Abstract
We have investigated the temporal relationship between the hemodynamic and histological/morphological progression in a rat model of pulmonary arterial hypertension that develops pulmonary arterial lesions morphologically indistinguishable from those in human pulmonary arterial hypertension. Adult male rats were injected with Sugen5416 and exposed to hypoxia for 3 wk followed by a return to normoxia for various additional weeks. At 1, 3, 5, 8, and 13 wk after the Sugen5416 injection, hemodynamic and histological examinations were performed. Right ventricular systolic pressure reached its maximum 5 wk after Sugen5416 injection and plateaued thereafter. Cardiac index decreased at the 3∼5-wk time point, and tended to further decline at later time points. Reflecting these changes, calculated total pulmonary resistance showed a pattern of progressive worsening. Acute intravenous fasudil markedly reduced the elevated pressure and resistance at all time points tested. The percentage of severely occluded small pulmonary arteries showed a similar pattern of progression to that of right ventricular systolic pressure. These small vessels were occluded predominantly with nonplexiform-type neointimal formation except for the 13-wk time point. There was no severe occlusion in larger arteries until the 13-wk time point, when significant numbers of vessels were occluded with plexiform-type neointima. The Sugen5416/hypoxia/normoxia-exposed rat shows a pattern of chronic hemodynamic progression similar to that observed in pulmonary arterial hypertension patients. In addition to vasoconstriction, nonplexiform-type neointimal occlusion of small arteries appears to contribute significantly to the early phase of pulmonary arterial hypertension development, and plexiform-type larger vessel occlusion may play a role in the late deterioration.
Keywords: vasoconstriction, neointimal occlusion, plexiform
a serious limitation in investigating the pathogenesis of pulmonary arterial hypertension (PAH, World Health Organization group 1 pulmonary hypertension) (19) is the limited availability of lung tissue samples from patients. It is essentially impossible to obtain serial lung tissue samples for detailed assessment of the lung vascular morphology/pathobiology. In most cases, only a single lung specimen becomes available at the time of transplant or at autopsy. Our understanding of human PAH pathogenesis is heavily dependent on these “snapshot” data of very late-stage patients. Although findings from human samples provide important information, we need to be cautious about the interpretation of these data. It is generally not possible to determine whether the snapshot findings are the cause or the consequence of PAH. It is also unclear whether the findings from end-stage patients reflect those at the time of diagnosis when therapies are started. For instance, we do not know how numerous the plexiform lesions are at the time of diagnosis. Furthermore, because most of the end-stage patients have received various treatments, appropriate interpretation of the obtained results is very difficult to make in many cases (20). This limitation will never be overcome unless novel technologies, such as noninvasive lung histology assessment, become available. Alternatively, an experimental model that precisely simulates PAH might be able to solve this problem. Unfortunately, however, such a model has not heretofore been available (16, 21).
It is now widely accepted that multiple “hits” are required for the development of PAH (24), except for that associated with congenital heart disease (at least when the shunt is nonrestricted and post-tricuspid). It is supposed there are numerous potential hits, including genetic (such as mutation of bone morphogenetic protein receptor type 2) and environmental (such as infection and inflammation) factors. One apparent and seemingly most important fact is that almost all PAH patients exhibit a very similar phenotype, featuring progressive severe pulmonary hypertension (chronic deterioration), plexogenic arteriopathy, and resistance to therapies, independent of the cause or the combination of hits.
We have recently shown that the Sugen5416/hypoxia/normoxia-exposed (SU/Hx/Nx) rat, a multiple-hit model of PAH, develops severe pulmonary hypertension, which is accompanied by the development of plexogenic arteriopathy that is morphologically indistinguishable from that of human PAH (1). However, the temporal hemodynamic and arteriopathic profiles of this model have not been described. The purpose of this study, therefore, was to investigate the time course of hemodynamic changes to determine whether the model has a chronic hemodynamic deterioration pattern similar to that of human PAH. We also examined acute effects of a potent novel class of vasodilator, fasudil (Rho kinase inhibitor), as well as the progression in pulmonary vascular remodeling, i.e., medial wall thickening and plexiform and nonplexiform type neointimal vascular occlusion, in an attempt to identify factors involved in the hemodynamic deterioration.
METHODS
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of South Alabama. All studies in animals were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Adult male Sprague-Dawley rats weighing 160–200 g were injected subcutaneously with Sugen5416 (20 mg/kg; Cayman) and exposed to normobaric hypoxia (10% O2) for 3 wk. They were then returned to normoxia (21% O2) for additional various weeks (up to 10 wk after exposure to hypoxia: SU/Hx/Nx rats). We examined normal control rats and five groups (1, 3, 5, 8, and 13 wk after the Sugen5416 injection) of SU/Hx/Nx rats.
Hemodynamic Measurements in Catheterized Rats
All rats were placed on controlled heating pads after they were anesthetized with pentobarbital sodium (30 mg/kg ip). Hemodynamic measurements were performed under normoxic conditions as previously described with minor modifications (1). Briefly, polyvinyl catheters (PV-1; internal diameter: 0.28 mm) were inserted into the right ventricle (RV) via the right jugular vein for measurement of RV systolic pressure (RVSP) under anesthesia. RVSP instead of pulmonary arterial pressure was measured, because the pulmonary artery (PA) could not be routinely catheterized in these very hypertensive (RVSP > 100 mmHg) rats that have a severely altered structure of the RV chamber. In some cases (at least 1 from each time point) in which the PA was catheterized, we confirmed that there was no difference between RVSP and systolic pulmonary arterial pressure. A microtipped P-V catheter (1.4 Fr; Millar Instruments) was inserted into the left ventricle (LV) through the carotid artery to measure cardiac output (CO). RVSP, heart rate (HR), and LV systolic pressure (LVSP) were continuously recorded by an MPVS-300 system with PowerLab/4SP, A/D converter (AD Instruments), and a personal computer. After baseline hemodynamic measurements, all rats received a bolus intravenous injection of the Rho kinase inhibitor and vasodilator fasudil (10 mg/kg) via the left jugular vein to test for acute reversibility of the pulmonary hypertension. Twenty minutes after the injection, rats were euthanized with an overdose of pentobarbital sodium, and heart and lungs were collected for histological evaluation and RV/LV + septum (RV/LV + S) weight ratio measurement. Lungs were inflated with formalin-agarose mixture at a constant pressure of 20 cm H2O before the fixation for morphological and histological analyses. Cardiac index (CI) was calculated by dividing CO by body weight. Total pulmonary vascular resistance index (TPRI) was estimated by dividing RVSP by CI (11). Although HRs were monitored to be consistent (>300 beats/min), the possibility that pentobarbital had minor cardiovascular suppressive effects cannot be excluded (17).
Histopathology
The inflated lungs were fixed in 10% formalin overnight (1). The left lobe was blocked and paraffin embedded. All sections were cut at 5 μm and were stained with hematoxylin and eosin or Verhoeff-Van Gieson.
Immunohistochemistry
Immunohistochemical staining for von Willebrand Factor (vWF; Dako) and α-smooth muscle actin (SMA; Abcam) was performed using the Vectastain Universal Quick kit (Vector Laboratories) (1).
Morphological/Histological Analyses
Pulmonary artery occlusion rate.
A quantitative analysis of PA luminal obstruction was performed as described previously with minor modifications (12). Briefly, we counted all small pulmonary arteries [outer diameter (OD) < 200 μm] per whole left lobe cross section including the hilum from six groups of rats (1 section/rat) by investigators who were unaware of the source of the sections. Vessels were assessed for occlusive lesions on Verhoeff-van Gieson-stained slides and scored as follows: no evidence of neointimal formation (grade 0); partial (< 50%) luminal occlusion (grade 1); and severe-luminal occlusion (> 50%; grade 2).
Types of severe (grade 2) PA occlusion.
The types of vessel occlusion were classified by the pattern of vWF-positive cell distribution as follows: vWF-positive cells were found only in the circularly arranged innermost layer of the vessel (nonplexiform-type) or they showed a complex/disorganized plexus/channel-like pattern (plexiform-type).
Medial wall thickness.
In each tissue section all circular muscular arteries with an OD between 50 and 200 μm were analyzed at ×400. We excluded vessels <50 μm because most normal PAs are nonmuscular and two elastic laminae could not be identified. Distance between internal and external elastic laminae was expressed as medial thickness/OD as described previously (13).
Statistical Analysis
Values shown are means ± SE. ANOVA with Bonferroni post hoc test was used for comparisons among the experimental groups (time point) or Student's t-test for comparisons between before and after fasudil was used. Differences were considered significant at P < 0.05.
RESULTS
RV Hypertrophy
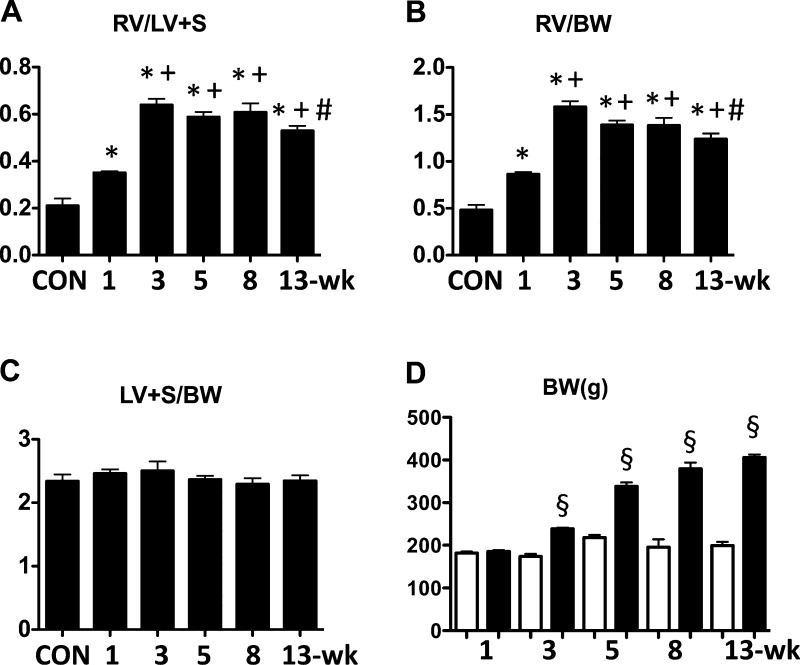
The RV/LV + S and RV/body weight ratios of the PAH rats were elevated significantly as early as the 1-wk time point, increased to a maximum by 3 wk, and then declined slightly out to 13 wk (Fig. 1, A and B). There was no increase in LV + S/body weight ratio at any time point (Fig. 1C). Body weight data are shown in Fig. 1D.
Fig. 1.
Time course changes in heart weight ratios and body weight in Sugen5416/hypoxia/normoxia-exposed rats at 1, 3, 5, 8, and 13 wk after the Sugen5416 injection. A: right ventricle/left ventricle + septum (RV/LV + S). B: right ventricle/body weight (RV/BW). C: left ventricle + septum/body weight (LV + S/BW). Con, normal control rats. D: body weights before (white) and after (black) exposure to Sugen5416/hypoxia/normoxia. Values are means ± SE of n = 5–9. *P < 0.05 vs. Con group; +P < 0.05 vs. 1-wk group; #P < 0.05 vs. 3-wk group; §P < 0.05 vs. before.
Baseline Hemodynamic Measurements
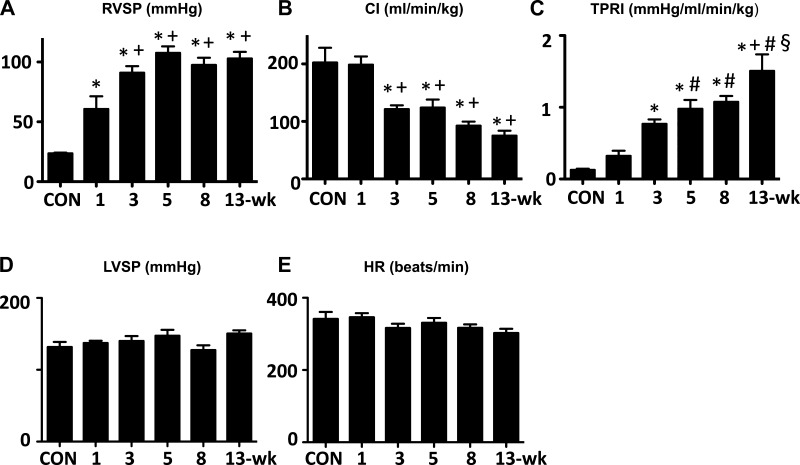
RVSP initially increased time dependently, and it appeared to reach its maximum (>100 mmHg) around 5 wk after Sugen5416 injection and stayed at about the same high level thereafter (Fig. 2A). At the 3- to 5-wk time point, cardiac index (CI) decreased to ∼50% of normal, and tended to further decrease at the 8- and 13-wk time points (Fig. 2B). Reflecting the increases in RVSP and the reductions in CI, estimated TPRI showed a progressive increase from 1 to 13 wk (Fig. 2C). No significant changes were observed in either LVSP (Fig. 2D) or HR (Fig. 2E) over time. There was no elevation in LV end-diastolic pressure at any time (ranging 0.2–3.0 mmHg in average).
Fig. 2.
Temporal changes in hemodynamic parameters in Sugen5416/hypoxia/normoxia-exposed rats at 1, 3, 5, 8, and 13 wk after the Sugen5416 injection. A: right ventricular systolic pressure (RVSP). B: cardiac index (CI). C: total pulmonary resistance index (TPRI). D: left ventricular systolic pressure (LVSP). E: heart rate (HR). Values are means ± SE of n = 6–10. *P < 0.05 vs. Con; +P < 0.05 vs. 1-wk group; #P < 0.05 vs. 3-wk group; §P < 0.05 vs. 5-wk group.
Acute Reversibility of Pulmonary Hypertension by Fasudil
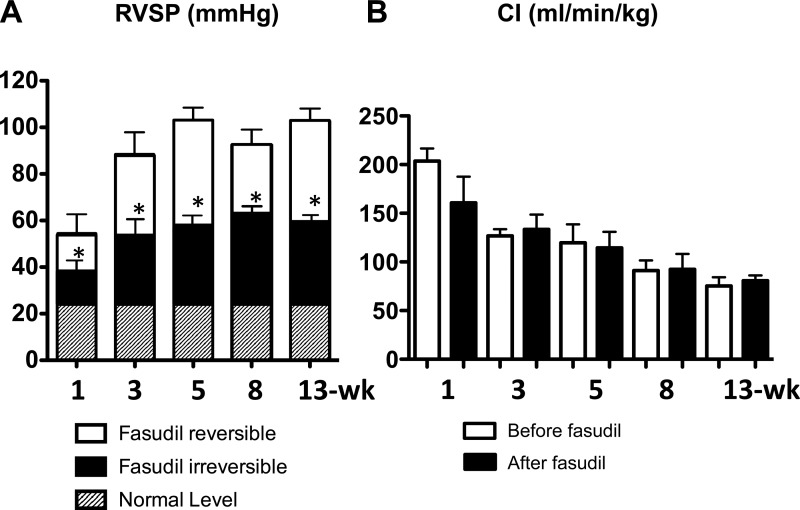
Consistent with our previous report (12), acute intravenous injection of fasudil immediately and markedly reduced RVSP (fasudil reversible component) at earlier (1-, 3-, and 5-wk) time points. Interestingly, fasudil was similarly effective in acutely reducing the high RVSP in later (8- and 13-wk) time points (Fig. 3A). CI was not changed acutely by fasudil (Fig. 3B) and calculated TPRI had the same trend as RVSP.
Fig. 3.
Acute effects of fasudil (10 mg/kg iv) on RVSP (A) and CI (B) at the 1-, 3-, 5-, 8-, and 13-wk time point Sugen5416/hypoxia/normoxia-exposed rats. A: white bar (fasudil-reversible component) indicates part of the elevated RVSP that was acutely reversed by fasudil. B: CI before (white) and after (black) fasudil. Values are means ± SE of n = 5–8. *P < 0.05 vs. before fasudil.
Pulmonary Arterial Occlusion
We assessed a total of 3,634 vessels in 23 left lobes from 6 groups of rats (n = 4 each, except for n = 3 in control group). Mean vessel numbers/lobe examined were 127, 116, 202, 191, 181, and 155, respectively, for control, 1-, 3-, 5-, 8-, and 13-wk animals. Although we carefully identified PAs based on their relationship with accompanying airways, PA numbers might have been underestimated in control and 1-wk animals, when there was little or no medial wall thickening, because of the difficulty in distinguishing PA from pulmonary vein in small nonmuscular vessels. Three ranges of vessel size were examined:
OD < 50 μm.
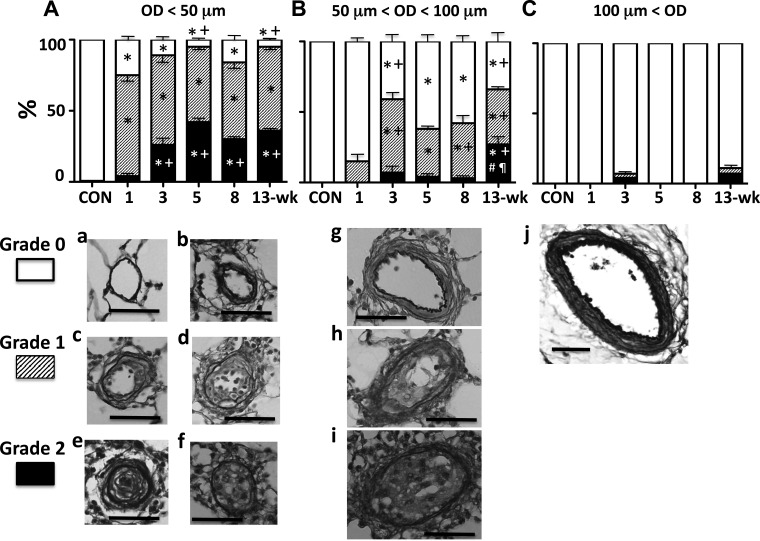
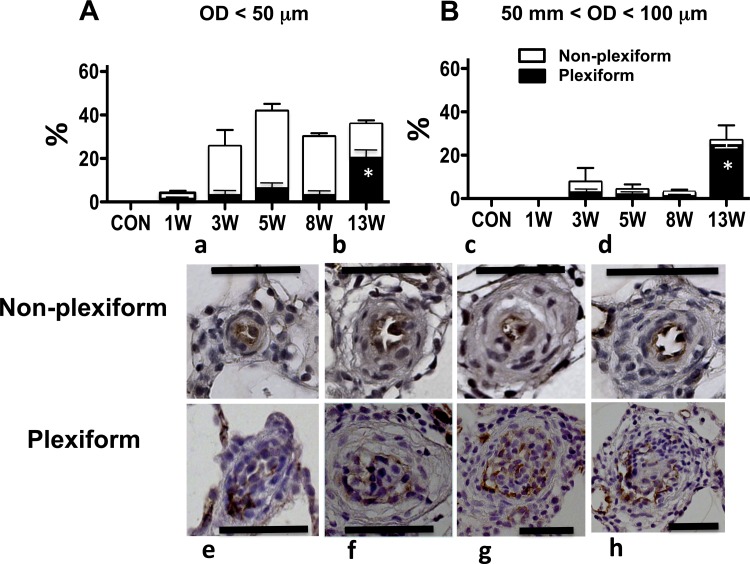
Many PAs in this size range (mostly nonmuscular in control rats) developed at least some degree of luminal occlusion caused by neointimal cell reaction (bulging)/proliferation as early as the 1-wk time point (Fig. 4A). The density (%) of severely occluded PAs (grade 2) increased over time initially (∼5-wk), which is in agreement with our previous observation (12). However, the density apparently peaked at the 5-wk time point and did not further increase in later (8- and 13-wk) time points. This pattern of progression was similar to that of RVSP. As shown in Fig. 5A, these severely occluded small PAs (grade 2) were predominantly with the nonplexiform type of neointima [Fig. 5, a–d until the very late stage (13-wk) when significantly more small PAs were occluded with the plexiform type lesions (Fig. 5, e–h)].
Fig. 4.
Top: percentage (mean) of grade 0 (no luminal occlusion; white), 1 (<50% occlusion; shaded), and 2 (>50% occlusion; black) pulmonary arteries of outer diameter (OD). A: <50 μm. B: between 50 and 100 μm. C: >100 μm at various time points. Values are means ± SE. *P < 0.05 vs. Con; +P < 0.05 vs. 1-wk group; #P < 0.05 vs. 3-wk group; ¶P < 0.05 vs. 8-wk group. Bottom: representative photomicrographs of Verhoeff-van Gieson-stained pulmonary arterial cross sections of corresponding OD. Grade 0: a, b, g, and j; grade 1: c, d, and h; grade 2: e, f, and i. Scale bars indicate 50 μm.
Fig. 5.
Top: percentage (mean) of grade 2 (>50% occlusion) pulmonary arteries of outer diameter (OD). A: <50 μm. B: between 50 and 100 μm at various time points. White and black bars indicate nonplexiform and plexiform types of occlusions, respectively. Bottom: representative photomicrographs of von Willebrand Factor-stained pulmonary arterial cross sections of nonplexiform (a, b, c, and d) and plexiform type (e, f, g, and h) of occlusions. Scale bars indicate 50 μm. *P < 0.05 vs. other time points. W, weeks.
50 < OD < 100 μm.
In this range of vessels (partially to fully muscular in control rats), there were PAs that had some degree of luminal occlusion (grade 1), but the density of occluded vessels was much less (up to 50% less) than that observed in the smaller PAs at any time point (Fig. 4B). Notably, there was no significant number of severely occluded PAs (grade 2) until the very late (13-wk) time point, when most of the occluded vessels were with the plexiform type of neointima (Fig. 5B).
OD > 100 μm.
This size range of vessels was mostly fully muscularized in controls (Fig. 4C). Very few occlusive changes, if any, were observed in these vessels at any time point of PAH.
Medial wall thickness.
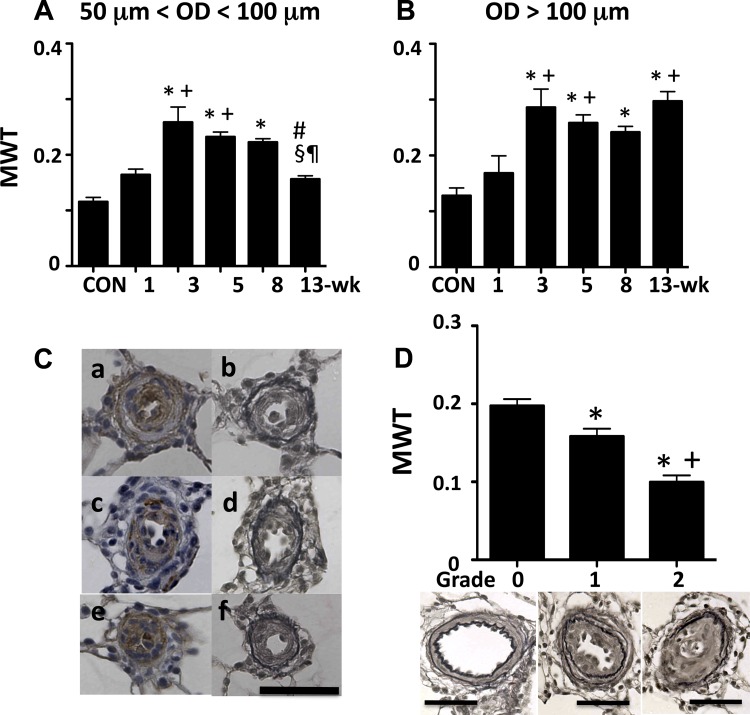
For 50 < OD < 100 μm (Fig. 6A), the medial wall thickness (MWT) increased significantly as early as at the 1-wk time point and further increased and peaked at the 3-wk time point. It stayed at the similarly high level thereafter until it decreased significantly (to a normal range) at the 13-wk time point.
Fig. 6.
A and B: medial wall thickness (MWT) at various time points of pulmonary arteries of OD between 50 and 100 μm (A) and >100 μm (B). Values are means ± SE. *P < 0.05 vs. CON; +P < 0.05 vs. 1-wk group; #P < 0.05 vs. 3-wk group; §P < 0.05 vs. 5-wk group; ¶P < 0.05 vs. 8-wk group. C: small pulmonary arteries from a 13-wk rat. a and b, c and d, and e and f are pairs of serial sections of the same artery stained with α-smooth muscle actin and Verhoeff-van Gieson, respectively. Scale bar indicates 50 μm. D: MWT at the 13-wk time point of pulmonary arteries of OD between 50 and 100 μm, and representative photomicrographs showing occlusion grade 0, 1, and 2 of pulmonary arteries (Verhoeff-van Gieson stained). Values are means ± SE. *P < 0.05 vs. grade 0 and +P < 0.05 vs. grade 1. Scale bars indicate 50 μm.
For 100 < OD < 200 μm (Fig. 6B), the pattern of change in MWT was very similar to that observed in smaller PAs, except for the lack of significant decline at the very late (13-wk) time point.
Neomuscularization.
We attempted to evaluate another possible contributing factor to the elevated RVSP/TPRI, i.e., neomuscularization of nonmuscular pulmonary arterioles (10), by staining lungs with SMA. However, because of the following findings, it appeared impossible to assess this factor by the SMA staining. We frequently found SMA-positive cells in the intima of larger as well as small (<50 μm) PAs in this model (Fig. 6C). In many severely occluded small PAs, there are SMA-positive cells in the intima and relatively weak SMA-positive cells in the thin media. In addition, at least in some cases, the SMA-positive intimal cells appeared to also be positive for endothelial markers (double positive for endothelial and smooth muscle cell markers). Based on these observations, it is speculated that the origin of the SMA-positive cells in the intima may not be smooth muscle cells but more likely endothelial cells (apoptosis-resistant endothelial cells may have undergone endothelial-mesenchymal transition) or circulating or resident progenitor cells. We therefore concluded that the SMA staining might not be suitable for the assessment of muscularization/medial thickness of PAs in this model as well as possibly in human PAH. We believe that it is very important to perform elastic fiber specific staining, such as VVG, to determine media and intima, and that the commonly used SMA staining may not be appropriate to identify smooth muscle cells and the media of severely remodeled pulmonary arteries in PAH.
Relationship Between Vascular Occlusion and MWT
Because there was a decrease in MWT and an increase in vascular occlusion rate in PAs of OD between 50 and 100 μm at the 13-wk time point, we examined their relationship. As shown in Fig. 6D, it appeared that the more severe intimal occlusion the PA developed, the less MWT it had.
DISCUSSION
Temporal Profile of Hemodynamic Parameters
Although there are virtually no data available until the diagnosis is made, it is generally thought that in human PAH there is a progressive increase in pulmonary arterial resistance/pressure from the time of onset to diagnosis. It is clinically well known that a majority of patients already have high levels of pulmonary arterial pressure at the time of diagnosis, and the pressure usually stays high and relatively stable until PAH progresses to very advanced end-stages. CO is usually below normal levels, which makes patients symptomatic, at the time of diagnosis, and it decreases gradually over time (years). Reflecting these parameters, pulmonary vascular resistance increases progressively during this period. The above description represents a generally accepted typical chronic progression/deterioration pattern of PAH (7). Our study demonstrated that the SU/Hx/Nx-exposed rat model of PAH showed a very similar chronic severe hemodynamic progression/deterioration pattern (see Fig. 2, A–C) to that of human PAH, in addition to having the “plexogenic arteriopathy” (1). These findings are of particular importance for a preclinical model of PAH, because conventional animal models of PAH fail to have this pattern as well as the occlusive arteriopathy. For instance, one of the most frequently used models, i.e., monocrotaline-injected rats, shows generally a rather acute progression leading to death at very early stages of PAH with MWT being the characteristic vascular remodeling (4, 21). Based on the comparison of hemodynamic progression profile of SU/Hx/Nx-exposed rats with human PAH, the 3∼5-wk time point, when RVSP peaks and CI begins to decline in the rat model, may correspond roughly to the time of diagnosis in humans. We, therefore, propose that an experimental chronic treatment should start from this time point or later in SU/Hx/Nx-exposed rats.
Relationship Between PA Pressure and RV Hypertrophy
It is well established that in conventional animal models of pulmonary hypertension, the RV/LV + S weight ratio, which reflects RV hypertrophy, correlates well with pulmonary arterial pressure (15). We have found in this study that the correlation stands in the SU/Hx/Nx PAH model until the very late stage, because there was a close similarity in the pattern of changes in RVSP and RV/LV + S, except for the 13-wk time point, where RV/LV + S decreased slightly while RVSP did not (Figs. 1A and 2A). This discrepancy may indicate the beginning of RV decompensation. In fact, CI tends to further decline at this time point.
Factors Contributing to the Increased RVSP/TPRI
Vasoconstriction.
We have previously shown that Rho kinase-mediated vasoconstriction is substantially involved in the elevated RVSP in the SU/Hx/Nx model of PAH at 2- and 5-wk time points (12). This study has extended the observation to even later time points when more severe and complicated pulmonary vascular remodeling, i.e., all features of plexogenic pulmonary arteriopathy, including occlusive concentric laminar and plexiform lesions, has developed (1). We found surprisingly that the vasodilator fasudil similarly and effectively reversed (by ∼50%) the elevated RVSP and TPRI at later (8- and 13-wk) time points. These results suggest that Rho kinase-mediated vasoconstriction, which was not fully reversed by conventional vasodilators such as inhaled nitric oxide and prostacyclin analog infusion in our previous study (12), plays a central role in the elevated vascular resistance of the severely remodeled pulmonary vasculature of this PAH model. Although an acute intravenous infusion of a low dose of fasudil significantly reduces pulmonary vascular resistance in PAH patients (6, 8, 9), the observed reductions have been rather moderate. This is likely because higher doses were not examined due to severe systemic hypotension. As we previously proposed (12), a pulmonary selective drug delivery is required to test the maximum pulmonary vasodilatory effects of Rho kinase inhibitors in human PAH.
Neointimal vascular luminal occlusion.
A majority (>70∼80%) of small (OD < 50 μm) PAs had some degree (grades 1 and 2) of occlusive lesions at all time points. The pattern of increase in density of severely (grade 2) obliterated vessels, which was caused mainly by nonplexiform type occlusion, was very similar to that of RVSP over time (Fig. 4A, black bars, vs. Fig. 2A). With consideration for the severity and number of affected vessels, these observations suggest that the severe nonplexiform type neointimal lesions in these small PAs in earlier time points may contribute significantly to the elevated RVSP. In contrast with the earlier time points, significant numbers of vessels were severely occluded with plexiform type neointima in the size range between 50 and 100 μm OD at the 13-wk time point. Because there was no further increase in either the fasudil-reversible component or the small PA occlusion density at this late time point, the formation of severe occlusive plexiform type neointimal lesions in these middle-sized PAs may be involved in the further increase in TPRI at this very late stage. Finally, there was very little, if any, neointimal lesion formation in larger PAs (OD > 100 μm). This is consistent with findings in human PAH, i.e., the occlusive lesions are found predominantly in small (pre- and intra-acinar) PAs (14).
MWT.
We found significant increases in MWT after the 3-wk time point in both sizes (50 μm < OD < 100 μm and OD > 100 μm) of PA examined. Although the increase in MWT is significant, its role in the elevated RVSP/TPRI is not obvious for the following reasons: 1) It is unclear whether the thickening is inward or outward. There is evidence that the latter is the case in conventional rat models (5, 22). If that is also true in this model, then the direct role of this factor in reducing vessel luminal area might be minimal. 2) Because the degree of the thickening in the fasudil-dilated PA is rather moderate (MWT increased from 0.11 to 0.26 and from 0.13 to 0.29 in medium and larger PA, respectively) as compared with the severe occlusion caused by neointimal lesions, the contribution of MWT to the reduction in luminal area might be much less than that of neointimal occlusion, even if the thickening is inward. In addition, we surprisingly observed that MWT decreased to a normal range in medium sized PA at the 13-wk time point. We asked if this decrease in MWT might be related to another particular finding in this size of PA at this time point, i.e., the emergence of grade 2 occlusive lesions. We, therefore, examined the relationship between MWT and occlusion grade and found that the more severe the intimal occlusion the less the MWT. This agrees with the “medial atrophy” concept that severe intimal fibrosis may produce secondary atrophy of the media (23), and this may explain at least partly why a recent study found only marginal increases in MWT in advanced stage PAH lung samples (20).
Other Factors
Vessel rarefaction.
Although our study was not specifically designed to assess this factor, we did not observe any obvious reductions in PA number in our histological/morphological analyses (see results). Additional studies are needed to define the role of vessel rarefaction in the elevated RVSP/TPRI.
Thromboemboli.
We also did not specifically look for thromboembolic lesions in this study. However, thromboemboli do not seem to be a major player in elevating RVSP/PVRI in this model, because few, if any, obvious thromboembolic-like lesions (a-/oligo-cellular occlusive lesion formation) were observed.
Perivascular Cell Accumulation
Consistent with findings in human PAH lungs (18, 20), there was marked cell accumulation around the remodeled PAs at all time points except for the 1-wk time point in the PAH rat lungs [unpublished (3)]. We observed that various cell types, including lymphocytes, macrophages, mast cells, and progenitor marker positive cells, were involved in this perivascular deposition, and the quantity as well as the quality of these cells appeared to change time dependently. Further detailed analyses are needed to identify the phenotypes and roles of the accumulated perivascular cells in the vascular remodeling.
Survival
The mortality rate of the undisturbed (resting) Sprague-Dawley PAH rats here in Mobile, AL is low; despite the fact that CI is severely impaired, only a few rats have died of apparent right heart failure out of ∼300 rats that were exposed to Sugen5416 and various durations of hypoxia (1–3 wk) and normoxia (2–10 wk). However, it has been reported that the mortality rate significantly increases after the PAH rats are exposed to treadmill exercise (4). Systematic exercise tolerance tests are needed in the future to determine the relationship between the impaired cardiac function and survival/mortality of this PAH model with and without treatment. Interestingly, we have recently observed that in contrast with Sprague-Dawley rats, Su/Hx/Nx-induced severe PAH in Fischer 344 rats is associated with early death (within 6 to 8 wk) due apparently to an acute cardiac decompensation (2). The severity of pulmonary hypertension is similar in Sprague-Dawley and Fischer 344 rats, and it is currently unknown what accounts for the difference in mortality between the two strains or for the survival benefit of genetic deletion of the TRPC4 channel in Fischer 344 rats.
Conclusion
This study demonstrated that SU/Hx/Nx rats exhibited a pattern of chronic progressive hemodynamic PAH deterioration that closely resembles that of human PAH. Our results also suggest that Rho kinase-mediated vasoconstriction plays a central role in the elevated RVSP/TPRI in this model even at very late stages when a full range of plexogenic arteriopathy has developed, and that nonplexiform type neointimal luminal occlusion of arterioles (OD < 50 μm) appears to contribute significantly to the early phase of PAH progression, and that plexiform type larger vessel occlusion (50 < OD < 100 μm) may play a role in the late deterioration in this rat model of PAH. Taken together, the SU/Hx/Nx-exposed PAH rat possesses major characteristics of human PAH, demonstrating that this is a more appropriate preclinical model of occlusive PAH than the conventional chronically hypoxic and monocrotaline-injected models. This study provides basic temporal hemodynamic and histological data of this PAH model, which identify therapeutic targets over time, for future mechanistic studies of PAH pathogenesis as well as evaluation of drug efficacy.
GRANTS
This work was supported by a grant from the National Heart, Lung, and Blood Institute (HL-106101) and by funds from the Department of Pharmacology and Center for Lung Biology, University of South Alabama.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.T. and M.O. conception and design of research; M.T., A.A., K.D.O., S.G., Y.M., K.O., K.A., and M.O. performed experiments; M.T., K.D.O., S.G., Y.M., K.O., K.A., and M.O. analyzed data; M.T., A.A., K.A., M.O., and I.F.M. interpreted results of experiments; M.T. and M.O. prepared figures; M.T. and M.O. drafted manuscript; M.T., A.A., K.D.O., S.G., Y.M., K.O., K.A., M.O., and I.F.M. approved final version of manuscript; M.O. and I.F.M. edited and revised manuscript.
REFERENCES
- 1.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 121: 2747–2754, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Alzoubi A, Almalouf P, Toba M, O'Neill K, Qian X, Francis M, Taylor MS, Alexeyev M, McMurtry IF, Oka M, Stevens T. TRPC4 inactivation confers a survival benefit in severe pulmonary arterial hypertension. Am J Physiol 183: 1779–1788, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzoubi A, Toba M, Abe K, Bauer N, Fagan FA, McMurtry I, Oka M. Dehydroepiandrosterone inhibits the infiltration of proliferative bone marrow-derived hematopoietic stem cells into the perivascular space of remodeled pulmonary arteries of SU5416/Normoxia/Hypoxia-exposed rats (Abstract). Am J Respir Crit Care Med 183: A3422, 2011 [Google Scholar]
- 4.Bogaard HJ, Natarajan R, Mizuno S, Abbate A, Chang PJ, Chau VQ, Hoke NN, Kraskauskas D, Kasper M, Salloum FN, Voelkel NF. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med 182: 652–660, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Cahill E, Rowan SC, Sands M, Banahan M, Ryan D, Howell K, McLoughlin P. The pathophysiological basis of chronic hypoxic pulmonary hypertension in the mouse: vasoconstrictor and structural mechanisms contribute equally. Exp Physiol 97: 796–806, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Fukumoto Y, Matoba T, Ito A, Tanaka H, Kishi T, Hayashidani S, Abe K, Takeshita A, Shimokawa H. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart 91: 391–392, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galie N, Manes A, Palzzini Negro L, Romanazzi S, Branzi A. Pharmacological impact on right ventricular remodelling in pulmonary arterial hypertension. Eur Heart J 9 (suppl H): H68–H74, 2007 [Google Scholar]
- 8.Ishikura K, Yamada N, Ito M, Ota S, Nakamura M, Isaka N, Nakano T. Beneficial acute effects of rho-kinase inhibitor in patients with pulmonary arterial hypertension. Circ J 70: 174–178, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Li F, Xia W, Yuan S, Sun R. Acute inhibition of Rho-kinase attenuates pulmonary hypertension in patients with congenital heart disease. Pediatr Cardiol 30: 363–366, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Meyrick B, Reid L. The effect of continued hypoxia on rat pulmonary arterial circulation. An ultrastructural study. Lab Invest 38: 188–200, 1978 [PubMed] [Google Scholar]
- 11.Nahrendorf M, Hu K, Fraccarollo D, Hiller KH, Haase A, Bauer WR, Ertl G. Time course of right ventricular remodeling in rats with experimental myocardial infarction. Am J Physiol Heart Circ Physiol 284: H241–H248, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Oka M, Karoor V, Homma N, Nagaoka T, Sakao E, Golembeski SM, Limbird J, Imamura M, Gebb SA, Fagan KA, McMurtry IF. Dehydroepiandrosterone upregulates soluble guanylate cyclase and inhibits hypoxic pulmonary hypertension. Cardiovasc Res 74: 377–387, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder RM. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol 43: 25S–32S, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Rabinovitch M, Gamble W, Nadas AS, Miettinen OS, Reid L. Rat pulmonary circulation after chronic hypoxia: hemodynamic and structural features. Am J Physiol Heart Circ Physiol 236: H818–H827, 1979 [DOI] [PubMed] [Google Scholar]
- 16.Ryan J, Bloch K, Archer SL. Rodent models of pulmonary hypertension: harmonisation with the world health organisation's categorisation of human PH. Int J Clin Pract Suppl 172: 15–34, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Saha DC, Saha AC, Malik G, Astiz ME, Rackow EC. Comparison of cardiovascular effects of tiletamine-zolazepam, pentobarbital, and ketamine-xylazine in male rats. J Am Assoc Lab Anim Sci 46: 74–80, 2007 [PubMed] [Google Scholar]
- 18.Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, Scheed A, Ritter C, Dahal BK, Vater A, Klussmann S, Ghofrani HA, Weissmann N, Klepetko W, Banat GA, Seeger W, Grimminger F, Schermuly RT. Immune/inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 897–908, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 54: S43–S54, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 261–272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297: L1013–L1032, 2009 [DOI] [PubMed] [Google Scholar]
- 22.van Suylen RJ, Smits JF, Daemen MJ. Pulmonary artery remodeling differs in hypoxia- and monocrotaline-induced pulmonary hypertension. Am J Respir Crit Care Med 157: 1423–1428, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Yamaki S, Wagenvoort CA. Plexogenic pulmonary arteriopathy: significance of medial thickness with respect to advanced pulmonary vascular lesions. Am J Pathol 105: 70–75, 1981 [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan JX, Rubin LJ. Pathogenesis of pulmonary arterial hypertension: the need for multiple hits. Circulation 111: 534–538, 2005 [DOI] [PubMed] [Google Scholar]