Abstract
Peroxisome proliferator-activated receptor-γ (PPARγ) and Rho-kinase (ROCK) regulate smooth muscle cell (SMC) proliferation and contribute to vascular remodeling in adult pulmonary hypertension. Whether these pathways interact to contribute to the development of vascular remodeling in persistent pulmonary hypertension of the newborn (PPHN) remains unknown. We hypothesized that ROCK-PPARγ interactions increase SMC proliferation resulting in vascular remodeling in experimental PPHN. Pulmonary artery SMCs (PASMCs) were harvested from fetal sheep after partial ligation of the ductus arteriosus in utero (PPHN) and controls. Cell counts were performed daily for 5 days with or without PPARγ agonists and ROCK inhibition. PPARγ and ROCK protein expression/activity were measured by Western blot in normal and PPHN PASMCs. We assessed PPARγ-ROCK interactions by studying the effect of ROCK activation on PPARγ activity and PPARγ inhibition (siRNA) on ROCK activity and PASMC proliferation. At baseline, PPHN PASMC cell number was increased by 38% above controls on day 5. ROCK protein expression/activity were increased by 25 and 34% and PPARγ protein/activity decreased by 40 and 50% in PPHN PASMC. ROCK inhibition and PPARγ activation restored PPHN PASMC growth to normal values. ROCK inhibition increased PPARγ activity by 50% in PPHN PASMC, restoring PPARγ activity to normal. In normal PASMCs, ROCK activation decreased PPARγ activity and PPARγ inhibition increased ROCK activity and cell proliferation, resulting in a PPHN hyperproliferative PASMC phenotype. PPARγ-ROCK interactions regulate SMC proliferation and contribute to increased PPHN PASMC proliferation and vascular remodeling in PPHN. Restoring normal PPARγ-ROCK signaling may prevent vascular remodeling and improve outcomes in PPHN.
Keywords: pulmonary artery smooth muscle cells (PASMC), peroxisome proliferator-activated gamma (PPARγ), Rho-kinase (ROCK), persistent pulmonary hypertension of the newborn (PPHN), vascular remodeling
persistent pulmonary hypertension of the newborn (PPHN) is a clinical syndrome characterized by elevated pulmonary vascular resistance (PVR) that persists after birth (31). Mechanisms contributing to increased PVR in PPHN include increased vascular tone, hypertensive vascular remodeling, and impaired angiogenesis (17, 20). Hypertensive pulmonary vascular remodeling is often present in newborns dying with severe PPHN in the first days of life, suggesting that intrauterine mechanisms contribute to the pathogenesis of PPHN (14, 27, 36, 45). Although inhaled nitric oxide (iNO) is effective in treating many newborns with PPHN, PPHN with vascular remodeling is often refractory to nitric oxide (NO) therapy (20, 38a), making it essential to study novel therapies that may be more effective in this setting. Strategies that can decrease pulmonary artery smooth muscle cell (PASMC) proliferation and prevent vascular remodeling will improve outcomes for infants with severe PPHN. However, mechanisms that regulate PASMC proliferation during development and contribute to vascular remodeling in severe PPHN remain poorly understood.
Past studies have shown that partial ligation of the ductus arteriosus (DA) in late gestation fetal sheep provides a useful animal model for studying the pathogenesis and treatment of PPHN (4). In this model, partial DA ligation increases pulmonary artery pressure without causing sustained elevations of pulmonary blood flow or hypoxemia, and PVR remains elevated at birth (4). Along with changes in vascular tone, DA ligation causes vascular remodeling and severe PPHN with decreased responsiveness to iNO therapy (4, 36, 51). How hemodynamic stress induced by hypertension disrupts normal PASMC signaling and function increasing proliferation and producing vascular remodeling in PPHN is unknown.
Rho kinase (ROCK) signaling is a complex pathway responsible for cellular proliferation, migration, differentiation and gene expression in diverse vascular beds (9). ROCK activity has been shown to regulate smooth muscle cell (SMC) contraction and vascular tone in systemic and pulmonary circulations (6, 8). In adult animal models of pulmonary hypertension, enhanced ROCK activity increases vascular tone, mediates calcium sensitization, and contributes to hypertensive remodeling (2, 9, 41, 50). During lung development, ROCK activity maintains high pulmonary vascular tone and contributes to the myogenic response in the fetal lung (42, 48). We have reported that increased ROCK activity alters pulmonary artery endothelial cell (PAEC) growth and impairs angiogenesis in experimental PPHN (19). Whether high ROCK activity increases PASMC proliferation and contributes to vascular remodeling in PPHN is unknown.
Peroxisome proliferator-activated receptor (PPAR) is an early phylogenic member of the nuclear receptor superfamily, consisting of three isotypes: PPARα, PPARδ, and PPARγ (1, 12, 15, 29). PPARγ is abundantly expressed in vascular smooth muscle and endothelial cells in the normal lung (1, 52) and regulates SMC proliferation (52). Selective PPARγ deletion in SMCs causes pulmonary hypertension and increased muscularization of distal vessels (22), and activation of PPARγ decreases proliferation of PASMC in vitro (16, 22). In adult models of pulmonary hypertension, PPARγ agonists prevent the development of vascular remodeling and pulmonary hypertension (PH) (10, 11, 21, 40). This effect may be developmentally regulated as conflicting reports have been published in the neonate where PPARγ agonists improved air space growth, but had no effect on hypoxia-induced pulmonary vascular remodeling (39). Decreased PPARγ signaling contributes significantly to PASMC proliferation in adult models of PH, but whether alterations in PPARγ signaling contribute to increased PASMC proliferation and vascular remodeling in PPHN is unclear.
Previous reports have demonstrated that PPARγ agonists produce vasodilation through inhibition of ROCK (5a, 26, 49), but whether PPARγ-ROCK interactions regulate SMC proliferation in PPHN remains unknown. Therefore, we hypothesized that increased PASMC ROCK expression and activity decreases PPARγ signaling, which increases SMC proliferation and contributes to vascular remodeling in PPHN. In this study we report increased proliferation, increased ROCK activity, and decreased PPARγ signaling in PPHN PASMCs and demonstrate that ROCK-PPARγ interactions contribute to vascular remodeling in experimental PPHN.
METHODS
Isolation and culture of fetal ovine PAECs.
All procedures and protocols were reviewed and approved by the Animal Care and Use Committee at the University of Colorado Health Sciences Center, Aurora, CO. Fetal sheep from mixed-breed Columbia-Rambouillet pregnant ewes were used for all studies. Left and right pulmonary arteries were isolated at 135–140 days gestation (term = 147 days), from late-gestation normal fetal sheep (n = 4), and from fetal sheep that had undergone partial ligation of the DA in utero at 125–130 days gestation, 7–10 days prior to euthanasia (PPHN) (n = 4) as previously described (18).
SMC culture methods.
Primary cultures of fetal PASMCs were prepared from conduit arteries isolated from late-gestation fetal lambs (135–140 days gestation; term = 147 days). The adventitia was gently removed under sterile conditions and the vessel was washed in sterile phosphate-buffered saline (PBS). The vessel was sliced longitudinally and placed with the intima side down in a petri dish containing 0.5% collagenase (Worthington, Lakewood, NJ; catalog no. LS004174) for 10 min at 37°C. The intimal surface was then gently removed with a cell scraper. The remaining portion of the vessel was cut into small (1–2 mm) pieces and washed with HBSS (Fisher Scientific, Pittsburgh, PA; catalog no. 50-983-203), sodium bicarbonate (NaHCO3) (20 mM), and HEPES (Fisher Scientific, catalog no. BP299-1) (10 mM) for 30 min in the 37°C hybridization oven. Fragments were placed in a SMC digest that consisted of 7.5 ml HBSS with 40 μM Ca2+, 4.0 mg elastase (Roche, catalog no. 100907), 4.07 mg type 2 collagenase (Worthington, catalog no. LS04174 CLS-2), 15 mg albumin (Sigma, St. Louis, MO; catalog no. A9647), and 147 μl soybean trypsin inhibitor (10 mg/ml) (Worthington, catalog no. LSO 3570) for 2 h in a 37°C hybridization oven. The digest was filtered through a 100-μm cell strainer, washed with 5 ml of 10% FBS (Sigma, catalog no. F6178)/DMEM (Fisher Scientific catalog no. MT-15-018-CV) with 1% l-glutamine and 1% antibiotic-antimycotic and spun at 250 g for 6 min. The supernatant was removed and the pellet resuspended in 10% FBS/DMEM with 1% l-glutamine (Invitrogen, Grand Island, NY; catalog no. 25030081) and 1% antibiotic-antimycotic (Invitrogen, catalog no. 15240062) and transferred to the culture flask. Cells were maintained in culture containing 10% FBS, 1% antibiotic-antimycotic, and 1% l-glutamine, until they reached confluence. PASMC identity was confirmed by morphology and immunostaining with antibodies against α-smooth muscle actin, calponin, caldesmon, and desmin. All PASMC isolates demonstrate greater than 95% positive staining for the above SMC markers, indicating an absence of contamination with fibroblasts or endothelial cells. PASMC from four normal and PPHN animals were utilized for all experiments, with experiments performed at passage 3–6. Utilizing passage 3–6 cells allows for expansion and freezing of clones and subsequent use for multiple experiments.
siRNA transfection.
PPARγ siRNA (Ovine) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA; catalog no. SC-156097) and siRNA transfection was performed per manufacturers' recommendations and protocol. PPARγ siRNA is an ovine-specific pool of three target-specific 19–25 nt siRNAs designed to knock down PPARγ gene expression. Briefly, in a six-well tissue culture plate, 5 × 105 cells per well were plated in 2 ml antibiotic-free normal growth medium supplemented with 10% FBS, at 37°C in 5% CO2 incubator and allowed to adhere overnight. Growth medium was removed and transfection reagents (siRNA and Scramble) were added to duplicate wells for a final volume of 1 ml. Duplicate wells were utilized, allowing for molecular analysis of transfection efficacy and utilization of transfected cells for experiments outlined below. PPARγ siRNA (4 μM) and control siRNA (Santa Cruz Biotechnology, catalog no. SC-37007) were mixed with equal parts of transfection reagent (Santa Cruz Biotechnology, catalog no. SC-29528) and added to the appropriate wells for 6 h. Multiple doses of siRNA (0–8 μM) were tested in preliminary experiments and 4 μM was siRNA utilized because this dose produced the greatest knockdown of PPARγ expression and activity without causing apoptosis. Two control wells were also prepared receiving only antibiotic-free normal growth medium supplemented with FBS. After the 6-h incubation, normal growth medium with 20% FBS was added to each well without removal of the transfection mixture. Cells were incubated for an additional 18–24 h and subsequently passaged for study. After passage, there was no further exposure to siRNA, and transfected cells were handled, utilizing the same conditions as controls. Western blot analysis was performed after passage on whole cell lysates and revealed a 37% decrease in total PPARγ protein expression and 32% decrease in the ratio of phosphorylated to total PPARγ protein (PPARγ activity) after siRNA treatment.
Cell growth.
Fetal PASMCs from normal (n = 4) and PPHN lambs (n = 4) at passage 4 were plated at 2 × 105 cells/well in a six-well plate and allowed to adhere overnight. Duplicate wells were plated for PASMC derived from each animal, and PASMCs were grown in DMEM supplemented with 10% FBS to initially determine whether under standard cell culture conditions differences exist between normal and PPHN PASMC proliferation. Daily cell counts were performed on duplicate wells for 5 days with a hemocytometer and experiments were repeated twice by the same methods to ensure reproducibility. The effect of PPARγ agonists rosiglitazone (Rosi 50 μM) (Cayman Chemical, Ann Arbor, MI; catalog no. 71740) and 15-deoxy-delta12,14-prostaglandin J2 (15d-PG-J2; 10 μM) (Cayman Chemical, catalog no. 18570), PPARγ siRNA (4 μM) and ROCK inhibition (y-27632 5 μM) (Cayman Chemical, catalog no. 129830-38-2) on cell growth were compared between PASMC from normal and PPHN fetal sheep. To determine the optimal dose for each agent, Western blot analysis was performed on normal and PPHN PASMC lysates for PPARγ protein expression and activity (total and phosphorylated PPARγ) (Rosi, 15d-PG-J2, and PPARγ siRNA) and ROCK protein expression and activity (total and phosphorylated MYPT-1) (y-27632). We utilized the lowest dose of each agent for which the desired effect was seen. Growth studies were performed in DMEM supplemented with 5% serum, because this was the lowest serum concentration that supported fetal PASMC survival and proliferation. Medium was changed daily to ensure that degradation of drugs did not occur in culture.
MTT assay.
The MTT assay measures cell proliferation of metabolically active cells by their mitochondrial activity. The tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (MTT, a yellow chemical) is taken up by live cells and reduced or converted by mitochondrial reductase into a water insoluble purple formazan dye crystals. The amount of conversion can be measured with an ELISA reader. Briefly, MTT (Sigma, catalog no. M2128) was dissolved at 5 mg/ml in 1 × PBS, and 10,000 PASMC from normal and PPHN fetal sheep were seeded per well of 96-well plates. MTT stock was added to each well at 1:10 dilution and incubated at 37°C for 6 h. The 96-well plates were spun at 220 g for 5 min, after which the supernatant was discarded and 150 μl of 100% acidic isopropanol was added to each well. Optical density was determined with a microplate spectrophotometer at a wavelength of 570 nm.
Western blot analysis.
PASMC from normal and PPHN fetal sheep were grown on 150 mm cloning dishes in DMEM supplemented with 5% serum. At confluence, cells were washed with ice cold PBS × 2 and lysed in radioimmunoprecipitation (RIPA) buffer [PBS, 1% Nonidet P-40, 0.5%, sodium deoxycholate, 0.1% SDS, PMSF (10 mg/ml), aprotinin (16 μl/ml), and sodium orthovanadate (1 mM)]. Cell lysates were scraped off the dishes, sonicated, and centrifuged at 10,000 g for 30 min at 4°C. The supernatant was removed and protein content in the supernatant was determined by the bicinchoninic acid assay [Pierce Biotechnology (catalog no. 23225) Rockford, IL], with bovine serum albumin as the standard. PASMC cell lysates were collected from four normal and PPHN animals and 20 μg of protein sample per lane was resolved by SDS-polyacrylamide gel electrophoresis. Duplicate samples for each clone of cells or animal and condition were loaded; where samples from four normal and four PPHN animals could not be loaded on the same polyacrylamide gel, samples from one normal and one PPHN animal were loaded, allowing for comparison. Proteins from the gel were then transferred to nitrocellulose membrane.
PPARγ/p-PPARγ.
After blocking, the blots were incubated overnight at 4°C with anti-PPAR gamma (catalog no. ab70405, Abcam, Cambridge, MA, 1:750 dilution), anti-p-PPARγ antibody (ab60953, Abcam; 1:750 dilution) diluted in 5% bovine serum albumin. Following overnight incubation blots were washed and subsequently incubated for 1 h at room temperature with goat anti-rabbit horseradish peroxidase (HRP)-conjugate secondary antibody (catalog no. SC2054, Santa Cruz Biotechnology 1:2,000). Bands of interest were visualized by enhanced chemiluminescence (ECL kit, Amersham Pharmacia Biotech, Buckinghamshire, UK or 2% ECL Advance Amersham Pharmacia Biotech) and identified by molecular weight as designated by the manufacturer for the protein of interest. PPAR-γ activity is expressed as the ratio of phosphorylated (p)-PPARγ/β actin to PPARγ/β actin.
ROCKII.
Blots were blocked for 30 min in 5% nonfat dry milk dissolved in buffer (10 mM Tris·HCl, 150 mM NaCl, 0.05% Tween-20, pH 8.0) Blots were incubated for 2 h at room temperature with anti-ROCK-II/ROCK∝ (BD610624 BD Biosciences, San Jose, CA) (1:500) diluted in 5% nonfat dry milk in buffer. After washing, blots were incubated for 1 h at room temperature with goat anti-mouse HRP-conjugated secondary antibody (Chemicon, Billerica, MA) (1:10,000). Bands of interest were visualized by enhanced chemiluminescence (ECL+ kit; Amersham Pharmacia Biotech, Buckinghamshire, UK), identified by molecular weight.
Phospho-MYPT-1/MYPT-1.
Blots were blocked for 60 min with 5% nonfat dry milk dissolved in TBS with 0.5% Tween 20 (10 mM Tris·HCl, 150 mM NaCl, 0.5% Tween-20, pH 8.0). Blots were then incubated overnight with p-MYPT1 (Thr853) antibody (no. 4563 Cell Signaling, Danvers, MA) (1:1,000) and MYPT-1 (Cell Signaling) (1:1,000). After washing, blots were incubated for 1 h at room temperature with goat anti-rabbit HRP-conjugated secondary (Santa Cruz Biotechnology, SC2054). Bands of interest were visualized by enhanced chemiluminescence as previously described (18). ROCK activity is expressed as the ratio of p-MYPT-1/β actin to MYPT-1/β actin.
All blots were stripped and reprobed with an antibody to β-actin (Sigma, A5316) as a loading control. Densitometry was performed using NIH Image (version 1.61) and changes in protein expression were analyzed after normalization for β-actin expression. For all figures representative blots are shown.
Study design.
To assess ROCK-PPARγ interactions, at confluence, PASMCs were treated with angiotensin II (ANGII; 100 nM) and thromboxane A2 (TXA2; 1 μM) to assess the effect of ROCK activation on PPARγ protein expression and activity. Y-27632 (5 μM) treatment was used to assess the effect of ROCK inhibition on PPARγ protein expression and activity. PPARγ knockdown with specific siRNA was used to assess the effect of PPARγ inhibition on ROCK protein expression and activity. Western blots were performed for PPARγ/p-PPARγ; after 24 h of exposure to ROCK activators and inhibitors and for ROCKII, p-MYPT-1, and total MYPT-1 after treatment with PPARγ siRNA.
Statistical analysis.
Data are presented as means ± SE. Statistical analysis was performed with the Prism 4 software package (GraphPad Software, San Diego, CA). Statistical comparisons were made by analysis of variance for proliferation assays and Western blot analysis with Bonferroni posttest analysis. Unpaired t-test was used for MTT assay, proliferation assays, and Western blot analysis where appropriate. P < 0.05 was considered significant.
RESULTS
Increased proliferation of PASMC from PPHN fetal sheep.
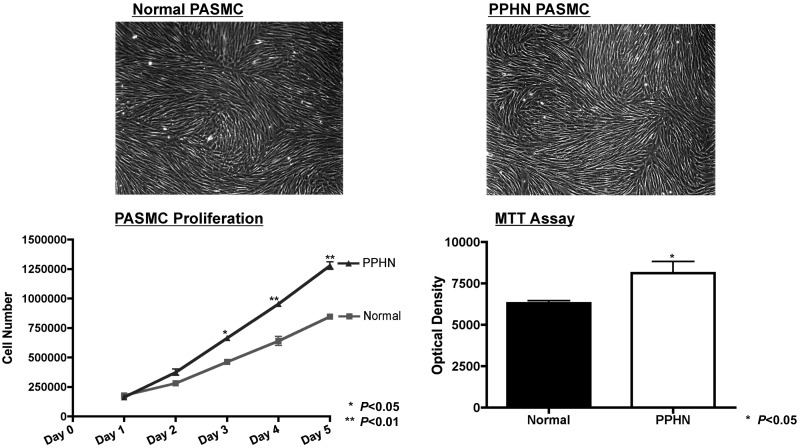
Growth of normal and PPHN PASMC was assessed by performing daily cell counts for 5 days and MTT assay. Compared with controls, growth of PPHN PASMC increased by 44% on day 3 (P < 0.05), 49% on day 4 (P < 0.01), and 51% on day 5 (P < 0.01). By MTT assay, growth of PPHN PASMC increased by 29% (P < 0.05) (Fig. 1). Despite significant differences in proliferation, normal and PPHN PASMC demonstrate identical cell morphology (Fig. 1).
Fig. 1.
Increased proliferation of pulmonary artery smooth muscle cells (PASMCs) from pulmonary hypertension of the newborn (PPHN) sheep. Growth of normal and PPHN PASMC was assessed by performing daily cell counts for 5 days and MTT assay. Under baseline conditions, growth of PPHN PASMC was increased compared with controls. Despite significant differences in proliferation, normal and PPHN PASMC demonstrate identical cell morphology.
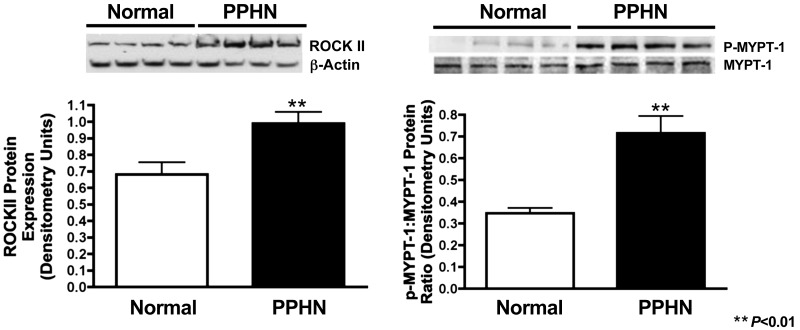
Increased ROCK protein expression and activity in PPHN PASMC.
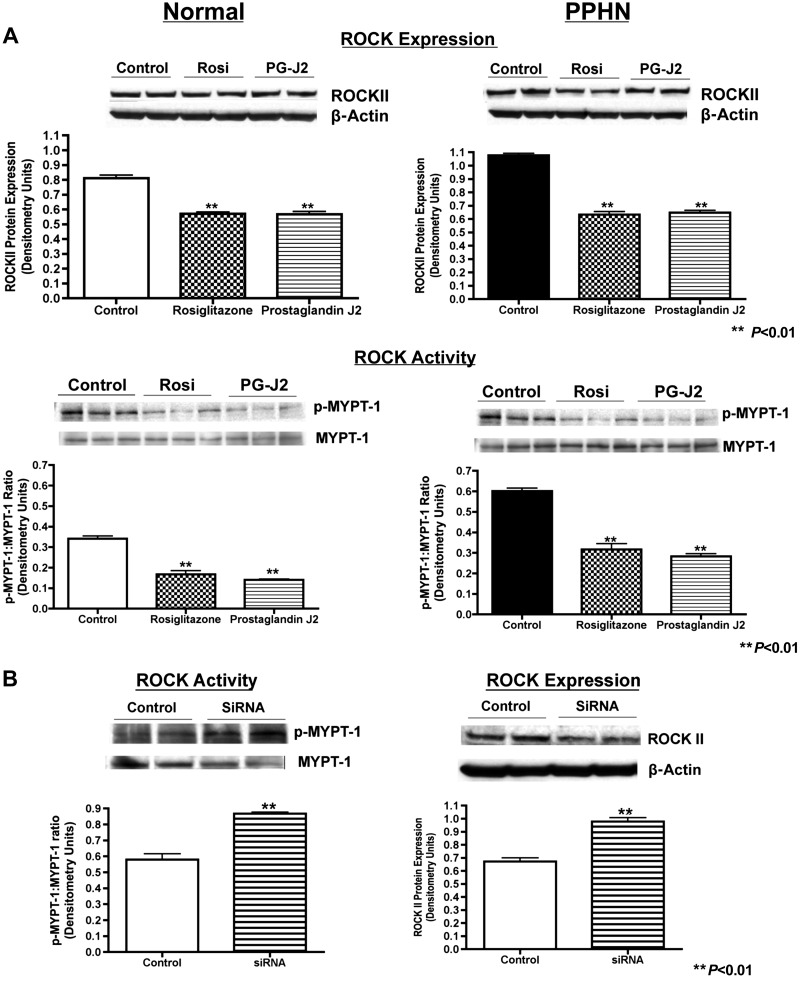
Western blot analysis on PASMC whole cell lysates from normal and PPHN fetal sheep demonstrates increased ROCK protein expression and activity. Compared with normal controls, ROCK protein expression (ROCKII) increased by 34% (P < 0.01) and activity (p-MYPT-1-to-MYPT-1 ratio) was 2× greater (P < 0.01) in PPHN PASMC (Fig. 2).
Fig. 2.
Increased ROCK protein expression and activity in normal and PPHN PASMC. Western blot analysis on PASMC whole cell lysates from normal and PPHN fetal sheep demonstrate increased ROCK protein expression (ROCKII) and activity (p-MYPT-1-to-MYPT-1 ratio). For all figures representative blots are shown.
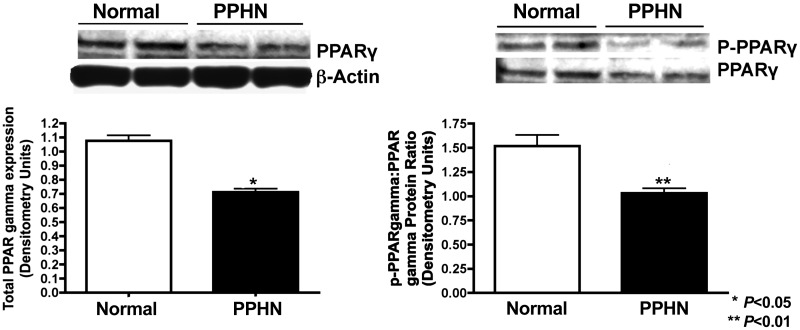
Decreased PPARγ protein expression and activity in PPHN PASMC.
Western blot analysis on PASMC whole cell lysates from normal and PPHN fetal sheep demonstrate decreased PPARγ protein expression and activity in PPHN PASMC. Compared with controls, total PPARγ decreased by 27% (P < 0.05) and PPARγ activity (p-PPARγ-to-PPARγ ratio) decreased by 32% (P < 0.01) in PPHN PASMC (Fig. 3).
Fig. 3.
PPARγ protein expression and activity in normal and PPHN PASMC. Western blot analysis on PASMC whole cell lysates from normal and PPHN fetal sheep demonstrate decreased PPARγ protein expression and activity (p-PPARγ-to-PPARγ ratio) in PPHN PASMC. For all figures representative blots are shown.
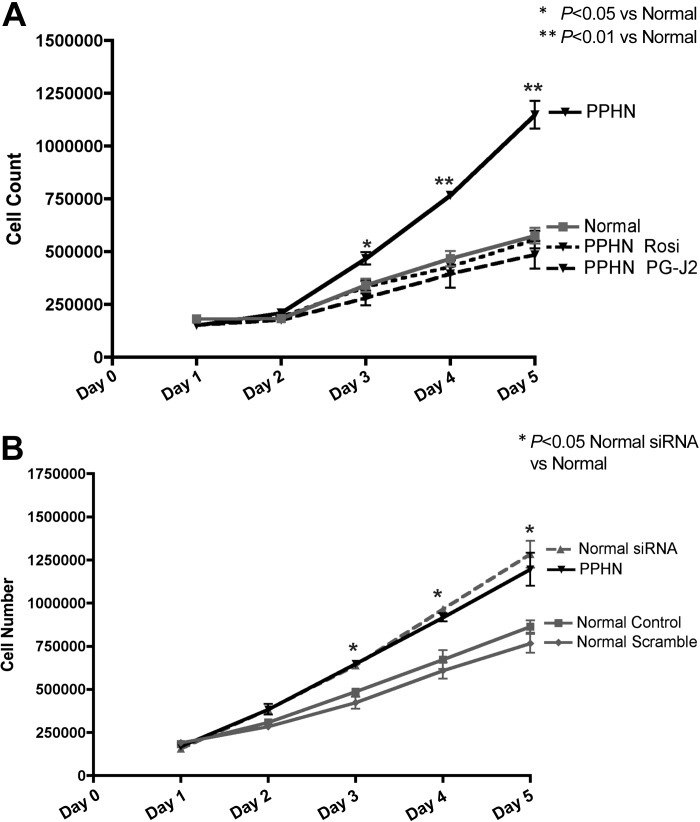
Effect of PPARγ activation and inhibition on PASMC proliferation.
Growth of PPHN PASMCs was assessed in response to PPARγ agonists (Rosi 50 μM and 15d-PG-J2, 10 μM) (Fig. 4A), and growth of normal PASMCs was assessed in response to PPARγ inhibition (PPARγ siRNA 4 μM) (Fig. 4B) by performing daily cell counts for 5 days. Both Rosi and 15d-PG-J2 decreased proliferation of PPHN PASMC, restoring growth to similar values seen in normal PASMC. Rosi and 15d-PG-J2 decreased proliferation by 42% (P < 0.05) and 46% (P < 0.05) on day 3, by 50% (P < 0.01) and 52% (P < 0.01) on day 4, and 51% (P < 0.01) and 55% (P < 0.01) on day 5 (Fig. 4A). PPARγ siRNA increased proliferation of normal PASMC producing a hyperproliferative phenotype in normal PASMC as observed in PPHN PASMC. Normal PASMC growth increased by 31% (P < 0.05) on day 3, 44% on day 4 (P < 0.01), and 49% on day 5 (P < 0.01) (Fig. 4B).
Fig. 4.
Effect of PPARγ activation and inhibition on PASMC proliferation. Growth of PPHN PASMC was assessed in response to PPARγ agonists [rosiglitazone (Rosi), 50 μM, and 15-deoxy-delta12,14-prostaglandin J2 (PG-J2), 10 mM] (A) and normal PASMC assessed in response to PPARγ inhibition (PPARγ siRNA 4 μM) (B). Treatment with Rosi and PG-J2 decreased proliferation in PPHN PASMC restoring growth to similar values seen in normal PASMC (A). PPARγ siRNA increased proliferation of normal PASMC, producing a phenotype in normal PASMC similar to what was seen in PPHN PASMC. Scramble siRNA had no effect on PASMC proliferation.
Effect of PPARγ activation and inhibition on ROCK protein expression and activity.
Effect of PPARγ activation on ROCK protein expression and activity was assessed by using PPARγ agonists (Rosi 50 μM and 15d-PG-J2, 10 μM) in normal and PPHN PASMC. Rosi decreased ROCK expression by 30% (Fig. 5A) and activity (ratio of p-MYPT-1 to MYPT-1) by 51% (Fig. 5A) and 15d-PG-J2 decreased ROCK expression by 30% (Fig. 5A) and activity by 58% (Fig. 5A) in normal PASMC. In PPHN PASMC, Rosi decreased ROCK expression by 41% (Fig. 5A) and activity by 47% (Fig. 5A) and 15d-PG-J2 decreased ROCK expression by 40% (Fig. 5A), and activity by 53%. (Fig. 5A). In normal fetal PASMC, PPARγ siRNA increased ROCK protein expression (ROCKII) by 46% (P < 0.01) and ROCK activity (p-MYPT-1-to-MYPT-1 ratio) by 50% (P < 0.01) (Fig. 5B).
Fig. 5.
Effect of PPARγ agonists on ROCK protein expression and activity in normal and PPHN PASMC and PPARγ inhibition on ROCK expression and activity in normal PASMC. As shown, PPARγ agonists (Rosi 50 μM and PG-J2 10 mM) decreased protein expression (ROCKII) and activity (p-MYPT-1-to-MYPT-1 ratio) in normal and PPHN PASMC (A). In normal PASMC, PPARγ siRNA increased ROCK protein expression (ROCKII) and activity (p-MYPT-1-to-MYPT-1 ratio) (B). For all figures representative blots are shown.
Effect of ROCK inhibition on PPARγ activity and proliferation in normal and PPHN PASMC.
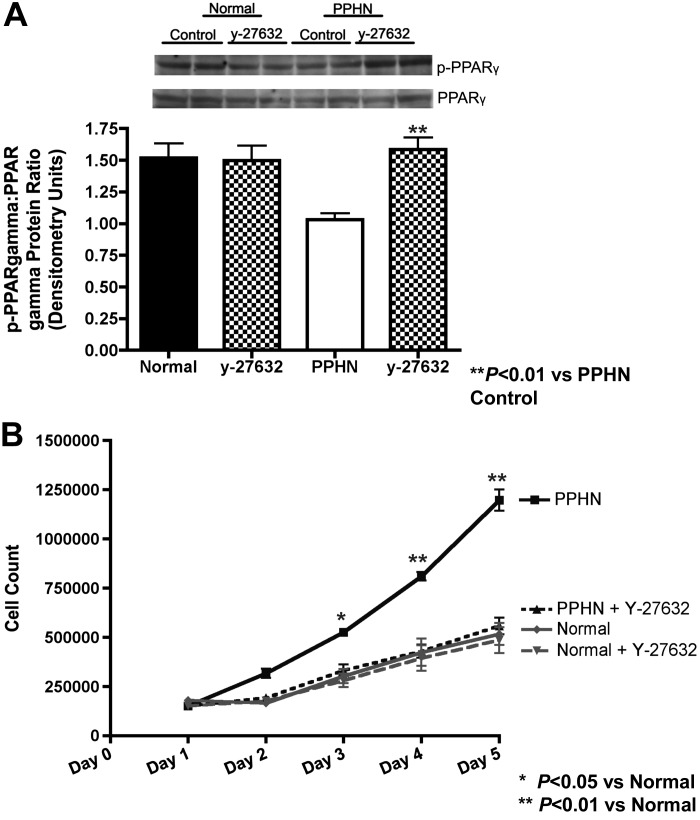
PPARγ activity was measured in whole cell lysates from normal and PPHN fetal PASMC in the presence and absence of ROCK inhibitor (y-27632, 5 μM) (Fig. 6A). At baseline, PPARγ activity (p-PPARγ-to-PPARγ ratio) was decreased by 32% (P < 0.01) in PPHN PASMC and increased with y-27632 treatment. Y-27632 increased PPARγ activity by 54% in PPHN PASMC (Fig. 6A), restoring PPARγ activity to normal values. ROCK inhibition had no effect on PPARγ activity in normal PASMC. Growth of normal and PPHN PASMC was assessed in the presence and absence of ROCK inhibitor (y-27632) (Fig. 6B). ROCK inhibition decreased growth of PPHN PASMC by 37% on day 3 (P < 0.05), 47% on day 4 (P < 0.01) and 53% on day 5 P < 0.01 (Fig. 6B). Y-27632 restored proliferation of PPHN PASMCs to similar values seen in normal untreated controls. Growth of normal PASMC was unchanged in response to ROCK inhibition.
Fig. 6.
Effect of ROCK inhibition on PPARγ activity and proliferation in normal and PPHN PASMC. At baseline PPARγ activity was decreased in PPHN PASMCs and increased with ROCK inhibition [y-27632 (5 mM)], restoring PPARγ activity to normal values (A). For all figures representative blots are shown. ROCK inhibition decreased growth of PPHN PASMC (B) and restored proliferation of PPHN PASMC to similar values seen in normal untreated controls. PPARγ activity and growth in normal PASMC was unchanged in response to ROCK inhibition.
Effect of ROCK activation on PPARγ activity in normal and PPHN PASMC.
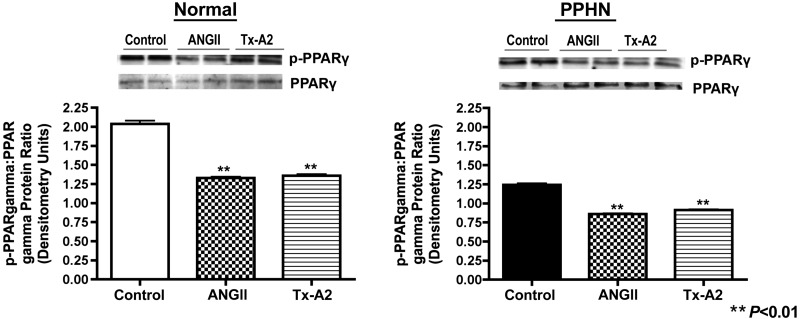
The effect of ROCK activation (ANGII, 100 nM, and TXA2, 1 μM) on PPARγ activity was measured by Western blot analysis on whole cell lysates from normal and PPHN fetal PASMC. ANGII and TXA2 decreased PPARγ activity (p-PPARγ-to-PPARγ ratio) in normal PASMC by 35% (P < 0.01) and 33% (P < 0.01) and PPHN PASMC by 31% (P < 0.01) and 27% (P < 0.05), respectively (Fig. 7).
Fig. 7.
Effect of ROCK activation on PPARγ activity in normal and PPHN PASMC. ROCK activation with angiotensin II (ANGII; 100 nM) and thromboxane A2 (TXA2, 1 μM) decreased PPARγ activity (p-PPARγ-to-PPARγ ratio) in normal and PPHN PASMC. For all figures representative blots are shown.
DISCUSSION
Pulmonary hypertensive vascular remodeling with SMC hyperplasia contributes to impaired responsiveness to vasodilator therapy and poor outcomes in PPHN. We found that isolated PASMC from fetal sheep with experimental PPHN demonstrate a higher intrinsic growth rate than PASMC from normal fetal sheep and that this persistent hyperproliferative phenotype is partly due to enhanced ROCK activity. We also report that decreased PPARγ signaling increases ROCK activity, which enhances normal fetal PASMC proliferation. In addition, PPARγ activation decreases ROCK activity in PPHN PASMC and decreases cell growth to similar rates measured in controls. Overall, these findings suggest that PPARγ-ROCK interactions contribute to high intrinsic rates of proliferation in PASMC from experimental PPHN.
Past studies have suggested multifactorial etiologies to the development of vascular remodeling in PPHN. Increased pulmonary blood flow in utero, hypoxia, or hyperoxia result in the release of various mediators including endothelin-1, platelet activating factor, reactive oxygen species, PDGF, transforming growth factor-β (TGF-β), as well as increased RhoA/ROCK activity (17). These mediators act to promote pulmonary vascular remodeling and SMC growth. Other mediators may inhibit vascular remodeling such as the NO/cGMP, VEGF, and PPARγ pathways (17). Thus stimuli suppressing these inhibitory signaling mechanisms also contribute to pulmonary vascular remodeling (17). The balance between stimulatory/ROCK-mediated and inhibitory/PPARγ-mediated pathways is essential for the prevention of vascular remodeling in PPHN; however, little is known about how these signaling pathways interact and their relative contribution to the development of vascular remodeling in PPHN.
Prior studies of isolated PASMC in PH have addressed whether changes in PASMC proliferation contribute to the development of vascular remodeling. PASMC from adult patients with idiopathic and inherited PH have demonstrated increased proliferation under basal conditions in vitro (13, 16) and treatment with PPARγ agonist Rosi restored PASMC growth to normal (16). Similarly, PASMC isolated from fawn-hooded rats, which spontaneously develop PH with vascular remodeling after exposure to mild hypoxia, also demonstrate increased PASMC proliferation in vitro (25). ROCK inhibition in this model prevents the development of vascular remodeling and PH (38). ROCK and PPARγ are key regulators of PASMC proliferation (2, 9, 52), and prior studies of ROCK and PPARγ in adult models of experimental PH have demonstrated that excessive ROCK activation and decreased PPARγ signaling contribute to the pathogenesis of vascular remodeling (2, 3, 10, 11, 21). In these models, chronic ROCK inhibition and activation of PPARγ improved survival and prevented the development of vascular remodeling and PH (2, 3, 10, 11, 40). These studies confirm the contribution of ROCK and PPARγ to the development of vascular remodeling in adult PH as well as their importance in the regulation of SMC proliferation.
To date there are no human studies evaluating whether changes in ROCK and PPARγ expression contribute to the development of neonatal PPHN. Although adult studies clearly implicate ROCK and PPARγ in the pathogenesis of PH, in neonates the contribution of these cell-signaling pathways to the development of PPHN is less clear. Bleomycin exposure in neonatal rat pups increases ROCK protein expression and activity, resulting in increased medial thickening and right ventricular hypertrophy (35), and ROCK inhibition prevents the development of vascular remodeling and PH (30). In our study we found increased ROCK protein expression and activity in PPHN PASMC, as well as rescue of the hyperproliferative PASMC phenotype in vitro with ROCK inhibition, confirming the importance of ROCK in regulating PASMC proliferation and its contribution to vascular remodeling in PPHN. Neonatal studies evaluating the efficacy of PPARγ agonists have focused on these agents in experimental rodent models of bronchopulmonary dysplasia (39, 44), demonstrating improved alveolarization in these models (39, 44), with less effect on preventing vascular remodeling (39). In our study we found decreased PPARγ protein expression in PPHN PASMC and confirm the importance of PPARγ signaling in regulating PASMC growth. PPARγ silencing in normal PASMC increases PASMC proliferation, producing a phenotype similar to what is seen in PPHN PASMC. Treatment of PPHN PASMC with the PPARγ agonists Rosi and 15d-PG-J2 decreased proliferation of PPHN PASMC, restoring the PPHN PASMC growth characteristics to normal. These results confirm the importance of ROCK and PPARγ in the pathogenesis of pulmonary hypertension in neonates and provides the rationale and background for performing human studies.
Although our findings are consistent with studies of ROCK and PPARγ in adult PH, developmental differences exist with respect to the contribution of ROCK and PPARγ to the pathogenesis of PH. In adult models of pulmonary hypertension due to chronic hypoxia, inhibition of Rho-kinase activity prevents pulmonary hypertension and inhibits angiogenesis (23). Unlike these findings in the adult lung, experimental pulmonary hypertension in fetal sheep is associated with increased PAEC ROCK expression and impaired angiogenesis (19). In this model, ROCK inhibition improves PAEC function and angiogenesis in vitro (19). In addition, ROCK signaling plays an important role in lung branching morphogenesis during development and inhibition of ROCK during critical periods of development may potentiate lung hypoplasia (33). With respect to PPARγ agonists, in rodent models of chronic hypoxia, similar developmental differences exist since Rosi prevented vascular remodeling in the adult but not the neonate (10, 11, 39). To date, animal studies evaluating the safety and efficacy of ROCK inhibitors in vivo, in experimental bronchopulmonary dysplasia, have not demonstrated any adverse effects on neonatal lung development (30). In contrast, the clinical use of PPARγ agonists has been limited by many of its adverse effects and toxicities (7). The developmental differences that exist with respect to the contribution of ROCK and PPARγ to the pathogenesis of PH, as well as the potential toxicities of ROCK inhibitors and PPARγ agonists during critical stages of lung development, make it essential to understand the role ROCK and PPARγ play in the pathogenesis of PPHN in a developmentally relevant model. Our data extend these observations by demonstrating increased PASMC proliferation in the developing lung after prolonged exposure to hemodynamic stress and changes in ROCK and PPARγ signaling that contribute to this in vitro hyperproliferative phenotype. This is the first study in PPHN to demonstrate a change in PASMC phenotype that persists in vitro, after partial ligation of the DA in utero. ROCK activity and protein expression were increased and PPARγ protein expression and activity decreased in PPHN PASMC, and inhibition of ROCK and activation of PPARγ restored proliferation by PPHN PASMC to normal values, suggesting that increased ROCK or decreased PPARγ is responsible for the increased PPHN PASMC proliferation. However, the separate and interactive effects of these two pathways in the regulation of PASMC proliferation in PPHN have not been previously studied.
Both ROCK and PPARγ regulate SMC proliferation (2, 9, 50) and contribute to the pathogenesis of PH (2, 3, 10, 11, 21, 40), but whether these pathways interact to produce vascular remodeling in PPHN is unknown. This is the first study of SMCs isolated from the pulmonary vasculature from an experimental model of PPHN, demonstrating that ROCK-PPARγ interactions contribute to increased PASMC proliferation and vascular remodeling in PPHN. ROCK activity and PASMC proliferation were significantly increased and PPARγ expression significantly decreased in PPHN PASMC and activation of ROCK with ANGII and TXA2 decreased PPARγ expression. ROCK inhibition decreased PPHN PASMC proliferation and increased PPARγ expression in PPHN but not normal PASMC, restoring proliferation by PPHN PASMC to normal values. Since ROCK and PPARγ are important regulators of PASMC proliferation, the possibility exists that the phase of the cell cycle is responsible for determining the expression of ROCK and PPARγ rather than a pathological process. However, our findings support the hypothesis that increased PASMC ROCK expression and activity are responsible for decreased PPARγ signaling and increased PPHN PASMC proliferation since the effect of ROCK inhibition on PASMC proliferation and PPARγ expression was specific to PPHN PASMC, decreasing the likelihood that ROCK and PPARγ expression was determined by the proliferative phase of the cell. Published reports have implicated ANGII as important regulator of PPARγ expression in vascular SMCs, through secretion of TGF-β1 and via phosphorylation of p38 mitogen-activated protein kinase and histone deacetylase 3 (21, 46). Our study and others confirm the importance of ANGII in the regulation of ROCK protein expression and activity (5a) and suggest that ANGII, through activation of ROCK and secretion of TGF-β1, is responsible for decreased PPARγ expression and the link between ROCK and PPARγ in PPHN PASMC.
Prior reports suggest that PPARγ may play an important role in the regulation of ROCK (5a, 49). In isolated aortic SMC, pioglitazone (PPARγ agonist) prevented activation of ROCK at baseline and in response to ROCK agonists (ANGII) (5a). Wakino et al. (49) expanded on this in vitro finding demonstrating the efficacy of pioglitazone, in decreasing ROCK activity in vivo, in spontaneously hypertensive rats (SHR). The SHR develop systemic hypertension that occurs in the setting of increased ROCK activity. The PPARγ agonist pioglitazone decreased ROCK activity and restored blood pressure to normal (49). Contrasting this observation, Crossno et al. (11) demonstrated the efficacy of PPARγ agonists in preventing vascular remodeling and right ventricular hypertrophy after chronic hypoxia but still found equal elevations in mean pulmonary arterial pressure. Fasudil (ROCK inhibitor) acutely normalized pulmonary arterial pressure, suggesting that PPARγ agonists were ineffective in preventing ROCK-mediated vasoconstriction. Our studies confirm cross talk between PPARγ and ROCK as silencing of PPARγ with siRNA, increased ROCK activity, and activation of PPARγ decreased ROCK activity. These findings support prior observations and suggest a role for PPARγ agonists in regulating ROCK activity (5a, 49). Because concerns exist with respect to the side effects of PPARγ agonists, inhibition of ROCK may be an effective strategy for modulating PPARγ activity, restoring PASMC proliferation to normal and preventing vascular remodeling in PPHN.
Potential limitations of this study include the use of fetal PASMC harvested from relatively large vessels and that differences may exist in the behavior of these cells compared with more distal PASMCs. Although more distal PASMC may better represent where vascular remodeling occurs during development, studying organ-specific cells from a developmentally relevant model of PPHN and the use of multiple clones of cells from different animals are strengths of our study. Another potential limitation is the fact that the efficacy of ROCK inhibitors and PPARγ agonists was only measured in vitro. Our in vitro studies provide mechanistic insights into the pathogenesis of vascular remodeling in severe PPHN and lay the foundation for future studies to address whether modulators of these pathways can prevent vascular remodeling in vivo in experimental PPHN. Because controversy exists as to the efficacy of PPARγ agonists in preventing vascular remodeling after exposure to chronic hypoxia in neonatal rats pups, future experiments exploring whether chronic inhibition of ROCK or activation of PPARγ can prevent vascular remodeling in experimental PPHN in vivo are crucial. To date there are no studies of PPARγ and ROCK in human neonatal tissue; this study provides the rationale for pursuing these important cell-signaling pathways in future studies of human tissues. The use of serum as well as passaged cells for the cell proliferation and molecular studies may potentially produce a hyperproliferative PASMC phenotype in vitro that differs significantly from the in vivo phenotype. This experimental model of PPHN is characterized by vascular remodeling in vivo, and this study provides insights into mechanisms responsible for vascular remodeling in this model. Although the use of serum is a limitation of the study, despite identical cell culture conditions, striking phenotypic differences exist between normal and PPHN PASMC with respect to cellular function and cell-signaling pathways. The possibility exists that the effect of PPARγ agonists on PASMC signaling and cellular function are secondary to off-target effects, rather than enhancement of PPARγ signaling. At the doses utilized, preliminary studies demonstrated enhanced PPARγ activity with both Rosi and 15d-PG-J2. Furthermore we demonstrate similar effects on cell signaling and function with the use of the two agents Rosi and 15d-PG-J2, minimizing the possibility that the effect of these agents was secondary to off-target effects.
In conclusion, we found that PASMC from fetal sheep with PPHN have high intrinsic rates of proliferation and that high ROCK activity is partly responsible for this persistent hyperproliferative phenotype in vitro. In addition, we found that PPARγ activation decreases ROCK activity and PASMC proliferation and restores proliferation by PPHN PASMC to growth rates that are similar to normal fetal PASMC and that inhibition of PPARγ signaling in normal PASMC increases ROCK activity and enhances proliferation. Overall, these findings suggest that cross talk between ROCK and PPARγ signaling pathways play a key role in the regulation of fetal PASMC growth and contributes to high proliferation of PPHN PASMC. We speculate that therapies aimed at inhibition of ROCK or activation of PPARγ would be effective in preventing vascular remodeling and improving outcomes in severe PPHN.
GRANTS
This study was supported by Entelligence Young Investigator Award and by National Heart, Lung, and Blood Institute Grants 5K08HL102261 and R01 HL068702-05A2.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.G., N.T., and G.J.S. conception and design of research; J.G., N.T., G.J.S., and G.B.R. performed experiments; J.G., G.J.S., and S.H.A. analyzed data; J.G., N.T., G.B.R., and S.H.A. interpreted results of experiments; J.G. prepared figures; J.G. drafted manuscript; J.G. and S.H.A. edited and revised manuscript; J.G. and S.H.A. approved final version of manuscript.
REFERENCES
- 1.Abbott BD, Wood CR, Watkins A, Das KP, Lau CS. peroxisome proliferator-activated receptors alpha, beta, and gamma mRNA and protein expression in human fetal tissues. PPAR Res 2010: 1–19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, Hattori T, Nakashima Y, Kaibuchi K, Sueishi K, Takeshita A. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res 94: 385–393, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Abe K, Tawara S, Oi K, Hizume T, Uwatoku T, Fukumoto Y, Kaibuchi K, Shimokawa H. Long-term inhibition of Rho-kinase ameliorates hypoxia-induced pulmonary hypertension in mice. J Cardiovasc Pharmacol 48: 280–285, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Abman SH, Shanley PF, Accurso FJ. Failure of postnatal adaptation of the pulmonary circulation after chronic intrauterine pulmonary hypertension in fetal lambs. J Clin Invest 83: 1849–1858, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ameshima S, Golpon H, Cool CD, Chan D, Vandivier RW, Gardai SJ, Wick M, Nemenoff RA, Geraci MW, Voelkel NF. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res 92: 1162–1169, 2003 [DOI] [PubMed] [Google Scholar]
- 5a.Atkins KB, Irey B, Xiang N, Brosius FC., 3rd A rapid, PPAR-γ-dependent effect of pioglitazone on the phosphorylation of MYPT. Am J Physiol Cell Physiol 296: C1151–C1161, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bi D, Nishimura J, Niiro N, Hirano K, Kanaide H. Contractile properties of the cultured vascular smooth muscle cells: the crucial role played by RhoA in the regulation of contractility. Circ Res 96: 890–897, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Bortolini M, Wright MB, Bopst M, Balas B. Examining the safety of PPAR agonists-current trends and future prospects. Expert Opin Drug Saf 12: 65–79, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Bussemaker E, Pistrosch F, Forster S, Herbrig K, Gross P, Passauer J, Brandes RP. Rho kinase contributes to basal vascular tone in humans: role of endothelium-derived nitric oxide. Am J Physiol Heart Circ Physiol 293: H541–H547, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Chapados R, Khotaro A, Ihida-Stansbury K, McKean D, Gates AT, Kern M, Merklinger S, Elliott J, Plant A, Shimokawa H, Jones PL. ROCK controls matrix synthesis in vascular smooth muscle cells: coupling vasoconstriction to vascular remodeling. Circ Res 99: 837–844, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Crossno JT, Jr, Morris KG, Jr, Klemm DJ. Attenuation of hypoxia-induced pulmonary artery remodeling by a peroxisome proliferator-activated receptor-gamma agonist. Chest 128, Suppl 6: 580S, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Crossno JT, Jr, Garat CV, Reusch JE, Morris KG, Dempsey EC, McMurtry IF, Stenmark KR, Klemm DJ. Rosiglitazone attenuates hypoxia-induced pulmonary arterial remodeling. Am J Physiol Lung Cell Mol Physiol 292: L885–L897, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 20: 649–688, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Dewachter L, Adnot S, Guignabert C, Tu L, Marcos E, Fadel E, Humbert M, Dartevelle P, Simonneau G, Naeije R, Eddahibi S. Bone morphogenetic protein signaling in heritable versus idiopathic pulmonary hypertension. Eur Respir J 34: 1100–1110, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Downing GJ, Thibeault DW. Pulmonary vasculature changes associated with idiopathic closure of the ductus arteriosus and hydrops fetalis. Pediatr Cardiol 15: 71–75, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahlit W. Control of the peroxisomal β-oxidation pathway by a novel family of nuclear hormone receptors. Cell 68: 879–887, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Falcetti E, Hall SM, Phillips PG, Patel J, Morrell NW, Haworth SG, Clapp LH. Smooth muscle proliferation and role of the prostacyclin (IP) receptor in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 182: 1161–1170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Y, Raj JU. Regulation of the pulmonary circulation in the fetus and newborn. Physiol Rev 90: 1291–1335, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Gien J, Seedorf GJ, Balasubramaniam V, Markham N, Abman SH. Intrauterine pulmonary hypertension impairs angiogenesis in vitro: role of vascular endothelial growth factor-nitric oxide signaling. Am J Respir Crit Care Med 176: 1146–1153, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gien J, Seedorf GJ, Balasubramaniam V, Tseng N, Markham N, Abman SH. Chronic intrauterine pulmonary hypertension increases endothelial cell Rho kinase activity and impairs angiogenesis in vitro. Am J Physiol Lung Cell Mol Physiol 295: L680–L687, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman AP, Tasker RC, Haworth SG, Sigston PE, Macrae DJ. Four patterns of response to inhaled nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics 98: 706–713, 1996 [PubMed] [Google Scholar]
- 21.Gong K, Xing D, Li P, Aksut B, Ambalavanan N, Yang Q, Nozell SE, Oparil S, Chen YF. Hypoxia induces downregulation of PPAR-γ in isolated pulmonary arterial smooth muscle cells and in rat lung via transforming growth factor-β signaling. Am J Physiol Lung Cell Mol Physiol 301: L899–L907, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest 118: 1846–1857, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyvelin JMHK, Nichol A, Costello CM, Preston RJ, McLoughlin P. Inhibition of Rho-kinase attenuates hypoxia-induced angiogenesis in the pulmonary circulation. Circ Res 97: 185–191, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347: 645–649, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Janakidevi K, Tiruppathi C, Del Vecchio PJ, Pinheiro JMB, Malik AB. Growth characteristics of pulmonary artery smooth muscle cells from fawn-hooded rats. Am J Physiol Lung Cell Mol Physiol 268: L465–L470, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Je HD, Park SY, Barber AL, Sohn UD. The inhibitory effect of rosiglitazone on agonist-induced or spontaneous regulation of contractility. Arch Pharmacol Res (Seoul) 30: 461–468, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Keller RL, Tacy TA, Hendricks-Munoz K, Xu J, Moon-Grady AJ, Neuhaus J, Moore P, Nobuhara KK, Hawgood S, Fineman JR. Congenital diaphragmatic hernia: endothelin-1, pulmonary hypertension, and disease severity. Am J Respir Crit Care Med 182: 555–561, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laudet V, Hanni C, Coll J, Catzefilis F, Stehelin D. Evolution of the nuclear receptor gene superfamily. EMBO J 11: 1003–1013, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee AH, Dhaliwal R, Kantores C, Ivanovska J, Gosal K, McNamara PJ, Letarte M, Jankov RP. ROCK inhibitor prevents bleomycin-induced injury in neonatal rats independent of effects on lung inflammation. Am J Respir Cell Mol Biol 2013. August 15 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Levin DL, Heymann MA, Kitterman JA, Gregory GA, Phibbs RH, Rudolph AM. Persistent pulmonary hypertension of the newborn infant. J Pediatr 89: 626–630, 1976 [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Tian XY, Mao G, Fang X, Fung ML, Shyy JY, Huang Y, Wang N. Peroxisome proliferator-activated receptor-γ ameliorates pulmonary arterial hypertension by inhibiting 5-hydroxytryptamine 2B receptor. Hypertension 60: 1471–1478, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Lohn M, Steioff K, Bleich M, Busch AE, Ivashchenko Y. Inhibition of Rho-kinase stimulates nitric oxide-independent vasorelaxation. Eur J Pharmacol 507: 179–186, 2005 [DOI] [PubMed] [Google Scholar]
- 34.McMurtry IF, Bauer NR, Fagan KA, Nagaoka T, Gebb SA, Oka M. Hypoxia and Rho/Rho-kinase signaling. Lung development versus hypoxic pulmonary hypertension. Adv Exp Med Biol 543: 127–137, 2003 [PubMed] [Google Scholar]
- 35.McNamara PJ, Murthy P, Kantores C, Teixeira L, Engelberts D, van Vliet T, Kavanagh BP, Jankov RP. Acute vasodilator effects of Rho-kinase inhibitors in neonatal rats with pulmonary hypertension unresponsive to nitric oxide. Am J Physiol Lung Cell Mol Physiol 294: L205–L213, 2008 [DOI] [PubMed] [Google Scholar]
- 36.McQueston JA, Kinsella JP, Ivy DD, McMurtry IF, Abman SH. Chronic pulmonary hypertension in utero impairs endothelium-dependent vasodilation. Am J Physiol Heart Circ Physiol 268: H288–H294, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Murphy JD, Vawter GF, Reid LM. Pulmonary vascular disease in fatal meconium aspiration. J Pediatr 104: 758–762, 1984 [DOI] [PubMed] [Google Scholar]
- 38.Nagaoka T, Gebb SA, Karoor V, Homma N, Morris KG, McMurtry IF, Oka M. Involvement of RhoA/Rho kinase signaling in pulmonary hypertension of the fawn-hooded rat. J Appl Physiol 100: 996–1002, 2006 [DOI] [PubMed] [Google Scholar]
- 38a.Neonatal Inhaled Nitric Oxide Study Group (NINOS) Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. Pediatrics 99: 838–845, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Nicola T, Ambalavanan N, Zhang W, James ML, Rehan V, Halloran B, Olave N, Bulger A, Oparil S, Chen YF. Hypoxia-induced inhibition of lung development is attenuated by the peroxisome proliferator-activated receptor-γ agonist rosiglitazone. Am J Physiol Lung Cell Mol Physiol 301: L125–L134, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol 42: 482–490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Parker TA, Roe G, Grover TR, Abman SH. Rho kinase activation maintains high pulmonary vascular resistance in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 291: L976–L982, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Porta NF, Steinhorn RH. Pulmonary vasodilator therapy in the NICU: inhaled nitric oxide, sildenafil, and other pulmonary vasodilating agents. Clin Perinatol 39: 149–164, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rehan VK, Wang Y, Patel S, Santos J, Torday JS. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, prevents hyperoxia-induced neonatal rat lung injury in vivo. Pediatr Pulmonol 41: 558–569, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Sluiter I, van der Horst I, van der Voorn P, Boerema-de Munck A, Buscop-van Kempen M, de Krijger R, Tibboel D, Reiss I, Rottier RJ. Premature differentiation of vascular smooth muscle cells in human congenital diaphragmatic hernia. Exp Mol Pathol 94: 195–202, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Subramanian V, Golledge J, Heywood EB, Bruemmer D, Daugherty A. Regulation of peroxisome proliferator-activated receptor-γ by angiotensin II via transforming growth factor- β1-activated p38 mitogen-activated protein kinase in aortic smooth muscle cells. Arterioscler Thromb Vasc Biol 32: 397–405, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tourneux P, Chester M, Grover T, Abman SH. Fasudil inhibits the myogenic response in the fetal pulmonary circulation. Am J Physiol Heart Circ Physiol 295: H1505–H1513, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wakino S, Hayashi K, Kanda T, Tatematsu S, Homma K, Yoshioka K, Takamatsu I, Saruta T. Peroxisome proliferator-activated receptor gamma ligands inhibit Rho/Rho kinase pathway by inducing protein tyrosine phosphatase SHP-2. Circ Res 95: e45–e55, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Wang Z, Jin N, Ganguli S, Swartz DR, Li L, Rhoades RA. Rho-kinase activation is involved in hypoxia-induced pulmonary vasoconstriction. Am J Respir Cell Mol Biol 25: 628–635, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Wild LM, Nickerson PA, Morin FC., 3rd Ligating the ductus arteriosus before birth remodels the pulmonary vasculature of the lamb. Pediatr Res 25: 251–257, 1989 [DOI] [PubMed] [Google Scholar]
- 52.Hsueh WA, Jackson S, Law RE. Control of vascular cell proliferation and migration by PPAR-γ: a new approach to the macrovascular complications of diabetes. Diabetes Care 24: 392–393, 2001 [DOI] [PubMed] [Google Scholar]