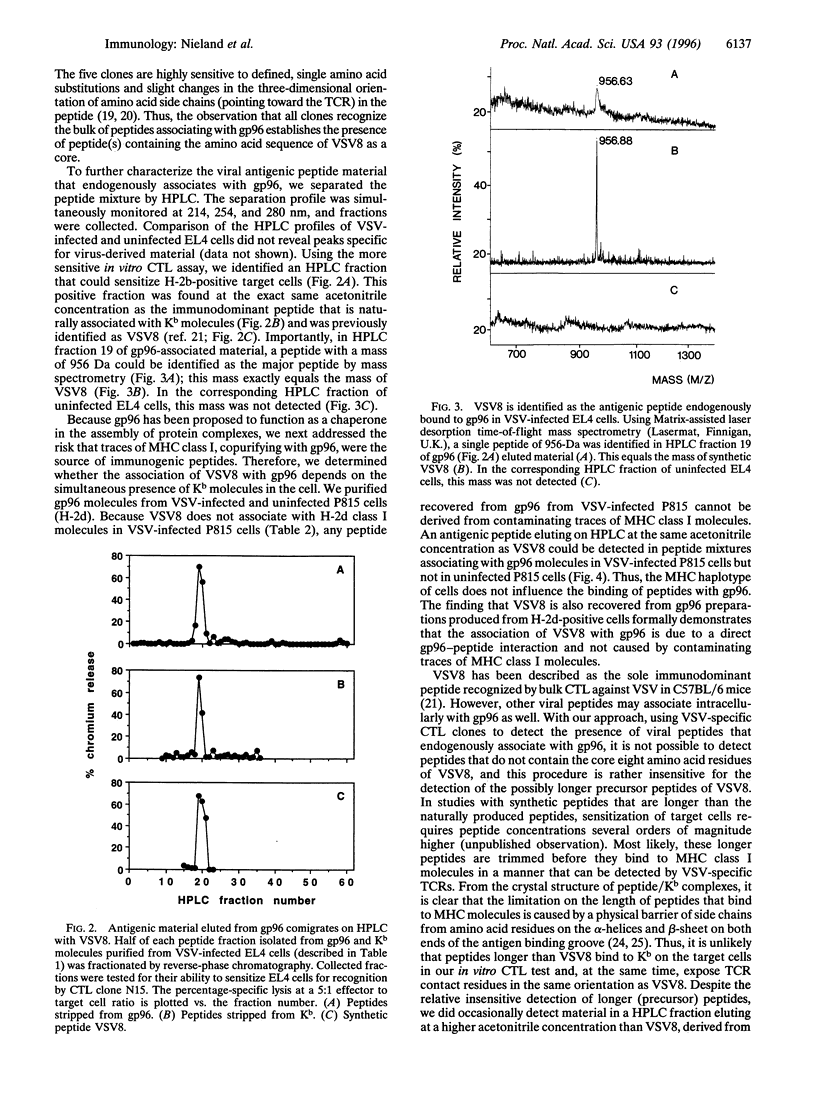
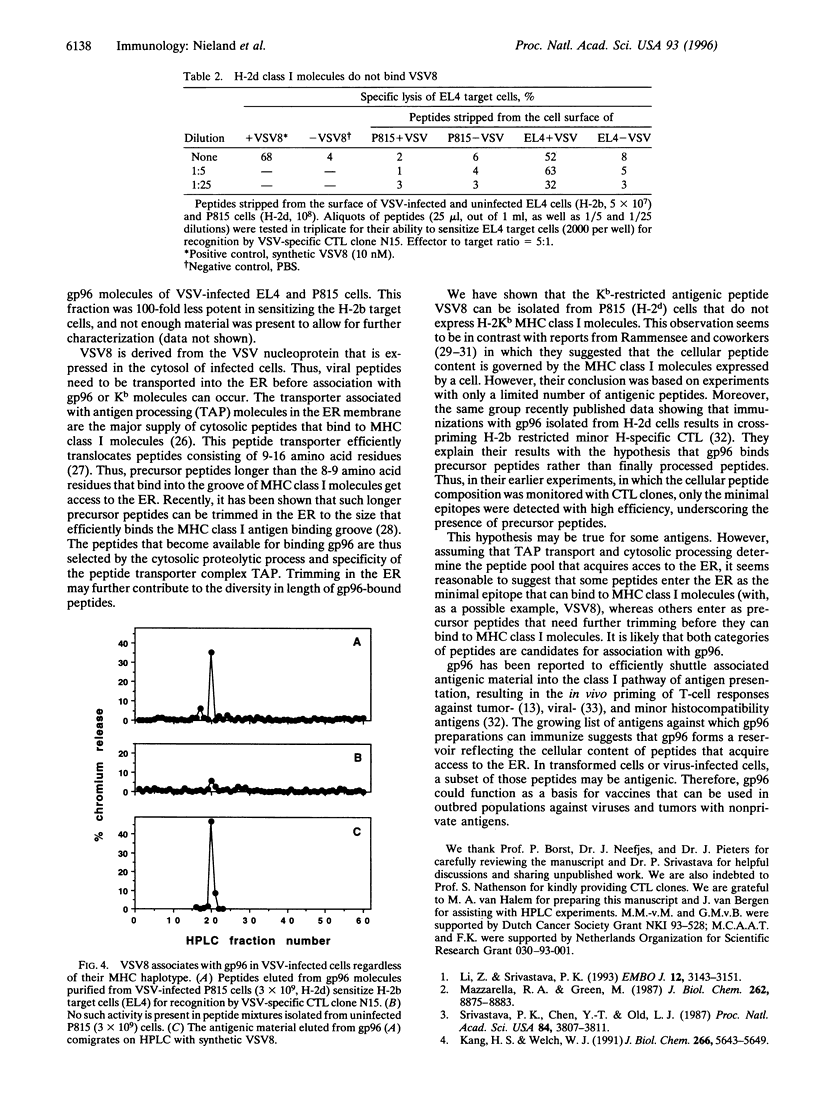
Abstract
Heat shock protein gp96 primes class I restricted cytotoxic T cells against antigens present in the cells from which it was isolated. Moreover, gp96 derived from certain tumors functions as an effective vaccine, causing complete tumor regressions in in vivo tumor challenge protocols. Because tumor-derived gp96 did not differ from gp96 isolated from normal tissues, a role for gp96 as a peptide carrier has been proposed. To test this hypothesis, we analyzed whether such an association of antigenic peptides with gp96 occurs in a well-defined viral model system. Here we present the full characterization of an antigenic peptide that endogenously associates with the stress protein gp96 in cells infected with vesicular stomatitis virus (VSV). This peptide is identical to the immunodominant peptide of VSV, which is also naturally presented by H-2Kb major histocompatibility complex class I molecules. This peptide associates with gp96 in VSV-infected cells regardless of the major histocompatibility com- plex haplotype of the cell. Our observations provide a biochemical basis for the vaccine function of gp96.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold D., Faath S., Rammensee H., Schild H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J Exp Med. 1995 Sep 1;182(3):885–889. doi: 10.1084/jem.182.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T., Willis A., Cerundolo V., Townsend A. Processing of major histocompatibility class I-restricted antigens in the endoplasmic reticulum. J Exp Med. 1995 Apr 1;181(4):1481–1491. doi: 10.1084/jem.181.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Rammensee H. G. Cellular peptide composition governed by major histocompatibility complex class I molecules. Nature. 1990 Nov 15;348(6298):248–251. doi: 10.1038/348248a0. [DOI] [PubMed] [Google Scholar]
- Franksson L., Petersson M., Kiessling R., Kärre K. Immunization against tumor and minor histocompatibility antigens by eluted cellular peptides loaded on antigen processing defective cells. Eur J Immunol. 1993 Oct;23(10):2606–2613. doi: 10.1002/eji.1830231034. [DOI] [PubMed] [Google Scholar]
- Fremont D. H., Matsumura M., Stura E. A., Peterson P. A., Wilson I. A. Crystal structures of two viral peptides in complex with murine MHC class I H-2Kb. Science. 1992 Aug 14;257(5072):919–927. doi: 10.1126/science.1323877. [DOI] [PubMed] [Google Scholar]
- Imarai M., Goyarts E. C., van Bleek G. M., Nathenson S. G. Diversity of T cell receptors specific for the VSV antigenic peptide (N52-59) bound by the H-2Kb class I molecule. Cell Immunol. 1995 Jan;160(1):33–42. doi: 10.1016/0008-8749(95)80006-5. [DOI] [PubMed] [Google Scholar]
- Kang H. S., Welch W. J. Characterization and purification of the 94-kDa glucose-regulated protein. J Biol Chem. 1991 Mar 25;266(9):5643–5649. [PubMed] [Google Scholar]
- Koch G. L., Macer D. R., Wooding F. B. Endoplasmin is a reticuloplasmin. J Cell Sci. 1988 Jul;90(Pt 3):485–491. doi: 10.1242/jcs.90.3.485. [DOI] [PubMed] [Google Scholar]
- Kozutsumi Y., Segal M., Normington K., Gething M. J., Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988 Mar 31;332(6163):462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- Lewis M. J., Turco S. J., Green M. Structure and assembly of the endoplasmic reticulum. Biosynthetic sorting of endoplasmic reticulum proteins. J Biol Chem. 1985 Jun 10;260(11):6926–6931. [PubMed] [Google Scholar]
- Li Z., Srivastava P. K. Tumor rejection antigen gp96/grp94 is an ATPase: implications for protein folding and antigen presentation. EMBO J. 1993 Aug;12(8):3143–3151. doi: 10.1002/j.1460-2075.1993.tb05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machold R. P., Andrée S., Van Kaer L., Ljunggren H. G., Ploegh H. L. Peptide influences the folding and intracellular transport of free major histocompatibility complex class I heavy chains. J Exp Med. 1995 Mar 1;181(3):1111–1122. doi: 10.1084/jem.181.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki R. G., Old L. J., Srivastava P. K. Human homologue of murine tumor rejection antigen gp96: 5'-regulatory and coding regions and relationship to stress-induced proteins. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5658–5662. doi: 10.1073/pnas.87.15.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzarella R. A., Green M. ERp99, an abundant, conserved glycoprotein of the endoplasmic reticulum, is homologous to the 90-kDa heat shock protein (hsp90) and the 94-kDa glucose regulated protein (GRP94). J Biol Chem. 1987 Jun 25;262(18):8875–8883. [PubMed] [Google Scholar]
- Melnick J., Aviel S., Argon Y. The endoplasmic reticulum stress protein GRP94, in addition to BiP, associates with unassembled immunoglobulin chains. J Biol Chem. 1992 Oct 25;267(30):21303–21306. [PubMed] [Google Scholar]
- Melnick J., Dul J. L., Argon Y. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature. 1994 Aug 4;370(6488):373–375. doi: 10.1038/370373a0. [DOI] [PubMed] [Google Scholar]
- Momburg F., Roelse J., Hämmerling G. J., Neefjes J. J. Peptide size selection by the major histocompatibility complex-encoded peptide transporter. J Exp Med. 1994 May 1;179(5):1613–1623. doi: 10.1084/jem.179.5.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino M. A., Jr, Srivastava P. K., Oettgen H. F., DeLeo A. B. Expression of a shared tumor-specific antigen by two chemically induced BALB/c sarcomas. Cancer Res. 1987 Oct 1;47(19):5074–5079. [PubMed] [Google Scholar]
- Ramakrishnan M., Tugizov S., Pereira L., Lee A. S. Conformation-defective herpes simplex virus 1 glycoprotein B activates the promoter of the grp94 gene that codes for the 94-kD stress protein in the endoplasmic reticulum. DNA Cell Biol. 1995 May;14(5):373–384. doi: 10.1089/dna.1995.14.373. [DOI] [PubMed] [Google Scholar]
- Rötzschke O., Falk K., Deres K., Schild H., Norda M., Metzger J., Jung G., Rammensee H. G. Isolation and analysis of naturally processed viral peptides as recognized by cytotoxic T cells. Nature. 1990 Nov 15;348(6298):252–254. doi: 10.1038/348252a0. [DOI] [PubMed] [Google Scholar]
- Schaiff W. T., Hruska K. A., Jr, McCourt D. W., Green M., Schwartz B. D. HLA-DR associates with specific stress proteins and is retained in the endoplasmic reticulum in invariant chain negative cells. J Exp Med. 1992 Sep 1;176(3):657–666. doi: 10.1084/jem.176.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K., Imarai M., van Bleek G. M., Joyce S., Nathenson S. G. Vesicular stomatitis virus antigenic octapeptide N52-59 is anchored into the groove of the H-2Kb molecule by the side chains of three amino acids and the main-chain atoms of the amino terminus. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3135–3139. doi: 10.1073/pnas.89.7.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. K., Chen Y. T., Old L. J. 5'-structural analysis of genes encoding polymorphic antigens of chemically induced tumors. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3807–3811. doi: 10.1073/pnas.84.11.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. K., DeLeo A. B., Old L. J. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc Natl Acad Sci U S A. 1986 May;83(10):3407–3411. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. K., Udono H., Blachere N. E., Li Z. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39(2):93–98. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- Suto R., Srivastava P. K. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995 Sep 15;269(5230):1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- Townsend A., Trowsdale J. The transporters associated with antigen presentation. Semin Cell Biol. 1993 Feb;4(1):53–61. doi: 10.1006/scel.1993.1007. [DOI] [PubMed] [Google Scholar]
- Udono H., Levey D. L., Srivastava P. K. Cellular requirements for tumor-specific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+ T cells in vivo. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3077–3081. doi: 10.1073/pnas.91.8.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udono H., Srivastava P. K. Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90, and hsp70. J Immunol. 1994 Jun 1;152(11):5398–5403. [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Wallny H. J., Rötzschke O., Falk K., Hämmerling G., Rammensee H. G. Gene transfer experiments imply instructive role of major histocompatibility complex class I molecules in cellular peptide processing. Eur J Immunol. 1992 Mar;22(3):655–659. doi: 10.1002/eji.1830220307. [DOI] [PubMed] [Google Scholar]
- Wiech H., Buchner J., Zimmermann R., Jakob U. Hsp90 chaperones protein folding in vitro. Nature. 1992 Jul 9;358(6382):169–170. doi: 10.1038/358169a0. [DOI] [PubMed] [Google Scholar]
- Zhang W., Young A. C., Imarai M., Nathenson S. G., Sacchettini J. C. Crystal structure of the major histocompatibility complex class I H-2Kb molecule containing a single viral peptide: implications for peptide binding and T-cell receptor recognition. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8403–8407. doi: 10.1073/pnas.89.17.8403. [DOI] [PMC free article] [PubMed] [Google Scholar]