Abstract
The singly coded gene O-linked-β-N-acetylglucosamine (O-GlcNAc) transferase (Ogt) resides on the X chromosome and is necessary for embryonic stem cell viability during embryogenesis. In mature cells, this enzyme catalyzes the posttranslational modification known as O-GlcNAc to various cellular proteins. Several groups, including our own, have shown that acute increases in protein O-GlcNAcylation are cardioprotective both in vitro and in vivo. Yet, little is known about how OGT affects cardiac function because total body knockout (KO) animals are not viable. Presently, we sought to establish the potential involvement of cardiomyocyte Ogt in cardiac maturation. Initially, we characterized a constitutive cardiomyocyte-specific (cm)OGT KO (c-cmOGT KO) mouse and found that only 12% of the c-cmOGT KO mice survived to weaning age (4 wk old); the surviving animals were smaller than their wild-type littermates, had dilated hearts, and showed overt signs of heart failure. Dysfunctional c-cmOGT KO hearts were more fibrotic, apoptotic, and hypertrophic. Several glycolytic genes were also upregulated; however, there were no gross changes in mitochondrial O2 consumption. Histopathology of the KO hearts indicated the potential involvement of endoplasmic reticulum stress, directing us to evaluate expression of 78-kDa glucose-regulated protein and protein disulfide isomerase, which were elevated. Additional groups of mice were subjected to inducible deletion of cmOGT, which did not produce overt dysfunction within the first couple of weeks of deletion. Yet, long-term loss (via inducible deletion) of cmOGT produced gradual and progressive cardiomyopathy. Thus, cardiomyocyte Ogt is necessary for maturation of the mammalian heart, and inducible deletion of cmOGT in the adult mouse produces progressive ventricular dysfunction.
Keywords: cardiac function, hypertrophy, remodeling, metabolism, O-linked-β-N-acetylglucosamine transferase, uridine diphospho-N-acetylglucosamine:polypeptide β-N-acetylglucosaminyltransferase
the postpartum transition affects each organ system differently. In the cardiovascular system, many changes must occur for the heart to withstand the higher hemodynamic afterload of adulthood. These changes include the early separation of the circulatory system into left and right paths. This is, in part, accomplished by closure of the anatomic shunts, which confines the pulmonary circulation to the newly ventilated lungs. The myocardium must also undergo further maturation in response to the increased workload caused by rising systemic resistance. In the mouse, like the human, this postnatal development is accomplished by both hyperplasia and hypertrophy. Yet, many of the mechanisms regulating aspects of this transition remain unknown.
O-linked-β-N-acetylglucosamine (O-GlcNAc) is an important cellular signal (3, 11, 22, 24). The high-energy donor for this modification, UDP-GlcNAc, is derived from glucose via the hexosamine biosynthetic pathway. UDP-GlcNAc is added to serine and threonine residues by the evolutionarily conserved enzyme O-GlcNAc transferase (OGT), where it remains until removed by another evolutionarily conserved enzyme, O-GlcNAcase. This modification is induced during acute stress events, and augmentation is cytoprotective (43). Recently, we and others have shown O-GlcNAc to be acutely cardioprotective both in vitro (5, 21, 23, 25, 26) and in vivo (17, 44). There is also increasing evidence showing that its dysregulation is involved in chronic diseases such as diabetes (6, 12, 29, 35), Alzheimer's disease (7, 37), and heart failure (39).
Located on the X chromosome, Ogt is also required for embryonic stem cell viability (27). Traditional germline deletion causes postimplantation embryo termination by embryonic day 5. Due to this early lethality, we know little regarding how O-GlcNAc signaling affects cardiac function. O-GlcNAc has been previously shown to be involved in both cardiac hypertrophy (2, 20, 39) and cell cycle functions (4, 35, 36). Thus, we hypothesized that OGT is essential in the maturing heart, which we investigated through the use of a cell-specific approach to remove OGT from the cardiomyocyte.
MATERIALS AND METHODS
Echocardiographic assessment.
Transthoracic echocardiography of the left ventricle (LV) was performed as previously described (39) with adjustments for the Vevo 770 (Toronto, ON, Canada) echocardiography system. Body temperature was maintained at 36.5–37.5°C using a rectal thermometer interfaced with a servo-controlled heat lamp. Mice were anesthetized with 2% isoflurane, maintained under anesthesia with 1.5% isoflurane, and then examined. Using the Vevo rail system, the mouse was placed chest up on an examination board interfaced with the Vevo 770. The board was outfitted with ECG electrodes for all limbs. Next, depilatory cream was applied to the mouse's chest and wiped clean to remove all hair in the area of interest. The 707-B (30 MHz) scan head was used to obtain two-dimensional images (100 frames/s) of the parasternal long axis. M-modes were taken from the same images. The probe was then rotated to acquire a short-axis view of the heart. Beginning at the level of the papillary muscles and moving apically, serial two-dimensional images were taken every millimeter. All measurements were taken using the Vevo 770's rail system to maintain probe placement and allow for minute adjustments of position. LV internal diameters during diastole and systole (LVIDd and LVIDs, respectively) were determined from long-axis M-modes along with heart rate (HR). LV fractional shortening (FS) was calculated as follows: FS (in %) = [(LVIDd − LVIDs)/LVIDd] × 100. Diastolic and systolic volumes were acquired by applying Simpson's rule of disks to serially acquired short-axis images. Stroke volume (SV) was calculated as follows: SV = diastolic volume − systolic volume. Ejection fraction (EF) was calculated as follows: EF (in %) = (SV/diastolic volume) × 100. Cardiac output was determined as SV × HR. Relative wall thickness was calculated as follows: (diastolic posterior wall thickness + diastolic anterior wall thickness)/LVIDd. The velocity of circumferential shortening corrected for HR (Vcfc) was calculated as follows: Vcfc = (FS/ejection time)/. All mice used in this study were on a C57BL/6J background. All animal procedures were performed in accordance with National Institutes of Health guidelines and approved by the Animal Care and Use Committee of University of Louisville.
Protein isolation, Western blot analysis, and histology.
Protein isolation, immunoblot analysis, and histological techniques were performed using previously published protocols (17, 21, 23, 25, 26, 39). Wheat germ agglutinin (WGA; Texas red-X conjugate, Invitrogen, Carlsbad, CA) was used to measure cardiomyocyte cross-sectional area. TUNEL (Promega, Madison, WI) was used to quantify apoptosis. WGA and TUNEL were imaged with a Nikon A1 Confocal microscope. Sections were stained with Mayer's hematoxylin (Electron Microscopy Sciences, Hatfield, PA) and eosin Y alcoholic solution (Sigma-Aldrich, St. Louis, MO) for histopathological assessment. Sections were imaged with a Nikon D5-Fi2 camera mounted on a Nikon Eclipse Ni light microscope. Fast green (EMD, Gibbstown, NJ) and Sirius red (Roboz, Washington, DC) were used to quantitate fibrosis. Antibodies used for Western blot analysis were OGT (2 μg, Sigma-Aldrich), O-GlcNAc (1 μg, Covance, Princeton, NJ), tubulin (2 μg, Sigma-Aldrich), actin (0.4 μg, Sigma-Aldrich), GAPDH (2.5 μg, Santa Cruz Biotechnology, Dallas, TX), cytochrome c oxidase subunit IV (5 μg, Cell Signaling, Beverly, MA), 78-kDa glucose-regulated protein (Grp78; 1:1,000, Cell Signaling), and protein disulfide isomerase (PDI; 1:1,000, Cell Signaling). Amido black was used to stain for total protein on Grp78 and PDI blots. Briefly, the membrane was stripped in 1× SDS-PAGE running buffer containing 100 mM DTT for 1 h, washed with distilled water, and then reactivated in 100% methanol. Twenty milliliters of 0.03% amido black 10B (Bio-Rad, Berkeley, CA) protein stain in 3% acetic acid was added to the blot. After removal of the amido black stain, the blot was destained with 3% glacial acetic acid. The membrane was then washed further in water and allowed to dry before being imaged.
RT-PCR and quantitative real-time PCR.
Total RNA from snap-frozen LV sections or isolated cardiomyocytes was extracted with TRIzol (Invitrogen). Total RNA levels were quantified using the ratio of absorbance at 260–280 nm with a NanoDrop 1000 Spectrophotometer (Thermo Scientific, Waltham, MA). To verify the purity of the sample, the absorbance ratio from 260 to 230 nm was used. We limited the use of RNA with a 260- to 230-nm ratio of >1.8. cDNA (20-μl final volume) was then synthesized from total RNA (500 ng) following the protocol in the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The relative level of mRNA transcripts was quantified by real-time PCR using fast SYBR Green (Applied Biosystems). The data generated were normalized to 18S rRNA cycle threshold (CT) values using the ΔΔCT comparative method. All primers used are shown in Table 1.
Table 1.
Primers used in the quantitative RT-PCR analysis
| Primer Name | Primer Sequence (5′-3′) |
|---|---|
| ANP | |
| Antisense | 5′-AAGCTGTTGCAGCCTAGTCC-3′ |
| Sense | 5′-CCTGTGTACAGTGCGGTGTC-3′ |
| ATP 5o | |
| Antisense | 5′-AAGGAAACGCTGTGGTCACT-3′ |
| Sense | 5′-CTATGCAACCGCCCTGTACT-3′ |
| BNP | |
| Antisense | 5′-CGATCCGGTCTATCTTCTGC-3′ |
| Sense | 5′-GGAAATGGCTCAGAGACAGC-3′ |
| COX IV | |
| Antisense | 5′-CAGCCAGAACCAGATGACAG-3′ |
| Sense | 5′-GGAGGTGGTGTCCCTACTGA-3′ |
| CPT1 | |
| Antisense | 5′-ATTTGCCGTAGAGGCTGAGA-3′ |
| Sense | 5′-CAAAGGTCGCTTCTTCAAGG-3′ |
| CPT2 | |
| Antisense | 5′-GATCCTTCATCGGGAAGTCA-3′ |
| Sense | 5′-TCCTCGATCAAGATGGGAAC-3′ |
| GAPDH | |
| Antisense | 5′-TCCTTGGAGGCCATGTGGGCCAT-3′ |
| Sense | 5′-TGATGACATCAAGAAGGTGGTGAAG-3′ |
| GFAT1 | |
| Antisense | 5′-GGACCGACTTCTGGTGGTAA-3′ |
| Sense | 5′-CCTCGTGATGTTTGCTCTCA-3′ |
| GFAT2 | |
| Antisense | 5′-TAAGAAGATGACCCGGTTGG-3′ |
| Sense | 5′-TCGGGGTACGAAGCAAATAC-3′ |
| GLUT1 | |
| Antisense | 5′-CACATACATGGGCACAAAGC-3′ |
| Sense | 5′-CTTTGTGTCTGCCGTGCTTA-3′ |
| GLUT4 | |
| Antisense | 5′-CTCAAAGAAGGCCACAAAGC-3′ |
| Sense | 5′-GAGTTAGCTGGGGTGGAACA-3′ |
| HEK1 | |
| Antisense | 5′-GAAGTCTCCCAGGCAGTCAG-3′ |
| Sense | 5′-GCTCAGAAAAGGGGGATTTC-3′ |
| HEK2 | |
| Antisense | 5′-TGAGGAGGATGCTCTGGTCT-3′ |
| Sense | 5′-CACTCCAGATGGCACAGAGA-3′ |
| MCAD | |
| Antisense | 5′-CCGCTGACCCATGTTTAGTT-3′ |
| Sense | 5′-GGTGTACAGGGGTGCAGACT-3′ |
| O-GlcNAcase | |
| Antisense | 5′-TGCTCAGCTTCTTCCACTGA-3′ |
| Sense | 5′-TGGAAGACCTTGGGTTATGG-3′ |
| OGT | |
| Antisense | 5′-ACGAAGATAAGCTGCCACAG-3′ |
| Sense | 5′-CTGTCACCCTTGACCCAAAT-3′ |
| PFK1 | |
| Antisense | 5′-TGGATAAGGCCAATCCTCAC-3′ |
| Sense | 5′-AGGTGACCAAAGACGTGACC-3′ |
| PGC-1α | |
| Antisense | 5′-GTGTGGTTTGCATGGTTCTG-3′ |
| Sense | 5′-AAACTTGCTAGCGGTCCTCA-3′ |
| PGC-1β | |
| Antisense | 5′-CCGGAGAGATTTCGGATGTA-3′ |
| Sense | 5′-CGTATTTGAGGACAGCAGCA-3′ |
| 18S rRNA | |
| Antisense | 5′-CCTCCAATGGATCCTCGTTA-3′ |
| Sense | 5′-AAACGGCTACCACATCCAAG-3′ |
| α-MHC | |
| Antisense | 5′-ACTCCGTGCGGATGTCAA-3′ |
| Sense | 5′-AACCAGAGTTTGAGTGACAGAATG-3′ |
| β-MHC | |
| Antisense | 5′-CTGGAAGGTGCTGTCCCCAGAT-3′ |
| Sense | 5′-CCTTGGTCCTTCAAGAGCTG-3′ |
ANP, atrial natriuretic peptide; ATP 5o, ; BNP, brain natriuretic peptide; COX IV, cytochrome c oxidase subunit IV; CPT, carnitine palmitoyltransferase; GFAT, glutamine fructose-6-phosphate amidotransferase; GLUT, glucose transporter; HEK, hexokinase; MCAD, medium-chain acyl-CoA dehydrogenase; O-GlcNAc, O-linked β-N-acetylglucosamine; OGT, O-GlcNAc transferase; PFK, phosphofructokinase; PGC, peroxisome proliferator-activated receptor-γ coactivator; MHC, myosin heavy chain.
Mitochondrial isolation.
Hearts were harvested from wild-type (WT) and constitutive cardiomyocyte-specific (cm)OGT (c-cmOGT) knockout (KO) mice, immediately rinsed with PBS, and placed in a Kontes Duall homogenizer (Kontes Glass, Vineland, NJ) containing 4 ml sucrose buffer A (300 mmol/l sucrose, 10 mmol/l Tris·HCl, 2 mmol/l EGTA, and 5 mg/ml BSA; pH 7.4) on ice. Hearts were homogenized using 12 strokes, and the homogenate was centrifuged at 2,000 g for 2 min at 4°C to remove cell debris. The supernatant was further centrifuged at 10,000 g for 5 min at 4°C to sediment impure mitochondria. Mitochondria were then washed twice with 1 ml of sucrose buffer A (minus BSA), purified by the addition of 19% Percoll (Sigma-Aldrich), and then centrifuged at 14,000 g for 10 min at 4°C. The mitochondrial pellet was then washed twice with 0.5 ml sucrose buffer B (300 mmol/l sucrose and 10 mmol/l Tris·HCl; pH 7.4). The purified mitochondrial pellet was resuspended in 0.5 ml sucrose buffer B. The mitochondrial protein concentration was determined using Bio-Rad protein assay buffer.
Mitochondrial O2 consumption.
The Seahorse Flux Analyzer (North Billerica, MA) was used to measure the O2 consumption of isolated mitochondria, similar to previously described reports of intact cells (31, 33). During sensor calibration, isolated mitochondria were seeded in a 50-μl volume of isolation buffer containing 5 μg protein/well in XF24 V7 cell culture microplates. After centrifugation of the plates at 200 g for 4 min at 4°C, 575 μl of respiration buffer (215 mmol/l mannitol, 75 mmol/l sucrose, 0.1% BSA, 20 mmol/l HEPES, 2 mmol/l MgCl2, and 2.5 mmol/l KH2PO4; pH 7.2) at 37°C were gently added to each well for a final volume of 625 μl/well at the beginning to the experiment. Plates were immediately placed into the calibrated seahorse XF24 flux analyzer for mitochondrial bioenergetics analyses. Pyruvate plus malate plus ADP, oligomycin, FCCP, and rotenone plus succinate were injected sequentially through ports A–D in Seahorse Flux Pak cartridges to yield final concentrations of 5 mmol/l pyruvate, 2.5 mmol/l malate, 1 mmol/l ADP, 1 μg/ml oligomycin, 1 μmol/l FCCP, 100 nmol/l rotenone, and 10 mmol/l succinate, respectively.
Statistical analyses.
Where appropriate, a two-tailed Student's t-test was performed for group comparisons. To determine differences from expected values, a Pearson's χ2-test was used. In all cases, P values of <0.05 were accepted as significant.
RESULTS
Ablation of cardiomyocyte Ogt increases perinatal mortality.
To determine if Ogt plays a role in postpartum cardiac function, we produced a c-cmOGT KO mouse. This was accomplished by crossing homozygous Ogt floxed mice (39) with an α-myosin heavy chain (MHC)-driven Cre recombinase transgenic line (1). The progeny were expected to have a Mendelian segregation of the transgenic/floxed gene (Fig. 1A). Female mice were expected at the same ratio with respect to the transgene but would only be heterozygous for the floxed allele. A 50:50 ratio with respect to sex and transgene was also expected but was not obtained. The actual results show (Fig. 1B) that significantly (P < 0.05) fewer male pups survived compared with female pups (41% to 59%, respectively) and significantly (P < 0.05) more of the surviving male mice were WT compared with c-cmOGT KO mice (88% to 12%, respectively). Surviving c-cmOGT KO male mice had visible signs of abnormal development. Female mice showed less severe non-Mendelian segregation (60% WT and 40% c-cmOGT KO mice, P < 0.05).
Fig. 1.
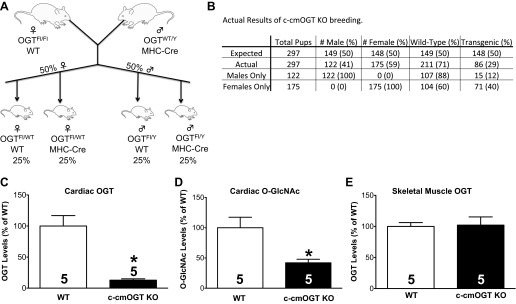
Breeding strategy and limited postnatal viability. A: homozygous O-linked-β-N-acetylglucosamine (O-GlcNAc) transferase (Ogt)-floxed (Fl) female mice were crossed with Ogt wild-type (WT) myosin heavy chain (MHC)-Cre transgenic male mice. This cross should have resulted in an even distribution of the MHC transgene (i.e., 50%) across both sexes. All male mice would be hemizygous (effectively homozygous) for the Ogt-floxed allele, whereas all female mice would be heterozygous. B: loss of Ogt did not produce the predicted Mendelian segregation. Tissues were harvested from 4-wk-old animals and subjected to biochemical analysis. C: expression of cardiac OGT was significantly reduced in constitutive cardiomyocyte-specific OGT (c-cmOGT) knockout (KO) mice. D: total cardiac O-GlcNAcylated protein levels were also significantly reduced compared with WT mice. E: to confirm the tissue specificity of Ogt ablation, skeletal muscle was removed and probed for OGT. No change was observed. *P < 0.05 vs. WT mice.
Due to the high mortality (95%) in adult (12 wk old) male c-cmOGT KO mice, we investigated male mice immediately after weaning. At this early age (4–5 wk), there was still a large disparity between WT and c-cmOGT KO mice, although more survived. We initially determined the efficiency and restriction of Ogt ablation by harvesting and snap freezing or preserving the tissues from all male weanlings. Tissues were then used for Western blot analysis or histological assessment.
Cardiac OGT protein levels were significantly reduced in c-cmOGT KO mice compared with WT mice (P < 0.05; representative image shown in Fig. 1C). The functional readout of OGT activity, total O-GlcNAcylated protein, was determined via Western blot analysis. Ablation of OGT significantly reduced total O-GlcNAcylated proteins in the heart (P < 0.05; Fig. 1D). Finally, we investigated the cardiac restriction of the transgene using a related tissue, skeletal muscle. Unlike the heart, levels of OGT in skeletal muscle were not altered (Fig. 1E), confirming that our transgene was restricted to cardiomyocytes. Taken together, these data show that we produced a c-cmOGT KO animal.
To ensure that the deleterious effects on cardiac function were not attributable to the MHC-Cre transgene itself, we examined an additional cardiomyocyte-specific Cre transgenic mouse. Male NKX2.5-Cre mice were crossed with homozygous Ogt-floxed mice to generate another line of c-cmOGT KO mice. As with MHC-Cre transgenic mice, there were similar trends in non-Mendelian distribution with respect to sex and transgene expression. Of the 21 male offspring, only 2 male offspring were Nkx positive; of the 34 female offspring, only 11 femal offspring were Nkx positive. These data support our conclusion that it is Ogt ablation (and not the transgene itself) that leads to severe abnormalities in cardiac maturation in c-cmOGT KO mice.
Cardiomyocyte ablation of Ogt induces gross abnormalities.
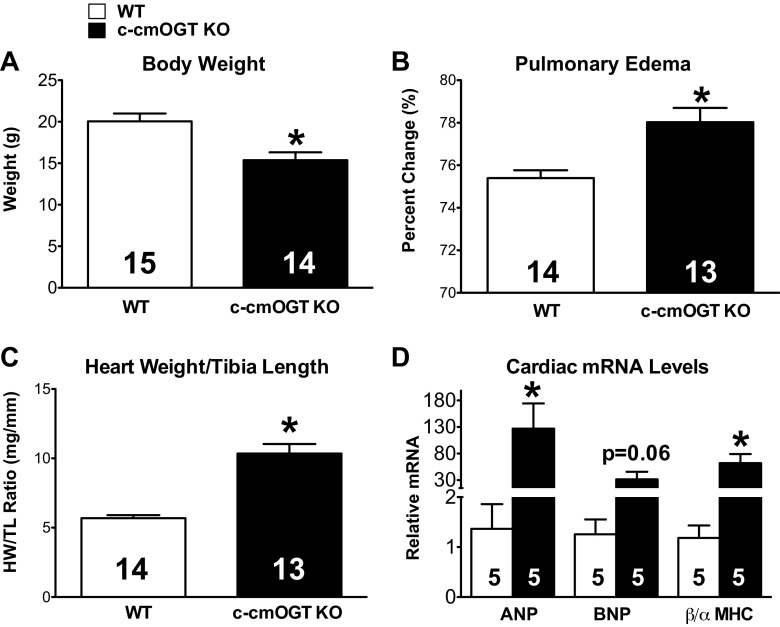
Cardiomyocyte ablation of Ogt also induced noticeable physical abnormalities. Mice were significantly smaller (P < 0.05; Fig. 2A) and severely frail, which are likely due to the robust depression in ventricular function discussed below. c-cmOGT KO mice had a significant amount of pulmonary edema, indicative of increased LV end-diastolic pressure (P < 0.05; Fig. 2B), as well as more massive hearts (P < 0.05; Fig. 2C), as evidenced by an elevated heart weight-to-tibia length ratio. We also assessed the expression of classic markers of heart failure by quantitative RT-PCR. Atrial natriuretic peptide and brain natriuretic peptide levels showed significant or trending (P < 0.05 and P = 0.06, respectively; Fig. 2D) elevation compared with WT animals. The ratio of β-MHC to α-MHC expression was also significantly (P < 0.05) elevated in c-cmOGT KO mice (Fig. 2D). Constitutive absence of cardiomyocyte OGT induced molecular markers classically associated with heart failure.
Fig. 2.
Gross phenotype of c-cmOGT KO mice. Constitutive ablation of cardiomyocyte Ogt led to gross physical changes. A: c-cmOGT KO mice weighed significantly less than their WT littermates. B: ablation of Ogt led to pulmonary edema. C: c-cmOGT KO mice had enlarged hearts. HW/TL ratio, heart weight-to-tibia length ratio. D: several classic markers of heart failure [atrial natriuretic peptide (ANP) and β-MHC] were also significantly elevated in c-cmOGT KO mice. The brain natriuretic peptide (BNP) elevation trended toward a difference but was P = 0.06. *P < 0.05 vs. WT mice.
Cardiomyocyte-specific loss of Ogt induces severe dilated cardiomyopathy.
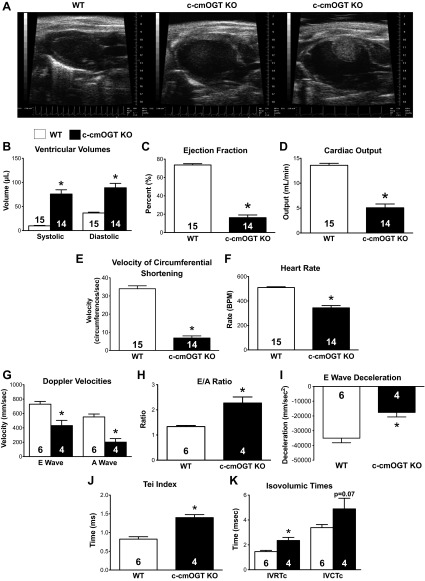
Immediately after weaning (4–5 wk), all surviving male c-cmOGT KO mice were subjected to echocardiography along with an equal number of WT littermates. c-cmOGT KO mice had severely dilated ventricles, a 23% prevalence of fibrous, and resolved clots (∼20 μl in volume), and 85% of c-cmOGT KO mice did not show a P wave in their echocardiography system-derived ECG recordings (Fig. 3A). c-cmOGT KO echocardiograms revealed increases in both systolic and diastolic volumes (P < 0.05; Fig. 3B). Although no initial difference in the diastolic wall thickness was determined, there was a significant reduction in the relative wall thickness. Systolic walls were thinner in the KO group and did not thicken as much as in the WT group (data not shown). The ventricular dilation led to significantly reduced SV and EF (P < 0.05; Fig. 3C) in c-cmOGT KO mice compared with WT mice. c-cmOGT KO mice also had significantly (P < 0.05) reduced cardiac output (Fig. 3D) and significantly (P < 0.05) reduced Vcfc, which is a load-dependent index of cardiac contractility (Fig. 3E). Along with these systolic defects, there was also significant (P < 0.05) bradycardia (Fig. 3F) and diastolic dysfunction. Doppler surveys of the mitral valve revealed that both passive (E wave) and active (A wave) filling of the ventricle were impaired (P < 0.05; Fig. 3G) and that transgenic hearts were being filled mostly by passive filling, as measured by the E-to-A ratio (P < 0.05; Fig. 3H). This filling was hindered by an increased ventricular stiffness, as evidenced by a decrease in the E wave deceleration (P < 0.05; Fig. 3I) (28). c-cmOGT KO mice had a significantly elevated Tei index (P < 0.05; Fig. 3J), which represents an additional index of cardiac performance. This increase in the Tei index was due to a prolongation of both isovolumic relaxation and isovolumic contraction times after correction for HR (IVRTc and IVCTc; P < 0.05 and P = 0.07, respectively; Fig. 3K). These data suggest that c-cmOGT KO mice have dilated cardiomyopathy with systolic and diastolic dysfunction.
Fig. 3.
Cardiac ablation of Ogt induces cardiomyopathy. By echocardiography, c-cmOGT KO mice were found to have severe dilated cardiomyopathy (representative echocardiograms are shown in A). B: ablation of cardiac Ogt led to increases in both systolic and diastolic volumes. c-cmOGT KO mice were also found to have decreased ejection fraction (C) and lowered cardiac output (D). E: c-cmOGT KO mice had a slower velocity of shortening, which is a measure of cardiac contractility. F: c-cmOGT KO mice showed a significant reduction in heart rate [in beats/min (BPM)] that was not remedied with anesthetic alterations. G: Doppler survey of the mitral valve revealed that passive (E wave) and active (A wave) filling of the ventricle was impaired. H: ventricles of c-cmOGT KO were filled mostly by passive filling, as denoted by the E-to-A ratio. I: the E wave deceleration time was reduced, suggesting a less compliant ventricle. J: the myocardial performance index (the Tei index) was also increased, suggesting poorer function in c-cmOGT KO mice. K: c-cmOGT KO mice had prolonged isovolumic relaxation and contraction times after correction for heart rate (IVRTc and IVCTc, respectively). *P < 0.05 vs. WT mice.
Cardiomyocyte ablation of Ogt induces pathological remodeling.
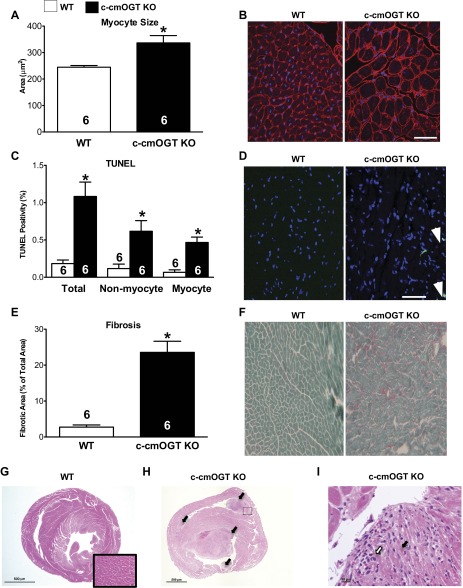
Cardiomyocytes in c-cmOGT KO hearts were significantly (P < 0.05) larger than their WT counterparts (Fig. 4, A and B), as determined by WGA staining. There was also a significant increase in TUNEL-positive nuclei in the c-cmOGT KO group compared with the WT group (Fig. 4, C and D); this increase was present in myocytic and nonmyocytic cell types. Finally, we quantified fibrosis by fast green-Sirius red staining. As indicated by the decreased E wave deceleration, c-cmOGT KO mice had significantly more interstitial fibrosis compared with their WT littermates (P < 0.05; Fig. 4, E and F). c-cmOGT KO mice had developed fibrotic, dilated cardiomyopathy.
Fig. 4.
Ventricular remodeling in c-cmOGT KO mice. After echocardiography, hearts were removed for histological assessment. c-cmOGT KO mice demonstrated more hypertrophy than their WT counterparts, as determined by wheat germ agglutinin staining (A); representative images are shown in B. Scale bar = 50 μm. C: constitutive ablation of Ogt led to an increase in the rate of TUNEL positivity in the hearts of c-cmOGT KO mice. This increase occurred in both myocyte and nonmyocyte cell fractions; representative images are shown in D. White arrows indicate TUNEL-positive nuclei. Scale bar = 50 μm. c-cmOGT KO mice also had an increase in interstitial fibrosis, as determined by fast green-sirius red staining (E); representative images are shown in F. G: cross-section of the myocardium from a WT (female) mouse showing intact cardiomyocytes (inset). H: multifocal areas of extensive papillary muscle necrosis (arrows) were present within both the right and left ventricular chambers of male c-cmOGT KO mice. I: higher-magnification image of the boxed region from H showing coagulative necrosis of cardiomyocytes (black arrow) and infiltrating neutrophils (white arrow). *P < 0.05 vs. WT mice.
Evaluation of routine hemotoxylin-eosin-stained myocardial sections from (homozygous) male c-cmOGT KO mice revealed multiple foci of marked to severe coagulative necrosis of the papillary muscle within the dilated ventricular chambers. In less severe cases, diffuse dissecting interstitial fibrosis separated the degenerating and atrophic cardiomyocytes. The mural cardiomyocytes showed variable amounts of degenerative and regenerative responses, including cytoplasmic vacuolization and “nuclear rowing,” respectively. No inflammatory response was present within the walls (Fig. 4, H and I). In some instances, hypereosinophilic staining was observed in areas corresponding to the ER, which is suggestive of protein accumulation.
Cardiomyocyte-specific loss of Ogt results in elevated ER stress.
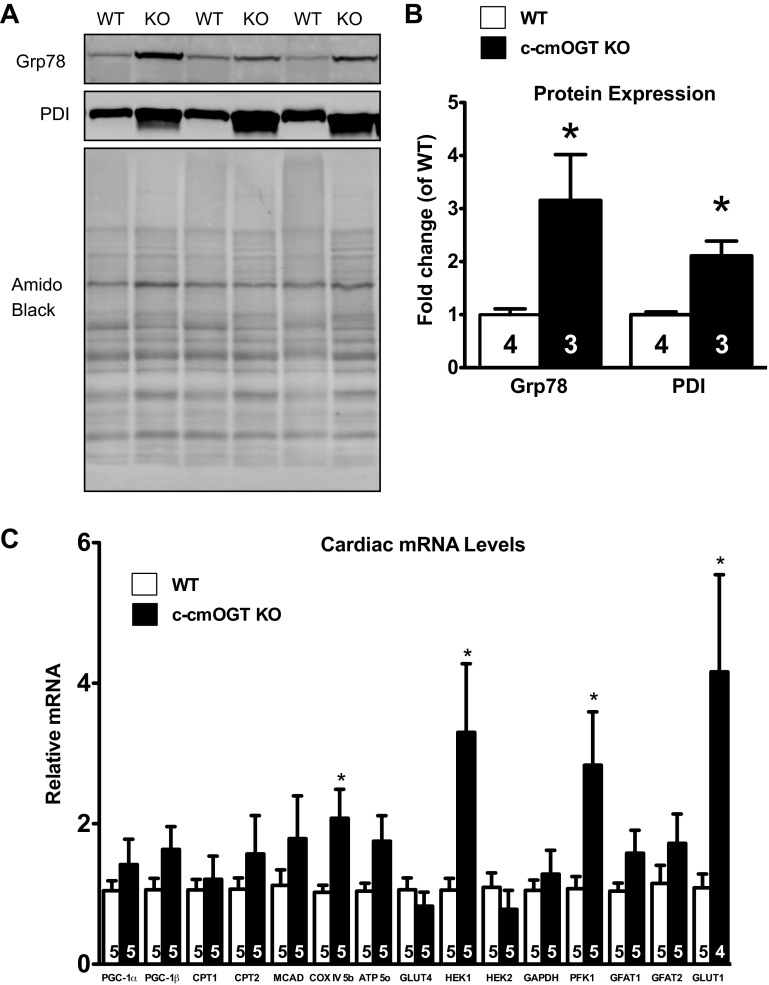
We have (23) previously demonstrated a relationship between ER stress and O-GlcNAcylation. Based on our previous work and results from the blinded histopathology (see above), we queried whether there was any evidence of ER stress in c-cmOGT KO hearts. To this end, several ER stress markers were evaluated by immunoblot analysis, including Grp78 and PDI (Fig. 5A). Protein levels of Grp78 and PDI were quantified and found to be significantly higher in c-cmOGT KO mice compared with WT mice (P < 0.05; Fig. 5B).
Fig. 5.
Cardiomyocyte Ogt deletion produces endoplasmic reticulum (ER) stress and alterations in metabolic gene expression. After echocardiography, hearts were removed and subjected to protein analysis and quantitative RT-PCR for metabolic genes. A: Western blot analysis showing elevated proteins associated with ER stress. Top, 78-kDa glucose-regulated protein (Grp78); middle, protein disulfide isomerase (PDI); bottom, amido black. B: quantification verified the significantly increased levels of Grp78 and PDI in c-cmOGT KO hearts. C: c-cmOGT KO mice had elevated levels of cytochrome-c oxidase (COX), hexokinase (HEK)1, phosphofructokinase (PFK)1, and glucose transporter (GLUT)1. PGC, peroxisome proliferator-activated receptor-γ coactivator; CPT, carnitine palmitoyltransferase; MCAD, medium-chain acyl-CoA dehydrogenase; ATP 5o, ATP synthase, subunit 5; GFAT, glutamine fructose-6-phosphate amidotransferase. *P < 0.05 vs. WT mice.
Cardiomyocyte-specific loss of Ogt alters the transcription of metabolism genes.
O-GlcNAcylation of proteins is known as a metabolic signal as well as a stress response. OGT itself has been shown to interact with peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α to regulate FoxO-1, which is involved with glucose metabolism in the liver (12, 13, 16). Some patients with heart failure have a downregulation of PGC-1α (34), and we (39) have previously implicated a dysregulation in PGC-1α-dependent signaling in the exacerbation of infarct-induced heart failure in Ogt-deficient mice. With this in mind, we addressed whether or not constitutive cardiomyocyte ablation of OGT induced an altered metabolic phenotype. We screened several PGC-dependent and glycolytic genes via quantitative RT-PCR and found that there was a significant increase in the amount of cytochrome c oxidase subunit IV, hexokinase, and phosphofructokinase (P < 0.05; Fig. 5C). There was also a significant (P < 0.05) increase in the amount of glucose transporter 1, suggesting that there may have been an increase in glucose metabolism, both aerobically and anaerobically.
To determine if there was an alteration in mitochondrial oxidative capacity, we extended our investigation to the level of mitochondrial function. After isolating a mitochondrial fraction, we used the Seahorse XF system to determine total mitochondrial O2 consumption. Isolated mitochondria were healthy and stable. We first determined O2 consumption derived from complex I by the addition of pyruvate, malate, and ADP. There was no difference in complex I-driven respiration. We next added oligomycin to determine the amount of uncoupled O2 consumption; again, there was no difference between the groups. Maximal respiration was determined by the addition of FCCP, which uncouples proton transport from ADP phosphorylation. There was no difference in maximal respiration between WT and c-cmOGT KO mice. Finally, we added rotenone, a complex I inhibitor, and succinate, a substrate of complex II, to determine substrate II-driven O2 consumption; again, there was no difference between the two groups (data not shown). Despite changes in several transcript levels, ablation of Ogt did not affect mitochondrial O2 consumption in isolated mitochondria. The possibility remains that there were, indeed, defects in metabolism not identified by this one assay.
Ogt heterozygosity causes gradual progressive cardiomyopathy.
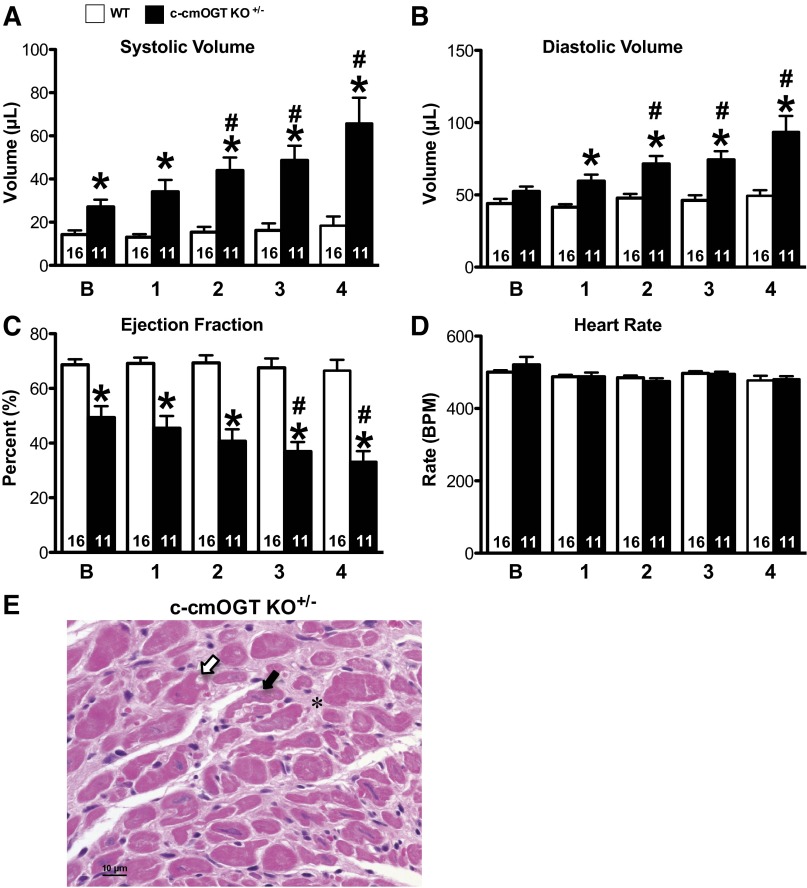
We also examined the impact of Ogt heterozygosity in female heterozygous-floxed/MHC-Cre transgenic (c-cmOGT KO+/−) mice and their nontransgenic (WT) littermates to ascertain if partial ablation of Ogt induced a cardiac phenotype. Adult (2–4 mo old) female mice were subjected to serial echocardiographic analysis. c-cmOGT KO+/− mice were found to have increased systolic (P < 0.05; Fig. 6A) and diastolic (P < 0.05; Fig. 6B) volumes. These increases in volume led to a reduction in EF (P < 0.05; Fig. 6C), although HR was unchanged (Fig. 6D). Not only did c-cmOGT KO+/− mice have a dilated cardiomyopathic phenotype at baseline, but also the dilation was persistent and progressed in severity throughout the course of observation.
Fig. 6.
Heterozygous female mice exhibit delayed cardiomyopathy and degeneration of cardiomyocytes. Baseline echocardiographic assessment was performed on 2- to 4-mo-old female mice and repeated monthly for 4 mo (x-axis labels).Heterozygous c-cmOGT KO+/− female mice had significantly larger systolic (A) and diastolic (B) volumes. C: ejection fraction was significantly reduced and decreased significantly over time. D: heart rate was not altered and remained stable throughout the 4-wk study. E: myocardial cross-section from a heterozygous female mouse. There were multifocal areas of cardiomyocyte degeneration with nuclear pyknosis (white arrow), cardiomyocyte atrophy (black arrow), and diffuse interstitial fibrosis (*). *P < 0.05 vs. time-matched control mice; #P < 0.05 vs. baseline of the same genotype.
Hearts from heterozygous female mice were also analyzed by hematoxylin-eosin staining. In heterozygous female mice, papillary muscle necrosis was observed, but not in all cases. In one case, chronic diffuse fibrosis, degeneration, and mild regeneration of cardiomyocytes were observed compared with other sections in which the lesions were more necrotizing and acute (Fig. 6E). As with homozygous male mice, hypereosinophilic staining was observed in areas corresponding to the ER in some heterozygous female mice. In another heterozygous female mouse, however, there was no evidence of fibrosis or degeneration.
OGT deletion in adult cardiomyocytes produces delayed cardiomyopathy.
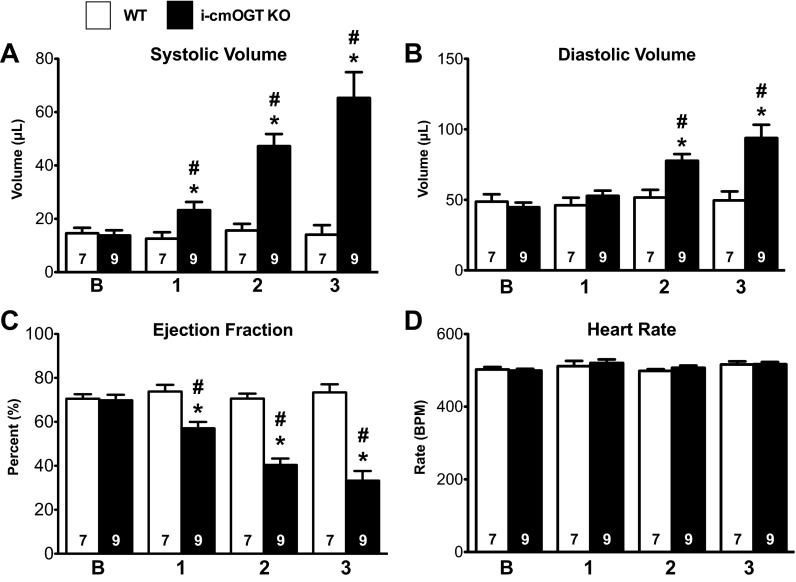
We next explored if cardiomyocyte OGT is necessary in the maintenance of cardiac function in the mature, fully developed adult myocardium. Homozygous Ogt floxed mice with the MerCreMer transgene (i-cmOGT KO mice) or without the MerCreMer transgene (WT mice) were given tamoxifen and followed for 3 mo with echocardiography. Beginning 1 mo after the induction of the transgene, i-cmOGT KO mice were found to have slightly increased systolic volumes (P < 0.05; Fig. 7A). This systolic dilation increased over the next 2 mo of the study. Diastolic volume increased later, but followed the same trend (Fig. 7B). Cardiac function, as measured by EF (P < 0.05; Fig. 7C), was also significantly (P < 0.05) reduced at 1 mo after induction of the transgene and continued to fall over the course of the study. HR (Fig. 7D) remained unchanged at all time points. Thus, Ogt expression is necessary for the long-term maintenance of cardiac function in the otherwise naïve heart.
Fig. 7.
Cardiomyocyte Ogt is also required for cardiac maintenance. Homozygous Ogt floxed mice with the MerCreMer transgene (i-cmOGT KO mice) were induced with tamoxifen, given a baseline echo after a tamoxifen washout period, and followed via echocardiography monthly for 3 mo. i-cmOGT KO mice were found to have slightly increased systolic (A) and diastolic (B) volumes after 1 mo of Ogt ablation; these increases were persistent and progressive throughout the course of study. C and D: the progressive expansion of ventricular volumes led to a reduction in ejection fraction (C), whereas heart rate remained constant (D). *P < 0.05 vs. WT mice; #P < 0.05 vs. baseline of the same genotype.
DISCUSSION
The goal of the present study was to elucidate the role of Ogt in the heart during maturation. Homozygous c-cmOGT KO mice were significantly less viable and exhibited severe cardiac dysfunction, which was evident by 4 wk of age, when all transgenic mice had overt signs of heart failure. Moreover, there were necrotizing and acute lesions, substantial papillary muscle necrosis, and apoptosis and fibrosis, both of which were exacerbated compared with WT mice and compared with our previously reported ligation-induced heart failure values (39). Loss of cardiomyocyte OGT expression also led to ER stress and the induction of several glycolytic genes. Thus, cardiomyocyte Ogt is absolutely necessary for normal cardiac function after parturition, whereas in the adult heart, loss of cardiomyocyte Ogt produces more gradual cardiomyopathy.
In addition to systolic dysfunction, c-cmOGT KO mice exhibited diastolic dysfunction. Ventricular filling was impaired, and IVRTc and IVCTc were greatly increased. The impairment in ventricular filling was likely due to the dilation and large increase in fibrosis; however, the increases in IVRTc and IVCTc could be due to alterations in intracellular Ca2+ handling. It is important to note that the mice also had significant bradycardia, although this could not explain the multiple severe contractile and fibrotic defects observed. It is possible that the bradycardia was secondary to the robust cardiac fibrosis and dysfunction; however, we cannot rule out a direct relationship between Ogt deficiency and electrical abnormalities in these mice. It is also possible, and even likely, that more than one pathological process is occurring simultaneously.
The data presented here describe the role of OGT in the immature heart and implicate several diverse pathways through which O-GlcNAc signaling may affect cardiac function or size. Others (14) have suggested that O-GlcNAcylation was partly responsible for the dysregulation of Ca2+ cycling in the diabetic myocardium. While our data do not directly address this issue, they do add weight to the hypothesis that O-GlcNAcylation of specific proteins could alter Ca2+ cycling. We show that in the absence of OGT (and therefore reduced O-GlcNAc signaling), the heart spends increased time in IVRTc and IVCTc. Cardiomyocyte hypertrophy could be indirectly related to alterations in intracellular Ca2+ handling, and, while there was evidence of hypertrophy in Ogt-deficient hearts, it was not as great compared with other cardiomyopathies or heart failure (39). Our previous studies have demonstrated a role for O-GlcNAcylation in the development of cardiomyocyte hypertrophy. On face value, this seems paradoxical, yet it is quite conceivable that other, more robust prohypertrophic stimuli outweighed the putative antihypertrophic loss of Ogt.
Another possible mechanism may involve the intense remodeling that occurs within these animals. Inflammation is a hallmark of heart failure (10), and chronic inflammation can induce heart failure with a predisposition to fibrosis (18). Previous reports have shown that O-GlcNAcylation is involved in reducing inflammatory responses by attenuating cell adhesion molecule expression (40) and by inhibiting NF-κB and TNF-α signaling (15, 41, 42). If OGT constitutively limits inflammation, these responses would likely be exacerbated without cardiomyocyte OGT. This increase in inflammation could explain the high TUNEL positivity found in OGT-competent nonmyocytic cells of c-cmOGT KO mice. Another explanation for this could be an alteration in the secretome of the myocytes, which could influence fibroblast survival and activity. Yet another possibly linked explanation could be that fibroblast turnover led to the high nonmyocytic TUNEL rate.
Along with these multifaceted, deleterious alterations in cardiac function and structure, we also found an augmentation of glycolytic genes (19). This increase agrees with past reports of metabolic changes in the failing heart. Unlike these previous reports, there was no significant decrease in the amount of PGC-dependent genes. This was quite surprising, as in most models of cardiac injury and stress, there is a distinct reduction in the expression of genes required for fatty acid metabolism (34). While there was an alteration in glycolytic enzymes, there was no effect on O2 consumption in isolated mitochondria, which is decreased in other cardiomyopathies (8).
ER stress is a major determinant of cell survival (9, 30, 32, 38). Based on the present histopathology and previous work from this laboratory (23), we hypothesized that the absence of OGT may be associated with ER stress. Previous work concluded that OGT overexpression reduces the maladaptive ER stress response. Conversely, we hypothesized that Ogt ablation should augment the observed ER stress response. Indeed, we observed elevated levels of both Grp78 and PDI, indicating an elevated ER stress response, within c-cmOGT KO hearts. Although causality cannot be established at this point, ER stress may be at least one contributing factor in the ventricular dysfunction and/or cardiomyocyte necrosis reported here.
Although the primary focus of this study was the constitutive deletion of Ogt, the findings from i-cmOGT KO mice are particularly intriguing. Ablation of Ogt in adult cardiomyocytes is largely unremarkable in terms of acute (<1 mo) effects; however, after several months, we note significant ventricular dysfunction. We (39) have previously shown that Ogt is not necessary for the maintenance of cardiac function in the adult heart over the course of a few weeks, although OGT activity seemed to be proadaptive during infarct-induced heart failure. On cursory inspection, it may appear that the present data (Fig. 7) run counter to such conclusions, but there are important differences to note. First, the present investigation of i-cmOGT KO mice took place over several months (not weeks, as before). Second, we used much more sensitive instrumentation here to interrogate ventricular function. Moreover, we were able to calculate ventricular volumes in the present study, which are more sensitive to small changes in ventricular geometry. Given the importance of Ogt, it is still quite remarkable that myocytes can live for extended periods of time in its absence.
In conclusion, in the present study, we provide direct genetic evidence showing that Ogt (and, by extension, O-GlcNAc signaling) plays a crucial role in the maturation of the cardiomyocyte. Cardiomyocyte Ogt ablation induces perinatal mortality and severe dilated cardiomyopathy in survivors. Long-term loss of Ogt in the mature adult heart produces progressive ventricular dysfunction. This study illuminates the significant and emerging role that Ogt and O-GlcNAc signaling play in the cardiomyocyte.
GRANTS
This work was supported by National Institutes of Health Grants R01-HL-083320, R01-HL-094419, P20-GM-103492 and P01-HL-078825. R. E. Brainard and L. J. Watson were American Heart Association- Great Rivers Affiliate Predoctoral Fellows.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.J.W., B.W.L., A.M.D., K.D.B., R.D.R., R.E.B., and T.D.C. performed experiments; L.J.W., B.W.L., K.D.B., R.D.R., T.D.C., and L.A. analyzed data; L.J.W., B.W.L., R.D.R., T.D.C., L.A., B.G.H., and S.P.J. interpreted results of experiments; L.J.W., B.W.L., A.M.D., R.D.R., and T.D.C. prepared figures; L.J.W. drafted manuscript; L.J.W., B.W.L., A.M.D., R.D.R., R.E.B., B.G.H., and S.P.J. edited and revised manuscript; L.J.W., B.W.L., R.D.R., R.E.B., T.D.C., B.G.H., and S.P.J. approved final version of manuscript; R.D.R., B.G.H., and S.P.J. conception and design of research.
ACKNOWLEDGMENTS
The authors acknowledge the support of the Diabetes and Obesity Center Imaging and Physiology Core (directed by S. P. Jones) and the expert technical assistance of Linda Harrison.
REFERENCES
- 1.Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest 100: 169–179, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belke DD. Swim-exercised mice show a decreased level of protein O-GlcNAcylation and expression of O-GlcNAc transferase in heart. J Appl Physiol 111: 157–162, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Butkinaree C, Park K, Hart GW. O-linked β-N-acetylglucosamine (O-GlcNAc): extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress. Biochim Biophys Acta 1800: 96–106, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, Vosseller K, Reginato MJ. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene 29: 2831–2842, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol Cell Physiol 291: C178–C187, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Copeland RJ, Bullen JW, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity. Am J Physiol Endocrinol Metab 295: E17–E28, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dias WB, Hart GW. O-GlcNAc modification in diabetes and Alzheimer's disease. Mol Biosyst 3: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol 551: 491–501, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glembotski CC. Endoplasmic reticulum stress in the heart. Circ Res 101: 975–984, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Hamid T, Guo SZ, Kingery JR, Xiang X, Dawn B, Prabhu SD. Cardiomyocyte NF-κB p65 promotes adverse remodelling, apoptosis, and endoplasmic reticulum stress in heart failure. Cardiovasc Res 89: 129–138, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart GW, Housley MP, Slawson C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446: 1017–1022, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Housley MP, Rodgers JT, Udeshi ND, Kelly TJ, Shabanowitz J, Hunt DF, Puigserver P, Hart GW. O-GlcNAc regulates FoxO activation in response to glucose. J Biol Chem 283: 16283–16292, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Housley MP, Udeshi ND, Rodgers JT, Shabanowitz J, Puigserver P, Hunt DF, Hart GW. A PGC-1α-O-GlcNAc transferase complex regulates FoxO transcription factor activity in response to glucose. J Biol Chem 284: 5148–5157, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, Dillmann WH. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res 96: 1006–1013, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Hwang SY, Shin JH, Hwang JS, Kim SY, Shin JA, Oh ES, Oh S, Kim JB, Lee JK, Han IO. Glucosamine exerts a neuroprotective effect via suppression of inflammation in rat brain ischemia/reperfusion injury. Glia 58: 1881–1892, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Issad T, Kuo M. O-GlcNAc modification of transcription factors, glucose sensing and glucotoxicity. Trends Endocrinol Metab 19: 380–389, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marban E. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation 117: 1172–1182, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-α. Circ Res 81: 627–635, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Lei B, Lionetti V, Young ME, Chandler MP, d'Agostino C, Kang E, Altarejos M, Matsuo K, Hintze TH, Stanley WC, Recchia FA. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol 36: 567–576, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Marsh SA, Dell'Italia LJ, Chatham JC. Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice. Amino Acids 40: 819–828, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ngoh GA, Facundo HT, Hamid T, Dillmann W, Zachara NE, Jones SP. Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circ Res 104: 41–49, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ngoh GA, Facundo HT, Zafir A, Jones SP. O-GlcNAc signaling in the cardiovascular system. Circ Res 107: 171–185, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ngoh GA, Hamid T, Prabhu SD, Jones SP. O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death. Am J Physiol Heart Circ Physiol 297: H1711–H1719, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ngoh GA, Jones SP. New insights into metabolic signaling and cell survival: the role of β-O-linkage of N-acetylglucosamine. J Pharmacol Exp Ther 327: 602–609, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ngoh GA, Watson LJ, Facundo HT, Dillmann W, Jones SP. Non-canonical glycosyltransferase modulates post-hypoxic cardiac myocyte death and mitochondrial permeability transition. J Mol Cell Cardiol 45: 313–325, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ngoh GA, Watson LJ, Facundo HT, Jones SP. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids 40: 895–911, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Donnell N, Zachara NE, Hart GW, Marth JD. Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol 24: 1680–1690, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohno M, Cheng CP, Little WC. Mechanism of altered patterns of left ventricular filling during the development of congestive heart failure. Circulation 89: 2241–2250, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Park K, Saudek CD, Hart GW. Increased expression of β-N-acetylglucosaminidase in erythrocytes from individuals with pre-diabetes and diabetes. Diabetes 59: 1845–1850, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao RV, Peel A, Logvinova A, del Rio G, Hermel E, Yokota T, Goldsmith PC, Ellerby LM, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett 514: 122–128, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Readnower RD, Brainard RE, Hill BG, Jones SP. Standardized bioenergetic profiling of adult mouse cardiomyocytes. Physiol Genomics 44: 1208–1213, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol 4: e374, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sansbury BE, Riggs DW, Brainard RE, Salabei JK, Jones SP, Hill BG. Responses of hypertrophied myocytes to reactive species: implications for glycolysis and electrophile metabolism. Biochem J 435: 519–528, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sihag S, Cresci S, Li AY, Sucharov CC, Lehman JJ. PGC-1α and ERRα target gene downregulation is a signature of the failing human heart. J Mol Cell Cardiol 46: 201–212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci 35: 547–555, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slawson C, Zachara NE, Vosseller K, Cheung WD, Lane MD, Hart GW. Perturbations in O-linked β-N-acetylglucosamine protein modification cause severe defects in mitotic progression and cytokinesis. J Biol Chem 280: 32944–32956, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Smet-Nocca C, Broncel M, Wieruszeski JM, Tokarski C, Hanoulle X, Leroy A, Landrieu I, Rolando C, Lippens G, Hackenberger CP. Identification of O-GlcNAc sites within peptides of the Tau protein and their impact on phosphorylation. Mol Biosyst 7: 1420–1429, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res 99: 275–282, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Watson LJ, Facundo HT, Ngoh GA, Ameen M, Brainard RE, Lemma KM, Long BW, Prabhu SD, Xuan YT, Jones SP. O-linked β-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci USA 107: 17797–17802, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xing D, Feng W, Nöt LG, Miller AP, Zhang Y, Chen YF, Majid-Hassan E, Chatham JC, Oparil S. Increased protein O-GlcNAc modification inhibits inflammatory and neointimal responses to acute endoluminal arterial injury. Am J Physiol Heart Circ Physiol 295: H335–H342, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xing D, Gong K, Feng W, Nozell SE, Chen YF, Chatham JC, Oparil S. O-GlcNAc modification of NFκB p65 inhibits TNF-α-induced inflammatory mediator expression in rat aortic smooth muscle cells. PLos One 6: e24021, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang WH, Park SY, Nam HW, Kim dH, Kang JG, Kang ES, Kim YS, Lee HC, Kim KS, Cho JW. NFκB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci USA 105: 17345–17350, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zachara NE, O'Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem 279: 30133–30142, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Zou L, Yang S, Champattanachai V, Hu S, Chaudry IH, Marchase RB, Chatham JC. Glucosamine improves cardiac function following trauma-hemorrhage by increased protein O-GlcNAcylation and attenuation of NF-κB signaling. Am J Physiol Heart Circ Physiol 296: H515–H523, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]