Abstract
Purinergic 2X (P2X) receptors on the endings of thin fiber afferents have been shown to play a role in evoking the exercise pressor reflex in cats. In this study, we attempted to extend this finding to decerebrated, unanesthetized rats whose femoral arteries were either freely perfused or were ligated 72 h before the start of the experiment. We first established that our dose of pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS; 10 mg/kg), a P2X receptor antagonist, attenuated the pressor response to α,β-methylene ATP (10 μg/kg), a P2X receptor agonist. We then compared the exercise pressor reflex before and after infusing PPADS into the arterial supply of the hindlimb muscles that were statically contracted. In rats with freely perfused femoral arteries, the peak pressor responses to contraction were not significantly attenuated by PPADS (before PPADS: 19 ± 2 mmHg, 13 min after PPADS: 17 ± 2 mmHg, and 25 min after PPADS: 17 ± 3 mmHg). Likewise, the cardioaccelerator and renal sympathetic nerve responses were not significantly attenuated. In contrast, we found that in rats whose femoral arteries were ligated PPADS significantly attenuated the peak pressor responses to contraction (before PPADS: 37 ± 5 mmHg, 13 min after PPADS: 27 ± 6 mmHg, and 25 min after PPADS: 25 ± 5 mmHg; P < 0.05). Heart rate was not significantly attenuated, but renal SNA was at certain time points over the 30-s contraction period. We conclude that P2X receptors play a substantial role in evoking the exercise pressor reflex in rats whose femoral arteries were ligated but play only a minimal role in evoking the reflex in rats whose femoral arteries were freely perfused.
Keywords: thin fiber muscle afferents; renal sympathetic activity; PPADS; α,β-methylene ATP; autonomic nervous system
the exercise pressor reflex arises from contracting skeletal muscles and functions to increase mean arterial pressure, heart rate, and peripheral vascular resistance (5, 28). The sensory arm of the reflex is comprised of thinly myelinated group III afferents, which are primarily mechanosensitive, and unmyelinated group IV afferents, which are primarily metabosensitive (17, 18, 28, 31). The distinction between mechanosensitive and metabosensitive is by no means total. For example, group IV afferents can be stimulated by mechanical distortion of their receptive fields, although the force applied is usually in the noxious range (18, 31). In addition, group III afferents can be stimulated by injection of putative muscle metabolites such as ATP and bradykinin; moreover, their responses to contraction, which mechanically distort their receptive fields, can be potentiated by muscle metabolites, such as bradykinin, cyclooxygenase metabolites of arachidonic acid, and ATP (11, 17, 30, 35, 36).
Several lines of evidence indicate that purinergic compounds, especially ATP, play a role in eliciting the exercise pressor reflex. Specifically, in both rats and dogs, the concentration of ATP in the muscle interstitial space is increased by static contraction (23, 32). The source of the ATP found in the interstitial space of contracting muscle is unclear, but most likely includes contracting myocytes, sympathetic postganglionic nerve endings, and red blood cells (7, 22). In addition, injection of ATP or its analogs into the arterial supply of skeletal muscle evokes reflex pressor, cardioaccelerator, and renal sympathetic nerve responses (9, 24). Moreover, injection of ATP or its analogs has been shown to stimulate both group III and IV muscle afferents in both cats and rats (11, 35). Finally, pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS)-induced blockade of purinergic 2 (P2) receptors on the endings of the group III and IV afferents has been shown to attenuate the pressor and cardioaccelerator responses to both injection of ATP analogs as well as to static contraction in decerebrated unanesthetized cats (10, 24). In contrast, blockade of purinergic 1 (P1) receptors had no effect on the reflex pressor and cardioaccelerator responses to either ATP injection or static contraction (10, 24).
P2X receptors have been identified on dorsal root ganglion (DRG) cells innervating hindlimb skeletal muscles of rats (25, 46). Moreover, the concentration of P2X receptors in these cells has been shown to be increased by ligating the ipsilateral femoral artery for 72 h (4, 46). In addition, the pressor response to femoral arterial injection of α,β-methylene ATP, a P2X receptor agonist, has been found to be greater in rats whose femoral artery was ligated for 72 h than in rats whose femoral artery was freely perfused (25). Likewise, the exercise pressor reflex has been found to be greater in rats whose femoral arteries were ligated 72 h before the experiment than in rats whose femoral arteries were either freely perfused or were ligated only 3 min before the contraction was initiated (41). When considered together, these findings suggest that P2X receptors play an important role in the generation of the exercise pressor reflex in rats whose femoral arteries are either freely perfused or ligated. We were prompted, therefore, to test the hypotheses that blockade of P2X receptors attenuated the exercise pressor reflex in both decerebrated unanesthetized rats whose arterial supply to their contracting hindlimb muscles was intact as well as in decerebrated unanesthetized rats whose arterial supply to their contracting muscles was ligated for 72 h before the start of the experiment.
METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Hershey Medical Center of Pennsylvania State University. Adult male Sprague-Dawley rats (n = 47; average weight was 454 ± 8 g) were used in these experiments. The rats were housed in a temperature-controlled room (24 ± 1°C) with a 12:12-h light-dark cycle and fed a standard diet and tap water ad libitum. In 24 rats, we ligated the left femoral artery 72 h before the experiment. Briefly, rats were anesthetized with isoflurane (2–3%) in 100% oxygen. Under sterile procedure, the left femoral artery was surgically exposed and ligated with suture (5–0, silk) just distal to the inguinal ligament. The incision was closed with surgical staples, and the rat was monitored for at least an hour to ensure that normal awareness and behavior had returned before it was returned to animal facility. Rats were then allowed to recover for 72 h. This technique has been shown to reduce blood flow capacity to about 10–20% of its exercise-induced maximum, while having little effect on resting blood flow (33, 47).
Surgical preparation.
On the day of the experiment, rats were anesthetized with isoflurane gas (2–3%) in oxygen. The trachea was cannulated, and the lungs were mechanically ventilated with the gas anesthetic until the decerebration was performed. Both carotid arteries and a jugular vein were cannulated (PE-50) to measure arterial blood pressure and to administer drugs and fluids, respectively. Although both carotid arteries were cannulated, the aortic baroreceptors were undisturbed and still fully functional. Arterial blood gases and pH were measured using an automated blood gas analyzer (ABL 8, Radiometer). Pco2 and arterial pH were maintained within normal ranges by adjusting ventilation and oxygen or through an intravenous administration of sodium bicarbonate (8.5%). Body temperature was maintained between 36.5 and 38.0°C by an isothermal heating pad and lamp. Arterial blood pressure was measured by attaching one carotid cannula to a Statham P23XL strain gauge. Heart rate was calculated beat to beat by a Gould Biotach. The left calcaneal bone was sectioned and was then attached to a force transducer (FT-10, Grass) to measure tension developed by the statically contracting triceps surae muscles.
In all rats, the tip of one of the carotid arterial catheters was advanced so that it was situated in the abdominal aorta just before it bifurcated into the common iliac arteries. The location of the tip was confirmed post mortem. In all rats, including those whose femoral arteries were ligated and those whose femoral arteries were patent, we placed a reversible snare around the right common iliac artery and vein. When tightened, the snare directed the injectate into the circulation of the left hindlimb.
A laminectomy was performed to expose the spinal cord and the lower lumbar roots (L2-L6). The rats were then secured in a Kopf spinal frame by clamps placed on the rostral lumbar vertebrae and the pelvis. Using the back skin, we formed a pool, which was filled with warm (37.0°C) mineral oil. The dura was then cut and reflected so that L4 and L5 ventral roots, which innervate the muscles of the hindlimb, could be identified and sectioned.
A precollicular decerebration was performed, and all neural tissue rostral to the section was removed (37, 41, 42). Bleeding was controlled, and the cranial vault was filled with gauze. Immediately after the decerebration, gas anesthesia was discontinued. The rats were allowed to stabilize for at least an hour before any experimental protocol was started.
Using a retroperitoneal approach, we exposed bundles from the left renal sympathetic nerve. The bundles were then glued (WPI Kwik-sil) onto a pair of thin stainless steel electrodes, which in turn were connected to a high impedance probe and then to an amplifier (Grass P511). Multiunit signals from the renal sympathetic nerve were filtered between 100 and 1 kHz and were displayed on a storage oscilloscope. At the end of each experiment, hexamethonium (20 mg/kg) was injected intravenously to abolish activity, thereby demonstrating that the activity was postganglionic in nature.
Experimental protocols.
Our first task was to establish that the dose of PPADS (10 mg/kg; 200 μl) used was effective in blocking P2X receptors. To accomplish this task, we measured the pressor responses to abdominal arterial injection of α,β-methylene ATP (10 μg/kg; 100 μl) before and after PPADS. Each injection of α,β-methylene ATP was performed as a bolus and required ∼2 s to complete. PPADS was slowly infused (20 μl/min) over a 10-min period into a carotid arterial catheter whose tip was placed in the abdominal aorta near its bifurcation into the external iliac arteries. α,β-Methylene ATP was first injected before PPADS and then injected again at 13 min and at 25 min after the start of PPADS infusion. This protocol was performed in seven rats whose femoral arteries were freely perfused as well as in six rats whose femoral arteries were ligated. In a second series of experiments, we followed the same protocol described above except that sterile water, the vehicle for PPADS, was slowly infused instead. During the infusion of PPADS and sterile water as well as during the injection of α,β-methylene ATP, we tightened the snare placed around the right external iliac artery and vein in an attempt to direct the infusate or injectate into the arterial circulation of the left hindlimb. A CMA 402 microdialysis pump was used to perform the infusions. The snare placed around the right external iliac artery and vein was released immediately after the completion of the infusion. Injection of α,β-methylene ATP into the abdominal aorta stimulated P2X3 receptors on all hindlimb sensory nerves, including those innervating skin, muscle, joint, and bone. Our purpose of injecting α,β-methylene ATP was to challenge the blockade of P2X receptors by PPADS to demonstrate the effectiveness of PPADS as an antagonist; our purpose was not to selectively stimulate thin fiber muscle afferents.
Once we had established that the dose of PPADS was effective in blocking the pressor response to α,β-methylene ATP, we examined its effect on the exercise pressor reflex in a separate group of rats. The left hindlimb muscles were statically contracted for 30 s by electrically stimulating (40 Hz; 0.1 ms) the cut peripheral ends of the L4 and L5 ventral roots. After waiting 10 min, we then slowly infused PPADS (10 mg/kg) into the carotid arterial catheter whose tip was placed in the abdominal aorta near its bifurcation. The infusion period was 10 min, identical to that used by Wang et al. (44), and it was followed by a 3-min interval after which the left hindlimb muscles were contracted again. This contraction is referred to as 13 min after PPADS. Subsequently, we contracted the hindlimb muscles at 25 min after the start of the infusion, and we referred to this contraction as 25 min after PPADS. Twenty-five minutes was chosen arbitrarily to add a second time point to shed some light on the time course of the antagonistic effect of PPADS on P2 receptors. All infusions were done with a CMA microdialysis pump. In addition, the snare placed around the right external iliac artery and vein was tightened during each infusion and was released 5 min after its completion.
Data analysis.
In all experiments, baseline as well as reflex changes in mean arterial pressure, heart rate, renal sympathetic nerve activity (RSNA), and developed tension were recorded continuously with a Spike 2 data acquisition system (CED, Cambridge) and stored on a computer hard drive (Dell). The data were analyzed in two ways. In the first, the peak pressor and cardioaccelerator responses to static contraction were compared before and after PPADS. In addition, renal sympathetic nerve activity was integrated and then summed for the entire 30-s contraction period and expressed as a percentage increase over the immediate 30-s period preceding contraction. In the second method, the time courses of the pressor and percent change in the integrated renal nerve responses were analyzed. Specifically, we plotted the pressor and integrated renal nerve responses for each second of the 30-s static contraction period and then compared them before and after PPADS. Mean arterial pressure is expressed in millimeters mercury and heart rate is in beats per minute. The tension-time index was calculated by integrating the area between the tension trace and the baseline level and is expressed in kilogram seconds.
All values are expressed as means ± SE. Statistical comparisons were performed with either a one- or two-way repeated-measures ANOVA. If the overall F value was significant, post hoc tests were performed with the Holm-Sidak's tests between individual means. The criterion for statistical significance was set as P < 0.05.
RESULTS
Freely perfused.
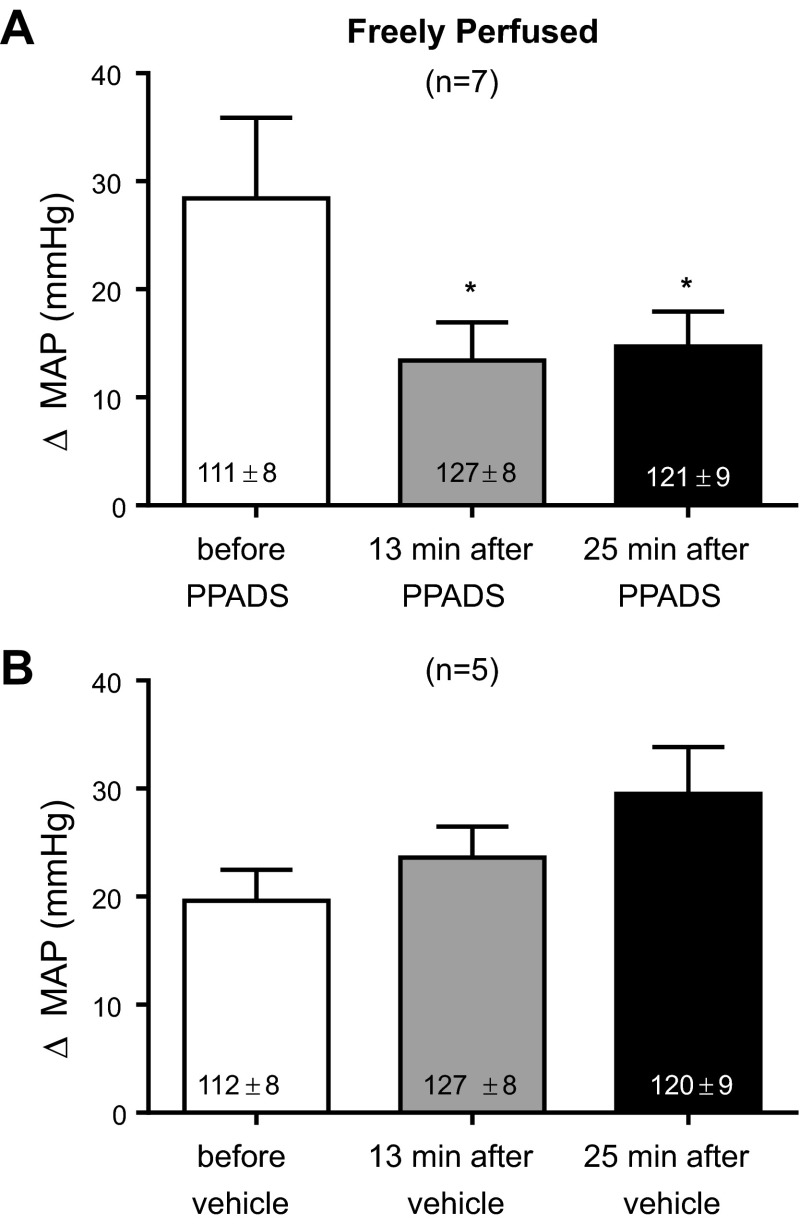
We first established that the dose of PPADS (10 mg/kg) used in our experiments blocked the pressor response to α,β-methylene ATP (10 μg/kg). Both substances were given through the carotid arterial catheter whose tip was placed in the abdominal aorta near its bifurcation. In eight rats whose left femoral arteries were freely perfused, we found that PPADS significantly attenuated the pressor response to α,β-methylene ATP by more than half at 13 and 25 min after the start of the infusion of the purinergic receptor antagonist (P < 0.05; Fig. 1). In addition, we found that injection of sterile water, which was the vehicle for PPADS, had no effect on the pressor response to α,β-methylene ATP (Fig. 1). The cardioaccelerator responses to α,β-methylene ATP were small, averaging no more than four beats per minute, and therefore were not analyzed.
Fig. 1.
Efficacy of the P2X receptor blockade in freely perfused rats. Pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS; 10 mg/kg; A) significantly attenuated the pressor response to α,β-methylene ATP (10 μg/kg), whereas sterile water (B), the vehicle, had no effect. MAP, mean arterial pressure. *P < 0.05, significantly smaller pressor response from the pressor response before PPADS.
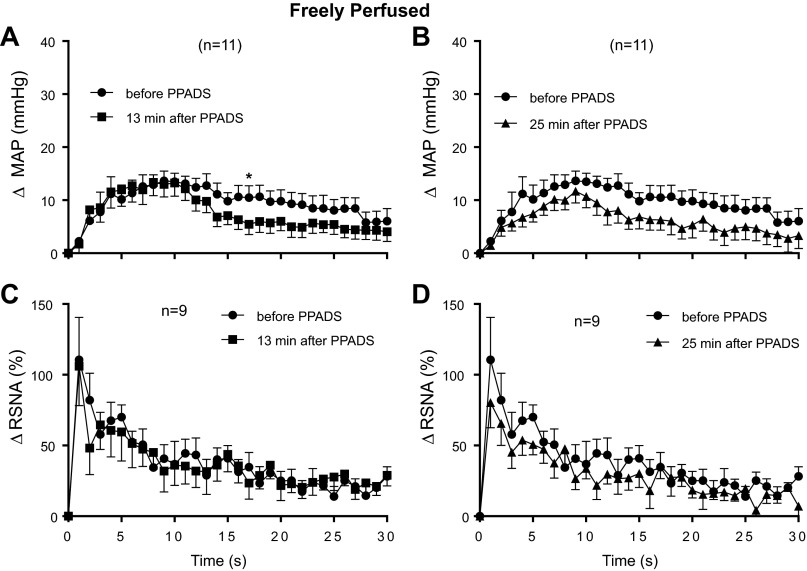
We next determined the effect of PPADS (10 mg/kg) on the exercise pressor reflex in 11 rats whose hindlimbs were freely perfused. None of the 11 rats were used in the experiments in which we injected α,β-methylene ATP into the abdominal aorta. Using the first method of analysis, we found that PPADS had no effect on the peak pressor and cardioaccelerator responses to static contraction of the hindlimb muscles in these 11 rats; this was the case at both 13 and 25 min after the start of the PPADS infusion, time points which were identical to those used to establish the efficacy of the P2 receptor blockade (Fig. 2). In addition, PPADS had no effect on RSNA when it was summed over the 30-s contraction period (Fig. 2). This too was the case at both 13 and 25 min after the start of the infusion. When viewed on individual basis, PPADS at 13 min attenuated the peak pressor component of the exercise pressor reflex by at least 5 mmHg in 5 of the 11 rats tested; likewise PPADS at 25 min attenuated the peak pressor component of the reflex by at least 5 mmHg in 4 of the 11 rats tested.
Fig. 2.
Peak pressor (A) and cardioaccelerator (B) responses to static contraction in freely perfused rats. C: increases in integrated renal sympathetic nerve activity (RSNA) averaged over the 30-s contraction period before and after infusion of PPADS (10 mg/kg). D: tension-time indexes (TTIs) after PPADS were not significantly different from those before PPADS. PPADS had no significant effect (P > 0.05) on the peak pressor, cardioaccelerator and renal nerve responses to static contraction in freely perfused rats. Likewise, PPADS had no effect on baseline mean arterial pressure or heart rate, the levels of which are shown inside the bars. Each of the increases for the pressor, cardioaccelerator, and integrated renal sympathetic nerve responses to contraction were significantly greater than their corresponding baseline values (P < 0.05).
Using the second method of analysis, we found that the main effect for PPADS 13 min after the start of its infusion had no significant effect (P = 0.39) on the time course of the pressor response to static contraction in “freely perfused rats” (Fig. 3A). Nevertheless, the overall interaction between PPADS and the contraction period of 30 s for the pressor response 13 min after PPADS infusion was significant (P = 0. 003). Post hoc tests, however, revealed only one significant point in time, namely at 17 s (Fig. 3A). Neither the main effect (P = 0.82) nor the overall interaction (P = 0.78) was significant for the time course of the RSNA response to contraction 13 min after the start of PPADS infusion (Fig. 3B).
Fig. 3.
Time courses of the average changes in pressor and renal sympathetic nerve responses to static contraction in freely perfused rats before and 13 min after PPADS infusion (A and B) as well as those before and 25 min after PPADS infusion (C and D). *P < 0.05, significant post hoc difference at 17 s between pressor responses before PPADS and 13 min after PPADS.
When analyzed as a main effect, PPADS, 25 min after the start of infusion, had no significant effect on the time course of either the pressor (P = 0.15) or RSNA responses to static contraction (P = 0.14) in freely perfused rats. Likewise, when analyzed for the interaction, PPADS 25 min after the start of infusion had no significant effect on the time course of either the pressor (P = 0.66) or RSNA (P = 0.90) responses to contraction in these rats (Fig. 3, C and D).
Ligated.
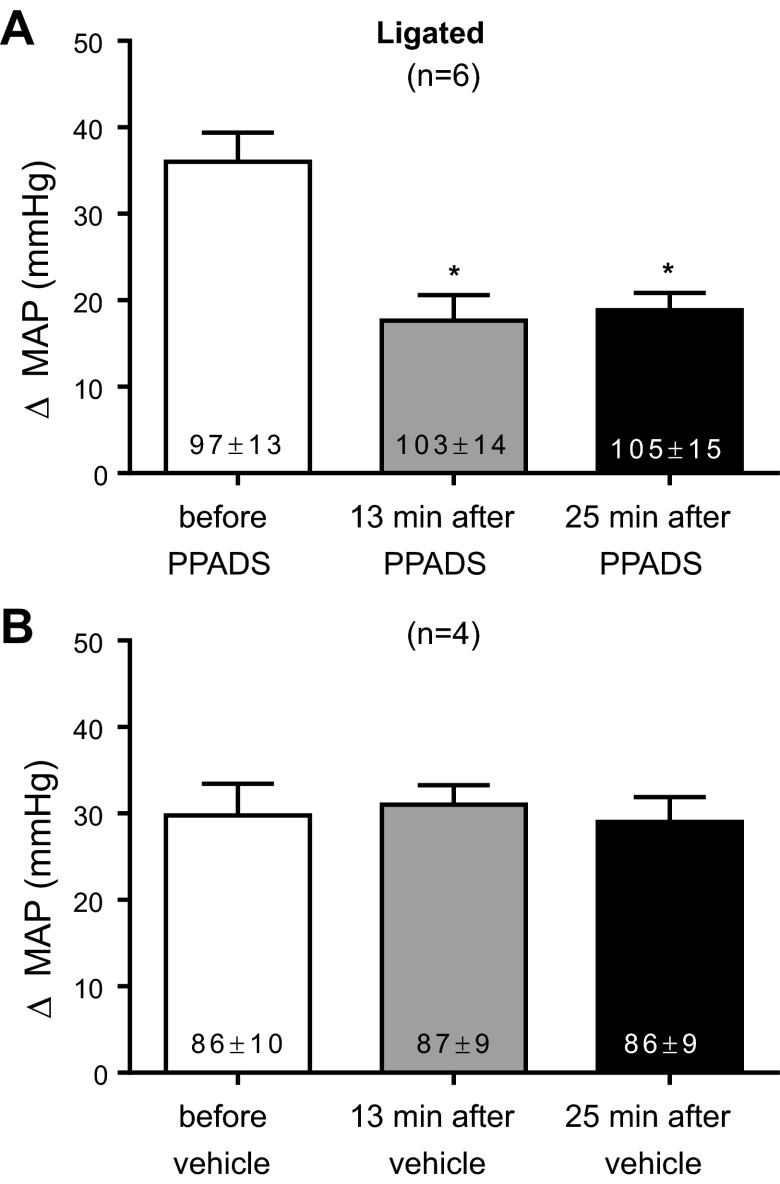
In six rats whose femoral arteries were ligated for 72 h before the start of the experiments, we established that the dose of PPADS (10 mg/kg) used in our experiments attenuated the pressor responses to α,β-methylene ATP (10 μg/kg). Both substances were given through the carotid arterial catheter whose tip was placed in the abdominal aorta near its bifurcation. PPADS significantly attenuated the pressor response to α,β-methylene ATP by more than half at 13 and 25 min after infusion of the purinergic receptor antagonist (P < 0.05; Fig. 4). The vehicle for PPADS, namely sterile water, had no effect on the pressor response to α,β-methylene ATP (Fig. 4). The cardioaccelerator responses to α,β-methylene ATP were small averaging no more than four beats per minute and therefore were not analyzed.
Fig. 4.
Efficacy of the P2X receptor blockade in ligated rats. PPADS (A; 10 mg/kg) significantly attenuated the pressor response to α,β-methylene ATP (10 μg/kg), whereas sterile water (B), the vehicle, had no effect. *P < 0.05, significantly smaller pressor response from the pressor response before PPADS.
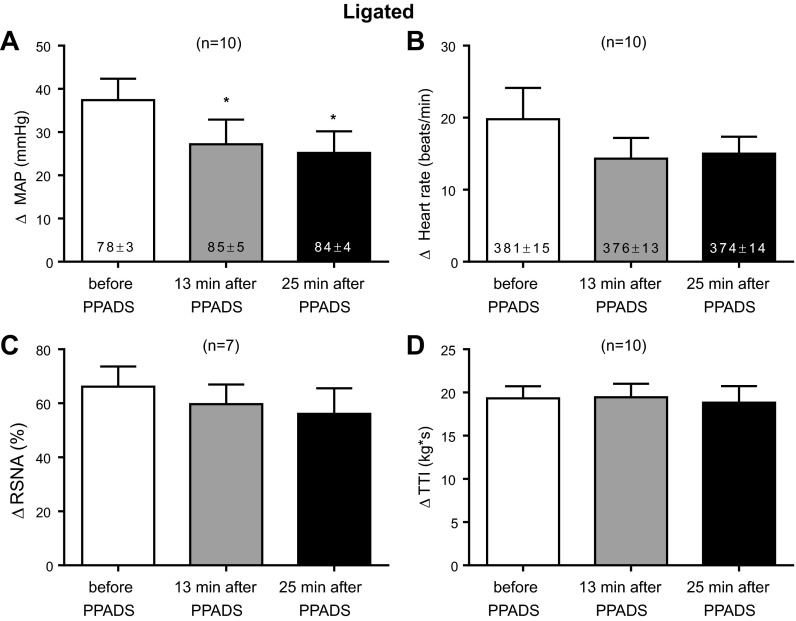
We then determined the effect of PPADS (10 mg/kg) on the exercise pressor reflex in 10 rats whose femoral arteries were ligated 72 h before the start of the experiment. None of the 10 rats were used in the experiments in which we injected α,β-methylene ATP into the abdominal aorta. Using the first method of analysis, we found that PPADS significantly attenuated (P < 0.05) the peak pressor responses to static contraction of the hindlimb muscles. This was the case both at 13 and 25 min after PPADS infusion, time points that were identical to those used to establish the efficacy of the P2 receptor blockade (Fig. 5). PPADS had no significant effect on the peak cardioaccelerator response or the renal sympathetic nerve response to static contraction when it was summed over the 30-s contraction period. When viewed on individual basis, PPADS at 13 min attenuated the peak pressor component of the exercise pressor reflex by at least 5 mmHg in 7 of the 10 ligated rats tested; likewise PPADS at 25 min attenuated the peak pressor component of the reflex by at least 5 mmHg in 8 of the 10 ligated rats tested.
Fig. 5.
Peak pressor (A) and cardioaccelerator (B) responses to static contraction in ligated rats. C: increases in integrated RSNA averaged over the 30 s contraction period before and after infusion of PPADS (10 mg/kg). D: TTIs after PPADS were not significantly different from those before PPADS. PPADS significantly decreased (P < 0.05) the peak pressor, but not the cardioaccelerator and renal nerve responses, to static contraction in ligated rats. PPADS had no effect on baseline mean arterial pressure or heart rate, the levels of which are shown inside the bars. *P < 0.05, significantly smaller pressor response from the pressor response before PPADS. Each of the increases for the pressor, cardioaccelerator, and integrated renal sympathetic nerve responses to contraction were significantly greater than their corresponding baseline values (P < 0.05).
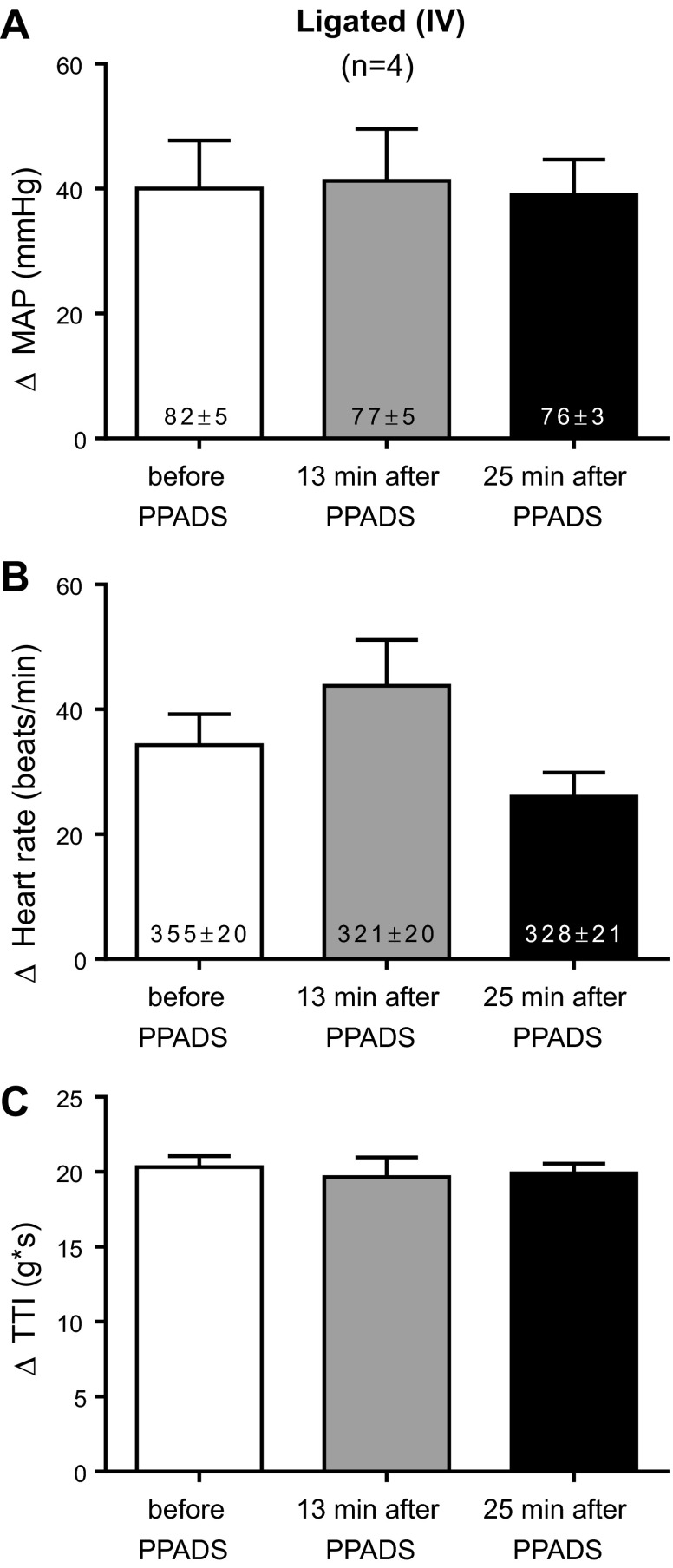
The possibility existed that PPADS, infused into the abdominal aorta over a 10-min period, circulated to the spinal cord or brainstem to exert its attenuating effect on the exercise pressor reflex. To control for this possibility, we infused PPADS (10 mg/kg) into the vena cava in four rats whose femoral arteries were ligated. We found that intravenous infusion of PPADS had no effect on the exercise pressor reflex (Fig. 6).
Fig. 6.
Peak pressor (A) and cardioaccelerator (B) responses to static contraction in ligated rats before and after intravenous infusion of PPADS (10 mg/kg). C: TTIs after PPADS were not significantly different from those before PPADS. PPADS had no effect on baseline mean arterial pressure or heart rate, which are shown inside the bars. Each of the increases for the pressor, cardioaccelerator and integrated renal sympathetic nerve responses to contraction were significantly greater than their corresponding baseline values (P < 0.05).
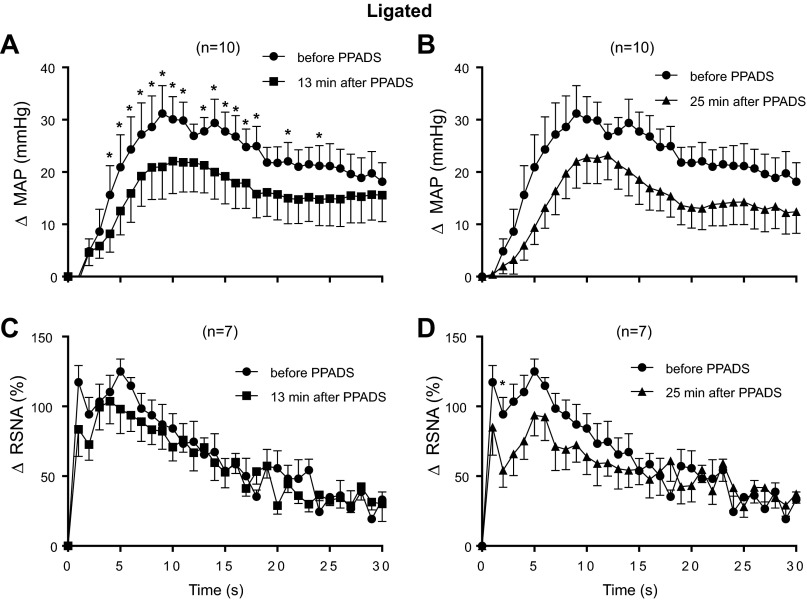
Using the second method of analysis and when analyzed as a main effect, we found that PPADS, 13 min after the start of its infusion, significantly attenuated the time course of the pressor response to static contraction over the 30-s contraction period (P = 0.02; Fig. 7A). Likewise, the overall interaction was significant (P = 0.04). Post hoc analysis of the interaction revealed that PPADS had an attenuating effect on the pressor response during 16 of the 30 s of static contraction (Fig. 7A). We also found that neither the main effect (P = 0.15) for PPADS nor its interaction (P = 0.60) over time was significant for RSNA response to contraction 13 min after the start of infusion of the P2X receptor antagonist (Fig. 7C).
Fig. 7.
Time courses of the average changes in pressor and renal sympathetic nerve responses to static contraction in ligated rats before and 13 min after PPADS infusion (A and B) as well as those before and 25 min after PPADS infusion (C and D). *P < 0.05, significant post hoc differences between pressor responses before PPADS and 13 min after PPADS.
When analyzed as a main effect, PPADS, 25 min after the start of infusion, significantly attenuated the time course of the pressor response to static contraction in the “ligated rats” (P = 0.01; Fig. 7B). The overall interaction between PPADS and the 30-s contraction period for the pressor response was not significant (P = 0.38; Fig. 7B). The main effect for the RSNA response to static contraction in the “ligated rats” was not significant (P = 0.45), but the interaction between PPADS and the 30-s contraction period was (P < 0.004; Fig. 7D). Post hoc analysis of the overall interaction revealed only one significant time point, namely at 2 s (Fig. 7D).
Difference in exercise pressor reflex between freely perfused and ligated rats.
Before infusing PPADS, a P2 receptor antagonist, we found that the pressor, cardioaccelerator, and renal sympathetic nerve responses to static contraction of the left hindlimb muscles were significantly greater in rats with ligated femoral arteries (n = 10) than in rats with patent (i.e., freely perfused) femoral arteries (n = 11; Figs. 2, 3, 5, and 6).
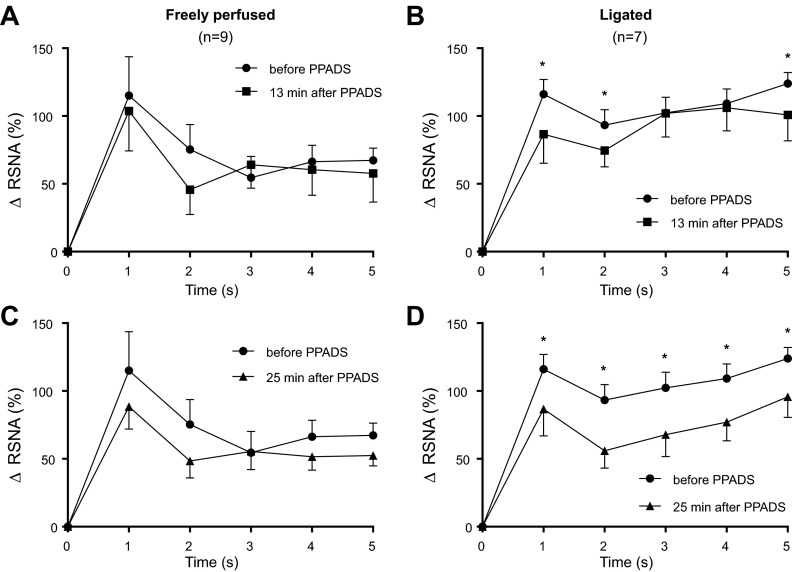
An index of mechanoreceptor activity.
PPADS had a significant effect on RSNA activity during the first 5 s of the contraction period in the rats with ligated femoral arteries. In contrast, the antagonist had no significant effect on RSNA during the first 5 s of the contraction period in the rats with freely perfused arteries (Fig. 8).
Fig. 8.
Increase in integrated RSNA during the first five s of static contraction in freely perfused rats (A and C) and in ligated rats (B and D) before and 13 min after PPADS infusion (A and B), as well as before and 25 min after PPADS infusion (C and D). *P < 0.05, significant decrease in activity after PPADS at its corresponding time point.
DISCUSSION
There are two types of purinergic receptors. The first, termed P1, is stimulated by adenosine and plays no role in eliciting the exercise pressor reflex in either cats (10, 27) or humans (26). The second, termed P2, is stimulated by ATP, and its blockade by PPADS in cats (10, 19, 20) and by pyridoxine in humans (6) attenuated the exercise pressor reflex. P2 receptors have been further classified into X and Y, with the former being ligand gated and the latter being G-protein gated (3). Chemical stimulation of somatic afferent P2X receptors in vivo evokes a reflex pressor response, whereas chemical stimulation of P2Y receptors does not (14). Consequently, P2X receptors are believed to be responsible for evoking the component of the exercise pressor reflex attributable to increases in the concentration of ATP in the interstitium of exercising muscle (9, 10, 14).
We found that PPADS-induced blockade of P2X receptors had minimal effect on the exercise pressor reflex in decerebrated rats with patent femoral arteries. In contrast, PPADS substantially reduced the reflex in decerebrated rats whose femoral arteries were ligated 72 h before the start of the experiment. The attenuating effect of PPADS on the exercise pressor reflex in the ligated rats was not caused by its circulation to either the spinal cord or brainstem because intravenous injection of the P2X receptor antagonist had no effect on the pressor responses to static contraction of the hindlimb muscles. The most likely explanation for our findings is that PPADS attenuated the exercise pressor reflex by blocking P2X receptors on the endings of group III and IV muscle afferents in rats whose femoral arteries were ligated.
Previously we reported that PPADS attenuated the exercise pressor reflex in decerebrated cats with freely perfused hindlimbs, whereas in the present study we report that the antagonist had little if any effect on the reflex in decerebrated rats with freely perfused hindlimbs (10, 20). One explanation for this difference may involve the number of purinergic receptors found on thin fiber afferents innervating the hindlimb muscles in rats and cats. Immunocytochemical methods have shown that there are few P2X3 receptors on the DRG cells innervating the hindlimb muscles of rats (2). Unfortunately, the number of P2X3 receptors on DRG cells innervating the hindlimb muscles of cats is not known and, as a consequence, our explanation must be considered to be speculation.
Blockade of P2X receptors with PPADS in our experiments was much more effective in attenuating the exercise pressor reflex in rats with ligated femoral arteries than it was in attenuating the reflex in rats with freely perfused arteries. This may have been caused by an occlusion-induced increase in the number of P2X receptors on the thin fiber muscle afferents evoking the reflex. For example, using immunocytochemistry, Xing et al. (46) found that occluding the femoral artery for 24 h increased the number of P2X3 receptors in DRG cells with unmyelinated fibers innervating the gastrocnemius muscles of rats. In patch-clamp studies, the magnitude of the current elicited by α,β-methylene ATP from DRG cells was greater in that taken from rats with occluded arteries than from that taken from rats with freely perfused arteries (46).
Group III mechanoreceptors contribute to the afferent arm of the exercise pressor reflex (12, 15, 16, 38). One can determine if a muscle metabolite, such as ATP, sensitized group III mechanoreceptors by measuring the muscle mechanoreflex (40), which is evoked by stretching the calcaneal tendon, before and after giving a purinergic antagonist, such as PPADS. However, calcaneal tendon stretch activates group III mechanoreceptors that are not activated by static contraction (13, 44). Consequently, the usefulness of tendon stretch as a maneuver to stimulate the same group III mechanoreceptors that are stimulated by static contraction is limited. A superior method of determining if ATP sensitized the group III mechanoreceptors participating in the exercise pressor reflex involves the recording of the renal sympathetic nerve component of the reflex before and after giving PPADS. Static contraction increases RSNA within 200 ms of its onset (43), and although speculative we think it is reasonable to attribute any renal sympathetic response to contraction that occurs within the first 2 s of contraction to the stimulation of group III mechanoreceptors (19). The first 2 s of contraction seems to be too short for ischemic metabolites, such as lactic acid and prostaglandins, to be either produced by the working muscles or, if they are produced, for their concentration to be high enough to stimulate group IV metaboreceptors, which require at least 5 and sometimes 30 s to respond to this stimulus (17, 18, 44). In contrast, ATP is released almost instantaneously at the onset of exercise with one possible source being red blood cells, which are mechanically distorted by the contractile process (39). The immediate release of ATP could contribute to the immediate sensitization of mechanoreceptors.
Our findings concerning the effects of PPADS on the first 2 s of the renal sympathetic nerve responses to static contraction in both rats with ligated femoral arteries and in rats with freely perfused femoral arteries can be interpreted with the above rationale in mind. Thus our finding that PPADS did not attenuate the renal sympathetic nerve responses to the first 2 s of static contraction in “freely perfused rats” is consistent with the possibility that the number of P2X receptors on the endings of group III mechanoreceptors was not sufficient to allow these thinly myelinated afferents to be sensitized by ATP released into the muscle interstitium during static contraction. In contrast, our finding that PPADS attenuated the renal sympathetic nerve responses to the first 2 s of contraction in “ligated” rats is consistent with the possibility that 72 h of femoral artery occlusion increased the number of P2X receptors on the endings of group III mechanoreceptors to a level that was sufficient to allow them to be sensitized by ATP during contraction. With regard to our latter finding, ligation of a femoral artery for only 24 h has been shown to increase the number of P2X3 receptors on the DRG cells that innervate the hindlimbs of rats (25). Moreover, ligation potentiated the pressor and renal sympathetic nerve responses to femoral arterial injection of α,β-methylene ATP, a P2X3 receptor antagonist (25).
Our finding that PPADS had no effect on the first 15 s of the static contraction period might be viewed as surprising in light of the recent report by Wang et al. (44) that PPADS significantly attenuated the responses of group III mechanoreceptors to static contraction in “sham” rats whose hindlimbs were freely perfused. Our method of infusing PPADS was identical to that used by Wang et al. (44) and therefore cannot serve as an explanation for the lack of consistency between our findings and those of Wang et al. One explanation may be that the PPADS-induced attenuation of group III afferent responses to static contraction reported by Wang et al. (44) was not sufficient to have an effect on the exercise pressor reflex.
The mechanism responsible for ligation-induced augmentation of the exercise pressor reflex remains to be determined. Nevertheless, there is evidence suggesting that growth factors may play an important role. For example, infusion of nerve growth factor (NGF) for 72 h into the femoral triangle of rats with freely perfused femoral arteries increased both the number of P2X3 positive cells as well as the expression of P2X3 receptor protein in the L4 and L5 DRG (25). Moreover, occlusion of the femoral artery for 48 h increased NGF levels in the L4–6 DRG (45). Furthermore, intrathecal infusion of both NGF as well as glial cell-derived neurotrophic factor (GDNF) increased the number of P2X3 positive cells in the lumbar dorsal ganglia of rats (34). Last, intrathecal infusion of GDNF completely reversed the decrease in P2X3 receptor protein expression induced by dorsal root section in primary afferent fibers synapsing in the L4–6 dorsal horns of rats (2). Although these lines of evidence are suggestive of growth factors playing a role in the femoral arterial ligation-induced exaggeration of the exercise pressor reflex in our experiments, it remains to be shown that blockade of NGF or GDNF receptors prevents this exaggeration.
Interpretation of our findings should be tempered with three limitations in mind. First, PPADS displays in vitro some antagonistic activity towards the P2Y receptor (21), a finding that has minimal impact on our conclusions because the P2Y receptor plays little role in evoking the exercise pressor reflex (14). Second, PPADS blocks all P2X receptors, and therefore our conclusions should not be limited to the P2X3 receptor, although this receptor appears to be prevalent on thin fiber somatic afferents (8), and its specific blockade on the peripheral endings of thin fiber afferents in cats has already been shown to attenuate the exercise pressor reflex (29). Third, our findings shed no light on the pressor and MSNA responses evoked by postcontraction circulatory occlusion. These responses, although a useful index, can be viewed as artificial because they do not simulate a mismatch between blood supply and demand during contraction, which is the relevant variable with regard to cardiovascular control by the nervous system during exercise.
In conclusion, we have shown that blockade of P2X receptors in rats whose femoral arteries were ligated for 72 h attenuated the exercise pressor reflex. In contrast, blockade of P2X receptors in rats whose femoral arteries were freely perfused had little effect on this reflex. Our findings in rats may be relevant to patients with peripheral artery disease. We note with interest that the exercise pressor reflex in patients with peripheral artery disease is exaggerated (1) just as it is in rats with ligated femoral arteries (41). Ligation of the femoral artery in rats is an established method of simulating the blood flow patterns found in exercising muscles of humans with peripheral artery disease (33, 47). We speculate that excessive stimulation by ATP of P2X receptors on the endings of group III and IV muscle afferents is responsible for the reports of pain and the exaggerated pressor response to exercise seen in patients with peripheral artery disease.
GRANTS
This work was supported by National Institutes of Health Grants HL-096570 and AR-059397.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.J.S. and M.P.K. conception and design of research; A.J.S. and K.Y. performed experiments; A.J.S. analyzed data; A.J.S. and M.P.K. interpreted results of experiments; A.J.S. prepared figures; A.J.S. and M.P.K. drafted manuscript; A.J.S., K.Y., and M.P.K. edited and revised manuscript; A.J.S., K.Y., and M.P.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Joyce Kim for technical assistance.
REFERENCES
- 1.Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology 50: 361–374, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci 12: 256–268, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Burnstock G, Kennedy C. Is there a basis for distinguishing two types of P2-purinoreceptor? Gen Pharmacol 16: 433–440, 1985 [DOI] [PubMed] [Google Scholar]
- 4.Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature 387: 505–508, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui J, Leuenberger UA, Blaha C, King NC, Sinoway LI. Effect of P2 receptor blockade with pyridoxine on sympathetic response to exercise pressor reflex in humans. J Physiol 589: 685–695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demolombe S, Escande D. ATP-binding cassette proteins as targets for drug discovery. Trends Pharmacol Sci 17: 273–275, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. Pharmacology of P2X channels. Pflügers Arch 452: 513–537, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Hanna RL, Hayes SG, Kaufman MP. α,β-Methylene ATP elicits a reflex pressor response arising from muscle in decerebrate cats. J Appl Physiol 93: 834–841, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Hanna RL, Kaufman MP. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J Appl Physiol 94: 1437–1445, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Hanna RL, Kaufman MP. Activation of thin-fiber muscle afferents by a P2X agonist in cats. J Appl Physiol 96: 1166–1169, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Hayes SG, Kaufman MP. Gadolinium attenuates exercise pressor reflex in cats. Am J Physiol Heart Circ Physiol 280: H2153–H2161, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Hayes SG, Kindig AE, Kaufman MP. Comparison between the effect of static contraction and tendon stretch on the discharge of group III and IV muscle afferents. J Appl Physiol 99: 1891–1896, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Hayes SG, McCord JL, Kaufman MP. Role played by P2X and P2Y receptors in evoking the muscle chemoreflex. J Appl Physiol 104: 538–541, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Hayes SG, McCord JL, Koba S, Kaufman MP. Gadolinium inhibits group III but not group IV muscle afferent responses to dynamic exercise. J Physiol 587: 873–882, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman MP. The exercise pressor reflex in animals. Exp Physiol 97: 51–58, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol 55: 105–112, 1983 [DOI] [PubMed] [Google Scholar]
- 18.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol 57: 644–650, 1984 [DOI] [PubMed] [Google Scholar]
- 19.Kindig AE, Hayes SG, Hanna RL, Kaufman MP. P2 antagonist PPADS attenuates responses of thin fiber afferents to static contraction and tendon stretch. Am J Physiol Heart Circ Physiol 290: H1214–H1219, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Kindig AE, Hayes SG, Kaufman MP. Blockade of purinergic 2 receptors attenuates the mechanoreceptor component of the exercise pressor reflex. Am J Physiol Heart Circ Physiol 293: H2995–H3000, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Lambrecht G. Agonists and antagonists acting at P2X receptors: selectivity profiles and functional implications. Naunyn Schmiedeberg Arch Pharmacol 362: 340–350, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Li J, Gao Z, Kehoe V, Xing J, King N, Sinoway L. Interstitial adenosine triphosphate modulates muscle afferent nerve-mediated pressor reflex. Muscle Nerve 38: 972–977, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol 95: 577–583, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Li J, Sinoway LI. ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol 283: H2636–H2643, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Li JD, Lu J, Xing J, Li J. Contribution of nerve growth factor to upregulation of P2X3 expression in DRG neurons of rats with femoral artery occlusion. Am J Physiol Heart Circ Physiol 301: H1070–H1079, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLean DA, Saltin B, Radegran G, Sinoway L. Femoral arterial injection of adenosine in humans elevates MSNA via central but not peripheral mechanisms. J Appl Physiol 83: 1045–1053, 1997 [DOI] [PubMed] [Google Scholar]
- 27.MacLean DA, Vickery LM, Sinoway L. Elevated interstitial adenosine concentrations do not activate the muscle reflex. Am J Physiol Heart Circ Physiol 280: H546–H553, 2001 [DOI] [PubMed] [Google Scholar]
- 28.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCord JL, Tsuchimochi H, Kaufman MP. P2X2/3 and P2X3 receptors contribute to the metaboreceptor component of the exercise pressor reflex in decerebrated cats. J Appl Physiol 109: 1416–1423, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mense S, Meyer H. Bradykinin-induced modulation of the response behavior of different types of feline group III and IV muscle receptors. J Physiol 398: 49–63, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mense S, Stahnke M. Responses in muscle afferent fibers of slow conduction velocity to contractions and ischemia in the cat. J Physiol 342: 383–397, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mo FM, Ballard HJ. The effect of systemic hypoxia on interstitial and blood adenosine, AMP, ADP, and ATP in dog skeletal muscle. J Physiol 536: 593–603, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol 287: H2434–H2447, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Ramer MS, Bradbury EJ, McMahon SB. Nerve growth factor induces P2X(3) expression in sensory neurons. J Neurochem 77: 864–875, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Reinohl J, Hoheisel U, Unger T, Mense S. Adenosine triphosphate as a stimulant for nociceptive and non-nociceptive muscle group IV receptors in the rat. Neurosci Lett 338: 25–28, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Rotto DM, Kaufman MP. Effects of metabolic products of muscular contraction on the discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988 [DOI] [PubMed] [Google Scholar]
- 37.Smith SA, Mitchell JH, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol 537: 961–970, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation 112: 2293–2300, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol 275: H1726–H1732, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effect of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol 65: 1539–1547, 1988 [DOI] [PubMed] [Google Scholar]
- 41.Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol 299: H106–H113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuchimochi H, McCord JL, Kaufman MP. Peripheral mu-opioid receptors attenuate the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol 299: H557–H565, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Victor RG, Rotto DM, Pryor SL, Kaufman MP. Stimulation of renal sympathetic activity by static contraction: evidence for mechanoreceptor-induced reflexes from skeletal muscle. Circ Res 64: 592–599, 1989 [DOI] [PubMed] [Google Scholar]
- 44.Wang HJ, Li YL, Gao L, Zucker IH, Wang W. Alteration in skeletal muscle afferents in rats with chronic heart failure. J Physiol 588: 5033–5047, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xing J, Lu J, Li J. Contribution of nerve growth factor to augmented TRPV1 responses of muscle sensory neurons by femoral artery occlusion. Am J Physiol Heart Circ Physiol 296: H1380–H1387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xing J, Lu J, Li J. Augmented P2X response and immunolabeling in dorsal root ganglion neurons innervating skeletal muscle following femoral artery occlusion. J Neurophysiol 109: 2161–2168, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol 278: H1966–H1973, 2000 [DOI] [PubMed] [Google Scholar]