Abstract
Although the development of abnormal myocardial mechanics represents a key step during the transition from hypertension to overt heart failure (HF), the underlying ultrastructural and cellular basis of abnormal myocardial mechanics remains unclear. We therefore investigated how changes in transverse (T)-tubule organization and the resulting altered intracellular Ca2+ cycling in large cell populations underlie the development of abnormal myocardial mechanics in a model of chronic hypertension. Hearts from spontaneously hypertensive rats (SHRs; n = 72) were studied at different ages and stages of hypertensive heart disease and early HF and were compared with age-matched control (Wistar-Kyoto) rats (n = 34). Echocardiography, including tissue Doppler and speckle-tracking analysis, was performed just before euthanization, after which T-tubule organization and Ca2+ transients were studied using confocal microscopy. In SHRs, abnormalities in myocardial mechanics occurred early in response to hypertension, before the development of overt systolic dysfunction and HF. Reduced longitudinal, circumferential, and radial strain as well as reduced tissue Doppler early diastolic tissue velocities occurred in concert with T-tubule disorganization and impaired Ca2+ cycling, all of which preceded the development of cardiac fibrosis. The time to peak of intracellular Ca2+ transients was slowed due to T-tubule disruption, providing a link between declining cell ultrastructure and abnormal myocardial mechanics. In conclusion, subclinical abnormalities in myocardial mechanics occur early in response to hypertension and coincide with the development of T-tubule disorganization and impaired intracellular Ca2+ cycling. These changes occur before the development of significant cardiac fibrosis and precede the development of overt cardiac dysfunction and HF.
Keywords: hypertension, T-tubules, ventricular mechanics, heart failure, calcium transients
hypertension (HTN) is the most common modifiable risk factor for heart failure (HF) (14, 43). During the transition from HTN to HF, along with the development of left ventricular (LV) hypertrophy (LVH), maladaptive myocardial mechanics (such as reduced longitudinal diastolic tissue velocities and reduced peak systolic strain) can occur and represent a form of “subclinical” (stage B) HF (1, 15). HTN leads to pressure overload, resulting in variable levels of LVH, fibrosis, and ischemia, eventually leading to overt cardiac dysfunction and HF (14). The Multi-Ethnic Study of Atherosclerosis, which used cardiac magnetic resonance tagging to measure regional strain as an indicator of systolic and diastolic myocardial mechanics, found that abnormalities in strain occur with concentric LV remodeling (35) and LVH (8). In addition, using tissue Doppler and speckle-tracking echocardiography, several studies (18, 20, 28, 34, 36, 40) have found that abnormalities in myocardial mechanics occur in patients with HTN, before more evident signs of cardiac dysfunction.
Given the importance of preventing HF in the general population, further investigation of the abnormal myocardial mechanics that occur during the transition from HTN to HF is critical. Improved understanding of the ultrastructural and cellular changes that underlie abnormalities in cardiac structure and function, including myocardial mechanics, would therefore provide valuable insights into the pathophysiological basis of the transition from HTN to HF. At the cellular level, transverse (T)-tubule disruption is a feature common to animal models of HF as well as human HF (23). T-tubule disruption also occurs in response to an acute, sudden rise in afterload, before the development of overt HF (42). This critical change in cell ultrastructure alters the ability of Ca2+ to influx through the L-type Ca2+ channel to induce Ca2+ release from the sarcoplasmic reticulum (SR), since the efficiency of this process is highly sensitive to the spatial organization of the Ca2+-release unit (41). Given the integral role of Ca2+ cycling in normal myocyte function and the abnormalities in excitation-contraction coupling that occur in conjunction with HTN (6), we hypothesized that T-tubule disorganization and the resultant abnormalities in Ca2+ signaling underlie the abnormalities in myocardial mechanics that occur early in response to HTN, before the development of overt HF.
Here, we report a comprehensive investigation of the relationships between subclinical changes in myocardial mechanics, assessed using advanced echocardiographic imaging techniques, and the statistical dynamics of changes in cell ultrastructure and function that arise in response to HTN in the spontaneously hypertensive rat (SHR), a model of gradual-onset HTN that ultimately leads to HF.
MATERIALS AND METHODS
Research strategy.
The goal of these experiments was to use several approaches to investigate how T-tubule disruption influences intracellular Ca2+ cycling and, in turn, how these cellular changes affect overall cardiac function and myocardial mechanics during the transition from HTN to HF. This combination of approaches required several measurements, including T-tubule organization among a large population of myocytes, Ca2+ transient characteristics among a similarly large cell population, and whole heart function through advanced echocardiographic analysis. All animal experiments were conducted according to a protocol approved by the Institutional Animal Care and Use Committee of Northwestern University.
Wistar-Kyoto (WKY) control rats and SHRs (Charles River Laboratories, Wilmington, MA) were used in this study to investigate the early changes in cardiac function in response to HTN, the development of hypertrophy, and the early stages of HF. Each rat was studied using echocardiographic imaging just before euthanization to provide a detailed analysis of cardiac structure and function in vivo. The heart was then removed, and T-tubule organization was measured using a modification of the approach used by Wei and colleagues (42) in which di-4-ANEPPS was used to stain T-tubule membranes, which were then imaged using confocal microscopy of large numbers of myocytes in the subepicardium of the intact rat LV. Finally, the heart was loaded with the Ca2+-sensitive dye fluo-4 AM to measure Ca2+ cycling in a large number of cells in the same cardiac region using confocal imaging (41).
Echocardiography and blood pressure measurement in WKY rats and SHRs.
Blood pressure was measured in awake animals using the tail-cuff method according to published guidelines with a CODA noninvasive blood pressure monitor (Kent Scientific, Torrington, CT), which uses volume-pressure recording technology (19). Next, each rat was lightly anesthetized in a chamber containing 1–2% isoflurane and supplemental O2 with inhalation via a nosecone to maintain a heart rate of >250 beats/min and to maintain spontaneous respiration. An experienced research sonographer specializing in small animal echocardiography then performed comprehensive two-dimensional (2-D), M-mode, Doppler, tissue Doppler, and speckle-tracking echocardiography using a GE Vivid 7 echocardiography machine with an i13L 11- to 14-MHz small animal linear transducer (GE Medical, Milwaukee, WI) as previously described (30, 32).
Standard parasternal long- and short-axis as well as apical four- and five-chamber 2-D and M-mode views were obtained. In each view, at least six cardiac cycles were captured at high frame rates (>14 frames/cardiac cycle, ∼60–80 frames/s). LV dimensions, wall thickness, mass, ejection fraction (EF), fractional shortening, and comprehensive diastolic function (including mitral inflow velocities, deceleration time, isovolumic relaxation time, and longitudinal mitral annular tissue velocities via tissue Doppler imaging) were measured offline using GE EchoPAC software (GE Medical). Speckle-tracking echocardiographic analysis was also performed using EchoPAC to calculate circumferential and radial midwall strain (in the short-axis view through the papillary muscles) and longitudinal strain (in the parasternal long-axis view). Strain was determined at the peak of systole, averaged across three cardiac cycles. We determined the duration of systole using Doppler flow profiles across the aortic valve. By measuring the time from the onset of QRS to the end of ejection (via the transaortic flow), we were able to determine the time point of end systole in the strain curves, and we used this information to determine peak systolic strain. Global strain values were obtained by averaging regional (segmental) strains (9, 30, 32).
Measurement of T-tubule organization in myocytes of the intact rat heart.
We measured T-tubule organization in a manner similar to those previously reported in isolated myocytes and in the intact rat heart (41, 42). This approach is based on staining the intact heart using di-4-ANEPPS (8–10 μM) and then recording z-stacks using confocal microscopy (excitation at 488 nm, emission at >520 nm, ×40 Zeiss Apochromat water-immersion objective with numerical aperture = 1.2, pixel size = 0.45 μm). Each rat was anesthetized, and the heart was removed and cannulated on a Langendorff apparatus located on the stage of an inverted Zeiss LSM510 confocal microscope. Perfusion was adjusted so that pressure was maintained at 60–100 mmHg, and the heart was placed horizontally in an experimental chamber with a glass bottom so that the LV faced downward toward the objective. Temperature was maintained at 26°C. The solution was then recirculated, and di-4-ANEPPS was added to the superfusate. As described in a previous publication (41), contraction was abolished by a combination of cytochalasin D (60 μM) and blebbistatin (25 μM, Sigma) to the recirculating solution. z-Stacks were recorded from 20–30 LV subepicardial sites in the midmyocardial region so that at least 100 myocytes could be imaged.
T-tubule organization was measured by extracting an image from the center of each cell from the z-stack, omitting the cell membrane, and analyzing each image file using customized Matlab software to obtain a mean power spectrum from the fast Fourier transform (FFT) of each longitudinal pixel row of the myocyte image. With the length of the T-tubules expected to be 2.5 μm, the spatial frequency of the T-tubules was expected to be 0.4 μm−1. Highly organized T-tubule structures should therefore have a distinct peak in the power spectrum near the 0.4-μm−1 frequency. Thus, T-tubule organization was estimated as the total area of the power spectrum in the 0.33- to 0.5-μm−1 frequency band (band 1) relative to the total areas under band 1 and the adjacent 0.2- to 0.33-μm−1 band (band 2). Band 2 includes both background and the signal for disorganized tubules which increases during remodeling, so that the OI reflects both the loss of organized T-tubules and the increase in poorly organized tubules. The range of this proposed organization index (OI) can be as high as 0.95 in a cell with a nearly perfect T-tubule network, where the area of band 1 will be significantly greater than the area of band 2 but rarely lower than ∼0.5 when T-tubules are nearly absent and bands 1 and 2 are nearly equal. This approach avoids complications in interpretation when only the peak area is measured, which does not account for differences in cell dimensions or scan quality. Note also that OI was measured in at least 100 myocytes from each heart to obtain a reliable average and SD for that heart.
Measurement of Ca2+ transients.
Measurements of Ca2+ transients were made in a subset of the WKY rats and SHRs in each age group. After three-dimensional imaging was completed, the heart was perfused with fresh Tyrode solution for 10 min, at which point recirculation was again initiated. Fluo-4 AM was added in three increments (15, 12, and 10 μM, 20 min each), the heart was washed with fresh solution for 10 min, and the solution was again recirculated with cytochalasin D and blebbistatin. Hooked stimulating electrodes were placed in the LV apex so that the heart could be paced at a basic cycle length of 700 ms. A pseudo-ECG was also recorded using AgCl2 wires placed on either side of the heart (Bioamplifier 8, WPI). Intracellular Ca2+ transients were recorded in the transverse direction by placing the scan line across multiple cells (typically 10–12 cells) or along the long axis of one myocyte in the same midmyocardial region from which z-stacks were recorded. Because of spectral overlap between di-4-ANEPPS and fluo-4, this approach did not allow the quantification of either resting or peak Ca2+ release but did allow measurements of the kinetics of Ca2+ cycling, particularly those related to Ca2+ release. For the experiments described in this study, we focused on the time to peak (TTP) of the Ca2+ transient, which is the parameter most sensitive to changes in T-tubule organization and altered triggering of Ca2+-induced Ca2+ release from the SR. To take a representative sample from each heart, Ca2+ transients from ∼50 myocytes were measured to obtain an average of TTP, which was then related to mean or SD for OI in the same heart.
Measurement of cardiac fibrosis.
Hearts from WKY rats and SHRs of different ages were perfused with 2% paraformaldehyde, embedded in paraffin, and sectioned (4 μm). Three contiguous transverse sections were cut in the midmyocardium and stained with Masson trichrome stain to determine extent of cardiac fibrosis. The free wall of the LV was mapped with a series of bright-field images (×4) for all three sections. Images were analyzed using customized Matlab software in which thresholding was used to distinguish myocytes from regions of fibrosis, which was measured as the percentage of total LV free wall area in each image and averaged from all three sections for each heart.
Statistical analysis.
All data are presented as means ± SD. For ease of displaying and comparing blood pressure, heart rate, heart and body weight, and echocardiographic data, we divided rats into the following three age groups: 2–6, 7–11, and 12–17 mo. Next, we compared SHRs with WKY rats for each parameter within each age group using t-tests. Within each type of rat, we evaluated the association between age and each parameter using linear regression with the physiological or echocardiographic parameter as the dependent variable and age (entered into the model as a continuous variable) as the independent variable. Finally, we pooled data from all rats and performed linear regression analysis for each echocardiographic parameter with age (continuous variable) and rat type as independent variables so that we could determine whether rat type (i.e, HTN) was associated with each parameter independent of age.
For T-tubule and Ca2+ transient data, event histograms were calculated for the number of events for each bin width for each parameter presented. Both the mean and variability (SD) of parameters such as T-tubule OI were compared with echocardiographic and Ca2+ transient data using correlation coefficients.
We performed intra- and interobserver variability analyses of the speckle-tracking analyses on 10 randomly selected rats (n = 5 WKY rats and n = 5 SHRs) to calculate the reproducibility of our methods. All measurements were made in a blinded fashion. The intraclass correlation, mean bias, and coefficient of variation for these analyses are shown in Table 1.
Table 1.
Reproducibility of speckle-tracking echocardiography parameters
| Intraobserver Reliability |
Interobserver Reliability |
||||||
|---|---|---|---|---|---|---|---|
| Parameter | Mean ± SD | ICC (95% CI) | Mean bias (95% CI) | CV, % | ICC (95% CI) | Mean bias (95% CI) | CV, % |
| Global longitudinal strain, % units | −13.9 ± 2.0 | 0.90 (0.78, 0.99) | −0.65 (−1.18, −0.13) | 5.2 | 0.85 (0.68, 0.99) | −0.54 (−1.19, 0.12) | 6.2 |
| Global circumferential strain, % units | −19.4 ± 2.5 | 0.82 (0.61, 0.99) | 0.28 (−0.34, 0.89) | 7.9 | 0.84 (0.66, 0.99) | −0.85 (0.52, 1.93) | 5.9 |
| Global radial strain, % units | 60.3 ± 7.9 | 0.91 (0.79, 0.99) | 2.0 (0.1, 3.9) | 4.2 | 0.88 (0.73, 0.99) | 2.1 (−0.1, 4.4) | 5.3 |
n =10. ICC, intraclass correlation; CI, confidence interval; CV, coefficient of variation.
Two-sided P values of <0.05 were considered statistically significant. We corrected for multiple comparisons using the false discovery rate method. False discovery rate Q values were calculated using the Benjamini-Hochberg method (2). All statistical analyses were performed using Stata (version 12.0, StataCorp, College Station, TX).
RESULTS
Abnormal myocardial mechanics occur early in the SHR, before signs of global cardiac dysfunction and HF.
We observed several changes in cardiac structure and function during prolonged exposure to HTN in the SHR (Table 2), before the development of HF. Similar changes occurred with aging in WKY rats, but these changes were delayed and less profound compared with SHRs. After controlling for age and body weight on linear regression analysis, SHRs had higher heart weight [β-coefficient: 0.31 g (95% confidence interval: 0.14–0.48 g), P < 0.001] compared with WKY rats. As previously described (37), prolonged exposure to HTN in the SHR resulted in increases in LV wall thickness and mass, which were associated with abnormalities in mitral inflow indicative of the development of diastolic dysfunction by 12–17 mo of age in the SHR. However, our analysis of diastolic tissue velocities in the SHR demonstrated that early diastolic tissue velocity (e′) decreased much earlier (as early as 7 mo of age) in the SHR.
Table 2.
Blood pressure, heart rate, body weight, and cardiac structure/function by age in WKY rats versus SHRs
| WKY Rats |
SHRs |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | 2–6 mo of age | 7–11 mo of age | 12–17 of age | P Value* | 2–6 mo of age | 7–11 mo of age | 12–17 mo of age | P Value* |
| n | 14 | 11 | 9 | 21 | 37 | 14 | ||
| Systolic blood pressure, mmHg | 166 ± 21 | 166 ± 24 | 164 ± 23 | 0.66 | 200 ± 21‡ | 196 ± 24‡ | 218 ± 26‡ | 0.030† |
| Diastolic blood pressure, mmHg | 117 ± 18 | 119 ± 23 | 115 ± 22 | 0.82 | 154 ± 24‡ | 150 ± 28‡ | 180 ± 29‡ | 0.006† |
| Mean arterial pressure, mmHg | 133 ± 18 | 134 ± 22 | 131 ± 22 | 0.88 | 168 ± 22‡ | 164 ± 27‡ | 192 ± 28‡ | 0.062† |
| Heart rate, beats/min | 347 ± 37 | 333 ± 32 | 316 ± 29 | 0.31 | 364 ± 33 | 325 ± 40 | 316 ± 24 | 0.001 |
| Body weight, g | 296 ± 78 | 393 ± 33 | 430 ± 51 | <0.001 | 340 ± 65 | 396 ± 36 | 433 ± 48 | 0.001 |
| LV mass, g | 0.78 ± 0.16 | 1.01 ± 0.07 | 1.10 ± 0.16 | <0.001 | 0.94 ± 0.13‡ | 1.15 ± 0.11‡ | 1.32 ± 0.24‡ | <0.001† |
| Septal wall thickness, mm | 1.65 ± 0.17 | 1.85 ± 0.18 | 1.95 ± 0.28 | 0.001 | 1.88 ± 0.16‡ | 2.11 ± 0.19‡ | 2.28 ± 0.35‡ | <0.001† |
| Posterior wall thickness, mm | 1.66 ± 0.15 | 1.87 ± 0.25 | 1.93 ± 0.25 | 0.005 | 1.86 ± 014‡ | 2.12 ± 0.24‡ | 2.19 ± 0.30‡ | <0.001† |
| LV end-diastolic dimension, mm | 6.59 ± 0.89 | 7.73 ± 0.86 | 7.68 ± 1.04 | 0.004 | 7.00 ± 0.59‡ | 7.92 ± 0.47 | 8.57 ± 1.16 | <0.001† |
| LV end-systolic dimension, mm | 3.06 ± 0.35 | 3.72 ± 0.71 | 3.87 ± 1.00 | 0.007 | 3.50 ± 0.46‡ | 4.15 ± 0.63 | 5.18 ± 0.84‡ | <0.001† |
| LV ejection fraction, % | 87.4 ± 3.9 | 87.4 ± 4.7 | 85.0 ± 6.3 | 0.28 | 85.6 ± 4.7 | 82.8 ± 4.9‡ | 75.1 ± 5.8‡ | <0.001† |
| Fractional shortening, % | 52.5 ± 5.3 | 52.7 ± 6.5 | 50.2 ± 7.7 | 0.44 | 50.1 ± 5.4 | 47.3 ± 4.73‡ | 39.7 ± 5.5‡ | <0.001† |
| E velocity, cm/s | 10.7 ± 1.4 | 10.8 ± 0.9 | 9.1 ± 1.9 | 0.021 | 11.8 ± 1.7‡ | 11.1 ± 1.5 | 11.2 ± 3.2 | 0.35† |
| A velocity, cm/s | 6.7 ± 2.0 | 7.7 ± 1.1 | 6.3 ± 2.1 | 0.69 | 7.4 ± 2.1 | 5.9 ± 1.7‡ | 5.5 ± 2.6 | 0.014 |
| E-to-A ratio | 1.7 ± 0.4 | 1.4 ± 0.3 | 1.6 ± 0.8 | 0.76 | 1.8 ± 0.8 | 2.0 ± 0.5‡ | 2.8 ± 2.0 | 0.022† |
| E deceleration time, ms | 46.6 ± 3.4 | 46.8 ± 5.1 | 48.6 ± 11.5 | 0.52 | 42.8 ± 4.4‡ | 47.4 ± 4.1 | 42.1 ± 10.1 | 0.89 |
| Isovolumic relaxation time, ms | 21.9 ± 2.1 | 21.5 ± 1.0 | 23.8 ± 5.3 | 0.22 | 21.6 ± 2.0 | 22.0 ± 3.4 | 34.1 ± 9.3‡ | <0.001 |
| Tissue Doppler e′ velocity, cm/s | 0.64 ± 0.19 | 0.76 ± 0.19 | 0.56 ± 0.18 | 0.49 | 0.70 ± 0.14 | 0.57 ± 0.17‡ | 0.54 ± 0.13 | 0.002 |
| Tissue Doppler s′ velocity, cm/s | 0.46 ± 0.05 | 0.46 ± 0.04 | 0.41 ± 0.03 | 0.08 | 0.46 ± 0.05 | 0.44 ± 0.05 | 0.41 ± 0.06 | 0.015† |
| E-to-e′ ratio | 20.7 ± 4.9 | 17.0 ± 3.9 | 18.4 ± 4.6 | 0.18 | 19.5 ± 3.5 | 21.3 ± 4.6‡ | 23.2 ± 4.3‡ | 0.015† |
| Global longitudinal strain, % | −14.1 ± 1.4 | −13.4 ± 1.7 | −11.7 ± 2.5 | 0.005 | −12.9 ± 1.3‡ | −10.8 ± 1.6‡ | −9.5 ± 2.2‡ | <0.001† |
| Global circumferential strain, % | −18.8 ± 2.8 | −15.9 ± 3.0 | −15.5 ± 4.2 | 0.017 | −15.6 ± 2.3‡ | −14.0 ± 3.1 | −12.4 ± 2.5‡ | 0.002† |
| Global radial strain, % | 53.7 ± 13.0 | 47.5 ± 15.5 | 38.2 ± 15.5 | 0.017 | 53.1 ± 10.1 | 40.3 ± 15.6 | 34.8 ± 10.4 | <0.001 |
Values are means ± SD. WKY rats, Wistar-Kyoto rats; SHRs, spontaneously hypertensive rats; LV, left ventricular; E, early diastolic mitral velocity; A, late (atrial) diastolic mitral velocity; e′, early mitral annular tissue velocity; s′, systolic mitral annular tissue velocity. All listed significant P values remained significant after accounting for the false discovery rate.
P value for linear trend across ages (i.e., linear regression analysis with age entered as a continuous independent variable and each parameter as the dependent variable).
SHR (i.e., hypertension)-associated parameter (P < 0.05) after adjustment for age.
P < 0.05 for the comparison of SHRs vs. WKY rats of the same age group.
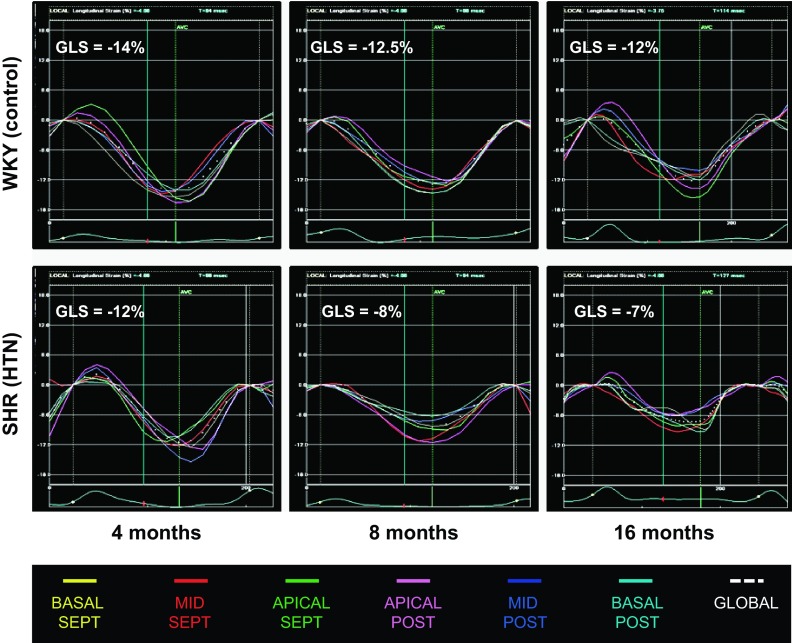
Analysis of global LV systolic function revealed that in the SHR, slight reductions in EF occurred by 7–11 mo, with more significant decreases occurring by 12–17 mo. Conversely, using speckle-tracking echocardiography, we found that reductions in global circumferential strain occurred early in the SHR; decreased circumferential strain was present even at young ages (2–6 mo) in SHRs compared with WKY control rats. Both global circumferential strain and global radial strain decreased continuously with prolonged exposure to HTN in the SHR (Table 2). Global radial strain decreased dramatically between 2–6 and 7–11 mo of age, before the large decrease in global LV EF. However, there was no difference in global radial strain between SHRs and WKY rats in each age group. Global longitudinal strain was more sensitive to HTN exposure (Table 2 and Fig. 1). Global longitudinal strain was lower in SHRs compared with WKY rats at each time point, and the overall decline in global longitudinal strain was steeper with aging in SHRs compared with WKY rats.
Fig. 1.
Speckle-tracking analysis of global longitudinal strain in spontaneously hypertensive rat (SHR) and Wistar-Kyoto (WKY) hearts in vivo. GLS, global longitudinal strain. GLS decreased with aging and prolonged exposure to hypertension in the SHR.
All of the aforementioned changes in the SHR occurred before the development of HF; none of the SHRs in the present study (2–17 mo of age) had symptoms or signs of overt HF such as lethargy, dyspnea, or increased lung weight, all of which occur later in SHRs (∼20–24 mo of age).
T-tubule disorganization occurs early in response to gradual-onset HTN, before overt cardiac dysfunction and symptomatic HF.
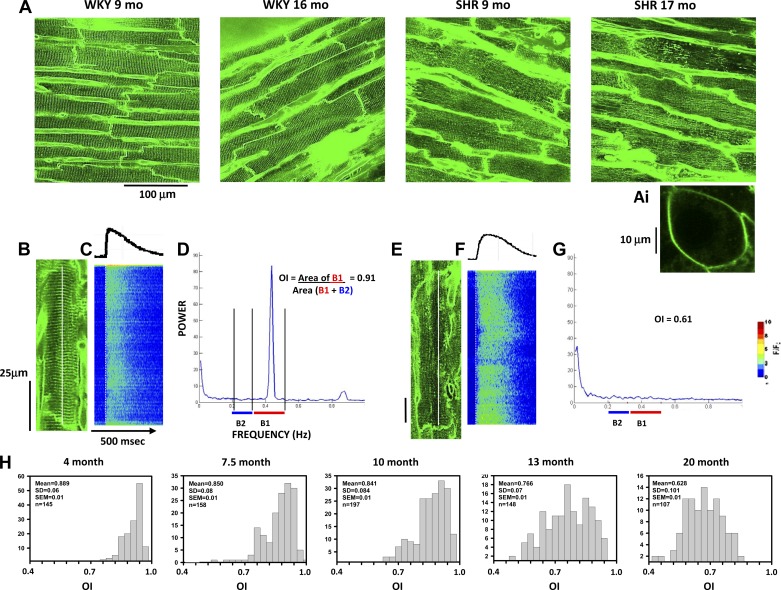
Figure 2 shows the changes in T-tubule organization during disease progression in the SHR model. Figure 2A shows representative 2-D images of di-4-ANEPPS staining of the mid-LV subepicardium taken from z-stacks showing T-tubule organization in multiple myocytes at different ages in both WKY rats and SHRs. At 9 mo, T-tubules were highly organized in all cells in the WKY heart and nearly as well organized at 17 mo. In contrast, some myocytes showed poor T-tubule organization in the 9-mo-old SHR; T-tubule disorganization was apparent in nearly all myocytes in the 17-mo-old SHR. For purposes of comparison, Fig. 2A,i shows di-4-ANEPPS staining of an HL-1 atrial tumor cell. These cells have no T-tubules but show strong staining of the sarcolemma, demonstrating the sarcolemmal specificity with this membrane dye and that there was no internal staining of any intracellular organelles.
Fig. 2.
Transverse (T)-tubule disruption in WKY rats and SHRs with age. A: representative images obtained from WKY rats and SHRs at different ages using di-4-ANEPPS staining. B–D: two-dimensional (2-D) image of a myocyte from a 9-mo-old WKY rat (B) with the accompanying Ca2+ transient (C) and fast Fourier transform (FFT) derived from the image in A from that cell (D). A,i: staining of an HL-1 atrial tumor cell for purposes of comparison. Band 1 (B1) and 2 (B2) are shown below the graph to indicate how the organization index (OI) was calculated. E–G: 2-D image (E), Ca2+ transient (F), and FFT graph (G) for a poorly organized myocyte in a 9-mo-old SHR. H: representative event histograms of OI values measured in SHR hearts at different stages of disease development.
Figure 2B shows an example of a myocyte from a 9-mo-old WKY rat with highly organized T-tubules whose Ca2+ transient increased synchronously and rapidly along the entire cell length (measured along the white line in the 2-D image; see the average intensity profile above the line scan image, Fig. 2C). The T-tubule OI in that myocyte was high (0.91), as measured from the FFT in Fig. 2D. In contrast, a myocyte from a 16-mo-old SHR with no obvious T-tubules showed almost no peak in band 1, and the OI derived from the FFT (Fig. 2, E–G) was 0.61. The line-scan image in Fig. 2F shows the fractionation of Ca2+ release along the cell length with many cellular regions showing delayed release while others showed a fairly rapid release.
Figure 2H shows representative examples of the distribution of OI measurements in large numbers of myocytes from five different SHR hearts representing different ages. There were several critical changes in the distribution of OIs with age, the most obvious of which was a general shift to lower OI values as SHRs aged. In addition, there was an increase in the width of the distribution (OI SD) with increasing age, demonstrating that not only is there an increase in the number of myocytes with poor T-tubule organization but that the variability in OI is also increased.
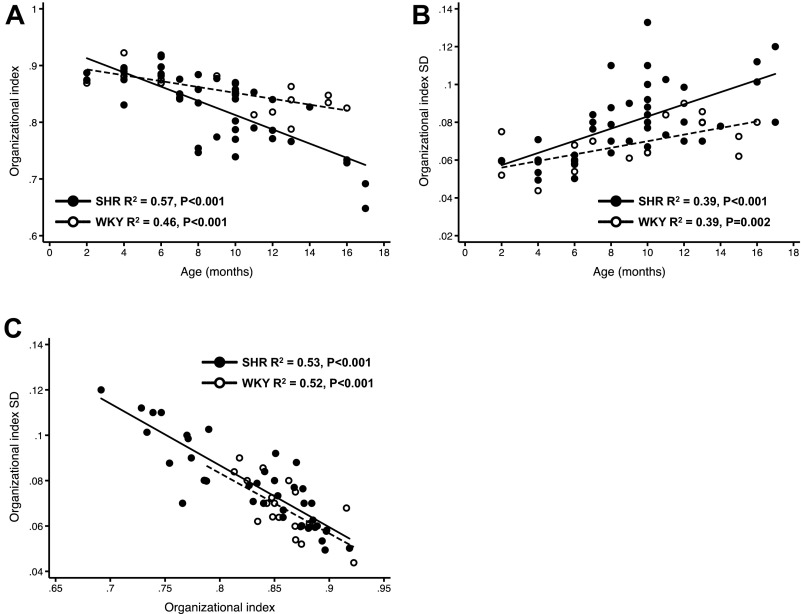
When the changes in OI and OI SD with age were compared between WKY rats and SHR, there was an exaggerated age-related decrease in OI in SHRs (Fig. 3A). OI decreased in normal WKY hearts as well, but this was much less than in SHRs over the same time period. The most striking result shown in Fig. 3A is that mean OI decreased with increasing age in SHRs. Furthermore, the cell-to-cell variability in OI (OI SD; Fig. 3B) increased with age and showed a steeper relationship with age in SHRs compared with WKY rats. Finally, these changes in OI occurred despite the fact that there were no signs of symptomatic HF or overt cardiac dysfunction, indicating that T-tubule disorganization (decreased OI) and heterogeneity (increased OI SD) occur early in the SHR, before the development of severe cardiac hypertrophy and dilation (and HF).
Fig. 3.
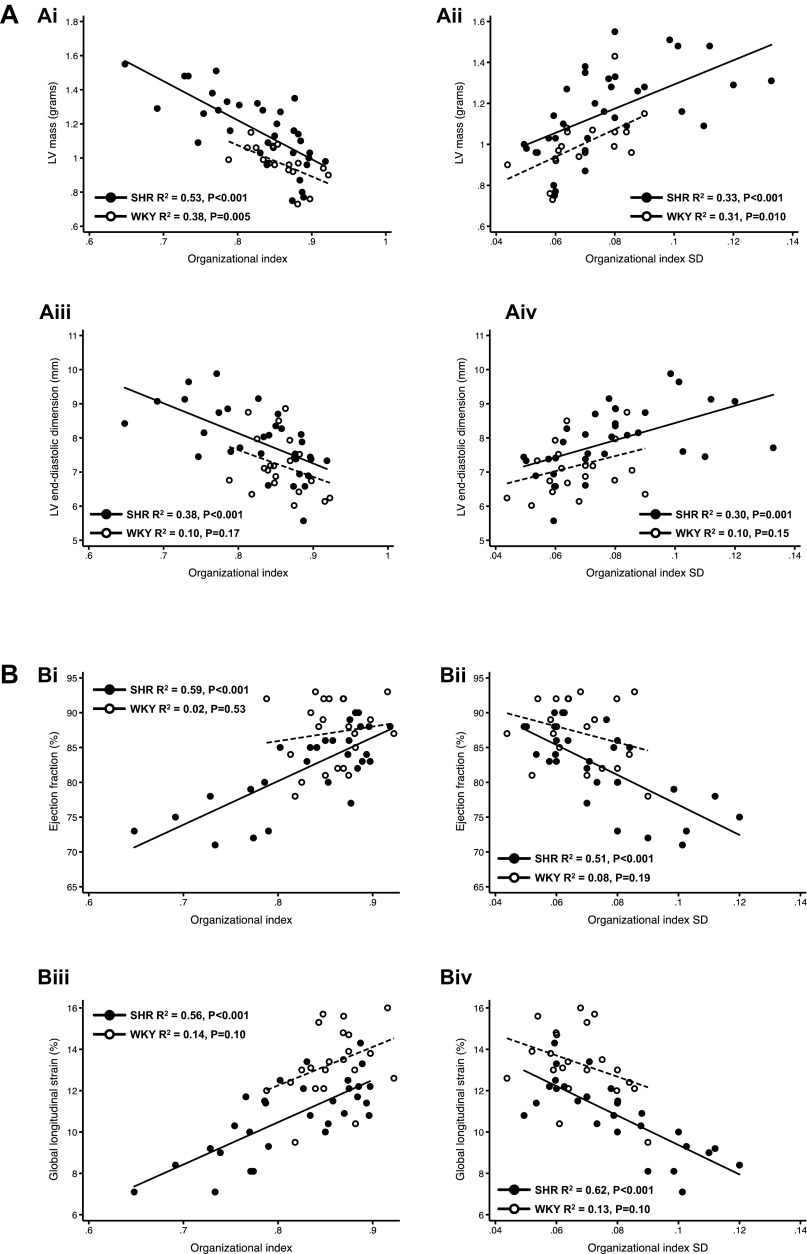
Summary of the relationships between OI and age (A), OI variability and age (B), and OI and OI variability (C) for WKY rats (n = 22) and SHRs (n = 41).
T-tubule disorganization results in abnormal Ca2+ release early in the SHR.
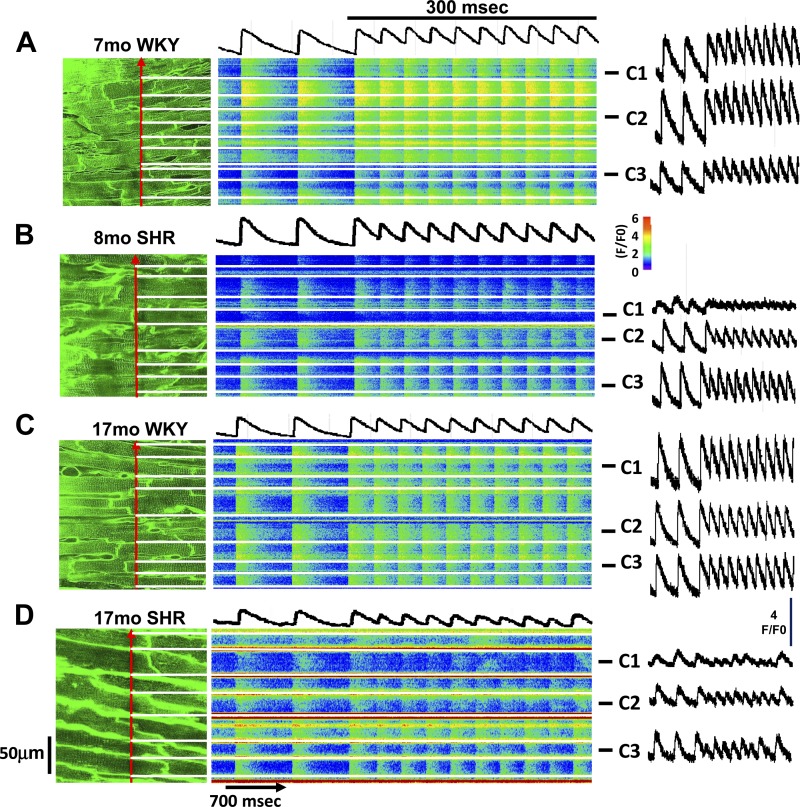
Figure 4 shows typical recordings of intracellular Ca2+ transients obtained in WKY rats and SHRs at both young and old ages. Figure 4A shows a 2-D image of T-tubules (di-4-ANEPPS staining) from a 7-mo-old WKY rat with the associated transverse recording of Ca2+ transients from the cells indicated by the position of the scan line (red arrow). Transients in all myocytes rose simultaneously during both basal pacing (basic cycle length: 700 ms) and during rapid pacing at a basic cycle length of 300 ms. The mean fluorescence profile for three representative cells (cells C1–C3) showed that Ca2+ transients were quite similar among these myocytes. Note that T-tubule organization was high in all myocytes in the 2-D image in Fig. 4A, left. In contrast, Fig. 4B shows that in an 8-mo-old SHR, there were some cells whose Ca2+ transient rose slowly and was low in magnitude (cell C1) compared with the other cells in this recording site, including representative cells (cells C2–C3). Myocytes with poor Ca2+ release also had poor T-tubule organization in the 2-D image (Fig. 4B, left). Figure 4C shows results from an older WKY rat, in which all myocytes still showed a rapid and simultaneous rise, just as in the younger WKY rat (Fig. 4A), with all myocytes showing good T-tubule organization. However, nearly all myocytes in a SHR of the same age (Fig. 4D) showed low T-tubule organization and a corresponding slow rise and generally poor release, especially during rapid pacing.
Fig. 4.
Relationship between OI and time to peak (TTP) of the Ca2+ transient in myocytes of intact 7- and 17-mo-old WKY hearts versus 8- and 17-mo-old SHR hearts. The relationship between T-tubule organization and Ca2+ release during aging in WKY rats and SHRs is shown. Left: 2-D images of each recording site stained with di-4-ANEPPS to show T-tubule organization. The red arrow shows the position of the scan line during the line-scan imaging shown in the middle images. Horizontal white lines indicate cell borders. The traces above each line scan show the mean fluorescence intensity during line-scan recording of all cells in the image. Traces on the right show average fluorescence of three selected myocytes (cells C1–C3) from each recording site. Recordings were obtained from a 7-mo-old WKY rat (A), an 8-mo-old SHR (B), a 17-mo-old WKY rat (C), and a 17-mo-old SHR (D).
These results demonstrate that there are some myocytes even in young SHRs that have poor T-tubule organization and, as a result, have abnormal Ca2+ cycling (slow rise and small magnitude). In the same heart, however, most myocytes have high T-tubule organization with resulting fast and large Ca2+ release. With age, more cells show T-tubule disruption with a resulting increase in the number of myocytes showing impaired Ca2+ cycling.
When the TTP of Ca2+ transients was measured in large numbers of myocytes in several hearts, there was a distinct pattern that emerged, which reflected the progressive decrease in OI with age (Fig. 5). Although slight changes were seen with aging in WKY rats, these changes in Ca2+ transients were very abnormal with aging in chronic HTN (SHRs). Figure 5C shows the distribution of TTP values in an 8-mo-old SHR. Although there was clustering of TTP values at short times, indicating a large number of cells with normal, rapid Ca2+ release, there was also a large proportion of cells with slower Ca2+ release, as predicted by the increasing number of cells with low OI even at this early age. At a much older age (17 mo; Fig. 5D), another SHR heart showed that very few myocytes demonstrated a short TTP and that there was a much broader distribution of TTP values, again reflecting the fact that OI values for myocytes at this age are both lower and more variable.
Fig. 5.
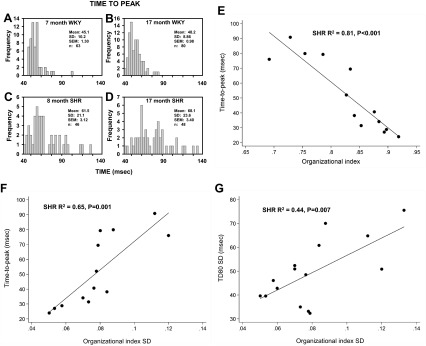
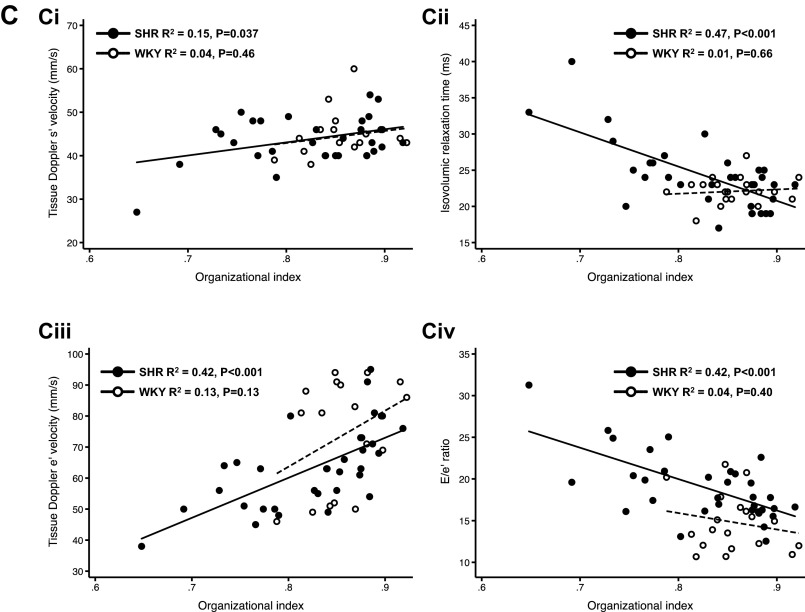
TTP of the Ca2+ transient in WKY rats versus SHRs and the relationship between T-tubule organization, TTP of the Ca2+ transient, and Ca2+ transient duration in SHRs. A–D: event histograms of TTP for 7- and 17-mo-old WKY rats and for 8- and 17-mo-old SHRs. E: relationship between mean OI and TTP in SHR hearts (n = 13). F: relationship between OI SD and TTP in SHR hearts (n = 13). G: relationship between OI SD and Ca2+ transient duration (TD80 SD) in SHR hearts (n = 15).
When the average TTP for each heart was plotted as a function of mean OI for that heart (Fig. 5E), there was a close relationship, demonstrating that the TTP slowed markedly as OI fell. Moreover, as the variability in OI (OI SD) increased, there was an accompanying increase in the average TTP (Fig. 5F), reflecting the influence that high intercellular variability in OI has to slow overall Ca2+ release. Figure 5G shows the association of OI SD with Ca2+ transient duration, which suggests that T-tubule organization relates to both Ca2+ release and Ca2+ uptake.
T-tubule disorganization occurs in concert with the development of LVH and underlies abnormalities in myocardial mechanics.
Comprehensive analysis of the association of T-tubule organization (at the cellular level) and whole heart structure and function (as assessed using echocardiography) demonstrated that there was a close correlation between T-tubule disorganization and abnormalities in cardiac structure and function, including systolic and diastolic cardiac mechanics, particularly in the SHR (Fig. 6). Tubule disorganization (reduced OI) and the heterogeneity of T-tubule disorganization (increased OI SD) correlated well with increased LV mass and LV end-diastolic dimension, reduced EF and global longitudinal strain, and worse diastolic function (decreased e′, increased isovolumic relaxation time, and increased E-to-e′ ratio, which estimates LV filling pressures). OI and OI SD were also associated with cell width as a marker of individual myocyte hypertrophy (data not shown).
Fig. 6.
Summary of the relationships between OI and OI SD and echocardiographic measurements, including myocardial mechanics, in both WKY rats (n = 20–21) and SHRs (n = 28–33), showing that indicators of abnormal cardiac structure and function, including myocardial mechanics, are a function of OI and OI SD. Relationships between OI and OI SD and indicators of cardiac structure, systolic function, and diastolic function are shown. A: OI and OI SD correlated with left ventricular (LV) mass (i and ii) and LV end-diastolic dimension (iii and iv) in SHRs (i and iii) and WKY rats (ii and iv). However, the strength and significance of the correlation between OI and OI SD with LV end-diastolic dimension were greater in SHRs compared with WKY rats. B: OI and OI SD correlated with ejection fraction (EF; i and ii) and GLS (iii and iv) in SHRs (i and iii) but not in WKY rats (ii and iv). In SHRs, as OI and OI SD worsened, indicating progressive T-tubule disruption, there was a linear decrease in EF and GLS. C: in SHRs, OI was also associated with tissue velocity (s′; i), isovolumic relaxation time (ii), early diastolic tissue velocity (e′; a marker of LV relaxation; iii), and the E-to-e′ ratio (a marker of LV filling pressures; iv). These relationships, which are not present in WKY rats, demonstrate that increasing T-tubule disruption (i.e., reduced OI) is associated with worse (impaired) LV relaxation and higher LV filling pressures, both indicative of progressive diastolic dysfunction.
T-tubule disorganization and abnormal myocardial mechanics occur before the onset of myocardial fibrosis.
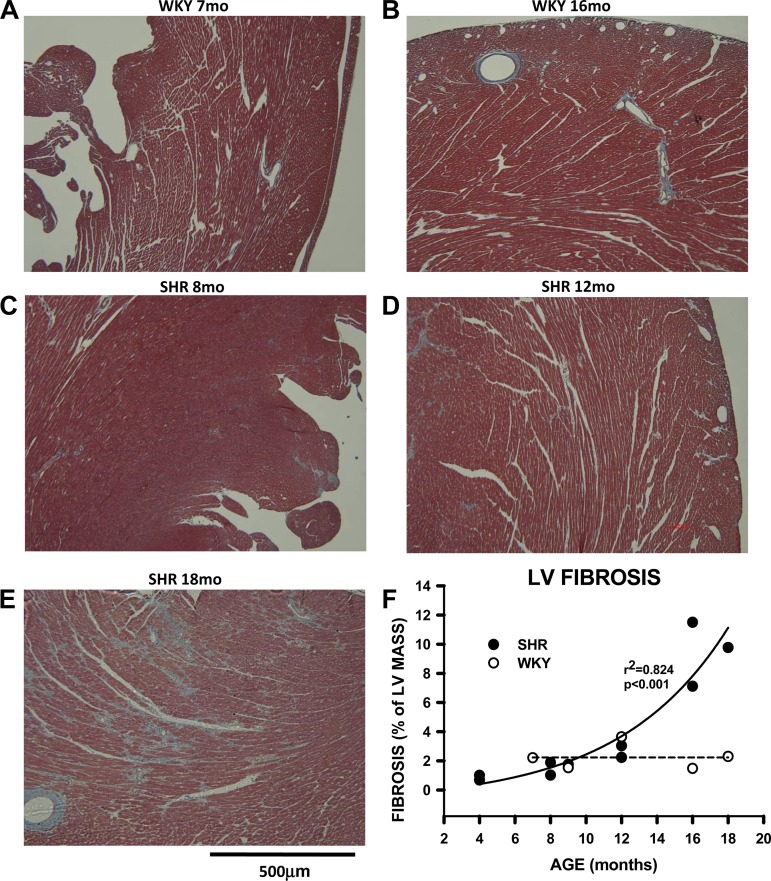
Figure 7 shows examples of sections from WKY and SHR hearts at different ages (blue indicates fibrosis). At 7 mo and even at 16 mo, there was little evidence of fibrosis in WKY hearts. In contrast, significant fibrosis can be seen beginning at 12 mo in SHRs and becomes more apparent at 18 mo. The scatterplot in Fig. 7 shows the development of fibrosis during aging in both groups. Up to about 12 mo, the percentage of the LV free wall with fibrosis was similar for both WKY rats (open circles) and SHRs (filled circles). After 12 mo, fibrosis increased dramatically in the SHR. These results demonstrate that decreases in OI, increases in OI SD, and abnormalities in systolic and diastolic myocardial mechanics are all present well before the onset of significant fibrosis in the SHR.
Fig. 7.
Onset of cardiac fibrosis during the development of hypertensive disease. A–E: images showing trichrome staining of the LV free wall from WKY and SHR hearts ranging from 7 to 18 mo of age. F: LV fibrosis of WKY and SHR hearts at different ages.
DISCUSSION
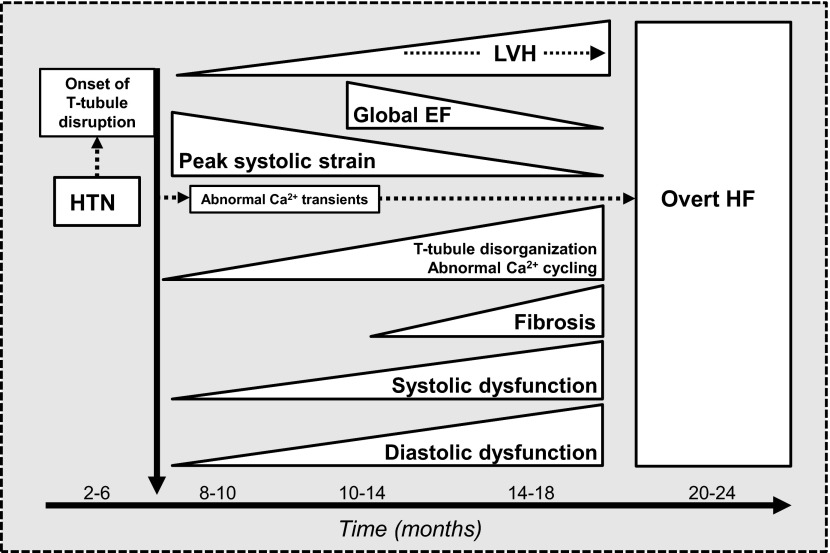
Here, we have described the results of a large and comprehensive study of the development of HF in a rat model of chronic HTN that closely mimics human HTN. By combining advanced in vivo echocardiographic imaging techniques (speckle-tracking and tissue Doppler imaging) with state-of-the-art confocal imaging, we were able to determine the ultrastructural and cellular basis for the abnormalities in myocardial mechanics that occur during the transition from HTN to HF. In addition, we definitively show here that even with chronic, slowly progressive HTN, T-tubule disruption and defects in Ca2+ cycling are apparent at an early stage, before the development of cardiac fibrosis, overt cardiac dysfunction, and symptomatic HF, as shown in Fig. 8. Finally, we show that HTN-induced T-tubule disruption in a growing population of cardiomyocytes, with resultant defective Ca2+ cycling, is the cellular mechanism responsible for the early reductions in systolic and diastolic myocardial mechanics. Thus, in response to HTN, the most common cause of HF, subclinical systolic dysfunction and diastolic dysfunction occur simultaneously. Over time, with the involvement of a growing proportion of myocytes demonstrating T-tubule remodeling and defective Ca2+ cycling, cardiomyocyte death gradually occurs and myocardial tissue is replaced by fibrosis, thereby worsening both systolic and diastolic dysfunction, ultimately leading to overt, symptomatic HF.
Fig. 8.
Development of heart failure (HF) in response to hypertension (HTN). T-tubule disruption and subsequent Ca2+ cycling defects occur very early in response to HTN. With the development of LV hypertrophy (LVH), these defects in Ca2+ cycling lead to simultaneous reductions in systolic and diastolic myocardial mechanics, which, in turn, lead to the development of overt HF (with either preserved or reduced LV EF).
Our work has important implications in understanding the clinical course of the development of HF: 1) in a controlled model of HTN in rats, we have replicated the finding in humans that subclinical abnormalities in both systolic and diastolic myocardial mechanics occur in response to HTN and before the development of overt clinical HF; 2) although the syndromes of HF with reduced and preserved EF are distinct clinically, abnormalities in both systole and diastole are intimately intertwined, with both likely occurring to varying degrees in the individual patient; and 3) T-tubule disruption in response to stress is likely a common mechanism by which HF risk factors cause early, subclinical cardiac dysfunction and therefore underlie stage B (asymptomatic) HF and the progression to stage C (symptomatic) HF.
T-tubule disruption and cardiac function.
The role of T-tubule disruption in the pathophysiology of abnormal triggering of Ca2+ release in myocytes from failing hearts has been one of the most important recent observations about the cellular mechanisms underlying HF. Initial studies (13, 23) found that T-tubule disruption is present late in animals and humans with overt HF. These observations led to the question of whether T-tubule disruption is a cause or consequence of HF. An investigation of transverse aortic constriction (TAC) in rats (associated with an acute 80-mmHg rise in afterload) found that T-tubule disruption occurred in response to a severe, sudden increase in afterload and precedes the development of cardiac dysfunction and HF (42). However, the clinical significance of the TAC model is unknown since human HTN is much more gradual (7, 12, 29); thus, before our study, the role of T-tubule disruption in the transition from HTN to HF was still unclear.
The SHR has important advantages over many other experimental models of HF in that it more closely mimics the clinical progression of hypertensive patients (and therefore may be more clinically relevant) than other models that use highly invasive procedures to induce cardiac dysfunction and failure over a short period (7, 12, 29). We took advantage of the slow progression of HTN-induced HF in the SHR to investigate how T-tubule disruption in the myocyte leads to abnormal cellular Ca2+ cycling and myocardial mechanics, which might explain how the HTN-induced heart disease progresses from early, asymptomatic stages to more advanced dysfunction and, finally, overt HF. Our results show that the observed abnormalities in diastolic and systolic myocardial mechanics (tissue Doppler e′ and systolic strain, respectively) occur earlier than previously recognized and coincide with the onset of LV hypertrophy in the SHR, well before the development of overt signs of cardiac dysfunction.
The fact that OI decreased with age in SHRs to a greater degree than in WKY rats explains why there is a decline in systolic (e.g., global longitudinal strain) and diastolic (e.g., e′) parameters in SHRs compared with WKY rats. As T-tubule organization decreases, there is less synchronous contraction within each myocyte, a critical observation that has been reported previously and confirmed here (21, 22, 25, 39). However, the fact that declining overall OI is associated with an increase in variability of OI is also likely to have a major contribution to declining cardiac function since the result will be a reduction in the efficiency of both contraction and relaxation. Most importantly, it is the population behavior across many myocytes in each heart that is critical to understanding how essentially cellular events provide the mechanism for HF development; the direct relationship between decline in both systolic and diastolic mechanics and T-tubule remodeling that involves a progressively increasing number of cells explains how disease progression occurs during prolonged HTN.
The molecular mechanism underlying T-tubule disruption in response to HTN is currently unknown but is an area of active investigation. Wei and colleagues (42) found evidence of a downregulation of junctophilin (JP)-2 (a member of the JP family of proteins, known to be important in the formation of T-tubules) in a TAC model of HTN. Whether JP-2 downregulation occurs in more gradual forms of HTN (e.g., SHRs) requires further study.
T-tubules and SR Ca2+ release.
We found that Ca2+ release was highly sensitive to both the mean and variability of T-tubule organization. This was in fact predicted in previous studies (10, 11), which reported a functional separation between triggering and release sites in myocytes from failing hearts well before T-tubule disruption was known to occur. We expected that T-tubule disruption would be responsible for a reduction in triggering of Ca2+ release. Note, however, that it is not possible using this statistical approach to separate out the contribution of mean OI from OI variability, given their highly linear relationship (Fig. 3C). It is possible, even likely, that a combination of small average decreases and increased variability in OI may both contribute to the critical slowing in TTP. We have also found that there is an increase in heterogeneity of Ca2+ release within myocytes during disease development, probably resulting from T-tubule disruption in different cellular regions (17). As described above, these regional differences in the timing of Ca2+ release within the cell will delay both contraction and relaxation as those regions of the cell showing delayed Ca2+ release will also relax later. As shown in our study, these cellular abnormalities in myocyte Ca2+ handling coincide with abnormal whole heart mechanics.
Relationship of cardiac fibrosis with T-tubule organization and myocardial mechanics.
Another important observation in our study is the finding that fibrosis increases as OI decreases. This is consistent with the idea that fibrosis occurs as T-tubule organization in individual myocytes falls below a critical level at which point Ca2+ cycling is so severely altered that diastolic Ca2+ rises, as has been previously reported. This increase in diastolic Ca2+ occurs as the result of several impairments in excitation-contraction coupling (31), including hyperphosphorylation of ryanodine receptors (RyRs), causing leak from the SR, in addition to slow reuptake of Ca2+ into the SR by a reduction in sarco(endo)plasmic reticulum Ca2+-ATPase function. The resulting rise in diastolic Ca2+ concentration is then thought to induce both apoptosis and necrosis, possibly involving cell death as the result of mitochondrial apoptotic pathways (5, 26, 27). The result is that myocytes with progressively reduced OI eventually die and are replaced by fibroblasts that deposit collagen and other matrix proteins, producing scarring in place of functioning myocardium.
Although prior studies (9, 16, 32, 33, 38) have found that abnormalities in myocardial mechanics correlate with cardiac fibrosis, our findings suggest abnormal myocardial mechanics occur before the development of significant cardiac fibrosis and, therefore, can signal cardiac dysfunction even earlier, at a time when T-tubule disorganization and abnormal Ca2+ cycling are just developing in myocytes in response to HTN.
Strengths and limitations.
The strengths of the present investigation include the comprehensive phenotyping of cellular and whole heart structure and function (with state-of-the-art confocal microscopy and speckle-tracking echocardiography) in a large sample of SHRs across a wide range of ages. The use of the SHR instead of a TAC model of HTN is also a strength of the study given the fact that the TAC-induced abrupt rise in afterload (followed by sustained HTN), although useful for the study of cardiac hypertrophy, does not mimic human forms of HTN-induced HF (12). The difference between acute-onset (TAC) and gradual-onset (SHR) HTN is not trivial, since a sudden, severe increase in afterload may create a nonphysiological stress on cardiomyocytes, thereby disrupting T-tubules (42). Thus, our study of SHRs more closely mimics human hypertensive heart disease and provides a comprehensive investigation of the cellular and ultrastructural basis for abnormal myocardial mechanics in a highly clinically relevant model of gradual-onset HTN.
Despite these strengths, our study should be interpreted in the context of certain limitations, including the lack of endocardial confocal imaging, which would improve our ability to relate myocyte abnormalities to longitudinal strain (which corresponds to subendocardial fibers). We were only able to perform confocal imaging of cell ultrastructure and Ca2+ transients on the epicardial surface of the LV, thus limiting our ability to extrapolate cellular results, even from large cell populations, to predict overall cardiac function. Another important limitation relates to the inability to reverse T-tubule disorganization and thereby demonstrate a cause-and-effect relationship between T-tubule disorganization, defects in Ca2+ cycling, and abnormal myocardial mechanics. However, there are currently two reports of interventions, both pharmacological (4) and physiological (24), that are known to stabilize T-tubules so that they do not become disorganized in response to stress. Finally, although T-tubule disorganization is the most likely reason for abnormalities in Ca2+ transients observed in our study, we cannot exclude the possibility that abnormalities in the RyR contributed to the abnormal Ca2+ transients. However, in a study of early SHRs, there were no differences in RyR density or phosphorylation of RyR (3); thus, it is unlikely that RyR abnormalities accounted for our findings.
Conclusions.
Subclinical abnormalities in myocardial mechanics occur early in response to HTN and coincide with the development of T-tubule disorganization and impaired Ca2+ cycling. These changes occur before the development of significant cardiac fibrosis and precede the development of overt cardiac dysfunction and HF. These data provide a novel ultrastructural and cellular mechanism for the development of abnormal myocardial mechanics in the setting of HTN, and they suggest that pharmacological amelioration of T-tubule disorganization may be an important therapeutic target for the prevention of HF in at-risk patients. Moreover, our results demonstrate, for the first time, that the T-tubule remodeling at the cellular level is in fact the physiological mechanism for early development of altered myocardial mechanics and that the progression of disease to overt HF occurs as a result of the fact that more cells transition from normal to abnormal T-tubule organization during maintained HTN. This is a critical observation that explains why both systolic and diastolic dysfunction develop early and progress to HF, and it raises the concept that the molecular basis for T-tubule remodeling during HTN should be the target of clinical intervention aimed at arresting and/or reversing T-tubule disruption.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants R01-HL-107577 (to S. J. Shah) and R01-HL-093490 (to R. Arora).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.J.S., G.L.A., D.K.G., L.B., S.M., B.K., A.I., R.A., J.N., and J.A.W. conception and design of research; S.J.S., D.K.G., M.J.O., A.F.N., D.S., N.C., N.B., S.R., L.B., S.M., B.K., D.W., B.R., M.W., L.A., T.M., M.R., M.K., J.N., and J.A.W. analyzed data; S.J.S., G.L.A., D.K.G., A.F.N., N.C., S.R., D.W., B.R., M.W., L.A., M.K., J.N., and J.A.W. interpreted results of experiments; S.J.S., M.J.O., D.W., B.R., M.K., and J.A.W. prepared figures; S.J.S., D.K.G., and J.A.W. drafted manuscript; S.J.S., G.L.A., R.A., J.N., and J.A.W. edited and revised manuscript; S.J.S., G.L.A., D.K.G., M.J.O., A.F.N., D.S., N.C., N.B., S.R., L.B., B.K., D.W., B.R., A.I., M.W., L.A., T.M., M.R., M.K., R.A., J.N., and J.A.W. approved final version of manuscript; G.L.A., D.K.G., D.S., N.B., L.B., S.M., B.K., A.I., and J.A.W. performed experiments.
REFERENCES
- 1.Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, Burnett JC, Jr, Rodeheffer RJ. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 115: 1563–1570, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B: 289–300, 1995 [Google Scholar]
- 3.Chen-Izu Y, Ward CW, Stark W, Jr, Banyasz T, Sumandea MP, Balke CW, Izu LT, Wehrens XH. Phosphorylation of RyR2 and shortening of RyR2 cluster spacing in spontaneously hypertensive rat with heart failure. Am J Physiol Heart Circ Physiol 293: H2409–H2417, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Chen B, Li Y, Jiang S, Xie YP, Guo A, Kutschke W, Zimmerman K, Weiss RM, Miller FJ, Anderson ME, Song LS. β-Adrenergic receptor antagonists ameliorate myocyte T-tubule remodeling following myocardial infarction. FASEB J 26: 2531–2537, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, Berretta R, Potts ST, Marsh JD, Houser SR. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res 97: 1009–1017, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Delbridge LM, Connell PJ, Morgan TO, Harris PJ. Contractile function of cardiomyocytes from the spontaneously hypertensive rat. J Mol Cell Cardiol 28: 723–733, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Doggrell SA, Brown L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res 39: 89–105, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Edvardsen T, Rosen BD, Pan L, Jerosch-Herold M, Lai S, Hundley WG, Sinha S, Kronmal RA, Bluemke DA, Lima JA. Regional diastolic dysfunction in individuals with left ventricular hypertrophy measured by tagged magnetic resonance imaging–the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J 151: 109–114, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr 23: 351–369, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Gomez AM, Guatimosim S, Dilly KW, Vassort G, Lederer WJ. Heart failure after myocardial infarction: altered excitation-contraction coupling. Circulation 104: 688–693, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science 276: 800–806, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Houser SR, Margulies KB, Murphy AM, Spinale FG, Francis GS, Prabhu SD, Rockman HA, Kass DA, Molkentin JD, Sussman MA, Koch W. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res 111: 131–150, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim M, Gorelik J, Yacoub MH, Terracciano CM. The structure and function of cardiac t-tubules in health and disease. Proc Biol Sci 278: 2714–2723, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izzo JL, Jr, Gradman AH. Mechanisms and management of hypertensive heart disease: from left ventricular hypertrophy to heart failure. Med Clin North Am 88: 1257–1271, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Jessup M, Brozena S. Heart failure. N Engl J Med 348: 2007–2018, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Kang SJ, Lim HS, Choi BJ, Choi SY, Hwang GS, Yoon MH, Tahk SJ, Shin JH. Longitudinal strain and torsion assessed by two-dimensional speckle tracking correlate with the serum level of tissue inhibitor of matrix metalloproteinase-1, a marker of myocardial fibrosis, in patients with hypertension. J Am Soc Echocardiogr 21: 907–911, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Kapur S, Aistrup GL, Sharma R, Kelly JE, Arora R, Zheng J, Veramasuneni M, Kadish AH, Balke CW, Wasserstrom JA. Early development of intracellular calcium cycling defects in intact hearts of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 299: H1843–H1853, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kosmala W, Plaksej R, Strotmann JM, Weigel C, Herrmann S, Niemann M, Mende H, Stork S, Angermann CE, Wagner JA, Weidemann F. Progression of left ventricular functional abnormalities in hypertensive patients with heart failure: an ultrasonic two-dimensional speckle tracking study. J Am Soc Echocardiogr 21: 1309–1317, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. Recommendations for blood pressure measurement in humans and experimental animals. Part 2: blood pressure measurement in experimental animals: a statement for professionals from the subcommittee of professional and public education of the American Heart Association council on high blood pressure research. Hypertension 45: 299–310, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Leggio M, Sgorbini L, Pugliese M, Mazza A, Bendini MG, Fera MS, Giovannini E, Leggio F. Systo-diastolic ventricular function in patients with hypertension: an echocardiographic tissue doppler imaging evaluation study. Int J Cardiovasc Imaging 23: 177–184, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR. Reduced synchrony of Ca2+ release with loss of T-tubules–a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res 62: 63–73, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Louch WE, Mork HK, Sexton J, Stromme TA, Laake P, Sjaastad I, Sejersted OM. T-tubule disorganization and reduced synchrony of Ca2+ release in murine cardiomyocytes following myocardial infarction. J Physiol 574: 519–533, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyon AR, MacLeod KT, Zhang Y, Garcia E, Kanda GK, Lab MJ, Korchev YE, Harding SE, Gorelik J. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci USA 106: 6854–6859, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyon AR, Nikolaev VO, Miragoli M, Sikkel MB, Paur H, Benard L, Hulot JS, Kohlbrenner E, Hajjar RJ, Peters NS, Korchev YE, Macleod KT, Harding SE, Gorelik J. Plasticity of surface structures and β2-adrenergic receptor localization in failing ventricular cardiomyocytes during recovery from heart failure. Circ Heart Fail 5: 357–365, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meethal SV, Potter KT, Redon D, Munoz-del-Rio A, Kamp TJ, Valdivia HH, Haworth RA. Structure-function relationships of Ca spark activity in normal and failing cardiac myocytes as revealed by flash photography. Cell Calcium 41: 123–134, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Muth JN, Bodi I, Lewis W, Varadi G, Schwartz A. A Ca2+-dependent transgenic model of cardiac hypertrophy: a role for protein kinase Cα. Circulation 103: 140–147, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest 117: 2431–2444, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narayanan A, Aurigemma GP, Chinali M, Hill JC, Meyer TE, Tighe DA. Cardiac mechanics in mild hypertensive heart disease: a speckle-strain imaging study. Circ Cardiovasc Imaging 2: 382–390, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Patten RD, Hall-Porter MR. Small animal models of heart failure: development of novel therapies, past and present. Circ Heart Fail 2: 138–144, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Peng Y, Popovic ZB, Sopko N, Drinko J, Zhang Z, Thomas JD, Penn MS. Speckle tracking echocardiography in the assessment of mouse models of cardiac dysfunction. Am J Physiol Heart Circ Physiol 297: H811–H820, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Pieske B, Houser SR. [Na+]i handling in the failing human heart. Cardiovasc Res 57: 874–886, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Popovic ZB, Benejam C, Bian J, Mal N, Drinko J, Lee K, Forudi F, Reeg R, Greenberg NL, Thomas JD, Penn MS. Speckle-tracking echocardiography correctly identifies segmental left ventricular dysfunction induced by scarring in a rat model of myocardial infarction. Am J Physiol Heart Circ Physiol 292: H2809–H2816, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Popovic ZB, Kwon DH, Mishra M, Buakhamsri A, Greenberg NL, Thamilarasan M, Flamm SD, Thomas JD, Lever HM, Desai MY. Association between regional ventricular function and myocardial fibrosis in hypertrophic cardiomyopathy assessed by speckle tracking echocardiography and delayed hyperenhancement magnetic resonance imaging. J Am Soc Echocardiogr 21: 1299–1305, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Poulsen SH, Andersen NH, Ivarsen PI, Mogensen CE, Egeblad H. Doppler tissue imaging reveals systolic dysfunction in patients with hypertension and apparent “isolated” diastolic dysfunction. J Am Soc Echocardiogr 16: 724–731, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Rosen BD, Edvardsen T, Lai S, Castillo E, Pan L, Jerosch-Herold M, Sinha S, Kronmal R, Arnett D, Crouse JR, 3rd, Heckbert SR, Bluemke DA, Lima JA. Left ventricular concentric remodeling is associated with decreased global and regional systolic function: the Multi-Ethnic Study of Atherosclerosis. Circulation 112: 984–991, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Rovner A, de las Fuentes L, Waggoner AD, Memon N, Chohan R, Davila-Roman VG. Characterization of left ventricular diastolic function in hypertension by use of Doppler tissue imaging and color M-mode techniques. J Am Soc Echocardiogr 19: 872–879, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Rysa J, Leskinen H, Ilves M, Ruskoaho H. Distinct upregulation of extracellular matrix genes in transition from hypertrophy to hypertensive heart failure. Hypertension 45: 927–933, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Saito M, Okayama H, Yoshii T, Higashi H, Morioka H, Hiasa G, Sumimoto T, Inaba S, Nishimura K, Inoue K, Ogimoto A, Shigematsu Y, Hamada M, Higaki J. Clinical significance of global two-dimensional strain as a surrogate parameter of myocardial fibrosis and cardiac events in patients with hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging 13: 617–623, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci USA 103: 4305–4310, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsioufis C, Chatzis D, Dimitriadis K, Stougianos P, Kakavas A, Vlasseros I, Tousoulis D, Stefanadis C, Kallikazaros I. Left ventricular diastolic dysfunction is accompanied by increased aortic stiffness in the early stages of essential hypertension: a TDI approach. J Hypertens 23: 1745–1750, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Wasserstrom JA, Sharma R, Kapur S, Kelly JE, Kadish AH, Balke CW, Aistrup GL. Multiple defects in intracellular calcium cycling in whole failing rat heart. Circ Heart Fail 2: 223–232, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Wei S, Guo A, Chen B, Kutschke W, Xie YP, Zimmerman K, Weiss RM, Anderson ME, Cheng H, Song LS. T-tubule remodeling during transition from hypertrophy to heart failure. Circ Res 107: 520–531, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol 47: 76–84, 2006 [DOI] [PubMed] [Google Scholar]