Abstract
Quantum dot (QD) imaging is a powerful tool for studying signaling pathways as they occur. Here we employ this tool to study adhesion molecule expression with lung inflammation in vivo. A key event in pulmonary inflammation is the regulation of vascular endothelial cell adhesion molecule-1 (VCAM), which drives activated immune cell adherence. The induction of VCAM expression is known to be associated with reactive oxygen species (ROS) production, but the exact mechanism or the cellular source of ROS that regulates VCAM in inflamed lungs is not known. NADPH oxidase 2 (NOX2) has been reported to be a major source of ROS with pulmonary inflammation. NOX2 is expressed by both endothelial and immune cells. Here we use VCAM-targeted QDs in a mouse model to show that NOX2, specifically endothelial NOX2, induces VCAM expression with lung inflammation in vivo.
Keywords: nicotinamide adenine dinucleotide phosphate oxidase 2, platelet endothelial cell adhesion molecule-1, lipopolysaccharide, reactive oxygen species, redox signaling
the onset of lung inflammation is characterized by activation and recruitment of neutrophils from systemic circulation into the lung. Neutrophils circulating in the blood first attach to the lung endothelium and then migrate through the vascular wall into the tissue where they release reactive oxygen species (ROS) required for clearance of tissue invasive bacteria. Crucial to this entire process is the initial association and adherence of neutrophils to the lung endothelium that reportedly occurs via low-affinity adhesion molecules (9, 11, 25). Vascular cell adhesion molecule-1 (VCAM) is one of the inducible cell adhesion molecules that regulate this association (17). VCAM, a 110-kDa member of the immunoglobulin gene superfamily, is not constitutively expressed on endothelial cells. VCAM is central to lung inflammation, since it both recruits and anchors phagocytes (i.e., monocytes, macrophages, neutrophils, and mast cells, among others) to the pulmonary endothelium (16, 24). These recruited cells release oxidants that further amplify injury and inflammation. This then leads to an exaggerated inflammatory response, and potentially causes endothelial damage and eventual pulmonary dysfunction (23). The contribution of VCAM to the inflammatory process is thought to result from its induction following exposure to inflammatory stimuli. However, the mechanisms underlying VCAM induction during inflammation in pulmonary endothelium are still unclear.
Studies on lung inflammation using bacterial toxins, such as lipopolysaccharide (LPS), show that NADPH oxidases (NOX) regulate key inflammatory signal transduction pathways involved in lung inflammation (12, 16, 28). Of the large NOX family, comprising NOX1–5 and Dual oxidase 1–2, NOX2 appears to be the major source for inflammation-associated ROS production (13, 22, 30). Until recently, the source of activated NOX2 with inflammation was thought to be polymorphonuclear leukocytes and neutrophils (3, 18, 30, 35), but recent evidence points to a role for NOX2 from other cell types such as endothelial cells (6). The cellular sources for NOX2, either leucocytes or endothelium, that participate in induction of VCAM expression are undetermined (18). While endothelial NOX2 appears to be activated during lung inflammation, the physiological significance of this remains unclear (6, 19). Thus, the aim of the present study is to investigate the role of NOX2 in the regulation of VCAM expression during pulmonary inflammation using models with LPS administered in vitro and in vivo.
In contrast to studies where VCAM expression was monitored in lung homogenate or fixed lung sections, both of which do not provide spatial information (32, 34, 37), we chose quantum dot (QD) technology, wherein fluorescent QDs functionalized with antibodies toward adhesion molecules were used to detect VCAM expression in vitro and in vivo. Here we created QDs modified with endothelial-specific antibodies, either platelet endothelial cell adhesion molecule-1 (PECAM) as a stable endothelial marker, or VCAM as a proadhesive endothelial activation marker of lung inflammation in mice.
MATERIALS AND METHODS
All chemicals and materials are from Thermo Fisher Scientific, unless otherwise noted. PECAM null mice were a gift from the DeLisser lab (University of Pennsylvania) (5). LPS (O111:B4), BSA, apocynin, and sephacryl 400-S resin are from Sigma. Antibodies targeting PECAM (MEC 13.3) and VCAM (429) and anti-rat IgG were purchased from BD Biosciences. Antibodies targeting His tag were purchased from Qiagen. Acetylated low-density lipoprotein AlexaFluor488 (AcLDL488) and ITK QD Amino [polyethylene glycol (PEG)] 525s or 655s, along with cell culture reagents and NuPAGE supplies, are from Life Technologies. The protein A/G (pAG) is a product of BioVision. Coupling reagents, sulfo-N-hydroxysuccinimide (sulfo-NHS), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), and Zeba Desalting Columns, were purchased from Thermo Scientific Pierce. Size exclusion centrifugal filters and Amicon Ultra-2 Centrifugal filters were from Millipore. Aqua Polymount was purchased from Polysciences.
QD Coupling Reactions
Conjugation conditions and adapter protein coupling were adapted from previously developed synthesis methods (2, 3, 8, 38). Briefly, one molar equivalent of QDs (525s or 655s, described by Life Technologies literature to be between 10 and 20 nm in diameter) (10) was allowed to stir for ∼10 min at room temperature (RT) with 10 molar equivalents of pAG (1 mg/ml). Approximately 1,000 molar equivalents of sulfo-NHS (2 mg/ml) dissolved in borate buffer were then added and allowed to stir for another 10 min. The QD mixture was then mixed for 3 h with 1,000 molar equivalents of EDC (2 mg/ml) at RT. The conjugates were separated and concentrated using an equilibrated Zeba Desalting Column (2,000 g, 2 min, 3 times) and a 100-kDa MWCO Amicon Ultra-2 Centrifugal filter (2,000 g, 20 min, 3 washes following initial spin concentration). Conjugate concentrations were determined using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific) with designated extinction coefficients for QD525 (ε = 130,000 M−1/cm at 488 nm) and QD655 (ε = 2,900,000 M−1/cm at 488 nm) (10). One molar equivalent of either QD-pAG(525 or 655) was coupled to five molar equivalents of either anti-VCAM or anti-PECAM, respectively, for 1 h with constant stirring. Excess antibody was removed by gravity flow column chromatography using sephacryl 400-S resin with borate buffer (pH 8.5) as vehicle. Fractions were collected, combined, and concentrated using a 100-kDa MWCO Amicon Ultra-2 Centrifugal filter (7,000 g, 20 min, 3 washes following initial spin concentration). Both concentration and conjugation of the QDV and QDP conjugates were determined as described previously, wherein molar extinction coefficients were used to determine concentrations, and electrophoretic migration changes were used to assess the presence of conjugate surface modification (20, 21). QD surface functionalization was assessed by standard procedures. Electrophoretic analyses demonstrated altered conjugate migration through the agarose gel consistent with surface modification. Confirmation of functionalization was shown by detection of the His tag signal associated with the pAG in conjugated QDs. Upon excitation with an ultraviolet source lamp, the QD conjugates displayed characteristic Gaussian-shaped emission spectra with no observable bathochromic shifts.
Animal Use
Animal use was reviewed and approved by the University of Pennsylvania Institutional Animal Care and Use Committee. C57Bl/6 and NOX2 null mice weighing ∼20–25 g were obtained from Jackson Labs (Bar Harbor, ME). All protocols were performed in accordance with the National Institutes of Health Guidelines.
Generation of Transgenic Endothelial-Specific NOX2-Expressing Mice
Endothelial-specific NOX2-expressing mice were bred by our laboratory as described earlier (4). To target NOX2 to the endothelium, a mouse in which human gp91phox was expressed from the endothelial-specific promoter Tie2 was used. These mice showed human gp91phox (NOX2) transgene expression only in the endothelial cell population and mouse gp91phox expression in other tissues. No human NOX2 was detected in the nonendothelial cell population. These mice were gifted to us from Keith M. Channon's laboratory (1). Male endothelial NOX2 expressers were mated with NOX2 null female mice in our animal facility. Because the NOX2 gene is X-linked, the male progeny that carry the transgene have human NOX2 in endothelial cells while all other cells are NOX2 null (i.e., this is a mouse NOX2 null but has human NOX2 expressed in its endothelium).
In these mice PCR showed the presence of the human NOX2 transgene (225-bp product) and mouse mutant (NOX2 null) genes. The following primers were used: for human NOX2 transgene, forward and reverse primers were CTGAGACTCATCCCAGCCAGTGAGGTAG and GTCACACCCTTCGCATCCATTCTCAAGTCAGT, respectively. The expected band size is 225 bp (J. K. Bendall, personal communication). For wild-type (mouse NOX2) and mutant (NOX2 null) genes, the common forward primer was CTAAGAGAAACTCCTCTGCTGTGAAG, and the reverse primers were CGCACTGGAACCCCTGAGAAAGG [for wild type (WT)] and GTTCTAATTCCATCAGAAGCTTATCG (for NOX2 null). As expected these mice generated in our laboratory express human NOX2 in their endothelium but lack NOX2 (human or mouse) expression in their remaining tissues, including neutrophils (4).
Cell Culture Experiments
REN, REN-PECAM, and MS-1 cells were each maintained and propagated in their cell type-specific growth medium under normal conditions. For all experiments, cells were plated on cover slips and allowed to propagate until ∼80% confluent. For assays to detect PECAM, cells were treated with 25 nM QD655 conjugates (QDP, QDr, QD-pAG, and QD) in basal growth medium for 1 h at 37°C to assess QDP specificity for PECAM. MS-1 cells were additionally treated with endothelial marker AcLDL488 (10 μg/ml) simultaneously with QD655 conjugates. For VCAM targeting, MS-1 cells were treated with 10 μg/ml LPS for 24 h in complete growth medium and incubated with 25 nM QD525 conjugates (QDV, QD-pAG, and QD) for 1 h at 37°C, washed three times in DPBS with Ca2+ and Mg2+ (DPBS+), then incubated with 25 nM QDP for 1 h at 37°C. For assays examining VCAM expression in the presence of a NOX2 activity inhibitor (apocynin), MS-1 cells were treated with 100 μM apocynin for 1 h in complete growth medium. This was followed by incubation with LPS for 24 h in the presence of apocynin, followed by QDV and QDP treatment as previously described. For all in vitro experiments, live labeling with QDs was followed by multiple washes (3×, DPBS+), fixation with 4% paraformaldehyde (PFA), washing [3×, DPBS without Ca2+ and Mg2+ (DPBS−)], and staining with nuclei marker DAPI (1:5,000) for 5 min. Finally, cover slips were washed again in DPBS− and mounted on glass slides with Aqua Polymount.
Animal Experiments
QD labeling of lungs ex vivo.
Mice were anaesthetized, and LPS was introduced via intratracheal instillation. Saline was used for surgical shams. Mice were allowed to recover and maintained in the vivarium with access to food and water. At 24 h post-LPS treatment, mice were anesthetized, and the trachea was cannulated. The lungs were ventilated before and during harvesting of the intact lungs and heart. The intact lungs were perfused using phenol red-free medium (RPMI 1640 supplemented with 3% BSA, and filtered with a sterile 0.22-μm filter and warmed to 37°C). QDs or dyes were injected via bolus in the pulmonary artery of the isolated lung. For PECAM detection, both QDP and endothelial dye (AcLDL488) were used. Before injecting AcLDL488 and QDP, the lungs were equilibrated with perfusion buffer. A reservoir with total volume of 60 ml of perfusion buffer was used throughout the experiment. Following equilibration, AcLDL488 (15 μl in 200 μl perfusion buffer) was injected over 10 min via bolus and allowed to perfuse for an additional 10 min. Following this, 25 nM QDP or QD-pAG(655) in 200 μl of perfusion buffer was injected via bolus over 10 min and also allowed to perfuse for an additional 10 min. The lung was maintained under constant ventilation throughout the labeling period. Upon completion of the labeling experiments, both the trachea and pulmonary artery were infused with 4% PFA. The lung was then allowed to continue to fix overnight in 4% PFA at 4°C. Next 2% agarose was instilled in the trachea, and the lung tissue was sectioned using a vibratome to obtain 100- to 150-μm sections. The sections were then washed (3×, DPBS−) and labeled with DAPI (1:5,000) for ∼5 min. Sections were washed three times, again, in DPBS− and mounted on slides with Aqua Polymount. For dual detection of VCAM and PECAM, the lungs were harvested, equilibrated, and ventilated as described for AcLDL488 and QDP. Both QDV and QDP (25 nM of either QDV or QDP in separate 200-μl perfusion buffer aliquots) were injected separately via bolus over 10 min under constant ventilation. Each injection was allowed to perfuse for 10 min before proceeding. The lungs were fixed, sectioned, and stained with DAPI for imaging as described above.
QD labeling of lungs in live animals.
Mice were injected intratracheally with LPS or saline, as previously described above. At 24 h postinstillation, mice were anaesthetized and injected, separately, in the right and left jugular veins, with 1 nM QDV in sterile saline followed by 1 nM QDP in sterile saline after 10 min. At 10 min after the second injection of QDP, the lungs were harvested, flushed with DPBS−, fixed, and processed for imaging as described for ex vivo lungs.
Imaging and Analyses
All tissue sections and cells were imaged using a Zeiss Meta 510 laser scanning confocal and multiphoton microscope with Zeiss 2007 software (Zeiss). DAPI was excited using a 750-nm two-photon excitation source. In cells, the excitation sources were 488 nm for both AcLDL488 and QDV conjugates and 594 nm for QDP conjugates. All QDP conjugates labeling tissue sections were excited at 375 nm. Both AcLDL488 and QDV conjugates were excited in tissue sections using a 488-nm laser. All images were analyzed using ImageJ (26). All data were acquired in triplicate from at least three independent experiments for each condition. For each sample (cells or lungs) images were acquired from several fields since there was some variation within every lung and culture dish. Statistics were performed on the several (at least 3) independent experiments (mice or cells). All statistics and graphing were performed using GraphPad Prism (version 5.0.4) software. The PDB used in the reaction schematic was PDBID:2JWD (27), and was rendered using PyMol software (29).
RESULTS
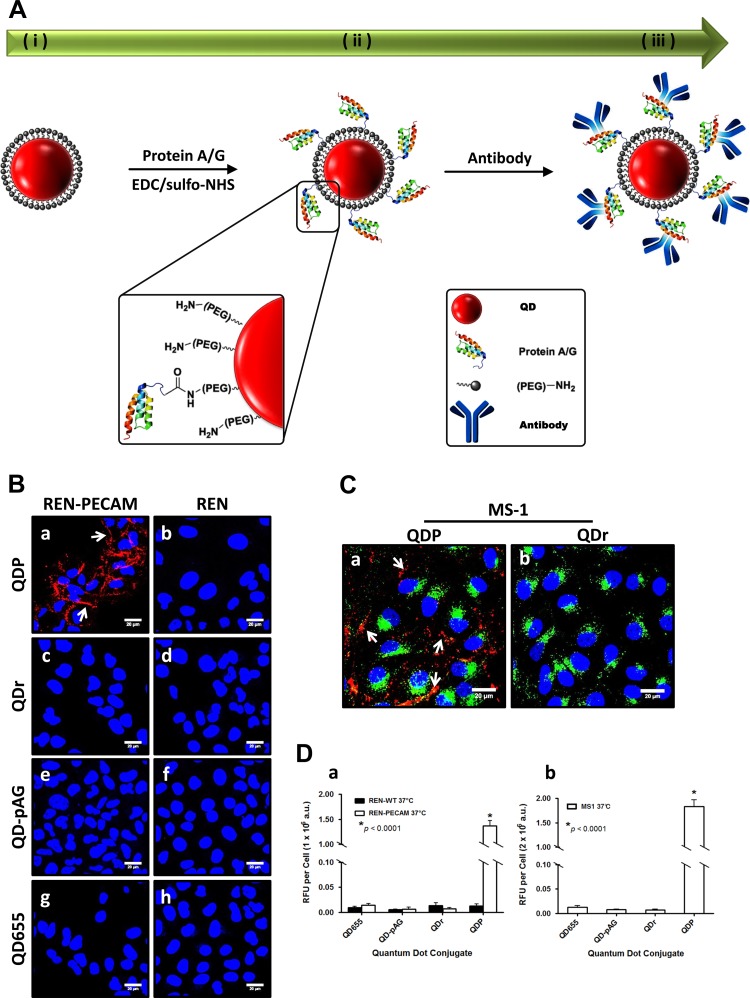
PECAM and VCAM targeting QD conjugate syntheses proceeded by a two-step process (Fig. 1A). QDs were first cross-linked to high-affinity antibody-binding recombinant pAG by EDC/sulfo-NHS amide condensation (QD-pAG). Next, antibodies directed at extracellular epitopes on either PECAM or VCAM were coupled to QD-pAGs. Functionality of QDs was confirmed by standard procedures (see materials and methods). Anti-PECAM QD (QDP) specificity was evaluated in vitro using a high PECAM expression cell line, specifically REN-PECAM (31). REN cells, a PECAM null line, were used as negative controls. REN-PECAM cells treated with QDP (red) showed fluorescence along the cell periphery (7) (Fig. 1Ba). Unconjugated QDs (QD655), QD-pAGs, and nontargeted anti-rat IgG-coupled QDs (QDr) showed no fluorescence in REN-PECAM cells (Fig. 1B, c, e, and g), supporting QDP specificity for PECAM. Similar results were observed in another PECAM-expressing cell line, mouse endothelial MS-1 cells (Fig. 1C). QDP binding was significantly higher in PECAM-expressing cells than nontargeted QD conjugates and indicated QDP was specific for PECAM in vitro (Fig. 1D).
Fig. 1.
Quantum dots (QDs) targeting cell adhesive molecules, platelet endothelial cell adhesion molecule-1 (PECAM) and vascular endothelial cell adhesion molecule-1 (VCAM), enable fluorescence visualization of target proteins in cells. A: schematic representing QD coupling reactions for the creation of targeted QD conjugates. QDP conjugates detect PECAM in PECAM-expressing cells REN-PECAM (B) and MS-1 (C) cells in vitro at 37°C. REN-PECAM and MS-1 cells demonstrate specific labeling by QDP (red) (Ba, Ca), whereas naïve REN cells, which lack PECAM, demonstrate none (Bb). MS-1 cells were simultaneously treated with endothelial-specific dye, acetylated low-density lipoprotein AlexaFluor488 (AcLDL488, green). Arrows indicate PECAM labeled at the cellular junctions by QDP. Nontargeted QDs (QDr, QD-pAG and QD655) show no fluorescence (B, b–h). Blue is DAPI stain showing the nuclei. Fluorescence quantitation of conjugates targeting PECAM expression in each cell line at the specified conditions (D, a and b). EDC, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide; QDP, anti-PECAM QD; QD-pAG, adapter protein functionalized QDs without antibody; QD655, unconjugated QDs; Qdr, nontargeted anti-rat IgG-coupled QDs. Each image is representative of experiments performed in triplicate (*P < 0.0001, ANOVA, means ± SE; scale bar is 20 μm).
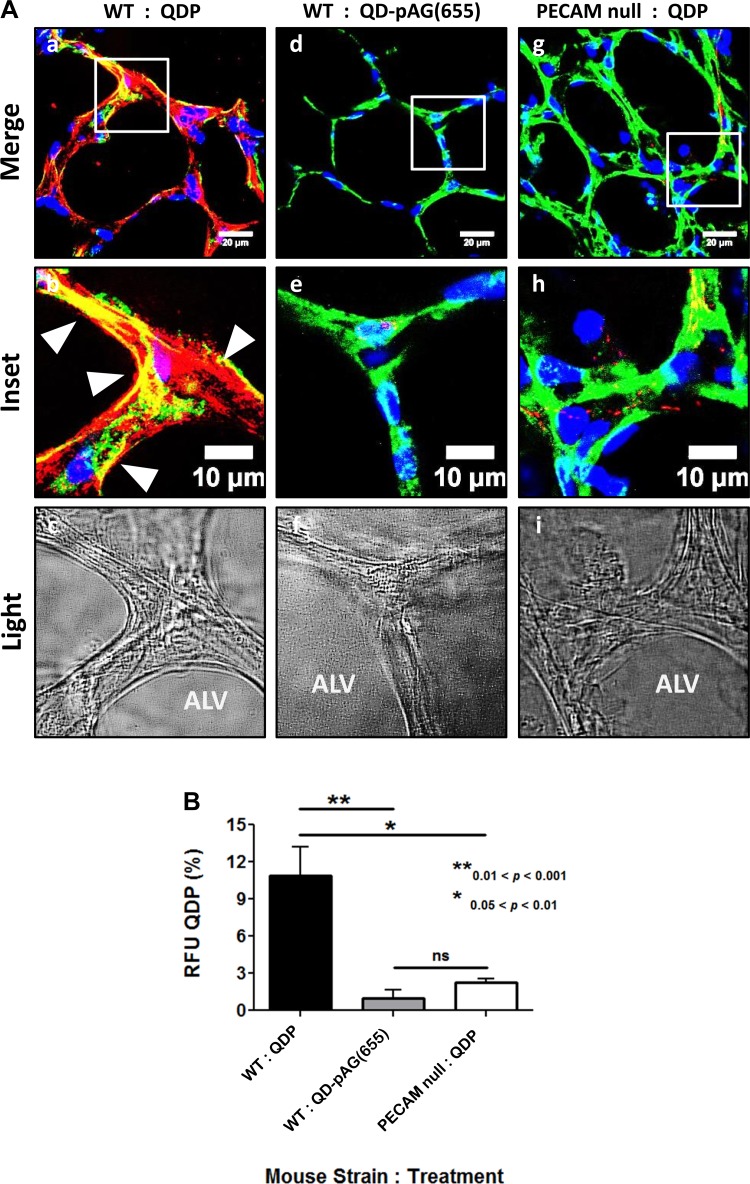
QDP binding efficacy was evaluated by comparing ex vivo lungs from WT and PECAM null mice. Isolated perfused mouse lungs were labeled with QDP and AcLDL488, a green endothelial-specific dye (Fig. 2). AcLDL488 was chosen because it binds scavenger receptors on endothelial cells (33). We observed colocalization between QDP and AcLDL488 in naïve WT lungs (Fig. 2, a and b). Some regions of QDP binding were not correlated with AcLDL488. Lavender et al. (14) reported higher acetylated low density lipoprotein scavenger receptor activity where endothelium lines smooth muscle layers, such as in larger vessels, arteries, and arterioles. PECAM, however, is expressed throughout lung endothelium. Presumably, smaller capillaries and vessels lacking a smooth muscle layer show little AcLDL488 stain, but are still PECAM positive. Very low and random QDP fluorescent signals were observed in PECAM null lungs. These arise possibly from nonspecific QDP adherence (Fig. 2, g and h), which may be due to high concentration of QDP that we chose to use in ex vivo experiments to approach saturation and thus minimize heterogeneous labeling of the endothelium to achieve a complete snapshot of the intricate pulmonary endothelium. Of note, diffuse signals of similar intensity were also observed in the lungs exposed to QD-pAG(655)s lacking anti-PECAM antibodies (Fig. 2, d and e) pointing to a minor degree of nonspecific binding at very high concentrations. However, these signals are markedly lower compared with that observed in WT mouse lungs perfused with QDP, affirming its specificity for PECAM (Fig. 2j). Based on these findings in naïve tissue, we concluded QDPs bind to PECAM in lung endothelium ex vivo.
Fig. 2.
QDP detects PECAM ex vivo. A: lung tissue from either wild-type (WT) or PECAM null mice was labeled with QDP (red) and AcLDL488 (green). Isolated WT mouse lungs, perfused with QDP (A, a and b), show significant PECAM in naïve tissue, whereas PECAM null lungs (A, g and h) lack the red fluorescence signal. Arrowheads highlight areas of colocalization (yellow) between QDP and AcLDL488. Colocalization with endothelial marker AcLDL488 demonstrates PECAM specificity. The nontargeted QD-pAG(655) (A, d and e) showed no fluorescence, indicating minimal nonspecific binding. B: quantitation of QDP signal reveals significantly higher PECAM detection in WT vs. PECAM null ex vivo mouse lungs. Phase-contrast (light) image of the lung section shows the alveolus (Alv) (A, c, f, and i). The regions between the alveoli are the vascularized regions where PECAM expression is observed. Blue is DAPI stain showing the nuclei. NS, not significant. **0.01 < P < 0.001, *0.05 < P < 0.01 ANOVA, Tukey's t-test, mean ± SE; scale bar is 20 μm (a, d, and g) and 10 μm (b–c, e, f, and h–i).
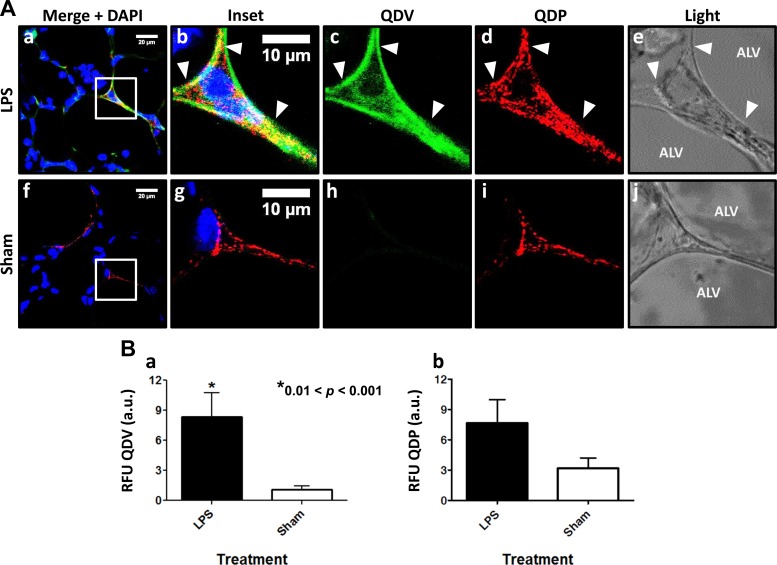
Anti-VCAM QD (QDV) binding was evaluated in an LPS model of lung inflammation. QDVs and QDPs were injected via systemic administration. Lung sections showed fluorescence from both QDV and QDP (Fig. 3A, a–d). QDV (green) detection revealed VCAM induction by LPS challenge. VCAM expression in LPS-treated mice was approximately eightfold higher than in sham animals (Fig. 3Ba). QDP fluorescence was also observed in lungs from LPS-treated mice. Presumably disruption of endothelial cell-cell junctions in these lungs allows better access to PECAM (Fig. 3Ad). Nevertheless, quantification of QDP fluorescence from several sections obtained from each lung and summarizing the data from n = 3 mice showed that PECAM expression in LPS-treated vs. naïve mice was not statistically different (Fig. 3Bb). Colocalization between QDV and QDP provided evidence that both probes remained specific in vivo.
Fig. 3.
QDV detects VCAM in vivo. Lipopolysaccharide (LPS) was instilled in mice it, and 24 h later QDs were injected in vivo. Sham animals were injected with saline followed by QD conjugates 24 h later. A: VCAM expression was observed in LPS-treated (A, a–e) vs. sham controls (A, f–j). Arrowheads highlight areas of colocalization between QDP (red) and QDV (green). Phase-contrast images (A, e and j) show the alveolus and the region between alveoli. B: QDV in LPS-treated mice is 8-fold higher than in sham animals (a). Blue is DAPI stain showing the nuclei. QDP signal in both LPS-treated and sham mice is not statistically different but reveals a slightly higher amount is detected in treated animal tissue possibly due to increased accessibility (b). Values are representative of at least 15–20 sections cut/lung with 3–4 sections randomly selected for imaging and analysis. For each section, 3 microscopic images were obtained *0.01<P < 0.001 (P = 0.0075), 2-tailed unpaired t-test, mean ± SE; scale bar is 10 μm.
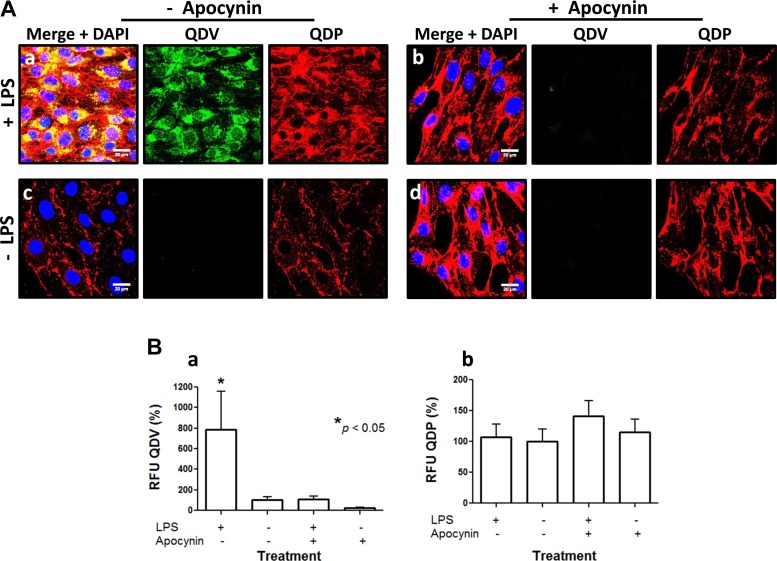
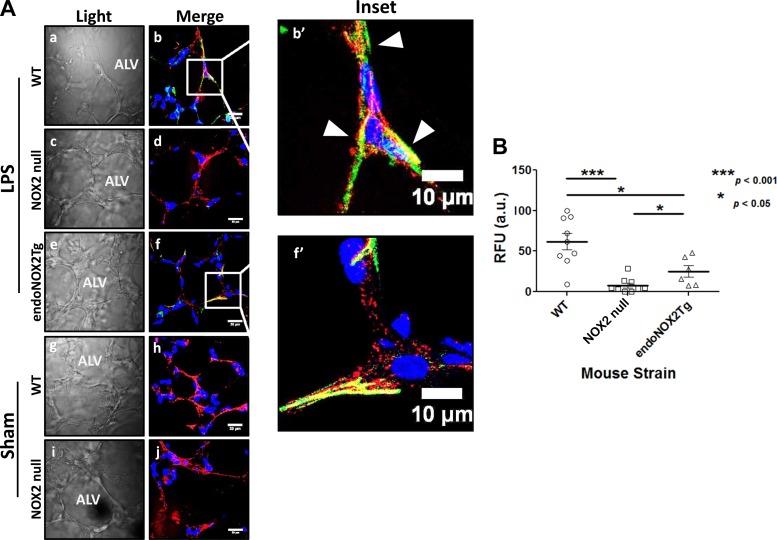
QDV specific binding to VCAM was supported by in vitro experiments. We observed QDV binding in LPS-treated cells but not in untreated cells (Fig. 4A, a–c). Pretreatment with the NOX2 inhibitor apocynin prevented QDV binding to cells challenged with LPS treatment (Fig. 4A). Quantification of the fluorescent signal emanating from live cells showed that QDV increased significantly in LPS-treated cells compared with untreated cells and that pretreatment with apocynin significantly reduced this signal increase. In untreated cells, apocynin alone had no significant effect on QDV expression (Fig. 4B). The QDP signal was not significantly altered in naïve, LPS-challenged, or apocynin-treated cells. However, the QDV signal revealed a direct association between NOX2 activity and VCAM expression in response to the proinflammatory agonist. To further investigate the mechanism of ROS/NOX2 involvement in this response, we used an ex vivo model of isolated perfused lungs from naïve and LPS-challenged mice (Fig. 5). We reasoned that such a model would be better suited to study the mechanistic link between NOX2-produced ROS and VCAM because it would limit the binding of QDs to the lung endothelium without the confounding effects of QD interaction with the systemic endothelium and other nonpulmonary cells. WT inflamed endothelium exhibited an eightfold increased surface expression of VCAM. Colocalization between QDV and QDP, along with no observed fluorescence in interstitial alveoli, indicated VCAM expression was associated with the pulmonary endothelium (Fig. 5A, b and b′). Lungs from NOX2 null showed almost no QDV (green) fluorescence under the same conditions (Fig. 5Ac). Thus, endothelial VCAM expression appeared stunted in the absence of NOX2 activity (Fig. 5B). No VCAM expression, determined by lack of green fluorescence, was detected in the surgical sham lungs (WT, Fig. 5Ah and NOX2 null, Fig. 5Aj).
Fig. 4.
LPS-induced VCAM expression is NADPH oxidase 2 (NOX2) dependent. QDVs reveal that LPS-stimulated VCAM expression in MS-1 cells is attenuated by the NOX2 activity inhibitor apocynin. A: MS-1 cells labeled live with QDP (red) and QDV (green) 24 h post-LPS treatment show PECAM and VCAM labeling in LPS-treated cells (Aa). Arrowheads highlight areas of colocalization (yellow) between QDP and QDV. Pretreatment with 100 μM apocynin 1 h before and during LPS treatment abolishes VCAM induction stimulation. Only QDP fluorescence is observed, indicating only PECAM detection (Ab). QDV failed to detect VCAM in the absence of LPS (A, c and d). Blue is DAPI stain showing the nuclei. B: quantitation of fluorescence signal from the QDs demonstrates the significant increase in QDV-detected VCAM following LPS treatment (a). Apocynin reduced QDV in naïve cells, but it is not significantly different from untreated naïve cells. PECAM detection is unchanged (b). QDV, anti-VCAM QD. *P < 0.05, ANOVA, mean ± SE; scale bar is 20 μm.
Fig. 5.
Endothelial NOX2 activation is sufficient to induce VCAM expression in vivo with LPS treatment. At 24 h posttreatment, lungs were labeled with QDV (green) and QDP (red) ex vivo. Fluorescence signal from WT, NOX2 null, and lung from transgenic mice expressing endothelial NOX2 on a NOX2 null background (endoNOX2Tg) was obtained. Arrowheads highlight areas of colocalization (yellow) between QDP and QDV. Qualitative analyses of fluorescence emanating from QDV indicate LPS-induced VCAM expression in WT (A, b and b′) is higher in stimulated WT tissue than WT surgical sham (Ah) and NOX2 null (A, d and j); however, expression levels of VCAM in endoNOX2Tg lung tissue were intermediate between WT and NOX2 null lungs (A, f and f′). Scale bar is 20 μm (A, a–j) and 10 μm (A, b′ and f′). B: quantitation shows an 8-fold higher incidence of VCAM detection in LPS-stimulated WT vs. sham and NOX2 null, but only a 3-fold higher incidence of VCAM detection in WT over endoNOX2Tg. Blue is DAPI stain showing the nuclei. For each mouse lung at least 15–20 sections were cut of which several sections (4–6) were randomly selected for imaging. For each section, 3 microscopic images were obtained. ***P < 0.001 (P = 0.0003) between WT and NOX2 null, *P < 0.05 (P = 0.0256) between WT and endoNOX2Tg and between NOX2 null and endoNOX2Tg, using 2-tailed Mann-Whitney-Wilcoxon test, mean ± SE.
We employed transgenic mice expressing endothelial NOX2 on a NOX2 null background (endoNOX2Tg) (4) to determine whether the source for VCAM-inducing NOX2-derived ROS is either endothelial or nonendothelial (Fig. 5C, f and f′). LPS-challenged endoNOX2Tg showed significantly higher VCAM expression than NOX2 null, but also significantly lower than WT. In fact, VCAM expression in endoNOX2Tg was intermediate between WT and NOX2 null. Approximately threefold less QDV was present in endoNOX2Tg than LPS-challenged WT (Fig. 5D). This suggested endothelial NOX2 activation in pulmonary endothelium was sufficient to induce VCAM expression due to LPS challenge. These findings point to the role endothelial NOX2 plays in endotoxin-induced VCAM expression.
DISCUSSION
We employed a QD molecular imaging platform to monitor VCAM expression with pulmonary inflammation. The advantage of QDs over standard antibody-based fluorescence staining of fixed tissue is that injection of QDs in vivo or ex vivo allows for visualization of a “snapshot” of VCAM dynamics within the vascular lumen of an inflamed lung. The QDs we used consist of a cadmium selenide (CdSe) semiconductor core (∼10–50 atoms in diameter) with a zinc sulfide (ZnS) capping layer. The core/shell structure is coated with an amphiphilic polymer with an amine-terminated PEG layer, which facilitates conjugation of the adapter protein pAG. The adapter protein was then used to couple antibodies, directed either toward VCAM or PECAM, to the surface of the QDs.
We then used these QDs to detect cellular adhesion molecules in a model of LPS-stimulated pulmonary inflammation ex vivo and in vitro. Studies performed elsewhere on systemic organs have shown that LPS stimulates VCAM, especially in renal inflammation via activation of NOX (15). Our interests focused on the regulation of VCAM expression by NOX2/ROS with lung inflammation. We found that with LPS treatment NOX2 activation plays a major role in VCAM induction since NOX2 deficiency (achieved either by the use of knockouts or by the putative NOX2 inhibitor apocynin) results in lack of increased VCAM expression.
In general, the use of QDs for detection of proteins in vivo is not without caveats. Prime among them are cytotoxicity due to the heavy metal constituents, nonspecific binding due to electrostatic interactions with biological moieties, and characteristic QD blinking behavior (i.e., periods of no emission followed by periods of emission) that may make it technically challenging to track QD locations/movements bound to single proteins in live cells and organs. In this study, we attempted to circumvent cytotoxicity by killing mice injected with QDs within 30 min of exposure; however, the potential for cytotoxicity in long-term tracking of proteins in vivo may pose a problem. Recent reports, though, on nonhuman primates showed almost no adverse response to intravenous injection of CdSe/cadmium sulfide/ZnS QDs and have somewhat reduced these concerns (36). Furthermore, our data revealed little nonspecific binding as ascertained by the use of adapter protein functionalized QDs without antibody, QD-pAGs. Because we did not perform real-time tracking in vivo, characteristic QD blinking was not a factor in our studies.
Based on the QDV fluorescence signal, we concluded VCAM expression is dependent on NOX2 in inflamed lungs. Although neutrophils have long been considered the source of NOX2 in LPS-mediated lung inflammation, endothelial NOX2 activation is now being increasingly accepted as a major contributor to the inflammation process (6, 19). To assess the role of endothelial NOX2 in VCAM regulation, we used endoNOX2Tg mice which express human NOX2 (gp91 subunit) in endothelial cells on a mouse NOX2 null background. Activation of NOX2 requires the assembly of membrane-bound components (NOX2 or gp91phox and p22phox) with cytosolic components (Rac 1, p47phox, p67phox). Work by Bendall et al. (1) shows that endothelial NOX2-overexpressing mice [i.e., mice expressing human and mouse gp91phox in endothelium on a mouse gp91phox (WT) background] showed higher NOX2 activity and superoxide production (in aortic sections) upon stimulation compared with WT mice (mouse gp91phox alone). This indicated that human NOX2 (gp91phox) could assemble with mouse cytosolic components to activate NOX2, since otherwise the ROS production in endothelium of NOX2 overexpressors would be comparable to that produced by WT mouse endothelium. Of note, the homology of human vs. mouse gp91phox (protein or nucleotide) is ∼90%. Basic BLAST program to align the nucleotide and amino acid sequences of human and mouse NOX2 (gp91phox) revealed an ∼88–90% homology in both nucleotides and amino acids.
In the endoNOX2Tg mice, fluorescent signals from QDV demonstrated VCAM expression in lungs with inflammation, indicating that endothelial ROS is sufficient to drive VCAM expression. However, VCAM expression in endoNOX2Tg mice was significantly lower (3-fold lower) compared with WT lungs. This VCAM induction difference could arise from several factors. One possibility is a difference in NOX2 activation between the human and mouse subunits. WT mice possess mouse NOX2 alone, whereas the endoNOX2Tg mice express human NOX2 (and lack mouse NOX2). Another possibility is the potential for nonendothelial NOX2 contribution in WT mice. Higher VCAM expression in endoNOX2Tg compared with sham or NOX2 null animals points to the essential participation by endothelial ROS in the induction of VCAM with inflammation.
We have recently shown that endothelial NOX2 regulates vascular remodeling with systemic ischemia (6). Taken together, our study supports that NOX2, specifically endothelial NOX2, may induce VCAM expression within an LPS model of lung inflammation. Our findings indicate that further investigation into the endothelial NOX2 role in VCAM expression may help in the development of strategies for preventing the progression of pulmonary inflammation.
These results have broader implications in the use of a targeted QD molecular imaging platform as a tool to monitor individual signaling proteins or events in vivo. By providing spatial resolution and sensitivity, targeted QDs can discern cell type and tissue location of the signaling molecules being studied. In addition to binding specificity ex vivo, our QDs reached their targets via systemic administration. This indicates potential application for real-time imaging and monitoring in vivo.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-075587 and NIH-T32-HL-07748-17 and by a McCabe Foundation Award.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.L.O., V.R.M., and S.C. conception and design of research; R.L.O., N.H., K.J.Y., and B.J.Z. performed experiments; R.L.O. and S.C. analyzed data; R.L.O., S.I.F., A.B.F., and S.C. interpreted results of experiments; R.L.O. and S.C. prepared figures; R.L.O. and S.C. drafted manuscript; R.L.O., N.H., K.J.Y., S.I.F., B.J.Z., A.B.F., V.R.M., and S.C. approved final version of manuscript; V.R.M. and S.C. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank the John Morgan mouse housing facility at the University of Pennsylvania and Daniel Gonder for help with maintaining and genotyping the endothelial-specific NOX2-expressing mice. We are grateful to Keith Channon of the University of Oxford for the gift of male NOX2 overexpressing mice and Dr. Horace DeLisser, University of Pennsylvania, for providing the PECAM null mice.
REFERENCES
- 1.Bendall JK, Rinze R, Adlam D, Tatham AL, de Bono J, Channon KM. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res 100: 1016–1025, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bentzen EL, Tomlinson ID, Mason J, Gresch P, Warnement MR, Wright D, Sanders-Bush E, Blakely R, Rosenthal SJ. Surface modification to reduce nonspecific binding of quantum dots in live cell assays. Bioconjugate Chem 16: 1488–1494, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Boots AW, Gerloff K, Bartholomé R, van Berlo D, Ledermann K, Haenen GRMM, Bast A, van Schooten FJ, Albrecht C, Schins RPF. Neutrophils augment LPS-mediated pro-inflammatory signaling in human lung epithelial cells. Biochim Biophys Acta 1823: 1151–1162, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Browning E, Wang H, Hong N, Yu K, Buerk DG, Debolt K, Gonder D, Sorokina E, Patel P, Deleon D, Feinstein SI, Fisher AB, Chatterjee S. Mechanotransduction drives post ischemic revascularization through KATP channel closure and production of reactive oxygen species (Abstract). Antioxid Redox Signal 12: 12, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan GS, Andrew DP, Takimoto H, Kaufman SA, Yoshida H, Spellberg J, de la Pompa JL, Elia A, Wakeham A, Karan-Tamir B, Muller WA, Senaldi G, Zukowski MM, Mak TW. Genetic evidence for functional redundancy of platelet/endothelial cell adhesion molecule-1 (PECAM-1): CD31-deficient mice reveal PECAM-1-dependent and PECAM-1-independent functions. J Immunol 162: 3022–3030, 1999 [PubMed] [Google Scholar]
- 6.Gandhirajan RK, Meng S, Chandramoorthy HC, Mallilankaraman K, Mancarella S, Gao H, Razmpour R, Yang XF, Houser SR, Chen J, Koch WJ, Wang H, Soboloff J, Gill DL, Madesh M. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J Clin Invest 123: 887–902, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garnacho C, Shuvaev V, Thomas A, McKenna L, Sun J, Koval M, Albelda S, Muzykantov V, Muro S. RhoA activation and actin reorganization involved in endothelial CAM-mediated endocytosis of anti-PECAM carriers: critical role for tyrosine 686 in the cytoplasmic tail of PECAM-1. Blood 111: 3024–3033, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldman ER, Anderson GP, Tran PT, Mattoussi H, Charles PT, Mauro JM. Conjugation of luminescent quantum dots with antibodies using an engineered adaptor protein to provide new reagents for fluoroimmunoassays. Anal Chem 74: 841–847, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol 8: 142–152, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Invitrogen Qdot ITK Carboxyl Quantum Dots. [Google Scholar]
- 11.Lafferty E, Qureshi S, Schnare M. The role of toll-like receptors in acute and chronic lung inflammation (Abstract). J Inflammation 7: 57, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Lassègue B, San Martín A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110: 1364–1390, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavender MD, Pang Z, Wallace CS, Niklason LE, Truskey GA. A system for the direct co-culture of endothelium on smooth muscle cells. Biomaterials 26: 4642–4653, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee IT, Shih RH, Lin CC, Chen JT, Yang CM. Role of TLR4/NADPH oxidase/ROS-activated p38 MAPK in VCAM-1 expression induced by lipopolysaccharide in human renal mesangial cells (Abstract). Cell Commun Signal 10: 33, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee IT, Yang CM. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem Pharmacol 84: 581–590, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Liu G, Place AT, Chen Z, Brovkovych VM, Vogel SM, Muller WA, Skidgel RA, Malik AB, Minshall RD. ICAM-1-activated Src and eNOS signaling increase endothelial cell surface PECAM-1 adhesivity and neutrophil transmigration. Blood 120: 1942–1952, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markovic N, McCaig LA, Stephen J, Mizuguchi S, Veldhuizen RAW, Lewis JF, Cepinskas G. Mediators released from LPS-challenged lungs induce inflammatory responses in liver vascular endothelial cells and neutrophilic leukocytes. Am J Physiol Gastrointest Liver Physiol 297: G1066–G1076, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Menden H, Tate E, Hogg N, Sampath V. LPS-mediated endothelial activation in pulmonary endothelial cells: role of Nox2-dependent IKK-beta phosphorylation. Am J Physiol Lung Cell Mol Physiol 304: L445–L455, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orndorff RL, Rosenthal SJ. Neurotoxin quantum dot conjugates detect endogenous targets expressed in live cancer cells. Nano Lett 9: 2589–2599, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Orndorff RL, Warnement MR, Mason JN, Blakely RD, Rosenthal SJ. Quantum dot ex vivo labeling of neuromuscular synapses. Nano Lett 8: 780–785, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Pendyala S, Usatyuk PV, Gorshkova IA, Garcia JG, Natarajan V. Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal 11: 841–860, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol 15: 164–187, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raeburn CD, Calkins CM, Zimmerman MA, Song Y, Ao L, Banerjee A, Meng X, Harken AH. Vascular cell adhesion molecule–1 expression is obligatory for endotoxin-induced myocardial neutrophil accumulation and contractile dysfunction. Surgery 130: 319–325, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Rahman I, MacNee W. Oxidative stress and regulation of glutathione in lung inflammation. Eur Respir J 16: 534–554, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Rasband WS. ImageJ. Bethesda, MD: National Institutes of Health, 1997–2012 [Google Scholar]
- 27.Robertson A, Horne J, Thomas B, Ellisdon AM, Scanlon MJ, Bottomley SP. PDB ID: 2JWD; Polyglutamine length-dependent midfolding is confined to the Poly-Q region. In press [Google Scholar]
- 28.Sato K, Kadiiska MB, Ghio AJ, Corbett J, Fann YC, Holland SM, Thurman RG, Mason RP. In vivo lipid-derived free radical formation by NADPH oxidase in acute lung injury induced by lipopolysaccharide: a model for ARDS. FASEB J 16: 1713–1720, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Schrοedinger L. The Pymol Molecular Graphics System, Version 1.1. DeLano Scientific, 2003–2008 [Google Scholar]
- 30.Segal BH, Davidson BA, Hutson AD, Russo TA, Holm BA, Mullan B, Habitzruther M, Holland SM, Knight PR. Acid aspiration-induced lung inflammation and injury are exacerbated in NADPH oxidase-deficient mice. Am J Physiol Lung Cell Mol Physiol 292: L760–L768, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Shuvaev VV, Tliba S, Nakada M, Albelda SM, Muzykantov VR. Platelet-endothelial cell adhesion molecule-1-directed endothelial targeting of superoxide dismutase alleviates oxidative stress caused by either extracellular or intracellular superoxide. J Pharmacol Exp Ther 323: 450–457, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Smeding L, Plotz FB, Lamberts RR, van der Laarse WJ, Kneyber MC, Groeneveld AB. Mechanical ventilation with high tidal volumes attenuates myocardial dysfunction by decreasing cardiac edema in a rat model of LPS-induced peritonitis. Respir Res 13: 1465–9921, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Voyta JC, Via DP, Butterfield CE, Zetter BR. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol 99: 2034–2040, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu K, Tian S, Zhou H, Wu Y. Statins protect human endothelial cells from TNF-induced inflammation via ERK5 activation. Biochem Pharmacol 85: 1753–1760, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Yamada M, Gomez JC, Chugh PE, Lowell CA, Dinauer MC, Dittmer DP, Doerschuk CM. Interferon-gamma production by neutrophils during bacterial pneumonia in mice. Am J Respir Crit Care Med 183: 1391–1401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye L, Yong KT, Liu L, Roy I, Hu R, Zhu J, Cai H, Law WC, Liu J, Wang K, Liu J, Liu Y, Hu Y, Zhang X, Swihart MT, Prasad PN. A pilot study in non-human primates shows no adverse response to intravenous injection of quantum dots. Nat Nano 7: 453–458, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Zhu T, Wang DX, Zhang W, Liao XQ, Guan X, Bo H, Sun JY, Huang NW, He J, Zhang YK, Tong J, Li CY. Andrographolide protects against LPS-induced acute lung injury by inactivation of NF-kappaB. PLoS One 8: 21, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zrazhevskiy P, Gao X. Quantum dot imaging platform for single-cell molecular profiling (Abstract). Nat Commun 4: 1619, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]