Abstract
BACKGROUND & AIMS
A high-fat diet (HFD) can cause serious health problems, including alteration of gastrointestinal transit, the exact mechanism of which is not clear. Several microRNAs (miRNAs) are involved in energy homeostasis, lipid metabolism, and HFD-induced weight gain. We investigated the role of miRNAs in HFD-induced damage to the enteric nervous system.
METHODS
Male mice were fed a HFD (60% calories from fat) or regular diets (18% calories from fat) for 11 weeks. Mice on regular diets and HFDs were given intraperitoneal injections of Mir375 inhibitor or a negative control. Body weights, food intake, stool indices, and gastrointestinal transit (following Evans blue gavage) were measured. An enteric neuronal cell line (IM-PEN) and primary enteric neurons were used for in vitro studies.
RESULTS
A HFD delayed intestinal transit, which was associated with increased apoptosis and loss of neurons from colonic myenteric neurons. Mice fed a low-palmitate HFD did not develop a similar phenotype. Palmitate caused apoptosis of enteric neuronal cells associated with mitochondrial dysfunction and endoplasmic reticulum stress. Palmitate significantly increased the expression of Mir375 in vitro; transfection of cells with a Mir375 inhibitor prevented the palmitate-induced enteric neuronal cell apoptosis. Mir375 expression was increased in myenteric ganglia of mice fed HFD, and associated with decreased levels of Mir375 target mRNAs, including Pdk1. Systemic injection of a Mir375 inhibitor for 5 weeks prevented HFD-induced delays in intestinal transit and morphologic changes.
CONCLUSIONS
HFD delay colonic transit, partly by inducing apoptosis in enteric neuronal cells. This effect is mediated by Mir375 and is associated with reduced levels of Pdk1. Mir375 might be targeted to increase survival of enteric neurons and gastrointestinal motility.
Keywords: hyperlipidemia, micrornas, motility, gastrointestinal
INTRODUCTION
MicroRNAs (miRNAs) are extensively studied for their roles in intracellular mechanisms including differentiation, proliferation and apoptosis in a wide range of eukaryotic organisms.1, 2 miRNAs are short non-coding RNAs (19–23 nucleotides), which act by post-transcriptional modulation of protein-coding genes through repressing mRNA translation or promoting their degradation. Each miRNA can affect multiple target genes. This makes them a potential key to understand the pathogenesis of chronic multifactorial diseases such as cardiovascular diseases, diabetes, obesity and cancer.3–5
Obesity has been an area of interest for miRNA research.6, 7 Studies suggest several miRNAs that are involved in energy homeostasis, lipid metabolism, adipogenesis and high-fat diet (HFD) induced weight gain.8, 9 In the broadest sense, obesity is the result of an energy intake exceeding energy expenditure, which is mainly caused by increased consumption of dietary fats.10 Although there is no clear association between obesity and delayed gastrointestinal (GI) transit, HFD is directly associated with dysmotility syndromes such as altered gastric emptying and constipation.11 However, most studies focus on the central and hormonal regulations of the GI tract and very few have examined the direct effects of HFD on the neurons of enteric nervous system (ENS). Hyperlipidemia has been shown to damage neurons in central nervous system by activating apoptotic pathways and disrupting normal cell proliferation.12 HFD upregulates apoptotic genes in hypothalamic neurons as early as 3 days after starting the diet.12 In this scenario, the saturated FFA palmitate is considered the major toxic compound. Palmitate can induce endoplasmic reticulum (ER) stress and apoptosis in various cell types by affecting calcium homeostasis and triggering the production of free radicals.13 Furthermore, FFAs can differentially regulate gene expression and activate different intracellular pathways.14 The role of intracellular accumulation of long chain FFAs in the pathogenesis of several disorders such as diabetes and cardiomyopathy has been reported previously, and recent studies suggest involvement of miRNAs. 15, 16 Studies on β-cells suggest that at least part of the detrimental effects of palmitate is mediated by alterations in the level of miRNAs, such as Mir375.15, 16 miRNAs also affect neuronal cell survival in pathologic conditions such as neurodegenerative diseases (i.e. Mir433 in Parkinson’s disease and Mir9 in Huntington’s disease).17 Therefore, studying the role of miRNAs will help us to understand the mechanisms of HFD-induced enteric neuronal cell damage and the pathophysiology of HFD-induced GI dysmotility in human. In this study, our aim was to investigate the direct toxic effects of palmitate on enteric neurons and understand the role of miRNAs in the HFD-induced damage to ENS.
We report that the saturated FFA palmitate modulates miRNAs expression in the enteric neurons, which is associated with apoptosis and organelle damage. Additionally, we provide in vivo evidence on the role of miRNA in GI transit and ENS neuronal survival.
MATERIALS AND METHODS
Animal protocol for high-fat diet experiment
In vivo experiments were performed using CF1 or C57BL/6 male mice. The Institutional Animal Care and Use Committee at Emory University approved animal handling and procedures. Eight-week-old male mice (Jackson Laboratory, Bar Harbor, ME) were put on HFD (60% calories from fat, TD.06414) or regular diet (RD, 18% calories from fat; TD.2018) for 11 weeks (5 mice per group). HFD and RD were purchased from Harlan Laboratories Inc. (Madison, WI). HFD consists of 8.0% Palmitate vs. 0.7% in RD (Supplementary Table 1). To examine the role of palmitate in the HFD induced transit changes we conducted a separate experiment for 6 weeks with three groups (n=4): low-palmitate-HFD (LP-HFD, Harlan Laboratories, Supplementary Table 1), HFD and RD. In addition twenty week old ob/ob and wild type male mice (n=4) were used as a genetic obesity model to evaluate motility and compare it to HFD induced motility changes.
Animal protocol for miRNA mimic injection experiment
Nine-week-old CF1 male mice were randomized into treatment and control groups (6 each) and were injected intraperitoneally twice with chemically synthesized Mir375 mirVana™ miRNA mimic and its negative control (Ambion) with 3 days interval. In vivo-jetPEI® was purchased from Polyplus-transfection (Illkirch, France). In vivo-jetPEI®/miRNA complexes were prepared according to the manufacturer’s protocol.
Animal protocol for miRNA inhibitor injection experiment
Eight-week old CF1 male mice were used in 5 groups: HFD or RD fed mice, receiving intraperitoneal injection of Locked-Nucleic Acid (LNA) modified Mir375 inhibitor (Custom miRCURY™ LNA Inhibitor probe, in-vivo, Exiqon) or its negative control, the fifth group received HFD and vehicle injection (Saline). The animals were dosed for three consecutive days, receiving daily doses of 7.5 mg/kg, and then a weekly maintenance injection for four additional weeks.
Primary enteric neurons and IM-PEN cell line culture
The immorto postnatal enteric neuronal (IM-PEN) cell line was established and characterized as described previously.18 For primary cell culture, p75-expressing cells were isolated from E14.5 rat embryos as previously described.19 In another method, the gut was isolated from embryos, diced and lysed by 0.125% Trypsin, and layered on newborn calf serum and centrifuged at 350G for 8 min at room temperature. Cells were then seeded on gelatin coated plates with DMEM:F12 medium, which was changed to modified N2 medium after 24 hours. Palmitate (Sigma Aldrich, St. Louis, MO) was used in 0.1–1 mM concentrations. This range is within the physiologically elevated limits observed in humans and animals, which can induce insulin resistance and hyperlipidemia related complications.20 Palmitate was dissolved in isopropanol to obtain a concentration of 100 mM. The required volume of the stock was added to the medium. The concentration of the vehicle, isopropanol, was less than 1% in the final culture media.
Mir375 inhibitor transfection
Mir375 inhibitor (AntimiR375) and negative control miRNA inhibitors were obtained commercially (Life technologies, USA). Lipofectamine 2000 transfection reagent was used to transfect cells according to the manufacturer’s protocol. Apoptosis rate was measured as described 24 hours after adding palmitate in cells treated with Mir375 inhibitor or negative control.
Statistical analysis
Results were analyzed using the 2-tailed Student’s t test or Mann-Whitney test. One-way analysis of variance (ANOVA) was used for more than two groups followed by the Tukey’s Post Hoc test. Data was analyzed using the SigmaPlot 11.0. P value ≤ 0.05 was considered statistically significant. Data is expressed as mean ± SEM.
All additional methods are included in the Supplementary Methods section.
RESULTS
High-fat diet causes delayed intestinal transit
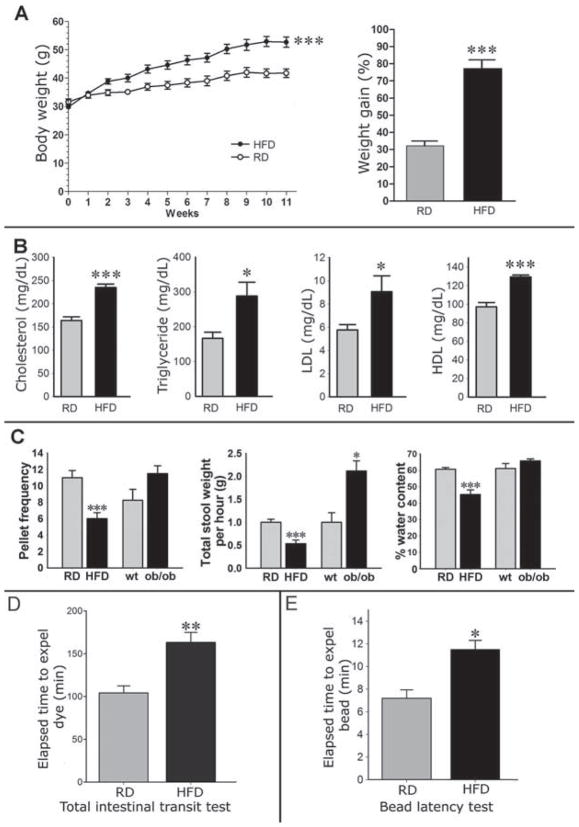
After 11 weeks, the HFD group gained significantly more weight compared with RD group (Figure 1A). This was associated with a significant increase in serum cholesterol, triglyceride, LDL, and HDL in HFD compared with RD (Figure 1B). Mice fed aHFD had lower pellet frequency (P<0.01), stool wet weight (P<0.01), dry weight (P<0.05), water content (P<0.001) and water percentage (P<0.001) compared with RD group, mimicking a constipation phenotype (Figure 1C). Mice on HFD also had a significantly smaller pellet size compared to RD (0.39 ± 0.01 vs. 0.53 ± 0.01 mm, P< 0.001). Food consumption normalized for body weight was not significantly different between HFD and RD. Total intestinal transit time measured by Evans blue gavage was greater in the HFD group indicating significantly slower transit in HFD treated mice compared with the RD (P<0.01, Figure 1D). Finally, bead latency test was longer in the HFD group indicating that HFD caused slower colonic propulsion in mice compared with RD (P<0.05, Figure 1E).
Figure 1. Mice fed a HFD develop higher body weight and lipid profile and slower GI motility.
(A) Body weight and percentage weight gain in mice fed a HFD or RD for 11 weeks. (B) Serum lipid concentrations were determined in the same groups of mice as in figure. 1A after overnight fasting. (C) The stool characteristics in the HFD and RD mice, and in ob/ob mice. (D) Comparison of the mean transit times of gastrointestinal transit marker between mice fed a HFD or RD showing significantly slower whole-intestinal transit in HFD (greater transit time). (E) Colonic bead propulsion test revealing slower colonic transit (higher time to expel) in HFD compared with RD. HFD indicates high-fat diet; RD, regular diet. Results presented as mean ± SEM. *P<0.05, ** P<0.01, ***P<0.001, n=5.
To tease out the effect of high-fat diet vs. obesity on intestinal transit, we measured stool indices and transit time in genetically obese mice, the ob/ob mice. These mice did not exhibit the delayed intestinal transit. The stool frequency, total wet weight and percentage water content in the ob/ob mice were similar or increased compared to the wild type mice (Figure 1C). The total intestinal transit time by Evans blue gavage was similar in ob/ob mice compared to the wild type mice fed a RD (Supplementary Figure 1A).
Palmitate contributes to High-fat diet induced delayed intestinal transit
Mice fed the low-palmitate high-fat diet (LP-HFD) for six weeks did not develop similar delayed transit phenotype as observed in the HFD. LP-HFD mice had significantly higher pellet frequency, total wet weight and water content, compared to the HFD (Supplementary Figure 2A). In addition, Evans blue transit time and determination of the GC showed a significantly faster intestinal transit in LP-HFD mice compared with the HFD (Supplementary Figure 2B–C).
High-fat diet decreases the number of neurons in proximal colonic myenteric plexus by inducing apoptosis
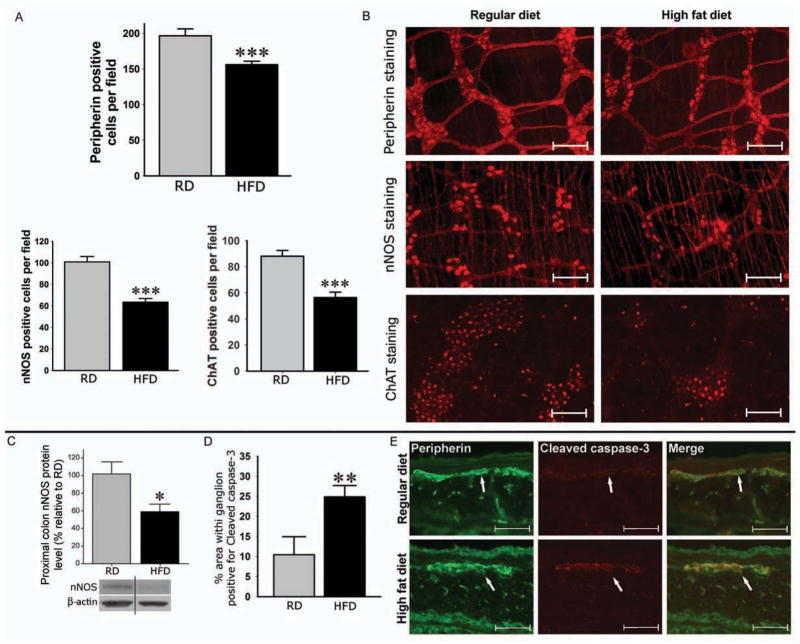
Whole mount IF staining of proximal colon showed a significant reduction in the total number of neurons (Peripherin) in HFD group, which was associated with a significant reduction in the number of nitrergic neurons (nNOS) and cholinergic neurons (ChAT) in the HFD compared with the RD group (Figure 2A and 2B). Furthermore, Western blot analysis of the proximal colon tissue lysates showed a significant decrease in the protein level of nNOS in HFD samples compared with RD (Figure 2C). The observed decrease in enteric neurons was associated with a significant increase in neuronal cell apoptosis in the myenteric ganglia of HFD group compared with RD group (Figure 2D–E). In ob/ob mice, the number of nitrergic neurons was not significantly different from that in wild type mice. (Supplementary Figure 1B). Further, the LP-HFD mice did not show reduction in the number of nitrergic neurons compared with RD fed mice (Supplementary Figure 2D).
Figure 2. HFD decreases number of myenteric neurons in the proximal colon by inducing apoptosis.
(A) Whole mount preparations of proximal colon from mice on HFD and RD for 11 weeks stained for peripherin, nNOS and ChAT. (B) Representative photographs of peripherin, nNOS and ChAT stained neurons. Scale bar: 100 μm. (C) Proximal colon of mice fed a HFD or RD for 11 weeks assessed for nNOS protein. (D) Proximal colon sections stained for Cleaved caspase-3 and peripherin. (E) Representative immunofluorescence staining photos. Original magnifications: 20x. Scale bar: 50 μm. Results presented as mean ± SEM, *P<0.05, **P<0.01, ***P<0.001, n=5.
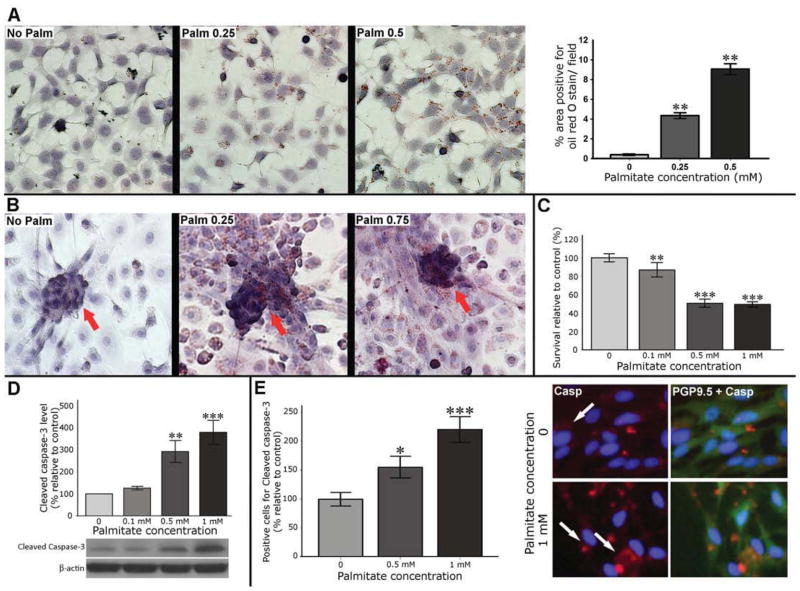
Palmitate induces cytoplasmic lipid accumulation and apoptosis in enteric neurons in vitro
Neurons do not store lipids under physiologic conditions, and cytoplasmic accumulation of lipids is mainly observed in neurodegenerative disorders, which is accompanied by significant cytotoxicity.21 Our results of Oil red O staining in enteric neurons showed a dose-dependent cytoplasmic accumulation of lipid droplets after palmitate exposure (P<0.01, Figure 3A–B). Cell viability measured by MTS assay showed a dose-dependent decrease in the enteric neuronal cell viability after 24 hours of palmitate exposure (Figure 3C). This effect was associated with a significant and dose-dependent increase in the Cleaved caspase-3 protein level in IM-PEN cell line and primary cell culture (Figure 3D–E).
Figure 3. Palmitate is taken up by enteric neuronal cells and induces cell death.
(A) Representative microphotographs of neuronal cell line treated with palmita te and stained with oil red O. (B) Primary cell cultures of enteric neurons treated with palmitate with arrows indicate lipid deposits in the neuronal clusters. (C) MTS survival assay in enteric neurons after 24 hours of palmitate exposure. (D) Western blot analysis of Cleaved caspase-3 protein level in enteric neuronal cell lines. Graph depicts the average of five separate experiments. (E) IF staining of primary enteric neuronal cells. Arrows indicate cells positive for Cleaved caspase-3. Results presented as mean ± SEM, *P<0.05, ** P<0.01, ***P<0.001.
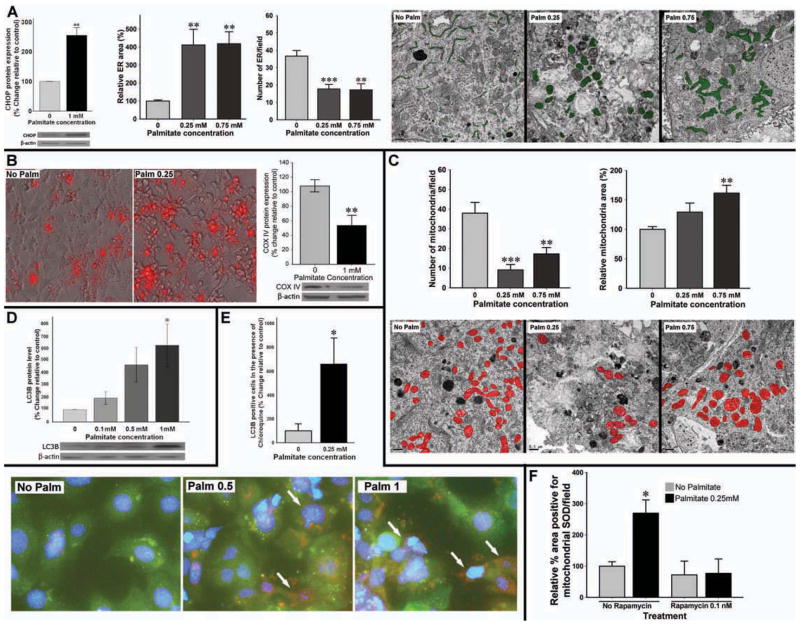
Palmitate causes mitochondrial and ER dysfunction in enteric neurons
To understand the mechanisms of palmitate-induced enteric neuronal cell apoptosis, we measured indices of ER and mitochondrial function in enteric neuronal cells. Palmitate significantly increased levels of CHOP protein (an index of PERK pathway activation in ER stress) in enteric neurons (Figure 4A). Electron microscopic (EM) study of primary enteric neurons confirmed morphological changes consistent with ER stress (Figure 4A). Palmitate exposure resulted in a significant ER swelling (more than four folds increase in size) and decrease in the number of ER (P<0.01). We then studied mitochondrial function in enteric neuronal cells and observed that palmitate significantly increases mitochondrial superoxide dismutase (SOD) (using MitoSOX kit, Invitrogen) as depicted in figure 4B. Palmitate also caused a significant decrease in the mtDNA-encoded protein COX IV, a marker of mitochondrial mass (Figure 4B). This observation was further confirmed by ultra-structural study showing a dose-dependent decrease in the number of mitochondria (Figure 4C) accompanied by a significant mitochondrial swelling. These results suggest that severe organelle damage including mitochondrial oxidative stress and swelling and ER stress are possible underlying mechanisms leading to palmitate-induced neuronal cell death.
Figure 4. Palmitate induces ER stress and mitochondrial damage and causes up-regulation of autophagy as a survival mechanism.
(A) Palmitate significantly increases CHOP levels in enteric neuronal cell line, and Ultra-structure of ER in primary en teric neurons treated with palmitate shows a marked ER swelling, associated with a dose-dependent decrease in the number of ER. Representative Electron micrographs in which ER area is traced in green. (B) Representative photographs of enteric neurons treated with palmitate for 24 hours and stained for mitochondrial SOD marker (MitoSOX) to assess oxidative stress. Western blot analysis also shows a decrease in the COX IV as a marker of mitochondrial mass. (C) Ultra-structural study of the number and area occupied by mitochondria (traced in red) in primary enteric neuronal culture. Scale bar: 0.5 μm. (D) Western blot assessing LC3B protein level after enteric neurons are exposed to palmitate. Graph depicts the average of five separate experiments. (E) Autophagy flux measured by IF analysis of IM-PEN enteric neuronal cell line stained for LC3B after palmitate treatment in the presence of chloroquine. Bottom panel is high magnification representative photos of primary enteric neurons showing the cells positive for LC3B as red. (F) The level of mitochondrial SOD in the enteric neurons pre-treated with rapamycin followed by exposure to palmitate. Results presented as mean ± SEM, *P<0.05, **P<0.01, ***P<0.001.
Palmitate stimulates the autophagy pathway in enteric neurons as a protective mechanism
Autophagy is a cellular catabolic mechanism to degrade its dysfunctional and unwanted components. As shown in figure 4D enteric neurons treated with palmitate for 24 hours showed a strong dose-dependent increase in the expression of LC3B. Autophagic flux was determined by pretreatment with the lysosomal inhibitor chloroquine. As shown in figure 4E, autophagic flux is induced in response to palmitate. To study whether the autophagy is a protective cellular mechanism, we used rapamycin, a macrolide antibiotic and autophagy inducer by inhibiting mammalian target of rapamycin (mTOR). Treatment with 0.1 nM rapamycin for 2 hours before adding palmitate prevented the palmitate induced mitochondrial SOD increase (Figure 4F). These results demonstrate that autophagy induction can protect enteric neurons against the detrimental effects of palmitate.
Palmitate increases the level of miRNAs involved in cell death
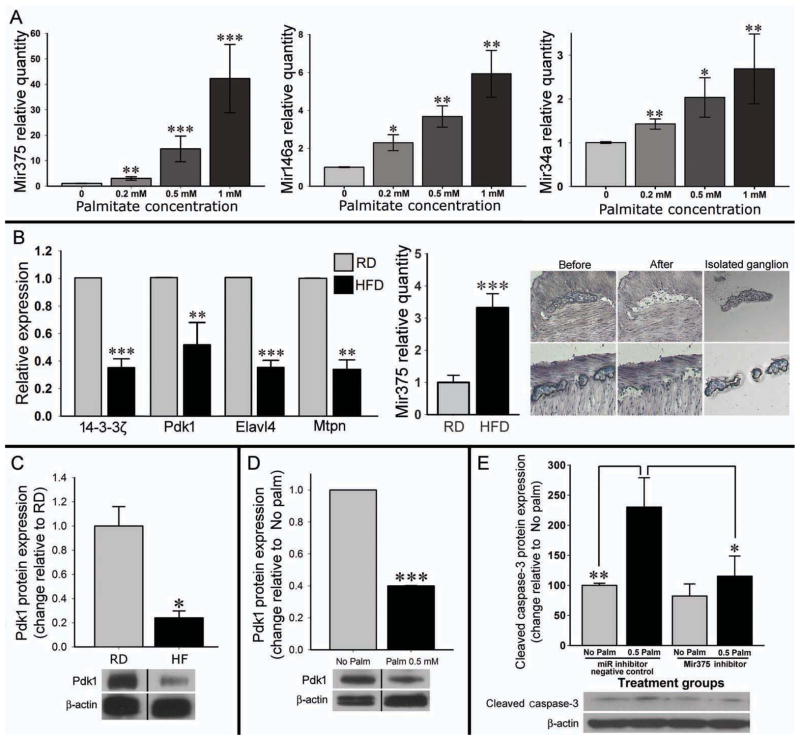
To investigate the mechanisms of palmitate induced enteric neuronal cell damage we studied the role of Mir375, Mir146a and Mir34a.16, 22 Palmitate caused a significant increase in the expression of these miRNAs amongst which Mir375 showed the most robust increase (Figure 5A). Next, we investigated the expression of Mir375 and four of its target mRNAs (14-3-3ζ, Pdk1, Elavl4 and Mtpn) in the myenteric ganglia isolated by LCM. Mir375 expression was significantly increased in enteric ganglia captured from HFD fed mice but not in those from RD (Figure 5B). Expressions of all four Mir375 target mRNAs were significantly decreased in the ganglia of HFD compared with RD mice (Figure 5B). Furthermore, unlike HFD, ob/ob mice showed no increase in the level of Mir375 in their myenteric ganglia (Supplementary Figure 1C). Next, by Western blot analysis we observed a significant decrease in Pdk1 protein in homogenates from HFD colon (Figure 5C), which could contribute to the observed neuronal cell apoptosis. A similar decrease in Pdk1 protein was also observed in enteric neuronal cells treated with palmitate (Figure 5D).
Figure 5. In vitro palmitate increases microRNA expressions and Mir375 target mRNAs decrease in myenteric ganglia of the HFD fed mice.
(A) Quantitative RT-PCR analysis of Mir375, Mir146a and Mir34a in the enteric neuronal cell line after treating with palmitate. (B) The expression of Mir375 and its target mRNAs in the myenteric ganglia isolated by LCM from the proximal colon of the mice treated with RD or HFD. Right panel shows how myenteric ganglion was isolated by LCM for the PCR. n= at least 3 (C) The decrease in Pdk1 mRNA in myenteric neurons was associated with a significant decrease in the protein level of Pdk1 protein in the proximal colon. (D) Similar decrease was observed in the Pdk1 protein level after palmitate treatment in enteric neuronal cells. (E) In vitro Mir375 inhibitor prevents the detrimental effect of palmitate as evident by preventing the increase of Cleaved caspase-3 in neuronal cells. HFD indicates high-fat diet; RD, regular diet. Results presented as mean ± SEM, *P<0.05, **P<0.01, ***P<0.001.
To further investigate the role of Mir375 we transfected enteric neuronal cells with a Mir375 specific inhibitor or its negative control for 24 hours followed by palmitate treatment (0.5 mM) for 24 hours. Western blot analysis for Cleaved Caspase-3 showed a significant reduction in palmitate-induced apoptosis when the Mir375 is inhibited compared with negative control miRNA inhibitor (Figure 5E).
Systemic injection of Mir375 induces apoptosis in enteric neurons and slows GI transit
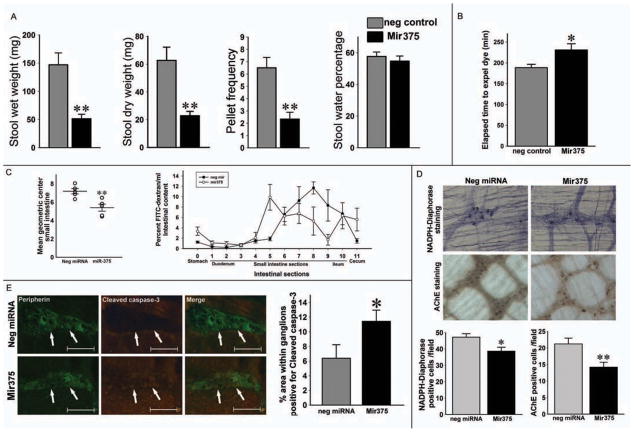
To test the hypothesis of Mir375 involvement in the detrimental effects of HFD on GI transit, we administered intraperitoneal exogenous Mir375. As shown in figure 6A, Mir375 injected mice showed a significantly lower stool wet weight (P<0.01), dry weight (P<0.01), and pellet frequency (P<0.01). Daily food intake per mouse was not different between groups (data not shown). Total intestinal transit measurement by Evans blue gavage also showed significantly slower transit in Mir375 injected mice compared with negative control (P< 0.05, Figure 6B). Determination of the GC showed a significantly slower intestinal transit in Mir375-injected mice compared with the negative control miRNA (P<0.01) and segmental distribution of the FITC-dextran confirmed that the distance traversed by the marker for the Mir375 was significantly shorter (Figure 6C). Whole mount staining showed a decrease in the number of nitrergic and cholinergic neurons in the proximal portion of colon in Mir375 injected mice compared with negative control group (Figure 7D). Using Cleaved caspase-3 and peripherin staining we observed a significant increase in apoptosis in the myenteric neurons in Mir375 injected group compared with the negative control group (Figure 7E). These data confirm the detrimental effect of Mir375 on enteric neuronal cell survival in the myenteric ganglia, which can contribute to the delayed intestinal transit.
Figure 6. Systemic Mir375 injection causes GI dysmotility and delayed transit, which is associated by myenteric neuronal loss and apoptosis.
(A) Stool indices of Mir375 and control miRNA injected mice. (B) Comparison of the mean transit times of Evans blue. (C) Geometric center determination for small intestine. Distribution of FITC-dextran marker 1 hour after gavage displaying slower transit in Mir375 injected mice. (D) Whole mount preparations of proximal colon from Mir375 and control miRNA injected mice stained for NADPH-diaphorase and AChE. (E) Analysis of proximal colon sections stained for Cleaved caspase-3 and peripherin. Original magnifications: 20x. Scale bar: 50 μm. Results presented as mean ± SEM, *P<0.05, **P<0.01, n=6.
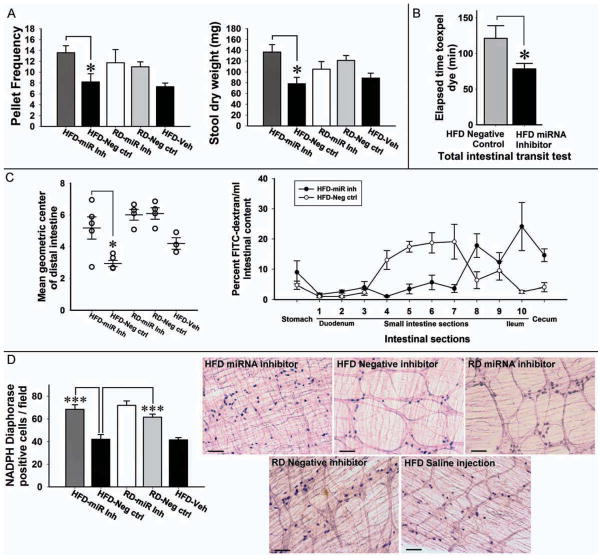
Figure 7. Systemic Mir375 inhibitor prevents the development of delayed intestinal transit and protects against the myenteric neuronal loss.
(A) Stool indices of the mice fed HFD or RD, treated with Mir375 inhibitor or negative control or vehicle. (B) Comparison of the mean transit times of Evans blue. (C) Geometric center determination for small intestine and distribution of FITC-dextran marker 1 hour after gavage suggesting slower intestinal transit. (D) Whole mount preparation of proximal colon for NADPH-diaphorase. HFD indicates high-fat diet; RD, regular diet. Original magnifications: 10x. Scale bar: 100 μm. Results presented as mean ± SEM, *P<0.05, ***P<0.001. n=3–5
Systemic injection of Mir375 inhibitor prevents HFD-induced changes in intestinal transit and enteric neurons
To test the hypothesis that Mir375 modulates HFD-induced delayed transit time and enteric neuronal apoptosis, we examined the effect of systemic administration of LNA modified Mir375 inhibitor or its negative control in the mice fed a HFD or RD for five weeks. Inhibition of Mir375 resulted in prevention of delayed intestinal motility as seen by an increase in stool pellet frequency (P<0.05) and dry weight (P<0.05) compared to HFD mice treated with the negative control (Figure 7A). HFD mice treated with Mir375 inhibitor had significantly faster total intestinal transit measured by Evans blue gavage compared to mice treated with negative control (P< 0.05, Figure 7B). Determination of the GC showed faster intestinal transit in Mir375 inhibitor injected mice compared with the negative control, which was differentially faster in the distal part of the small intestine. FITC-dextran intestinal distribution showed that in HFD fed mice intestinal transit was faster in Mir375 injected mice compared to negative controls (Figure 7C). Finally whole mount staining confirmed a significant decrease in the number of nitrergic neurons in the proximal colon of the HFD fed mice, which was prevented in the Mir375 inhibitor treated HFD fed mice (Figure 7D).
DISCUSSION
In the present study, we investigated the mechanisms of enteric neuronal cell damage caused by HFD and excess FFAs. We showed that HFD-induced loss of enteric neurons could contribute to delayed intestinal transit. Furthermore, palmitate decreased enteric neuronal cell viability through mitochondrial damage and ER stress. Finally, we suggested the role of Mir375 in mediating the detrimental effects of HFD by downregulating the pro-survival protein Pdk1 translation.
The available literature regarding the chronic effects of HFD on the ENS and neuromuscular function is relatively sparse and sometimes controversial.23 Acute exposure to HFD or hyperglycemia slows gastric emptying and GI tract motility through modulation of GI hormones (including CCK, GLP-1, PYY, and ghrelin) but not much is known about the pathophysiology of chronic HFD and its intracellular mechanisms.24–26 Our aim was to investigate the possible direct role of HFD on the ENS in the small intestine and colon. To our knowledge this is the first study to investigate the role of microRNAs in the HFD induced GI motility disorders.
miRNAs are present in human peripheral blood and unlike mRNAs, are remarkably stable and resistant to RNase activity. This makes them suitable candidates for therapeutic purposes.
Promising results in preclinical studies have led to miRNA-directed therapies in human. Currently more than 100 ongoing trials incorporating miRNAs are under way.3, 27
The role of miRNAs in palmitate-induced cell damage has been described in several tissues such as hepatocytes and pancreatic islet cells.15 Mir375, in particular, is studied in palmitate mediated lipoapoptosis and suppression of several types of cancers including esophageal, gastric and head and neck via apoptotic pathways.28, 29
High-fat diets rich in saturated FFA such as palmitate increase oxidative stress in various tissues such as brain and gastric and intestinal mucosa.30, 31 Mitochondria as the major source of ROS are susceptible to FFAs accumulation and the ROS-induced lipid peroxidation.32 It has been suggested that FFAs in beta cells must be metabolized to long chain fatty acyl-CoA to exert toxicity, and this effect can be reduced by activation of fatty acid oxidation in mitochondria.33 In our study, we observed a significant increase in mitochondrial SOD following palmitate exposure, suggesting a mitochondrial oxidative stress overload. Interestingly, mitochondrial dysfunction is also a major contributor in neurodegeneration in ENS and the GI related symptoms in neurodegenerative disorders.34 Our results confirmed a significant decrease in the mitochondrial quantity measured by COX IV protein level, as well as ultra-structural changes consistent with mitochondrial dysfunction. Palmitate triggers ER stress by altering the distribution of ER chaperones, leading to accumulation of misfolded proteins.35 It is suggested that autophagy is activated for cell survival after ER stress occurs.36 However, if extensive ER stress is sustained, the function of ER cannot be restored, which may leads to apoptosis and removal of the affected cells.37 Autophagy plays crucial role in eukaryotic cells homeostasis. It acts by forming a double-membrane structure called autophagosome or autophagic vacuole to sequester cytoplasm and unwanted organelles.
Additionally, autophagy is also involved in lipid droplet removal. This can be another mechanism involved in palmitate-induced autophagy activation, as we showed for the first time that enteric neuronal cells accumulate lipid after palmitate treatment. The term “macrolipophagy” was coined to describe the involvement of selective autophagy in the delivery of lipid droplets for lysosomal degradation, suggesting a novel function for autophagy in cellular lipid handling.38 Therefore, not only ER stress can induce autophagy, but also lipid accumulation itself may contribute to autophagy induction, perhaps as a rescue mechanism. In this study, pretreatment of neuronal cells with the autophagy inducer rapamycin significantly decreases mitochondrial SOD production. This suggests that autophagy may be a protective mechanism in enteric neurons against the palmitate induced oxidative stress and organelle damage.
To investigate the possible mechanisms involved in HFD induced enteric neuronal cell damage, we studied three possible miRNAs and observed a robust increase in the Mir375, which is tightly related to cell survival and differentiation, both with palmitate treatment in vitro and in myenteric ganglia isolated from the proximal colon of HFD fed mice.
Tsukamoto et al. reported that Mir375 promotes apoptosis in gastric carcinoma cell line and decreases cell viability via targeting Pdk1 and 14-3-3ζ mRNAs. Pdk1 plays a central role in many signaling pathways including activation of PI3K/Akt, through which cell proliferation, differentiation and apoptosis are regulated39, and our results showed that Mir375 upregulation in HFD directly targets Pdk1 protein expression (Figure 5C). Another target downstream to Mir375 is Mtpn, which encodes the myotrophin (V1) protein.16 Myotrophin promotes dimerization of NF-κB subunits and downregulates NF-κB transcriptional activity.40 This is consistent with the fact that HFD triggers chronic inflammation in the majority of tissues including neurons, and that palmitate in particular is a potent inducer of inflammation.41, 42 We observed a significant decrease in Mtpn gene transcription in enteric neurons isolated from colonic myenteric ganglia (Figure 5B). To confirm the above-mentioned detrimental role of Mir375 on enteric neurons in vitro, we transfected cultured enteric neurons with Mir375 inhibitor before exposing them to palmitate. As expected, cells transfected with Mir375 inhibitor showed significantly less apoptosis in response to palmitate. In order to investigate the role of Mir375 in vivo, we conducted two experiments. We used systemic injection of Mir375 to prove its direct toxic effects on enteric neurons, and confirmed that high circulating levels of Mir375 could induce enteric neuronal cell apoptosis, associated with lower number of neurons and delayed intestinal transit. We observed approximately a two-fold increase in apoptosis in the Mir375 injected mice compared to negative control. Finally, by systemic injection of Mir375 inhibitor to the mice fed a HFD for five weeks we prevented the development of detrimental effects of HFD on intestinal transit and enteric neurons number, providing direct evidence for the role of Mir375. Future studies will focus on dissecting the precise mechanisms of action of Mir375 on modulating ER stress and mitochondrial function. Due to the pro-survival nature of Mir375 and its overexpression in certain cancers, prior to its therapeutic use for gastrointestinal motility disorders further studies will need to be done to assess its role in cancer development.
In conclusion, our results suggest that HFD can induce apoptosis in enteric neurons through the effect of the FFA palmitate. Palmitate activates oxidative stress and ER stress in enteric neurons, leading to apoptosis and neuronal cell loss. We showed that exogenous systemic Mir375 could mimic the HFD induced GI dysmotility symptoms, while inhibition of Mir375 in HFD fed mice prevents the neuronal damage and development of the phenotype. Our results suggest Mir375 as a possible future therapeutic target for clinical GI dysmotility.
Supplementary Material
Acknowledgments
Grant support: This research was funded by the NIH grant number NIH-RO1-DK080684 and VA-Merit Award.
We thank Ms. Hong Yi of the Integrated Microscopy and Micro analytical Facility at Emory University for performing the electronic microscopy.
Abbreviations
- AChE
acetylcholine esterase
- ANOVA
One-way analysis of variance
- BSA
Bovine serum albumin
- CCK
cholecystokinin
- ChAT
Choline Acetyltransferase
- CHOP
CAAT/enhancer-binding protein-homologous protein
- COX IV
Cytochrome c oxidase IV
- Ct
cycle threshold
- Elavl4
embryonic lethal, abnormal vision, Drosophila-like 4
- EM
Electron microscope
- ENS
enteric nervous system
- ER
endoplasmic reticulum
- FBS
Fetal bovine serum
- FFAs
Free fatty acids
- FITC-dextran
fluorescein isothiocyanate-dextran
- GC
geometric center
- GDNF
glial cell line-derived neurotrophic factor
- GI
gastrointestinal
- HFD
High-fat diet
- IF
Immunofluorescence
- IM-PEN
immorto postnatal enteric neuronal
- LC3B
light chain 3-B
- LCM
Laser Capture Microdissection
- LP-HFD
Low-palmitate HFD
- miRNAs
MicroRNAs
- Ms
Mouse
- mTOR
Mammalian target of rapamycin
- Mtpn
myotrophin
- MTS
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- nNOS
Neuronal nitric oxide synthase
- nPTB
neuronal polypyrimidine tract binding
- PCR
polymerase chain reaction
- Pdk1
3-phosphoinositide-dependent protein kinase-1
- PEI
Polyethylenimines
- PERK
protein kinase RNA-like endoplasmic reticulum kinase
- PGP9.5
Protein gene product 9.5
- Rb
Rabbit
- RD
Regular diet
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- wt
wild type
Footnotes
Conflict of interest: The authors have no conflict of interests to declare
- Behtash Ghazi Nezami was involved in the study concept and design, acquisition of data, statistical analysis, analysis and interpretation of data, drafting of the manuscript.
- Simon M. Mwangi was involved in the study concept and design, acquisition of data, analysis and interpretation of data
- Sabrina Jeppsson was involved in the acquisition of data, analysis and interpretation of data
- Mallappa Anitha was involved in the acquisition of data, analysis and interpretation of data
- Shadi S. Yarandi was involved in the analysis and interpretation of data, drafting of the manuscript
- Jai Eun Lee was involved in the acquisition of data
- Alton B Farris III was involved in the study concept and design
- Shanthi Srinivasan obtained funding and was involved in the study supervision, study concept and design, drafting of the manuscript, critical revision of the manuscript for important intellectual content
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 2.Ivey KN, Srivastava D. MicroRNAs as regulators of differentiation and cell fate decisions. Cell Stem Cell. 2010;7:36–41. doi: 10.1016/j.stem.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Nana-Sinkam SP, Croce CM. Clinical applications for microRNAs in cancer. Clin Pharmacol Ther. 2013;93:98–104. doi: 10.1038/clpt.2012.192. [DOI] [PubMed] [Google Scholar]
- 4.Natarajan R, Putta S, Kato M. MicroRNAs and diabetic complications. J Cardiovasc Transl Res. 2012;5:413–422. doi: 10.1007/s12265-012-9368-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali AS, Ali S, Ahmad A, et al. Expression of microRNAs: potential molecular link between obesity, diabetes and cancer. Obes Rev. 2011;12:1050–1062. doi: 10.1111/j.1467-789X.2011.00906.x. [DOI] [PubMed] [Google Scholar]
- 6.Williams MD, Mitchell GM. MicroRNAs in insulin resistance and obesity. Exp Diabetes Res. 2012;2012:484696. doi: 10.1155/2012/484696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ono K. MicroRNA links obesity and impaired glucose metabolism. Cell Res. 2011;21:864–866. doi: 10.1038/cr.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Q, Gao Z, Alarcon RM, et al. A role of miR-27 in the regulation of adipogenesis. Febs J. 2009;276:2348–2358. doi: 10.1111/j.1742-4658.2009.06967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn J, Lee H, Chung CH, et al. High fat diet induced downregulation of microRNA-467b increased lipoprotein lipase in hepatic steatosis. Biochem Biophys Res Commun. 2011;414:664–669. doi: 10.1016/j.bbrc.2011.09.120. [DOI] [PubMed] [Google Scholar]
- 10.Astrup A, Dyerberg J, Selleck M, et al. Nutrition transition and its relationship to the development of obesity and related chronic diseases. Obes Rev. 2008;9 (Suppl 1):48–52. doi: 10.1111/j.1467-789X.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- 11.Bertrand RL, Senadheera S, Tanoto A, et al. Serotonin availability in rat colon is reduced during a Western diet model of obesity. Am J Physiol Gastrointest Liver Physiol. 2012;303:G424–434. doi: 10.1152/ajpgi.00048.2012. [DOI] [PubMed] [Google Scholar]
- 12.Moraes JC, Coope A, Morari J, et al. High-fat diet induces apoptosis of hypothalamic neurons. PLoS One. 2009;4:e5045. doi: 10.1371/journal.pone.0005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei Y, Wang D, Topczewski F, et al. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 14.Duplus E, Forest C. Is there a single mechanism for fatty acid regulation of gene transcription? Biochem Pharmacol. 2002;64:893–901. doi: 10.1016/s0006-2952(02)01157-7. [DOI] [PubMed] [Google Scholar]
- 15.Lovis P, Roggli E, Laybutt DR, et al. Alterations in microRNA expression contribute to fatty acid-induced pancreatic beta-cell dysfunction. Diabetes. 2008;57:2728–2736. doi: 10.2337/db07-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Xu X, Liang Y, et al. miR-375 enhances palmitate-induced lipoapoptosis in insulin-secreting NIT-1 cells by repressing myotrophin (V1) protein expression. Int J Clin Exp Pathol. 2010;3:254–264. [PMC free article] [PubMed] [Google Scholar]
- 17.Packer AN, Xing Y, Harper SQ, et al. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington’s disease. J Neurosci. 2008;28:14341–14346. doi: 10.1523/JNEUROSCI.2390-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anitha M, Joseph I, Ding X, et al. Characterization of fetal and postnatal enteric neuronal cell lines with improvement in intestinal neural function. Gastroenterology. 2008;134:1424–1435. doi: 10.1053/j.gastro.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anitha M, Gondha C, Sutliff R, et al. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344–356. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopaschuk GD, Collins-Nakai R, Olley PM, et al. Plasma fatty acid levels in infants and adults after myocardial ischemia. Am Heart J. 1994;128:61–67. doi: 10.1016/0002-8703(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 21.Paul CA, Boegle AK, Maue RA. Before the loss: neuronal dysfunction in Niemann-Pick Type C disease. Biochim Biophys Acta. 2004;1685:63–76. doi: 10.1016/j.bbalip.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Kong L, Zhu J, Han W, et al. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol. 2011;48:61–69. doi: 10.1007/s00592-010-0226-0. [DOI] [PubMed] [Google Scholar]
- 23.Baudry C, Reichardt F, Marchix J, et al. Diet-induced obesity has neuroprotective effects in murine gastric enteric nervous system: involvement of leptin and glial cell line-derived neurotrophic factor. The Journal of physiology. 2012;590:533–544. doi: 10.1113/jphysiol.2011.219717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clegg ME, McKenna P, McClean C, et al. Gastrointestinal transit, post-prandial lipaemia and satiety following 3 days high-fat diet in men. Eur J Clin Nutr. 2011;65:240–246. doi: 10.1038/ejcn.2010.235. [DOI] [PubMed] [Google Scholar]
- 25.Park JH, Kwon OD, Ahn SH, et al. Fatty diets retarded the propulsive function of and attenuated motility in the gastrointestinal tract of rats. Nutr Res. 2013;33:228–234. doi: 10.1016/j.nutres.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Zhou SY, Lu YX, Owyang C. Gastric relaxation induced by hyperglycemia is mediated by vagal afferent pathways in the rat. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1158–1164. doi: 10.1152/ajpgi.00067.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bader AG. miR-34 - a microRNA replacement therapy is headed to the clinic. Front Genet. 2012;3:120. doi: 10.3389/fgene.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong KL, Kwong DL, Chan TH, et al. MicroRNA-375 inhibits tumour growth and metastasis in oesophageal squamous cell carcinoma through repressing insulin-like growth factor 1 receptor. Gut. 2012;61:33–42. doi: 10.1136/gutjnl-2011-300178. [DOI] [PubMed] [Google Scholar]
- 29.Hui AB, Lenarduzzi M, Krushel T, et al. Comprehensive MicroRNA profiling for head and neck squamous cell carcinomas. Clin Cancer Res. 2010;16:1129–1139. doi: 10.1158/1078-0432.CCR-09-2166. [DOI] [PubMed] [Google Scholar]
- 30.Bagchi D, Carryl OR, Tran MX, et al. Stress, diet and alcohol-induced oxidative gastrointestinal mucosal injury in rats and protection by bismuth subsalicylate. Journal of applied toxicology : JAT. 1998;18:3–13. doi: 10.1002/(sici)1099-1263(199801/02)18:1<3::aid-jat461>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 31.Stranahan AM, Cutler RG, Button C, et al. Diet-induced elevations in serum cholesterol are associated with alterations in hippocampal lipid metabolism and increased oxidative stress. Journal of neurochemistry. 2011;118:611–615. doi: 10.1111/j.1471-4159.2011.07351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schrauwen P, Schrauwen-Hinderling V, Hoeks J, et al. Mitochondrial dysfunction and lipotoxicity. Biochim Biophys Acta. 2010;1801:266–271. doi: 10.1016/j.bbalip.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 33.El-Assaad W, Buteau J, Peyot ML, et al. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144:4154–4163. doi: 10.1210/en.2003-0410. [DOI] [PubMed] [Google Scholar]
- 34.Viader A, Wright-Jin EC, Vohra BP, et al. Differential regional and subtype-specific vulnerability of enteric neurons to mitochondrial dysfunction. PLoS One. 2011;6:e27727. doi: 10.1371/journal.pone.0027727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karaskov E, Scott C, Zhang L, et al. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology. 2006;147:3398–3407. doi: 10.1210/en.2005-1494. [DOI] [PubMed] [Google Scholar]
- 36.Jeffrey KD, Alejandro EU, Luciani DS, et al. Carboxypeptidase E mediates palmitate-induced beta-cell ER stress and apoptosis. Proc Natl Acad Sci U S A. 2008;105:8452–8457. doi: 10.1073/pnas.0711232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jing G, Wang JJ, Zhang SX. ER stress and apoptosis: a new mechanism for retinal cell death. Exp Diabetes Res. 2012;2012:589589. doi: 10.1155/2012/589589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Ouaamari A, Baroukh N, Martens GA, et al. miR-375 targets 3′-phosphoinositide-dependent protein kinase-1 and regulates glucose-induced biological responses in pancreatic beta-cells. Diabetes. 2008;57:2708–2717. doi: 10.2337/db07-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knuefermann P, Shi SP, Chen P, et al. Myotrophin/V-1 does not act as an extracellular signal to induce myocyte hypertrophy. Tex Heart Inst J. 2006;33:281–289. [PMC free article] [PubMed] [Google Scholar]
- 41.Kim F, Tysseling KA, Rice J, et al. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol. 2005;25:989–994. doi: 10.1161/01.ATV.0000160549.60980.a8. [DOI] [PubMed] [Google Scholar]
- 42.Little JP, Madeira JM, Klegeris A. The saturated fatty acid palmitate induces human monocytic cell toxicity toward neuronal cells: exploring a possible link between obesity-related metabolic impairments and neuroinflammation. J Alzheimers Dis. 2012;30 (Suppl 2):S179–183. doi: 10.3233/JAD-2011-111262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.