Abstract
The execution and maintenance of all brain functions are dependent on a continuous flow of blood to meet the metabolic needs of the tissue. To ensure the delivery of resources required for neural processing and the maintenance of neural homeostasis, the cerebral vasculature is elaborately and extensively regulated by signaling from neurons, glia, interneurons, and perivascular nerves. Hypertension is associated with impaired neurovascular regulation of the cerebral circulation and culminates in neurodegeneration and cognitive dysfunction. Here, we review the physiological processes of neurovascular signaling in the brain and discuss mechanisms of hypertensive neurovascular dysfunction.
Keywords: astrocyte, cerebral blood flow, hypertension, neurovascular coupling, parenchymal arteriole
this article is part of a collection on Hypertension and Novel Modulators of Vascular Tone. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
The brain has a high basal metabolic rate and limited capacity to store energy. As a result, the execution and maintenance of all brain functions are dependent on a continuous flow of blood that is at all times and under all conditions sufficient to meet the metabolic needs of the tissue. The cerebral vasculature, as the infrastructure by which the brain receives the resources necessary to support neural processing and maintain the milieu necessary for neural homeostasis, is elaborately and extensively regulated by signaling from neurons, glia, interneurons, and perivascular nerves.
The brain is particularly vulnerable to hypertensive injury. Hypertension leads to neurodegeneration and cognitive disability and has been causally linked to dementia and Alzheimer's disease (23, 64). It is increasingly evident that hypertensive neurodegeneration is a consequence of the damaging effects of high blood pressure on the cerebral vasculature. Chronic and even acute exposure to elevated intravascular pressure sets in motion a number of pathological mechanisms that disrupt the neurovascular regulation of cerebral blood flow (CBF). To understand how these mechanisms interfere with neurovascular signaling in the brain, the physiological mechanisms of these processes must first be understood. In this review, we provide an overview of neurovascular signaling in the brain and discuss mechanisms of hypertensive neurovascular dysfunction.
Neurovascular Regulation of CBF: Anatomy of the Cerebral Circulation
The cerebral vasculature consists of pial arteries located at the base and on the surface of the brain and their downstream tributaries. Pial arteries branch and narrow into pial arterioles, which also run along the brain surface. Pial arteries and arterioles are innervated by nerve fibers arising from the superior cervical, sphenopalatine/otic, and trigeminal ganglia (67). At various points, pial arteries and arterioles form perpendicular branches that dive from the surface parent artery into the brain parenchymal tissue, called “parenchymal arterioles” (also referred to as “penetrating arterioles”). The point of entry of the arteriole into the brain parenchyma is surrounded by a small, cerebrospinal fluid-filled pocket called the Virchow-Robin space. Innervation by extrinsic nerve fibers terminates at the Virchow-Robin space, beyond which parenchymal arterioles are directly contacted, or “innervated,” by glial and neuronal cell types. Parenchymal arterioles are predominantly surrounded by “endfoot” terminals of astrocyte projections, but there is evidence to suggest they are also contacted directly by interneurons (24, 173). Brain capillaries are enveloped by astrocytic endfeet as well, with pericytes occasionally interposed between the endfoot and capillary wall. The neurovascular regulation of CBF occurs primarily at pial arteries and parenchymal arterioles; however, neurovascular signaling through contractile pericytes has been suggested to regulate capillary diameter as well (53, 68).
Neurovascular Regulation of Pial Artery Tone by Extrinsic Perivascular Nerves
The sympathetic nerves arising from the superior cervical ganglion that innervate pial arteries and arterioles on the brain surface modulate vascular tone through the release of the contractile substances norepinephrine (NE) and neuropeptide Y (NPY) (45, 67). Parasympathetic nerve fibers from the sphenopalatine/otic ganglia innervating these vessels release the vasodilators acetylcholine (ACh), vasoactive intestinal peptide (VIP), and nitric oxide (NO), and fibers from the trigeminal ganglion release calcitonin gene-related peptide, substance P, and pituitary adenylate-cyclase activating polypeptide, to name a few (6, 45, 67). One role of vasoconstrictor release from sympathetic nerves is to shift the range of arterial blood pressures over which CBF remains relatively constant, or “autoregulates,” to higher pressures. (45). Parasympathetically released vasodilators restore resting tone after vasoconstriction and have been implicated in pathological conditions associated with vasodilation, such as migraine (176). For a thorough review of cerebrovascular regulation by extrinsic nerves, see Hamel (67).
Neurovascular Coupling: Matching CBF to Neuronal Activity
The major function of neurovascular signaling in the brain is to precisely coordinate perfusion in space and time to match the metabolic needs of the neurons. In most, if not all, tissues, an increase in cellular metabolism is accompanied by an increase in blood flow. When this occurs, it is known as functional hyperemia, a phenomenon in the brain that has been appreciated for well over a century and is vital for neural processing and viability (116, 142). The process by which functional hyperemia occurs in the brain, referred to as “neurovascular coupling” (NVC), involves the rapid communication to the cerebral vasculature of signals arising from active neurons. Upon receipt, these signals cause vasodilation and increase local CBF to a level sufficient to support and maintain neuronal function. The vascular targets for CBF regulation in NVC are parenchymal arterioles as well as pial arteries and arterioles. A role for pericyte regulation of capillary diameter in NVC has also been proposed (53, 68); however, the evidence supporting this role is limited.
NVC is studied in vivo by quantifying vascular diameter or hemodynamic responses to the activation of neurons by a sensory or electrical stimulus. Commonly, this is done in the retina by stimulation with light and in the cerebral cortex using contralateral whisker stimulation, fore-/hindlimb stimulation, or electrical stimulation of basal forebrain afferents. Insights into the molecular mechanisms underlying NVC have also been obtained by examination of the neurovascular unit (neurons, astrocytes, and microvessels) in brain slices by observing vascular responses to electrical depolarization of neurons or localized release of putative mediators induced by photolytic cleavage of molecular cages containing the compounds. Both in vivo and brain slice approaches are often used together to exploit their respective strengths and mitigate their individual weaknesses. In vivo experiments lack control over the extracellular environment and can be confounded by changes in circulating factors and the presence of various anesthetics. Brain slice experiments permit better optical resolution and the control of the extracellular milieu without compounding systemic effects, but the blood vessels are not subjected to physiological flow or pressure. Collectively, these approaches have demonstrated that NVC is a multilayered, interactive process of extraordinary complexity that is intricately sculpted and finely tuned. Moreover, the mechanisms that mediate NVC may vary in a neuronal network-specific manner.
Astrocytes in NVC
Substantial evidence supports a role for astrocytes in NVC. Astrocytic endfeet, which wrap around parenchymal arterioles within the brain, are vasoregulatory centers that modulate vascular tone. Other projections (as many as 160,000) from astrocytes terminate at neuronal synapses, creating a “tripartite” synapse consisting of presynaptic and postsynaptic neurons and the astrocytic terminal (75). As a result of this configuration, astrocytes are positioned to monitor and modify synaptic activity. The current paradigm is that the engagement of neurons and consequent generation of action potentials result in the synaptic release of neurotransmitters that stimulate astrocytes to generate inositol 1,4,5-trisphosphate (IP3), initiating an IP3 receptor (IP3R)-dependent Ca2+ wave that propagates through the astrocyte to the perivascular endfoot (Fig. 1). It is generally thought that the astrocytic response to neuronal activity is mediated by glutamate binding to G protein-coupled group I metabotropic glutamate receptors (mGluRs), mGluR5 and (to a lesser extent) mGluR1 (62, 136, 188). This view is consistent with the fact that these isoforms signal through Gq and trigger activation of the enzyme phospholipase C (PLC)-β to hydrolyze membrane phosphoinositides and generate IP3 (1, 12, 114, 169). In a recent study (160), it was reported that mGluR5 is expressed in astrocytes from young (<2 wk postnatal) but not adult mice. The authors of this study suggested instead that the predominant isoform in adult mouse and human astrocytes is mGluR3, which negatively regulates the adenylate cyclase/cAMP/PKA pathway and has been linked to adenosine release and cGMP turnover (5, 115, 139, 182). Because mGluR3 agonists are not Gq-coupled and therefore presumably do not increase intracellular Ca2+ concentration ([Ca2+]i) in astrocytes, which experimental evidence suggests is a requirement for astrocyte-mediated vasoregulation (41, 62, 158, 159, 187), it is unlikely that mGluR3 directly mediates NVC, although it could conceivably modulate it. Ultimately, molecular evidence for developmental stage-dependent expression of mGluR5 must be weighed against clear functional evidence showing that type I mGluR agonists induce an increase in astrocyte Ca2+ and promote dilation of brain arterioles in adult mice (62, 136). Nevertheless, while the preponderance of evidence continues to support a central role for Gq-coupled mGluRs in translating neuronal activity into an elevation in astrocytic endfoot Ca2+ and for astrocytic endfoot Ca2+ in controlling vascular diameter, recently raised questions about the developmental regulation of mGluR subtype expression (160) and the relative contribution of astrocyte Ca2+ signaling to NVC (126, 164) highlight the importance of continued research in this area. Other pathways through which neurotransmitters released by active neurons might mediate NVC by stimulating astrocytic Ca2+ signaling will be discussed below in Neurons in NVC.
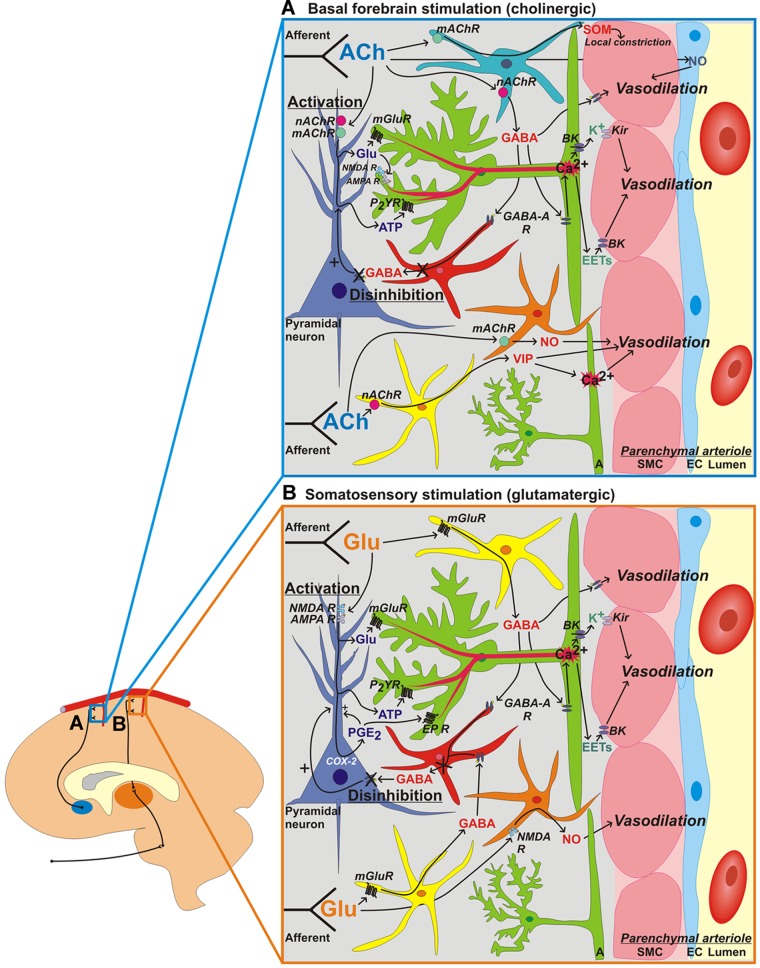
Fig. 1.
Neuronal network-specific mechanisms of neurovascular coupling. Signaling mechanisms engaged in the cortex to promote vasodilation and increase cerebral blood flow in response to activation of cholinergic basalocortical afferents via basal forebrain stimulation (A) or activation of glutamatergic thalamocortical afferents via somatosensory stimulation (B). Parenchymal arterioles are depicted at the right of each image. Blue, endothelial cell (EC); pink, smooth muscle cell (SMC); green, astrocyte (A); purple, pyramidal neuron; red, GABA inhibitory interneuron; yellow, vasoactive intestinal peptide (VIP)/choline acyltransferase interneuron; orange, nitric oxide (NO) synthase/neuropeptide Y (NPY) interneuron; turquoise, somatostatin (SOM) interneuron. Neurotransmitters released from ascending afferent neurons are shown in blue for A and orange for B. Mediators derived from pyramidal neurons are shown in purple, interneurons in red, astrocytes in green, and vascular ECs in dark blue. R, receptor; mAChR, muscarinic ACh receptor; nAChR, nicotinic ACh receptor; mGluR, metabotropic glutamate (Glu) receptor; NMDA, N-methyl-d-aspartate; AMPA, α-amino-hydroxy-5-methyl-4-isoxazolepropionic acid; BK, large-conductance Ca2+-actived K+ channel; Kir, inward rectifier K+ channel; P2YR, P2Y receptor; EET, epoxyeicosatrienoic acid; EP, PGE2 receptor; COX, cyclooxygenase.
Neuronally evoked astrocytic Ca2+ signals seem to be primarily mediated by IP3R-dependent endoplasmic reticulum (ER) Ca2+ release. In brain slices, flash photolysis of caged IP3 in astrocytic endfeet increases endfoot [Ca2+]i (158). Moreover, depletion of ER Ca2+ stores with cyclopiazonic acid greatly attenuates (by 90%) the increase in astrocytic Ca2+ induced by activating neurons with electrical field stimulation (EFS), whereas ryanodine does not, indicating that ryanodine receptor-mediated ER Ca2+ release is not involved (158). With respect to Ca2+ entry pathways, the L-type voltage-dependent Ca2+ channel (VDCC) blocker nifedipine has been shown to reduce astrocytic endfoot [Ca2+]i to EFS (55). This result likely reflects inhibition of VDCCs in cortical neurons and not in astrocytes (9, 31, 148). Although functional VDCCs have been identified in cultured and freshly isolated astrocytes from neonates (132), Carmignoto et al. (22) did not observe VDCC currents in astrocytes in situ. Moreover, the dramatic reduction in activity-induced astrocyte Ca2+ signals after ER Ca2+ store depletion with cyclopiazonic acid argues against a significant contribution of astrocytic VDCCs. Ca2+ entry into astrocytic endfeet does seem to be involved in NVC, as recent evidence from our laboratory indicates that Ca2+ entry through transient receptor potential vanilloid 4 (TRPV4) channels contributes to the astrocytic endfoot Ca2+ increase to neuronal activation (41).
Neuronal depolarization by EFS in brain slices or somatosensory stimulation in vivo typically generates a single, relatively sustained elevation in astrocyte [Ca2+]i that gradually decays (55, 158, 177). In contrast, Pasti et al. (133) reported that pharmacological stimulation of mGluRs with 1-aminocyclopentane-1,3-dicarboxylic acid (t-ACPD) induced oscillatory Ca2+ signals in astrocytes in brain slices; however, higher concentrations of t-ACPD elicited a single rise in astrocyte [Ca2+]i with characteristics similar to those seen with EFS in slices and in vivo. Notably, in vivo two-photon imaging demonstrated that the rise in astrocyte [Ca2+]i during somatosensory activation decays and returns to baseline before termination of the stimulus (177). The implications of this observation are not clear, but it could be indicative of Ca2+-dependent inhibition of IP3Rs or some form of negative feedback from astrocytes to neurons. The astrocytic Ca2+ response to neural activation is strongly correlated with neuronal activity and is dependent on stimulus frequency. In the somatosensory cortex in vivo, both neuronal activity (measured by local field potential recording) and astrocyte [Ca2+]i exhibit a bimodal dependence on stimulus frequency, increasing to a peak response and then declining with further increases in stimulus frequency (177).
A rise in endfoot [Ca2+]i is critical for astrocyte-dependent vasodilation (159). Yet, the relative contribution of astrocyte-mediated, Ca2+-dependent signaling to the overall hyperemic response to neural activation is an area of ongoing controversy. Using a mouse knockout model of the IP3R believed to be the primary subtype in astrocytes (IP3R2), two very recent reports by Nizar et al. (126) and Takata et al. (164) suggested that astrocytic IP3Rs and Ca2+ signaling are not required for full elaboration of neuronal activity-induced vasodilation in the mouse cortex. However, another recent report (72) using the same knockout model provided compelling evidence showing that evoked vasodilation is lost. It is noteworthy that in all of these studies, Ca2+ signaling was examined in the astrocyte soma and not in the astrocytic processes. Data derived largely from knockout mouse strains should be considered cautiously due to possible compensation during development. Indeed, it is difficult to imagine that any major cell type, including astroctyes, could function without IP3R-dependent Ca2+ signaling. Although IP3R2 is the major isoform in astrocytes, other isoforms have been reported (e.g., IP3R1) (83). Moreover, in the case of Nizar et al., the results were based largely on the use of a Ca2+ indicator with a Kd of 3 μM, which is roughly an order of magnitude greater than previously published estimates of activity-induced increases in astrocytic [Ca2+]i. In any case, endfoot-restricted increases in [Ca2+]i produced by photolysis of caged Ca2+ evoke arteriolar dilation and increase CBF (62, 163), and there is substantial experimental evidence showing that neuronal activity initiates an increase in astrocytic [Ca2+]i that, upon reaching the endfoot, activates Ca2+-dependent pathways to release vasoactive substances that elicit vasodilation. The exact complement of vasoactive substances released from astrocytes to modulate vascular tone and local CBF has not been fully clarified; however, some key mediators have emerged.
One of the first proposed (and still widely touted) astrocyte-derived vasoactive mediators of NVC is PGE2. Early evidence for a role for PGE2 in NVC was provided by a report (49) showing that PGE2 dilates cerebral arteries in vivo. Consistent with this supposition, a subsequent study (188) reported that glutamate stimulates Ca2+-dependent PGE2 release in cultured astrocytes. Nonselective inhibition of cyclooxygenase (COX)-1 and COX-2 by indomethacin attenuates the increase in CBF to electrical hindpaw and whisker stimulation (7, 93); selective inhibition of COX-1 with SC-560 prevents the increase in CBF to endfoot Ca2+ uncaging (163); and both SC-560 and the COX-2 inhibitor NS-398 attenuate the CBF response to whisker stimulation (93, 98, 125, 187). However, recent evidence suggests that COX metabolism in neurons, not astrocytes, is responsible for prostanoid-mediated effects during NVC in the somatosensory cortex (93). Moreover, single cell RT-PCR experiments found mRNA for COX-1 in only 10% of astrocytes examined in the rat whisker barrel cortex and did not detect COX-2 at all (93). In contrast, COX-2 is expressed constitutively in populations of pyramidal neurons within the cortex that are engaged during NVC (14, 93, 185). Importantly, Dabertrand et al. (32) recently demonstrated that isolated cortical parenchymal arterioles with myogenic tone do not dilate, but instead constrict, to PGE2. Reports (54, 109) of increased CBF to PGE2 in vivo as well as observed effects of COX inhibition on NVC may reflect an effect of PGE2 on neurons or astrocytes, both of which express PGE2 (EP) receptors. Indeed, PGE2 has been shown to stimulate intracellular Ca2+ release in cultured hippocampal astrocytes (147). It should also be noted that studies identifying a COX-dependent effect on NVC were modeled using somatosensory stimuli, in which the stimulus is communicated to the somatosensory cortex by ascending glutamatergic afferents. However, in the case of basal forebrain stimulation, which involves cortical activation by incoming cholinergic afferents, or light stimulation in the retina, which involves purinergic afferents, COX inhibition has no effect on NVC (92, 113). These observations support the concept that neuronal COX contributes to NVC and does so in a network-specific manner. An alternate explanation is that PGI2, rather than PGE2, released from neurons or possibly the endothelium may be responsible for COX-dependent effects on NVC, as PGI2 robustly dilates parenchymal arterioles (32). These results convincingly demonstrate that, while neuronally released PGE2 may be involved in NVC at some level, PGE2 is not an astrocyte-derived mediator of parenchymal arteriolar dilation during NVC.
Other mediators likely to be released by astrocytes during NVC are epoxyeicosatrienoic acids (EETs; Fig. 1). EETs are generated by cytochrome P-450 epoxygenase metabolism of arachidonic acid. The rise in endfoot [Ca2+]i to neuronal activity stimulates the Ca2+-dependent form of phospholipase A2 (PLA2) to hydrolyze membrane phospholipids and release arachidonic acid. Cultured astrocytes express cytochrome P-450 2C11 epoxygenase and have been shown to produce arachidonic acid and EETs in response to glutamate (2, 154). Parenchymal arterioles reportedly do not dilate to mGluR stimulation in cortical brain slices treated with the PLA2 inhibitor arachidonyl trifluoromethyl ketone (69). Similarly, parenchymal arterioles in brain slices from mice lacking the gene for cytosolic PLA2-α are insensitive to mGluR stimulation (72). However, PLA2 inhibition may have a direct effect on parenchymal arterioles; methyl arachidonyl fluorophosphonate has been shown to dilate arterioles in brain slices, thereby decreasing vasodilatory capacity (41). With the lack of a conditional and tissue-specific knockout model, it is difficult to determine the precise role of astrocytic endfoot PLA2 in NVC. Selective inhibition of EET formation attenuates parenchymal arteriolar dilation to mGluR stimulation and suppresses the increase in CBF elicited by glutamate, the mGluR agonist (S)-3,5-dihydroxyphenylglycine, whisker stimulation, and electrical stimulation of basal forebrain afferents (2, 13, 92, 99, 151). Pial arteries have been reported to dilate to 5,6-EET, 8,9-EET, and 11,12-EET regioisomers through activation of large-conductance Ca2+-sensitive K+ (BKCa) channels in arterial smooth muscle cells (SMCs) (60). It has also been reported that 11,12-EET dilates pial arteries through activation of TRPV4 channels in vascular SMCs (42, 43), and 5,6-EET-mediated dilation involves COX-mediated formation of dilatory oxygen radicals (48). However, an examination of the effect of EETs on parenchymal arterioles with physiological myogenic tone is lacking; thus, EETs remain to be validated as mediators of astrocyte-dependent arteriolar dilation in NVC.
Another astrocyte-derived mediator of NVC is K+ (Fig. 1). The increase in astrocytic endfoot [Ca2+]i that accompanies neuronal activity increases the open probability of endfoot BKCa channels, resulting in the release of K+ into the spatially restricted perivascular cleft between the endfoot and parenchymal arteriolar smooth muscle (56). An elevation of external K+ concentration ([K+]o) from the physiological concentration of 3 mM to 8–15 mM causes a rapid and profound dilation of parenchymal arterioles that can be prevented by inhibition of strong inward rectifier K+ (Kir) channels (56, 62). This moderate local increase in [K+]o in the perivascular space activates Kir channels on arteriolar SMCs to cause SMC hyperpolarization, deactivation of VDCCs, and relaxation (56). The elevation in [K+]o and membrane potential hyperpolarization activate Kir channels by driving internal blocking polyamines from the channel pore [for reviews, see Quayle et al (122) and Nelson and Quayle (140)]. The ensuing increase in K+ conductance moves the smooth muscle membrane potential in parenchymal arterioles (−44 mV at 40 mmHg) to the predicted K+ equilibrium potential for 8 mM [K+]o (−76 mV) (56). Inhibition of BKCa channels or Kir channels attenuates arteriolar dilation to EFS-induced neuronal depolarization in brain slices and reduces the increase in CBF to whisker stimulation in vivo (56, 61, 62). Importantly, the effects of BKCa and Kir channel inhibitors are not additive, indicating that these elements act in series (56, 62). Moreover, electrical stimulation in brain slices failed to suppress SMC Ca2+ in parenchymal arterioles from mice lacking the BKCa channel α1-subunit (Kcnma−/−), despite a normal increase in astrocytic endfoot [Ca2+]i (56).
It has very recently been suggested that astrocytes release d-serine and glutamate during NVC, stimulating endothelial NO synthase (eNOS)-dependent dilation of parenchymal arterioles (155). Photolysis of caged Ca2+ and stimulation with t-ACPD in cortical astrocytes in brain slices stimulates the astrocytic release of d-serine and glutamate, reportedly dilating parenchymal arterioles through eNOS-dependent suppression of the synthesis of the vasoconstrictor fatty acid 20-HETE. However, these interesting results require further validation, since experiments were performed in young mice (2–3 wk old), arterioles were not preconstricted and therefore had little to no resting tone, and the magnitude and time course of dilations were much smaller and longer, respectively, than previously reported responses to Ca2+ uncaging and t-ACPD stimulation in brain slices and, most importantly, in vivo (62, 163).
The recent controversy regarding mGluR subtype expression and Ca2+ signaling notwithstanding, astrocytes clearly play a role in NVC in that neuronal activity triggers Ca2+ signals in astrocytic processes that have been demonstrated to stimulate the release of EETs and K+, and possibly other mediators, that dilate parenchymal arterioles and increase local CBF.
The Glia Limitans in NVC
NVC does not occur solely at the level of parenchymal arterioles. To sustain elevations in local CBF during neuronal activation, upstream arterial segments must also dilate. The glia limitans is a specialized population of astrocytes that line the pial surface of the brain and signal to pial arteries and arterioles to promote dilation of these larger-caliber vessels upstream of parenchymal arterioles during NVC. Much as astrocytic endfeet are positioned between neurons and parenchymal arterioles, the glia limitans is positioned between cortical neurons and pial arterioles, allowing the glia limitans to communicate signals arising from neuronal populations to the pial circulation.
Neurovascular signaling through the glia limitans to the pial vasculature appears to involve mechanisms that are both similar to and distinct from those that couple astrocytes to parenchymal arterioles. Neuronal activation produces a rapid dilation of local pial arterioles that can be prevented by selective disruption of the glia limitans by the gliotoxin l-α-aminoadipic acid (l-AAA) (124, 183, 184). As is the case for astrocytes surrounding parenchymal arterioles, astrocytes of the glia limitans have been reported to dilate pial arterioles through astrocytic BKCa channels and smooth muscle Kir channels acting in series. Pial arteriolar dilation in response to in vivo sciatic nerve stimulation (SNS) is nonadditively reduced by ∼60% after local inhibition of BKCa channels with paxilline and Kir channels with Ba2+ (130). The mechanism of engagement of BKCa channels is presumably through an elevation in [Ca2+]i, but Ca2+ imaging studies in glia limitans during NVC in vivo or in brain slices are notably lacking.
Inhibition of adenosine receptors has been reported to attenuate the increase in cortical CBF to whisker stimulation by 39% (38) and blunt the increase in cerebellar blood flow to parallel fiber stimulation by 45% (96). Whereas dilation of parenchymal arterioles to astrocytic Ca2+ uncaging in vivo is not sensitive to inhibition of adenosine A1 and A2 receptors (163), pial arteriolar dilation to SNS is reduced by about half by inhibition of A2 receptors (130), suggesting that adenosine-mediated hyperemia during NVC occurs at the level of pial arterioles. This attenuation of pial arteriolar dilation to SNS via adenosine A2 receptors is not further augmented with BKCa or Kir channel inhibition, suggesting an interaction or convergence between adenosine signaling and the BKCa-Kir pathway in this response. It has been suggested that this interaction may occur through adenosine A2 receptor-mediated K+ channel phosphorylation via PKA (130), but this has not been confirmed. Adenosine strongly dilates pial arteries through direct activation of A2 receptors on SMCs (44, 87). Adenosine A2 receptor-dependent pial arteriolar dilation during NVC appears to be primarily mediated by AMP and adenosine generated by the enzymatic conversion of released ATP (174). Although active neurons release ATP, the source of ATP that contributes to pial arteriole dilation during NVC is likely glia limitans astrocytes, since dilation of pial arterioles to SNS is almost entirely prevented by l-AAA, and astrocytes readily release ATP in response to an increase in [Ca2+]i (17, 66, 183).
There is also evidence showing that the glia limitans mediates neurovascular signaling to pial arterioles through the release of carbon monoxide (CO). CO, which is generated endogenously by heme oxygenase-mediated metabolism of heme, dilates pial arterioles through activation of BKCa channels on SMCs (76). Glutamate and ADP applied to the cortical brain surface have been demonstrated to increase heme oxygenase-2-dependent CO production and dilate pial arterioles in newborn pigs in brain slices and in vivo, effects that are eliminated by disruption of the glia limitans with l-AAA (79, 94, 95). The majority of studies identifying CO as a glia limitans-derived neurovascular signaling molecule have been performed in neonates, so it is unclear what role CO plays in neurovascular signaling in the adult brain.
Neurons in NVC
After engagement by afferent pathways, cortical excitatory neurons and interneurons release substances that can directly or indirectly influence vascular tone to modulate CBF. It is evident that the mediators released vary according to the neuronal network activated and the frequency of the stimulus (Fig. 1) (50). In other words, signaling mechanisms mediating NVC are neuronal network dependent as well as being stimulus frequency dependent.
Signaling from afferent and cortical excitatory neurons in NVC.
Ascending afferent neurons arising from subcortical areas of the brain that terminate in the cerebral cortex activate cortical neurons via the release of network-specific neurotransmitters that have intrinsic vasoactive properties. These neurotransmitters include, among others, ACh and serotonin (5-HT), which have the potential to dilate and constrict parenchymal arterioles directly, respectively (33, 175). The basal forebrain is the major source of cholinergic input to the cortex, and some basal forebrain afferents have been shown to project directly to intracerebral microvessels, including parenchymal arterioles, capillaries, and possibly veins (171). The cortical hyperemic response to basal forebrain stimulation has been shown to partially depend on local ACh release and non-neuronal NO, leading to the suggestion that ACh released from these afferents during NVC directly stimulates vasodilation through activation of eNOS (see Fig. 1A, top) (186). However, a role for direct neurovascular signaling from ascending subcortical afferents during NVC is unclear, as it is now understood that ACh- and NO-dependent effects may occur at the level of pyramidal neurons and interneurons (92). Subcortical afferent activity is critical for the elaboration of NVC insofar as it activates cortical neurons, but the CBF response to somatosensory stimulation has been attributed to neural processing within the cortex rather than to direct signaling from incoming subcortical afferents (57).
Activation of cortical pyramidal neurons by afferent signals results in the synaptic release of the excitatory neurotransmitter glutamate (Fig. 1). The astrocyte-mediated portion of the hyperemic response to neuronal activity has, to this point, been largely attributed to glutamate binding to astrocytic Gq-coupled mGluRs. Notwithstanding the recent study by Sun et al. (160) calling this paradigm into question, there is abundant evidence in support of a role for astrocytic mGluRs in NVC. Astrocytes also express N-methyl-d-aspartate (NMDA) and α-amino-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) ionotropic glutamate receptors (iGluRs), and the interaction of glutamate with these receptors produces astrocytic Ca2+ signals (100, 101, 150). However, cortical application of NMDA or AMPA receptor inhibitors does not suppress the astrocytic Ca2+ response to whisker stimulation in vivo (177). In contrast, in the brain stem nucleus tractus solitarius, AMPA blockade prevents the rise in [Ca2+]i in astrocytes in response to electrical stimulation of vagal nerve afferents (110). NMDA and AMPA receptor antagonists have also been shown to reduce the cortical hyperemic response to basal forebrain stimulation by ∼30% (Fig. 1A); this effect was not further augmented by EET inhibition, suggesting it occurs upstream of astrocytes (92). These findings indicate that the involvement of astrocytic iGluRs in NVC responses is network dependent.
Whereas NMDA/AMPA receptor inhibition does not block the astrocytic Ca2+ response to whisker stimulation, NMDA receptor inhibition has been reported to decrease the associated CBF response (93). It has been hypothesized that this effect occurs by preventing the activation of cortical pyramidal neurons by glutamate (Fig. 1B), suggesting that for this neuronal network, the effect of NMDA receptor antagonism on CBF response reflects diminished excitatory neuronal activation. If true, however, suppressed activation of pyramidal neurons would be expected to attenuate downstream astrocytic Ca2+ responses as well. A possible explanation for this discrepancy, namely, an effect of NMDA receptor blockade on whisker stimulation-induced CBF increases in the absence of an effect on astrocytic Ca2+, may be that synaptically released glutamate activates NMDA receptors on NOS-expressing interneurons, stimulating the release of NO, which dilates parenchymal arterioles (Fig. 1B, bottom). The role of interneurons in NVC will be discussed in detail below.
Another neurotransmitter released by activated excitatory neurons is ATP (91, 131, 180). ATP stimulates astrocytic Ca2+ signals through the activation of G protein-coupled metabotropic P2Y purinergic receptors, stimulating the PLC-β-dependent formation of IP3 and release of Ca2+ into the cytosol through IP3Rs (Fig. 1) (25, 51, 58). ATP-induced increases in astrocytic [Ca2+]i have been linked to NVC in the olfactory bulb and retina, although it should be noted that in the olfactory bulb, this mechanism was associated with vasoconstriction, making its physiological relevance questionable (123, 167).
A recent study has demonstrated that adenosine, a powerful dilator of cerebral arterioles (78), is released from electrically activated postsynaptic CA1 neurons (102). While postsynaptic adenosine release in this study was functionally correlated with synaptic depression, it could potentially also contribute to the vasodilatory response to neuronal activity.
Signaling from interneurons in NVC.
Interneurons also play a significant role in neurovascular signaling. Interneurons are believed to act as integrators of neuronal activity and have been demonstrated to make direct contact with cortical microvessels and astrocytic processes (24, 26, 173). Depending on the type and frequency of the stimulus, incoming afferent signals in the cortex engage specific populations of GABA interneurons, which release vasoactive substances capable of directly dilating or constricting arterioles as well as neurotransmitters that indirectly affect vascular tone through the modulation of excitatory neurons or astrocytes. These populations include interneurons containing VIP, parvalbumin, somatostatin (SOM) and/or NPY, choline acyltransferase (which synthesizes ACh), and NOS/NPY. Cauli et al. (24) found that direct electrical stimulation of individual VIP or NOS/NPY interneurons dilated parenchymal arterioles in brain slices, whereas direct stimulation of SOM interneurons produced vasoconstriction. Perfusion with VIP or the NO donor diethylamine-NONOate potently dilated preconstricted parenchymal arterioles in brain slices, supporting the potential for a direct action of these substances on vascular tone; however, arterioles in brain slices did not constrict when perfused with SOM. Of interest, constrictions observed with electrical stimulation of SOM interneurons were highly localized to points of contact between arterioles and interneurons, creating a vascular sphincter-like effect. This observation supports a model in which localized constriction accompanies dilation during NVC, redirecting blood flow to more specifically target activated networks (Fig. 1B, top). SOM and VIP have also been found to increase [Ca2+]i in astrocytic endfeet, indicating that the release of these substances from interneurons may contribute to NVC indirectly through an effect on astrocytes (158).
Basal forebrain stimulation initiates cholinergic-dependent activation of not only COX-2 pyramidal neurons but also SOM and NOS/NPY GABA interneurons (Fig. 1A) (24). It is thought that activation of these interneurons contributes to NVC through released GABA, which binds to GABA-A receptors in downstream GABAergic inhibitory interneurons, relieving GABA-mediated inhibition of pyramidal neuron activity and thereby increasing excitatory neuronal activity and, consequently, local CBF (88). Whisker stimulation, which is communicated to the cortex via glutamatergic thalamocortical afferents, engages VIP/choline acyltransferase interneurons (Fig. 1B). As with basal forebrain stimulation, the role of these interneurons in the hyperemic response to whisker stimulation is attributed to GABA-A receptor-mediated alleviation of suppression of pyramidal neuron activity by parvalbumin and calbindin inhibitory interneurons rather than through a direct effect of VIP or ACh on vascular tone (93). Moreover, GABA released from GABAergic interneurons can induce Ca2+ signaling in astrocytes through stimulation of astrocytic GABA-A receptors. GABA-A receptor inhibition reportedly attenuates the CBF increase to basal forebrain stimulation upstream of EET formation, suggesting an astrocyte-mediated effect (92). GABA can also directly dilate parenchymal arterioles (albeit to a limited extent) in brain slices, suggesting multiple contributions to NVC of GABA signaling from interneurons (52, 111). The cortex also receives serotonergic afferent input from brain stem nuclei, and stimulation of cortical interneurons expressing 5-HT3A receptors with the agonist 1-(3-chlorophenyl)biguanide hydrochloride induces NO-mediated dilation and NPY-mediated constriction of parenchymal arterioles (135).
It has long been appreciated that NO contributes to both resting CBF and NVC. Inhibition of NOS has been shown to reduce the increase in CBF in the cortex induced by stimulation of the sciatic nerves (127), basal forebrain (127), hindpaw (86), or facial whiskers (37) and in the cerebellum by stimulation of parallel fibers (74); in each case, the reduction was ∼50%. Moreover, tissue NO has been shown to rapidly increase in the somatosensory cortex of rats in response to electrical forepaw stimulation and is temporally correlated with an increase in CBF (16). It is thought that neurons are largely the source of NO in NVC, insofar as CBF responses to neuronal activation are blunted in the presence of the neuronal NOS inhibitor 7-nitroindazole (28, 153) and in mice lacking neurtonal NOS but not in eNOS-deficient mice (4, 86, 103). More specifically, studies (112, 141, 146, 170, 172) have suggested that NO is released during neuronal activation by NOS-expressing GABA interneurons in response to stimulation of muscarinic ACh receptors (mAChRs) by ACh released from cholinergic afferents (Fig. 1A), stimulation of NMDA receptors by glutamate released from glutamatergic afferents (Fig. 1B), or stimulation of α-/β-adrenoreceptors by NE released from adrenergic afferents. Additionally, NOS expression has been reported in CA1 pyramidal neurons in the hippocampus, so it is possible that NO may be produced and released by excitatory neurons as well (179). NO production by eNOS in vascular endothelial cells in response to mAChR stimulation by neuronally released ACh may also contribute to the CBF response to neuronal activation (47). Moreover, the effect of NOS inhibition on NVC may be partly due to an effect on the baseline diameter of parenchymal arterioles. NOS inhibition significantly reduces resting CBF in mice and rats (70, 138) and has been shown to constrict parenchymal arterioles in rats by 14–36% (29, 84, 165). This vasoconstriction, or increase in vascular tone, could alter the responsiveness of parenchymal arterioles to subsequent incoming signals during NVC. Indeed, evidence has been presented showing that the magnitude of stimulus-evoked dilation of parenchymal arterioles in brain slices depends on their resting tone (13), indicating that an effect of NOS inhibition on resting arteriolar tone may contribute to altered NVC responses.
Pericytes in NVC
Pericytes are contractile cells that are adjacent to, and embedded in the basal lamina of, capillaries. Pericyte projections wrap around capillaries and are capable of regulating capillary diameter by contracting or relaxing. Cultured pericytes contract to 5-HT (82) and NE (106) and relax to NO (65), adenosine (107), VIP (106), and PGI2 (39), all of which can be released by activated neuronal networks [for a review, see Hamilton et al. (68)]. In cerebellar slices, NE stimulates pericyte-mediated constriction of capillaries, and subsequent application of glutamate relaxes pericytes to cause capillary dilation (134). Moreover, GABA receptor inhibition contracts pericytes and reduces capillary diameter in the whole retina, suggesting a relaxing effect of GABA signaling on pericyte tone. The contractile ability of pericytes has been confirmed in vivo and shown to produce alterations in red blood cell flow in capillaries (53). However, Fernandez-Klett et al. (53) found that cortical capillary diameter in vivo did not change in response to an increase in neuronal activity induced by the GABA-A receptor antagonist bicuculline; also, while an increase in capillary diameter was observed during cortical spreading depolarization, it was found to occur passively as a result of upstream arteriolar dilation. These findings indicate that, while neurovascular signaling through pericytes to capillaries may occur under certain circumstances, it does not contribute significantly to NVC. Nevertheless, pericytes play a critical role in maintaining the blood-brain barrier, which is vital for maintaining the milieu required for neurovascular regulatory mechanisms to function properly.
Neurovascular Dysfunction in Hypertension
Some reports (46, 85, 117, 121) have indicated that resting CBF is reduced in hypertension and progressively declines over time, whereas others (30, 71, 145) have found that resting global and regional CBF in hypertension is unchanged from that in normotensive controls. The observation of unchanged resting CBF in hypertension is likely attributable to myogenic autoregulation, which resets to higher pressure levels in hypertension but remains functional over a similar magnitude of pressure change (10, 156, 157). Impaired NVC (impaired ability to increase local CBF in response to elevated neuronal activity) has been observed in hypertension and may be the critical consideration in linking hypertension to cognitive dysfunction. Still, the impact of hypertension on neurovascular signaling and regulation in the brain has not been extensively investigated, and mechanistic insights have only begun to emerge in the last decade. It is unclear whether hypertension itself impairs neurovascular regulation or if ANG II signaling is the real culprit behind the apparent hypertensive neurovascular dysfunction. Available evidence, though limited, suggests the latter may be true. Hypertension induced acutely by intravenous ANG II infusion, or chronically by subcutaneous ANG II infusion via osmotic pumps, blunted the rise in cortical CBF to whisker stimulation in mice by 65% (81). However, direct cortical application of ANG II produced the same attenuation in NVC without increasing arterial pressure. Similarly, chronic, subpressor infusion of ANG II disrupts NVC before the development of hypertension (20). Moreover, chronic infusion of phenylephrine raises arterial pressure to the same extent as ANG II infusion but has no effect on the CBF response to whisker stimulation, suggesting it is not hypertension but ANG II that disrupts NVC (81). Impaired NVC in retinal arterioles has also been demonstrated in monkeys subjected to an ANG II-dependent, two-kidney/two-clip model of hypertension (59). ANG II is centrally involved in the pathogenesis of many forms of human hypertension, as evidenced by the prevalence and efficacy of antihypertensive therapies that target the renin-angiotensin-aldosterone system (34). Therefore, even if impaired NVC is specific to ANG II signaling, it is highly relevant to human hypertension.
NVC has not been well studied in genetic hypertension. Clozel et al. (30) found that the increase in CBF (measured by radioactive microspheres) to bicuculline-induced seizure activity was blunted in spontaneously hypertensive rats (SHRs) compared with normotensive control rats and that the blunted hyperemic response in SHRs was normalized by treatment with the angiotensin-converting enzyme inhibitor cilazapril. This observation suggests that even in genetic hypertension, ANG II signaling contributes to neurovascular dysfunction; however, a bicuculline-induced seizure is not a physiological condition. A very recent study by Calcinaghi et al. (18) examined physiological NVC in the SHR model. Using laser speckle imaging, they found that the peak and duration of the somatosensory cortical CBF response to whisker stimulation were attenuated in SHRs at 20 and 40 wk of age compared with normotensive control rats. Impairment of NVC in 40-wk-old SHRs was not reversed by 10-wk treatment with an antihypertensive drug [verapamil (VDCC antagonist) or losartan (ANG II receptor antagonist)] to normalize blood pressure, suggesting that other factors may be involved in altered NVC at this stage of hypertension (17). Much additional work is required to characterize neurovascular function in genetic and other models of hypertension to obtain a clearer understanding of NVC phenotypes and the underlying mechanisms central to NVC dysfunction in these models.
The disruption of NVC by ANG II is dependent on ANG II type 1 receptor (AT1R) signaling and downstream ROS production by NADPH oxidase (Nox), an enzyme that generates O2·− (80). AT1Rs and gp91phox, a subunit of Nox, were found to localize to endothelial cells and adventitia, but not SMCs, of mouse parenchymal arterioles, suggesting that impairment of NVC by ANG II is due to O2·− formation in the vascular endothelium (80). Capone et al. (19) reported that ANG II-stimulated ROS formation by Nox is dependent on a permissive role of constitutive COX-1-derived PGE2 from microglia acting on prostaglandin EP1 receptors in vascular endothelial cells; however, the mechanism of interaction between these pathways is unknown. In chronic, slow pressor ANG II-dependent hypertension, cerebral microvascular oxidative stress is also induced by increased vascular endothelin-1 production as a result of ANG II-stimulated arginine vasopressin release from the hypothalamic paraventricular nucleus (21).
The mechanisms by which vascular oxidative stress interrupts neurovascular signaling in the brain are not known. The simplest explanation would be that this effect relates to a loss of NO bioavailability and impaired vasodilation resulting from increased scavenging of NO through a reaction with O2·−, because, as discussed above, NO clearly plays a significant role in NVC. However, the source of NO produced during NVC (in the somatosensory cortex, at least) is believed to be neurons, specifically NOS interneurons, as opposed to the vascular endothelium (4). The NO donor S-nitroso-N-acetyl penicillamine induces an increase in CBF in ANG II hypertensive mice, indicating that hypertensive cerebral arterioles retain their NO responsiveness and should still be able to dilate to neuronally released NO (81). While an eNOS-dependent dilation mediated by ACh released from active cholinergic neurons has been proposed, and this could account for impaired vasodilation during NVC (186), a direct vascular action of ACh during NVC has not been experimentally validated and does not likely make a large contribution to the overall response, particularly in noncholinergic afferent neuronal networks. Therefore, a loss of endothelium-derived NO per se is unlikely to play a large role in ANG II-dependent impairment of NVC.
It is likely that the effect of ANG II hypertension on NVC is not so much due to a loss of endothelial NO bioavailability as it is to the formation of other highly reactive species. In addition to vascular oxidative stress, ANG II-dependent hypertension produces cerebral vascular nitrosative stress, dramatically increasing the levels of 3-nitrotyrosine, a marker for ONOO− (63). Cerebral vascular ONOO− formed in response to ANG II was found to be produced by the reaction of eNOS-derived NO with Nox2-derived O2·− (63). Pretreatment of the somatosensory cortex with a scavenger or decomposition catalyst of ONOO− prevents the effect of ANG II on the hyperemic response to whisker stimulation (63). This observation suggests that the ANG II-mediated attenuation in NVC is entirely dependent on ONOO−. The pathways targeted by ONOO− that produce neurovascular dysfunction are unknown, although ONOO− has been demonstrated to disrupt vasodilatory responses in pial arteries to decreased intraluminal pressure, calcitonin gene-related peptide, and an ATP-sensitive K+ channel opener (35). Notably, in this latter study, ONOO− did not alter pial artery dilation to K+. ONOO− was also found to constrict pial arteries at low concentrations but dilate at concentrations of >1 μM (105). Furthermore, ONOO− mediates the cerebral vascular dysfunction associated with aging through activation of poly(ADP-ribose) polymerase (36). ONOO− is a highly reactive and potentially destructive molecule that can compromise the function of cellular proteins through nitrosylation (97). A critical area for future research will be elucidating the cellular targets of ONOO− in the cerebral vasculature in ANG II hypertension.
Therefore, it should be noted that, although the loss of endothelial NO as a vasodilator may not be responsible for disrupted NVC in hypertension, endothelial NO provides a substrate for the formation of damaging radicals that may compromise the structural and functional integrity of the cerebral circulation. Moreover, endothelial NO makes a significant contribution to resting cerebral vascular tone and CBF, and reduced endothelial NO bioavailability in the cerebral vasculature could promote chronic hypoperfusion of the brain in hypertension (84, 104).
Hypertension and Cerebral Vascular Structure and Reactivity
Although the attenuation of NVC by ANG II can be observed acutely and in the absence of hypertension, chronic hypertension compromises cerebral vascular structural integrity and reactivity as well [for a recent review, see Pires et al. (137)]. Chronic hypertension induces vascular hypertrophy with increases in extracellular matrix deposition and inward remodeling as well as an associated decrease in lumen diameter in pial and parenchymal arteries and arterioles (3, 11, 27, 40, 119, 168). Pial arteries, measured in vivo through a closed cranial window, are more constricted at rest and after hypercapnic challenge in SHRs than in normotensive rats (77). It stands to reason that the maximum pial artery diameter elicited by neuronal stimulation may also be diminished in hypertension, but this has not been studied. Impaired NVC at the level of pial arteries/arterioles in hypertension could have a significant impact on cerebral homeostasis, as upstream dilation is necessary to sustain adequate parenchymal perfusion. Hypertension also increases blood-brain barrier permeability, primarily at the level of parenchymal arterioles (118–120, 129, 143). These changes produce arterial necrosis and cerebral edema, which clearly have disastrous consequences on vascular reactivity and neurovascular signaling in the brain, although empirical evidence to this effect is limited (27, 118, 128, 152). In addition to endothelial dysfunction (which is a universal hallmark of hypertensive cerebral arteries), impaired vascular reactivity of pial arteries to 5-HT and high K+ concentration has been demonstrated in SHRs (181), and isolated pial artery SMCs from hypertensive animals were found to be more sensitive to the VDCC agonist Bay K 8644 (15, 178). Furthermore, ATP-mediated dilation of carotid arteries (73) and K+-mediated dilation of pial arteries (108) are reportedly diminished in SHRs, suggesting that these dilatory pathways may also be impaired in vascular segments (pial and parenchymal arterioles) involved in CBF responses to neural activity.
Hypertension and Astrocytes, Neurons, and Pericytes
Other cell types that make up the neurovascular unit in the brain exhibit pathological changes in hypertension that are, in most cases, secondary to the loss of structural integrity of the cerebral vasculature. The progression of hypertension in genetically hypertensive SHRs and stroke-prone SHRs is associated with pericyte degeneration, which correlates with increased blood-brain barrier permeability and astrocyte hypertrophy and fibrosis (3, 144, 161). Tagami et al. (161) found that fibrotic astrocytes in stroke-prone SHRs were localized adjacent to open interendothelial junctions, suggesting that the loss of blood-brain barrier integrity promotes astrocyte fibrosis (161). Furthermore, neuron number is decreased in SHRs, and dead neurons are observed adjacent to fibrotic astrocytes (144, 162). Exposure of cultured astrocytes to endothelial cell-conditioned medium from stroke-prone SHRs stimulates greater astrocyte proliferation than that from normotensive rats, indicating that endothelial cells release mitogenic, proproliferative signals in response to high intravascular pressure.
In ANG II-dependent hypertension, ANG II may have direct effects on neurons and astrocytes. In medullary and cerebellar, but not cortical or hypothalamic, astrocytes from neonatal mice, ANG II stimulates a PLC/IP3-mediated increase in Ca2+ concentration via AT1Rs as well as PGI2 release (166). These findings suggest that ANG II can have region-specific effects on astrocyte function; however, cautious interpretation is warranted, as responses in neonates are not typically accurate representations of adult responses. Furthermore, chronic ANG II infusion has been found to increase O2·− formation in neurons in addition to the cerebral vasculature, suggesting that the inhibitory effect of ANG II on NVC may also reflect effects on neurons.
Summary
Neurovascular regulation of CBF is a highly dynamic, complex, coordinated process involving the modulation of cerebral vascular tone by signaling within and between neuronal subpopulations (extrinsic perivascular nerves, excitatory neurons, and interneurons), astrocytes, and pericytes. Neurovascular signaling matches substrate delivery by the blood to metabolic demand, maintains the proper milieu for neural processing, and protects brain cells from damage by physical forces and neurohumoral factors within the vasculature. The destructive effects of hypertension on the brain illustrate the critical importance of neurovascular signaling to brain function. Hypertension initiates a chain of events that starts with structural and functional breakdown of the cerebral vasculature and leads to disruption of neurovascular unit microanatomy, loss of neurovascular regulation, and, ultimately, neurodegeneration. As insights are made into the physiological mechanisms of neurovascular regulation, significant gaps remain to be filled in our understanding of the mechanisms linking hypertension to neurovascular dysfunction in the brain. A major imperative moving forward is to elucidate the mechanisms by which vascular oxidative stress impairs neurovascular signaling and to better understand the role of the endothelium in neurovascular signaling. It is also necessary to further characterize NVC responses in genetic and other non-ANG II-dependent hypertensive models and to investigate NVC in human hypertensive subjects using blood oxygen level-dependent functional MRI and other dynamic imaging modalities. Impaired blood oxygen level-dependent hemodynamic signals elicited in response to neural activation in poststroke human subjects (90) or human subjects with extracerebral and intracerebral artery disease (stenosis) (69) have been reported, but these investigations have not been made in hypertensive patients. A more thorough understanding of neurovascular dysfunction in hypertension will further illuminate the role of ANG II signaling versus other mechanisms and identify optimal therapeutic strategies for preventing or reversing hypertensive neuropathy. Insights into neurovascular dysfunction in hypertension may also shed light on pathogenic mechanisms of other forms of small vessel disease of the brain, including cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Evidence of increased age-related white matter lesions in hypertensive patients expressing common variants of Notch3, the gene associated with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy pathology (149), suggests that hypertension may provide a permissive background for the manifestation of genetic small vessel disease-associated neuropathy, or vice versa.
It is increasingly clear that preservation of the brain requires preservation of vascular function and regulation, not only in hypertension but also in other progressive neurodegenerative diseases, such as Alzheimer's disease and dementia, as well as acute neurologic pathologies like subarachnoid hemorrhage and stroke (8, 23, 64, 89, 90). A thorough understanding of cerebral vascular physiology and neurovascular signaling in the brain is essential to identifying therapeutic neuroprotection strategies.
GRANTS
This work was supported by National Institutes of Health Grants P01-HL-095488, R37-DK-053832, R01-HL-44455, R01-HL-098243, and T32 HL07944, the Fondation Leducq for the Transatlantic Network of Excellence on the Pathogenesis of Small Vessel Disease of the Brain, and the Totman Medical Research Trust.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.M.D. prepared figures; K.M.D. drafted manuscript; K.M.D. and M.T.N. edited and revised manuscript; M.T.N. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. David Hill-Eubanks for editorial comments.
REFERENCES
- 1.Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem 267: 13361–13368, 1992 [PubMed] [Google Scholar]
- 2.Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR. Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke 28: 1066–1072, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Amenta F, Strocchi P, Sabbatini M. Vascular and neuronal hypertensive brain damage: protective effect of treatment with nicardipine. J Hypertens Suppl 14: S29–S35, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Ayata C, Ma J, Meng W, Huang P, Moskowitz MA. l-NA-sensitive rCBF augmentation during vibrissal stimulation in type III nitric oxide synthase mutant mice. J Cereb Blood Flow Metab 16: 539–541, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Baba A, Saga H, Hashimoto H. Inhibitory glutamate response on cyclic AMP formation in cultured astrocytes. Neurosci Lett 149: 182–184, 1993 [DOI] [PubMed] [Google Scholar]
- 6.Baeres FM, Moller M. Origin of PACAP-immunoreactive nerve fibers innervating the subarachnoidal blood vessels of the rat brain. J Cereb Blood Flow Metab 24: 628–635, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Bakalova R, Matsuura T, Kanno I. The cyclooxygenase inhibitors indomethacin and Rofecoxib reduce regional cerebral blood flow evoked by somatosensory stimulation in rats. Exp Biol Med 227: 465–473, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Baker WB, Sun Z, Hiraki T, Putt ME, Durduran T, Reivich M, Yodh AG, Greenberg JH. Neurovascular coupling varies with level of global cerebral ischemia in a rat model. J Cereb Blood Flow Metab 33: 97–105, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barres BA, Chun LL, Corey DP. Calcium current in cortical astrocytes: induction by cAMP and neurotransmitters and permissive effect of serum factors. J Neurosci 9: 3169–3175, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barry DI, Strandgaard S, Graham DI, Braendstrup O, Svendsen UG, Vorstrup S, Hemmingsen R, Bolwig TG. Cerebral blood flow in rats with renal and spontaneous hypertension: resetting of the lower limit of autoregulation. J Cereb Blood Flow Metab 2: 347–353, 1982 [DOI] [PubMed] [Google Scholar]
- 11.Baumbach GL, Walmsley JG, Hart MN. Composition and mechanics of cerebral arterioles in hypertensive rats. Am J Pathol 133: 464–471, 1988 [PMC free article] [PubMed] [Google Scholar]
- 12.Bernstein M, Behnisch T, Balschun D, Reymann KG, Reiser G. Pharmacological characterisation of metabotropic glutamatergic and purinergic receptors linked to Ca2+ signalling in hippocampal astrocytes. Neuropharmacology 37: 169–178, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Blanco VM, Stern JE, Filosa JA. Tone-dependent vascular responses to astrocyte-derived signals. Am J Physiol Heart Circ Physiol 294: H2855–H2863, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breder CD, Dewitt D, Kraig RP. Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol 355: 296–315, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruner CA, Webb RC. Increased vascular reactivity to Bay K 8644 in genetic hypertension. Pharmacology 41: 24–35, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Buerk DG, Ances BM, Greenberg JH, Detre JA. Temporal dynamics of brain tissue nitric oxide during functional forepaw stimulation in rats. Neuroimage 18: 1–9, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Butt AM. ATP: a ubiquitous gliotransmitter integrating neuron-glial networks. Semin Cell Dev Biol 22: 205–213, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Calcinaghi N, Wyss MT, Jolivet R, Singh A, Keller AL, Winnik S, Fritschy JM, Buck A, Matter CM, Weber B. Multimodal imaging in rats reveals impaired neurovascular coupling in sustained hypertension. Stroke 44: 1957–1964, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Capone C, Faraco G, Anrather J, Zhou P, Iadecola C. Cyclooxygenase 1-derived prostaglandin E2 and EP1 receptors are required for the cerebrovascular dysfunction induced by angiotensin II. Hypertension 55: 911–917, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capone C, Faraco G, Park L, Cao X, Davisson RL, Iadecola C. The cerebrovascular dysfunction induced by slow pressor doses of angiotensin II precedes the development of hypertension. Am J Physiol Heart Circ Physiol 300: H397–H407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capone C, Faraco G, Peterson JR, Coleman C, Anrather J, Milner TA, Pickel VM, Davisson RL, Iadecola C. Central cardiovascular circuits contribute to the neurovascular dysfunction in angiotensin II hypertension. J Neurosci 32: 4878–4886, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmignoto G, Pasti L, Pozzan T. On the role of voltage-dependent calcium channels in calcium signaling of astrocytes in situ. J Neurosci 18: 4637–4645, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carnevale D, Mascio G, D'Andrea I, Fardella V, Bell RD, Branchi I, Pallante F, Zlokovic B, Yan SS, Lembo G. Hypertension induces brain beta-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension 60: 188–197, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cauli B, Tong XK, Rancillac A, Serluca N, Lambolez B, Rossier J, Hamel E. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci 24: 8940–8949, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centemeri C, Bolego C, Abbracchio MP, Cattabeni F, Puglisi L, Burnstock G, Nicosia S. Characterization of the Ca2+ responses evoked by ATP and other nucleotides in mammalian brain astrocytes. Br J Pharmacol 121: 1700–1706, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chedotal A, Cozzari C, Faure MP, Hartman BK, Hamel E. Distinct choline acetyltransferase (ChAT) and vasoactive intestinal polypeptide (VIP) bipolar neurons project to local blood vessels in the rat cerebral cortex. Brain Res 646: 181–193, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Chester EM, Agamanolis DP, Banker BQ, Victor M. Hypertensive encephalopathy: a clinicopathologic study of 20 cases. Neurology 28: 928–939, 1978 [DOI] [PubMed] [Google Scholar]
- 28.Cholet N, Seylaz J, Lacombe P, Bonvento G. Local uncoupling of the cerebrovascular and metabolic responses to somatosensory stimulation after neuronal nitric oxide synthase inhibition. J Cereb Blood Flow Metab 17: 1191–1201, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Cipolla MJ, Bullinger LV. Reactivity of brain parenchymal arterioles after ischemia and reperfusion. Microcirculation 15: 495–501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clozel JP, Kuhn H, Hefti F. Effects of cilazapril on the cerebral circulation in spontaneously hypertensive rats. Hypertension 14: 645–651, 1989 [DOI] [PubMed] [Google Scholar]
- 31.D'Ascenzo M, Vairano M, Andreassi C, Navarra P, Azzena GB, Grassi C. Electrophysiological and molecular evidence of L-(Cav1), N-(Cav2.2), and R- (Cav2.3) type Ca2+ channels in rat cortical astrocytes. Glia 45: 354–363, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Dabertrand F, Hannah RM, Pearson JM, Hill-Eubanks DC, Brayden JE, Nelson MT. Prostaglandin E2, a postulated astrocyte-derived neurovascular coupling agent, constricts rather than dilates parenchymal arterioles. J Cereb Blood Flow Metab 33: 479–482, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dacey RG, Jr, Bassett JE. Cholinergic vasodilation of intracerebral arterioles in rats. Am J Physiol Heart Circ Physiol 253: H1253–H1260, 1987 [DOI] [PubMed] [Google Scholar]
- 34.De Silva TM, Faraci FM. Effects of angiotensin II on the cerebral circulation: role of oxidative stress. Front Physiol 3: 484, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeWitt DS, Mathew BP, Chaisson JM, Prough DS. Peroxynitrite reduces vasodilatory responses to reduced intravascular pressure, calcitonin gene-related peptide, and cromakalim in isolated middle cerebral arteries. J Cereb Blood Flow Metab 21: 253–261, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Didion SP, Kinzenbaw DA, Schrader LI, Faraci FM. Heterozygous CuZn superoxide dismutase deficiency produces a vascular phenotype with aging. Hypertension 48: 1072–1079, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Dirnagl U, Lindauer U, Villringer A. Role of nitric oxide in the coupling of cerebral blood flow to neuronal activation in rats. Neurosci Lett 149: 43–46, 1993 [DOI] [PubMed] [Google Scholar]
- 38.Dirnagl U, Niwa K, Lindauer U, Villringer A. Coupling of cerebral blood flow to neuronal activation: role of adenosine and nitric oxide. Am J Physiol Heart Circ Physiol 267: H296–H301, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Dodge AB, Hechtman HB, Shepro D. Microvascular endothelial-derived autacoids regulate pericyte contractility. Cell Motil Cytoskeleton 18: 180–188, 1991 [DOI] [PubMed] [Google Scholar]
- 40.Dorrance AM, Rupp NC, Nogueira EF. Mineralocorticoid receptor activation causes cerebral vessel remodeling and exacerbates the damage caused by cerebral ischemia. Hypertension 47: 590–595, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Dunn KM, Hill-Eubanks DC, Liedtke WB, Nelson MT. TRPV4 channels stimulate Ca2+-induced Ca2+ release in astrocytic endfeet and amplify neurovascular coupling responses. Proc Natl Acad Sci USA 110: 6157–6162, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Earley S. Endothelium-dependent cerebral artery dilation mediated by transient receptor potential and Ca2+-activated K+ channels. J Cardiovasc Pharmacol 57: 148–153, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res 97: 1270–1279, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Edvinsson L, Fredholm BB. Characterization of adenosine receptors in isolated cerebral arteries of cat. Br J Pharmacol 80: 631–637, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edvinsson L, Krause DN. Cerebral Blood Flow and Metabolism. Philadelphia, PA: Lippincott, Williams & Wilkins, 2002 [Google Scholar]
- 46.Efimova IY, Efimova NY, Triss SV, Lishmanov YB. Brain perfusion and cognitive function changes in hypertensive patients. Hypertens Res 31: 673–678, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Elhusseiny A, Hamel E. Muscarinic–but not nicotinic–acetylcholine receptors mediate a nitric oxide-dependent dilation in brain cortical arterioles: a possible role for the M5 receptor subtype. J Cereb Blood Flow Metab 20: 298–305, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Ellis EF, Police RJ, Yancey L, McKinney JS, Amruthesh SC. Dilation of cerebral arterioles by cytochrome P-450 metabolites of arachidonic acid. Am J Physiol Heart Circ Physiol 259: H1171–H1177, 1990 [DOI] [PubMed] [Google Scholar]
- 49.Ellis EF, Wei EP, Kontos HA. Vasodilation of cat cerebral arterioles by prostaglandins D2, E2, G2, and I2. Am J Physiol Heart Circ Physiol 237: H381–H385, 1979 [DOI] [PubMed] [Google Scholar]
- 50.Enager P, Piilgaard H, Offenhauser N, Kocharyan A, Fernandes P, Hamel E, Lauritzen M. Pathway-specific variations in neurovascular and neurometabolic coupling in rat primary somatosensory cortex. J Cereb Blood Flow Metab 29: 976–986, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Fam SR, Gallagher CJ, Kalia LV, Salter MW. Differential frequency dependence of P2Y1- and P2Y2- mediated Ca2+ signaling in astrocytes. J Neurosci 23: 4437–4444, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fergus A, Lee KS. GABAergic regulation of cerebral microvascular tone in the rat. J Cereb Blood Flow Metab 17: 992–1003, 1997 [DOI] [PubMed] [Google Scholar]
- 53.Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci USA 107: 22290–22295, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fiebich BL, Schleicher S, Spleiss O, Czygan M, Hull M. Mechanisms of prostaglandin E2-induced interleukin-6 release in astrocytes: possible involvement of EP4-like receptors, p38 mitogen-activated protein kinase and protein kinase C. J Neurochem 79: 950–958, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res 95: e73–81, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci 9: 1397–1403, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Franceschini MA, Nissila I, Wu W, Diamond SG, Bonmassar G, Boas DA. Coupling between somatosensory evoked potentials and hemodynamic response in the rat. Neuroimage 41: 189–203, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fumagalli M, Brambilla R, D'Ambrosi N, Volonte C, Matteoli M, Verderio C, Abbracchio MP. Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia 43: 218–203, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Garner A, Ashton N, Tripathi R, Kohner EM, Bulpitt CJ, Dollery CT. Pathogenesis of hypertensive retinopathy. An experimental study in the monkey. Br J Ophthalmol 59: 3–44, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol Heart Circ Physiol 263: H519–H525, 1992 [DOI] [PubMed] [Google Scholar]
- 61.Gerrits RJ, Stein EA, Greene AS. Ca2+-activated potassium (KCa) channel inhibition decreases neuronal activity-blood flow coupling. Brain Res 948: 108–116, 2002 [DOI] [PubMed] [Google Scholar]
- 62.Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci USA 107: 3811–3816, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler Thromb Vasc Biol 27: 303–309, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: 2672–2713, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haefliger IO, Zschauer A, Anderson DR. Relaxation of retinal pericyte contractile tone through the nitric oxide-cyclic guanosine monophosphate pathway. Invest Ophthalmol Vis Sci 35: 991–997, 1994 [PubMed] [Google Scholar]
- 66.Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology 57: 343–346, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol 100: 1059–1064, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics: 2, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hamzei F, Knab R, Weiller C, Rother J. The influence of extra- and intracranial artery disease on the BOLD signal in FMRI. Neuroimage 20: 1393–1399, 2003 [DOI] [PubMed] [Google Scholar]
- 70.Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial SKCa and IKCa channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab 31: 1175–1186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harper SL. Effects of antihypertensive treatment on the cerebral microvasculature of spontaneously hypertensive rats. Stroke 18: 450–456, 1987 [DOI] [PubMed] [Google Scholar]
- 72.He L, Linden DJ, Sapirstein A. Astrocyte inositol triphosphate receptor type 2 and cytosolic phospholipase A2 alpha regulate arteriole responses in mouse neocortical brain slices. PLos One 7: e42194, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hongo K, Nakagomi T, Kassell NF, Sasaki T, Lehman M, Vollmer DG, Tsukahara T, Ogawa H, Torner J. Effects of aging and hypertension on endothelium-dependent vascular relaxation in rat carotid artery. Stroke 19: 892–897, 1988 [DOI] [PubMed] [Google Scholar]
- 74.Iadecola C, Li J, Ebner TJ, Xu X. Nitric oxide contributes to functional hyperemia in cerebellar cortex. Am J Physiol Regul Integr Comp Physiol 268: R1153–R1162, 1995 [DOI] [PubMed] [Google Scholar]
- 75.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci 10: 1369–1376, 2007 [DOI] [PubMed] [Google Scholar]
- 76.Jaggar JH, Leffler CW, Cheranov SY, Tcheranova D, Shuyu E, Cheng X. Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels. Circ Res 91: 610–617, 2002 [DOI] [PubMed] [Google Scholar]
- 77.Johansson BB, Auer LM, Sayama I. Reaction of pial arteries and veins to hypercapnia in hypertensive and normotensive rats. Stroke 16: 320–323, 1985 [DOI] [PubMed] [Google Scholar]
- 78.Kajita Y, Dietrich HH, Dacey RG., Jr Effects of oxyhemoglobin on local and propagated vasodilatory responses induced by adenosine, adenosine diphosphate, and adenosine triphosphate in rat cerebral arterioles. J Neurosurg 85: 908–916, 1996 [DOI] [PubMed] [Google Scholar]
- 79.Kanu A, Leffler CW. Roles of glia limitans astrocytes and carbon monoxide in adenosine diphosphate-induced pial arteriolar dilation in newborn pigs. Stroke 40: 930–935, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res 95: 1019–1026, 2004 [DOI] [PubMed] [Google Scholar]
- 81.Kazama K, Wang G, Frys K, Anrather J, Iadecola C. Angiotensin II attenuates functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol 285: H1890–H1899, 2003 [DOI] [PubMed] [Google Scholar]
- 82.Kelley C, D'Amore P, Hechtman HB, Shepro D. Vasoactive hormones and cAMP affect pericyte contraction and stress fibres in vitro. J Muscle Res Cell Motil 9: 184–194, 1988 [DOI] [PubMed] [Google Scholar]
- 83.Kesherwani V, Agrawal SK. Regulation of inositol 1,4,5-triphosphate receptor, type 1 (IP3R1) in hypoxic/reperfusion injury of white matter. Neurol Res 34: 504–511, 2012 [DOI] [PubMed] [Google Scholar]
- 84.Kimura M, Dietrich HH, Dacey RG., Jr Nitric oxide regulates cerebral arteriolar tone in rats. Stroke 25: 2224–2233, 1994 [DOI] [PubMed] [Google Scholar]
- 85.Kitagawa K. Cerebral blood flow measurement by PET in hypertensive subjects as a marker of cognitive decline. J Alzheimer's Dis 20: 855–859, 2010 [DOI] [PubMed] [Google Scholar]
- 86.Kitaura H, Uozumi N, Tohmi M, Yamazaki M, Sakimura K, Kudoh M, Shimizu T, Shibuki K. Roles of nitric oxide as a vasodilator in neurovascular coupling of mouse somatosensory cortex. Neurosci Res 59: 160–171, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Kleppisch T, Nelson MT. Adenosine activates ATP-sensitive potassium channels in arterial myocytes via A2 receptors and cAMP-dependent protein kinase. Proc Natl Acad Sci USA 92: 12441–12445, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kocharyan A, Fernandes P, Tong XK, Vaucher E, Hamel E. Specific subtypes of cortical GABA interneurons contribute to the neurovascular coupling response to basal forebrain stimulation. J Cereb Blood Flow Metab 28: 221–231, 2008 [DOI] [PubMed] [Google Scholar]
- 89.Koide M, Bonev AD, Nelson MT, Wellman GC. Inversion of neurovascular coupling by subarachnoid blood depends on large-conductance Ca2+-activated K+ (BK) channels. Proc Natl Acad Sci USA 109: E1387–E1395, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krainik A, Hund-Georgiadis M, Zysset S, von Cramon DY. Regional impairment of cerebrovascular reactivity and BOLD signal in adults after stroke. Stroke 36: 1146–1152, 2005 [DOI] [PubMed] [Google Scholar]
- 91.Larsson M, Sawada K, Morland C, Hiasa M, Ormel L, Moriyama Y, Gundersen V. Functional and anatomical identification of a vesicular transporter mediating neuronal ATP release. Cereb Cortex 22: 1203–1214, 2012 [DOI] [PubMed] [Google Scholar]
- 92.Lecrux C, Kocharyan A, Sandoe CH, Tong XK, Hamel E. Pyramidal cells and cytochrome P450 epoxygenase products in the neurovascular coupling response to basal forebrain cholinergic input. J Cereb Blood Flow Metab 32: 896–906, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lecrux C, Toussay X, Kocharyan A, Fernandes P, Neupane S, Levesque M, Plaisier F, Shmuel A, Cauli B, Hamel E. Pyramidal neurons are “neurogenic hubs” in the neurovascular coupling response to whisker stimulation. J Neurosci 31: 9836–9847, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leffler CW, Parfenova H, Fedinec AL, Basuroy S, Tcheranova D. Contributions of astrocytes and CO to pial arteriolar dilation to glutamate in newborn pigs. Am J Physiol Heart Circ Physiol 291: H2897–H2904, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li A, Xi Q, Umstot ES, Bellner L, Schwartzman ML, Jaggar JH, Leffler CW. Astrocyte-derived CO is a diffusible messenger that mediates glutamate-induced cerebral arteriolar dilation by activating smooth muscle cell KCa channels. Circ Res 102: 234–241, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li J, Iadecola C. Nitric oxide and adenosine mediate vasodilation during functional activation in cerebellar cortex. Neuropharmacology 33: 1453–1461, 1994 [DOI] [PubMed] [Google Scholar]
- 97.Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 364: 626–632, 1993 [DOI] [PubMed] [Google Scholar]
- 98.Liu X, Li C, Falck JR, Harder DR, Koehler RC. Relative contribution of cyclooxygenases, epoxyeicosatrienoic acids, and pH to the cerebral blood flow response to vibrissal stimulation. Am J Physiol Heart Circ Physiol 302: H1075–H1085, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu X, Li C, Gebremedhin D, Hwang SH, Hammock BD, Falck JR, Roman RJ, Harder DR, Koehler RC. Epoxyeicosatrienoic acid-dependent cerebral vasodilation evoked by metabotropic glutamate receptor activation in vivo. Am J Physiol Heart Circ Physiol 301: H373–H381, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lopez-Colome AM, Ortega A, Fragoso G, Trueba E. Excitatory amino acid receptors coupled to the phosphoinositide pathway in Bergmann glia. Neurochem Res 22: 305–312, 1997 [DOI] [PubMed] [Google Scholar]
- 101.Lopez-Colome AM, Ortega A, Romo-de-Vivar M. Excitatory amino acid-induced phosphoinositide hydrolysis in Muller glia. Glia 9: 127–135, 1993 [DOI] [PubMed] [Google Scholar]
- 102.Lovatt D, Xu Q, Liu W, Takano T, Smith NA, Schnermann J, Tieu K, Nedergaard M. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc Natl Acad Sci USA 109: 6265–6270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ma J, Ayata C, Huang PL, Fishman MC, Moskowitz MA. Regional cerebral blood flow response to vibrissal stimulation in mice lacking type I NOS gene expression. Am J Physiol Heart Circ Physiol 270: H1085–H1090, 1996 [DOI] [PubMed] [Google Scholar]
- 104.Ma J, Meng W, Ayata C, Huang PL, Fishman MC, Moskowitz MA. l-NNA-sensitive regional cerebral blood flow augmentation during hypercapnia in type III NOS mutant mice. Am J Physiol Heart Circ Physiol 271: H1717–H1719, 1996 [DOI] [PubMed] [Google Scholar]
- 105.Maneen MJ, Hannah R, Vitullo L, DeLance N, Cipolla MJ. Peroxynitrite diminishes myogenic activity and is associated with decreased vascular smooth muscle F-actin in rat posterior cerebral arteries. Stroke 37: 894–899, 2006 [DOI] [PubMed] [Google Scholar]
- 106.Markhotina N, Liu GJ, Martin DK. Contractility of retinal pericytes grown on silicone elastomer substrates is through a protein kinase A-mediated intracellular pathway in response to vasoactive peptides. IET Nanobiotechnol 1: 44–51, 2007 [DOI] [PubMed] [Google Scholar]
- 107.Matsugi T, Chen Q, Anderson DR. Adenosine-induced relaxation of cultured bovine retinal pericytes. Invest Ophthalmol Vis Sci 38: 2695–2701, 1997 [PubMed] [Google Scholar]
- 108.McCarron JG, Halpern W. Impaired potassium-induced dilation in hypertensive rat cerebral arteries does not reflect altered Na+,K+-ATPase dilation. Circ Res 67: 1035–1039, 1990 [DOI] [PubMed] [Google Scholar]
- 109.McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, Breyer RM, Andreasson K. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci 24: 257–268, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McDougal DH, Hermann GE, Rogers RC. Vagal afferent stimulation activates astrocytes in the nucleus of the solitary tract via AMPA receptors: evidence of an atypical neural-glial interaction in the brainstem. J Neurosci 31: 14037–14045, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Meier SD, Kafitz KW, Rose CR. Developmental profile and mechanisms of GABA-induced calcium signaling in hippocampal astrocytes. Glia 56: 1127–1137, 2008 [DOI] [PubMed] [Google Scholar]
- 112.Meng W, Tobin JR, Busija DW. Glutamate-induced cerebral vasodilation is mediated by nitric oxide through N-methyl-d-aspartate receptors. Stroke 26: 857–863, 1995 [DOI] [PubMed] [Google Scholar]
- 113.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci 26: 2862–2870, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miller S, Bridges RJ, Chamberlin AR, Cotman CW. Pharmacological dissociation of glutamatergic metabotropic signal transduction pathways in cortical astrocytes. Eur J Pharmacol 269: 235–241, 1994 [DOI] [PubMed] [Google Scholar]
- 115.Moldrich RX, Aprico K, Diwakarla S, O'Shea RD, Beart PM. Astrocyte mGlu(2/3)-mediated cAMP potentiation is calcium sensitive: studies in murine neuronal and astrocyte cultures. Neuropharmacology 43: 189–203, 2002 [DOI] [PubMed] [Google Scholar]
- 116.Mosso A. Sulla circolazione del cervello dell'uomo. Atti R Academia dei Lincei: 237–358, 1880 [Google Scholar]
- 117.Muller M, van der Graaf Y, Visseren FL, Mali WP, Geerlings MI. Hypertension and longitudinal changes in cerebral blood flow: the SMART-MR study. Ann Neurol 71: 825–833, 2012 [DOI] [PubMed] [Google Scholar]
- 118.Nag S. Cerebral changes in chronic hypertension: combined permeability and immunohistochemical studies. Acta Neuropathol (Berl) 62: 178–184, 1984 [DOI] [PubMed] [Google Scholar]
- 119.Nag S. Immunohistochemical localization of extracellular matrix proteins in cerebral vessels in chronic hypertension. J Neuropathol Exp Neurol 55: 381–388, 1996 [DOI] [PubMed] [Google Scholar]
- 120.Nag S, Robertson DM, Dinsdale HB. Morphological changes in spontaneously hypertensive rats. Acta Neuropathol (Berl) 52: 27–34, 1980 [DOI] [PubMed] [Google Scholar]
- 121.Nagata R, Kawabe K, Ikeda K. Olmesartan, an angiotensin II receptor blocker, restores cerebral hypoperfusion in elderly patients with hypertension. J Stroke Cerebrovasc Dis 19: 236–240, 2010 [DOI] [PubMed] [Google Scholar]
- 122.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol Cell Physiol 268: C799–C822, 1995 [DOI] [PubMed] [Google Scholar]
- 123.Newman EA. Calcium increases in retinal glial cells evoked by light-induced neuronal activity. J Neurosci 25: 5502–5510, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ngai AC, Ko KR, Morii S, Winn HR. Effect of sciatic nerve stimulation on pial arterioles in rats. Am J Physiol Heart Circ Physiol 254: H133–H139, 1988 [DOI] [PubMed] [Google Scholar]
- 125.Niwa K, Araki E, Morham SG, Ross ME, Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci 20: 763–770, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nizar K, Uhlirova H, Tian P, Saisan PA, Cheng Q, Reznichenko L, Weldy KL, Steed TC, Sridhar VB, Macdonald CL, Cui J, Gratiy SL, Sakadzic S, Boas DA, Beka TI, Einevoll GT, Chen J, Masliah E, Dale AM, Silva GA, Devor A. In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J Neurosci 33: 8411–8422, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Northington FJ, Matherne GP, Berne RM. Competitive inhibition of nitric oxide synthase prevents the cortical hyperemia associated with peripheral nerve stimulation. Proc Natl Acad Sci USA 89: 6649–6652, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ogata J, Fujishima M, Tamaki K, Nakatomi Y, Ishitsuka T, Omae T. Stroke-prone spontaneously hypertensive rats as an experimental model of malignant hypertension. I. A light- and electron-microscopic study of the brain. Acta Neuropathol (Berl) 51: 179–184, 1980 [DOI] [PubMed] [Google Scholar]
- 129.Olsen F. Increased permeability for plasma components of the cerebral vessels during acute angiotensin hypertension in rats. Acta Pathol Microbiol Scand [A] 85: 572–576, 1977 [DOI] [PubMed] [Google Scholar]
- 130.Paisansathan C, Xu H, Vetri F, Hernandez M, Pelligrino DA. Interactions between adenosine and K+ channel-related pathways in the coupling of somatosensory activation and pial arteriolar dilation. Am J Physiol Heart Circ Physiol 299: H2009–H2017, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pankratov Y, Lalo U, Verkhratsky A, North RA. Quantal release of ATP in mouse cortex. J Gen Physiol 129: 257–265, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Parpura V, Grubisic V, Verkhratsky A. Ca2+ sources for the exocytotic release of glutamate from astrocytes. Biochim Biophys Acta 1813: 984–991, 2011 [DOI] [PubMed] [Google Scholar]
- 133.Pasti L, Volterra A, Pozzan T, Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J Neurosci 17: 7817–7830, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature 443: 700–704, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Perrenoud Q, Rossier J, Ferezou I, Geoffroy H, Gallopin T, Vitalis T, Rancillac A. Activation of cortical 5-HT3 receptor-expressing interneurons induces NO mediated vasodilatations and NPY mediated vasoconstrictions. Front Neural Circuits 6: 50, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Petzold GC, Albeanu DF, Sato TF, Murthy VN. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron 58: 897–910, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol 304: H1598–H1614, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Prado R, Watson BD, Kuluz J, Dietrich WD. Endothelium-derived nitric oxide synthase inhibition. Effects on cerebral blood flow, pial artery diameter, and vascular morphology in rats. Stroke 23: 1118–1124, 1992 [DOI] [PubMed] [Google Scholar]
- 139.Prezeau L, Carrette J, Helpap B, Curry K, Pin JP, Bockaert J. Pharmacological characterization of metabotropic glutamate receptors in several types of brain cells in primary cultures. Mol Pharmacol 45: 570–577, 1994 [PubMed] [Google Scholar]
- 140.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev 77: 1165–1232, 1997 [DOI] [PubMed] [Google Scholar]
- 141.Rancillac A, Rossier J, Guille M, Tong XK, Geoffroy H, Amatore C, Arbault S, Hamel E, Cauli B. Glutamatergic control of microvascular tone by distinct GABA neurons in the cerebellum. J Neurosci 26: 6997–7006, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Roy CS, Sherrington CS. On the regulation of the blood-supply of the brain. J Physiol 11: 85–158, 1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sabbatini M, Strocchi P, Vitaioli L, Amenta F. Microanatomical changes of intracerebral arteries in spontaneously hypertensive rats: a model of cerebrovascular disease of the elderly. Mech Ageing Dev 122: 1257–1268, 2001 [DOI] [PubMed] [Google Scholar]
- 144.Sabbatini M, Tomassoni D, Amenta F. Hypertensive brain damage: comparative evaluation of protective effect of treatment with dihydropyridine derivatives in spontaneously hypertensive rats. Mech Ageing Dev 122: 2085–2105, 2001 [DOI] [PubMed] [Google Scholar]
- 145.Sadoshima S, Fujii K, Yao H, Kusuda K, Ibayashi S, Fujishima M. Regional cerebral blood flow autoregulation in normotensive and spontaneously hypertensive rats–effects of sympathetic denervation. Stroke 17: 981–984, 1986 [DOI] [PubMed] [Google Scholar]
- 146.Sammut S, Park DJ, West AR. Frontal cortical afferents facilitate striatal nitric oxide transmission in vivo via a NMDA receptor and neuronal NOS-dependent mechanism. J Neurochem 103: 1145–1156, 2007 [DOI] [PubMed] [Google Scholar]
- 147.Sanzgiri RP, Araque A, Haydon PG. Prostaglandin E2 stimulates glutamate receptor-dependent astrocyte neuromodulation in cultured hippocampal cells. J Neurobiol 41: 221–229, 1999 [PubMed] [Google Scholar]
- 148.Sayer RJ, Schwindt PC, Crill WE. Metabotropic glutamate receptor-mediated suppression of L-type calcium current in acutely isolated neocortical neurons. J Neurophysiol 68: 833–842, 1992 [DOI] [PubMed] [Google Scholar]
- 149.Schmidt H, Zeginigg M, Wiltgen M, Freudenberger P, Petrovic K, Cavalieri M, Gider P, Enzinger C, Fornage M, Debette S, Rotter JI, Ikram MA, Launer LJ, Schmidt R. CHARGE Consortium Neurology Working Group Genetic variants of the NOTCH3 gene in the elderly and magnetic resonance imaging correlates of age-related cerebral small vessel disease. Brain 134: 3384–3397, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shelton MK, McCarthy KD. Mature hippocampal astrocytes exhibit functional metabotropic and ionotropic glutamate receptors in situ. Glia 26: 1–11, 1999 [DOI] [PubMed] [Google Scholar]
- 151.Shi Y, Liu X, Gebremedhin D, Falck JR, Harder DR, Koehler RC. Interaction of mechanisms involving epoxyeicosatrienoic acids, adenosine receptors, and metabotropic glutamate receptors in neurovascular coupling in rat whisker barrel cortex. J Cereb Blood Flow Metab 28: 111–125, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shinkai H, Yoshida Y, Ooneda G. An electron microscopic study of plasmatic arterionecrosis in the human cerebral arteries. Virchows Arch A Pathol Anat Histol 369: 181–190, 1976 [DOI] [PubMed] [Google Scholar]
- 153.Stefanovic B, Schwindt W, Hoehn M, Silva AC. Functional uncoupling of hemodynamic from neuronal response by inhibition of neuronal nitric oxide synthase. J Cereb Blood Flow Metab 27: 741–754, 2007 [DOI] [PubMed] [Google Scholar]
- 154.Stella N, Tence M, Glowinski J, Premont J. Glutamate-evoked release of arachidonic acid from mouse brain astrocytes. J Neurosci 14: 568–575, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stobart JL, Lu L, Anderson HD, Mori H, Anderson CM. Astrocyte-induced cortical vasodilation is mediated by d-serine and endothelial nitric oxide synthase. Proc Natl Acad Sci USA 110: 3149–3154, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Strandgaard S. Autoregulation of cerebral blood flow in hypertensive patients. The modifying influence of prolonged antihypertensive treatment on the tolerance to acute, drug-induced hypotension. Circulation 53: 720–727, 1976 [DOI] [PubMed] [Google Scholar]
- 157.Strandgaard S, Olesen J, Skinhoj E, Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. Br Med J 1: 507–510, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Straub SV, Bonev AD, Wilkerson MK, Nelson MT. Dynamic inositol trisphosphate-mediated calcium signals within astrocytic endfeet underlie vasodilation of cerebral arterioles. J Gen Physiol 128: 659–669, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Straub SV, Nelson MT. Astrocytic calcium signaling: the information currency coupling neuronal activity to the cerebral microcirculation. Trends Cardiovasc Med 17: 183–190, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sun W, McConnell E, Pare JF, Xu Q, Chen M, Peng W, Lovatt D, Han X, Smith Y, Nedergaard M. Glutamate-dependent neuroglial calcium signaling differs between young and adult brain. Science 339: 197–200, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tagami M, Kubota A, Nara Y, Yamori Y. Detailed disease processes of cerebral pericytes and astrocytes in stroke-prone SHR. Clin Exp Hypertens A 13: 1069–1075, 1991 [DOI] [PubMed] [Google Scholar]
- 162.Tagami M, Nara Y, Kubota A, Fujino H, Yamori Y. Ultrastructural changes in cerebral pericytes and astrocytes of stroke-prone spontaneously hypertensive rats. Stroke 21: 1064–1071, 1990 [DOI] [PubMed] [Google Scholar]
- 163.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci 9: 260–267, 2006 [DOI] [PubMed] [Google Scholar]
- 164.Takata N, Nagai T, Ozawa K, Oe Y, Mikoshiba K, Hirase H. Cerebral blood flow modulation by Basal forebrain or whisker stimulation can occur independently of large cytosolic Ca2+ signaling in astrocytes. PLos One 8: e66525, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Takayasu M, Kajita Y, Suzuki Y, Shibuya M, Sugita K, Hidaka H. A role of nitric oxide in vasomotor control of cerebral parenchymal arterioles in rats. J Auton Nerv Syst 49, Suppl: S63-S66, 1994 [DOI] [PubMed] [Google Scholar]
- 166.Tallant EA, Higson JT. Angiotensin II activates distinct signal transduction pathways in astrocytes isolated from neonatal rat brain. Glia 19: 333–342, 1997 [PubMed] [Google Scholar]
- 167.Thyssen A, Hirnet D, Wolburg H, Schmalzing G, Deitmer JW, Lohr C. Ectopic vesicular neurotransmitter release along sensory axons mediates neurovascular coupling via glial calcium signaling. Proc Natl Acad Sci USA 107: 15258–15263, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tomassoni D, Mancinelli G, Mignini F, Sabbatini M, Amenta F. Quantitative image analysis of choroid and retinal vasculature in SHR: a model of cerebrovascular hypertensive changes? Clin Exp Hypertens 24: 741–752, 2002 [DOI] [PubMed] [Google Scholar]
- 169.Toms NJ, Roberts PJ. Group 1 mGlu receptors elevate [Ca2+]i in rat cultured cortical type 2 astrocytes: [Ca2+]i synergy with adenosine A1 receptors. Neuropharmacology 38: 1511–1517, 1999 [DOI] [PubMed] [Google Scholar]
- 170.Toussay X, Basu K, Lacoste B, Hamel E. Locus coeruleus stimulation recruits a broad cortical neuronal network and increases cortical perfusion. J Neurosci 33: 3390–3401, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vaucher E, Hamel E. Cholinergic basal forebrain neurons project to cortical microvessels in the rat: electron microscopic study with anterogradely transported Phaseolus vulgaris leucoagglutinin and choline acetyltransferase immunocytochemistry. J Neurosci 15: 7427–7441, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vaucher E, Linville D, Hamel E. Cholinergic basal forebrain projections to nitric oxide synthase-containing neurons in the rat cerebral cortex. Neuroscience 79: 827–836, 1997 [DOI] [PubMed] [Google Scholar]
- 173.Vaucher E, Tong XK, Cholet N, Lantin S, Hamel E. GABA neurons provide a rich input to microvessels but not nitric oxide neurons in the rat cerebral cortex: a means for direct regulation of local cerebral blood flow. J Comp Neurol 421: 161–171, 2000 [PubMed] [Google Scholar]
- 174.Vetri F, Xu H, Mao L, Paisansathan C, Pelligrino DA. ATP hydrolysis pathways and their contributions to pial arteriolar dilation in rats. Am J Physiol Heart Circ Physiol 301: H1369–H1377, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vollmer DG, Takayasu M, Dacey RG., Jr An in vitro comparative study of conducting vessels and penetrating arterioles after experimental subarachnoid hemorrhage in the rabbit. J Neurosurg 77: 113–119, 1992 [DOI] [PubMed] [Google Scholar]
- 176.Waeber C, Moskowitz MA. Migraine as an inflammatory disorder. Neurology 64: S9–S15, 2005 [DOI] [PubMed] [Google Scholar]
- 177.Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci 9: 816–823, 2006 [DOI] [PubMed] [Google Scholar]
- 178.Watts SW, Finta KM, Lloyd MC, Storm DS, Webb RC. Enhanced vascular responsiveness to Bay K 8644 in mineralocorticoid- and N-nitro-arginine-induced hypertension. Blood Press 3: 340–348, 1994 [DOI] [PubMed] [Google Scholar]
- 179.Wendland B, Schweizer FE, Ryan TA, Nakane M, Murad F, Scheller RH, Tsien RW. Existence of nitric oxide synthase in rat hippocampal pyramidal cells. Proc Natl Acad Sci USA 91: 2151–2155, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.White TD. Characteristics of neuronal release of ATP. Prog Neuropsychopharmacol Biol Psychiatry 8: 487–493, 1984 [DOI] [PubMed] [Google Scholar]
- 181.Winquist RJ, Bohr DF. Structural and functional changes in cerebral arteries from spontaneously hypertensive rats. Hypertension 5: 292–297, 1983 [DOI] [PubMed] [Google Scholar]
- 182.Wroblewska B, Wegorzewska IN, Bzdega T, Olszewski RT, Neale JH. Differential negative coupling of type 3 metabotropic glutamate receptor to cyclic GMP levels in neurons and astrocytes. J Neurochem 96: 1071–1077, 2006 [DOI] [PubMed] [Google Scholar]
- 183.Xu HL, Mao L, Ye S, Paisansathan C, Vetri F, Pelligrino DA. Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. Am J Physiol Heart Circ Physiol 294: H622–H632, 2008 [DOI] [PubMed] [Google Scholar]
- 184.Yaksh TL, Wang JY, Go VL. Cortical vasodilatation produced by vasoactive intestinal polypeptide (VIP) and by physiological stimuli in the cat. J Cereb Blood Flow Metab 7: 315–326, 1987 [DOI] [PubMed] [Google Scholar]
- 185.Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron 11: 371–386, 1993 [DOI] [PubMed] [Google Scholar]
- 186.Zhang F, Xu S, Iadecola C. Role of nitric oxide and acetylcholine in neocortical hyperemia elicited by basal forebrain stimulation: evidence for an involvement of endothelial nitric oxide. Neuroscience 69: 1195–1204, 1995 [DOI] [PubMed] [Google Scholar]
- 187.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 6: 43–50, 2003 [DOI] [PubMed] [Google Scholar]
- 188.Zonta M, Sebelin A, Gobbo S, Fellin T, Pozzan T, Carmignoto G. Glutamate-mediated cytosolic calcium oscillations regulate a pulsatile prostaglandin release from cultured rat astrocytes. J Physiol 553: 407–414, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]