Abstract
The very long-chain acyl-CoA dehydrogenase (VLCAD) enzyme catalyzes the first step of mitochondrial β-oxidation. Patients with VLCAD deficiency present with hypoketotic hypoglycemia and cardiomyopathy, which can be exacerbated by fasting and/or cold stress. Global VLCAD knockout mice recapitulate these phenotypes: mice develop cardiomyopathy, and cold exposure leads to rapid hypothermia and death. However, the contribution of different tissues to development of these phenotypes has not been studied. We generated cardiac-specific VLCAD-deficient (cVLCAD−/−) mice by Cre-mediated ablation of the VLCAD in cardiomyocytes. By 6 mo of age, cVLCAD−/− mice demonstrated increased end-diastolic and end-systolic left ventricular dimensions and decreased fractional shortening. Surprisingly, selective VLCAD gene ablation in cardiomyocytes was sufficient to evoke severe cold intolerance in mice who rapidly developed severe hypothermia, bradycardia, and markedly depressed cardiac function in response to fasting and cold exposure (+5°C). We conclude that cardiac-specific VLCAD deficiency is sufficient to induce cold intolerance and cardiomyopathy and is associated with reduced ATP production. These results provide strong evidence that fatty acid oxidation in myocardium is essential for maintaining normal cardiac function under these stress conditions.
Keywords: fatty acid oxidation, mitochondria, cardiac metabolism, cardiomyopathy, mouse, VLCAD, cold intolerance
fatty acids are the preferred substrate for ATP production in the mammalian heart. Very long-chain acyl-CoA dehydrogenase (VLCAD) catalyzes the first step of mitochondrial β-oxidation, the dehydrogenation of acyl-CoAs with 14 to 20 carbon-chain fatty acids. Mutations in the VLCAD gene are the most common inherited long-chain fatty acid oxidation (FAO) disorder, with an incidence currently estimated to be between 1/42,500 and 1/120,000 (3, 7, 27, 46). Affected individuals demonstrate a variety of clinical symptoms including nonketotic hypoglycemia, heart and liver lipidosis, encephalopathy, skeletal myopathy, cardiomyopathy, arrhythmias, and sudden death (2, 5, 7, 22, 34). Because VLCAD is highly expressed in the liver, heart, lung, brown adipose tissue (BAT), and skeletal muscles, global VLCAD deficiency causes multiple organ dysfunction and diverse clinical symptoms. Three phenotypes have been described: 1) a severe childhood form with no residual enzyme activity, typically presenting with cardiomyopathy and resulting in high mortality (2, 29, 41); 2) a milder childhood form with hypoketotic hypoglycemia as the main feature (2, 3) and 3) an adult presentation with intermittent skeletal myopathy mainly triggered by fasting or exercise (2).
Global VLCAD knockout (KO) mice recapitulate some features of human VLCAD deficiency. Adult KO mice demonstrate cardiomyopathy with increased numbers of degenerative fibers, collagen deposition, and vacuolated myocytes as well as increased lipid accumulation in cardiomyocytes (16), abnormal cardiac electrophysiological changes including facilitated induction of polymorphic ventricular tachycardia, abnormal intracellular Ca2+ homeostasis and dynamics in cardiomyocytes (45), and prolonged QT interval (20). Global VLCAD KO mice stressed by cold and/or fasting develop hypoglycemia, hypothermia, and severe bradycardia and become moribund within several hours (15). The contribution of different organs and tissues to development of cold intolerance in systemic VLCAD-deficient mice is unknown. In addition, little is known as to whether the cardiac phenotype in global VLCAD KO mice or in humans with VLCAD deficiency is due to the absence of VLCAD in heart or, alternatively, occurs secondary to pathophysiological changes in other organs.
In this study, we generated cardiac-specific VLCAD KO mice, assessed the phenotype, and investigated the mechanisms responsible. Our results demonstrate that cardiac-specific VLCAD deficiency induces dilated cardiomyopathy associated with reduced ATP production in cardiomyocytes. Furthermore, the present study also demonstrated that, upon exposure to cold, cardiac-specific VLCAD KO mice rapidly developed severe hypothermia, severe bradycardia, and depressed cardiac function, leading to a moribund state. These results highlight the key role of energy starvation induced by diminished FAO in the development of cardiomyopathy and cold intolerance.
MATERIALS AND METHODS
Generation of cardiac-specific VLCAD-deficient mice.
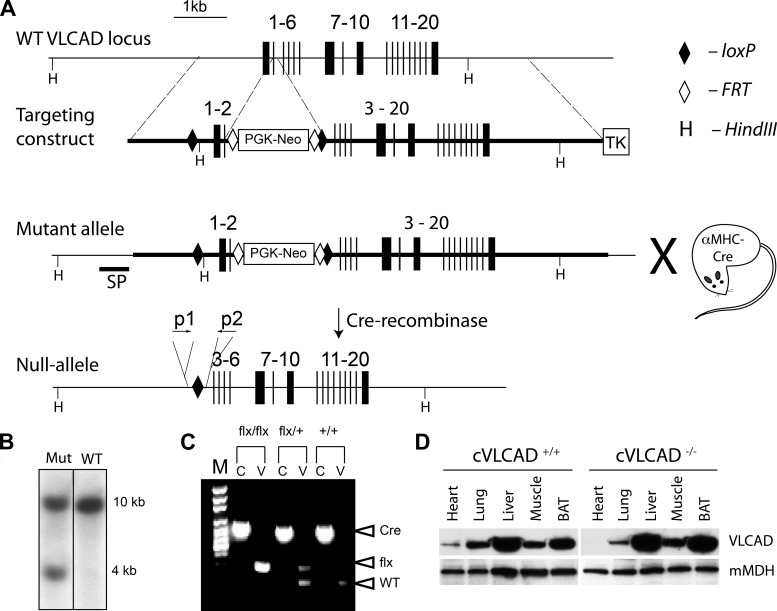
Genetic recombination was employed to assemble a gene-targeting vector (28). Homologous recombination in G4 ES cells (129S6/C57BL intercross) was performed by electroporating the linearized targeting vector containing a neomycin resistance cassette flanked with loxP sites positioned at 476-bp upstream of exon 1 and 46-bp downstream of exon 2 (Fig. 1A). Thymidine kinase was used for negative selection. Neomycin- and gancyclovir-resistant ES cell clones were screened by southern blot analysis of ES cell genomic DNA using a probe located outside of the 5′-homology arm (Fig. 1B). Correctly targeted ES cell clones were coaggregated with a tetraploid morula and implanted into pseudopregnant females. Male chimeras were crossed with C57BL/6J females (Jackson Laboratories) to produce F0 offspring, which was confirmed by Southern blot and PCR analysis. Table 1 shows primer sequences for these studies. Mice were further bred to homozygosity, and then VLCADflx/flx female mice were crossed with C57BL males expressing Cre recombinase driven by the α-myosin heavy chain (MHC) promoter (31). We chose α-MHC-Cre transgenic mice because this model lacks any detectable phenotype and has been widely used as a genetic tool to selectively inactivate genes specifically in cardiac muscle.
Fig. 1.
Generation of cardiac-specific very long-chain acyl-CoA dehydrogenase (VLCAD)-deficient mice. A: gene targeting strategy: exons 1 and 2 were flanked by loxP sites, and a neomycin (Neo) resistance cassette (PGK-Neo) and thymidine kinase gene (TK) flanked by FRT sites were inserted downstream of exon 2 for positive and negative selection, respectively. Mice carrying the mutant floxed allele were crossed with transgenic mouse carrying α-MHC-Cre. The resultant null-allele of the VLCAD gene is depicted. B: Southern blot for analysis of mutant floxed allele (Mut) and wild-type (WT) VLCAD alleles of HindIII fragments of genomic DNA from ES cells using the 32P-labeled DNA probe shown in A (SP). C: PCR for mouse genotyping: PCR-products amplified from Cre-recombinase-encoding DNA (Cre) and floxed VLCAD (flx) and WT VLCAD alleles were separated on an agarose gel; (c, PCR for Cre; v, PCR for VLCAD on agarose gel). D: Western blot analysis for VLCAD protein in the heart, lung, liver, skeletal muscle, and brown adipose tissue (BAT) of cVLCAD+/+ and cVLCAD−/− mice. Anti-mitochondrial malate dehydrogenase (mMDH) antibodies were used to control for protein loading. Please note that image in B is spliced.
Table 1.
DNA oligonucleotides used in PCR genotyping
| Amplicon |
||||
|---|---|---|---|---|
| ID | Sequence | Target | WT | Targeted |
| P1 | 5′-GTCTTCACTCTAGCAGGGTCTATG-3′ | VLCAD | 311 | 285 |
| P2 | 5′-GGGTGTACTCTAAGCGAATTCTGAG-3′ | |||
| P3 | 5′-GAGTTCCCCAAGTGAATGAAA-3′ | Cre-recombinase | 900 | N/A |
| P4 | 5′-CACGTACTGACGGTGGGAGAATGTT-3′ | |||
VCLAD, very long-chain acyl-CoA dehydrogenase; WT, wild type; N/A, not applicable.
Mice heterozygous for the floxed VLCAD allele and carrying the α-MHC-Cre transgene (VLCADflx/+/Cre) were identified by tail genomic DNA PCR analysis (Fig. 1C). Then, male and female VLCADflx/+/Cre mice were mated to generate cardiac-specific VLCAD KO (VLCADflx/flx/α-MHC-Cre, or cVLCAD−/−) mice, which were homozygous for the floxed VLCAD allele and also carried the α-MHC-Cre transgene. Copy numbers of the Cre transgene were analyzed with qPCR, and only mice with a single copy of the Cre-recombinase gene were used in subsequent experiments. Most experiments were performed on males; however, heart perfusion experiments and adenine nucleotide analyses were done both males and females. Mice were fed standard rodent chow containing 3.8% fat, 14.7% protein, and 64% carbohydrates (RHM1500; formulated by Purina LabDiet, St. Louis, MO).
All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of both the Cincinnati Children's Research Foundation and the Medical University of South Carolina.
Protein isolation and immunoblotting.
Tissues were homogenized in cold 0.5% RIPA solution (PBS, 1% NP40, and 0.1% SDS), with proteinase inhibitors PMSF (100 μg/ml) and aprotinin (30 μl/ml; Sigma). The lysates were centrifuged for 20 min at 12,000 g at 4°C, and the supernatants were collected. Protein concentration was determined using the DC protein assay (Bio-Rad). Five to twenty micrograms of protein per lane were loaded on a 4–20% gradient SDS gel, transferred to a PVDF membrane, and blocked with 5% nonfat milk. VLCAD (homemade), AMPK, and P-AMPK (Cell Signaling, Danvers, MA) were detected using specific antibodies against these proteins. Bands were visualized by chemiluminescence with ECL reagent (Amersham Pharmacia Biotech).
Culture of cardiomyocytes and mitochondrial respiration measurements.
Neonatal cardiac myocytes were isolated from the hearts of 1-day-old cVLCAD−/− and cVLCADflx/flx neonatal mice as previously described (25). Viable cells were counted with a hemocytometer and plated at 20,000–50,000 cells per well density on laminin-coated XF24 plates. Cells were cultured 72 h in a CO2 incubator at 37°C. The genotype of cardiomyocytes in each well was determined by PCR-genotyping of tail samples from the corresponding carcass. Two or three hours before measurements on XF24 extracellular flux analyzer (Seahorse Bioscience, Billerica, MA), cells were removed from the CO2 incubator and placed at 37°C in normal atmosphere. Thirty minutes before measurements, the medium was replaced with FX assay medium composed of 143 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 0.91 mM Na2HPO4, 2 mM glutamine, 2 mM carnitine, 2 mg/ml BSA, and 15 mg/ml phenol red, pH 7.4. Palmitate-BSA conjugate (5×, 2 mM) was prepared as described by the instrument manufacturer and loaded into the injection port A. In control experiments, 5× stock solution (0.17 mM) with fatty acid free BSA was loaded in port A. Stock solutions (10×) of oligomycin, FCCP and rotenone were prepared in FX assay medium and loaded into injection ports B, C, and D, respectively.
Preparation of permeabilized cardiac fibers and mitochondrial respiration measurements.
Cardiac fibers were prepared as described elsewhere (38). Fibers were permeabilized with 25 μg/ml saponin at 4°C for 30 min with gentle shaking. Oxygen consumption rates in saponin-permeabilized fibers were measured using the Oxygraph Instrument at 30°C and analyzed with O2View software (Hansatech Instruments, Norfolk, UK). Mitochondrial respiration medium (MitoMed) was composed of 110 mM sucrose, 60 mM lactobionic acid, 20 mM HEPES-K, 20 mM taurine, 10 mM KH2PO4, 3 mM MgCl2, 0.5 mM EGTA, and 1 mg/ml BSA at pH 7.4. Mitochondria were isolated from cardiac muscle, and mitochondrial ATPase activity was measured as described elsewhere (33).
Quantitative profiling of mitochondrial proteins using isobaric protein labeling and tandem mass spectrometry.
Mitochondria were isolated from mouse hearts as described earlier (33). Mitochondria from two VLCADflx/flx and two cVLCAD−/− hearts were subjected to quantitative protein profiling using the iTRAQ method (35) by following the vendor's instructions (ABSciex Framingham, MA). The general steps in the protocol included solubilization of the enriched mitochondria in Laemmli gel buffer, preparative separation of the proteins on a mini SDS-PAGE gel, in-gel trypsin digestion and recovery of the peptides, iTRAQ tagging of two control and two VLCAD KO samples with the iTRAQ 4-plex reagents, combining the peptides from the four samples, and separation of the tagged peptides into 14 fractions by strong cation exchange (SCX) and nanoLC-MSMS of the 14 SCX fractions followed by protein identification and quantitation of the collective data set using ProteinPilot (PP) and ProteinPilot Descriptive Statistics Template (PDST) software algorithms (ABSciex).
Echocardiographic study.
Murine transthoracic echocardiograms were performed as described previously (42).
Histology and electron microscopy.
Light microscopic histological analysis was performed on tissues fixed in 10% formalin, with 5-μm paraffin-embedded sections processed for hematoxylin and eosin staining. For electron microscopic examination, 6-mo-old mice were perfused, and hearts were harvested as described previously (37). Ultrathin sections were fixed overnight in glutaraldehyde, postfixed in osmium tetroxide and embedded in epoxy resin before examination by transmission electron microscopy (37).
Isolated perfused heart preparation and measurement of isovolumic contractile performance.
Hearts were isolated and perfused in the Langendorff mode as described previously (23). The hearts were excised from 5- to 6-mo-old cVLCAD+/+ (n = 13) and cVLCAD−/− (n = 14) mice, arrested in ice-cold buffer, and connected via the aorta to the perfusion cannula. Retrograde perfusion was performed at a constant coronary arterial perfusion pressure of 80 mmHg at 37°C with modified perfusion buffer containing 25 mM NaHCO3, 0.5 mM EDTA, 5.3 mM KCl, 1.2 mM MgSO4, 118 mM NaCl, 5.5 mM glucose, 2.5 mM CaCl2, and 0.4 mM mixed free fatty acids (palmitate, palmitoleic, linoleic, and oleic) carried in 1% BSA, 0.19 mM β-hydroxybutarate, 1 mM lactate, and 0.63 nM insulin. Right ventricular drainage was accomplished by incision of the pulmonary artery. Effluent from the Thebesian veins was drained by a thin polyethylene tube (PE-10) pierced through the apex of the left ventricle. A water-filled balloon made of polyvinylchloride film was inserted in the left ventricle and connected to a data acquisition system. After a 30-min stabilization, the balloon volume (BV), which produced a left ventricular end diastolic pressure (EDP) of 0 mmHg, was determined. The BV was then increased in 5-μl increments until 50 μl. The heart was allowed to stabilize at each BV for 40 s, and indexes of cardiac function were recorded. The BV was then adjusted to set EDP at ∼10 mmHg and held constant. Once the heart developed stable isovolumic contractions, the baseline function was recorded for 10 min. A set of hearts was freeze clamped for measuring nucleotides at baseline condition; another set of hearts was then subjected to a high workload by infusing dobutamine (final concentration: 300 nM) through side tubing driven by a digital console driver at 2% of coronary flow rate. After steady state was reached, cardiac function was measured for 15 min. Hearts were then freeze clamped for measuring nucleotides at high workload.
HLPC analysis of ATP, ADP, AMP, NAD, and NADH.
Hearts from both cVLCAD−/− mice and littermate controls (n = 5–8) were freeze clamped under liquid nitrogen after baseline perfusion or at the end of the high workload in response to 300 nM dobutamine, respectively. Frozen heart tissues (20–30 mg wet weight) were pulverized in a mortar under liquid nitrogen and extracted with 0.6 N perchloric acid. An aliquot of the homogenate was removed, and protein concentration was measured. After neutralization and centrifugation, the supernatant was passed through a 0.45-μm filter, diadenosine pentaphosphate (1 mM) was added, and the resulting solution was passed through a 0.2-μm filter. The supernatant (100 μl) was injected into a Waters HPLC system equipped with a Waters 1525 HPLC pump and a 2487 dual wavelength (UV/Vis) absorbance detector (Waters, Milford MA) to measure nucleotides. ATP, ADP, AMP, NAD, and NADH were separated using a Kromasil 250 × 4.6-mm, 5-mm-particle-size column at a flow rate of 0.8 ml/min through a step gradient from 100% of buffer A (10 mM tetrabutylammonium hydroxide, 10 mM KH2PO4, and 0.125% methanol, pH 7.0) to 100% of buffer B (2.8 mM tetrabutylammonium hydroxide, 100 mM KH2PO4, and 30% methanol, pH 5.5) over 90 min. Peaks were monitored by UV absorption at 256 nm and identified by comparison with the retention times of known standards. Nucleotide content was quantified using standard curves and normalized to the protein concentration in each sample.
Stress tests and open circuit indirect calorimetry.
Response to the combination of fasting and cold was evaluated in 2- and 6 mo-old male cVLCAD−/− and VLCAD+/+ mice (n ≥ 6 for each group). The mice were deprived of food overnight with free access to water. The next morning, the mice were housed in temperature-controlled metabolic chambers without bedding at 31 or 5°C, again with free access to water but not food.
The resting oxygen consumption and carbon dioxide production rates (V̇o2 and V̇co2, respectively) were measured in metabolic chambers every 10 min using the Oxymax system (Columbus Instrument, Columbus, OH) overnight for at least 20 h. Fresh air was delivered into chambers with an electric pump. Respiratory exchange ratio (RER), also known as the respiratory quotient, was calculated as V̇o2/V̇co2 using CLAX software (Columbus Instrument, Columbus, OH). Energy expenditure was calculated as energy expenditure = CV × V̇o2, where calorific value (CV) is calculated from the empirical formula, CV = 3.815 + 1. 232 RER. Complete oxidation of glucose would result in a RER of 1.00, palmitate −0.707, and a 50/50 diet would result in a RER of 0.85 (29a). Echocardiograms, rectal temperature, and blood glucose levels were obtained before and 75–90 min after exposure to cold on isoflurane-anesthetized mice. Body composition in live mice was studied with EchoMRI-100 analyzer.
Heart rates were continuously monitored using implanted G2-HR E-Mitter telemetry transmitters (Mini Mitter, Bend, OR). Transmitters were surgically implanted in mice (10), and signals were continuously recorded during a 24 h-period with a Mini Mitter RS-485 receiver.
Statistical analyses.
Data are expressed as means ± SE unless otherwise noted. Differences among genotypes and/or treatments were compared by two-tailed t-tests or one- or two-way factorial ANOVA followed by post hoc Bonferoni or Mann-Whitney tests, as appropriate. Probability values <0.05 were considered significantly different.
RESULTS
Generation of cardiac-specific VLCAD KO mice.
To gain further insight the role of very long fatty acid oxidation in the heart, we generated cardiac-specific VLCAD-deficient mice (Fig. 1, A–C). These cVLCAD−/− mice completely lacked any detectable VLCAD protein in the heart. Western blot analysis of protein extracts from heart, skeletal muscle, BAT, and liver confirmed that VLCAD protein was completely ablated in cardiac myocytes of cVLCAD−/− mice; but, in all other tested tissues, VLCAD protein level was not affected (Fig. 1D) and comparable to cVLCAD+/+ controls
Under standard laboratory conditions and diet, cVLCAD−/− mice were viable and fertile. There was no difference in long-term survival and fertility between cVLCAD−/− and controls, including heterozygous (VLCAD+/−-α-MHC-Cre or cVLCAD+/−), mice with wild-type (WT) VLCAD allele (VLCAD+/+-α-MHC-Cre or cVLCAD+/+), or mice with “floxed” VLCAD allele (VLCADflx/flx), indicating that the VLCADflx/flx allele itself and α-MHC-Cre transgene were innocuous. Therefore, in subsequent experiments, mice carrying the WT VLCAD allele and harboring the α-MHC-Cre transgene (cVLCAD+/+) were used as the control group. Body compositions (body fat mass, lean mass, and body water) were not significantly different between control and cVLCAD−/− groups (Table 2).
Table 2.
Body composition of 2-mo-old cVLCAD+/+ and cVLCAD−/− male mice analyzed by EchoMRI
| Body Fat Mass | Body Lean Mass | Free H2O | |
|---|---|---|---|
| cVLCAD+/+ (n = 20) | 1.92 ± 0.07 | 20.74 ± 0.27 | 17.66 ± 0.21 |
| cVLCAD−/− (n = 18) | 2.06 ± 0.15 | 21.51 ± 0.30 | 18.33 ± 0.24 |
| NS | NS | NS |
Values are means ± SE. NS, differences are not statistically significant (two-way ANOVA analysis).
Cardiac-specific VLCAD deficiency leads to cardiomyopathy and cardiac dysfunction.
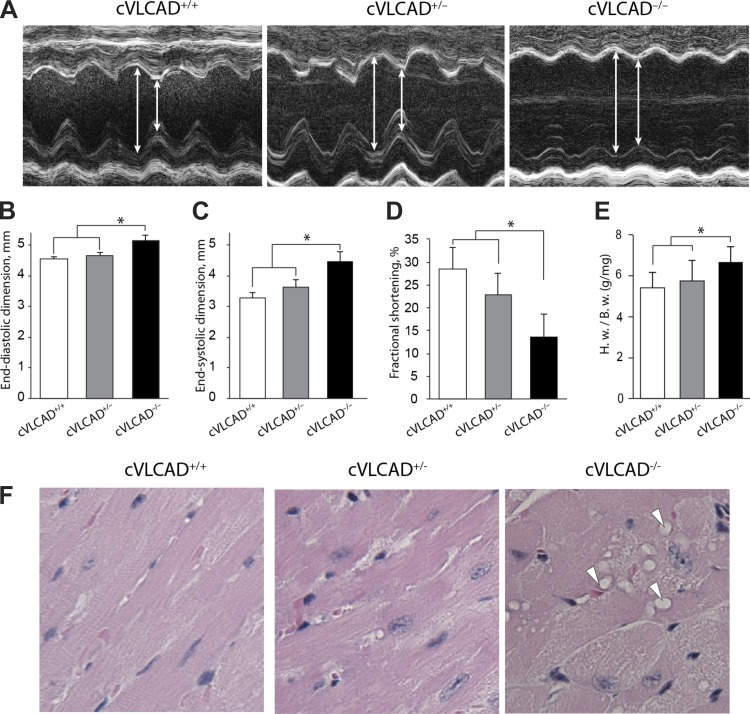
To determine whether cardiac-specific VLCAD KO is sufficient to induce cardiomyopathy, transthoracic M-mode echocardiography was performed in 3- and 6-mo-old cVLCAD−/−, cVLCAD+/−, and cVLCAD+/+ mice. There was no significant ventricular dilation or left ventricular systolic dysfunction in 3-mo-old mice (data not shown). However, in 6-mo-old cVLCAD−/− mice, left ventricular dilation was manifested by a significant increase in left ventricular end-diastolic and end-systolic dimensions and was accompanied by abnormal systolic performances with decreased left ventricular fractional shortening (Fig. 2, A–D). Other manifestations of dilated cardiomyopathy in the 6-mo-old cardiac-specific deficient mice were an increased heart weight-to-body weight ratio and abnormal cardiac histology in cVLCAD−/− mice compared with cVLCAD+/− and cVLCAD+/+ animals (Fig. 2E). Histological analysis revealed frequent vacuolations in cVLCAD−/− cardiomyocytes (Fig. 2F). However, in stark contrast to our previously reported global VLCAD KO, Oil Red O staining of frozen sections of heart muscle did not show an increase in fat accumulation in cVLCAD−/− cardiomyocytes (data not shown). These results demonstrate that cardiac-specific VLCAD deficiency is sufficient to induce dilated cardiomyopathy and depressed systolic function by 6 mo of age.
Fig. 2.
Evidence of dilated cardiomyopathy and depressed left ventricular systolic function in cardiac VLCAD-deficient mice. A: representative transthoracic M-mode echocardiographic tracings from cVLCAD+/+, cVLCAD+/−, and cVLCAD−/− mice. The left ventricle is dilated with reduced wall motion in cVLCAD−/− animals. The average heart rates of three groups were not significantly different. B–D: transthoracic M-mode echocardiographic measurements. There were significant increases in end-diastolic (B) and end-systolic dimensions (C) in cVLCAD−/− mice compared with cVLCAD+/− and cVLCAD+/+ mice. No significant difference was found between cVLCAD+/− and cVLCAD+/+ mice. The fractional shortening (FS) was lower in the cVLCAD−/− mice compared with cVLCAD+/− and cVLCAD+/+ mice, while there is no significant difference in FS values between cVLCAD+/− and cVLCAD+/+ groups. E: ratio of heart weight (mg) to body wt (g) was significantly greater in cVLCAD−/− than cVLCAD+/− mice and cVLCAD+/+ mice (B–E: n = 6 for each group; *P < 0.05). F: histological analysis of LV muscle of cardiac-specific VLCAD knockout mice. Hematoxylin and eosin staining (magnification ×100) revealed vacuolated myocytes (arrowheads) in cVLCAD−/− mouse heart, but no abnormalities were found in cVLCAD+/− or cVLCAD+/+ hearts.
Mitochondrial ultrastructure and oxidative function are preserved in VLCAD-deficient cardiomyocytes.
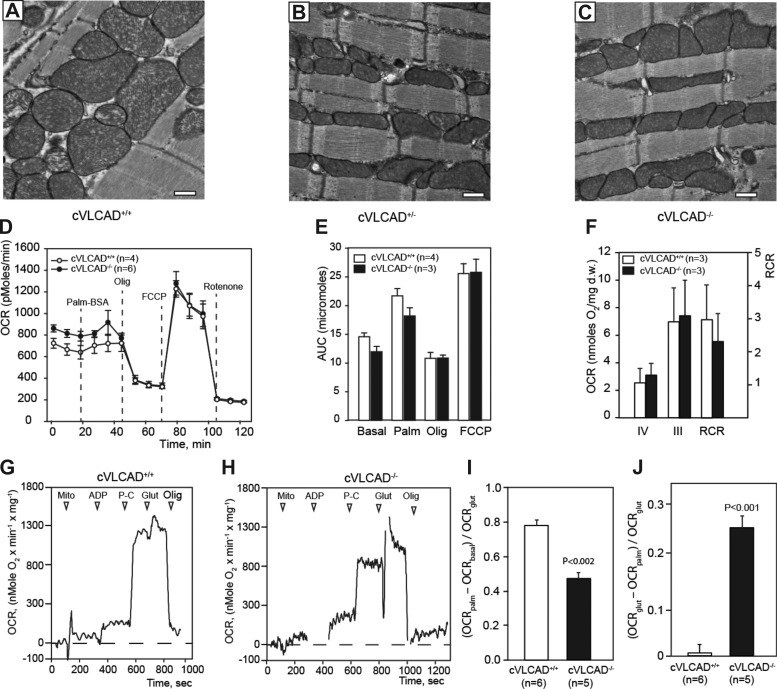
Electron microscopic analysis did not show any apparent differences in cardiac mitochondrial morphology and numbers between controls, cVLCAD+/− and cVLCAD−/− groups in 6-mo-old mice (Fig. 3, A–C).
Fig. 3.
Mitochondrial ultrastructure and function are preserved in cVLCAD−/− mice. A–C: electron micrographs of left ventricles of 6-mo-old control (cVLCAD+/+), heterozygous (cVLCAD+/−), and homozygous cardiac-specific VLCAD-knockout mice (cVLCAD−/−) mice (bar = 500 nm). D and E: mitochondrial oxygen consumption rates (OCR) in cultured cardiomyocytes. D: OCR traces, expressed as pmol O2/min in control (cVLCAD+/+) and mutant (cVLCAD−/−) cardiomyocytes and normalized to cell numbers. Vertical dashed lines indicate the time of addition palmitate-BSA complex (palm-BSA), oligomycin, FCCP, and rotenone. E: mitochondrial function was quantitated by calculated areas under curve (AUC) for each segment of the OCR tracing and expressed as pmol of O2 accumulated during respiration. F: mitochondrial function in saponin-permeabilized cardiac fibers from 6-mo-old WT and cVLCAD mice. State III respiration was measured in the presence of glutamate and malate. State IV respiration rate was measured after addition of 2 mM ADP. Respiration control ratio (RCR, right vertical axis) was calculated as VIII/VIV. G and H: Palmitoyl-l-carnitine (P-C) stimulation of oxygen consumption in control (G) and VLCAD-deficient cardiac mitochondria, isolated from 2-mo-old mice. I and J: quantification of palmitoyl-l-carnitine effect on oxygen consumption rates in mitochondria. Palm-BSA, palmitate-BSA conjugate; Mito, mitochondrial suspension (6–16 μg); Olig, oligomycin (2 μM). Significance values in I and J were calculated with one-way ANOVA with assumption of equal variances. Vertical bars indicate means ± SE; n = number of experiments per group.
To examine whether mitochondrial energy metabolism is affected by inactivation of the VLCAD gene, we analyzed mitochondrial respiration in neonatal cardiomyocytes, isolated mitochondria from hearts of 2-mo-old mice and saponin-permeabilized cardiac fibers from 6-mo-old mice. Oxygen consumption rates (OCR) were measured in cultured cardiomyocytes isolated from neonatal cVLCAD+/+ and cVLCAD−/− mice using an XF24 extracellular flux analyzer (Fig. 3, D and E). The basal and palmitate-stimulated rates were slightly, although not statistically significantly lower, in VLCAD-deficient cells compared with controls (P = 0.06, Single-factor ANOVA with Turkey posttest). There was no difference in maximal uncoupled respiration rates between VLCAD-deficient and control cardiomyocytes after administration of FCCP (Fig. 3E).
As noted above, cardiac abnormalities in cVLCAD mice only become apparent by 6 mo of age. Therefore, we determined whether mitochondrial function is preserved in older mice (Fig. 3F). Cardiac fibers from 6-mo-old cVLCAD−/− and control mice were permeabilized with saponin and placed in a respiration chamber with 2 ml of MitoMed at 30°C, and state IV respiration was measured in the presence of 5 mM glutamate and 5 mM malate. State III respiration was registered after addition of 2 mM ADP to the chamber. Subsequent addition of FCCP (4 μM) did not increase respiration rate further, an indication that ADP had already evoked the maximal respiration rate in cardiac fibers. Non-mitochondrial O2 consumption was registered after addition of 2 μM rotenone and 2 μM antimycin A.
Analysis showed that rates of state III and state IV respiration, as well as the respiratory control ratio (VIII/VIV), did not differ significantly between WT and cVLCAD−/− groups, indicating that mitochondrial function is largely intact in cardiac muscle of 6-mo-old cVLCAD−/− mice (Fig. 3F). Together, these results demonstrate that the mitochondrial electron transport chain is preserved in VLCAD-deficient mitochondria.
Next, we investigated whether VLCAD KO affected fatty acid oxidation in cardiac mitochondria. Mitochondria were isolated from hearts of 2-mo-old control and cVLCAD−/− mice, and respiration rates were measured using a Clark-type oxygen electrode (Fig. 3, G–J). Mitochondria were administered into 2 ml media containing MitoMed with 5 mM malate. Administration of 500 μM ADP had little effect on mitochondrial OCR (Fig. 3, G and H). Addition of 5 μM palmitoyl-l-carnitine (P-C) substantially stimulated respiration both in WT and VLCAD-deficient mitochondria; however, the response to P-C in VLCAD−/− mitochondria was significantly less than in WTs (Fig. 3I). Subsequent addition of 5 mM glutamate evoked near-maximal rates of respiration both in control and VLCAD-deficient mitochondria. However, the effect of glutamate on respiration rate was significantly higher in VLCAD-deficient mitochondria compared with controls (Fig. 3J), demonstrating that 5 μM P-C was insufficient to induce a near-maximal respiration in VLCAD-deficient mitochondria. Maximal respiratory rates of mitochondria were not affected by VLCAD-deficiency (Fig. 3, G and H), supporting our finding that mitochondrial oxidative phosphorylation is not impaired in VLCAD-deficient cardiac mitochondria.
VLCAD gene ablation did not result in significant changes in the abundances of energy-producing enzymes in cardiac mitochondria. Analysis of the proteomic landscape in cardiac mitochondria from 2-mo-old cVLCAD+/+ and cVLCAD−/− mice showed a 1.5-fold reduction of g-subunit of ATP synthase (Table 3). However, enzymatic activity of mitochondrial ATPase is not affected in cVLCAD−/− cardiac mitochondria (data not shown). The relative abundances of three Ca2+-handling enzymes (ATP2A2, CASQ2, and SRCA) were reduced in cVLCAD−/− cardiac mitochondria. These results are consistent with earlier report of alterations in Ca2+ homeostasis in cardiomyocytes from global VLCAD KO mice (45).
Table 3.
Differential summary of the mitochondrial proteomes of 2-mo-old control and VLCAD-deficient hearts
| Protein Name | Fold Change | Sequence Coverage, % |
|---|---|---|
| Downregulated in cVLCAD−/− cardiac mitochondria | ||
| Very long-chain specific acyl-CoA dehydrogenase (VLCAD) | 3.3 | 59.8 |
| Isoform ATP2A2A of endoplasmic reticulum calcium ATPase 2 (ATP2A2) | 1.6 | 30.3 |
| Calsequestrin-2 (CASQ2) | 1.6 | 22.2 |
| Sarcalumenin (SRCA) | 1.5 | 17.6 |
| ATP synthase subunit g, mitochondrial (ATP5L) | 1.5 | 48.5 |
| Upregulated in cVLCAD−/− cardiac mitochondria | ||
| Transmembrane protein (TMEM143) | 1.5 | 11.1 |
| Methylcrotonoyl-CoA carboxylase subunit alpha (MCCA) | 1.5 | 9.2 |
Proteins identified and quantified with 95% confidence and 1.5-fold-change levels (fold change is the average of the 2 replicates).
Reduced systolic and diastolic function in isolated hearts from cardiac-specific VLCAD KO mice.
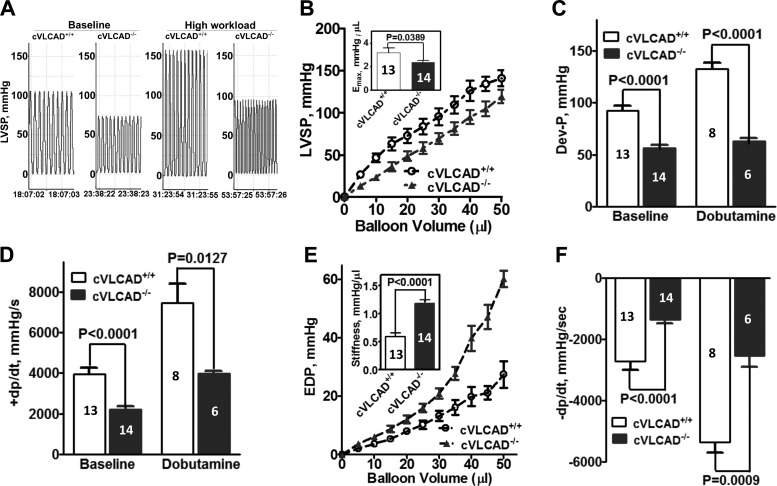
To further determine whether the absence of VLCAD in cardiomyocytes is sufficient to induce cardiac dysfunction independent of confounding variables present in the whole body setting such as available substrates or the neuro-hormonal environment, heart function was evaluated via Langendorff preparation in 5- to 6-mo-old cVLCAD+/+ and cVLCAD−/− mice. As shown in Fig. 4, at baseline, the hearts from cVLCAD−/− mice developed systolic dysfunction evidenced by decreases in the maximal left ventricular systolic pressure (Fig. 4, A and B), maximal systolic elastance (Fig. 4B), developed pressure by each contraction (Fig. 4C), and rate of change of pressure during isovolumic contraction (Fig. 4D) compared with cVLCAD+/+ mice. Compared with WT heart, stiffness (Fig. 4E) was markedly increased, whereas the rate of change of pressure during isovolumic relaxation (Fig. 4F) was significantly decreased in hearts from cVLCAD−/− mice, suggesting that diastolic dysfunction was also present. With high workload stress in response to dobutamine challenge, all parameters of cardiac work significantly increased in the cVLCAD+/+ and cVLCAD−/− hearts compared with baseline values for the same genotype, but a similar pattern of systolic and diastolic dysfunction persisted in VLCAD deficient hearts (Fig. 4, A, C, D, and F). There were no differences in heart rates between WT and VLCAD deficient hearts at baseline or with dobutamine challenge (data not shown). These data demonstrated that VLCAD in cardiac muscle is essential to maintain normal systolic and diastolic function.
Fig. 4.
Reduced systolic and diastolic function in isolated hearts from cardiac-specific VLCAD knockout mice. A: representative tracing of left ventricular systolic pressure (LVSP) from a cVLCAD+/+ and a cVLCAD−/− heart at baseline and with dobutamine challenge. B: LVSP at different balloon volume and maximal systolic elastance (Emax) which was defined by the overall slope of the LVSP-balloon volume curve. C: pressure developed by each contraction (Dev-P). D: +dP/dt, positive change in pressure over time during isovolumic contraction. E: LVEDP at different balloon volume and stiffness which was defined by the overall slope of the LVEDP-balloon volume curve. F: −dP/dt, negative change in pressure over time during isovolumic relaxation. Data shown are means ± SE; number of experiments per group is denoted in the corresponding bar.
Cardiac-specific VLCAD deficiency leads to death upon exposure to combined stresses of cold and fasting.
As previously reported, global VLCAD deficiency rapidly leads to hypothermia, hypoglycemia, and death during exposure to cold and fasting. However, the cardiac role in development of this cold-sensitive phenotype is unknown. We hypothesized that the heart was not involved in cold intolerance but rather that BAT was essential. To determine whether cardiac-specific VLCAD deficiency is sufficient to induce cold intolerance, cVLCAD−/− mice and littermate controls were challenged with the combined stresses of fasting and cold.
First, we investigated whether cVLCAD+/+ and cVLCAD−/− differ in their basal resting metabolic rates in a thermoneutral environment. The thermoneutral zone is defined as the temperature zone at which the lowest metabolic rate is observed. The thermoneutral zone for adult mice is 30–32°C (9). Mice from normal housing conditions, 24°C, free access to food and water, and a 12-h dark/light cycle were transferred into environmentally controlled metabolic chambers with ambient temperature of 31°C without food but with free access to water. Oxygen consumption and CO2 production rates were recorded over the following 20 h. In separate set of experiments heart rates were monitored using implanted telemetry devices.
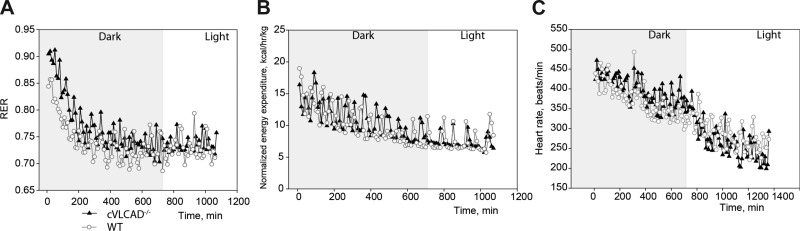
As expected, during fasting in the thermoneutral environment (+31°C), both control and cVLCAD−/− mice gradually shifted RER values from 0.9 to 0.7 (Fig. 5A), suggesting that, when fasted, mice preferably utilize fat as a metabolic fuel. This is accompanied by reduction of heart rate and energy expenditure (Fig. 5, B and C). In the thermoneutral zone cVLCAD−/− mice can survive without food for prolonged period of time (>48 h, data not shown) without any signs of distress. Thus fasting alone cannot evoke a lethal phenotype in cVLCAD−/− mice. Heart rates gradually decreased both in control and cVLCAD−/− mice when placed at 31°C.
Fig. 5.
Indirect calorimetry analysis of 2-mo-old fasted mice at the zone of thermoneutrality. Indirect calorimetric analysis of respiratory exchange ratio (RER; A) and energy expenditure (B) in cVLCAD+/+ and cVLCAD−/− mice during a 20-h fasting period at 31°C. C, Heart rates of cVLCAD+/+ and cVLCAD−/− mice were recorded in a separate set of experiments at 31°C using implanted telemetry devices. Dark and light periods of day cycle are shown. Representative experimental recordings from 2 animals of each genotype and each age group are presented (n = 4 per genotype).
Next, we investigated how mice responded to cold/fasting stress (Fig. 6). Metabolic response to cold was assessed in cVLCAD+/+ and cVLCAD−/− male mice of two age groups, 2 and 6 mo of age. Mice were deprived of food overnight with free access to water. In the morning, all mice were placed at 5°C in individual metabolic cages without bedding and food, but with free access to water.
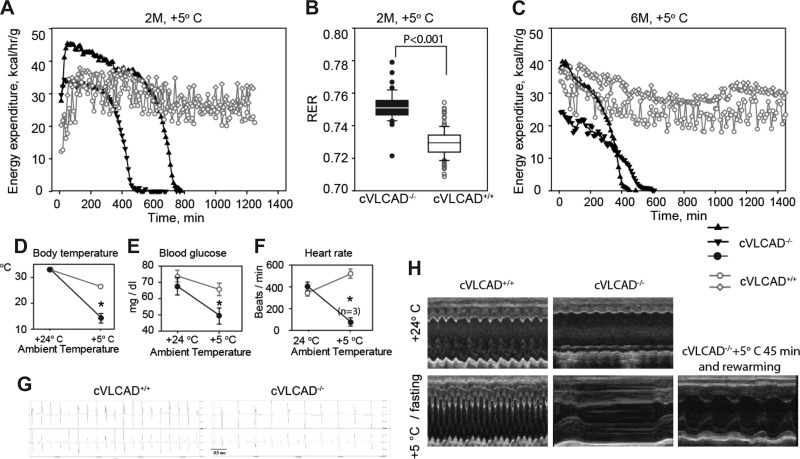
Fig. 6.
Phenotypes of cardiac-specific mouse upon exposure to the combined stresses of cold and fasting. A–C: representative indirect calorimetric analysis of energy expenditure and RER in cVLCAD+/+ and cVLCAD−/− mice at 2 mo (A and B) and 6 mo of age at +5°C. cVLCAD−/− mice become lethargic and die within 4–5 h upon cold exposure, if rewarming intervention is not timely (black triangles in A and C). Representative experimental recordings from two animals of each genotype and each age group are presented. A total of 8 mice per genotype were subjected to cold/fasting tests, and 4 mice per genotype were studied with indirect calorimetry for each age group. D–F: changes in body core temperature (D), blood glucose levels (E), and heart rates (F) in 2-mo-old cVLCAD+/+ and cVLCAD−/− mice before and after exposure to cold (n = 6, unless otherwise indicated). Recordings on D–G were obtained 2–5 min after mice were removed from the cold. H: representative transthoracic M-mode echocardiographic tracings of 6-mo-old mice before (top) and after (bottom) cold exposure (n = 6). One cVLCAD−/− mouse was removed after 45 min of exposure to cold, rewarmed, and echocardiography was performed (H, bottom right). *P < 0.05: precold exposure compared with postcold exposure.
When exposed to cold, all 2-mo-old cVLCAD−/− mice became moribund within 3–10 h and would have died without intervention (Fig. 6A). All control mice survived without showing any signs of distress during the entire session (20 h). Similarly, all 6-mo-old cVLCAD−/− mice became severely lethargic when exposed to cold and would have died if not rescued, but all controls survived for 24 h (Fig. 6C). Six-month-old cVLCAD−/− mice were more sensitive to cold and became lethargic more quickly (within 1–5 h), than 2-mo-old mutant mice (3–10 h). This may be related to developing cardiac dysfunction in the older group. RER values for 2-mo-old mice were calculated from measurements during the first 340 min of cold exposure. RER values were higher in cVLCAD−/− mice compared with controls from the same age group, reflecting a moderate, but statistically significant, reduction of fatty acid metabolism in cVLCAD−/− mice (Fig. 6B).
Body core temperature was measured in 2-mo-old mice before and after 45 min of exposure to cold with a rectal temperature probe. There are no significant differences in body temperature of cVLCAD−/− mice compared with control group prior to exposure to cold. However, after 45 min in the cold, the body temperature was significantly lower in the cVLCAD−/− mice compared with controls (14 ± 1.8°C in cVLCAD−/−; 24.6 ± 0.9°C in cVLCAD+/+; P < 0.04; Fig. 6D). Before cold stress, blood glucose concentrations were not significantly different between control and mutant groups. After 60 min of exposure to cold, blood glucose levels moderately decreased in the cVLCAD−/− mice (Fig. 6E).
Electrocardiograms were obtained from 2- mo-old group before and after exposure to cold. Control mice responded to cold with statistically significant increases of heart rate, from 348 ± 20 beats/min at room temperature to 509 ± 28 beats/min in cold (P = 0.0009). However, the impact of cold on the mutant group was the opposite: upon exposure to cold for 75 min, heart rates of cVLCAD−/− mice dropped from 416 ± 40 to 100 ± 22 beats/min (P = 0.0006) (Fig. 6F).
Echocardiography was performed on 6-mo-old mice before and after 45–60 min exposure to cold. Again, echocardiography revealed sharp increases in heart rates in cold exposed cVLCAD+/+ mice. In contrast, cVLCAD−/− mice developed severe bradycardia, and systolic function was drastically diminished in the cold environment (Fig. 6F), eventually requiring euthanasia in all 6 cVLCAD−/− mice, while all cVLCAD+/+ mice survived. Interestingly, intervention and rewarming of moribund cVLCAD−/− mouse after 45-min exposure to cold were sufficient to rescue and recover heart rate and function (Fig. 6H).
Impaired energy production in VLCAD-deficient cardiac muscle.
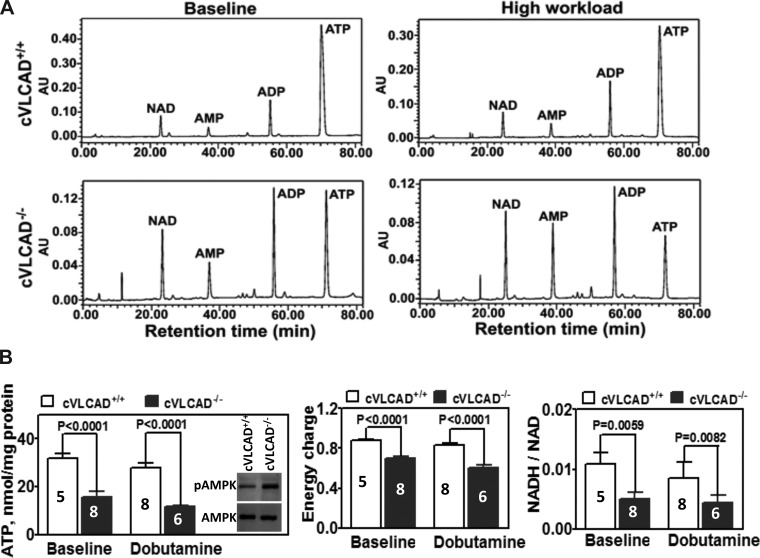
It is known that 70% of ATP in heart is generated via fatty acid β-oxidation. Therefore, we anticipated that VLCAD deficiency-induced impairment of FAO would lead to decreased ATP synthesis in the hearts of VLCAD deficient mice. To assess this, adenine nucleotide (ATP, ADP, and AMP) levels and NADH-to-NAD ratio were measured by HPLC analysis in the extracts of freeze-clamped hearts from 6-mo-old cVLCAD+/+ and cVLCAD−/− mice. No gender-based differences were observed in adenine nucleotide or NADH and NAD levels. Therefore, data were collapsed across gender. As shown in the representative chromatograms (Fig. 7A), compared with cVLCAD+/+ hearts, the ratio of ATP to ADP in cVLCAD−/− hearts was significantly decreased both at baseline and with high workload in response to dobutamine challenge. Furthermore, there were decreases in ATP content, calculated total energy charge, and the ratio of NADH to NAD in the cVLCAD−/− hearts compared with cVLCAD+/+ hearts (Fig. 7B). Similarly, reduction of ATP content and NADH-to-NAD ratio was observed in limited number of experiments on 2-mo-old mouse hearts (data not shown). Reduction of ATP content and increase of AMP in muscle lead to activation of AMP-dependent protein kinase, a master regulator of cell metabolism. As expected, Western blot analysis with phosphospecific antibodies to AMPK (Thr-172) demonstrated that phosphorylation state of AMPK was higher in VLCAD-deficient hearts from 2-mo-old mice compared with WT controls (Fig. 7B).
Fig. 7.
Reduction of ATP pool in the hearts from cardiac-specific VLCAD knockout mice. A: representative chromatograms that show reduced ATP/ADP ratio in KO hearts at baseline and with dobutamine challenge compared with those in WT hearts. B: absolute values of ATP content (left), calculated total energy charge (middle), and the ratio of NADH/NAD (right) in WT and KO hearts at baseline and with dobutamine challenge. Western blot insertion in B, left, demonstrates activation of AMPK in response to ATP level reduction in VLCAD-deficient cardiac muscle. Data shown are means ± SE; number of experiments per group is denoted in the corresponding bar.
Taken together, these findings demonstrate that inactivation of the VLCAD gene only in cardiac muscle is sufficient to induce severe cold intolerance in mice, accompanied by failure to maintain the body core temperature, severe bradycardia, and depressed cardiac function. Cold intolerance is unlikely to be caused by mitochondrial dysfunction or lower blood glucose but may be due to lower cardiac output resulting from severe bradycardia or/and arrhythmia in the setting of hypothermia.
DISCUSSION
The present study demonstrated that cardiac-specific VLCAD deficiency is sufficient to cause death in mice when exposed to cold and fasting, a surprising phenotype that is similar to our global VLCAD KO model (15, 43). We expected that, given normal expression of VLCAD in liver, muscle, and BAT, cVLCAD−/− mice would still be able to tolerate cold. However, when cVLCAD−/− mice were exposed to cold and fasting, the animals rapidly developed hypothermia, severe bradycardia, worsening cardiac dysfunction and lethargy, becoming moribund. In contrast, control mice exposed to identical stress survived, exhibiting tachycardia and increased systolic function, the normal mammalian adaptive response to catecholamine release induced by cold (32).
In mammals, mitochondria are critical for not only ATP production but also heat generation. UCP1 is an inner mitochondrial protein highly expressed in BAT and functions by abolishing the proton gradient across the inner mitochondrial membrane and uncoupling oxygen consumption from oxidative phosphorylation. This uncoupling of respiration from ATP production causes energy dissipation in the form of heat (8). Hypothermia has been documented in several mouse models with genetic modifications that impair brown adipose tissue development, mitochondrial biosynthesis and function, uncoupling, and FAO enzyme reaction (13, 14, 21, 26, 29, 44) (for review see Refs. 9, 39). In fact, cold intolerance in global VLCAD KO mice was attributed to impaired control of thermogenesis in BAT. However, we now demonstrate cold intolerance in the animals with only cardiac-specific loss of VLCAD. In this model, VLCAD protein levels were unaffected in liver or any principal thermogenic tissues, such as BAT and skeletal muscle.
At this point, the mechanisms responsible for severe bradycardia and worsening cardiac dysfunction in stressed cVLCAD−/− mice are unknown. It is unlikely that cold intolerance is caused by reduction of mitochondrial respiratory capacity. Electron microscopy findings demonstrated normal mitochondrial number and morphology in VLCAD-deficient cardiomyocytes. Notably, proteomic analysis did not show any major changes in expression of energy-producing enzymes in cardiac mitochondria of cVLCAD−/− mice. VLCAD-deficient cardiomyocytes, isolated mitochondria, and cardiac fibers from young and older mice have similar maximal respiration rates as WT controls. Similarly, OCRs were not affected in mitochondria and cardiac fibers (Fig. 3, E–H). These data suggest that energy-producing capacity (electron transport chain) of mitochondria is not affected by deficiency of VLCAD, although energy production from FAO is diminished.
In isolated mitochondria, we demonstrate that FAO is partially impaired in cardiac mitochondria. The remaining effect of palmitoyl-l-carnitine on VLCAD-deficient mitochondria may be attributed to the presence of long-chain acyl-CoA dehydrogenase (LCAD) in cardiac mitochondria because LCAD has overlapping substrate specificity with VLCAD. As a limitation of current study we recognize that palmitate-stimulated respiration activities in isolated mitochondria are insufficient for precise estimation of substrate oxidation rates in whole heart. It is plausible that LCAD has a lower affinity to P-C than VLCAD. Hence, the low concentration of P-C (5 μM) used in isolated mitochondria experiments may set conditions that reveal the impact of VLCAD deficiency (Fig. 3, G and H). While cytosolic and mitochondrial concentrations of long-chain fatty acids are unknown, one could speculate that local concentrations of acyl-CoAs at mitochondrial microcompartments are high enough to enable LCAD to compensate for VLCAD activity. Substrate utilization measurements and metabolic tracing are necessary for accurate analysis of metabolic fluxes in perfused hearts.
The plausible explanation of cold/fasting sensitivity in cVLCAD−/− mice is insufficient cardiac output and heat exchange due to depressed cardiac function. It has been documented that acute cold exposure increases blood flow and oxygen consumption in BAT (19). Acute revamp of blood flow requires mobilization of cardiac energy metabolism, particularly oxidation of fatty acids. It is feasible that when exposed to cold cVLCAD−/− mice are not able to increase blood flow through BAT due to impaired cardiac metabolism and depressed systolic function. Insufficient blood flow through BAT may result in inadequate heat exchange and eventually cause hypothermia and death. Indeed, the echocardiographic data from our study revealed that cVLCAD−/− mice developed severe sinus bradycardia and worsening systolic dysfunction with a significant decrease in body core temperature in the cold. Impaired FAO and energy output under stress conditions, rather than developing cardiomyopathy, are likely to be major causative factors in developing of cold intolerance in cVLCAD−/− mice. This hypothesis is supported by our experimental observation of cold intolerance in 2-mo-old mice, long before the cardiac phenotype becomes apparent. Moreover, mice with an inactivated tafazzin gene, another mouse model with suppressed systolic function (1), can survive in the cold for >18 h without signs of distress (33). Possible arrhythmia and altered Ca2+ homeostasis may also be contributing factors in worsening of cardiac output in cVLCAD−/− mice in the cold environment. It is important to mention that mice of two genetic backgrounds (C57BL6 and C57BL6 × FVB intercross) were subjected to cold stress and cVLCAD−/− mice demonstrated cold/fasting intolerance regardless of their genetic background.
We cannot exclude the possibility that hypothermia is secondary to predisposition of cVLCAD−/− mice to magnified parasympathetic response, altered kinetics of ionic channels, or reduced direct thermogenesis in cardiac muscle or epicardial adipose tissue (36). Since heart rate and rectal temperature assessment occurred at only two time points (before and then late after exposure to cold stress), our data are not sufficient to fully delineate that the interactions of hypothermia, bradycardia, and cardiac energetics. Future studies are needed to explore the molecular mechanisms involved in the potential abnormality of thermoregulation caused by defect of FAO in cardiomyocytes and other oxidative tissues.
In addition to cold/fasting intolerance, the present study identified that cardiac-specific VLCAD deficiency is sufficient to induce dilated cardiomyopathy by 6 mo of age. The notion that energy “starvation” is a significant contributor to the pathogenesis of cardiomyopathy and cardiac failure has long been entertained (24, 30). It is known that to support contractile activity, the human heart requires a large amount of ATP and that even subtle variations in the efficiency of energy generation or utilization may have a profound cumulative impact on cellular energy levels (18). Ninety percent of ATP is produced in mitochondria, and in healthy hearts, up to 70% of ATP is generated via FAO (4, 30, 40).
To determine the relationship of cardiac dysfunction with energy deficiency, we assessed contractile performance and measured ATP levels of isolated hearts in the Langendorff preparation at baseline and with dobutamine challenge. The Langendorff experiment not only identified decreased systolic and diastolic function in the hearts of cVLCAD−/− mice but also confirmed significant reduction of ATP synthesis in these hearts. Contrary to our findings, a recent study of global VLCAD KO mice demonstrated the ability of VLCAD deficient hearts perfused ex vivo to maintain normal contractile performance and metabolic fluxes (20). Discrepancies in results between these two studies may have resulted from possible differences in adaptive metabolic remodeling in hearts of global- and cardiac-specific VLCAD KO models. For example, cardiomyocytes from global VLCAD KO mice contained many fat droplets, a feature that we did not find in cVLCAD−/− model. It is plausible that extracardiac factors contributing into generation of these droplets. One may speculate that fat accumulations may serve as additional internal substrate pools for other ACADs, such as LCAD and disguise the effects of VLCAD ablation. Additionally, we did not find changes in expression level of tmem135 gene in our cVLCAD−/− mice (data not shown), which has been reported to be upregulated in global VLCAD KO hearts (17). Finally, we cannot rule out a possibility that replacement of first seven exons with neomycin-resistance cassette in global VLCAD KO model could affect an expression pattern of mir324, which is located in intron 11 in both mouse and human VLCAD genes. Unintentional interruption of the regulatory sequences of microRNA may unpredictably modify the observed phenotype of global VLCAD KO mice. Additional studies are needed to address these issues.
Cardiac functional and metabolic phenotypes of global VLCAD KO mice under basal conditions are milder than in humans (12, 16) (for review, see Ref. 39). This has been attributed to the capacity of LCAD to substitute for VLCAD in mice but not in humans (11, 20). This substrate overlap between VLCAD and LCAD in mice may be compensating for VLCAD deficiency during the first months of life and preventing acute metabolic decompensation, unlike the situation in children with null VLCAD gene mutations. For the same reason, cardiomyopathy may become manifest only by 6 mo of age in mouse models of VLCAD deficiency. Alternatively, the milder cardiac phenotype in VLCAD deficient mice may be associated with mouse diet. Our indirect calorimetry results demonstrate that on standard diet (3.8% fat, 14.7% protein, and 64% carbohydrates) mice mainly rely on carbohydrate metabolism. However, when fasted, mice shift their metabolic preferences toward fat (Fig. 5A). One may speculate that on a standard diet cardiac metabolism in mice is more glycolytic than in humans. However, if fed a high-fat diet for a prolonged period, VLCAD mutant mice would develop a more pronounced cardiac phenotype. Either way, the consequences of VLCAD deficiency may be magnified in human patients with mutations in the VLCAD gene and cold and fasting may be detrimental for patients with VLCAD deficiency.
Significant lipid deposition was found in the hearts of global VLCAD KO mice, and lipotoxicity has been implicated in the development of cardiomyopathy (16). However, Oil Red O staining of cVLCAD−/− heart tissue did not show significantly increased intracellular lipid deposition. A possible rationale for the discrepancy between global and cVLCAD−/− mice is that lipolysis and FAO in liver, skeletal muscle, and other organs remain intact in cVLCAD−/− mice; therefore, fatty acids can be oxidized in these tissues to prevent lipid accumulation in heart. The absence of any significant effect of VLCAD deficiency in hearts on body fat mass (Table 2) supports this hypothesis. Thus our data suggest that lipidosis is less important in the development of cardiomyopathy and cardiac dysfunction in cVLCAD−/− mice compared with global VLCAD KO mice.
In summary, our results indicate that cardiac-specific VLCAD deficiency is sufficient to induce dilated cardiomyopathy, concomitant with diminished energy production resulting from a defect in mitochondrial FAO. In addition, the presence of VLCAD in liver, skeletal muscle, BAT, and brain did not provide cVLCAD−/− mice protection against hypothermia, bradycardia, and severely depressed cardiac function with cold stress. These results add to the evidence that energy starvation in myocardium plays an important role in development of cardiomyopathy and that FAO in cardiomyocytes is critical for body temperature maintenance.
GRANTS
This study was partially supported by the Cincinnati Children's Research Foundation, National Heart, Lung, and Blood Institute Grant RO1-HL-108867 (to Z. Khuchua), and the Departments of Anesthesiology and Perioperative Medicine, College of Medicine, Medical University of South Carolina.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.X., H.H., J.J., C.T., C.P., Y.H., H.O., J.A.B., and Z.K. performed experiments; D.X., H.H., J.J., E.P., F.X.M., and Z.K. analyzed data; D.X., H.H., J.A.T., E.P., S.J., A.W.S., and Z.K. interpreted results of experiments; D.X., H.H., and Z.K. prepared figures; D.X., J.A.T., and Z.K. drafted manuscript; H.H., J.J., S.J., F.X.M., A.W.S., and Z.K. edited and revised manuscript; S.J., A.W.S., and Z.K. approved final version of manuscript; F.X.M., A.W.S., and Z.K. conception and design of research.
REFERENCES
- 1.Acehan D, Vaz F, Houtkooper RH, James J, Moore V, Tokunaga C, Kulik W, Wansapura J, Toth MJ, Strauss A, Khuchua Z. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem 286: 899–906, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andresen BS, Olpin S, Poorthuis BJ, Scholte HR, Vianey-Saban C, Wanders R, Ijlst L, Morris A, Pourfarzam M, Bartlett K, Baumgartner ER, deKlerk JB, Schroeder LD, Corydon TJ, Lund H, Winter V, Bross P, Bolund L, Gregersen N. Clear correlation of genotype with disease phenotype in very-long-chain acyl-CoA dehydrogenase deficiency. Am J Hum Genet 64: 479–494, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoyama T, Uchida Y, Kelley RI, Marble M, Hofman K, Tonsgard JH, Rhead WJ, Hashimoto T. A novel disease with deficiency of mitochondrial very-long-chain acyl-CoA dehydrogenase. Biochem Biophys Res Commun 191: 1369–1372, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation 116: 434–448, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Bennett MJ, Rinaldo P, Strauss AW. Inborn errors of mitochondrial fatty acid oxidation. Crit Rev Clin Lab Sci 37: 1–44, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Bertrand C, Largilliere C, Zabot MT, Mathieu M, Vianey-Saban C. Very long chain acyl-CoA dehydrogenase deficiency: identification of a new inborn error of mitochondrial fatty acid oxidation in fibroblasts. Biochim Biophys Acta 1180: 327–329, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Boss O, Muzzin P, Giacobino JP. The uncoupling proteins, a review. Eur J Endocrinol 139: 1–9, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 214: 242–253, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Cesarovic N, Jirkof P, Rettich A, Arras M. Implantation of radiotelemetry transmitters yielding data on ECG, heart rate, core body temperature and activity in free-moving laboratory mice. J Vis Exp 57: 3260, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chegary M, Brinke H, Ruiter JP, Wijburg FA, Stoll MS, Minkler PE, van Weeghel M, Schulz H, Hoppel CL, Wanders RJ, Houten SM. Mitochondrial long chain fatty acid beta-oxidation in man and mouse. Biochim Biophys Acta 1791: 806–815, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox KB, Liu J, Tian L, Barnes S, Yang Q, Wood PA. Cardiac hypertrophy in mice with long-chain acyl-CoA dehydrogenase or very long-chain acyl-CoA dehydrogenase deficiency. Lab Invest 89: 1348–1354, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellis JM, Li LO, Wu PC, Koves TR, Ilkayeva O, Stevens RD, Watkins SM, Muoio DM, Coleman RA. Adipose acyl-CoA synthetase-1 directs fatty acids toward beta-oxidation and is required for cold thermogenesis. Cell Metab 12: 53–64, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387: 90–94, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Exil VJ, Gardner CD, Rottman JN, Sims H, Bartelds B, Khuchua Z, Sindhal R, Ni G, Strauss AW. Abnormal mitochondrial bioenergetics and heart rate dysfunction in mice lacking very-long-chain acyl-CoA dehydrogenase. Am J Physiol Heart Circ Physiol 290: H1289–H1297, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Exil VJ, Roberts RL, Sims H, McLaughlin JE, Malkin RA, Gardner CD, Ni G, Rottman JN, Strauss AW. Very-long-chain acyl-coenzyme a dehydrogenase deficiency in mice. Circ Res 93: 448–455, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Exil VJ, Silva Avila D, Benedetto A, Exil EA, Adams MR, Au C, Aschner M. Stressed-induced TMEM135 protein is part of a conserved genetic network involved in fat storage and longevity regulation in caenorhabditis elegans. PLoS One 5: e14228, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrari R, Cargnoni A, Ceconi C. Anti-ischaemic effect of ivabradine. Pharmacol Res 53: 435–439, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Foster DO, Frydman ML. Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol 57: 257–270, 1979 [DOI] [PubMed] [Google Scholar]
- 20.Gelinas R, Thompson-Legault J, Bouchard B, Daneault C, Mansour A, Gillis MA, Charron G, Gavino V, Labarthe F, Des Rosiers C. Prolonged QT interval and lipid alterations beyond beta-oxidation in very long-chain acyl-CoA dehydrogenase null mouse hearts. Am J Physiol Heart Circ Physiol 301: H813–H823, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerra C, Koza RA, Walsh K, Kurtz DM, Wood PA, Kozak LP. Abnormal nonshivering thermogenesis in mice with inherited defects of fatty acid oxidation. J Clin Invest 102: 1724–1731, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hale DE, Bennett MJ. Fatty acid oxidation disorders: a new class of metabolic diseases. J Pediatr 121: 1–11, 1992 [DOI] [PubMed] [Google Scholar]
- 23.He H, Javadpour MM, Latif F, Tardiff JC, Ingwall JS. R-92L and R-92W mutations in cardiac troponin T lead to distinct energetic phenotypes in intact mouse hearts. Biophys J 93: 1834–1844, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res 95: 135–145, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Khuchua ZA, Qin W, Boero J, Cheng J, Payne RM, Saks VA, Strauss AW. Octamer formation and coupling of cardiac sarcomeric mitochondrial creatine kinase are mediated by charged N-terminal residues. J Biol Chem 273: 22990–22996, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Lee SH, Dobrzyn A, Dobrzyn P, Rahman SM, Miyazaki M, Ntambi JM. Lack of stearoyl-CoA desaturase 1 upregulates basal thermogenesis but causes hypothermia in a cold environment. J Lipid Res 45: 1674–1682, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Liebig M, Schymik I, Mueller M, Wendel U, Mayatepek E, Ruiter J, Strauss AW, Wanders RJ, Spiekerkoetter U. Neonatal screening for very long-chain acyl-coA dehydrogenase deficiency: enzymatic and molecular evaluation of neonates with elevated C14:1-carnitine levels. Pediatrics 118: 1065–1069, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res 13: 476–484, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathur A, Sims HF, Gopalakrishnan D, Gibson B, Rinaldo P, Vockley J, Hug G, Strauss AW. Molecular heterogeneity in very-long-chain acyl-CoA dehydrogenase deficiency causing pediatric cardiomyopathy and sudden death. Circulation 99: 1337–1343, 1999 [DOI] [PubMed] [Google Scholar]
- 29a.McLean JA, Tobin G. Animal and Human Calorimetry. Cambridge, UK: Cambridge Univ Press, 1987 [Google Scholar]
- 30.Murray AJ, Edwards LM, Clarke K. Mitochondria and heart failure. Curr Opin Clin Nutr Metab Care 10: 704–711, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, Molkentin JD. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res 98: 837–845, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Postolache T, Gautier S, Laloux B, Safar M, Benetos A. Positive correlation between the blood pressure and heart rate response to the cold pressor test and the environmental temperature in older hypertensives. Am J Hypertens 6: 376–381, 1993 [DOI] [PubMed] [Google Scholar]
- 33.Powers C, Huang Y, Strauss A, Khuchua Z. Diminished exercise capacity and mitochondrial bc1 complex deficiency in Tafazzin-knockdown mice. Front Physiol 4: 74, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinaldo P, Matern D, Bennett MJ. Fatty acid oxidation disorders. Annu Rev Physiol 64: 477–502, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics 3: 1154–1169, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F, Karas J, Optican R, Bahouth SW, Garrett E, Wolf RY, Carter RA, Robbins T, Wolford D, Samaha J. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab 94: 3611–3615, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Sadayappan S, Gulick J, Osinska H, Martin LA, Hahn HS, Dorn GW, Klevitsky R, 2nd, Seidman CE, Seidman JG, Robbins J. Cardiac myosin-binding protein-C phosphorylation and cardiac function. Circ Res 97: 1156–1163, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuh RA, Jackson KC, Khairallah RJ, Ward CW, Spangenburg EE. Measuring mitochondrial respiration in intact single muscle fibers. Am J Physiol Regul Integr Comp Physiol 302: R712–R719, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiekerkoetter U, Wood PA. Mitochondrial fatty acid oxidation disorders: pathophysiological studies in mouse models. J Inherit Metab Dis 33: 539–546, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stanley WC. Myocardial energy metabolism during ischemia and the mechanisms of metabolic therapies. J Cardiovasc Pharmacol Therap 9, Suppl 1: S31–45, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Strauss AW, Powell CK, Hale DE, Anderson MM, Ahuja A, Brackett JC, Sims HF. Molecular basis of human mitochondrial very-long-chain acyl-CoA dehydrogenase deficiency causing cardiomyopathy and sudden death in childhood. Proc Natl Acad Sci USA 92: 10496–10500, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka N, Dalton N, Mao L, Rockman HA, Peterson KL, Gottshall KR, Hunter JJ, Chien KR, Ross J., Jr. Transthoracic echocardiography in models of cardiac disease in the mouse. Circulation 94: 1109–1117, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Tucci S, Primassin S, Spiekerkoetter U. Fasting-induced oxidative stress in very long chain acyl-CoA dehydrogenase-deficient mice. FEBS J 277: 4699–4708, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Vergnes L, Chin R, Young SG, Reue K. Heart-type fatty acid-binding protein is essential for efficient brown adipose tissue fatty acid oxidation and cold tolerance. J Biol Chem 286: 380–390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werdich AA, Baudenbacher F, Dzhura I, Jeyakumar LH, Kannankeril PJ, Fleischer S, LeGrone A, Milatovic D, Aschner M, Strauss AW, Anderson ME, Exil VJ. Polymorphic ventricular tachycardia and abnormal Ca2+ handling in very-long-chain acyl-CoA dehydrogenase null mice. Am J Physiol Heart Circ Physiol 292: H2202–H2211, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Yamaguchi S, Indo Y, Coates PM, Hashimoto T, Tanaka K. Identification of very-long-chain acyl-CoA dehydrogenase deficiency in three patients previously diagnosed with long-chain acyl-CoA dehydrogenase deficiency. Pediatr Res 34: 111–113, 1993 [DOI] [PubMed] [Google Scholar]