Abstract
Alcohol-induced congenital heart defects are frequently among the most life threatening and require surgical correction in newborns. The etiology of these defects, collectively known as fetal alcohol syndrome, has been the focus of much study, particularly involving cellular and molecular mechanisms. Few studies have addressed the influential role of altered cardiac function in early embryogenesis because of a lack of tools with the capability to assay tiny beating hearts. To overcome this gap in our understanding, we used optical coherence tomography (OCT), a nondestructive imaging modality capable of micrometer-scale resolution imaging, to rapidly and accurately map cardiovascular structure and hemodynamics in real time under physiological conditions. In this study, we exposed avian embryos to a single dose of alcohol/ethanol at gastrulation when the embryo is sensitive to the induction of birth defects. Late-stage hearts were analyzed using standard histological analysis with a focus on the atrio-ventricular valves. Early cardiac function was assayed using Doppler OCT, and structural analysis of the cardiac cushions was performed using OCT imaging. Our results indicated that ethanol-exposed embryos developed late-stage valvuloseptal defects. At early stages, they exhibited increased regurgitant flow and developed smaller atrio-ventricular cardiac cushions, compared with controls (uninjected and saline-injected embryos). The embryos also exhibited abnormal flexion/torsion of the body. Our evidence suggests that ethanol-induced alterations in early cardiac function have the potential to contribute to late-stage valve and septal defects, thus demonstrating that functional parameters may serve as early and sensitive gauges of cardiac normalcy and abnormalities.
Keywords: cardiac function, ethanol, congenital heart defects
despite the fact that alcohol was identified as a teratogen many decades ago, recent data indicate that, in the United States, over 500,000 women per year report drinking during pregnancy, with one in five of this population admitting to binge drinking (20). Even low levels of prenatal alcohol (ethanol) exposure, such as in a single dose, can produce birth defects termed fetal alcohol syndrome (FAS). In addition to the originally identified growth retardation and craniofacial and neurological abnormalities (11, 38), as high as 54% of live-born children with FAS present with cardiac anomalies (18), e.g., valvuloseptal defects and pulmonary stenosis (24), that can lead to developmental challenges, ongoing medical care, and death. The mechanisms of alcohol-induced congenital heart defects (CHDs) remain largely unclear even though its etiology has been the focus of much study, especially the cellular and molecular mechanisms (4, 19, 49, 56). One aspect that has not been thoroughly investigated due to technological limitations is the role of abnormal cardiac function in the progression of FAS-related CHDs.
Altered cardiac function has been suggested to have an important role in cardiogenesis. When cardiac function (flow, excitation, contraction, calcium transients) is disrupted, this alone can lead to further cellular/molecular, functional, and structural anomalies. For example, abnormal calcium handling has been linked to CHDs (63), whereas alterations in myocardial contraction have been associated with cardiac structural defects in avian embryo models (13, 22). Several studies have suggested that biomechanical forces exerted by blood flow contribute to the normal and abnormal development of the heart (23, 27, 30–31, 39, 44, 46, 53, 66) through regulation of mechanosensitive gene and protein expression (2, 26–27, 43, 59). In one such study, vitelline vein ligation in avian embryos at looping heart stages altered blood flow in the outflow tract and induced ventricular septal defects, semilunar valve anomalies, and pharyngeal arch artery malformations at later stages (30). Recently, retrograde blood flow in the looping heart has been linked to abnormal valve development (62). Defects in cardiac conduction are proposed to cause CHDs by altering patterns of myocardial contraction, in turn affecting cardiac hemodynamics (9–10). Thus there exists a large body of evidence that highlights the importance of cardiovascular function in normal and abnormal heart development.
The few studies that have measured cardiac function in FAS animal models support the hypothesis that CHDs arising from ethanol exposure could have a significant contribution from abnormal function. Abnormal embryonic cardiac function, as well as abnormal valves, was identified through ultrasound and histology, respectively, at late stages (embryonic day 15.5) of mouse development after ethanol exposure at gastrulation (56). Functional and structural defects, including anomalies in heart volume/morphology and conduction, were observed in late-stage zebrafish after ethanol exposure, thus mimicking malformations occurring in patients with FAS (17). Yelin et al. (68) utilized optical imaging and found structural and contraction anomalies in the septated heart in late-stage Xenopus embryos exposed to alcohol (68). For early-stage functional analyses, previous groups used pioneering cinephotography studies to image Hamburger-Hamilton (HH) stage 19 chick embryos after ethanol exposure and found that cardiac output and contractility were reduced (5a, 55). These early imaging techniques, which involved volume estimations, did not have the resolution and in vivo structural information that is necessary to make consistently precise measurements. The aim of the present study was to use novel optical coherence tomography (OCT) technology to quantitatively and sensitively identify functional changes in early ethanol-exposed embryos and thereby identify early responses that may deflect the heart toward a trajectory leading to CHDs.
At the early looping stages that we are investigating, the heart is small (0.5–1.0 mm in length and about 0.3 mm in diameter), rapidly contracting (2.0–3.5 Hz for avian embryos), fragile, and undergoing dynamic three-dimensionally complex morphological changes. The heart beat is also very sensitive to environmental changes, such as oxygen concentration and temperature (28, 51, 62). These characteristics of the developing heart demand an imaging modality, such as OCT with high spatial resolution, fast imaging rate, easy setup, minimal invasiveness, and environmental control, to capture hemodynamic changes. In this study, we developed a quail embryo model of FAS and employed OCT to measure hemodynamics in the early-looping, ethanol-exposed avian heart in an environmental chamber under near physiological conditions to address to what extent cardiac function is altered by ethanol exposure at an early stage. This protocol could potentially be applied to a host of other disease models to assess whether changes in certain early functional parameters can be linked to late-stage abnormalities.
MATERIALS AND METHODS
Avian model.
Avian models have a clear advantage for the study of detailed morphology and function in vivo under physiological conditions at the early tubular and looping heart stages that we are targeting. The avian model also shares many molecular, structural, and functional similarities with human development (58). The quail embryo was utilized in this project because it is readily accessible to our biophotonic tools, easy to manipulate, and economical.
Ethics statement.
IACUC approval was not required for this study, which involves the use of avian embryos that were collected at embryonic day 8 at the latest. Avian embryos typically hatch at embryonic day 21. According to IACUC guidelines at Case Western Reserve University, the policy on the use of Avian Embryos states that, “If embryos will be sacrificed prior to 3 days before hatching, the research will not be subject to IACUC review.”
Ethanol exposure.
Fertilized quail eggs (Coturnix coturnix communis; Boyd's Bird, Pullman, WA) were incubated in a humidified incubator (G.Q.F. Manufacturing, Savannah, GA) at 38°C until HH stage 4–5 (gastrulation). At this stage, the embryo has been found to be vulnerable to induction of CHDs (56). Solutions were injected into the air space at the top of the egg using an insulin needle (28 gauge), and holes were sealed with tape before eggs were reincubated. Experimental eggs were injected a single time (acute exposure) with 40 μl of 50% ethanol, and control eggs were either injected with 40 μl of saline or left intact. Ethanol dosage was based on previously published protocols (56) as being equivalent to one binge drink episode in humans (4–5 standard drinks on one occasion) and reliably produced FAS-associated CHDs.
CHD analysis in late-stage embryos.
Cohorts of control and ethanol-exposed embryos were allowed to develop until HH stage 34–35, when the heart has acquired a mature, four-chambered morphology and developed valve leaflets. Hearts from the different exposure groups were dissected, fixed in formalin, and prepared for paraffin embedment. The paraffin sections were stained with hematoxylin and eosin (H&E) for histological analysis focusing on the valvular leaflets.
OCT imaging of early function.
For early-stage analyses, eggs were injected with saline or ethanol or left intact, after which they were reincubated until HH stage 12–13 (heart-looping stages). Embryos were placed in a shell-less culture in a sterilized 35-mm Petri dish (48) and photographed on the yolk using standard light stereomicroscopy. The embryos were then placed within a customized environmentally controlled OCT imaging chamber (35) to ensure physiological conditions of temperature and humidity and incubated until HH stage 19. This developmental stage corresponds to a time point when there is active remodeling of the primitive cardiac jelly, emergence of endocardial cushions, and appearance of mesenchymal cells within endocardial cushions. Previously, this early stage was inaccessible for detailed study in vivo. Heart rate variability was significantly higher at stages earlier than HH stage 19, thus preventing meaningful measurements of cardiac function at those time points using our present methods. Embryos were subsequently imaged using a custom-built, swept-source OCT system, employing a buffered Fourier Domain Mode-Locked laser (33–34, 36), which operates at a 117-kHz line rate. The axial resolution is 8 μm in tissue, and the lateral resolution is ∼10 μm (35). Thus OCT imaging offers high spatial resolution and extremely high temporal resolution while providing good penetration depth (1–3 mm in cardiac tissues) and Doppler flow sensing (32, 50). These characteristics make it well suited to assess morphology and hemodynamics in early looping-stage hearts. For our early-stage (HH stage 19) functional assays, pulsed Doppler traces were obtained from the left vitelline artery, where flow has been shown to reflect cardiac function in our previous study (28). Retrograde flow was estimated by integrating the Doppler signal over time, and the fraction of retrograde flow was normalized by dividing the negative flow by the positive flow.
OCT imaging of early structure.
At HH stage 19, embryos from control and ethanol-exposed groups were selected, and their hearts were dissected and fixed in 10% formalin. They were then imaged in 3-D using OCT for structural analysis. The image-processing program Amira (FEI Visualization Sciences Group, Burlington, MA) was used to segment the cardiac cushions and measure their volume.
Statistics.
The arterial Doppler waveforms for control embryos exhibited a notable shoulder, which was absent or severely reduced in the ethanol-exposed embryos. The percentage of embryos that displayed this phenotype was analyzed with Fisher's exact test (free online program, http://graphpad.com/quickcalcs/contingency1.cfm). Compared with the saline-treated embryos, ethanol-exposed embryos showed a significant increase in the absent/reduced shoulder phenotype (*P < 0.01). For retrograde flow percentages, the results from multiple embryos were averaged and plotted as a box-and-whisker plot (MatLab; MathWorks, Natick, MA). The comparison was made with ANOVA, and, after a significant difference was found, the Tukey-Kramer method (Tukey's HSD) was used for post hoc multiple comparisons (MatLab), with the result being graphically represented by the homogeneous subsets (*P < 0.05). For cushion volumes, multiple comparisons were performed using one-way ANOVA and Tukey's HSD post hoc test (MatLab) (*P < 0.05).
RESULTS
Ethanol-exposed embryos developed hearts with FAS-associated CHDs.
Ethanol was introduced at HH stage 4–5 (gastrulation stages) in the avian embryo. At this developmental time point, embryos have been reported to be susceptible to the induction of birth defects (7, 56). It remains unclear as to whether the ethanol has an immediate effect on developmental processes or whether it has a prolonged role in the induction of birth defects. Experimental animals in our study were injected with 40 μl of 50% ethanol. This dosage was modeled after a single binge exposure for humans (56) and created cardiac defects in our system while still allowing for survival. Control animals were injected with the same volume of saline or left uninjected. After injection, embryos were reincubated until HH stage 34–35, at which point hearts were dissected and processed for paraffin sectioning with H&E staining. This late developmental time point corresponds to a stage when the normal embryo has developed a fully septated heart with four chambers, as well as differentiating valve leaflets. All embryos were confirmed to be of the appropriate stage before histological analysis. Compared with control (uninjected and saline-treated) specimens, ethanol-exposed embryos developed smaller atrio-ventricular (AV) septal valves (Fig. 1, A and B), which have not been reported frequently in the literature. They also exhibited cardiac defects commonly associated with FAS, similar to those seen in animal models in previous studies (8, 56). These defects included thinner interventricular septae, malformed aortic valves, and ventricular wall thinning (Fig. 1, C–F). One of the ethanol-exposed embryos in this cohort displayed reduced eye growth. Other ethanol-exposed embryos that were not part of this cohort and thus not analyzed for heart defects also exhibited cranial folds that failed to fuse. The AV anomalies were of particular interest to us because of the frequency of the defect in the experimental animals as well as the paucity of information available concerning such defects in ethanol-exposure models. After establishing a quail embryo model of FAS, we then sought to determine whether abnormal cardiac function at earlier stages was detectable using the precision of OCT technology.
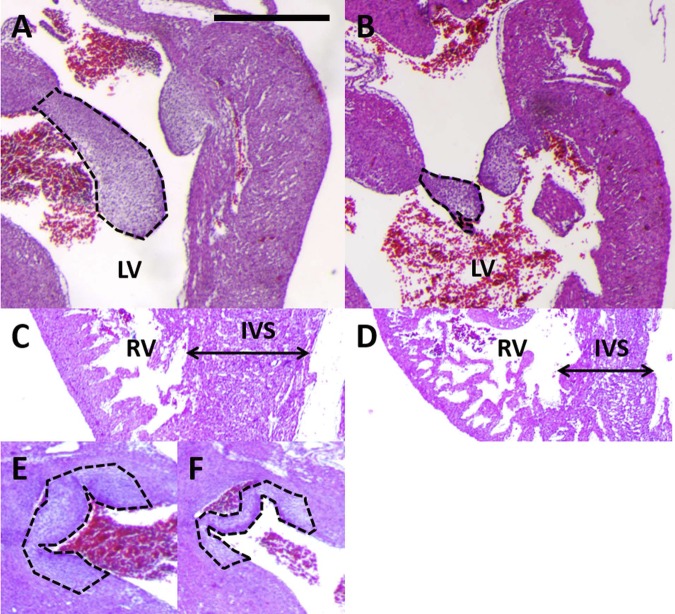
Fig. 1.
Late-stage congenital heart defects after ethanol exposure included smaller atrio-ventricular (AV) valves. A: control heart has large septal leaflet for left AV valve (outlined in black). Similar large AV leaflets were observed for all samples in this group (n = 4). LV, left ventricle. B: ethanol-exposed embryo developed a smaller AV septal leaflet (outlined in black). Small or malformed AV leaflets were observed for 5 of the embryos in this group (n = 7). C: control heart had a thick right ventricular (RV) wall and a thick interventricular septum (IVS). D: ethanol-exposed embryo had a thin ventricular wall and a thin interventricular septum. These defects were each seen in 3 embryos of the ethanol group (n = 7). E: control heart had thick, well-developed aortic valve leaflets (dashed black line). F: ethanol-exposed embryo had thinner, underdeveloped aortic valve leaflets (dashed black line). 3 embryos in the group exhibited this defect (n = 7). Scale bar = 100 μm.
Ethanol-exposed embryos developed structural abnormalities of the body axis at early stages.
As described previously, embryos were injected at HH stage 4–5 with ethanol/saline or left intact and then reincubated until HH stage 12–13. Intact embryos and egg contents were transferred into Petri dishes, placed in a tissue culture incubator, and incubated until HH stage 19, when they were imaged under the light stereomicroscope. At this stage, control embryos were morphologically normal compared with the Hamburger and Hamilton reference guide on avian development (29). Normal embryos have completed cervical flexure at this time point, and the trunk is relatively straight (Fig. 2A). Ethanol-exposed embryos fell into three categories of structural morphology (Table 1): normal, twisted, and super-twisted. Twisted embryos accounted for 49% of embryos that were alive at HH stage 19 after ethanol exposure (Table 1) and were observed to develop an S-shaped body, thus demonstrating abnormalities in overall embryo flexion/torsion (Fig. 2B). In addition, ∼13% of ethanol-exposed embryos were so severely twisted (super-twisted) (Fig. 2C, Supplemental Movie S1; supplemental material for this article is available online at the American Journal of Physiology Heart and Circulatory Physiology website) that the head was folded underneath the embryo and not visible on the surface. Overall, ethanol-exposed embryos did not exhibit a significant change in heart rate compared with controls at this time point; however, unlike controls, some developed abnormalities within the yolk sac vascular network (Table 1). Interestingly, the twisted body axis of the ethanol-exposed embryos was very similar to structural findings reported for the had zebrafish mutant (57). In these zebrafish embryos, Na,K-ATPase activity (which maintains membrane electrochemical gradients) was blocked, resulting in cardiac functional abnormalities as early as 24 h postfertilization and twisting of the body axis at 48 h postfertilization (57). Because ethanol exposure in our study was producing defects similar to those linked to an alteration of cardiovascular function, we hypothesized that ethanol exposure at gastrulation stages was impacting early cardiac function.
Fig. 2.
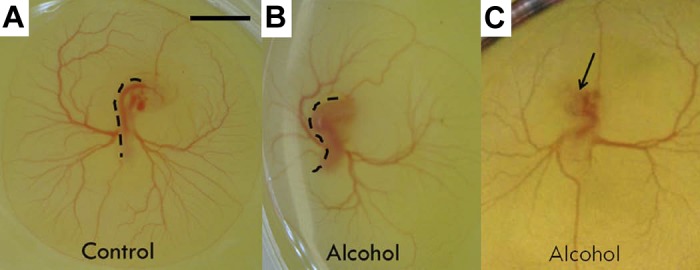
Hamburger-Hamilton (HH) stage 19 quail embryos on the yolk incubated in shell-less culture from different treatment groups. A: untreated embryo with relatively straight trunk and normal cranial and cervical flexure. B: alcohol/ethanol-treated embryo with S-shaped trunk (twisted). Dashed black lines delineate the abnormal shape of the embryo backbone. C: alcohol/ethanol-treated embryo with super-twisted body and head submerged underneath the body within the egg yolk. Black arrow denotes where head would normally have been located. Scale bar = 2 mm.
Table 1.
Embryo morphology of untreated, saline-injected, and ethanol-injected quail embryos at HH stage 19 (72 h)
| Phenotype | Untreated | Saline | Ethanol |
|---|---|---|---|
| Heart rate, bpm | 201 ± 26 (7) | 203 ± 28 (8) | 188 ± 36 (8) |
| Normal body | 100% (26) | 100% (31) | 38% (53) |
| Twisted | 0% (26) | 0% (31) | 49% (53) |
| Super-twisted | 0% (26) | 0% (31) | 13% (53) |
| Abnormal yolk sac vasculature | 0% (26) | 0% (31) | 15% (53) |
Applicable values are means ± SE. Numbers in parentheses represent total number of specimens from which percentages were calculated. HH, Hamburger-Hamilton. bpm, beats per minute.
Ethanol exposure created abnormal flow at early stages of cardiac cushion emergence.
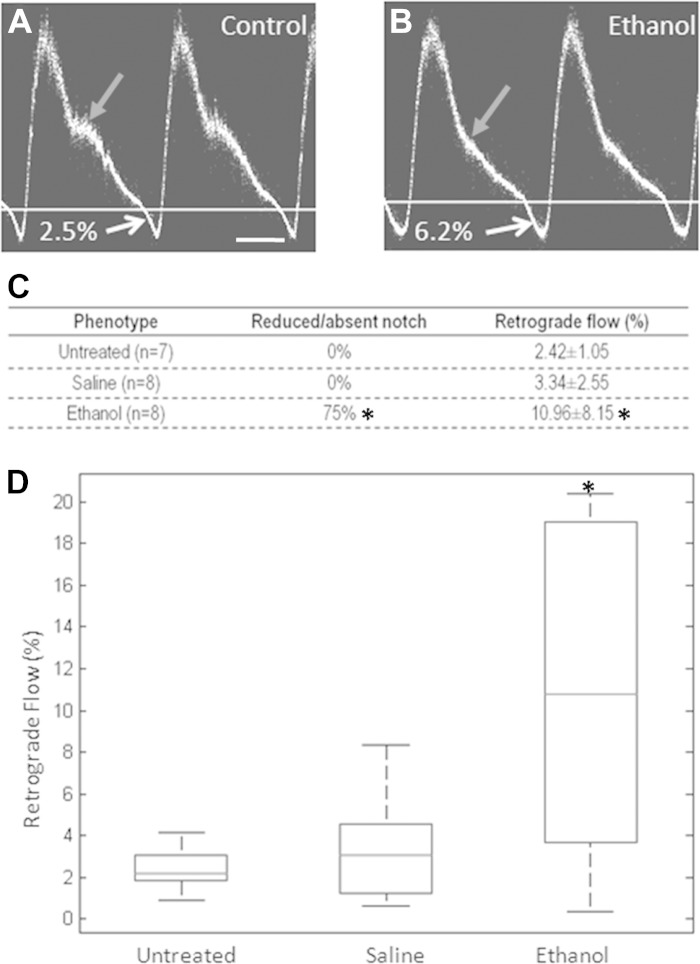
HH stage 19 corresponds to a time in embryonic development where the cardiac cushions are actively forming, in large part because of the epithelial-to-mesenchymal transition (EMT) of endocardial cells and the proliferation and migration of the resultant mesenchymal cells throughout the endocardial cushions (12). We also observed significant heart rate variability even in control embryos at stages earlier than HH stage 19, which prevented us from accurately measuring cardiac function at those time points with our present tools. At HH stage 19, embryos from different treatment groups were imaged ex ovo within a humidified, temperature-controlled OCT imaging chamber (35). For our studies, pulsed Doppler waveforms were obtained from the left vitelline arteries, which were previously shown by our group to accurately and repeatably reflect cardiac function (28). Doppler waveforms for uninjected embryos consisted of a positive peak with a shoulder and a small but noticeable negative peak (e.g., Fig. 3A). Our measurements suggested that uninjected embryos experienced a consistent but low level of retrograde flow (2.42 ± 1.05%), calculated as the ratio of the area of the negative peak to the area of the positive peak (Fig. 3C). Saline-injected embryos displayed similar Doppler waveform morphology to uninjected embryos (Fig. 3A) and had similar levels of retrograde flow (3.34 ± 2.55%) (Fig. 3C) compared with uninjected embryos. Ethanol-exposed embryos, however, had reduced or absent shoulders (e.g., Fig. 3B) and had significantly higher levels of retrograde flow (10.96 ± 8.15%, P = 0.0006) (Fig. 3, C and D) compared with both uninjected and saline-treated embryos. Thus, at these very early developmental stages, ethanol exposure led to abnormal retrograde flow. The absence of shoulders seen in the pulsed Doppler traces for the ethanol-exposed embryos may also be indicative of abnormally formed AV cushions. In previous studies, changes in retrograde flow were shown to affect cardiac cushion and valve formation in a zebrafish model (62). This led us to examine the AV cushions in detail using a structural assay.
Fig. 3.
Abnormal flow was observed in ethanol-exposed embryos at HH stage 19. A: pulsed Doppler waveform for controls (untreated, saline-treated) consisted of a positive peak with a prominent shoulder (gray arrow) and a negative peak. For this specimen, the percentage of retrograde flow was 2.5%, as calculated from the percentage ratio of the area of the negative peak to the area of the positive peak. Scale bar = 100 ms. B: pulsed Doppler waveform for ethanol-exposed embryos often had no shoulders (gray arrow). Retrograde flow for this embryo was 6.2%. C: incidence of abnormal shoulders and retrograde flow percentages for the different treatment groups. Ethanol-exposed embryos had reduced or absent shoulders in the Doppler waveforms and exhibited significantly higher levels of retrograde flow (P = 0.0006) compared with controls. D: box plot of retrograde flow percentages for each of the 3 treatment groups: untreated, saline-treated, and ethanol-treated groups. Box encompasses the 25th to 75th percentile, line within the box represents the median, and dashed lines represent the range. *P < 0.05.
At early stages, AV cushions in the ethanol-exposed embryo were smaller in volume.
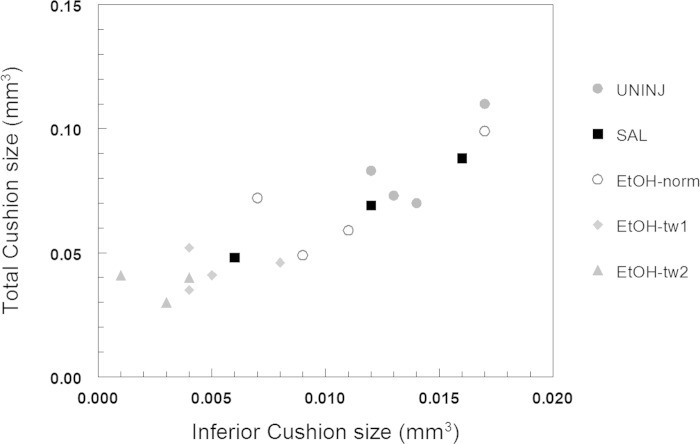
At HH stage 19 in the early embryonic heart, the superior and inferior AV cushions have emerged as swellings in the AV portion of the heart, with the increased deposition of “cardiac jelly” in localized regions (12). In addition, endocardial cushion cells will undergo EMT and migrate into and populate the cushion as proliferating and migrating mesenchymal cells. At this developmental time point, the AV cushions already serve as physical barriers to efficiently minimize regurgitant flow in the early heart tube. In our studies, OCT was used to image the embryonic heart, which, at this stage, is still relatively transparent due to the thin myocardium. 3-D OCT images were obtained of the cardiac cushions, specifically the inferior AV cushion and the contiguous superior AV/conotruncal cushion, and segmented (Amira image processing software, FEI Visualization Sciences Group; also used to create 3-D reconstructions) (Fig. 4, A and B). Cushion volumes were calculated using the measurement tools in Amira. Ethanol-exposed embryos developed smaller cushions compared with saline-treated and untreated controls (Fig. 4, C and D). At these early stages, therefore, reduced cushion sizes were noted at a time when there were also hemodynamic anomalies in the ethanol-exposed embryos. In addition, individual embryos were graphed in a scatter plot of inferior AV cushion volume vs. total cushion volume (Fig. 5). In the plot, ethanol-exposed embryos that were twisted, either moderately (EtOH-tw1) or severely (EtOH-tw2), were grouped together at the lower end of the spectrum of cushion volumes. On the other hand, ethanol-exposed embryos that were not twisted (EtOH-norm) had cushion sizes that were larger and thus comparable to those of the controls. This finding may point to a possible association between ethanol-induced twisting defects of the body axis and cushion dysmorphogenesis because the most severely twisted embryos developed the smallest cardiac cushions.
Fig. 4.

Ethanol-exposed embryos developed smaller cardiac cushion volumes compared with controls. A: 3-D reconstruction of cardiac cushions of control heart from optical coherence tomography images using Amira software. Purple cushion = inferior AV cushion, green cushion = fusion of superior AV cushion and conotruncal cushion. A, atrium; V, ventricle. B: 3-D reconstruction of cardiac cushions of ethanol-exposed heart. C: quantitation of endocardial cushion volumes among the 3 treatment groups: untreated, saline-treated, and ethanol-treated groups. *P < 0.05, showing statistical difference compared to saline-treated and untreated controls.
Fig. 5.
Scatter plot of inferior AV cushion volume vs. total cushion volume for ethanol-exposed, saline-treated, and uninjected embryos. The most severely twisted embryos in the ethanol-exposed group developed the smallest cardiac cushions. ●, uninjected (UNINJ), ■, saline (SAL), ○, ethanol-exposed embryos with normal trunk development (EtOH-norm), ⧫, ethanol-exposed embryos with moderately twisted trunks (EtOH-tw1), ▲, ethanol-exposed embryos with severely twisted trunks (EtOH-tw2).
DISCUSSION
Many previous studies have focused on the neurodevelopmental symptoms of FAS, but comparatively few have investigated cardiac birth defects associated with ethanol exposure. Analysis of the co-occurrence of Fetal Alcohol Spectrum of Defects (FASD; an umbrella term including the FAS diagnosis) and CHDs suggested that the prevalence rate for comorbid CHDs and FASD was 28.6% (6). Thus, with an average of four million US pregnancies per year, there will be ∼10,000 cases of alcohol-induced CHDs, far in excess of the few hundred cases expected by clinicians (6). Continued study of the mechanisms involved in the development of alcohol-induced cardiac birth defects is warranted to implement effective treatments and/or prevention strategies.
We aimed to determine whether ethanol-induced cardiac defects in a quail embryo model could be correlated with abnormalities in early cardiac function. Disturbed cardiac function has been associated with embryonic cardiac defects in other animal models, such as mouse, zebrafish, and Xenopus (17, 56, 68). It is likely that defects in cardiac function will produce global effects that can retard brain growth/differentiation, extraembryonic vascular growth, as well as vascular development in various organ systems. However, early cardiac function has not been assayed in a consistent, quantitative manner primarily because of limitations in technology. The capability of OCT imaging to make precise, real-time hemodynamic measurements enabled us to overcome this obstacle.
To undertake our studies, we established a quail model of FAS that mimicked a binge-drinking exposure at the stage of gastrulation, at which point a woman may not yet be aware of her pregnancy. The severity of the cardiac defects associated with acute ethanol exposure has previously been shown to vary depending on dosage and timing of the exposure (3). In studies that have introduced ethanol at stages after gastrulation in the avian embryo (8), researchers observed cardiac defects such as chamber dilatation and ventricular wall thinning. When alcohol was introduced at stages after gastrulation in the mouse model (14), embryos developed ventricular septal defects, double outlet right ventricle, and great vessel anomalies. Our late-stage defects are consistent with cardiac defects observed in the mouse model after ethanol exposure at gastrulation (56), including malformed aortic valves and heart wall thinning. We also observed smaller AV valves in the ethanol-exposed embryos, which have not been described in detail in the literature. The quail embryo model is also clinically relevant for the study of cardiac defects because the avian heart has proven to be similar to the human heart in morphology and physiology (58). In addition, we were able to make physiological cardiovascular measurements in vivo, using shell-less avian culture and an OCT system in an environmentally controlled chamber. This resolution of morphology and function under physiological conditions is not possible to achieve in in utero mouse embryo models using present technologies.
We also demonstrated that ethanol-exposed embryos exhibited structural defects in the bending and flexure of the trunk at early developmental stages, which has not been reported in earlier studies. This twisting of the body axis appeared to be quite similar to defects produced in zebrafish embryos with impaired cardiac function (57), suggesting that abnormal cardiac function could be linked to the progression of ethanol-induced birth defects. At this point, it remains unclear as to whether the heart is the primary site of action for the ethanol or whether the cardiac anomalies are secondary effects induced by another target (21, 47). For example, it may be that, after ethanol exposure, the body axis begins to twist, thereby exerting mechanical forces on the heart and thus influencing blood flow. An alternative scenario is that the heart loops abnormally due to ethanol exposure and induces the twisting of the body axis.
Ethanol-exposed embryos in the present study also exhibited increased retrograde flow and smaller AV cardiac cushions compared with controls. Recently, investigations using zebrafish showed that altering retrograde flow in the AV canal of the developing heart before and during valvulogenesis led to arrested valve development (62). In these studies, normal valve formation appeared to be mediated by the transcription factor klf-2a, whose gene expression was sensitive to intracardiac hemodynamic forces (62). The similarities between the two animal models suggest that ethanol exposure, which altered early cardiac function, may also be acting through klf-2a to produce cushion and valvular defects. Klf-2 has been used as a shear stress marker in several studies, where correlations were found between klf-2 expression levels and normal, low, disturbed, or oscillatory flow patterns (15, 25, 27, 42, 65). Future examination of klf2a expression and signaling, involving demonstrated downstream targets such as ERK5 (40), in cushion/prevalvular regions of the ethanol-exposed heart would shed more light on this molecular factor of ethanol-induced CHDs. Complementary investigations could involve the use of OCT imaging to make detailed shear stress measurements, as we have demonstrated previously (37, 52), at prevalvular sites after ethanol exposure.
Previously, researchers have focused on studying a variety of molecular mechanisms to determine potential candidates that can explain the development of FAS-associated birth defects. For example, the progression of alcohol-induced developmental defects, including cardiovascular anomalies, has been shown to be mediated by the retinoic acid signaling pathway (16, 41, 49, 61). Sonic hedgehog signaling has been found to be disrupted in the embryo after ethanol exposure (5, 45). In other studies, alcohol potentiated Wnt/β-catenin signaling, thus preventing normal cardiogenesis in FAS animal models (19, 56). Numerous groups have also investigated the role and/or deleterious effects of neural crest cell death in producing ethanol-associated cardiac defects (7–8, 54, 64), especially because neural crest ablation is known to alter the length of the outflow tract as well as cardiac looping, which in turn could influence trunk flexure (67). Therefore, alcohol may have a global effect on a multitude of signaling mechanisms, all of which are important pieces of the puzzle and may significantly impact embryonic heart development. If we approach the problem from a different perspective, by precisely assaying cardiovascular function at early stages and identifying certain checkpoints in defect development, we may be able to alter function directly and thus normalize molecular and cellular responses to rescue the defective FAS phenotype. Clinical trials have already shown that ethanol exposure can have direct vascular effects, including basal vasoconstriction and potentiation of vasodilation (60). One possible rescue strategy could involve the use of pharmaceutical drugs to directly modulate cardiovascular function to reduce if not block the progression of ethanol-induced CHDs. This could be a first step in implementing new therapeutic strategies based on the early and accurate diagnosis of cardiac birth defects.
GRANTS
This work was supported by the National Institutes of Health Grants R01HL083048 and R01HL095717.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: G.K., K.K.L., A.M.R., and M.W. conception and design of research; G.K., S.G., Y.Q.D., L.P., K.M., and Q.M. performed experiments; G.K., S.G., Y.Q.D., L.P., K.M., Q.M., M.W.J., A.M.R., and M.W. analyzed data; G.K., S.G., Y.Q.D., L.P., K.M., M.W.J., A.M.R., and M.W. interpreted results of experiments; G.K. and S.G. prepared figures; G.K. drafted manuscript; G.K., S.G., Y.Q.D., L.P., M.W.J., K.K.L., A.M.R., and M.W. edited and revised manuscript; G.K., S.G., Y.Q.D., L.P., K.M., Q.M., M.W.J., K.K.L., A.M.R., and M.W. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1.Abel EL. Fetal Alcohol Syndrome. Oradell, NJ: Medical Economics, 1990. [Google Scholar]
- 2.Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res 100: 1686–1695, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Bilotta J, Barnett JA, Hancock L, Saszik S. Ethanol exposure alters zebrafish development: a novel model of fetal alcohol syndrome. Neurotoxicol Teratol 26: 737–743, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Binkhorst M, Wortmann SB, Funke S, Kozicz T, Wevers RA, Morava E. Glycosylation defects underlying fetal alcohol spectrum disorder: a novel pathogenetic model. “When the wine goes in, strange things come out” - S. T. Coleridge, The Piccolomini. J Inherit Metab Dis 35: 399–405, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan D, Giles S. Sonic hedgehog expression is disrupted following in ovo ethanol exposure during early chick eye development. Reprod Toxicol 41: 49–56, 2013. [DOI] [PubMed] [Google Scholar]
- 5a.Bruyere HJ, Jr, Stith CE. Ethyl alcohol reduces cardiac output, stroke volume, and end diastolic volume in the embryonic chick. Teratology 49: 104–112, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Burd L, Deal E, Rios R, Adickes E, Wynne J, Klug MG. Congenital heart defects and fetal alcohol spectrum disorders. Congenit Heart Dis 2: 250–255, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Cartwright MM, Smith SM. Increased cell death and reduced neural crest cell numbers in ethanol-exposed embryos: partial basis for the fetal alcohol syndrome phenotype. Alcohol Clin Exp Res 19: 378–386, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Cavieres MF, Smith SM. Genetic and developmental modulation of cardiac deficits in prenatal alcohol exposure. Alcohol Clin Exp Res 24: 102–109, 2000. [PubMed] [Google Scholar]
- 9.Chi NC, Bussen M, Brand-Arzamendi K, Ding C, Olgin JE, Shaw RM, Martin GR, Stainier DY. Cardiac conduction is required to preserve cardiac chamber morphology. Proc Natl Acad Sci USA 107: 14662–14667, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chi NC, Shaw RM, Jungblut B, Huisken J, Ferrer T, Arnaout R, Scott I, Beis D, Xiao T, Baier H, Jan LY, Tristani-Firouzi M, Stainier DY. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol 6: e109, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarren SK, Smith DW. The fetal alcohol syndrome. Lamp 35: 4–7, 1978. [PubMed] [Google Scholar]
- 12.Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res 105: 408–421, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creazzo TL, Brotto MA, Burch J. Excitation-contraction coupling in the day 15 embryonic chick heart with persistent truncus arteriosus. Pediatr Res 42: 731–737, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Daft PA, Johnston MC, Sulik KK. Abnormal heart and great vessel development following acute ethanol exposure in mice. Teratology 33: 93–104, 1986. [DOI] [PubMed] [Google Scholar]
- 15.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2). Blood 100: 1689–1698, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Deltour L, Ang HL, Duester G. Ethanol inhibition of retinoic acid synthesis as a potential mechanism for fetal alcohol syndrome. FASEB J 10: 1050–1057, 1996. [PubMed] [Google Scholar]
- 17.Dlugos CA, Rabin RA. Structural and functional effects of developmental exposure to ethanol on the zebrafish heart. Alcohol Clin Exp Res 34: 1013–1021, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Flentke GR, Garic A, Amberger E, Hernandez M, Smith SM. Calcium-mediated repression of beta-catenin and its transcriptional signaling mediates neural crest cell death in an avian model of fetal alcohol syndrome. Birth Defects Res A Clin Mol Teratol 91: 591–602, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Floyd RL, Sidhu JS. Monitoring prenatal alcohol exposure. Am J Med Genet C Semin Med Genet 127C: 3–9, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Flynn ME, Pikalow AS, Kimmelman RS, Searls RL. The mechanism of cervical flexure formation in the chick. Anat Embryol (Berl) 184: 411–420, 1991. [DOI] [PubMed] [Google Scholar]
- 22.Godt RE, Fogaca RT, Nosek TM. Alterations of myocardial contraction associated with a structural heart defect in embryonic chicks. Adv Exp Med Biol 453: 453–458; discussion 459, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Granados-Riveron JT, Brook JD. The impact of mechanical forces in heart morphogenesis. Circ Cardiovasc Genet 5: 132–142, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Grewal J, Carmichael SL, Ma C, Lammer EJ, Shaw GM. Maternal periconceptional smoking and alcohol consumption and risk for select congenital anomalies. Birth Defects Res A Clin Mol Teratol 82: 519–526, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groenendijk BC, Hierck BP, Vrolijk J, Baiker M, Pourquie MJ, Gittenberger-de Groot AC, Poelmann RE. Changes in shear stress-related gene expression after experimentally altered venous return in the chicken embryo. Circ Res 96: 1291–1298, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Groenendijk BC, Stekelenburg-de Vos S, Vennemann P, Wladimiroff JW, Nieuwstadt FT, Lindken R, Westerweel J, Hierck BP, Ursem NT, Poelmann RE. The endothelin-1 pathway and the development of cardiovascular defects in the haemodynamically challenged chicken embryo. J Vasc Res 45: 54–68, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Groenendijk BC, Van der Heiden K, Hierck BP, Poelmann RE. The role of shear stress on ET-1, KLF2, and NOS-3 expression in the developing cardiovascular system of chicken embryos in a venous ligation model. Physiology (Bethesda) 22: 380–389, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Gu S, Jenkins MW, Peterson LM, Doughman YQ, Rollins AM, Watanabe M. Optical coherence tomography captures rapid hemodynamic responses to acute hypoxia in the cardiovascular system of early embryos. Dev Dyn 241: 534–544, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol 88: 49–92, 1951. [PubMed] [Google Scholar]
- 30.Hogers B, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Extraembryonic venous obstructions lead to cardiovascular malformations and can be embryolethal. Cardiovasc Res 41: 87–99, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421: 172–177, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, Fujimoto JG, et al. Optical coherence tomography. Science 254: 1178–1181, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huber R, Adler DC, Fujimoto JG. Buffered Fourier domain mode locking: Unidirectional swept laser sources for optical coherence tomography imaging at 370,000 lines/s. Opt Lett 31: 2975–2977, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Huber R, Wojtkowski M, Fujimoto JG. Fourier Domain Mode Locking (FDML): A new laser operating regime and applications for optical coherence tomography. Opt Express 14: 3225–3237, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Jenkins MW, Watanabe M, Rollins AM. Longitudinal imaging of heart development with optical coherence tomography. IEEE J 18: 1166–1175, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkins MW, Adler DC, Gargesha M, Huber R, Rothenberg F, Belding J, Watanabe M, Wilson DL, Fujimoto JG, Rollins AM. Ultrahigh-speed optical coherence tomography imaging and visualization of the embryonic avian heart using a buffered Fourier Domain Mode Locked laser. Opt Express 15: 6251–6267, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins MW, Peterson L, Gu S, Gargesha M, Wilson DL, Watanabe M, Rollins AM. Measuring hemodynamics in the developing heart tube with four-dimensional gated Doppler optical coherence tomography. J Biomed Opt 15: 066022, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet 302: 999–1001, 1973. [DOI] [PubMed] [Google Scholar]
- 39.Keller BB, Liu LJ, Tinney JP, Tobita K. Cardiovascular developmental insights from embryos. Ann NY Acad Sci 1101: 377–388, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Kim M, Kim S, Lim JH, Lee C, Choi HC, Woo CH. Laminar flow activation of ERK5 protein in vascular endothelium leads to atheroprotective effect via NF-E2-related factor 2 (Nrf2) activation. J Biol Chem 287: 40722–40731, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kot-Leibovich H, Fainsod A. Ethanol induces embryonic malformations by competing for retinaldehyde dehydrogenase activity during vertebrate gastrulation. Dis Model Mech 2: 295–305, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JS, Yu Q, Shin JT, Sebzda E, Bertozzi C, Chen M, Mericko P, Stadtfeld M, Zhou D, Cheng L, Graf T, MacRae CA, Lepore JJ, Lo CW, Kahn ML. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev Cell 11: 845–857, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Lee TC, Zhao YD, Courtman DW, Stewart DJ. Abnormal aortic valve development in mice lacking endothelial nitric oxide synthase. Circulation 101: 2345–2348, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Linask KK, Vanauker M. A role for the cytoskeleton in heart looping. ScientificWorldJournal 7: 280–298, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loucks EJ, Ahlgren SC. Deciphering the role of Shh signaling in axial defects produced by ethanol exposure. Birth Defects Res A Clin Mol Teratol 85: 556–567, 2009. [DOI] [PubMed] [Google Scholar]
- 46.Lucitti JL, Visconti R, Novak J, Keller BB. Increased arterial load alters aortic structural and functional properties during embryogenesis. Am J Physiol Heart Circ Physiol 291: H1919–H1926, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Manner J, Seidl W, Steding G. Correlation between the embryonic head flexures and cardiac development. An experimental study in chick embryos. Anat Embryol (Berl) 188: 269–285, 1993. [DOI] [PubMed] [Google Scholar]
- 48.Manner J, Thrane L, Norozi K, Yelbuz TM. High-resolution in vivo imaging of the cross-sectional deformations of contracting embryonic heart loops using optical coherence tomography. Dev Dyn 237: 953–961, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Marrs JA, Clendenon SG, Ratcliffe DR, Fielding SM, Liu Q, Bosron WF. Zebrafish fetal alcohol syndrome model: effects of ethanol are rescued by retinoic acid supplement. Alcohol 44: 707–715, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marschall S, Sander B, Mogensen M, Jorgensen TM, Andersen PE. Optical coherence tomography-current technology and applications in clinical and biomedical research. Anal Bioanal Chem 400: 2699–2720, 2011. [DOI] [PubMed] [Google Scholar]
- 51.McQuinn TC, Bratoeva M, Dealmeida A, Remond M, Thompson RP, Sedmera D. High-frequency ultrasonographic imaging of avian cardiovascular development. Dev Dyn 236: 3503–3513, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Peterson LM, Jenkins MW, Gu S, Barwick L, Watanabe M, Rollins AM. 4D shear stress maps of the developing heart using Doppler optical coherence tomography. Biomed Opt Express 3: 3022–3032, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reckova M, Rosengarten C, deAlmeida A, Stanley CP, Wessels A, Gourdie RG, Thompson RP, Sedmera D. Hemodynamics is a key epigenetic factor in development of the cardiac conduction system. Circ Res 93: 77–85, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Rovasio RA, Battiato NL. Role of early migratory neural crest cells in developmental anomalies induced by ethanol. Int J Dev Biol 39: 421–422, 1995. [PubMed] [Google Scholar]
- 55.Ruckman RN, Messersmith DJ, O'Brien SA, Getson PR, Boeckx RL, Morse DE. Chronic ethanol exposure in the embryonic chick heart: effect on myocardial function and structure. Teratology 37: 317–327, 1988. [DOI] [PubMed] [Google Scholar]
- 56.Serrano M, Han M, Brinez P, Linask KK. Fetal alcohol syndrome: cardiac birth defects in mice and prevention with folate. Am J Obstet Gynecol 203: 75.e7–75.e15, 2010. [DOI] [PubMed] [Google Scholar]
- 57.Shu X, Cheng K, Patel N, Chen F, Joseph E, Tsai HJ, Chen JN. Na,K-ATPase is essential for embryonic heart development in the zebrafish. Development 130: 6165–6173, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Smith SM, Flentke GR, Garic A. Avian models in teratology and developmental toxicology. Methods Mol Biol 889: 85–103, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tai LK, Zheng Q, Pan S, Jin ZG, Berk BC. Flow activates ERK1/2 and endothelial nitric oxide synthase via a pathway involving PECAM1, SHP2, and Tie2. J Biol Chem 280: 29620–29624, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Tawakol A, Omland T, Creager MA. Direct effect of ethanol on human vascular function. Am J Physiol Heart Circ Physiol 286: H2468–H2473, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Twal WO, Zile MH. Retinoic acid reverses ethanol-induced cardiovascular abnormalities in quail embryos. Alcohol Clin Exp Res 21: 1137–1143, 1997. [PubMed] [Google Scholar]
- 62.Vermot J, Forouhar AS, Liebling M, Wu D, Plummer D, Gharib M, Fraser SE. Reversing blood flows act through klf2a to ensure normal valvulogenesis in the developing heart. PLoS Biol 7: e1000246, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vittorini S, Storti S, Parri MS, Cerillo AG, Clerico A. SERCA2a, phospholamban, sarcolipin, and ryanodine receptors gene expression in children with congenital heart defects. Mol Med 13: 105–111, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang G, Bieberich E. Prenatal alcohol exposure triggers ceramide-induced apoptosis in neural crest-derived tissues concurrent with defective cranial development. Cell Death Dis 1: e46, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang N, Miao H, Li YS, Zhang P, Haga JH, Hu Y, Young A, Yuan S, Nguyen P, Wu CC, Chien S. Shear stress regulation of Kruppel-like factor 2 expression is flow pattern-specific. Biochem Biophys Res Commun 341: 1244–1251, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Yashiro K, Shiratori H, Hamada H. Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature 450: 285–288, 2007. [DOI] [PubMed] [Google Scholar]
- 67.Yelbuz TM, Waldo KL, Kumiski DH, Stadt HA, Wolfe RR, Leatherbury L, Kirby ML. Shortened outflow tract leads to altered cardiac looping after neural crest ablation. Circulation 106: 504–510, 2002. [DOI] [PubMed] [Google Scholar]
- 68.Yelin R, Yelin D, Oh WY, Yun SH, Boudoux C, Vakoc BJ, Bouma BE, Tearney GJ. Multimodality optical imaging of embryonic heart microstructure. J Biomed Opt 12: 064021, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.