Abstract
Moment-to-moment adjustment of cerebral blood flow (CBF) to neuronal activity via neurovascular coupling is essential for the maintenance of normal neuronal function. Increased oxidative stress that occurs with aging was shown to impair neurovascular coupling, which likely contributes to a significant age-related decline in higher cortical function, increasing the risk for vascular cognitive impairment. Resveratrol is a polyphenolic compound that exerts significant antiaging protective effects in large vessels, but its effects on the cerebromicrovasculature remain poorly defined. The present study was undertaken to investigate the capacity of resveratrol to improve neurovascular coupling in aging. In aged (24-mo-old) C57BL/6 mice Nω-nitro-l-arginine methyl ester-sensitive, nitric oxide-mediated CBF responses to whisker stimulation and to the endothelium-dependent dilator acethylcholine (ACh) were impaired compared with those in young (3-mo-old) mice. Treatment of aged mice with resveratrol rescued neurovascular coupling and ACh-induced responses, which was associated with downregulation of cortical expression of NADPH oxidase and decreased levels of biomarkers of oxidative/nitrative stress (3-nitrotyrosine, 8-isoprostanes). Resveratrol also attenuated age-related increases in reactive oxygen species (ROS) production in cultured cerebromicrovascular endothelial cells (DCF fluorescence, flow cytometry). In conclusion, treatment with resveratrol rescues cortical neurovascular coupling responses to increased neuronal activity in aged mice, likely by restoring cerebromicrovascular endothelial function via downregulation of NADPH oxidase-derived ROS production. Beneficial cerebromicrovascular effects of resveratrol may contribute to its protective effects on cognitive function in aging.
Keywords: senescence, oxidative stress, ROS, reactive hyperemia, vascular cognitive impairment, microvascular dysfunction
normal brain function is critically dependent on a continuous, tightly controlled supply of oxygen and glucose through cerebral blood flow (CBF). Although energetic demands of neurons are very high, the brain has very little reserve capacity. During periods of intense neuronal activity, there is a requirement for rapid increases in oxygen and glucose delivery. This is ensured by neurovascular coupling, a vital mechanism of regulation of CBF that maintains optimal microenvironment of cerebral tissue by adjusting local blood flow to local neuronal activity (3). Since higher processes in the brain occur almost instantly, moment-to-moment adjustment of CBF via neurovascular coupling is essential for the maintenance of normal neuronal function. There is strong evidence that neurovascular coupling is impaired in the elderly (12, 40, 43, 53), which likely contributes to a significant age-related decline in higher cortical function, including cognition (38) and gait coordination (39). Thus therapeutic interventions that improve neurovascular coupling in elderly patients have the potential to improve a range of age-related neurological deficits.
Neurovascular coupling and cerebral functional hyperemia depend on a coordinated interaction of activated neurons, astrocytes, and vascular endothelial and smooth muscle cells. Transduction of signals from neurons and astrocytes initiate regional vasodilation in the cerebral microcirculation, which involve production and release of nitric oxide (NO) by microvascular endothelial cells (14, 41, 55). Previous studies demonstrate that aging is associated with an increased production of NADPH oxidase-derived reactive oxygen species (ROS) in the cerebral microvasculature, which contribute to compromised neurovascular coupling in aged mice (28), likely by decreasing bioavailability of NO and promoting endothelial dysfunction (13, 28, 43, 53). On the basis of the aforementioned findings it is logical to hypothesize that pharmacological treatments that improve endothelial function will have the capacity to improve neurovascular coupling in aged individuals.
Recent studies provide strong evidence that treatment of laboratory rodents with resveratrol (3,4′,5-trihydroxystilbene), a plant-derived polyphenolic compound, exerts significant endothelial protective effects in the aorta and vessels from the peripheral circulation both during aging and in pathophysiological conditions that are associated with accelerated vascular aging (31, 46, 50, 51, 57). Importantly, resveratrol was shown to increase bioavailability of endothelium-derived NO and inhibit NADPH oxidases in large vessels (31, 33, 54, 56, 57). Although resveratrol has been shown to exert beneficial effects on the age-dependent decline in cognitive function (26, 58), its potential protective effect on the aged cerebral microvasculature has not been investigated.
The present study was designed to test the hypothesis that resveratrol treatment, similar to its documented vasoprotective effects in the aorta, can restore cerebromicrovascular endothelial function and thus improve neurovascular coupling in aged mice. To achieve this goal, aged mice were treated with resveratrol followed by tests for neurovascular coupling and cerebromicrovascular endothelial function. Markers of oxidative stress and expression of NADPH oxidase in the cerebral cortex were also assessed. To substantiate the in vivo findings, the effects of resveratrol on ROS production and expression of NADPH oxidase in activated cultured astrocytes and in cerebromicrovascular endothelial cells derived from aged animals were obtained in vitro.
MATERIALS AND METHODS
Animals, resveratrol treatment.
Young (3 mo, n = 30) and aged (24 mo, n = 30) male C57BL/6 mice were purchased from the aging colony maintained by the National Institute on Aging at Charles River Laboratories (Wilmington, MA). Animals were housed under specific pathogen-free barrier conditions in the Rodent Barrier Facility at University of Oklahoma Health Sciences Center under a controlled photoperiod (12-h light; 12-h dark) with unlimited access to water. Mice in each age cohort were assigned to two groups (n = 15 each group) and were fed a standard AIN-93G diet (ad libitum) or a standard diet plus resveratrol (200 mg·kg−1·day−1, for 10 days) following previously described protocols (31, 46, 50). All procedures were approved by the Institutional Animal Use and Care Committees of the University of Oklahoma Health Sciences Center.
Measurement of cerebral blood flow responses to whisker stimulation and pharmacological studies.
At the end of the treatment period, mice in each group were anesthetized with α-chloralose (50 mg/kg ip) and urethane (750 mg/kg ip), endotracheally intubated, and ventilated (MousVent G500; Kent Scientific, Torrington, CT). Rectal temperature was maintained at 37°C using a thermostatic heating pad (Kent Scientific).
End-tidal CO2 (including dead space) was maintained between 3.2 and 3.7% to keep blood gas values within the physiological range (PaCO2: 37.3 ± 1.9 mmHg; PaO2: 108 ± 3 mmHg). Mice were immobilized and placed on a stereotaxic frame (Leica Microsystems, Buffalo Grove, IL), the scalp and periosteum were pulled aside and the skull was removed over the barrel cortex, and the dura was gently removed. The cranial window was superfused with artificial cerebrospinal fluid (composition: 119 mM NaCl, 26.2 mM NaHCO3, 2.5 mM KCl, 1 mM NaH2PO4, 1.3 mM MgCl2, 10 mM glucose, and 2.5 mM CaCl2, pH 7.3, 37°C). The right femoral artery was cannulated for arterial blood pressure measurement (Living Systems Instrumentations, Burlington, VT). The blood pressure was within the physiological range throughout the experiments (in mmHg; young: 100 ± 2; aged: 103 ± 2.3; and aged + resveratrol: 108 ± 1.5). A laser Doppler probe (Transonic Systems, Ithaca, NY) was placed above the barrel cortex (1–1.5 mm posterior and 3–3.5 mm lateral to bregma), and to achieve the highest CBF response the right whiskers were stimulated for 1 min at 10 Hz from side to side. Changes in CBF (n = 7 in each group) were assessed above the left barrel cortex in three trials, separated by 5- to 10-min intervals. CBF responses to whisker stimulation were repeated in the presence of the NADPH oxidase inhibitor apocynin (3 × 10−4 mol/l) administered topically to the brain surface for 30 min (8, 57). In a separate series of experiments (n = 8 in each group), CBF responses to whisker stimulation and to topical administration of acetylcholine (ACh; 10−5 mol/l) and adenosine (5 × 10−5 mol/l) were obtained in the presence and absence of the NO synthase inhibitor Nω-nitro-l-arginine methyl ester (l-NAME; 10−4 mol/l, 20 min) and then in the presence of the muscarinic ACh receptor antagonist atropine (10−5 mol/l). Changes in CBF were expressed as percent (%) increase from the baseline value (16). The experimenter was blinded to the treatment of the animals. At the end of the experiments, the animals were transcardially perfused and decapitated. The brains were immediately removed, and pieces of the somatosensory and motor cortex were isolated and frozen for subsequent analysis.
Assessment of the effect of in vivo resveratrol treatment on markers of oxidative stress.
To characterize the effect of resveratrol treatment on cellular redox homeostasis in aging, 3-nitrotyrosine (a marker for peroxynitrite action) was assessed in homogenates of cortical samples using OxiSelect Protein Nitrotyrosine ELISA Kits (Cell Biolabs), according to the manufacturer's guidelines, as previously described (44).
As an additional marker of oxidative stress, the total tissue 8-isoprostane content was measured in cerebral cortical samples using the 8-isoprostane EIA kit (Cayman Chemicals) according to the manufacturer's guidelines, as previously described (32). In brief, samples were homogenized in 100 mmol/l potassium phosphate buffer (pH 7.4) containing 1 mmol/l EDTA and 0.005% BHT using a Precellys 24 tissue homogenizer with CK28 ceramic beads at 5,500 rpm for two cycles of 20 s. Samples were centrifuged, and the supernatant was collected. Fifty microliters of each sample were reserved for protein determination. An equal volume of 15% KOH was added to the remaining sample in each tube, and samples were incubated for 1 h at 40°C to hydrolyze 8-isoprostane esterified to phospholipids. Samples were neutralized by the addition of 1 mol/l HCl. Samples were then purified using 8-isoprostane affinity columns according to the manufacturer's guidelines, dried under nitrogen, and resuspended with EIA buffer before analysis. A standard curve was established by serial dilution of 8-isoprostane between 0.8 and 500 pg/ml using EIA Buffer as the matrix. The concentration of each sample was calculated from a logistic four-parameter fit of the standard concentrations vs. percent bound/maximum bound (%B/B0). Protein concentrations were determined using a Bradford assay.
Quantitative real-time RT-PCR.
A quantitative real-time RT-PCR technique was used to analyze mRNA expression for following genes in cortical samples of mice from each experimental group: Nox1, Nox2, Nox4, and Ncf1 (p47phox) using a Strategen MX3000 platform, as previously reported (4) (Table 1). In brief, total RNA was isolated with a Mini RNA Isolation Kit (Zymo Research, Orange, CA) and was reverse transcribed using Superscript III RT (Invitrogen) as described previously (4). Amplification efficiencies were determined using a dilution series of a standard vascular sample. Quantification was performed using the efficiency-corrected ΔΔCq method. The relative quantities of the reference genes Hprt, Ywhaz, B2m, and Actb were determined, and a normalization factor was calculated based on the geometric mean for internal normalization. Fidelity of the PCR reaction was determined by melting temperature analysis and visualization of the product on a 2% agarose gel.
Table 1.
Oligonucleotides for quantitative RT-PCR
| mRNA Targets | Description, Aliases | Species | Sense | Antisense |
|---|---|---|---|---|
| Nox1 | NADPH oxidase 1, mitogenic oxidase 1 | M. musculus | TTCACAGTTATTCATATCATTGC | GAGATAGGCTGGAGAGAAC |
| Nox2 | Cytochrome b-245, beta-polypeptide (Cybb), gp91phox | M. musculus | GAAGACAACTGGACAGGAACC | CCGACTCTGGCATTCACAC |
| Nox4 | NADPH oxidase 4 | M. musculus | GGCTTGTCCAGGCTTGATGTG | GCAAGGAAGTTCACTCAACATAGC |
| Ncf1 | Neutrophil cytosolic factor 1, p47phox | M. musculus | CTTTCATGTCTCTATTTCCATC | CGGAGTTACAGGCAAATG |
| Hprt | Hypoxanthine phosphoribosyltransferase 1 | M. musculus | TGCTGCGTCCCCAGACTTTTG | AGATAAGCGACAATCTACCAGAGG |
| Ywhaz | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta-polypeptide 1 | M. musculus | ACTGTCTTGTCACCAACCATTC | GGGCTGTAGAGAGGATGAGG |
| B2m | Beta-2-microglobulin | M. musculus | CGGTCGCTTCAGTCGTCAG | CAGTTCAGTATGTTCGGCTTCC |
| Actb | Beta-actin | M. musculus | AATAAGTGGTTACAGGAAGTC | ATGAAGTATTAAGGCGGAAG |
| Nox2 | Cytochrome b-245, beta-polypeptide (Cybb), gp91phox | R. norvegicus | TCTACTTCTACTGGCTGTG | TTTCTCCTCATCGTGGTG |
| Nox4 | NADPH oxidase 4 | R. norvegicus | TGCCTCCATCAAGCCAAG | TTCCAGTCATCCAGTAGAGTG |
| B2m | Beta-2-microglobulin | R. norvegicus | ATTCACACCCACCGAGAC | GGATCTGGAGTTAAACTGGTC |
| Hprt | Hypoxanthine phosphoribosyltransferase 1 | R. norvegicus | AAGACAGCGGCAAGTTGAATC | AAGGGACGCAGCAACAGAC |
| Ywhaz | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta-polypeptide 1 | R. norvegicus | CTTCTCTGTGTTCTACTATG | TATCAAGTTCAGCAATGG |
Assessment of the effect of in vitro resveratrol treatment on ROS production in cultured cerebromicrovascular endothelial cells and astrocytes.
To confirm the direct antioxidative effect of resveratrol in vitro in cells involved in neurovascular coupling, we assessed the effect of resveratrol on cellular ROS production in cultured primary cerebromicrovascular endothelial cells (CMVECs) and astrocytes. The establishment and characterization of the cell strains used have been recently reported (45, 49).
In brief, to establish primary cultures of CMVECs, the brains of male 3- and 24-mo-old F344xBN rats (obtained from the National Institute on Aging) were removed aseptically, rinsed in ice cold PBS and minced into ≈1-mm squares. The tissue was washed twice in ice cold 1× PBS by low-speed centrifugation (50 g, 2–3 min). The diced tissue was digested in a solution of collagenase (800 U/g tissue), hyaluronidase (2.5 U/g tissue), and elastase (3 U/g tissue) in 1ml PBS/100 mg tissue for 45 min at 37°C in a rotating humid incubator. The digested tissue was passed through a 100-um cell strainer. The single cell lysate was centrifuged for 2 min at 70 g. After the supernatant was removed, the pellet was washed twice in cold PBS supplemented with 2.5% fetal calf serum (FCS) and the suspension was centrifuged at 300 g for 5 min at 4C. To create an endothelial cell enriched fraction, the cell suspension was centrifuged using an OptiPrep gradient solution (Axi-Shield, PoC). Briefly, the cell pellet was resuspended in HBSS and mixed with 40% iodixanol thoroughly [final concentration: 17% (wt/vol) iodixanol solution; ρ = 1.096 g/ml]. Two milliliters of HBSS were layered on top and centrifuged at 400 g for 15 min at 20°C. Endothelial cells, which banded at the interface between HBSS and the 17% iodixanol layer, were collected. The endothelial cell enriched fraction was incubated for 30 min at 4°C in the dark with anti-CD31/PE (BD Biosciences, San Jose, CA) and anti-MCAM/FITC (BD Biosciences). After the cells were washed twice with MACS Buffer (Milltenyi Biotech, Cambridge, MA), anti-FITC magnetic beads labeled with anti-PE magnetic bead-labeled secondary antibodies were used for 15 min at room temperature. Endothelial cells were collected by magnetic separation using the MACS LD magnetic separation columns according to the manufacturer's guidelines (Milltenyi Biotech). The endothelial fraction was cultured on fibronectin-coated plates in endothelial growth medium (Cell Application, San Diego, CA) for 10 days. Endothelial cells were phenotypically characterized by flow cytometry (GUAVA 8HT; Merck Millipore, Billerica, MA). Briefly, antibodies against five different endothelial-specific markers were used (anti-CD31-PE, anti-erythropoietin receptor-APC, anti-VEGF R2-PerCP, anti-ICAM-fluorescein, and anti-CD146-PE) and isotype-specific antibody labeled fractions served as negative controls. Flow cytometric analysis showed that after the third cycle of immunomagnetic selection there were virtually no CD31, CD146, EpoR, and VEGFR2 cells in the resultant cell populations. All antibodies were purchased from R&D Systems (Minneapolis, MN). To assess the direct effects of resveratrol on endothelial ROS production, primary CMVECs derived from young and aged rats were treated with resveratrol in vitro (10 μmol/l, for 24 h).
Primary cultures of astrocytes were derived from postnatal days 2–5 F344 rat pups following previously described methods (2). Briefly, cortical tissue was enzymatically and mechanically digested, resuspended in growth media [DMEM containing 2% NuSerum, 10% fetal bovine serum, penicillin (10 U/ml), streptomycin (10 μg/ml), and l-glutamine (29.2 μg/ml)] at a density of 2.5 million cells/ml, and seeded on 50 μg/ml poly-d-lysine-coated T75-flasks. Fresh growth media was supplemented every 3–4 days. Oligodendrocytes and microglia were removed via immunopanning on day 10. Astrocytes were then trypsinized and passaged into a 96-well plate and grown in fresh growth media. Resulting cultures are ∼93% GFAP-positive astrocytes (2). To assess the direct effects of resveratrol on ROS production by astrocytes, primary astrocytes in culture were incubated for 24 h with resveratrol (10 μmol/l) and then activated by treatment with 10 ng/ml TNF-α, 10 ng/ml IL-1β, and 10 ng/ml IL-6 (for 4 h).
After the experimental period peroxide production in cultured CMVECs and astrocytes was assessed using the cell-permeant oxidative fluorescent indicator dye CM-H2DCFDA [5 (and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate-acetyl ester; Life Technology, Carlsbad, CA] as previously reported (7, 48). In brief, following the treatment period cells were incubated with CM-H2DCFDA (10 μM, at 37°C, for 30 min). CM-H2DCFDA fluorescence was assessed by flow cytometry as previously reported (7, 48) using the Guava easyCyte 8HT flow-cytometer (Millipore).
Statistical analysis.
Data were analyzed by two-way ANOVA followed by Tukey's post hoc test. P < 0.05 was considered statistically significant. Data are expressed as mean ± SE.
RESULTS
Treatment with resveratrol rescues NO mediation of neurovascular coupling in aged mice.
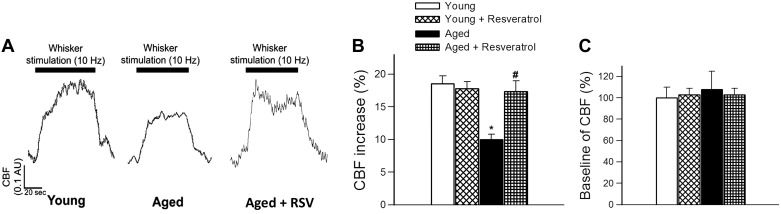
Changes in CBF in the whisker barrel cortex in response to contralateral whisker stimulation was significantly decreased in aged mice compared with young animals indicating impaired neurovascular coupling in aging (representative tracings are shown in Fig. 1A, summary data are shown in Fig. 1B) (28). We found that 10 days of treatment with resveratrol significantly increased CBF responses induced by contralateral whisker stimulation in aged mice, restoring neurovascular coupling to levels observed in young mice (Fig. 1B). Resveratrol treatment did not affect neurovascular coupling in young animals (Fig. 1B). Resveratrol treatment did not affect baseline CBF neither in young mice nor in aged mice (Fig. 1C).
Fig. 1.
Resveratrol (RSV) treatment rescues neurovascular coupling in aged mice. A: representative traces of cerebral blood flow (CBF) measured with a laser Doppler probe above the whisker barrel cortex during contralateral whisker stimulation (1 min, 10 Hz) in young (3 mo old), aged (24 mo old), and resveratrol-treated aged mice[0.1 arbitrary units (AU) corresponds to ∼5% increase in CBF from baseline]. B: summary data of the effect of resveratrol treatment (200 mg/kg, 10 days in food) on CBF responses to whisker stimulation and on baseline CBF (C) in young and aged mice. Data are means ± SE (n = 8 in each group). *P < 0.05 vs. young; #P < 0.05 vs. aged.
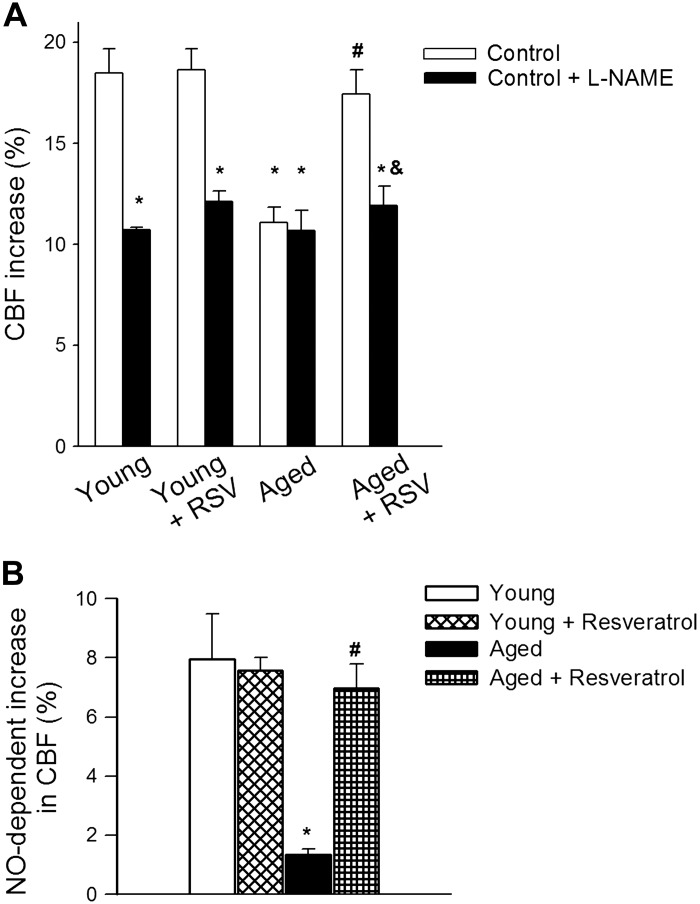
In young animals administration of the NO synthase inhibitor l-NAME significantly decreased CBF responses in the barrel cortex elicited by contralateral whisker stimulation, eliminating the differences between the age groups (Fig. 2A). In untreated aged animals administration of l-NAME was without effect (Fig. 2A). In contrast in resveratrol-treated aged mice l-NAME significantly decreased CBF responses elicited by whisker stimulation (Fig. 2A), suggesting that resveratrol treatment restored the NO mediation of neurovascular coupling in aged animals (Fig. 2B).
Fig. 2.
Resveratrol treatment rescues nitric oxide (NO) mediation of neurovascular coupling in aged mice. A: changes of CBF in response to whisker-stimulation in the absence and presence of the NO synthase inhibitor Nω-nitro-l-arginine methyl ester (l-NAME) above the barrel cortex in control and resveratrol-treated young (3 mo old) and aged (24 mo old) mice. B: l-NAME-sensitive component of CBF responses to whisker stimulation. Data are means ± SE (n = 7–8 in each group). *P < 0.05 vs. young; #P < 0.05 vs. aged; &P < 0.05 vs. aged + resveratrol.
Treatment with resveratrol reverses aging-induced impairment of ACh-induced, NO-mediated endothelial responses.
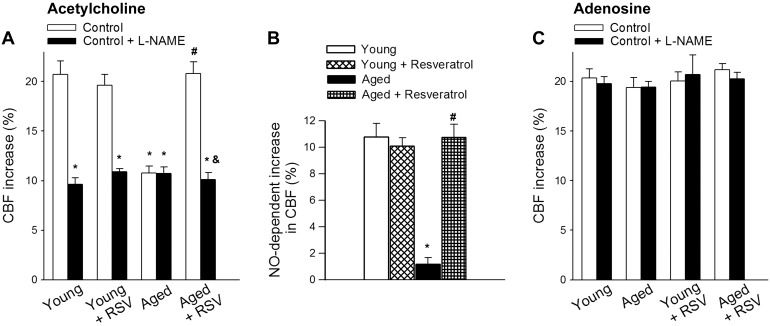
Topical application of the endothelium-dependent vasodilator agent ACh (10−5 mol/l) resulted in a significant increase in CBF in the barrel cortex of young mice (Fig. 3A). ACh-induced increases in CBF were significantly attenuated (∼50%) in aging mice (Fig. 3A). Treatment of aged mice with resveratrol significantly improved ACh-induced CBF responses, restoring responses to the level observed in young mice (Fig. 3A). Administration of ACh was reported to affect CBF through stimulation of endothelial NO production and by activation of neuronal muscarinic ACh receptors (14, 18, 55). To assess the endothelium-dependent component of ACh-induced responses, l-NAME was applied. l-NAME significantly inhibited ACh-induced CBF responses both in young animals and resveratrol-treated aged mice, whereas it did not exert a significant effect in untreated aged mice (Fig. 3, A and B). After administration of l-NAME, ACh-induced CBF responses did not differ among young, aged and resveratrol-treated aged mice (Fig. 3, A and B). Coadministration of l-NAME and the muscarinic ACh receptor antagonist atropine (5 × 10−5 mol/l) abolished the remaining ACh-induced CBF response in each experimental group (data not shown). CBF responses elicited by administration of adenosine (which predominantly acts on vascular smooth muscle cells) did not differ significantly among the experimental groups and were not affected significantly by administration of l-NAME or l-NAME plus atropine (Fig. 3, A–C).
Fig. 3.
Resveratrol treatment rescues acetylcholine-induced, endothelial NO-mediated CBF responses in aged mice. Changes of CBF are shown in response to the endothelium-dependent dilator acethylcholine (A) and the endothelium-independent dilator adenosine (C) in the absence and presence of the NO synthase inhibitor l-NAME above the barrel cortex in control and resveratrol-treated young (3 mo old) and aged (24 mo old) mice. B: l-NAME-sensitive component of acetylcholine-induced CBF responses. Data are means ± SE (n = 7–8 in each group). *P < 0.05 vs. young; #P < 0.05 vs. aged; &P < 0.05 vs. aged + resveratrol.
NADPH oxidase inhibitor apocynin reverses neurovascular dysfunction in aged mice.
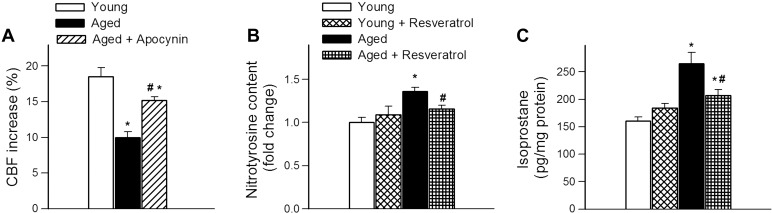
The CBF response in aged animals was significantly increased by treatment with the NADPH oxidase inhibitor apocynin (Fig. 4A) supporting the concept that increased production of ROS by NADPH oxidases play a central role in aging-induced cerebromicrovascular dysfunction.
Fig. 4.
Resveratrol treatment attenuates oxidative stress in aged mice. A: administration of the NADPH oxidase inhibitor apocynin improves CBF responses elicited by whisker-stimulation in aged (24 mo old) mice. CBF responses in young (3 mo old) mice are shown for comparison. Data are means ± SE (n = 6 in each group) *P < 0.05 vs. young; #P < 0.05 vs. aged control. B: cortical 3-nitrotyrosine content and 8-isoprostane level (C) in young, resveratrol-treated young, aged, and resveratrol-treated aged mice (n = 6 in each group). Data are means ± SE. *P < 0.05 vs. young control; #P < 0.05 vs. aged control.
Treatment with resveratrol attenuates aging-induced oxidative stress.
3-nitrotyrosine content in the cerebral cortex was significantly elevated in aged mice (Fig. 4B) consistent with increased oxidative/nitrosative stress in the aged brain (6, 23). Resveratrol treatment significantly reduced 3-nitrotyrosine content in the cortex of aged mice (Fig. 4B), as well as the level of 8-isoprostane. These results support the concept that antioxidative effects of resveratrol play a central role in its microvascular protective effects in aging.
In vitro treatment with resveratrol attenuates oxidative stress in aged microvascular endothelial cells and activated astrocytes.
To substantiate the antioxidative effects of resveratrol in vitro, we assessed the effects of resveratrol on cellular ROS production in cultured CMVECs derived from aged animals using the DCF fluorescence method. We found that ROS production in CMVECs derived from aged animals was significantly increased compared with that in CMVECs derived from young animals (Fig. 5, A and B). Resveratrol treatment significantly decreased ROS production in aged CMVECs, eliminating the difference between the two age groups (Fig. 5B). Aging is also associated with activation of astrocytes (36), and increased ROS production in activated astrocytes (37) may also affect the efficiency of neurovascular coupling. To determine whether resveratrol can also affect astrocytic ROS production, activated cultured astrocytes were treated with resveratrol. We found that resveratrol treatment significantly decreased ROS production in cytokine-stimulated astrocytes (Fig. 5C).
Fig. 5.
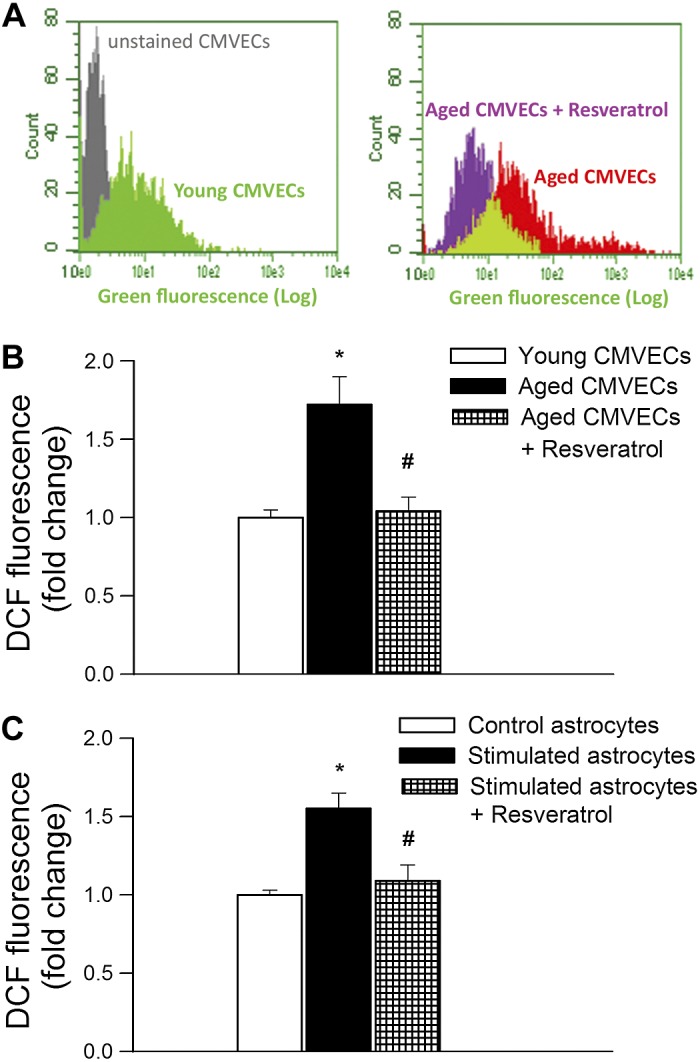
Resveratrol decreases oxidative stress in cultured cerebromicrovascular endothelial cells and astrocytes. A: representative figures showing flow cytometric analysis of DCF fluorescence [indicating reactive oxygen species (ROS) production] in primary cerebromicrovascular endothelial cells (CMVECs) derived from young and aged F344xBN rats. Shown is the effect of resveratrol treatment (10 μmol/l, for 24 h) on ROS production by aged CMVECs. Appropriate control (unstained cells) is also shown. B: resveratrol (10 μmol/l, for 24 h) attenuates increased ROS production in aged CMVECs. Data are means ± SE (n = 8 in each group). *P < 0.05 vs. young control; #P < 0.05 vs. aged control. C: resveratrol (10 μmol/l, for 24 h) attenuates increased ROS production in activated astrocytes. Primary astrocytes in culture were stimulated by treatment with 10 ng/ml TNF-α, 10 ng/ml IL-1β, and 10 ng/ml IL-6 (for 4 h) with and without resveratrol pretreatment (10 μmol/l, for 24 h). Cellular ROS production was measured using the DCF fluorescence method by flow cytometry. Data are means ± SE (n = 8 in each group). *P < 0.05 vs. control; #P < 0.05 vs. stimulated cells (no resveratrol).
Treatment with resveratrol attenuates expression of NADPH oxidases in the aged mouse brain.
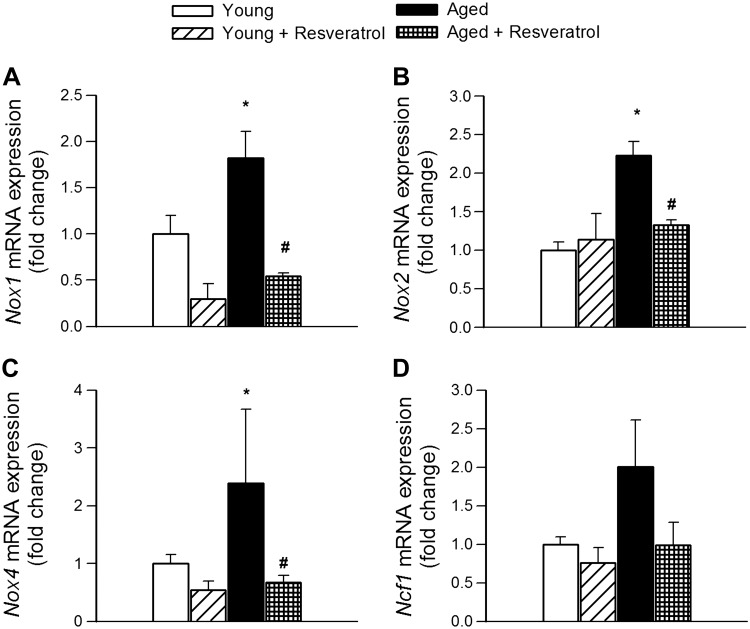
NADPH oxidases are important sources of ROS in the cerebral vasculature, whose increased expression and activity contribute to impaired neurovascular coupling (Fig. 4A) (28). We found that in the cortex of aged mice mRNA expression of the NADPH oxidase subunits Nox1, Nox2, and Nox4 were upregulated compared with young mice (Fig. 6, A–C), extending recent findings (28). Treatment of aged mice with resveratrol resulted in significant downregulation of these NADPH oxidase subunits, normalizing their expression to control levels (Fig. 6, A–C). The expression of Ncf1 also tended to increase in the aged brain and there was also a tendency for downregulation by resveratrol treatment, although these changes did not reach statistical significance (Fig. 6D).
Fig. 6.
Resveratrol treatment results in downregulation of NADPH oxidase expression in the aged mouse brain. Effect of resveratrol treatment (200 mg/kg po, for 10 days) on mRNA expression of NADPH oxidase subunits Nox1 (A), Nox2 (B), Nox4 (C), and Ncf1 (p47phox; D) in the cerebral cortex of young and aged mice. Data are means ± SE (n = 6 in each group). *P < 0.05 vs. young control; #P < 0.05 vs. aged control.
In vitro treatment with resveratrol downregulates expression of NADPH oxidases in aged microvascular endothelial cells.
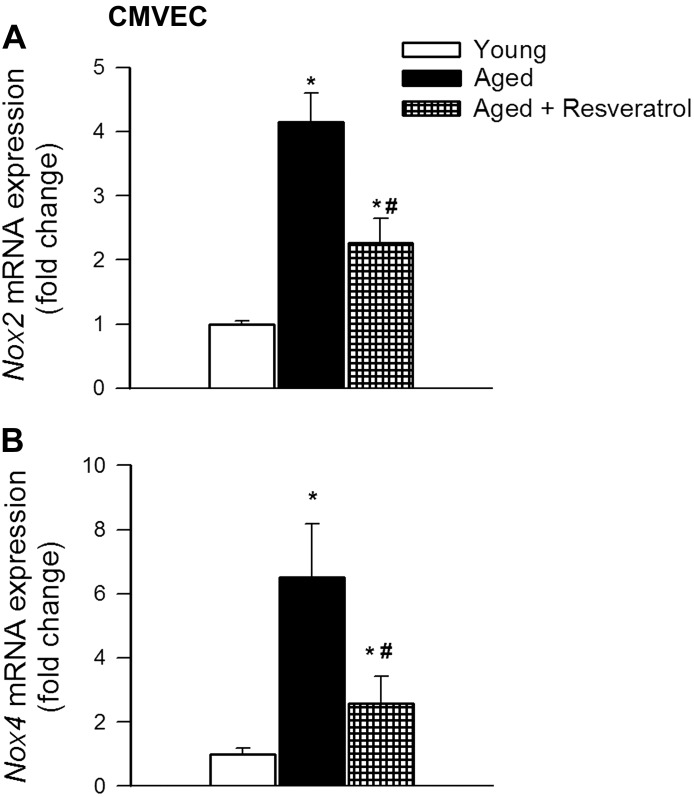
To extend our in vivo findings, a parallel in vitro analysis of the effect of resveratrol on expression of NADPH oxidases in aged CMVECs was undertaken. We found that in aged CMVECs mRNA expression of the NADPH oxidase subunits Nox2 and Nox4 were upregulated compared with young CMVECs (Fig. 7). Resveratrol treatment resulted in significant downregulation of these NADPH oxidase subunits in aged CMVECs (Fig. 7).
Fig. 7.
Resveratrol downregulates NADPH oxidase expression in aged CMVECs in culture. Shown are the effects of resveratrol treatment (10 μmol/l, for 24 h) on mRNA expression of NADPH oxidase subunits Nox2 (A) and Nox4 (B) in aged CMVECs. Data are means ± SE (n = 6 in each group). *P < 0.05 vs. young CMVECs; #P < 0.05 vs. untreated aged CMVECs.
DISCUSSION
This is the first study to demonstrate positive effects of resveratrol on neurovascular coupling responses in a mouse model of aging that recapitulates cerebromicrovascular alterations and deficits of higher cortical function present in elderly humans.
We demonstrate that aging leads to profound neurovascular dysregulation (28), characterized by impaired CBF responses induced by neuronal activity (Fig. 1). Age-related impairment of neurovascular coupling manifests in elderly patients (40, 43, 53) and has been causally linked to the decline in higher cortical functions including cognition (38, 39). Here, we demonstrate for the first time that aging-induced decrease in neurovascular coupling is restored by treatment with resveratrol (Fig. 1B). Restoration of a key homeostatic mechanism matching energy supply with the needs of active neuronal tissue likely has beneficial effect on brain function in aging. Thus it is logical to hypothesize that the significant improvement of neurovascular coupling contributes to the documented beneficial effect of resveratrol treatment on cognitive function that has been reported in aged rodents (19, 26, 58).
Our findings have important clinical relevance. In humans resveratrol is well tolerated with no reports of significant toxicity (5). Although the bioavalibility of resveratrol in parenchymal tissues is relatively low, the vascular endothelium, which is in direct contact with blood, is considered an ideal target for circulating resveratrol and its active metabolites (5). There is initial evidence that in humans short-term resveratrol administration results in dose-dependent increases in cerebral blood flow during task performance (17). In addition, epidemiological studies suggest that Mediterranean diets that are rich in resveratrol are associated with significantly reduced risk of age-related cognitive decline in humans (52). Recent evidence suggests that regular consumption of the cocoa-derived dietary polyphenol epicathecin (38) may also improve cognitive function in older individuals; thus further studies addressing whether in geriatric patients short-term and/or long-term dietary intake of resveratrol as a supplement can improve neurovascular coupling and reduce the incidence or severity of age-related cognitive decline appear critical (25).
There is growing experimental evidence that endothelial NO production has an important role in neurovascular coupling (14, 20, 41). This concept is supported by our observation that inhibition of NO synthesis dramatically reduces neurovascular coupling in young mice (Fig. 2A). The findings that in aged mice inhibition of endothelial NO synthesis did not decrease the CBF responses elicited by neuronal activity (Fig. 2A) suggest that cerebromicrovascular endothelial dysfunction underlies age-related dysregulation of neurovascular coupling (Fig. 2B) (28). The dilator capacity of smooth muscle cells in the aged cerebral vasculature is not impaired, as shown by the intact responses to adenosine (Fig. 3C) and the NO donor S-nitroso-N-acetyl-penicillamine (28). Resveratrol treatment restored NO mediation of neurovascular coupling in aged animals (Fig. 2A) supporting the concept that endothelial protective effects play a key role in preservation of neuronal function in aging. Additional evidence in support of this concept comes from the observations that resveratrol treatment rescued ACh-induced, endothelial NO-mediated cerebromicrovascular dilation in aged mice (Fig. 3A). Recovery of cerebromicrovascular endothelial function with resveratrol was anticipated based on its documented benefit in restoring endothelial function in large vessels of aged mice (31) and in mice with accelerated vascular aging (1, 57).
The mechanism by which aging impairs cerebromicrovascular endothelial function involves an increased breakdown of NO by elevated levels of ROS. Several lines of evidence support this concept. First, aging is associated with increased ROS production in microvessels both in the brain and other vascular beds (9, 28). Previous studies suggest that increased activity/expression of NADPH oxidases contribute significantly to aging-induced microvascular oxidative stress (28). Accordingly, our findings that acute inhibition of NADPH oxidases is able to rescue neurovascular coupling in aged mice (Fig. 4A) provides direct evidence for the role of increased production of NADPH oxidase-derived ROS in cerebromicrovascular endothelial dysfunction (Fig. 1), extending earlier findings of the laboratory of Iadecola et al. (28). Interestingly, in Aβ-overproducing APP mice CBF is compromised at least in part by enhanced generation of NADPH-derived ROS (29, 30). Second, we demonstrate that NADPH oxidase expression is significantly upregulated in the brain of aged mice (Fig. 6). Third, in the microcirculation of aged rodents endothelium-derived NO was shown to react with O2− forming ONOO−, thus decreasing the bioavailability of NO (9, 27). Aged mouse brains exhibit an increased 3-nitrotyrosine content (Fig. 4B), a biomarker of increased ONOO− formation, suggesting that impaired endothelial mediation of cerebromicrovascular dilation in aging is due to increased scavenging of vasodilator NO (28).
We propose that resveratrol treatment restores neurovascular coupling and endothelium-dependent cerebromicrovascular dilation in aged mice by attenuating oxidative stress. In support of this concept we demonstrate that resveratrol treatment effectively attenuates age-related cerebral oxidative/nitrative stress, as indicated by the significant decrease in nitrotyrosine content in the brain of aged mice (Fig. 4, B and C). Further, administration of resveratrol significantly attenuates ROS production in aged CMVECs (Fig. 5, A and B). Astrocytes are important conduits between neuronal and vascular activity. Important for the present discussion is that aging is also associated with activation of astrocytes (36) and increased ROS production in activated astrocytes (37) may also negatively impact the efficiency of neurovascular coupling. In that regard, it is significant that resveratrol treatment also inhibits ROS production in activated astrocytes in vitro (Fig. 5C). One can hypothesize that resveratrol treatment of aged mice in addition to its beneficial endothelial effects may also attenuate astrocyte-derived ROS generation, improving neurovascular coupling (e.g., by decreasing production of constrictor arachidonic acid metabolites).
The mechanisms by which resveratrol attenuates NADPH oxidase-derived ROS production are likely multifaceted. We demonstrate that resveratrol treatment results in a significant downregulation of NADPH oxidases in the aged mouse brain (Fig. 6). This finding was further confirmed by a parallel in vitro analysis of the effect of resveratrol in aged CMVECs (Fig. 7). Previously, we showed that resveratrol also downregulates NADPH oxidase in the aorta of aged mice as well (31). There are also studies showing resveratrol-induced downregulation of NADPH oxidases in the vasculature in pathological conditions associated with accelerated vascular aging (57). Interestingly, NOX4-containing NADPH oxidase is present in the mitochondria and likely contributes to age-related increases in mitochondrial oxidative stress (11). In that regard it is important that resveratrol was shown to effectively reduce mitochondrial oxidative stress (47). In addition, resveratrol may also directly inhibit NADPH oxidase activation via a SIRT1-dependent pathway (54). Finally, there is also strong evidence that resveratrol activates the transcription factor Nrf2 in the vascular wall, upregulating endogenous antioxidant systems, which likely act in concert to decrease cellular ROS levels produced by NADPH oxidases (46).
In conclusion, our results demonstrate that resveratrol exerts beneficial cerebromicrovascular effects in aged mice. Resveratrol rescued neurovascular coupling responses to increased neuronal activity in the aged cortex, which likely contributes to improvement of higher cortical function. Our findings, taken together with the results of earlier studies (26), point to benefits at several levels of cerebrovascular pathology of aging and to the potential use of resveratrol as therapy for prevention of aging-induced cognitive decline (19). Importantly, neurovascular coupling is compromised both in patients with Alzheimer's disease (AD) (15, 21, 22, 24, 34, 35) and in experimental models of AD (42), which is believed to accelerate clinical deterioration. Thus our findings are also likely relevant to the treatment of AD in elderly patients as well.
GRANTS
This work was supported by grants from the American Heart Association (to P. Toth, A. Csiszar, and Z. Ungvari), American Federation for Aging Research (to A. Csiszar), Oklahoma Center for the Advancement of Science and Technology (to A. Csiszar, Z. Ungvari, and W. E. Sonntag), Hungarian National Science Research Fund (OTKA) K 108444, Developing Competitiveness of Universities in the South Transdanubian Region, “Identification of new biomarkers . . .”, SROP-4.2.2.A-11/1/KONV-2012-0017 and “Complex examination of neuropeptide . . .” SROP-4.2.2.A-11/1/KONV-2012-0024 (to A. Koller and Z. Ungvari), National Institutes of Health (AG-031085 to A. Csiszar; AT-006526 to Z. Ungvari; and AG-038747, NS-056218, and P01-AG-11370 to W. E. Sonntag), and Ellison Medical Foundation (to W. E. Sonntag).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.T., Z.T., P.B., A.C., and Z.I.U. conception and design of research; P.T., S.T., Z.T., N.M.A., D.S., T.G., W.E.S., and A.C. performed experiments; P.T., N.M.A., D.S., A.C., and Z.I.U. analyzed data; P.T., Z.T., P.B., A.K., A.C., and Z.I.U. interpreted results of experiments; P.T., A.C., and Z.I.U. prepared figures; P.T. and A.C. drafted manuscript; P.T., S.T., Z.T., N.M.A., D.S., T.G., P.B., A.K., W.E.S., A.C., and Z.I.U. edited and revised manuscript; P.T., S.T., Z.T., N.M.A., D.S., T.G., P.B., A.K., W.E.S., A.C., and Z.I.U. approved final version of manuscript.
REFERENCES
- 1.Arrick DM, Sun H, Patel KP, Mayhan WG. Chronic resveratrol treatment restores vascular responsiveness of cerebral arterioles in type 1 diabetic rats. Am J Physiol Heart Circ Physiol 301: H696–H703, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashpole NM, Chawla AR, Martin MP, Brustovetsky T, Brustovetsky N, Hudmon A. Loss of calcium/calmodulin-dependent protein kinase II activity in cortical astrocytes decreases glutamate uptake and induces neurotoxic release of ATP. J Biol Chem 288: 14599–14611, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature 468: 232–243, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, Bass C, Sonntag WE, Ungvari Z, Csiszar A. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol Biol Med Sci 67: 313–329, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baur JA, Ungvari Z, Minor RK, Le Couteur DG, de Cabo R. Are sirtuins viable targets for improving healthspan and lifespan? Nat Rev Drug Discov 11: 443–461, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce-Keller AJ, White CL, Gupta S, Knight AG, Pistell PJ, Ingram DK, Morrison CD, Keller JN. NOX activity in brain aging: exacerbation by high fat diet. Free Radic Biol Med 49: 22–30, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csiszar A, Podlutsky A, Podlutskaya N, Sonntag WE, Merlin SZ, Philipp ER, Doyle K, Davila A, Recchia FA, Ballabh P, Pinto JT, Ungvari Z. Testing the oxidative stress hypothesis of aging in primate fibroblasts: is there a correlation between species longevity and cellular ROS production? J Gerontol A Biol Sci Med Sci 67: 841–852, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-α-induced activation of coronary arterial endothelial cells: role of NF-κB inhibition. Am J Physiol Heart Circ Physiol 291: H1694–H1699, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circ Res 110: 1109–1124, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabiani M, Gordon BA, Maclin EL, Pearson MA, Brumback-Peltz CR, Low KA, McAuley E, Sutton BP, Kramer AF, Gratton G. Neurovascular coupling in normal aging: a combined optical, ERP and fMRI study. Neuroimage pii: S1053–S8119, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 100: 328–335, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler Thromb Vasc Biol 27: 303–309, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Hock C, Villringer K, Muller-Spahn F, Wenzel R, Heekeren H, Schuh-Hofer S, Hofmann M, Minoshima S, Schwaiger M, Dirnagl U, Villringer A. Decrease in parietal cerebral hemoglobin oxygenation during performance of a verbal fluency task in patients with Alzheimer's disease monitored by means of near-infrared spectroscopy (NIRS)–correlation with simultaneous rCBF-PET measurements. Brain Res 755: 293–303, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res 95: 1019–1026, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Kennedy DO, Wightman EL, Reay JL, Lietz G, Okello EJ, Wilde A, Haskell CF. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am J Clin Nutr 91: 1590–1597, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Lecrux C, Kocharyan A, Sandoe CH, Tong XK, Hamel E. Pyramidal cells and cytochrome P450 epoxygenase products in the neurovascular coupling response to basal forebrain cholinergic input. J Cereb Blood Flow Metab 32: 896–906, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu GS, Zhang ZS, Yang B, He W. Resveratrol attenuates oxidative damage and ameliorates cognitive impairment in the brain of senescence-accelerated mice. Life Sci 91: 872–877, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Longden T, Nelson M. Recruitment of the vascular endothelium into neurovascular coupling. Proc Brit Pharmacol Soc (Online). http://www.pa2online.org/abstracts/vol9issue3abst062p.pdf 9 [2011]. [Google Scholar]
- 21.Machulda MM, Ward HA, Borowski B, Gunter JL, Cha RH, O'Brien PC, Petersen RC, Boeve BF, Knopman D, Tang-Wai DF, Ivnik RJ, Smith GE, Tangalos EG, Jack CR., Jr Comparison of memory fMRI response among normal, MCI, and Alzheimer's patients. Neurology 61: 500–506, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mentis MJ, Alexander GE, Krasuski J, Pietrini P, Furey ML, Schapiro MB, Rapoport SI. Increasing required neural response to expose abnormal brain function in mild vs. moderate or severe Alzheimer's disease: PET study using parametric visual stimulation. Am J Psychiatry 155: 785–794, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN, Uranga RM, Bruce-Keller AJ, Keller JN. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem 114: 1581–1589, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolakakis N, Hamel E. Neurovascular function in Alzheimer's disease patients and experimental models. J Cereb Blood Flow Metab 31: 1354–1370, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogle WO, Speisman RB, Ormerod BK. Potential of treating age-related depression and cognitive decline with nutraceutical approaches: a mini-review. Gerontology 59: 23–31, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci 1: 4, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab 27: 1908–1918, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, Carlson GA, Iadecola C. NADPH-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci 25: 1769–1777, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci USA 105: 1347–1352, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8: 157–168, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pepe M, Mamdani M, Zentilin L, Csiszar A, Qanud K, Zacchigna S, Ungvari Z, Puligadda U, Moimas S, Xu X, Edwards JG, Hintze TH, Giacca M, Recchia FA. Intramyocardial VEGF-B167 gene delivery delays the progression towards congestive failure in dogs with pacing-induced dilated cardiomyopathy. Circ Res 106: 1893–1903, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rakici O, Kiziltepe U, Coskun B, Aslamaci S, Akar F. Effects of resveratrol on vascular tone and endothelial function of human saphenous vein and internal mammary artery. Int J Cardiol 105: 209–215, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Rombouts SA, Barkhof F, Veltman DJ, Machielsen WC, Witter MP, Bierlaagh MA, Lazeron RH, Valk J, Scheltens P. Functional MR imaging in Alzheimer's disease during memory encoding. AJNR Am J Neuroradiol 21: 1869–1875, 2000 [PMC free article] [PubMed] [Google Scholar]
- 35.Rosengarten B, Paulsen S, Molnar S, Kaschel R, Gallhofer B, Kaps M. Acetylcholine esterase inhibitor donepezil improves dynamic cerebrovascular regulation in Alzheimer patients. J Neurol 253: 58–64, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci 34: 3–11, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Sheng WS, Hu S, Feng A, Rock RB. Reactive oxygen species from human astrocytes induced functional impairment and oxidative damage. Neurochem Res 10: 2148–2159, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorond FA, Hurwitz S, Salat DH, Greve DN, Fisher ND. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology 81: 904–909, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorond FA, Kiely DK, Galica A, Moscufo N, Serrador JM, Iloputaife I, Egorova S, Dell'Oglio E, Meier DS, Newton E, Milberg WP, Guttmann CR, Lipsitz LA. Neurovascular coupling is impaired in slow walkers: the MOBILIZE Boston Study. Ann Neurol 70: 213–220, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stefanova I, Stephan T, Becker-Bense S, Dera T, Brandt T, Dieterich M. Age-related changes of blood-oxygen-level-dependent signal dynamics during optokinetic stimulation. Neurobiol Aging 34: 2277–2286, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Stobart JL, Lu L, Anderson HD, Mori H, Anderson CM. Astrocyte-induced cortical vasodilation is mediated by d-serine and endothelial nitric oxide synthase. Proc Natl Acad Sci USA 110: 3149–3154, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tong XK, Lecrux C, Rosa-Neto P, Hamel E. Age-dependent rescue by simvastatin of Alzheimer's disease cerebrovascular and memory deficits. J Neurosci 32: 4705–4715, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Topcuoglu MA, Aydin H, Saka E. Occipital cortex activation studied with simultaneous recordings of functional transcranial Doppler ultrasound (fTCD) and visual evoked potential (VEP) in cognitively normal human subjects: effect of healthy aging. Neurosci Lett 452: 17–22, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Toth P, Tucsek Z, Sosnowska D, Gautam T, Mitschelen M, Tarantini S, Deak F, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Age-related autoregutory dysfunction and cerebromicrovascular injury in mice with angiotensin II induced hypertension. J Cereb Blood Flow Metab 33: 1732–1742, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tucsek Z, Gautam T, Sonntag WE, Toth P, Saito H, Salomao R, Szabo C, Csiszar A, Ungvari Z. Aging exacerbates microvascular endothelial damage induced by circulating factors present in the serum of septic patients. J Gerontol A Biol Sci Med Sci 68: 652–660, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol Heart Circ Physiol 299: H18–H24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ungvari Z, Labinskyy N, Mukhopadhyay P, Pinto JT, Bagi Z, Ballabh P, Zhang C, Pacher P, Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol 297: H1876–H1881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ungvari Z, Sosnowska D, Podlutsky A, Koncz P, Sonntag WE, Csiszar A. Free radical production, antioxidant capacity, and oxidative stress response signatures in fibroblasts from Lewis dwarf rats: effects of life span-extending peripubertal GH Treatment. J Gerontol A Biol Sci Med Sci 66: 501–510, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ungvari Z, Tucsek Z, Sosnowska D, Toth P, Gautam T, Podlutsky A, Csiszar A, Losonczy G, Valcarcel-Ares MN, Sonntag WE. Aging-induced dysregulation of Dicer1-dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J Gerontol A Biol Sci Med Sci 68:877–891, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ungvari ZI, Bailey-Downs L, Gautam T, Jimenez R, Losonczy G, Zhang C, Ballabh P, Recchia FA, Wilkerson DC, Sonntag WE, Pearson KJ, de Cabo R, Csiszar A. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am J Physiol Heart Circ Physiol 300: H1133–H1140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ungvari ZI, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith KE, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Valls-Pedret C, Lamuela-Raventos RM, Medina-Remon A, Quintana M, Corella D, Pinto X, Martinez-Gonzalez MA, Estruch R, Ros E. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis 29: 773–782, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Zaletel M, Strucl M, Pretnar-Oblak J, Zvan B. Age-related changes in the relationship between visual evoked potentials and visually evoked cerebral blood flow velocity response. Funct Neurol 20: 115–120, 2005 [PubMed] [Google Scholar]
- 54.Zarzuelo MJ, Lopez-Sepulveda R, Sanchez M, Romero M, Gomez-Guzman M, Ungvary Z, Perez-Vizcaino F, Jimenez R, Duarte J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: implications for vascular aging. Biochem Pharmacol 85: 1288–1296, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Zhang F, Xu S, Iadecola C. Role of nitric oxide and acetylcholine in neocortical hyperemia elicited by basal forebrain stimulation: evidence for an involvement of endothelial nitric oxide. Neuroscience 69: 1195–1204, 1995 [DOI] [PubMed] [Google Scholar]
- 56.Zhang H, Morgan B, Potter BJ, Ma L, Dellsperger KC, Ungvari ZI, Zhang C. Resveratrol improves left ventricular diastolic relaxation in type 2 diabetes by inhibiting oxidative/nitrative stress. Am J Physiol Heart Circ Physiol 299: H985–H994, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol improves endothelial function: role of TNFα and vascular oxidative stress. Arterioscler Thromb Vasc Biol 29: 1164–1171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao YN, Li WF, Li F, Zhang Z, Dai YD, Xu AL, Qi C, Gao JM, Gao J. Resveratrol improves learning and memory in normally aged mice through microRNA-CREB pathway. Biochem Biophys Res Commun 435: 597–602, 2013 [DOI] [PubMed] [Google Scholar]