Abstract
Previous studies have established integrins as cell surface receptors that mediate cardiomyocyte-extracellular matrix (ECM) attachments. This study sought to determine the contributions of the myocardial β1- and β3-integrin subunits to ventricular dilatation and coronary flow regulation using a blood-perfused isolated heart preparation. Furthermore, cardiomyocyte adhesion to collagen types I and IV, fibronectin, and laminin with and without a β1-integrin subunit neutralizing antibody was assessed during the course of remodeling secondary to a sustained cardiac volume overload, including the onset of heart failure. Isolated cardiomyocytes were obtained during the initial, compensated, and decompensated phases of remodeling resulting from an aortocaval fistula created in 8-wk-old male Sprague-Dawley rats. Blocking the β1-integrin subunit in isolated normal hearts produced ventricular dilatation, whereas this was not the case when the β3-subunit was blocked. Substantial reductions in cardiomyocyte adhesion coincided with the previously documented development of ventricular dilatation and decreased contractility postfistula, with the β1-integrin contribution to adhesion ranging from 28% to 73% over the course of remodeling being essentially substrate independent. In contrast, both integrin subunits were found to be involved in regulating coronary vascular resistance. It is concluded that marked reductions in integrin-mediated cardiomyocyte adhesion to the ECM play a significant role in the progression of adverse myocardial remodeling that leads to heart failure. Furthermore, although both the β1- and β3-integrin subunits were involved in regulating coronary vascular resistance, only inhibition of β1-integrin-mediated adhesion resulted in ventricular dilatation of the normal heart.
Keywords: β1- and β3-integrin subunits, ventricular remodeling, aortocaval fistula, extracellular matrix, heart failure, coronary flow, laminin, fibronectin, collagen types I and IV
integrins serve to maintain the three-dimensional spatial relationship of the myocardial components by mediating the attachment or adhesion of cardiomyocytes to the basement membrane and extracellular matrix (ECM). In so doing, integrins contribute to the transmission of cardiomyocyte contractile forces to the ventricular chamber (29, 30). Although numerous studies have established the importance of integrins as structural elements, there is still relatively little known about their functional role in the normal and diseased adult heart. However, changes in the interaction between cardiomyocyte integrins and the ECM represent a potential mechanism that may contribute to the adverse structural and functional alterations induced by sustained elevations in ventricular wall stress secondary to pressure and/or volume overload. Accordingly, the purpose of this study was to examine the hypothesis that alterations in cardiomyocyte adhesion are associated with the well-documented adverse ventricular remodeling events that occur over time following the creation of a sustained cardiac volume overload [i.e., aortocaval (AV) fistula model] that culminate in the development of heart failure (3, 6, 7, 10, 16, 17). To this end, the relative contributions of the myocardial β1- and β3-integrin subunits to changes in cardiomyocyte adhesion, ventricular dilatation, and regulation of coronary flow were determined in normal rat hearts. Furthermore, changes in isolated cardiomyocyte adhesion to collagen types I and IV, fibronectin, and laminin were assessed over the temporal course of myocardial remodeling secondary to sustained cardiac volume overload, including the onset of heart failure. These proteins were chosen because they represent, respectively, the predominant fibrillar collagen, basement membrane collagen, glycoprotein, and basal laminae components of the heart that mediate the interaction of the cardiomyocyte with the ECM. The results indicate that the acute disruption of cardiomyocyte adhesion to the ECM mediated by β1-integrins produces ventricular dilatation in the normal heart. Consistent with this observation, cardiomyocyte adhesion was significantly decreased during the first week of cardiac volume overload, followed by a subsequent rebound during the compensatory hypertrophic remodeling phase, evidenced by a significant increase in adhesion by 21 days postfistula. However, beyond 21 days, a steady decline in cardiomyocyte adhesion preceded the onset of overt heart failure.
MATERIALS AND METHODS
All experiments were performed using adult male Sprague-Dawley (Hsd:SD) rats housed under standard environmental conditions and maintained on commercial rat chow and tap water ad libitum. All studies conformed to the Guiding Principles for the Care and Use of Vertebrate Animals in Research and Training (American Physiological Society), and the protocol was approved by the University's Animal Care and Use Committee. Anesthesia for surgical procedures and subsequent euthanasia at the experimental end point was affected by pentobarbital sodium (50 mg/kg) administered via intraperitoneal injection. Postoperative analgesia was provided by buprenorphine HCl (0.025 mg/kg), administered subcutaneously to the rats at the time of surgery. The rats were alert and had resumed normal activity within 24 h of the survival surgical procedure.
Assessing the functional effect of β1- and β3-integrin subunit blockade in normal hearts.
The effects of neutralizing antibodies directed against β1- and β3-integrin subunits on left ventricular (LV) size and function of normal hearts from 8-wk-old rats was determined using our previously described blood perfused isolated heart preparation (6, 7). Briefly, arterial blood from the carotid artery of a support rat was pumped to a pressurized (90 mmHg) reservoir for retrograde perfusion of the extirpated heart, and the coronary venous effluent was then collected and returned to the support rat via a jugular vein catheter. After removal of the left atrial appendage, a latex balloon was inserted through the mitral valve orifice into the LV chamber. The proximal end of the balloon was connected via a short piece of tubing to a three-way stopcock that was used to adjust the balloon volume through one port while measuring LV pressure using a pressure transducer (Transpac IV; Abbott Critical Care Systems, North Chicago, IL) attached to the remaining port. Once the heart developed stable isovolumetric contractions, the unstressed LV volume corresponding to a LV end-diastolic pressure (LVEDP) of 0 mmHg (V0) was determined. Balloon volume was then increased in 10- to 20-μl increments until an LVEDP of 25 mmHg was attained. LVEDP and peak isovolumetric pressures were recorded following each increase in balloon volume; three to four such data sets were obtained per heart.
After these baseline pressure-volume relationships were obtained, the functioning normal heart was perfused with preimmunized IgG antibodies or antibodies directed against either the β1- or the β3-integrin subunit. This was accomplished by adding 1 ml of 0.9% sterile saline containing either the anti-β1-integrin subunit (0.156 mg/ml; 1:50 dilution; BD Biosciences, San Jose, CA), anti-β3-integrin subunit (0.156 mg/ml; 1:50 dilution; BD Biosciences, San Jose, CA), or preimmunized IgG (1 mg/ml; 1:50 dilution) to the blood in the pressurized perfusion reservoir. To assess the effect of the antibodies on coronary flow, coronary venous effluent was collected for 3 min immediately before and after administration of the antibody. The venous effluent containing the antibody was not returned to the support rat. A second set of pressure-volume relationships was obtained 30 min after perfusion with the antibody.
Surgical procedure for creating an AV fistula.
An infrarenal AV fistula was created as previously described in 8-wk-old male Sprague-Dawley rats (6). Briefly, a ventral abdominal laparotomy was performed to expose the aorta and caudal vena cava ∼1.5 cm below the renal arteries. Both vessels were then briefly occluded, and an 18-gauge needle was inserted into the aorta and advanced through the medial wall into the vena cava to create the fistula. The needle was then withdrawn and the aortic puncture site sealed with cyanoacrylate. Creation of a successful fistula was evident by the pulsatile flow of oxygenated blood into the vena cava. The musculature and skin incisions were closed by standard techniques with absorbable suture and autoclips, respectively.
LV cardiomyocyte isolation procedure.
Surgeries were performed in a total of 102 rats, which were divided into 10 groups (Fig. 1) consisting of 7 (n = 10)- and 56 (n = 10)-day sham-operated control groups and AV fistula groups at 1 (n = 10), 3 (n = 10), 5 (n = 10), 7 (n = 10), 21 (n = 10), 35 (n = 10), 56 (n = 10), and 140 (n = 12) days after creation of the fistula. Beginning at 56 days and beyond, any rats exhibiting clinical signs of overt heart failure were considered as a separate group (failing; n = 6), leaving a total of six rats in the 140-day postfistula group. Cardiomyocytes were then isolated using a modification of previously described methods (21, 34). At the experimental end point, the thoracic aorta was cannulated with a blunted 18-gauge needle, and the extirpated heart was attached to a water-jacketed modified Langendorff perfusion apparatus. The heart was initially perfused for 5 min to remove any remaining blood with a 37°C calcium-free modified Hank's Balanced Salt Solution of (in mM) 118.5 NaCl, 25.0 NaHCO3, 2.6 KCl, 1.2 KH2PO4, 1.2 MgSO4, 10.0 HEPES, and 11.1 Dextrose. Perfusion was then continued with a 37°C modified Joklik's MEM enzymatic solution of 10.0 mM HEPES, 25.0 mM NaHCO3, 1.2 mM MgSO4, and 331 U/mg Type II Collagenase (Worthington Biochemical, Lakewood, NJ) for 10–20 min, or until adequate enzymatic digestion had occurred. After enzymatic digestion, the right ventricle and atria were removed, and the LV was minced and resuspended in the 37°C modified Joklik's MEM collagenase solution containing 0.025 mM CaCl2. The LV tissue pieces were further digested via continuous manual agitation for 15 min followed by filtration through a 300-mm mesh filter. The filtered cells were collected, resuspended in Joklik's MEM, and placed in a 37°C water bath. The remaining tissue pieces were resuspended in collagenase solution, and the sequence of chemical and manual agitation, filtration, and resuspension was repeated until no additional viable cells were detected upon microscopic examination. The filtrates were then centrifuged at 20 g and resuspended in sequential washes containing 0.25 mM and 0.50 mM CaCl2. Finally, a hemocytometer was used to count cardiomyocytes and assess viability.
Fig. 1.
Schematic depicting the control and postfistula groups from which cardiomyocytes were obtained to assess their ability to adhere to extracellular matrix components.
Adhesion assay and β1-integrin subunit antibody adhesion-suppression.
Based upon the ex vivo isolated normal heart experiments, the β3-integrin subunit did not result in ventricular dilatation as occurred with the β1-integrin subunit administration. Thus, in the experiments to evaluate temporal changes in cardiomyocyte adhesion, only the contribution of the β1-integrin subunit was evaluated. Adhesion assays to ECM components were performed using a modification of the procedure described by Lundgren et al. (24). Falcon Primaria culture plates were coated with either type I collagen (50 μg/ml), type IV collagen (50 μg/ml), fibronectin (10 μg/ml), or laminin (10 μg/ml). All plates and substrates were purchased from BD Biosciences (San Jose, CA). The plates were stored at 4°C for 24 h. After this incubation, the plates were blocked with 1% bovine serum albumin (Sigma Chemical, St. Louis, MO) in a modified salt solution of (in mM) 137.0 NaCl, 4.7 KCl, 0.6 MgSO4, 1.2 CaCl2, and 10.0 HEPES for 30 min at 37°C. The blocking solution was then aspirated, and each plate was washed twice with the aforementioned salt solution, and again aspirated. Based on the cardiomyocyte counts obtained from the enzymatic isolation procedure, the isolated cardiomyocytes were brought to a final concentration of ∼20,000 cells per ml in media containing 1.0 mM CaCl2. Each plate was then seeded with ∼20,000 isolated, viable cardiomyocytes per ml (∼65 cells per mm2). Separate aliquots of the cardiomyocytes were incubated for 1 h at 37°C with β1-integrin subunit antibodies before seeding the plates. Plates seeded with both groups of cardiomyocytes were then incubated for 2 h at 37°C to allow cellular adhesion to occur. After the 2-h period, the media containing unbound cells was aspirated, and the plates were gently washed as described above. After the final wash, the salt solution containing 100 μl of reconstituted 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT; Sigma Chemical) was pipetted into each well containing the adhered cardiomyocytes and incubated for a further 3 h at 37°C. The mitochondrial dehydrogenase of viable cells cleaves the tetrazolium ring of MTT-producing purple formazan crystals, which are insoluble in aqueous solutions, but will dissolve in acidified isopropanol solution (10% Triton X-100, 0.1 N HCl, anhydrous isopropanol). After incubation, the acidified isopropanol was pipetted into each well to dissolve the formazan crystals produced to yield a purple solution, which was then analyzed spectrophotometrically at a wavelength of 570 nm. An increase or decrease in the number of cells adhering to the different ECM components resulted in a proportional change in the amount of formazan crystals formed. A standard curve was performed using serial dilutions of ∼20,000 cardiomyocytes/ml and the MTT assay. The absorbance of the plated cells was then compared with the standard curve to yield a percentage of cardiomyocytes adhered in each well.
Statistical analysis.
All results are presented as means ± SE. Statistical analyses were performed using SPSS 13 software (SPSS, Chicago, IL). Adhesion and antibody-induced adhesion suppression data were analyzed among the 10 groups (i.e., combined control, 1-, 3-, 5-, 7-, 21-, 35-, 56-, and 140-days postfistula and failing) by using one-way ANOVA. When a significant F value was obtained (P < 0.05), intergroup comparisons were made using Fisher's protected least significant difference method. LV volumes in the isolated normal hearts were adjusted to account for the volume displaced by the balloon material in the LV lumen. Pressure-volume relations generated for the balloon alone indicated that the contribution of the balloon to LVEDP was negligible over the range of experimental volumes. LVEDP-LVEDV data were fitted with a third-order polynomial regression, and V0 for each P-V curve was calculated as previously described (12). Using this regression, LVEDV was calculated at discrete EDP intervals (i.e., for each 2.5 mmHg) for graphical comparison. An index of intrinsic (i.e., neurohormonal influence removed) chamber contractility was obtained from systolic LV P-V data fit to a linear regression, and the slopes of these relations were statistically compared by analysis of covariance.
RESULTS
Assessment of integrin blockade on cardiac function in normal hearts.
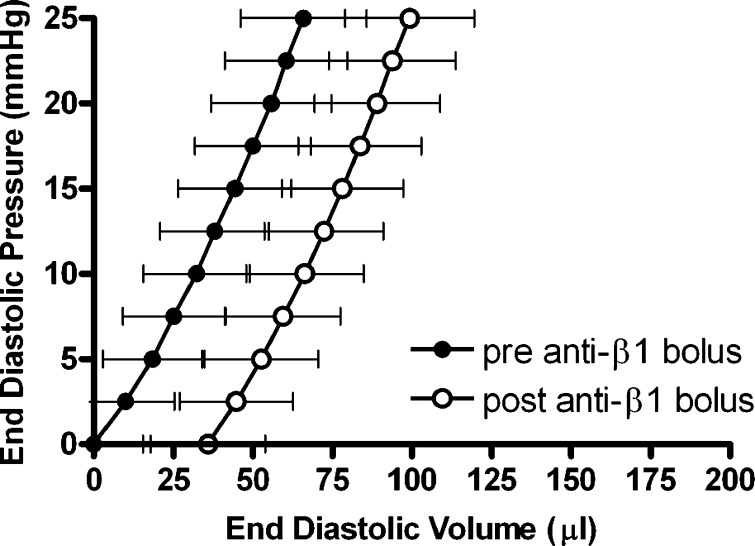
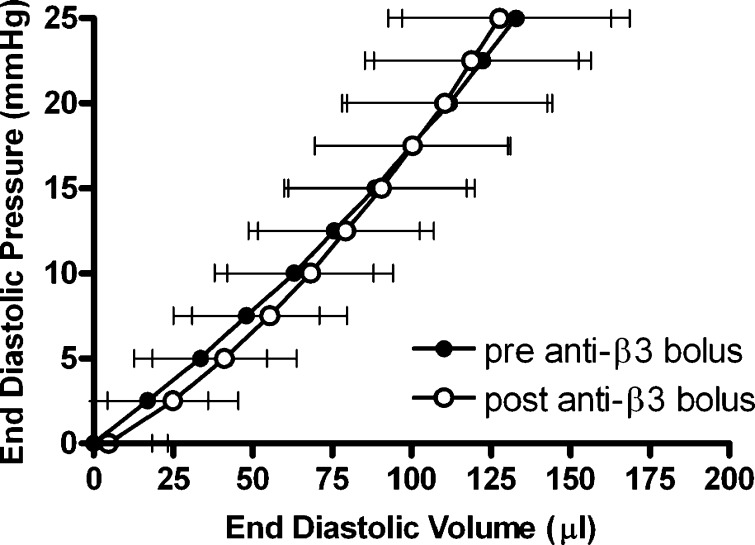
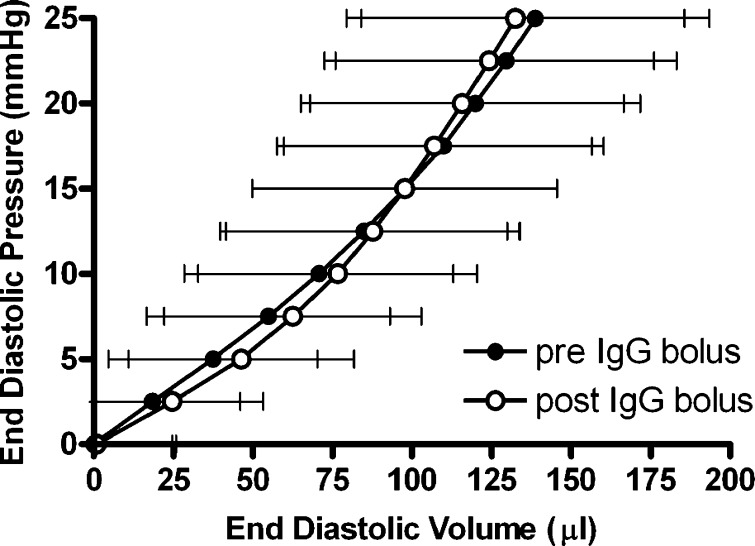
As shown in Table 1 and Fig. 2, the anti-β1-integrin subunit neutralizing antibody resulted in a significant increase in the unstressed LV volume (V0) (13.9%; p ≤ 0.05 vs. pre-anti-β1-integrin subunit). In contrast, the anti-β3-integrin subunit and preimmunized IgG had no effect on V0 (Table 1 and Figs. 3 and 4). Furthermore, none of the three antibody interventions had an effect on ventricular compliance as assessed by the change in the total volume required to raise LV end-diastolic pressure from 0 to 25 mmHg (ΔV0–25) or on myocardial contractility [i.e., the slope of the peak isovolumetric pressure vs. EDV (Pmax-V) relationship]. Finally, both the anti-β1- and anti-β3-integrin subunits produced a significant increase in coronary blood flow, whereas there was no change with the preimmunized IgG bolus (Table 1).
Table 1.
Pre- and Postantibody Comparisons of Isolated Heart Size, Compliance, Coronary Flow, and Contractility
| Anti-β1-Integrin Bolus |
Anti-β3-Integrin Bolus |
Preimmunized IgG Bolus |
||||
|---|---|---|---|---|---|---|
| Prebolus | Postbolus | Prebolus | Postbolus | Prebolus | Postbolus | |
| V0, μl | 257.7 ± 15.5 | 293.6 ± 19.8* | 214.4 ± 18.4 | 219.1 ± 18.5 | 199.1 ± 24.8 | 200.1 ± 24.9 |
| ΔV0–25, μl | 65.9 ± 13.4 | 63.4 ± 12.5 | 132.9 ± 24.3 | 123.0 ± 23.3 | 138.8 ± 42.2 | 131.6 ± 38.0 |
| Pmax-V slope, mmHg/μl | 0.89 ± 0.25 | 0.80 ± 0.20 | 0.63 ± 0.14 | 0.71 ± 0.14 | 0.75 ± 0.20 | 0.69 ± 0.14 |
| Coronary flow, ml/min | 2.0 ± 1.0 | 3.9 ± 2.4* | 3.5 ± 1.9 | 5.3 ± 2.8* | 4.0 ± 2.0 | 4.6 ± 2.4 |
Values are means ± SE. Cardiac dimension and functional parameters obtained from isolated heart preparation for the left ventricular (LV) volume at an end-diastolic pressure (EDP) of 0 mmHg (V0); the amount of volume required to increase EDP from 0 to 25 mmHg (V0–25); the slope of the linear peak isovolumetric pressure-volume relationship (Pmax-V), which serves as an assessment of intrinsic LV contractility; and coronary flow are shown.
P < 0.05 vs. prebolus.
Fig. 2.
Left ventricular end-diastolic pressure-volume relationships obtained in isolated hearts from normal rats pre- and post-anti-β1-integrin subunit bolus administration (dilution, 1:50). Data presented are means ± SE.
Fig. 3.
Left ventricular end-diastolic pressure-volume relationships obtained in isolated hearts from normal rats pre- and post-anti-β3-integrin subunit bolus administration (dilution, 1:50). Data presented are means ± SE.
Fig. 4.
Left ventricular end-diastolic pressure-volume relationships obtained in isolated hearts from normal rats pre- and post-preimmunized IgG bolus administration (dilution, 1:50). Data presented are means ± SE.
Cardiomyocyte adhesion response to cardiac volume overload with and without β1-antibody.
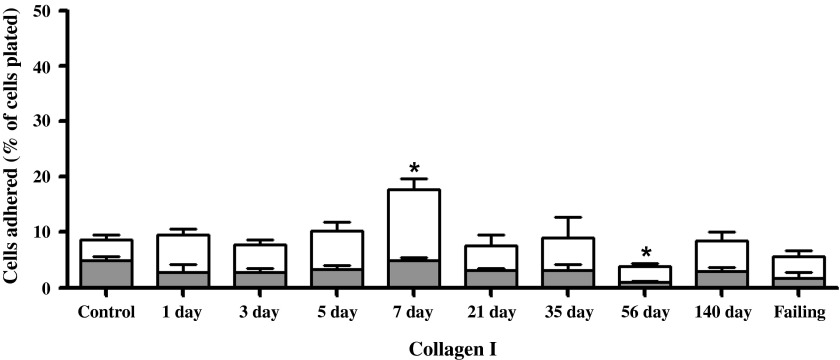
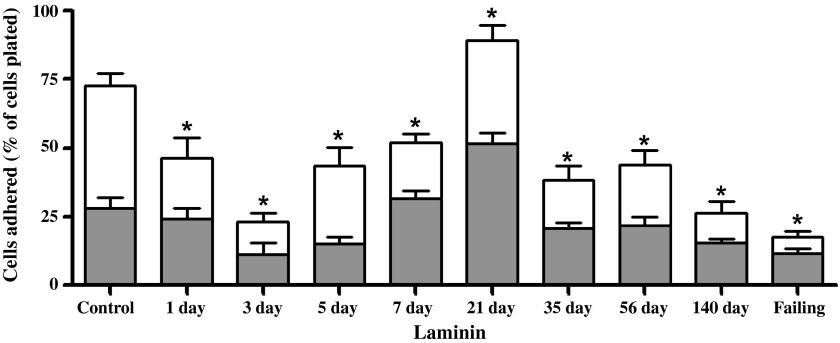
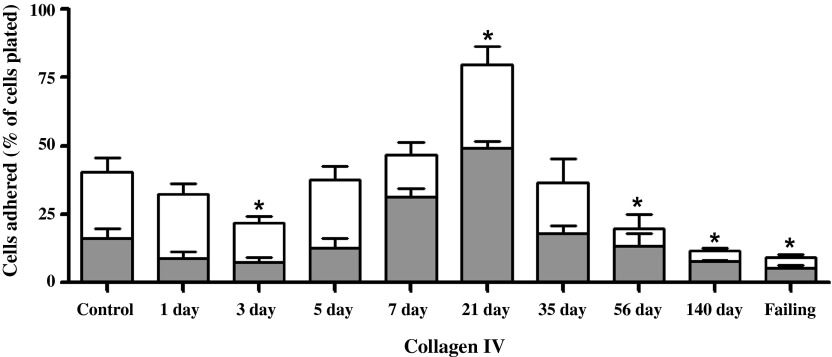
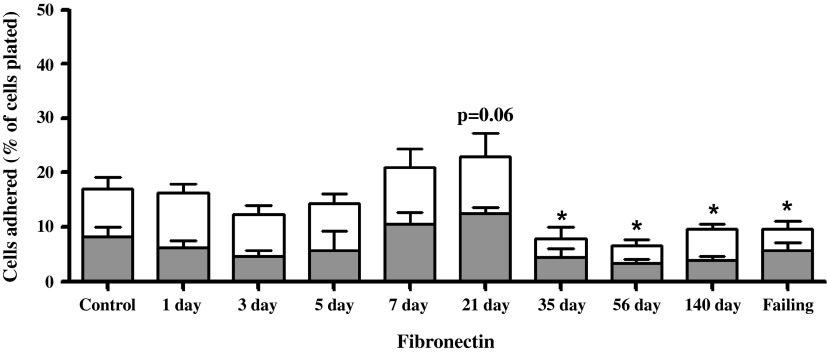
Between 8 and 10 wk, six rats exhibiting clinical signs of overt heart failure (i.e., acute increase in body weight exceeding 50 g, labored breathing, and lethargy) were considered as a separate group (failing), leaving a total of six compensated rats in the 140-day postfistula group. Because there was no statistical difference between the 1- and 8-wk sham groups, their results were combined. The adhesion of cardiomyocytes to individual substrates is depicted in Figs. 5–8 for the 10 experimental groups. Control cardiomyocyte adhesion was least for type I collagen (Fig. 5, ∼10%) and greatest for laminin (Fig. 8, ∼72% of the cells adhered). The approximate control adhesion values for type IV collagen (Fig. 6) and fibronectin (Fig. 7) were 40% and 17%, respectively. Volume overload produced similar effects on adhesion for each of the four substrates, in that at 3 days postfistula the percentage of cells adhered was less than the respective control values. The reduction in cardiomyocyte adhesion was most pronounced for laminin, with a 68% decrease in adhesion at 3 days postfistula relative to control. Similarly, adhesion to type IV collagen was 45% less than control at the 3-day time point. These reductions in adhesion attained significance in the laminin group at days 1, 3, 5, and 7 and in the type IV collagen group at day 3. The initial reduction in adhesion at 3 days postfistula was followed by a subsequent increase in adhesion to all substrates. By 7 days postfistula, adhesion to type I collagen was significantly elevated (105% increase relative to control, P < 0.05). Whereas, adhesion for type IV collagen and laminin significantly exceeded controls by 97% and 23%, respectively, at 21 days postfistula, with a similar trend also observed in the fibronectin group (P = 0.06). Thereafter, adhesion declined significantly below their respective control values for all substrates except type I collagen. In the case of collagen type IV and laminin, the lowest adhesion values relative to the other postfistula time points occurred in the heart failure group.
Fig. 5.
Left ventricular cardiomyocyte adhesion to type I collagen for various times postfistula and for a group with overt failure. Data presented as a percent of total cells plated. The shaded portion represents the residual adhesion following a preincubation with β1-integrin antibody. Means are ± SE. *P ≤ 0.05 vs. control.
Fig. 8.
Left ventricular isolated cardiomyocyte adhesion to laminin for various times postfistula and for a group with overt failure. Data presented as a percent of total cells plated. The shaded portion represents the residual adhesion following a preincubation with β1-integrin subunit antibody. Note change in y-axis scale from that in Figs. 5 and 7. Means are ± SE. *P ≤ 0.05 vs. control.
Fig. 6.
Left ventricular cardiomyocyte adhesion to type IV collagen for various times postfistula and for a group with overt failure. Data presented as a percent of total cells plated. The shaded portion represents the residual adhesion following a preincubation with β1-integrin antibody. Note change in y-axis scale from that in Figs. 5 and 7. Means are ± SE. *P ≤ 0.05 vs. control.
Fig. 7.
Left ventricular cardiomyocyte adhesion to fibronectin for various times postfistula and for a group with overt failure. Data presented as a percent of total cells plated. The shaded portion represents the residual adhesion following a preincubation with β1-integrin antibody. Means are ± SE. *P ≤ 0.05 vs. control.
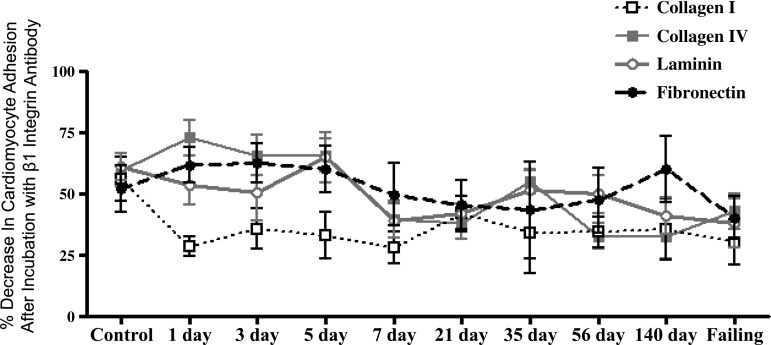
The adhesion following incubation with the β1-antibody for each substrate is depicted in Figs. 5–8 as the shaded regions. These data are also expressed as the percent reduction in the number of adherent cells following incubation with the β1-integrin subunit neutralizing antibody relative to the number of cells adhered without β1-integrin blockade (Fig. 9). Incubation of the cardiomyocytes with antibody directed against the β1-integrin subunit reduced adhesion in all groups, with the percent reduction in adhesion being similar over the time course of remodeling induced by volume overload for laminin, fibronectin, and collagen type IV. For control cardiomyocytes, as well as those obtained at 1, 3, and 5 days postfistula, incubation with the β1-integrin subunit antibody reduced adhesion by ∼60% for all substrates other than collagen type I. For the remainder of the time points (i.e., 7 days and beyond) the contribution of β1-integrins to cardiomyocyte adhesion was between 40% and 50%. With respect to type I collagen, it was only during the initial 1-, 3-, and 5-day postfistula time points that β1-mediated cardiomyocyte adhesion differed from that of the other substrates (i.e., 30% vs. 60%). Beyond 7 days postfistula, the contribution of the β1-integrin subunit to type I collagen cardiomyocyte adhesion was similar to that of the other substrates.
Fig. 9.
The percent reduction in the number of adhered left ventricular cardiomyocytes following β1-integrin blockade relative to the number of cardiomyocytes adhered without blockade for various times postfistula and for a group with overt failure. Means are ± SE.
DISCUSSION
Via their attachments to the cardiac ECM, cardiomyocyte integrins play an essential role in 1) transmitting contractile forces to the ventricular chamber, 2) maintaining ventricular size and shape, and 3) converting extracellular mechanical stimuli into intracellular biochemical signals (29–31). Although it has been shown that cardiomyocyte adhesion to laminin, fibronectin, and collagen type IV is diminished by 70–80% in pigs with tachycardia-induced heart failure (34), it has not been determined whether alterations in integrin adhesion occur during the adaptations following the imposition of a sustained elevation in myocardial stress. Accordingly, the purpose of this study was to determine the contribution of β1- and β3-integrin subunits to cardiomyocyte adhesion in normal hearts, as well as the temporal response in integrin-mediated cardiomyocyte adhesion during the initial, compensated, and failure stages of myocardial remodeling induced by a chronic cardiac volume overload (17). Specifically, cardiomyocyte adhesion to several major components of the ECM (i.e., collagen types I and IV, fibronectin, and laminin) were assessed over a 20-wk period, which included the onset of heart failure. The findings herein are the first to demonstrate a reduction in integrin-mediated adhesion contributing to the development of ventricular dilatation secondary to chronic cardiac volume overload.
The β1-integrin subunit has been shown to be critical for normal cardiac development, since its ablation in all tissues results in embryonic lethality (11). Moreover, β1-integrin gene excision in the adult cardiac myocyte has been shown to cause defects in mechanotransduction and adrenergic signal transduction responses (20, 29). However, the results herein further our understanding of the contribution of β1- and β3-integrin subunits to maintaining the structural integrity of the normal and stressed heart. Specifically, results from the experiments using hearts isolated from normal rats indicate that infusion of a β1-integrin neutralizing antibody caused significant acute ventricular dilatation (evidenced by a 36-μl increase in V0; Table 1 and Fig. 2). In contrast, no structural dilatation was observed following blockade of the β3-integrin subunit. However, our results do indicate that both the β1- and β3-integrin subunits are involved in the regulation of coronary vascular resistance; that is, antibodies to both integrin subunits resulted in significant coronary vasodilation and increased coronary flow.
Our observations are consistent with the few studies evaluating adhesion of adult cardiomyocytes to ECM components in normal and diseased hearts (1, 2, 34). These studies reported similar patterns of cardiomyocyte adhesion to collagen type IV, fibronectin, and laminin. Akin to our findings, Borg et al. (1) reported that cardiomyocytes did not form strong attachments to collagen type I, whereas Zellner et al. (34) unfortunately did not evaluate adhesion to collagens other than type IV. Although direct attachments between cardiomyocytes and the fibrillar collagen network in the heart have been described (28), the limited adhesion of adult cardiomyocytes to fibrillar collagen type I brings the existence of direct connections to the interstitial collagen struts into question; that is, the consistency of these findings suggest that it may be an indirect coupling via intermediate connections to basement membrane components, instead of a direct coupling between cardiomyocyte integrins and type I collagen that maintain cardiomyocyte alignment. However, an alternative explanation could simply be that the glycosylation of the fibrillar collagen substrates used herein and by others was not consistent with that occurring in vivo (1, 2, 34). Regardless, it is clear that β1-integrin heterodimers significantly contribute to cardiomyocyte adhesion to the ECM.
Our laboratory has extensively characterized the time course of adverse myocardial and cardiomyocyte remodeling occurring secondary to a sustained cardiac volume overload in male rats (3, 6, 7, 10). However, this is the first study to examine temporal differences in integrin-mediated cardiomyocyte adhesion in relation to volume overload-induced myocardial remodeling. Our previous studies have shown this remodeling occurs in three distinct phases (i.e., initial, compensated, and failing) (17). The initial phase (i.e., the first week postfistula) is characterized by increased matrix metalloproteinase (MMP) activity, decreased interstitial fibrillar collagen, and the onset of significant ventricular dilatation (6, 7). However, the studies herein implicate the decrease in cardiomyocyte adhesion to collagen types I and IV, fibronectin, and laminin (Figs. 5–8) as coinciding with the significant LV dilatation known to occur during the first week postfistula. With the assumption that the β1-antibody produced a similar reduction in cardiomyocyte ECM adhesion as was induced in control cardiomyocytes (i.e., ∼58% in Fig. 9), then one would predict that the 67% reduction in cardiomyocyte adhesion at 3 days postfistula would have resulted in an increased V0 of 42 μl. This is somewhat less than the previously reported increase in V0 at 1 wk postfistula of 65 μl (6). However, the remaining LV dilatation (i.e., 23 μl) can likely be attributed to the 40–50% reduction in ECM fibrillar collagen occurring between 3 and 5 days postfistula (3). In support of this supposition, Chancey et al. (8) reported an acute 28-μl increase in V0 following a 53% reduction in myocardial collagen volume fraction due to an increase in myocardial MMP activity resulting from chemically induced-mast cell degranulation. This reasoning would suggest that the increase in LV chamber size during the initial phase of cardiac volume overload was due to a combination of reduced cardiomyocyte adhesion to the ECM, as well as a decrease in myocardial fibrillar collagen.
In contrast, the period between 7 and 21 days postfistula consists of concentric compensatory hypertrophy, during which no additional LV dilatation develops and cardiac function is largely preserved (6, 16). This compensated phase is of variable duration, beginning between 7 and 14 days and continuing until symptoms of congestive heart failure develop. The hypertrophic response during this period of compensated remodeling was associated with a marked increase in cardiomyocyte adhesion to the ECM substrates. Concurrent with the increase in adhesion, the concentration of fibrillar collagen rebounded to near or above control levels, whereas MMP activity normalized (3). Interestingly, a study by Terracio et al. (32) found increased expression of β1α1- and β1α5-heterodimers was associated with the increased adhesion during the compensatory hypertrophy secondary to renovascular hypertension. A similar observation that β3-integrins are upregulated during induction of hypertrophy suggests the possibility that they may also contribute to increased cardiomyocyte adhesion (33). Therefore, a similar induction of integrin heterodimers not present in normal adult hearts is likely to underlie the increased adhesion during the compensatory phase of volume overload. Consistent with this supposition, the previously reported development of concentric hypertrophy (i.e., increase in LV weight with no structural dilatation) during the compensated phase of cardiac volume overload suggests that the myocardial remodeling at this point is similar to that occurring in hypertension (6, 16). Thus these sequential changes in adhesion might factor into why longstanding hypertension poses an increased risk of subsequently developing heart failure. This also points to the need for future studies to identify which β1- and/or β3-integrin heterodimers are involved in compensatory remodeling.
The significant, progressive reductions in cardiomyocyte adhesion to the ECM substrates, which developed after 21 days postfistula, coincide with the subsequent development of marked ventricular dilatation and symptomatic congestive heart failure (identified by edema, dyspnea, and lethargy). This transition to the decompensated phase is preceded by the striking reductions in integrin-mediated adhesion to the ECM substrates, whereas other remodeling events associated with the structural dilatation, such as significant increases in cardiomyocyte length (i.e., in series sarcomeric addition) and a significant reduction in myocardial contractility, appear to develop after the decreases in adhesion (7, 10). In contrast with the initial phase, the remodeling in these failing hearts occurred despite progressive hypertrophy and significant myocardial fibrosis (7), prominent features of hypertensive remodeling (16). Thus these findings implicate decreased integrin-mediated cardiomyocyte adhesion, possibly due to decreased receptor affinity or receptor density, as an underlying mechanism responsible for the marked ventricular dilatation and transition to heart failure occurring secondary to chronic cardiac volume overload.
A perspective as to the mechanism responsible for decreased integrin-mediated adhesion is suggested by results from our laboratory demonstrating a significant decrease in the number of cardiomyocytes adhering to laminin after a 30-min incubation with 50 ng/ml of TNF-α (4), suggesting that TNF-α may mediate an acute decrease in integrin receptor affinity. This could potentially underlie the findings herein, since we have previously shown that myocardial TNF-α is markedly elevated in males during the first week of volume overload postfistula (3, 19, 27). Recently, we established that estrogen attenuates myocardial remodeling in male rats subjected to chronic volume overload (13), with one potential mechanism for this cardioprotection being downregulation of TNF-α by estrogen (26). However, estrogen also induced an upregulation of β1-integrin in that study. The possibility that these two factors are related is suggested by Chou et al. (9), who found that TNF-α reduced the strength of fibroblast α2β1-integrin binding to collagen. Apparently, the same signaling pathway is also active in cardiomyocytes. It is also important to note that, in contrast with male and ovariectomized rats, TNF-α remains at control levels in intact female rat hearts during the initial phase of an AV fistula-induced cardiac volume overload (23), and intact females do not subsequently experience adverse remodeling or the development of heart failure (5, 12). This is due in part to differences in remodeling at the level of the cardiomyocyte. For example, cardiomyocytes in intact females undergo progressive, temporally proportional increases in cardiomyocyte length and cross-sectional area as early as 1 wk postfistula (21, 22), whereas in male rats a significant decrease in cardiomyocyte length relative to age-matched controls, together with a reduced number of sarcomeres per cell, was noted at 5 days postfistula (10). There was also a concurrent increase in the percentage of mononucleated cardiomyocytes from 11.6% to 18% at 5 days postfistula with no evidence of cardiomyocyte proliferation, indicating cardiomyocyte hyperplasia due to cytokinesis. Conversely, the dimensions of male cardiomyocytes do not significantly increase until after 35 days of cardiac volume overload (10). Accordingly, it would not be unreasonable to predict the existence of sex differences in the temporal response of cardiomyocyte adhesion to cardiac volume overload; yet this remains to be determined. The possibility that changes in integrin-mediated adhesion alter the dynamics of the mechanical load imposed on cardiomyocytes, leading to remodeling at the cellular level, is also consistent with what is known about integrin-mediated mechanotransduction (15).
In addition to TNF-α-mediated integrin inactivation, other possible mechanisms may contribute to the overall reduction in cardiomyocyte affinity for the ECM ligands, including a decrease in integrin receptor density or integrin receptor shedding (14, 25). The results using the β1-integrin subunit antibody indicate β1-integrins contribute 28% to 73% to total adhesion, depending on the substrate and the amount of time postfistula. Thus it should be stressed that although our findings indicate that neutralizing antibody to the β3-integrin subunit did not result in LV dilatation in the normal heart, the current study does not preclude a possible contribution of β3-integrin subunits to cardiomyocyte adhesion in hearts subjected to a sustained volume overload. Indeed this possibility is suggested by other studies, which indicate that the β3-integrin subunit may play an important role in the stressed myocardium (18, 33). For example, a study in which transverse aortic constriction was performed in β3-integrin knockout mice reported reduced compensatory hypertrophy, depressed ventricular function, and increased mortality (18). Accordingly, additional studies are needed to determine the mechanisms responsible for the residual cardiomyocyte adhesion following incubation with the β1-integrin subunit antibody. Nevertheless, it is clear from the results herein that reductions in β1-integrin-mediated cardiomyocyte adhesion to the ECM are an underlying cause of ventricular remodeling leading to ventricular dilatation and the development of heart failure.
In summary, the findings from the experiments using hearts isolated from normal rats indicate that both β1- and β3-integrin subunits are involved in the regulation of coronary vascular resistance. In contrast, only the β1-integrin subunit contributed to ventricular dilatation in these normal hearts. Consistent with this ex vivo observation, temporal reductions in cardiomyocyte adhesion secondary to a sustained cardiac volume overload precede the development of ventricular dilatation, increased compliance, and decreased myocardial contractility. The summation of our findings implicate both a reduction in cardiomyocyte adhesion and ECM collagen degradation independently contribute to the ventricular dilatation. Thus we conclude that progressive reductions in integrin-mediated cardiomyocyte adhesion to ECM substrates play a significant role in the progression of adverse myocardial remodeling and subsequent transition to heart failure.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute RO1 Grants HL-59981, HL-62228, and HL-73990 (to J. S. Janicki) and American Heart Association Grant SDG5310006 (to J. A. Stewart, Jr.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.A.S. performed experiments; J.A.S., J.D.G., G.L.B., and J.S.J. analyzed data; J.A.S. drafted manuscript; J.A.S., J.D.G., G.L.B., and J.S.J. edited and revised manuscript; J.A.S., J.D.G., G.L.B., and J.S.J. approved final version of manuscript; J.D.G., G.L.B., and J.S.J. interpreted results of experiments; J.D.G. and G.L.B. prepared figures; G.L.B. and J.S.J. conception and design of research.
ACKNOWLEDGMENTS
Present address of J. A. Stewart, Jr.: Dept. of Biological Sciences, Mississippi State Univ., Mississippi State, MS 39762 (e-mail: jas1144@msstate.edu).
Present address of J. D. Gardner: Dept. of Physiology, Louisiana State University Health Science Center-New Orleans, New Orleans, Louisiana (e-mail: jgardn@lsuhsc.edu).
REFERENCES
- 1.Borg TK, Rubin K, Lundgren E, Borg K, Obrink B. Recognition of extracellular matrix components by neonatal and adult cardiac myocytes. Dev Biol 104: 86–96, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Borg TK, Terracio L. Interaction of the extracellular matrix with cardiac myocytes during development and disease. In: Issues in Biomedicine, edited by Robinson T. Karger. Basel, 1991, p. 113–129 [Google Scholar]
- 3.Brower GL, Chancey AL, Thanigaraj S, Matsubara BB, Janicki JS. Cause and effect relationship between myocardial mast cell number and matrix metalloproteinase activity. Am J Physiol Heart Circ Physiol 283: H518–H525, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Brower GL, Du Y, Hill BC, McLarty JL, Janicki JS, Levick SP. Gender differences in tumor necrosis factor-α mediated ventricular dilatation. FASEB J 25: 62.–5., 2011 [Google Scholar]
- 5.Brower GL, Gardner JD, Janicki JS. Gender mediated cardiac protection from adverse ventricular remodeling is abolished by ovariectomy. Mol Cell Biochem 251: 89–95, 2003 [PubMed] [Google Scholar]
- 6.Brower GL, Henegar JR, Janicki JS. Temporal evaluation of left ventricular remodeling and function in rats with chronic volume overload. Am J Physiol Heart Circ Physiol 271: H2071–H2078, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Brower GL, Janicki JS. Contribution of ventricular remodeling to pathogenesis of heart failure in rats. Am J Physiol Heart Circ Physiol 280: H674–H683, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Chancey AL, Brower GL, Janicki JS. Cardiac mast cell-mediated activation of gelatinase and alteration of ventricular diastolic function. Am J Physiol Heart Circ Physiol 282: H2152–H2158, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Chou DH, Lee W, McCulloch CA. TNF-alpha inactivation of collagen receptors: implications for fibroblast function and fibrosis. J Immunol 156: 4354–4362, 1996 [PubMed] [Google Scholar]
- 10.Du Y, Plante E, Janicki JS, Brower GL. Temporal evaluation of cardiac myocyte hypertrophy and hyperplasia in male rats secondary to chronic volume overload. Am J Pathol 177: 1155–1163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fässler R, Meyer M. Consequences of lack of β1 integrin gene expression in mice. Genes Dev 9: 1896–1908, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Gardner JD, Brower GL, Janicki JS. Effects of dietary phytoestrogens on cardiac remodeling secondary to chronic volume overload in female rats. J Appl Physiol 99: 1378–1383, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Gardner JD, Murray DB, Voloshenyuk TG, Brower GL, Bradley JM, Janicki JS. Estrogen attenuates chronic volume overload induced structural and functional remodeling in male rat hearts. Am J Physiol Heart Circ Physiol 298: H497–H504, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldsmith EC, Carver W, McFadden A, Goldsmith JG, Price RL, Sussman M, Lorell BH, Cooper G, Borg TK. Integrin shedding as a mechanism of cellular adaptation during cardiac growth. Am J Physiol Heart Circ Physiol 284: H2227–H2234, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Harston RK, Kuppuswamy D. Integrins are the necessary links to hypertrophic growth in cardiomyocytes. J Signal Transduct 2011: 521742, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janicki JS, Brower GL. The role of myocardial fibrillar collagen in ventricular remodeling and function. J Card Fail 8: S319–S325, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Janicki JS, Brower GL, Gardner JD, Chancey AL, StewardJr JA. The dynamic interaction between matrix metalloproteinase activity and adverse myocardial remodeling. Heart Fail Rev 9: 33–42, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Johnston RK, Balasubramanian S, Kasiganesan H, Baicu CF, Zile MR, Kuppuswamy D. β3 Integrin-mediated ubiquitination activates survival signaling during myocardial hypertrophy. FASEB J 23: 2759–2771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levick SP, Gardner JD, Holland M, Hauer-Jensen M, Janicki JS, Brower GL. Protection from adverse myocardial remodeling secondary to chronic volume overload in mast cell deficient rats. J Mol Cell Cardiol 45: 56–61, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li R, Wu Y, Manso AM, Gu Y, Liao P, Israeli S, Yajima T, Nguyen U, Huang MS, Dalton ND, Peterson KL, Ross RS. β1 Integrin gene excision in the adult murine cardiac myocyte causes defective mechanical and signaling responses. Am J Pathol 180: 952–962, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z, Hilbelink DR, Crockett WB, Gerdes AM. Regional changes in hemodynamics and cardiac myocyte size in rats with aortocaval fistulas. 1. Developing and established hypertrophy. Circ Res 69: 52–58, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Hilbelink DR, Gerdes AM. Regional changes in hemodynamics and cardiac myocyte size in rats with aortocaval fistulas. 2. Long-term effects. Circ Res 69: 59–65, 1991 [DOI] [PubMed] [Google Scholar]
- 23.Lu H, Melendez GC, Levick SP, Janicki JS. Prevention of adverse cardiac remodeling to volume overload in female rats is the result of an estrogen-altered mast cell phenotype. Am J Physiol Heart Circ Physiol 302: H811–H817, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundgren E, Borg T, Mardh S. Isolation, characterization and adhesion of calcium-tolerant myocytes from the adult rat heart. J Mol Cell Cardiol 16: 355–362, 1984 [DOI] [PubMed] [Google Scholar]
- 25.Manso AM, Elsherif L, Kang SM, Ross RS. Integrins, membrane-type matrix metalloproteinases and ADAMs: potential implications for cardiac remodeling. Cardiovasc Res 69: 574–584, 2006 [DOI] [PubMed] [Google Scholar]
- 26.McLarty JL, Melendez GC, Levick SP, Bennett S, Sabo-Attwood T, Brower GL, Janicki JS. Estrogenic modulation of inflammation-related genes in male rats following volume overload. Physiol Genomics 44: 362–373, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murray DB, McMillan R, Brower GL, Janicki JS. ETA selective receptor antagonism prevents ventricular remodeling in volume-overloaded rats. Am J Physiol Heart Circ Physiol 297: H109–H116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson TF, Cohen-Gould L, Factor SM, Eghbali M, Blumenfeld OO. Structure and function of connective tissue in cardiac muscle: collagen types I and III in endomysial struts and pericellular fibers. Scanning Microsc 2: 1005–1015, 1988 [PubMed] [Google Scholar]
- 29.Ross RS. The extracellular connections: the role of integrins in myocardial remodeling. J Card Fail 8: S326–S331, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Ross RS, Borg TK. Integrins and the myocardium. Circ Res 88: 1112–1119, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Ross RS. Molecular and mechanical synergy: cross-talk between integrins and growth factor receptors. Cardiovasc Res 63: 381–390, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Terracio L, Rubin K, Gullberg D, Balog E, Carver W, Jyring R, Borg TK. Expression of collagen binding integrins during cardiac development and hypertrophy. Circ Res 68: 734–744, 1991 [DOI] [PubMed] [Google Scholar]
- 33.Willey CD, Balasubramanian S, Rodriguez Rosas MC, Ross RS, Kuppuswamy D. Focal complex formation in adult cardiomyocytes is accompanied by the activation of beta3 integrin and c-Src. J Mol Cell Cardiol 35: 671–683, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Zellner JL, Spinale FG, Eble DM, Hewett KW, Crawford FA., Jr Alterations in myocyte shape and basement membrane attachment with tachycardia-induced heart failure. Circ Res 69: 590–600, 1991 [DOI] [PubMed] [Google Scholar]