Abstract
Clinical evidence indicates that obstructive sleep apnea is more common and more severe in men compared with women. Sex differences in the vasoconstrictor response to hypoxemia-induced sympathetic activation might contribute to this clinical observation. In the current laboratory study, we determined sex differences in the acute physiological responses to maximal voluntary end-expiratory apnea (MVEEA) during wakefulness in healthy young men and women (26 ± 1 yr) as well as healthy older men and women (64 ± 2 yr). Mean arterial pressure (MAP), heart rate (HR), brachial artery blood flow velocity (BBFV, Doppler ultrasound), and cutaneous vascular conductance (CVC, laser Doppler flowmetry) were measured, and changes in physiological parameters from baseline were compared between groups. The breath-hold duration and oxygen-saturation nadir were similar between groups. In response to MVEEA, young women had significantly less forearm vasoconstriction compared with young men (ΔBBFV: 2 ± 7 vs. −25 ± 6% and ΔCVC: −5 ± 4 vs. −31 ± 4%), whereas ΔMAP (12 ± 2 vs. 16 ± 3 mmHg) and ΔHR (4 ± 2 vs. 6 ± 3 bpm) were comparable between groups. The attenuated forearm vasoconstriction in young women was not observed in postmenopausal women (ΔBBFV −21 ± 5%). We concluded that young women have blunted forearm vasoconstriction in response to MVEEA compared with young men, and this effect is not evident in older postmenopausal women. These data suggest that female sex hormones dampen neurogenic vasoconstriction in response to apnea-induced hypoxemia.
Keywords: sympathetic nervous system, blood flow, hypoxia, vascular resistance, chemoreflex
numerous population-based studies have demonstrated that obstructive sleep apnea (OSA) is more common in men than women (41, 54, 69). Furthermore, this difference is magnified in the clinical setting with estimates of the male:female ratio as high as 8:1 (55). The exact mechanisms underlying the apparent sex differences are unclear, but prior studies have suggested that upper airway anatomy, body fat distribution, reproductive hormones, and neurovascular control might all contribute to the higher prevalence of OSA in men (41, 44, 51). With regard to neurovascular control, it has been established that both obstructive apnea during sleep (39, 60, 72) and voluntary apnea during wakefulness (19, 22, 34, 40, 62) lead to a reduction in arterial oxygen saturation (SaO2), a rise in muscle sympathetic nerve activity (MSNA), reduced limb blood flow, and a transient rise in arterial blood pressure (BP). Whether there are sex differences in these acute physiological responses to voluntary apnea have not been evaluated. In fact, most previous apnea studies have only enrolled male participants (19, 39, 40, 46, 62, 72) or studied only a few women (25, 59, 60). Understanding the physiological responses to apnea is clinically valuable because heightened vasoconstriction and elevated BP are thought to be primary stimuli for adverse cardiovascular events (e.g., sudden cardiac death) in patients with OSA (15, 42).
The purpose of the present investigation was to determine whether young women have attenuated forearm vasoconstriction in response to maximal voluntary end-expiratory apnea (MVEEA) compared with age-matched men. We hypothesized that acute reductions in brachial artery blood flow velocity (BBFV, Doppler ultrasound) and cutaneous vascular conductance (CVC, laser Doppler flowmetry) would be blunted in young women compared with young men (i.e., less vasoconstriction at similar levels of hypoxemia). We also enrolled groups of older men and postmenopausal older women to test the hypothesis that forearm vasoconstrictor response to MVEEA would be augmented in older women compared with young women. To determine whether the observed sex differences in forearm vasoconstriction were unique to MVEEA or were generalizable to another sympathoexcitatory stimulus that raises BP, participants also underwent the cold pressor test. The present data indicate that young women have blunted vasoconstriction in both forearm skeletal muscle and the cutaneous circulation in response to MVEEA compared with young men; this effect is not evident in older postmenopausal women.
MATERIALS AND METHODS
Participants.
All study protocols were approved in advance by the Institutional Review Board of the Penn State Milton S. Hershey Medical Center and conformed to the Declaration of Helsinki. A total of 9 young men, 10 young (premenopausal) women, 9 older men, and 8 older (postmenopausal) women participated and provided written informed consent (Table 1). All young women were eumenorrheic and were studied in the early follicular phase (days 1–4) of the menstrual cycle, which is when both estradiol and progesterone levels are lowest in women (5, 45, 61). Six of the ten young women were using a combined estrogen and progestin for contraceptive purposes. None of the older women were taking hormone replacement therapy. It is important to note that serum estrogen is markedly greater in young women compared with young men (35); estrogen levels in older women (55–80 yr) are not different than older men. The young men were taller and heavier than the young women and also had a higher body mass index (all P < 0.05, Table 1). The older women were of comparable stature to the young women but had significantly higher resting BP (all P < 0.05, Table 1). All participants had supine resting BP below 120/80 mmHg, and all were nonasthmatic, nonobese, nonsmokers, not taking any prescription or vasoactive medication, and were in good health as determined by history and physical examination. All participants reported being physically active, but none were competitive athletes. Before enrollment, the older men and women all underwent a Bruce treadmill protocol with 12-lead EKG monitoring, which was read by a cardiologist to rule out heart disease. Participants refrained from caffeine, alcohol, and exercise for 24 h before the study and arrived to the laboratory in a semifasted state (i.e., 4–6 h after their last meal).
Table 1.
Anthropometric and baseline hemodynamic characteristics
| Young Men | Young Women | Older Women | Older Men | |
|---|---|---|---|---|
| Participants | 9 | 10 | 8 | 9 |
| Age, yr | 27 ± 1 | 26 ± 1 | 64 ± 2† | 66 ± 3‡ |
| Height, cm | 179 ± 3 | 166 ± 3* | 163 ± 4 | 179 ± 2 |
| Weight, kg | 80.8 ± 2.4 | 64.5 ± 3.7* | 67.0 ± 1.9 | 81.7 ± 3.0 |
| BMI | 25.2 ± 0.3 | 23.1 ± 0.8* | 25.3 ± 1.9 | 25.5 ± 0.8 |
| SBP, mmHg | 110 ± 2 | 107 ± 1 | 119 ± 2† | 118 ± 2‡ |
| DBP, mmHg | 67 ± 1 | 65 ± 21 | 73 ± 3† | 77 ± 2‡ |
| MAP, mmHg | 81 ± 1 | 78 ± 2 | 87 ± 3† | 90 ± 2‡ |
| HR, beats/min | 64 ± 2 | 61 ± 2 | 63 ± 5 | 59 ± 3 |
Values are means ± SE.
Significant difference from young men,
significant difference from young women,
significant difference from young men. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate.
Physiological measurements.
All experiments were performed in the supine posture in a dimly lit thermoneutral laboratory (22–25°C). Upon arrival to the laboratory, participants dressed in a high-density tube-lined suit (Med-Eng Systems, Ottawa, Ontario, Canada) that covered the entire body except for the feet, hands, head, and both forearms. Neutral water (34–35°C) was perfused through the suit to maintain mean skin temperature at a constant level and ensure the obtained responses were reflex mediated and not influenced by ambient temperature. After 15 min of quiet rest, baseline BP was obtained via automated oscillometry of the brachial artery (SureSigns Vs3; Philips, Andover, MA) in triplicate.
Beat-by-beat BP was measured by photoplethysmography (Finometer; FMS, Amsterdam, the Netherlands), and heart rate (HR) was measured via three-lead EKG (Cardiocap/5; GE Healthcare, Piscataway, NJ). Arterial O2 saturation (SaO2, %) was measured with a respiratory gas monitor (RGM 5250; Ohmeda, Madison, WI). Respiratory movement was monitored with a “pneumobelt”, a device using a pneumatic bellows that is interfaced to a pressure transducer and is prestretched by an adjustable strap placed around the chest so that its internal pressure varies as the volunteer breathes. Cutaneous blood flow flux was measured by three laser Doppler probes (Moor Instruments, Wilmington, DE) placed on the right ventral forearm with local temperature set at 34°C. Beat-by-beat mean BBFV was measured in the left arm by Doppler ultrasound (HDI 5000; ATL Ultrasound, Bothell, WA) as previously described (47, 67). Briefly, a 5–12-MHz linear transducer was placed over the left brachial artery, and the insonation angle was <60°. Mean BBFV was acquired in pulsed Doppler mode, and velocity waveforms were synchronized to a data acquisition system (PowerLab; ADInstruments, Colorado Springs, CO) by a Doppler audio transformer (23). Because our ultrasound does not allow for the simultaneous measurement of velocity and diameter, we chose to remain in Doppler mode for the duration of the apnea. In a separate series of studies (n = 3 young women and n = 3 young men), brachial artery diameter in response to MVEEA was found to not change in either group (see results). These data combined with a previous report (40) suggest that changes in velocity attributable to MVEEA are proportional to changes in absolute blood flow because diameter does not change.
MVEEA procedures.
Participants were familiarized to the MVEEA procedure by performing a practice trial to ensure they held their breath in expiration without performing Valsalva and Müeller maneuvers, consistent with prior reports (18, 40, 46, 56). After baseline cardiovascular parameters were obtained (∼3 min), participants were then asked using standard wording to perform an MVEEA in room air (21% oxygen), “Whenever you are ready, please perform a breath hold. Keep your body relaxed and hold your breath as long as you can, only breathe when you have to.” HR, mean arterial pressure (MAP), SaO2, BBFV, and skin blood flow flux were recorded continuously. Three separate apnea parameters were used during offline analysis: 1) an average of seconds 12–15 of MVEEA (i.e., cessation of lung inflation but only modest hypoxemia), 2) an average of the three cardiac cycles immediately before inspiration (i.e., the “asphyxic break point”), and 3) an average of the three cardiac cycles immediately after inspiration (i.e., usually coinciding with the lowest observed SaO2). These time points were chosen based on previously published studies from this laboratory (53) and others (39, 40, 56). Because expired gases were not measured in this study, the onset of inspiration and expiration was quantified by offline inspection of the pneumograph tracing. Two separate MVEEA trials were conducted for each subject (separated by 3 to 5 min), and an average is reported.
Cold pressor test procedures.
Seven young men, seven young women, seven older men, and seven older (postmenopausal) women underwent the cold pressor test after completing the MVEEA trials. The cold pressor test was chosen because it is a sympathoexcitatory stimulus that raises BP without altering arterial blood gases (i.e., it does not activate the arterial chemoreflex) (38, 65). Participants placed their left hand up to the styloid process into 1°C water, and the hand remained submerged for 2 min. Only HR, MAP, and skin blood flow flux (right ventral forearm) were measured during the cold pressor test. Data from the last 20 s of immersion were used as the peak physiological response. Thermal sensation (0 = neutral/no sensation of cold and 11 = unbearable cold) and pain perception (0 = no pain and 10 = unbearable pain) were obtained immediately after the hand was removed from the water (17, 21).
Data collection and statistical analysis.
Data were collected at 200 Hz by a PowerLab (ADInstruments) and were analyzed offline. The three forearm skin blood flow sites were averaged, and CVC was calculated as skin blood flow flux/MAP and then expressed as a percent change from baseline, which is a common method of data presentation for CVC (49, 50, 64). To compare BBFV and CVC in the same units, percent change was also calculated for BBFV. Changes in HR and MAP from baseline were determined in absolute physiological units. As stated above, the MVEEA data were analyzed at three distinct time points: 1) an average of seconds 12–15, 2) an average of the three cardiac cycles immediately before inspiration, and 3) an average of the three cardiac cycles immediately after inspiration. In this report, these time points are called “15 s of apnea”, “last three apnea”, and “first three inhale,” respectively.
All statistical analyses were conducted using IBM SPSS 21.0, and graphics were produced using Microsoft Excel and Adobe Illustrator CS5. Baseline anthropometric and hemodynamic parameters were determined with independent sample t-tests (Table 1). Physiological responses to MVEEA and the cold pressor tests were also compared between groups with independent sample t-tests. Bivariate correlations were conducted to relate CVC and BBFV responses, and intraclass correlations were used to determine test-retest reliability in the combined group of participants (comparing the first apnea performed to the second apnea performed). Significance was set at P < 0.05, and data are presented as means ± SE throughout.
RESULTS
MVEEA: young men vs. young women.
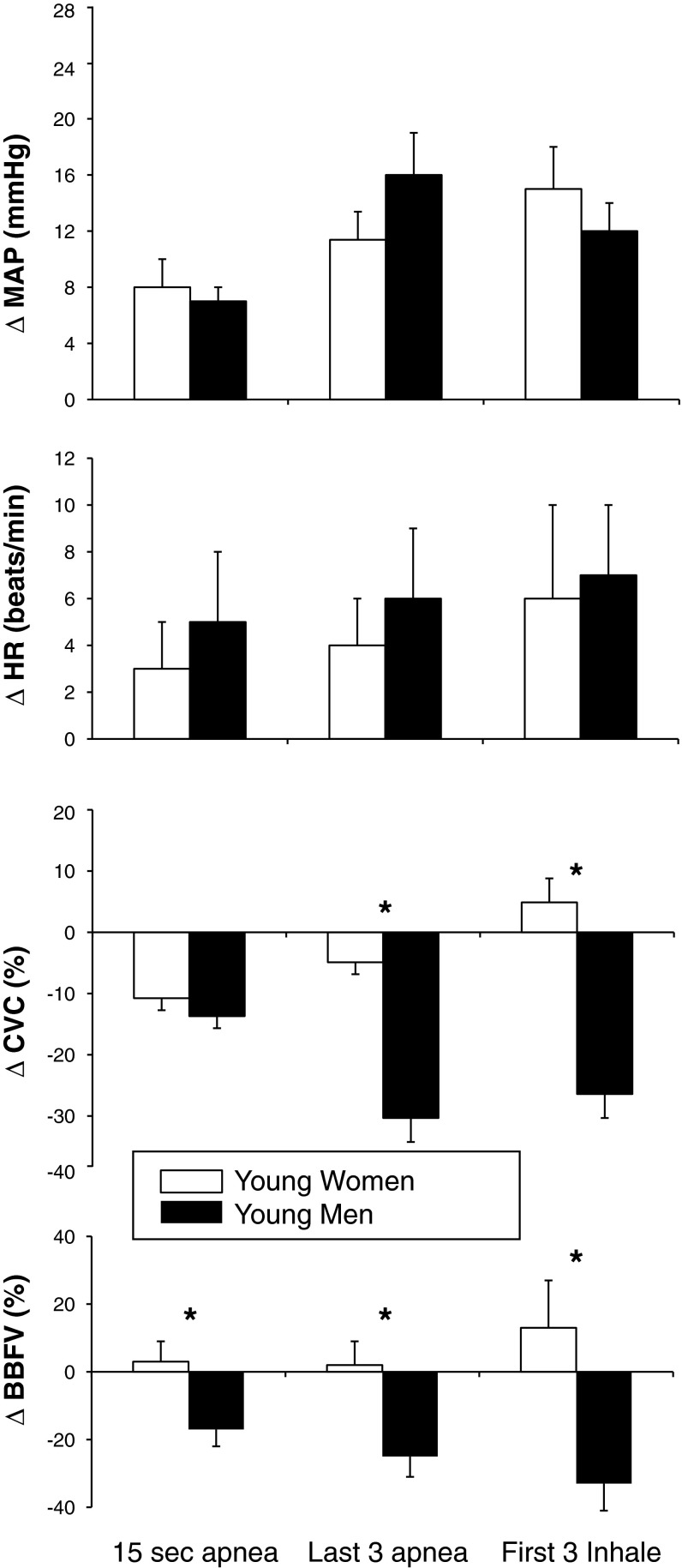
MVEEA duration was not different between young men (26 ± 3 s) and young women (23 ± 1 s, P = 0.262). Similarly, the SaO2 nadir was not different between young men (91 ± 1%) and young women (93 ± 1%, P = 0.188). As shown in Fig. 1, the ΔMAP and ΔHR were comparable between groups at the three separate time points. However, young women had significantly less ΔBBFV (i.e., less vasoconstriction) compared with young men at all time points (P = 0.033, 0.018, and 0.019, respectively). Young women also had a blunted ΔCVC (i.e., less vasoconstriction) during the last three cardiac cycles of apnea (P = 0.029) and the first three cardiac cycles of inhalation (P = 0.048) compared with young men. Representative recordings of MVEEA are included for one young man (Fig. 2) and one young woman (Fig. 3). In a secondary analysis, there were no significant differences in the physiological responses to MVEEA between young women using hormonal contraceptives (n = 6) and those not using hormonal contraceptives (n = 4).
Fig. 1.
Changes in mean arterial pressure (ΔMAP), heart rate (ΔHR), forearm cutaneous vascular conductance (ΔCVC), and brachial blood flow velocity (ΔBBFV) in response to maximal voluntary end-expiratory apnea (MVEEA) in young women (open bars, n = 10) and young men (solid bars, n = 9). Means ± SE, *P < 0.05 between groups at the indicated time point.
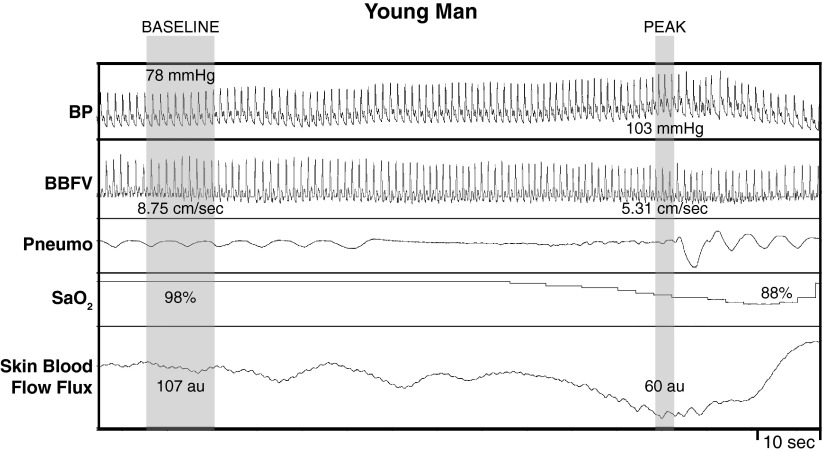
Fig. 2.
Representative recording of arterial blood pressure (BP), BBFV, respiratory movement (Pneumo), arterial oxygen saturation (SaO2), and skin blood flow flux in one young man during baseline and during MVEEA. In young male participants, MVEEA (as indicated by lack of respiration on Pneumo) elicited a strong pressor response with a decrease in BBFV, SaO2, and skin blood flow flux.
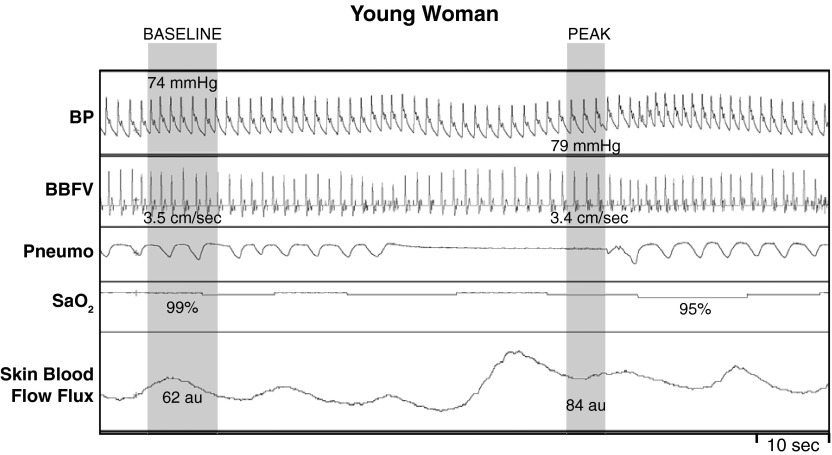
Fig. 3.
Representative recording of arterial BP, BBFV, Pneumo, SaO2, and skin blood flow flux in one young woman during baseline and during MVEEA. In young female participants, the apnea-induced hypoxemia elicited a moderate pressor response along with a minimal change in BBFV and skin blood flow flux.
In three young men and three young women, we measured brachial artery diameter in response to MVEEA (instead of measuring BBFV). Expectedly, men had larger brachial artery size at rest (P < 0.001). However, MVEEA did not influence brachial artery diameter in either men (from 3.7 ± 0.1 mm at rest to 3.6 ± 0.2 mm at peak) or women (from 3.0 ± 0.1 mm at rest to 3.1 ± 0.1 mm at peak). The percent change in diameter was also not different between men (−1.7 ± 0.8%) and women (1.0 ± 1.5%, P = 0.191). Thus the sex differences in BBFV (Fig. 1) are likely indicative of changes in absolute blood flow because brachial diameter was unchanged in response to MVEEA.
MVEEA: young women vs. older women.
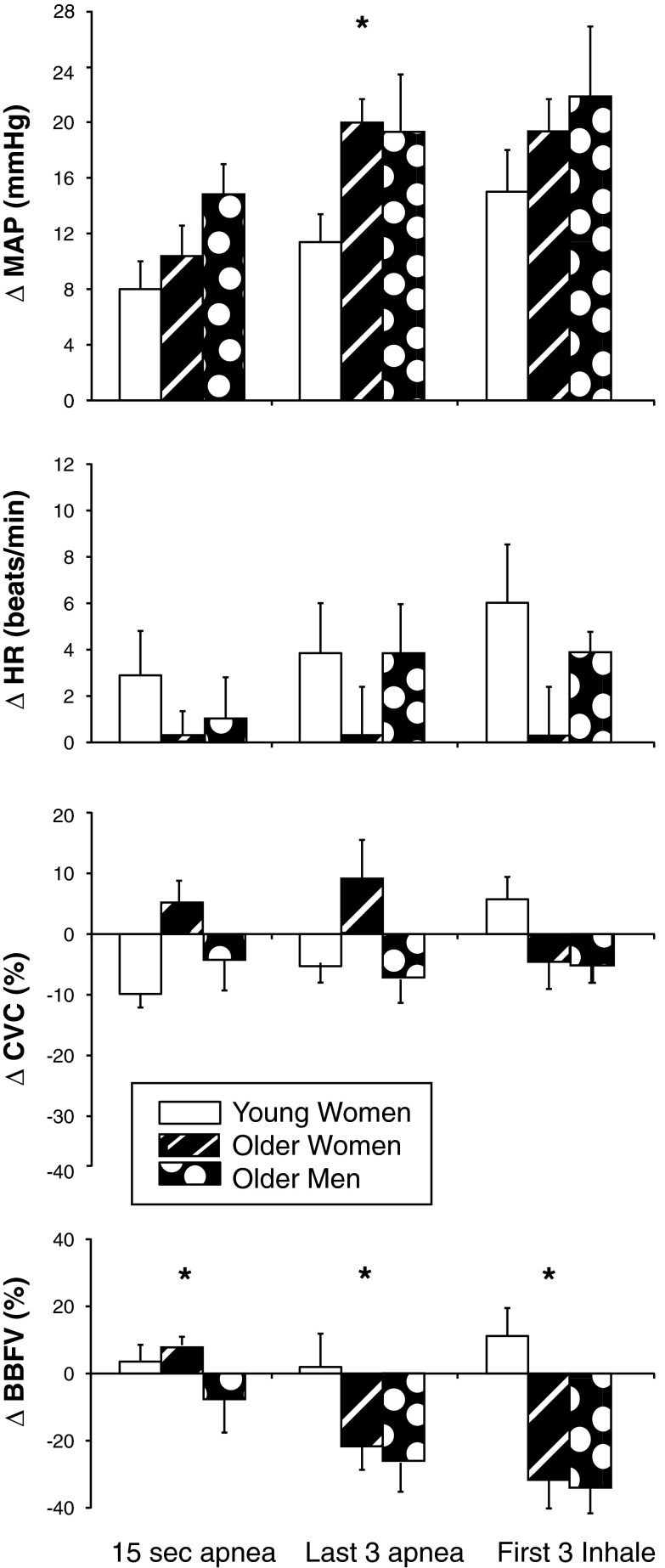
When comparing the young women to the older postmenopausal women, we found that there were no difference in breath-hold duration (28 ± 3 s, P = 0.183) or SaO2 nadir (94 ± 1%, P = 0.749). As shown in Fig. 4, the older women had an augmented ΔMAP during the last three cardiac cycles of apnea (P = 0.009), but ΔHR was not statistically different at any time point. The ΔBBFV was larger in the older women (i.e., more vasoconstriction) compared with the young women at 15 s of apnea (P = 0.026), last three cardiac cycles of apnea (P = 0.027), and first three cardiac cycles of inhalation (P = 0.027). Importantly, the forearm vasoconstrictor response to MVEEA in older women was remarkably similar to that observed in young men (i.e., both groups were expected to have low levels of female reproductive hormones).
Fig. 4.
Changes in ΔMAP, ΔHR, forearm ΔCVC, and ΔBBFV in response to MVEEA in young women (open bars, n = 10), postmenopausal older women (black dashed bars, n = 8), and older men (black bars with white dots, n = 9). Means ± SE, *P < 0.05 between young women and older women at the indicated time point. There were no differences between older women and older men.
MVEEA: older men vs. older women.
MVEEA duration was not different between older men (29 ± 3 s) and older women (28 ± 3 s, P = 0.483). Similarly, the SaO2 nadir was not different between older men (91 ± 2%) and older women (93 ± 1%, P = 0.156). As shown in Fig. 4, the physiological responses to MVEEA were not different between older men and older women.
MVEEA: correlations.
In addition to reporting mean data (above), we also used intraclass correlations to determine test-retest reliability in physiological response to MVEEA (comparing the first MVEEA to the second MVEEA). The breath-hold duration (Cronbach's α = 0.905, P < 0.001) and SaO2 nadir (Cronbach's α = 0.943, P < 0.001) both demonstrated high test-retest reliability, which indicates a similar level of hypoxemia within the same individual for different apnea trials. The ΔMAP at 15 s of apnea (Cronbach's α = 0.837, P < 0.001), last three apnea (Cronbach's α = 0.867, P < 0.001), and first three inhale (Cronbach's α = 0.888, P < 0.001) demonstrated moderate to strong test-retest reliability. In a similar way, the ΔHR at 15 s of apnea (Cronbach's α = 0.812, P < 0.001), last three apnea (Cronbach's α = 0.901, P < 0.001), and first three inhale (Cronbach's α = 0.918, P < 0.001) also demonstrated moderate to strong test-retest reliability. When analyzing ΔBBFV, we found that test-retest reliability was high at all time points (15 s of apnea: Cronbach's α = 0.820, last three apnea: Cronbach's α = 0.811, first three inhale: Cronbach's α = 0.893, all P < 0.001). In a similar way, test-retest reliability was moderate to high for CVC (Cronbach's α = 0.787, 0.766, and 0.771, respectively, all P < 0.001). Thus individuals with greater forearm vasoconstrictor responses for the first trial also had greater forearm vasoconstrictor responses during the second trial.
We conducted additional correlation analysis to determine the relationships between BBFV and CVC in response to MVEEA in the combined group of participants. During the last three cardiac cycles before inspiration, CVC and BBFV were positively related (R = 0.633, P = 0.001), and, during the first three cardiac cycle of inhalation, this relationship was also evident (R = 0.527, P = 0.008). Thus individuals with greater skeletal muscle vasoconstriction also had greater cutaneous vasoconstriction.
Cold pressor test.
As displayed in Table 2, immersion of the hand into 1°C water for 2 min increased MAP (P = 0.329) and HR (P = 0.869) to similar levels in young men and young women. However, young women exhibited cutaneous vasodilation, whereas young men had cutaneous vasoconstriction, and this comparison was statistically significant (P = 0.034).
Table 2.
Hemodynamic and cutaneous responses to the cold pressor test
| Young Men | Young Women | Older Women | Older Men | |
|---|---|---|---|---|
| Participants | 7 | 7 | 7 | 7 |
| ΔMAP, mmHg | 33 ± 4 | 27 ± 4 | 22 ± 4 | 19 ± 5 |
| ΔHR, beats/min | 6 ± 3 | 5 ± 3 | 5 ± 3 | 2 ± 2 |
| ΔCVC, % | −12 ± 5 | 35 ± 18* | −7 ± 4† | −9 ± 7 |
| Thermal sensation | 9 ± 1 | 9 ± 1 | 9 ± 1 | 9 ± 1 |
| Pain perception | 8 ± 2 | 9 ± 1 | 9 ± 1 | 9 ± 1 |
Values are means ± SE. Thermal sensation (0 = neutral/no sensation of cold and 11 = unbearable cold) and pain perception (0 = no pain and 10 = unbearable pain) were obtained immediately after the hand was removed from the water (17, 21).
Significant difference from young men,
significant difference from young women. CVC, cutaneous vascular conductance.
Older women had similar ΔMAP (P = 0.411) and ΔHR (P = 0.869) in response to the cold pressor test compared with young women. In contrast to the vasodilation observed in young women, older women experienced a modest cutaneous vasoconstriction (P = 0.034) such that CVC was comparable to young men. As shown in Table 2, ratings of hand pain and cold sensation were comparable in all groups.
DISCUSSION
In this study, we examined the acute physiological responses to MVEEA in healthy women and men. Along with HR and MAP, we measured forearm skeletal muscle blood flow velocity and cutaneous blood flow flux to MVEEA in young men, young women, older men, and postmenopausal older women. The major findings are that young women have blunted apnea-induced forearm vasoconstriction compared with young men, and this response is lost in older postmenopausal women. These findings are novel because previous apnea studies have either excluded female participants altogether (19, 39, 40, 46, 62, 72) or have combined men and women into one group for data analysis (25, 32, 34, 56, 59, 60). Importantly, none of these cited studies measured forearm blood flow. The present data provide evidence that sex modifies the vascular response to apnea-induced hypoxemia in healthy humans and might also stimulate future work in patients with OSA.
Both obstructive apnea during sleep and MVEEA during wakefulness activate the sympathetic nervous system (19, 26, 32, 40, 60). Specifically, Greaney et al. (18) observed a 51% increase in MSNA in response to MVEEA, Hardy et al. (19) noted a 94% increase in MSNA, and Leuenberger et al. (40) documented a ∼200% increase in MSNA in response to this same stimulus. The rise in MSNA and the resulting peripheral vasoconstriction are due to the combined effects of hypoxemia, hypercapnia, and cessation of lung inflation (46, 56, 59). With respect to sympathetically mediated vasoconstriction, Leuenberger et al. (40) demonstrated an 11% decrease in femoral artery blood flow in response to MVEEA as measured via Doppler ultrasound. In the present study, we observed a 20–30% decrease in BBFV in young men (depending on the time point), indicating forearm vasoconstriction (Figs. 1 and 4). We further the literature by demonstrating that young women have a blunted forearm vasoconstriction in response to MVEEA compared with young men. Because the apnea duration and SaO2 nadir were similar between groups, we suspect that afferent input to the chemoreflex is comparable between healthy young men and women. The observed sex difference in efferent responses (i.e., forearm blood flow) could be due to 1) reduced sympathetic outflow (i.e., MSNA), 2) impaired synthesis and/or release of norepinephrine and cotransmitters, 3) attenuated α-adrenergic responsiveness, 4) enhanced vasodilation due to activation of β-adrenergic receptors, nitric oxide, adenosine, and/or estrogen (all of which would dampen the chemoreflex-mediated vasoconstrictor signal), or 5) a combination of the aforementioned effects (4, 16, 36, 66). This process is undoubtedly complex and warrants further study to pharmacodissect the observed sex differences.
Reflex control of the cutaneous circulation is tightly regulated by the sympathetic nervous system (6, 30). Previous studies have demonstrated that cutaneous vasoconstriction occurs in response to voluntary apnea (1, 2, 28, 33). However, each of these cited studies had fundamental limitations, such as not maintaining mean skin temperature at a constant level (28), performing apnea during exercise (2), not allowing values to return to baseline (1), and the use of plethysmographic methods (33). In the present study, we determined the reflex cutaneous vascular responses to MVEEA (i.e., a nonthermal stimulus) under rigorously controlled laboratory conditions. The present data demonstrate clear sex differences in CVC during the last three cardiac cycles of apnea and also the first three cardiac cycles of inspiration (Figs. 1, 2, and 3). These findings are consistent with a study by Feger and Braune (12), which revealed sex differences in skin blood flow responses to inspiratory gasp. However, a short-duration inspiratory gasp is unlikely to elicit a reduction in SaO2 and rather exerts its cutaneous effects via involvement of lung-stretch receptors. Indeed, a recent publication by Seitz et al. (56) demonstrated that inspiratory apnea causes a brief sympathetic inhibition unrelated to chemoreceptor reflex mechanism. Given that cutaneous vasoconstriction is aimed at oxygen redistribution (1), these findings suggest men may be better suited to redistribute blood flow toward vital organs, such as the brain and heart during hypoxemia (29, 52). Thus cutaneous vasoconstriction during MVEEA teleologically makes sense.
OSA is not only more prevalent in men, but disordered breathing during sleep is also more common among postmenopausal women compared with premenopausal women with estimates ranging from 4% to 22% (57). Hence, we studied eight postmenopausal women and nine men of similar age to determine whether cutaneous and forearm skeletal muscle blood flow responses to MVEEA are different. Herein, we demonstrate postmenopausal women have an augmented pressor response to voluntary apnea as well as augmented forearm vasoconstriction compared with young women (Fig. 4). The most likely mechanism is that estrogen exerts direct vascular effects by inducing vasodilation through increased vascular nitric oxide production in young women (63). Although MVEEA is an intense stimulus for peripheral vasoconstriction, it may be counterbalanced by the vasodilatory effects of estrogen, resulting in minimal to no net change in BBFV in young women. In postmenopausal women, the vasodilatory effects of estrogen are less, allowing a more complete expression of vasoconstriction, as indicated in Fig. 4. Because we studied young women in the early follicular phase of the menstrual cycle (i.e., when serum sex hormones are lowest albeit still higher than levels in young men), the ability of progesterone to inhibit the vasodilatory effects of estrogen was likely low. Indeed, α-adrenergic responsiveness is lower in the early follicular phase compared with the midluteal phase (14), and this is consistent with a recent study showing that progesterone enhances cutaneous vasoconstriction in response to norepinephrine (66). Beyond the effects of female sex hormones on the vasculature, there is mounting evidence in humans and animal models that estradiol is sympathoinhibitory and progesterone is sympathoexcitatory (5). Taken together, the present data support the concept that young women have markedly different forearm vascular responses to MVEEA compared with either young men (Fig. 1) or older women (Fig. 4).
In addition to the observed sex differences detailed above, our correlation data provide two interesting findings that may inform future studies. First, ΔCVC and ΔBBFV were correlated such that larger cutaneous vasoconstriction was associated with larger skeletal muscle vasoconstriction. This may indicate that either CVC or BBFV is a valuable measurement to be used in future MVEEA studies despite the fact that skin and muscle blood flow are not controlled identically (6). Second, physiological responses to MVEEA are reproducible in subsequent trials. This might suggest that physiological responses to MVEEA measured in a laboratory setting (e.g., MAP, HR, BBFV, CVC) could translate into predictable hemodynamic outcomes during obstructive apnea during sleep. This speculation warrants further study.
A subset of participants also underwent the cold pressor test to evaluate whether the observed sex differences with apnea were attributable to a generalized inability of young women to vasoconstrict the forearm in responses to sympathetic stressors or whether sex differences were unique to MVEEA. The present data suggest that young women also have a blunted vasoconstrictor response to the cold pressor test. Indeed, young men and women had similar MAP and HR responses, but women had a smaller reduction in CVC compared with young men (and also compared with older women). It is thought that the reflex cutaneous vasoconstriction in response to the cold pressor test is mediated by α-adrenergic receptors (13), but vasoconstriction is not universally observed (8). With respect to sex differences, a previous study found that young women had an attenuated increase in BP and MSNA to isometric handgrip exercise compared with men (11). Other studies reported sex differences in the MSNA responses to orthostatic stress (58, 68). However, prior studies did not find sex differences in MSNA responses to the cold pressor test, but these studies did not measure skin blood flow (27, 31). Because MSNA is positively correlated to total peripheral resistance in young men, but not in young women (20), it is likely that there are also sex differences in the transduction of sympathetic nerve activity to cutaneous vasoconstriction as well. It is also possible that the myogenic response is different between young men and young women, such that a given stretch of a blood vessel results in an attenuated net vasoconstrictor response in women (43). This concept remains to be experimentally tested in human skin.
Recently, polysomnography studies have confirmed epidemiological findings that OSA is, not only more common, but also more severe in men than women (51). Our data suggest that differences in clinical expression of OSA may be in part related to altered vascular responses to apnea-induced hypoxemia. There is convincing evidence that OSA is associated with an increased risk of ischemic heart disease (37), myocardial infarction (24), stroke (10), arrhythmia (15), and mortality (3). The strongest evidence supports an independent causal link between OSA and arterial hypertension (70). The cyclical rise in BP during sleep is believed to result from apnea-induced peripheral vasoconstriction mediated by repetitive activation of the sympathetic nervous system (40). MVEEA similarly activates the sympathetic nervous system to induce peripheral vasoconstriction and a transient rise in BP (32, 34, 62). Considering that there are sex differences in many aspects of BP control (71), we believe that future studies should enroll both men and women with OSA to examine the underlying mechanisms and potential therapeutic targets.
Limitations.
In the present study, we did not measure serum estrogen or progesterone. However, we studied young women in the early follicular phase (days 1–4), which is the point at which estrogen and progesterone are lowest (5, 45, 61). Because sex differences in vascular responses to MVEEA were not observed in older women (i.e., a group of women expected to have low levels of female sex hormones), it is reasonable to suspect that female sex hormones had an effect in vivo. Some of the young women were using hormonal contraception, but the physiological responses to MVEEA were not significantly different compared with women who were not using hormonal contraception. In consideration that hormonal contraceptives have differing mechanisms of action (e.g., androgenic vs. antiandrogenic), this area of research is indeed complex. A previous study found that postmenopausal women undergoing hormone replacement therapy (n = 907) had a lower prevalence of sleep-disordered breathing compared with women not taking hormone replacement (n = 1,945) (57). An interesting follow-up study would be to evaluate whether vascular responses to apnea are influenced by hormone replacement therapy. It is important to note that MVEEA is not a true OSA per se because it does not involve arousal from sleep or upper airway collapse (i.e., both of which may engage the sympathetic nervous system) (7, 9, 48). However, we believe there is similar underlying physiology between MVEEA and OSA, allowing us to make useful comparisons between men and women (25).
Conclusions.
The present data indicate that young (premenopausal) women have blunted forearm vasoconstriction in response to MVEEA compared with young men. This presumably favorable characteristic of young women is lost in postmenopausal older women. Thus sex can have a profound effect on vascular responses to sympathetic stress in healthy humans. Whether these effects are also present in patients with OSA requires further study.
GRANTS
This work was supported by National Institutes of Health Grants P01 HL096570 (LIS), UL1 RR033184 (LIS), and UL1 TR000127 (LIS).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.M.P. and M.D.M. conception and design of research; H.M.P., M.J.H., A.J.R., and M.D.M. performed experiments; H.M.P., M.J.H., A.J.R., and M.D.M. interpreted results of experiments; H.M.P. and M.D.M. drafted manuscript; H.M.P., M.J.H., A.J.R., and M.D.M. edited and revised manuscript; H.M.P., M.J.H., A.J.R., and M.D.M. approved final version of manuscript; M.J.H. and M.D.M. analyzed data; M.D.M. prepared figures.
ACKNOWLEDGMENTS
The authors are grateful for the nursing support provided by Cheryl Blaha, Jessica Mast, Todd Nicklas, and Aimee Caufmann and the engineering support provided by Dr. Michael Herr. Gratitude is also extended to Anne Muller for preparing the graphics for this study and to Dr. Lawrence Sinoway and Dr. Urs Leuenberger for clinical oversight and funding support. Finally, the authors acknowledge the administrative guidance of Kris Gray and Jen Stoner.
REFERENCES
- 1.Andersson J, Schagatay E. Arterial oxygen desaturation during apnea in humans. Undersea Hyperb Med 25: 21–25, 1998 [PubMed] [Google Scholar]
- 2.Andersson JP, Liner MH, Fredsted A, Schagatay EK. Cardiovascular and respiratory responses to apneas with and without face immersion in exercising humans. J Appl Physiol 96: 1005–1010, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Bliwise DL, Bliwise NG, Partinen M, Pursley AM, Dement WC. Sleep apnea and mortality in an aged cohort. Am J Public Health 78: 544–547, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blumenthal RS, Heldman AW, Brinker JA, Resar JR, Coombs VJ, Gloth ST, Gerstenblith G, Reis SE. Acute effects of conjugated estrogens on coronary blood flow response to acetylcholine in men. Am J Cardiol 80: 1021–1024, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Carter JR, Fu Q, Minson CT, Joyner MJ. Ovarian cycle and sympathoexcitation in premenopausal women. Hypertension 61: 395–399, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc 78: 603–612, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Chouchou F, Pichot V, Pepin JL, Tamisier R, Celle S, Maudoux D, Garcin A, Levy P, Barthelemy JC, Roche F. Sympathetic overactivity due to sleep fragmentation is associated with elevated diurnal systolic blood pressure in healthy elderly subjects: the PROOF-SYNAPSE study. Eur Heart J 34: 2122–2131; 2131a, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Cui J, Shibasaki M, Low DA, Keller DM, Davis SL, Crandall CG. Heat stress attenuates the increase in arterial blood pressure during the cold pressor test. J Appl Physiol 109: 1354–1359, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dempsey JA, Xie A, Patz DS, Wang D. Physiology in medicine: obstructive sleep apnea pathogenesis and treatment—considerations beyond airway anatomy. J Appl Physiol 116: 3–12, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyken ME, Somers VK, Yamada T, Ren ZY, Zimmerman MB. Investigating the relationship between stroke and obstructive sleep apnea. Stroke 27: 401–407, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Ettinger SM, Silber DH, Collins BG, Gray KS, Sutliff G, Whisler SK, McClain JM, Smith MB, Yang QX, Sinoway LI. Influences of gender on sympathetic nerve responses to static exercise. J Appl Physiol 80: 245–251, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Feger J, Braune S. Measurement of skin vasoconstrictor response in healthy subjects. Auton Neurosci 120: 88–96, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Frank SM, Raja SN. Reflex cutaneous vasoconstriction during cold pressor test is mediated through alpha-adrenoceptors. Clin Auton Res 4: 257–261, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Freedman RR, Girgis R. Effects of menstrual cycle and race on peripheral vascular alpha-adrenergic responsiveness. Hypertension 35: 795–799, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Gami AS, Somers VK. Implications of obstructive sleep apnea for atrial fibrillation and sudden cardiac death. J Cardiovasc Electrophysiol 19: 997–1003, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Gilligan DM, Badar DM, Panza JA, Quyyumi AA, Cannon RO., 3rd Acute vascular effects of estrogen in postmenopausal women. Circulation 90: 786–791, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Glickman-Weiss EL, Hearon CM, Nelson AG, Robertson RJ. A thermal perception scale for use during resting exposure to cold air. Percept Mot Skills 79: 547–560, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Greaney JL, Ray CA, Prettyman AV, Edwards DG, Farquhar WB. Influence of increased plasma osmolality on sympathetic outflow during apnea. Am J Physiol Regul Integr Comp Physiol 299: R1091–R1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy JC, Gray K, Whisler S, Leuenberger U. Sympathetic and blood pressure responses to voluntary apnea are augmented by hypoxemia. J Appl Physiol 77: 2360–2365, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Hart EC, Joyner MJ, Wallin BG, Charkoudian N. Sex, ageing and resting blood pressure: gaining insights from the integrated balance of neural and haemodynamic factors. J Physiol 590: 2069–2079, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Havenith G, van de Linde EJ, Heus R. Pain, thermal sensation and cooling rates of hands while touching cold materials. Eur J Appl Physiol Occup Physiol 65: 43–51, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Heistad DD, Abbound FM, Eckstein JW. Vasoconstrictor response to simulated diving in man. J Appl Physiol 25: 542–549, 1968 [DOI] [PubMed] [Google Scholar]
- 23.Herr MD, Hogeman CS, Koch DW, Krishnan A, Momen A, Leuenberger UA. A real-time device for converting Doppler ultrasound audio signals into fluid flow velocity. Am J Physiol Heart Circ Physiol 298: H1626–H1632, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet 336: 261–264, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Imadojemu VA, Gleeson K, Gray KS, Sinoway LI, Leuenberger UA. Obstructive apnea during sleep is associated with peripheral vasoconstriction. Am J Respir Crit Care Med 165: 61–66, 2002 [DOI] [PubMed] [Google Scholar]
- 26.James JE, Daly Mde B. Cardiovascular responses in apnoeic asphyxia: role of arterial chemoreceptors and the modification of their effects by a pulmonary vagal inflation reflex. J Physiol 201: 87–104, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarvis SS, VanGundy TB, Galbreath MM, Shibata S, Okazaki K, Reelick MF, Levine BD, Fu Q. Sex differences in the modulation of vasomotor sympathetic outflow during static handgrip exercise in healthy young humans. Am J Physiol Regul Integr Comp Physiol 301: R193–R200, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jay O, Christensen JP, White MD. Human face-only immersion in cold water reduces maximal apnoeic times and stimulates ventilation. Exp Physiol 92: 197–206, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Jiang ZL, He J, Yamaguchi H, Tanaka H, Miyamoto H. Blood flow velocity in common carotid artery in humans during breath-holding and face immersion. Aviat Space Environ Med 65: 936–943, 1994 [PubMed] [Google Scholar]
- 30.Johnson JM, Yen TC, Zhao K, Kosiba WA. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am J Physiol Heart Circ Physiol 288: H1573–H1579, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Jones PP, Spraul M, Matt KS, Seals DR, Skinner JS, Ravussin E. Gender does not influence sympathetic neural reactivity to stress in healthy humans. Am J Physiol Heart Circ Physiol 270: H350–H357, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Katragadda S, Xie A, Puleo D, Skatrud JB, Morgan BJ. Neural mechanism of the pressor response to obstructive and nonobstructive apnea. J Appl Physiol 83: 2048–2054, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Kawakami Y, Natelson BH, DuBois AR. Cardiovascular effects of face immersion and factors affecting diving reflex in man. J Appl Physiol 23: 964–970, 1967 [DOI] [PubMed] [Google Scholar]
- 34.Khayat RN, Przybylowski T, Meyer KC, Skatrud JB, Morgan BJ. Role of sensory input from the lungs in control of muscle sympathetic nerve activity during and after apnea in humans. J Appl Physiol 97: 635–640, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Khosla S, Melton LJ, 3rd, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 83: 2266–2274, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Kneale BJ, Chowienczyk PJ, Brett SE, Coltart DJ, Ritter JM. Gender differences in sensitivity to adrenergic agonists of forearm resistance vasculature. J Am Coll Cardiol 36: 1233–1238, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Koskenvuo M, Kaprio J, Partinen M, Langinvainio H, Sarna S, Heikkila K. Snoring as a risk factor for hypertension and angina pectoris. Lancet 1: 893–896, 1985 [DOI] [PubMed] [Google Scholar]
- 38.Kregel KC, Seals DR, Callister R. Sympathetic nervous system activity during skin cooling in humans: relationship to stimulus intensity and pain sensation. J Physiol 454: 359–371, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leuenberger U, Jacob E, Sweer L, Waravdekar N, Zwillich C, Sinoway L. Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J Appl Physiol 79: 581–588, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Leuenberger UA, Hardy JC, Herr MD, Gray KS, Sinoway LI. Hypoxia augments apnea-induced peripheral vasoconstriction in humans. J Appl Physiol 90: 1516–1522, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Lin CM, Davidson TM, Ancoli-Israel S. Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev 12: 481–496, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Jimenez F, Sert Kuniyoshi FH, Gami A, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. Chest 133: 793–804, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Lott ME, Hogeman C, Herr M, Bhagat M, Sinoway LI. Sex differences in limb vasoconstriction responses to increases in transmural pressures. Am J Physiol Heart Circ Physiol 296: H186–H194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macey PM, Kumar R, Woo MA, Yan-Go FL, Harper RM. Heart rate responses to autonomic challenges in obstructive sleep apnea. PLoS One 8: e76631, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Morgan BJ, Denahan T, Ebert TJ. Neurocirculatory consequences of negative intrathoracic pressure vs. asphyxia during voluntary apnea. J Appl Physiol 74: 2969–2975, 1993 [DOI] [PubMed] [Google Scholar]
- 47.Muller MD, Drew RC, Cui J, Blaha CA, Mast JL, Sinoway LI. Effect of oxidative stress on sympathetic and renal vascular responses to ischemic exercise. Physiol Rep 1: 1–13, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muller MD, Mast JL, Cui J, Heffernan MJ, McQuillan PM, Sinoway LI. Tactile stimulation of the oropharynx elicits sympathoexcitation in conscious humans. J Appl Physiol 115: 71–77, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muller MD, Sauder CL, Ray CA. Melatonin attenuates the skin sympathetic nerve response to mental stress. Am J Physiol Heart Circ Physiol 305: H1382–H1386, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muller MD, Sauder CL, Ray CA. Mental stress elicits sustained and reproducible increases in skin sympathetic nerve activity. Physiol Rep 1: 1–8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med 161: 1465–1472, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Pan AW, He J, Kinouchi Y, Yamaguchi H, Miyamoto H. Blood flow in the carotid artery during breath-holding in relation to diving bradycardia. Eur J Appl Physiol Occup Physiol 75: 388–395, 1997 [DOI] [PubMed] [Google Scholar]
- 53.Patel H, Mast JL, Sinoway LI, Muller MD. Effect of healthy aging on renal vascular responses to local cooling and apnea. J Appl Physiol 115: 90–96, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quintana-Gallego E, Carmona-Bernal C, Capote F, Sanchez-Armengol A, Botebol-Benhamou G, Polo-Padillo J, Castillo-Gomez J. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respir Med 98: 984–989, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med 149: 722–726, 1994 [DOI] [PubMed] [Google Scholar]
- 56.Seitz MJ, Brown R, Macefield VG. Inhibition of augmented muscle vasoconstrictor drive following asphyxic apnoea in awake human subjects is not affected by relief of chemical drive. Exp Physiol 98: 405–414, 2013 [DOI] [PubMed] [Google Scholar]
- 57.Shahar E, Redline S, Young T, Boland LL, Baldwin CM, Nieto FJ, O'Connor GT, Rapoport DM, Robbins JA. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 167: 1186–1192, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Shoemaker JK, Hogeman CS, Khan M, Kimmerly DS, Sinoway LI. Gender affects sympathetic and hemodynamic response to postural stress. Am J Physiol Heart Circ Physiol 281: H2028–H2035, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Smith ML, Niedermaier ON, Hardy SM, Decker MJ, Strohl KP. Role of hypoxemia in sleep apnea-induced sympathoexcitation. J Auton Nerv Syst 56: 184–190, 1996 [DOI] [PubMed] [Google Scholar]
- 60.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96: 1897–1904, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stachenfeld NS, Silva C, Keefe DL, Kokoszka CA, Nadel ER. Effects of oral contraceptives on body fluid regulation. J Appl Physiol (1985) 87: 1016–1025, 1999 [DOI] [PubMed] [Google Scholar]
- 62.Steinback CD, Breskovic T, Frances M, Dujic Z, Shoemaker JK. Ventilatory restraint of sympathetic activity during chemoreflex stress. Am J Physiol Regul Integr Comp Physiol 299: R1407–R1414, 2010 [DOI] [PubMed] [Google Scholar]
- 63.Sudhir K, Jennings GL, Funder JW, Komesaroff PA. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women. Hypertension 28: 330–334, 1996 [DOI] [PubMed] [Google Scholar]
- 64.Tew GA, George KP, Cable NT, Hodges GJ. Endurance exercise training enhances cutaneous microvascular reactivity in post-menopausal women. Microvasc Res 83: 223–228, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Victor RG, Leimbach WN, Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension 9: 429–436, 1987 [DOI] [PubMed] [Google Scholar]
- 66.Wenner MM, Taylor HS, Stachenfeld NS. Progesterone enhances adrenergic control of skin blood flow in women with high but not low orthostatic tolerance. J Physiol 589: 975–986, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilson TE, Sauder CL, Kearney ML, Kuipers NT, Leuenberger UA, Monahan KD, Ray CA. Skin-surface cooling elicits peripheral and visceral vasoconstriction in humans. J Appl Physiol 103: 1257–1262, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Yang H, Cooke WH, Reed KS, Carter JR. Sex differences in hemodynamic and sympathetic neural firing patterns during orthostatic challenge in humans. J Appl Physiol 112: 1744–1751, 2012 [DOI] [PubMed] [Google Scholar]
- 69.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235, 1993 [DOI] [PubMed] [Google Scholar]
- 70.Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, Skatrud J. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med 157: 1746–1752, 1997 [PubMed] [Google Scholar]
- 71.Zimmerman MA, Sullivan JC. Hypertension: what's sex got to do with it? Physiology (Bethesda) 28: 234–244, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zou D, Grote L, Eder DN, Peker Y, Hedner J. Obstructive apneic events induce alpha-receptor mediated digital vasoconstriction. Sleep 27: 485–489, 2004 [DOI] [PubMed] [Google Scholar]