Abstract
Therapy-related myelodysplastic syndrome (t-MDS) and therapy-related acute myeloid leukemia (t-AML) are late complications of cytotoxic therapies used to treat malignant, and increasingly, non-malignant conditions. Although distinct clinical, morphologic, and genetic features can be recognized, these disorders should be seen as part of a single disease spectrum recognized by the WHO in a singular classification, therapy-related myeloid neoplasms (t-MNs). Etiologic factors for t-MNs remain elusive, but ongoing research has characterized risk factors which vary between patient subgroups and exposures. Agents that damage DNA directly, interfere with DNA repair, and suppress the immune system’s ability to detect malignant cells increase the risk of t-MNs. As in primary MDS and de novo AML, prognosis and treatment strategies rely on patient characteristics as well as cytogenetics. However, the overall outcome for patients with t-MNs remains poor. Here we review our current understanding of t-MNs as they are most often encountered by the practicing clinician.
Keywords: Therapy-related, myeloid, neoplasm, leukemia, myelodysplastic syndrome
Introduction
Therapy-related myeloid neoplasms (t-MNs) are an increasingly common and often lethal late complication of cytotoxic treatment for a primary cancer or a non-malignant disease. Although t-MNs can be subdivided into therapy-related myelodysplastic syndrome (t-MDS), therapy-related acute myeloid leukemia (t-AML), and therapy-related myelodysplastic/myeloproliferative neoplasms based on disease parameters, all of these presentations are best considered to be within the spectrum of a single disease entity.[1] At present, etiologic factors remain elusive, but ongoing research is beginning to characterize risk factors for t-MNs which are vary between individual patient subgroups and exposures. As in de novo AML, prognosis and treatment strategies are based on patient characteristics as well as on cytogenetics. However, the overall outcome for the majority of patients with t-MNs remains poor.[2–4] Here we review our current understanding of t-MNs as they are most often encountered by the practicing clinician.
Definition and Recognized Subgroups
t-MNs have been defined by the World Health Organization (WHO) as myeloid neoplasms, including the spectrum of MDS, AML, and overlap myelodysplastic/myeloproliferative neoplasms, occurring at any time after exposure to DNA damaging agents in a patient who previously had a non-myeloid disorder.[1] Thus, patients relapsing with MDS after treatment for AML or a chronic myeloproliferative neoplasm, for example, are not included. Patients exposed to environmental hematotoxins such as benzene are also not included although the mechanisms of malignant transformation may be similar to those active in t-MN. The implicated cytotoxic exposures include traditional cytotoxic chemotherapeutic agents and radiation therapy, given mostly for malignant but also for some non-malignant diseases. Classically, two specific subtypes of t-MNs are recognized in association with different classes of cytotoxic exposures. The first subtype, associated with exposure to alkylating agents (such as cyclophosphamide, melphalan, mechlorethamine, or nitrosureas), is characterized by a longer latency of 3 to greater than 10 years, a preceding myelodysplastic phase, deletions or loss of chromosomes 5 or 7 or both (sometimes as part of complex karyotypes), and frequent somatic loss of TP53.[5] Both radiation therapy and antimetabolites (such as azathioprine) are also associated with t-MN, usually with characteristics similar to those arising after alkylating agents.[5, 6] The second subtype, associated with exposure to topoisomerase-II inhibitors (such as etoposide, doxorubicin, or mitoxantrone), is characterized by a shorter latency of only a few months up to about 3 years, often lacks a preceding myelodysplastic phase, presents with acute leukemia, and features balanced chromosomal rearrangements especially involving chromosome bands 11q23 or 21q22.[5] Of note, therapy-related acute promyelocytic leukemia with a typical t(15;17) has been reported in patients treated with mitoxantrone for multiple sclerosis.[7] With the increasing use of multiagent chemotherapeutic regimens for many malignancies, many cancer survivors have been exposed to several mechanistic classes or modalities of cytotoxic agents, making it more difficult to implicate a single causative agent or the time course over which the critical DNA damage occurred.
Epidemiology
At present, t-MNs account for 10–20% of all malignant myeloid diagnoses.[1] With current estimates of 13.7 million cancer survivors in the United States alone and expectations of 18 million survivors by 2022,[8] the population of individuals at risk is growing, and a continued increase in the incidence of t-MNs is expected. The development of t-MNs appears to be independent of the specific primary disease for which cytotoxic therapy was prescribed. Thus, in the setting of prior cytotoxic therapy, these myeloid neoplasms are best labeled “therapy-related” and not “secondary leukemia” or “second malignancies.”
Efforts to understand the risk factors for the development of t-MN have identified various populations at risk among patients treated for different neoplastic and non-neoplastic diseases as well as among patients with the same primary disease but who received different treatment regimens. Cases of t-MNs are seen among survivors of both solid tumors as well as hematologic malignancies.[2, 3] The largest published series of t-MN cases at a single center was published by our group at the University of Chicago.[3] It included 306 individuals diagnosed with t-MN between the early 1970s through 2001; 55% of patients had a prior hematologic malignancy, 38% had a prior solid tumor, and 6% had been treated for non-malignant diseases. Among these, individuals with prior Hodgkin lymphoma (45%) and breast cancer (28%) accounted for the largest subgroups within those with hematologic malignancies and solid tumors, respectively. In a second, more recent series from Germany which included cases diagnosed between 1993 and 2008, 71% of t-MN cases had a prior solid tumor and 27.5% had a prior hematologic malignancy, with breast cancer and non-Hodgkin lymphoma accounting for the largest subsets within these two groups.[2] This shift in primary cancer diagnoses likely reflects the improvements seen in survival from solid tumors overall [8] as well as changes in the primary therapy given for various malignancies.
Treatment-specific risk factors for the development of t-MN have been identified among individuals with the same prior cancer. Survivors of breast cancer and Hodgkin lymphoma (HL), who account for a large proportion of t-MN cases, have been well studied. Among those treated for breast cancer, younger age at the time of exposure, higher dose intensity of cytotoxic treatments, concomitant treatment with radiation, and adjuvant use of hematopoietic growth factors together with cytotoxic therapy to assist in blood count recovery are factors associated with an increased risk of t-MN.[9, 10] Changes that have evolved in the primary treatment of HL provide another example of treatment-related risk factors and some insight into the apparent decrease in t-MN cases among HL survivors in more recent series.[2, 3] Among HL survivors who received treatments that are now only historical, usually consisting of alkylating agent-based chemotherapy including mechlorethamine and procarbazine with or without extended field radiation therapy, the incidence of t-MN was 2% to 6.7%.[11–14] This incidence was even higher among those who also received long-term exposure to oral alkylating agents for maintenance treatment.[15] In contrast, those treated using current regimens (e.g., ABVD, standard BEACOPP, or Stanford V) and more limited radiation fields have a t-MN incidence of only 0–0.3%, even after more than 10 years of follow-up.[11, 12, 14]
The role of hematopoietic growth factors, specifically granulocyte colony-stimulating factor (G-CSF) in elevating the incidence of t-MN merits special attention. A recent meta-analysis examining data from 25 trials in which individuals were randomized to chemotherapy with or without G-CSF for various malignancies found an increased relative risk of t-MN in those treated with G-CSF (relative risk 1.9; 95% confidence interval 1.19 to 3.07; p=.007).[16] It has been hypothesized that G-CSF may drive damaged hematopoietic stem cells into cell division before they have had a chance to repair the genetic injuries from cytotoxic therapy. Thus, clinical epidemiology provides some clues into the role of various exposures in the etiology of t-MN, and these data advise caution for the use of G-CSF in combination with chemotherapy when not clearly necessary.
Etiologic factors
By definition, t-MNs occur after cytotoxic exposures. These neoplasms are thought to be the direct consequence of mutational events induced by the prior therapy. However, the exact role of the cytotoxic exposure in the development of t-MN remains unclear. The possibilities include: 1) a mutational event or series of mutations entirely due to the cytotoxic exposure; 2) an entirely stochastic event (i.e. happening by chance); 3) a host susceptible to mutagenic events only in the setting of a specific exposure; and 4) a host susceptible to development of myeloid neoplasms regardless of exposures.
Evidence in support of the pivotal role of cytotoxic agents includes the characteristic recurring cytogenetic abnormalities induced by specific cytotoxic exposures with unique mechanisms of action such as the association of topoisomerase-II inhibitors (e.g., etoposide) with balanced chromosomal rearrangements involving the MLL gene at chromosome band 11q23 and the association of alkylating agents and radiation with deletions involving chromosomes 5 and/or 7.[5] In addition, epidemiologic data showing an increased incidence of t-MNs in those treated with dose-intense regimens containing both alkylating agents and topoisomerase-II inhibitors (such as Adriamycin, cyclophosphamide, and paclitaxel for breast cancer) plus G-CSF versus standard dosing and in those treated with combined modality chemoradiotherapy versus chemotherapy alone suggest a role for the specific exposures.[10]
However, accumulating evidence is beginning to elucidate the potential role for various germline genetic factors in an individual’s susceptibility to t-MNs. Evidence exists for a role for both common as well as rare highly penetrant germline genetic variants in specific subsets of t-MN patients. The common variants that have been shown to play a role in t-MN susceptibility include those that alter drug metabolism such as inactivating variants in the gene NQ01, whose protein product reduces substrates such as the hematotoxin benzene to prevent formation of damaging reactive oxygen species[17] as well as variants in the glutathione S-transferase family of enzymes which detoxify electrophiles (such as alkylation metabolites)[18] and polymorphisms in TP53 and MDM2, genes important in the DNA damage response.[19]
In most cases, individuals with t-MN have been diagnosed with two separate malignancies. Thus, a role for inherited cancer susceptibility in these individuals seems a logical possibility. In addition, the functions of many of the genes known to be involved in hereditary cancer susceptibility, such as BRCA1 and BRCA2, are in various DNA repair pathways, which play an important role in maintaining DNA integrity in the face of damaging exposures whether natural environmental or iatrogenic and therapeutic. In addition, several of these genes are also known to cause Fanconi anemia, a disorder featuring an 800-fold risk of eventual development of leukemia when two abnormal copies are inherited.[20] Other cancer susceptibility genes, such as those responsible for the telomere biology disorder dyskeratosis congenita, are also known to predispose individuals to both solid tumors as well MDS and acute leukemia.[21] Therefore, some individuals with t-MNs may actually have a shared susceptibility to both tumor types.
Several small series examining the incidence of germline variants in various cancer susceptibility genes in t-MN patients have added support to this theory. In fact, germline mutations in BRCA1, BRCA2, and TP53, which cause hereditary breast and ovarian cancer and Li Fraumeni syndrome, respectively, have been identified in a few patients who developed t-MNs following treatment for breast cancer.[22, 23] Schulz et al found germline mutations in these 3 genes plus BARD1, a gene known to be involved in breast cancer susceptibility, in 9 of 53 (17%) t-MN patients, with the majority (6 of 14; 43%) occurring in those with a prior breast cancer.[24] Thus, accumulating evidence suggests a role for germline mutations in cancer susceptibility genes in augmenting the risk of t-MN, especially for breast cancer survivors. Further investigations into the incidence of mutations in these and other cancer susceptibility genes in various t-MN subgroups may help identify those at highest risk such that they could be identified prior to treatment of their primary cancer. This would provide an opportunity to alter the primary treatment if appropriate and improve patient counseling and surveillance.
Clinical presentation and evaluation
Patients with t-MNs generally present with clinical symptoms similar to patients presenting with de novo MDS and AML. Typically, those who develop t-MNs featuring abnormalities of chromosomes 5 and 7 will have a latency of 3 to 10 years from their initial cytotoxic exposure and usually have a myelodysplastic phase whereas those with t-MNs featuring balanced chromosomal rearrangements often present with AML within a few months to 3 years after starting chemotherapy. Common symptoms include fatigue, generalized malaise, easy bruising, bleeding or petechiae, and infections, all of which are related to deficits in normal hematopoiesis. Other patients may have minimal symptoms but are found to have blood count abnormalities including macrocytosis or mild to moderate cytopenias on routine blood counts during a follow-up visit. Signs heralding the emergence of t-MN may mimic relapse of the primary disease, especially multiple myeloma, or myelosuppression from active treatment ongoing for the primary disease, such as metastatic breast or ovarian cancer. However, when these findings are encountered in a patient who has received cytotoxic agents, the clinical suspicion for t-MN must be high and the appropriate work-up pursued (Table 1).
Table 1.
Recommended evaluation at the time of diagnosis of a therapy-related myeloid neoplasm.
| Recommended at Diagnosis | Significance |
|---|---|
1. Detailed history and physical examination with particular attention to:
|
Identify clinical factors that will impact t-MN prognosis (e.g., age & performance status), the potential for treatment-related complications (e.g. cardiomyopathy) and choice of therapeutic regimen (e.g. candidacy for stem cell transplantation) |
2. Complete blood count with differential
|
Assess cytopenias, t-MN status, and need for transfusion support |
| 3. Complete metabolic panel, prothrombin time, activated partial thromboplastin time, lactate dehydrogenase and uric acid levels | Assess baseline renal and hepatic function as well as t-MN related electrolyte or coagulation abnormalities |
4. Bone marrow aspirate and trephine biopsy including:
|
Assess t-MN status and identify factors essential for prognosis and treatment |
| 5. HLA typing (for fit patients) | Assess hematopoietic stem cell donor options |
| 6. Measurement of cardiac ejection fraction (for patients fit enough to consider intensive treatment approach) | Assess baseline cardiac function and need for treatment modification of anthracycline-based regimens |
| 7. Computed tomography or other imaging modality (for restaging of prior malignancy, if appropriate) | Assess candidacy for aggressive treatment approach especially when considering hematopoietic stem cell transplantation |
| 8. Pulmonary function testing (for patients fit enough to consider intensive treatment approach) | Consider in those with symptoms or previous therapies with known significant pulmonary toxicity |
Essential elements of the diagnostic evaluation include: a detailed medical history and physical examination with particular attention to patient age, comorbidities, and performance status, details of specific agent exposures and their cumulative doses, evidence of any organ dysfunction related to the previous cancer or its treatment, and the remission status of the previous cancer. A detailed family history is also essential to rule out the potential role of a hereditary cancer susceptibility syndrome as discussed above. The recommended laboratory evaluation should include a complete blood count with differential, complete metabolic panel, lactate dehydrogenase and uric acid levels, prothrombin time and activated partial thromboplastin time. A careful review of the peripheral blood smear will often reveal dysplastic changes in the granulocytes as well as megaloblastoid erythroid changes. We recommend that medically fit patients should have HLA-typing performed at diagnosis to expedite identification of a potential stem cell donor for an eventual allogeneic transplant. A bone marrow aspirate with a trephine biopsy should be performed and should include immunophenotyping as well as full metaphase cytogenetic analysis.[25] FISH (fluorescent in situ hybridization) assays using a limited panel of probes are not adequate, as critical chromosomal rearrangements such as inv(3) or t(3;3) will be missed. The value of molecular diagnostic testing in t-MNs, such as assessments of mutations in FLT3, NPM1, CEBPA, or c-KIT, is currently unclear and is the subject of ongoing research. A complete diagnostic work-up is key for assessing prognosis as well as determining the most appropriate treatment for an individual patient.
Key determinants of prognosis in t-MNs include patient age, performance status, and karyotype.[2, 3] The prognostic significance of karyotype in t-MNs is similar to that in de novo MDS and AML.[2] However, the key difference between de novo and therapy-related disease is the proportion of patients that will be found to have abnormal and often complex karyotypes. Clonal cytogenetic abnormalities are found in 75–90% of t-MN cases whereas they are seen in only about half of de novo AML cases.[2, 3] Cases of t-MN are also more likely to have adverse karyotypes at diagnosis, most often featuring a complex karyotype or deletions or loss of chromosomes 5 and/or 7, than de novo cases (46–70% vs 20%, respectively).[3, 26] These differences in karyotype likely reflect differences in underlying etiology and tumor biology, but importantly, also contribute to the adverse prognosis of the majority of patients with t-MNs.
Treatment approach and outcomes
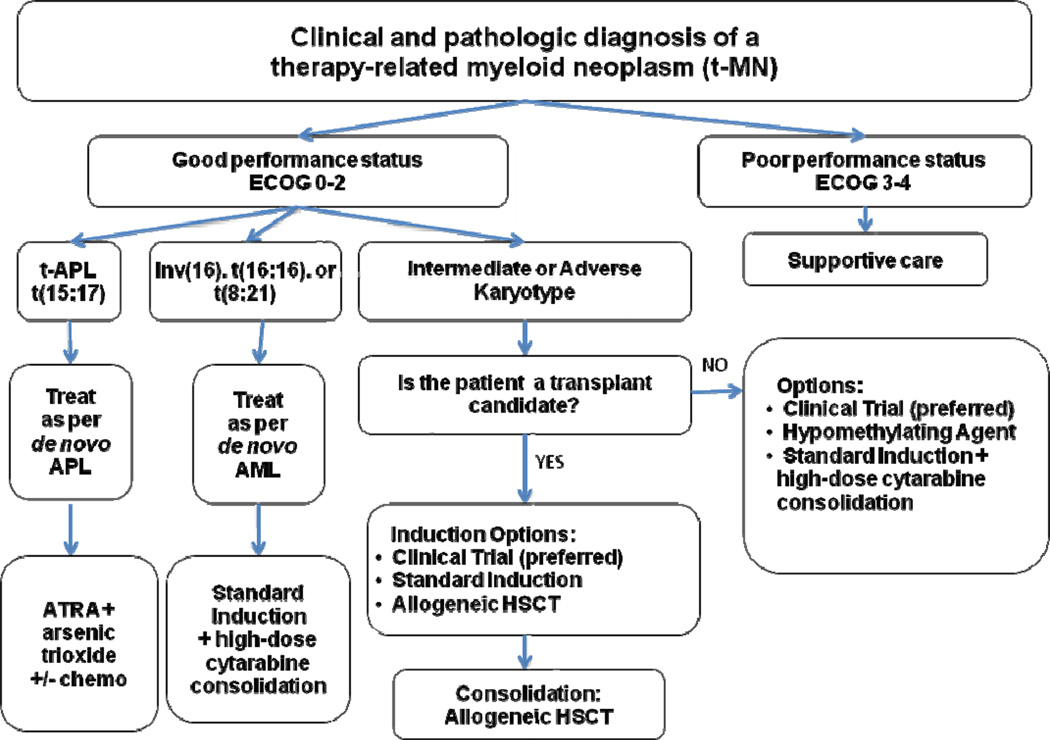
The treatment of patients with t-MNs presents some challenges unique from those seen in de novo myeloid malignancies. This patient group often has a greater number of comorbidities and decreased organ reserve, including less hematopoietic reserve, related to their primary disease and their previous cytotoxic exposures, making treatment-related complications more common. For all patients with t-MNs, the initial approach should include a determination of performance status (Figure 1). Those with a poor performance status at baseline should be offered supportive care measures as they are unlikely to benefit from intensive treatments. Those with an adequate performance status should first be offered participation in a clinical trial whenever possible, as this patient subgroup has historically been excluded from participation in trials. As a result, evidence-based treatment that is specific for t-MNs is sorely needed. All medically fit patients who are potential candidates for allogeneic hematopoietic stem cell transplantation (HSCT) should undergo HLA-typing at diagnosis. This allows early identification of potential donor options, as this remains the only potentially curative option for the majority of t-MN patients with the exception of those with a favorable karyotype.
Figure 1.
Treatment algorithm for individuals with therapy-related myeloid neoplasms.
Treatment algorithm for medically fit patients
For patients determined to have an adequate performance status and for whom a clinical trial is not available, we recommend treatment based on the karyotype.
Favorable karyotype
The small subset of t-MN patients who present with a favorable karyotype, specifically inversion (16), translocation (16;16), and translocation (8;21), should be treated similarly to patients with de novo AML featuring these same rearrangements. Treatment usually consists of standard AML induction chemotherapy followed by high-dose cytarabine consolidation treatment. Individuals with t-MNs with these favorable karyotypes achieve remissions and disease-free survival using conventional regimens at rates similar to those with de novo AML.[27] However, their overall survival is decreased, possibly due to more treatment-related complications or comorbidities, or in some cases the persistence of their primary malignancy.[2]
Therapy-related acute promyelocytic leukemia (t-APL)
This subset of t-MNs also falls within the favorable karyotype subset. However, as in de novo APL cases, this myeloid malignancy is treated separately due to its unique response to agents targeting the fusion protein PML-RARα generated by the chromosomal translocation t(15;17). We recommend that patients with t-APL be treated similarly to de novo cases of APL using regimens containing tretinoin (all-trans retinoic acid; ATRA) and arsenic trioxide, as the expected complete remission (CR) and overall survival rates are similar.[28, 29] An area of active investigation is whether cytotoxic chemotherapy can be avoided in t-APL patients by using only arsenic trioxide, either alone or together with ATRA, in order to avoid additional chemotherapy-related toxicities in this group of previously treated patients.[30] The recent report of excellent outcomes of patients with low and intermediate risk de novo APL treated with ATRA plus arsenic trioxide without cytotoxic chemotherapy adds support for the likely success of this approach in t-APL as well.[31]
Intermediate and Adverse Karyotypes
For this subgroup, an assessment of each patient’s candidacy for an allogeneic HSCT and early identification of potential donors are essential, as HSCT remains the only curative option for these patients. For those deemed to be transplant candidates, initial treatment with standard induction chemotherapy can be attempted and may be expected to achieve a similar rate of CRs as de novo cases with the same karyotypes.[2] Those achieving a CR should proceed directly to transplant if a donor is available as further cycles of chemotherapy only raise the significant risk of additional morbidity and mortality in this population with limited reserve.[32] Alternatively, patients with t-MN, especially those with t-MN in a myelodysplastic phase with few bone marrow blasts (≤5%) or with marked marrow hypocellularity, can proceed directly to a myeloablative allogeneic HSCT to avoid the risks associated with induction chemotherapy.[32, 33]
Small series of HSCT using both myeloablative and nonmyeloablative regimens for t-MN patients report long-term survival for approximately 20–47% of patients.[2, 32, 34, 35] Outcomes are worse for patients older than 60 years of age and for those with adverse cytogenetics.[2, 32, 34, 35] The risk of relapse is higher in those not in remission at the time of transplantation.[33] In one recent large series, the cumulative incidence of relapse for younger patients (less than 60 years old) after transplantation was similar between t-MN and de novo cases.[2] However, there was a marked increase in the cumulative incidence of death from all causes in the t-MN subset (36% at 4 years versus 17% in de novo cases), probably reflecting their more limited organ reserve and pre-existing comorbidities. Thus, for fit patients with few bone marrow or peripheral blood blasts or with chemosensitive disease, an allogeneic HSCT can be recommended, but this procedure will be curative for only a subset. For patients who are medically fit but lack a histocompatible donor or for those who are unwilling or deemed ineligible for a transplant, initial treatment options include standard intensive induction chemotherapy, or alternatively, treatment with a hypomethylating agent. Azacitidine or decitabine may extend survival and reduce transfusion requirements even if a CR is not achieved. For those who achieve a remission after a standard induction regimen and remain in good health, consolidation with high-dose cytarabine can be considered although this has not been very effective in patients with adverse karyotypes.
Outcomes
Although the expected rate of complete remission for patients with t-MNs is similar to that for de novo cases with the same karyotype, with current treatment approaches the median overall survival for patients with t-MNs continues to be within the range of only 6–9.7 months.[2–4] Improved outcomes for these patients will likely depend on a greater understanding of the etiology of t-MN and susceptible populations such that novel therapies with efficacy and limited toxicity as well as preventative strategies can be realized for this specific group of patients.
Summary
Therapy-related myeloid neoplasms (t-MNs) are an increasingly common and often lethal late complication of cytotoxic treatment for a primary cancer or a non-malignant disease. The incidence of t-MN is increasing as more individuals survive treatment for a primary cancer diagnosis. Although t-MNs can be subdivided into therapy-related myelodysplastic syndrome (t-MDS), therapy-related acute myeloid leukemia (t-AML), and therapy-related myelodysplastic/myeloproliferative neoplasms based on disease parameters, all of these presentations are considered to be within the spectrum of a single disease entity. Eventually, all lead to life-threatening pancytopenia from bone marrow failure. As in de novo AML, prognosis and treatment strategies rely on patient characteristics as well as on cytogenetics. Patient characteristics of primary importance include their performance status, which reflects age and co-morbidities, the status of the primary disease, and the presence of complications from any prior therapy. Clinicians should encourage patients with t-MNs to enroll on clinical trials whenever possible as these patients were often excluded in the past and this has limited progress in improving outcomes. Until novel therapies specific to this subgroup are defined, these patients should be treated on trials designed for other MDS and AML patients with similar cytogenetic abnormalities. Patients who have an HLA-matched donor should be considered for allogeneic HSCT, although patients with favorable karyotypes may do reasonably well with conventional intensive chemotherapy alone. Research priorities should focus on identifying the underlying etiology and risk factors, in order to identify the most susceptible populations and to implement novel prevention and treatment strategies.
Acknowledgements
This work was supported in part by grants CA40046 (RAL), CA14599 (RAL), and CA139160-03 (JEC) from the National Cancer Institute of the National Institutes of Health, USA, and from the Cancer Research Foundation (JEC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors report no conflicts of interest.
References
- 1.Vardiman JW, Arber DA, Brunning RD, et al. Classification of Tumours of Haematopoietic and Lymphoid Tissues. Fourth ed. Lyon, France: IARC Press; 2008. pp. 127–129. [Google Scholar]
- 2.Kayser S, Dohner K, Krauter J, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117(7):2137–2145. doi: 10.1182/blood-2010-08-301713. [DOI] [PubMed] [Google Scholar]
- 3.Smith SM, Le Beau MM, Huo D, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102(1):43–52. doi: 10.1182/blood-2002-11-3343. [DOI] [PubMed] [Google Scholar]
- 4.Takeyama K, Seto M, Uike N, et al. Therapy-related leukemia and myelodysplastic syndrome: a large-scale Japanese study of clinical and cytogenetic features as well as prognostic factors. Internat J Hematol. 2000;71(2):144–152. [PubMed] [Google Scholar]
- 5.Godley LA, Larson RA. Therapy-related myeloid leukemia. Semin Oncol. 2008;35(4):418–429. doi: 10.1053/j.seminoncol.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larson RA. Cytogenetics, not just previous therapy, determines the course of therapy-related myeloid neoplasms. J Clin Oncol. 2012;30(19):2300–2302. doi: 10.1200/JCO.2011.41.1215. [DOI] [PubMed] [Google Scholar]
- 7.Pascual AM, Tellez N, Bosca I, et al. Revision of the risk of secondary leukaemia after mitoxantrone in multiple sclerosis populations is required. Mult Scler. 2009;15(11):1303–1310. doi: 10.1177/1352458509107015. [DOI] [PubMed] [Google Scholar]
- 8.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 9.Le Deley MC, Suzan F, Cutuli B, et al. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J Clin Oncol. 2007;25(3):292–300. doi: 10.1200/JCO.2006.05.9048. [DOI] [PubMed] [Google Scholar]
- 10.Smith RE, Bryant J, DeCillis A, et al. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol. 2003;21(7):1195–1204. doi: 10.1200/JCO.2003.03.114. [DOI] [PubMed] [Google Scholar]
- 11.Advani RH, Hoppe RT, Rosenberg SA, et al. Incidence of secondary leukemia/myelodysplasia in Hodgkin's disease with three generations of therapy at Stanford University. ASCO 2006 Proceedings. J Clin Oncol. 2006;24(18S) Abstract#7516. [Google Scholar]
- 12.Brusamolino E, Baio A, Orlandi E, et al. Long-term events in adult patients with clinical stage IA-IIA nonbulky Hodgkin's lymphoma treated with four cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine and adjuvant radiotherapy: a single-institution 15-year follow-up. Clin Cancer Research. 2006;12(21):6487–6493. doi: 10.1158/1078-0432.CCR-06-1420. [DOI] [PubMed] [Google Scholar]
- 13.Brusamolino E, Gotti M, Fiaccadori V. The risk of therapy-related myelodysplasia/acute myeloid leukemia in Hodgkin lymphoma has substantially decreased in the ABVD era abolishing mechlorethamine and procarbazine and limiting volumes and doses of radiotherapy. Med J Hematol Infect Dis. 2012;4(1):e2012022. doi: 10.4084/MJHID.2012.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delwail V, Jais JP, Colonna P, et al. Fifteen-year secondary leukaemia risk observed in 761 patients with Hodgkin's disease prospectively treated by MOPP or ABVD chemotherapy plus high-dose irradiation. Br J Haematol. 2002;118(1):189–194. doi: 10.1046/j.1365-2141.2002.03564.x. [DOI] [PubMed] [Google Scholar]
- 15.Brusamolino E, Anselmo AP, Klersy C, et al. The risk of acute leukemia in patients treated for Hodgkin's disease is significantly higher aft [see bined modality programs than after chemotherapy alone and is correlated with the extent of radiotherapy and type and duration of chemotherapy: a case-control study. Haematologica. 1998;83(9):812–823. [PubMed] [Google Scholar]
- 16.Lyman GH, Dale DC, Wolff DA, et al. Acute myeloid leukemia or myelodysplastic syndrome in randomized controlled clinical trials of cancer chemotherapy with granulocyte colony-stimulating factor: a systematic review. J Clin Oncol. 2010;28(17):2914–2924. doi: 10.1200/JCO.2009.25.8723. [DOI] [PubMed] [Google Scholar]
- 17.Larson RA, Wang Y, Banerjee M, et al. Prevalence of the inactivating 609C-->T polymorphism in the NAD(P)H:quinone oxidoreductase (NQO1) gene in patients with primary and therapy-related myeloid leukemia. Blood. 1999;94(2):803–807. [PubMed] [Google Scholar]
- 18.Allan JM, Wild CP, Rollinson S, et al. Polymorphism in glutathione S-transferase P1 is associated with susceptibility to chemotherapy-induced leukemia. Proceed Nat Acad Sci USA. 2001;98(20):11592–11597. doi: 10.1073/pnas.191211198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis NA, Huo D, Yildiz O, et al. MDM2 SNP309 and TP53 Arg72Pro interact to alter therapy-related acute myeloid leukemia susceptibility. Blood. 2008;112(3):741–749. doi: 10.1182/blood-2007-11-126508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood. 2003;101(3):822–826. doi: 10.1182/blood-2002-05-1498. [DOI] [PubMed] [Google Scholar]
- 21.Alter BP, Giri N, Savage SA, et al. Cancer in dyskeratosis congenita. Blood. 2009;113(26):6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Link DC, Schuettpelz LG, Shen D, et al. Identification of a novel TP53 cancer susceptibility mutation through whole-genome sequencing of a patient with therapy-related AML. JAMA. 2011;305(15):1568–1576. doi: 10.1001/jama.2011.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin MGJM, Shao J, et al. BRCA1 and BRCA2 Nucleotide Variants in Young Women with Therapy Related Acute Myeloid Leukemias. Blood. 2009;114 Abstract 1102. [Google Scholar]
- 24.Schulz E, Valentin A, Ulz P, et al. Germline mutations in the DNA damage response genes BRCA1, BRCA2, BARD1 and TP53 in patients with therapy related myeloid neoplasms. J Med Genet. 2012;49(7):422–428. doi: 10.1136/jmedgenet-2011-100674. [DOI] [PubMed] [Google Scholar]
- 25.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 26.Schoch C, Kern W, Schnittger S, et al. Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004;18(1):120–125. doi: 10.1038/sj.leu.2403187. [DOI] [PubMed] [Google Scholar]
- 27.Quesnel B, Kantarjian H, Bjergaard JP, et al. Therapy-related acute myeloid leukemia with t(8;21), inv(16), and t(8;16): a report on 25 cases and review of the literature. J Clin Oncol. 1993;11(12):2370–2379. doi: 10.1200/JCO.1993.11.12.2370. [DOI] [PubMed] [Google Scholar]
- 28.Beaumont M, Sanz M, Carli PM, et al. Therapy-related acute promyelocytic leukemia. J Clin Oncol. 2003;21(11):2123–2137. doi: 10.1200/JCO.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 29.Pulsoni A, Pagano L, Lo Coco F, et al. Clinicobiological features and outcome of acute promyelocytic leukemia occurring as a second tumor: the GIMEMA experience. Blood. 2002;100(6):1972–1976. doi: 10.1182/blood-2001-12-0312. [DOI] [PubMed] [Google Scholar]
- 30.Ravandi F. Therapy-related acute promyelocytic leukemia. Haematologica. 2011;96(4):493–495. doi: 10.3324/haematol.2011.041970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo Coco F, Avvisati G, Orlando SM, et al. ATRA and arsenic trioxide versus ATRA and idarubicin for newly diagnosed, non high-risk acute promyelocytic leukemia: Results of the phase III, prospective, randomized, intergroup APL0406 study by the Italian-German cooperative groups GIMEMA-SAL-AMLSG. Blood. 2012;120:6. Abstract#6. [Google Scholar]
- 32.Anderson JE, Gooley TA, Schoch G, et al. Stem cell transplantation for secondary acute myeloid leukemia: evaluation of transplantation as initial therapy or following induction chemotherapy. Blood. 1997;89(7):2578–2585. [PubMed] [Google Scholar]
- 33.Litzow MR, Tarima S, Perez WS, et al. Allogeneic transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood. 2010;115(9):1850–1857. doi: 10.1182/blood-2009-10-249128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yakoub-Agha I, de La Salmoniere P, Ribaud P, et al. Allogeneic bone marrow transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia: a long-term study of 70 patients-report of the French society of bone marrow transplantation. J Clin Oncol. 2000;18(5):963–971. doi: 10.1200/JCO.2000.18.5.963. [DOI] [PubMed] [Google Scholar]
- 35.Zinke-Cerwenka W, Valentin A, Posch U, et al. Reduced-intensity allografting in patients with therapy-related myeloid neoplasms and active primary malignancies. Bone Marrow Transplant. 2011;46(12):1540–1544. doi: 10.1038/bmt.2011.165. [DOI] [PubMed] [Google Scholar]