Abstract
Contraction regulates heart development via a complex mechanotransduction process controlled by various mechanical forces. Here, we exploit zebrafish embryos as an in vivo animal model to discern the contribution from different mechanical forces and identify the underlying mechanotransductive signaling pathways of cardiogenesis. We treated 2 days postfertilization zebrafish embryos with Blebbistatin, a myosin II inhibitor, to stop cardiac contraction, which induces a response termed cessation of contraction-induced cardiomyocyte (CM) enlargement (CCE). Accompanying the CCE, lateral fusion of myofibrils was attenuated within CMs. The CCE can be blunted by loss of blood in tail-docked zebrafish but not in cloche mutant fish, suggesting that transmural pressure rather than shear stress is accountable for the chamber enlargement. By screening a panel of small molecule inhibitors, our data suggested essential functions of phosphoinositide 3-kinase signaling and protein synthesis in CCE, which are independent of the sarcomere integrity. In summary, we defined a unique CCE response in genetically tractable zebrafish embryos. A panel of assays was established to verify the contribution from extrinsic forces and interrogate underlying signaling pathways.
Keywords: cardiogenesis, contraction, mechanotransduction, sarcomere
the heart is a unique component of the circulatory system that constantly maintains physiological blood pressure through regulation of the strength and frequency of contractions. During cardiogenesis, de novo assembly of sarcomeres is an important differentiation process within cardiomyocytes (CMs) that provides the structural basis to initiate contraction. At the same time, the developing CMs gradually increase in size—a process called developmental hypertrophy. Accumulating evidence suggests that contraction imposes an important epigenetic influence on the process of heart development (1, 3, 12, 16). As the force generator, the actomyosin movement within CMs may impose a cell-autonomous influence on the development of individual CMs through internal stress (38). In addition, circulating blood flow, driven by the contracting heart, will reciprocally impose nonautonomous effects on CMs through extrinsic forces, including transmural pressure that is perpendicular to the ventricular wall and shear stress that is parallel to the ventricular wall (3, 30). The endocardium plays an important role in transmitting signaling events initiated by shear stress. It is hypothesized that shear stress is sensed by cilia on the endocardium surface, which secretes molecules to activate mechanotransductive signaling in the neighboring CMs (13). To elucidate the mechanotransduction underlying contraction-regulated heart development, cell-culture systems, such as those on stretch chambers, have been adapted as simple models (8, 20, 32). Along this direction, induced pluripotent stem cells (iPSCs), reprogrammed by exogenous overexpression of octamer-binding transcription factor 4, sex-determining region Y-box 2 (Sox2), Kruppel-like factor 4 (Klf4), and cMyc, have also been used to elucidate cell-autonomous mechanotransductive signaling (35). However, because of the integrated contribution from intrinsic and extrinsic factors and complex interactions between epigenetic signaling and genetic transcriptional circuits, in vivo vertebrate models are clearly needed to resolve functions of contraction in cardiogenesis in a native physiological context.
Zebrafish embryos have the potential to serve as an in vivo vertebrate model to elucidate the complicated relationships among heart contraction, sarcomere assembly, and heart formation (2, 31, 36). The simple and transparent fish heart allows characterization of mechanical force distribution in the whole organ; definition of deformation at the cell, tissue, and organ levels; and generation of system-wide signaling networks, which are important for achieving a global mechanistic view (16, 39, 40). In fast-developing zebrafish embryos, sarcomeres appear as narrow, nascent myofibrils, ∼22 h postfertilization (hpf), consisting of assembled thin and thick filaments. The neighboring nascent myofibrils will then fuse laterally and align with each other to form mature sarcomeres, ∼48 hpf, as represented by the expansion of Z-dots to striated Z-discs (18, 26, 39). This lateral fusion process of sarcomere maturation presumably supports stronger and more coordinated heart contraction in 48-hpf embryos (4). Throughout normal developmental stages, the zebrafish heart transforms from a tube into two distinct chambers after a looping and ballooning process, in which outer-curvature (OC) cells in the ventricle are elongated and enlarged, but inner-curvature cells remain rounded and small (1). Fish embryos harboring mutations in sarcomere genes have been reported to manifest severe chamber malformation. For example, the ventricular chamber was enlarged in ventricle myosin heavy chain mutant fish but deflated in atrial myosin heavy chain mutant and α-actinin2 morphant fish (1, 6, 39). Detailed studies of these mutant fish lines suggested that integrity of sarcomere function in combination with blood flow dynamics regulates heart development through mechanotransduction (1). However, the precise relationship among contraction, sarcomere integrity, blood flow, and cardiac morphogenesis remains elusive.
Here, we report a unique cessation of contraction-induced CM enlargement (CCE) response in zebrafish embryos. This is possible because fish embryos can survive without a functional circulation system for more than 24 h, with sufficient oxygen acquired by diffusion through the skin (29). We found that cessation of contraction imposed a consistent influence on the lateral fusion of the sarcomere and a developmental stage-dependent effect on ventricular chamber size. Further studies indicated that CCE can be ascribed to nonautonomous signaling due to increased preload but not endocardium-based shear stress. Chemical screening uncovered a critical role of the phosphoinositide 3-kinase (PI3K) signaling pathway in this mechanotransductive process. Our results present a unique and convenient assay in zebrafish that can be used to interrogate further functions of contraction in cardiogenesis.
MATERIALS AND METHODS
Zebrafish husbandry.
The embryos and adult fish were raised and maintained under standard laboratory conditions. The animal experiments were approved by the Mayo Clinic College of Medicine Institutional Animal Care and Use Committee (protocol #A15611). Wik strain zebrafish larvae were used for most experiments. Cloche mutant fish were generously supplied by Dr. Calum MacRae (Brigham and Women's Hospital, Boston, MA).
Assessment of ventricular chamber volume.
The ventricular chamber volume was measured as described previously (39). Briefly, the transgenic(titin:membrane green fluorescent protein) [Tg(titin:mGFP)] fish, which express a GFP signal in the cardiac and skeletal muscle, were incubated in E3 water containing 20 mmol/l 2,3-butanedione monoxime (BDM; Sigma, St. Louis, MO) and 0.4% tricaine (Sigma) for 5 min. Under these conditions, heart-beating was stopped, and the heart was then fixed at the end of the diastolic stage. The fish embryos were embedded in 3% methyl cellulose (Fisher Scientific, Pittsburgh, PA), with orientations adjusted to expose the ventricle laterally to the objective lens. A Zeiss Axioplan 2 microscope was used for imaging. The ventricular chamber volume was calculated by the formula: volume = (4/3) πab2 (where a = long axis length, and b = short axis length between the myocardial borders of ventricles). At least 10 fish in each individual group were measured to calculate the average ventricular chamber volume. Relative Blebbistatin (BLE)-induced ventricular chamber enlargement was calculated by the formula: (VBLE − VDMSO)/VDMSO (where VBLE = ventricular chamber volume in BLE-treated fish, and VDMSO = ventricular chamber volume in DMSO-treated fish).
Calculation of cardiac function parameters.
After developing to the indicated developmental stage, the fish larvae were embedded directly in 3% methyl cellulose, with orientations adjusted to expose the ventricle to the objective lens of the Axioplan 2 microscope. The heart contractions were documented in a movie of 10 s. Shortening fraction (SF) was calculated by the formula: SF = (length at diastole − length at systole)/(length at diastole) × 100. At least 10 fish were used to calculate cardiac function for each individual group. Heart rate was measured by counting heart beats in the whole movie and then dividing by the length of the movie.
Stem cell culture and differentiation.
iPSC lines were generated from Gt(ROSA)26Sortm(rtT1 × M2)Jae Col1a1tm3(tetO-Pou5f1,-Sox2,-Klf4,-Myc)Jae/J mouse embryonic fibroblasts (MEFs), harvested at 14.5 days postcoitum. MEFs were plated onto inactivated feeders and cultured for 24 h in DMEM (Gibco, Life Technologies, Carlsbad, CA), supplemented with 10% FBS, penicillin G, streptomycin, L-glutamine (Invitrogen, Grand Island, NY), sodium pyruvate (Lonza, Basel, Switzerland), and nonessential amino acids (Mediatech, Herndon, VA). Cells were then induced to pluripotency through the addition of 2 μg/ml doxycycline and cultured in EmbryoMax DMEM (EMD Millipore, Billerica, MA), supplemented with 15% embryonic stem cell-qualified FBS, penicillin G, streptomycin, L-glutamine, sodium pyruvate, nonessential amino acids, 2-mercaptoethanol (Sigma), and leukemia inhibitory factor (EMD Millipore).
Spontaneous differentiation of iPSCs was performed using an embryoid body (EB)-based system. EBs containing ∼2,000 cells were generated via centrifugation of iPSCs into an AggreWell plate (Stemcell Technologies, Vancouver, BC, Canada), according to the manufacturer's instructions. Differentiating EBs were maintained in DMEM, supplemented with 20% FBS, penicillin G, streptomycin, L-glutamine, sodium pyruvate, nonessential amino acids, and 2-mercaptoethanol. Beating activity was monitored closely using light microscopy in both BLE-treated and control groups at 6 h post-treatment on day 15 of differentiation. For enhanced image quality of iPSC-derived CMs (iPSC-CMs), parallel experiments were conducted in which the EBs from both treatment and control groups were dissociated via collagenase treatment at day 11 and replated in a monolayer on gelatin-coated culture plates.
Cessation of cardiac contraction by BLE treatment.
The fish embryos were kept in a 28°C incubator until 22 hpf for 1-day postfertilization (dpf) embryos and 44 hpf for 2-dpf embryos. After being deprived of the chorion by protease treatment (Sigma), the fish embryos were bathed in E3 water containing 8 μmol/l BLE (Sigma). Within 10 min, cardiac contractions were stopped completely.
To obtain the time course of ventricular chamber change during and after BLE treatment, 44-hpf fish embryos were divided into three different groups after dechorionation. One group of fish was bathed in E3 water containing 0.1% DMSO, and the other two groups of fish were incubated with 8 μmol/l BLE. After an 8-h incubation, the BLE solution was replaced with fresh E3 water in one BLE treatment group. All three groups were maintained for another 15 h. The ventricular volume was measured at the end of every hour within the 8-h treatment and every 2 h after the treatment.
To assess the dose-response curve of chamber-size change to BLE treatment, the 44-hpf dechorionated fish larvae were treated with DMSO and 0.5 μmol/l, 1 μmol/l, 2 μmol/l, 5 μmol/l, and 10 μmol/l BLE for 8 h and then imaged to document cardiac contraction and ventricular morphology.
To assess the cell-autonomous nature of BLE-induced cardiac phenotypes, murine iPSCs were differentiated in vitro and treated with 0 μmol/l, 2 μmol/l, 8 μmol/l, and 25 μmol/l BLE for 8 h at day 15 of differentiation in biological triplicates. Following treatments with BLE, both treated cells and untreated controls were fixed in 4% paraformaldehyde for 10 min at 37°C, permeabilized with 0.1% Triton X-100, blocked for 3 h, and stained with α-actinin (Sigma) and then with Alexa Fluor 568 (goat antimouse; Invitrogen) as a secondary antibody.
Measurement of CM size and Z-disc length.
To measure the CM size, immunostaining with anti-β-catenin antibody (Sigma) and antimyocyte enhancer factor-2 (anti-Mef2) antibody (Santa Cruz Biotechnology, Santa Cruz, CA) was performed in isolated embryonic zebrafish hearts, according to the protocol described previously (40). Briefly, after isolated from living, anesthetized zebrafish embryos, the heart was held on a glass slide and fixed with 4% formaldehyde for 15 min. The heart sample was then blocked with 10% sheep serum and penetrated by 0.5% Triton X-100 in PBS solution before incubation with primary antibody and antimouse IgG1 or antirabbit IgG (heavy + light chains) antibody, conjugated with Alexa Fluor dye. The nuclear and cell-surface stains of CMs were shown clearly in the stained heart. Approximately 10 cells in the OC region of each individual heart were selected for measuring cell-surface area using AxioVision software. Measurements from five fish for each group were calculated to acquire the average and SD of CM size. Relative BLE-induced cell enlargement was calculated by the formula: (CBLE − CDMSO)/CDMSO (where CBLE = cell-surface area in BLE-treated fish, and CDMSO = cell-surface area in DMSO-treated fish).
The Z-disc length is defined as the length perpendicular to the long axis of the CM, as described before (39). To measure the Z-disc length of individual fish hearts, anti-α-actinin antibody (Sigma) was used to stain the Z-disc. Approximately three to five Z-discs in each myofibril and 10 myofibrils from each fish were measured to obtain the average Z-disc length of individual fish. A total of five fish in each group was assessed to calculate the average and SD of Z-disc length.
CM size and sarcomeric Z-disc length in iPSC-CMs were measured via α-actinin (1:500; Sigma) staining at day 15 of in vitro differentiation and visualized at low and high magnification, respectively. For each treatment group, 30 CMs from biological triplicates were selected for size and Z-disc measurements and 10 Z-disc measurements/cell to obtain an average for each CM.
Measurement of CM thickness.
After the anesthetized Tg(titin:mGFP) fish were frozen in tissue-freezing medium from Electron Microscopy Sciences (Hatfield, PA), the fish were cut transversely from head to somite using Research Cryostat Leica CM3050 S. Immunostaining was performed on the sections with anti-GFP (Invitrogen), anti-β-catenin (Sigma), and antivinculin (Sigma) antibodies, as described before (34). The heart, distinguished by the GFP signal, was imaged with the Zeiss Axioplan 2 microscope. According to the outline of CMs revealed by the β-catenin signal, the diameter of each individual CM along the ventricle radius was measured as cell thickness. Only CMs with clear outline staining were chosen for measurement. Five fish in each group were measured for calculating the average and SD of CM thickness.
Screen of small molecule inhibitors.
Sixteen inhibitors for known signaling pathways were screened (Table 1). Fish embryos at 44 hpf were pretreated with the small-molecule inhibitors for 1 h, followed by cotreatment with 8 μmol/l BLE and the same small molecule inhibitor for another 8 h. The ventricular chamber size was measured at the end of treatment in at least 10 fish/group. For each small molecule inhibitor, three different concentrations were tested with the highest concentration listed in Table 1.
Table 1.
Summary of the small molecule inhibitors used in screen for attenuation of BLE-induced chamber enlargement
| Target | Drug | Concentration | IC50 (from Vendor Website) |
|---|---|---|---|
| IGF-1R | NVP-ADW742 | 25 μmol/l | 0.17 μmol/l |
| FGFR1 | SU5402 | 20 μmol/l | 10–20 μmol/l |
| JNK | SP600125 | 100 μmol/l | 40 nmol/l |
| MEK1/2 | U0126 | 40 μmol/l | 72 nmol/l/58 nmol/l |
| AKT1/AKT2 | AKTi | 3 μmol/l | 58 nmol/l/210 nmol/l |
| mTOR | Rapamycin | 2 μmol/l | 0.05 nmol/l |
| GSK3 | SB-216763 | 50 μmol/l | 10 μmol/l |
| GSK3β | LiCl | 100 mmol/l | 1 mmol/l |
| PI3K | LY294002 | 30 μmol/l | 1.4 μmol/l |
| PI3K | Wortmannin | 2 μmol/l | 2–4 nmol/l |
| Calcineurin | Cyclosporin A | 17 μmol/l | 5 nmol/l |
| Endosome/lysosome | Chloroquine (CQ) | 500 μmol/l | 0.566 μmol/l |
| Proteasome | MG-132 | 100 μmol/l | 100 nmol/l |
| HDAC | Trichostatin (TSA) | 0.5 μmol/l | 1.8 nmol/l |
| Transcription | Actinomycin D | 8 μmol/l | 0.4 nmol/l |
| Translation | Cycloheximide | 71 μmol/l (20 μg/ml) | 3.5 μmol/l |
BLE, Blebbistatin; R, receptor; mTOR, mammalian target of rapamycin; PI3K, phosphoinositide 3-kinase; HDAC, histone deacetylase.
Western blotting.
Western blot was performed, as described previously (9). Anti-Akt antibody (#9272; Cell Signaling Technology, Danvers, MA) and antiphospho-Akt antibody (#4058; Cell Signaling Technology) were used to assess Akt and phospho-Akt levels, respectively.
Cutting tail operation.
At the indicated developmental stages, the fish larvae were bathed in E3 water containing 0.4% tricaine and 0.1 mg/ml heparin for 10 min before surgery to delay formation of blood clots. The fish tail was clipped by the tips of two syringe needles on the posterior part of cloaca. When the fish tail was removed by moving the two syringe needles quickly, the blood started flowing out from the cut site of the tail until clots formed. After surgery, the fish were put back into fresh E3 water and incubated at 28°C for 24 h before imaging.
Statistics.
The values shown in the graphs are means ± SD. Normality was tested for each set of data using JMP software. If the data fit a normal distribution, then the Student's t-test or Dunnett's test was performed between the control group and the treatment group. If the data were not distributed normally, then the Mann-Whitney-Wilcoxon test was performed between the control group and the treatment group. P < 0.01 is regarded as significant.
RESULTS
Contraction oppositely regulates ventricular chamber size at different stages of cardiogenesis.
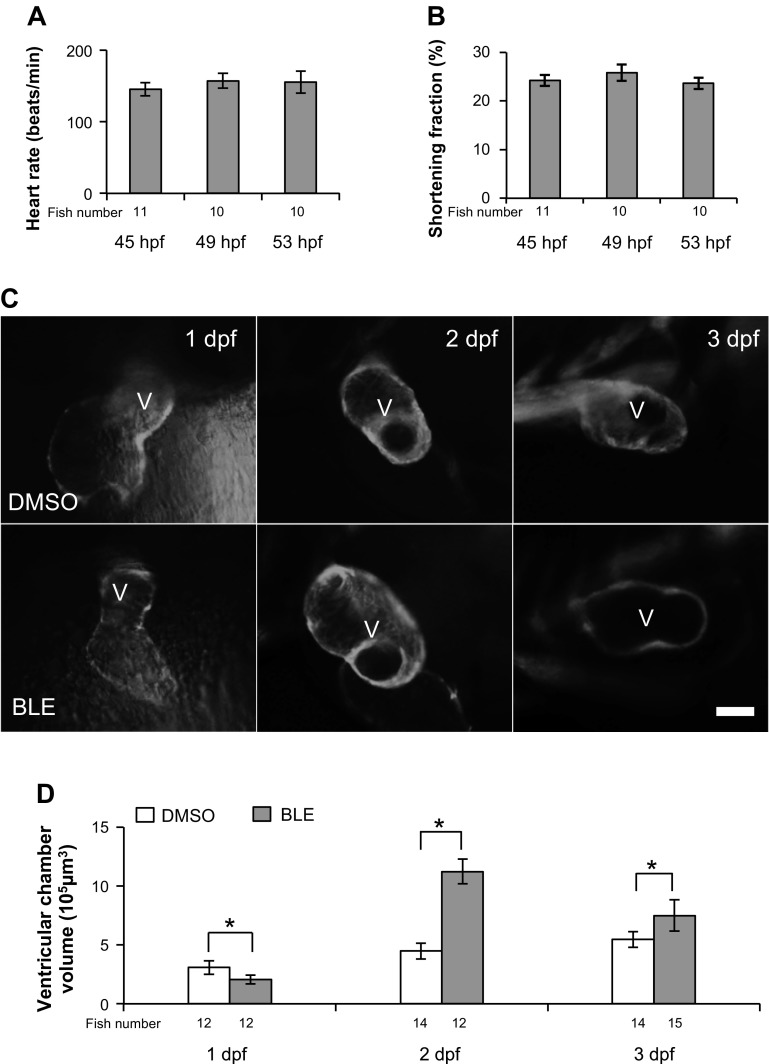
In zebrafish, the embryonic heart starts its peristaltic beating ∼22 hpf. More coordinated beating can be observed at 2 dpf, when the heart rate stabilizes ∼152 (±5) beats/min, and SF reaches ∼24% (±0.9%; Fig. 1, A and B). Concomitantly, the ventricle chamber formation begins from 1 dpf, and the chamber volume increases from 3 × 105 μm3 at 1 dpf to 5.4 × 105 μm3 at 3 dpf (Fig. 1, C and D). To assess how contraction regulates physiological chamber enlargement during cardiogenesis, we reduced cardiac contraction by incubating fish with BLE, a myosin II inhibitor that uncouples excitation contraction (23). We noted a developmental stage-dependent effect. If 1-dpf embryos were treated with BLE for 8 h, then the ventricle chamber was smaller than the untreated control. In contrast, if 2- or 3-dpf embryos were treated with BLE for 8 h, then the ventricular chamber was enlarged significantly (Fig. 1, C and D).
Fig. 1.
Cessation of contraction impairs ventricular chamber formation. Measurement of heart rate (A) and shortening fraction (SF; B) in larval fish at 45 h postfertilization (hpf), 49 hpf, and 53 hpf. C: Blebbistatin (BLE) treatment modifies ventricular chamber morphology differentially at different developmental stages. The various ventricle changes were revealed by green fluorescent protein (GFP) signal in 1-day postfertilization (dpf; 32 hpf), 2-dpf (52 hpf), and 3-dpf (80 hpf) transgenic(titin:membrane GFP) [Tg(titin:mGFP)] fish embryos. The fish embryos were treated with DMSO or 8 μmol/l BLE for 8 h. Ventral view of 1-dpf fish with head upward and lateral view of 2- and 3-dpf embryo with head leftward. V, ventricle; original scale bar, 50 μm. D: the measurement of ventricle chamber size in C. The ventricular volume is reduced in 1-dpf embryos but increased significantly in 2- and 3-dpf embryos after BLE treatment. *P < 0.01.
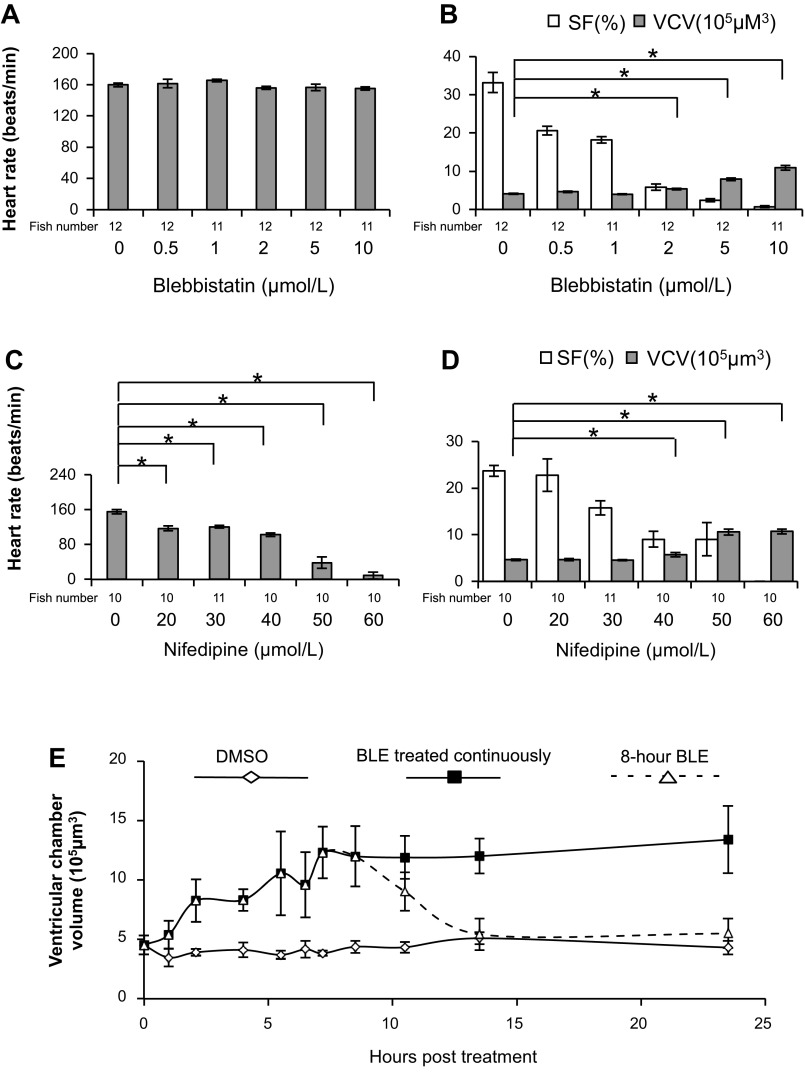
To test the specificity of this phenotype, we assessed the dose response of BLE-induced ventricular chamber enlargement in 2-dpf embryos. As expected, embryos treated with BLE manifest a dose-dependent reduction of SF without a dramatic change in heart rate (Fig. 2, A and B). When treated with 8 μmol/l BLE, the heart only twitches with the electrical impulses, resulting in completely halted blood circulation. The ventricular chamber volume is not affected when SF is 10–30% but is increased dramatically when SF is <10% (Fig. 2B). To validate that this chamber enlargement is due to cessation of contraction, we treated embryos with Nifedipine, a calcium ion channel inhibitor that reduces both the heart rate and SF in a dose-dependent manner (Fig. 2, C and D). Consistent with BLE treatment, Nifedipine treatment also induced a significantly enlarged ventricle chamber when SF was reduced to <10% (Fig. 2D). Similar observations were made with BDM, another myosin II inhibitor that affects both contractility and cardiac action potential (data not shown) (21).
Fig. 2.
The effect of BLE or Nifedipine treatment on cardiac function and morphology. A–D: heart function, SF, and ventricular chamber volume (VCV) were assessed in 52-hpf fish embryos after the fish were treated by BLE or Nifedipine at indicated concentrations for 8 h. A: BLE treatment does not affect heart rate in 2-dpf fish embryos. B: BLE treatment in 2-dpf embryo fish reduces SF but increases VCV in a dose-dependent manner. C: Nifedipine treatment dose dependently decreases heart rate in 2-dpf fish embryos. D: Nifedipine treatment reduces SF but increases ventricular chamber size in a dose-dependent manner. E: the time course of ventricular chamber change in 2-dpf embryos. The 44-hpf fish embryos were treated with 8 μmol/l BLE for 8 h (dashed line with open triangle), 8 μmol/l BLE for 23 h (solid line with solid rectangle), or DMSO (solid line with open rhombus). *P < 0.01.
By following the time course of contraction-regulated ventricular chamber-size changes in 2-dpf embryos (Fig. 2E), we found that the ventricular chamber size responds quickly and reversibly to cessation of contraction. The ventricular chamber size starts to increase within 1 h of treatment with BLE and reaches a plateau ∼8 h post-treatment, when the chamber volume has almost tripled compared with the untreated fish. When contraction was resumed by washing out the BLE solution, the ventricular chamber size reduces to normal, and major cardiac functional indices, such as ejection fraction, return to the normal levels in ∼6 h (Fig. 2E).
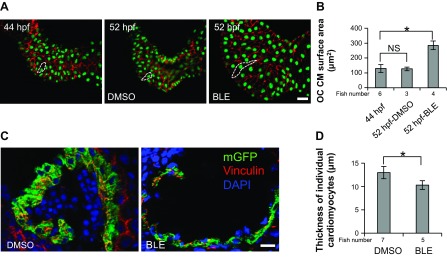
At the cellular level, we measured the surface area of individual CMs in dissected hearts, whose nuclei and borders were defined by immunostaining using Mef2 and β-catenin antibodies, respectively (Fig. 3A). The surface area of CMs in the OC region increased by approximately twofold from 44 hpf to 52 hpf in BLE-treated embryos, a change that is much more dramatic than physiological enlargement in DMSO-treated control embryos, which did not show detectable chamber enlargement during such a short time window (Fig. 3, A and B). By contrast, the thickness of ventricle CMs is reduced, as quantified by measuring their diameter in cross-sectioned hearts or measuring wall thickness in live Tg(titin:mGFP) fish (Fig. 3, C and D). If an ellipsoid shape is assumed for individual CMs, then the total volume of individual cells can also be considered to increase (data not shown) (7). Together, our data demonstrate that cessation of contraction in 2-dpf zebrafish embryos incurs a reversible enlargement of the ventricular chamber, with individual CMs becoming larger but thinner.
Fig. 3.
Changed ventricle chamber size is ascribed to cell-shape change of individual cardiomyocytes (CMs). A: the nucleus and outline of CMs were revealed by myocyte enhancer factor-2 (green) and β-catenin (red) antibody staining in 2-dpf fish embryos, before (44 hpf) and after 8-h DMSO or 8 μmol/l BLE treatment (52 hpf). The outline of representative CMs in each group is indicated by white, dashed lines. Original scale bar, 20 μm. B: measurements of CM size in A show that CM size is increased significantly by BLE treatment, whereas having no dramatic change from 44 hpf to 52 hpf in DMSO-treated fish. OC, outer curvature; NS, nonsignificant; *P < 0.01. C: outline and nuclear staining of CMs in cross sections of 2-dpf Tg(titin:mGFP) fish were revealed by mGFP (green), 4′,6′-diamidino-2-phenylindole (DAPI; blue), and vinculin (red) staining after the fish were treated with DMSO or 8 μmol/l BLE for 8 h. Original scale bar, 20 μm. D: the measurement of thickness of individual CMs in C indicates significantly reduced CM thickness in BLE-treated fish. *P < 0.01.
Contraction is required for lateral fusion of developing sarcomeres.
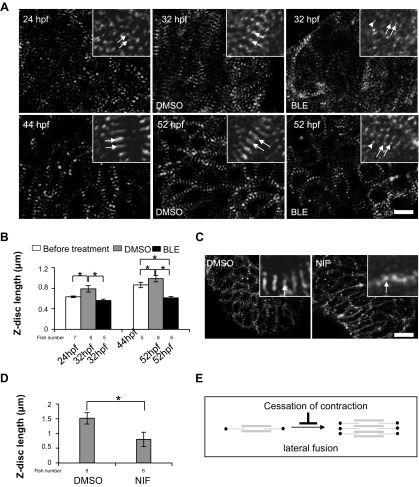
At the structural level, increasingly, mature sarcomeres accompany gradually increased heart contraction during zebrafish cardiogenesis (18, 27). From 24 hpf to 52 hpf, the stronger and coordinated heart beat is accompanied by a lateral fusion and cross-registration process to form bundled sarcomeres, as reflected by the fusion of punctate Z-dots to form longer Z-discs with gradually increased length (Fig. 4, A and B). We found that cessation of contraction by BLE treatment from 24 hpf to 32 hpf prevents the initiation of lateral fusion. As a consequence, sarcomeres remain at a rudimentary stage that manifests as thinner myofibrils with punctate Z-dots at 32 hpf. Some sarcomeric components even failed to integrate into the sarcomere, as reflected by randomly diffused α-actinin dots in the cytoplasm (Fig. 4A). Furthermore, cessation of contraction by BLE treatment from 44 hpf to 52 hpf disrupts the sarcomere structure, reverting Z-discs to Z-bodies with a length comparable with those in a 24-hpf fish embryo (Fig. 4, A and B). Thus BLE treatment disassembled the myofibrils into more primitive structures, indicating that contraction is required for the maintenance of intact sarcomeres (Fig. 4, A and B). Similarly, sarcomeres in fish treated with Nifedipine also show the lateral fusion defect, as reflected by a significantly reduced Z-disc length (Fig. 4, C and D). Together, our data suggest that cardiac contraction is required for both initiating and maintaining the lateral fusion of sarcomeres (Fig. 4E).
Fig. 4.
Contraction is required primarily for the lateral growth of Z-discs. A: the Z-discs in CMs revealed by α-actinin staining in 1-dpf (top) and 2-dpf (bottom) fish embryos, before and after the fish were treated with DMSO and 8 μmol/l BLE for 8 h. The 2 typical striated Z-dots or Z-discs in each group are indicated by arrows, whereas the randomly dispersed α-actinin dots, which do not assemble to myofibrils, are indicated by arrowheads. Original scale bar, 10 μm. B: Z-disc length, defined as the length of the Z-disc along the axis perpendicular to the myofibril, is increased during development but reduced significantly by BLE treatment. *P < 0.01. C: α-actinin staining in fish hearts for 2-dpf zebrafish embryos after the fish were treated by DMSO or 60 mM Nifedipine (NIF) for 8 h. The Z-disc becomes significantly shorter in Nifedipine-treated fish compared with DMSO-treated fish. Original scale bar, 20 μm. D: the length of the Z-disc is reduced dramatically by Nifedipine treatment. *P < 0.01. E: the schematic diagram illustrates the lateral fusion step in sarcomere assembly attenuated by cessation of contraction.
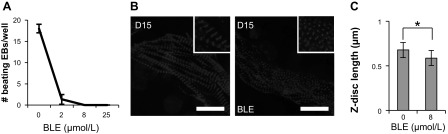
To validate our observation in fish embryos, we examined the impact of contraction on sarcomere maturation within iPSC-CMs during in vitro cardiogenesis. Similar to the zebrafish embryos, 8-h treatment with BLE stops iPSC-CM beating in a dose-dependent manner (Fig. 5A). When iPSC-CMs, at day 15 of differentiation, were treated with 8 μmol/l BLE to stop spontaneous contractions completely, Z-disc length was reduced significantly, indicating a lateral fusion defect in the myofibril (Fig. 5, B and C). Consistent with our observations in BLE-treated live fish, this in vitro data support an essential intrinsic function of contraction for sarcomere assembly in differentiating CMs without physiological blood flow demands.
Fig. 5.
Cessation of contraction in induced pluripotent stem cell-derived CMs (iPSC-CMs) disrupts sarcomere structure. A: beating activity (as measured by visibly contracting regions/well) of iPSC-CMs decreases in a dose-dependent manner following treatment with increasing concentrations of BLE. iPSCs were differentiated for 15 days in vitro in 6-well culture plates and treated with indicated concentrations of BLE. EBs, embryoid bodies. B: disrupted sarcomeric phenotype in iPSC-CMs upon 8 h treatment with 8 μmol/l BLE relative to control at day 15 (D15) of in vitro differentiation. Representative CM images from different treatment groups are shown. Z-discs are stained with an α-actinin antibody, and nuclei are stained with DAPI. Original scale bars, 20 μm. C: quantification of Z-disc length from B; n = 30 CMs/treatment group (10 cells across 3 biological triplicates and 10 Z-disc measurements/cell). *P < 0.01.
CCE response is due to increased preload.
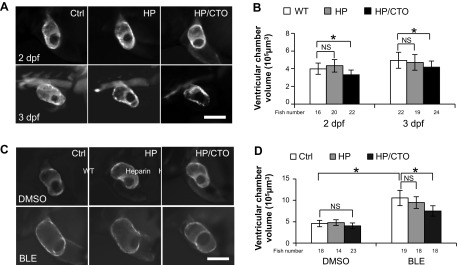
The initiation of cardiac contraction drives the development of a circulation system in the developing embryo. The resulting blood flow has been demonstrated to be an important epigenetic factor that affects heart development, including shape change, cushion development, and the trabeculation process (16). Therefore, we hypothesized that when contraction is inhibited, the continued return of venous blood might increase the preload and contribute to CCE. To test this hypothesis, we disrupted the circulation system by cutting the fish tail to release the blood and reduce the preload (Supplemental Movies 1 and 2). Heparin, an anticoagulation compound that delays blood clotting, was added to ensure more complete blood removal. The tail-docked fish embryos can survive the blood removal with normal heart function for 1 day after surgery (Supplemental Movies 3 and 4, and data not shown). We found that our cutting tail operation (CTO) prevents physiological, ventricular enlargement in both 1- and 2-dpf embryos, 24 h after tail cutting (Fig. 6, A and B). This is unlike the opposing effects that cessation of contraction had on ventricular chamber size at these two different developmental stages, indicating the consistent role of preload in inducing chamber enlargement during development. Interestingly, 2-dpf tail-docked fish, which have not shown the reduced chamber size, 8 h after tail cutting, have significantly blunted BLE-induced chamber enlargement (Fig. 6, C and D), supporting the contribution of preload derived from blood pressure to CCE. In contrast, the sarcomere structure remains normal in tail-docked fish, as indicated by the maintenance of broad Z-discs instead of punctate Z-dots (data not shown), suggesting that the absence of blood pressure did not affect sarcomere structure.
Fig. 6.
Increased preload contributes to both physiological and cessation of contraction-induced cardiac hypertrophy. A: the ventricle chamber is revealed by GFP signal in 2- and 3-dpf Tg(titin:mGFP) fish after no treatment [control (Ctrl)], heparin treatment (HP), and cutting tail operation (CTO), respectively. For 2-dpf embryos, CTO was performed at 32 hpf, and the image was documented at 44 hpf. For 3-dpf embryo, CTO was performed at 52 hpf, and the image was documented at 72 hpf. Original scale bar, 100 μm. B: the measurement of VCV showed significant reduction in ventricular volume after CTO but not heparin treatment. WT, wild-type; *P < 0.01. C: the 2-dpf Tg(titin:mGFP) fish embryos were treated with DMSO or 8 μmol/l BLE for 8 h after no treatment, heparin treatment, or CTO. The ventricular morphology was revealed by GFP signal in fish ventricles. CTO was performed at 44 hpf immediately before treatment, and image was captured at 52 hpf. Original scale bar, 100 μm. D: the measurement of volume of cardiac ventricle in C. The increase in ventricle volume after BLE treatment was attenuated significantly by CTO. *P < 0.01.
The CCE response remains normal in cloche mutant fish without endocardium.
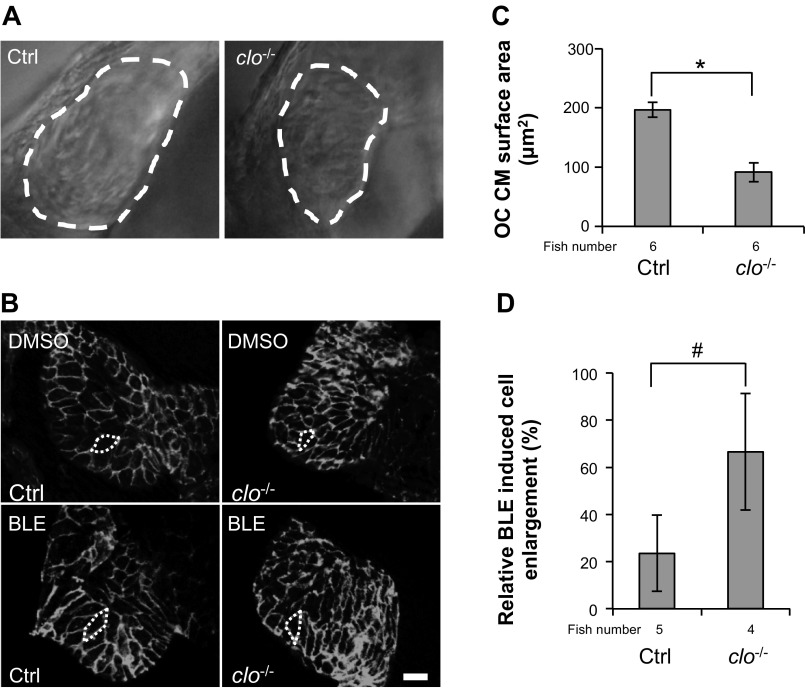
Mechanistically, blood flow could regulate ventricular CMs directly via transmural pressure that is perpendicular to the heart wall or indirectly via shear stress that is in parallel with the heart wall. In the latter circumstance, it is believed that shear stress activates chemical signaling in the endocardium, which secretes factors that indirectly activate downstream signaling in neighboring CMs (13). To determine the function of the endocardium in CCE, we conducted BLE treatment in cloche mutant fish with a completely depleted endocardium (33). Despite the significantly smaller CM surface area in cloche mutant fish (Fig. 7, A–C), BLE treatment incurs an even stronger cell-enlargement response in mutant fish (66% increase of cell size) relative to control, sibling fish (24% increase of cell size; Fig. 7, B and D). Therefore, our observation strongly suggests that endocardium-based mechanotransductive signaling is dispensable and possibly even inhibitory for CCE in zebrafish at 2 dpf.
Fig. 7.
CM remodeling is independent of the endocardium. A: the ventricular chamber outlined by the white, dashed lines is smaller in cloche mutant fish than in control fish. clo−/−, cloche mutants. B: the outline of ventricular CMs was revealed by β-catenin staining in 2-dpf cloche mutant fish and control fish after the fish were treated with either DMSO or 8 μmol/l BLE for 8 h. The treatment started at 44 hpf, and heart samples were collected at 52 hpf. Original scale bar, 20 μm. C and D: quantification of cell size of CMs in B. BLE treatment elicits a higher degree of cell enlargement in cloche mutants than in control fish (D) despite the smaller cell size in cloche mutants than control fish in the DMSO treatment condition quantified in C. *P < 0.01; #P < 0.05.
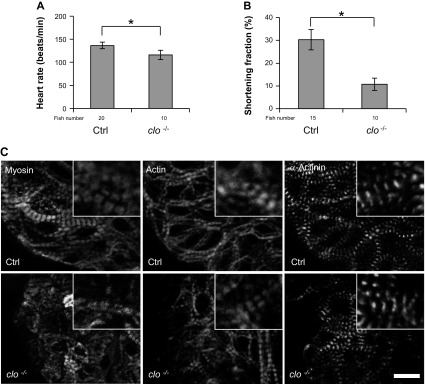
We did notice an attenuated physiological ventricular chamber enlargement in cloche mutants from 1 to 2 dpf (Fig. 7C). Both heart rate and SF are reduced significantly, whereas the lateral fusion of myofibrils in the CMs is maintained (Fig. 8). It remains to be determined whether the defective physiological heart enlargement is ascribed directly to a depleted endocardium or indirectly to the subsequent contraction defect due to lack of endocardium.
Fig. 8.
Cardiac function is impaired, but lateral fusion of myofibrils is not affected in cloche mutants. A and B: both heart rate and SF are reduced significantly in 52-hpf cloche mutants compared with control fish. *P < 0.01. C: the thick filament, thin filament, and Z-disc are revealed by immunostaining with anti-myosin antibody (Myosin), phalloidin (Actin), and anti-α-actinin antibody, respectively, in hearts isolated from 52-hpf cloche mutants and control fish. The lateral fusion of sarcomeres is intact in cloche mutants. Original scale bar, 5 μm.
CCE requires protein synthesis and PI3K signaling.
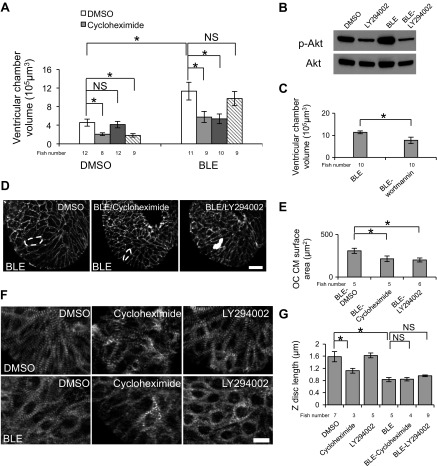
The unique CCE response in zebrafish embryos provides an experimental platform to assess related molecular events and mechanotransductive signaling pathways. The permeability of zebrafish embryos enables a chemical genetic approach that has been proven to be productive in uncovering signaling pathways (28, 41). Accordingly, we performed a ventricular chamber size-based small molecule inhibitor screen to interrogate the potential molecular mechanism of CCE (Table 1), focusing on inhibitors of general transcription/translation and inhibitors of known mechanotransduction signaling pathways. We found that the translation inhibitor cycloheximide can reduce the ventricular volume and CM size effectively in DMSO- and BLE-treated fish (Fig. 9, A, D, and E). The inhibitory effect is enhanced with increased concentrations of cycloheximide, and the ratio of ventricular chamber enlargement is reduced from 1.5- to 0.7-fold in 40 μmol/l cycloheximide-treated fish, indicating that CCE is prevented (Fig. 10A).
Fig. 9.
Protein synthesis and the phosphoinositide 3-kinase signaling pathway are essential for chamber enlargement induced by cessation of contraction. Fish [43 hpf Tg(titin:mGFP)] were pretreated with indicated small molecule inhibitors for 1 h and then cotreated with BLE and the same small molecule inhibitor for another 8 h before subjected to ventricular chamber-size measurement. A: the chamber enlargement induced by BLE treatment was attenuated significantly by cotreatment with cycloheximide and LY294002 but not SU5402. Cycloheximide or SU5402 treatment alone reduces ventricular chamber size in DMSO-treated fish. B: LY294002 effectively inhibits phospho-Akt (p-Akt), as shown by Western blot. Note the activation of phospho-Akt in BLE-treated fish. C: the chamber enlargement induced by BLE treatment was attenuated significantly by cotreatment with wortmannin. The data are plotted as the average and SD of 3 replicate experiments. D and E: CM size, which is increased significantly by BLE treatment, was recovered by cycloheximide and LY294002 treatments. The outline of CMs was revealed by β-catenin staining in cardiac ventricles. The representative OC CM is indicated by the white, dashed lines. Original scale bar, 10 μm. F and G: cycloheximide and LY294002 could not rescue sarcomere lateral fusion in BLE-treated fish hearts. The Z-discs in cardiac ventricles are revealed by α-actinin antibody staining in 2-dpf embryonic fish after treatment with the indicated small molecule for 8 h. The sarcomere lateral fusion is disrupted by BLE treatment but not rescued by cotreatment with the indicated small molecule inhibitor. Original scale bar, 10 μm. Dunnett's test was used for statistical comparison. *P < 0.01.
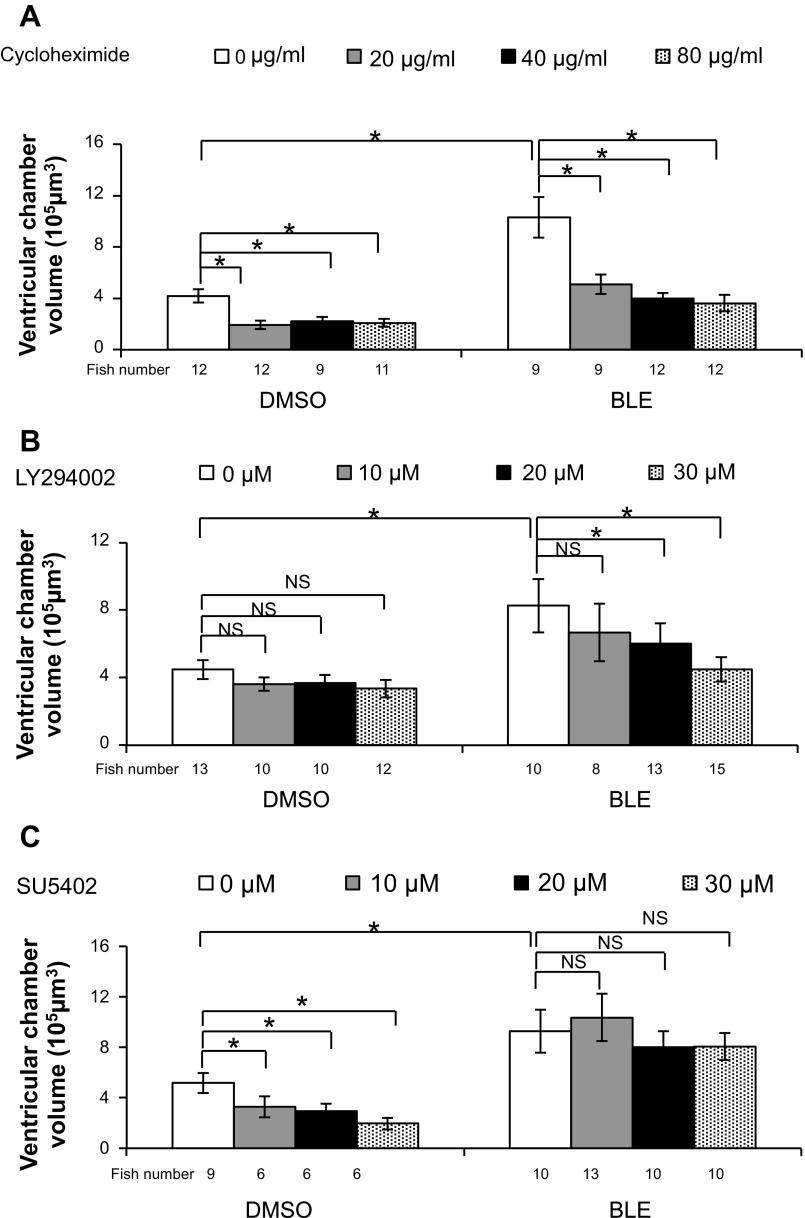
Fig. 10.
Dose-dependent effect of small molecule inhibitors on cardiac hypertrophy. Tg(titin:mGFP) fish (43 hpf) were pretreated with indicated small molecule inhibitors for 1 h and then cotreated with BLE and the same small molecule inhibitor for another 8 h before subjected to ventricular chamber-size measurement. A: the ventricle enlargement induced by BLE treatment is attenuated by 20, 40, and 80 μg/ml cycloheximide in a dose-dependent manner. The cycloheximide treatment also significantly inhibits developmental hypertrophy in DMSO-treated fish. B: the inhibitory effect of LY294002 on BLE-induced ventricular chamber enlargement is dependent on the concentration of LY294002. Quantification of chamber size shows that a high concentration of LY294002 (20 and 30 μmol/l) significantly suppresses BLE-induced chamber enlargement. C: SU5402, a FGF inhibitor, significantly reduces ventricular chamber size in DMSO-treated fish but not in BLE-treated fish. Dunnett's test was used for statistical comparison. *P < 0.01.
We then tested a panel of inhibitors for known signaling pathways involved in cell growth and mechanotransduction. We identified LY294002, a potent inhibitor of PI3K, which significantly attenuates CCE in a dose-dependent fashion (Figs. 9, A, D, and E, and 10B). Specifically, without affecting ventricular chamber size in DMSO-treated fish, LY294002 reduces the ventricular volume increase ratio from 1.5- to 0.3-fold in BLE-treated fish (Fig. 10B). The effectiveness of LY294002 has been confirmed by reduced levels of phospho-Akt, a PI3K downstream target, via Western blotting (Fig. 9B). To verify the specificity of LY294002, we tested wortmannin, another PI3K inhibitor, and found that wortmannin also significantly inhibits the CCE response (Fig. 9C). In contrast, we did not detect any suppressive effect from the mammalian target of rapamycin (mTOR) inhibitor (rapamycin), JNK inhibitor (SP600125), and MAPK inhibitor (U0126; data not shown). Together, our data suggested a unique function of the PI3K signaling pathway in mediating this particular mechanotransduction event.
In contrast to LY294002 and cycloheximide, SU5402—a FGF inhibitor—reduces ventricular chamber size in DMSO-treated fish as substantially as cycloheximide but does not interfere with CCE (Figs. 9A and 10C). With respect to the sarcomere structure, cycloheximide and LY294002 did not show any rescue effect on lateral fusion of myofibrils or Z-disc length despite substantially restoring the chamber-size (Fig. 9, F and G), supporting the conclusion that contraction regulates heart morphogenesis and sarcomere integrity via different mechanisms.
DISCUSSION
CCE response and ventricle chamber morphogenesis.
In this manuscript, we report a unique cessation of contraction-induced response in sarcomere maturation and cellular hypertrophy during cardiogenesis. Mechanistically, the enlarged CM size could be a consequence of disrupted sarcomere integrity (1), a decrease in internal cellular stress that would otherwise inhibit cell enlargement (5), and/or an increased blood pressure. By developing assays to assess these different contributors, our results suggest that the CCE response is at least partially ascribed to a nonautonomous signaling event induced by increased preload. When the heart stops beating, the continued return of venous blood to the heart would increase the preload immediately and sustainably, which sequentially causes chamber dilation. The fact that emptying the circulation of blood mitigated the effects of BLE to cause dilation supports this nonautonomous effect. We also show that the nonautonomous signaling event is independent of the endocardium. Without an endocardium in cloche fish, the influence from blood flow can still be transduced to the CM to incur morphological changes (14, 37) (Fig. 7). Therefore, we speculate that blood pressure and circumferential stretch, rather than shear stress, are the predominant mechanical forces that drive the nonautonomous branch of this mechanotransduction event (13).
Of note, we found that CTO cannot completely rescue the ventricular chamber size in BLE-treated fish. This observation could be explained partially by the incomplete removal of the blood and/or the recovery of the circulation system (22) but also suggests the autonomous contributions, including altered cell stiffness and sarcomere structure. Disruption of sarcomere integrity accompanies CCE and has been considered as an important intrinsic contributor to contraction-regulated cell-shape changes (1). However, our data suggested that sarcomere assembly and CCE might be regulated by contraction via distinct mechanisms. In line with this statement, cessation of contraction consistently impacts sarcomere assembly at 1 dpf and 2 dpf compared with the opposing effects of BLE treatment on chamber size at these two different developmental stages. Moreover, the rescuing effects of PI3K inhibitors seem restricted to CCE but not sarcomere defects. To assess directly whether contraction also regulates CM size via a cell-autonomous mechanism, we measured cell-surface area in iPSC-CMs. Although we indeed detected a mild increase of CM surface area (data not shown), the nonsynchronized iPSC-CMs make it difficult to draw any conclusion on the intrinsic effect of contraction inhibition on CM size in vitro. More synchronized differentiation of iPSC-CMs and/or a lineage-directed in vitro culturing system are needed to validate this conclusion. Overall, we favor the model that loss of contraction and consequential increased preload trigger a remodeling process that shifts the whole heart into a new mechanical homoeostasis, to which both autonomous and nonautonomous signaling events contribute.
Interestingly, we noted a stage-dependent effect of cessation of contraction on the ventricular chamber size. In contrast to decreasing ventricular chamber size in fish embryos treated at 1 dpf, cessation of contraction incurred an excessive enlargement of the ventricle in fish embryos treated at 2 dpf (Fig. 1). We speculate that nonautonomous mechanotransduction might account for the different influences of cessation of contraction on chamber size at different developmental stages. Cessation of contraction in 1-dpf embryos collapses the initiation of circulation and thus reduces the preload to the heart (10). In contrast, the preload to the heart has already reached a high level by 2 dpf. As a consequence, cessation of contraction might increase this preload further, which thereby induces the enlargement of individual CMs. Ultimately, to test these possibilities, new technologies to measure the magnitude of mechanical force in the embryonic zebrafish heart need to be developed.
Functions of contraction on sarcomere assembly.
Consistent with results from previous in vitro studies, our studies validate an essential function of contraction in regulating sarcomere assembly. Unlike the more severe phenotype manifested as completely disrupted sarcomere striations in nonbeating cells (8, 11), our results, in an in vivo vertebrate model, indicate that sarcomeres are still assembled to striated dots in the absence of contraction. Therefore, we conclude that contraction is not required for assembly of a striated sarcomere structure before the stage of nascent myofibril but is required for a later maturation step, i.e., lateral fusion of the myofibril (18, 39). In addition to ablating de novo lateral fusion of the Z-disc, cessation of contraction degrades previously assembled Z-discs at 2 dpf, underscoring the essential role of contraction for both promoting and maintaining myofibril lateral fusion within the heart. The impact of contraction on sarcomere assembly and integrity is likely a cell-autonomous event, as a similar disruptive effect of cessation of contraction on sarcomere assembly was detected in day 15 iPSC-CMs. Normal sarcomere structure was noted in both tail-docked and cloche mutant fish, suggesting that neither circulation system nor endocardium is required for sarcomere assembly in CMs.
Potential mechanisms for how contraction regulates sarcomere assembly have been proposed. A crucial midstep has been proposed to be the assembly of focal adhesions and costameres that is regulated by mechanical stretch (32). Because of its rapid and dramatic sarcomere structure change in response to cessation of contraction, our CCE model could be an accessible in vivo vertebrate model to test these and other hypotheses.
CCE can be used as an in vivo system to study mechanotransduction.
We explored the CCE response as an in vivo model to dissect mechanotransductive signaling pathways involved in regulating the growth and differentiation of CMs. Initial chemical screening prompted several interesting discoveries regarding molecular mechanisms of mechanotransductive signaling, as detailed below. First, previous studies in in vitro systems suggest that MAPK and PI3K-Akt signaling pathways are activated by stretch in CMs (19, 24). Our observation that inhibition of the PI3K-Akt but not MAPK signaling pathway attenuated CCE underscores distinct roles of PI3K and MAPK in different mechanotransduction events. Given the known function of PI3K in mechanotransduction and the function of preload on CCE, as suggested by our CTO treatment, we postulated that cessation of contraction increases preload, which sequentially induces the PI3K-Akt signaling pathway to incur an enlargement of ventricular volume. Second, despite the fact that mTOR is a downstream signaling branch of PI3K signaling and a prominent pathway to control organ size (25), results from our inhibitor screen using rapamycin indicated that mTOR does not confer the effects of PI3K signaling during CCE. Third, we found that protein synthesis is required for CCE, supporting that CCE is a mechanotransduction event in which gene translation is involved. This statement is supported by a previous study demonstrating that mechanical force can regulate protein synthesis (15). It is interesting to note that cycloheximide attenuates CCE and also reduces the chamber size in DMSO-treated fish. This observation suggests that protein synthesis is not only involved in mechanotransduction but also is required for ventricular enlargement during normal development. Fourth, SU5402, an inhibitor of FGF signaling that has been well established to govern early heart development, only reduces chamber size in DMSO-treated fish but does not interfere with CCE. Therefore, FGF signaling might represent an intrinsic program that is required for ventricular enlargement during normal development but not involved in mechanotransduction.
Some unique features of zebrafish embryos make it possible to observe the CCE response in an in vivo setting. Fish embryos can survive either cessation of contraction or a disrupted circulation system for an extended time. Because of their small body size, sufficient oxygen can diffuse into the whole body, which is quite difficult for many other in vivo models (29). Moreover, the single layer of CMs during early cardiogenesis maximizes the organ response to the dynamic mechanical forces from blood flow (17). Indeed, the BLE-induced ventricle enlargement can still be noted in embryos treated at day 3 or 4 but not in older fish (data not shown). This unique assay in zebrafish embryos provides an in vivo model to interrogate mechanisms of mechanotransduction. In the present manuscript, we demonstrated the feasibility of discerning different contributors, including the epigenetic program regulated by contraction from the intrinsic genetic program that drives physiological heart growth. Because of a spectrum of available genetic tools and the possibility to conduct large-scale chemical screens, it is anticipated that studies in fish embryos and iPSCs will continue to deepen our understanding of mechanotransduction in the heart.
GRANTS
Support for this work was provided, in part, by funds from the National Institutes of Health (NIH; HL-81753 to X. Xu and OD-007015 to T. J. Nelson), NIH Predoctoral Training Program in Molecular Pharmacology (T32GM072474 to K. A. Hartjes), and the Mayo Foundation (to X. Xu).
DISCLOSURES
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Author contributions: J.Y., K.A.H., and X.X. conception and design of research; J.Y. and K.A.H. performed experiments; J.Y., K.A.H., T.J.N., and X.X. analyzed data; J.Y., K.A.H., T.J.N., and X.X. interpreted results of experiments; J.Y. and K.A.H. prepared figures; J.Y. drafted manuscript; J.Y., K.A.H., T.J.N., and X.X. edited and revised manuscript; J.Y., K.A.H., T.J.N., and X.X. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Calum MacRae for sharing the cloche mutant fish. We also thank Dr. Xing Li for his advice on statistical analysis.
REFERENCES
- 1.Auman HJ, Coleman H, Riley HE, Olale F, Tsai HJ, Yelon D. Functional modulation of cardiac form through regionally confined cell shape changes. PLoS Biol 5: e53, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakkers J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc Res 91: 279–288, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartman T, Hove J. Mechanics and function in heart morphogenesis. Dev Dyn 233: 373–381, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Borisov AB, Kontrogianni-Konstantopoulos A, Bloch RJ, Westfall MV, Russell MW. Dynamics of obscurin localization during differentiation and remodeling of cardiac myocytes: obscurin as an integrator of myofibrillar structure. J Histochem Cytochem 52: 1117–1127, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Cai S, Pestic-Dragovich L, O'Donnell ME, Wang N, Ingber D, Elson E, De Lanerolle P. Regulation of cytoskeletal mechanics and cell growth by myosin light chain phosphorylation. Am J Physiol Cell Physiol 275: C1349–C1356, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Huang W, Dahme T, Rottbauer W, Ackerman MJ, Xu X. Depletion of zebrafish essential and regulatory myosin light chains reduces cardiac function through distinct mechanisms. Cardiovasc Res 79: 97–108, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coelho-Filho OR, Shah RV, Mitchell R, Neilan TG, Moreno H, Jr, Simonson B, Kwong RY, Rosenzweig A, Das S, Jerosch-Herold M. Quantification of cardiomyocyte hypertrophy by cardiac magnetic resonance: implications on early cardiac remodeling. Circulation 128: 1225–1233, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Deyne PG. Formation of sarcomeres in developing myotubes: role of mechanical stretch and contractile activation. Am J Physiol Cell Physiol 279: C1801–C1811, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Ding Y, Sun X, Huang W, Hoage T, Redfield M, Kushwaha S, Sivasubbu S, Lin X, Ekker S, Xu X. Haploinsufficiency of target of rapamycin attenuates cardiomyopathies in adult zebrafish. Circ Res 109: 658–669, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forouhar AS, Liebling M, Hickerson A, Nasiraei-Moghaddam A, Tsai HJ, Hove JR, Fraser SE, Dickinson ME, Gharib M. The embryonic vertebrate heart tube is a dynamic suction pump. Science 312: 751–753, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Fujita H, Nedachi T, Kanzaki M. Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes. Exp Cell Res 313: 1853–1865, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Granados-Riveron JT, Brook JD. The impact of mechanical forces in heart morphogenesis. Circ Cardiovasc Genet 5: 132–142, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 10: 53–62, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hierck BP, Van der Heiden K, Alkemade FE, Van de Pas S, Van Thienen JV, Groenendijk BC, Bax WH, Van der Laarse A, Deruiter MC, Horrevoets AJ, Poelmann RE. Primary cilia sensitize endothelial cells for fluid shear stress. Dev Dyn 237: 725–735, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Hornberger TA, Esser KA. Mechanotransduction and the regulation of protein synthesis in skeletal muscle. Proc Nutr Soc 63: 331–335, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421: 172–177, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Hu N, Sedmera D, Yost HJ, Clark EB. Structure and function of the developing zebrafish heart. Anat Rec 260: 148–157, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Huang W, Zhang R, Xu X. Myofibrillogenesis in the developing zebrafish heart: a functional study of tnnt2. Dev Biol 331: 237–249, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, Shyy JY, Chien S. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci USA 98: 1042–1046, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jani K, Schock F. Molecular mechanisms of mechanosensing in muscle development. Dev Dyn 238: 1526–1534, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Jou CJ, Spitzer KW, Tristani-Firouzi M. Blebbistatin effectively uncouples the excitation-contraction process in zebrafish embryonic heart. Cell Physiol Biochem 25: 419–424, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopp R, Pelster B, Schwerte T. How does blood cell concentration modulate cardiovascular parameters in developing zebrafish (Danio rerio)? Comp Biochem Physiol A Mol Integr Physiol 146: 400–407, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of Blebbistatin inhibition of myosin II. J Biol Chem 279: 35557–35563, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Lal H, Verma S, Golden H, Foster D, Holt A, Dostal D. Molecular signaling mechanisms of myocardial stretch: implications for heart disease. In: Mechanosensitivity in Cells and Tissues: Mechanosensitivity of the Heart, edited by Kamkin A, Kiseleva I. Berlin, Germany: Springer, 2010, p. 55–81. [Google Scholar]
- 25.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 149: 274–293, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin YF, Swinburne I, Yelon D. Multiple influences of blood flow on cardiomyocyte hypertrophy in the embryonic zebrafish heart. Dev Biol 362: 242–253, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malone MH, Sciaky N, Stalheim L, Hahn KM, Linney E, Johnson GL. Laser-scanning velocimetry: a confocal microscopy method for quantitative measurement of cardiovascular performance in zebrafish embryos and larvae. BMC Biotechnol 7: 40, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peal DS, Peterson RT, Milan D. Small molecule screening in zebrafish. J Cardiovasc Transl Res 3: 454–460, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Rombough P. Gills are needed for ionoregulation before they are needed for O(2) uptake in developing zebrafish, Danio rerio. J Exp Biol 205: 1787–1794, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Santhanakrishnan A, Miller LA. Fluid dynamics of heart development. Cell Biochem Biophys 61: 1–22, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Sehnert AJ, Stainier DY. A window to the heart: can zebrafish mutants help us understand heart disease in humans? Trends Genet 18: 491–494, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Sharp WW, Simpson DG, Borg TK, Samarel AM, Terracio L. Mechanical forces regulate focal adhesion and costamere assembly in cardiac myocytes. Am J Physiol Heart Circ Physiol 273: H546–H556, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Stainier DY, Weinstein BM, Detrich HW, >III, Zon LI, Fishman MC. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development 121: 3141–3150, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Sun X, Hoage T, Bai P, Ding Y, Chen Z, Zhang R, Huang W, Jahangir A, Paw B, Li YG, Xu X. Cardiac hypertrophy involves both myocyte hypertrophy and hyperplasia in anemic zebrafish. PLoS One 4: e6596, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teramura T, Takehara T, Onodera Y, Nakagawa K, Hamanishi C, Fukuda K. Mechanical stimulation of cyclic tensile strain induces reduction of pluripotent related gene expressions via activation of Rho/ROCK and subsequent decreasing of AKT phosphorylation in human induced pluripotent stem cells. Biochem Biophys Res Commun 417: 836–841, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Tu S, Chi NC. Zebrafish models in cardiac development and congenital heart birth defects. Differentiation 84: 4–16, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol 10: 34–43, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J, Xu X. alpha-Actinin2 is required for the lateral alignment of Z discs and ventricular chamber enlargement during zebrafish cardiogenesis. FASEB J 26: 4230–4242, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J, Xu X. Immunostaining of dissected zebrafish embryonic heart. J Vis Exp e3510, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zon LI, Peterson RT. In vivo drug discovery in the zebrafish. Nat Rev Drug Discov 4: 35–44, 2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.