Abstract
Angiotensin (ANG)-(1–12) excites neurons via ANG II type 1 receptors (AT1Rs), which are present in the caudal ventrolateral medullary depressor area (CVLM). We hypothesized that microinjections of ANG-(1–12) into the CVLM may elicit decreases in mean arterial pressure (MAP), heart rate (HR), and sympathetic nerve activity. This hypothesis was tested in urethane-anesthetized adult male Wistar rats. Microinjections of ANG-(1–12) into the CVLM elicited decreases in MAP, HR, and greater splanchnic nerve activity (GSNA). ANG-(1–12)-induced responses consisted of initial (first 1–8 min) and delayed (8–24 min) phases. Prior microinjections of losartan, A-779, and captopril into the CVLM blocked initial, delayed, and both phases of ANG-(1–12) responses, respectively. Blockade of GABA receptors in the rostral ventrolateral medullary pressor area (RVLM) attenuated cardiovascular responses elicited by microinjections of ANG-(1–12) into the ipsilateral CVLM. Microinjections of ANG-(1–12) into the CVLM potentiated the reflex decreases and attenuated the reflex increases in GSNA elicited by intravenous injections of phenylephrine and sodium nitroprusside, respectively. These results indicate that microinjections of ANG-(1–12) into the CVLM elicit decreases in MAP, HR, and GSNA. Initial and delayed phases of these responses are mediated via ANG II and ANG-(1–7), respectively; the effects of ANG II and ANG-(1–7) are mediated via AT1Rs and Mas receptors, respectively. Captopril blocked both phases of ANG-(1–12) responses, indicating that angiotensin-converting enzyme is important in mediating these responses. GABA receptors in the RVLM partly mediate the cardiovascular responses to microinjections of ANG-(1–12) into the CVLM. Microinjections of ANG-(1–12) into the CVLM modulate baroreflex responses.
Keywords: angiotensin II receptors, baroreflex, GABAergic neurons, Mas receptors, sympathetic nerve activity
angiotensin (ANG)-(1–12), a new ANG, has been recently identified (29). In brain tissue, the concentration of ANG-(1–12) is about five times greater than ANG II (29). Unlike ANG II, renin is not involved in the formation of ANG-(1–12) (15, 40), prompting the suggestion that ANG-(1–12) may act as a renin-independent alternative substrate for the formation of ANG II in several organs (40). In the periphery, ANG-(1–12) may exert its actions via a rapid conversion to ANG II because pressor responses elicited by intravenous injections of ANG-(1–12) in the rat were blocked by prior injections of an ANG type 1 receptor (AT1R) antagonist (29). Because it is known that ANG I and ANG II can be converted into ANG-(1–7) (41), there is a possibility that ANG-(1–7) may be formed from ANG-(1–12) in the brain. The receptors via which ANG-(1–7) exerts its actions [Mas receptors (MasRs)] have been identified in medullary brain tissue (14).
We have previously reported that ANG-(1–12) elicits cardiovascular actions in the central nervous system as well. In a previous study (10), microinjections of ANG-(1–12) into the nucleus tractus solitarius (NTS) elicited decreases in mean arterial pressure (MAP), heart rate (HR), and sympathetic nerve activity (SNA). In the rostral ventrolateral medullary pressor area (RVLM) and hypothalamic paraventricular and arcuate nuclei, microinjections of ANG-(1–12) elicited increases in MAP, HR, and SNA (4, 5, 11).
This laboratory was the first to report the critical role that the caudal ventrolateral medullary depressor area (CVLM) plays as a relay nucleus in medullary baroreflex pathways (28, 43–46). Peripheral baroreceptor, chemoreceptor, and cardiopulmonary afferents are known to make their first synapse in the NTS. Second-order NTS neurons project to CVLM neurons and use an excitatory amino acid (probably glutamate) as a neurotransmitter. The CVLM neurons involved in the baroreflex are GABA-containing neurons, and they project to the RVLM (16, 17, 25, 34, 37). The presence of AT1Rs has been reported in the CVLM, and microinjections of ANG II into the CVLM decrease blood pressure (BP) (3, 8). Microinjections of ANG-(1–7) into the CVLM have been reported to elicit depressor responses (2). Furthermore, microinjections of ANG II and ANG-(1–7) into the CVLM have been reported to modulate baroreflex sensitivity (3). Direct application of ANG-(1–12) has an excitatory effect on neurons (10, 31). Based on this information, we hypothesized that microinjections of ANG-(1–12) into the CVLM may elicit decreases in BP, HR, and SNA and modulate baroreflex function. This hypothesis was tested in anesthetized Wistar rats.
MATERIALS AND METHODS
General procedures.
Adult male Wistar rats weighing 300–360 g (Charles River Laboratories, Wilmington, MA) were used in this study. Animals were housed under controlled conditions with light and dark cycles set at 12 h each. Food and water were available to the rats freely. The Institutional Animal Care and Use Committee of Rutgers New Jersey Medical School approved the protocols of this study, and experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to prevent the suffering of the animals and minimize their discomfort.
The general procedures used in this study were similar to those previously published by us (23). Inhalation of isoflurane (2–3% in 100% oxygen) was used to initially anesthetize the rats. A cannula was placed in the trachea, and artificial ventilation was administered using a rodent ventilator (model 683, Harvard Apparatus, Holliston, MA). End-tidal CO2 was maintained at 3.5–4.5% by adjusting the frequency and tidal volume on the ventilator. The femoral artery on one side was cannulated to monitor BP. MAP and HR were derived electronically from BP waves. One of the femoral veins was cannulated and urethane (1.2–1.4 g/kg) was injected intravenously in eight to nine aliquots at 2-min intervals; the total volume of the anesthetic solution injected into the animal over a period of 16–18 min was 0.4–0.45 ml. As soon as urethane administration was completed, isoflurane inhalation was stopped. The absence of a BP response and/or withdrawal of the limb in response to pinching of a hindpaw were used as tests to ensure that the level of anesthesia was adequate. A temperature controller (model TCAT-2AC, Physitemp Instruments, Clifton, NJ) was used to maintain the rectal temperature at 37 ± 0.5°C. All recordings were stored on a computer hard drive using a data-acquisition system obtained from Cambridge Electronic Design (CED; Cambridge, UK). At the conclusion of the experiment, a high dose of urethane (2 g/kg iv) was injected into the rats to euthanize them, and an incision was made in one of the intercostal muscles to produce pneumothorax; the cessation of a heart beat indicated that euthanasia was complete.
Microinjection technique.
We have reported the details of this technique in a previous publication (22). Rats were placed in a prone position in a stereotaxic instrument (David Kopf Instruments, Tajunga, CA), and the bite bar was fixed 18 mm below the interaural line. The dorsal medulla was exposed. Multibarreled glass micropipettes (tip size: 20–40 μm) were used for microinjections. In the majority of experiments, micropipettes were introduced into the CVLM perpendicularly; using this approach, the coordinates for microinjections into the CVLM were 0.7–1.3 mm rostral to the calamus scriptorius, 1.9–2.1 mm lateral to the midline, and 2.9–3.1 mm deep from the dorsal medullary surface. When the protocol required microinjections into the CVLM as well as the RVLM in the same experiment, micropipettes were inserted into the RVLM perpendicularly and at an angle into the CVLM (80° angle pointing rostrally). Using this approach, the coordinates for the microinjections into the CVLM were 0.3–0.8 mm rostral to the calamus scriptorius, 1.9–2.1 mm lateral to the midline, and 3.0–3.1 mm deep from the dorsal medullary surface, and the coordinates for microinjections into the RVLM were 2.0–2.4 mm rostral to the calamus scriptorius, 2.0–2.3 mm lateral to the midline, and 3.1–3.3 mm deep from the dorsal medullary surface. CVLM and RVLM sites were always identified first by microinjections of l-glutamate (l-Glu; 5 mM). The duration of microinjection was 10 s. Controls for microinjections consisted of artificial cerebrospinal fluid (aCSF; pH 7.4). In most of the experiments, microinjections into the CVLM and RVLM were unilateral. In experiments testing the baroreflex, microinjections into the CVLM were bilateral. The volume of all microinjections into the CVLM and RVLM was 100 nl.
Vagotomy.
Both of the vagus nerves were identified bilaterally low in the neck and sectioned at a level just caudal to the site where the superior laryngeal nerves branched off from these nerves. A stabilization period of 45–60 min was allowed after vagotomy.
Greater splanchnic and renal nerve recording.
The greater splanchnic nerve (GSN) and one of the renal nerve branches (RN) were exposed retroperitoneally in separate experiments (21, 30). The GSN was sectioned at its junction with the celiac ganglion, and the RN was sectioned at its entry into the kidney. In each case, a small segment was desheathed, and its activity was recorded using a bipolar silver wire hook electrode. The activity of the whole GSN (GSNA) and RN (RSNA) was amplified (×10,000–20,000), filtered (100–5,000 Hz), digitized, and stored on a computer hard drive. Digitized signals were full wave rectified and integrated over consecutive 1-s intervals using the Spike 2 program (CED). When the nerve recording was completed, the GSN or RN was sectioned rostral to the recording site, and the remaining activity was considered to be the noise level, which was subtracted from the GSNA or RSNA amplitude. A bolus injection (10 μg/kg iv) of l-phenylephrine (PE) was always used to test the barosensitivity of recorded nerve activities; PE elicited an increase in systemic BP and reflex inhibition of nerve activity.
Evaluation of baroreflex function.
The effect of microinjections of ANG-(1–12) in the CVLM on baroreflex sensitivity was tested as follows. Changes in HR and GSNA elicited by intravenous bolus injections of PE (7.5 μg/kg; the concentration of the solution was 30 μg/ml) were recorded, ANG-(1–12) (0.5 mM) was microinjected into the CVLM bilaterally, and intravenous injections of PE were repeated 2 and 12 min later. The interval between the microinjections of ANG-(1–12) into the CVLM on two sides was 1 min. Bilateral microinjections of aCSF (100 nl) into the CVLM were used as controls for microinjections of ANG-(1–12). Baroreflex sensitivity was expressed by the ratios of the maximal change in pulse index (PI; in ms) to maximal change in MAP (in ms/mmHg) (3) and maximal percent change in GSNA to maximal change in MAP (in %/mmHg). PI was calculated using the following formula: PI = 60,000/HR (3). In another group of rats, the same protocol for testing baroreflex sensitivity was used except that intravenous injections of sodium nitroprusside (SNP; 7.5 μg/kg iv; the concentration of the solution was 30 μg/ml) were used instead of PE.
Histological identification of microinjection sites.
When the experiment was completed, diluted green retrobeads IX (1: 50) were microinjected into the CVLM and RVLM to mark the microinjection sites. Animals were perfused and fixed with 2% paraformaldehyde, and serial sections of the medulla were cut (40 μm) in a vibratome and mounted on slides. Microinjection sites were identified under a microscope. Sections were then photographed and compared with a standard atlas (33).
Drugs and chemicals.
The following drugs and chemicals were used in this study: ANG-(1–12), A-779 [d-Ala7-ANG I/II (1–7); MasR antagonist] (36), captopril [angiotensin-converting enzyme (ACE) inhibitor] (26), CGP-52432 (GABAB receptor antagonist) (24), chymostatin (chymase inhibitor) (19), gabazine (GABAA receptor antagonist) (18), green retrobeads IX, isoflurane, l-Glu, PE, losartan (selective AT1R antagonist), PD-123319 [ANG type 2 receptor (AT2R) antagonist] (7), SNP, and urethane. All solutions for the microinjections were freshly prepared in aCSF. The concentration of drugs refers to their salts where applicable. The concentrations of A-779, captopril, chymostatin, losartan, l-Glu, PD-123319, and urethane used were selected from published literature (10, 20, 23, 42). The vendors for the different drugs and chemicals were as follows: ANG-(1–12) and A-779 were from American Peptide (Sunnyvale, CA), green retrobeads IX were from Lumafluor (Durham, NC), CGP-52432, gabazine, and PD-123319 were from Tocris Bioscience (Ellisville, MO), and isoflurane was from Piramal Critical Care (Bethlehem, PA). All other drugs and chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Statistical analyses.
Maximum changes in MAP and HR in response to microinjections of different drugs were expressed as means ± SE. Two-way ANOVA followed by Tukey-Kramer's multiple test was used to assess the statistical significance of the difference between the following responses elicited from the CVLM: microinjections of different concentrations of ANG-(1–12), ANG-(1–12)-induced responses after aCSF and A-779, ANG-(1–12)-induced responses after aCSF and losartan, and ANG-(1–12)-induced responses after aCSF and captopril. The significance of differences in baroreflex responses before and after microinjections of either aCSF or ANG-(1–12) into the CVLM was determined by one-way ANOVA followed by Tukey-Kramer's multiple test. Baroreflex sensitivity was expressed by the following ratios: changes in PI to changes in MAP and changes in GSNA to changes in MAP. Changes in MAP were induced by either intravenous injections of PE or SNP. Student's unpaired t-test was used for comparison of maximal decreases in MAP, HR, GSNA, and RSNA induced by microinjections of ANG-(1–12) into the CVLM in different groups of rats. For analyses of GSNA and RSNA, the integrated signals obtained just before the microinjections of ANG-(1–12) into the CVLM were averaged over a period of 60 s. When responses to these treatments were maximal, integrated signals were averaged over a period of 60–90 s. Percent changes in GSNA and RSNA elicited by these treatments were calculated. In all cases, differences were considered significant at P values of <0.05.
RESULTS
In urethane-anesthetized Wistar rats (n = 85), baseline values for MAP and HR were 103.2 ± 1.3 mmHg and 343.3 ± 4.7 beats/min, respectively.
Concentration responses of ANG-(1–12) in the CVLM.
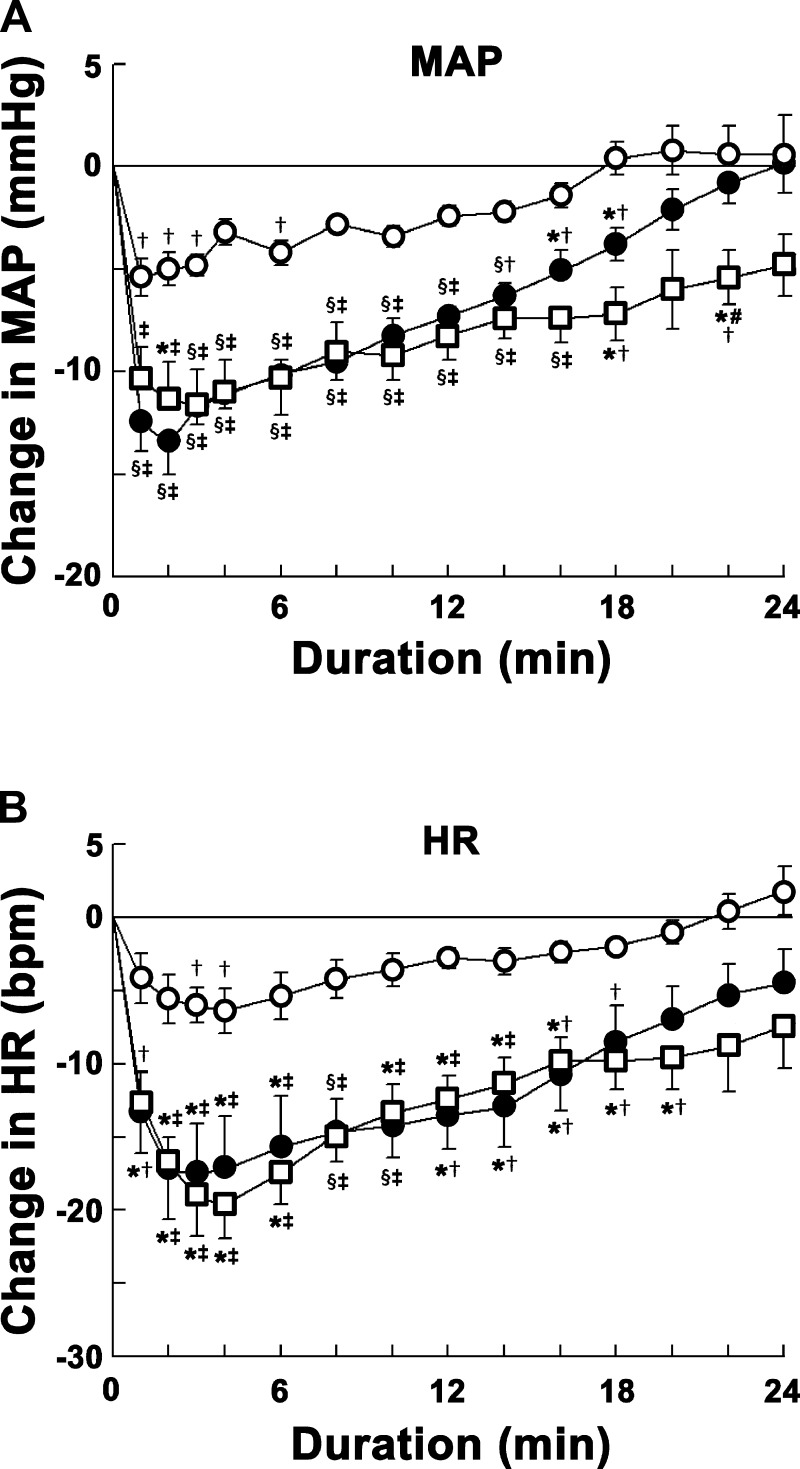
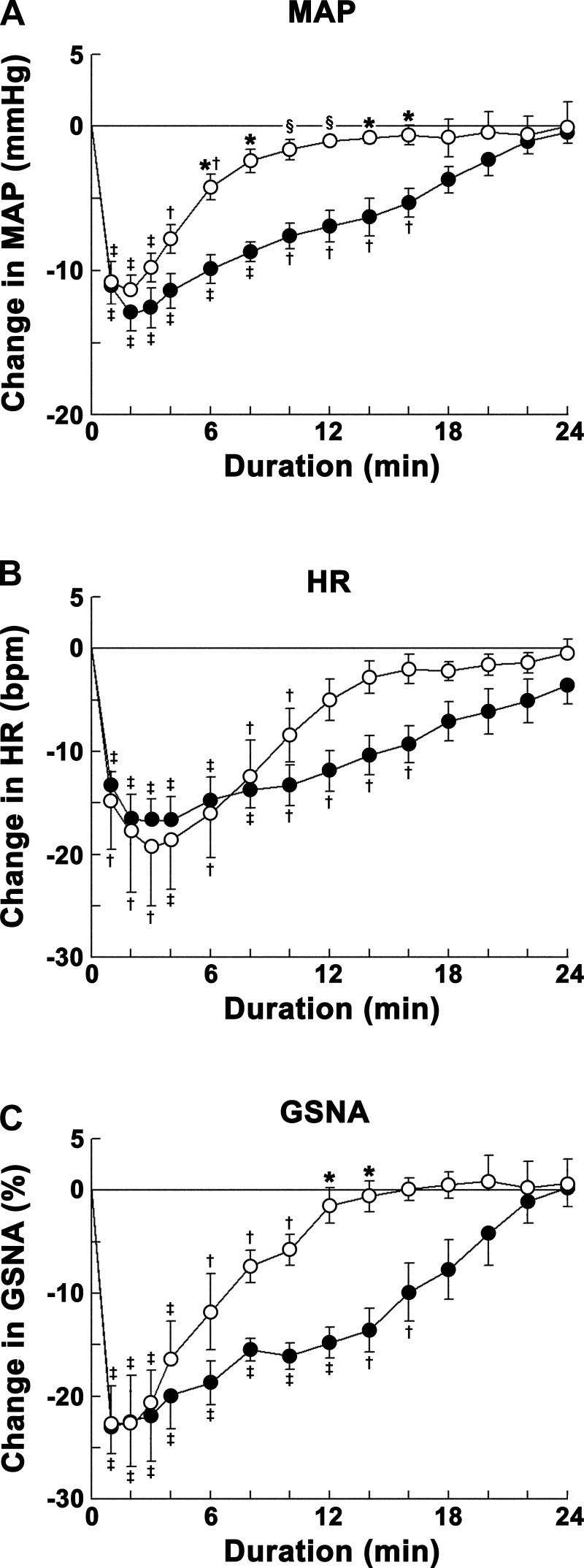
The CVLM was first identified by microinjections of l-Glu (5 mM); decreases in MAP (24.1 ± 2.8 mmHg) and HR (17.2 ± 3.4 beats/min) were observed. The time course of concentration responses of microinjections of ANG-(1–12) into the CVLM is shown in Fig. 1. The concentrations of ANG-(1–12) used were 0.25, 0.5, and 1 mM. ANG-(1–12) microinjections elicited decreases in MAP. Maximal decreases in MAP were elicited within 1–3 min. The decreases in MAP elicited by the 1 mM concentration were significantly greater than those elicited by the 0.25 mM concentration at 2- to 18- and 22-min time points and by the 0.5 mM concentration at the 22-min time point after microinjections. The decreases in MAP elicited by the 0.5 mM concentration were significantly greater than those elicited by the 0.25 mM concentration at 1- to 18-min time points after microinjections (Fig. 1A).
Fig. 1.
Concentration-response of angiotensin (ANG)-(1–12) into the caudal ventrolateral medullary depressor area (CVLM). Open circles show responses to microinjections of 0.25 mM ANG-(1–12) (n = 5); solid circles show responses to microinjections of 0.5 mM ANG-(1–12) (n = 6); open squares show responses to microinjections of 1 mM ANG-(1–12) (n = 5). A: decreases in mean arterial pressure (MAP) elicited by microinjections of ANG-(1–12). Each concentration of ANG-(1–12) elicited maximal decreases in MAP within 1–3 min. Comparison of depressor responses at one of these time points (e.g., 2 min) showed that the responses elicited by 0.5 and 1 mM concentrations (13.5 ± 1.5 and 11.4 ± 1.9 mmHg, respectively) were not significantly different (P > 0.05) but that responses were significantly (P < 0.01–0.05) greater than the responses elicited by the 0.25 mM concentration (5.0 ± 0.8 mmHg) at this time point. The depressor responses elicited by the 1 mM concentration lasted longer (>24 min) than those elicited by 0.25 and 0.5 mM concentrations (within 24 min). B: decreases in HR [in beats/min (bpm)] elicited by microinjections of ANG-(1–12) into the CVLM. Each concentration elicited maximal decreases in HR within 3–4 min. Comparison of bradycardic responses at one of these time points (e.g., 3 min) showed that the responses elicited by 0.5 and 1.0 mM concentrations (17.5 ± 3.4 and 19.2 ± 2.6 beats/min, respectively) were not significantly (P > 0.05) different but that responses were significantly (P < 0.05) greater than those elicited by the 0.25 mM concentration (6.0 ± 1.2 beats/min) at this time point. In this and other figures, the volume of all microinjections was 100 nl; all values are expressed as means ± SE. *P < 0.05 compared with the 0.25 mM concentration (all comparisons refer to corresponding time points); §P < 0.01 compared with the 0.25 mM concentration; #P < 0.05 compared with the 0.5 mM concentration; †P < 0.05 and ‡P < 0.01 compared with baseline (0-min time point).
Maximal decreases in HR were elicited within 3–4 min by these concentrations of ANG-(1–12). The decreases in HR elicited by the 1 mM concentration were significantly greater than those elicited by the 0.25 mM concentration at 2- to 20-min time points. There was no significant difference in HR responses elicited by 1 and 0.5 mM concentrations at any time point. The decreases in HR elicited by the 0.5 mM concentration were significantly greater than those elicited by the 0.25 mM concentration at 1- to 16-min time points after microinjections (Fig. 1B).
The 0.5 mM concentration of ANG-(1–12) was selected for further study in other groups of rats because responses elicited by this concentration were not significantly different from the 1 mM concentration at most of the time points. The onset, peak, and duration of MAP responses elicited by the 0.5 mM concentration of ANG-(1–12) were 8.1 ± 1.4 s, 1.5 ± 0.2 min, and 23.1 ± 1.8 min, respectively.
Effect of microinjections of ANG-(1–12) into the CVLM on SNA.
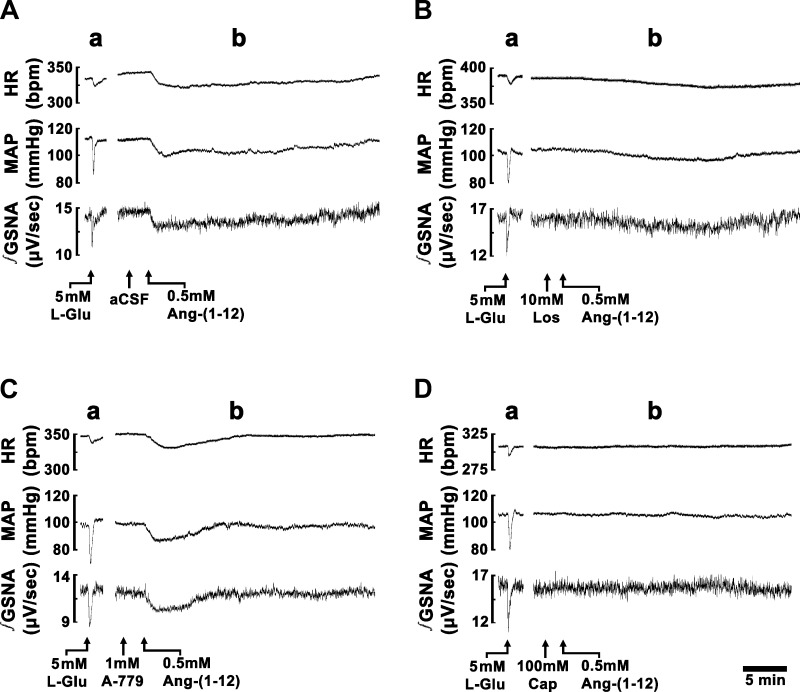
Typical tracings of the effects of ANG-(1–12) (0.5 mM) on HR, MAP, and integrated GSNA are shown in Fig. 2A. A microinjection of l-Glu (5 mM) into the CVLM elicited decreases in HR, MAP, and GSNA (Fig. 2A,a). Five minutes later, a microinjection of aCSF into the CVLM elicited no HR, MAP, and GSNA responses (Fig. 2A,b). Two minutes later, a microinjection of ANG-(1–12) (0.5 mM) into the CVLM elicited decreases in HR, MAP, and GSNA (Fig. 2A,b). Group data for the decreases in MAP, HR, and GSNA elicited by microinjections of ANG-(1–12), after microinjections of aCSF at the same site, are shown in Figs. 3–5. Maximal decreases in MAP, HR, and GSNA in this group of rats were 13.3 ± 1.3 mmHg, 17.4 ± 2.3 beats/min, and 24.3 ± 3.7%, respectively.
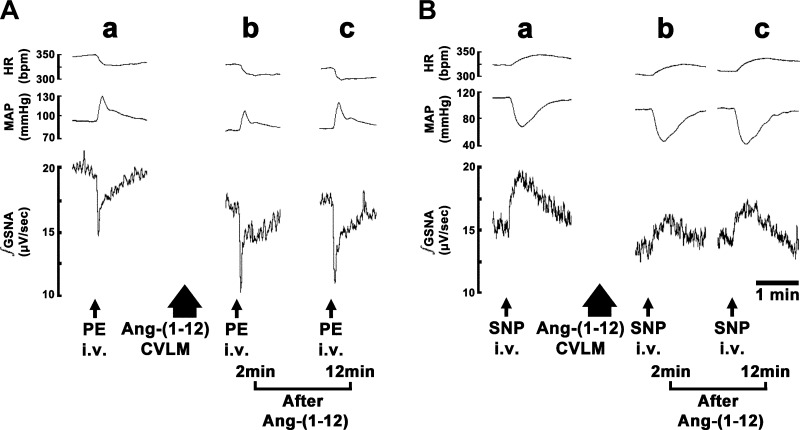
Fig. 2.
Traces showing the effect of microinjections of ANG-(1–12) into the CVLM. In each A–D, the top trace shows HR (in beats/min), the middle trace shows MAP (in mmHg), and the bottom trace shows integrated greater splanchnic nerve activity (∫GSNA; in μV/s). Tracings in A–D are from different rats. The CVLM was first identified by microinjections of l-glutamate (l-Glu; 5 mM); decreases in HR, MAP, and GSNA were observed (A–D,a). Tracings of the responses to microinjections of ANG-(1–12) (0.5 mM) are shown in A–D,b. A: responses of ANG-(1–12) after microinjection of artificial cerebrospinal fluid (aCSF) at the same site. B: responses of ANG-(1–12) after microinjection of losartan (Los; 10 mM) at the same site. C: responses of ANG-(1–12) after microinjection of A-779 (1 mM, Mas receptor antagonist) at the same site. D: responses of ANG-(1–12) after microinjection of captopril (Cap; 100 mM) at the same site.
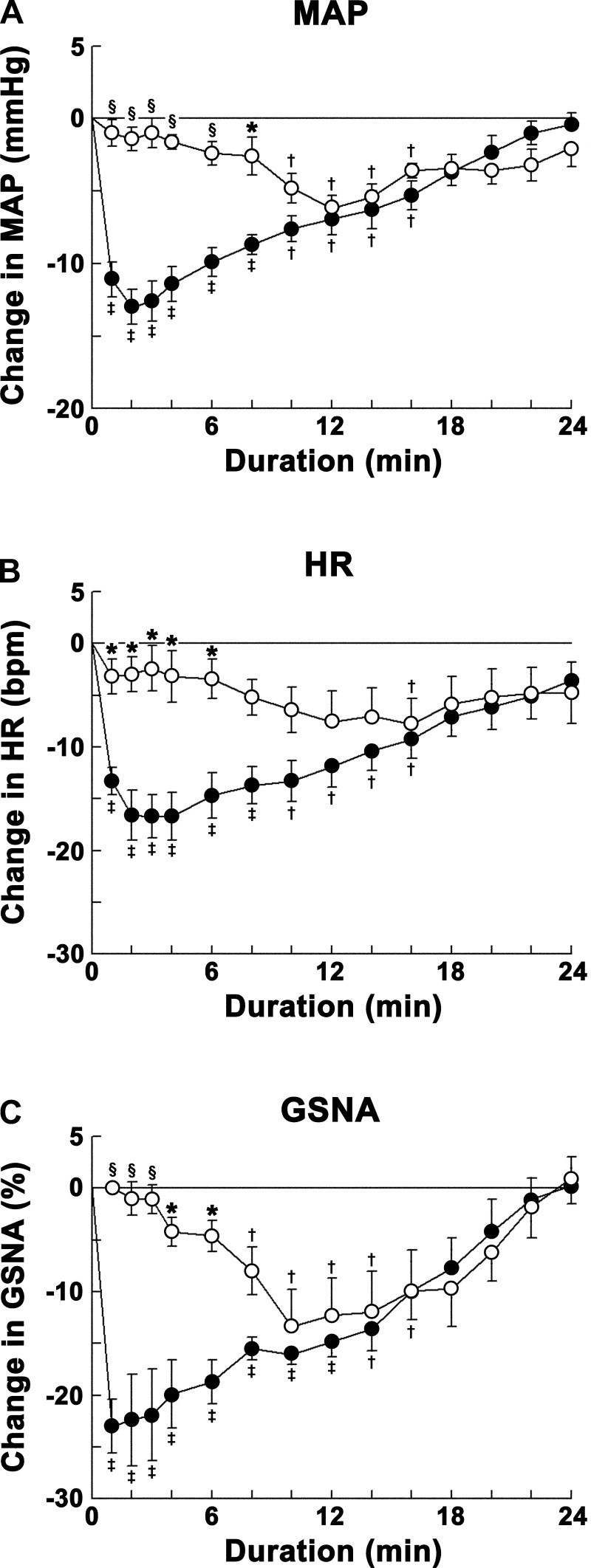
Fig. 3.
Group data showing the effects of ANG type 1 receptor blockade on ANG-(1–12)-induced responses. Decreases in MAP (A), HR (B), and GSNA (C) were elicited by microinjections of ANG-(1–12) (0.5 mM) into the CVLM. Open circles show responses to microinjections of ANG-(1–12) into the CVLM after microinjections of Los (10 mM) into the same site (n = 5); solid circles show responses to microinjections of ANG-(1–12) into the CVLM after microinjections of aCSF into the same site (n = 7). The initial phase (∼1–8 min) of ANG-(1–12) responses was abolished by microinjection of Los. Comparison of responses at one of these time points (e.g., 2 min) showed that the decreases in MAP (1.4 ± 0.8 mmHg), HR (3.0 ± 1.7 beats/min), and GSNA (1.0 ± 1.6%) elicited by microinjections of ANG-(1–12) after microinjections of Los were significantly smaller (P < 0.01–0.05) than the decreases in MAP (13.0 ± 1.2 mmHg), HR (16.7 ± 2.1 beats/min), and GSNA (22.4 ± 4.4%) elicited by microinjections of ANG-(1–12) after microinjections of aCSF. *P < 0.05 compared with responses elicited by microinjections of ANG-(1–12) after microinjections of aCSF (all comparisons refer to corresponding time points); §P < 0.01 compared with responses elicited by microinjections of ANG-(1–12) after microinjections of aCSF; †P < 0.05 and ‡P < 0.01 compared with baseline (0-min time point).
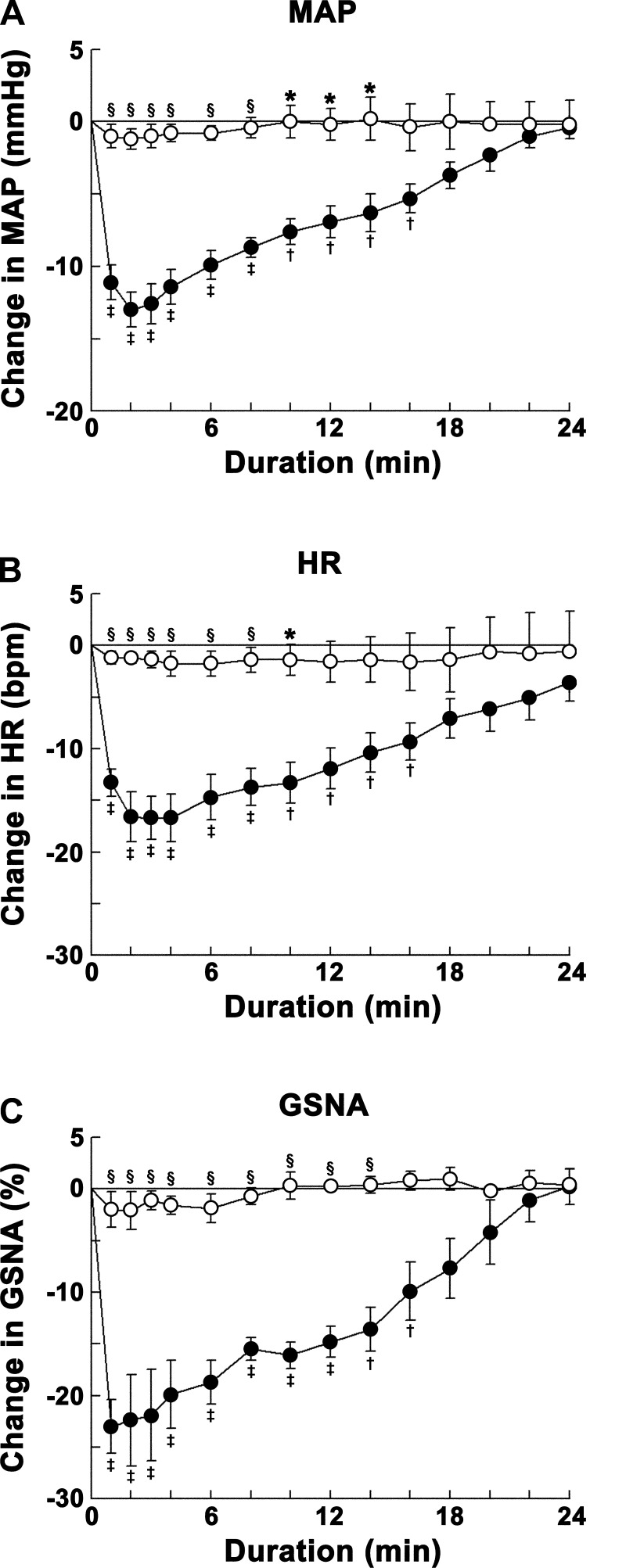
Fig. 5.
Group data showing the effects of angiotensin-converting enzyme (ACE) inhibition on ANG-(1–12)-induced responses. Decreases in MAP (A), HR (B), and GSNA (C) were elicited by microinjections of ANG-(1–12) (0.5 mM) into the CVLM. Open circles show responses to microinjections of ANG-(1–12) into the CVLM after microinjections of Cap (100 mM) into the same site (n = 5); solid circles show responses to microinjections of ANG-(1–12) into the CVLM after microinjections of aCSF into the same site (n = 7). Both initial (∼1–8 min) and delayed (∼8–24 min) phases of ANG-(1–12) responses were blocked by microinjection of Cap. Comparison of responses at one of these time points in the initial phase (e.g., 2 min) showed that the decreases in MAP (1.2 ± 0.7 mmHg), HR (1.2 ± 0.5 beats/min), and GSNA (2.1 ± 1.8%) elicited by microinjections of ANG-(1–12) after microinjections of Cap were significantly smaller (P < 0.01) than the decreases in MAP (13.0 ± 1.2 mmHg), HR (16.7 ± 2.1 beats/min), and GSNA (22.4 ± 4.4%) elicited by microinjections of ANG-(1–12) after microinjections of aCSF. Comparison of responses at one of these time points in the delayed phase (e.g., 10 min) showed that the changes in MAP (0.0 ± 1.1 mmHg), HR (−1.4 ± 1.5 beats/min), and GSNA (+0.3 ± 1.3%) elicited by microinjections of ANG-(1–12) after microinjections of Cap were significantly smaller (P < 0.01–0.05) than the decreases in MAP (7.6 ± 0.9 mmHg), HR (13.3 ± 2.0 beats/min), and GSNA (16.1 ± 2.0%) elicited by microinjections of ANG-(1–12) after microinjections of aCSF. *P < 0.05 compared with responses elicited by microinjections of ANG-(1–12) after microinjections of aCSF (all comparisons refer to corresponding time points); §P < 0.01 compared with responses elicited by microinjections of ANG-(1–12) after microinjections of aCSF; †P < 0.05 and ‡P < 0.01 compared with baseline (0-min time point).
The effects of ANG-(1–12) (0.5 mM) on RSNA were tested in another group of rats (n = 5). The maximal decreases in MAP, HR, and RSNA elicited by microinjections of ANG-(1–12) into the CVLM were 12.8 ± 1.6 mmHg, 21.6 ± 4.3 beats/min, and 29.5 ± 4.8%, respectively. These responses were not significantly different (P > 0.05) from the decreases in MAP, HR, and GSNA elicited by microinjections of ANG-(1–12) (0.5 mM) into the CVLM (see values above).
Effect of AT1R blockade on ANG-(1–12)-induced responses in the CVLM.
A typical tracing of the effect of AT1R blockade in the CVLM on ANG-(1–12)-induced responses is shown in Fig. 2B. A microinjection of l-Glu into the CVLM elicited decreases in HR, MAP, and GSNA (Fig. 2B,a). Five minutes later, a microinjection of losartan (10 mM) into the CVLM elicited no HR, MAP, and GSNA responses (Fig. 2B,b). After an interval of 2 min, ANG-(1–12) (0.5 mM) was microinjected at the same site; the initial decreases in HR, MAP, and GSNA elicited by microinjections of ANG-(1–12) were blocked, but the delayed phase of decreases in these variables persisted (Fig. 2B,b).
Group data for this experiment are shown in Fig. 3. Microinjections of ANG-(1–12) (0.5 mM) into the CVLM, 2 min after the microinjections of losartan (10 mM) at the same site, did not elicit the initial decreases in MAP observed when ANG-(1–12) was microinjected after control microinjections of aCSF. However, after ∼10 min, the decreases in MAP elicited by ANG-(1–12) after microinjections of losartan were similar to the decreases in MAP elicited by ANG-(1–12) after microinjections of aCSF. Thus, the decreases in MAP elicited by ANG-(1–12) after microinjections of losartan at the same site were significantly (P < 0.01–0.05) reduced at 1- to 8-min time points compared with the decreases in MAP elicited by ANG-(1–12) after microinjections of aCSF into the CVLM at the same time points (Fig. 3A).
Microinjections of losartan blocked the initial decrease in HR elicited by microinjections of ANG-(1–12) observed when ANG-(1–12) was microinjected after aCSF. However, after 8 min, the decreases in HR elicited by ANG-(1–12) were not statistically different in the two groups of rats. Thus, the decreases in HR elicited by ANG-(1–12) after microinjections of losartan at the same site were significantly (P < 0.05) reduced at 1- to 6-min time points compared with the decreases in HR elicited by ANG-(1–12) after microinjections of aCSF into the CVLM at the same time points (Fig. 3B).
The initial decreases in GSNA elicited by microinjections of ANG-(1–12) into the CVLM, which were observed when ANG-(1–12) was microinjected after aCSF, were blocked by prior microinjections of losartan. However, after 8 min, the decreases in GSNA elicited by ANG-(1–12) were not statistically different in the two groups of rats. Thus, the decreases in GSNA elicited by ANG-(1–12) after microinjections of losartan at the same site were significantly (P < 0.01–0.05) reduced at 1- to 6-min time points compared with the decreases in GSNA elicited by ANG-(1–12) after microinjections of aCSF into the CVLM at the same time points (Fig. 3C).
The results described above show that prior microinjections of losartan into the CVLM abolished the decreases in MAP, HR, and GSNA elicited by microinjections of ANG-(1–12) at the same site during the initial 1–8 min. This phase of ANG-(1–12) responses (referred to as the initial phase in this report) was mediated via the activation of AT1Rs.
In a separate group of rats (n = 4), the blocking effect of losartan on initial depressor effects of ANG-(1–12) lasted for at least 30 min. In these experiments, losartan (10 mM) was microinjected into the CVLM, and, 30 min later, ANG-(1–12) (0.5 mM) was microinjected into the same site; the initial depressor responses (for the first ∼4 min) to ANG-(1–12) were blocked by losartan.
Effect of AT2R blockade on ANG-(1–12)-induced responses in the CVLM.
In this experiment, the effect of blockade of AT2Rs in the CVLM was studied only on the initial (maximal) responses elicited by microinjections of ANG-(1–12) into the CVLM. Microinjections of PD-123319 (5 mM) into the CVLM did not alter depressor and bradycardic responses elicited by microinjections of ANG-(1–12) (0.5 mM) at the same site. The maximal decreases in MAP (14.2 ± 1.2 mmHg) and HR (16.2 ± 1.9 beats/min) elicited by ANG-(1–12) in this group of rats (n = 5) were not significantly different (P > 0.05) compared with the maximal decreases in MAP (13.3 ± 1.3 mmHg) and HR (17.4 ± 2.3 beats/min) elicited by microinjections of the same concentration of ANG-(1–12) into the CVLM after control microinjections of aCSF (n = 7). Microinjections of PD-123319 (5 mM) into the CVLM elicited small decreases (5.0 ± 1.8 mmHg) in MAP and no change in HR. The maximum decrease in MAP was elicited by PD-123319 within 1–2 min, and this level stabilized for ∼10 min. ANG-(1–12) (0.5 mM), microinjected into the CVLM 2 min after PD-123319, elicited decreases in MAP as well as HR, which peaked at 1–3 min. Therefore, AT2Rs did not contribute to the maximal MAP and HR effects of ANG-(1–12).
Effect of MasR blockade on ANG-(1–12)-induced responses in the CVLM.
A typical tracing of the effect of MasR blockade in the CVLM on ANG-(1–12)-induced responses is shown in Fig. 2C. A microinjection of l-Glu into the CVLM elicited decreases in HR, MAP, and GSNA (Fig. 2C,a). Five minutes later, a microinjection of A-779 (1 mM) into the CVLM elicited no HR, MAP, and GSNA responses (Fig. 2C,b). After an interval of 2 min, the duration of HR, MAP, and GSNA responses elicited by microinjection of ANG-(1–12) into the CVLM was reduced (Fig. 2C,b).
Group data for this experiment are shown in Fig. 4. The duration of decreases in MAP elicited by microinjections of ANG-(1–12) (0.5 mM) into the CVLM, after microinjection of A-779 (1 mM) at the same site, was smaller compared with the duration of decreases in MAP elicited by microinjections of ANG-(1–12) (0.5 mM) into the CVLM after control microinjection of aCSF at the same site. Thus, the decreases in MAP elicited by ANG-(1–12) after microinjections of A-779 were significantly (P < 0.01–0.05) reduced at 6- to 16-min time points compared with the decreases in MAP elicited by ANG-(1–12) after microinjections of aCSF into the CVLM at the same time points (Fig. 4A).
Fig. 4.
Group data showing the effects of Mas receptor blockade on ANG-(1–12)-induced responses. Decreases in MAP (A), HR (B), and GSNA (C) were elicited by microinjections of ANG-(1–12) (0.5 mM) into the CVLM. Open circles show responses to microinjections of ANG-(1–12) into the CVLM after microinjections of A-779 (1 mM) into the same site (n = 5); solid circles show responses to microinjections of ANG-(1–12) into the CVLM after microinjections of aCSF into the same site (n = 7). The delayed phase (∼8–24 min) of ANG-(1–12) responses was abolished by microinjection of A-779. Comparison of responses at one of these time points (e.g., 12 min) showed that the decreases in MAP (1.0 ± 0.4 mmHg) and GSNA (1.5 ± 1.7%) elicited by microinjections of ANG-(1–12) after microinjections of A-779 were significantly smaller (P < 0.01–0.05) than the decreases in MAP (6.9 ± 1.1 mmHg) and GSNA (14.8 ± 1.5%) elicited by microinjections of ANG-(1–12) after microinjections of aCSF. However, HR responses to ANG-(1–12) were not significantly different between the groups in which aCSF and A-779 were used. *P < 0.05 compared with responses elicited by microinjections of ANG-(1–12) after microinjections of aCSF (all comparisons refer to corresponding time points); §P < 0.01 compared with responses elicited by microinjections of ANG-(1–12) after microinjections of aCSF; †P < 0.05 and ‡P < 0.01 compared with baseline (0-min time point).
The duration of decreases in HR elicited by microinjections of ANG-(1–12) into the CVLM, after microinjection of A-779 at the same site, appeared to be reduced compared with the duration of decreases in HR elicited by microinjections of ANG-(1–12) (0.5 mM) into the CVLM after microinjection of aCSF at the same site. However, statistical analysis showed that the two responses were not significantly (P > 0.05) different at all time points (Fig. 4B).
The duration of decreases in GSNA elicited by microinjections of ANG-(1–12) into the CVLM, after microinjection of A-779 at the same site, was reduced compared with the duration of decreases in GSNA elicited by microinjections of ANG-(1–12) into the CVLM after microinjection of aCSF at the same site. Thus, the decreases in GSNA elicited by ANG-(1–12) after microinjections of A-779 at the same site were significantly (P < 0.05) reduced at 12- to 14-min time points compared with the decreases in MAP elicited by ANG-(1–12) after microinjections of aCSF into the CVLM at the same time points (Fig. 4C).
As described above, the decreases in MAP, HR, and GSNA elicited by ANG-(1–12) during the first 1–8 min was referred to as the initial phase of responses, which were mediated via AT1Rs. The decreases in MAP, HR, and GSNA elicited by microinjections of ANG-(1–12) into the CVLM during the later phase (8–24 min) are referred to as the delayed phase in this report. The results described above showed that prior microinjections of A-779 into the CVLM reduced the durations of decreases in MAP, HR, and GSNA elicited by microinjections of ANG-(1–12) at the same site. We thus concluded that the duration of ANG-(1–12) responses was reduced by blocking the delayed phase (∼8–24 min) of ANG-(1–12) responses using A-779. The delayed phase of ANG-(1–12) responses is mediated via MasRs.
The maximal decreases in MAP, HR, and GSNA elicited by microinjections of ANG-(1–12) into the CVLM after microinjections of A-779 into the same site were not significantly (P > 0.05) different from the maximal decreases in these variables elicited by ANG-(1–12) into the CVLM after microinjections of aCSF (Fig. 4, A–C).
Effect of ACE inhibition on ANG-(1–12)-induced responses in the CVLM.
A typical tracing of the effect of ACE inhibition in the CVLM on ANG-(1–12)-induced responses is shown in Fig. 2D. A microinjection of l-Glu into the CVLM elicited decreases in HR, MAP, and GSNA (Fig. 2D,a). Five minutes later, a microinjection of captopril (100 mM) into the CVLM elicited no HR, MAP, and GSNA responses (Fig. 2D,b). After an interval of 2 min, the decreases in HR, MAP, and GSNA elicited by microinjection of ANG-(1–12) into the CVLM were blocked (Fig. 2D,b).
Group data for this experiment are shown in Fig. 5. The decreases in MAP (Fig. 5A), HR (Fig. 5B), and GSNA (Fig. 5C) elicited by microinjections of ANG-(1–12) (0.5 mM) into the CVLM after microinjections of captopril (100 mM) at the same site were blocked compared with the decreases in the same variables elicited by microinjections of ANG-(1–12) into the CVLM after control microinjections of aCSF at the same site.
The results of ACE inhibition on ANG-(1–12) responses were different from those observed with losartan; captopril blocked the entire decreases in MAP, HR, and GSNA, whereas losartan blocked only the initial phase of the decreases in these variables elicited by ANG-(1–12). Microinjections of captopril (100 mM) into the CVLM did not alter baseline MAP and HR.
Effect of chymase inhibition on ANG-(1–12)-induced responses in the CVLM.
In this experiment, the effect of chymase inhibition was studied only on the initial (maximal) responses elicited by microinjections of ANG-(1–12) into the CVLM. ANG-(1–12)-induced cardiovascular responses in the CVLM were attenuated by prior microinjections of chymostatin (1 mM) at the same site. The maximal decreases in MAP (6.2 ± 0.5 mmHg) and HR (6.2 ± 1.8 beats/min) elicited by ANG-(1–12) (0.5 mM) in this group of rats (n = 5) were significantly smaller (P < 0.01–0.05) compared with the decreases in MAP (13.3 ± 1.3 mmHg) and HR (17.4 ± 2.3 beats/min) elicited by microinjections of the same concentration of ANG-(1–12) into the CVLM after control microinjections of aCSF (n = 7). Microinjections of chymostatin (1 mM) into the CVLM elicited no changes in baseline MAP and HR.
Effect of bilateral vagotomy on ANG-(1–12)-induced responses in the CVLM.
In this experiment, the effect of bilateral vagotomy was studied only on the initial (maximal) responses elicited by microinjections of ANG-(1–12) into the CVLM. Bilateral vagotomy did not alter depressor and bradycardic responses elicited by microinjections of ANG-(1–12) (0.5 mM) into the CVLM. The maximal decreases in MAP (14.0 ± 1.5 mmHg) and HR (19.4 ± 3.6 beats/min) elicited by ANG-(1–12) in this group of rats (n = 5) were not significantly different (P > 0.05) compared with the maximal decreases in MAP (13.3 ± 1.3 mmHg) and HR (17.4 ± 2.3 beats/min) elicited by microinjections of the same concentration of ANG-(1–12) into the CVLM after control microinjections of aCSF (n = 7). Bilateral vagotomy elicited increases in baseline HR (42.0 ± 4.2 beats/min) but no significant changes in MAP.
Effect of GABA receptor blockade in the RVLM on ANG-(1–12)-induced responses in the CVLM.
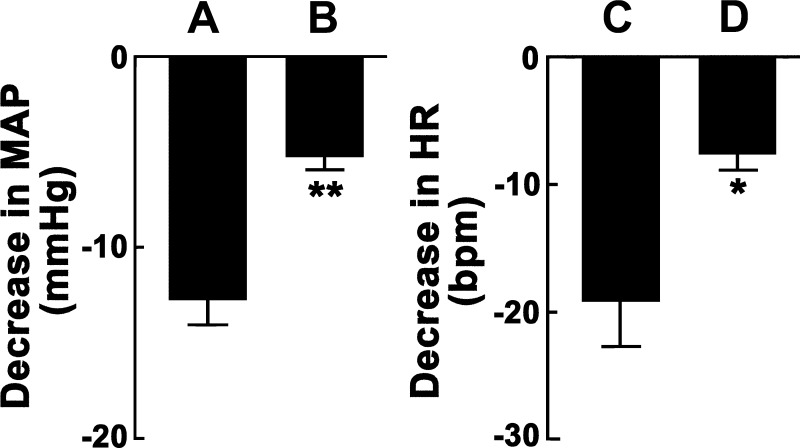
In this experiment, the effect of blockade of GABA receptors in the RVLM was studied only on the initial (maximal) responses elicited by microinjections of ANG-(1–12) into the CVLM. The effect of GABA receptor blockade in the RVLM on ANG-(1–12)-induced responses in the ipsilateral CVLM is shown in Fig. 6. In one group of rats (n = 5), aCSF was microinjected into the RVLM, which was previously identified by microinjections of l-Glu (5 mM). Seven to ten minutes later, microinjections of ANG-(1–12) (0.5 mM) into the ipsilateral CVLM elicited decreases in MAP (Fig. 6A). The same protocol was used in another group of rats (n = 5) except that GABAA and GABAB receptors in the RVLM were blocked by combined microinjections of gabazine (2 mM) and CGP-52432 (10 mM), respectively. In this group of rats, microinjections of ANG-(1–12) (0.5 mM) into the ipsilateral CVLM elicited significantly smaller (P < 0.01) decreases in MAP (Fig. 6B) compared with the decreases in MAP observed when aCSF was microinjected into the RVLM (Fig. 6A). Comparison of HR responses showed that blockade of GABA receptors in the RVLM significantly attenuated the decreases in HR elicited by microinjections of ANG-(1–12) into the ipsilateral CVLM; microinjections of ANG-(1–12) (0.5 mM) into the CVLM elicited significantly smaller (P < 0.05) decreases in HR (Fig. 6D) compared with the decreases in HR (Fig. 6C) observed when aCSF was microinjected into the RVLM. Blockade of GABA receptors in the RVLM elicited increases in baseline MAP (34.6 ± 2.8 mmHg) and HR (19.6 ± 4.7 beats/min), which peaked after 7–10 min.
Fig. 6.
Effect of GABA receptor blockade in the rostral ventrolateral medullary pressor area (RVLM) on ANG-(1–12)-induced cardiovascular responses elicited from the CVLM. A: microinjections of ANG-(1–12) (0.5 mM) into the CVLM, after microinjections of aCSF into the ipsilateral RVLM as a control, elicited decreases in MAP (12.8 ± 1.2 mmHg, n = 5). B: decreases in MAP (5.2 ± 0.7 mmHg) elicited by microinjections of ANG-(1–12) (0.5 mM) into the CVLM were significantly (**P < 0.01) attenuated by prior combined microinjections of gabazine (2 mM) and CGP-52432 (10 mM) into the ipsilateral RVLM (n = 5). C: microinjections of ANG-(1–12) (0.5 mM) into the CVLM, after microinjections of aCSF into the ipsilateral RVLM, elicited decreases in HR (19.2 ± 3.4 beats/min; the same group of rats as in A). D: decreases in HR (7.6 ± 1.3 beats/min) elicited by microinjections of ANG-(1–12) into the CVLM were significantly (*P < 0.05) attenuated by prior combined microinjections of gabazine and CGP-52432 into the ipsilateral RVLM (the same group of rats as in B).
Effect of microinjections of ANG-(1–12) into the CVLM on the baroreflex.
The baroreceptor reflex was tested using protocols previously published in the literature (3). Typical tracings for the changes in HR, MAP, and GSNA elicited by bolus intravenous injections of PE or SNP are shown in Fig. 7. A bolus injection of PE (7.5 μg/kg iv) elicited a pressor response and reflex decreases in HR and GSNA (Fig. 7A,a). Two and twelves minutes after bilateral microinjections of ANG-(1–12) (0.5 mM) into the CVLM, the same injections of PE elicited augmented reflex decreases in GSNA but not HR (Fig. 7A,b and c). On the other hand, a bolus injection of SNP (7.5 μg/kg iv) elicited a depressor response and reflex increases in HR and GSNA (Fig. 7B,a). Two and twelves minutes after bilateral microinjections of ANG-(1–12) into the CVLM, the same injections of SNP elicited attenuated increases in GSNA but not HR (Fig. 7B,b and c). The 2-min interval after microinjections of ANG-(1–12) was selected to test baroreflex responses to intravenous PE and SNP because the effects of ANG-(1–12) mediated through AT1Rs were maximal at this time interval (see Fig. 3A). The 12-min time interval after microinjections of ANG-(1–12) was selected to test baroreflex responses to PE and SNP because the effects of ANG-(1–12) mediated through MasRs were maximal at this time interval (see Fig. 4A).
Fig. 7.
Tracings showing the effects of microinjections of ANG-(1–12) into the CVLM on the baroreflex. In A and B, the top trace shows HR (in beats/min), the middle trace shows MAP (in mmHg), and the bottom trace shows ∫GSNA (in μV/s). A: baroreceptor stimulation by phenylephrine (PE). A bolus injection of PE (7.5 μg/kg iv) elicited an increase in MAP and reflex decreases in HR and GSNA (a). The decrease in GSNA induced by PE injection was potentiated 2 min (b) and 12 min (c) after bilateral microinjections of ANG-(1–12) into the CVLM. B: baroreceptor unloading by sodium nitroprusside (SNP). A bolus injection of SNP (7.5 μg/kg iv) elicited a decrease in MAP and reflex increases in HR and GSNA (b). The increase in GSNA induced by SNP injection was attenuated 2 min (b) and 12 min (c) after bilateral microinjections of ANG-(1–12) into the CVLM.
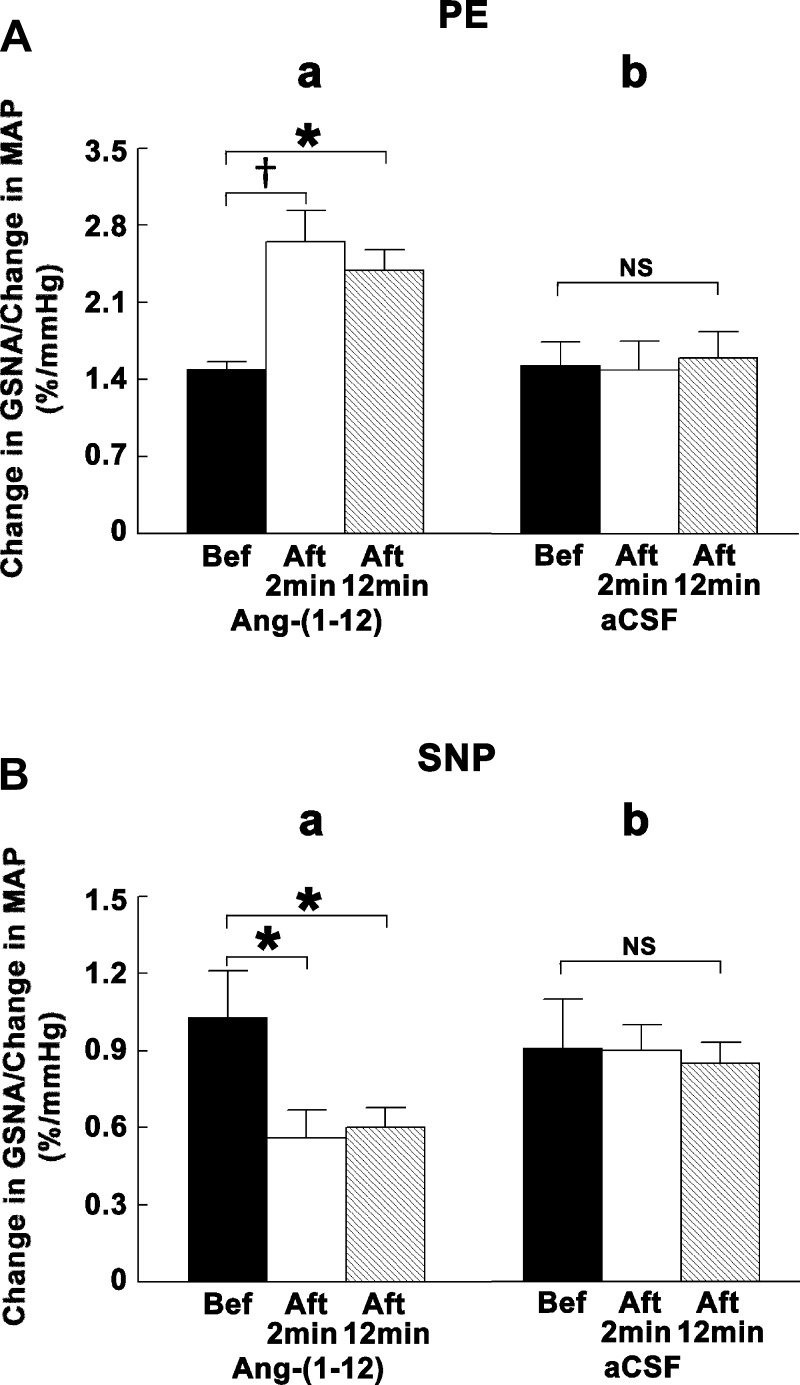
Group data for this experiment are shown in Fig. 8. The ratio of changes in GSNA to changes in MAP elicited by bolus intravenous injections of PE was significantly (P < 0.01–0.05) increased by bilateral microinjections of ANG-(1–12) into the CVLM at both 2- and 12-min time intervals after microinjections of ANG-(1–12), whereas microinjections of aCSF did not alter this ratio (Fig. 8A). On the other hand, the ratio of changes in GSNA to changes in MAP elicited by bolus intravenous injections of SNP was significantly (P < 0.05) decreased by bilateral microinjections of ANG-(1–12) into the CVLM at both 2- and 12-min time intervals after microinjections of ANG-(1–12), whereas microinjections of aCSF did not alter this ratio (Fig. 8B).
Fig. 8.
Group data showing the effects of microinjections of ANG-(1–12) into the CVLM on the baroreflex. In A and B, the solid bars show reflex GSNA responses to PE or SNP before bilateral microinjections of either ANG-(1–12) (a) or aCSF (b) into the CVLM, open bars show reflex GSNA responses to PE or SNP 2 min after microinjections of either ANG-(1–12) (a) or aCSF (b) into the CVLM, and hatched bars show reflex GSNA responses to PE or SNP 12 min after microinjections of either ANG-(1–12) (a) or aCSF (b) into the CVLM. A: baroreceptor stimulation by PE (7.5 μg/kg iv). a: Bilateral microinjections of ANG-(1–12) (0.5 mM) into the CVLM significantly potentiated reflex GSNA responses (n = 5). The ratios of changes in GSNA to changes in MAP elicited by PE injections before, 2 min after, and 12 min after microinjections of ANG-(1–12) into the CVLM were 1.50 ± 0.06, 2.65 ± 0.28, and 2.39 ± 0.18%/mmHg, respectively. b: Bilateral microinjections of aCSF (100 nl) into the CVLM did not alter reflex GSNA responses (n = 4). The same ratios elicited by PE injections before, 2 min after, and 12 min after microinjections of aCSF into the CVLM were 1.53 ± 0.21, 1.49 ± 0.26, and 1.60 ± 0.24%/mmHg, respectively. B: baroreceptor unloading by SNP (7.5 μg/kg iv). a: Bilateral microinjections of ANG-(1–12) into the CVLM significantly attenuated reflex GSNA responses (n = 5). The same ratios elicited by SNP injections before, 2 min after, and 12 min after microinjections of ANG-(1–12) into the CVLM were 1.03 ± 0.18, 0.56 ± 0.11, and 0.60 ± 0.08%/mmHg, respectively. b: Bilateral microinjections of aCSF into the CVLM did not alter reflex GSNA responses (n = 4). The same ratios elicited by SNP injections before, 2 min after, and 12 min after microinjections of aCSF into the CVLM were 0.91 ± 0.19, 0.90 ± 0.10, and 0.85 ± 0.08%/mmHg, respectively. *P < 0.05; †P < 0.01.
Bilateral microinjections of ANG-(1–12) into the CVLM did not alter HR responses to the baroreflex; the ratio of changes in PI (derived from HR) to changes in MAP elicited by bolus intravenous injections of either PE or SNP was not altered (P > 0.05) by microinjections of ANG-(1–12) into the CVLM (n = 5 for each injection). The ratios of changes in PI to changes in MAP elicited by PE injections were 0.35 ± 0.03, 0.38 ± 0.04, and 0.36 ± 0.04 ms/mmHg before, 2 min after, and 12 min after microinjections of ANG-(1–12) into the CVLM, respectively. The same ratios elicited by SNP injections were 0.30 ± 0.04, 0.32 ± 0.07, and 0.30 ± 0.06 ms/mmHg before, 2 min after, and 12 min after microinjections of ANG-(1–12) into the CVLM, respectively.
Histological identification of microinjection sites in the CVLM and RVLM.
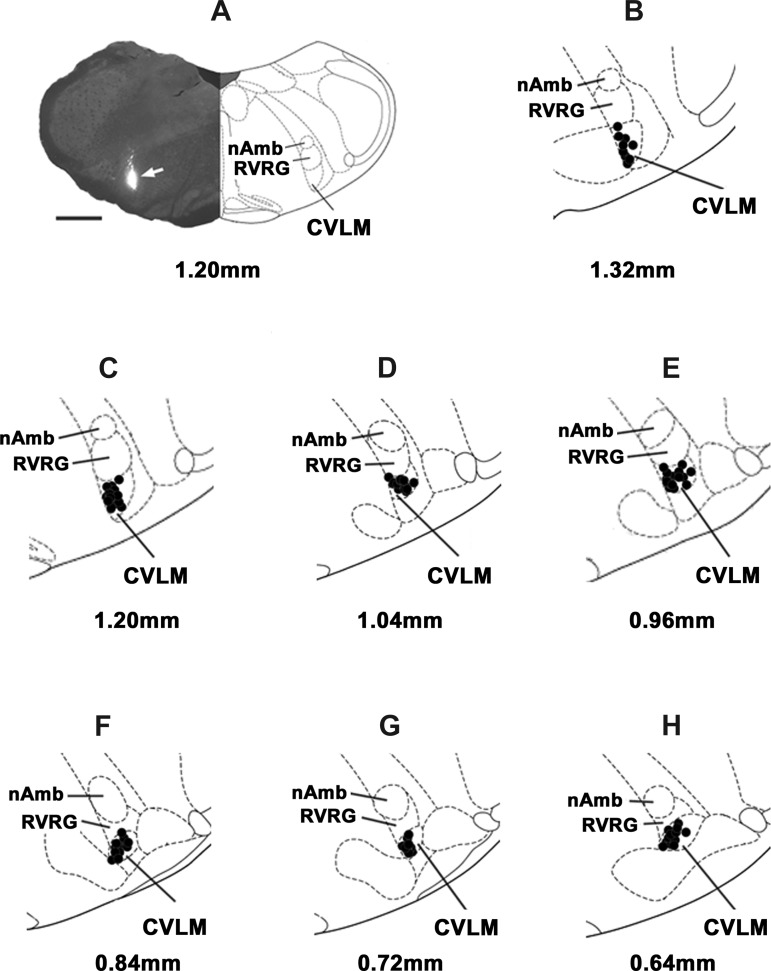
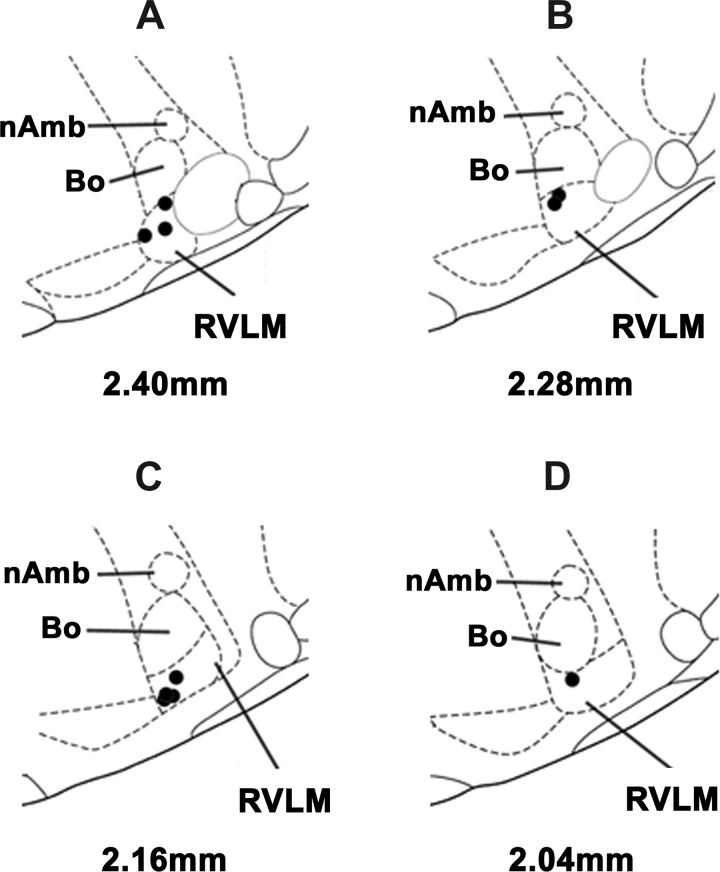
A typical CVLM site, marked with green retrobeads IX (1:50), is shown in Fig. 9A (arrow). Figure 9, B–H, shows composite diagrams of the CVLM microinjection sites, which were located 0.64–1.32 mm rostral to the calamus scriptorius, 1.9–2.3 mm lateral to the midline, and 2.8–3.2 mm deep from the dorsal medullary surface. Figure 10, A–D, shows composite diagrams of the RVLM microinjection sites, which were located 2.04–2.40 mm rostral to the calamus scriptorius, 2.0–2.3 mm lateral to the midline, and 3.0–3.3 mm deep from the dorsal medullary surface.
Fig. 9.
Histological identification of microinjection sites in the CVLM. A: a typical microinjection site (arrow) in the CVLM marked with green retrobeads IX (100 nl). The site was located at a level 1.20 mm rostral to the calamus scriptorius. Scale bar = 1 mm. B–H: composite diagrams of CVLM sections at levels 1.32–0.64 mm rostral to the CS. In the diagrams showing microinjection sites, the solid circle represents the center of one microinjection site in one animal. However, some of the microinjection sites overlapped. nAmb, nucleus ambiguus; RVRG, rostral ventral respiratory group.
Fig. 10.
Histological identification of microinjection sites in the RVLM. A–D: composite diagrams of RVLM sections at levels 2.40–2.04 mm rostral to the calamus scriptorius. Bo, Botzinger complex.
DISCUSSION
The major findings of the study are as follows: 1) microinjections of ANG-(1–12) into the CVLM elicited decreases in MAP, HR, and GSNA; 2) the initial phase of these responses was mediated via AT1Rs but not AT2Rs; 3) the delayed phase of these responses was mediated via MasRs; 4) entire ANG-(1–12)-induced responses were blocked by an ACE inhibitor; 5) chymase partly mediated ANG-(1–12)-induced responses; 6) ANG-(1–12) responses elicited from the CVLM were partly mediated via GABA receptors in the RVLM; and 7) administration of ANG-(1–12) into the CVLM modulated the sympathetic baroreflex response.
Distortion of brain tissue at the microinjection site or any other nonspecific effects were not responsible for the cardiovascular responses elicited by ANG-(1–12) in the CVLM because microinjections of aCSF at the same site did not elicit any cardiovascular responses.
The duration of the decreases in MAP elicited by microinjections of ANG-(1–12) (23–24 min for the 0.5 mM concentration) into the CVLM in our study was greater than the duration of decreases in MAP reported for microinjections of ANG II (∼5 min for 0.4 mM) into the same brain area (2). Because many actions of ANG-(1–12) have been reported to be mediated via ANG II (6, 29), the durations of actions of the two ANGs are expected to be similar. This discrepancy can be explained as follows: ANG-(1–12) microinjected into the CVLM can be converted to ANG II as well as ANG-(1–7) (41). Microinjections of ANG II into the CVLM have been reported to elicit a decrease in MAP (2). In our study, ANG-(1–12) microinjected into the CVLM elicited decreases in MAP, HR, and GSNA. The time course of ANG-(1–12) responses showed two phases: an initial phase (the first 1–8 min) and a delayed phase (8–24 min). The delayed phase of ANG-(1–12) effects was blocked by prior microinjections of A-779 (MasR antagonist) at the same site, whereas the initial phase was abolished by prior microinjections of losartan (AT1R antagonist) at the same site. Based on these results, we concluded that the initial phase of ANG-(1–12)-induced responses was mediated via the formation of ANG II from ANG-(1–12), whereas the delayed phase was mediated via the formation of ANG-(1–7) from ANG-(1–12). Thus, we concluded that the longer duration of action of ANG-(1–12), compared with ANG II effects, can be ascribed to the formation of ANG-(1–7) from ANG-(1–12), which may be relatively delayed because of the requirement of several additional metabolic steps (41). In our study, the delayed phase of ANG-(1–12) effects was revealed after the blockade of AT1Rs by losartan. In these experiments, the onset of the delayed phase of ANG-(1–12) responses was not due to fading of the losartan effect because in separate experiments we observed that the blockade effect of losartan at AT1Rs lasted for at least 30 min.
Several pathways have been implicated in the metabolism of ANG-(1–12) (41). Based on our results using captopril, we concluded that ACE is the key enzyme involved in the metabolism of ANG-(1–12) in the CVLM. Chymase is also involved in the metabolism of ANG-(1–12) because chymostatin attenuated the responses to ANG-(1–12) in the CVLM.
The decreases in MAP, HR, and GSNA elicited by microinjections of ANG-(1–12) into the CVLM may be mediated via GABAergic CVLM neurons projecting to the RVLM because the cardiovascular responses elicited by ANG-(1–12) were attenuated by blockade of GABAA and GABAB receptors in the RVLM. We (10) have previously shown that direct microapplication of ANG-(1–12) to single NTS neurons results in an increase in their extracellular firing rate, which was blocked by prior microapplication of either ACE and chymase inhibitors or an AT1R antagonist. ANG-(1–12) may have similar actions on CVLM neurons. The conversion of ANG-(1–12) to ANG II may result in the stimulation of GABAergic CVLM neurons via AT1Rs. Activation of GABAergic projections from the CVLM to the RVLM may decrease the activity of presympathetic RVLM neurons, causing a decrease in the activity of sympathetic preganglionic neurons in the intermediolateral cell column. Consequently, there is a decrease in the sympathetic outflow, resulting in decreases in MAP and HR (17, 37). Bradycardic responses elicited by microinjections of ANG-(1–12) into the CVLM were not mediated via the excitation of vagal nerves because these responses were not altered by vagotomy.
The medullary circuits involved in the baroreflex are well established (17, 37). Based on this scheme, our results regarding the effect of ANG-(1–12) on baroreflex responses can be explained as follows. The increase in MAP, elicited by intravenous injections of PE, increased the activity of baroreceptor afferents, which, in turn, resulted in the release of glutamate in the NTS, causing increased activity of second-order neurons in the medial subnucleus of the NTS (mNTS). Activation of mNTS neuronal projections resulted in the release of glutamate in the CVLM, causing the activation of GABAergic neurons. Activation of GABAergic CVLM neurons resulted in the release GABA in the RVLM and a decrease in the activity of presympathetic neurons located in the RVLM, causing a decrease in SNA (GSNA in our study). Microinjections of ANG-(1–12) into the CVLM increased the excitability of neurons in this nucleus (10, 31). The ANG-(1–12)-induced increased excitability of CVLM neurons resulted in exaggerated decreases in GSNA in response to baroreceptor activation by intravenous injections of PE. On the other hand, the decrease in MAP, elicited by intravenous injections of SNP, decreased the activity of baroreceptor afferents, which, in turn, resulted in decreased release of glutamate in the NTS, causing a decrease in the activity of second-order mNTS neurons. Decreased activity of mNTS neurons resulted in decreased excitatory input to CVLM neurons and the release of GABA in the RVLM was decreased, causing disinhibition of presympathetic RVLM neurons. Consequently, there was an increase in GSNA. As described aboved, microinjections of ANG-(1–12) into the CVLM increased the excitability of CVLM neurons. Therefore, the reduction of CVLM neuronal activity in response to decreased excitatory input from the baroreceptor afferents and mNTS neurons, caused by decreased MAP induced by intravenous SNP injections, was attenuated. As described above, the reduction in the activity of CVLM neurons caused a reflex increase in the activity of RVLM presympathetic neurons. When ANG-(1–12) microinjections in the CVLM attenuated the reduction of CVLM GABAergic neuronal activity induced by intravenous injections of SNP, reflex activation of RVLM neurons was reduced and GSNA responses were attenuated.
Perspectives
In the CVLM, ANG II or AT1Rs have been reported to play a role in diverse physiological or pathophysiological situations. For example, in the CVLM, ANG II and AT1Rs mediate depressor and bradycardic responses (2, 8, 9, 27, 38), inhibition of the baroreflex (3, 32, 35, 39), stress responses (13, 32), and vasopressin release (1). In this report, we showed that microinjections of ANG-(1–12) elicit cardiovascular responses, which are mediated via its conversion to ANG II and ANG-(1–7). However, it has been suggested that ANG-(1–12) may be endogenously formed via a renin-independent pathway (40). There is an increase in the activity of the brain renin-angiotensin system in spontaneously hypertensive rats, salt-deprived rats, or rats with heart failure (12, 47). It is possible that brain levels of ANG-(1–12) are also increased in these pathological situations. It will be possible to design studies in which the physiological or pathophysiological role of ANG-(1–12) can be studied when the enzyme that forms ANG-(1–12) from angiotensinogen is identified. Information regarding the role of ANG-(1–12) in various physiological and pathophysiological functions is still evolving. Our results in this study provide a platform on which further studies on the role of ANG-(1–12) in the CVLM in diverse physiological and pathophysiological functions can be investigated.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants HL-024347 and HL-076248 (to H. N. Sapru).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.K., K.K., and H.N.S. conception and design of research; T.K. and K.K. performed experiments; T.K., K.K., and H.N.S. analyzed data; T.K., K.K., and H.N.S. interpreted results of experiments; T.K. and K.K. prepared figures; T.K., K.K., and H.N.S. drafted manuscript; T.K. and H.N.S. edited and revised manuscript; H.N.S. approved final version of manuscript.
REFERENCES
- 1.Allen AM, Mendelsohn FAO, Gieroba ZJ, Blessing WW. Vasopressin release following microinjection of angiotensin II into the caudal ventrolateral medulla oblongata in the anaesthetized rabbit. J Neuroendocrinol 2: 867–873, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Alzamora AC, Santos RA, Campagnole-Santos MJ. Hypotensive effect of ANG II and ANG-(1–7) at the caudal ventrolateral medulla involves different mechanisms. Am J Physiol Regul Integr Comp Physiol 283: R1187–R1195, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Alzamora AC, Santos RA, Campagnole-Santos MJ. Baroreflex modulation by angiotensins at the rat rostral and caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 290: R1027–R1034, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Arakawa H, Chitravanshi VC, Sapru HN. The hypothalamic arcuate nucleus: a new site of cardiovascular action of angiotensin-(1–12) and angiotensin II. Am J Physiol Heart Circ Physiol 300: H951–H960, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arakawa H, Kawabe K, Sapru HN. Angiotensin-(1–12) in the rostral ventrolateral medullary pressor area of the rat elicits sympathoexcitatory responses. Exp Physiol 98: 94–108, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold AC, Isa K, Shaltout HA, Nautiyal M, Ferrario CM, Chappell MC, Diz DI. Angiotensin-(1–12) requires angiotensin converting enzyme and AT1 receptors for cardiovascular actions within the solitary tract nucleus. Am J Physiol Heart Circ Physiol 299: H763–H771, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blankley CJ, Hodges JC, Klutchko SR, Himmelsbach RJ, Chucholowski A, Connolly CJ, Neergaard SJ, Van Nieuwenhze MS, Sebastian A, Quin J, 3rd, Essenberg AD, Cohen DM. Synthesis and structure-activity relationships of a novel series of non-peptide angiotensin II receptor binding inhibitors specific for the AT2 subtype. J Med Chem 34: 3248–3260, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Bourassa EA, Sved AF, Speth RC. Anteroposterior distribution of AT1 angiotensin receptors in caudal brainstem cardiovascular regulatory centers of the rat. Brain Res 1306: 69–76, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chai CY, Chen SY, Lin AMY, Tseng CJ. Angiotensin II activates pressor and depressor sites of the pontomedulla that react to glutamate. Clin Exp Pharmacol Physiol 23: 415–423, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Chitravanshi VC, Sapru HN. Cardiovascular responses elicited by a new endogenous angiotensin in the nucleus tractus solitarius of the rat. Am J Physiol Heart Circ Physiol 300: H230–H240, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chitravanshi VC, Proddutur A, Sapru HN. Cardiovascular actions of angiotensin-(1–12) in the hypothalamic paraventricular nucleus of the rat are mediated via angiotensin II. Exp Physiol 97: 1001–1017, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dampney RAL, Horiuchi J, Tagawa T, Fontes MA, Potts PD, Polson JW. Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol Scand 177: 209–218, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Davern PJ, Chen D, Head GA, Chavez CA, Walther T, Mayorov DN. Role of angiotensin II type 1A receptors in cardiovascular reactivity and neuronal activation after aversive stress in mice. Hypertension 54: 1262–1268, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Diz DI, Garcia-Espinosa MA, Gallagher PE PE, Ganten D, Ferrario CM, Averill DB. Angiotensin-(1–7) and baroreflex function in nucleus tractus solitarii of (MRen2)27 transgenic rats. Cardiovasc Pharmacol 51: 542–548, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrario CM, Varagic J, Habibi J, Nagata S, Kato J, Chappell MC, Trask AJ, Kitamura K, Whaley-Connell A, Sowers JR. Differential regulation of angiotensin-(1–12) in plasma and cardiac tissue in response to bilateral nephrectomy. Am J Physiol Heart Circ Physiol 296: H1184–H1192, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon FJ, Sved AF. Neurotransmitters in central cardiovascular regulation: glutamate and GABA. Clin Exp Pharmacol Physiol 29: 522–524, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Hamann M, Desarmenien M, Desaulles E, Bader MF, Feltz P. Quantitative evaluation of the properties of a pyridazinyl GABA derivative (SR 95531) as a GABAA competitive antagonist, an electrophysiological approach. Brain Res 442: 287–296, 1998 [DOI] [PubMed] [Google Scholar]
- 19.He S, Gaca MD, McEuen AR, Walls AF. Inhibitors of chymase as mast cell-stabilizing agents: contribution of chymase in the activation of human mast cells. J Pharmacol Exp Ther 291: 517–523, 1999 [PubMed] [Google Scholar]
- 20.Kasamatsu K, Chitravanshi VC, Sapru HN. Depressor and bradycardic responses to microinjections of endomorphin-2 into the nucleus tractus solitarius are mediated via ionotropic glutamate receptors. Am J Physiol Regul Integr Comp Physiol 287: R715–R728, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Kawabe T, Chitravanshi VC, Kawabe K, Sapru HN. Cardiovascular function of a glutamatergic projection from the hypothalamic paraventricular nucleus to the nucleus tractus solitarius in the rat. Neuroscience 153: 605–617, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawabe T, Kawabe K, Sapru HN. Cardiovascular responses to chemical stimulation of the hypothalamic arcuate nucleus in the rat: role of the hypothalamic paraventricular nucleus. PLos One 7: e45180, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawabe T, Kawabe K, Sapru HN. Tonic γ-aminobutyric acid-ergic activity in the hypothalamic arcuate nucleus is attenuated in the spontaneously hypertensive rat. Hypertension 62: 281–287, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanza M, Fassio A, Gemignani A, Bonanno G, Raiteri M. CGP 52432: A novel potent and selective GABAB autoreceptor antagonist in rat cerebral cortex. Eur J Pharmacol 237: 191–195, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Machado BH. Neurotransmission of the cardiovascular reflexes in the nucleus tractus solitarii of awake rats. Ann NY Acad Sci 940: 179–196, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Migdalof BH, Antonaccio MJ, McKinstry DN, Singhvi SM, Lan SJ, Egli P, Kripalani KJ. Captopril: pharmacology, metabolism and disposition. Drug Metab Rev 15: 841–869, 1984 [DOI] [PubMed] [Google Scholar]
- 27.Muratani H, Averill DB, Ferrario CM. Effect of angiotensin II in ventrolateral medulla of spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol 260: R977–R984, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Murugaian J, Sundaram K, Krieger AJ, Sapru HN. Electrolytic lesions in the ventrolateral medullary depressor area abolish depressor responses to the electrical stimulation of the aortic nerve. Brain Res 499: 371–377, 1989 [DOI] [PubMed] [Google Scholar]
- 29.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun 350: 1026–1031, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T, Bhatt S, Sapru HN. Cardiovascular responses to hypothalamic arcuate nucleus stimulation in the rat: role of sympathetic and vagal efferents. Hypertension 54: 1369–1375, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Oshima N, Kumagai H, Iigaya K, Onimaru H, Kawai A, Nishida Y, Saruta T, Itoh H. Baro-excited neurons in the caudal ventrolateral medulla (CVLM) recorded using the whole-cell patch-clamp technique. Hypertens Res 35: 500–506, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Palma-Rigo K, Bassi JK, Nguyen-Huu TP, Jackson KL, Davern PJ, Chen D, Elghozi JL, Thomas WG, Allen AM, Head GA. Angiotensin 1A receptors transfected into caudal ventrolateral medulla inhibit baroreflex gain and stress responses. Cardiovasc Res 96: 330–339, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (6th ed.). London: Academic, 2007 [Google Scholar]
- 34.Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens 20: 1675–1688, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Saigusa T, Iriki M, Arita J. Brain angiotensin II tonically modulates sympathetic baroreflex in rabbit ventrolateral medulla. Am J Physiol Heart Circ Physiol 271: H1015–H1021, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Santos RA, Campagnole-Santos MJ, Baracho NC, Fontes MA, Silva LC, Neves LA, Oliveira DR, Caligiorne SM, Rodrigues AR, Gropen CARV, Jr, Carvalho WS, Silva ACSE, Khosla MC. Characterization of a new angiotensin antagonist selective for angiotensin-(1–7): evidence that the actions of angiotensin-(1–7) are mediated by specific angiotensin receptors. Brain Res Bull 35: 293–298, 1994 [DOI] [PubMed] [Google Scholar]
- 37.Sapru HN. Glutamate circuits in selected medullo-spinal areas regulating cardiovascular function. Clin Exp Pharmacol Physiol 29: 491–496, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Sasaki S, Dampney RA. Tonic cardiovascular effects of angiotensin II in the ventrolateral medulla. Hypertension 15: 274–283, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Sesoko S, Muratani H, Takishita S, Teruya H, Kawazoe N, Fukiyama K. Modulation of baroreflex function by angiotensin II endogenous to the caudal ventrolateral medulla. Brain Res 671: 38–44, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1–12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol 294: H2242–H2247, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Varagic J, Trask AJ, Jessup JA, Chappell MC, Ferrario CM. New angiotensins. J Mol Med (Berl) 86: 663–671, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang HJ, Zhang FF, Zhang Y, Gao XY, Wang W, Zhu GQ. AT1 receptor in paraventricular nucleus mediates the enhanced cardiac sympathetic afferent reflex in rats with chronic heart failure. Auton Neurosci 121: 56–63, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Willette RN, Barcas PP, Krieger AJ, Sapru HN. Vasopressor and depressor areas in the rat medulla: identification by l-glutamate microinjections. Neuropharmacology 22: 1071–1079, 1983 [DOI] [PubMed] [Google Scholar]
- 44.Willette RN, Krieger AJ, Barcas PP, Sapru HN. Medullary GABA receptors and the regulation of blood pressure in the rat. J Pharmacol Exp Ther 226: 893–899, 1983 [PubMed] [Google Scholar]
- 45.Willette RN, Barcas PP, Krieger AJ, Sapru HN. Endogenous GABAergic mechanisms in the medulla and the regulation of blood pressure. J Pharmacol Exp Ther 230: 34–39, 1984 [PubMed] [Google Scholar]
- 46.Willette RN, Punnen S, Krieger AJ, Sapru HN. Interdependence of rostral and caudal ventrolateral medullary areas in the control of blood pressure. Brain Res 321: 169–174, 1984 [DOI] [PubMed] [Google Scholar]
- 47.Wright JW, Harding JW. Regulatory role of brain angiotensins in the control of physiological and behavioral responses. Brain Res Rev 17: 227–262, 1992 [DOI] [PubMed] [Google Scholar]