochondrial DNA (mtDNA), released following cell injury from modest elevations in total peripheral resistance, leads to TLR9 activation in VSMCs, ECs, and immune cells (e.g., monocytes and macrophages). This can subsequently activate a positive feedback loop, exacerbating further cell injury and/or death and promoting vascular remodeling and hypertension.
Clinical Implications
It is well known that resistance arteries are the major contributors to total peripheral resistance and thus blood pressure regulation, due to large composite cross-sectional area and sympathetic innervation. As such, deleterious alterations to their structure can promote the development and progression of hypertension (129). Moreover, stiffening of conduit vessels influences blood pressure regulation through propagation of blood flow to the downstream arterial tree (e.g., turbulent or laminar blood flow and subsequent vascular adaptations to shear stress). In fact, aortic stiffening is a precursor to the development of hypertension (63), is associated with inflammation in patients with untreated essential hypertension (90), and is a strong independent predictor of cardiovascular morbidity in hypertension (77). Therefore, investigations of the molecular mechanisms that contribute to global vascular remodeling throughout the arterial tree should be conducted.
Although the etiology of essential hypertension is heterogeneous and has not been fully elucidated, immune system activation and chronic inflammation have been recently acknowledged as a significant contributor to the hypertensive process (1, 34, 46, 55, 118, 136). The interaction between DAMPs and TLRs on vascular tissues may be the starting point for the development of vascular dysfunction and remodeling, the adaptive immune system response, and the genesis and establishment of hypertension (Fig. 2).
Perspectives
The discovery of TLRs has guided the field of immunology to an era of accelerated advancement and has created exciting therapeutic and experimental possibilities for targeting infections and noninfectious inflammatory diseases (14). The involvement of TLRs in the pathogenesis of hypertension, as described in the current review, extends these therapeutic possibilities above and beyond the current challenges in immunology. TLRs may be the missing link between host-derived “dangerous” molecules (DAMPs such as mtDNA), low-grade inflammation, vascular remodeling, and hypertension. Moreover, activation of TLRs may be a necessary precursor of adaptive immune system involvement in the development of hypertension. The etiology of essential hypertension is multifactorial, and a plethora of endogenous molecules have been previously linked to the development this disease (Table 1), further increasing the complexity its pathophysiology. Paradoxically, TLR signaling presents a low complexity system (i.e., 10 TLRs in humans, 4 adaptor molecules, and 2 downstream inflammatory transcription factors are required for most invading and host-derived molecule recognition) (14). The low complexity of TLR signaling and the existence of specific inhibitors and antagonists for components of the TLR signaling may lead to exciting and novel therapeutic interventions for hypertension and other hypertensive disorders.
GRANTS
This study was supported in part by the American Heart Association (No. 13PRE14080019), the National Institutes of Health (R01 HL-071138 and R01 DK-083685), the Society for Women's Health Research, the Preeclampsia Foundation (Vision Grant), the CNPq, Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil, and the Naito Foundation, Japan.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.G.M. and S.G. prepared figures; C.G.M. drafted manuscript; C.G.M., S.G., C.F.W., K.M.S., T.M., and R.C.W. edited and revised manuscript; C.G.M., S.G., C.F.W., K.M.S., T.M., and R.C.W. approved final version of manuscript.
REFERENCES
- 1.Abboud FM, Harwani SC, Chapleau MW. Autonomic neural regulation of the immune system: implications for hypertension and cardiovascular disease. Hypertension 59: 755–762, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamo R, Sokol S, Soong G, Gomez MI, Prince A. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and toll-like receptor 2 as well as toll-like receptor 5. Am J Respir Cell Mol Biol 30: 627–634, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science 272: 54–60, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Hoshino K. Myeloid differentiation factor 88-dependent and -independent pathways in toll-like receptor signaling. J Infect Dis 187, Suppl 2: S356–S363, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Ameziane N, Beillat T, Verpillat P, Chollet-Martin S, Aumont MC, Seknadji P, Lamotte M, Lebret D, Ollivier V, de Prost D. Association of the Toll-like receptor 4 gene Asp299Gly polymorphism with acute coronary events. Arterioscler Thromb Vasc Biol 23: e61–e64, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Anderson KV, Jurgens G, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell 42: 779–789, 1985 [DOI] [PubMed] [Google Scholar]
- 7.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277: 15028–15034, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Ba D, Takeichi N, Kodama T, Kobayashi H. Restoration of T cell depression and suppression of blood pressure in spontaneously hypertensive rats (SHR) by thymus grafts or thymus extracts. J Immunol 128: 1211–1216, 1982 [PubMed] [Google Scholar]
- 9.Baiersdorfer M, Schwarz M, Seehafer K, Lehmann C, Heit A, Wagner H, Kirschning CJ, Koch-Brandt C. Toll-like receptor 3 mediates expression of clusterin/apolipoprotein J in vascular smooth muscle cells stimulated with RNA released from necrotic cells. Exp Cell Res 316: 3489–3500, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Bao W, Behm DJ, Nerurkar SS, Ao Z, Bentley R, Mirabile RC, Johns DG, Woods TN, Doe CP, Coatney RW, Ohlstein JF, Douglas SA, Willette RN, Yue TL. Effects of p38 MAPK inhibitor on angiotensin II-dependent hypertension, organ damage, and superoxide anion production. J Cardiovasc Pharmacol 49: 362–368, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57: 469–476, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol 7: 49–56, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Behr TM, Nerurkar SS, Nelson AH, Coatney RW, Woods TN, Sulpizio A, Chandra S, Brooks DP, Kumar S, Lee JC, Ohlstein EH, Angermann CE, Adams JL, Sisko J, Sackner-Bernstein JD, Willette RN. Hypertensive end-organ damage and premature mortality are p38 mitogen-activated protein kinase-dependent in a rat model of cardiac hypertrophy and dysfunction. Circulation 104: 1292–1298, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430: 257–263, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Bomfim GF, Dos Santos RA, Oliveira MA, Giachini FR, Akamine EH, Tostes RC, Fortes ZB, Webb RC, Carvalho MH. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci (Lond) 122: 535–543, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bomfim GF, Szasz T, Carvalho MH, Webb RC. The Toll way to hypertension: role of the innate immune response. Endocrinol Metabol Syndrome S: 8, 2011 [Google Scholar]
- 17.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 25: 280–288, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Buss C, Opitz B, Hocke AC, Lippmann J, van Laak V, Hippenstiel S, Krull M, Suttorp N, Eitel J. Essential role of mitochondrial antiviral signaling, IFN regulatory factor (IRF)3, and IRF7 in Chlamydophila pneumoniae-mediated IFN-beta response and control of bacterial replication in human endothelial cells. J Immunol 184: 3072–3078, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Cartwright N, McMaster SK, Sorrentino R, Paul-Clark M, Sriskandan S, Ryffel B, Quesniaux VF, Evans TW, Mitchell JA. Elucidation of toll-like receptor and adapter protein signaling in vascular dysfunction induced by gram-positive Staphylococcus aureus or gram-negative Escherichia coli. Shock 27: 40–47, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol 57: 1511–1522, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee P, Chiasson VL, Kopriva SE, Young KJ, Chatterjee V, Jones KA, Mitchell BM. Interleukin 10 deficiency exacerbates toll-like receptor 3-induced preeclampsia-like symptoms in mice. Hypertension 58: 489–496, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee P, Weaver LE, Doersch KM, Kopriva SE, Chiasson VL, Allen SJ, Narayanan AM, Young KJ, Jones KA, Kuehl TJ, Mitchell BM. Placental Toll-like receptor 3 and Toll-like receptor 7/8 activation contributes to preeclampsia in humans and mice. PLos One 7: e41884, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem 45: 7–17, 1999 [PubMed] [Google Scholar]
- 24.Cheng XW, Song H, Sasaki T, Hu L, Inoue A, Bando YK, Shi GP, Kuzuya M, Okumura K, Murohara T. Angiotensin type 1 receptor blocker reduces intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Hypertension 57: 981–989, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure and National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Chuang T, Ulevitch RJ. Identification of hTLR10: a novel human Toll-like receptor preferentially expressed in immune cells. Biochim Biophys Acta 1518: 157–161, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Cole JE, Navin TJ, Cross AJ, Goddard ME, Alexopoulou L, Mitra AT, Davies AH, Flavell RA, Feldmann M, Monaco C. Unexpected protective role for Toll-like receptor 3 in the arterial wall. Proc Natl Acad Sci USA 108: 2372–2377, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colleoni F, Lattuada D, Garretto A, Massari M, Mando C, Somigliana E, Cetin I. Maternal blood mitochondrial DNA content during normal and intrauterine growth restricted (IUGR) pregnancy. Am J Obstet Gynecol 203: 365.e1–365.e6, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol 298: R1089–R1097, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtiss LK, Tobias PS. The toll of Toll-like receptors, especially toll-like receptor 2, on murine atherosclerosis. Curr Drug Targets 8: 1230–1238, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Dajnowiec D, Langille BL. Arterial adaptations to chronic changes in haemodynamic function: coupling vasomotor tone to structural remodelling. Clin Sci (Lond) 113: 15–23, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Dauphinee SM, Voelcker V, Tebaykina Z, Wong F, Karsan A. Heterotrimeric Gi/Go proteins modulate endothelial TLR signaling independent of the MyD88-dependent pathway. Am J Physiol Heart Circ Physiol 301: H2246–H2253, 2011 [DOI] [PubMed] [Google Scholar]
- 33.de Graaf R, Kloppenburg G, Kitslaar PJ, Bruggeman CA, Stassen F. Human heat shock protein 60 stimulates vascular smooth muscle cell proliferation through Toll-like receptors 2 and 4. Microbes Infect 8: 1859–1865, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Dzielak DJ. The immune system and hypertension. Hypertension 19: I36–I44, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Eissler R, Schmaderer C, Rusai K, Kuhne L, Sollinger D, Lahmer T, Witzke O, Lutz J, Heemann U, Baumann M. Hypertension augments cardiac Toll-like receptor 4 expression and activity. Hypertens Res 34: 551–558, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol 87: 989–999, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Folkow B. Physiological aspects of primary hypertension. Physiol Rev 62: 347–504, 1982 [DOI] [PubMed] [Google Scholar]
- 38.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447: 972–978, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Foster SL, Medzhitov R. Gene-specific control of the TLR-induced inflammatory response. Clin Immunol 130: 7–15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frantz S, Ertl G, Bauersachs J. Mechanisms of disease: Toll-like receptors in cardiovascular disease. Nat Clin Pract Cardiovasc Med 4: 444–454, 2007 [DOI] [PubMed] [Google Scholar]
- 41.George PM, Badiger R, Shao D, Edwards MR, Wort SJ, Paul-Clark MJ, Mitchell JA. Viral Toll Like Receptor activation of pulmonary vascular smooth muscle cells results in endothelin-1 generation; relevance to pathogenesis of pulmonary arterial hypertension. Biochem Biophys Res Commun 426: 486–491, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Giachini FR, Sullivan JC, Lima VV, Carneiro FS, Fortes ZB, Pollock DM, Carvalho MH, Webb RC, Tostes RC. Extracellular signal-regulated kinase 1/2 activation, via downregulation of mitogen-activated protein kinase phosphatase 1, mediates sex differences in desoxycorticosterone acetate-salt hypertension vascular reactivity. Hypertension 55: 172–179, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Girerd X, London G, Boutouyrie P, Mourad JJ, Safar M, Laurent S. Remodeling of the radial artery in response to a chronic increase in shear stress. Hypertension 27: 799–803, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Goulopoulou S, Matsumoto T, Bomfim GF, Webb RC. Toll-like receptor 9 activation: a novel mechanism linking placenta-derived mitochondrial DNA and vascular dysfunction in pre-eclampsia. Clin Sci (Lond) 123: 429–435, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goulopoulou S, Matsumoto T, Webb RC. TLR-9 activation potentiates the role of ERK1/2 in thromboxane A2-induced contractions in uterine but not in resistance arteries. FASEB J 26: 870.9, 2012 [Google Scholar]
- 46.Granger JP. An emerging role for inflammatory cytokines in hypertension. Am J Physiol Heart Circ Physiol 290: H923–H924, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamann L, Glaeser C, Hamprecht A, Gross M, Gomma A, Schumann RR. Toll-like receptor (TLR)-9 promotor polymorphisms and atherosclerosis. Clin Chim Acta 364: 303–307, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Harwani SC, Chapleau MW, Legge KL, Ballas ZK, Abboud FM. Neurohormonal modulation of the innate immune system is proinflammatory in the prehypertensive spontaneously hypertensive rat, a genetic model of essential hypertension. Circ Res 111: 1190–1197, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410: 1099–1103, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Heagerty AM, Aalkjaer C, Bund SJ, Korsgaard N, Mulvany MJ. Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertension 21: 391–397, 1993 [DOI] [PubMed] [Google Scholar]
- 52.Hennessy EJ, Parker AE, O′Neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov 9: 293–307, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Heo SK, Yun HJ, Noh EK, Park WH, Park SD. LPS induces inflammatory responses in human aortic vascular smooth muscle cells via Toll-like receptor 4 expression and nitric oxide production. Immunol Lett 120: 57–64, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Hua Z, Hou B. TLR signaling in B-cell development and activation. Cell Mol Immunol 10: 103–106, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension 38: 581–587, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Ishida M, Ishida T, Thomas SM, Berk BC. Activation of extracellular signal-regulated kinases (ERK1/2) by angiotensin II is dependent on c-Src in vascular smooth muscle cells. Circ Res 82: 7–12, 1998 [DOI] [PubMed] [Google Scholar]
- 57.Itagaki K, Adibnia Y, Sun S, Zhao C, Sursal T, Chen Y, Junger W, Hauser CJ. Bacterial DNA induces pulmonary damage via TLR-9 through cross-talk with neutrophils. Shock 36: 548–552, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji Y, Liu J, Wang Z, Liu N. Angiotensin II induces inflammatory response partly via toll-like receptor 4-dependent signaling pathway in vascular smooth muscle cells. Cell Physiol Biochem 23: 265–276, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Ji YY, Liu JT, Liu N, Wang ZD, Liu CH. PPARalpha activator fenofibrate modulates angiotensin II-induced inflammatory responses in vascular smooth muscle cells via the TLR4-dependent signaling pathway. Biochem Pharmacol 78: 1186–1197, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Ji YY, Wang ZD, Liu JT, Liu N. Angiotensin II induces toll-like receptor 4 expression and myeloperoxidase activity in RAW264.7 cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 24: 1037–1039, 2008 [PubMed] [Google Scholar]
- 61.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11: 1173–1179, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Jin B, Sun T, Yu XH, Yang YX, Yeo AE. The effects of TLR activation on T-cell development and differentiation. Clin Dev Immunol 2012: 836485, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 308: 875–881, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 59: 324–330, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11: 373–384, 2010 [DOI] [PubMed] [Google Scholar]
- 66.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med 13: 460–469, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Kim J, Lee YR, Lee CH, Choi WH, Lee CK, Kim J, Bae YM, Cho S, Kim B. Mitogen-activated protein kinase contributes to elevated basal tone in aortic smooth muscle from hypertensive rats. Eur J Pharmacol 514: 209–215, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Kim S, Iwao H. Stress and vascular responses: mitogen-activated protein kinases and activator protein-1 as promising therapeutic targets of vascular remodeling. J Pharm Sci 91: 177–181, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Kim SC, Stice JP, Chen L, Jung JS, Gupta S, Wang Y, Baumgarten G, Trial J, Knowlton AA. Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ Res 105: 1186–1195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim TH, Ku SK, Bae JS. Inhibitory effects of kaempferol-3-O-sophoroside on HMGB1-mediated proinflammatory responses. Food Chem Toxicol 50: 1118–1123, 2012 [DOI] [PubMed] [Google Scholar]
- 71.Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol 4: 249–258, 2004 [DOI] [PubMed] [Google Scholar]
- 72.Kolch W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol 6: 827–837, 2005 [DOI] [PubMed] [Google Scholar]
- 73.Kuhns DB, Priel DA, Gallin JI. Induction of human monocyte interleukin (IL)-8 by fibrinogen through the toll-like receptor pathway. Inflammation 30: 178–188, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Lafaille JJ. The role of helper T cell subsets in autoimmune diseases. Cytokine Growth Factor Rev 9: 139–151, 1998 [DOI] [PubMed] [Google Scholar]
- 75.Langille BL, O′Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science 231: 405–407, 1986 [DOI] [PubMed] [Google Scholar]
- 76.Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol 5: 190–198, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37: 1236–1241, 2001 [DOI] [PubMed] [Google Scholar]
- 78.Lee GL, Chang YW, Wu JY, Wu ML, Wu KK, Yet SF, Kuo CC. TLR 2 induces vascular smooth muscle cell migration through cAMP response element-binding protein-mediated interleukin-6 production. Arterioscler Thromb Vasc Biol 32: 2751–2760, 2012 [DOI] [PubMed] [Google Scholar]
- 79.Lee YR, Lee CK, Park HJ, Kim H, Kim J, Kim J, Lee KS, Lee YL, Min KO, Kim B. c-Jun N-terminal kinase contributes to norepinephrine-induced contraction through phosphorylation of caldesmon in rat aortic smooth muscle. J Pharmacol Sci 100: 119–125, 2006 [DOI] [PubMed] [Google Scholar]
- 80.Lenhard SC, Nerurkar SS, Schaeffer TR, Mirabile RC, Boyce RW, Adams DF, Jucker BM, Willette RN. p38 MAPK inhibitors ameliorate target organ damage in hypertension: Part 2. Improved renal function as assessed by dynamic contrast-enhanced magnetic resonance imaging. J Pharmacol Exp Ther 307: 939–946, 2003 [DOI] [PubMed] [Google Scholar]
- 81.Li T, Wang Y, Liu C, Hu Y, Wu M, Li J, Guo L, Chen L, Chen Q, Ha T, Li C, Li Y. MyD88-dependent nuclear factor-kappaB activation is involved in fibrinogen-induced hypertrophic response of cardiomyocytes. J Hypertens 27: 1084–1093, 2009 [DOI] [PubMed] [Google Scholar]
- 82.Liang CF, Liu JT, Wang Y, Xu A, Vanhoutte PM. Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4. Arterioscler Thromb Vasc Biol 33: 777–784, 2013 [DOI] [PubMed] [Google Scholar]
- 83.Liu N, Liu J, Ji Y, Lu P. Toll-like receptor 4 signaling mediates inflammatory activation induced by C-reactive protein in vascular smooth muscle cells. Cell Physiol Biochem 25: 467–476, 2010 [DOI] [PubMed] [Google Scholar]
- 84.Liu N, Liu JT, Ji YY, Lu PP. C-reactive protein triggers inflammatory responses partly via TLR4/IRF3/NF-kappaB signaling pathway in rat vascular smooth muscle cells. Life Sci 87: 367–374, 2010 [DOI] [PubMed] [Google Scholar]
- 85.Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R. Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum 52: 2936–2946, 2005 [DOI] [PubMed] [Google Scholar]
- 86.Lundberg IE. The role of cytokines, chemokines, and adhesion molecules in the pathogenesis of idiopathic inflammatory myopathies. Curr Rheumatol Rep 2: 216–224, 2000 [DOI] [PubMed] [Google Scholar]
- 87.Lundgren Y, Hallback M, Weiss L, Folkow B. Rate and extent of adaptive cardiovascular changes in rats during experimental renal hypertension. Acta Physiol Scand 91: 103–115, 1974 [DOI] [PubMed] [Google Scholar]
- 88.Luttrell DK, Luttrell LM. Not so strange bedfellows: G-protein-coupled receptors and Src family kinases. Oncogene 23: 7969–7978, 2004 [DOI] [PubMed] [Google Scholar]
- 89.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension 46: 1118–1122, 2005 [DOI] [PubMed] [Google Scholar]
- 91.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res 108: 1133–1145, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinez-Lemus LA, Hill MA, Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 24: 45–57, 2009 [DOI] [PubMed] [Google Scholar]
- 93.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res 107: 263–270, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mathur S, Walley KR, Wang Y, Indrambarya T, Boyd JH. Extracellular heat shock protein 70 induces cardiomyocyte inflammation and contractile dysfunction via TLR2. Circ J 75: 2445–2452, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Matzinger P. The danger model: a renewed sense of self. Science 296: 301–305, 2002 [DOI] [PubMed] [Google Scholar]
- 96.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol 12: 991–1045, 1994 [DOI] [PubMed] [Google Scholar]
- 97.Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol 11: 221–230, 2011 [DOI] [PubMed] [Google Scholar]
- 98.Mazzolai L, Duchosal MA, Korber M, Bouzourene K, Aubert JF, Hao H, Vallet V, Brunner HR, Nussberger J, Gabbiani G, Hayoz D. Endogenous angiotensin II induces atherosclerotic plaque vulnerability and elicits a Th1 response in ApoE−/minus mice. Hypertension 44: 277–282, 2004 [DOI] [PubMed] [Google Scholar]
- 99.McAllister-Lucas LM, Jin X, Gu S, Siu K, McDonnell S, Ruland J, Delekta PC, Van Beek M, Lucas PC. The CARMA3-Bcl10-MALT1 signalosome promotes angiotensin II-dependent vascular inflammation and atherogenesis. J Biol Chem 285: 25880–25884, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McCarthy CG, Goulopoulou S, Wenceslau CF, Matsumoto T, Webb RC. Chronic Toll-like receptor 9 activation mediates heightened vascular contractility via attenuated NOS activity in isolated aortic segments. FASEB J 27: 878.6, 2013 [Google Scholar]
- 101.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388: 394–397, 1997 [DOI] [PubMed] [Google Scholar]
- 102.Meredith PA, Ostergren J. From hypertension to heart failure—are there better primary prevention strategies? J Renin Angiotensin Aldosterone Syst 7: 64–73, 2006 [DOI] [PubMed] [Google Scholar]
- 103.Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol 25: 1213–1219, 2005 [DOI] [PubMed] [Google Scholar]
- 104.Mitchell GF. Arterial stiffness and wave reflection: biomarkers of cardiovascular risk. Artery Res 3: 56–64, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mitchell GF, Lacourciere Y, Ouellet JP, Izzo JL, Jr, Neutel J, Kerwin LJ, Block AJ, Pfeffer MA. Determinants of elevated pulse pressure in middle-aged and older subjects with uncomplicated systolic hypertension: the role of proximal aortic diameter and the aortic pressure-flow relationship. Circulation 108: 1592–1598, 2003 [DOI] [PubMed] [Google Scholar]
- 106.Morange PE, Tiret L, Saut N, Luc G, Arveiler D, Ferrieres J, Amouyel P, Evans A, Ducimetiere P, Cambien F, Juhan-Vague I, Study Group PRIME TLR4/Asp299Gly, CD14/C-260T, plasma levels of the soluble receptor CD14 and the risk of coronary heart disease: The PRIME Study. Eur J Hum Genet 12: 1041–1049, 2004 [DOI] [PubMed] [Google Scholar]
- 107.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 136: 2348–2357, 1986 [PubMed] [Google Scholar]
- 108.Muller DN, Dechend R, Mervaala EM, Park JK, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H, Luft FC. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats. Hypertension 35: 193–201, 2000 [DOI] [PubMed] [Google Scholar]
- 109.Mulvany MJ. Small artery remodeling in hypertension. Curr Hypertens Rep 4: 49–55, 2002 [DOI] [PubMed] [Google Scholar]
- 110.Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, Schiffrin EL, Heistad DD. Vascular remodeling. Hypertension 28: 505–506, 1996 [PubMed] [Google Scholar]
- 111.Nerurkar SS, Olzinski AR, Frazier KS, Mirabile RC, O′Brien SP, Jing J, Rajagopalan D, Yue TL, Willette RN. P38 MAPK inhibitors suppress biomarkers of hypertension end-organ damage, osteopontin and plasminogen activator inhibitor-1. Biomarkers 12: 87–112, 2007 [DOI] [PubMed] [Google Scholar]
- 112.Netea MG, Wijmenga C, O′Neill LA. Genetic variation in Toll-like receptors and disease susceptibility. Nat Immunol 13: 535–542, 2012 [DOI] [PubMed] [Google Scholar]
- 113.Ok SH, Jeong YS, Kim JG, Lee SM, Sung HJ, Kim HJ, Chang KC, Kwon SC, Sohn JT. c-Jun NH2-terminal kinase contributes to dexmedetomidine-induced contraction in isolated rat aortic smooth muscle. Yonsei Med J 52: 420–428, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485: 251–255, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Olzinski AR, McCafferty TA, Zhao SQ, Behm DJ, Eybye ME, Maniscalco K, Bentley R, Frazier KS, Milliner CM, Mirabile RC, Coatney RW, Willette RN. Hypertensive target organ damage is attenuated by a p38 MAPK inhibitor: role of systemic blood pressure and endothelial protection. Cardiovasc Res 66: 170–178, 2005 [DOI] [PubMed] [Google Scholar]
- 116.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA 97: 13766–13771, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279: 7370–7377, 2004 [DOI] [PubMed] [Google Scholar]
- 118.Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J 53: 258–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22: 153–183, 2001 [DOI] [PubMed] [Google Scholar]
- 120.Pryshchep O, Ma-Krupa W, Younge BR, Goronzy JJ, Weyand CM. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation 118: 1276–1284, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Putnam K, Shoemaker R, Yiannikouris F, Cassis LA. The renin-angiotensin system: a target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am J Physiol Heart Circ Physiol 302: H1219–H1230, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rather LJ. Disturbance of function (functio laesa): the legendary fifth cardinal sign of inflammation, added by Galen to the four cardinal signs of Celsus. Bull N Y Acad Med 47: 303–322, 1971 [PMC free article] [PubMed] [Google Scholar]
- 123.Rodriguez-Iturbe B, Ferrebuz A, Vanegas V, Quiroz Y, Mezzano S, Vaziri ND. Early and sustained inhibition of nuclear factor-kappaB prevents hypertension in spontaneously hypertensive rats. J Pharmacol Exp Ther 315: 51–57, 2005 [DOI] [PubMed] [Google Scholar]
- 124.Sachdev U, Cui X, McEnaney R, Wang T, Benabou K, Tzeng E. TLR2 and TLR4 mediate differential responses to limb ischemia through MyD88-dependent and independent pathways. PLos One 7: e50654, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Safar ME, Girerd X, Laurent S. Structural changes of large conduit arteries in hypertension. J Hypertens 14: 545–555, 1996 [DOI] [PubMed] [Google Scholar]
- 126.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 107: 2864–2869, 2003 [DOI] [PubMed] [Google Scholar]
- 127.Sagan L. On the origin of mitosing cells. J Theor Biol 14: 255–274, 1967 [DOI] [PubMed] [Google Scholar]
- 128.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 177: 1272–1281, 2006 [DOI] [PubMed] [Google Scholar]
- 129.Schiffrin EL. Vascular remodeling in hypertension: mechanisms and treatment. Hypertension 59: 367–374, 2012 [DOI] [PubMed] [Google Scholar]
- 130.Schiffrin EL. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am J Hypertens 17: 1192–1200, 2004 [DOI] [PubMed] [Google Scholar]
- 131.Schultz K, Murthy V, Tatro JB, Beasley D. Endogenous interleukin-1α promotes a proliferative and proinflammatory phenotype in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 292: H2927–H2934, 2007 [DOI] [PubMed] [Google Scholar]
- 132.Shao J, Nangaku M, Miyata T, Inagi R, Yamada K, Kurokawa K, Fujita T. Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension 42: 31–38, 2003 [DOI] [PubMed] [Google Scholar]
- 133.Smith MF, Jr, Mitchell A, Li G, Ding S, Fitzmaurice AM, Ryan K, Crowe S, Goldberg JB. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-kappa B activation and chemokine expression by epithelial cells. J Biol Chem 278: 32552–32560, 2003 [DOI] [PubMed] [Google Scholar]
- 134.Song R, Zeng Q, Ao L, Yu JA, Cleveland JC, Zhao KS, Fullerton DA, Meng X. Biglycan induces the expression of osteogenic factors in human aortic valve interstitial cells via Toll-like receptor-2. Arterioscler Thromb Vasc Biol 32: 2711–2720, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Speer T, Rohrer L, Blyszczuk P, Shroff R, Kuschnerus K, Krankel N, Kania G, Zewinger S, Akhmedov A, Shi Y, Martin T, Perisa D, Winnik S, Muller MF, Sester U, Wernicke G, Jung A, Gutteck U, Eriksson U, Geisel J, Deanfield J, von Eckardstein A, Luscher TF, Fliser D, Bahlmann FH, Landmesser U. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 38: 754–768, 2013 [DOI] [PubMed] [Google Scholar]
- 136.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol 78: 539–552, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stacey KJ, Young GR, Clark F, Sester DP, Roberts TL, Naik S, Sweet MJ, Hume DA. The molecular basis for the lack of immunostimulatory activity of vertebrate DNA. J Immunol 170: 3614–3620, 2003 [DOI] [PubMed] [Google Scholar]
- 138.Su X, Ao L, Shi Y, Johnson TR, Fullerton DA, Meng X. Oxidized low density lipoprotein induces bone morphogenetic protein-2 in coronary artery endothelial cells via Toll-like receptors 2 and 4. J Biol Chem 286: 12213–12220, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sun S, Sursal T, Adibnia Y, Zhao C, Zheng Y, Li H, Otterbein LE, Hauser CJ, Itagaki K. Mitochondrial DAMPs increase endothelial permeability through neutrophil dependent and independent pathways. PLos One 8: e59989, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Takahashi M, Suzuki E, Takeda R, Oba S, Nishimatsu H, Kimura K, Nagano T, Nagai R, Hirata Y. Angiotensin II and tumor necrosis factor-α synergistically promote monocyte chemoattractant protein-1 expression: roles of NF-κB, p38, and reactive oxygen species. Am J Physiol Heart Circ Physiol 294: H2879–H2888, 2008 [DOI] [PubMed] [Google Scholar]
- 141.Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S. Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol 169: 10–14, 2002 [DOI] [PubMed] [Google Scholar]
- 142.Tang PC, Qin L, Zielonka J, Zhou J, Matte-Martone C, Bergaya S, van Rooijen N, Shlomchik WD, Min W, Sessa WC, Pober JS, Tellides G. MyD88-dependent, superoxide-initiated inflammation is necessary for flow-mediated inward remodeling of conduit arteries. J Exp Med 205: 3159–3171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem 279: 17079–17084, 2004 [DOI] [PubMed] [Google Scholar]
- 144.Tinsley JH, Chiasson VL, Mahajan A, Young KJ, Mitchell BM. Toll-like receptor 3 activation during pregnancy elicits preeclampsia-like symptoms in rats. Am J Hypertens 22: 1314–1319, 2009 [DOI] [PubMed] [Google Scholar]
- 145.Touyz RM, El Mabrouk M, He G, Wu XH, Schiffrin EL. Mitogen-activated protein/extracellular signal-regulated kinase inhibition attenuates angiotensin II-mediated signaling and contraction in spontaneously hypertensive rat vascular smooth muscle cells. Circ Res 84: 505–515, 1999 [DOI] [PubMed] [Google Scholar]
- 146.Touyz RM, He G, Deng LY, Schiffrin EL. Role of extracellular signal-regulated kinases in angiotensin II-stimulated contraction of smooth muscle cells from human resistance arteries. Circulation 99: 392–399, 1999 [DOI] [PubMed] [Google Scholar]
- 147.Touyz RM, He G, Wu XH, Park JB, Mabrouk ME, Schiffrin EL. Src is an important mediator of extracellular signal-regulated kinase 1/2-dependent growth signaling by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients. Hypertension 38: 56–64, 2001 [DOI] [PubMed] [Google Scholar]
- 148.Valente AJ, Yoshida T, Murthy SN, Sakamuri SS, Katsuyama M, Clark RA, Delafontaine P, Chandrasekar B. Angiotensin II enhances AT1-Nox1 binding and stimulates arterial smooth muscle cell migration and proliferation through AT1, Nox1, and interleukin-18. Am J Physiol Heart Circ Physiol 303: H282–H296, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Veiko NN, Konorova IL, Neverova ME, Fidelina OV, Mkrtumova NA, Ershova ES, Kon′kova MS, Postnov AI. Delayed appearance of hypertension in spontaneously hypertensive rat (SHR) injected with CpG-rich DNA early in ontogenesis. Biomed Khim 56: 686–699, 2010 [DOI] [PubMed] [Google Scholar]
- 150.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328: 228–231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wagner H, Bauer S. All is not Toll: new pathways in DNA recognition. J Exp Med 203: 265–268, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang X, Coriolan D, Murthy V, Schultz K, Golenbock DT, Beasley D. Proinflammatory phenotype of vascular smooth muscle cells: role of efficient Toll-like receptor 4 signaling. Am J Physiol Heart Circ Physiol 289: H1069–H1076, 2005 [DOI] [PubMed] [Google Scholar]
- 153.Yang X, Murthy V, Schultz K, Tatro JB, Fitzgerald KA, Beasley D. Toll-like receptor 3 signaling evokes a proinflammatory and proliferative phenotype in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 291: H2334–H2343, 2006 [DOI] [PubMed] [Google Scholar]
- 154.Yang ZC, Wang KS, Wu Y, Zou XQ, Xiang YY, Chen XP, Li YJ. Asymmetric dimethylarginine impairs glucose utilization via ROS/TLR4 pathway in adipocytes: an effect prevented by vitamin E. Cell Physiol Biochem 24: 115–124, 2009 [DOI] [PubMed] [Google Scholar]
- 155.Yogi A, Callera GE, Montezano AC, Aranha AB, Tostes RC, Schiffrin EL, Touyz RM. Endothelin-1, but not Ang II, activates MAP kinases through c-Src independent Ras-Raf dependent pathways in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 27: 1960–1967, 2007 [DOI] [PubMed] [Google Scholar]
- 156.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 303: 1522–1526, 2004 [DOI] [PubMed] [Google Scholar]
- 157.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464: 104–107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhou MS, Schulman IH, Chadipiralla K, Raij L. Role of c-Jun N-terminal kinase in the regulation of vascular tone. J Cardiovasc Pharmacol Ther 15: 78–83, 2010 [DOI] [PubMed] [Google Scholar]