Abstract
Chronic hypoxia (CH) associated with respiratory disease results in elevated pulmonary vascular intracellular Ca2+ concentration, which elicits enhanced vasoconstriction and promotes vascular arterial remodeling and thus has important implications in the development of pulmonary hypertension (PH). Store-operated Ca2+ entry (SOCE) contributes to this elevated intracellular Ca2+ concentration and has also been linked to acute hypoxic pulmonary vasoconstriction (HPV). Since our laboratory has recently demonstrated an important role for acid-sensing ion channel 1 (ASIC1) in mediating SOCE, we hypothesized that ASIC1 contributes to both HPV and the development of CH-induced PH. To test this hypothesis, we examined responses to acute hypoxia in isolated lungs and assessed the effects of CH on indexes of PH, arterial remodeling, and vasoconstrictor reactivity in wild-type (ASIC1+/+) and ASIC1 knockout (ASIC1−/−) mice. Restoration of ASIC1 expression in pulmonary arterial smooth muscle cells from ASIC1−/− mice rescued SOCE, confirming the requirement for ASIC1 in this response. HPV responses were blunted in lungs from ASIC1−/− mice. Both SOCE and receptor-mediated Ca2+ entry, along with agonist-dependent vasoconstrictor responses, were diminished in small pulmonary arteries from control ASIC−/− mice compared with ASIC+/+ mice. The effects of CH to augment receptor-mediated vasoconstrictor and SOCE responses in vessels from ASIC1+/+ mice were not observed after CH in ASIC1−/− mice. In addition, ASIC1−/− mice exhibited diminished right ventricular systolic pressure, right ventricular hypertrophy, and arterial remodeling in response to CH compared with ASIC1+/+ mice. Taken together, these data demonstrate an important role for ASIC1 in both HPV and the development of CH-induced PH.
Keywords: store-operated Ca2+ entry, capacitative Ca2+ entry, pulmonary vascular remodeling, degenerin/epithelial Na+ channel, hypoxic pulmonary vasoconstriction, receptor-mediated vasoconstriction, acid-sensing ion channels
acid-sensing ion channels (ASICs) belong to the degenerin/epithelial Na+ channel (ENaC) family. There are four ASIC genes in mammals (ASIC1–ASIC4), which encode at least six distinct ASIC subunits termed ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4 (35). Three subunits form voltage-insensitive proton-gated cation channels (Na+ > K+), which can be homomeric or heteromeric. Homomeric ASIC1a and heteromeric ASIC1a/2b also form Ca2+-permeable channels (42, 52, 53), suggesting that these channels play an important role in intracellular signaling and excitability. Although ASICs are widely distributed in the central and peripheral nervous systems, multiple subunits for both ENaC and ASICs are present in vascular smooth muscle and endothelial cells from a variety of vascular beds, with emerging roles in the mechanotransduction of the myogenic response, flow-induced shear stress response, endothelial cell cortical stiffness, and cellular migration (6–9, 11, 12, 17, 28, 47). Recently, our laboratory (13, 14) has shown that ASIC1 is an important mediator of store-operated Ca2+ entry (SOCE) in pulmonary vascular smooth muscle in both physiological and pathophysiological settings.
Intracellular Ca2+ is an important second messenger that can mediate contraction, migration, proliferation, and gene expression in pulmonary arterial smooth muscle cells (PASMCs). Alveolar hypoxia is a fundamental physiological stimulus for pulmonary vasoconstriction, and accumulating evidence indicates that hypoxic pulmonary vasoconstriction is mediated, in part, by SOCE (26, 43, 44, 48). In addition, increases in basal intracellular Ca2+ concentration [Ca2+]i, agonist-induced Ca2+ entry, and sensitivity of the contractile myofilaments to Ca2+ all contribute to vasoconstriction and vascular remodeling in several models of pulmonary hypertension as well as in patients with idiopathic pulmonary hypertension (19, 37). Although our laboratory (13) has shown ASIC1 contributes to enhanced receptor-mediated vasoconstriction and SOCE in small pulmonary arteries after 4-wk exposure to chronic hypoxia (CH), it is unknown whether this increase in ASIC1-mediated Ca2+ influx contributes to the development of pulmonary hypertension. In this study, we tested the working hypothesis that ASIC1 contributes to vasoconstrictor and vascular remodeling components of CH-induced pulmonary hypertension using an ASIC1 knockout (ASIC1−/−) mouse model.
METHODS
Animals and CH Exposure Protocol
ASIC1−/− mice were kindly provided by Dr. M. J. Welsh and Dr. J. A. Wemmie [University of Iowa, Iowa City, IA (50)]. Mice were bred on a C57BL/6 background, and ASIC1 wild-type (ASIC1+/+) and ASIC1−/− male and female mice (∼12 wk old) were used equally for each protocol. Disruption of ASIC1 was confirmed by PCR and agarose gel electrophoresis using a three-primer system to detect both wild-type and disrupted alleles. Animals designated for exposure to CH were housed in a hypobaric chamber with barometric pressure maintained at ≈380 mmHg for 4 wk. Age-matched control mice were housed at ambient barometric pressure (≈630 mmHg in Albuquerque, NM). All protocols used in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of New Mexico School of Medicine.
Generation of Primary PASMC Cultures and Transfection
To determine the requirement of ASIC1 in SOCE, PASMCs from ASIC1−/− mice were transfected with commercially available Ultimate Open Reading Frame (ORF) human (h)ASIC1. First, this ORF was recombined into Lumio Gateway expression vectors (Invitrogen) containing the human cytomegalovirus immediate-early promoter to yield expression of hASIC1. Plasmid DNA was sequenced to verify the correct orientation of hASIC1 (MCLAB). Mouse ASIC1 and hASIC1 have >98% homology. In addition, overexpression of hASIC1 has effectively been used in a transgenic mouse model (51), verifying the compatibility of hASIC1 in mouse tissue. PASMCs were isolated according to Yuan et al. (55) from intrapulmonary arteries of ASIC1+/+ and ASIC1−/− mice. PASMCs were cultured until confluent (∼5–8 days) at 37°C with 6% CO2. Approximately one million PASMCs from ASIC1−/− mice were transfected with 5 μg hASIC1 plasmid in 100 μl Ingenio electroporation reagent (Mirus Bio) using Nucleofector 2b (Lonza). After transfection, PASMCs were seeded 3–5 days before experiments. Verification of hASIC1 expression was accomplished by PCR and immunofluorescence.
RT-PCR.
Total RNA was prepared from PASMCs using TRIzol extraction, and 1 μg total RNA was reverse transcribed to cDNA using the Transcriptor First-Strand cDNA Synthesis kit (Roche). Specific primers were used to detect transcripts for ASIC1 and β-actin (Table 1) (33). PCR was performed with the iCycler PCR system (Bio-Rad) using Taq polymerase. Two microliters of the first-strand cDNA mixture was amplified by annealing at 59°C for 30 s, extending at 68°C for 1 min, and denaturing at 94°C for 30 s. PCR products were electrophoresed through a 3% agarose gel and stained with ethidium bromide for visualization under ultraviolet light.
Table 1.
Primers used for RT-PCR
| Primer Pair Sequence | Product Size, bp | |
|---|---|---|
| ASIC1 | ||
| Sense | 5′-TGGCCCACATCTTCTCCTAC-3′ | 251 |
| Antisense | 5′-CTCCCCAGCATGATACAGGT-3′ | |
| β-Actin | ||
| Sense | 5′-AGTGTGACGTTGACATCCGT-3′ | 244 |
| Antisense | 5′-GACTCATCGTACTCCTGCTT-3′ |
ASIC1, acid-sensing ion channel 1.
Immunofluorescence.
Transfected and nontransfected PASMCs from ASIC1+/+ and ASIC1−/− mice were fixed with 2% paraformaldehyde and incubated overnight at 4°C with rabbit anti-smooth muscle-22α (1:200, Abcam) and goat anti-ASIC1 (1:50, Santa Cruz Biotechnology). For immunofluorescence labeling, PASMCs were incubated for 1 h at room temperature with donkey anti-rabbit Alexa fluor 647 and donkey anti-goat DyLight 549. Images were acquired using a confocal microscope (TCS SP5, Leica).
Store-operated Ca2+ entry.
Transfected and nontransfected PASMCs from ASIC1+/+ and ASIC1−/− mice were incubated with fura-2 AM [2 μM and 0.05% pluronic acid in physiological saline solution (PSS), Molecular Probes] for 30 min at 32°C. Fura-2-loaded PASMCs were alternately excited at 340 and 380 nm at a frequency of 1 Hz with an IonOptix Hyperswitch dual excitation light source (IonOptix), and the respective 510-nm emissions were detected with a photomultiplier tube. PASMCs were superfused (5 ml/min at 37°C) with Ca2+-free, HEPES-based PSS [containing (in mM) 130 NaCl, 4 KC1, 1.2 MgSO4, 4 NaHCO3, 10 HEPES, 1.18 KH2PO4, 6 glucose, and 3 EGTA; pH adjusted to 7.4 with NaOH] containing 50 μM diltiazem to prevent Ca2+ entry through L-type voltage-gated Ca2+ channels and 10 μM cyclopiazonic acid (CPA) to deplete intracellular Ca2+ stores and prevent Ca2+ reuptake through sarco(endo)plasmic reticulum Ca2+-ATPase. Changes in [Ca2+]i were determined upon repletion of HEPES-based PSS containing 1.8 mM CaCl2 in the continued presence of diltiazem and CPA.
Hypoxic Pulmonary Vasoconstrictor Responses in the Isolated Perfused Mouse Lung
The contribution of ASIC1 to changes in pulmonary vascular resistance in response to acute hypoxia was assessed in isolated, saline-perfused lungs from ASIC1+/+ and ASIC1−/− mice. Mice were anesthetized with pentobarbital sodium (200 mg/kg ip). The trachea was cannulated with a 22-gauge blunt needle stub, and the lungs were ventilated with a positive-pressure mouse ventilator (model 845, Harvard Apparatus) at a frequency of 90 breaths/min and a tidal volume of 250 μl with a warmed and humidified normoxic gas mixture (21% O2, 6% CO2, and balance N2) at peak inspiratory and end-expiratory pressures of 9 and 2.5 cmH2O, respectively. After a median sternotomy, heparin (100 U/20 g) was injected directly into the right ventricle (RV), and the pulmonary artery was cannulated with a 22-gauge feeding needle. The preparation was immediately perfused with NaHCO3-based PSS [containing (in mM) 129.8 NaCl, 5.4 KCl, 0.83 MgSO4, 19 NaHCO3, 1.8 CaCl2, 0.5 NaH2PO4, and 5.5 glucose] containing 4% (wt/vol) albumin (Sigma), 300 μM NG-nitro-l-arginine (Sigma), and 30 μM meclofenamate (Sigma) at 0.6 ml/min with a peristaltic pump (Ismatec). NG-nitro-l-arginine and meclofenamate were added to minimize potential complicating influences of endogenous nitric oxide and prostaglandins on vascular reactivity. The perfusion rate was gradually increased to 30 ml·min−1·kg body wt−1 and maintained at this rate for the duration of the experiment. Pulmonary arterial pressure was measured with a P75 transducer linked to a PLUGSYS TAM-A amplifier (Harvard Apparatus). Data were stored and processed with a computer-based data acquisition-analysis system (AT-CODAS, DATAQ Instruments). After a 30-min stabilization period, the lung was ventilated with a hypoxic gas mixture (6% CO2 and balance N2) until the peak hypoxic response was reached. After the return to baseline arterial resistance with normoxic ventilation, changes in arterial resistance to depolarizing concentrations of KCl (20–30 mM) were assessed to verify lung viability.
Cannulation and Fura-2 Loading of Small Pulmonary Arteries
To determine changes in pulmonary vasoreactivity and SOCE, small intrapulmonary arteries were cannulated and pressurized for simultaneous dimensional and [Ca2+]i analysis as previously described (14). Briefly, mice were anesthetized with pentobarbital sodium (200 mg/kg ip). The left lung was removed, and small intrapulmonary arteries (fourth to fifth order) were dissected free, transferred to a vessel chamber (Living Systems), and secured to tapered glass pipettes with a single strand of silk ligature. After cannulation, the artery was pressurized with a servo-controlled peristaltic pump (Living Systems) to 12 mmHg. Any artery that failed to maintain pressure upon switch off of the servo-controller was discarded. The vessel chamber was superfused with HEPES-based PSS (5 ml/min at 37°C). Red-wavelength, bright-field images were obtained using an Eclipse TS100 microscope (Nikon) and IonOptix CCD100M camera to measure inner diameter, and dimensional analysis was performed by IonOptix Ion Wizard software (IonOptix). Arteries were incubated, abluminally, with fura-2 AM (2 μM and 0.05% pluronic acid in PSS, Molecular Probes) for 45 min at room temperature, as previously described (14), and fura-2 fluorescence was detected as described above.
SOCE.
In addition to the method described above for PASMCs, SOCE was additionally quantified by quenching of fura-2 fluorescence with Mn2+, which enters cells as a Ca2+ surrogate. As previously described (13), fura-2-loaded pulmonary arteries were excited at 360 nm, and emission light was recorded at 510 nm. Store-operated channels were activated by superfusing the vessel with Ca2+-free PSS (without EGTA) containing diltiazem (50 μM) and CPA (10 μM) for 15 min. MnCl2 (500 μM) was added to the superfusate, and the percent change in fura-2 fluorescence 10 min after MnCl2 was calculated from the baseline fluorescence intensity at time 0.
Small pulmonary artery vasoconstrictor reactivity.
Receptor-mediated vasoconstrictor reactivity was assessed by superfusion (5 ml/min at 37°C) of cumulative concentrations of UTP (10−7–10−3.5 M, Sigma-Aldrich) or endothelin-1 (ET-1; 10−11–10−7 M, Sigma-Aldrich). Depolarization-mediated vasoconstriction was determined using depolarizing concentrations of equal molar KCl (10−1.75–10−1.00 M, Sigma-Aldrich).
ASIC1 Expression
Lung fixation and immunofluorescence.
Mice were anesthetized with pentobarbital sodium (200 mg/kg ip). After a median sternotomy, heparin (100 U/20 g) was injected directly into the RV, and the pulmonary artery was cannulated with a 22-gauge feeding needle. The preparation was immediately perfused with 25 ml of 0.1 M PBS containing 10−4 M papaverine to maximally dilate the vasculature and flush the circulation of blood. The lungs were then perfused with 25 ml fixative (0.1 M PBS containing 4% sucrose, 4% paraformaldehyde, and 10−4 M papaverine) at a pressure of 50 cmH2O above the hilum, and the trachea inflated to a pressure of 25 cmH2O. Along with the vasodilator papaverine, this transmural distending pressure of 25 cmH2O causes adequate capillary distension with minimal risk of rupture to observe structural changes in the pulmonary vasculature independent of changes in vascular tone. The trachea was ligated with 4-0 silk, and the lungs were immersed in fixative overnight, dehydrated, and then mounted in paraffin.
Sections were cut (5 μm thick) and mounted onto Superfrost Plus slides (Fisher Scientific). Antibody-antigen binding was enhanced by an antigen retrieval method in which sections were held just below boiling for 15 min with 10 mM Tris (pH 9.0) buffer (containing 1 mM EDTA + 0.05% Tween 20). Sections were incubated with goat anti-ASIC1 (1:50, Santa Cruz Biotechnology) and rabbit anti-smooth muscle α-actin (1:200, Abcam) overnight at 4°C. ASIC1 and α-actin were detected with donkey anti-goat DyLight 549 and anti-rabbit Alexa fluor 647 (1:100, 1 h, Jackson ImmunoResearch). Sections were mounted with FluoroGel (Electron Microscopy Sciences), and images were acquired using a confocal microscope (TCS SP5, Leica). Fluorescence images were digitally inverted using ImageJ (National Institutes of Health) to provide better contrast and visibility of immunofluorescence.
Western blot analysis.
Intrapulmonary arteries (<500 μm) were homogenized in 10 mM Tris·HCl homogenization buffer (containing 255 mM sucrose, 2 mM EDTA, 12 μM leupeptin, 1 μM pepstatin A, and 0.3 μM aprotinin) and centrifuged at 10,000 g for 10 min at 4°C to remove insoluble debris (13). Sample protein concentrations were determined by the Bradford method (Bio-Rad). Pulmonary artery lysates (20 μg) were separated by SDS-PAGE (7.5% Tris·HCl) and transferred to polyvinylidene difluoride membranes. Blots were blocked for 1 h with 5% milk and then incubated overnight at 4°C with rabbit anti-ASIC1 (1:500, Abcam) and subsequently for 1 h with rabbit anti-GAPDH (1:5,000, Abcam). For immunochemical labeling, blots were incubated with goat anti-rabbit IgG-horseradish peroxidase (1:3,000, 1 h, Bio-Rad). Blots were exposed to chemiluminescence-sensitive film (Kodak), and quantification of ASIC1 and GAPDH bands was accomplished by densitometric analysis of scanned images (Gel Logic, Care Stream).
Measurement of RV Pressure, Heart Rate, RV Hypertrophy, and Polycythemia
RV systolic pressure (RVSP) was measured as an index of pulmonary arterial pressure in anesthetized mice (2% isoflurane and 98% O2 gas mixture). An upper transverse laparotomy was performed to expose the diaphragm. A 25-gauge needle, connected to a pressure transducer (model P23 XL, Spectramed), was inserted into the RV via a closed-chest transdiaphragmatic approach, and the output amplified by a Gould Universal amplifier. All data were recorded, and heart rate was calculated with a computer-based data-acquisition system (AT-CODAS, DATAQ Instruments). Fulton's index, expressed as the ratio of RV weight to left ventricular plus septal (LV + S) weight, was used to assess RV hypertrophy. Polycythemia was assessed by measuring hematocrit from blood samples collected in microcapillary tubes after direct cardiac puncture at the time of lung isolation.
Assessment of Arterial Remodeling
Lung sections were prepared for immunofluorescence from parraffin-embedded lung tissue as described above. Sections were incubated with rabbit anti-smooth muscle α-actin (1:200, Abcam) overnight at 4°C. Smooth muscle α-actin was detected by incubating the slides with DyLight 549-donkey anti-rabbit (1:400, 3.5 h, Jackson ImmunoResearch). Twenty images from the right and left lobes were randomly collected per animal using a ×20 objective on a confocal microscope (TCS SP5, Leica). Images were thresholded using MetaMorph Imaging software (Molecular Devices). Numbers of fully muscularized arteries were counted per animal. Regions of interest (ROIs) were drawn around each fully muscularized artery. The percent thresholded area to total ROI area was calculated for each artery as the percent muscularization. Arterial diameter was calculated based on the circumference of the ROI. Fluorescence images were digitally inverted to provide better contrast and visibility of immunofluorescence.
Calculations and Statistics
All data are expressed as means ± SE. n values of numbers of animals in each group unless otherwise stated. A t-test, one-way ANOVA, or two-way ANOVA was used to make comparisons when appropriate. If differences were detected by ANOVA, individual groups were compared with the Student-Newman-Keuls test. P values of <0.05 were accepted as significant for all comparisons.
RESULTS
Restoration of ASIC1 Expression in PASMCs From ASIC1−/− Mice Rescues SOCE
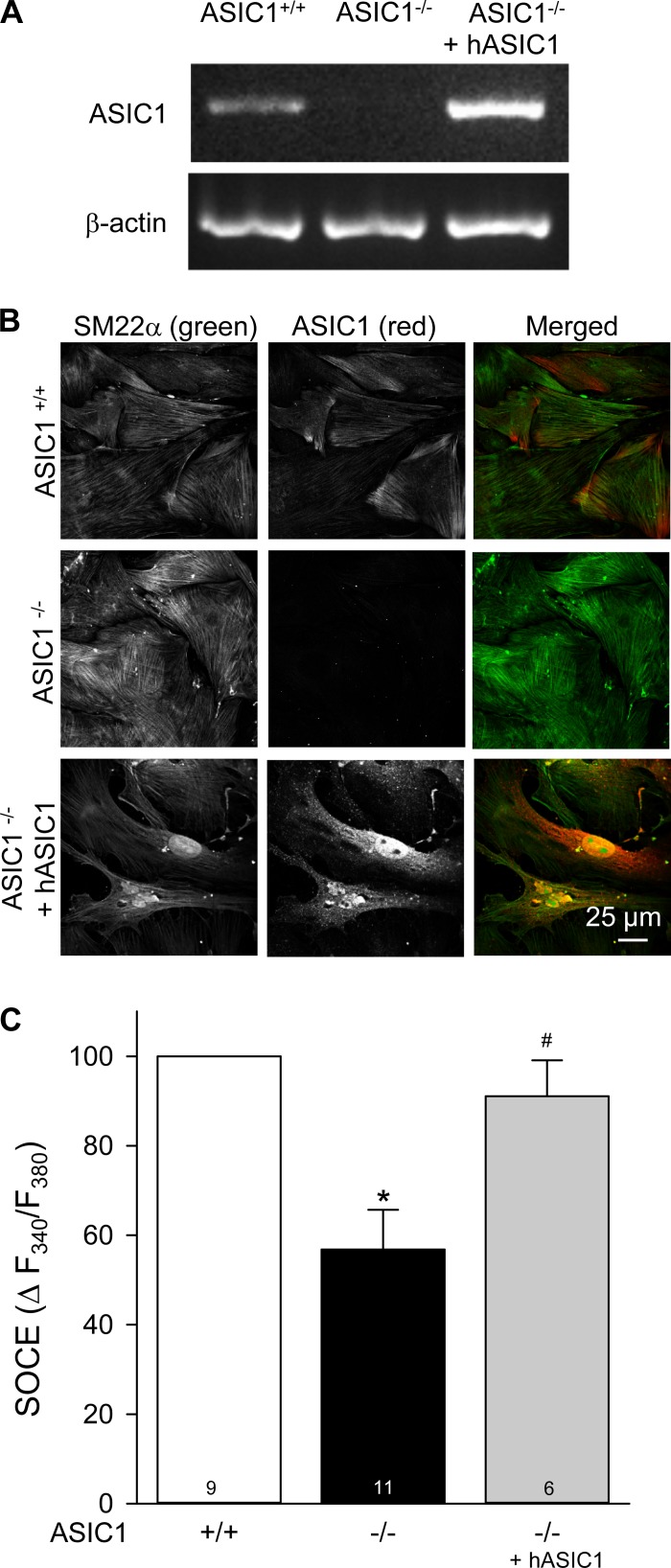
Transfection of hASIC1 restored ASIC1 expression in PASMCs from ASIC1−/− mice (Fig. 1, A and B). Similar to what we have observed with pharmacological inhibitors of ASIC1 (13, 14), SOCE was significantly reduced in primary cultured PASMCs from ASIC1−/− mice compared with ASIC1+/+ mice (Fig. 1C). Transfection of the hASIC1 gene into PASMCs from ASIC1−/− mice restored SOCE to a level similar to that in PASMCs from ASIC1+/+ mice, demonstrating that ASIC1, per se, is an essential component of SOCE.
Fig. 1.
Restoration of acid-sensing ion channel 1 (ASIC1) in pulmonary arterial smooth muscle cells (PASMCs) from ASIC1 knockout (ASIC1−/−) mice rescues store-operated Ca2+ entry (SOCE). A: RT-PCR for ASIC1 and β-actin in PASMCs from ASIC1+/+ and ASIC1−/− mice transfected with human (h)ASIC1. B: smooth muscle-22α (SM22α; green) and ASIC1 (red) immunofluorescence in PASMCs from ASIC1+/+ and ASIC1−/− mice. PASMCs from ASIC1−/− mice were additionally transfected with hASIC1. C: summary data showing SOCE-induced changes (Δ) in intracellular Ca2+ concentration ([Ca2+]i) [expressed as changes in the 340-to-380-nm fluorescence ratio (ΔF340/F380)] in PASMCs isolated from ASIC1+/+ and ASIC1−/− mice. PASMCs from ASIC1−/− mice were additionally transfected with hASIC1. All experiments were performed in the presence of cyclopiazonic acid (CPA; 10 μM) and diltiazem (50 μM). Values are means ± SE; numbers of animals (n values) are indicated in bars. *P < 0.05 vs. PASMCs from ASIC1+/+ mice; #P <0.05 vs. PASMCs from ASIC1−/− mice.
Hypoxic Pulmonary Vasoconstriction Is Attenuated in Isolated Perfused Lungs From ASIC1−/− Mice
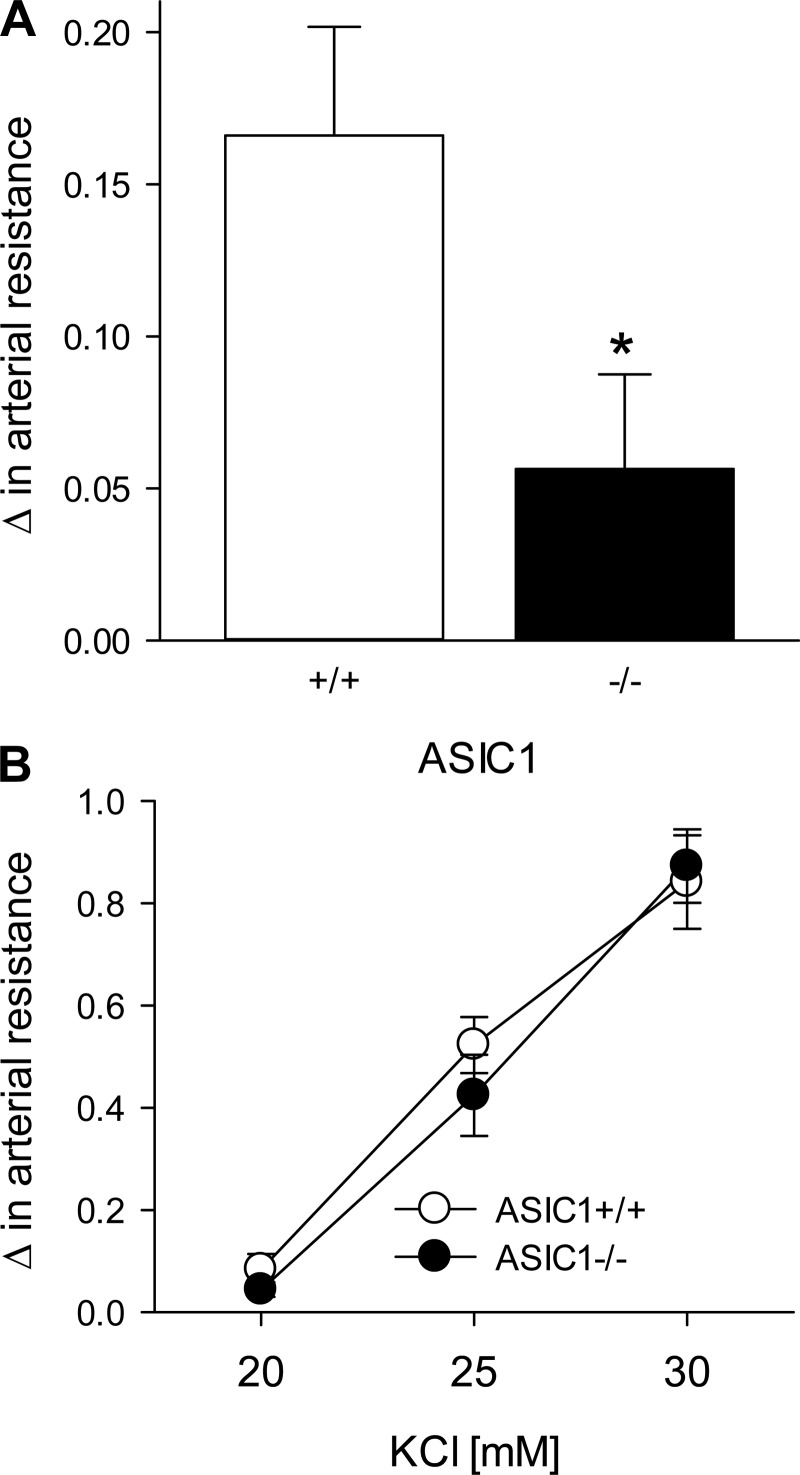
Baseline arterial resistance under normoxic ventilation was not different between isolated perfused lungs from ASIC1+/+ and ASIC1−/− mice (0.32 ± 0.03 and 0.32 ± 0.20 mmHg·kg·min·ml−1, respectively). Hypoxic ventilation increased pulmonary arterial resistance in lungs from both ASIC1+/+ and ASIC1−/− mice; however, the increase in pulmonary arterial resistance was significantly less in lungs from ASIC1−/− mice (Fig. 2A). In contrast, changes in pulmonary arterial resistance to increasing concentrations of KCl were similar between groups (Fig. 2B), indicating that depolarization-induced changes in arterial resistance are not impaired in ASIC1−/− mice. These data imply that ASIC1 is directly involved in hypoxic pulmonary vasoconstriction (HPV).
Fig. 2.
Hypoxic pulmonary vasoconstriction is blunted in ASIC1−/− mice. A and B: changes in pulmonary arterial resistance (in mmHg·ml−1·min·kg) to hypoxia (A) and KCl (20–30 mM; B) in isolated lungs from ASIC1+/+ and ASIC1−/− mice. All experiments were conducted in the presence of NG-nitro-l-arginine (300 μM) and meclofenamate (30 μM). Data are expressed as means ± SE; n = 5–7 animals/group. *P ≤ 0.05 vs. normoxic ventilation.
Enhanced SOCE in Small Pulmonary Arteries After CH Is Absent in ASIC1−/− Mice
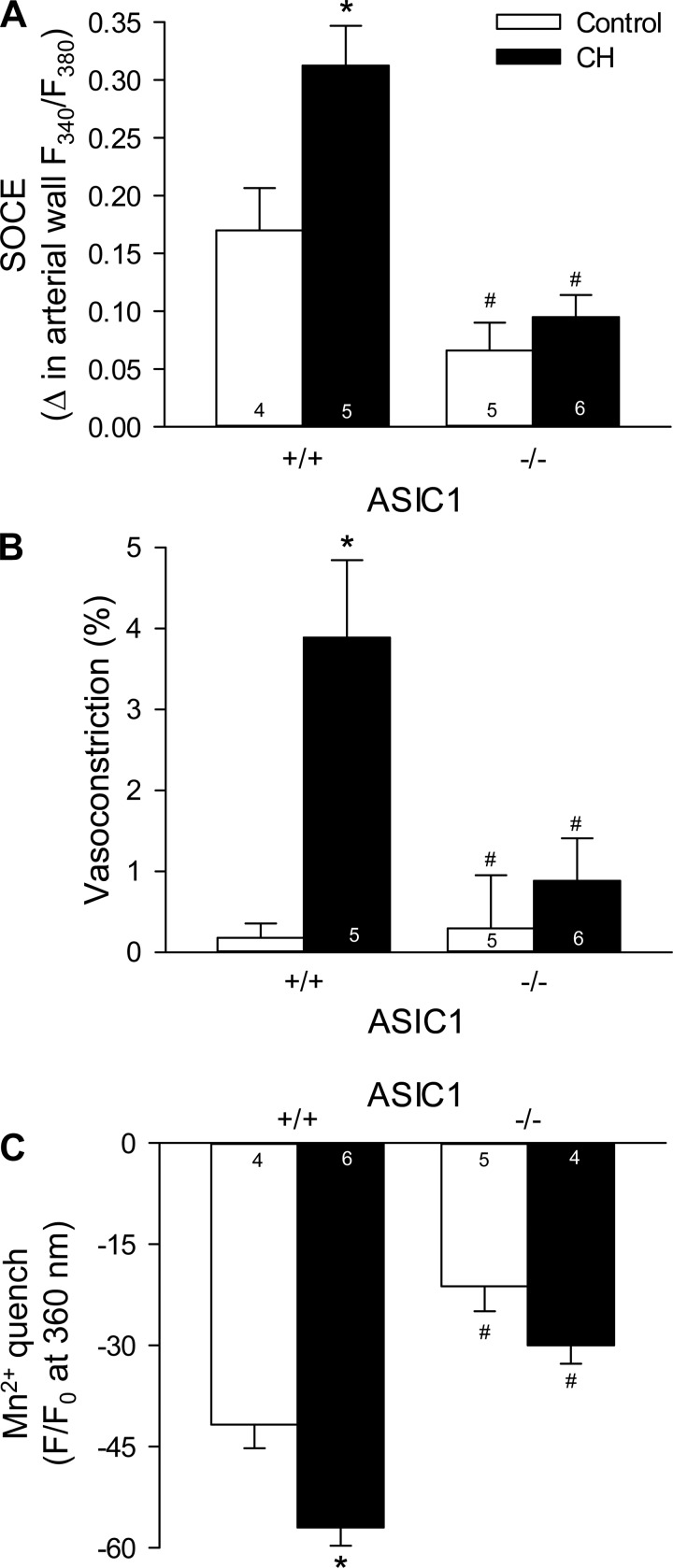
ASIC1−/− mice exhibited diminished SOCE in isolated small pulmonary arteries compared with arteries from ASIC1+/+ mice. CH augmented SOCE in pulmonary arteries from ASIC1+/+ mice (Fig. 3A), which was accompanied by a modest constriction (Fig. 3B). In contrast, CH was without effect on SOCE in ASIC1−/− mice (Fig. 3). These data, along with previous observations from our laboratory (13), demonstrate the involvement of ASIC1 in SOCE in control animals and the augmentation of this response after exposure to CH.
Fig. 3.
ASIC1 contributes to augmented SOCE after chronic hypoxia (CH) in isolated small pulmonary arteries. SOCE-induced changes in arterial wall [Ca2+]i (ΔF340/F380; A), vasoconstriction (percent baseline diameter; B), and magnitude of Mn2+ quenching 10 min after MnCl2 (500 μM) administration (C). All experiments were performed in the presence of CPA (10 μM) and diltiazem (50 μM). F, fluorescence intensity; F0, baseline fluorescence intensity at time 0. Values are means ± SE; n values are indicated in bars. *P < 0.05 vs. the control group; #P < 0.05 vs. the corresponding ASIC1+/+ artery.
Enhanced Receptor-Mediated, But Not Depolarization-Dependent, Vasoconstrictor and Arterial Wall [Ca2+]i Responses After CH Are Impaired in ASIC1−/− Mice
Baseline fura-2 ratios were greater in arteries isolated from CH mice compared with control mice (Table 2); however, there were no significant differences between arteries from ASIC1+/+ and ASIC1−/− mice. Baseline vessel inner diameters and in situ fura-2 calibrations were also not different between groups (Table 2). This indicates that the change in fura-2 fluorescence between control and CH arteries is not likely mediated by differences in vessel size or fura-2 loading between groups. In addition, this increased basal vessel wall [Ca2+]i was abolished by an incubation in Ca2+-free PSS (data not shown), suggesting that CH increases basal [Ca2+]i through a mechanism independent of ASIC1.
Table 2.
Baseline small pulmonary artery inner diameter and fura-2 ratios as well as in situ fura-2 calibrations after intraluminal pressurization to 12 mmHg
| ASIC1+/+ Group |
ASIC1−/− Group |
|||
|---|---|---|---|---|
| Control | CH | Control | CH | |
| Baseline inner diameter, μm | 131 ± 9 | 118 ± 8 | 123 ± 12 | 141 ± 9 |
| Baseline fura-2 ratio, F340/F380 | 0.56 ± 0.02 | 0.66 ± 0.03* | 0.55 ± 0.04 | 0.67 ± 0.05* |
| Low calibration, F340/F380 | 0.43 ± 0.02 | 0.42 ± 0.04 | 0.43 ± 0.03 | 0.42 ± 0.03 |
| High calibration, F340/F380 | 1.11 ± 0.16 | 1.16 ± 0.16 | 0.92 ± 0.12 | 1.03 ± 0.19 |
Data are expressed as means ± SE; n = 11–14 animals depending on the group. For in situ calibrations, fura-2 ratios were collected from arteries at the end of the experiment by incubation with the Ca2+ ionophore ionomycin (10 μM) in either Ca2+-free or normal physiological saline solution (1.8 mM CaCl2) for low and high calibration, respectively. CH, chronic hypoxia; F340/F380, 340-to-380-nm fluoresence ratio.
P < 0.05 vs. the corresponding control group.
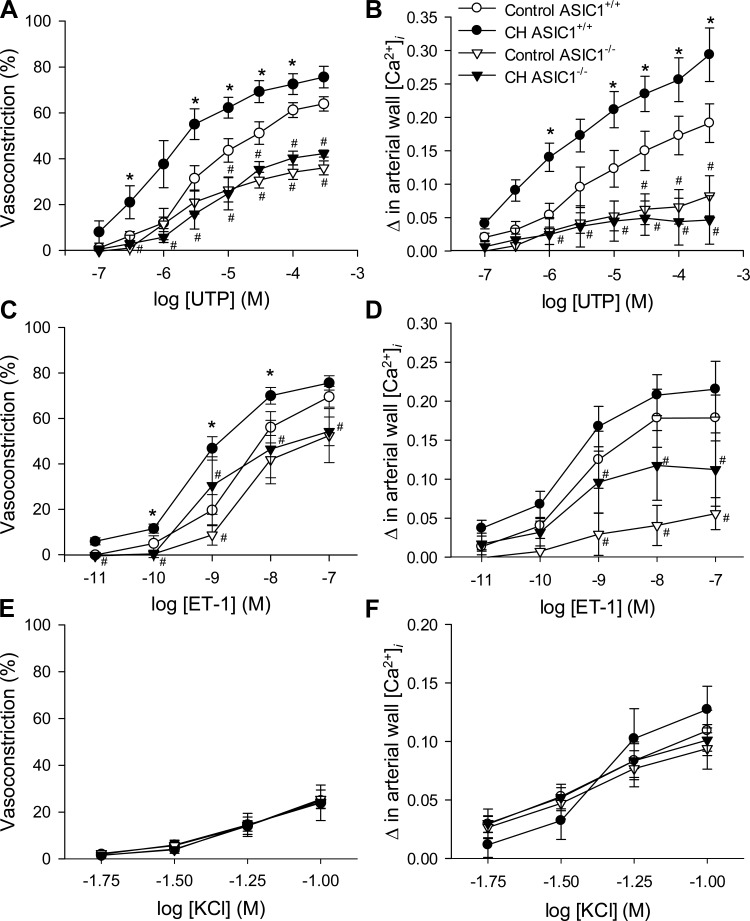
UTP-induced vasoconstrictor responses (Fig. 4A) and changes in arterial wall [Ca2+]i (Fig. 4B) were augmented in arteries from ASIC1+/+ CH mice compared with control mice. Similar increases in vasoconstrictor reactivity were observed in response to ET-1 (Fig. 4C). Vasoconstrictor responses and changes in arterial wall [Ca2+]i to UTP and ET-1 in arteries from both control and CH ASIC1−/− mice were largely blunted compared with ASIC1+/+ mice (Fig. 4, A–D). Furthermore, exposure to CH did not augment vasoconstrictor reactivity in arteries from ASIC1−/− mice. In contrast to receptor-dependent vasoconstriction, depolarization-induced vasoconstriction (Fig. 4E) and changes in arterial wall [Ca2+]i (Fig. 4F) were not different between groups, indicating that vascular reactivity per se is not impaired in ASIC1−/− mice.
Fig. 4.
ASIC1 contributes to enhanced receptor-mediated vasoconstriction after CH in small pulmonary arteries. A–F: vasoconstriction (percent baseline diameter; A, C, and E) and changes in arterial wall [Ca2+]i (ΔF340/F380; B, D, and F) to UTP (10−7 to 3 × 10−4 M; A and B), endothelin-1 (ET-1; 10−11 to 10−7 M; C and D), and depolarizing concentrations of KCl (10−1.75 to 10−1.00 M; E and F) in small pulmonary arteries from control and CH ASIC1+/+ and ASIC1−/− mice. Values are means ± SE; n = 5–7 animals/group. *P ≤ 0.05 vs. the control group; #P <0.05 vs. the corresponding ASIC1+/+ artery.
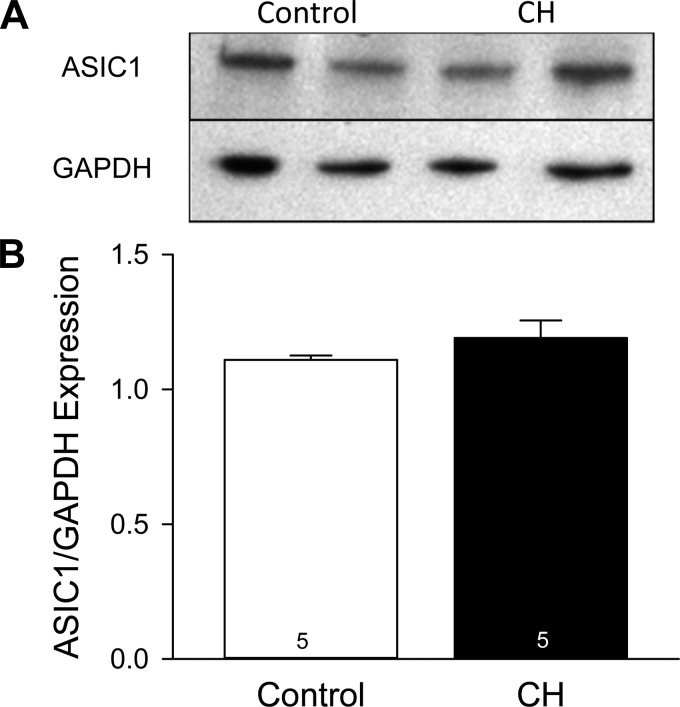
CH Does Not Alter ASIC1 Protein Levels in Small Pulmonary Arteries
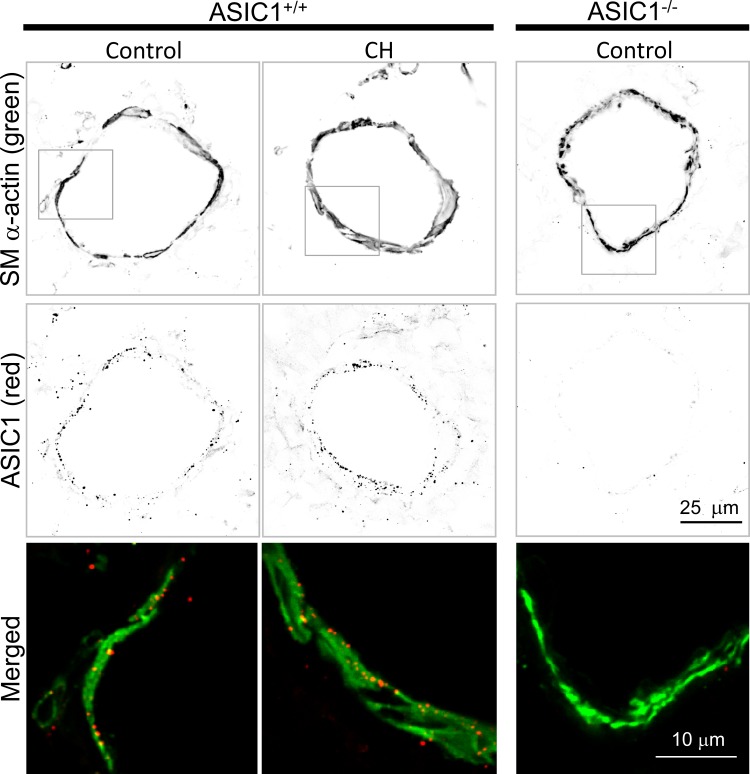
ASIC1 was detected as punctate fluorescence within the vascular smooth muscle and endothelial cell layers from ASIC1+/+ but not ASIC1−/− lung sections (Fig. 5). Exposure to CH did not alter ASIC1 protein (Fig. 6) or mRNA (data not shown) expression in isolated pulmonary arteries from ASIC1+/+ mice, suggesting that an increase in ASIC1 protein expression is not required to elicit enhanced ASIC1-dependent Ca2+ influx.
Fig. 5.
ASIC1 protein expression in small pulmonary arteries. Smooth muscle α-actin (SM α-actin) and ASIC1 immunofluorescence in ASIC1+/+ and ASIC1−/− lung sections demonstrate the presence of ASIC1 in small pulmonary arteries (∼70–100 μm). Fluorescence images were digitally inverted using ImageJ to provide better contrast and visibility of immunofluorescence. The bottom images represent digitally zoomed fluorescence overlay images corresponding to the boxes in the images of smooth muscle α-actin (green; top) and ASIC1 (red; middle).
Fig. 6.
CH does not alter ASIC1 protein levels in small pulmonary arteries. A: representative Western blots of ASIC1 and GAPDH (20 μg protein/lane). B: summary data for Western blot analysis of ASIC1/GAPDH protein expression. Values are means ± SE; n values are indicated in bars.
ASIC1−/− Mice Exhibit Blunted CH-Induced Pulmonary Hypertension
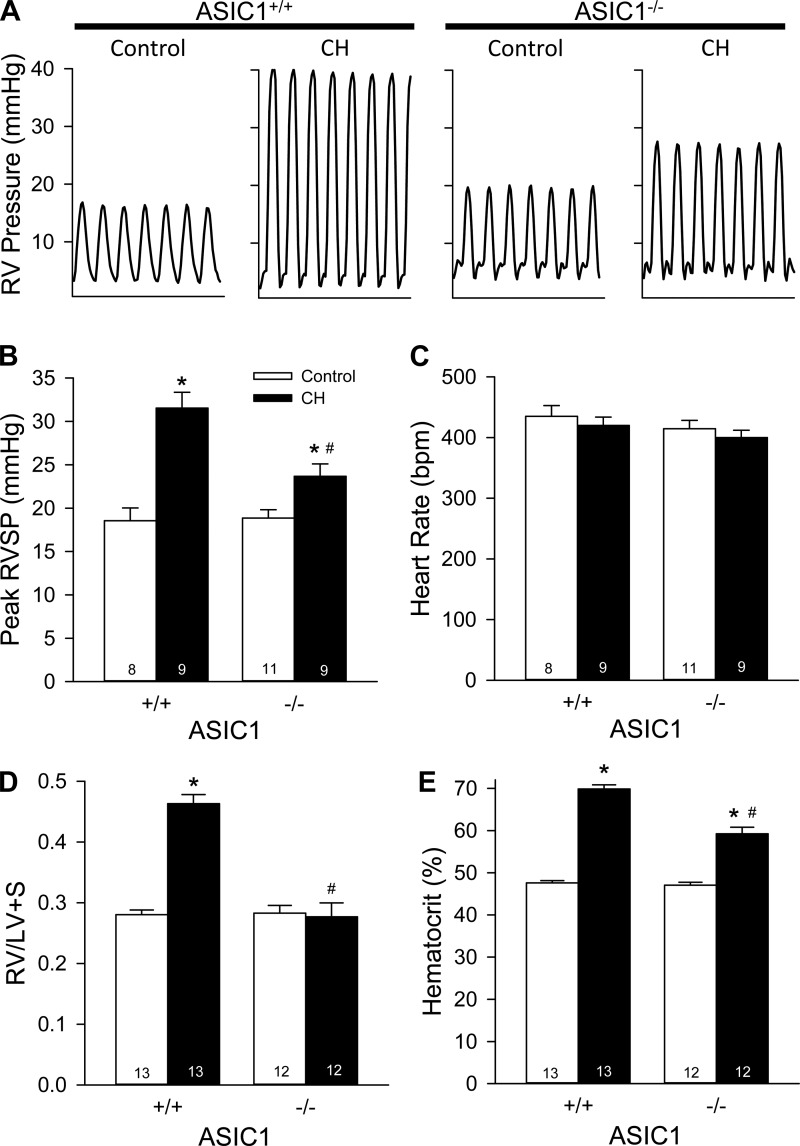
RVSP was similar in ASIC1+/+ and ASIC1−/− control mice. After 4 wk of exposure to CH, ASIC1+/+ and ASIC1−/− mice exhibited increased RVSP compared with their control littermates; however, RVSP was significantly lower in ASIC1−/− mice compared with ASIC1+/+ mice (Fig. 7, A and B). Heart rate was not different between groups (Fig. 7C). RV hypertrophy was evident in ASIC1+/+ mice, as indicated by a greater RV heart weight after CH leading to increased RV-to-(LV + S) ratios (Table 3 and Fig. 7D). Interestingly, ASIC1−/− mice did not develop RV hypertrophy (Fig. 7D). ASIC1+/+ and ASIC1−/− mice both exhibited polycythemia after CH, as indicated by a significantly greater hematocrit compared with control animals. However, the degree of polycythemia was significantly less in ASIC1−/− CH mice compared with ASIC1+/+ CH mice (Fig. 7E). These data suggest ASIC1 contributes to CH-induced pulmonary hypertension, RV hypertrophy, and polycythemia.
Fig. 7.
ASIC1 contributes to CH-induced pulmonary hypertension. A: representative traces of transdiaphragmatic measurements of right ventricular (RV) pressure. B and C: summary data for RV systolic pressure (RVSP; B) and heart rate [HR; in beats/min (bpm); C] in anesthetized control and CH ASIC1+/+ and ASIC1−/− mice. D and E: ratio of RV to left ventricular plus septal (LV + S) heart weight (D) and hematocrit (in %; E) in ASIC1+/+ and ASIC1−/− mice exposed to control or CH conditions. Values are means ± SE; n = 8–13 animals/group. *P < 0.05 vs. the control group; # P < 0.05 vs. the corresponding ASIC1+/+ mice.
Table 3.
Mean body weight, right ventricular weight, and left ventricular plus septal heart weight in ASIC1+/+ and ASIC1−/− mice exposed to control or CH conditions
| ASIC1+/+ Group |
ASIC1−/− Group |
|||
|---|---|---|---|---|
| Control | CH | Control | CH | |
| Body weight, g | 24.5 ± 1.3 | 24.1 ± 1.0 | 24.4 ± 0.8 | 24.9 ± 0.7 |
| Right ventricular weight, mg | 21.9 ± 1.1 | 32.4 ± 2.3* | 20.4 ± 0.6 | 21.6 ± 1.3† |
| Left Ventricular plus septal weight, mg | 84.8 ± 4.1 | 81.2 ± 4.3 | 75.6 ± 2.6 | 79.0 ± 4.0 |
Values are means ± SE; n = 12–13 animals/group.
P < 0.05 vs. the control group;
P < 0.05 vs. corresponding ASIC1+/+ mice.
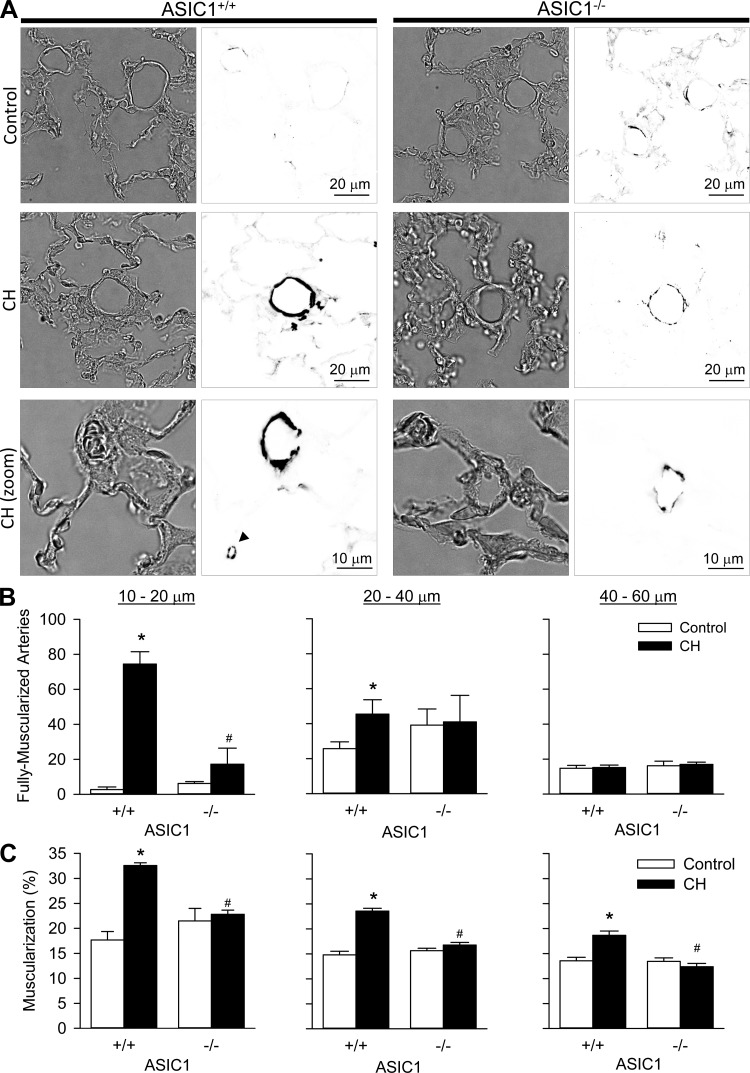
Mice Have Diminished Small Pulmonary Arterial Remodeling After CH
CH increased the number of fully muscularized arteries (Fig. 8, A and B) in both the 10- to 20- and 20- to 40-μm diameter ranges in ASIC+/+ but not ASIC−/− mice. In contrast, CH was without effect on the number of fully muscularized arteries in the 40- to 60-μm diameter range in either group, as most arteries of this size are muscularized even under control conditions. Furthermore, the degree of muscularization (Fig. 8C) was greater in small pulmonary arteries from CH versus control ASIC1+/+ mice over all three ranges of vessel diameters. Such medial hypertrophy was absent, however, in arteries from ASIC1−/− mice. There were no differences in the degree of muscularization in arteries of >60-μm outer diameter (data not shown). Taken together, these data suggest ASIC1 contributes to small pulmonary arterial remodeling after CH.
Fig. 8.
ASIC1 contributes to small pulmonary artery remodeling after CH. A: representative bright-field (left) or smooth muscle α-actin immunofluorescence (right) images of lung sections from control and CH ASIC1+/+ and ASIC1−/− mice. Bottom images show higher-magnification images of ∼10-μm arteries from CH ASIC1+/+ and ASIC1−/− mice. The arrow bottom middle image indicates an alveolar duct smooth muscle cell. Fluorescence images were digitally inverted to provide better contrast and visibility of immunofluorescence. B: numbers of fully muscularized arteries according to arterial diameter (10–20, 20–40, and 40–60 μm) from 20 random images/lung section. C: percent muscularization calculated as percent thresholded smooth muscle α-actin area divided by total arterial wall area. Values are means ± SE; n = 4 animals/group. *P ≤ 0.05 vs. the control group; #P <0.05 vs. corresponding ASIC1+/+ mice.
DISCUSSION
Our laboratory (14) has previously shown that ASIC1 contributes to SOCE in small pulmonary arteries. Furthermore, we (13) have also demonstrated that ASIC1 is required for the enhanced SOCE and receptor-mediated vasoconstriction after exposure to CH. Several studies (20, 21, 39, 43, 48, 49) have showm that SOCE is an important component of acute hypoxic vasoconstrictor responses as well as the enhanced vasoconstrictor responsiveness seen with pulmonary hypertension. However, it is unknown whether ASIC1-mediated Ca2+ influx contributes to hypoxic vasoconstriction or the development of pulmonary hypertension. The goal of this study was to determine the contribution of ASIC1 to pulmonary arterial remodeling and vasoconstrictor components of CH-induced pulmonary hypertension in an ASIC1−/− mouse model. The major findings from this study are that 1) restoration of ASIC1 in PASMCs from ASIC1−/− mice rescues SOCE; 2) acute HPV is attenuated in ASIC1−/− mice; 3) ASIC1-dependent increases in SOCE and receptor-mediated vasoconstriction after CH are not contingent upon an increase in ASIC1 protein expression; and 4) ASIC1−/− mice exhibit diminished RVSP, RV hypertrophy, hematocrit, and pulmonary arterial remodeling compared with ASIC1+/+ mice. The results from this study demonstrate an important role for ASIC1 in HPV and the development of CH-induced pulmonary hypertension and further suggest that this requirement for ASIC1 is independent of changes in protein expression.
Alveolar hypoxia is a fundamental physiological stimulus for pulmonary vasoconstriction, which is an important physiological response that diverts blood flow to better ventilated areas of the lung, thereby matching perfusion to ventilation and facilitating arterial blood oxygenation. However, prolonged HPV contributes to the vasoconstrictor component of CH-induced pulmonary hypertension. Although HPV has been recognized and studied for over a century, the mechanism(s) that mediates this response remain controversial (for a review, see Ref. 40). Accumulating evidence indicates that HPV is mediated by a release of Ca2+ from the sarcoplasmic reticulum and secondary SOCE (26, 43, 44, 48). Consistent with this, we found that changes in pulmonary arterial resistance in response to hypoxia were blunted in isolated lungs from ASIC1−/− mice compared with ASIC1+/+ mice. Although this is a novel finding with regard to the pulmonary vasculature, ASICs have been shown to contribute to carotid body chemoreception and lung chemoreceptor hypersensitivity after CH (22, 41). While ASIC1 may be present in lung sensory neurons, a physiological role of ASIC1 in PASMCs is suggested by our functional experiments in multiple isolated preparations in which neural feedback is absent. Nonetheless, a role for ASIC in HPV is in agreement with emerging evidence for ASIC involvement in many hypoxia-induced pathological processes associated with cancer, arthritis, intervertebral disk degeneration, and ischemic brain injury (54).
In contrast to the beneficial role of HPV to match ventilation and perfusion, chronic global hypoxia is a critical pathological factor associated with the development of pulmonary hypertension resulting from airway obstruction (chronic obstructive pulmonary disease and sleep apnea), diffusion impairment (interstitial lung disease), developmental lung abnormalities, or high-altitude exposure (World Health Organization Group III). In addition to HPV, other mechanisms of vasoconstriction and arterial remodeling also contribute to the development of pulmonary hypertension. Amiloride (and various amiloride analogs), which can inhibit ASICs, has been shown to protect against the development of pulmonary hypertension by inhibiting PASMC proliferation and migration (3, 31). Quinn et al. (31) demonstrated that subcutaneous infusion of different amiloride analogs inhibits PASMC proliferation and CH-induced pulmonary hypertension in rats (14-day CH) and reported that this effect of amiloride was a result of Na+/H+ exchange inhibition. Chan et al. (3) reported that continuous infusion of an amiloride analog, phenamil, decreases pulmonary hypertension by inhibiting ASIC, promoting the differentiated, contractile PASMC phenotype, and reducing cell growth and migration. In contrast to our work, however, Chan et al. (3) found that phenamil increased SOCE. It is unclear from the above study the exact target of phenamil since 1) amiloride did not have the same effect as phenamil in these studies and 2) amiloride (and analogs) are known to inhibit numerous ion channels and transporters (18). Consequently, the role of ASIC1 in the development of pulmonary hypertension and the mechanism by which ASIC1 contributes to this response are unclear.
In addition to HPV, our data also suggest ASIC1 is required for increased receptor-mediated vasoconstrictor reactivity in CH-induced pulmonary hypertension. Consistent with responses we have previously observed in rats (13), SOCE and receptor-mediated vasoconstriction were augmented in isolated pulmonary arteries from ASIC1+/+ (C57BL/6) mice exposed to CH. Furthermore, SOCE and receptor-mediated vasoconstriction, but not depolarization-induced vasoconstriction, were largely blunted in pulmonary arteries from ASIC1−/− mice. Members of the ENaC/ASIC family are considered to be voltage insensitive (16) and, therefore, would not be expected to respond to depolarizing stimuli (i.e., KCl). These present data illustrate the specificity of ASIC1-dependent Ca2+ entry to contribute to hypoxia and receptor-dependent responses and further suggest that the decreased vasoconstrictor responsiveness in arteries from ASIC1−/− is not a result of a generalized decrease in vasoreactivity. In addition, both ASIC1+/+ and ASIC1−/− mice show increased pulmonary arterial resting [Ca2+]i levels after exposure to CH. This finding is also in agreement with previous data showing that ASIC1 inhibition had no effect on CH-induced increases in resting [Ca2+]i levels in isolated pulmonary arteries from rats. It is thought that this increase in basal PASMC [Ca2+]i with pulmonary hypertension is caused by Ca2+ influx that is independent of voltage-gated Ca2+ channels (13, 20, 36, 46). Therefore, it appears that higher basal [Ca2+]i after CH is a result of increased activity of other nonselective cation channels, possibly one or more of the classical/canonical transient receptor potential (TRPC) family members. Nevertheless, these data demonstrate a contribution of ASIC1 to the elevated SOCE and vasoconstriction in CH-induced pulmonary hypertension that are conserved between rats and mice.
There is, however, at least one aspect that is different between our findings in rats and mice. In rats, we (13) have previously shown that pulmonary arterial ASIC1 protein expression is augmented but mRNA expression is unchanged after 4-wk CH. We also examined mRNA expression at earlier time points of CH with the rationale that ASIC1 mRNA may increase early during CH exposure and subside by 4-wk CH; however, mRNA levels did not change during the course of CH exposure (unpublished observations). In contrast, neither ASIC1 protein expression nor mRNA levels were different between pulmonary arteries from normoxic and CH ASIC1+/+ mice. Although these data suggest that an increase in ASIC1 expression is not required for enhanced ASIC1-mediated SOCE after CH, ASIC1 is still a fundamental component of the SOCE response. This is corroborated by our present findings demonstrating that transfection of the hASIC1 gene into PASMCs from ASIC1−/− mice restored SOCE to a level similar to that in PASMCs from ASIC1+/+ mice. These data demonstrate that ASIC1, per se, is an essential component of SOCE.
There are several possibilities by which CH may lead to enhanced ASIC1-dependent SOCE. First, it is possible that CH increases the proportion of ASIC1 at the membrane and that this leads to enhanced SOCE. Second, CH may increase ASIC1 activity by altering the kinetics of the channel itself or by improving the coupling of ASIC1 to up- or downstream effectors. ASIC1 activity can be regulated by second messengers such as PKC and ROS (1, 2, 5, 10, 56), which are known to contribute to the development of pulmonary hypertension. Consistent with this possibility, we (27, 38) have previously shown that PKC can inhibit SOCE in pulmonary arteries and pulmonary endothelial sheets. Another potential regulator of ASIC1 activity is the RhoA/Rho kinase pathway, which mediates pulmonary vasoconstriction and is largely responsible for increased pulmonary vascular resistance in several models of pulmonary hypertension (24). Although there is little evidence linking RhoA/Rho kinase with ASIC1, several studies (23, 30) have shown that small GTPases, including RhoA, are involved in the regulation of both gating and localization of the related family member, ENaC, to the apical plasma membrane. Whether ASICs are similarly regulated by RhoA remains an interesting question that we are currently addressing. Such a finding would be consistent with RhoA/Rho kinase activating a Ca2+ signal; however, it is alternatively possible that the Ca2+ signal mediated by ASIC1 activates the RhoA/Rho kinase pathway (32, 45). Under this circumstance, ASIC1 activity and plasma membrane channel number may be unchanged after CH; rather, ASIC1 might play a necessary role in activating other molecules and signaling pathways that lead to increased vasoconstrictor responses, arterial remodeling, and pulmonary hypertension.
Here, we showed a direct role of ASIC1 in the development of pulmonary hypertension. RVSP was largely blunted in ASIC1−/− mice compared with ASIC1+/+ mice, and ASIC1−/− mice displayed minimal RV hypertrophy and pulmonary arterial remodeling. Although mice typically exhibit modest changes in vascular remodeling after CH, our data clearly demonstrated an increase in the number of fully muscularized arteries (10- to 40-μm diameter) after CH in ASIC1+/+ mice, suggesting the distal extension of smooth muscle into previously nonmuscularized, precapillary arteries. In addition, we observed medial thickening of muscularized arteries after CH only in ASIC1+/+ mice, which can be attributed to hypertrophy and proliferation of vascular smooth muscle cells. Although medial thickening was present in ASIC1+/+ mice after CH, we do not know the extent to which this thickening may impinge upon the luminal diameter or contribute to increased pulmonary vascular resistance. We expect the lack of RV hypertrophy to largely result from diminished active pulmonary vasoconstriction. Indeed, we found that increases in pulmonary arterial resistance to acute hypoxia were attenuated in isolated lungs from ASIC1−/− mice. However, we cannot discount a possible direct role for ASIC1 in cardiac hypertrophy independent of its contribution to increased pulmonary vascular resistance. Nonetheless, these data suggest ASIC1 contributes to the pulmonary arterial remodeling component of pulmonary hypertension and are consistent with other reports (9, 15, 34) of ASIC1 involvement in migration and proliferation in a variety of cells, including vascular smooth muscle cells.
Interestingly, we also found that the development of polycythemia was blunted in ASIC1−/− mice. Although the role of ASIC1 in erythropoiesis is unclear, it is possible that Ca2+ influx through ASIC1 contributes to the activation of transcription factors such as hypoxia-inducible factor. Hypoxia-inducible factor plays a key role in the hypoxic regulation of erythropoiesis, and its activation has been shown to be dependent on an increase in [Ca2+]i (25, 29). In addition, TRPC2 mediates Ca2+ influx in erythroid cells upon stimulation of the erythropoietin receptor by erythropoietin (4). This rise in [Ca2+]i is essential for the proliferation and differentiation of erythroid progenitors and precursors. Although ASIC1 could be playing a similar role as TRPC2 to promote proliferation and differentiation of erythroid cells, evidence for a role of ASIC1 in hematopoietic cells is currently lacking.
In summary, the present study provides evidence showing that ASIC1-mediated Ca2+ entry in PASMCs is an important constituent to both the active vasoconstrictor and vascular remodeling components of pulmonary hypertension. Alongside many other Ca2+-permeable channels, ASIC1 contributes to the altered PASMC Ca2+ homeostasis and vasoconstriction in the hypertensive pulmonary circulation. Although ASICs are classically known for their activation by extracellular H+, recent data suggest that a variety of extracellular and intracellular signaling modalities, many of which are poorly defined, can modulate ASIC activity. A key area of future research relies on determining interactions between ASICs and proximal or distal signaling mediators and, furthermore, how pathological changes to these signaling pathways influence the function of these channels in vascular tissue.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-92598 (to N. L. Jernigan), HL-111084 (to N. L. Jernigan), HL-88192 (to T. C. Resta), and HL-95640 (to B. R. Walker).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.H.N., D.A.O., L.M.H., B.F.B., and N.L.J. performed experiments; C.H.N., D.A.O., L.M.H., T.C.R., B.R.W., and N.L.J. edited and revised manuscript; C.H.N., D.A.O., L.M.H., T.C.R., B.R.W., and N.L.J. approved final version of manuscript; D.A.O. and N.L.J. analyzed data; D.A.O., T.C.R., B.R.W., and N.L.J. interpreted results of experiments; T.C.R., B.R.W., and N.L.J. conception and design of research; N.L.J. prepared figures; N.L.J. drafted manuscript.
ACKNOWLEDGMENTS
ASIC1 knockout mice were kindly provided by Dr. M. J. Welsh and Dr. J. A. Wemmie (University of Iowa, Iowa City, IA). Additionally, the authors thank Tamara Howard for assistance with the preparation of lung sections for immunofluorescence and Kelley Merrick for assistance with genotyping animals.
REFERENCES
- 1.Bashari E, Qadri Y, Zhou Z, Kapoor N, Anderson S, Meltzer R, Fuller C, Benos D. Two PKC consensus sites on human acid-sensing ion channel 1b differentially regulate its function. Am J Physiol Cell Physiol 296: C372–C384, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berdiev BK, Xia J, Jovov B, Markert JM, Mapstone TB, Gillespie GY, Fuller CM, Bubien JK, Benos DJ. Protein kinase C isoform antagonism controls BNaC2 (ASIC1) function. J Biol Chem 277: 45734–45740, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Chan MC, Weisman AS, Kang H, Nguyen PH, Hickman T, Mecker SV, Hill NS, Lagna G, Hata A. The amiloride derivative phenamil attenuates pulmonary vascular remodeling by activating NFAT and the bone morphogenetic protein signaling pathway. Mol Cell Biol 31: 517–530, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu X, Cheung JY, Barber DL, Birnbaumer L, Rothblum LI, Conrad K, Abrasonis V, Chan YM, Stahl R, Carey DJ, Miller BA. Erythropoietin modulates calcium influx through TRPC2. J Biol Chem 277: 34375–34382, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Chung W, Farley J, Drummond H. ASIC-like currents in freshly isolated cerebral artery smooth muscle cells are inhibited by endogenous oxidase activity. Cell Physiol Biochem 27: 129–138, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drummond HA, Gebremedhin D, Harder DR. Degenerin/epithelial Na+ channel proteins: components of a vascular mechanosensor. Hypertension 44: 643–648, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Gannon KP, Vanlandingham LG, Jernigan NL, Grifoni SC, Hamilton G, Drummond HA. Impaired pressure-induced constriction in mouse middle cerebral arteries of ASIC2 knockout mice. Am J Physiol Heart Circ Physiol 294: H1793–H1803, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Grifoni SC, Gannon KP, Stec DE, Drummond HA. ENaC proteins contribute to VSMC migration. Am J Physiol Heart Circ Physiol 291: H3076–H3086, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Grifoni SC, Jernigan NL, Hamilton G, Drummond HA. ASIC proteins regulate smooth muscle cell migration. Microvasc Res 75: 202–210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Z, Huang C, Fu H, Jin Y, Wu W, Xiong Q, Xie N, Long L, Chen J, Wang F. Disruption of PICK1 attenuates the function of ASICs and PKC regulation of ASICs. Am J Physiol Cell Physiol 299: C1355–C1362, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Jeggle P, Callies C, Tarjus A, Fassot C, Fels J, Oberleithner H, Jaisser F, Kusche-Vihrog K. Epithelial sodium channel stiffens the vascular endothelium in vitro and in Liddle mice. Hypertension 61: 1053–1059, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Jernigan NL, Drummond HA. Myogenic vasoconstriction in mouse renal interlobar arteries: role of endogenous β and γENaC. Am J Physiol Renal Physiol 291: F1184–F1191, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Jernigan NL, Herbert LM, Walker BR, Resta TC. Chronic hypoxia upregulates pulmonary arterial ASIC1: a novel mechanism of enhanced store-operated Ca2+ entry and receptor-dependent vasoconstriction. Am J Physiol Cell Physiol 302: C931–C940, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jernigan NL, Paffett ML, Walker BR, Resta TC. ASIC1 contributes to pulmonary vascular smooth muscle store-operated Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 297: L271–L285, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapoor N, Bartoszewski R, Qadri YJ, Bebok Z, Bubien JK, Fuller CM, Benos DJ. Knockdown of ASIC1 and epithelial sodium channel subunits inhibits glioblastoma whole cell current and cell migration. J Biol Chem 284: 24526–24541, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Kim EC, Ahn DS, Yeon SI, Lim M, Lee YH. Epithelial Na+ channel proteins are mechanotransducers of myogenic constriction in rat posterior cerebral arteries. Exp Physiol 97: 544–555, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Kleyman T, Cragoe EJ. Amiloride and its analogs as tools in the study of ion transport. J Membr Biol 105: 1–21, 1988 [DOI] [PubMed] [Google Scholar]
- 19.Kuhr FK, Smith KA, Song MY, Levitan I, Yuan JX. New mechanisms of pulmonary arterial hypertension: role of Ca2+ signaling. Am J Physiol Heart Circ Physiol 302: H1546–H1562, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin MJ, Leung GP, Zhang WM, Yang XR, Yip KP, Tse CM, Sham JS. Chronic hypoxia-induced upregulation of store-operated and receptor-operated Ca2+ channels in pulmonary arterial smooth muscle cells: a novel mechanism of hypoxic pulmonary hypertension. Circ Res 95: 496–505, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Liu XR, Zhang MF, Yang N, Liu Q, Wang RX, Cao YN, Yang XR, Sham JS, Lin MJ. Enhanced store-operated Ca2+ entry and TRPC channel expression in pulmonary arteries of monocrotaline-induced pulmonary hypertensive rats. Am J Physiol Cell Physiol 302: C77–C87, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, He L, Dinger B, Fidone SJ. Chronic hypoxia-induced acid-sensitive ion channel expression in chemoafferent neurons contributes to chemoreceptor hypersensitivity. Am J Physiol Lung Cell Mol Physiol 301: L985–L992, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mastroberardino L, Spindler B, Forster I, Loffing J, Assandri R, May A, Verrey F. Ras pathway activates epithelial Na+ channel and decreases its surface expression in Xenopus oocytes. Mol Biol Cell 9: 3417–3427, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMurtry IF, Abe K, Ota H, Fagan KA, Oka M. Rho kinase-mediated vasoconstriction in pulmonary hypertension. Adv Exp Med Biol 661: 299–308, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Mottet D, Michel G, Renard P, Ninane N, Raes M, Michiels C. Role of ERK and calcium in the hypoxia-induced activation of HIF-1. J Cell Physiol 194: 30–44, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Ng LC, Wilson SM, Hume JR. Mobilization of sarcoplasmic reticulum stores by hypoxia leads to consequent activation of capacitative Ca2+ entry in isolated canine pulmonary arterial smooth muscle cells. J Physiol 563: 409–419, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paffett ML, Riddle MA, Kanagy NL, Resta TC, Walker BR. Altered protein kinase C regulation of pulmonary endothelial store- and receptor-operated Ca2+ entry after chronic hypoxia. J Pharmacol Exp Ther 334: 753–760, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pérez FR, Venegas F, González M, Andrés S, Vallejos C, Riquelme G, Sierralta J, Michea L. Endothelial epithelial sodium channel inhibition activates endothelial nitric oxide synthase via phosphoinositide 3-kinase/Akt in small-diameter mesenteric arteries. Hypertension 53: 1000–1007, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Pisarcik S, Maylor J, Lu W, Yun X, Undem C, Sylvester JT, Semenza GL, Shimoda LA. Activation of hypoxia-inducible factor-1 in pulmonary arterial smooth muscle cells by endothelin-1. Am J Physiol Lung Cell Mol Physiol 304: L549–L561, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pochynyuk O, Medina J, Gamper N, Genth H, Stockand JD, Staruschenko A. Rapid translocation and insertion of the epithelial Na+ channel in response to RhoA signaling. J Biol Chem 281: 26520–26527, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Quinn DA, Du HK, Taylor TB, Hales CA. Amiloride analogs inhibit chronic hypoxic pulmonary hypertension. Am J Respir Crit Care Med 157: 1263–1268, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Ratz PH, Berg KM, Urban NH, Miner AS. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am J Physiol Cell Physiol 288: C769–C783, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Rios EJ, Fallon M, Wang J, Shimoda LA. Chronic hypoxia elevates intracellular pH and activates Na+/H+ exchange in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 289: L867–L874, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Rooj AK, McNicholas CM, Bartoszewski R, Bebok Z, Benos DJ, Fuller CM. Glioma-specific cation conductance regulates migration and cell cycle progression. J Biol Chem 287: 4053–4065, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherwood TW, Frey EN, Askwith CC. Structure and activity of the acid-sensing ion channels. Am J Physiol Cell Physiol 303: C699–C710, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimoda LA, Sham JS, Shimoda TH, Sylvester JT. L-type Ca2+ channels, resting [Ca2+]i, and ET-1-induced responses in chronically hypoxic pulmonary myocytes. Am J Physiol Lung Cell Mol Physiol 279: L884–L894, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Shimoda LA, Wang J, Sylvester JT. Ca2+ channels and chronic hypoxia. Microcirculation 13: 657–670, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Snow JB, Kanagy NL, Walker BR, Resta TC. Rat strain differences in pulmonary artery smooth muscle Ca2+ entry following chronic hypoxia. Microcirculation 22: 1–12, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song M, Makino A, Yuan JX. STIM2 contributes to enhanced store-operated Ca2+ entry in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. J Cell Mol Med 14: 1890–1903, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev 92: 367–520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan ZY, Lu Y, Whiteis CA, Simms AE, Paton JF, Chapleau MW, Abboud FM. Chemoreceptor hypersensitivity, sympathetic excitation, and overexpression of ASIC and TASK channels before the onset of hypertension in SHR. Circ Res 106: 536–545, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature 386: 173–177, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Shimoda L, Weigand L, Wang W, Sun D, Sylvester J. Acute hypoxia increases intracellular [Ca2+] in pulmonary arterial smooth muscle by enhancing capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol 288: L1059–L1069, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Shimoda LA, Sylvester JT. Ca2+ responses of pulmonary arterial myocytes to acute hypoxia require release from ryanodine and inositol trisphosphate receptors in sarcoplasmic reticulum. Am J Physiol Lung Cell Mol Physiol 303: L161–L168, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Weigand L, Foxson J, Shimoda LA, Sylvester JT. Ca2+ signaling in hypoxic pulmonary vasoconstriction: effects of myosin light chain and Rho kinase antagonists. Am J Physiol Lung Cell Mol Physiol 293: L674–L685, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Weigand L, Lu W, Sylvester JT, Semenza GL, Shimoda LA. Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ Res 98: 1528–1537, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Wang SU, Meng FE, Mohan S, Champaneri B, Gu Y. Functional ENaC channels expressed in endothelial cells: a new candidate for mediating shear force. Microcirculation 16: 276–287, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Ward J, Robertson T, Aaronson P. Capacitative calcium entry: a central role in hypoxic pulmonary vasoconstriction? Am J Physiol Lung Cell Mol Physiol 289: L2–L4, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Weigand L, Foxson J, Wang J, Shimoda L, Sylvester J. Inhibition of hypoxic pulmonary vasoconstriction by antagonists of store-operated Ca2+ and nonselective cation channels. Am J Physiol Lung Cell Mol Physiol 289: L5–L13, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron 34: 463–477, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Wemmie JA, Coryell MW, Askwith CC, Lamani E, Leonard AS, Sigmund CD, Welsh MJ. Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior. Proc Natl Acad Sci USA 101: 3621–3626, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118: 687–698, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci USA 101: 6752–6757, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yingjun G, Xun Q. Acid-sensing ion channels under hypoxia. Channels (Austin); 10.4161/chan.25223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan XJ, Goldman WF, Tod ML, Rubin LJ, Blaustein MP. Ionic currents in rat pulmonary and mesenteric arterial myocytes in primary culture and subculture. Am J Physiol Lung Cell Mol Physiol 264: L107–L115, 1993 [DOI] [PubMed] [Google Scholar]
- 56.Zha X, Wang R, Collier D, Snyder P, Wemmie J, Welsh M. Oxidant regulated inter-subunit disulfide bond formation between ASIC1a subunits. Proc Natl Acad Sci USA 106: 3573–3578, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]