Abstract
Patent foramen ovale (PFO) percutaneous closure has previously been an accepted intervention for the prevention of recurrent cryptogenic stroke on the basis of observational studies. However, randomized trials have been lacking until now. Three recently published randomized trials (CLOSURE I, PC and RESPECT) do not demonstrate the superiority of this intervention versus optimal medical therapy, therefore making this practice questionable. Nonetheless, these trials have had certain pitfalls, mainly a lower than initially estimated number of patients recruited, therefore lacking sufficient statistical power. On the other hand, different closure devices were used in the three trials. In two of them (PC and RESPECT), the Amplatzer PFO Occluder was used and the STARflex device was used in the other one (CLOSURE I). Taken altogether, a meta-analysis of these three trials does not demonstrate a statistically significant benefit of percutaneous PFO closure (1.9% vs 2.9%; P = 0.11). However, if we analyze only the PC and RESPECT trials together, in which the Amplatzer PFO Occluder was used, a statistically significant benefit of percutaneous PFO closure is observed (1.4% vs 3.0%, P = 0.04). In conclusion, our interpretation of these trials is that the use of a dedicated, specifically designed Amplatzer PFO device could possibly reduce the risk of stroke in patients with PFO and cryptogenic stroke. This consideration equally applies to patients who have no contraindications for anticoagulant or antithrombotic therapy.
Keywords: Patent, Foramen, Ovale, Closure, Percutaneous, Device, Cryptogenic, Stroke, Risk
Core tip: Percutaneous patent foramen ovale (PFO) closure has been used for the prevention of recurrent cryptogenic stroke on the basis of observational studies; however, recent randomized trials do not support its use for this indication. A detailed analysis of these randomized trials could suggest that when the Amplatzer PFO Occluder is used, the risk of stroke is reduced.
COMMENTARY ON HOT TOPICS
Patent foramen ovale (PFO) is present in a very high proportion of healthy subjects but as its frequency is higher in patients that have suffered a cryptogenic stroke, PFO has been accepted as a potential cause of stroke, especially in younger patients and in the presence of atrial septal aneurysm[1-3]. As a result, percutaneous closure of PFO has been performed in some patients that have suffered a cryptogenic stroke and in whom a PFO has been demonstrated. The indications of this procedure have been widely debated. Guidelines have been conservative, accepting this strategy only for patients with recurrent stroke despite antithrombotic therapy[4], but this procedure has also been performed in many patients after a first stroke, mainly in younger patients and in those with a concomitant atrial septal aneurysm.
Non-randomized studies suggested that the recurrence of stroke in patients with cryptogenic stroke was lower if a percutaneous closure of PFO was performed, compared with patients that remained on medical therapy alone[2,5,6]. However, the main limitation for a wider acceptance of percutaneous closure has been the absence of randomized trials[4].
Last year, the final results of the CLOSURE I trial were published. In this study, 909 patients between 18 and 60 years of age with a cryptogenic stroke (72%) or transient ischemic attack (TIA) (28%) and a PFO were randomized to percutaneous closure using the STARflex (NMT Medical Inc.,) device in addition to medical treatment (aspirin 81 or 325 mg daily for two years and clopidogrel for the first six months) or to medical treatment alone (aspirin 325 mg daily and/or warfarin for a target INR 2.0-3.0) and followed-up for two years[7]. This study was negative, since the primary endpoint at 2 years (stroke or TIA, death from any cause during the first 30 d, or death from neurological causes between 31 d and 2 years) was not reduced with percutaneous closure (5.5% vs 6.8% in the medical therapy group; P = 0.37). Moreover, the risk of stroke at 2 years was similar between both groups of patients (2.9% with percutaneous closure vs 3.1% with medical treatment; P = 0.79). The CLOSURE I had some limitations, such as a much lower than initially intended number of patients recruited (909 instead of 1600)[8], patients with either stroke or TIA were included, three of twelve (25%) strokes occurred within 30 d after the procedure, other possible causes of stroke became apparent in patients who had recurrences, patients with prothrombotic disorders were excluded, and randomization was not locally blind. Another possible explanation for the negative results is the relatively short follow-up period[9].
Nonetheless, these results were very discouraging, especially for interventional cardiologists. On top of this, two other negative randomized trials regarding the same issue but using a device specifically designed for PFO closure (Amplatzer PFO Occluder, St Jude Medical) have been published in March of this year[10,11]. The RESPECT trial[10] randomized 980 patients to medical treatment or PFO closure using the Amplatzer PFO Occluder. The primary endpoint was the occurrence of recurrent ischemic stroke or early death in patients 18-60 years of age. The intention-to-treat analysis was negative (HR = 0.49, 95%CI: 0.22-1.11, P = 0.08), but due to a high dropout rate in the medical treatment group, the between-group difference was significant in the rate of recurrent stroke in the pre-specified per-protocol cohort (HR = 0.37, 95%CI: 0.14-0.96, P = 0.03) and in the as-treated cohort (HR = 0.27, 95%CI: 0.10-0.75, P = 0.007).
The PC trial randomized patients with a PFO and ischemic stroke, TIA or a peripheral thromboembolic event to undergo closure of the PFO with the Amplatzer PFO Occluder or to receive medical therapy. The primary endpoint was a composite of death, nonfatal stroke, TIA or peripheral embolism and was not reduced with percutaneous closure (HR = 0.63, 95%CI: 0.24-1.62, P = 0.34). Non-fatal stroke occurred in 1 patient (0.5%) in the closure group and 5 patients (2.4%) in the medical therapy group (HR = 0.20, 95%CI: 0.02-1.72, P = 0.14).
A simplistic interpretation of these three trials could lead us to conclude definitively that percutaneous closure of PFO is not effective in reducing the risk of stroke in patients with cryptogenic stroke. Since these trials have been flawed by marked difficulties in patient recruitment, it is evident that each of them individually will probably lack sufficient power to prove any possible differences. In this sense, if we perform a pooled analysis from the 3 trials, including 2303 patients overall, percutaneous closure of PFO does not reduce the incidence of stroke (1.9% vs 2.9%, P = 0.11; Figure 1). However, if we include only the 2 trials in which an Amplatzer PFO Occluder device, specifically designed for PFO, was used, percutaneous closure was associated with a significant reduction in the incidence of stroke (1.4% vs 3.0% P = 0.04; Figure 2).
Figure 1.
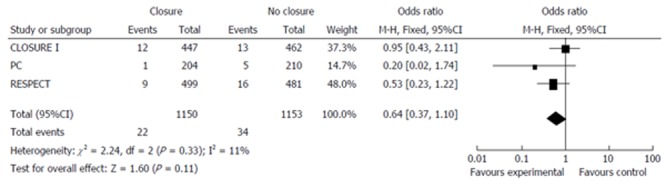
Meta-analysis of all three randomized trials.
Figure 2.
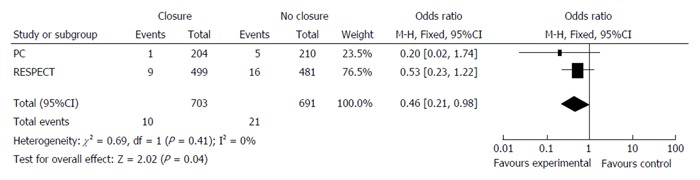
Meta-analysis of the two trials using an Amplatzer Patent Foramen Ovale Occluder.
Possible explanations for these differences may be the following: the STARFlex closure system has been associated with a significantly higher thrombosis rate at 30 d than the Amplatzer PFO Occluder device in two different studies, 3.6% vs 0%, P < 0.01 and 5.7% vs 0%, P < 0.05[12,13], and the incidence of atrial fibrillation[14] has also been documented more frequently at 30 d with STARFlex (4.5% vs 1.3%; P = 0.02). Also, a lower rate of periprocedural complications in the PC and respect trials could partly explain the better results of percutaneous closure in the PC and RESPECT trials.
Our interpretation of these trials is that the use of a dedicated, specifically designed Amplatzer PFO device could possibly reduce the risk of stroke in patients with PFO and cryptogenic stroke. Therefore, although present evidence does not support PFO closure for the prevention of recurrent cryptogenic stroke, a detailed analysis of recent randomized trials can make us consider that the door for PFO closure might not be entirely closed. This consideration equally applies to patients who have no contraindications for anticoagulant or antithrombotic therapy.
Footnotes
P- Reviewers: Alzand BSN, Lehmann L, Tagarakis G, Teragawa H S- Editor: Qi Y L- Editor: Roemmele A E- Editor: Liu SQ
References
- 1.Overell JR, Bone I, Lees KR. Interatrial septal abnormalities and stroke: a meta-analysis of case-control studies. Neurology. 2000;55:1172–1179. doi: 10.1212/wnl.55.8.1172. [DOI] [PubMed] [Google Scholar]
- 2.Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, Coste J. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345:1740–1746. doi: 10.1056/NEJMoa011503. [DOI] [PubMed] [Google Scholar]
- 3.Luermans JG, Budts W, Ten Berg JM, Plokker HW, Suttorp MJ, Post MC. Comparison of outcome after patent foramen ovale closure in older versus younger patients. EuroIntervention. 2011;7:209–215. doi: 10.4244/EIJV7I2A35. [DOI] [PubMed] [Google Scholar]
- 4.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal S, Bajaj NS, Kumbhani DJ, Tuzcu EM, Kapadia SR. Meta-analysis of transcatheter closure versus medical therapy for patent foramen ovale in prevention of recurrent neurological events after presumed paradoxical embolism. JACC Cardiovasc Interv. 2012;5:777–789. doi: 10.1016/j.jcin.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Kitsios GD, Dahabreh IJ, Abu Dabrh AM, Thaler DE, Kent DM. Patent foramen ovale closure and medical treatments for secondary stroke prevention: a systematic review of observational and randomized evidence. Stroke. 2012;43:422–431. doi: 10.1161/STROKEAHA.111.631648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–999. doi: 10.1056/NEJMoa1009639. [DOI] [PubMed] [Google Scholar]
- 8.Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M, et al. Study design of the CLOSURE I Trial: a prospective, multicenter, randomized, controlled trial to evaluate the safety and efficacy of the STARFlex septal closure system versus best medical therapy in patients with stroke or transient ischemic attack due to presumed paradoxical embolism through a patent foramen ovale. Stroke. 2010;41:2872–2883. doi: 10.1161/STROKEAHA.110.593376. [DOI] [PubMed] [Google Scholar]
- 9.Wahl A, Jüni P, Mono ML, Kalesan B, Praz F, Geister L, Räber L, Nedeltchev K, Mattle HP, Windecker S, et al. Long-term propensity score-matched comparison of percutaneous closure of patent foramen ovale with medical treatment after paradoxical embolism. Circulation. 2012;125:803–812. doi: 10.1161/CIRCULATIONAHA.111.030494. [DOI] [PubMed] [Google Scholar]
- 10.Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, Marks DS, Tirschwell DL. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092–1100. doi: 10.1056/NEJMoa1301440. [DOI] [PubMed] [Google Scholar]
- 11.Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083–1091. doi: 10.1056/NEJMoa1211716. [DOI] [PubMed] [Google Scholar]
- 12.Taaffe M, Fischer E, Baranowski A, Majunke N, Heinisch C, Leetz M, Hein R, Bayard Y, Büscheck F, Reschke M, et al. Comparison of three patent foramen ovale closure devices in a randomized trial (Amplatzer versus CardioSEAL-STARflex versus Helex occluder) Am J Cardiol. 2008;101:1353–1358. doi: 10.1016/j.amjcard.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 13.Krumsdorf U, Ostermayer S, Billinger K, Trepels T, Zadan E, Horvath K, Sievert H. Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1,000 consecutive patients. J Am Coll Cardiol. 2004;43:302–309. doi: 10.1016/j.jacc.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 14.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]


