Abstract
Mena, a member of the Ena/VASP family of actin regulatory proteins, modulates microfilaments and interacts with cytoskeletal proteins associated with heart failure. Mena is localized at the intercalated disc (ICD) of adult cardiac myocytes, colocalizing with numerous cytoskeletal proteins. Mena's role in the maintainence of mechanical myocardial stability at the cardiomyocyte ICD remains unknown. We hypothesized that Mena may modulate signals from the sarcolemma to the actin cytoskeleton at the ICD to regulate the expression and localization of connexin 43 (Cx43). The small GTPase Rac1 plays a pivotal role in the regulation of actin cytoskeletal reorganization and mediating morphological and transcriptional changes in cardiomyocytes. We found that Mena is associated with active Rac1 in cardiomyocytes and that RNAi knockdown of Mena increased Rac1 activity significantly. Furthermore, Mena knockdown increased Cx43 expression and altered Cx43 localization and trafficking at the ICD, concomitant with faster intercellular communication, as assessed by dye transfer between cardiomyocyte pairs. In mice overexpressing constitutively active Rac1, left ventricular Mena expression was increased significantly, concomitant with lateral redistribution of Cx43. These results suggest that Mena is a critical regulator of the ICD and is required for normal localization of Cx43 in part via regulation of Rac1.
Keywords: Mena, connexin 43, heart failure
heart failure (HF) is a devastating disease with a poor clinical prognosis. Gene expression analyses have revealed associations between expression patterns and HF pathogenesis (4). Previously, we reported altered expression of the mammalian homologue of Drosophila enabled, Mena, among the top-ranked genes that could blindly predict HF (5). Mena gene and protein expression is upregulated in both human and animal HF models (3). Normal cardiac expression of Mena is downregulated from neonate to adult. Therefore, increased Mena in HF corresponds to fetal gene activation, a hallmark of the altered molecular response in HF.
Mena belongs to the Ena/VASP (vasodilator-stimulated phosphoprotein) family of proteins that regulate cell movement and shape by controlling the geometry of the actin filament network. In the adult heart, Mena is associated with focal adhesion sites and is localized to the intercalated disc (ICD) of cardiomyocytes (10). Cell membranes of two adjacent cardiomyocytes are extensively intertwined in this region, bound together by desmosomes, adherens junction (cadherin, catenins, and vinculin), and gap junction (connexins) proteins (19). Recent data suggest that Mena may interact with ICD proteins in the heart; the EVH1 domain of Mena binds to vinculin (6), and Mena colocalizes with cadherin, as well connexin 43 (Cx43), the major ventricular isoform (7).
Disruption and alterations of the cytoskeleton contribute to cardiac remodeling in HF (12). Alterations of ICD proteins, which affect both their structural integrity and transmission of intracellular signals, promote dilated cardiomyopathy in mice (9). Gap junctions facilitate impulse propagation between adjacent myocytes for synchronized cardiac activity. Cx43 remodeling, including changes in protein expression, distribution, and turnover, contributes to nonuniformity in conduction and arrhythmia (16). We have shown recently that genetic ablation of Mena results in mild cardiac dysfunction and conduction abnormalities, accompanied by ICD destabilization, and Cx43 remodeling (3). Currently, the role of Mena in maintaining mechanical myocardial stability and cardiac conduction remains poorly understood.
The regulation of cell shape, migration, proliferation, and survival requires a balance between cell adhesion and cell motility signals. The Rho family of small GTPase proteins (Rho, Rac, and Cdc42) plays a pivotal role in coordinating these processes (8). Rac1 is active when GTP bound, and increased Rac1 activation is implicated in hypertrophic remodeling and cardiomyopathy (21). Given our prior Mena studies, we sought to investigate a direct role for Mena-dependent regulation of the ICD, specifically in the cardiomyocyte. We hypothesized that Mena modulates signals from cardiac sarcolemma to the actin cytoskeleton at the ICD that control Cx43 expression and localization via the small GTPase Rac1. We report that Mena associates with active Rac1 in cardiomyocytes and that Mena knockdown increases Rac1 activity. Reduced Mena expression altered localization of Cx43, increased Cx43 protein expression, and enhanced intercellular coupling between adjacent cardiomyocytes. These results suggest Mena as a critical regulator of the ICD at the intersection of Cx43 and Rac1.
MATERIALS AND METHODS
Neonatal rat ventricular cardiomyocyte isolation.
Care and experimentation with rats was performed under the auspices of the animal protocol that was approved by the Cincinnati Children's Research Foundation's Institutional Animal Care and Use Committee. Primary cultures from 1- to 2-day-old Sprague-Dawley rats were performed and maintained as described previously (18). Ventricles were minced and dissociated via enzymatic digestion, and nonmyocytes were removed by preplating. Myocytes were cultured on collagen IV-coated plates in M199 supplemented with 5% serum and antibiotics. After overnight incubation, cells were rinsed and replaced with fresh media and additives as indicated for specific experiments.
Animal studies.
Explanted hearts from wild-type and transgenic mice with constitutively active Rac1 under the control of the α-myosin heavy chain promoter Rac1ET (21) were kindly provided by Dr. Mark Sussman (University of California, San Diego, CA). The hearts of six controls and four transgenics were used to evaluate protein expression by Western analysis and confocal immunofluorescence.
Mena gene silencing with siRNA transfection.
Cardiomyocyte transfection with predesigned siRNA targeting rat Mena mRNA (Dharmacon-Smart pool) or scrambled (SCR) siRNA to a final concentration of 50 nM was performed using Lipofectamine RNAimax (Invitrogen). Cardiomyocytes were transfected in antibiotic-free medium containing Opti-MEM (Invitrogen) for 5 h. The cells were harvested after 72 h of transfection for experimentation.
Rac1 GTPase pulldown assay.
Fresh lysates containing protease inhibitors were cleared by centrifugation. Active Rac1 was precipitated with the p21-binding domain of human p21-activated kinase 1 (Pak1) fused to glutathione-S-transferase (GST)-Pak1 (Thermo Fisher). Pulldown fraction was separated by SDS-PAGE and immunoblotted with Rac1 antibody, followed by horseradish peroxidase and enhanced chemiluminescence detection.
Cell death assays.
Apoptotic cells were detected using the Alexa 488-labeled dUTP nick end labeling (TUNEL; Invitrogen). TUNEL-positive nuclei and propidium iodide counterstain were visualized using a Nikon confocal microscope. In parallel, whole cell lysates were subjected to SDS-PAGE and immunoblotting for antiapototic Bcl2 and proapototic Bax proteins.
Immunoblotting.
Cells were lysed in mammalian protein extraction reagent buffer (Pierce) containing protease and phosphatase inhibitors. Equal amounts of protein were subjected to SDS-PAGE and immunoblotting. Signal intensity was quantified using National Institutes of Health Image J software. The arbitrary pixel densities of each protein were normalized to GAPDH. Mouse Mena monoclonal antibody was developed in the laboratory of Dr. Frank Gertler (Massachusetts Institute of Technology, Cambridge, MA). Cx43 antibody was purchased from Sigma, and anti-Bcl2 and Bax were purchased from Santa Cruz Biotechnology.
Immunofluorescence.
Cardiomyocytes were fixed in 4% paraformaldehyde, permeabilized, and stained with Cx43 and Alexa Fluor secondary antibody. α-Actinin or phalloidin detection for F-actin and 4,6-diamidino-2-phenylindole (DAPI) for nuclei were used in parallel. Cells were visualized with an Olympus/Nikon confocal microscope.
Fluorescence recovery after photobleaching.
Cell-to-cell communication via functional gap junction channels was assessed by gap-fluorescence recovery after photobleaching (FRAP) assay. Experiments were performed in a humidified chamber maintained at 37°C and 5% CO2. Live cardiomyocytes were loaded with 10 μm of 5,6 carboxyfluorescein diacetate (CFDA; Invitrogen) for 10 min. FRAP was recorded using an epifluorescence microscope (Olympus IX81) equipped with an Argon laser and charge-coupled device camera. After prebleach image acquisition, a single cell was bleached with maximal laser intensity. Dye recovery was acquired using a sample interval of 15 s for 20 time points. Fluorescence recovery was measured in the bleached cell using Slidebook and analyzed using Clampfit version 10.0. Postbleach intensity measurements of all samples were corrected using the equation Fn = (Ft − F0)/(F300 − F0), where Fn is the normalized fluorescence intensity, Ft is fluorescence intensity between 0 and 300 s, F0 is the fluorescence measured from the first postbleach image, and F300 is the fluorescent signal obtained at 300 s postbleach. The time constant of half-maximal recovery, tau, was estimated by fitting the normalized data to a single exponential fit equation.
Statistics.
Numerical data are presented as means ± SE. Analysis was performed using GraphPad Prism 4.0 (GraphPad, La Jolla, CA). Differences between treatment groups were analyzed by Student's t-test. P ≤ 0.05 was considered statistically significant.
RESULTS
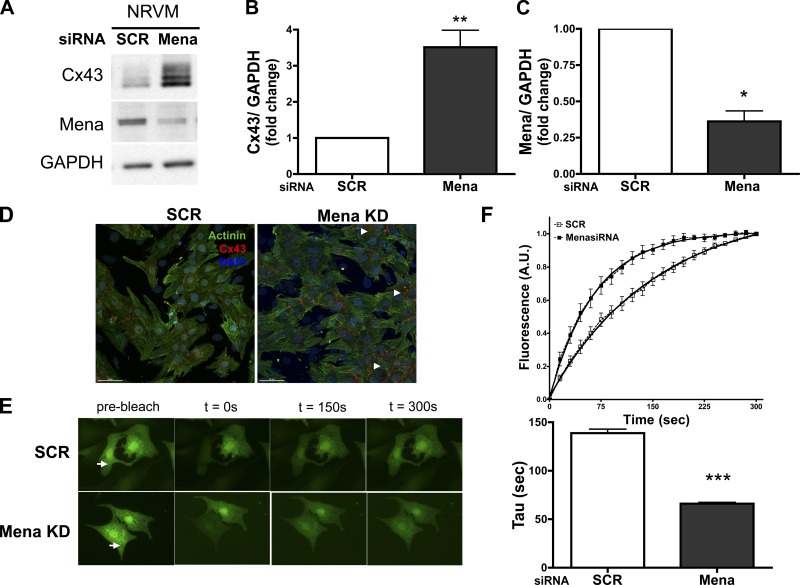
In this study, we explored a direct role for Mena in a cardiomyocyte-specific manner and determined mechanisms of Mena-mediated effects on the ICD. Cardiomyocytes were transfected with either Mena or SCR siRNA for 72 h. Knockdown of Mena by 63% tripled total Cx43 expression compared with control in cardiomyocytes (Fig. 1, A–C). The spatial localization of Cx43 in cardiomyocytes that were visualized using confocal microscopy (Fig. 1D) revealed that Cx43 is present primarily at cell-to-cell borders between adjacent cardiomyocytes in SCR cells. In contrast, Cx43 staining intensity is increased following Mena knockdown and, although present at cell-to-cell borders, is mislocalized along with aggregation within the cytoplasm of the cardiomyocyte. These results are in accordance with our prior findings in global Mena−/− mice (3) that display altered conduction, suggesting a direct role for Mena in cardiomyocytes and its contribution to disruption of Cx43 function and localization. As a consequence of increased Cx43 aggregation at cell borders observed by confocal microscopy, we predicted alterations in gap junction intercellular communication. Dye transfer assays of adjacent cardiomyocytes in the presence of the gap junction permeant fluorescent tracer 5,6-CFDA demonstrated faster fluorescence recovery of the bleached cell in Mena knockdown cardiomyocytes compared with SCR (Fig. 1, E and F). Mena siRNA cardiomyocytes significantly reduced the time constant of recovery tau twofold, indicating faster dye transfer between cardiomyocyte pairs.
Fig. 1.
Mena knockdown affects connexin 43 (Cx43) expression and localization and enhances intercellular communication in cardiomyocytes. Neonatal rat ventricular myocytes (NRVMs) were transfected with either scramble (SCR) or Mena siRNA (Mena KD). A–C: representative Western blot (A) and densitometry data (B and C) are summarized for Mena and Cx43 expression. *P ≤ 0.05, **P ≤ 0.01 vs. SCR; n = 5. Transfection efficiency was >63%. D: transfected cardiomyocytes are immunostained for Cx43 (red), actin (green), and 4,6-diamidino-2-phenylindole (DAPI; nuclei, blue) and Alexa Fluor secondary antibodies. Arrowheads indicate areas of Cx43 aggregation within the cytoplasm of cardiomyocytes. Scale bar, 10 μm. E: fluorescent images of cardiomyocytes after SCR or Mena siRNA before bleaching (arrow, prebleach) and after t = 0, 150 s, and 300 s of fluorescence recovery. F: time course plot of bleached cell fluorescence intensity is shown with fitted data for calculation of time constant of recovery (solid line) and the plot for time constant, tau (***P ≤ 0.0001 vs. SCR). Data were obtained from 4 separate experiments.
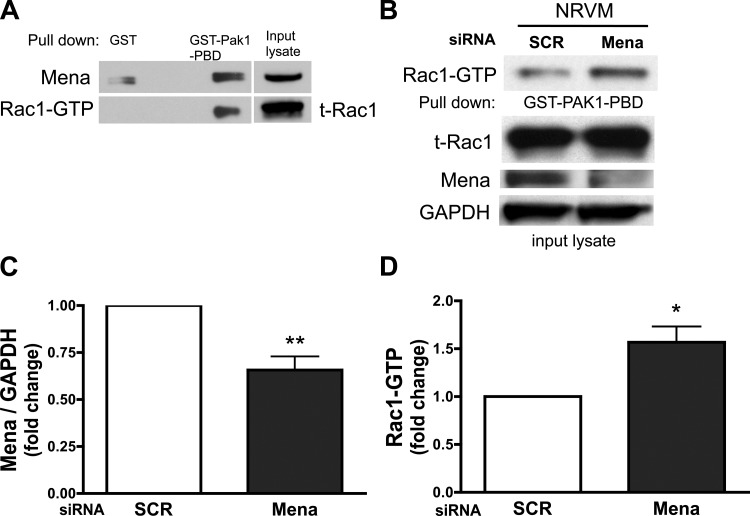
The Rho family of small GTPases is a major regulator of cellular junctions and the actin cytoskeleton. In particular, Rac1 has been proposed to signal through Ena/VASP (15). To delineate a role for Rac1 in the process of Mena-mediated regulation of the myocardial gap junction protein Cx43, the association of Rac1-GTP with Mena in cardiomyocytes was investigated using an in vitro GST pulldown assay. Mena is immunodetected in the pulldown lysate, suggesting that Mena interacts with active Rac1 in cardiomyocytes, although this interaction may be indirect, possibly occurring through Rac1 binding protein (Fig. 2A). To further explore the Mena-Rac1 interaction, we determined whether loss of Mena contributed directly to changes in Rac1 activity in cardiomyocytes. Knockdown of Mena significantly increases active Rac1-GTP twofold in cardiomyocytes compared with control (Fig. 2, B–D). These data suggest that Mena may modulate Rac1 activity in addition to alterations in Cx43 expression and localization in cardiomyocytes.
Fig. 2.
Mena associates with activated Rac1, and loss of Mena in cardiomyocytes significantly increases Rac1 activity. A: cardiomyocytes were incubated with GST-Pak1-p21 binding domain (PBD) or glutathione-S-transferase (GST) alone. After pulldown, active Rac1 (GTP bound) was detected by immunoblotting. Active Rac1 interacts with PBD domain of Pak1 and is absent in GST alone control lane. Mena is detected simultaneously in the same blot in Rac1-GTP lane. Whole input lysate was probed for total Rac1 (t-Rac1) and Mena. B: representative immunoblot is shown for Rac1-GTP, endogenous t-Rac1, Mena, and GAPDH in whole lysates. Lysates were subjected to GST-Pak1-PBD pulldown and SDS-PAGE gel electrophoresis. C and D: densitometry data is summarized for Mena (C) and Rac1-GTP (D). *P ≤ 0.05, **P ≤ 0.01 vs. SCR; n = 5, MenasiRNA transfection efficiency was 65%.
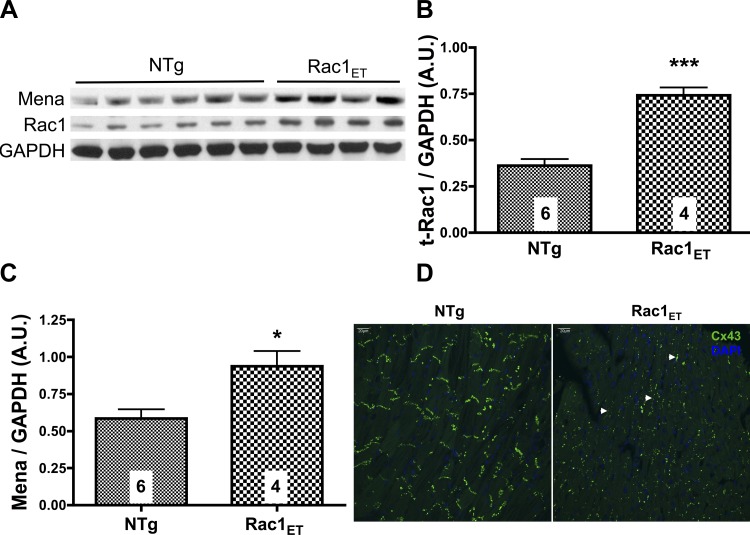
To further characterize the interaction of Rac1, Mena, and Cx43, we studied transgenic mouse hearts with cardiomyocyte-specific overexpression of constitutively active Rac1 (Rac1ET) (21). The Rac1ET hearts have a 30-fold upregulation of Rac1 activity compared with nontransgenic (NTg) wild-type hearts (1). The expression of total Rac1 was evaluated, revealing a significant increase in Rac1ET ventricular lysates compared with NTg (Fig. 3, A and B). Mena protein expression is also significantly upregulated in Rac1ET ventricular lysates compared with NTg (Fig. 3C). Because Rac1 transgenic mice display a cardiomyopathy phenotype, increases in Mena expression in this model are consistent with our previous findings of Mena upregulation in human and several animal models of HF (3). We next examined the effect of active Rac1 overexpression on Cx43 localization (Fig. 3D). NTg left ventricular (LV) sections show that Cx43 immunolabeling is localized to the ICD region perpendicular to the longitudinal axis of the myocytes. In contrast, the distribution of Cx43 is affected at the longitudinal cell termini in LV sections from Rac1ET mice. Cx43 is remodeled to the lateral borders (Cx43 lateralization) parallel to the long axis of adjacent cardiomyocytes.
Fig. 3.
Constitutively active Rac1 transgenic (Rac1ET) cardiac overexpression increases Mena expression and Cx43 lateralization. A: left ventricular (LV) tissue lysates from nontransgenic (NTg) and Rac1ET mice were subjected to gel electrophoresis. Representative immunoblot is shown for Mena, t-Rac1, and GAPDH. B and C: densitometry data is summarized for t-Rac1 and Mena expression; *P ≤ 0.05, ***P ≤ 0.0001 vs. NTg. No. of animals/group is indicated in the bar chart. D: LV tissue sections from NTg (left) and Rac1ET (right) were immunostained with Cx43 and DAPI. Cx43 is typically localized perpendicular to the longitudinal axis of cardiomyocytes at the intercalated disc (ICD). Arrowheads point to areas of Cx43 lateralization showing localization parallel to the long axis of cardiomyocytes. Myocyte orientation is visualized from background green fluorescence. Confocal projection stack is 8 μm at ×40 magnification.
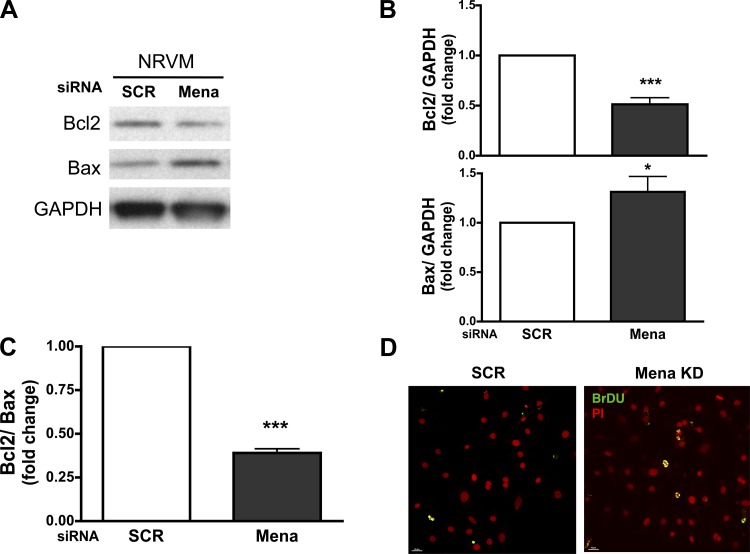
To determine whether increased Rac1 activation contributes directly to apoptosis in Mena-deficient cardiomyocytes, we evaluated the expression of apoptotic proteins Bax and Bcl2 using Western analysis. Bcl2 is significantly decreased in Mena siRNA knockdown cardiomyocytes. In contrast, the expression of Bax, a proapoptotic molecule, is significantly elevated. The fraction of Bcl2 signal relative to Bax expression shows a significant decrease following Mena knockdown in cardiomyocytes (Fig. 4, A–C). In association with the upregulation of apoptotic activity, we evaluated the presence of cells positive for nicked DNA using a TUNEL assay in cardiomyocytes transfected with SCR or Mena siRNA. As shown in Fig. 4D, cardiomyocytes transfected with Mena siRNA show an increase in TUNEL-positive cells that is indicative of increased cell death compared with SCR transfected cells.
Fig. 4.
Mena KD triggers apoptotic cell death in cardiomyocytes. Cardiomyocytes were transfected with SCR or Mena KD. A: representative immunoblot showing Bcl2, Bax, and GAPDH in whole lysates. B and C: densitometry data are summarized for Bcl2 and Bax (B) and Bcl2/Bax ratio (C); *P ≤ 0.05 and ***P ≤ 0.0001 vs. SCR; n = 4. Mena KD transfection efficiency was 65%. D: representative confocal image of TUNEL staining of cardiomyocytes in fixed cells following transfection. Merged images of double-labeling immunofluorescence for bromodeoxyuridine (BrdU) and propidium iodide are identified in yellow color, which detects apoptotic cells. Scale bar, 10 μm.
DISCUSSION
The present study addresses the cardiomyocyte-specific role of Mena and whether Mena is essential for maintenance of ICD stability and modulation of the gap junction protein Cx43 for normal cardiac function. Our key findings are that 1) knockdown of Mena in neonatal rat ventricular cardiomyocytes significantly increases Cx43 expression and alters normal cellular distribution of Cx43; 2) Mena associates with active Rac1-GTP in vitro, and loss of Mena increases Rac1 activity in cardiomyocytes; 3) Cx43 is redistributed to the lateral borders of the myocytes in mice expressing cardiomyocyte-restricted, constitutively active Rac1ET; and 4) knockdown of Mena triggers cardiomyocyte apoptosis.
Previously, we reported that global Mena knockout mice have mild cardiac dysfunction and arrhythmia associated with remodeling of Cx43 expression and localization (3). Consistent with this observation, in this study we report increased expression and altered localization of Cx43 expression following Mena knockdown concomitant with faster dye transfer between adjacent cardiomyocytes (Fig. 1). During the initial hypertrophic phase of HF, after acute injury or stretch, cardiac Cx43 expression is acutely modulated in the development of this adaptive response (22). This early compensatory phase is also accompanied by alterations of cytoskeletal proteins and reexpression of sarcomeric proteins. We also observed increased Mena protein expression in response to acute cyclic mechanical stretch in neonatal cardiomyocytes (data not shown). These data suggest a very tight interplay between mechanical and electrical activities. Mena's localization in the ICD and the response during hypertrophy and HF suggest its critical role in regulating this macromolecular complex and as an initial adaptive response gene.
One of the key findings in our study is that knockdown of Mena results in altered Cx43 localization and intercellular accumulation within the cytoplasm of cardiomyocytes. The half-life of Cx43 is 1–3 h (17); its delivery to the membrane, maintenance at the ICD, and internalization are dynamic processes. Accordingly, Cx43 hemichannels are packaged into vesicles and targeted to reach the plasma membrane via microtubules to form gap junction plaques at cell-to-cell junctions. The actin microfilament is also implicated in regulation of intracellular vesicular transport. In a recent study (20), Cx43 was demonstrated to colocalize with nonsarcomeric actin, and inhibition of actin significantly reduced delivery of Cx43 hemichannels to the plasma membrane. In fact, since microtubules are highly dynamic structures, Cx43 vesicles appeared to utilize actin filaments as transition points in the cytoplasm en route to the plasma membrane. Loss of Mena can impact these fundamental processes in maintaining actin organization, thus altering intracellular trafficking and maintenance of Cx43. Given Mena's role in actin polymerization and the importance of actin in Cx43 channel trafficking, we speculate that alterations in the Cx43 localization and aggregation may be due in part to effects on actin dynamics mediated by Mena.
The balance of cell adhesion and motility is important for an orchestrated function of the actin cytoskeleton. Rho, Rac1, and Cdc42 are well-established regulators of cellular junctions and the actin cytoskeleton. Increases in Rac1 activity have been implicated in mediating cardiac hypertrophy and in response to acute mechanical stretch (8). VASP (11) and Mena (13) are thought to be among several cytoskeletal-remodeling proteins such as Wiskott-Aldrich syndrome protein (WASP), Ras GTPase-activating-like protein 1 (IQGAP1), and Pak1 that are influenced by Rac1. Specifically in cardiomyocytes, the regulation of cytoskeletal dynamics and their contribution to cardiovascular pathology in the context of Rac1 are still areas of open investigation. We demonstrate here that Mena is associated with active Rac1 in cardiomyocytes and that Mena knockdown increases Rac1 activation in cardiomyocytes (Fig. 2). Although our data demonstrate that Mena associates with Rac1-GTP, we cannot rule out the possibility of an indirect interaction via a Rac1 binding partner. Data from the Rac1-GTP myocardial transgenic mice, which display cardiomyopathy and altered focal adhesions, further suggest a comodulatory role for Mena and Rac1-GTP (21).
Previously, in Rac1ET-overexpressing mice, it was shown that increased Rac1 activity was associated with changes in Cx43 expression (2). We show a redistribution of Cx43 to lateral borders of myocytes in the left ventricle of Rac1ET-overexpressing mice, suggesting that, in addition to modulating gap junction expression, increased Rac1 activity can also impact Cx43 localization. Rac1 has been implicated in modulating Cx43 expression and localization. Based on our findings, we suggest that modulating Mena may affect pathways in Rac1 signaling, thereby modulating Cx43 maintenance and localization at the ICD. Rac1 has also been shown to modulate induction of apoptosis in the heart. Increased Rac1 activation following β-adrenergic stimulation in cultured adult cardiomyocytes (14) resulted in apoptotic cell death. In our study, increases in Rac1 activity following Mena knockdown were associated with apoptosis in cardiomyocytes (Fig. 4) and are consistent with our findings in Mena knockout mice (unpublished data). Increased Cx43 expression and intercellular communication were observed with loss of Mena; transfer of apoptotic signals via gap junctions may mitigate cell survival in the setting of Mena knockdown, contributing to HF pathophysiology. In our most recent study of transgenic cardiac Mena-overexpressing mice (4a), we demonstrated that turning off the Mena gene prior to pathological cardiac injury mitigated hypertrophy in the setting of reduced cardiac function. It will be of interest to determine whether maintaining appropriate levels of Mena will mitigate cellular apoptosis and thus improved cell survival.
In summary, we demonstrate that the actin regulatory protein Mena is a critical regulator of the ICD at the intersection of the Rac1 and Cx43 in neonatal rat ventricular cardiomyocytes. Mena's role in the actin cytoskeleton along with other proteins may modulate the signaling domains contributing to this observation. Indeed, it appears that a complex interplay of cytoskeletal elements regulates the ICD and maintenance of gap junction. Future investigations regarding the role of Mena in gap junction regulation, particularly in the adult cardiomyocyte, may lead to development of methods to enhance and preserve the ICD, possibly mitigating myocardial conduction abnormalities.
GRANTS
This study was supported by American Heart Association 10POST4150039 (R. Ram) and National Heart, Lung, and Blood Institute Grants R01-HL-089885 and R01-HL-091475 (B. C. Blaxall).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.R., R.T.D., and B.C.B. contributed to the conception and design of the research; R.R., A.P.W., and K.V. performed the experiments; R.R., A.P.W., K.V., R.T.D., and B.C.B. analyzed the data; R.R., A.P.W., K.V., R.T.D., and B.C.B. interpreted the results of the experiments; R.R. and R.T.D. prepared the figures; R.R. drafted the manuscript; R.R., A.P.W., K.V., R.T.D., and B.C.B. edited and revised the manuscript; R.R., R.T.D., and B.C.B. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We acknowledge the support of Dr. Mark Sussman for providing us Rac1ET transgenic mice hearts, Dr. Frank Gertler for providing us Mena antibody and careful review of the manuscript, Tamlyn Thomas, Dr. Keigi Fujiwara, Samantha Lomber, and Linda Groom for their expert technical guidance, and Drs. Stephen Belmonte and Emily Schulz for their valuable insight.
REFERENCES
- 1.Adam O, Frost G, Custodis F, Sussman MA, Schafers HJ, Bohm M, Laufs U. Role of Rac1 GTPase activation in atrial fibrillation. J Am Coll Cardiol 50: 359–367, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Adam O, Lavall D, Theobald K, Hohl M, Grube M, Ameling S, Sussman MA, Rosenkranz S, Kroemer HK, Schäfers HJ, Böhm M, Laufs U. Rac1-induced connective tissue growth factor regulates connexin 43 and N-cadherin expression in atrial fibrillation. J Am Coll Cardiol 55: 469–480, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Aguilar F, Belmonte SL, Ram R, Noujaim SF, Dunaevsky O, Protack TL, Jalife J, Todd Massey H, Gertler FB, Blaxall BC. Mammalian enabled (Mena) is a critical regulator of cardiac function. Am J Physiol Heart Circ Physiol 300: H1841–H1852, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrans JD, Allen PD, Stamatiou D, Dzau VJ, Liew CC. Global gene expression profiling of end-stage dilated cardiomyopathy using a human cardiovascular-based cDNA microarray. Am J Pathol 160: 2035–2043, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Belmonte SL, Ram R, Mickelsen DM, Gertler FB, Blaxall BC. Cardiac overexpression of Mammalian enabled (Mena) exacerbates heart failure in mice. Am J Physiol Heart Circ Physiol 305: H875–H884, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaxall BC, Spang R, Rockman HA, Koch WJ. Differential myocardial gene expression in the development and rescue of murine heart failure. Physiol Genomics 15: 105–114, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Boeda B, Briggs DC, Higgins T, Garvalov BK, Fadden AJ, McDonald NQ, Way M. Tes, a specific Mena interacting partner, breaks the rules for EVH1 binding. Mol Cell 28: 1071–1082, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Eigenthaler M, Engelhardt S, Schinke B, Kobsar A, Schmitteckert E, Gambaryan S, Engelhardt CM, Krenn V, Eliava M, Jarchau T, Lohse MJ, Walter U, Hein L. Disruption of cardiac Ena-VASP protein localization in intercalated disks causes dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 285: H2471–H2481, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 420: 629–635, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Ferreira-Cornwell MC, Luo Y, Narula N, Lenox JM, Lieberman M, Radice GL. Remodeling the intercalated disc leads to cardiomyopathy in mice misexpressing cadherins in the heart. J Cell Sci 115: 1623–1634, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Gambaryan S, Hauser W, Kobsar A, Glazova M, Walter U. Distribution, cellular localization, and postnatal development of VASP and Mena expression in mouse tissues. Histochem Cell Biol 116: 535–543, 2001 [DOI] [PubMed] [Google Scholar]
- 11.García Arguinzonis MI, Galler AB, Walter U, Reinhard M, Simm A. Increased spreading, Rac/p21-activated kinase (PAK) activity, and compromised cell motility in cells deficient in vasodilator-stimulated phosphoprotein (VASP). J Biol Chem 277: 45604–45610, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Heling A, Zimmermann R, Kostin S, Maeno Y, Hein S, Devaux B, Bauer E, Klovekorn WP, Schlepper M, Schaper W, Schaper J. Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ Res 86: 846–853, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Higashi M, Ishikawa C, Yu J, Toyoda A, Kawana H, Kurokawa K, Matsuda M, Kitagawa M, Harigaya K. Human Mena associates with Rac1 small GTPase in glioblastoma cell lines. PLoS One 4: e4765, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito M, Adachi T, Pimentel DR, Ido Y, Colucci WS. Statins inhibit beta-adrenergic receptor-stimulated apoptosis in adult rat ventricular myocytes via a Rac1-dependent mechanism. Circulation 110: 412–418, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Kang N, Yaqoob U, Geng Z, Bloch K, Liu C, Gomez T, Billadeau D, Shah V. Focal adhesion assembly in myofibroblasts fosters a microenvironment that promotes tumor growth. Am J Pathol 177: 1888–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kostin S. Zonula occludens-1 and connexin 43 expression in the failing human heart. J Cell Mol Med 11: 892–895, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci USA 99: 10446–10451, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oxford EM, Musa H, Maass K, Coombs W, Taffet SM, Delmar M. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ Res 101: 703–711, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Severs NJ. The cardiac gap junction and intercalated disc. Int J Cardiol 26: 137–173, 1990 [DOI] [PubMed] [Google Scholar]
- 20.Smyth JW, Vogan JM, Buch PJ, Zhang SS, Fong TS, Hong TT, Shaw RM. Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ Res 110: 978–989, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sussman MA, Welch S, Walker A, Klevitsky R, Hewett TE, Price RL, Schaefer E, Yager K. Altered focal adhesion regulation correlates with cardiomyopathy in mice expressing constitutively active rac1. J Clin Invest 105: 875–886, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhuang J, Yamada KA, Saffitz JE, Kleber AG. Pulsatile stretch remodels cell-to-cell communication in cultured myocytes. Circ Res 87: 316–322, 2000 [DOI] [PubMed] [Google Scholar]